- 1Department of Medical, Taixing People's Hospital, Taixing, China
- 2School of Public Health, Wuhan University, Wuhan, China
- 3Department of Health Promotion, XiaoGan Center For Disease Control and Prevention, Xiaogan, China
- 4Department of Medical Optics, Hospital of Stomatology Wuhan University, Wuhan, China
Background: Respiratory health is closely related to immune system function, and diet can also influence immune homeostasis. Diet, an important part of a healthy lifestyle, is also linked to respiratory health. We aimed to explore the relationship between different dietary patterns and the risk of chronic respiratory diseases (CRDs), including chronic bronchitis (CB), emphysema, and asthma.
Method: A total of 23,042 adults from the United States were selected from the National Health and Nutrition Examination Survey (NHANES) dataset between 2007 and 2018. Diet quality was assessed using 2-day, 24-hour dietary recall data and quantified as the Healthy Eating Index-2020 (HEI-2020), the Dietary Inflammation Index (DII), the Mediterranean Dietary Index (MEDI), and the Dietary Approaches to Stop Hypertension Index (DASHI). Binary logistic regression models, restricted cubic splines (RCS), and the weighted quartile sum (WQS) models were used to assess the relationship between diet quality and the risk of CB, emphysema, and asthma.
Results: In logistic regression analyses of the four dietary indices with the three chronic respiratory diseases, it was consistently observed that higher dietary quality scores were linked to a reduced risk of respiratory disease. These consistent trends were also evident in the assessments of the dose–response relationship between dietary quality score and the risk of respiratory disease. Furthermore, evaluations of the combined effects of dietary components across different dietary indices in the risk of chronic respiratory disease yielded results consistent with the logistic regression models. Notably, high-quality protein, minerals, and fiber-rich fruits and vegetables emerged as the food groups making the most significant contributions to health across different dietary indices.
Conclusion: Low-quality diets, lacking in high-quality protein, minerals, and fruits and vegetables rich in dietary fiber, are associated with a higher risk of chronic respiratory disease, regardless of the dietary index used to measure diet quality.
1 Introduction
Chronic respiratory diseases are significant public health issues in the United States (U.S.) and even globally, with chronic bronchitis (CB) (1), emphysema (2), and asthma (3) being the most common (4, 5). Approximately 8% of U.S. adults have asthma (6). CB and emphysema are both manifestations of chronic obstructive pulmonary disease (COPD), which affects more than 15 million people in the U.S. and is the fourth leading cause of death in the U.S. and the third leading cause of death globally (6–9).
The human respiratory tract is constantly exposed to harmful microorganisms and air pollutants, and the immune system responds to these pests to protect the host. However, the production of an unbalanced inflammatory response by the immune system may itself promote tissue damage and ultimately lead to acute and chronic respiratory diseases (10–12). When damage to the respiratory tract occurs, the respiratory epithelium produces a series of mediators that protect respiratory health by directly killing microorganisms, activating tissue-resident immune cells, and recruiting leukocytes from the bloodstream. The mediators are composed of a large number of leukocytes, including innate lymphocytes (ILCs), which are actively involved in the pathogenesis of chronic respiratory diseases (13, 14). Regarding the prevention and management of chronic respiratory diseases (12, 15), notable treatment guidelines emphasize the significance of adopting a healthy lifestyle as a pivotal measure to prevent diseases and enhance patients’ quality of life. Consequently, beyond the use of relevant therapeutic medications, prioritizing a healthy lifestyle is of paramount importance (16, 17).
Diet is an important part of a healthy lifestyle, and in recent decades, there has been an increasing number of studies on diet and chronic diseases (18–21), such as cognitive, metabolic, and cardiovascular diseases. Diet is also closely related to immunity. Dietary fat provides calories and ATP in the non-specific immunity of leukocytes (22). Diet and nutritional status are also major regulators of memory T-cell biology and organismal health (23). Studies have shown that healthy diets contribute to the stability of immune system function, while unhealthy diets such as high-salt diets and high-calorie diets can induce immune dysfunction (24, 25). The Healthy Eating Index (HEI) serves as a valuable indicator of dietary quality, aligning with the Dietary Guidelines for Americans (DGA) (26). In addition to the HEI, other indices contribute to our understanding of diet’s effects on health. The Dietary Inflammation Index (DII) gauges the influence of diet on the body’s inflammatory response (27). Meanwhile, the Mediterranean Dietary Index (MEDI) and the Dietary Approaches to Stop Hypertension Index (DASHI) have been used to represent the Mediterranean dietary pattern and the Dietary Approaches to Stop Hypertension (DASH) dietary pattern to assess the quality of healthy diets (28, 29). These dietary indices (DII, MEDI, and DASHI) better reflect the dietary intake of the population and are highly correlated with the human health levels and the risk of chronic diseases. However, there are no studies related to different dietary patterns and chronic respiratory diseases in U.S. populations. A healthy diet contributes to the homeostasis of the immune system. At the same time, the immune system is closely linked to respiratory health. However, few studies have examined the relationship between diet and respiratory and lung health. We hypothesized that improvements in dietary quality would contribute to the reduction of the risk of chronic respiratory diseases.
The National Health and Nutrition Examination Survey (NHANES) database is a large continuous cross-sectional survey in the U.S. We used this large, representative database of U.S. residents to explore the relationship between different dietary pattern indices and three chronic respiratory diseases. Because of the importance of chronic respiratory health and the indispensable role of diet in daily life, the study of dietary and respiratory health correlations explored in this study has strong public health implications.
2 Materials and methods
2.1 Study sample
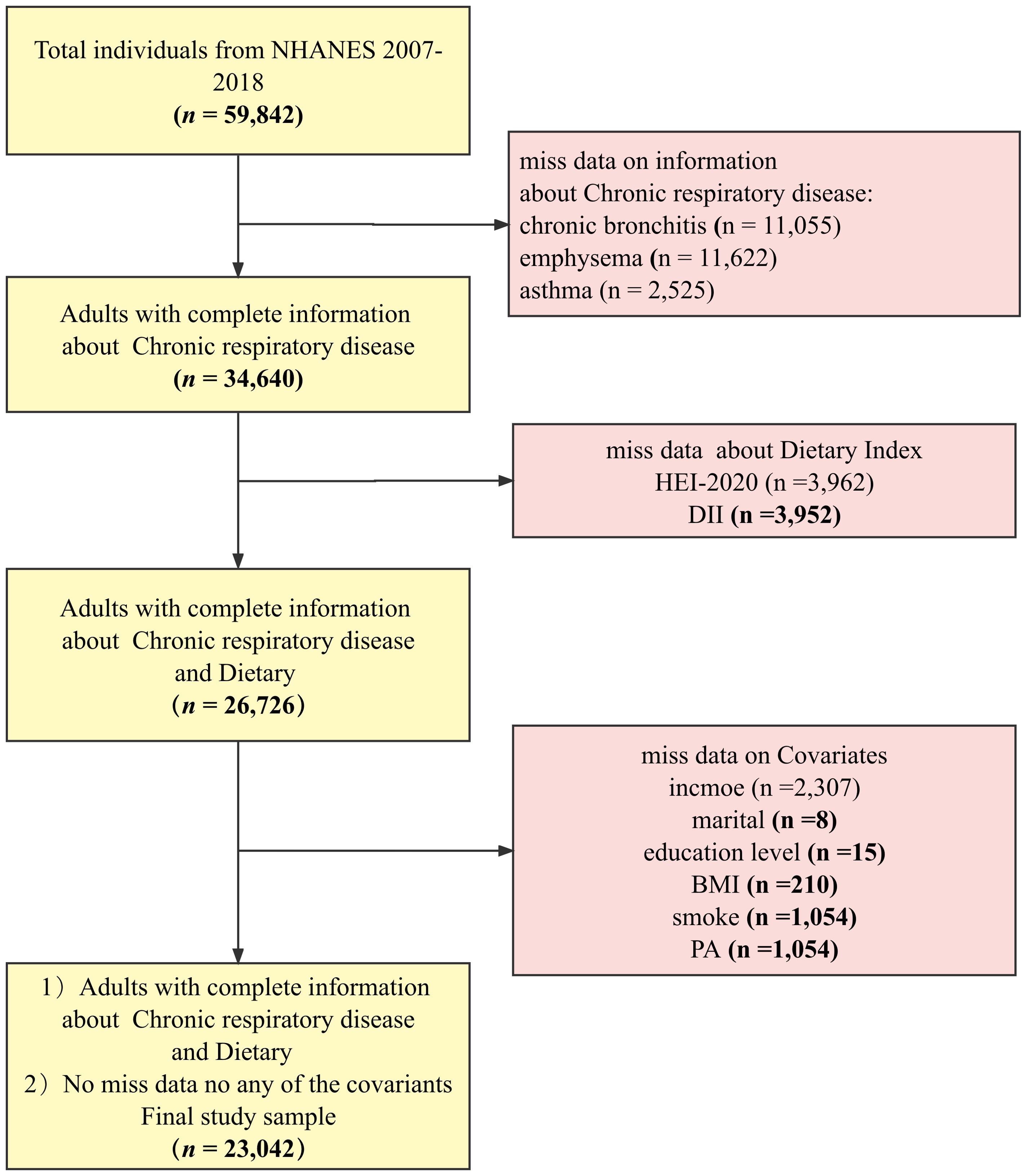
The NHANES database is a regularly conducted cross-sectional study from the Centers for Disease Control and Prevention’s (CDC’s) National Center for Health Statistics (NCHS) that investigates nutritional intake and health-related conditions of populations in the U.S (30). The NHANES utilizes a complex, multistage sampling design to make the survey results well-representative of populations across the U.S. The data for this study were obtained from NHANES 2007–2018. Data with complete demographics (gender, age, education, and family income), behavior (BMI, physical activity, smoking, and drinking status), chronic disease data (diabetes and hypertension), and dietary data were included in the sample. The final sample (n = 23,042) was weighted to represent 160 million non-institutionalized adult U.S. population, and the process can be seen in Figure 1.
