
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Immunol. , 29 August 2024
Sec. Cancer Immunity and Immunotherapy
Volume 15 - 2024 | https://doi.org/10.3389/fimmu.2024.1467306
This article is part of the Research Topic The Role of the Microbiome in Modulating Response and Adverse Events in Solid Tumor Immunotherapy Treated Patients View all 5 articles
Background: Immune checkpoint inhibitors have demonstrated promising therapeutic outcomes in recurrent/metastatic (R/M) Head and Neck Squamous Cell Carcinoma (HNSCC), prompting numerous clinical trials to investigate the safety and efficacy of this approach in neoadjuvant therapy. This systematic review aims to consolidate and analyze the findings from various clinical trials combining neoadjuvant immunotherapy for HNSCC, with the goal of identifying the most effective neoadjuvant immunotherapy regimen.
Methods: The system conducted searches across electronic databases including PubMed, Embase, the Cochrane Library and Web of science from their inception to July 1, 2024. The primary focus was on evaluating efficacy (particularly pathological complete response (pCR), major pathological response (MPR), and overall response rate (ORR)) and safety (primarily assessed by grade 3-4 treatment-related adverse reactions).
Results: A total of 1943 patients from 32 studies were analyzed. Combining neoadjuvant immunotherapy with chemotherapy or radiotherapy demonstrated superiority over neoadjuvant immunotherapy alone in terms of the MPR rate, while showing no statistically significant difference in the pCR rate. Furthermore, the combination of neoadjuvant immunotherapy with chemotherapy or radiotherapy exhibited a lower CR rate compared to neoadjuvant immunotherapy with radiotherapy alone, but a higher PR rate and SD rate. Apart from the neoadjuvant immunotherapy group in isolation, there were no statistically significant differences in grade ≥3 treatment-related adverse events (TRAEs) and immune-related adverse events (irAEs) among the other three combination therapy groups.
Conclusion: This systematic review and meta-analysis indicate that patients with locally advanced HNSCC might benefit from neoadjuvant immunotherapy, particularly when used in conjunction with chemotherapy or radiotherapy. Nonetheless, additional data is required to definitively confirm its efficacy.
Systematic Review Registration: https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=553753, identifier CRD42024553753.
Head and neck squamous cell carcinoma (HNSCC) arises in the mucosal epithelium of the oral cavity, pharynx, and larynx, representing the most prevalent form of cancer within the head and neck. This region is anatomically intricate, serving crucial roles in essential functions such as eating, speaking, and breathing (1). The majority of HNSCC patients receive a diagnosis of localized or locally advanced disease, with standard treatment typically involving a combination of radiotherapy, surgery, and possibly chemotherapy tailored to individual risk levels (2). However, individuals diagnosed with locally advanced HNSCC face a significant risk of both local recurrence (approximately 15-40%) and distant metastasis, with a 5-year overall survival rate of only 50% (3). While platinum-based chemotherapy, like the Docetaxel + cisplatin + 5-fluorouracil (5-FU) regimen, is the standard neoadjuvant treatment for HNSCC patients, research indicates that these strategies may not always effectively extend patient survival or prevent progression due to insensitivity or resistance to these chemotherapeutic agents (4–6). Novel treatment approaches are essential to enhance survival rates or lessen the burden of conventional therapies.
Recently, the academic community has increasingly acknowledged the efficacy of immune checkpoint inhibitors, specifically monoclonal antibodies targeting programmed cell death-1 (PD-1) and programmed cell death ligand-1 (PD-L1), in managing relapsed or metastatic HNSCCs. Preclinical studies indicate that neoadjuvant PD-1/PD-L1 pathway blockade may be more effective than adjuvant blockade, leveraging tumor antigens within the preoperative immune environment for enhanced efficacy (7, 8). In a phase Ib study, the effectiveness and safety of neoadjuvant immunoradiotherapy in patients with locally advanced HNSCC were highlighted, demonstrating an MPR of 86%, a complete pathologic response of 67%, and a clinical-to-pathologic downstaging rate of 90% (9). Several current trials investigating neoadjuvant immunotherapy for HNSCC, focusing on single or dual immunotherapy, as well as combinations with chemotherapy or radiotherapy, have displayed encouraging outcomes (10–12).
This meta-analysis endeavors to gather findings from current clinical studies to evaluate the effectiveness and safety of various neoadjuvant immunotherapy combination treatments for managing locally advanced HNSCC, offering additional clinical treatment alternatives.
This systematic review adheres to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (13). The comprehensive protocol has been registered online with the International Prospective Register of Systematic Reviews (PROSPERO: CRD42024553753). As this review and meta-analysis did not involve the use of individual patient data, it was not subject to institutional review board approval.
We systematically searched databases including PubMed, Embase, the Cochrane Library and Web of science for relevant studies published before July 2024 concerning neoadjuvant immunotherapy in patients with HNSCC (refer to Supplementary Materials for the search strategy). Additionally, we sought unpublished data from ongoing clinical trials on neoadjuvant immunotherapy in HNSCC patients presented at major international oncology conferences such as the American Society of Clinical Oncology and the European Society of Oncology Medicine.
