- 1Department of Urology, Affiliated Xiaoshan Hospital, Hangzhou Normal University, Hangzhou, China
- 2Department of Science and Education, Affiliated Xiaoshan Hospital, Hangzhou Normal University, Hangzhou, China
- 3Physical Examination Center, Affiliated Xiaoshan Hospital, Hangzhou Normal University, Hangzhou, China
Background: The predictive accuracy of the preoperative neutrophil-to-lymphocyte ratio (NLR) on the prognosis of patients with non-muscle-invasive bladder cancer (NMIBC) with intravesical Bacillus Calmette–Guérin immunotherapy (BCG) after transurethral resection of the bladder tumor (TURBT) remains unknown. Therefore, the current study performed a systematic review and meta-analysis to examine the relationship between preoperative NLR and the prognosis of patients with NMIBC with intravesical BCG immunotherapy.
Methods: For this systematic review and meta-analysis, articles were retrieved from PubMed, Cochrane Library, Web of Science, and Embase databases from their inception to 14 May 2024. The role of NLR in predicting recurrence and progression in NMIBC was determined using pooled hazard ratios (HRs) and 95% confidence intervals (CIs).
Results: Seven articles were included in this meta-analysis, involving 4,187 patients. An elevated NLR was significantly associated with recurrence (HR = 2.67, 95% CI = 1.34–5.32, P < 0.001) and progression (HR = 1.72, 95% CI = 1.13–2.60, P = 0.004) in patients with NMIBC with intravesical BCG immunotherapy.
Conclusion: This meta-analysis demonstrated that elevated preoperative NLR levels were significantly associated with recurrence and disease progression in patients with NMIBC who underwent intravesical BCG immunotherapy after TURBT.
Systematic review registration: https://inplasy.com/inplasy-2024-7-0058/, identifier 202470058.
Introduction
Bladder cancer (BCa) is one of the most commonly diagnosed malignancies worldwide and the most common malignancy of the urologic system, with approximately 573,000 new cases and 213,000 deaths according to the Global Cancer Statistics 2020 (1). On the basis of the depth of tumor invasion, BCa can be classified as non–muscle-invasive bladder cancer (NMIBC) and muscle-invasive bladder cancer (MIBC). In approximately 70% of the patients diagnosed with BCa, the disease is limited to the mucosa [stage Ta, carcinoma in situ (CIS)] or submucosa (stage T1) (2, 3). The gold standard for the treatment of NMIBC is transurethral resection of the bladder tumor (TURBT) followed by intravesical instillation therapy and then undergoing a second surgery if necessary (3). Bacillus Calmette–Guérin (BCG) has been shown to be the most effective intravesical immunotherapy for preventing the recurrence of NMIBC by inducing an immune response in the bladder to attack cancer cells (4–7). However, decades have passed since BCG was suggested as a treatment for NMIBC, but immunotherapy for NMIBC has not progressed much (8). The intravesical BCG immunotherapy is based on an attenuated non-pathogenic strain of Mycobacterium bovis that was originally used as a vaccine against tuberculosis (9–11). NMIBC typically shows a favorable prognosis with a 5-year overall survival (OS) rate of approximately 90%; still 30%–80% of cases exhibit recurrences and 45% of cases progress to muscle invasion were observed within 5 years (12). The clinical results of intravesical BCG immunotherapy are promising, but a subset of patients has shown unwanted results, such as the lack of clinical response, tumor recurrence, and tumor progression (13). Therefore, predicting BCG failure, tumor recurrence, and progression may facilitate timely radical cystectomy or combination therapy and improve the survival rate of patients. Currently, the European Organization for Research and Treatment of Cancer (EORTC) model is widely utilized to assess the risk of recurrence and progression of NMIBC treated with intravesical BCG immunotherapy. The scoring system has proven useful in clinical practice, but the accuracy of the EORTC model requires further improvement (14, 15). At present, other personalized models and optimization schemes have been proposed, but their performance has not been clinically verified (16, 17). One approach to address this challenge is to optimize the general treatment, particularly the initial patient condition (18). Consequently, easily accessible and objective predictors are needed to improve the risk classification and prediction of recurrence and progression of NMIBC.
Several studies have suggested that some inflammatory markers may be associated with the prognosis of intravesical BCG immunotherapy for NMIBC. The neutrophil-to-lymphocyte ratio (NLR) is a biomarker for assessing inflammatory status and is easily obtainable in clinical practice (19). Many systematic reviews and meta-analyses have effectively integrated the findings of multiple studies, yielding valuable insights into the complex relationships between NLR and solid tumors [e.g., breast cancer (20), cervical cancer (21), esophageal cancer (22), colorectal cancer (23), prostate cancer (24), and pancreatic cancer (25)]. The evidence consistently suggests that elevated NLR levels are significantly associated with poor prognosis in patients who develop these specific cancer types. Furthermore, NLR levels have been found to be independent predictors of the prognosis of BCa. In a meta-analysis of 17 studies reported by Xingxing Tang et al., elevated NLR was found to predict poor clinical outcomes in patients with BCa, including OS, recurrence-free survival (RFS), progression-free survival (PFS), and cancer-specific survival (CSS) (26). Another meta-analysis of six studies confirmed that an elevated preoperative NLR predicted worse RFS and PFS in patients with NMIBC treated with TURBT (27).
Despite previous studies having reported the relationship between NLR and survival outcomes in patients with NMIBC, no comprehensive meta-analysis has been conducted on patients with NMIBC with intravesical BCG immunotherapy. Patients receiving intravesical BCG immunotherapy after TURBT represent independent treatment types, and several studies have reported that preoperative NLR levels can predict the efficacy of intravesical BCG immunotherapy and disease relapse or progression in patients with NMIBC. Based on these previous findings, this meta-analysis aimed to systematically evaluate the relationship between preoperative NLR and the prognostic value of postoperative intravesical BCG immunotherapy in patients with NMIBC.
Materials and methods
Study registration
The present systematic review and meta-analysis was conducted on the basis of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guideline (28). The protocol of this review has been registered online on INPLASY (202470058).
Search strategy
Relevant studies were systematically searched on PubMed, Cochrane Library, Web of Science, and Embase databases from inception to 14 May 2024, with no language limitations. The search terms combined the suggested words by Medical Subject Heading (MeSH) with other related words. The search query in PubMed was as follows: ((((Neutrophil-to-Lymphocyte Ratio[Title/Abstract]) OR (Neutrophil to Lymphocyte Ratio[Title/Abstract])) OR (NLR[Title/Abstract])) AND (((((((((Non-Muscle Invasive Bladder Neoplasms[MeSH Terms]) OR (Non Muscle Invasive Bladder Neoplasms[Title/Abstract])) OR (NMIBC[Title/Abstract])) OR (Non-Muscle-Invasive Bladder Cancer[Title/Abstract])) OR (Bladder Cancer, Non-Muscle-Invasive[Title/Abstract])) OR (Bladder Cancers, Non-Muscle-Invasive[Title/Abstract])) OR (Cancer, Non-Muscle-Invasive Bladder[Title/Abstract])) OR (Cancers, Non-Muscle-Invasive Bladder[Title/Abstract])) OR (Non-Muscle Invasive Bladder Cancer[Title/Abstract]))) AND (((((Bacillus Calmette-Guerin[Title/Abstract]) OR (Bacillus Calmette Guerin[Title/Abstract])) OR (Bacillus Calmette-Guérin[Title/Abstract])) OR (Bacillus Calmette Guérin[Title/Abstract])) OR (BCG[Title/Abstract])). Additional relevant research was identified by manually reviewing the references cited in the captured articles.
Inclusion and exclusion criteria
Studies were included if they met the following criteria: (1) articles reporting the relationship between NLR and the prognosis of patients with NMIBC receiving intravesical BCG immunotherapy after surgery, using the formula NLR = neutrophil count/lymphocyte count; (2) groups were classified according to their preoperative NLR levels; (3) specific endpoints of interest, including recurrence and progression; and (4) availability of hazard ratios (HRs) or odds ratios (ORs) with 95% confidence intervals (CIs) or the ability to calculate them from the data presented in the articles. The exclusion criteria consisted of the following: (1) case reports, conference abstracts, editorials, and reviews; (2) studies with insufficient information and extractable data on the treatment and main outcomes of patients with NMIBC; (3) animal studies; (4) duplicates, or surveys investigating the same sample; and (5) the full text was unavailable.