2.2 Diet quality
Our analysis is grounded in data from four widely recognized dietary pattern indices that serve as proxies for dietary quality: the HEI-2020, the DII, the MEDI, and the DASHI. The HEI-2020 evaluates adherence to the DGA 2020–2025, encompassing 13 components (31): adequacy components (total vegetables, greens and beans, total fruits, whole fruits, whole grains, dairy, total protein foods, seafood, plant proteins, and fatty acids) and moderation components (sodium, refined grains, saturated fats, and added sugars). Each component is weighted differently and has a distinct maximum score. HEI-2020 scores range from 0 to 100, with higher scores indicating superior diet quality. The DII was crafted to gauge the inflammatory impact of diets on the body. Given the constraints of the NHANES database, our study incorporated 28 dietary components for DII calculations, including vitamin A, vitamin C, vitamin D, B vitamins, proteins, dietary fiber, unsaturated fatty acids, and others. The DII is derived by calculating the z-value, which is the difference between the world average intake and the individual’s actual intake, divided by the standard deviation. To mitigate bias, this value is converted into percentile scores, doubled, and then adjusted by subtracting 1.0 to center the distribution to approximately 0. These centered scores are subsequently multiplied by the Inflammatory Effects Score, and the food parameters are summed for a comprehensive assessment. For detailed methodology, refer to the original research (32). The MEDI score, adapted from the Mediterranean diet scale by Trichopoulou et al. (33), comprises eight dietary components, namely, vegetables, fruits, nuts, whole grains, legumes, fish, the monounsaturated-to-saturated fat ratio (MtSR), and red and processed meats. A higher MEDI score signifies better diet quality (34). The DASHI measures the adherence to the DASH dietary patterns, and we have utilized the version validated by Mellen et al (35), which includes nine dietary components (total fat, saturated fat, protein, fiber, cholesterol, calcium, magnesium, sodium, and potassium). Higher DASHI scores are indicative of better diet quality. Supplementary Table 1 provides further details on these dietary indices. For weighted Scott-Rao chi-square tests and weighted logistic regressions, the dietary index was treated as both a continuous and a categorical variable. The dietary index scores were categorized into quartiles, designated as Q1 (reference group), Q2, Q3, and Q4 (36).
2.3 Chronic respiratory diseases
Three chronic respiratory diseases (CB, emphysema, and asthma) were selected as outcome variables in this study.
2.3.1 CB
All subjects were asked “Has a doctor or other health professional ever told you that you had chronic bronchitis?” and then categorized into non-patients (reference group) and patients according to the answers (37).
2.3.2 Emphysema
All subjects were asked “Has a doctor or other health professional ever told you that you had emphysema?” and then categorized into non-patients (reference group) and patients according to the answers (37).
2.3.3 Asthma
All subjects were asked “Has a doctor or other health professional ever told you that you had asthma?” and then categorized into non-patients (reference group) and patients according to the answers (37).
2.4 Covariates
Based on the relevant studies, the relevant covariates were also selected to correct the model in this study.
1. Demographic variables include gender (male and female), age (<40, between ≥40 and <60, and ≥60 years), race (non-Hispanic white, non-Hispanic black, Mexican, and other), education level (less than high school, high school graduate/GED or equivalent, and above), marital status (married or living with partner, divorced or widowed, and never married), and income level (1.3 < pir, 3.5 ≤ pir < 3.5, and 3.5 ≤ pir) (38–40).
2. Lifestyle variables include BMI, smoking, drinking, and physical exercise (PA). We used serum cotinine levels to reflect smoking status with tertile definition, and alcohol use data from dietary surveys to reflect drinking status. Metabolic equivalent (MET) was calculated to reflect physical activity (39, 41).
3. Disease covariates include hypertension and diabetes. Hypertension is defined using self-reporting in conjunction with blood pressure levels. Diabetes was defined using self-reporting in conjunction with plasma fasting glucose, glycohemoglobin, and oral glucose tolerance test (OGTT) (42, 43).
2.5 Statistical analysis
Baseline characteristics across various groups were analyzed utilizing chi-square tests for categorical variables and t-tests for continuous variables. Binary logistic regression models were applied to assess the correlation between distinct dietary index scores and the likelihood of developing chronic respiratory diseases, encompassing CB, emphysema, and asthma. The dose–response relationship between the scores of different dietary indices and the prevalence of chronic respiratory diseases was scrutinized with the aid of RCS. The WQS regression model was employed to evaluate the synergistic effects of dietary components from the various indices on the risk of chronic respiratory diseases. Baseline characteristics across various groups were analyzed utilizing chi-square tests for categorical variables and t-tests for continuous variables. Binary logistic regression models were applied to assess the correlation between distinct dietary index scores and the likelihood of developing chronic respiratory diseases, encompassing CB, emphysema, and asthma. The dose–response relationship between the scores of different dietary indices and the prevalence of chronic respiratory diseases was scrutinized with the aid of RCS. The WQS regression model was employed to evaluate the synergistic effects of dietary components from the various indices on the risk of chronic respiratory diseases (44).
The study sample was weighted and analyzed using the recommended appropriate methodology for a study population sample with a complex sampling design. This study also used sensitivity analyses with unweighted samples and gender subgroups.
All statistical tests were two-sided, and significance was considered at p < 0.05. All statistical analyses were performed with R (version 4.1.2). RCS was implemented with the R package “rms” (version 6.3-0). WQS was implemented with the R package “gWQS” (version 3.0.4).
3 Results
3.1 Characteristics of the baseline
Baseline characteristics are shown in Table 1. After screening the sample, a total sample of n = 23,042 was included, of which 47.3% were male and 52.7% were female; the mean score of HEI-2020 was 54.01 ± 13.42, the mean score of DII was 1.39 ± 1.59, the mean score of MEDI was 5.70 ± 1.01, and the mean score of DASHI was 3.52 ± 1.15. As seen in the baseline characterization between the healthy population and the chronic respiratory disease population, adults with chronic respiratory disease were more likely to be older, have lower income, be obese, be less physically active, have chronic illnesses, and have lower dietary quality.
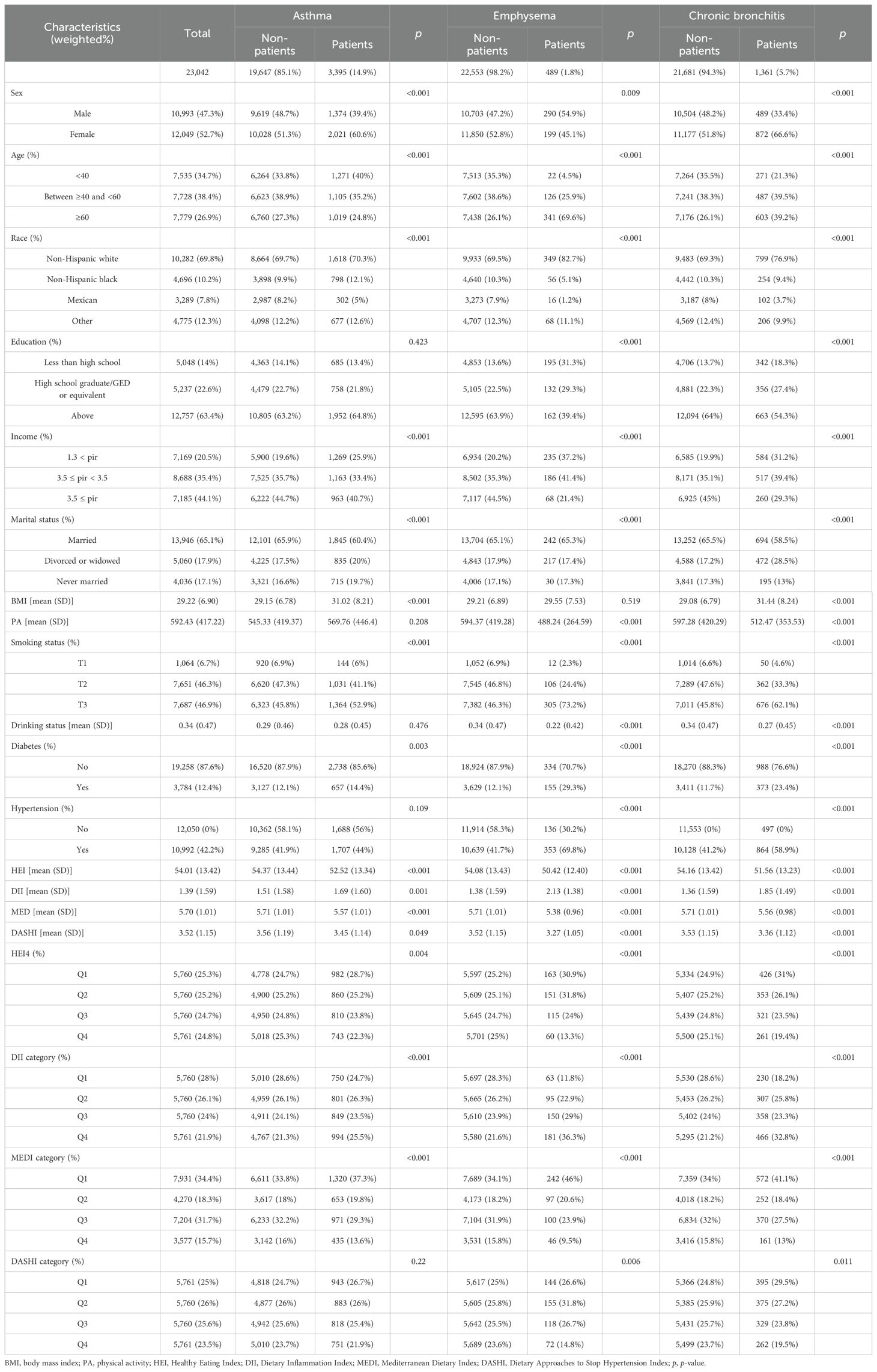
Table 1. The baseline population characteristics among U.S. adults by the three chronic respiratory diseases.
