
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol., 18 November 2024
Sec. Cancer Immunity and Immunotherapy
Volume 15 - 2024 | https://doi.org/10.3389/fimmu.2024.1466704
This article is part of the Research TopicNeuro-Immune Interplay: Unraveling the Complexities of Neurological Complications and ImmunologyView all 7 articles
 Sapir Bar Mucha1†
Sapir Bar Mucha1† Ayal Rozenberg1,2†
Ayal Rozenberg1,2† Lilach Gutter Kapon3
Lilach Gutter Kapon3 Alon Gorenshtein4
Alon Gorenshtein4 Esther Ganelin-Cohen5,6
Esther Ganelin-Cohen5,6 Rachel Ben Hayun1
Rachel Ben Hayun1 Nataliya Yarovinsky1
Nataliya Yarovinsky1 Shahar Shelly1,2*
Shahar Shelly1,2*Objective: It is unknown whether delay in diagnosis affects morbidity reportedly in paraneoplastic syndromes (PNS). We aimed to explore various aspects of PNS, including prevalence, clinical characteristics, diagnostic criteria, and treatment outcomes.
Methods: We studied n-PNS diagnosis between 2016 to 2023, and included only patients with positive onconeural antibodies, who developed cancer, and exhibited a recognizable PNS phenotype.
Results: We identified 12 patients with positive Abs and co-occurring cancer, most prevalent PNS antibodies included anti-GAD65, anti-Recoverin and anti-Yo. The most common phenotypes were limbic encephalitis (n=5, 42%) and encephalomyelitis (n=4,33%). Cancer preceded neurological presentation in 6 cases. Among the 6 patients who initially presented with n-PNS, median time from neurological presentation to oncologic diagnosis was 73 days, as five of them (83%) were diagnosed with cancer during oncological evaluation prompted by the PNS diagnosis or suspicion. Lymphoma was the most frequent cancer (n=3, 25%), followed by lung cancer (n=2, 17%), and ovarian cancer (n=2, 17%). Among patients who received immunotherapy as n-PNS treatment (n=9, 75%), steroids were a part of the management at 78% (n=7). Another immunotherapy used included plasmapheresis (n=5, 55%) and steroid sparing immunosuppressant (n=2, 29%). Four (33%) patients had short term therapeutic benefit with improvement or stabilization at mRS ≤ 4. Median Disability-adjusted life years (DALYs), as disease burden value, was 13 years. Death occurred in 9 of the 12 patients, with most cases deaths attributed to cancer progression. Compering to the expected median survival by type and stage of tumor, from 9 deceased patients, 56% (n=5) died younger than expected. Median survival was 410 days (range 29-2738 days), and 152 days since the appearance of n-PNS (range 8-1434 days). There were no differences in survival between patients who initially presented with n-PNS versus cancer (p=0.39).
Conclusion: In up to 8 years of follow up, there was no difference in mortality among patients who presented initially n-PNS. There was a significant decline in the quality of life, most face substantial disability and functional impairment long term.
Paraneoplastic syndromes are infrequent clinical manifestations characterized as diverse autoimmune disorders, displaying a distinct set of clinical features that emerge alongside neoplastic disease process (1–5). Prior to the identification of novel antibodies, paraneoplastic syndromes were thought to affect between 1% and 7.4% of cancer patients (6). Recent advancements in antibody detection have revised these figures, showing that these syndromes now affect an estimated 10-15% of individuals with cancer (1, 7). Paraneoplastic syndromes can manifest as the initial clinical presentation of undetected cancer, during treatment for a newly diagnosed cancer, or as an indication of cancer relapse (1). These syndromes are associated with a range of affected systems, including neurologic, endocrine, dermatologic, musculoskeletal, hematologic, renal, and other systems (6).
The characteristics of neurological symptoms classified the syndrome as paraneoplastic neurologic syndrome (PNS). The most frequently associated with PNS tumors are lung carcinoma, breast cancer, ovarian cancer, and lymphomas (8). Awareness of clinical manifestation and diagnosis of PNS has greatly increased in recent years. In 2021, new diagnostic criteria for PNS were established by the PNS-Care panel (8). Epidemiology trends demonstrated that the incidence of PNS is increasing over time. This trend is likely attributable to increased awareness and the advancement of detection techniques, including the identification of numerous new neuronal antibodies and correlation to existing immunoglobulins like oligoclonal bands (9, 10).
Recent studies highlight a significant increase in morbidity and mortality among patients with PNS (11–13). These findings indicate that these patients often experience survival rates that fall short of expectations based on the type and stage of their cancer, affecting approximately two-thirds of cases (12). Furthermore, PNS patients are observed to accumulate higher total disability-adjusted life years (DALYs) compared to those afflicted with other diseases (11). Despite the diverse outcomes associated with PNS, research on their prevalence and specific risk factors, such as types of tumors, associated antibodies, phenotypes, and treatments, remains scarce with only a few studies addressing these issues (11–13).
In this study, our objective was to identify the risk factors associated with early mortality compared to the median survival expected based on the correlation with tumor type and stage (8). Additionally, we aimed to investigate the risk factors contributing to mortality specifically due to complications arising from PNS. To reach this goal, we made a comparison for many demographic and medical history parameters, clinical presentation parameters, test results, treatment management, quality of follow-up, and accepted scores such as modified Rankin Scale (mRS) and Clinical Dementia Rating (CDR).
