- 1Department of Thoracic Surgery, Jining No. 1 People’s Hospital, Jining, Shandong, China
- 2Institute of Thoracic Surgery, Jining Institute of Medical Sciences, Jining, Shandong, China
- 3School of Clinical Medicine, Jining Medical University, Jining, Shandong, China
- 4Department of Thoracic Surgery, Huzhou Central Hospital, Huzhou, Zhejiang, China
Background: The study was conducted in order to investigate whether neoadjuvant immunotherapy combined with chemotherapy can bring survival benefits to patients with locally advanced resectable esophageal squamous cell carcinoma (ESCC) in the real world.
Methods: We retrospectively analysed patients with locally advanced resectable ESCC who underwent surgery at the Jining First People’s Hospital from April 2020 to April 2022. Based on their medical history, the enrolled patients were divided into a neoadjuvant immunochemotherapy plus surgery group (nICT group) and a surgery-only group (S group). Primary endpoints were the two-year overall survival (OS) and disease-free survival (DFS) rates. Secondary endpoints were the safety and efficacy of neoadjuvant immunochemotherapy for patients with locally advanced esophageal cancer, and compared the surgery and postoperative outcomes between the two groups.
Results: A total of 47 patients in the nICT group and 73 patients in the S group were included for further analysis, the stage of the nICT group was more advanced than that of the S group. In the group nICT, 8 patients (17%) achieved the complete pathological response (pCR), 29 patients (61.7%) achieved major pathological response (MPR), including 6 patients (12.8%) with a primary tumor achieving pCR but had residual tumor cells in the lymph nodes (pT0N+), and the treatment-related AES was manageable. The surgery and postoperative outcomes were comparable in both groups. The two-year OS and DFS rates for the nICT group were 91.5% and 85.5% respectively, while those for the S group were 71.2% and 68.5%, and Kaplan–Meier survival analysis and log-rank test revealed significant differences in DFS and OS between the two groups. Patients who achieved MPR in the nICT group showed better DFS and OS, while the Three-cycle subgroup did not exhibit any survival benefit compared to the Two-cycle subgroup.
Conclusions: Neoadjuvant sintilimab combined with chemotherapy has promising efficacy and safety in the treatment of locally advanced resectable ESCC. The treatment modality has the potential to become a standard therapy for locally advanced resectable ESCC.
Introduction
Esophageal cancer (EC) has become one of the common malignant tumours in the world. According to the global cancer statistics in 2022, the number of new patients with EC reached 511,000 and the number of deaths reached 445,000 (1). China is an area with a high incidence of EC. Although the incidence and mortality of EC in China are declining, they are still the main malignant tumours threatening the health of Chinese residents. According to the estimation of the prevalence of malignant tumours in China in 2015, the incidence and mortality of EC ranked sixth and fourth among all malignant tumours respectively (2). In China, more than 90% of EC is esophageal squamous cell carcinoma (ESCC). The simple surgical treatment of EC has low OS and high recurrence rate in five years (3–5). A clinical trial in Japan (JCOG9907) confirmed that neoadjuvant chemotherapy (NCT) can significantly improve the five-year survival rate (55% vs 43%) of resectable stage II and III EC compared with postoperative adjuvant chemotherapy (6). The 5010 study in China showed that patients treated with neoadjuvant chemoradiotherapy (NCRT) have a five-year survival rate of 60% (7). The ten-year OS rate of CROSS study showed that the neoadjuvant radiotherapy and chemotherapy plus surgery group had an OS of 38%, while the surgery alone group had an OS of 25%, and the risk of death was reduced by 40% (8). However, 40%–50% of patients still have tumour recurrence, and distant metastasis is the most common (8, 9). In general, NCRT or NCT combined with surgical resection can improve the survival rate of patients with locally advanced resectable EC, but the prognosis of this group of patients is still not ideal (10). It is still necessary to explore more effective modes of treatment for EC so as to improve the long-term survival of EC patients and reduce recurrence and metastasis.
In recent years, PD-1 inhibitor has become a highly popular immunotherapy for cancer. By blocking the interaction between PD-1 and PD-L1, it can restore the anti-tumour activity of T lymphocytes, enhance the immune response and reduce the proliferation and metastasis of tumour cells. With the breakthrough progress of PD-1 inhibitor and PD-L1 inhibitor in the immunotherapy of melanoma, non-small cell lung cancer, kidney cancer and other tumours, the research on EC was gradually launched, and initial results were achieved (11). At present, immunotherapy for advanced EC has moved from the second line to the first line (12–14). Immunochemotherapy has become the standard first-line treatment for advanced EC, which can bring significant survival benefits to patients (14). Whether neoadjuvant immunochemotherapy can bring more opportunities to cure patients with locally advanced resectable EC has become a key research direction of EC.
Currently, multiple clinical studies on neoadjuvant immunotherapy for EC are being conducted, and they have achieved surprising results (15–17). Sintilimab is a fully humanised recombinant IgG4 monoclonal antibody targeting PD-1 (18). It has a relatively low treatment cost and is considered safe and effective in clinical settings. While multiple studies have affirmed the clinical efficacy of neoadjuvant sintilimab in the treatment of EC (19–21), data on its effectiveness and safety in real-world settings remains insufficient. Currently, clinicians still have concerns about the efficacy and safety of neoadjuvant immunochemotherapy, such as whether it increases the risk of disease progression, whether treatment-related adverse reactions are controllable, whether it increases the difficulty of surgery or increases the risk of postoperative complications, and it is crucial that there is still lack of long-term follow-up results. These clinical questions require further exploration, and thus, we have designed and conducted the current study.
In this study, we conducted a real-world retrospective analysis of 120 ESCC patients who received neoadjuvant immunochemotherapy (nICT) followed by surgery or surgery alone. The primary aim was to evaluate whether neoadjuvant immunotherapy combined with chemotherapy can bring survival benefits to patients with locally advanced ESCC in the real world.
Methods
Patients
This retrospective analysis was conducted on the patients with ESCC who received surgical treatment in Jining First People’s Hospital from April 2020 to April 2022. The study has been reviewed and approved by the Medical Ethics Committee of Jining First People’s Hospital.
The inclusion criteria were: the diagnosis of ESCC by pathological biopsy under electronic gastroscope; cTNM staging; cT2-4aN0-2M0 (8th edition of International Union for Cancer Control TNM classification); being without dysfunction of important organs such as heart, brain, lung, liver and kidney and without other serious diseases before treatment; an ECOG score of 0–1; if the patient is receiving preoperative neoadjuvant therapy, the neoadjuvant therapy scheme is combination chemotherapy with sintilimab.
The exclusion criteria were: age less than 18 years or greater than 80 years; the cancer is combined with other malignant tumours; the patient has received neoadjuvant chemotherapy or neoadjuvant radiochemotherapy; the patient has a history of intraperitoneal surgery, intrathoracic surgery and esophageal surgery; the patient cannot tolerate one-lung ventilation; the patient has an immunodeficiency disease, infectious disease, or another serious systemic disease requiring systemic immunosuppressive treatment.
All enrolled patients were divided into a neoadjuvant immunochemotherapy plus surgery group (nICT group) and a surgery-only group (S group) based on their treatment plan.