This analysis included clinical trials investigating immunotherapy as a neoadjuvant intervention in HNSCC patients without distant metastases. Patients with potentially curable primary lesions in the oral cavity, oropharynx, hypopharynx, and larynx (excluding the nasopharynx) were considered. Two researchers (CL and MZL) independently screened and extracted articles for potential inclusion. In cases of disagreement, a discussion or consultation with a third researcher was conducted to determine study inclusion. Data were meticulously documented and stored in an Excel spreadsheet. Parameters were extracted in a standardized format, including details such as the first author, publication year, approval number, study design (single-arm or randomized controlled trial), pathological stage, treatment regimen, sample size, age distribution, gender ratio, pathological complete response (pCR), major pathological response (MPR), R0 resection rate, incidence of grade 3 or higher treatment-related adverse events (TRAEs), complete response (CR), partial response (PR), overall response rate (ORR), stable disease (SD), disease control rate (DCR), and other relevant factors.
The meta-analysis was conducted utilizing non-comparative binary data from RevMan software version 5.4 (Cochrane Collaboration), given that the majority of studies were single-arm clinical trials. Effect indicators such as odds ratios (ORs) and their corresponding 95% confidence intervals (CIs) were employed. Subgroup analysis was carried out based on different combination treatment approaches. Statistical heterogeneity was assessed using the Cochran Q chi-square test and the inconsistency index. In cases where study heterogeneity was low (P > 0.1, I2 < 50%), a fixed-effect model was applied. Conversely, if significant heterogeneity was present, the random-effects model was utilized.
The two reviewers utilized the MINORS scale to evaluate the study quality. This scale is specifically tailored for assessing non-randomized studies and comprises 8 criteria, each rated on a scale of 0-2, resulting in a total score of 16. Studies scoring between 13-16 points were classified as high-quality, those scoring 9-12 points were deemed moderate quality (and included in the final analysis and data extraction), while studies scoring below 9 points were regarded as low quality and therefore excluded from the analysis.
The PRISMA diagram illustrating the selection process is detailed in Figure 1. Following the search strategy, a total of 1649 studies were screened, with 88 duplicates removed. Among the 32 selected studies, encompassing 1943 patients, all met the criteria for inclusion in the final meta-analysis. Notably, four of these studies were in the form of conference abstracts. The meta-analysis comprised 23 single-arm clinical studies and 9 randomized controlled trials, categorized based on different combination therapy modalities: 11 (10, 14–23) studies focused on neoadjuvant immunotherapy alone (NI), 12 (1, 11, 24–33) studies on neoadjuvant immunotherapy combined with chemotherapy (NICT), 5 (9, 34–37) studies on neoadjuvant immunotherapy combined with radiotherapy (NIRT), and 4 (12, 38–40) studies on neoadjuvant immunotherapy combined with chemoradiotherapy (NICRT). Table 1 summarizes the key characteristics of the included studies, while the main outcomes are presented in Supplementary Table 1. Additionally, Supplementary Table 2 indicates an overall low risk of bias across the included studies.