This is a retrospective database study conducted in a tertiary referral center Rambam HealthCare Campus (RHC). The RHC institutional review board approved this study, and all patients consented to the use of their medical records for research purposes.
We retrospectively identified all patients who underwent serum or CSF onconeural antibody panel testing at RHC 1/11/2016 to 6/3/2023. The presence of 18 unique antibodies were identified by immunofluorescence (IF) or immunoblot (IB) assays, as detailed in Table 1. Figure 1 (flowchart) shows the study design. We included in the study patients who: diagnosed with either a confirmed malignancy or a probable malignancy with high certainty which could not be confirmed due to the patient’s fragile state; and exhibited a recognizable PNS phenotype as determined by a board-certified neurologist (Table 2). We excluded all patients with other causes of their symptoms.
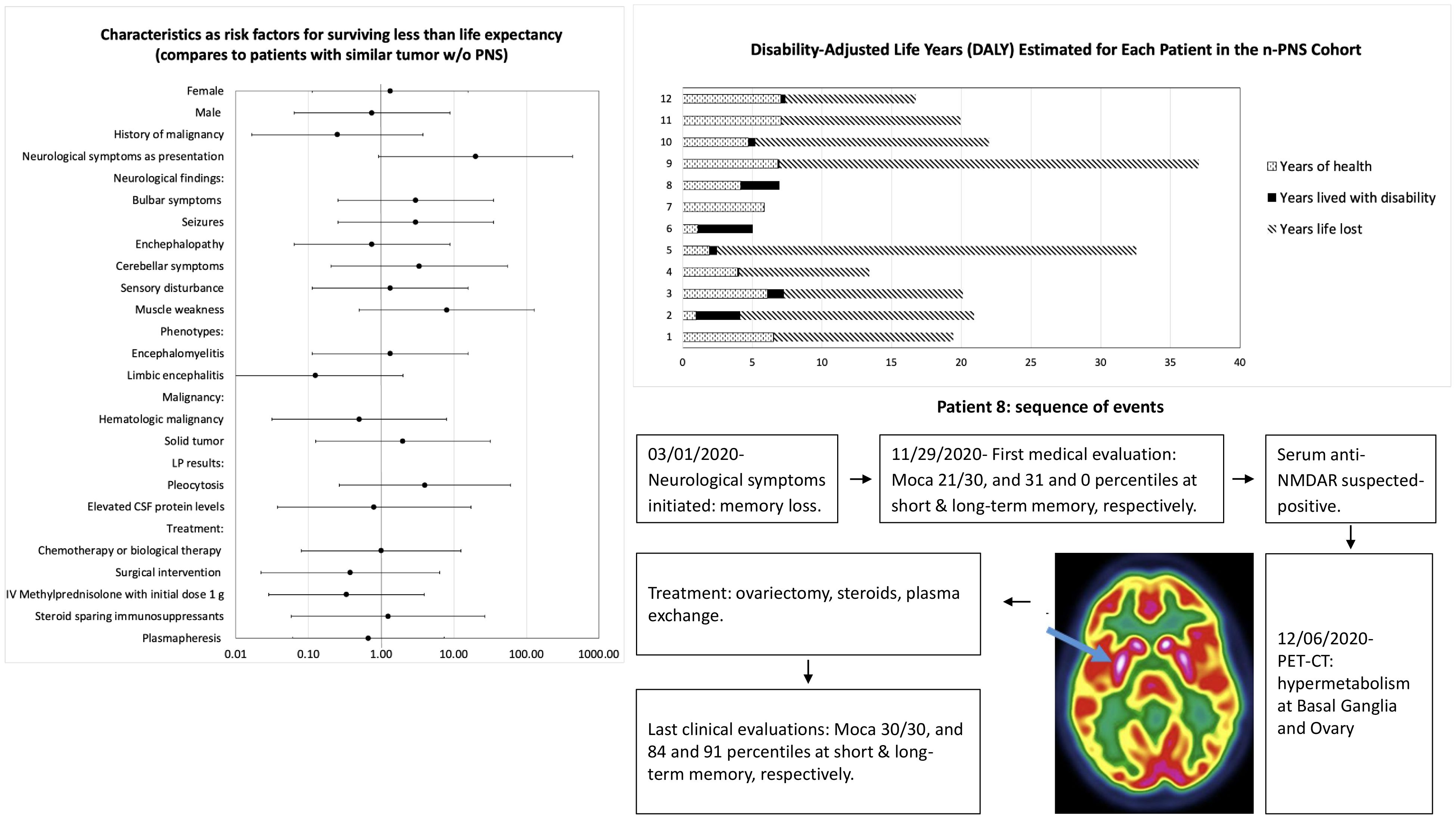
Figure 1. patients (n=535) underwent serum or CSF onconeural antibody panel testing at Rambam Health center between 1/11/2016-6/3/2023. We excluded 488 due to negative antibody test results. 47 patients had non-negative antibody test results included “suspected-positive” and “low-positive” results. 35 excluded due to lack of cancer diagnosis or recognizable PNS phenotype. 12 patients eventually developed cancer and exhibited a recognizable PNS phenotype.
Clinical information obtained through an electronic record retrieval system until 31/12/2023. Patients labeled as smokers if smoking is mentioned in their medical records in the past or at present. The time of PNS diagnosis is defined as the time of receiving the oncological antibodies test result. The time of cancer diagnosis was defined as the time of biopsy for patients who underwent biopsy with pathological findings. In one case, the patient did not undergo a biopsy procedure due to oncologic guidance that recommended initiating treatment without biopsy, in that case, the time of cancer diagnosis was defined as the time imaging was performed.