Study selection and data extraction
In order to determine suitability of the articles, the titles and abstracts screened were independently by two investigators (JH and LL). Subsequently, the full texts were examined. Non-English articles were translated using Google Translate if necessary. In cases of replicated publications, the study with the most extensive information was considered. Discrepancies in study inclusion between the two independent investigators were settled by consulting a third party (DM). Data from the articles were extracted and recorded in a Microsoft Excel spreadsheet (Microsoft Corporation, Redmond, WA) by two investigators (JH and LL) independently, including the following: the name of first author; the year of study publication; study region (country); number of study centers; mean or median age; sample size; tumor stage; follow-up time (months); NLR cutoff; cutoff selection; and survival outcome, HRs, or ORs with 95% CIs.
Quality assessment
Two investigators (JH and LL) were involved in the quality evaluation using the Newcastle–Ottawa Scale (NOS), and a third party (DM) acted as adjudicator in case of disagreement. A higher score indicated a higher study quality, showing a positive correlation between the two. Studies with scores higher than 6 were considered of high quality.
Study outcomes and statistical analysis
Meta-analysis was conducted using Stata 16.0 software (StataCorp). HRs and 95% CIs were computed as the combined effect size to assess the relationship between pre-treatment NLR and recurrence and progression. The Q test was used to analyze the heterogeneity among the results of the included studies. I2 was utilized to quantitatively assess the magnitude of heterogeneity. Based on I2 statistics, heterogeneity was categorized as high (above 75%), moderate (25% to 75%), and low (below 25%) (29). When I2 >50% and/or P < 0.05, the random model was used; otherwise, the fixed model was used. Potential sources of heterogeneity were identified through subgroup analyses. If grouped data on subgroup categories were not available, then they were excluded. In addition, the meta-analysis was also subjected to a sensitivity analysis in order to eliminate the effect of individual study data on survival outcomes. The publication bias was evaluated using Egger’s test. P < 0.05 was regarded statistically significant.
Results
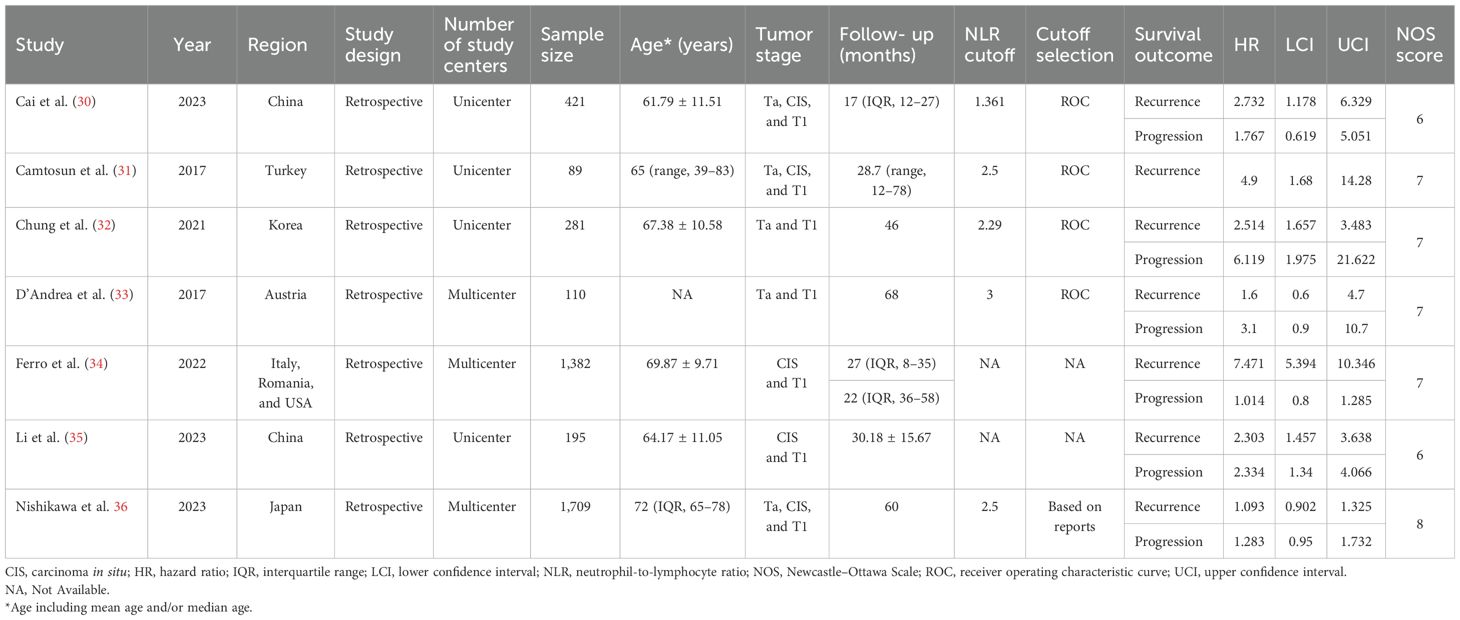
As a whole, 115 publications were retrieved by searching PubMed (n = 31), Web of Science (n = 39), Cochrane Library (n = 1), and Embase (n = 44). After removing duplicate articles, 52 articles remained for further screening. After reading the titles and abstracts of these publications, 18 studies were identified for full-text screening. In the meta-analysis, a total of seven retrospective studies published between 2017 and 2023 and involving 4,187 participants were ultimately included (30–36). The Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) flowchart of the study selection is described in Figure 1. Four studies were conducted in Asian, and five studies were conducted in non-Asian countries. The sample size of the included articles ranged from 89 to 1,709. In terms of tumor type, the seven studies focused on NMIBC and all were patients who underwent intravesical BCG immunotherapy after TURBT. The pathological stage was Ta and/or T1, and five studies reported concurrent CIS. The cutoff values for NLR ranged from 1.361 to 3. Furthermore, all seven studies evaluated the association of preoperative NLR with tumor recurrence, and six studies evaluated the association of NLR with tumor progression. All studies scored at least six points on the NOS, indicating a high overall quality. Table 1 shows an overview of the baseline data and quality evaluations of the studies.
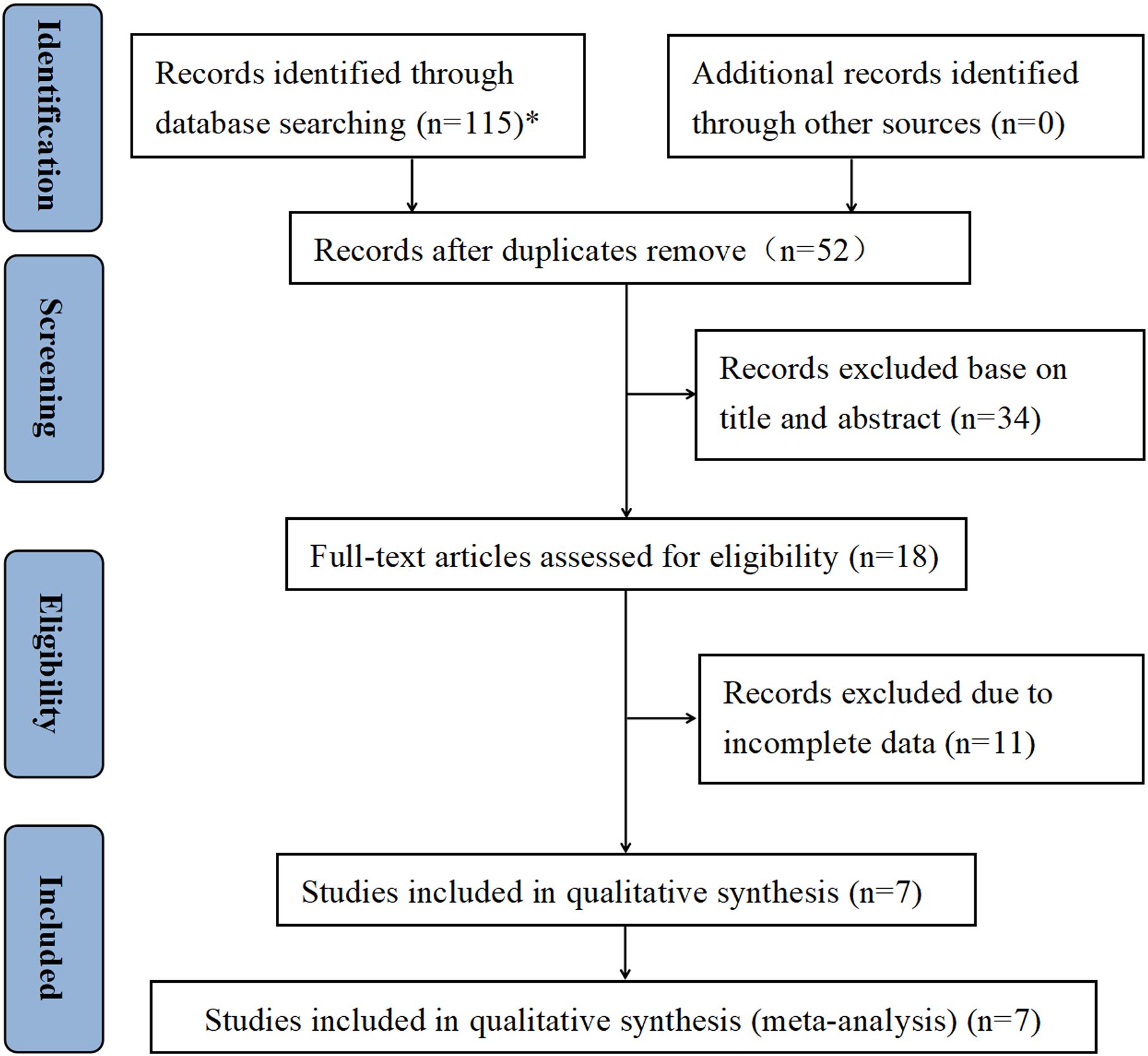
Figure 1. PRISMA diagram showing the study selection process. *PubMed (n = 31), Web of Science (n = 39), Cochrane Library (n = 1), and Embase (n = 44).