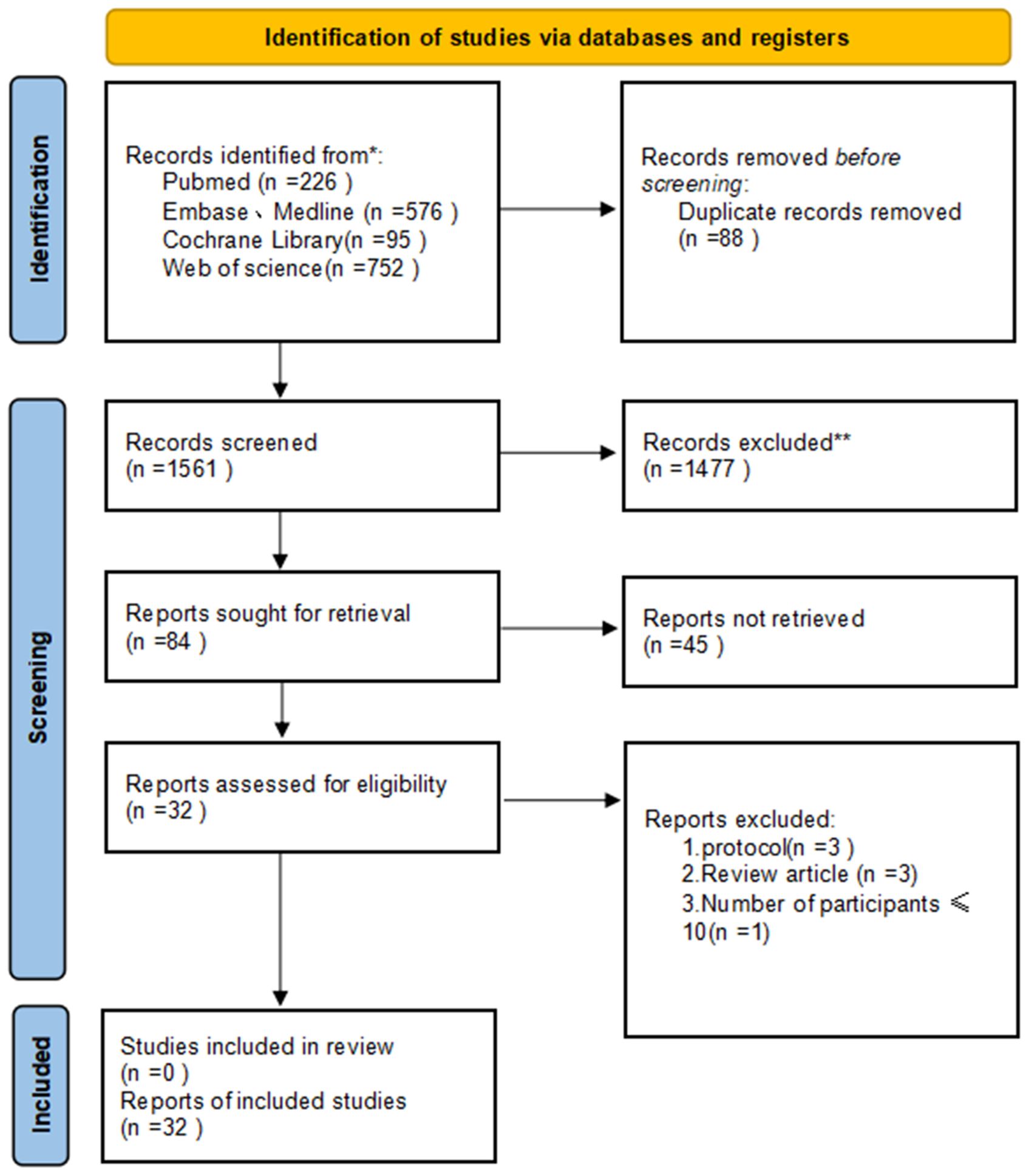
Figure 1. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) diagram of the study selection.
This study primarily assessed the efficacy of neoadjuvant immunotherapy by analyzing MPR and pCR rates. Across the enrolled studies, MPR rates varied widely from 2.9% to 92.9%. Among the 17 qualifying studies, subgroup analysis revealed a notably higher MPR rate in the NIRT group (OR=0.76, 95% CI: 0.60-0.91, P< 0.0001, I2 = 97.3%, Figure 2A) compared to the NI and NICT groups. Furthermore, the 15 studies that reported pCR rates (ranging from 16.7% to 68.2%) indicated that both the NIRT and NICT groups had higher pCR rates than the NI group, although this difference did not reach statistical significance (P=0.54, I2 = 0%, Figure 2B).
Outcome metrics (CR, PR, ORR, SD, DCR) for assessing imaging in clinical trials of antineoplastic agents were performed using Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. Among the included studies, subgroup analysis revealed a higher CR rate in NICRT than in the NICT and NIRT groups (OR=0.65, 95% CI: 0.31-0.99, P= 0.009, I2 = 78.8%, Figure 3A). Meanwhile, the PR rate in the NICT group was higher than the other three groups (OR=0.61, 95% CI: 0.48-0.73, P= 0.0002, I2 = 85.2%, Figure 3B). When evaluating ORR, the NICRT group exhibited a slightly higher ORR rate (OR=0.84, 95% CI: 0.64-1.05, P=0.17, I2 = 40%, as shown in Figure 4A) compared to the other three groups, although this variance did not reach statistical significance. Regarding the SD rate assessment, the NIRT and NI groups demonstrated higher rates overall compared to the other groups (P<0.00001, I2 = 94.3%, Figure 4B). Notably, in evaluating the DCR, it was observed that three studies in the NICT group and one study in the NIRT group achieved a 100% DCR rate.
The R0 resection rate and surgical resection rate serve as crucial metrics for evaluating the efficacy of neoadjuvant immunotherapy. Across the included studies, the average R0 resection rate in the NI group stood at 98.9%, surpassing the rates of 93.3% in the NICT group and 90% in the NIRT group. Moreover, the surgical resection rates in the NI and NICT groups were similar, with a non-significant difference (P=0.51, I2 = 0%, Figure 5).
The safety profile of neoadjuvant immunotherapy was evaluated based on the occurrence of grade 3-5 treatment-related adverse events (TRAEs) as outlined in the National Cancer Institute Common Terminology Criteria for Adverse Events (NCICTCAE16; version 4.0). Among the included clinical studies, 21 reported the frequency of grade 3 and higher adverse events. Subgroup analysis revealed a higher incidence of grade ≥3 TRAEs in the NICRT group compared to the other three groups (OR=0.65, 95% CI: 0.31-0.99, P=0.009, I2 = 78.8%, Figure 6A). Furthermore, 7 studies were analyzed for the occurrence of grade ≥3 immune-related adverse events (irAEs), showing that the incidence was higher in the NI group than in the other three groups (OR=0.36, 95% CI: 0.24-0.48, P=0.002, I2 = 80.1%, Figure 6B).
