- 1Kidney Institute, Department of Nephrology, Shanghai Changzheng Hospital, Naval Medical University (Second Military Medical University), Shanghai, China
- 2Department of Radiology, Shanghai Changzheng Hospital, Naval Medical University, Shanghai, China
- 3Department of Health Management Center, Shanghai Changzheng Hospital, Naval Medical University, Shanghai, China
- 4Department of Hepatic Surgery, Fudan University Shanghai Cancer Center, Shanghai, China
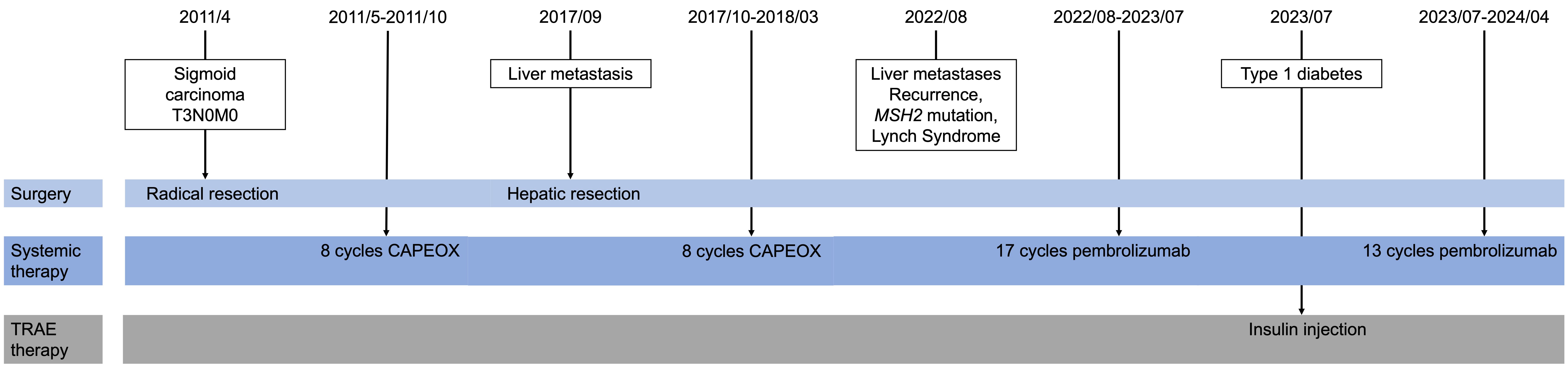
Pembrolizumab and other immunotherapies have become central in treating metastatic colon cancer, particularly effective in patients with mismatch repair deficiencies. We report a case involving a man who initially underwent radical surgery for sigmoid colon cancer on April 27, 2011, followed by hepatic tumor resection on September 21, 2017. Post-surgery, he received eight cycles of adjuvant chemotherapy with the CAPEOX regimen and was regularly monitored through CT and MRI scans. On August 24, 2022, liver metastases were detected, and he was diagnosed with Lynch syndrome (LS) due to germline mutation in the MSH2 and EPCAM genes. He commenced treatment with 200mg of pembrolizumab intravenously every three weeks on September 2, 2022, and demonstrated a sustained response. However, after 17 cycles, he developed a treatment related adverse event (TRAE) of pancreatic endocrine dysfunction, leading to type 1 diabetes, managed with subcutaneous insulin injections. After 30 cycles of treatment, no evidence of disease was observed. This case underscores the significant clinical benefits of first-line pembrolizumab in managing hepatic metastasis in colonic carcinoma associated with LS, despite the occurrence of TRAEs. It raises critical questions regarding the optimal duration of immunotherapy following a complete or partial response and whether treatment should be discontinued upon the emergency of TRAEs. Continued research and forthcoming clinical trials with checkpoint inhibitors are expected to refine treatment protocols for LS-associated carcinoma.
Introduction
Pembrolizumab is the classic immune checkpoint inhibitor (ICI) and has various roles in advanced malignancies including the colon, lung, kidney, and bladder as well as lymphomas and melanoma. The unexpected response rate accelerates the approval of pembrolizumab monotherapy by US Food and Drug Administration (FDA) in 2017 (1).
