- 1Adeno-Associated Virus Biology Section, National Institute of Dental and Craniofacial Research, National Institutes of Health, Bethesda, MD, United States
- 2Critical Care Medicine and Pulmonary Branch, National Heart, Lung and Blood Institute, National Institutes of Health, Bethesda, MD, United States
- 3Sjögren’s Disease Clinic, National Institute of Dental and Craniofacial Research, National Institutes of Health, Bethesda, MD, United States
- 4Muscle Disease Section, National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Institutes of Health, Bethesda, MD, United States
- 5Department of Neurology, Johns Hopkins University School of Medicine, Baltimore, MD, United States
- 6Environmental Autoimmunity Group, National Institute of Environmental Health Sciences, National Institutes of Health, Bethesda, MD, United States
- 7Salivary Disorders Unit, National Institute of Dental and Craniofacial Research, National Institutes of Health, Bethesda, MD, United States
Autoantibodies are important for the diagnosis of autoimmune interstitial lung disease (ILD). Standard immunoassays have limitations, including their qualitative nature and/or a narrow dynamic range of detection, hindering the usefulness of autoantibodies as biomarkers of disease activity. Here, the luciferase immunoprecipitation system (LIPS) was evaluated for measuring myositis-specific and other lung-related autoantibodies in 25 subjects with idiopathic inflammatory myopathies (IIM), 26 with Sjögren’s disease (SjD), and 10 healthy volunteers. LIPS detected a broad dynamic range of autoantibodies, to MDA5, Jo-1, PL12, KS, U1-70K, and Ro52, and matched seropositivity status with established immunoassays. Robust anti-MDA5 autoantibodies in four IIM-ILD patients had a median value of 1,134,000 LU (IQR 473,000-2,317,000), which was 500 times higher than in 21 seronegative IIM patients. Markedly elevated anti-Jo-1 autoantibodies in five IIM-ILD patients demonstrated a median value of 1,177,000 LU (IQR: 604,000-2,520,000), which was 1000-fold higher than in seronegative patients. Robust anti-Ro52 and other anti-tRNA-synthetase autoantibodies were detected in a subset of IIM-ILD subjects. In SjD, only anti-U1-70K and KS autoantibodies were identified in ILD patients with a prevalence of 30% and 20%, respectively. In longitudinal samples of five IIM-ILD patients, anti-Jo-1 autoantibody levels paralleled clinical improvement of lung function. LIPS can accurately quantify autoantibody levels as biomarkers for treatment response in patients with autoimmune ILD.
Introduction
Interstitial lung disease (ILD) represents a diverse group of disorders sharing radiographic, physiologic, and histopathologic pulmonary manifestations (1). In systemic autoimmune diseases such as idiopathic inflammatory myopathies (IIM) and Sjögren’s disease (SjD), ILD is a frequent manifestation associated with high morbidity and mortality (2). Autoantibodies play an important role in the diagnosis of these diseases and have contributed to more specific clinical characterization of these patients (3–6). For example, patients with anti-PL7 and anti-PL12 autoantibodies present with more severe lung involvement than those with anti-Jo1 (6). Anti-MDA5 autoantibodies confer an increased risk of rapidly progressive ILD (5). A high prevalence of sicca symptoms has been reported in patients with anti-KS autoantibodies (7).
Recent evidence also suggests autoantibodies are not only useful biomarkers (8, 9), but they play an important role in the disease pathogenesis of IIM (10, 11) and IIM-ILD (8, 12). For example, treatment with CAR-T CD19 cells can reduce autoantibody titers and induce sustained remission in patients with anti-synthetase syndrome (13). Additionally, IIM patients who respond to rituximab therapy also experience a reduction in autoantibody levels (14). Importantly, research quantifying autoantibody levels as relevant disease biomarkers has been limited by several factors. While immunoprecipitation (IP) is considered the gold standard test, this immunoassay is costly, slow to generate results, unable to differentiate between autoantibodies against autoantigens with similar molecular weights, and does not provide autoantibody levels. Standard assays, including ELISA, Western blots, and line blot immunoassays (LIA) also have limitations in accurately determining autoantibody-positive and -negative patients, and/or the accurate quantification of autoantibody levels (15). For instance, a recent study by Loganathan et al. highlighted the failure of standard assays to detect non-Jo-1 antisynthetase autoantibodies, such as PL7, OJ, and KS, with high sensitivity and specificity compared to IP (16).
Our group developed Luciferase immunoprecipitation systems (LIPS) as a simple, quantitative immunoassay that employs light-emitting proteins in an immunoprecipitation format for the detection of autoantibodies against both linear and conformational epitopes (17). We have shown in multiple studies that LIPS has high sensitivity and specificity for detecting and quantifying autoantibodies associated with a multitude of autoimmune conditions, including systemic sclerosis (18), systemic lupus erythematosus (SLE) (19), and SjD (17, 20). In particular, LIPS has shown to provide a wide range of anti-Ro52 autoantibody levels (21, 22), which is a myositis-associated autoantibody that has been associated with IIM-ILD (23–27). In this study, LIPS was used to detect and quantify myositis-specific and myositis-associated autoantibodies in patients with IIM and SjD.
Materials and methods
Study design and patient characteristics
Sera were collected at the National Institutes of Health Clinical Research Center from patients enrolled in institutional review board-approved protocols (NIEHS: 94-E-0165, 11-E-0072, 07-E-0012, 05-E-N200; NIDCR: 15-D-0051, 11-D-0172) after informed consent was obtained. Since the focus of this study was to identify biomarkers of ILD, the IIM group was pre-selected to include a large percentage of patients with known ILD. The study included cross-sectional serum samples from 10 healthy volunteers (NV), 25 patients with IIM (21 with ILD), and 26 SjD patients (10 with ILD). The IIM patient group (n=25) were enrolled based on the criteria of Bohan and Peter (28) for myositis, in which 14 met definite EULAR-ACR criteria (29). Among the IIM-ILD subjects, 42% (9/21) had identifiable ILD subtypes based on lung biopsy and/or high-resolution computed tomography (HRCT) reports. Specifically, six subjects had nonspecific interstitial pneumonia (NSIP), two subjects had cryptogenic organizing pneumonia/bronchiolitis obliterans organizing pneumonia (COP/BOOP), and one subject had both NSIP and Organizing Pneumonia (OP) on lung biopsy. All the SjD participants used in this study met the 2016 American College of Rheumatology Sjögren’s Disease Classification Criteria including assessment of focal lymphocytic (range 0-12). Among the ten SjD-ILD cases, 70% (7/10) had identifiable ILD subtypes. Three SjD-ILD subjects had non-specific fibrosis, two had NSIP, one with acute interstitial pneumonitis and one with lymphocytic interstitial pneumonitis. All participants in this study were comprehensively evaluated, including for ILD, and had clinical autoantibody testing. IIM patients also had a physician global activity (PGA) score, on a 10 cm visual analog scale (30).
In addition to cross-sectional patient samples, anti-Ro52, and anti-Jo-1 autoantibody levels were analyzed in 5 IIM patients with longitudinal samples. Clinical and lung function measurements, including forced vital capacity, percent predicted (FVC %) and diffusing capacity of the lungs for carbon monoxide percent predicted (DLCO), for all available serial samples for these patients were also included in analysis.
LIPS measurement of autoantibody levels in the cohort
The LIPS immunoassay was used in a 96-well format to detect autoantibodies to both conformational and linear epitopes of protein antigens (17). The technology involves the use of custom luciferase-antigen fusion proteins employed in a fluid-phase immunoassay which provides high sensitivity, specificity, and a wide dynamic range of detection. Several previously described luciferase-fusion proteins for LIPS were used against known autoantigens including Ro52, Ro60, CENP-A, U1-70K, Jo-1, IFN-α, IFN-ω, KCNRG, BPIFB1 and TRIM38 (19, 31, 32). For this study, new autoantigen fusion proteins were constructed for detecting autoantibodies against MDA5 (IFIH-1), PL7 (TARS1), PL12 (AARS1), EJ (GARS1), KS (NARS1), Ha (YARS1), OJ (IARS1), Zo (FARS1), ABLIM (actin-binding LIM protein 1) and CDH5R (cadherin-5). cDNAs were amplified from either existing plasmid clones or generated as synthetic DNA fragments (Twist Bioscience) and were then cloned as C-terminal fusion proteins with Renilla luciferase except for CDH5R, which were generated as N-terminal fusion with Gaussia luciferase. The exact amino acids (aa) used for the new target autoantigens are as follows: MDA5/IFIH1 (NP_071451.2) with a N-terminal protein fragment spanning aa 2-577 and a C-terminal fragment spanning aa 578-1105), PL7/TARS1 (NP_001245366.1); aa 281-723, PL12/AARS1 (NP_001596.2); aa 516-968, EJ/GARS1 (NP_002038.2); aa 149-739, KS/NARS1 (NP_004530.1); aa 1-548, Ha/YARS1 (NP_003671.1); aa 2-528, OJ/IARS1 (NP_001365515.1); aa 679-1211, Zo/FARS-beta subunit (NP_005678.3); aa 2-598, ABLIM (NP_001309817.1.2); aa 2-455, and CDHR5 (NP_001165439.2); aa 1-520. Bacterial cultures containing these plasmids were expanded and plasmid DNA was prepared using a Qiagen Midi kit. The purified plasmids were then used for DNA sequencing to confirm construct integrity and for transfection of mammalian cells.