Association of preoperative NLR and recurrence in patients with NMIBC with intravesical BCG immunotherapy
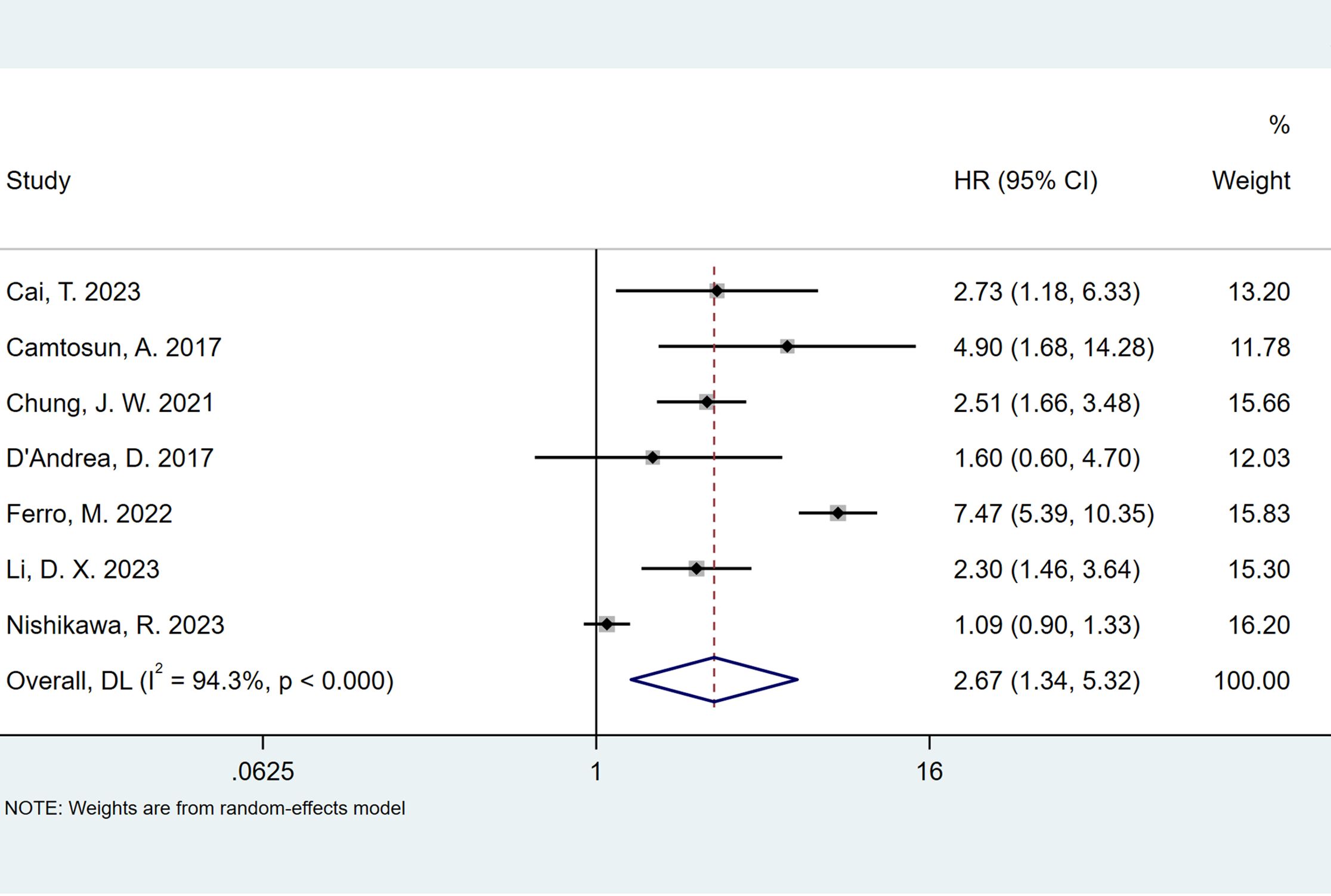
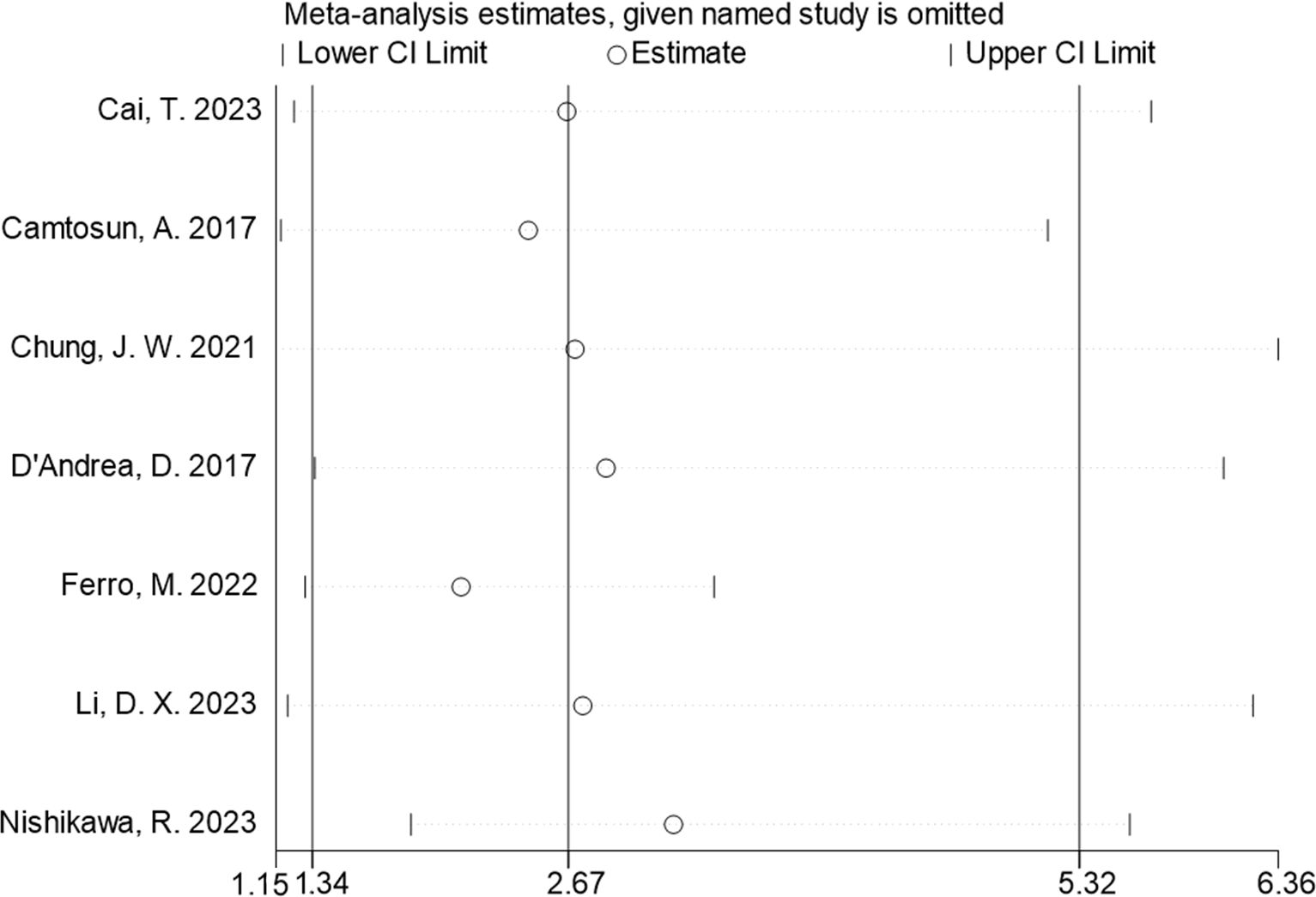
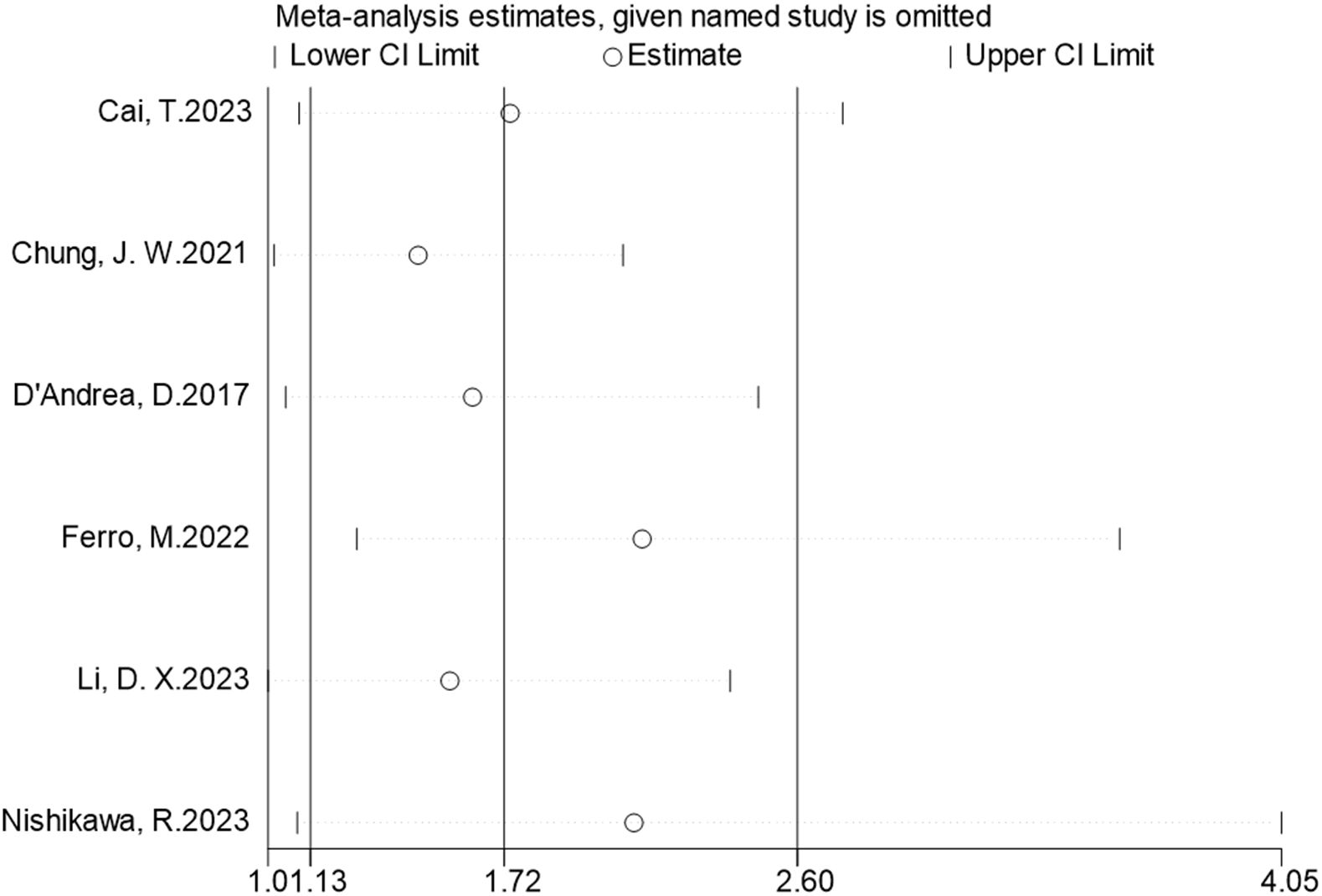
The seven studies all reported the predictive value of preoperative NLR in the risk of tumor recurrence. A random effects model (I2 = 94.3%, P < 0.001) was employed and the pooled analysis showed that patients with elevated NLR tended to have a higher recurrence risk (HR = 2.67, 95% CI = 1.34–5.32) (Figure 2). The sensitivity analysis indicated that removing individual studies did not affect the results of this study; hence, the results of the above random effects were stable and reliable, as shown in Figure 3. Then, the source of heterogeneity