3.2 Healthier dietary index scores are associated with a lower risk of chronic respiratory disease
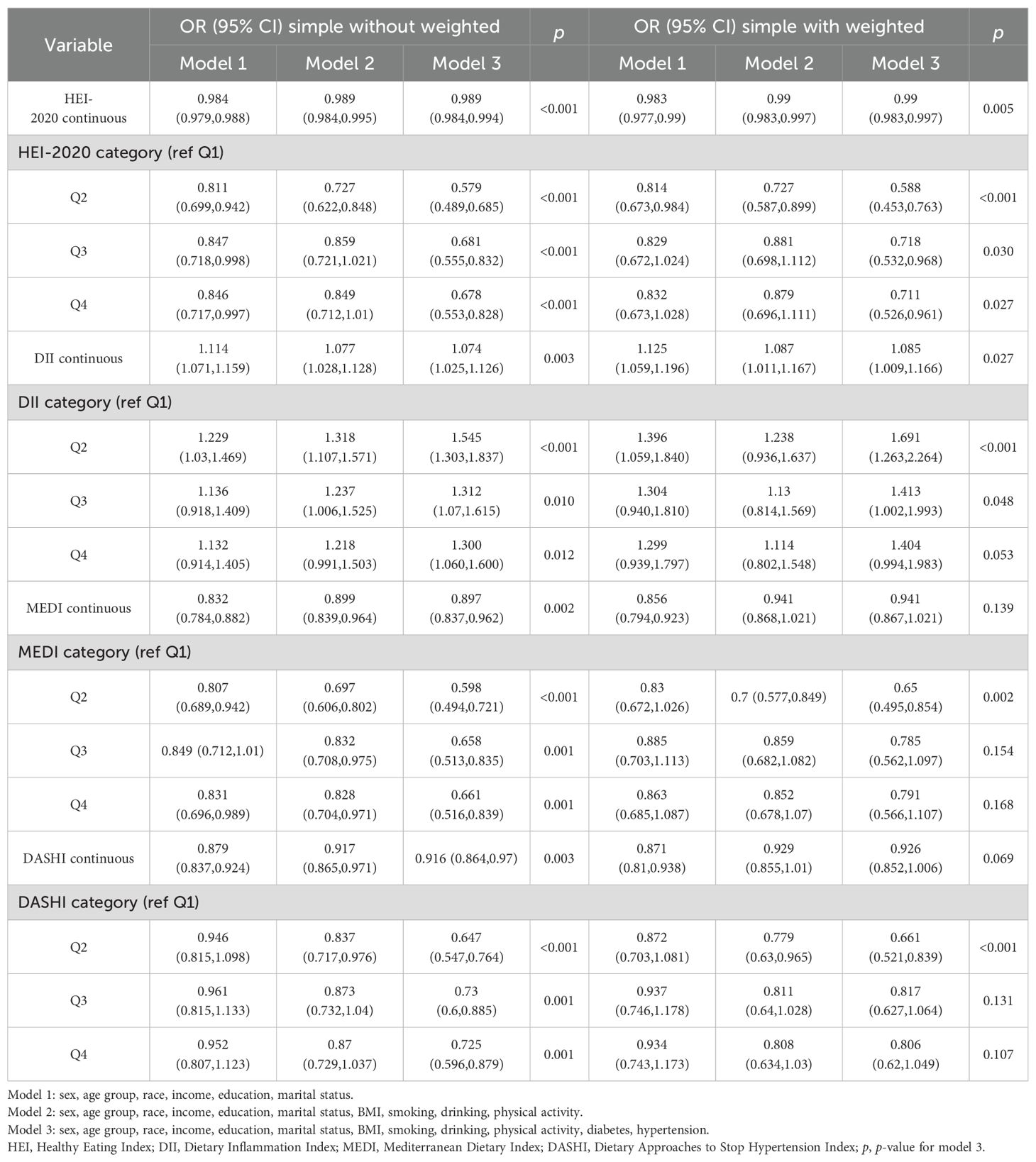
A multifactorial stepwise logistic regression model was employed to examine the association between different dietary pattern index scores and the risk of chronic respiratory diseases. The stepwise logistic regression model used three covariate models. Model 1 was adjusted for demographic variables (including gender, age, race, education level, marital status, and income level), Model 2 was adjusted for demographic variables and lifestyle variables (including BMI, smoking, drinking, and PA), and Model 3 was adjusted for demographic variables, lifestyle variables, and disease covariates (hypertension and diabetes). After stepwise correction for covariates, the results of the risk-related regressions of the four dietary index scores on asthma, emphysema, and CB in the weighted sample are shown in Tables 2–4, respectively. In Table 2, HEI-2020, MEDI, and DASHI (both continuous and quartile categorical variables) all showed stable significant correlations with the risk of asthma. In Table 3, HEI-2020, MEDI (both continuous and quartile categorical variables), quartile categorical variables of DII scores, and continuous variables of DASHI scores all showed stable significant correlations with risk of emphysema. In Table 4, only HEI-2020 (both continuous and quartile categorical variables) showed stable significant correlations with risk of CB [OR of HEI-2020 continuous:0.99 (0.983,0.997), p < 0.001; OR of HEI-2020 Q4 vs. Q1: 0.711 (0.526,0.961), p = 0.027].
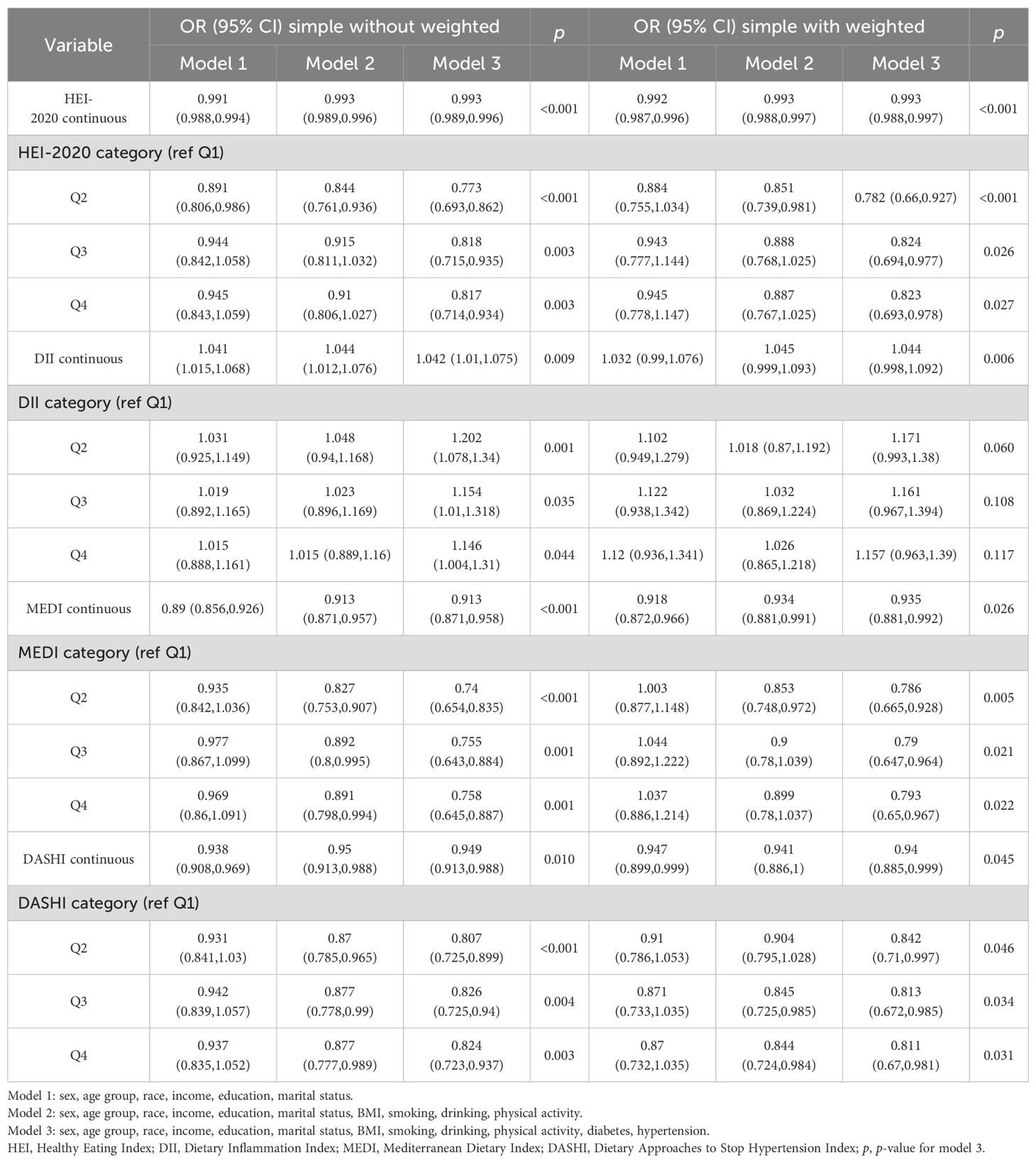
Table 2. Relationship between different dietary indices and asthma among adults aged 20 years or older.
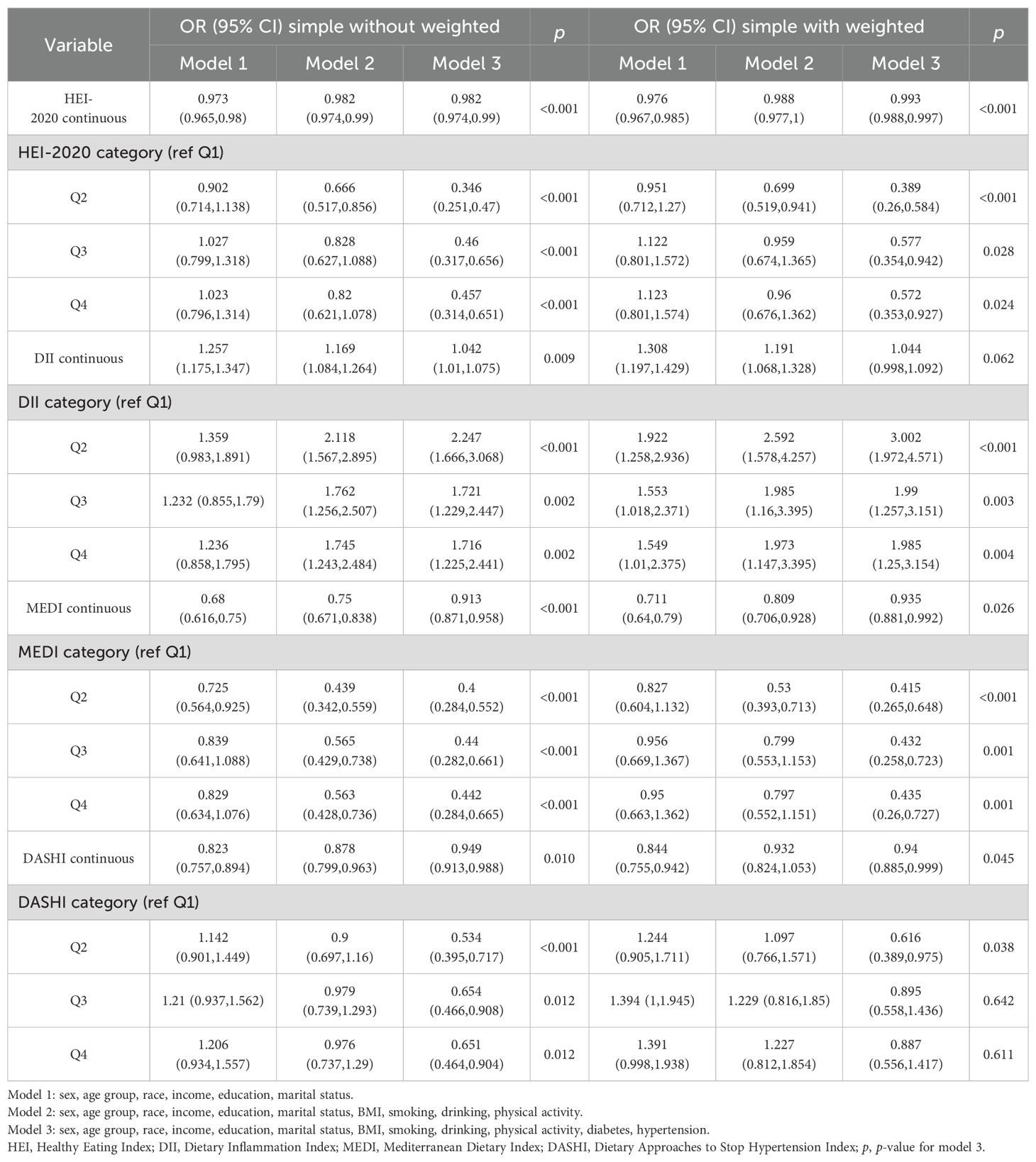
Table 3. Relationship between different dietary indices and emphysema among adults aged 20 years or older.