Treatment regimen
All enrolled patients underwent enhanced CT scans of the neck, chest and abdomen as well as neck ultrasound to determine their clinical staging. In addition, position emission tomography (PET) was prescribed when necessary. Patients received the above examinations every two cycles before esophagectomy in the nICT group.
The doses of sintilimab administered were fixed doses of 200 mg every three weeks. The adjuvant chemotherapy regimens included a combination of cisplatin (80mg/m2) and albumin-bound paclitaxel (200mg/m2) or a single drug regimen of albumin paclitaxel every three weeks. The chemotherapy regimens and doses adjusted by patients’ general condition. The number of neoadjuvant treatment cycles was between two and four.
Patients in the nICT group underwent esophagectomy four to six weeks after neoadjuvant immunochemotherapy. The minimally invasive McKeown esophagectomy was the primary surgical approach in two groups. Ivor Lewis esophagectomy and Sweet esophagectomy were performed on some patients who were expected to have challenges in surgery. Under difficult circumstances, minimally invasive surgery could be transferred to thoracotomy. Enlarged two-field lymphadenectomy was regularly conducted, and standard three-field lymphadenectomy was performed in patients with suspected swollen lymph nodes in the neck. Gastric tubes were used for the reconstruction of digestive tract. All operations were conducted by experienced surgeons with more than 50 cases annually, which ensured the surgery’s quality.
Postoperative adjuvant treatment was administered according to postoperative pathological results, the recovery condition of each patient and treatment-related adverse events (TRAEs). Multidisciplinary team discussion was also necessary.
Endpoints and assessments
Primary endpoints were the two-year OS and DFS rates. Secondary endpoints were the safety and efficacy of neoadjuvant immunochemotherapy and the following surgery, including: Treatment-related adverse events (TRAEs) during neoadjuvant therapy, complete pathological response (pCR) rate and major pathological response (MPR) rate in the nICT group, and the surgery and postoperative outcomes were compared between the two groups. In addition, we conducted an exploratory analysis to determine whether patients in the nICT group who achieved MPR or who had undergone the three-cycle neoadjuvant therapy had better survival benefits.
Follow-up were conducted through outpatient clinics or phone calls. The follow-up time for patients in the nICT group was calculated from the date of receiving immunochemotherapy, while for the S group, it was calculated from the date of surgery completion. All patients in the two groups were followed up with every three months in the first two years and every six months in the third year after the operation. The content of the follow-up included the patient’s general condition, recent re-examination results and other information. The deadline for the follow-up visits was May 2024.
Patient demographics, treatment pattern, intraoperative outcomes and postoperative complications such as anastomotic leakage, pleural effusion, pulmonary infection and ICU admission and 30-day mortality were recorded. Treatment-related adverse events (TRAEs) were collected from each patient’s medical records. TRAEs were graded according to the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0 of the US National Cancer Institute. Evaluate lymph node response according to the Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST 1.1), and assess esophageal lesion relief through esophageal barium swallowing radiography before and after neoadjuvant treatment, including complete remission (CR), partial remission (PR), disease progression (PD) and disease stability (SD). Finally, a comprehensive assessment was conducted. (The specific evaluation details see Supplementary Material). Objective response rate (ORR) was defined as the proportion of patients who achieved CR or PR and disease control rate (DCR) was defined as the proportion of patients who achieved CR, PR, or SD. PCR is defined as the absence of evidence of tumour cells in the surgical specimen, including lymph nodes. MPR is defined as the presence of ≤10% tumour cells in the surgical specimen. OS is defined as the time from the start of follow-up to the date of death from any cause or the date of the last follow-up. DFS is defined as the time from the start of follow-up to the date of disease recurrence or death from any cause.
Statistical analysis
The continuous variables were expressed as median (quartile difference) and the categorical variables were expressed as numbers (percentage). Differences in categorical variables and continuous variables were compared with the x2 test or Fisher’s exact test and t-test respectively. OS and DFS were calculated using the Kaplan–Meier method and were then compared by the log-rank test. Statistical analyses were performed using SPSS 27.0 (IBM Corp.) and R (v.4.4.0). P<0.05 (two-sided) was considered statistically significant.
Results
Patient selection and baseline characteristics
According to the inclusion criteria, a total of 47 patients in the nICT group and 73 patients in the S group were included for further analysis. Baseline demographic and clinicopathological characteristics are summarised in Table 1. There were no significant differences between the two groups in age, smoking, drinking habits, comorbidities or tumour location. No significant gender difference was detected between the two groups, both in which men were predominant: 72.3% and 82.2%, respectively (p =0.201). There were significant differences between the two groups in the clinical N stage, clinical T stage and clinical TNM stage. In the nICT group, cT4a, cN2 and IVA accounted for 42.6%, 19.1% and 42.6% respectively, and the proportion of cT4a, cN2 and IVA is 19.2%, 1.4% and 19.2% respectively in the S group. Obviously, the stage of the nICT group was more advanced than that of the S group.
In the nICT group, all patients received sintilimab combined with chemotherapy, 45 patients received sintilimab combined with albumin-bound paclitaxel and cisplatin and two patients received sintilimab combined with albumin-bound paclitaxel. 33 cases received two cycles and 14 cases received three cycles.
Effectiveness and safety of the neoadjuvant treatment
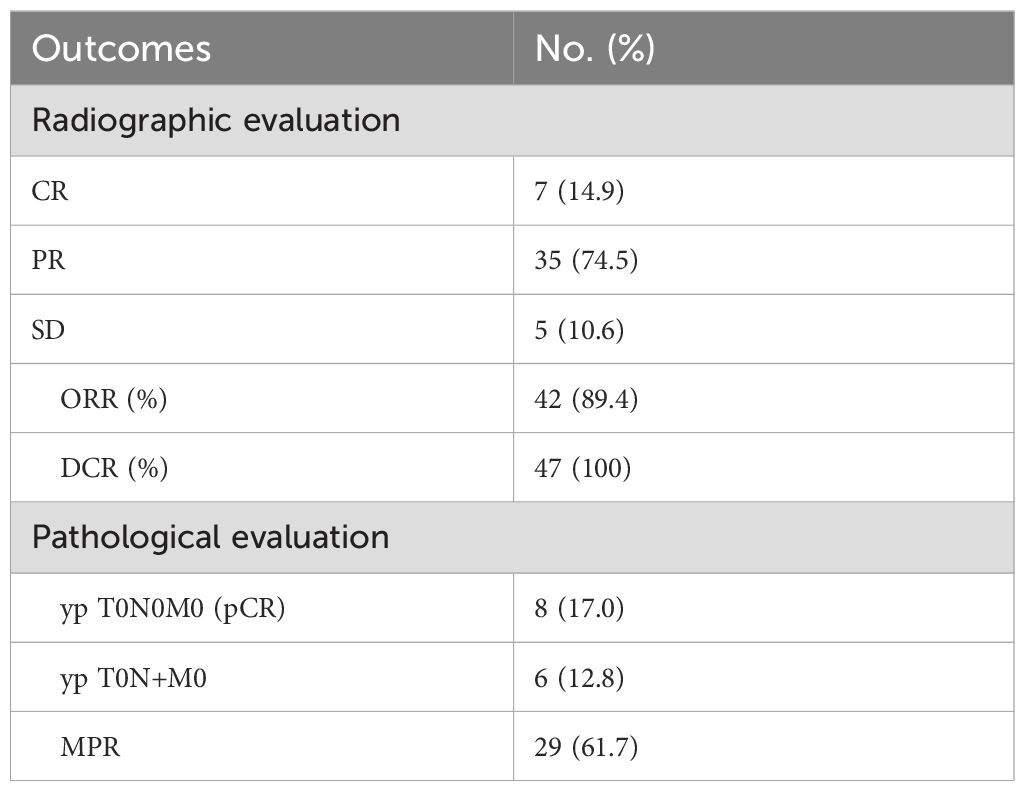
Radiographic response evaluation was performed in group nICT before surgery. Of the 47 patients, seven (14.9%) achieved CR, 35 (74.5%) achieved PR and five patients (10.6%) achieved SD. The ORR and DCR were 89.4% and 100%, respectively. Pathological evaluation was conducted after operation. 8 patients (17%) achieved the complete pathological response (pCR), 29 patients (61.7%) achieved major pathological response (MPR), including 6 patients (12.8%) with a primary tumor achieving pCR but had residual tumor cells in the lymph nodes (pT0N+) (Table 2).