Despite revisiting the study search, selection, and inclusion criteria, heterogeneity persisted without reduction. To ensure that the outcomes were not unduly impacted by any specific group, a sensitivity analysis was conducted by rearranging the included studies out of sequence. In the examination of individual studies on MPR, the NI group emerged as a key contributor to heterogeneity, despite not carrying the largest weight among all studies. Notably, heterogeneity significantly decreased upon excluding studies from the NI group, yet no statistically significant variance in MPR rates was observed between the NICT and NIRT groups (P=0.31, I2 = 1.5%, Supplementary Figure 1). Similarly, the NICRT group played a pivotal role in the heterogeneity of PR and SD. Following their exclusion, the PR and SD rates in the remaining three groups did not exhibit statistically significant differences (P=0.84, I2 = 0%, Supplementary Figure 2; P=0.40, I2 = 0%, Supplementary Figure 3).
Furthermore, during the sensitivity analysis investigating the safety of neoadjuvant immunotherapy, the NI group was identified as a source of heterogeneity for both the incidence of grade ≥3 TRAEs and irAEs. Upon excluding the NI group, it was revealed that the incidence of grade ≥3 TRAEs and grade ≥3 irAEs within the remaining three groups also did not show statistically significant differences (P=0.18, I2 = 41.2%, Supplementary Figure 4; P=0.28, I2=21.9%, Supplementary Figure 5).
Neoadjuvant therapy using immune checkpoint inhibitors has shown promise across a range of cancer types, including melanoma (41), non-small cell lung cancer (42), and bladder cancer (43). PD-1 inhibitors, specifically nivolumab and pembrolizumab, have been sanctioned for treating recurrent/metastatic HNSCC, showcasing extended OS in contrast to chemotherapy (44–46). Ongoing clinical trials have investigated neoadjuvant immunotherapies, either as standalone treatments or in combination with other medications. This meta-analysis represents the pioneering effort to assess the effectiveness and safety of various neoadjuvant immunotherapy combinations in treating patients with locally advanced HNSCC. Drawing from 32 concise studies involving 1,943 patients, our analysis quantitatively amalgamates the efficacy and safety data concerning neoadjuvant immunotherapy. Through direct subgroup analyses and sensitivity assessments, we observed that both the NICT group (OR=0.62, 95% CI: 0.41-0.84) and the NIRT group (OR=0.76, 95% CI: 0.60-0.91) surpassed the NI group (OR=0.11, 95% CI: 0.05-0.17) in achieving a higher MPR rate. However, there was no statistically significant variance between the NICT and NIRT groups. No statistically significant difference was observed in the pCR rates among the NI, NICT, and NIRT groups upon calculation. When evaluating the clinical imaging outcome metrics, we observed that the NICRT group (OR=0.65, 95% CI: 0.31-0.99) outperformed the NICT group (OR=0.11, 95% CI: 0.02-0.19) and the NIRT group (OR=0.10, 95% CI: -0.01-0.21) in terms of achieving a CR rate. However, there was no statistically significant difference in the PR rate and SD rate among the NI, NICT, and NIRT groups, although they remained higher than the NICRT group. When examining the ORR, while there were numerical discrepancies among the four groups, no statistical differences were detected. ORR serves as a valuable clinical parameter for assessing tumor treatment response through imaging; however, it has limitations, especially in the context of immunotherapy. Inflammatory pseudotumor presents histologically as a benign process characterized by acute and chronic inflammatory cells, exhibiting similar imaging features (47). This occurrence is frequently observed in patients undergoing immunotherapy, attributed to the immune impact of PD-1 inhibitors. The solid mass comprises both tumor and immune cells, resulting in a skewed assessment of ORR. Once more, there was no statistically significant variance in surgical resection rates between the NI and NICT groups.
Furthermore, this meta-analysis evaluating the safety of various neoadjuvant immunotherapies revealed that the NI group exhibited significantly lower rates of grade ≥3 TRAEs compared to the other three groups, while showing notably higher rates of grade ≥3 irAEs than the other three groups. However, no statistical differences were found between the NICT, NIRT, and NICRT groups concerning both grade ≥3 TRAEs and grade ≥3 irAEs. Treatment-related deaths, attributed to general disease, site conditions, and vascular rupture, were identified in a single study. In this study, two patients in the avelumab group experienced such events, while one patient in the placebo group passed away due to acute respiratory failure (12). Other largely controllable adverse events, including hypothyroidism, fatigue, nausea, diarrhea, oral and non-oral pain, rash/psoriasis, myalgia, constipation, cough, elevated creatinine, dyspnea, back spasms, and hypertension, as well as immune-related colitis, hyperbilirubinemia, thrombocytopenia, and proteinuria, did not lead to severe adverse consequences or increased postoperative mortality rates.The main clinical outcomes for patients with tumors are overall survival (OS) and progression-free survival (PFS), both crucial measures assessing the clinical benefits achieved by the patient. In a study by Xia Li et al., the 2-year PFS was 27% (95% CI: 18-36%) in the NI group and 44% (95% CI: 32-56%) in the NICT group, showing a statistically significant difference (P = 0.041). The 2-year OS rates in the NI and NICT groups were 61% (95% CI: 52-70%) and 70% (95% CI: 60-80%), with no statistically significant difference (P = 0.681) (27). The studies included in our meta-analysis had relatively brief follow-up durations. Consequently, the identification of superior treatment options would be facilitated by the availability of randomized controlled trials (RCTs) reporting clinical outcomes over three to five years.