3.3 Dose–response relationship between dietary index scores and risk of chronic respiratory disease
RCS was used to explore the dose–response relationship between different dietary pattern index scores and the risk of three chronic respiratory diseases. The results of RCS demonstrated a significant dose–response relationship between HEI-2020 and the risk of asthma without concomitant nonlinear relationships (Figure 2). The results of RCS also demonstrated a significant dose–response relationship between all four dietary indices and the risk of emphysema without nonlinear relationships in Figure 3. Similarly, the four dietary index scores had a significant dose–response relationship with the risk of CB in Figure 4. There was no nonlinear relationship between them.
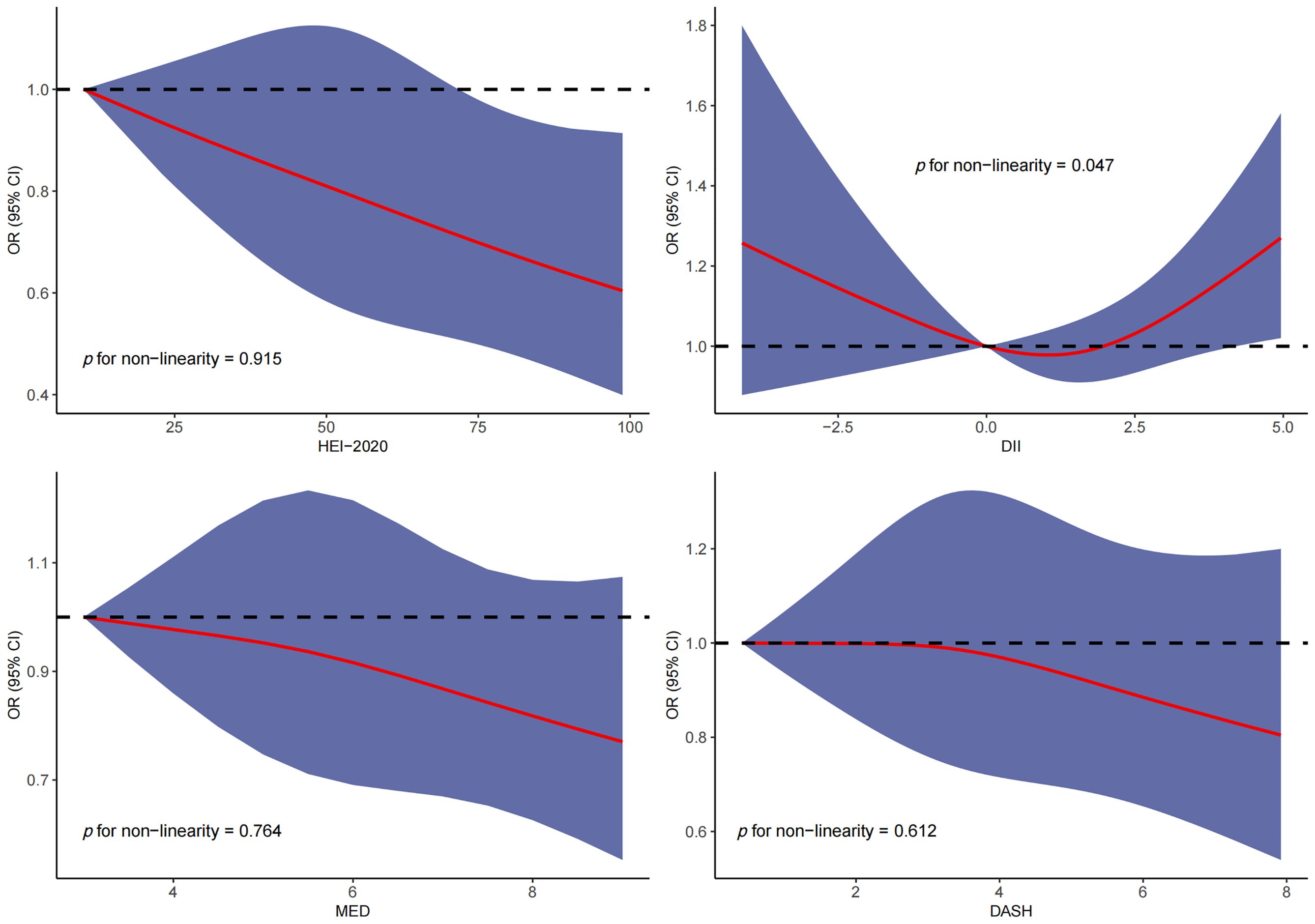
Figure 2. Dose–response relationship results between four dietary indices (HEI-2020, DII, MEDI, and DASHI) and OR ratios in the RCS-asthma model.
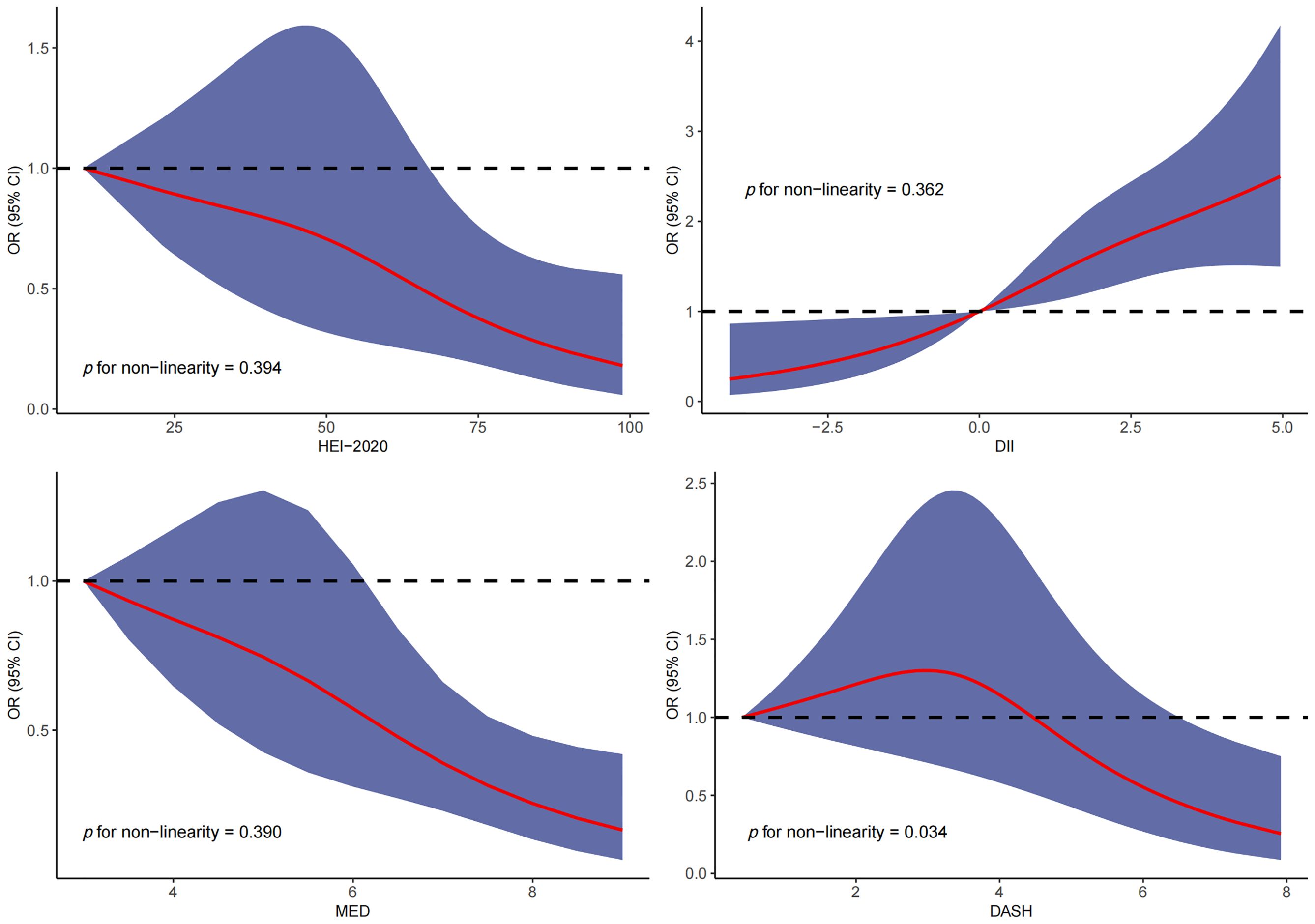
Figure 3. Dose–response relationship results between four dietary indices (HEI-2020, DII, MEDI, and DASHI) and OR ratios in the RCS-emphysema model.
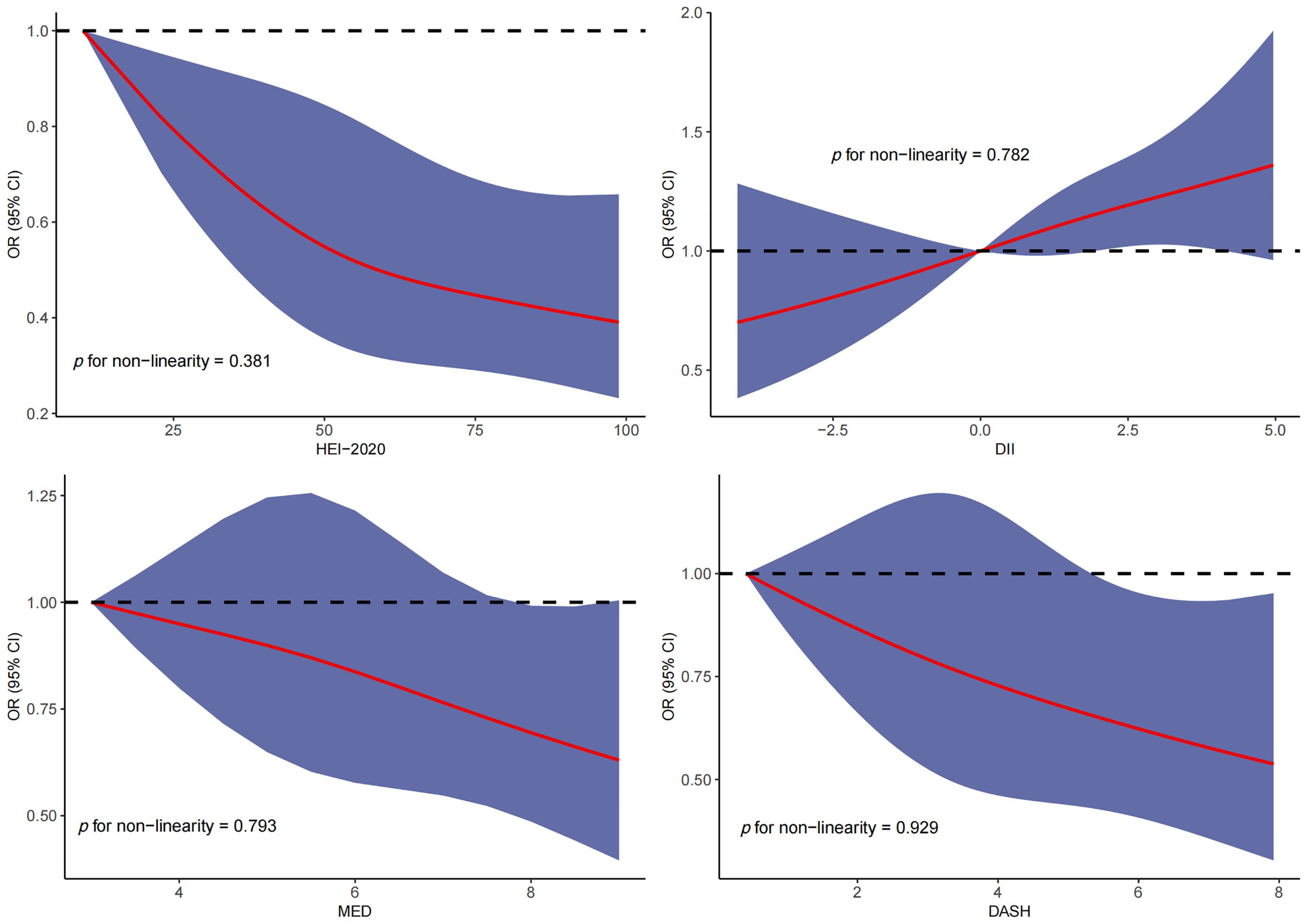
Figure 4. Dose–response relationship results between four dietary indices (HEI-2020, DII, MEDI, and DASHI) and OR ratios in the RCS-chronic bronchitis (CB) model.