Autoantibodies were detected in patient serum or cerebrospinal fluid by transfected cell-based immunofluorescence assay (EUROIMMUN, Lubeck, Germany) (14).
The modified Rankin Scale (mRS) was used to assess clinical outcome. The mRS is an ordinal 6-point scoring system that measures neurological disability and has been widely applied to evaluate acute and long-term outcomes in patients with autoimmune encephalitis. mRS score was calculated at 3 time points: before onset, at onset, and at last follow-up. Oncological and neurological outcomes, as reported in the last medical records, were also mentioned in this study and included cancer status and neurological symptoms status as assessed by the attending physicians. Mortality and causes of mortality were reported as noted in medical records. Survival in each case was also compared to the expected median survival by tumor type and stage. We have tested the following variables, morbidity: We examined factors contributing to severe morbidity, as defined by a modified Rankin Scale (mRS) score exceeding a specified threshold (mRS > 3). Therapeutic Benefit: Our analysis will assess determinants of therapeutic benefit, characterized by either improvement or stabilization of patients with a pre-treatment mRS score of ≤4 or improvement in cases with a pre-treatment mRS score of 5 (15). Tumor-Related Survival: We investigated factors influencing tumor-related survival, including the correlation between early mortality and the expected median survival based on tumor type and stage. Mortality due to PNS complications: factors contributing to mortality resulting from complications related to paraneoplastic syndromes (PNS).
Another modality used for morbidity and mortality estimations was a hybrid model of disability-adjusted life years (DALYs), calculated as the summation of incidence-based years of life lost (YLL) and prevalence-based years lived with disability (YLDs) (16). YLL was defined as the standard expected years of life lost based on the age at death from the World Health Organization Global Burden of Disease. Using extensive population-based survey data, Global Burden of Disease (GBD) has provided nonfatal burden estimates including disability weights for neurologic conditions (17, 18). Our data referred to the categories motor impairment, motor and cognitive impairment, epilepsy, major depressive disorder, and intellectual disability, which were applied to individuals in our PNS cohort. For each patient in the cohort, YLD was defined as the cumulative years from neurologic syndrome onset to death, last follow-up, or end of the study period, multiplied by the assigned disability weight.
Group differences were established using Wilcoxon statistics between the means of continuous variables. Survival was analyzed, using Kaplan–Meier, and Cox regression analysis following further grouping of our patients into those who died or survived. P< 0.05 was regarded as statistically significant.
Out of 535 patients, 47 (9%) tested “positive”, “suspected-positive” or “low-positive” for PNS-related antibodies, but only 12 who developed cancer with a clear PNS phenotype were included for analysis. According to the PNS-care score by Graus et al. (5), 42% (n=5) received a definite diagnosis, 33% (n=4) probable, and 25% (n=3) possible. Two possible cases exhibiting lymphoma and presenting with antibodies not listed in Graus et al. criteria were included in the analysis due to their clinically evident neurological phenotypes, which are associated with PNS. All specific test results are shown in Table 2. The follow-up period from symptoms onset until the last medical record or death ranged from 29 days to 7.5 years.
Demographic data and medical history were collected and summarized in Table 3. 42% of all patients were females. The median age at symptoms presentation was 70 years (range 29-81), and the median age at the last follow-up or at death was 73.5 years (range 32-81). Among all patients, almost half (42%, n=5) were smokers at presentation. The history of other malignancy diseases was noted at 33% (n=4) which were bladder transitional cell carcinoma (TCC), renal cell carcinoma, chronic myelogenous leukemia, and larynx malignancy. Coexisting Immunosuppression was reported at 17% (n=2) while in one case due to azathioprine as inflammatory bowel disease treatment, and in the other case due to treatment for a kidney transplant.
The most common neurological complaints were generalized weakness and diplopia, reported in 25% of patients each. Other neurological complaints at presentation included muscle weakness, dysphagia, walking difficulty, paresthesia, loss of consciousness, delirium, muscle pain, vertigo, headache, generalized seizures, cognitive decline, aggression, fall and head injury, and memory decline. Each of these symptoms was reported by at least one patient, with frequencies ranging from 8% to 17%. Neurological examination findings are summarized in Table 3 with the most common findings including muscle weakness in 50% (n=6) of patients, followed by seizures and delirium in 42% (n=5) of patients. All patients in this study were classified as having “high-risk” phenotypes according to the updated criteria for PNS (5). The most observed phenotype was limbic encephalitis, present in 42% of patients (n=5), followed by encephalomyelitis, observed in 33% of patients (n=4). The less commonly observed phenotypes were rapidly progressive cerebellar syndrome, present in 17% of patients (n=2), and sensory neuropathy, observed in 8% of patients (n=1).
PNS was the initial presentation in half of the patients, while the other half had oncologic symptoms prior to the onset of PNS symptoms. The median time from neurological presentation to oncologic diagnosis was 73 days (range 23-373). Among the six patients who initially presented with PNS, five (83%) were diagnosed with cancer during oncological evaluation prompted by the PNS diagnosis or suspicion. In one case, (P6) neurological symptoms were initially misdiagnosed as a psychiatric disorder, leading to a delayed diagnosis of cancer, a year after the onset of neurological symptoms (positive antibodies for anti-Yo). Among the 6 patients whose first presentation was oncological symptoms, data about the time of oncological symptoms initiation was available in 4 patients. Median time from oncological symptoms initiation or cancer diagnosis to neurological symptoms initiation was 664 days (range 36-2728), and to PNS diagnosis was 973.5 days (range 69-2737).