As previously described (33), plasmids for the mammalian expression vectors encoding different luciferase autoantigen fusion protein constructs were transfected into Cos1 cells with Lipofectamine 2000, and cell lysates were harvested 48 hours later to obtain crude cell extracts. For autoantibody testing, serum samples were diluted 1:10 in assay buffer A (20 mM Tris, pH 7.5, 150 mM NaCl, 5 mM MgCl2, 1% Triton X-100) and diluted aliquots (10 μl) were then evaluated using a 96-well plate format. For these tests, serum (equivalent to 1 μl of serum), 40 μl of buffer A and 50 μl of Cos1 cell extract containing 107 light units (LU) of a particular luciferase-antigen extract were used. After incubation at room temperature for one-hour, a microtiter filter plate containing protein A/G beads captured the IgG antibody-antigen complexes during a one-hour incubation. The antibody-antigen-bead complexes were then washed eight times with buffer A and twice with PBS on a microtiter filter plate to remove unbound antigens. After the final wash, LU were measured in a Berthold LB 960 Centro microplate luminometer (Berthold Technologies, Bad Wildbad). Coelenterazine substrate (Promega) was used for the detection of Renilla luciferase and Gaussia luciferase reporter activity and the Nano-Glo® substrate (Promega) was used for nanoluciferase (33).
Comparison of LIPS with other immunoassays
The serological results for the LIPS assay for the anti-MDA5 and anti-aminoacyl-tRNA-synthetase autoantibodies in the IIM group were compared with testing at the University of Pittsburgh or OMRF by immunoprecipitation and immunoprecipitation-immunoblot (34). The Ro52 LIPS results were compared with anti-Ro52 autoantibody testing by ELISA as described (23). Except for KS testing, all samples were evaluated by LIPS without knowledge of this serological immunoreactivity information. Post-hoc comparison with clinical testing was compared where applicable.
Statistical analysis
GraphPad Prism software (San Diego, CA) was used for analyzing the autoantibody levels in this study. Autoantibody levels, expressed as median log (10) LU and 25-75% interquartile range (IQR), were calculated, and presented as antilog values. The non-parametric Mann-Whitney U statistical test was used for comparison of autoantibody levels in the different subject groups. Calculations of sensitivity and specificity for the different LIPS assays involved cut-off limits derived from previous studies (18) or from the mean LU value plus five standard deviations based on values obtained from the normal controls.
A colored heatmap was used to compare the relative autoantibody levels between the different patients for each of the different seropositive antigens. The mean plus five standard deviations cutoff value based on the healthy volunteers was first subtracted from the autoantibody levels for each antibody-serum pair. The resulting value was then divided by the corresponding standard deviation for the specific autoantibody to yield a relative level of the autoantibody above these baseline values and was then color-coded from pink to dark black.
Results
Characteristics of the subjects in the cohort with and without ILD
The cohort of subjects studied included 26 with SjD, 25 with IIM, along with 10 healthy volunteers as a reference group (Table 1). Ten (38%) of the SjD and 21 (84%) of the IIM patients had ILD, respectively. Among the IIM group, 15 patients were classified with adult dermatomyositis (ADM) (one also had overlapping autoimmune thyroid disease), 6 with adult polymyositis (APM), 3 participants with juvenile dermatomyositis (JDM) (one with overlapping scleroderma), and one with juvenile polymyositis (JPM) overlapping with SLE. Within the SjD group, determination of the focus score, a marker of inflammation within the salivary gland, showed that the mean focus score in the SjD without and with ILD was 4.8 and 3.0, respectively.
Detection of aminoacyl-tRNA synthetase autoantibodies by LIPS
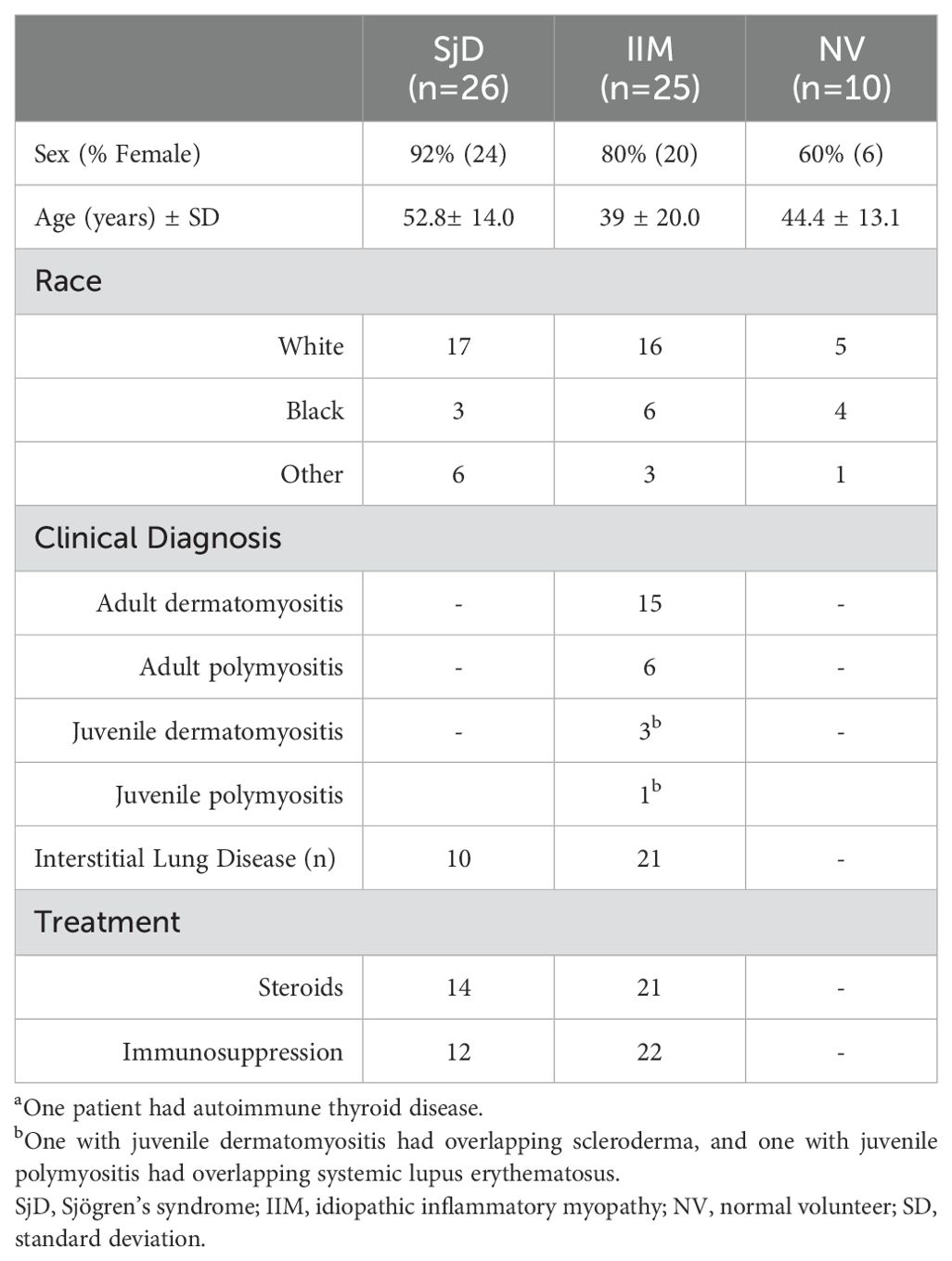
Autoantibodies against eight different aminoacyl-tRNA-synthetases were tested in the cohort. Among the anti-aminoacyl-tRNA-synthetase autoantibodies examined, anti-Jo-1 autoantibody was the most prevalent in our IIM cohort, with 5 patients (20%) displaying seropositivity. The median level for anti-Jo-1 autoantibodies was 1,176,600 LU and included a wide dynamic range (IQR: 604,000-1,940,000). In contrast, seronegative IIM patients exhibited a significantly lower median anti-Jo-1 autoantibody level of 1,586 LU (IQR: 930-3490) (Figure 1A). Analysis of anti-PL7 autoantibodies identified two seropositive IIM patients (Figure 1B). Notably, one patient exhibited high autoantibody levels, while another displayed levels near the cut-off value. The assessment of other tRNA-synthetase autoantibodies revealed one patient with anti-PL12 autoantibodies (Figure 1C), one with anti-Zo autoantibodies (Figure 1D), and one patient with anti-KS autoantibodies (Figure 1E). Of note, none of the patients within the IIM cohort exhibited anti-Ha (Figure 1F), anti-EJ, or anti-OJ autoantibodies (data not shown). Although independent testing did not examine anti-Zo autoantibodies, overall, the tRNA-synthetase autoantibody profile determined by OMRF testing closely matched the results from LIPS (Supplementary Table 1). However, one APM-ILD subject who had both anti-Jo-1 and anti-PL7 autoantibodies in the LIPS assay, was only positive for anti-Jo-1 autoantibodies by the OMRF immunoprecipitation assay. In the SjD cohort, one patient without clinical signs of ILD was positive for anti-Jo-1 autoantibodies (Figure 1A), and two patients with SjD-ILD were positive for anti-KS autoantibodies (Figure 1E).