Surgical resection typically stands as the primary option for locally advanced HNSCC (3). A notable ORR post-neoadjuvant therapy indicates a reduced tumor burden, making it conducive for surgical intervention. The scope of surgical resection is guided by pre-neoadjuvant imaging assessments. Further exploration is warranted to ascertain if post-treatment imaging can inform adjustments to the surgical approach and if patients achieving CR can be managed with radiotherapy alone, bypassing surgery. HPV infection serves as a significant oncogenic factor in HNSCC and is recognized as a positive prognostic indicator for the survival of HNSCC patients undergoing conventional chemotherapy and radiotherapy. Through transcriptomic analysis of 280 HNSCC cases from the TCGA database, it was observed that HPV-positive tumors demonstrated heightened immunogenicity compared to HPV-negative tumors, characterized by increased infiltration of activated CD8+ T cells (1). This underscores the role of HPV infection in stimulating the immune response. Nevertheless, the extent to which HPV-infected patients may derive greater benefits from immunotherapy remains largely unexplored.
The meta-analysis faced several limitations. Firstly, a portion of the data included was derived from ongoing trials or conference abstracts. Secondly, the absence of key indicators in the studies and the absence of randomized clinical trials were significant drawbacks. Moreover, the diversity in treatment protocols, use of different immunotherapeutic agents, variations in primary tumor sites, HPV status, and patient characteristics all contributed to heterogeneity, potentially diminishing the robustness of the conclusions. Furthermore, the assessment of treatment safety should encompass surgical complexity and postoperative complications. Lastly, the systematic reporting of long-term prognostic factors like OS was lacking.
This systematic review and meta-analysis indicate that patients with locally advanced HNSCC might benefit from neoadjuvant immunotherapy, particularly when used in conjunction with chemotherapy or radiotherapy. Nonetheless, additional data is required to definitively confirm its efficacy.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
CL: Conceptualization, Formal Analysis, Investigation, Methodology, Software, Validation, Writing – original draft, Writing – review & editing. ML: Conceptualization, Data curation, Formal Analysis, Methodology, Software, Validation, Writing – review & editing. XL: Methodology, Project administration, Software, Supervision, Validation, Visualization, Writing – review & editing. TS: Methodology, Project administration, Supervision, Validation, Writing – review & editing. YW: Project administration, Resources, Supervision, Validation, Writing – review & editing. CS: Project administration, Supervision, Validation, Writing – review & editing. WZ: Project administration, Supervision, Validation, Visualization, Writing – review & editing. BW: Project administration, Supervision, Validation, Visualization, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1467306/full#supplementary-material
Supplementary Figure 1 | Sensitivity analysis of MPR.
Supplementary Figure 2 | Sensitivity analysis of PR.
Supplementary Figure 3 | Sensitivity analysis of SD.
Supplementary Figure 4 | Sensitivity analysis of ≥3 TRAEs.
Supplementary Figure 5 | Sensitivity analysis of ≥3 irAEs.
1. Wu D, Li Y, Xu P, Fang Q, Cao F, Lin H, et al. Neoadjuvant chemo-immunotherapy with camrelizumab plus nab-paclitaxel and cisplatin in resectable locally advanced squamous cell carcinoma of the head and neck: a pilot phase II trial. Nat Commun. (2024) 15:2177. doi: 10.1038/s41467-024-46444-z
2. Machiels JP, René Leemans C, Golusinski W, Grau C, Licitra L, Gregoire V. Squamous cell carcinoma of the oral cavity, larynx, oropharynx and hypopharynx: EHNS-ESMO-ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol: Off J Eur Soc Med Oncol. (2020) 31:1462–75. doi: 10.1016/j.annonc.2020.07.011
4. Chaukar D, Prabash K, Rane P, Patil VM, Thiagarajan S, Ghosh-Laskar S, et al. Prospective phase II open-Label randomized controlled trial to compare mandibular preservation in upfront surgery with neoadjuvant chemotherapy followed by surgery in operable oral cavity cancer. J Clin Oncol: Off J Am Soc Clin Oncol. (2022) 40:272–81. doi: 10.1200/JCO.21.00179