Table 2 categorizes cancer diagnoses by PNS antibody type. Hematological cancer was the most frequent with the following positive antibodies: anti-Recoverin (n=2), anti-GAD65 (n=1), and anti-MA2 (n=1). Data about ancillary tests that led to cancer diagnosis was available for 11 patients, and from 10 of them, a biopsy was done. The most common findings observed at radiology imaging were lymphadenopathy at 91% of cases, skeletal lesions at 45%, and lung consolidation or lesion at 36%. Positive biopsies with malignant histopathological findings were identified in 9 patients. At one patient (P11), the biopsy was not indicative of malignancy, probably due to clinical misdiagnosis. Noteworthy, in cases in which PNS was the first presentation, the median time from neurological presentation to first body screening imaging was 48 days (range 4-372), and the median time to biopsy was 59 days (range 23-289). In terms of oncologic staging, most patients (75%, n=9) were found to have advanced disease.
CSF analysis showed elevated protein levels in 9 out of 11 cases (median CSF protein 70 mg/dl, range 32-232 mg/dl). Patients with normal levels of CSF protein levels had antibodies for NMDAR and Sox1. Pleocytosis was reported at 64% (n=7) of patients (median of 9 cells/mm3, range 0-208 cells/mm3). Patient (P9) with extreme pleocytosis of 208 cells/mm3 had antibodies for CV2. Pathological CSF cytology was reported at 36% (n=4) and showed monoclonal lymphocytosis in one case, polyclonal lymphocytosis in two cases, and pleocytosis with increased red blood cells in one case (P9). The median time from neurological presentation to first brain imaging was 0 days (range 0-262 days), and only two had significant delays (P2 at 91 days and P8 at 262 days due to delay in patient consumption of medical services).
Additional tests were performed including EEG in 6 cases and EMG at 4 cases. Four cases showed exceptional EEG recordings showed slow background activity and in one case epileptiform discharges were obtained. Moreover, these four cases also showed abnormal EMG recording findings which were one or more of the following: axonal sensory and motor polyneuropathy (n=2), demyelinating neuropathy (n=2), ganglionopathy (n=1), and myopathy (n=1). The median time from neurological presentation to the first EEG was 49 days (range 1-334), and to the first EMG was 4 days (range 2-314).
Data on cancer treatment was available for 11 patients. Of these, 27% (n=3) did not receive treatment due to poor prognosis (n=2) or undiagnosed progression and sudden clinical deterioration (n=1). The remaining 8 patients received treatment with a median initiation time of 29 days (range 0-68 days). Chemotherapy or biological therapy was the most common treatment (n=7), followed by surgery (n=3), and radiotherapy (n=1). Stable or responsive disease was observed in 38% (n=3) of treated patients, while 63% (n=5) showed disease progression. Among the 3 patients who underwent surgery, only one showed improvement in PNS symptoms post-operatively. In terms of chemotherapy and neurological symptoms, 3 patients received chemotherapy prior to neurological symptoms, with only one patient developing symptoms shortly after, fifteen days after treatment initiation.
Data on PNS treatment was available for 12 patients. Of these, 25% (n=3) did not receive PNS treatment due to proximity to death (n=2) or misdiagnosis (n=1). Among the 9 patients treated for PNS, 67% (n=6) initiated treatment before receiving positive antibody results, with a median time of -10 days (range -23 to 14 days). Steroids were used in 78% (n=7) of cases, with protocols including 1000mg (n=6), and 500mg (n=1) initial doses of IV Methylprednisolone, as a first-line therapy it was given in 5 cases; and Prednisone at a dose of 60 mg as a first-line therapy (n=1). Steroid sparing immunosuppressants or biological treatment, as additional treatment lines, were given in 2 cases, including Rituximab, Cyclophosphamide, and azathioprine. Plasmapheresis was administered in 5 cases, with 2 as a complementary treatment to steroids.
The follow-up period from symptoms onset until last medical record or death ranged from 29 days to 7.5 years. Out of 11 patients with available oncological follow-up data, proper oncological follow-up visits were formed at 45% (n=5); lack of follow-up due to recent oncologic diagnosis with poor prognosis was reported at 18% (n=2); and lack of proper follow-up was reported at 36% (n=4). The reason for all non-optimal follow-up visits was poor compliance for scheduling or attending routine follow-up visits (n=4), and even poor compliance for further ambulatory evaluation when abnormal findings emerged (n=3).
Of the 12 patients included in the study who had available neurological follow-up data, only 25% (n=3) received proper neurological follow-up visits. Lack of follow-up was reported in 42% (n=5) of patients due to a recent PNS diagnosis with poor prognosis, while 33% (n=4) of patients reported lack of proper follow-up. The reasons for non-optimal follow-up visits included poor compliance for scheduling or attending routine follow-up visits (n=1), poor compliance for further ambulatory evaluation when abnormal findings emerged (n=1), misdiagnosis (n=2), and/or lack of communication between medical units (n=1).