Mismatch repair deficiencies (dMMRs) are predictive of response to immunotherapy in metastatic colorectal cancers. Because of the dMMRs, there are more mutations in DNA to pass unrepaired during cell division, thereby promoting tumor growth. The most familiar mismatch repair genes include MLH1, MSH2, MSH6, PMS2, or EPCAM (deletions of the epithelial cell adhesion molecule gene). It is reported that MSH2 or MLH1 accounts for almost 60% to 80% of all Lynch syndrome (LS)-associated cancers, others are attributed to MSH6 or PMS2, rarely, to EPCAM (2). A small subset of patients accumulate transcriptional errors in regions with repetitive nucleotide sequences throughout the genome (microsatellite instabilities, MSI). This microsatellite unstable phenotype is termed dMMRs/microsatellite instability-high (MSI-H).
LS is one of the most prevalent hereditary cancer syndromes and accounts for some 3% of unselected patients with colorectal or endometrial cancer and 10–15% of those with DNA mismatch repair (MMR)-deficient tumors. Extracolonic malignancies are also common, including endometrial, which is the most common, but also tumors of the upper gastrointestinal tract, urinary tract, ovarian, hepatobiliary tract, pancreas, and brain (3). It is characterized as an autosomal dominant cancer genetic predisposition syndrome due to a germline mutation in one of the mismatch repairs mentioned above.
Although dMMRs/MSI-H are new indications for pembrolizumab, our experience with it and other checkpoint inhibitors in MMR-deficient tumors is still limited. Herein we report a case of a patient with hepatic metastasis of colonic carcinoma and LS due to MSH2 and EPCAM genes mutation who is enjoying an ongoing, prolonged treatment response of almost two years to single-agent pembrolizumab, even though he underwent treatment related adverse event (TRAE) during the treatment period of monotherapy.
Case description
A 60-year-old Asian man with a 15-year of hypertension and no other past medical history, initially presented in April, 2011. He had bright red blood per rectum (BRBPR) for months initially attributed to hemorrhoids and about 5kg weight loss during the past half a year. His family history was positive for colon cancer in his mother (diagnosed in the 50s) and one sister died of colon cancer in her 20s. He underwent a colonoscopy examination and was diagnosed with sigmoid carcinoma. He was surgically staged as Stage IIA (T3N0M0, with no metastatic disease on subsequent computed tomography (CT) imaging). The postoperative pathological assessment revealed mucinous adenocarcinoma localized to the sigmoid colon. He received a course of adjuvant chemotherapy with eight cycles of adjuvant chemotherapy following the CAPEOX regimen, involving Oxaliplatin (130 mg/m2 IV on day 1) and Capecitabine (1000 mg/m2 PO twice daily for 14 days every three weeks), which was complete in October 2011. Despite a family history suggestive of colorectal cancer predisposition, genetic testing for dMMR and MSI-H was not performed at the time of the initial diagnosis. This was partly due to the limited availability and awareness of genetic testing for LS in China in 2011.
In the intervening years, he underwent routine surveillance follow-ups with no evidence of disease on annual clean colonoscopies every year and magnetic resonance imaging (MRI) until October 2016.
In September, 2017, he was diagnosed with hepatic tumor and underwent hepatic tumor resection, disclosing postoperative pathology indicative of metastatic adenocarcinoma. Immunohistochemistry demonstrated deficient mismatch repair proteins, with loss of both MSH2, MLH, and MSH6. In the aftermath of the surgical intervention, an additional eight cycles of CAPEOX regimen were administered (Figure 1).