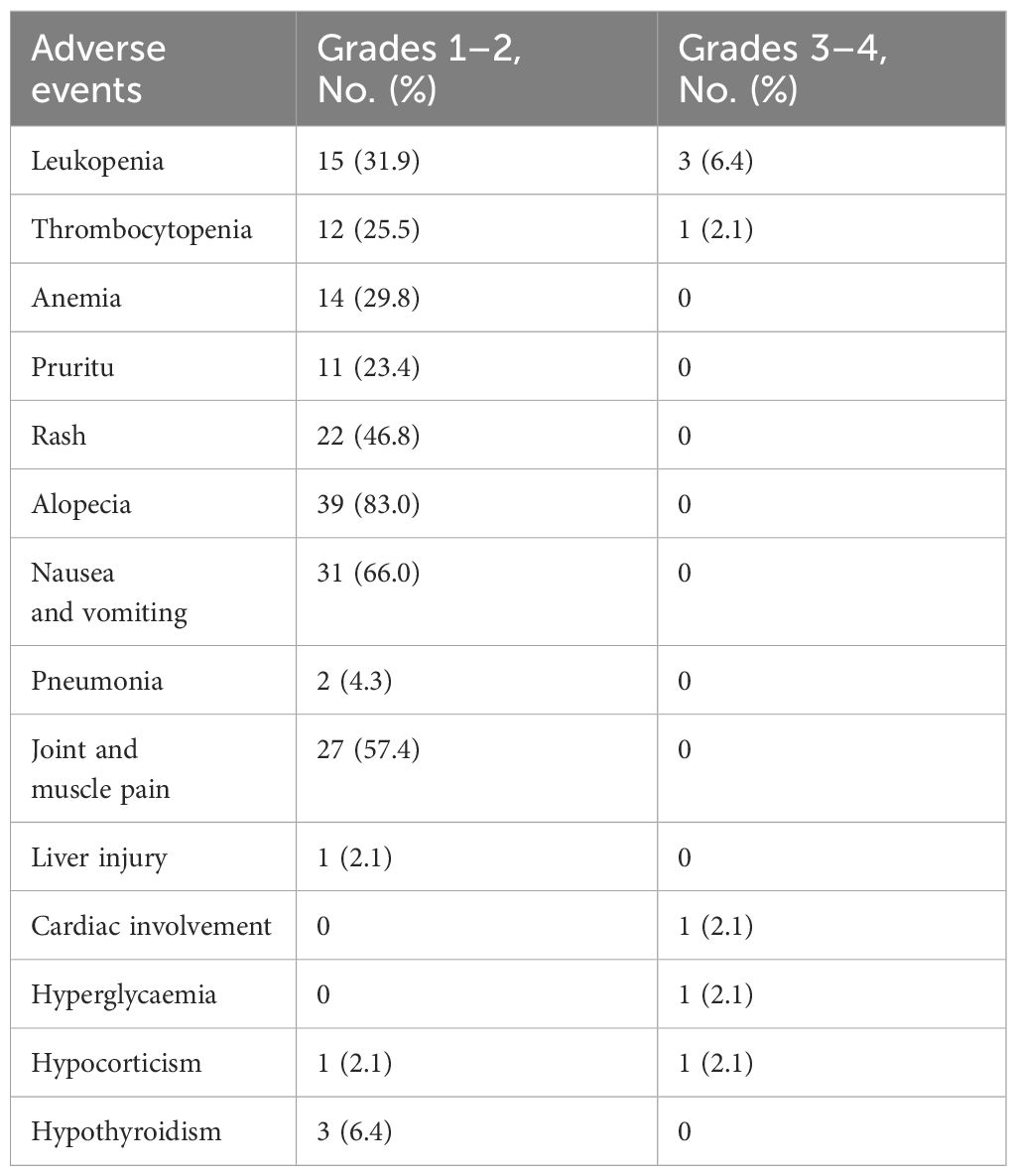
TRAEs related to drugs before surgery were observed in 42 (89.4%) patients in the nICT group. The common sintilimab-related adverse reactions are skin reactions, including pruritus (11 of 47, 23.4%), rash (22 of 47, 46.8%) and most of them are minor adverse events of grade 1–2. Others were pneumonia (2 of 47, 4.3%) and hypocorticism (2 of 47, 4.3%), among which grade 3–4 adverse events were lower. Adverse reactions related to chemotherapy included common haematological toxicity. Gastrointestinal discomfort and alopecia also had a high incidence, but most of them were grade 1–2 adverse events. Grade 3–4 adverse events were leukopenia in three cases (6.4%) and thrombocytopenia in one case (2.1%) (Table 3).