Disease burden was calculated by the disability-adjusted life years (DALYs) model (Figure 2). Total DALYs for 12 patients with PNS and cancer were 156.4 years, based on total years of life lost (YLL) for patients dying between June 2018 and May 2023 (n=9) of 151 years, plus years lived with disability (YLD) for all patients (n=12) of 5.4 years. Median DALYs were 13 (range 0.008–30), median YLL were 12.8 (range 9.3–30.1), and median YLD were 0.18 (range 0.008–2.58), with individual DALYs, Figure 3. Final oncological status was available for 10 patients. 30% (n=3) of them showed a decrease in cancer size, with complete remission reported in 2 cases, while 70% (n=7) showed an increase in size and dissemination of cancer. Neurological symptoms progression as reported in medical records were available for all patients. 42% (n=5) of them showed stability or improvement in neurological symptoms from the worst presentation to the last medical record, while 58% (n=7) showed worsening symptoms. Stable or improved symptoms were reported in 56% (n=5), one patient (P8) showed full recovery with normal neurological examination and 30/30 at the Montreal Cognitive Assessment score, 2 patients showed partial recovery, and 2 patients had stable symptoms. Out of these patients, 2 patients were treated with one modality (plasmapheresis or Methylprednisolone alone), while others were treated with several modalities.
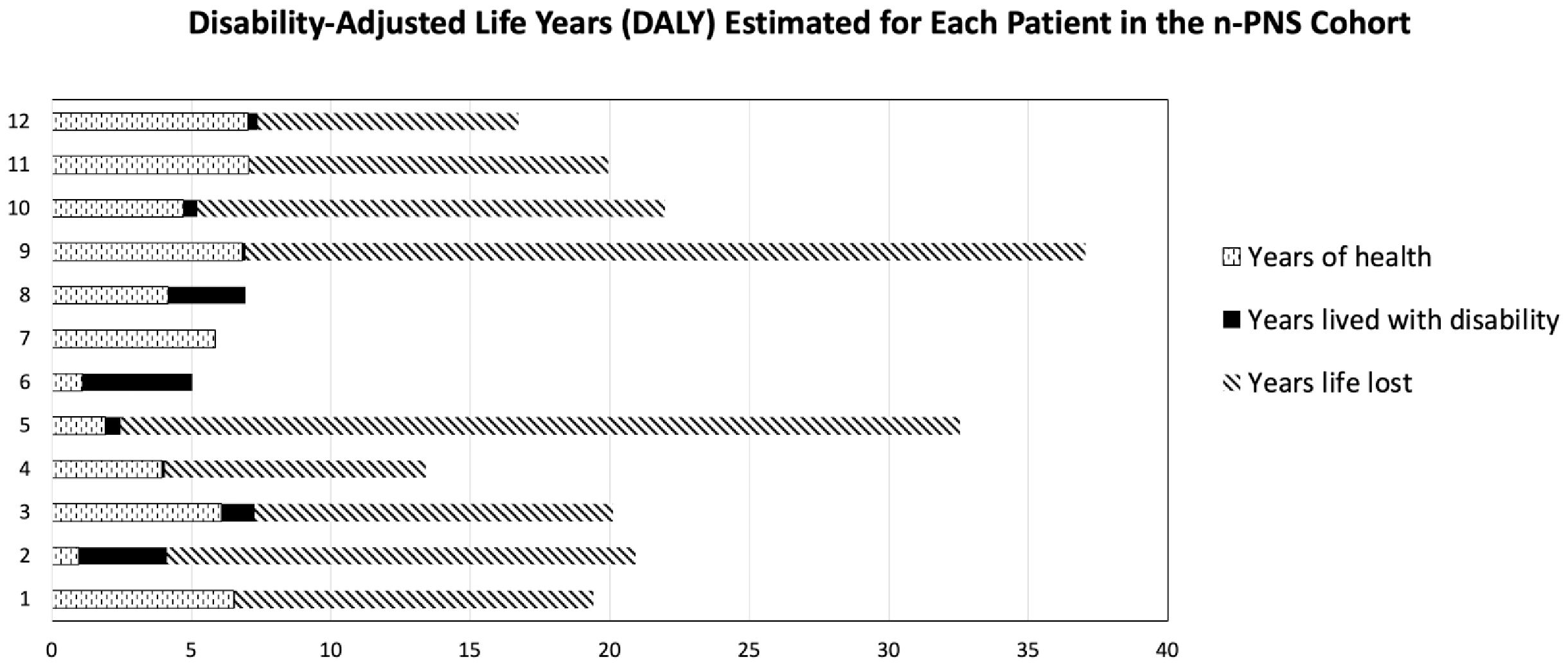
Figure 2. Disability-adjusted life years (DALYs) for each of the 12 patients, based on total years of life lost (YLL) plus years lived with disability (YLD).
Out of all patients, 33% (n=4) had a therapeutic benefit, two patients had improvement at mRS (P2) had an initial score of 4 and final score of 1; P8 had an initial score of 2 and final score of 0), and two patients had unchanged mRS (P6 had an initial and final score of 3; P7 had an initial and final score of 1). The PNS associated antibodies in patients who were presented with therapeutic benefits were anti-Recoverin, anti-Yo, anti-Sox1, and anti-NMDAR.
Death occurred in 75% (n=9) out of all patients, with 67% (n=6) of deaths attributed to cancer progression. The causes of death were one or a combination of the following: respiratory failure in (n=4), multisystem failure in (n=3), septic shock in (n=2), pneumonia (n=2), and unknown cause (n=3). Median survival, as time from first presentation to last medical record or death, was 410 days (range 29-2738 days). Median survival from neurological symptoms initiation to last medical record or death, was 152 days (range 8-1434 days).