3.4 Mixed effects of 13 dietary components on chronic respiratory disease
Table 5 shows the mixed effects of the dietary components of the four kinds of dietary index on the risk of chronic respiratory disease in the model of WQS.
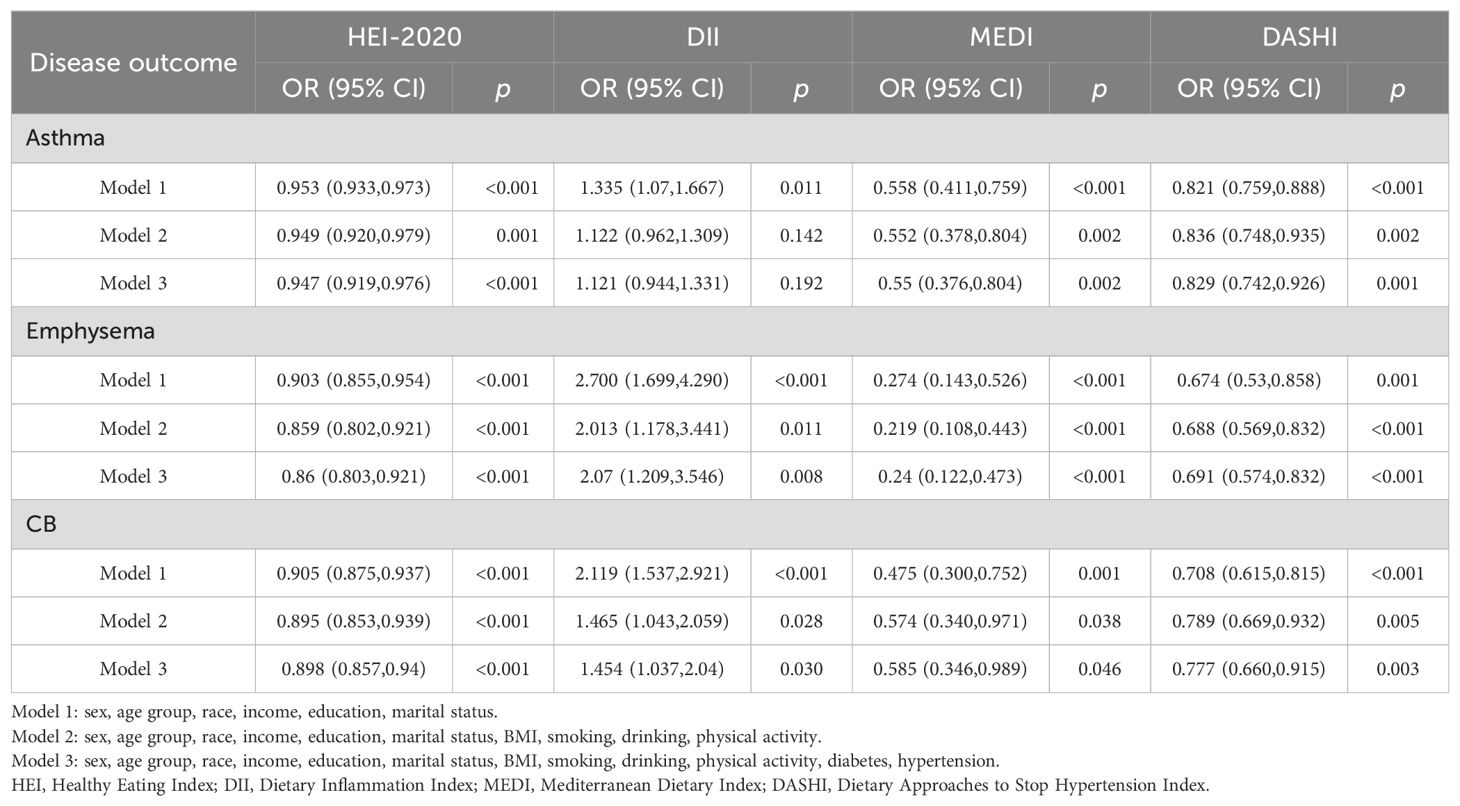
Table 5. Relationship between the mixed effects of the dietary components of the HEI-2020 and chronic respiratory disease among adults aged 20 years or older.
After stepwise inclusion of covariates, HEI-2020, MEDI, and DASHI exhibited significant health mixing effects on the risk of asthma in the WQS-asthma model [HEI-2020 OR: 0.947 (0.919,0.976); MEDI OR: 0.55 (0.376,0.804); DASHI OR: 0.829 (0.742,0.926)]. HEI-2020, DII, MEDI, and DASHI have shown significant mixing effects on the risk of emphysema in the WQS-emphysema model [HEI-2020 OR: 0.86 (0.803,0.921); DII OR: 2.07 (1.209,3.546); MEDI OR: 0.24 (0.122,0.473); DASHI OR: 0.691 (0.574,0.832)]. In the WQS-CB model, HEI-2020, DII, MEDI, and DASHI have shown significant mixing effects on the risk of CB [HEI-2020 OR: 0.898 (0.857,0.94); DII OR: 1.454 (1.037,2.04); MEDI OR: 0.585 (0.346,0.989); DASHI OR: 0.777 (0.660,0.915)].
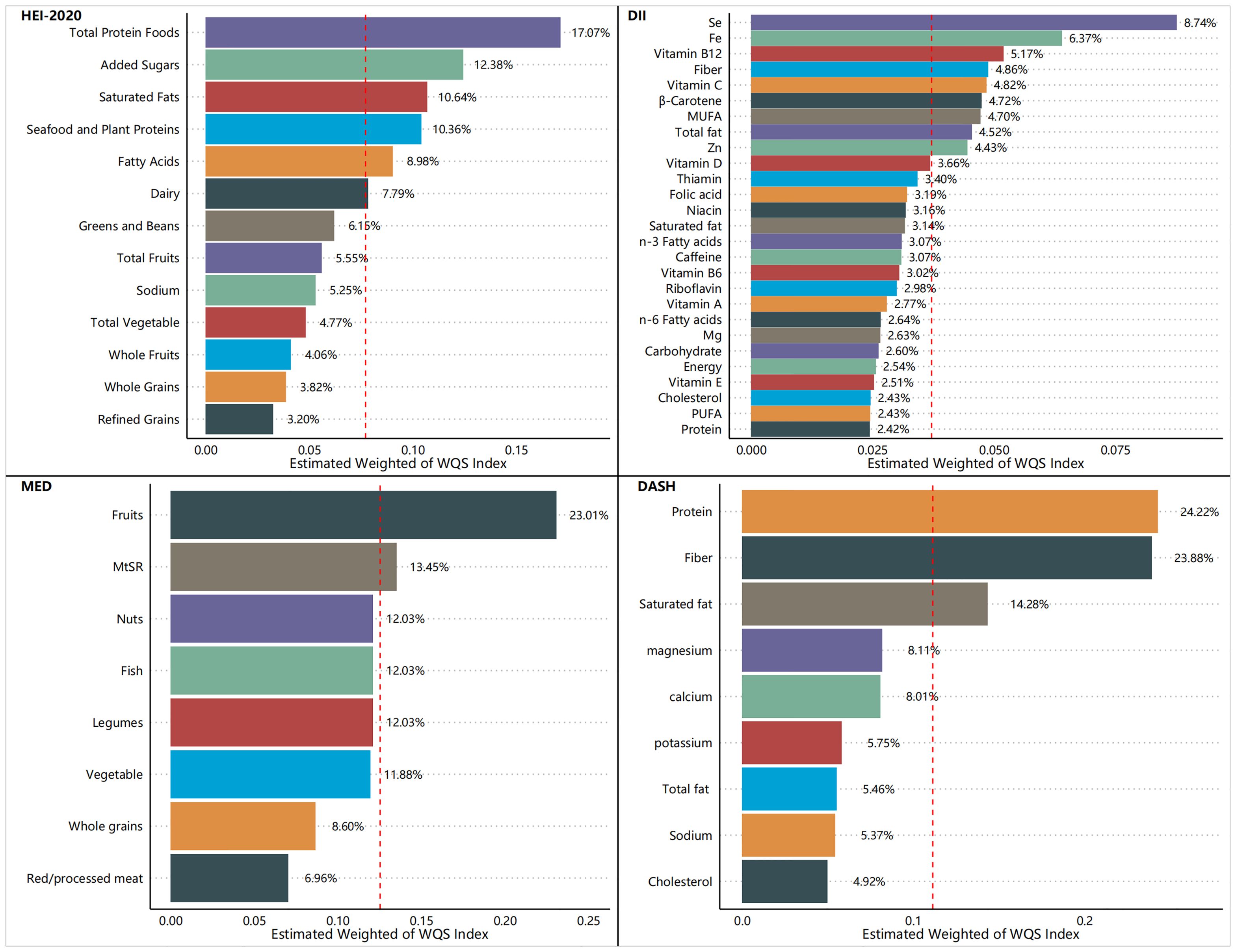
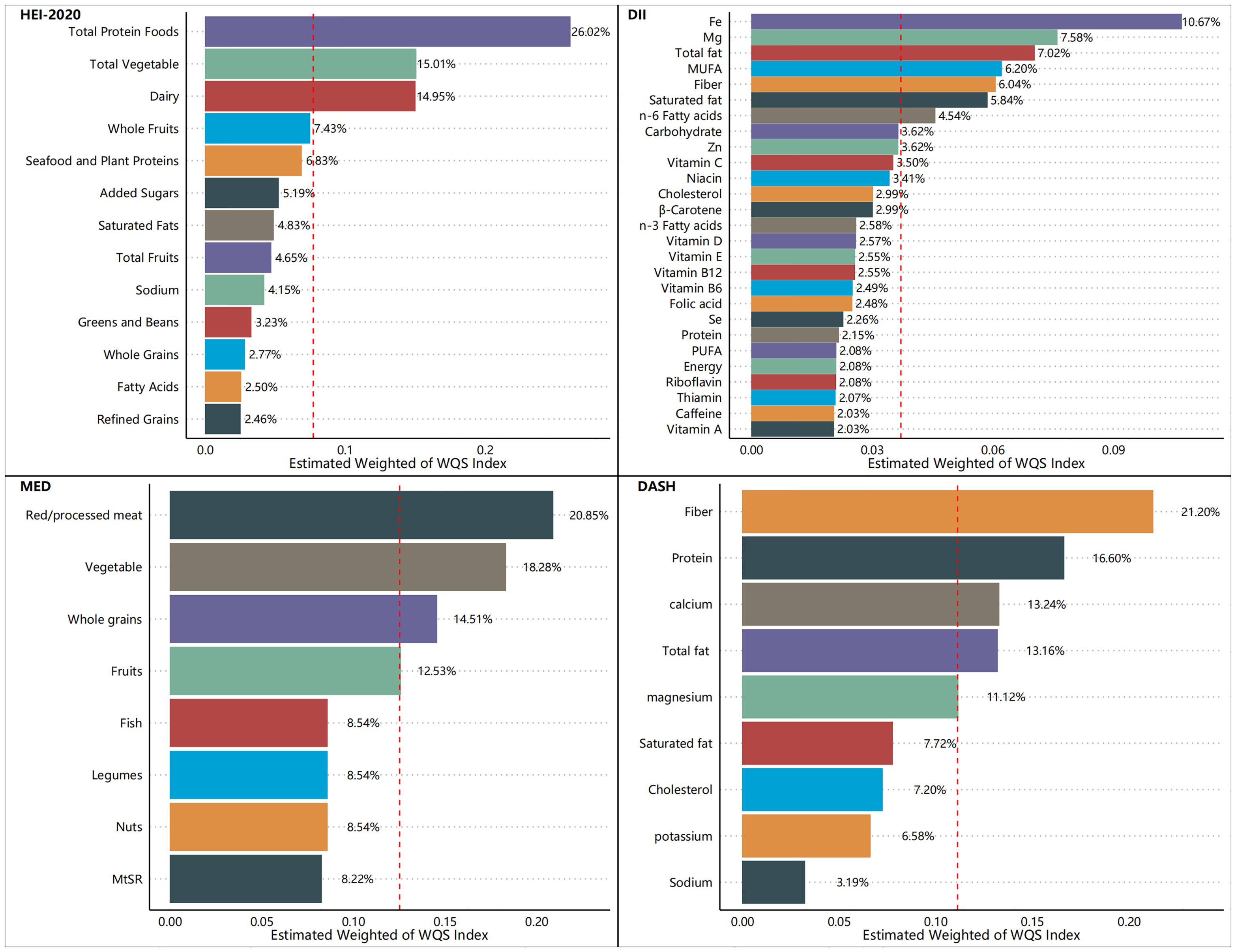
Figures 5–7 show the results of the mixed effects of dietary components from various dietary indices on the risk of CRD within the WQS model. In the WQS-asthma model, the most contributing dietary components in HEI-2020, DII, MEDI, and DASHI were total protein foods (17.07%), Se (8.74%), fruits (23.01%), and proteins (24.22%), respectively (Figure 5). In the WQS of the emphysema model, the dietary components that contributed the most to HEI-2020, DII, MEDI, and DASHI were seafood and plant protein (19.07%), Fe (9.30%), whole grains (30.95%), and dietary fiber (60.45%), respectively (Figure 6). In the WQS-CB model, the dietary components that contributed the most to HEI-2020, DII, MEDI, and DASHI were seafood and plant protein (26.02%), Fe (10.67%), red/processed meat (20.85%), and dietary fiber (21.20%), respectively (Figure 7).
3.5 Subgroup analysis of the correlation between dietary index scores and chronic respiratory diseases
We also performed subgroup analyses by gender and found that the relationship between dietary index scores and CRD risk varied by gender. In comparison to female patients, the significant relationship between high dietary quality and the low risk of CRD in male patients was more stable across dietary scores and across population proportions (Supplementary Tables 2−4).
The RCS was also used to explore the dose–response relationship between dietary index scores and risk of chronic respiratory disease by gender (Supplementary Figures 1−6). It was found that the dose–response relationship between the continuous dietary quality scores and the risk of CRD was more significant in men compared to women, while the dose–response relationship between the four dietary quality scores and the risk of emphysema was the most significant and stable in men, and there was no nonlinear relationship between them.
4 Discussion
Our research encompassed a sample of 23,042 U.S. adults, which, after appropriate weighting, is representative of approximately 160 million non-institutionalized U.S. adults. Three positive rating indicators (HEI-2020, MEDI, and DASHI) and one negative rating indicator (DII) were used together to assess the relationship between dietary quality and respiratory health. Higher healthy diet scores (HEI-2020, MEDI, and DASHI) and lower unhealthy diet scores (DII) were also found to be associated with a lower risk of respiratory disease in the results. This suggests a strong relationship between dietary quality and respiratory health, as well as a consistent relationship between different dietary scores and respiratory health, and the robustness of the results of this study. Moreover, this correlation appears to be robust, holding steady across variations in sample demographics and differing definitions of dietary quality. Among the dietary indices evaluated, the HEI-2020 consistently demonstrated the most pronounced health benefits, with both its continuous and quartile scores significantly correlating with a lower risk of the aforementioned respiratory diseases in both unweighted and weighted samples. The restricted cubic spline (RCS) analysis revealed significant inverse relationships between the three health-promoting dietary indices (HEI-2020, MEDI, and DASHI) and respiratory disease risk, while a significant positive association was observed with the DII. These findings were corroborated by the outcomes of logistic regression analyses, which further substantiated the stability and reliability of our results. The Weighted Quantile Sum (WQS) model also identified a salutary interaction between the three dietary indices (HEI-2020, MEDI, and DASHI) and the risk of respiratory disease, in contrast to an adverse interaction observed between the DII score and respiratory disease risk. Additionally, we conducted an in-depth analysis to determine the individual