Surgery and postoperative safety
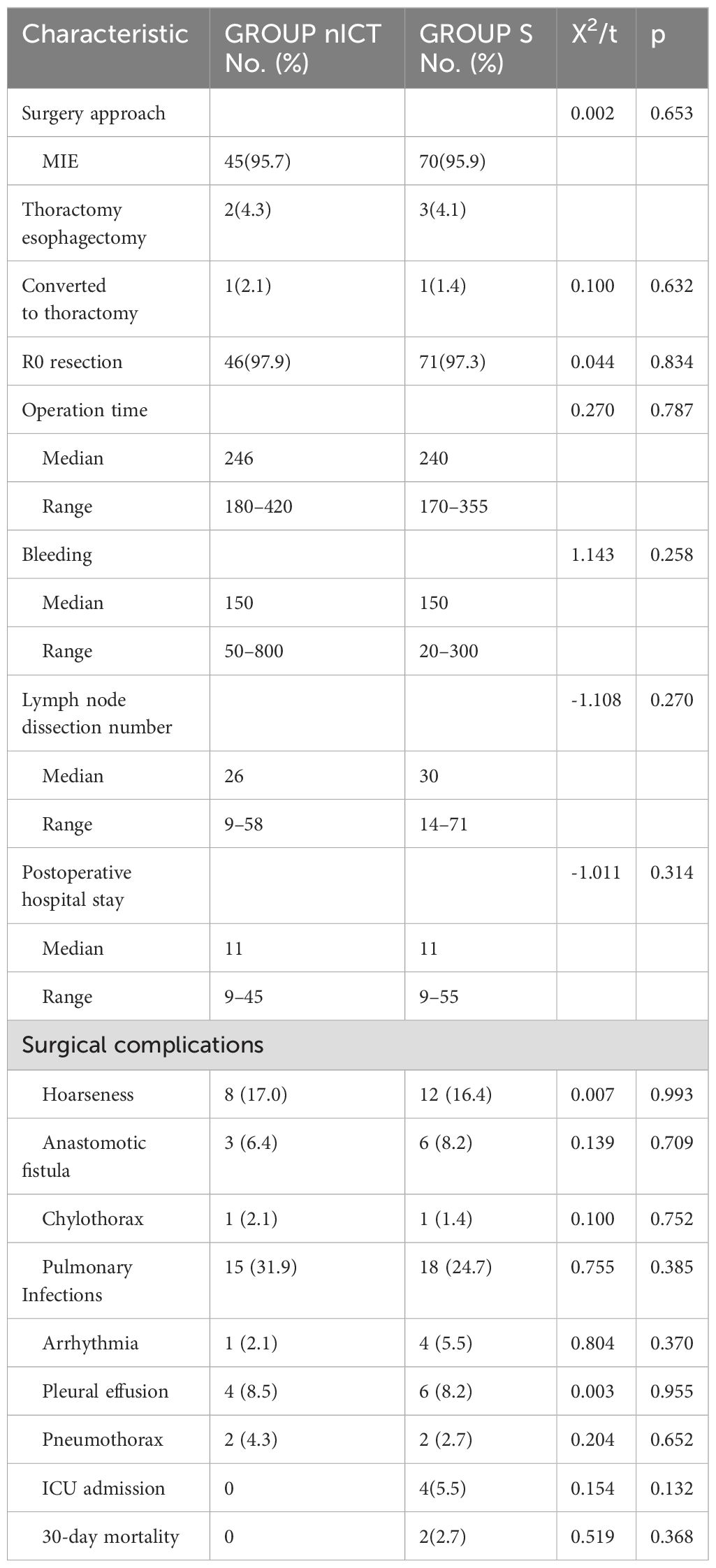
No surgery was delayed beyond six weeks after the final neoadjuvant therapy in group nICT. The percentages of minimally invasive esophagectomy (MIE) were 95.7% and 95.9% in the nICT group and group S respectively, while one case in each group was converted to thoracotomy (p = 0.632). Radical resection (R0) was achieved in 46 (97.9%) and 71 (97.3%) patients in the nICT group and group S respectively (p=0.834). No exploratory surgery was performed in either group. No significant difference was found between the two groups in the operation time (p=0.787), intraoperative blood loss (p=0.258), postoperative hospital stay (p=0.314) or lymph node dissection number (p=0.270) (Table 4).
Postoperative surgical complications within 30 days are summarised in Table 4. The incidence of hoarseness, anastomotic fistula, tracheoesophageal fistula, pulmonary infections, arrhythmia, pleural effusion, pneumothorax and chylothorax were comparable in both groups. One case of chylothorax in each group was successfully treated by conservative treatment. Four cases of ICU admission and two cases of 30-day mortality were observed in the S group, while no ICU admission and no 30-day mortality occurred in the nICT group. The causes of death of the two patients in the S group were tracheoesophageal fistula and pneumonia.
Follow-up
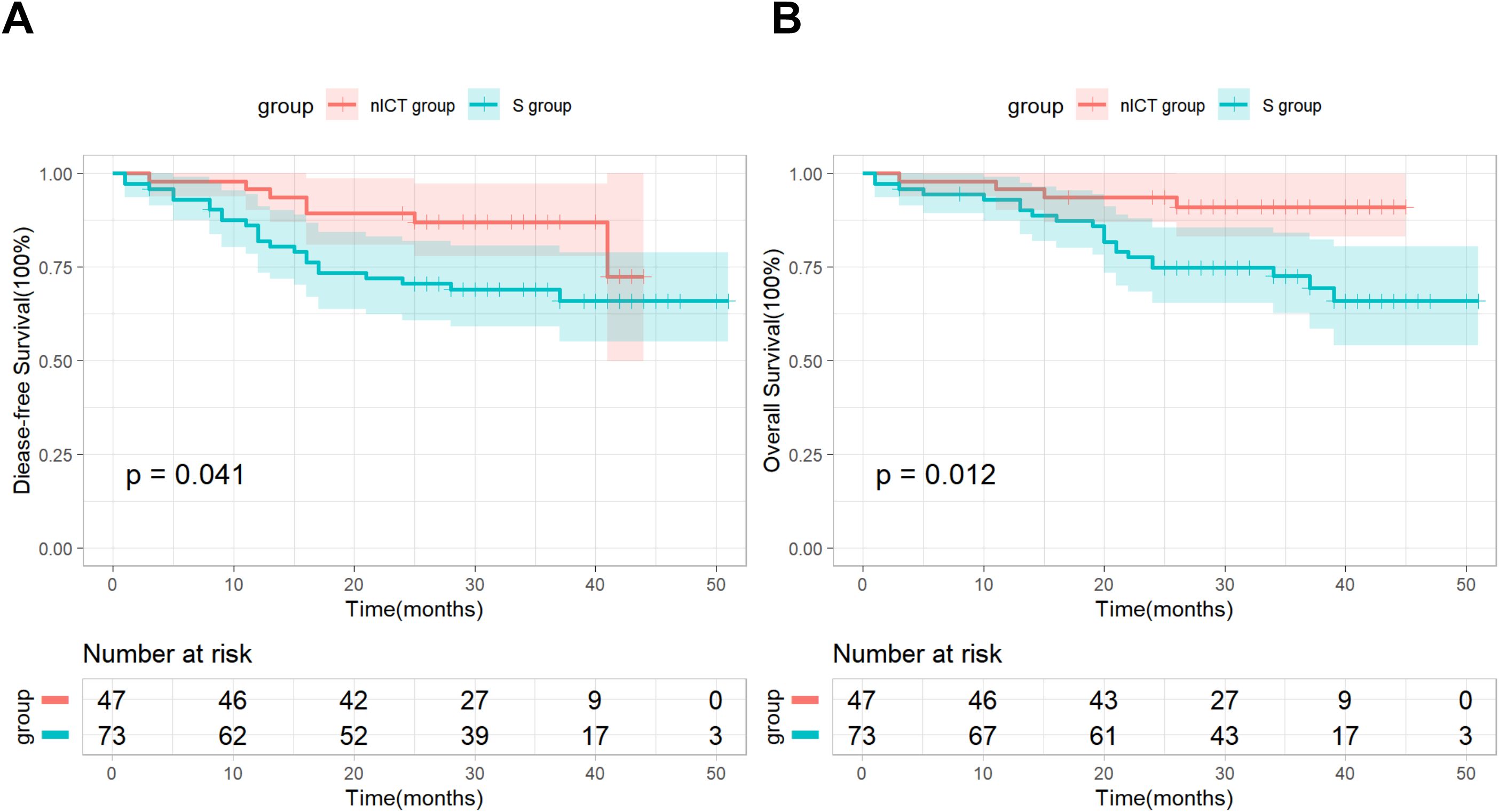
As of the cut-off date of May 2024, all patients had been followed up with for more than two years. The median follow-up time was 31 months (interquartile range: 27.0–37.0) in the nICT group, while the median follow-up time was 34 months (interquartile range: 29.5–42.0) in the S group. The median OS and DFS have not yet been reached in both groups. Specifically, the two-year OS and DFS rates for the nICT group were 91.5% and 85.5%, while the two-year OS and DFS rates for the S group were 71.2% and 68.5%. Kaplan–Meier survival analysis and log-rank test revealed significant differences in DFS and OS between the two groups (Figure 1).