To isolate the decline in survival due to PNS only, survival from the first presentation was compared to the expected median survival by type and stage of tumor. All living patients (n=3) had yet to reach the expected survival of their tumors. Of the remaining patients (n=9), 56% (n=5) died younger than the expected median survival, and the rest (n=4) exceeded it. Notably, the survival of three individuals exceeded the expected median by 2 times. Their associated antibodies were anti-Recoverin with T cell lymphoma, multiple antibodies (anti-Yo, anti-ZIC4, anti-NMDAR) with TCC, and anti-GAD65 with leukemia. Table 4 details the survival by tumor diagnosis and the respective expected median survival. There were no statistical differences between patients who were presented with cancer versus PNS, p=0.36.
At last, analysis was performed on all demographic, clinical, testing and outcomes that was collected, in order to seek risk factors for surviving less than life expectancy at patients with PNS, as detailed at Figure 4. Statistically, we did not find any parameter that can be defined as risk factor for poor outcome.
Figure 4. Analysis characteristics as risk factors for surviving less than life expectancy by odds ratio.
PNS are rare with diverse clinical manifestations. We aimed to explore various aspects of PNS, including prevalence, clinical characteristics, diagnostic criteria, and treatment outcomes. We found the prevalence of PNS at 9% (47 out of 535), highlighting an upward trend likely due to improved recognition of many new neural autoantibody markers (19). The most common tumors in our study were lung carcinoma, breast cancer, ovarian cancer, and lymphomas, aligning with previous literature (8). In our cohort, limbic encephalitis and encephalomyelitis were the most common phenotypes observed, underscoring the need to consider these conditions in the differential diagnosis of neurological symptoms in cancer patients.
In some of our cases, PNS was the initial clinical manifestation, leading to delayed cancer diagnoses as neurological symptoms were initially misinterpreted. This emphasizes the need for heightened clinical suspicion and awareness recognizing these symptoms. We showed n-PNS significantly impacted quality of life, with most patients (74%) having substantial disability and functional impairment, affecting their daily lives (Figures 2, 3). Moreover, the mortality burden of PNS and cancer is considerable, with a survival rate below the median expected based on tumor type and stage for 42% of our cohort (Table 4). Outcomes varied depending on the antibodies present. We show the impact of specific antibodies on the prognosis of PNS patients. Anti-Recoverin, anti-NMDAR, and anti-GAD65 antibodies were associated with better survival and manageable neurological outcomes, suggesting a less aggressive disease course or more effective response to treatment. Conversely, anti-Yo and CV2 antibodies were linked to poorer outcomes, reflecting the severe nature of the diseases they accompany
Specifically, anti-Recoverin antibodies were associated with a notably better prognosis. Two patients with these antibodies had survival times exceeding the expected median by more than double, even with aggressive malignancies such as T-cell lymphoma. Cancer-associated retinopathy with recoverin-specific cytotoxic T lymphocytes can recognize and target cancer cells expressing recoverin (20, 21). In our cohort, clinically patients with anti-Recoverin antibodies presented with encephalomyelitis instead of the more commonly associated cancer-associated retinopathy (22). Anti-NMDAR antibodies are typically linked to anti-NMDAR encephalitis, often associated with ovarian teratoma (8).
In our study, two patients with these antibodies had normal CSF protein levels and experienced improved neurological outcomes with stable disease progression, suggesting these antibodies as indicators of manageable disease trajectories. Anti-GAD65 antibodies were observed in a patient who exceeded the median expected survival for leukemia, presenting with limbic encephalitis, a common manifestation associated with high anti-GAD65 titers (23). Patients with low titers of anti-GAD65, part of our inclusion criteria, had concurrent anti-Hu and anti-SOX1 antibodies, with notable findings of cognitive change. Anti-Yo antibodies were linked to poorer prognosis. Patients with these antibodies typically had aggressive cancers and significant neurological impairments. For example, two patients with anti-Yo antibodies showed shorter survival times compared to the expected median. The nature of anti-Yo, an intracellular neuronal antigen, leads to immune-mediated neuronal death, making therapeutic interventions less effective once significant neuronal loss occurs. Lastly, a patient with CV2 antibodies had extreme pleocytosis and experienced rapid cancer progression with worsening neurological symptoms, resulting in shorter survival. CV2 antibodies are strongly associated with small-cell lung cancer or thymoma (24), although our patient had poorly differentiated carcinoma involving multiple organs. We also compared protein CSF levels with patients who exceeded expected survival had higher median CSF protein levels compared to those with less favorable outcomes. Pleocytosis was more frequently observed when CSF studies were conducted early in the disease (25), suggesting these patients were in earlier stages and benefited from timely treatment interventions.
Several limitations should be acknowledged. The retrospective nature of our study might introduce selection bias, and the relatively small sample size limits the generalizability of our findings. Additionally, relying on medical records for data collection could lead to incomplete or missing information.