contributions of various dietary components to the risk of respiratory disease, considering their proportion relative to the total dietary mass. When the dietary index was decomposed into its constituent food elements, the specific dietary components of the DII were examined for their influence on respiratory disease risk. In the context of asthma risk, the food groups that predominantly influenced the scores of the dietary indices (HEI-2020, DII, MEDI, and DASHI) were total protein foods (17.07%), selenium (Se, 8.74%), fruits (23.01%), and proteins (24.22%), respectively. Regarding the risk of emphysema, the food groups that had the most significant impact on the dietary indices scores were seafood and plant protein (19.07%), iron (Fe, 9.30%), whole grains (30.95%), and dietary fiber (60.45%), respectively. For the risk of CB, the food groups that were most influential to the scores of the dietary indices were seafood and plant protein (26.02%), iron (Fe, 10.67%), red/processed meat (20.85%), and dietary fiber (21.20%), respectively. These findings underscore the substantial health potential of proteins, particularly high-quality sources such as plant and seafood proteins, minerals, fruits, and vegetables, which may be beneficial in mitigating the risk of respiratory diseases.
In 2014, the European Respiratory Society released a statement highlighting the importance of nutritional assessment and treatment for COPD (45). The role of nutrition in managing chronic respiratory diseases has gained widespread recognition in recent years (46–48). Western dietary patterns, notably marked by an elevated intake of preserved and processed meats, are correlated with a heightened risk of chronic respiratory diseases. This correlation could be due to the inflammatory consequences of excessive sugar, salt, and nitrite consumption from processed foods (49, 50). Furthermore, an array of studies have reported the positive impact of the DII, DASH, and the Mediterranean diet on respiratory health (51–55). In their study on the effects of plant-based diets, Alwarith et al. discovered that such diets were linked to a decrease in asthma attacks and improvement in symptoms. These effects may be attributed to the modulation of cytokine release, mitigation of free radical damage, and modulation of immune responses, all of which play a role in the onset and progression of asthma (56). These findings are consistent with the results of our study.
Proteases and antiproteases are secreted by the respiratory epithelium and are involved in the immune homeostatic balance of the respiratory tract. Alterations in protease/antiprotease balance can lead to the development of lung diseases such as emphysema or COPD (57). Supplementation of the dietary route with nutritive antioxidants, such as flavonoids, may enhance respiratory mucosal responses and/or prevent infections, reducing the risk of respiratory disease development (58). This also demonstrates the benefits of fruit and vegetable intake for respiratory health. Malnutrition has been found to be associated with poor quality of survival in COPD patients in different observational studies (59, 60). Nutritional therapy has notably demonstrated its efficacy in sustaining and enhancing muscle strength and exercise tolerance among malnourished COPD patients (61). Studies have pinpointed low body weight and diminished fat-free mass (FFM) as ominous prognostic indicators in COPD patients, with evidence suggesting that a diet rich in high-quality protein or essential amino acid supplementation can bolster the fat-free body weight index and elevate arterial oxygen saturation levels (62, 63). A study delving into protein absorption and utilization among COPD patients has uncovered that inadequate protein intake, systemic inflammation, and hypertension are predictors of a reduced postabsorptive protein balance, which correlates with diminished daily physical performance (64). Individuals suffering from COPD frequently present with clinical malnutrition, particularly protein deficiency, which can precipitate dyspnea or skeletal muscle dysfunction (65–67). The significance of minerals, such as selenium (Se) and iron (Fe), in respiratory health has been underscored by recent research (68, 69), aligning with findings that a deficiency in these minerals in the DII can heighten the inflammatory potential of one’s diet. Concurrently, two extensive cohort studies in the U.S. have correlated a higher consumption of marine fish with a decreased risk of developing COPD (70). Moreover, additional research has indicated that the intake of whole grains or dietary fiber may also mitigate the risk of chronic respiratory diseases (65, 71, 72), potentially due to the fiber’s ability to modulate the gut microbiota and augment short-chain fatty acid production, which, in turn, regulates neutrophil activity and mitigates allergic responses (73). Collectively, these studies underscore the advantageous impact of protein, mineral, fruit, and vegetable consumption on the preservation of respiratory health.