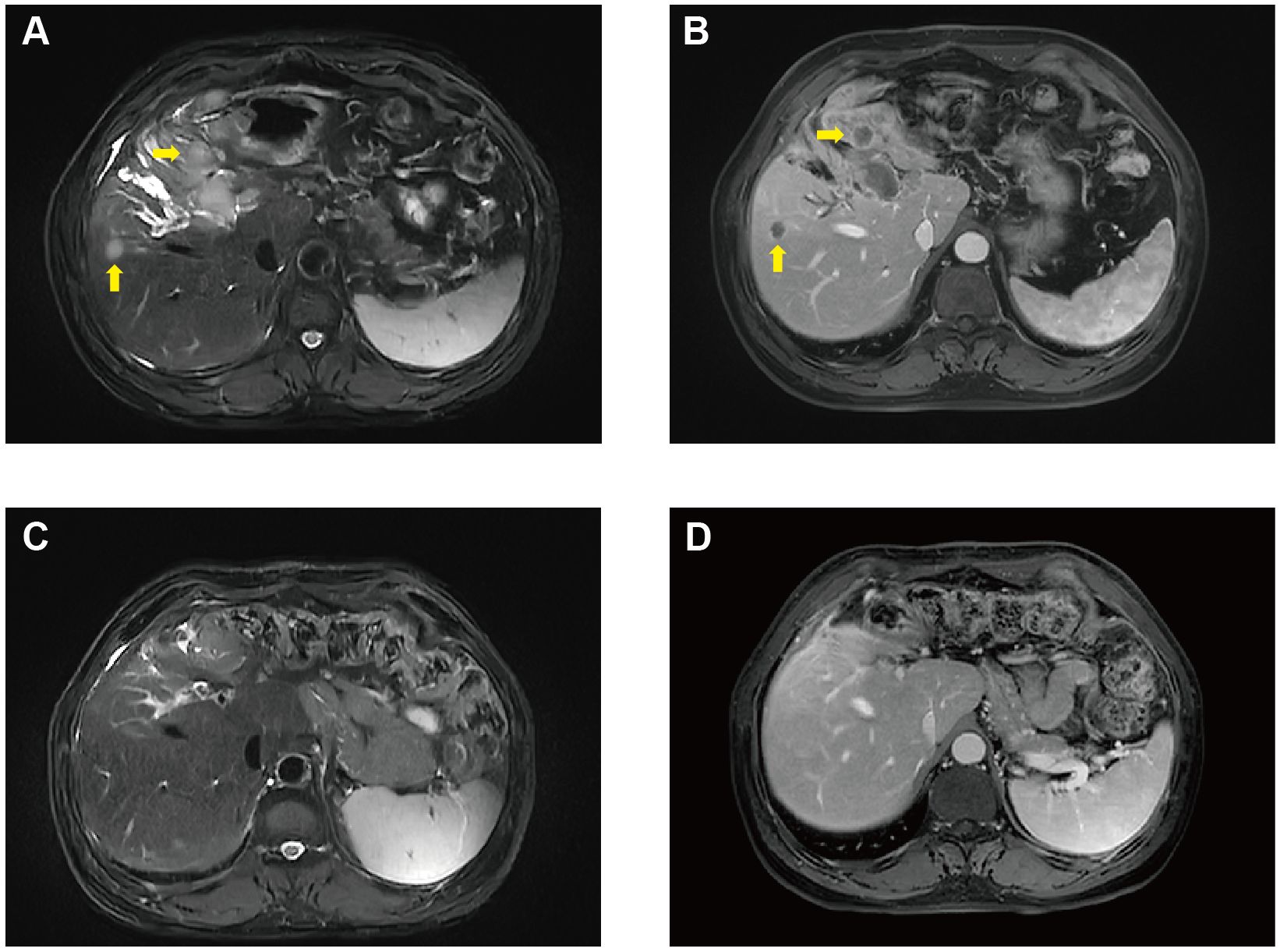
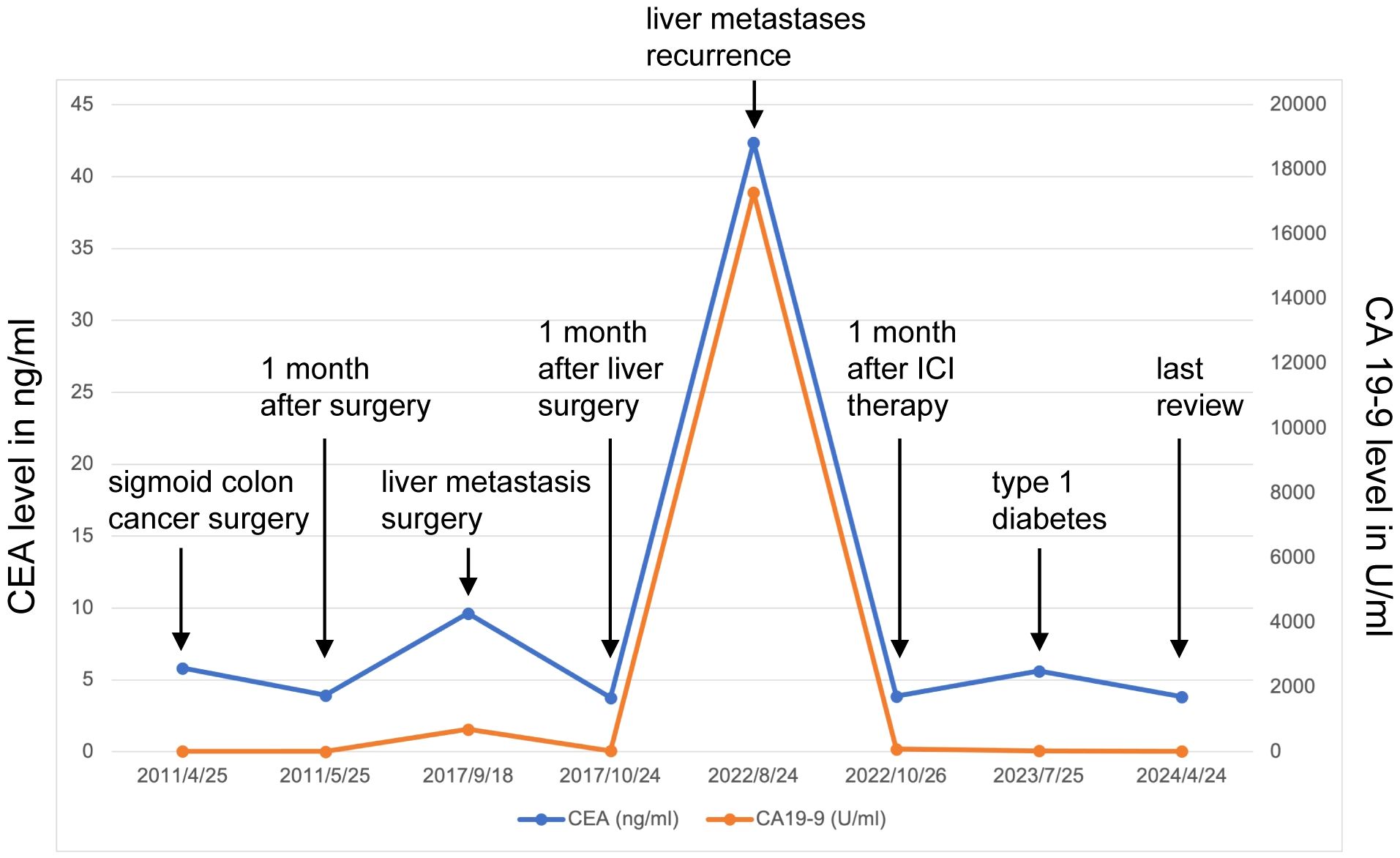
Unfortunately, in August 2022, he was diagnosed with liver metastasis with abdominal MRI on routine physical examination, which unveiled a spectrum of intrahepatic complexities, including multiple metastatic lesions, intrahepatic bile duct dilation, metastasis to the porta hepatis lymph nodes, and a potential tumor thrombus in the left branch of the portal vein (Figures 2A, B). Physical examination was unremarkable. Laboratory results showed an evidently increase of carbohydrate antigen 19-9 (CA19-9) and carcinoembryonic antigen (CEA) (Figure 3), which were 17288U/ml (0-39) and 42.37ug/L (0-5), respectively. The alpha fetal protein (AFP) was normal with 2.7ug/L (0-20). Further information revealed that one of his brothers developed carcinoma of the bladder in his mid-50s and died of glioblastoma. Another brother developed colon cancer in his 60s. His niece also developed colon cancer and papillary thyroid carcinoma in the past 5 years. Ultimately, he was diagnosed with LS based on germline testing done on peripheral blood DNA which showed mutation in MSH2 and EPCAM, with a TMB of 35.8 mutations/megabase, with no alterations in KRAS or BRAF identified. And microsatellite instability testing was positive.
Because of the known tumor MMR deficiency, he subsequently underwent immunotherapy of anti-programmed death 1 (PD-1) antibodies pembrolizumab at a dose of 200 mg intravenously every three weeks. Only after 3 cycles of treatment of pembrolizumab, the laboratory results and clinical status of the patient improved tremendously (Figure 3). However, after 17 cycles of treatment, he attended our clinic because of diabetic ketoacidosis. His data at onset, including extremely low serum C-peptide (0.08 ng/mL), fulfilled the diagnostic criteria for fulminant type 1 diabetes. Using multiple daily insulin injection therapy, his glycemic control has been maintained.
He continued pembrolizumab treatment and aside from immunotherapy-induced type 1 diabetes, he tolerated treatment well. He received a total of 30 cycles of pembrolizumab. CA19-9 and CEA remained within normal limits (Figure 3) and surveillance radiographic changes in May, 2024 showed no evidence of metastatic disease, indicating a remarkable response when compared to the MRI before the initiation of immunotherapy (Figures 2C, D). He was also being followed with yearly flex sigmoidoscopy, upper endoscopy every year, and oncology clinic visits with laboratory test index every nine weeks. Immunotherapy was optimally tolerated and the patient reported an active life and returned to his full-time employment.