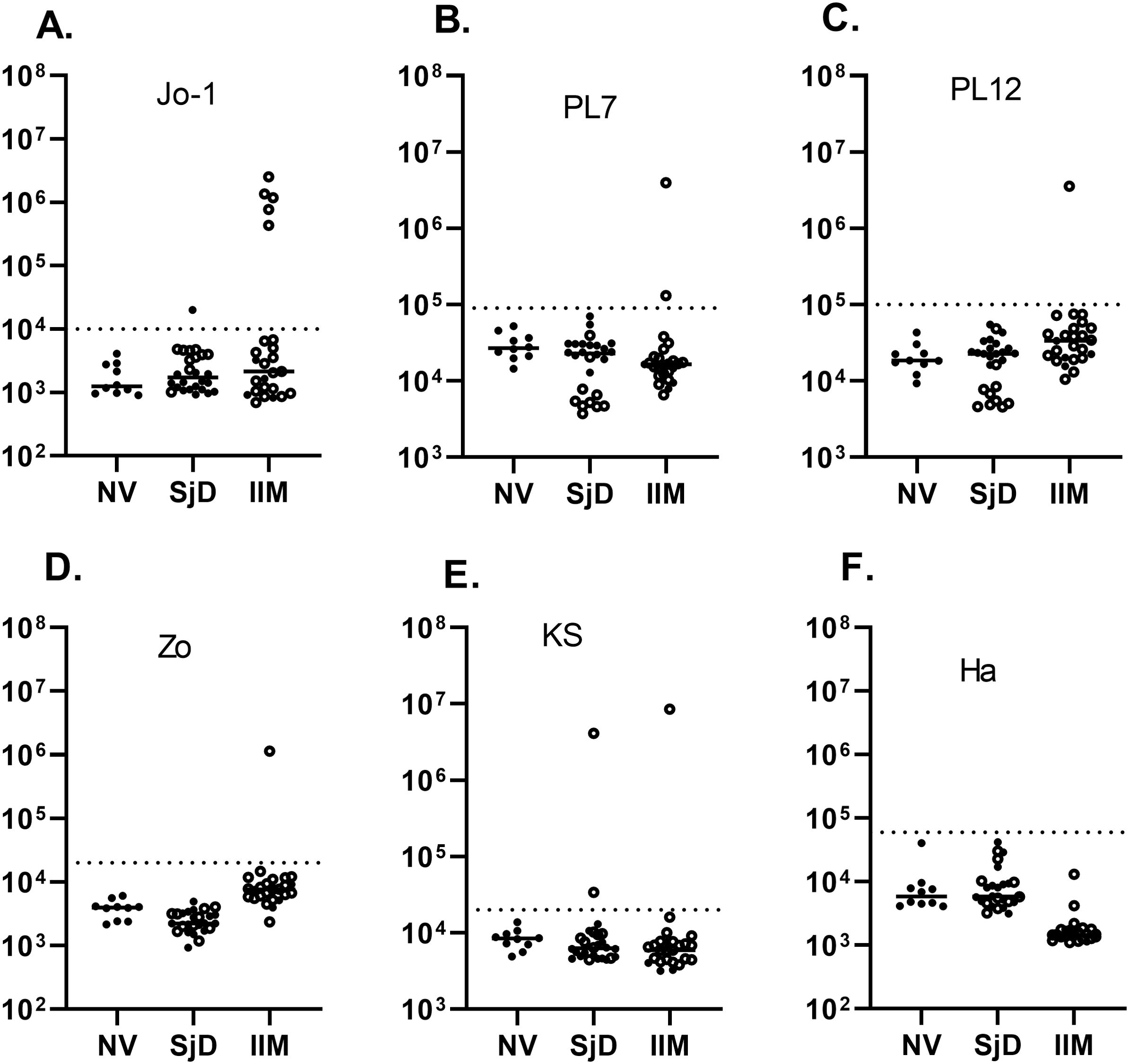
Figure 1. Autoantibodies against a panel of aminoacyl-tRNA synthetases. Autoantibody levels by LIPS were determined against six aminoacyl-tRNA synthetases including (A) Jo-1, (B) PL7, (C) PL12, (D) Zo, (E) KS, and (F) Ha. Each symbol represents a sample from subjects who were healthy volunteers (NV) or diagnosed with SjD (Sjogren’s disease) and IIM (idiopathic inflammatory myopathy). SjD and IIM subjects with interstitial lung disease are shown by the open circles. Autoantibody levels are plotted in light units on a log10 scale, and the dashed lines represent the cut-off level for determining seropositive autoantibodies for each antigen, as described in the Methods.
Detection of anti-MDA5 autoantibodies by LIPS
LIPS was also used to measure anti-MDA5 autoantibodies in the IIM and SjD cohorts. Based on the relatively large protein size of the MDA5 autoantigen, encoded by the IFIH1 gene, two protein fragments spanning the N-terminal, designated MDA5-Δ1 (aa 2-577), and C-terminal halves of the protein, designated MDA5A-Δ2 (578–1105), were generated and separately tested. Using cut-off values derived from the normal volunteers, seropositive MDA5-Δ1 autoantibodies were detected in 4 (16%) patients from the IIM group and none of SjD patients (Figure 2A). The median value for MDA5-Δ1 autoantibodies was 1,134,000 LU (IQR 473,000-2,316,000) in those four patients, which was approximately 100 times higher than the remaining 21 seronegative patients (median of 2,279 LU, IQR 1,147-3,472). Analysis for autoantibodies against the MDA5-Δ2 protein fragment showed similar results, identifying the same four IIM subjects as seropositive (Figure 2B). The comparison between the LIPS assay and the MDA5 IP-immunoblot test (OMRF) detected the same seropositive samples amongst the JDM and ADM samples (Supplementary Table 1).
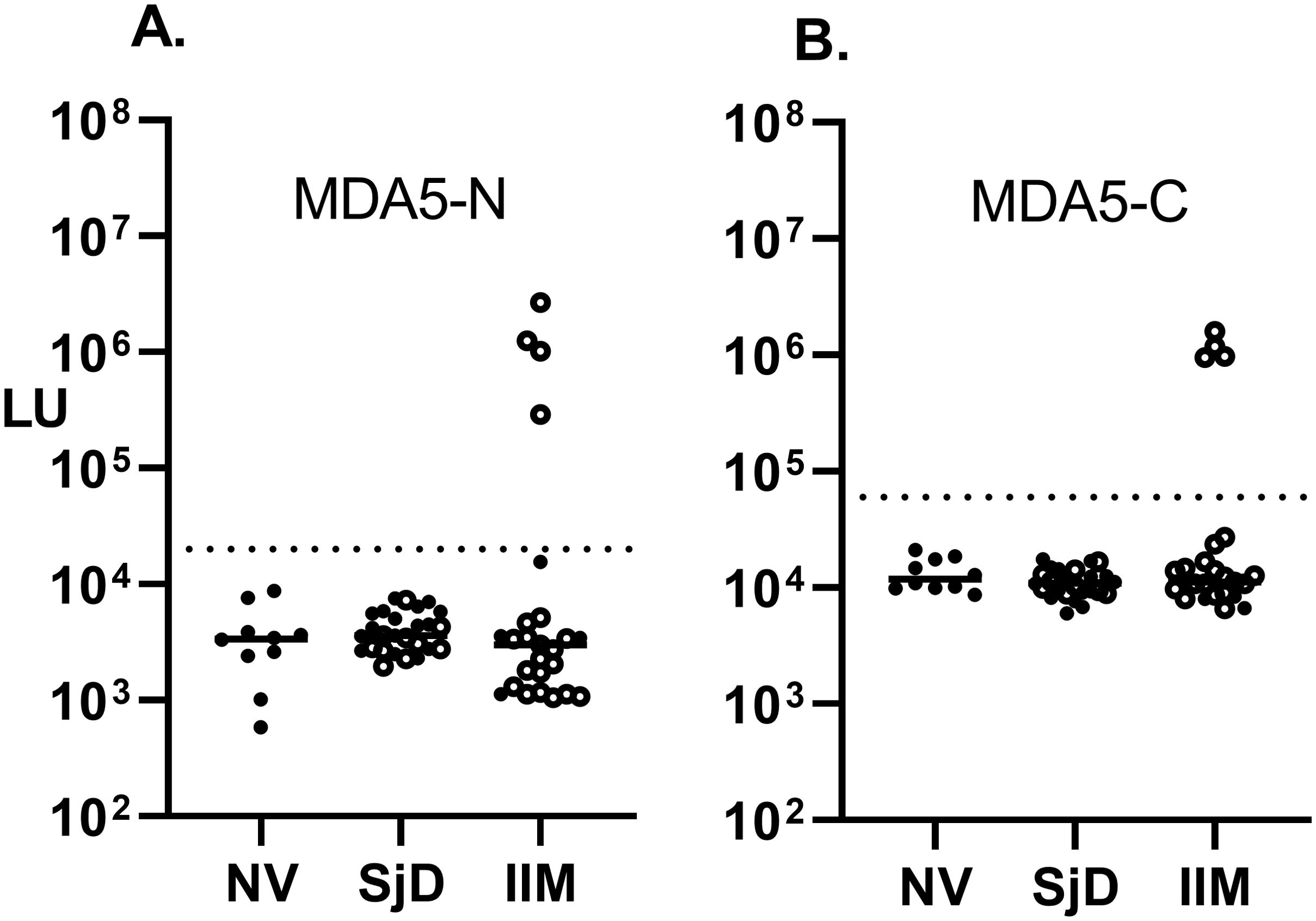
Figure 2. Detection of autoantibodies against the N- and C-terminal protein fragments of the MDA5 (IFIH1) autoantigen. Autoantibody levels against the (A) N-terminal (MDA5-N) and (B) C-terminal half of MDA5 protein (MDA5-C) were measured by LIPS in the cohort. Each symbol represents a sample from subjects who were healthy volunteers (NV) or diagnosed with SjD (Sjogren’s disease) and IIM (idiopathic inflammatory myopathy). Autoantibody levels are plotted in light units on a log10 scale, and the dashed lines represent the cut-off level for determining seropositive autoantibodies, as described in the Methods.