In conclusion, our study contributes to the evolving understanding of PNS by shedding light on its prevalence, clinical characteristics, and diagnostic challenges. PNS remains a complex and often underdiagnosed condition that demands increased awareness among healthcare providers. Multidisciplinary collaboration between neurologists, oncologists, and other specialists is crucial for improving the management and outcomes of PNS patients. Further research is warranted to delineate the risk factors contributing to morbidity and mortality in this patient population, ultimately guiding more effective treatment strategies. Future research should focus on conducting prospective studies with larger cohorts to further elucidate the risk factors associated with unfavorable PNS outcomes. Additionally, efforts should be made to establish standardized treatment guidelines that address the unique challenges posed by PNS.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
The studies involving humans were approved by Rambam HealthCare Campus institutional review board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
SB: Conceptualization, Data curation, Investigation, Methodology, Validation, Writing – original draft. AR: Conceptualization, Data curation, Investigation, Methodology, Validation, Writing – original draft. LG: Data curation, Writing – review & editing. AG: Data curation, Investigation, Writing – review & editing. EG-C: Investigation, Methodology, Validation, Writing – review & editing. RB: Data curation, Writing – review & editing. NY: Data curation, Writing – review & editing. SS: Conceptualization, Data curation, Formal analysis, Methodology, Writing – original draft.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
We utilized GPT (specifically OpenAI’s GPT-4 model) for grammatical corrections and minor language refinements in our submission.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Gilligan M, McGuigan C, McKeon A. Paraneoplastic neurologic disorders. Curr Neurol Neurosci Rep. (2023) 23:67–82. doi: 10.1007/s11910-023-01250-w
2. Krasowski MD, Dolezal A, Steussy BW, Gailey MP, Darbro BW. Data on the utilization of paraneoplastic syndrome autoantibody testing at an academic medical center. Data Brief. (2021) 39:107578. doi: 10.1016/j.dib.2021.107578
3. Barahman M, Shamsaei G, Kashipazha D, Bahadoram M, Akade E. Paraneoplastic neurological syndromes of small cell lung cancer. Postep Psychiatr Neurol. (2024) 33:80–92. doi: 10.5114/ppn.2024.141157
4. Zhao J, Bhatnagar V, Ding L, Atay SM, David EA, McFadden PM, et al. A systematic review of paraneoplastic syndromes associated with thymoma: Treatment modalities, recurrence, and outcomes in resected cases. J Thorac Cardiovasc Surg. (2020) 160:306–314.e314. doi: 10.1016/j.jtcvs.2019.11.052
5. Candler PM, Hart PE, Barnett M, Weil R, Rees JH. A follow up study of patients with paraneoplastic neurological disease in the United Kingdom. J Neurol Neurosurg Psychiatry. (2004) 75:1411–5. doi: 10.1136/jnnp.2003.025171
6. Soomro Z, Youssef M, Yust-Katz S, Jalali A, Patel AJ, Mandel J. Paraneoplastic syndromes in small cell lung cancer. J Thorac Dis. (2020) 12:6253–63. doi: 10.21037/jtd.2020.03.88
7. Sardina Gonzalez C, Martinez Vivero C, Lopez Castro J. Paraneoplastic syndromes review: The great forgotten ones. Crit Rev Oncol Hematol. (2022) 174:103676. doi: 10.1016/j.critrevonc.2022.103676
8. Graus F, Vogrig A, Muniz-Castrillo S, Antoine JCG, Desetret V, Dubey D, et al. Updated diagnostic criteria for paraneoplastic neurologic syndromes. Neurol Neuroimmunol Neuroinflamm. (2021) 8:e1014. doi: 10.1212/NXI.0000000000001014
9. Vogrig A, Gigli GL, Segatti S, Corazza E, Marini A, Bernardini A, et al. Epidemiology of paraneoplastic neurological syndromes: a population-based study. J Neurol. (2020) 267:26–35. doi: 10.1007/s00415-019-09544-1
10. Rozenberg A, Shelly S, Vaknin-Dembinsky A, Friedman-Korn T, Benoliel- Berman T, Spector P, et al. Cognitive impairments in autoimmune encephalitis: the role of autoimmune antibodies and oligoclonal bands. bands. Front. Immunol. (2022) 27. doi: 10.3389/fimmu.2024.1405337
11. Shah S, Flanagan EP, Paul P, Smith CY, Bryant SC, Devine MF, et al. Population-based epidemiology study of paraneoplastic neurologic syndromes. Neurol Neuroimmunol Neuroinflamm. (2022) 9:e1124. doi: 10.1212/NXI.0000000000001124
12. Chan AM, Baehring JM. Paraneoplastic neurological syndromes: a single institution 10-year case series. J Neurooncol. (2019) 141:431–9. doi: 10.1007/s11060-018-03053-3
13. Giammello F, Galletta K, Grillo F, Brizzi T, Cavallaro M, Mormina E, et al. Paraneoplastic neurological syndromes of the central nervous system: a single institution 7-year case series. Acta Neurol Belg. (2023) 123:1355–69. doi: 10.1007/s13760-023-02232-y
14. Gultekin SH, Rosenfeld MR, Voltz R, Eichen J, Posner JB, Dalmau J. Paraneoplastic limbic encephalitis: neurological symptoms, immunological findings and tumour association in 50 patients. Brain. (2000) 123:1481–94. doi: 10.1093/brain/123.7.1481
15. Pozarowszczyk N, Kurkowska-Jastrzebska I, Sarzynska-Dlugosz I, Nowak M, Karlinski M. Reliability of the modified Rankin Scale in clinical practice of stroke units and rehabilitation wards. Front Neurol. (2023) 14:1064642. doi: 10.3389/fneur.2023.1064642
16. Schroeder SA. Incidence, prevalence, and hybrid approaches to calculating disability-adjusted life years. Popul Health Metr. (2012) 10:19. doi: 10.1186/1478-7954-10-19
17. Salomon JA, Haagsma JA, Davis A, Noordhout CM, Polinder S, Havelaar AH, et al. Disability weights for the Global Burden of Disease 2013 study. Lancet Glob Health. (2015) 3:e712–723. doi: 10.1016/S2214-109X(15)00069-8
18. Stuckler D, King L, Robinson H, McKee M. WHO’s budgetary allocations and burden of disease: a comparative analysis. Lancet. (2008) 372:1563–9. doi: 10.1016/S0140-6736(08)61656-6
19. Patel A, Meng Y, Najjar A, Lado F, Najjar S. Autoimmune encephalitis: A physician’s guide to the clinical spectrum diagnosis and management. Brain Sci. (2022) 12:1130.12. doi: 10.3390/brainsci12091130
20. Maeda A, Ohguro H, Nabeta Y, Hirohashi Y, Sahara H, Maeda T, et al. Identification of human antitumor cytotoxic T lymphocytes epitopes of recoverin, a cancer-associated retinopathy antigen, possibly related with a better prognosis in a paraneoplastic syndrome. Eur J Immunol. (2001) 31:563–72. doi: 10.1002/1521-4141(200102)31:2<563::AID-IMMU563>3.0.CO;2-D
21. Maeda A, Maeda T, Liang Y, Yenerel M, Saperstein DA. Effects of cytotoxic T lymphocyte antigen 4 (CTLA4) signaling and locally applied steroid on retinal dysfunction by recoverin, cancer-associated retinopathy antigen. Mol Vis. (2006) 12:885–91.