Discussion
We present a case of a complete metabolic and serologic remission in a patient with dMMR/MSI-H metastatic colorectal cancer (mCRC) and Lynch syndrome under treatment with pembrolizumab monotherapy after radical surgery for sigmoid colon cancer on April 27, 2011, and hepatic tumor resection on September 21, 2017, respectively. Following 8 cycles of adjuvant chemotherapy with the CAPEOX regimen after each operation.
Up to 30% of CRC are inherited, and known syndromes account for only 2-5% of all cases. The two most common syndromes are LS and familial adenomatous polyposis (FAP) (4). LS is diagnosed using clinical criteria like the Amsterdam Criteria II and Revised Bethesda Guidelines, tumor testing for MSI and immunohistochemistry to detect MMR protein deficiencies, and genetic testing of peripheral blood DNA to identify germline mutations in MMR genes (MLH1, MSH2, MSH6, PMS2, EPCAM). Next-generation sequencing (NGS) enhances sensitivity and specificity. A detailed family history helps identify LS patterns, and genetic counseling is recommended (5). LS is characterized by germline mutations in the MMR genes that cause genomic instability and therefore tumorigenesis. Due to the loss of expression of these proteins, mutations in microsatellite regions (repetitive DNA sequences) accumulate, resulting in MSI and a high tumor mutational burden (TMB).
Recent advancements have unveiled new diagnostic methods. Liquid biopsy techniques analyzing circulating tumor DNA (ctDNA) offer a non-invasive diagnostic and monitoring tool, detecting MSI and MMR deficiencies from blood samples. Emerging evidence highlights the role of microRNAs (miRNAs) in LS pathogenesis, where overexpression of miR-155, miR-21, and miR-137 may induce MSI or modulate MMR gene expression. These miRNAs could serve as biomarkers and therapeutic targets, enhancing precision medicine approaches for LS. Ongoing research into the miRNAs involvement in LS provides insights into novel diagnostic and therapeutic strategies, promising to refine the management of this hereditary cancer syndrome (6).
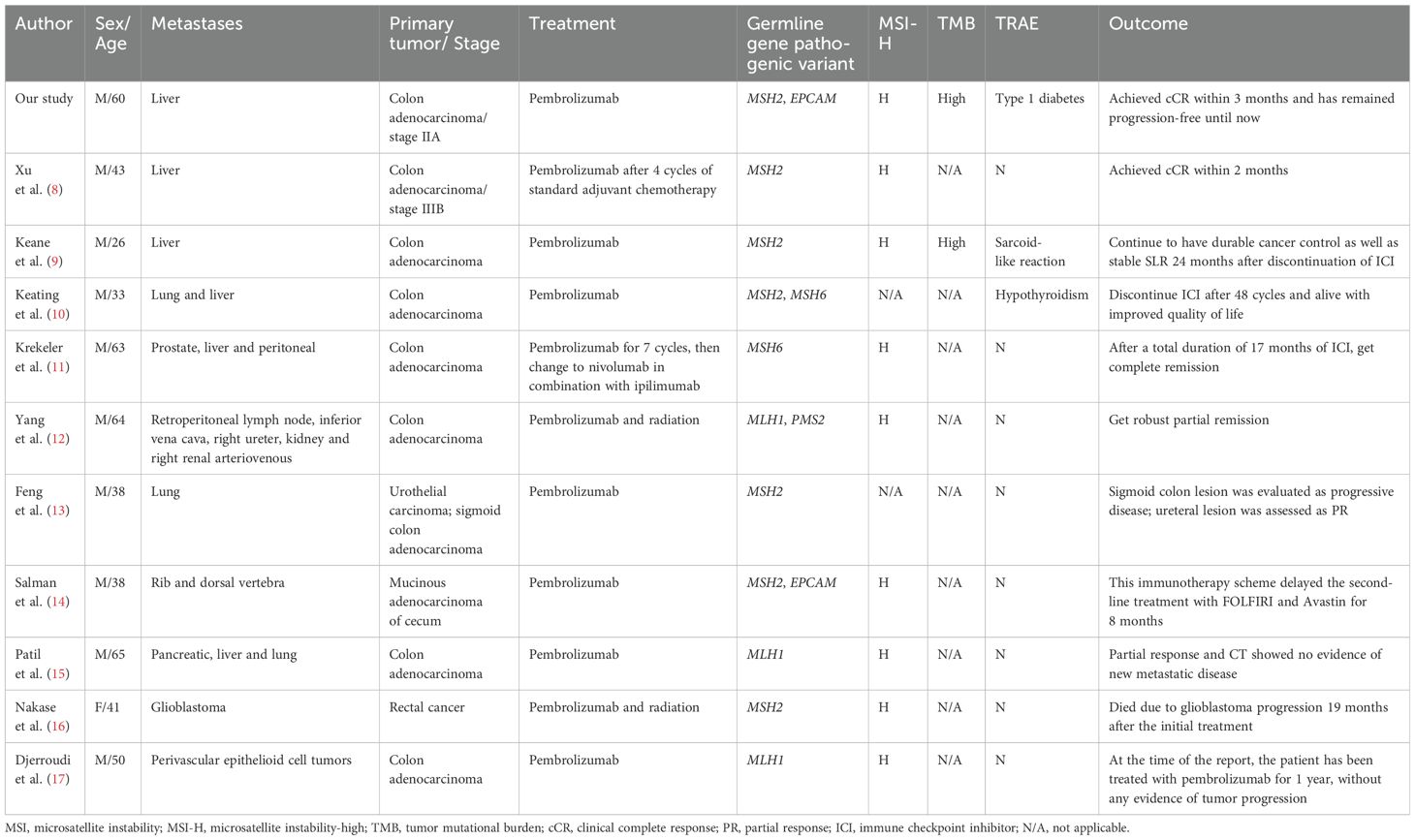
Treatment of mCRC with dMMR/MSI-H has traditionally been challenging due to the poor response to conventional chemotherapeutic therapy, just like in our case (7). However, the long-term remissions induced by ICIs in many types of cancers have opened up the possibility of a broader use of immunotherapy in less immunogenic but genetically heterogeneous tumors. For mCRC, pembrolizumab has been approved as the preferred option and nivolumab, alone or in combination with ipilimumab as an