Autoantibodies against other targets in the IIM and SjD cohorts
In an exploratory fashion, the IIM and SjD cohorts were analyzed for autoantibodies against a panel of antigens associated with multiple autoimmune diseases, including autoimmune-associated lung disease. Anti-Ro52, a myositis-associated autoantibody, was identified in 48% (12/25) of the IIM and 61% (16/26) of the SjD subjects. These numbers increased when only patients with ILD were examined, showing as positive in 57% (12/21) of IIM-ILD and in 70% (7/10) of the SjD-ILD (Figure 3A). The median value for anti-Ro52 autoantibodies in the seropositive IIM and SjD subjects was 1,827,000 LU (IQR 640,000-2,500,000) compared to the remaining seronegative patients (median of 13,300 LU, IQR (4918-32,480). Ro52 autoantibody detection by LIPS exactly matched the available seropositive Ro52 status in the IIM subgroup determined by ELISA. Anti-Ro60 autoantibodies demonstrated a high prevalence in the non-ILD SjD (63%) and SjD-ILD (80%) patients but were only present in 5 (16%) patients in the IIM cohort (Figure 3B). TRIM38 autoantibodies, a Ro52-related molecule, were present in 30% of the non-ILD SjD cohort, 20% of the SjD-ILD cohort, and 5% of IIM-ILD patients (Figure 3C). Anti-CENP-A autoantibody, associated with systemic sclerosis, was only positive in two non-ILD SjD and one SJD-ILD patients (Figure 3D). None of the IIM patients were seropositive for anti-CENP-A autoantibody. Anti-U1-70K autoantibodies, associated with mixed connective tissue disease and overlap myositis, were seropositive in three SjD-ILD and one IIM-ILD patients (Figure 3E). In the IIM group, U1-70K autoantibody detection matched the OMRF immunoprecipitation seropositive status (Supplementary Table 1) Lastly, interferon-ω autoantibodies, associated with viral pulmonary disease, were detected in one IIM subject at relatively high levels of over 200 times higher than the controls, and at low levels in another IIM individual (Figure 3F). Further testing for anti-interferon-α5 autoantibodies revealed seropositivity exclusively in the one IIM individual exhibiting elevated levels of auto-IFN-ω autoantibodies, but in no other samples (data not shown). Autoantibody testing against two other autoantigens, KCNRG and BPIFB1, found in autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED) patients with autoimmune pneumonia, revealed no seropositivity in any of the SjD or IIM subjects (data not shown). Similarly, autoantibodies to two other antigens, ABLIM (35) and CDHR5 (36), associated with pulmonary disease in two recent studies identified by phage immunoprecipitation sequencing (PhIP-Seq) were seronegative by LIPS in the cohort (data not shown).
Figure 3. Autoantibodies against six known pulmonary-associated autoantigens by LIPS assay. Autoantibody levels against six known autoantigens including (A) Ro52, (B) Ro60, (C) TRIM38, (D) CENP-A, (E) U1-70K, and (F) IFN-omega determined by LIPS. Each symbol represents a sample from individual healthy subjects (NV) or patients with SjD and IIM. Autoantibody levels are plotted in light units on a log10 scale, and the dashed lines represent the cut-off level for determining seropositive autoantibodies for each antigen as described in the Methods.
Heatmap analysis of autoantibody immunoreactivity
To further understand the heterogeneous immunoreactivity seen in the IIM and SjD patients without and with ILD, a heatmap analysis was generated. From the LIPS testing for 21 autoantibodies, 13 target antigens were seropositive (Figures 4A, B). A color code was used to denote the relative number of standard deviations in the autoantibody levels in the disease case sera above the corresponding cut-off value for the healthy controls. In the case of IIM (Figure 4), the type of myositis and the presence of ILD is listed along with the corresponding physician global score (PGS) of disease activity. All IIM patients without ILD (n=4) showed no seropositivity to any of the autoantigens measured in the study (Figure 4). In contrast, 17 of the 21 IIM-ILD sera showed heterogenous seropositivity to at least one autoantigen in the panel. The relative number of autoantibodies or level of seropositivity seen in the IIM patients did not correlate with the overall physician global disease activity. The most informative myositis-specific autoantibodies were anti-Jo-1 autoantibodies, present in three APM and two ADM patients, followed by anti-MDA5 autoantibodies, present in two ADM and two JDM patients (Figure 4A). Of note, seropositivity against the five aminoacyl tRNA-synthetases (Jo1, PL7, PL12, Zo and KS) in the IIM-ILD patients did not overlap with the MDA5 seropositive patients. Ro52 seropositive IIM cases were much more likely to harbor anti-MDA-5 and anti-synthetase autoantibodies (Fisher’s exact test P=0.035). Interestingly, the only IIM patient who had high levels U1-70K autoantibodies had the clinical diagnosis of JPM/SLE and had high levels of autoantibodies against both IFN-α and IFN-ω. This patient was seronegative for the eight other anti-synthetase autoantibodies measured in the study. While anti-CENP-A autoantibody (associated with scleroderma), and anti-Ro-60 (associated with SjD) were not informative in the IIM cohort, anti-Ro-52 autoantibody was present in 52% (n=11) of IIM-ILD patients.
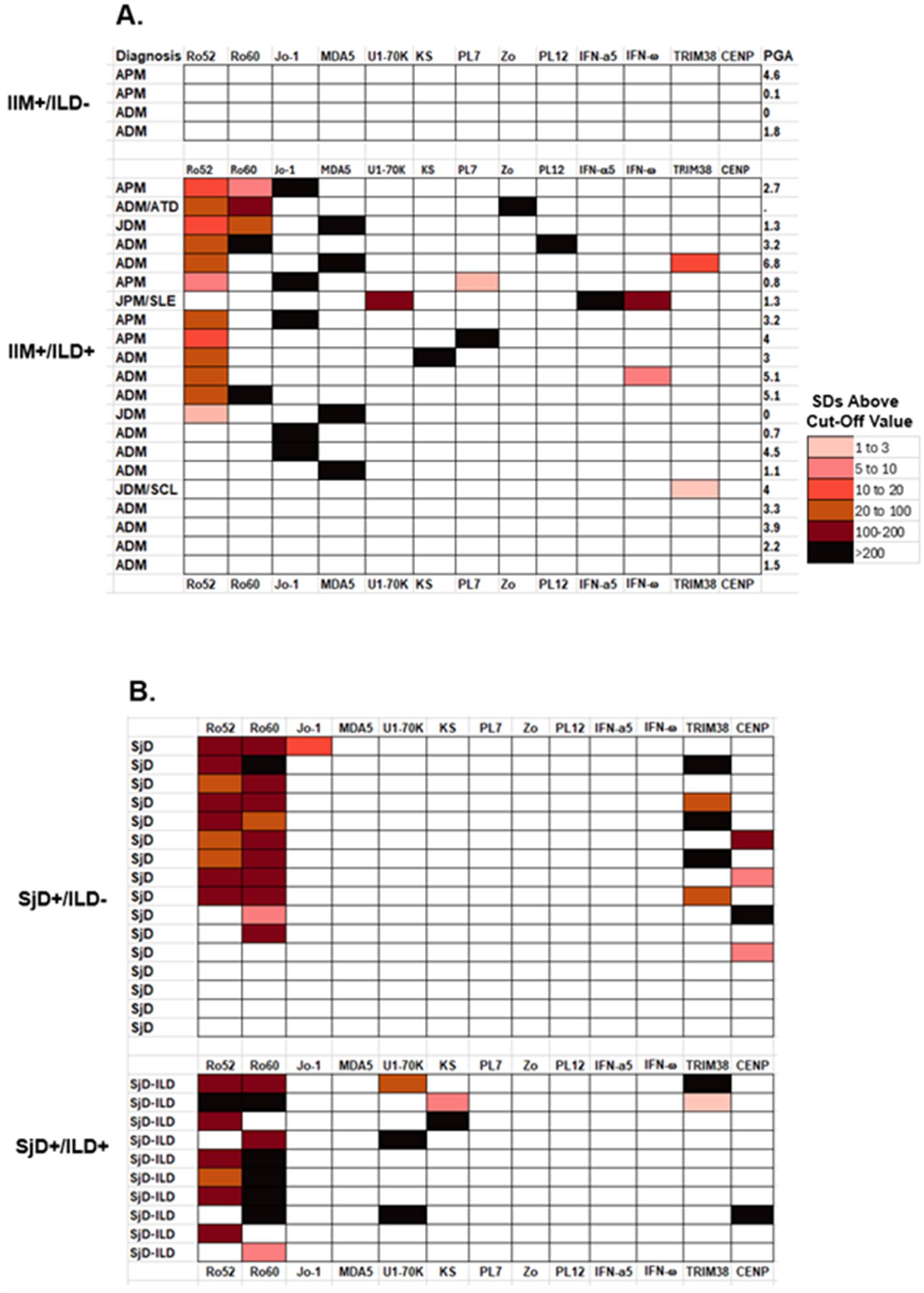
Figure 4. Heatmap analysis of autoantibodies in the IIM and SjD cases. Heatmap analysis shows the seropositivity observed in (A) IIM patients with and without ILD and (B) in the SjD cases with and without ILD. Autoantibody levels against 13 of the target proteins are shown where there is seropositivity in at least one subject. Only the results with N-terminal fragment for MDA5 are shown. Color coding denotes relative autoantibody levels in standard deviations above the baseline cut-off value. Autoantibody levels in the patients ranged from low levels (pink) to extremely high autoantibody levels (black). The clear boxes represent seronegative responses with the autoantigens in each subject. Physician global activity score (PGA) is shown for the IIM subjects on the left side of the panel.