was assessed on the basis of ethnicity (Asian and non-Asian), number of study centers (unicenter and multicenter), sample size (<300 subjects and ≥300 subjects), and tumor stage (with concomitant CIS and without concomitant CIS). Moreover, subgroup analyses demonstrated that preoperative NLR remained a significant prognostic factor for recurrence and failed to find the source of heterogeneity, as shown in Table 2. The subgroup analyses did not explain the source of heterogeneity. Subsequently, Egger’s tests were conducted to evaluate publication bias, showing no statistically significant publication bias (P = 0.355).
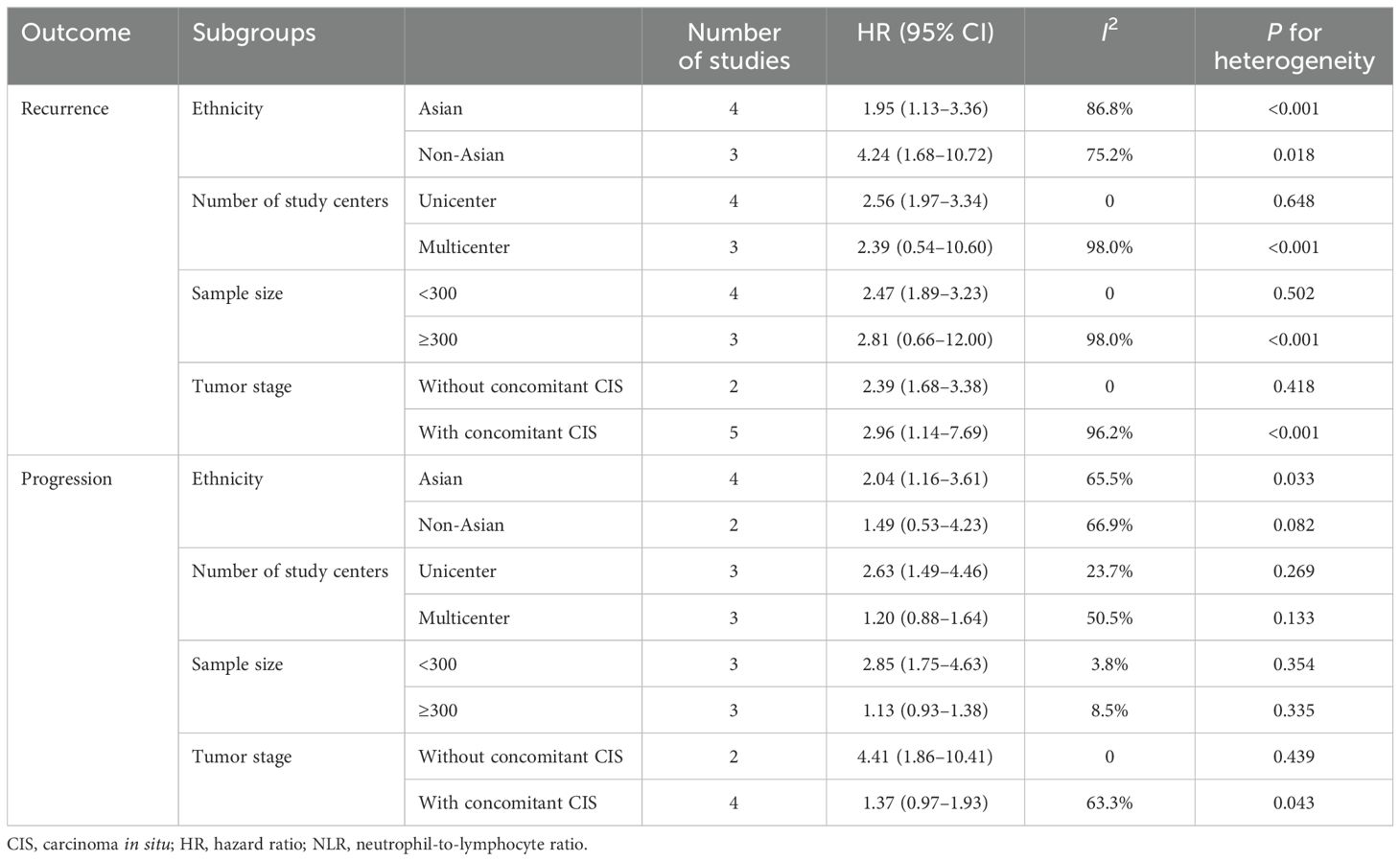
Table 2. Subgroup analyses for the prognostic role of NLR for recurrence and progression in patients with NMIBC.