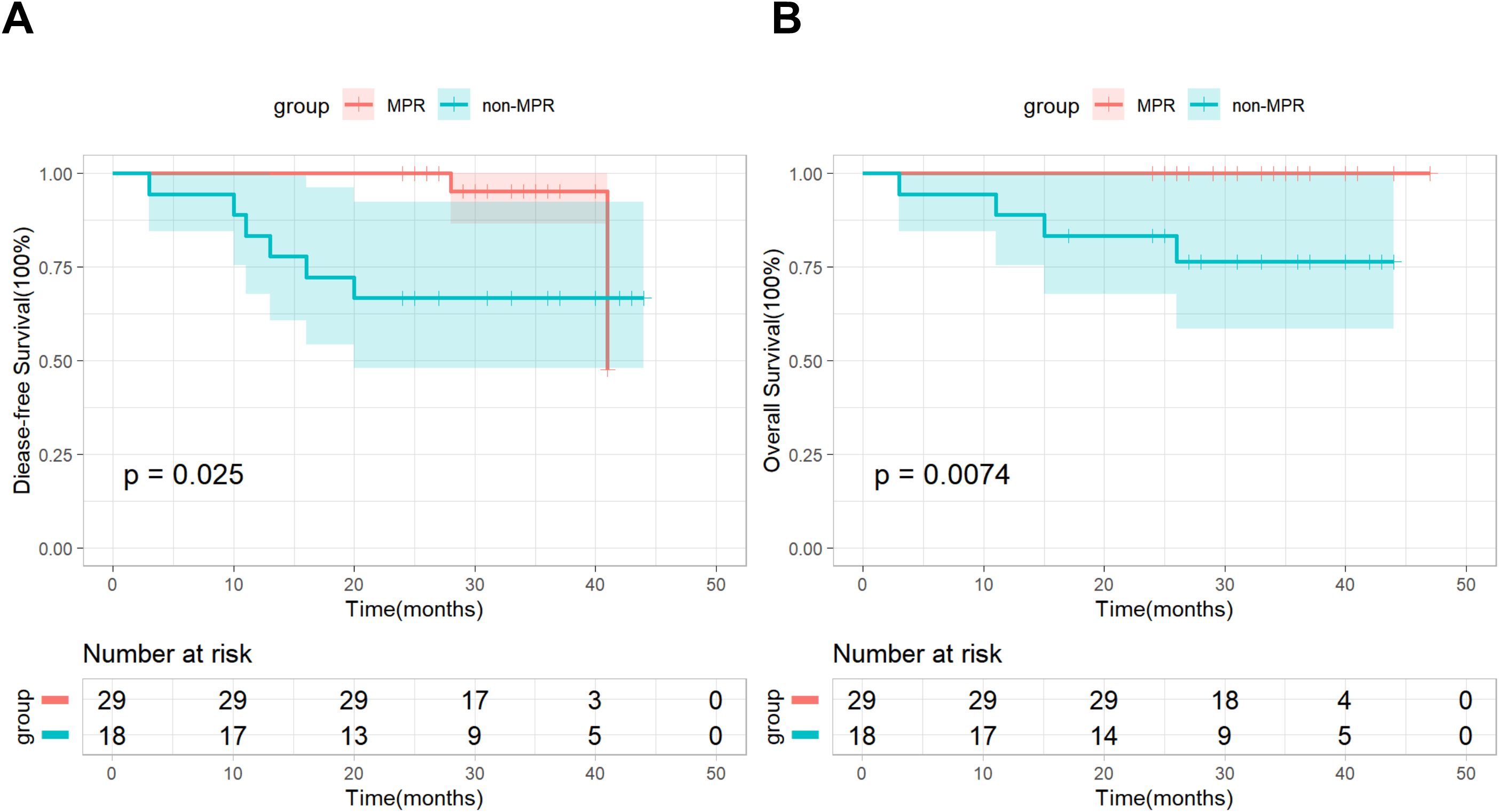
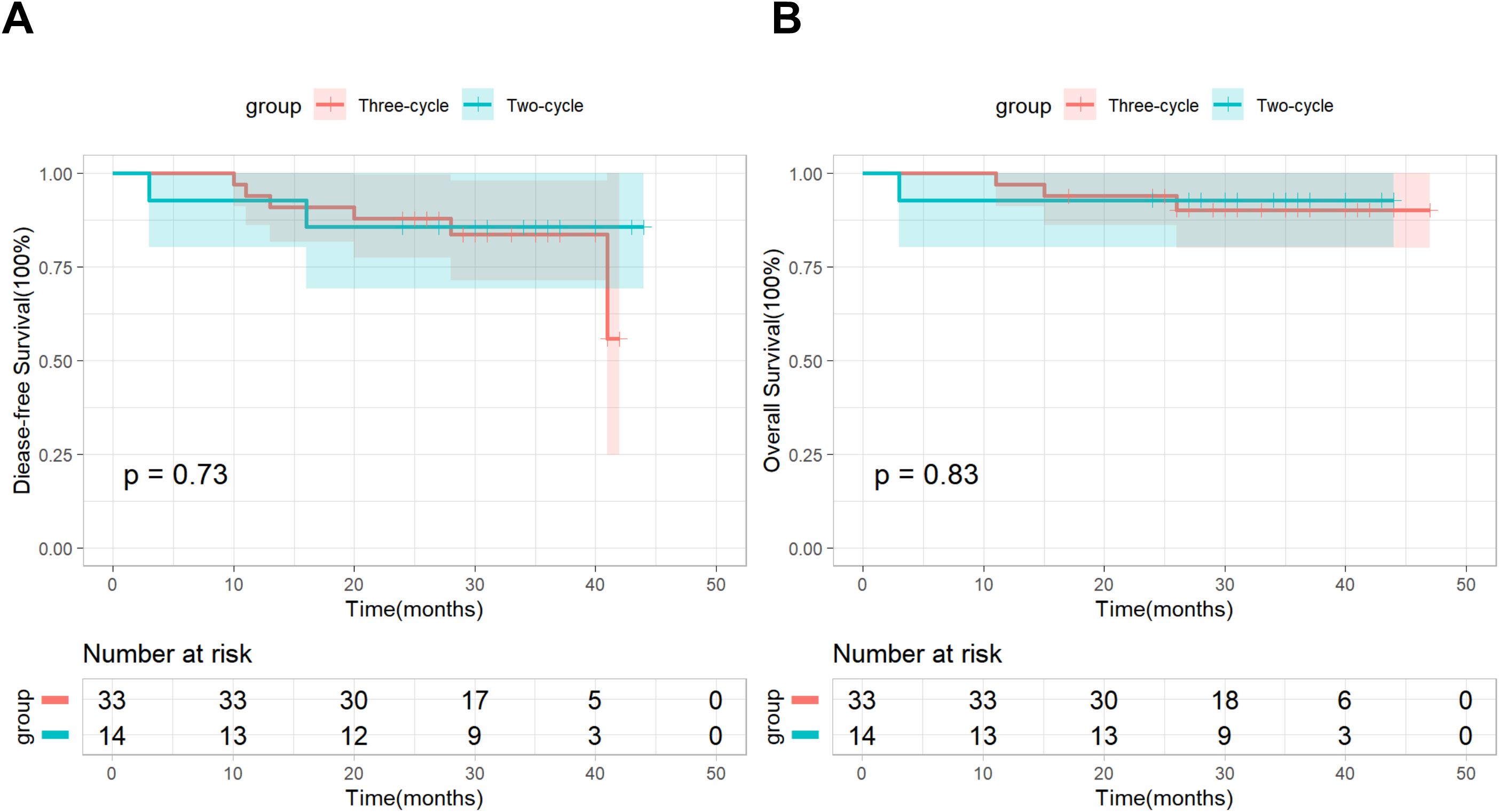
In addition, based on postoperative pathological results, we conducted a subgroup analysis by dividing patients in the nICT group into an MPR subgroup (n=29) and a non-MPR subgroup (n=18). As of the follow-up cutoff date, all patients (100%) in the MPR subgroup were still alive, and recurrence was observed in only 2 (6.9%) patients, Kaplan-Meier survival analysis combined with the log-rank test demonstrated significant differences in both DFS and OS between the two subgroups (Figure 2). Based on the number of neoadjuvant therapy cycles, patients in the nICT group were divided into a Two-cycle subgroup (n=33) and a Three-cycle subgroup (n=14), the Kaplan–Meier survival analysis and log-rank test revealed no significant differences in DFS and OS between the two subgroups (Figure 3).