Analysis of the autoantibody seropositivity in the SjD patients showed less immunoreactivity to the full panel of autoantigens (Figure 4B). All SjD patients, with and without ILD, showed high levels of Ro52 and Ro60 autoantibodies, whereas only five patients were positive for CENP-A autoantibodies. TRIM38 autoantibodies, associated with increased severity of salivary damage in other published cohorts (32, 37), were identified in 30% (n=5) of non-ILD SjD and 20% (n=2) of SjD-ILD patients and were not useful for identifying patients with ILD. Two autoantibodies showed significant seropositivity in the SjD-ILD group only. Three ILD patients had anti-U1-70K autoantibodies and two had anti-KS autoantibodies, while none of the SjD patients without ILD was seropositive for these two autoantibodies. The two SjD-ILD patients with anti-KS autoantibodies were also positive for anti-Ro52 autoantibodies (Figure 4B). In the SjD without ILD group, only one patient had low levels of anti-Jo-1 autoantibodies, who had recognized clinical muscle weakness, but had normal serum creatine kinase levels.
Measuring Jo-1 autoantibodies longitudinally in treated IIM-ILD patients
Based on prior reports of association of autoantibody levels with disease activity, LIPS was used to evaluate changes in anti-Jo-1 autoantibody levels in IIM patients with sequential samples who had been receiving rituximab immunosuppressive treatment and had clinical and lung function data available. As shown in Figure 5A, four of the five subjects were Jo-1 seropositive (pt #5 was Jo-1 seronegative). While the anti-Jo-1 autoantibodies decreased over time, two patients (Pt #1 and Pt #3) showed a marked decrease, with over a 3.5-fold decline in Jo-1 autoantibody levels over the course of one year. The decline in anti-Jo-1 autoantibodies modestly tracked the clinical improvement in pulmonary function testing, in which FVC% (Figure 5B) and DLCO% (Figure 5C) remained stable or increased in the four anti-Jo1 seropositive patients. Conversely, creatine kinase levels (Figure 5D) and physician global activity score (Figure 5E) decreased in the patients and tracked with the decline in anti-Jo-1 autoantibody levels.
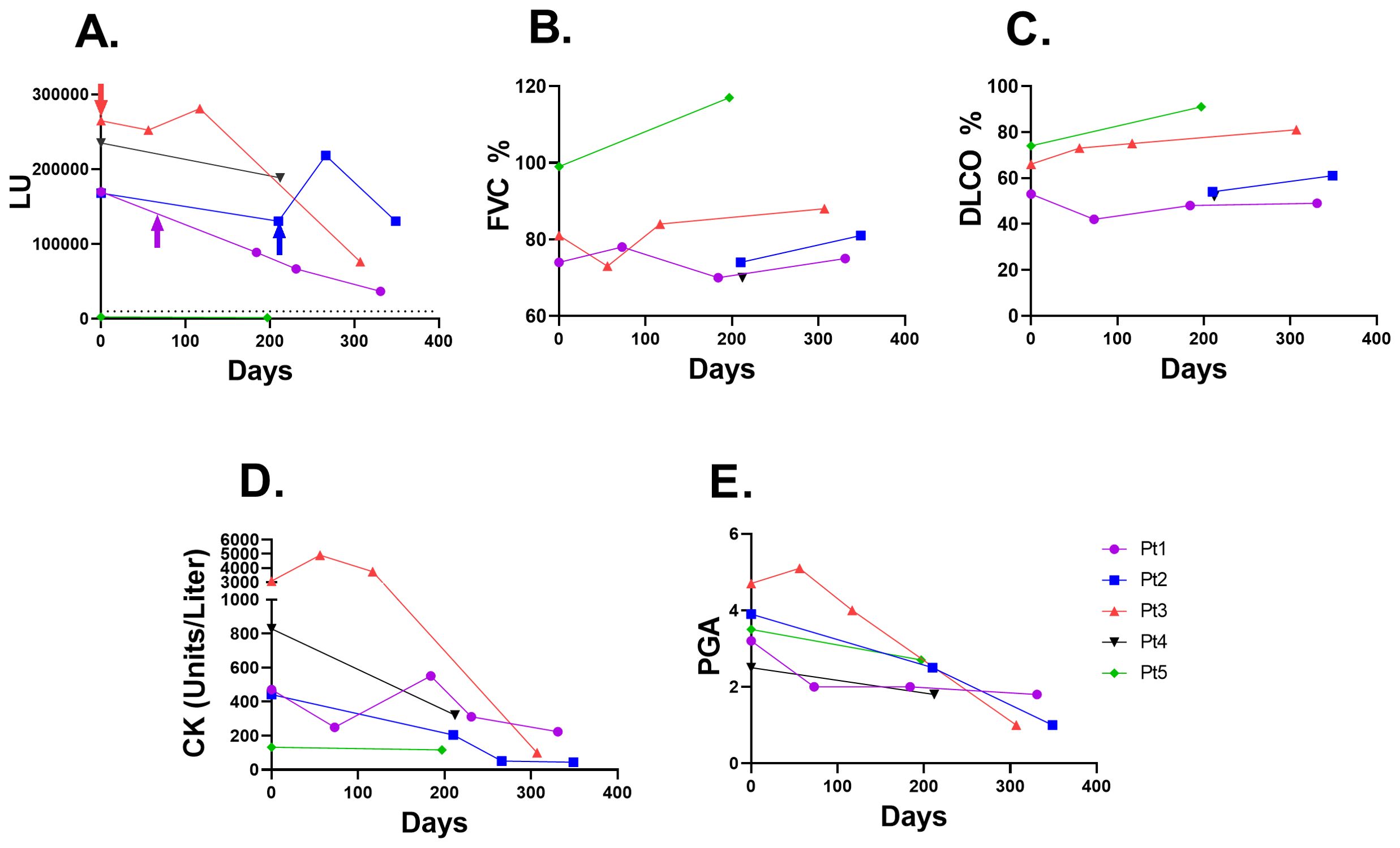
Figure 5. Jo-1 autoantibody trajectory following rituximab immunosuppressive treatment in IIM-ILD patients. (A) Jo-1 autoantibody levels (LU) are shown over time in five IIM-ILD patients. Three of the patients (Pt 1-3) received rituximab therapy, in which the time of rituximab administration is shown by the arrow. The cut-off for Jo-1 seropositivity is shown by the dotted line. Patient #5 was seronegative for Jo-1 autoantibodies. (B) The corresponding lung function of forced vital capacity (FVC) percent changes (C) Diffusion capacity of the lungs for carbon monoxide (DLCO) changes over time (D) Creatine kinase (CK) levels and (E) Physician global activity (PGA) score for each of the serial samples from the patients shown in panel (A).