Association of preoperative NLR and progression in patients with NMIBC with intravesical BCG immunotherapy
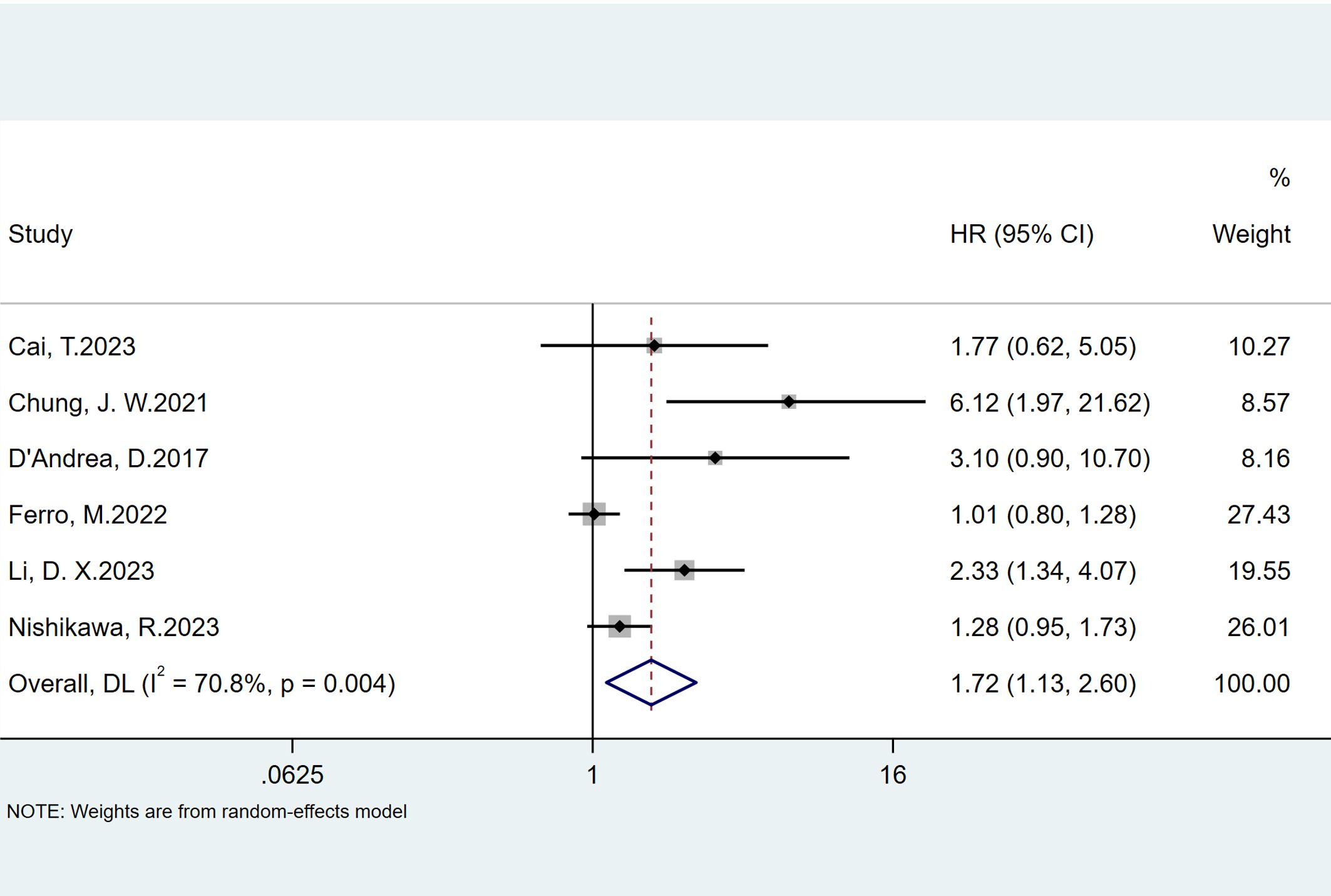
Six studies reported the predictive value of preoperative NLR in the risk of tumor progression; the pooled analysis showed that patients with elevated NLR tended to have a higher progression risk (HR = 1.72, 95% CI = 1.13–2.60), based on a random effects model (I2 = 70.8%, P = 0.004) (Figure 4). The sensitivity analysis revealed that the arbitrary deletion of studies did not affect the results, indicating that the results of the above random effects are stable and reliable, as shown in Figure 5. Further subgroup analysis revealed that the number of study centers and the sample size might be the main source of heterogeneity (Table 2). Egger’s tests were conducted to evaluate publication bias, showing the possibility of publication bias (P = 0.023). However, discussing publication bias in a meta-analysis containing less than 10 studies presents a challenge (37). The number of studies with publication bias included in the above analysis was relatively small, so the trim-and-fill method cannot be used to assess publication bias.
Discussion
Our research findings indicate that elevated preoperative NLR levels are a promising and cost-effective prognostic biomarker for recurrence and progression in patients with NMIBC with intravesical BCG immunotherapy. To the extent of our knowledge, this is the first meta-analysis to comprehensively examine the relationship between preoperative NLR levels and the risk of recurrence and progression in patients with NMIBC who underwent intravesical BCG immunotherapy. In patients with NMIBC receiving intravesical BCG immunotherapy after TURBT, the combined results showed a significant correlation between preoperative NLR and recurrence and progression. The increase in preoperative NLR levels independently increased the risk of recurrence and progression in patients with NMIBC receiving intravesical BCG immunotherapy after TURBT. These findings provide valuable insights into the potential of NLR as a prognostic marker for patients with NMIBC receiving intravesical BCG immunotherapy, which can guide the follow-up of patients with NMIBC.
TURBT and intravesical BCG immunotherapy are recommended as treatments for patients with intermediate- and high-risk NMIBC to reduce the risk of recurrence and progression of NMIBC (3, 38). The EORTC risk score is used to evaluate the risk of recurrence and progression of NMIBC receiving intravesical BCG immunotherapy after TURBT (15). Jae Wook Chung et al. reported that the combined use of preoperative NLR and EORTC systems could improve the predictive accuracy of disease progression (32). Hence, NLR represents a practical, cost-effective, and non-invasive biomarker that has been widely used to predict poor prognosis of tumors. The NLR was calculated using the formula below: NLR = neutrophil count/lymphocyte count. As a result, an elevated NLR could be caused by increased neutrophils and/or decreased lymphocytes. The predictive role of NLR in cancer can be explained by several potential mechanisms.