Discussion
Although surgery following neoadjuvant chemoradiotherapy represents the standard treatment protocol for locally advanced ESCC, neoadjuvant radiotherapy may induce edema and fibrosis in the tissues surrounding the tumor, thereby elevating the surgical complexity. Furthermore, it may also augment the risk of postoperative bleeding and increase fluid leakage in patients. Consequently, the proportion of preoperative concurrent chemoradiotherapy conducted in China remains relatively low (22). Additionally, findings from several clinical trials and retrospective studies indicate that the clinical efficacy of neoadjuvant chemotherapy falls significantly short of that of neoadjuvant chemoradiotherapy (23, 24). The treatment of neoadjuvant immunochemotherapy followed by surgery brought more chances to cure patients with locally advanced resectable EC, which has become the focus of many studies on locally advanced EC. Most of these studies are single-arm, small-sample clinical studies, while other large prospective randomised controlled clinical studies have not yet yielded results, and there is no definitive conclusion regarding whether there is a long-term survival benefit. Therefore, real-world evidence can also serve as a good reference. Thus, we conducted this real-world retrospective study to investigate the efficacy, safety and the two-year follow-up results of neoadjuvant sintilimab combined with chemotherapy for locally advanced resectable ESCC. In the study that enrolled a relatively large population, several encouraging results were identified, which are consistent with previous studies.
Compared to the S group, the proportion of cT4a and cN2 patients was significantly higher in the nICT group, and the stage of the nICT group was more advanced than that of the S group. However, the surgical data did not reflect that the patients in the nICT group had advanced tumours. Compared to the S group, the percentages of MIE, rates of conversion to thoracotomy and radical resection rates did not increase in the nICT group. No significant difference was found between the two groups in the operation time, intraoperative blood loss, postoperative hospital stay or lymph node dissection number. In the study, a median of 26(9 to 58) lymph nodes were dissected in the nICT group, while in NEOCRTEC5010 study, the median lymph node dissection number was 20(15 to 27) in nCRT group (25). The R0 resection rate was 97.9% (46/47) in the nICT group, which is comparable to the NEOCRTEC5010 study (25). In our surgical practice, most patients in the nICT group had no obvious fibrosis or dense adhesions in the esophageal bed. These findings suggest that the neoadjuvant sintilimab combined with chemotherapy effectively downstaged the tumour and reduced the difficulty of surgery.
The postoperative outcomes shows that the incidence of hoarseness, anastomotic fistula, tracheoesophageal fistula, pulmonary infections, arrhythmia, pleural effusion, pneumothorax and chylothorax were comparable in both groups. The incidence of anastomotic fistula in the nICT group was 6.4%, which was lower than that previously reported in the CROSS study (22%) (26) and NEOCRTEC5010 study (8.6%) (25). The neoadjuvant immunotherapy did not increase postoperative mortality, which was consistent with the previous reports (27). Two cases of 30-day mortality were observed in the S group, and the causes of death were tracheoesophageal fistula and pneumonia, which may be related to severe tumour invasion and long operation time. In general, neoadjuvant sintilimab combined with chemotherapy was well-tolerated and safe. The neoadjuvant immunochemotherapy should be prescribed to more patients with locally advanced resectable ESCC.
TRAEs related to drugs before surgery were observed in 42 (89.4%) patients in the nICT group, and TRAEs of grade 3–4 were observed in 14.9% of patients, which is significantly lower than the 54.3% reported in the NEOCRTEC5010 nCRT group (25). In the study, the adverse reactions related to sintilimab were consistent with those reported in previous studies (21).
Recently, a number of small-sample clinical trial of neoadjuvant immunotherapy combined with chemotherapy for locally advanced EC have been published successively. The pCR rate of each study is between 18.8% and 50%, and the MPR rate is 43.8%–72.4% (28–35). In the study, the pCR rate was 17%, the reason for the low pCR rate in this study may be that many patients had advanced tumours and that most of the patients received two cycles of neoadjuvant therapy. Adding neoadjuvant therapy cycles may increase the pCR rate, but it may also increase adverse drug reactions and surgical complications. It is noteworthy that the MPR rate was 61.7% in this study, including 6 patients (12.8%) with a primary tumor achieving pCR but had residual tumor cells in the lymph nodes (pT0N+), which is comparable to that in other clinical trials, indicating a good response of locally advanced resectable ESCC to neoadjuvant sintilimab.