Discussion
There is an increasing interest in developing serological biomarkers for screening and monitoring patients with ILD. In this study, we demonstrate the utility of the fluid-phase LIPS assay to detect and quantify a panel of over 14 different autoantibodies, including myositis-specific and myositis-associated autoantibodies in patients with IIM and additional autoantibodies in SjD. The results obtained by LIPS with the IIM patient group for myositis-specific autoantibodies were nearly identical to those obtained via immunoprecipitation by two reference laboratories. First, the detection of anti-MDA-5, -Jo-1, -PL12, and -KS autoantibodies exactly matched the seropositivity status of these other established immunoassays. However, for some of the other targets, there were some discrepancies. While LIPS detected the same sera with PL7 autoantibodies, it also detected an additional seropositive PL7 signal in a Jo-1-positive patient that was not detected by standard immunoprecipitation. While this may be a false positive anti-PL7 autoantibody result, given the rarity of having two anti-synthetase autoantibodies in the same patient, it may also be accurate and reflect the increased sensitivity of the LIPS assay. Moreover, dual anti-tRNA synthetase autoantibody positivity has been reported in a recent study using a multiplex bead array assay (38). LIPS also detected a single Zo seropositive case that was not tested by immunoprecipitation. Beyond providing seropositive/seronegative status, LIPS also provided a wide dynamic range of myositis-specific autoantibody levels that often ranged from 5000 to 3 million LU, a span of three log10, in which many of the seropositive autoantibody subjects often had values 100 times higher than the seronegative cases. A recent study identified 16 anti-tRNA synthetase autoantibodies in 72 IIM patients, including the detection of novel autoantibodies that had not been reported in standard assays in patients previously classified as myositis-specific autoantibody negative (38). While the multiplex bead array technology used in that study allowed the identification of novel autoantibodies, it did not quantify autoantibody levels. Nevertheless, these results highlight the possibility that additional anti-synthetase autoantibodies might be included to expand the LIPS assay to measure and quantify autoantibodies in IIM.
Among SjD patients, LIPS demonstrated the presence of U1-70K and KS autoantibodies only in patients with ILD. This is a clinically important finding given that ILD is underdiagnosed in SjD (39). U1-70K autoantibodies, associated with mixed connective tissue, were detected in 3 of the 10 SjD-ILD subjects and none of the SjD subjects without ILD. U1-70K autoantibodies have been found to be a superior marker of mixed connective tissue disease (40) and may be useful in the evaluation of patients with SjD. In our study, LIPS identified and quantified anti-KS autoantibodies in 2 SjD patients, both with ILD. One SjD-ILD subject had quite high levels of KS autoantibodies, and the other subject had lower levels that might be expected to be missed by Western blotting or other immunoassays. While neither of the two KS seropositive SjD-ILD cases reported elevated levels of serum creatine kinase, one individual exhibited muscle weakness. The finding of KS seropositive autoantibodies associated with SjD-ILD is consistent with a recent study by Hosono et al., who screened a cohort of patients with IIM-ILD for KS autoantibodies and detected 9% (16/177 patients) as positive, in which 4 patients had SjD (7). The most striking finding was that half of the anti-KS-positive patients had sicca symptoms, which is one of the key diagnostic criteria for the diagnosis of SS. Four of these patients with sicca symptoms were diagnosed as SjD, which is an uncommon clinical feature in anti-synthetase syndrome. Also, this study found that these patients with KS autoantibodies had less severe myositis and ILD, without muscle weakness and elevated serum creatine kinase levels, and had a relatively favorable prognosis (7). Several case reports have also found KS autoantibodies in SjD. In another study, however, only 1 of 19 patients with anti-KS autoantibodies had SjD (41). The finding that 20% of SjD-ILD had anti-KS autoantibodies in our study may be due to the higher level of sensitivity by LIPS than existing immunoassays. Based on the results, the anti-U1-70K and anti-KS autoantibodies should be further explored as potentially useful markers to be included in the evaluation of patients with SjD, particularly as they might increase the recognition of ILD in this population.
Despite the promising detection and quantification of autoantibodies using LIPS, 30% of the IIM-ILD and 50% of the SjD-ILD subjects did not harbor known autoantibody biomarkers associated with ILD. While there are several factors that could explain this phenomenon (stage of disease, immunosuppressive medications, restricted HLA alleles, among other covariates), it is likely that other autoantibodies that we did not test for, particularly other anti-tRNA-synthetases autoantibodies, are present in these subjects (42). Of note, most of these patients were negative for other autoantibodies associated with autoimmunity and/or lung autoimmunity in other conditions (KCNRG, BPIFB1, TRIM38, CENP-A, interferon-ω, ABLIM, and CDHR) suggesting some specificity for ILD in the context of SjD.
Autoantibodies to TRIM38, a Ro52-related molecule, which have been associated with increased immune infiltrates in the salivary gland and more severe disease in SjD disease (32, 37), were not significantly increased in prevalence in SjD-ILD, highlighting how the immune attack on the lung and salivary gland can occur independently of each other. Interestingly, one JPM/SLE subject who was seronegative for anti-synthetase and anti-MDA5 autoantibodies had anti-U1-70K autoantibodies with high levels of IFN-ω autoantibodies and to a lesser extent with interferon-α autoantibodies. Autoantibodies to interferon-ω and interferon-α are now well-recognized to be associated with high morbidity with lung damage in older male patients infected with SARS-CoV-2 (43) and even contribute to poor outcomes in patients with influenza (44). Interestingly, this patient with anti-interferon autoantibodies had moderate severity of lung disease with dyspnea at rest, cough, pulmonary function tests consistent with restrictive lung disease and the lung biopsy revealed non-specific interstitial pneumonia. Although we did not test the serum of this patient for its capacity to neutralize interferon-ω activity (due to lack of available sample), the relative levels of ant-interferon-ω autoantibodies detected by LIPS are consistent with the possibility that the autoantibodies present in this subject are neutralizing. Future studies screening for a wider range of anti-interferon and other anti-cytokine autoantibodies and their inhibitory effects in a larger cohort of IIM-ILD subjects is warranted.
Previous studies using LIPS have revealed that levels of pathogenic autoantibodies can be used to monitor disease activity during treatment including in patients with membranous nephropathy (45) and Graves’ disease (18). In the present study, we quantified anti-Jo1 autoantibody levels in a small number of subjects with longitudinal data. Our results showed that anti-Jo-1 autoantibody levels decreased over time in patients treated with immunosuppressive therapy whose lung function stabilizes or improves over time. While this has been suggested in a few previous reports in patients with IIM (14, 46–48) and scleroderma (49, 50), the wide dynamic range of levels identified via LIPS may allow us to better assess the role of autoantibody levels as markers of disease activity and treatment response using a highly sensitive, specific, and inexpensive screening tool.
In summary, our study demonstrates LIPS can accurately detect and quantify numerous myositis-specific, myositis-associated, and other lung-related autoantibodies and can quantify a wide range of autoantibody levels in patients with IIM and SjD. Given the known challenges with standard assays measuring non-Jo-1 autoantibodies (16), LIPS is a promising assay that may have a significant role in clinical practice. Furthermore, in SjD, LIPS led to the identification of other relevant non-SjD-related autoantibodies which reflect important clinical features of disease activity for ILD. This will facilitate the characterization of clinically relevant subsets of patients. Limitations of our study include the small sample sizes of each patient group and the lack of concomitant measures of lung function or symptoms on the day of blood sampling. It is also possible that we underestimated the seroprevalence of a few of the anti-synthetase autoantibodies due to the use of protein fragments instead of full-length protein, but to evaluate will require testing a larger cohort of samples. Future studies applying LIPS to evaluate a wider range of autoantibodies in a large cohort of deeply phenotyped ILD subjects over time are warranted to assess whether quantitative autoantibody levels can be useful biomarkers in the stratification, disease monitoring, and treatment responses in patients with autoimmune ILD.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Institutional review board-approved protocols from NIEHS, National Institutes of Health (94-E-0165, 11-E-0072, 07-E-0012, 05-E-N200) and NIDCR, National Institutes of Health (15-D-0051, 11-D-0172). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
PB: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. JH: Conceptualization, Writing – original draft, Writing – review & editing, Data curation, Investigation, Supervision, Formal analysis. ZK: Data curation, Resources, Writing – review & editing. MB: Data curation, Resources, Writing – review & editing. IP-F: Formal analysis, Investigation, Writing – review & editing. AM: Formal analysis, Investigation, Writing – review & editing. JC: Funding acquisition, Resources, Supervision, Writing – review & editing. PN: Writing – review & editing, Data curation, Formal analysis. FM: Resources, Writing – review & editing. AS: Data curation, Resources, Writing – review & editing, Investigation. KS: Resources, Writing – review & editing, Data curation. BW: Conceptualization, Investigation, Resources, Supervision, Writing – review & editing. LR: Conceptualization, Investigation, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by intramural research awards of the National Institute of Dental and Craniofacial Research (1ZIADE000695 and Z01-DE000704), of the National Institute of Environmental Health Sciences (Z01-ES101081) and of the National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Institutes of Health. PN was funded with Federal funds from the National Institutes of Health, Department of Health and Human Services, under contract 75N95021D00012.