5. Zorat PL, Paccagnella A, Cavaniglia G, Loreggian L, Gava A, Mione CA, et al. Randomized phase III trial of neoadjuvant chemotherapy in head and neck cancer: 10-year follow-up. J Natl Cancer Inst. (2004) 96:1714–7. doi: 10.1093/jnci/djh306
6. Paccagnella A, Orlando A, Marchiori C, Zorat PL, Cavaniglia G, Sileni VC, et al. Phase III trial of initial chemotherapy in stage III or IV head and neck cancers: a study by the Gruppo di Studio sui Tumori della Testa e del Collo. J Natl Cancer Inst. (1994) 86:265–72. doi: 10.1093/jnci/86.4.265
7. Friedman J, Moore EC, Zolkind P, Robbins Y, Clavijo PE, Sun L, et al. Neoadjuvant PD-1 immune checkpoint blockade reverses functional immunodominance among tumor antigen-Specific T cells. Clin Cancer Res: an Off J Am Assoc Cancer Res. (2020) 26:679–89. doi: 10.1158/1078-0432.CCR-19-2209
8. Blank CU, Rozeman EA, Fanchi LF, Sikorska K, van de Wiel B, Kvistborg P, et al. Neoadjuvant versus adjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma. Nat Med. (2018) 24:1655–61. doi: 10.1038/s41591-018-0198-0
9. Leidner R, Crittenden M, Young K, Xiao H, Wu Y, Couey MA, et al. Neoadjuvant immunoradiotherapy results in high rate of complete pathological response and clinical to pathological downstaging in locally advanced head and neck squamous cell carcinoma. J Imunother Cancer. (2021) 9. doi: 10.1136/jitc-2021-002485
10. Kim CG, Hong MH, Kim DH, Lim SM, Lee B, Bang YJ, et al. Preoperative durvalumab (D) with or without tremelimumab (T) for resectable head and neck squamous cell carcinoma (HNSCC): Updated results with high dimensional profiling of circulating immune cells. J Clin Oncol. (2022) 40. doi: 10.1200/JCO.2022.40.16_suppl.6072
11. Zinner R, Johnson JM, Tuluc M, Curry JM, Luginbuhl A, Fundakowski CC, et al. Neoadjuvant nivolumab (N) plus weekly carboplatin (C) and paclitaxel (P) in resectable locally advanced head and neck cancer. J Clin Oncol. (2020) 38. doi: 10.1200/JCO.2020.38.15_suppl.6583
12. Lee NY, Ferris RL, Psyrri A, Haddad RI, Tahara M, Bourhis J, et al. Avelumab plus standard-of-care chemoradiotherapy versus chemoradiotherapy alone in patients with locally advanced squamous cell carcinoma of the head and neck: a randomised, double-blind, placebo-controlled, multicentre, phase 3 trial. Lancet Oncol. (2021) 22:450–62. doi: 10.1016/S1470-2045(20)30737-3
13. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. Open Med: peer-rev independent open-access J. (2009) 3:e123–130. doi: 10.1371/journal.pmed.1000097
14. Ferrarotto R, Bell D, Rubin ML, Hutcheson KA, Johnson JM, Goepfert RP, et al. Impact of neoadjuvant durvalumab with or without tremelimumab on CD8(+) tumor lymphocyte density, safety, and efficacy in patients with oropharynx cancer: CIAO trial results. Clin Cancer Res: an Off J Am Assoc Cancer Res. (2020) 26:3211–9. doi: 10.1158/1078-0432.CCR-19-3977
15. Uppaluri R, Campbell KM, Egloff AM, Zolkind P, Skidmore ZL, Nussenbaum B, et al. Neoadjuvant and adjuvant pembrolizumab in resectable locally advanced, human papillomavirus-Unrelated head and neck cancer: A multicenter, phase II trial. Clin Cancer Res: an Off J Am Assoc Cancer Res. (2020) 26:5140–52. doi: 10.1158/1078-0432.CCR-20-1695
16. Zuur L, Vos JL, Elbers JB, Krijgsman O, Qiao X, van der Leun A, et al. Neoadjuvant nivolumab and nivolumab plus ipilimumab induce (near-) complete responses in patients with head and neck squamous cell carcinoma: The IMCISION trial. Ann Oncol. (2020) 31:S1169–9. doi: 10.1016/j.annonc.2020.08.2270
17. Ferrarotto R, Amit M, Nagarajan P, Rubin ML, Yuan Y, Bell D, et al. Pilot phase II trial of neoadjuvant immunotherapy in locoregionally advanced, resectable cutaneous squamous cell carcinoma of the head and neck. Clin Cancer Res: an Off J Am Assoc Cancer Res. (2021) 27:4557–65. doi: 10.1158/1078-0432.CCR-21-0585
18. Ferris RL, Spanos WC, Leidner R, Gonçalves A, Martens UM, Kyi C, et al. Neoadjuvant nivolumab for patients with resectable HPV-positive and HPV-negative squamous cell carcinomas of the head and neck in the CheckMate 358 trial. J Imunother Cancer. (2021) 9. doi: 10.1136/jitc-2021-002568
19. Knochelmann HM, Horton JD, Liu S, Armeson K, Kaczmar JM, Wyatt MM, et al. Neoadjuvant presurgical PD-1 inhibition in oral cavity squamous cell carcinoma. Cell Rep Med. (2021) 2. doi: 10.1016/j.xcrm.2021.100426
20. Vos JL, Elbers JBW, Krijgsman O, Traets JJH, Qiao X, van der Leun AM, et al. Neoadjuvant immunotherapy with nivolumab and ipilimumab induces major pathological responses in patients with head and neck squamous cell carcinoma. Nat Commun. (2021) 12:7348.