Consistent with previous studies demonstrating the survival benefits of neoadjuvant immunotherapy in EC (15, 36, 37), our study found significant differences in OS and DFS between the two groups after more than two years of follow-up. In the study, the 2-year OS and DFS rates for the nICT group were 91.5% and 85.5%, which is close to the results of the PERFECT study (2-year OS and PFS rates was 92%, 85%) (38) and far higher than the SCALE-1 study (2-year OS and PFS rates was 78%, 63.8%) (16), indicating that reducing radiotherapy in neoadjuvant therapy regimen for ESCC may also achieve good survival benefit. In a Phase II clinical trial on neoadjuvant camrelizumab combined with chemotherapy for locally advanced ESCC, the 2-year OS rate and DFS rate were 97.6% and 92.3%, respectively (39). The reason for these higher rates compared to our results may be that 95.7% of the patients in that study received three cycles of neoadjuvant therapy and all patients were clinically staged as II-III. However,only 14 patients (29.8%) received three cycles of neoadjuvant therapy, and 42.6% of the patients were clinically staged as IVA in our study. Furthermore, there may be variations in the efficacy of different neoadjuvant drugs. In the study, patients in the Three-cycle subgroup of the nICT group did not demonstrate significant survival benefits compared to the Two-cycle subgroup, which could potentially be attributed to the relatively small number of patients who underwent the Three-cycle neoadjuvant therapy. Whether increasing the number of neoadjuvant therapy cycles would enhance survival benefits remains unknown, and this is a direction that requires further research. Recently, the NICE study (40) released the two-year follow-up results of neoadjuvant camrelizumab combined with chemotherapy for clinical N2-3 ESCC: the study showed that distant recurrence remains the primary recurrence pattern, and MPR may be associated with a lower recurrence rate and better survival, in addition, the 2-year OS and RFS rates were 78.1% and 67.9%, respectively. The recurrence pattern in the nICT group of this study is consistent with the NICE study: among the 6 patients who recurred, 4 had distant recurrence and 2 had local recurrence. Compared to the NICE study, the study showed better survival benefits, which may be due to the following reasons: all patients enrolled in the NICE study were stage III-IV, while 31.9% of the patients in this study were stage II; there are differences in neoadjuvant treatment regimens and adjuvant treatment strategies. In the study, patients in the nICT group who achieved MPR also demonstrated better survival benefits, this suggests that MPR could potentially serve as an effective indicator for predicting the long-term prognosis of locally advanced resectable ESCC treated with neoadjuvant immunotherapy combined with chemotherapy. Furthermore, all patients in the MPR subgroup survived, regardless of whether they achieved pCR or not, and only two patients who did not achieve pCR experienced recurrence, this might suggest that ESCC patients can derive significant survival benefits as long as they achieve MPR after neoadjuvant immunotherapy (even if pCR is not attained). It is worth noting that postoperative adjuvant therapy in the study was not mandatory and was administered based on factors such as the patient’s pathological results, tolerance to chemotherapy drugs, the patient compliance and other considerations. In the study, ten patients (21.3%) in the nICT group received adjuvant immunochemotherapy, 2 (4.3%) received adjuvant radiotherapy, and 1 (2.1%) received adjuvant chemoradiotherapy after surgery. In the S group, 19 patients (26.0%) received adjuvant chemotherapy and 4 (5.6%) received adjuvant radiotherapy after surgery. The postoperative adjuvant therapy in both groups may have also influenced the survival outcomes of the study, but there is a significant discrepancy in postoperative schemes, which cannot be compared for analysis. Of course, our findings need to be validated through larger randomized controlled trials and further follow-up. In conclusion, neoadjuvant immunotherapy combined with chemotherapy for locally advanced ESCC has shown remarkable survival benefits, and long-term results still require further follow-up.
However, there are also some limitations: (1) the patients enrolled in this study were all from a single centre, and our results need to be further validated through multi-centre, randomised controlled trials with a larger sample size; (2) this study did not further explore relevant tumour markers, whose expression levels may affect treatment outcomes.
Conclusions
Neoadjuvant sintilimab combined with chemotherapy has promising efficacy and safety in the treatment of locally advanced resectable ESCC, and during the follow-up period of over two years, it exhibited significant survival benefits, this treatment modality has the potential to become a standard therapy for locally advanced resectable ESCC.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Committee of the first People’s Hospital in Jining. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because the study was retrospective, and it fulfills all the conditions for exemption from informed consent.
Author contributions
LC: Data curation, Investigation, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. JH: Data curation, Software, Validation, Writing – original draft, Writing – review & editing. HM: Conceptualization, Supervision, Validation, Writing – original draft, Writing – review & editing. SZ: Data curation, Investigation, Software, Writing – original draft, Writing – review & editing. KZ: Data curation, Investigation, Methodology, Writing – original draft, Writing – review & editing. DY: Investigation, Methodology, Writing – original draft, Writing – review & editing. JS: Conceptualization, Writing – original draft, Writing – review & editing. HL: Investigation, Methodology, Writing – original draft, Writing – review & editing. RS: Methodology, Writing – original draft, Writing – review & editing. HC: Conceptualization, Funding acquisition, Project administration, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The project is supported by the Dr. Foundation of Jining First People’s Hospital (2022-BS-003), Wu Jieping Medical Foundation (320.6750.2021-16-54) and Key R&D Program of Jining (2023YXNS197).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1453176/full#supplementary-material
Abbreviations
EC, esophageal cancer; ESCC, esophageal squamous cell carcinoma; nICT group, neoadjuvant immunochemotherapy plus surgery group; S group, surgery-only group; OS, overall survival; DFS, disease-free survival; NCT, neoadjuvant chemotherapy; NCRT, neoadjuvant chemoradiotherapy; nICT, neoadjuvant immunochemotherapy; PET, position emission tomography; CR, complete remission; PR, partial remission; PD, disease progression; SD, disease stability; ORR, objective response rate; DCR, disease control rate; TRAEs, Treatment-related adverse events; pCR, complete pathological response; MPR, major pathological response.
References
1. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
2. Cao W, Chen HD, Yu YW, Li N, Chen WQ. Changing profiles of cancer burden worldwide and in China: a secondary analysis of the global cancer statistics 2020. Chin Med J (Engl). (2021) 134:783–91. doi: 10.1097/CM9.0000000000001474
3. Lin Y, Totsuka Y, He Y, Kikuchi S, Qiao Y, Ueda J, et al. Epidemiology of esophageal cancer in Japan and China. J Epidemiol. (2013) 23:233–42. doi: 10.2188/jea.je20120162