alternative option for patients with dMMR/MSI-H disease, independently of their eligibility for intensive chemotherapy. The KEYNOTE-177 study showed that in patients with dMMR/MSI-H mCRC, pembrolizumab significantly improves progression-free survival (PFS), objective response rate (ORR), and complete response (CR) rate, doubling median PFS from 8.2 months to 16.5 months, extending ORR from 33.1% to 43.8%, and increasing CR rate from 3.9% to 11.1%, comparing with the standard chemotherapy (3). Previous studies about mCRC treated with pembrolizumab were summarized in Table 1, and most of the cases got CR or PR (8–17). A clinical study by Le et al. also highlighted the effectiveness of ICIs in dMMR/MSI-H colorectal cancer, corroborating our findings. Additionally, cases reported by André et al. provided evidence of durable responses and manageable adverse events of Pembrolizumab in MSI-H CRC, similar to our patient’s experience (18, 19).
Although the response to ICIs has not yet been fully understood. One approach to explanation is as follows: the increased rate of neoantigens can cause an immune response and an upregulation of PD-1 ligands within the immunogenic tumor environment (20).
The most recent guidelines from the National Comprehensive Cancer Network (NCCN) and the European Society for Medical Oncology (ESMO) recommend the use of pembrolizumab as a first-line treatment for patients with dMMR/MSI-H metastatic colorectal cancer (21, 22). These guidelines emphasize the importance of genetic testing for LS and other mismatch repair deficiencies to guide immunotherapy decisions. Moreover, the guidelines suggest close monitoring for treatment-related adverse events and advocate for a multidisciplinary approach to manage these patients effectively.
In our case, the patient with LS received PD-1 inhibitor monotherapy (pembrolizumab) after liver metastases. He achieved clinical complete response (cCR) until now, while the TRAE also occurred after 17 cycles of pembrolizumab. A body of studies reported that the TRAEs are predictive biomarkers for ICI efficacy, including bystander inflammation of off-target organs due to activated infiltrating T cells. ICI-associated diabetes could be caused by the autoreactive CD8+ T-cells, which are infiltrating pancreatic islets and destroy insulin-producing beta cells (23). Most of the patients present with diabetic ketoacidosis characterized by absent C-peptide, causing the decline of the insulin production, as was observed in our patient. He is still receiving pembrolizumab treatment every 3 weeks as maintenance therapy under regularly insulin treatment.