21. Hanna GJ, O’Neill A, Shin K-Y, Wong K, Jo VY, Quinn CT, et al. Neoadjuvant and adjuvant nivolumab and lirilumab in patients with recurrent, resectable squamous cell carcinoma of the head and neck. Clin Cancer Res. (2022) 28:468–78. doi: 10.1158/1078-0432.CCR-21-2635
22. Ju W-t, Xia R-h, Zhu D-w, Dou S-j, Zhu G-p, Dong M-j, et al. A pilot study of neoadjuvant combination of anti-PD-1 camrelizumab and VEGFR2 inhibitor apatinib for locally advanced resectable oral squamous cell carcinoma. Nat Commun. (2022) 13. doi: 10.1038/s41467-022-33080-8
23. Wise-Draper TM, Gulati S, Palackdharry S, Hinrichs BH, Worden FP, Old MO, et al. Phase II clinical trial of neoadjuvant and adjuvant pembrolizumab in resectable local-Regionally advanced head and neck squamous cell carcinoma. Clin Cancer Res: an Off J Am Assoc Cancer Res. (2022) 28:1345–52. doi: 10.1158/1078-0432.CCR-21-3351
24. Zinner R, Johnson JM, Tuluc M, Curry JM, Luginbuhl A, Fundakowski C, et al. Neoadjuvant nivolumab (N) plus weekly carboplatin (C) and paclitaxel (P) outcomes in HPV(-) resectable locally advanced head and neck cancer. Ann Oncol. (2020) 31:S682–2. doi: 10.1016/j.annonc.2020.08.1083
25. Hecht M, Gostian AO, Eckstein M, Rutzner S, von der Grün J, Illmer T, et al. Safety and efficacy of single cycle induction treatment with cisplatin/docetaxel/durvalumab/tremelimumab in locally advanced HNSCC: first results of CheckRad-CD8. J Imunother Cancer. (2020) 8. doi: 10.1136/jitc-2020-001378
26. Hellwig K, Ellmann S, Eckstein M, Wiesmueller M, Rutzner S, Semrau S, et al. Predictive value of multiparametric MRI for response to single-Cycle induction chemo-Immunotherapy in locally advanced head and neck squamous cell carcinoma. Front Oncol. (2021) 11. doi: 10.3389/fonc.2021.734872
27. Li X, Fang Q, Du W, Zhang X, Dai L, Qiao Y. Induction chemotherapy combined with immunotherapy in locally advanced head and neck squamous cell carcinoma. BMC Cancer. (2021) 21:622. doi: 10.1186/s12885-021-08373-8
28. Hecht M, Eckstein M, Rutzner S, von der Grün J, Illmer T, Klautke G, et al. et al. Induction chemoimmunotherapy followed by CD8+ immune cell-based patient selection for chemotherapy-free radioimmunotherapy in locally advanced head and neck cancer. J Imunother Cancer. (2022) 10. doi: 10.1136/jitc-2021-003747
29. Huang X, Liu Q, Zhong G, Peng Y, Liu Y, Liang L, et al. et al. Neoadjuvant toripalimab combined with gemcitabine and cisplatin in resectable locally advanced head and neck squamous cell carcinoma (NeoTGP01): An open label, single-arm, phase Ib clinical trial. J Exp Clin Cancer Res. (2022) 41. doi: 10.1186/s13046-022-02510-2
30. Zhang Z, Wu B, Peng G, Xiao G, Huang J, Ding Q, et al. Neoadjuvant chemoimmunotherapy for the treatment of locally advanced head and neck squamous cell carcinoma: A single-arm phase 2 clinical trial. Clin Cancer Res: an Off J Am Assoc Cancer Res. (2022) 28:3268–76. doi: 10.1158/1078-0432.CCR-22-0666
31. Wang K, Gui L, Lu H, He X, Li D, Liu C, et al. Efficacy and safety of pembrolizumab with preoperative neoadjuvant chemotherapy in patients with resectable locally advanced head and neck squamous cell carcinomas. Front Immunol. (2023) 14:1189752. doi: 10.3389/fimmu.2023.1189752
32. Wang H, Wang X, Zhou X, Li Y, Wang P, Zhang X, et al. Neoadjuvant PD-1 inhibitor combined with Nab-paclitaxel and cisplatin in resectable locally advanced head and neck squamous cell carcinoma (NCT05522985): A randomized, controlled, open label, phase II clinical trial. Ann Oncol. (2023) 34:S571–2. doi: 10.1016/j.annonc.2023.09.2040
33. Wang HL, Yue K, Wu YS, Duan YS, Jing C, Wang XD. [Phase II clinical trial of PD-1 inhibitor combined with chemotherapy for locally advanced resectable oral squamous cell carcinoma]. Zhonghua er bi yan hou tou jing wai ke za zhi = Chin J Otorhinolaryngol Head Neck Surg. (2024) 59:335–42.