The development, invasion, proliferation, and metastasis of BCa could be promoted by many cell types. The microenvironment of cancer cells is altered by the recruitment and activation of stromal, adipocyte, fibroblast, inflammatory, and mesenchymal progenitors (39, 40). The potential predictive role of NLR in carcinogenesis and tumor invasiveness may be attributed to the interaction with other cell populations to produce cytokines and effector molecules as the number of neutrophils increases (41). In the tumor environment, neutrophils play a role in controlling immune responses by polarizing to antitumorigenic or protumorigenic phenotypes (42, 43). They are rapidly recruited to pathogen signals, including chemotactic mediators, lipid mediators, chemokines, and cytokines, to mediate host defense (44). The major pro-tumor activities include cancer cell proliferation, cell invasion, metastasis, extracellular matrix remodeling, angiogenesis, lymphangiogenesis, and suppression of antitumor immune surveillance (45). All of these contribute to tumor formation and development (46, 47). In addition, lymphocytes play a role in immune defense by promoting the death of cytotoxic cells and inhibiting the proliferation and migration of tumor cells (48, 49). Improvements in therapeutic outcomes are associated with lymphocyte infiltration in tumor tissues. In contrast, when lymphocyte numbers in the tumor microenvironment decrease, the anti-tumor ability decreases, leading to immune tolerance and tumor escape (50). Thus, elevated NLR levels resulting from elevated neutrophil count and decreased lymphocyte count are often associated with poor prognosis in patients with cancer. Consequently, patients with elevated NLR levels should receive more aggressive therapy and a closer follow-up to ensure the timely detection of tumor recurrence and progression. It is worth popularizing in clinical practice as a cost-effective and practical biomarker that could lead to a turning point in the treatment of patients with BCG-unresponsive NMIBC. The recommended treatment for BCG-unresponsive disease remains radical cystectomy and urinary diversion (3, 51). Therefore, to order to meet patients who desire a bladder-sparing approach or are too frail for major surgery, intravesical chemotherapy, chemo-hyperthermia, immunotherapy, inflammation-targeted agents, and gene therapy receive more attention; novel molecular therapeutic targets including Programmed Death-1 (PD-1)/Programmed Death-ligand 1 (PD-L1) and Cytotoxic T-lymphocyte-associated Protein 4 (CTLA-4) also receive attention (52–55).
Nonetheless, the relationship between the systemic NLR and the local bladder tumor infiltration of neutrophils and lymphocytes remains unknown. Wael Abdo Hassan et al. performed a pathological examination for tumor grade and stage and for tumor-infiltrating neutrophils and lymphocytes, and determined the NLR at the tissue level. They reported a significant increase in neutrophil count in high-grade BCa cases compared to that in low-grade cases. Moreover, a significant increase in neutrophil count and a decrease in CD8 lymphocytes were observed in MIBC cases compared to that in NMIBC cases, indicating the tumor-promoting effect of neutrophils and the possible role of CD8 lymphocytes in hindering the progression to muscle invasion. In addition, significantly higher NLR was found in MIBC cases compared to that in the NMIBC cases in high-grade neoplasms. NLR was more likely to be associated with the progression of tumor invasion compared to that in the tumor grade (56). The above findings indicated that the systemic NLR was correlated with tumor-infiltrating NLR at the tissue level. However, further study is required to confirm it.
However, there are several limitations that should be acknowledged in this meta-analysis. Firstly, because all the included studies were retrospective, confounding factors may affect the accuracy of our findings. Although the participants included were patients with NMIBC, there were differences in the basic characteristics, clinical stage, histological subtypes, and degree of tumor invasion and treatment of patients with NMIBC in the included literature. For example, there are obvious differences in the prognosis of different histological subtypes. According to previous literature reports, the risk of recurrence and progression of high-grade lesions is significantly higher than that of low-grade (57, 58). CIS is considered a precursor of invasive high-grade cancer, and 54% of patients will progress to myometrial invasion without treatment (59). The re-TURBT and upfront BCG may also affect the tumor prognosis (60, 61). According to the European Association of Urology (EAU) guidelines, lymph vessel invasion (LVI), CIS in prostatic urethra, and EAU risk groups also determined the different prognosis, but only one literature in the included studies mentioned LVI and CIS in prostatic urethra (58, 62, 63). Due to the relatively small sample size, all confounding factors could not be considered in the subgroup analysis. Further prospective studies should be conducted to validate the current findings and produce stronger evidence. Secondly, the criteria for choosing cutoff values differ across studies, which may introduce selection bias related to factors such as ethnicity or clinical setting. Careful consideration of standardized methods or cutoff values tailored to different patient populations or clinical settings has significant value. Thirdly, the publication bias needs to be considered. Finally, a significant heterogeneity in recurrence and progression was found. However, a subgroup analysis was performed and found a potential source of heterogeneity.
Conclusion
This meta-analysis demonstrated that elevated preoperative NLR levels were significantly associated with recurrence and disease progression in patients with NMIBC who underwent intravesical BCG immunotherapy after TURBT. As a cost-effective and practical biomarker, NLR can serve as a useful tool to guide the management and follow-up of patients with NMIBC. However, further large-scale prospective validation studies are needed to confirm these findings.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
JH: Data curation, Formal analysis, Writing – original draft. LL: Data curation, Formal analysis, Writing – original draft. DM: Investigation, Methodology, Supervision, Writing – original draft. RH: Supervision, Validation, Writing – review & editing. FG: Conceptualization, Methodology, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
Thanks for the contribution of the participants and Home for Researchers editorial team (www.home-for-researchers.com) for language editing service.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