Of course, our case also raises a question about the duration of immunotherapy, especially those with processable TRAEs. In our case, pembrolizumab was administered for 2 years. The median duration of treatment with Pembrolizumab is 11.1 months, with the longest duration reaching 30.6 months; however, the majority of patients complete their treatment within two years (24). Ongoing clinical trials with checkpoint inhibitors in dMMR/MSI-H mCRC are needed to generate more data on treatment outcomes. Meanwhile, we believe that our observation is informative for clinicians taking care of patients with mCRC because of their genetic predisposition to LS, and get a potentially significant clinical benefit of checkpoint inhibitors used as single agents early in the dMMR/MSI-H hepatic metastasis of colonic carcinoma and LS.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
CX: Writing – original draft. DZ: Methodology, Writing – original draft. XW: Writing – original draft. LJ: Writing – original draft. YL: Writing – original draft. SZ: Writing – original draft. JL: Writing – original draft. LC: Writing – original draft. MR: Writing – original draft. DX: Writing – original draft. QL: Writing – original draft. YF: Writing – review & editing. SM: Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This paper was supported by the National Nature Science Foundation of China (grant NO.81800681).
Acknowledgments
All authors thank the patient and his family for their support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sinicrope FA. Lynch syndrome-associated colorectal cancer. N Engl J Med. (2018) 379:764–73. doi: 10.1056/NEJMcp1714533
2. Jin Z, Sinicrope FA. Mismatch repair-deficient colorectal cancer: building on checkpoint blockade. J Clin Oncol. (2022) 40:2735–50. doi: 10.1200/JCO.21.02691
3. Boukouris AE, Theochari M, Stefanou D, Papalambros A, Felekouras E, Gogas H, et al. Latest evidence on immune checkpoint inhibitors in metastatic colorectal cancer: A 2022 update. Crit Rev Oncol Hematol. (2022) 173:103663. doi: 10.1016/j.critrevonc.2022.103663
4. Taieb J, Svrcek M, Cohen R, Basile D, Tougeron D, Phelip JM. Deficient mismatch repair/microsatellite unstable colorectal cancer: Diagnosis, prognosis and treatment. Eur J Cancer. (2022) 175:136–57. doi: 10.1016/j.ejca.2022.07.020
5. Umar A, Boland CR, Terdiman JP, Syngal S, de la Chapelle A, Ruschoff J, et al. Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst. (2004) 96:261–8. doi: 10.1093/jnci/djh034
6. Ascrizzi S, Arillotta GM, Grillone K, Carida G, Signorelli S, Ali A, et al. Lynch syndrome biopathology and treatment: the potential role of microRNAs in clinical practice. Cancers (Basel). (2023) 15(15):3930. doi: 10.3390/cancers15153930
7. Shulman K, Barnett-Griness O, Friedman V, Greenson JK, Gruber SB, Lejbkowicz F, et al. Outcomes of chemotherapy for microsatellite instable-high metastatic colorectal cancers. JCO Precis Oncol. (2018) 2:PO.17.00253. doi: 10.1200/PO.17.00253
8. Xu Y, Li Q, Zhao J, Ni X, Li P, Hu W. Case report: Complete response to pembrolizumab in a liver metastatic colon adenocarcinoma patient with a novel likely pathogenic germline MSH2 mutation. Front Immunol. (2022) 13:1064488. doi: 10.3389/fimmu.2022.1064488
9. Keane F, Yogiaveetil E, Kezlarian B, Lagratta M, Segal NH, Abou-Alfa G, et al. Immune checkpoint blockade induced sarcoid-like reaction mimicking progression of disease in a patient with microsatellite instable colorectal cancer: case report and review of the literature. J Gastrointest Oncol. (2024) 15:500–7. doi: 10.21037/jgo
10. Keating M, Giscombe L, Tannous T, Hartshorn K. Prolonged treatment response to pembrolizumab in a patient with pretreated metastatic colon cancer and lynch syndrome. Case Rep Oncol Med. (2019) 2019:3847672. doi: 10.1155/2019/3847672
11. Krekeler C, Wethmar K, Mikesch JH, Kerkhoff A, Menck K, Lenz G, et al. Complete metabolic response to combined immune checkpoint inhibition after progression of metastatic colorectal cancer on pembrolizumab: A case report. Int J Mol Sci. (2023) 24(15):12056. doi: 10.3390/ijms241512056