Acknowledgments
We thank Drs. Ira Targoff and Chester Oddis for myositis autoantibody testing. We thank the clinical support staff of the NIEHS Environmental Autoimmunity Group and the NIDCR Sjögren’s Disease Clinic.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1462242/full#supplementary-material
Abbreviations
CK, Creatine kinase; DLCO, diffusing capacity of the lungs for carbon monoxide; FVC, forced vital capacity percent; ILD, interstitial lung diseases; IIM, idiopathic inflammatory myopathies; IQR, interquartile range; SjD, Sjogren’s disease; LIPS, luciferase immunoprecipitation systems assay system; LU, light units; NV, normal volunteer; ATD, autoimmune thyroid disease; SLE, systemic lupus erythematosus; SCL, scleroderma.
References
1. Wijsenbeek M, Cottin V. Spectrum of fibrotic lung diseases. N Engl J Med. (2020) 383:958–68. doi: 10.1056/NEJMra2005230
2. Panagopoulos P, Goules A, Hoffmann-Vold AM, Matteson EL, Tzioufas A. Natural history and screening of interstitial lung disease in systemic autoimmune rheumatic disorders. Ther Adv Musculoskelet Dis. (2021) 13:1759720X211037519. doi: 10.1177/1759720X211037519
3. Galindo-Feria AS, Notarnicola A, Lundberg IE, Horuluoglu B. Aminoacyl-tRNA synthetases: on anti-synthetase syndrome and beyond. Front Immunol. (2022) 13:866087. doi: 10.3389/fimmu.2022.866087
4. Andersson H, Aalokken TM, Gunther A, Mynarek GK, Garen T, Lund MB, et al. Pulmonary involvement in the antisynthetase syndrome: A comparative cross-sectional study. J Rheumatol. (2016) 43:1107–13. doi: 10.3899/jrheum.151067
5. Sato S, Hoshino K, Satoh T, Fujita T, Kawakami Y, Fujita T, et al. RNA helicase encoded by melanoma differentiation-associated gene 5 is a major autoantigen in patients with clinically amyopathic dermatomyositis: Association with rapidly progressive interstitial lung disease. Arthritis Rheumatol. (2009) 60:2193–200. doi: 10.1002/art.24621
6. Pinal-Fernandez I, Casal-Dominguez M, Huapaya JA, Albayda J, Paik JJ, Johnson C, et al. A longitudinal cohort study of the anti-synthetase syndrome: increased severity of interstitial lung disease in black patients and patients with anti-PL7 and anti-PL12 autoantibodies. Rheumatol (Oxford). (2017) 56:999–1007. doi: 10.1093/rheumatology/kex021
7. Hosono Y, Ishii A, Izumi Y, Sasaki N, Nogi S, Yamada C, et al. New aspects of clinical and immunological characteristics in patients with anti-asparaginyl tRNA synthetase (anti-KS) autoantibody. Mod Rheumatol. (2023) 34:122–8. doi: 10.1093/mr/road011
8. Galindo-Feria AS, Wang G, Lundberg IE. Autoantibodies: pathogenic or epiphenomenon. Best Pract Res Clin Rheumatol. (2022) 36:101767. doi: 10.1016/j.berh.2022.101767
9. Choi MY, Satoh M, Fritzler MJ. Update on autoantibodies and related biomarkers in autoimmune inflammatory myopathies. Curr Opin Rheumatol. (2023) 35:383–94. doi: 10.1097/BOR.0000000000000957
10. Pinal-Fernandez I, Munoz-Braceras S, Casal-Dominguez M, Pak K, Torres-Ruiz J, Musai J, et al. Pathological autoantibody internalisation in myositis. Ann Rheum Dis. (2024). doi: 10.1136/ard-2024-225773
11. Pinal-Fernandez I, Milisenda JC, Pak K, Munoz-Braceras S, Casal-Dominguez M, Torres-Ruiz J, et al. Transcriptional derepression of CHD4/NuRD-regulated genes in the muscle of patients with dermatomyositis and anti-Mi2 autoantibodies. Ann Rheum Dis. (2023) 82:1091–7. doi: 10.1136/ard-2023-223873
12. Gayed C, Uzunhan Y, Cremer I, Vieillard V, Hervier B. Immunopathogenesis of the anti-synthetase syndrome. Crit Rev Immunol. (2018) 38:263–78. doi: 10.1615/CritRevImmunol.v38.i4
13. Muller F, Taubmann J, Bucci L, Wilhelm A, Bergmann C, Volkl S, et al. CD19 CAR T-cell therapy in autoimmune disease - A case series with follow-up. N Engl J Med. (2024) 390:687–700. doi: 10.1056/NEJMoa2308917
14. Aggarwal R, Oddis CV, Goudeau D, Koontz D, Qi Z, Reed AM, et al. Autoantibody levels in myositis patients correlate with clinical response during B cell depletion with rituximab. Rheumatol (Oxford). (2016) 55:991–9. doi: 10.1093/rheumatology/kev444
15. Damoiseaux J, Vulsteke JB, Tseng CW, Platteel ACM, Piette Y, Shovman O, et al. Autoantibodies in idiopathic inflammatory myopathies: Clinical associations and laboratory evaluation by mono- and multispecific immunoassays. Autoimmun Rev. (2019) 18:293–305. doi: 10.1016/j.autrev.2018.10.004
16. Loganathan A, Zanframundo G, Yoshida A, Faghihi-Kashani S, Bauer Ventura I, Dourado E, et al. Agreement between local and central anti-synthetase antibodies detection: results from the Classification Criteria of Anti-Synthetase Syndrome project biobank. Clin Exp Rheumatol. (2024) 42:277–87. doi: 10.55563/clinexprheumatol/s14zq8
17. Burbelo PD, Lebovitz EE, Notkins AL. Luciferase immunoprecipitation systems for measuring antibodies in autoimmune and infectious diseases. Transl Res. (2015) 165:325–35. doi: 10.1016/j.trsl.2014.08.006
18. Burbelo PD, Gordon SM, Waldman M, Edison JD, Little DJ, Stitt RS, et al. Autoantibodies are present before the clinical diagnosis of systemic sclerosis. PloS One. (2019) 14:e0214202. doi: 10.1371/journal.pone.0214202
19. Ching KH, Burbelo PD, Tipton C, Wei C, Petri M, Sanz I, et al. Two major autoantibody clusters in systemic lupus erythematosus. PloS One. (2012) 7:e32001. doi: 10.1371/journal.pone.0032001
20. Burbelo PD, Leahy HP, Issa AT, Groot S, Baraniuk JN, Nikolov NP, et al. Sensitive and robust luminescent profiling of anti-La and other autoantibodies in Sjogren’s syndrome. Autoimmunity. (2009) 42:515–24. doi: 10.1080/08916930902911738
21. Burbelo PD, Ching KH, Issa AT, Loftus CM, Li Y, Satoh M, et al. Rapid serological detection of autoantibodies associated with Sjögren’s syndrome. J Trans Med. (2009) 7:83. doi: 10.1186/1479-5876-7-83
22. Burbelo PD, Ching KH, Han BL, Bush ER, Reeves WH, Iadarola MJ. Extraordinary antigenicity of the human Ro52 autoantigen. Am J Transl Res. (2010) 2:145–55.