BCa, bladder cancer; BCG, Bacillus Calmette–Guérin; CI, confidence interval; CIS, carcinoma in situ; CSS, cancer-specific survival; EORTC, European Organization for Research and Treatment of Cancer; HR, hazard ratio; MeSH, Medical Subject Heading; NLR, neutrophil-to-lymphocyte ratio; NMIBC, non–muscle-invasive bladder cancer; NOS, Newcastle–Ottawa Scale; OS, overall survival; PFS, progression-free survival; RFS, recurrence-free survival; TURBT, transurethral resection of the bladder tumor.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Lenis AT, Lec PM, Chamie K, Mshs MD. Bladder cancer: A review. JAMA. (2020) 324:1980–91. doi: 10.1001/jama.2020.17598
3. Babjuk M, Burger M, Capoun O, Cohen D, Compérat EM, Dominguez Escrig JL, et al. European association of urology guidelines on non-muscle-invasive bladder cancer (Ta, T1, and carcinoma in situ). Eur Urol. (2022) 81:75–94. doi: 10.1016/j.eururo.2021.08.010
4. Böhle A, Jocham D, Bock PR. Intravesical bacillus Calmette-Guerin versus mitomycin C for superficial bladder cancer: a formal meta-analysis of comparative studies on recurrence and toxicity. J Urol. (2003) 169:90–5. doi: 10.1016/s0022-5347(05)64043-8
5. Han RF, Pan JG. Can intravesical bacillus Calmette-Guérin reduce recurrence in patients with superficial bladder cancer? A meta-analysis of randomized trials. Urology. (2006) 67:1216–23. doi: 10.1016/j.urology.2005.12.014
6. Shelley MD, Kynaston H, Court J, Wilt TJ, Coles B, Burgon K, et al. A systematic review of intravesical bacillus Calmette-Guérin plus transurethral resection vs transurethral resection alone in Ta and T1 bladder cancer. BJU Int. (2001) 88:209–16. doi: 10.1046/j.1464-410x.2001.02306.x
7. Shelley MD, Wilt TJ, Court J, Coles B, Kynaston H, Mason MD. Intravesical bacillus Calmette-Guérin is superior to mitomycin C in reducing tumour recurrence in high-risk superficial bladder cancer: a meta-analysis of randomized trials. BJU Int. (2004) 93:485–90. doi: 10.1111/j.1464-410x.2003.04655.x
8. Morales A, Eidinger D, Bruce AW. Intracavity bacillus Calmette-Guerin in the treatment of superficial bladder tumors. J Urol. (1976) 116:S142–5. doi: 10.1016/j.juro.2016.10.101
9. Herr HW, Laudone VP, Badalament RA, Oettgen HF, Sogani PC, Freedman BD, et al. Bacillus Calmette-Guérin therapy alters the progression of superficial bladder cancer. J Clin Oncol. (1988) 6:1450–5. doi: 10.1200/JCO.1988.6.9.1450
10. Simons MP, O’Donnell MA, Griffith TS. Role of neutrophils in BCG immunotherapy for bladder cancer. Urol Oncol. (2008) 26:341–5. doi: 10.1016/j.urolonc.2007.11.031
11. Redelman-Sidi G, Glickman MS, Bochner BH. The mechanism of action of BCG therapy for bladder cancer–a current perspective. Nat Rev Urol. (2014) 11:153–62. doi: 10.1038/nrurol.2014.15
12. van Rhijn BW, Burger M, Lotan Y, Solsona E, Stief CG, Sylvester RJ, et al. Recurrence and progression of disease in non-muscle-invasive bladder cancer: from epidemiology to treatment strategy. Eur Urol. (2009) 56:430–42. doi: 10.1016/j.eururo.2009.06.028
13. Anastasiadis A, de Reijke TM. Best practice in the treatment of nonmuscle invasive bladder cancer. Ther Adv Urol. (2012) 4:13–32. doi: 10.1177/1756287211431976
14. Busato Júnior WF, Almeida GL, Ribas CA, Ribas Filho JM, De Cobelli O. EORTC risk model to predict progression in patients with non-muscle-invasive bladder cancer: is it safe to use in clinical practice. Clin Genitourin Cancer. (2016) 14:176–82. doi: 10.1016/j.clgc.2015.09.005
15. Xylinas E, Kent M, Kluth L, Pycha A, Comploj E, Svatek RS, et al. Accuracy of the EORTC risk tables and of the CUETO scoring model to predict outcomes in non-muscle-invasive urothelial carcinoma of the bladder. Br J Cancer. (2013) 109:1460–6. doi: 10.1038/bjc.2013.372
16. Lazebnik T. Cell-level spatio-temporal model for a bacillus calmette-guérin-based immunotherapy treatment protocol of superficial bladder cancer. Cells. (2022) 11:2372. doi: 10.3390/cells11152372
17. Savchenko E, Bunimovich-Mendrazitsky S. Investigation toward the economic feasibility of personalized medicine for healthcare service providers: the case of bladder cancer. Front Med (Lausanne). (2024) 11:1388685. doi: 10.3389/fmed.2024.1388685
18. Ylösmäki E, Fusciello M, Martins B, Feola S, Hamdan F, Chiaro J, et al. Novel personalized cancer vaccine platform based on Bacillus Calmette-Guèrin. J Immunother Cancer. (2021) 9:e002707. doi: 10.1136/jitc-2021-002707
19. Forget P, Khalifa C, Defour JP, Latinne D, Van Pel MC, De Kock M. What is the normal value of the neutrophil-to-lymphocyte ratio. BMC Res Notes. (2017) 10:12. doi: 10.1186/s13104-016-2335-5
20. Ethier JL, Desautels D, Templeton A, Shah PS, Amir E. Prognostic role of neutrophil-to-lymphocyte ratio in breast cancer: a systematic review and meta-analysis. Breast Cancer Res. (2017) 19:2. doi: 10.1186/s13058-016-0794-1
21. Zou P, Yang E, Li Z. Neutrophil-to-lymphocyte ratio is an independent predictor for survival outcomes in cervical cancer: a systematic review and meta-analysis. Sci Rep. (2020) 10:21917. doi: 10.1038/s41598-020-79071-x
22. Li B, Xiong F, Yi S, Wang S. Prognostic and clinicopathologic significance of neutrophil-to-lymphocyte ratio in esophageal cancer: an update meta-analysis. Technol Cancer Res Treat. (2022) 21:15330338211070140. doi: 10.1177/15330338211070140
23. Naszai M, Kurjan A, Maughan TS. The prognostic utility of pre-treatment neutrophil-to-lymphocyte-ratio (NLR) in colorectal cancer: A systematic review and meta-analysis. Cancer Med. (2021) 10:5983–97. doi: 10.1002/cam4.4143
24. Tang L, Li X, Wang B, Luo G, Gu L, Chen L, et al. Prognostic value of neutrophil-to-lymphocyte ratio in localized and advanced prostate cancer: A systematic review and meta-analysis. PloS One. (2016) 11:e0153981. doi: 10.1371/journal.pone.0153981
25. Zhou Y, Wei Q, Fan J, Cheng S, Ding W, Hua Z. Prognostic role of the neutrophil-to-lymphocyte ratio in pancreatic cancer: A meta-analysis containing 8252 patients. Clin Chim Acta. (2018) 479:181–9. doi: 10.1016/j.cca.2018.01.024
26. Tang X, Du P, Yang Y. The clinical use of neutrophil-to-lymphocyte ratio in bladder cancer patients: a systematic review and meta-analysis. Int J Clin Oncol. (2017) 22:817–25. doi: 10.1007/s10147-017-1171-5
27. Vartolomei MD, Porav-Hodade D, Ferro M, Mathieu R, Abufaraj M, Foerster B, et al. Prognostic role of pretreatment neutrophil-to-lymphocyte ratio (NLR) in patients with non-muscle-invasive bladder cancer (NMIBC): A systematic review and meta-analysis. Urol Oncol. (2018) 36:389–99. doi: 10.1016/j.urolonc.2018.05.014
28. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
29. Ades AE, Lu G, Higgins JP. The interpretation of random-effects meta-analysis in decision models. Med Decis Making. (2005) 25:646–54. doi: 10.1177/0272989x05282643
30. Cai T, Lu J, Lin Z, Lup M, Liang H, Qin Z, et al. Intravesical instillation of bacillus Calmette-Guerin for non-muscle invasive bladder cancer: outcomes of 421 patients in a single center. Nan Fang Yi Ke Da Xue Xue Bao. (2023) 43:488–94. doi: 10.12122/j.issn.1673-4254.2023.03.21
31. Camtosun A, Celik H, Altintas R, Topcu I, Tasdemir C. Does neutrophil-to-lymphocyte ratio has a value in predicting BCG recurrence in bladder tumor patients. BioMed Res. (2017) 28:36–40.