This study has certain strengths; most notably, it utilized a multi-model sensitivity analysis design: (1) four dietary score approaches (HEI-2020, DII, MEDI, and DASHI) were used to define dietary quality; (2) the study population samples were weighted and transformed into different population proportions, unweighted and weighted samples, and subgroups were analyzed by gender; and (3) dietary index scores were also classified into categorical continuous variables and quartiles of the variables, to make the results more realistic and reliable. The second is the use of a large U.S. Nutrition and Health Survey database, which is reliable and representative, and lastly, the use of the latest version of the HEI, HEI-2020, which is based on DGA 2020–2025, which is representative of the U.S. population’s dietary intake, and dietary indices of three mainstream nutritional studies.
In addition, there are shortcomings in this study, the largest being that this was a cross-sectional study and could not validate the causal link between dietary quality and the risk of chronic respiratory disease. Secondly, unknown confounders and potential model overfitting problems are also a shortcoming of this study. Third, the inability to standardize the dietary index components is also one of the shortcomings of this study.
The study found that high dietary quality scores were associated with low chronic respiratory risk, suggesting that improving the quality of the diet itself by following different healthy dietary patterns, especially the dietary patterns represented by the HEIs from the DGA 2020–2025 recommendations, could help prevent the occurrence and exacerbation of chronic respiratory diseases.
5 Conclusion
Low-quality diets, lacking in high-quality protein, minerals, and fruits and vegetables rich in dietary fiber, are associated with a higher risk of chronic respiratory disease, regardless of the dietary index used to measure diet quality.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: The NHANES dataset is publicly available online, accessible at cdc.gov/nchs/nhanes/index.htm.
Author contributions
HL: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. XT: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft. XG: Data curation, Investigation, Methodology, Writing – original draft. MZZ: Data curation, Formal analysis, Writing – original draft. MJZ: Data curation, Methodology, Writing – original draft. JN: Data curation, Formal analysis, Writing – original draft. SF: Data curation, Methodology, Writing – original draft. HZ: Data curation, Methodology, Software, Writing – original draft. YS: Data curation, Formal analysis, Methodology, Writing – original draft. XD: Methodology, Supervision, Writing – original draft. JL: Data curation, Methodology, Writing – original draft. XY: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1457860/full#supplementary-material
References
1. Kim V, Criner GJ. Chronic bronchitis and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. (2013) 187:228–37. doi: 10.1164/rccm.201210-1843CI
2. Kheradmand F, Zhang Y, Corry DB. Contribution of adaptive immunity to human COPD and experimental models of emphysema. Physiol Rev. (2023) 103:1059–93. doi: 10.1152/physrev.00036.2021
3. Sockrider M, Fussner L. What is asthma? Am J Respir Crit Care Med. (2020) 202:P25–6. doi: 10.1164/rccm.2029P25
4. Christenson SA, Smith BM, Bafadhel M, Putcha N. Chronic obstructive pulmonary disease. Lancet. (2022) 399:2227–42. doi: 10.1016/S0140-6736(22)00470-6
5. Papi A, Brightling C, Pedersen SE, Reddel HK. Asthma. Lancet. (2018) 391:783–800. doi: 10.1016/S0140-6736(17)33311-1
6. Gaffney AW, Hawks L, Bor D, White AC, Woolhandler S, McCormick D, et al. National trends and disparities in health care access and coverage among adults with asthma and COPD: 1997-2018. Chest. (2021) 159:2173–82. doi: 10.1016/j.chest.2021.01.035
7. André S, Conde B, Fragoso E, Boléo-Tomé JP, Areias V, Cardoso J. COPD and cardiovascular disease. Pulmonology. (2019) 25:168–76. doi: 10.1016/j.pulmoe.2018.09.006
8. Soriano JB, Abajobir AA, Abate KH, Abera SF, Agrawal A, Ahmed MB, et al. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Respir Med. (2017) 5:691–706. doi: 10.1016/S2213-2600(17)30293-X
9. Iyer AS, Sullivan DR, Lindell KO, Reinke LF. The role of palliative care in COPD. Chest. (2022) 161:1250–62. doi: 10.1016/j.chest.2021.10.032
10. Lareau SC, Fahy B, Meek P, Wang A. Chronic obstructive pulmonary disease (COPD). Am J Respir Crit Care Med. (2019) 199:P1–2. doi: 10.1164/rccm.1991P1
11. Brusselle GG, Provoost S, Bracke KR, Kuchmiy A, Lamkanfi M. Inflammasomes in respiratory disease: from bench to bedside. Chest. (2014) 145:1121–33. doi: 10.1378/chest.13-1885
12. Fazleen A, Wilkinson T. Early COPD: current evidence for diagnosis and management. Ther Adv Respir Dis. (2020) 14:1753466620942128. doi: 10.1177/1753466620942128
13. Bullone M, Carriero V, Bertolini F, Folino A, Mannelli A, Di Stefano A, et al. Elevated serum IgE, oral corticosteroid dependence and IL-17/22 expression in highly neutrophilic asthma. Eur Respir J. (2019) 54. doi: 10.1183/13993003.00068-2019
14. Hsu AT, Gottschalk TA, Tsantikos E, Hibbs ML. The role of innate lymphoid cells in chronic respiratory diseases. Front Immunol. (2021) 12:733324. doi: 10.3389/fimmu.2021.733324
15. Halpin DMG, Criner GJ, Papi A, Singh D, Anzueto A, Martinez FJ, et al. Global initiative for the diagnosis, management, and prevention of chronic obstructive lung disease. The 2020 GOLD science committee report on COVID-19 and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. (2021) 203:24–36. doi: 10.1164/rccm.202009-3533SO
16. Mirza S, Clay RD, Koslow MA, Scanlon PD. COPD guidelines: A review of the 2018 GOLD report. Mayo Clin Proc. (2018) 93:1488–502. doi: 10.1016/j.mayocp.2018.05.026
17. Cosío BG, Hernández C, Chiner E, Gimeno-Santos E, Pleguezuelos E, Seijas N, et al. Spanish COPD guidelines (GesEPOC 2021): non-pharmacological treatment update. Arch Bronconeumol. (2022) 58:345–51. doi: 10.1016/j.arbres.2021.08.010
18. Tian T, Zhang J, Xie W, Ni Y, Fang X, Liu M, et al. Dietary quality and relationships with metabolic dysfunction-associated fatty liver disease (MAFLD) among United States adults, results from NHANES 2017-2018. Nutrients. (2022) 14. doi: 10.3390/nu14214505
19. Wang K, Zhao Y, Nie J, Xu H, Yu C, Wang S. Higher HEI-2015 score is associated with reduced risk of depression: result from NHANES 2005-2016. Nutrients. (2021) 13. doi: 10.3390/nu13020348
20. Shan Z, Li Y, Baden MY, Bhupathiraju SN, Wang DD, Sun Q, et al. Association between healthy eating patterns and risk of cardiovascular disease. JAMA Intern Med. (2020) 180:1090–100. doi: 10.1001/jamainternmed.2020.2176
21. Deng M-G, Nie J-Q, Li Y-Y, Yu X, Zhang Z-J. Higher HEI-2015 scores are associated with lower risk of sleep disorder: results from a nationally representative survey of United States adults. Nutrients. (2022) 14. doi: 10.3390/nu14040873
22. Lee AH, Dixit VD. Dietary regulation of immunity. Immunity. (2020) 53:510–23. doi: 10.1016/j.immuni.2020.08.013
23. Collins N. Dietary regulation of memory T cells. Int J Mol Sci. (2020) 21. doi: 10.3390/ijms21124363
24. Iyer SS, Chatraw JH, Tan WG, Wherry EJ, Becker TC, Ahmed R, et al. Protein energy malnutrition impairs homeostatic proliferation of memory CD8 T cells. J Immunol. (2012) 188:77–84. doi: 10.4049/jimmunol.1004027
25. Lin T-Y, Jiang D, Chen W-R, Lin JS, Zhang X-Y, Chen C-H, et al. Trained immunity induced by high-salt diet impedes stroke recovery. EMBO Rep. (2023) 24:e57164. doi: 10.15252/embr.202357164
26. Phillips JA. Dietary guidelines for americans, 2020-2025. Workplace Health Saf. (2021) 69:395. doi: 10.1177/21650799211026980
27. Phillips CM, Chen L-W, Heude B, Bernard JY, Harvey NC, Duijts L, et al. Dietary inflammatory index and non-communicable disease risk: A narrative review. Nutrients. (2019) 11. doi: 10.3390/nu11081873
28. Schwingshackl L, Hoffmann G. Diet quality as assessed by the Healthy Eating Index, the Alternate Healthy Eating Index, the Dietary Approaches to Stop Hypertension score, and health outcomes: a systematic review and meta-analysis of cohort studies. J Acad Nutr Diet. (2015) 115. doi: 10.1016/j.jand.2014.12.009
29. Dominguez LJ, Di Bella G, Veronese N, Barbagallo M. Impact of mediterranean diet on chronic non-communicable diseases and longevity. Nutrients. (2021) 13. doi: 10.3390/nu13062028
31. Shams-White MM, Pannucci TE, Lerman JL, Herrick KA, Zimmer M, Meyers Mathieu K, et al. Healthy eating index-2020: review and update process to reflect the dietary guidelines for americans,2020-2025. J Acad Nutr Diet. (2023) 123:1280–8. doi: 10.1016/j.jand.2023.05.015
32. Shivappa N, Steck SE, Hurley TG, Hussey JR, Hébert JR. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. (2014) 17:1689–96. doi: 10.1017/S1368980013002115
33. Trichopoulou A, Costacou T, Bamia C, Trichopoulos D. Adherence to a Mediterranean diet and survival in a Greek population. N Engl J Med. (2003) 348:2599–608. doi: 10.1056/NEJMoa025039
34. Fung TT, Rexrode KM, Mantzoros CS, Manson JE, Willett WC, Hu FB. Mediterranean diet and incidence of and mortality from coronary heart disease and stroke in women. Circulation. (2009) 119:1093–100. doi: 10.1161/CIRCULATIONAHA.108.816736
35. Mellen PB, Gao SK, Vitolins MZ, Goff DC Jr. Deteriorating dietary habits among adults with hypertension: DASH dietary accordance, NHANES 1988-1994 and 1999-2004. Arch Internal Med. (2008) 168:308–14. doi: 10.1001/archinternmed.2007.119