23. Sabbagh S, Pinal-Fernandez I, Kishi T, Targoff IN, Miller FW, Rider LG, et al. Anti-Ro52 autoantibodies are associated with interstitial lung disease and more severe disease in patients with juvenile myositis. Ann Rheum Dis. (2019) 78:988–95. doi: 10.1136/annrheumdis-2018-215004
24. Xing X, Li A, Li C. Anti-Ro52 antibody is an independent risk factor for interstitial lung disease in dermatomyositis. Respir Med. (2020) 172:106134. doi: 10.1016/j.rmed.2020.106134
25. Pepper E, Vilar L, Ward IM. Clinical characteristics and prognostic value of ro52/SSA antibodies in idiopathic inflammatory myopathies. J Clin Rheumatol. (2023) 29:347–53. doi: 10.1097/RHU.0000000000002015
26. Shao C, Sun Y, Huang H, Zhang Z, Pan R, Xu K, et al. Myositis specific antibodies are associated with isolated anti-Ro-52 associated interstitial lung disease. Rheumatol (Oxford). (2022) 61:1083–91. doi: 10.1093/rheumatology/keab488
27. Lv C, You H, Xu L, Wang L, Yuan F, Li J, et al. Coexistence of anti-ro52 antibodies in anti-MDA5 antibody-positive dermatomyositis is highly associated with rapidly progressive interstitial lung disease and mortality risk. J Rheumatol. (2023) 50:219–26. doi: 10.3899/jrheum.220139
28. Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts). N Engl J Med. (1975) 292:344–7. doi: 10.1056/NEJM197502132920706
29. Bottai M, Tjarnlund A, Santoni G, Werth VP, Pilkington C, de Visser M, et al. EULAR/ACR classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups: a methodology report. RMD Open. (2017) 3:e000507. doi: 10.1136/rmdopen-2017-000507
30. Rider LG, Werth VP, Huber AM, Alexanderson H, Rao AP, Ruperto N, et al. Measures of adult and juvenile dermatomyositis, polymyositis, and inclusion body myositis: Physician and Patient/Parent Global Activity, Manual Muscle Testing (MMT), Health Assessment Questionnaire (HAQ)/Childhood Health Assessment Questionnaire (C-HAQ), Childhood Myositis Assessment Scale (CMAS), Myositis Disease Activity Assessment Tool (MDAAT), Disease Activity Score (DAS), Short Form 36 (SF-36), Child Health Questionnaire (CHQ), physician global damage, Myositis Damage Index (MDI), Quantitative Muscle Testing (QMT), Myositis Functional Index-2 (FI-2), Myositis Activities Profile (MAP), Inclusion Body Myositis Functional Rating Scale (IBMFRS), Cutaneous Dermatomyositis Disease Area and Severity Index (CDASI), Cutaneous Assessment Tool (CAT), Dermatomyositis Skin Severity Index (DSSI), Skindex, and Dermatology Life Quality Index (DLQI). Arthritis Care Res (Hoboken). (2011) 63 Suppl 11:S118–57. doi: 10.1002/acr.20532
31. Ferre EMN, Break TJ, Burbelo PD, Allgauer M, Kleiner DE, Jin D, et al. Lymphocyte-driven regional immunopathology in pneumonitis caused by impaired central immune tolerance. Sci Transl Med. (2019) 11. doi: 10.1126/scitranslmed.aav5597
32. Burbelo PD, Teos LY, Herche JL, Iadarola MJ, Alevizos I. Autoantibodies against the Immunoglobulin-Binding Region of Ro52 Link its Autoantigenicity with Pathogen Neutralization. Sci Rep. (2018) 8:3345. doi: 10.1038/s41598-018-21522-7
33. Burbelo PD, Ching KH, Klimavicz CM, Iadarola MJ. Antibody profiling by luciferase immunoprecipitation systems (LIPS). J Vis Exp. 2009 32:1549.
34. Targoff IN, Mamyrova G, Trieu EP, Perurena O, Koneru B, O’Hanlon TP, et al. A novel autoantibody to a 155-kd protein is associated with dermatomyositis. Arthritis Rheumatol. (2006) 54:3682–9. doi: 10.1002/art.22164
35. Yoon YM, Velez TE, Upadhyay V, Vazquez SE, Lee CT, Selvan KC, et al. Antigenic responses are hallmarks of fibrotic interstitial lung diseases independent of underlying etiologies. medRxiv. (2023). doi: 10.1101/2023.05.08.23289640
36. Upadhyay V, Yoon YM, Vazquez SE, Velez TE, Jones KD, Lee CT, et al. Phage immunoprecipitation-sequencing reveals CDHR5 autoantibodies in select patients with interstitial lung disease. ACR Open Rheumatol. (2024). doi: 10.1002/acr2.11696
37. Wolska N, Rybakowska P, Rasmussen A, Brown M, Montgomery C, Klopocki A, et al. Brief report: patients with primary Sjogren’s syndrome who are positive for autoantibodies to tripartite motif-containing protein 38 show greater disease severity. Arthritis Rheumatol. (2016) 68:724–9. doi: 10.1002/art.39497
38. Preger C, Notarnicola A, Hellstrom C, Wigren E, Fernandes-Cerqueira C, Kvarnstrom M, et al. Autoantigenic properties of the aminoacyl tRNA synthetase family in idiopathic inflammatory myopathies. J Autoimmun. (2023) 134:102951. doi: 10.1016/j.jaut.2022.102951
39. Gupta S, Ferrada MA, Hasni SA. Pulmonary manifestations of primary Sjogren’s syndrome: underlying immunological mechanisms, clinical presentation, and management. Front Immunol. (2019) 10:1327. doi: 10.3389/fimmu.2019.01327
40. Hof D, Cheung K, de Rooij DJ, van den Hoogen FH, Pruijn GJ, van Venrooij WJ, et al. Autoantibodies specific for apoptotic U1-70K are superior serological markers for mixed connective tissue disease. Arthritis Res Ther. (2005) 7:R302–9. doi: 10.1186/ar1490
41. Aiko N, Yamakawa H, Iwasawa T, Takemura T, Okudela K, Kitamura H, et al. Clinical, radiological, and pathological features of anti-asparaginyl tRNA synthetase antibody-related interstitial lung disease. Respir Investig. (2020) 58:196–203. doi: 10.1016/j.resinv.2019.12.003
42. Vulsteke JB, Derua R, Dubucquoi S, Coutant F, Sanges S, Goncalves D, et al. Mass spectrometry-based identification of new anti-Ly and known antisynthetase autoantibodies. Ann Rheum Dis. (2023) 82:546–55. doi: 10.1136/ard-2022-222686
43. Bastard P, Rosen LB, Zhang Q, Michailidis E, Hoffmann HH, Zhang Y, et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science. (2020) 370. doi: 10.1126/science.abd4585
44. Zhang Q, Pizzorno A, Miorin L, Bastard P, Gervais A, Le Voyer T, et al. Autoantibodies against type I IFNs in patients with critical influenza pneumonia. J Exp Med. (2022) 219. doi: 10.1084/jem.20220514
45. Burbelo PD, Olson SW, Keller JM, Joshi M, Schwartz DM, Chuang YJ, et al. Prediagnostic appearance of thrombospondin type-1 domain 7A autoantibodies in membranous nephropathy. Kidney360. (2023) 4:217–25. doi: 10.34067/KID.0005112022
46. Notarnicola A, Preger C, Lundstrom SL, Renard N, Wigren E, Van Gompel E, et al. Longitudinal assessment of reactivity and affinity profile of anti-Jo1 autoantibodies to distinct HisRS domains and a splice variant in a cohort of patients with myositis and anti-synthetase syndrome. Arthritis Res Ther. (2022) 24:62. doi: 10.1186/s13075-022-02745-6
47. Pinal-Fernandez I, Mecoli CA, Casal-Dominguez M, Pak K, Hosono Y, Huapaya J, et al. More prominent muscle involvement in patients with dermatomyositis with anti-Mi2 autoantibodies. Neurology. (2019) 93:e1768–e77. doi: 10.1212/WNL.0000000000008443
48. Tiniakou E, Pinal-Fernandez I, Lloyd TE, Albayda J, Paik J, Werner JL, et al. More severe disease and slower recovery in younger patients with anti-3-hydroxy-3-methylglutaryl-coenzyme A reductase-associated autoimmune myopathy. Rheumatol (Oxford). (2017) 56:787–94. doi: 10.1093/rheumatology/kew470
49. Ananyeva LP, Garzanova LA, Koneva OA, Starovoytova MN, Desinova OV, Ovsyannikova OB, et al. Anti-topoisomerase 1 antibody level changes after B cell depletion therapy in systemic sclerosis. Dokl Biochem Biophys. (2023) 511:212–8. doi: 10.1134/S1607672923700266
Keywords: interstitial lung disease, myositis-specific autoantibody, myositis-associated autoantibody, idiopathic inflammatory myopathies, Sjögren’s disease
Citation: Burbelo PD, Huapaya JA, Khavandgar Z, Beach M, Pinal-Fernandez I, Mammen AL, Chiorini JA, Noroozi Farhadi P, Miller FW, Schiffenbauer A, Sarkar K, Warner BM and Rider LG (2024) Quantification of autoantibodies using a luminescent profiling method in autoimmune interstitial lung disease. Front. Immunol. 15:1462242. doi: 10.3389/fimmu.2024.1462242
Received: 09 July 2024; Accepted: 30 September 2024;
Published: 25 October 2024.
Edited by:
Jan Stępniak, Medical University of Lodz, PolandReviewed by:
Gerson D. Keppeke, Universidad Católica del Norte, ChileDespina Michailidou, University of Oklahoma Health Sciences Center, United States
Copyright © 2024 Burbelo, Huapaya, Khavandgar, Beach, Pinal-Fernandez, Mammen, Chiorini, Noroozi Farhadi, Miller, Schiffenbauer, Sarkar, Warner and Rider. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peter D. Burbelo, YnVyYmVsb3BAbmlkY3IubmloLmdvdg==; Julio A. Huapaya, anVsaW8uaHVhcGF5YWNhcnJlcmFAbmloLmdvdg==
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work and share last authorship
 Peter D. Burbelo
Peter D. Burbelo Julio A. Huapaya
Julio A. Huapaya Zohreh Khavandgar
Zohreh Khavandgar Margaret Beach3
Margaret Beach3 Iago Pinal-Fernandez
Iago Pinal-Fernandez Andrew L. Mammen
Andrew L. Mammen John A. Chiorini
John A. Chiorini Payam Noroozi Farhadi
Payam Noroozi Farhadi Frederick W. Miller
Frederick W. Miller Adam Schiffenbauer
Adam Schiffenbauer Kakali Sarkar
Kakali Sarkar Blake M. Warner
Blake M. Warner Lisa G. Rider
Lisa G. Rider