34. Darragh LB, Knitz MM, Hu J, Clambey ET, Backus J, Dumit A, et al. A phase I/Ib trial and biological correlate analysis of neoadjuvant SBRT with single-dose durvalumab in HPV-unrelated locally advanced HNSCC. Nat Cancer. (2022) 3:1300–17. doi: 10.1038/s43018-022-00450-6
35. Shen P, Qiao B, Jin N, Wang S. Neoadjuvant immunoradiotherapy in patients with locally advanced oral cavity squamous cell carcinoma: a retrospective study. Invest New Drugs. (2022) 40:1282–9. doi: 10.1007/s10637-022-01293-9
36. Johnson JM, Vathiotis IA, Harshyne LA, Ali A, Bar Ad V, Axelrod R, et al. et al. Nivolumab and ipilimumab in combination with radiotherapy in patients with high-risk locally advanced squamous cell carcinoma of the head and neck. J Imunother Cancer. (2023) 11. doi: 10.1136/jitc-2023-007141
37. Mell LK, Torres-Saavedra P, Wong S, Chang S, Kish JA, Minn AJ, et al. Radiotherapy with durvalumab vs. Cetuximab in patients with locoregionally advanced head and neck cancer and a contraindication to cisplatin: phase II results of NRG-HN004. Int J Radiat Oncol Biol Phys. (2022) 114:1058. doi: 10.1016/j.ijrobp.2022.09.003
38. Powell SF, Gold KA, Gitau MM, Sumey CJ, Lohr MM, McGraw SC, et al. Safety and efficacy of pembrolizumab with chemoradiotherapy in locally advanced head and neck squamous cell carcinoma: A phase IB study. J Clin Oncol: Off J Am Soc Clin Oncol. (2020) 38:2427–37. doi: 10.1200/JCO.19.03156
39. Tao Y, Auperin A, Sun X, Sire C, Martin L, Coutte A, et al. Avelumab-cetuximab-radiotherapy versus standards of care in locally advanced squamous-cell carcinoma of the head and neck: The safety phase of a randomised phase III trial GORTEC 2017-01 (REACH). Eur J Cancer. (2020) 141:21–9. doi: 10.1016/j.ejca.2020.09.008
40. Machiels JP, Tao Y, Licitra L, Burtness B, Tahara M, Rischin D, et al. Pembrolizumab plus concurrent chemoradiotherapy versus placebo plus concurrent chemoradiotherapy in patients with locally advanced squamous cell carcinoma of the head and neck (KEYNOTE-412): a randomised, double-blind, phase 3 trial. Lancet Oncol. (2024) 25:572–87.
41. Amaria RN, Reddy SM, Tawbi HA, Davies MA, Ross MI, Glitza IC, et al. Neoadjuvant immune checkpoint blockade in high-risk resectable melanoma. Nat Med. (2018) 24:1649–54.
42. Forde PM, Chaft JE, Smith KN, Anagnostou V, Cottrell TR, Hellmann MD, et al. Neoadjuvant PD-1 blockade in resectable lung cancer. New Engl J Med. (2018) 378:1976–86. doi: 10.1056/NEJMoa1716078
43. Necchi A, Anichini A, Raggi D, Briganti A, Massa S, Lucianò R, et al. Pembrolizumab as neoadjuvant therapy before radical cystectomy in patients with muscle-Invasive urothelial bladder carcinoma (PURE-01): an open-Label, single-Arm, phase II study. J Clin Oncol: Off J Am Soc Clin Oncol. (2018) 36:3353–60. doi: 10.1200/JCO.18.01148
44. Ferris RL, Blumenschein G Jr., Fayette J, Guigay J, Colevas AD, Licitra L, et al. Nivolumab for recurrent squamous-Cell carcinoma of the head and neck. New Engl J Med. (2016) 375:1856–67. doi: 10.1056/NEJMoa1602252
45. Seiwert TY, Burtness B, Mehra R, Weiss J, Berger R, Eder JP, et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial. Lancet Oncol. (2016) 17:956–65. doi: 10.1016/S1470-2045(16)30066-3
46. Burtness B, Harrington KJ, Greil R, Soulières D, Tahara M, de Castro G Jr., et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet (London England). (2019) 394:1915–28.
Keywords: HNSCC, neoadjuvant immunotherapy, efficacy, safety, meta-analysis
Citation: Liu C, Li M, Liu X, Shi T, Wang Y, Sui C, Zhang W and Wang B (2024) Evaluating the efficacy and safety of different neoadjuvant immunotherapy combinations in locally advanced HNSCC: a systematic review and meta-analysis. Front. Immunol. 15:1467306. doi: 10.3389/fimmu.2024.1467306
Received: 19 July 2024; Accepted: 12 August 2024;
Published: 29 August 2024.
Edited by:
Michael G. White, University of Texas MD Anderson Cancer Center, United StatesReviewed by:
Priyanka S. Rana, Case Western Reserve University, United StatesCopyright © 2024 Liu, Li, Liu, Shi, Wang, Sui, Zhang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bowen Wang, eXRzeXl3YndAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.