32. Chung JW, Kim JW, Lee EH, Chun SY, Park DJ, Byeon KH, et al. Prognostic significance of the neutrophil-to-lymphocyte ratio in patients with non-muscle invasive bladder cancer treated with intravesical bacillus calmette-guérin and the relationship with the CUETO scoring model. Urol J. (2021) 19:281–8. doi: 10.22037/uj.v18i.6765
33. D’Andrea D, Moschini M, Gust K, Abufaraj M, Özsoy M, Mathieu R, et al. Prognostic role of neutrophil-to-lymphocyte ratio in primary non-muscle-invasive bladder cancer. Clin Genitourin Cancer. (2017) 15:e755–64. doi: 10.1016/j.clgc.2017.03.007
34. Ferro M, Tătaru OS, Musi G, Lucarelli G, Abu Farhan AR, Cantiello F, et al. Modified glasgow prognostic score as a predictor of recurrence in patients with high grade non-muscle invasive bladder cancer undergoing intravesical bacillus calmette-guerin immunotherapy. Diagnost (Basel). (2022) 12:586. doi: 10.3390/diagnostics12030586
35. Li DX, Wang XM, Feng DC, Han P. Neutrophil-to-lymphocyte ratio (NLR) during induction is a better predictor than preoperative NLR in non-muscle-invasive bladder cancer receiving Bacillus Calmette-GuÉRin. Asian J Surg. (2023) 46:1348–51. doi: 10.1016/j.asjsur.2022.08.108
36. Nishikawa R, Miyake M, Morizane S, Shimizu R, Teraoka S, Honda M, et al. C-reactive protein as a prognostic predictor for non-muscle invasive bladder cancer after intravesical bacillus Calmette-Guérin therapy: A Japan Urological Oncology Group study analysis. Int J Urol. (2023) 30:299–307. doi: 10.1111/iju.15106
37. Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. (2011) 343:d4002. doi: 10.1136/bmj.d4002
38. Malmström PU, Sylvester RJ, Crawford DE, Friedrich M, Krege S, Rintala E, et al. An individual patient data meta-analysis of the long-term outcome of randomised studies comparing intravesical mitomycin C versus bacillus Calmette-Guérin for non-muscle-invasive bladder cancer. Eur Urol. (2009) 56:247–56. doi: 10.1016/j.eururo.2009.04.038
39. van der Horst G, Bos L, van der Pluijm G. Epithelial plasticity, cancer stem cells, and the tumor-supportive stroma in bladder carcinoma. Mol Cancer Res. (2012) 10:995–1009. doi: 10.1158/1541-7786.mcr-12-0274
40. Chaffer CL, Brennan JP, Slavin JL, Blick T, Thompson EW, Williams ED. Mesenchymal-to-epithelial transition facilitates bladder cancer metastasis: role of fibroblast growth factor receptor-2. Cancer Res. (2006) 66:11271–8. doi: 10.1158/0008-5472.can-06-2044
41. Galdiero MR, Bonavita E, Barajon I, Garlanda C, Mantovani A, Jaillon S. Tumor associated macrophages and neutrophils in cancer. Immunobiology. (2013) 218:1402–10. doi: 10.1016/j.imbio.2013.06.003
42. Giese MA, Hind LE, Huttenlocher A. Neutrophil plasticity in the tumor microenvironment. Blood. (2019) 133:2159–67. doi: 10.1182/blood-2018-11-844548
43. Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. (2009) 16:183–94. doi: 10.1016/j.ccr.2009.06.017
44. Kruger P, Saffarzadeh M, Weber AN, Rieber N, Radsak M, von Bernuth H, et al. Neutrophils: Between host defence, immune modulation, and tissue injury. PloS Pathog. (2015) 11:e1004651. doi: 10.1371/journal.ppat.1004651
45. Mantovani A, Cassatella MA, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol. (2011) 11:519–31. doi: 10.1038/nri3024
46. Jaillon S, Ponzetta A, Di Mitri D, Santoni A, Bonecchi R, Mantovani A. Neutrophil diversity and plasticity in tumour progression and therapy. Nat Rev Cancer. (2020) 20:485–503. doi: 10.1038/s41568-020-0281-y
47. Swierczak A, Mouchemore KA, Hamilton JA, Anderson RL. Neutrophils: important contributors to tumor progression and metastasis. Cancer Metastasis Rev. (2015) 34:735–51. doi: 10.1007/s10555-015-9594-9
48. Paijens ST, Vledder A, de Bruyn M, Nijman HW. Tumor-infiltrating lymphocytes in the immunotherapy era. Cell Mol Immunol. (2021) 18:842–59. doi: 10.1038/s41423-020-00565-9
49. Chen KJ, Zhou L, Xie HY, Ahmed TE, Feng XW, Zheng SS. Intratumoral regulatory T cells alone or in combination with cytotoxic T cells predict prognosis of hepatocellular carcinoma after resection. Med Oncol. (2012) 29:1817–26. doi: 10.1007/s12032-011-0006-x
50. Gooden MJ, de Bock GH, Leffers N, Daemen T, Nijman HW. The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br J Cancer. (2011) 105:93–103. doi: 10.1038/bjc.2011.189
51. Alfred Witjes J, Max Bruins H, Carrión A, Cathomas R, Compérat E, Efstathiou JA, et al. European association of urology guidelines on muscle-invasive and metastatic bladder cancer: summary of the 2023 guidelines. Eur Urol. (2024) 85:17–31. doi: 10.1016/j.eururo.2023.08.016
52. Claps F, Pavan N, Ongaro L, Tierno D, Grassi G, Trombetta C, et al. BCG-unresponsive non-muscle-invasive bladder cancer: current treatment landscape and novel emerging molecular targets. Int J Mol Sci. (2023) 24:12596. doi: 10.3390/ijms241612596
53. Jennifer G, Vinay P. Pembrolizumab for non–muscle-invasive bladder cancer—A costly therapy in search of evidence. JAMA Oncol. (2021) 7:501–2. doi: 10.1001/jamaoncol.2020.6142
54. Rouanne M, Bajorin DF, Hannan R, Galsky MD, Williams SB, Necchi A, et al. Rationale and outcomes for neoadjuvant immunotherapy in urothelial carcinoma of the bladder. Eur Urol Oncol. (2020) 3:728–38. doi: 10.1016/j.euo.2020.06.009
55. Gao J, Navai N, Alhalabi O, Siefker-Radtke A, Campbell MT, Tidwell RS, et al. Neoadjuvant PD-L1 plus CTLA-4 blockade in patients with cisplatin-ineligible operable high-risk urothelial carcinoma. Nat Med. (2020) 26:1845–51. doi: 10.1038/s41591-020-1086-y
56. Hassan WA, ElBanna AK, Noufal N, El-Assmy M, Lotfy H, Ali RI. Significance of tumor-associated neutrophils, lymphocytes, and neutrophil-to-lymphocyte ratio in non-invasive and invasive bladder urothelial carcinoma. J Pathol Transl Med. (2023) 57:88–94. doi: 10.4132/jptm.2022.11.06
57. Cambier S, Sylvester RJ, Collette L, Gontero P, Brausi MA, van Andel G, et al. EORTC nomograms and risk groups for predicting recurrence, progression, and disease-specific and overall survival in non-muscle-invasive stage ta-T1 urothelial bladder cancer patients treated with 1-3 years of maintenance bacillus calmette-guérin. Eur Urol. (2016) 69:60–9. doi: 10.1016/j.eururo.2015.06.045
58. Sylvester RJ, Rodríguez O, Hernández V, Turturica D, Bauerová L, Bruins HM, et al. European association of urology (EAU) prognostic factor risk groups for non-muscle-invasive bladder cancer (NMIBC) incorporating the WHO 2004/2016 and WHO 1973 classification systems for grade: an update from the EAU NMIBC guidelines panel. Eur Urol. (2021) 79:480–8. doi: 10.1016/j.eururo.2020.12.033
60. Beijert IJ, Hentschel AE, Bründl J, Compérat EM, Plass K, Rodríguez O, et al. Second TURB, restaging TURB or repeat TURB in primary T1 non-muscle invasive bladder cancer: impact on prognosis. Int Urol Nephrol. (2024) 56:1323–33. doi: 10.1007/s11255-023-03867-9
61. Soria F, Rosazza M, Livoti S, Dutto D, Colucci F, Sylvester RJ, et al. Repeat transurethral resection (TUR) + Bacillus calmette-guérin (BCG) versus upfront induction BCG after TUR in high-risk non-muscle-invasive bladder cancer: feasibility phase of a randomized controlled study. Eur Urol Focus. (2023). doi: 10.1016/j.euf.2023.10.019
62. Kim HS, Kim M, Jeong CW, Kwak C, Kim HH, Ku JH. Presence of lymphovascular invasion in urothelial bladder cancer specimens after transurethral resections correlates with risk of upstaging and survival: a systematic review and meta-analysis. Urol Oncol. (2014) 32:1191–9. doi: 10.1016/j.urolonc.2014.05.008
63. Palou J, Sylvester RJ, Faba OR, Parada R, Peña JA, Algaba F, et al. Female gender and carcinoma in situ in the prostatic urethra are prognostic factors for recurrence, progression, and disease-specific mortality in T1G3 bladder cancer patients treated with bacillus Calmette-Guérin. Eur Urol. (2012) 62:118–25. doi: 10.1016/j.eururo.2011.10.029
Keywords: non-muscle-invasive bladder cancer, bacillus Calmette-Guérin, neutrophil-tolymphocyte ratio, recurrence, progression, systematic review, meta-analysis
Citation: Huang J, Lin L, Mao D, Hua R and Guan F (2024) Prognostic value of neutrophil-to-lymphocyte ratio in patients with non–muscle-invasive bladder cancer with intravesical Bacillus Calmette–Guérin immunotherapy: a systematic review and meta-analysis. Front. Immunol. 15:1464635. doi: 10.3389/fimmu.2024.1464635
Received: 16 July 2024; Accepted: 03 October 2024;
Published: 23 October 2024.
Edited by:
Zhiming Li, Sun Yat-sen University Cancer Center (SYSUCC), ChinaReviewed by:
Francesco Claps, The Netherlands Cancer Institute (NKI), NetherlandsTeddy Lazebnik, University College London, United Kingdom
María Marcela Barrio, Fundación Cáncer, Argentina
Jiajia Huang, Sun Yat-sen University Cancer Center (SYSUCC), China
Copyright © 2024 Huang, Lin, Mao, Hua and Guan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Feifei Guan, emp4c2hnZmZAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Jiaguo Huang1†
Jiaguo Huang1† Feifei Guan
Feifei Guan