12. Yang J, Bi F, Gou H. Complete pathologic response after concurrent treatment with pembrolizumab and radiotherapy in metastatic colorectal cancer: A case report. Onco Targets Ther. (2021) 14:2555–61. doi: 10.2147/OTT.S298333
13. Feng Y, Cao Y, Yuan M, Chen R, Ji X, Hu X. Different responses to anti-programmed cell death protein 1 (PD-1) immunotherapy in a patient with Lynch syndrome and metachronous urothelial and colon cancer: A case report. Oncol Lett. (2019) 18:5085–90. doi: 10.3892/ol
14. Salman P, Panay S, Fernandez R, Mahave M, Soza-Ried C. Evidence of response to pembrolizumab in a patient with Lynch syndrome-related metastatic colon cancer. Onco Targets Ther. (2018) 11:7295–300. doi: 10.2147/OTT
15. Patil NR, Khan GN. Exceptional response to A single cycle of immunotherapy in a lynch syndrome patient with metastatic pancreatic adenocarcinoma. Am J Case Rep. (2020) 21:e923803. doi: 10.12659/AJCR.923803
16. Nakase K, Matsuda R, Sasaki S, Nakagawa I. Lynch syndrome-associated glioblastoma treated with concomitant chemoradiotherapy and immune checkpoint inhibitors: case report and review of literature. Brain Tumor Res Treat. (2024) 12:70–4. doi: 10.14791/btrt.2023.0042
17. Djerroudi L, Masliah-Planchon J, Brisse HJ, El Zein S, Helfre S, Tzanis D, et al. Metastatic Malignant perivascular epithelioid cell tumors with microsatellite instability within lynch syndrome successfully treated with anti-PD1 pembrolizumab. JCO Precis Oncol. (2023) 7:e2200627. doi: 10.1200/PO.22.00627
18. Andre T, Shiu KK, Kim TW, Jensen BV, Jensen LH, Punt C, et al. Pembrolizumab in microsatellite-Instability-High advanced colorectal cancer. N Engl J Med. (2020) 383:2207–18. doi: 10.1056/NEJMoa2017699
19. Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. (2017) 357:409–13. doi: 10.1126/science.aan6733
20. Roudko V, Cimen Bozkus C, Greenbaum B, Lucas A, Samstein R, Bhardwaj N. Lynch syndrome and MSI-H cancers: from mechanisms to “Off-the-shelf” Cancer vaccines. Front Immunol. (2021) 12:757804. doi: 10.3389/fimmu.2021.757804
21. Benson AB, Venook AP, Adam M, Chang G, Chen YJ, Ciombor KK, et al. Colon cancer, version 3.2024, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2024) 22(2 D):e240029. doi: 10.6004/jnccn.2024.0029
22. Yoshino T, Cervantes A, Bando H, Martinelli E, Oki E, Xu RH, et al. Pan-Asian adapted ESMO Clinical Practice Guidelines for the diagnosis, treatment and follow-up of patients with metastatic colorectal cancer. ESMO Open. (2023) 8:101558. doi: 10.1016/j.esmoop.2023.101558
23. Tsang ES, Walker EJ, Carnevale J, Fisher GA, Ko AH. Durable response after immune checkpoint inhibitor-related diabetes in mismatch repair-deficient pancreatic cancer. Immunotherapy. (2021) 13:1249–54. doi: 10.2217/imt-2021-0008
Keywords: immune-checkpoint inhibitors, Lynch syndrome, metastatic colorectal cancer, mismatch repair deficiencies, treatment related adverse event
Citation: Xue C, Zhu D, Wang X, Jiao L, Lu Y, Zhang S, Lv J, Cui L, Ruan M, Xu D, Liu Q, Feng Y and Mei S (2024) Durable response to pembrolizumab in hepatic metastasis from colonic carcinoma with Lynch syndrome: a case report. Front. Immunol. 15:1455907. doi: 10.3389/fimmu.2024.1455907
Received: 28 June 2024; Accepted: 07 August 2024;
Published: 23 August 2024.
Edited by:
Pierosandro Tagliaferri, Magna Græcia University, ItalyReviewed by:
Caterina Riillo, University Medical Center Utrecht, NetherlandsGiulio Caridà, Magna Græcia University of Catanzaro, Italy
Copyright © 2024 Xue, Zhu, Wang, Jiao, Lu, Zhang, Lv, Cui, Ruan, Xu, Liu, Feng and Mei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yun Feng, ZHJmZW5neXVuQDE2My5jb20=; Shuqin Mei, bWVpc2h1cWluMzIyQDE2My5jb20=
†These authors have contributed equally to this work
 Cheng Xue
Cheng Xue Dongqing Zhu2†
Dongqing Zhu2† Yun Feng
Yun Feng Shuqin Mei
Shuqin Mei