22. Dutta Majumder P, Marchese A, Pichi F, Garg I, Agarwal A. An update on autoimmune retinopathy. Indian J Ophthalmol. (2020) 68:1829–37. doi: 10.4103/ijo.IJO_786_20
23. Boronat A, Sabater L, Saiz A, Dalmau J, Graus F. GABA(B) receptor antibodies in limbic encephalitis and anti-GAD-associated neurologic disorders. Neurology. (2011) 76:795–800. doi: 10.1212/WNL.0b013e31820e7b8d
24. Yu Z, Kryzer TJ, Griesmann GE, Kim K, Benarroch EE, Lennon VA. CRMP-5 neuronal autoantibody: marker of lung cancer and thymoma-related autoimmunity. Ann Neurol. (2001) 49:146–54. doi: 10.1002/1531-8249(20010201)49:2<146::AID-ANA34>3.0.CO;2-E
25. Psimaras D, Carpentier AF, Rossi C, Euronetwork PNS. Cerebrospinal fluid study in paraneoplastic syndromes. J Neurol Neurosurg Psychiatry. (2010) 81:42–5. doi: 10.1136/jnnp.2008.159483
26. Awad MM, Leonardi GC, Kravets S, Euronetwork PNS. Impact of MET inhibitors on survival among patients with non-small cell lung cancer harboring MET exon 14 mutations: a retrospective analysis. Lung Cancer. (2019) 133:96–102. doi: 10.1016/j.lungcan.2019.05.011
27. Gianni L, Eiermann W, Semiglazov V, Lluch A, Tjulandin S, Zambetti M, et al. Neoadjuvant and adjuvant trastuzumab in patients with HER2-positive locally advanced breast cancer (NOAH): follow-up of a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet Oncol. (2014) 15:640–7. doi: 10.1016/S1470-2045(14)70080-4
28. Oliver KE, Brady WE, Birrer M, Gershenson DM, Fleming G, Copeland LJ, et al. An evaluation of progression free survival and overall survival of ovarian cancer patients with clear cell carcinoma versus serous carcinoma treated with platinum therapy: An NRG Oncology/Gynecologic Oncology Group experience. Gynecol Oncol. (2017) 147:243–9. doi: 10.1016/j.ygyno.2017.08.004
29. Abramson JS, Feldman T, Kroll-Desrosiers AR, Muffly LS, Winer E, Flowers CR, et al. Peripheral T-cell lymphomas in a large US multicenter cohort: prognostication in the modern era including impact of frontline therapy. Ann Oncol. (2014) 25:2211–7. doi: 10.1093/annonc/mdu443
30. Pardanani A. How I treat patients with indolent and smoldering mastocytosis (rare conditions but difficult to manage). Blood. (2013) 121:3085–94. doi: 10.1182/blood-2013-01-453183
31. Lowrance WT, Breau RH, Chou R, Chapin BF, Crispino T, Dreicer R, et al. Advanced prostate cancer: AUA/ASTRO/SUO guideline PART I. J Urol. (2021) 205:14–21. doi: 10.1097/JU.0000000000001375
Keywords: paraneoplastic syndrome, cancer related neurological symptoms, encephalitis, diagnosis, long term follow-up, prognosis
Citation: Bar Mucha S, Rozenberg A, Gutter Kapon L, Gorenshtein A, Ganelin-Cohen E, Ben Hayun R, Yarovinsky N and Shelly S (2024) Long term survival and outcomes in patients with paraneoplastic neurologic syndromes. Front. Immunol. 15:1466704. doi: 10.3389/fimmu.2024.1466704
Received: 18 July 2024; Accepted: 25 October 2024;
Published: 18 November 2024.
Edited by:
Pankaj Gaur, Georgetown University, United StatesReviewed by:
Kumar Vaibhav, Augusta University, United StatesCopyright © 2024 Bar Mucha, Rozenberg, Gutter Kapon, Gorenshtein, Ganelin-Cohen, Ben Hayun, Yarovinsky and Shelly. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shahar Shelly, c19zaGVsbHlAcm1jLmdvdi5pbA==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.