36. Nie J, Deng M-G, Wang K, Liu F, Xu H, Feng Q, et al. Higher HEI-2015 scores are associated with lower risk of gout and hyperuricemia: Results from the national health and nutrition examination survey 2007-2016. Front Nutr. (2022) 9:921550. doi: 10.3389/fnut.2022.921550
37. Rahman HH, Niemann D, Munson-McGee SH. Association between asthma, chronic bronchitis, emphysema, chronic obstructive pulmonary disease, and lung cancer in the US population. Environ Sci pollut Res Int. (2023) 30:20147–58. doi: 10.1007/s11356-022-23631-3
38. Deng M-G, Liu F, Liang Y, Chen Y, Nie J-Q, Chai C, et al. Associations of serum zinc, copper, and selenium with sleep disorders in the American adults: Data from NHANES 2011-2016. J Affect Disord. (2023) 323:378–85. doi: 10.1016/j.jad.2022.11.088
39. Wang K, Xia F, Li Q, Luo X, Wu J. The associations of weekend warrior activity patterns with the visceral adiposity index in US adults: repeated cross-sectional study. JMIR Public Health Surveill. (2023) 9:e41973. doi: 10.2196/41973
40. Patel JS, Oh Y, Rand KL, Wu W, Cyders MA, Kroenke K, et al. Measurement invariance of the patient health questionnaire-9 (PHQ-9) depression screener in U.S. adults across sex, race/ethnicity, and education level: NHANES 2005-2016. Depress Anxiety. (2019) 36:813–23. doi: 10.1002/da.22940
41. Liu F, Nie J, Deng M-G, Yang H, Feng Q, Yang Y, et al. Dietary flavonoid intake is associated with a lower risk of diabetic nephropathy in US adults: data from NHANES 2007-2008, 2009-2010, and 2017-2018. Food Funct. (2023) 14:4183–90. doi: 10.1039/d3fo00242j
42. Wu M, Si J, Liu Y, Kang L, Xu B. Association between composite dietary antioxidant index and hypertension: insights from NHANES. Clin Exp Hypertens. (2023) 45:2233712. doi: 10.1080/10641963.2023.2233712
43. Qiu Z, Chen X, Geng T, Wan Z, Lu Q, Li L, et al. Associations of serum carotenoids with risk of cardiovascular mortality among individuals with type 2 diabetes: results from NHANES. Diabetes Care. (2022) 45:1453–61. doi: 10.2337/dc21-2371
44. Chen L, Sun Q, Peng S, Tan T, Mei G, Chen H, et al. Associations of blood and urinary heavy metals with rheumatoid arthritis risk among adults in NHANES, 1999-2018. Chemosphere. (2022) 289:133147. doi: 10.1016/j.chemosphere.2021.133147
45. Schols AM, Ferreira IM, Franssen FM, Gosker HR, Janssens W, Muscaritoli M, et al. Nutritional assessment and therapy in COPD: a European Respiratory Society statement. Eur Respir J. (2014) 44:1504–20. doi: 10.1183/09031936.00070914
46. Marín-Hinojosa C, Eraso CC, Sanchez-Lopez V, Hernández LC, Otero-Candelera R, Lopez-Campos JL. Nutriepigenomics and chronic obstructive pulmonary disease: potential role of dietary and epigenetics factors in disease development and management. Am J Clin Nutr. (2021) 114:1894–906. doi: 10.1093/ajcn/nqab267
47. Schols AMWJ. The 2014 ESPEN Arvid Wretlind Lecture: Metabolism & nutrition: Shifting paradigms in COPD management. Clin Nutr. (2015) 34:1074–9. doi: 10.1016/j.clnu.2015.09.005
48. Beijers RJHCG, Steiner MC, Schols AMWJ. The role of diet and nutrition in the management of COPD. Eur Respir Rev. (2023) 32. doi: 10.1183/16000617.0003-2023
49. van Iersel LEJ, Beijers RJHCG, Gosker HR, Schols AMWJ. Nutrition as a modifiable factor in the onset and progression of pulmonary function impairment in COPD: a systematic review. Nutr Rev. (2022) 80:1434–44. doi: 10.1093/nutrit/nuab077
50. Guilleminault L, Williams EJ, Scott HA, Berthon BS, Jensen M, Wood LG. Diet and asthma: is it time to adapt our message? Nutrients. (2017) 9. doi: 10.3390/nu9111227
51. Gutiérrez-Carrasquilla L, Sánchez E, Hernández M, Polanco D, Salas-Salvadó J, Betriu À, et al. Effects of mediterranean diet and physical activity on pulmonary function: A cross-sectional analysis in the ILERVAS project. Nutrients. (2019) 11. doi: 10.3390/nu11020329
52. Marx W, Veronese N, Kelly JT, Smith L, Hockey M, Collins S, et al. The dietary inflammatory index and human health: an umbrella review of meta-analyses of observational studies. Adv Nutr. (2021) 12:1681–90. doi: 10.1093/advances/nmab037
53. Ardestani ME, Onvani S, Esmailzadeh A, Feizi A, Azadbakht L. Adherence to dietary approaches to stop hypertension (DASH) dietary pattern in relation to chronic obstructive pulmonary disease (COPD): A case-control study. J Am Coll Nutr. (2017) 36:549–55. doi: 10.1080/07315724.2017.1326858
54. Vardavas CI, Flouris AD, Tsatsakis A, Kafatos AG, Saris WHM. Does adherence to the Mediterranean diet have a protective effect against active and passive smoking? Public Health. (2011) 125:121–8. doi: 10.1016/j.puhe.2010.11.012
55. Neelakantan N, Koh W-P, Yuan J-M, van Dam RM. Diet-quality indexes are associated with a lower risk of cardiovascular, respiratory, and all-cause mortality among chinese adults. J Nutr. (2018) 148:1323–32. doi: 10.1093/jn/nxy094
56. Alwarith J, Kahleova H, Crosby L, Brooks A, Brandon L, Levin SM, et al. The role of nutrition in asthma prevention and treatment. Nutr Rev. (2020) 78:928–38. doi: 10.1093/nutrit/nuaa005
57. Meyer M, Jaspers I. Respiratory protease/antiprotease balance determines susceptibility to viral infection and can be modified by nutritional antioxidants. Am J Physiol Lung Cell Mol Physiol. (2015) 308:L1189–201. doi: 10.1152/ajplung.00028.2015
58. Salo PM, Mendy A, Wilkerson J, Molsberry SA, Feinstein L, London SJ, et al. Serum antioxidant vitamins and respiratory morbidity and mortality: a pooled analysis. Respir Res. (2022) 23:150. doi: 10.1186/s12931-022-02059-w
59. Nguyen HT, Collins PF, Pavey TG, Nguyen NV, Pham TD, Gallegos DL. Nutritional status, dietary intake, and health-related quality of life in outpatients with COPD. Int J Chron Obstruct Pulmon Dis. (2019) 14:215–26. doi: 10.2147/COPD.S181322
60. Ingadottir AR, Beck AM, Baldwin C, Weekes CE, Geirsdottir OG, Ramel A, et al. Association of energy and protein intakes with length of stay, readmission and mortality in hospitalised patients with chronic obstructive pulmonary disease. Br J Nutr. (2018) 119:543–51. doi: 10.1017/S0007114517003919
61. Collins PF, Stratton RJ, Elia M. Nutritional support in chronic obstructive pulmonary disease: a systematic review and meta-analysis. Am J Clin Nutr. (2012) 95:1385–95. doi: 10.3945/ajcn.111.023499
62. Dal Negro RW, Aquilani R, Bertacco S, Boschi F, Micheletto C, Tognella S. Comprehensive effects of supplemented essential amino acids in patients with severe COPD and sarcopenia. Monaldi Arch Chest Dis. (2010) 73:25–33. doi: 10.4081/monaldi.2010.310
63. Engelen MPKJ, Rutten EPA, De Castro CLN, Wouters EFM, Schols AMWJ, Deutz NEP. Supplementation of soy protein with branched-chain amino acids alters protein metabolism in healthy elderly and even more in patients with chronic obstructive pulmonary disease. Am J Clin Nutr. (2007) 85:431–9. doi: 10.1093/ajcn/85.2.431
64. Cruthirds CL, Deutz NEP, Harrykissoon R, Zachria AJ, Engelen MPKJ. A low postabsorptive whole body protein balance is associated with markers of poor daily physical functioning in Chronic Obstructive Pulmonary Disease. Clin Nutr. (2022) 41:885–93. doi: 10.1016/j.clnu.2022.02.018
65. Rondanelli M, Faliva MA, Peroni G, Infantino V, Gasparri C, Iannello G, et al. Food pyramid for subjects with chronic obstructive pulmonary diseases. Int J Chron Obstruct Pulmon Dis. (2020) 15:1435–48. doi: 10.2147/COPD.S240561
66. Tramontano A, Palange P. Nutritional state and COPD: effects on dyspnoea and exercise tolerance. Nutrients. (2023) 15. doi: 10.3390/nu15071786
67. Hussain SNA, Sandri M. Role of autophagy in COPD skeletal muscle dysfunction. J Appl Physiol (1985). (2013) 114:1273–81. doi: 10.1152/japplphysiol.00893.2012
68. Shakoor H, Feehan J, Al Dhaheri AS, Ali HI, Platat C, Ismail LC, et al. Immune-boosting role of vitamins D, C, E, zinc, selenium and omega-3 fatty acids: Could they help against COVID-19? Maturitas. (2021) 143:1–9. doi: 10.1016/j.maturitas.2020.08.003
69. Gozzi-Silva SC, Teixeira FME, Duarte AJ da S, Sato MN, Oliveira L de M. Immunomodulatory role of nutrients: how can pulmonary dysfunctions improve? Front Nutr. (2021) 8:674258. doi: 10.3389/fnut.2021.674258
70. Varraso R, Barr RG, Willett WC, Speizer FE, Camargo CA. Fish intake and risk of chronic obstructive pulmonary disease in 2 large US cohorts. Am J Clin Nutr. (2015) 101:354–61. doi: 10.3945/ajcn.114.094516
71. Kaluza J, Harris H, Wallin A, Linden A, Wolk A. Dietary fiber intake and risk of chronic obstructive pulmonary disease: A prospective cohort study of men. Epidemiology. (2018) 29:254–60. doi: 10.1097/EDE.0000000000000750
72. Tabak C, Smit HA, Heederik D, Ocké MC, Kromhout D. Diet and chronic obstructive pulmonary disease: independent beneficial effects of fruits, whole grains, and alcohol (the MORGEN study). Clin Exp Allergy. (2001) 31:747–55. doi: 10.1046/j.1365-2222.2001.01064.x
Keywords: HEI-2020, DII, Mediterranean diet, DASH diet, chronic respiratory diseases, NHANES
Citation: Li H, Tang X, Guo X, Zhang M, Zhang M, Nie J, Fang S, Zhang H, Shi Y, Dai X, Li J and Yin X (2024) Association of dietary patterns with chronic respiratory health among U.S. adults. Front. Immunol. 15:1457860. doi: 10.3389/fimmu.2024.1457860
Received: 01 July 2024; Accepted: 19 November 2024;
Published: 06 December 2024.
Edited by:
Weicheng Hu, Yangzhou University, ChinaReviewed by:
Guadalupe Rivera-Torruco, Vitalant Research Institute, United StatesXiaojian Jiang, Huaiyin Normal University, China
Muniyappan Madesh, Yangzhou University, China
Copyright © 2024 Li, Tang, Guo, Zhang, Zhang, Nie, Fang, Zhang, Shi, Dai, Li and Yin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xin Yin, eWlueGluXzg4MDYwNUAxNjMuY29t
†These authors have contributed equally to this work
 Hui Li
Hui Li XiaoLi Tang1†
XiaoLi Tang1† XinWei Guo
XinWei Guo JiaQi Nie
JiaQi Nie Hong Zhang
Hong Zhang