4. He Y, Li D, Shan B, Liang D, Shi J, Chen W, et al. Incidence and mortality of esophagus cancer in China, 2008-2012. Chin J Cancer Res. (2019) 31:426–34. doi: 10.21147/j.issn.1000-9604.2019.03.04
5. Guo X, Mao T, Gu Z, Ji C, Fang W. Clinical study on postoperative recurrence in patients with pN1 esophageal squamous cell carcinoma. Thorac Cancer. (2015) 6:146–50. doi: 10.1111/1759-7714.12155
6. Ando N, Kato H, Igaki H, Shinoda M, Ozawa S, Shimizu H, et al. A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907). Ann Surg Oncol. (2012) 19:68–74. doi: 10.1245/s10434-011-2049-9
7. Yang H, Liu H, Chen Y, Zhu C, Fang W, Yu Z, et al. Long-term efficacy of neoadjuvant chemoradiotherapy plus surgery for the treatment of locally advanced esophageal squamous cell carcinoma: the NEOCRTEC5010 randomized clinical trial. JAMA Surg. (2021) 156:721–29. doi: 10.1001/jamasurg.2021.2373
8. Eyck BM, van Lanschot J, Hulshof M, van der Wilk BJ, Shapiro J, van Hagen P, et al. Ten-year outcome of neoadjuvant chemoradiotherapy plus surgery for esophageal cancer: the randomized controlled CROSS trial. J Clin Oncol. (2021) 39:1995–2004. doi: 10.1200/JCO.20.03614
9. Leng X, He W, Yang H, Chen Y, Zhu C, Fang W, et al. Prognostic impact of postoperative lymph node metastases after neoadjuvant chemoradiotherapy for locally advanced squamous cell carcinoma of esophagus: from the results of NEOCRTEC5010, a randomized multicenter study. Ann Surg. (2021) 274:e1022–29. doi: 10.1097/SLA.0000000000003727
10. Vagliasindi A, Franco FD, Degiuli M, Papis D, Migliore M. Extension of lymph node dissection in the surgical treatment of esophageal and gastroesophageal junction cancer: seven questions and answers. Future Oncol. (2023) 19:327–39. doi: 10.2217/fon-2021-0545
11. Moujaess E, Haddad FG, Eid R, Kourie HR. The emerging use of immune checkpoint blockade in the adjuvant setting for solid tumors: a review. Immunotherapy. (2019) 11:1409–22. doi: 10.2217/imt-2019-0087
12. Kato K, Cho BC, Takahashi M, Okada M, Lin CY, Chin K, et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. (2019) 20:1506–17. doi: 10.1016/S1470-2045(19)30626-6
13. Huang J, Xu J, Chen Y, Zhuang W, Zhang Y, Chen Z, et al. Camrelizumab versus investigator’s choice of chemotherapy as second-line therapy for advanced or metastatic oesophageal squamous cell carcinoma (ESCORT): a multicentre, randomised, open-label, phase 3 study. Lancet Oncol. (2020) 21:832–42. doi: 10.1016/S1470-2045(20)30110-8
14. Sun JM, Shen L, Shah MA, Enzinger P, Adenis A, Doi T, et al. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Lancet. (2021) 398:759–71. doi: 10.1016/S0140-6736(21)01234-4
15. Yin J, Yuan J, Li Y, Fang Y, Wang R, Jiao H, et al. Neoadjuvant adebrelimab in locally advanced resectable esophageal squamous cell carcinoma: a phase 1b trial. Nat Med. (2023) 29:2068–78. doi: 10.1038/s41591-023-02469-3
16. Jiang N, Zhang J, Guo Z, Wu Y, Zhao L, Kong C, et al. Short-course neoadjuvant radiotherapy combined with chemotherapy and toripalimab for locally advanced esophageal squamous cell carcinoma (SCALE-1): a single-arm phase Ib clinical trial. J Immunother Cancer. (2024) 12:e008229. doi: 10.1136/jitc-2023-008229
17. Li C, Zhao S, Zheng Y, Han Y, Chen X, Cheng Z, et al. Preoperative pembrolizumab combined with chemoradiotherapy for oesophageal squamous cell carcinoma (PALACE-1). Eur J Cancer. (2021) 144:232–41. doi: 10.1016/j.ejca.2020.11.039
18. Zhang L, Mai W, Jiang W, Geng Q. Sintilimab: A promising anti-tumor PD-1 antibody. Front Oncol. (2020) 10:594558. doi: 10.3389/fonc.2020.594558
19. Duan H, Wang T, Luo Z, Wang X, Liu H, Tong L, et al. A multicenter single-arm trial of sintilimab in combination with chemotherapy for neoadjuvant treatment of resectable esophageal cancer (SIN-ICE study). Ann Transl Med. (2021) 9:1700. doi: 10.21037/atm-21-6102
20. Zhang Z, Hong ZN, Xie S, Lin W, Lin Y, Zhu J, et al. Neoadjuvant sintilimab plus chemotherapy for locally advanced esophageal squamous cell carcinoma: a single-arm, single-center, phase 2 trial (ESONICT-1). Ann Transl Med. (2021) 9:1623. doi: 10.21037/atm-21-5381
21. Zhang Z, Ye J, Li H, Gu D, Du M, Ai D, et al. Neoadjuvant sintilimab and chemotherapy in patients with resectable esophageal squamous cell carcinoma: A prospective, single-arm, phase 2 trial. Front Immunol. (2022) 13:1031171. doi: 10.3389/fimmu.2022.1031171
22. Zhang G, Zhang C, Sun N, Xue L, Yang Z, Fang L, et al. Neoadjuvant chemoradiotherapy versus neoadjuvant chemotherapy for the treatment of esophageal squamous cell carcinoma: a propensity score-matched study from the National Cancer Center in China. J Cancer Res Clin Oncol. (2022) 148:943–54. doi: 10.1007/s00432-021-03659-7
23. Klevebro F, Alexandersson VDG, Wang N, Johnsen G, Jacobsen AB, Friesland S, et al. A randomized clinical trial of neoadjuvant chemotherapy versus neoadjuvant chemoradiotherapy for cancer of the oesophagus or gastro-oesophageal junction. Ann Oncol. (2016) 27:660–67. doi: 10.1093/annonc/mdw010
24. Samson P, Robinson C, Bradley J, Lockhart AC, Puri V, Broderick S, et al. Neoadjuvant chemotherapy versus chemoradiation prior to esophagectomy: impact on rate of complete pathologic response and survival in esophageal cancer patients. J Thorac Oncol. (2016) 11:2227–37. doi: 10.1016/j.jtho.2016.07.031
25. Yang H, Liu H, Chen Y, Zhu C, Fang W, Yu Z, et al. Neoadjuvant chemoradiotherapy followed by surgery versus surgery alone for locally advanced squamous cell carcinoma of the esophagus (NEOCRTEC5010): A phase III multicenter, randomized, open-label clinical trial. J Clin Oncol. (2018) 36:2796–803. doi: 10.1200/JCO.2018.79.1483
26. van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge HM, Wijnhoven BP, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. (2012) 366:2074–84. doi: 10.1056/NEJMoa1112088
27. Sihag S, Ku GY, Tan KS, Nussenzweig S, Wu A, Janjigian YY, et al. Safety and feasibility of esophagectomy following combined immunotherapy and chemoradiotherapy for esophageal cancer. J Thorac Cardiovasc Surg. (2021) 161:836–43. doi: 10.1016/j.jtcvs.2020.11.106
28. Shang X, Zhao G, Liang F, Zhang C, Zhang W, Liu L, et al. Safety and effectiveness of pembrolizumab combined with paclitaxel and cisplatin as neoadjuvant therapy followed by surgery for locally advanced resectable (stage III) esophageal squamous cell carcinoma: a study protocol for a prospective, single-arm, single-center, open-label, phase-II trial (Keystone-001). Ann Transl Med. (2022) 10:229. doi: 10.21037/atm-22-513
29. Liu J, Yang Y, Liu Z, Fu X, Cai X, Li H, et al. Multicenter, single-arm, phase II trial of camrelizumab and chemotherapy as neoadjuvant treatment for locally advanced esophageal squamous cell carcinoma. J Immunother Cancer. (2022) 10:e004291. doi: 10.1136/jitc-2021-004291
30. Liu J, Li J, Lin W, Shao D, Depypere L, Zhang Z, et al. Neoadjuvant camrelizumab plus chemotherapy for resectable, locally advanced esophageal squamous cell carcinoma (NIC-ESCC2019): A multicenter, phase 2 study. Int J Cancer. (2022) 151:128–37. doi: 10.1002/ijc.33976
31. Yang W, Xing X, Yeung SJ, Wang S, Chen W, Bao Y, et al. Neoadjuvant programmed cell death 1 blockade combined with chemotherapy for resectable esophageal squamous cell carcinoma. J Immunother Cancer. (2022) 10:e003497. doi: 10.1136/jitc-2021-003497
32. Xing W, Zhao L, Zheng Y, Liu B, Liu X, Li T, et al. The sequence of chemotherapy and toripalimab might influence the efficacy of neoadjuvant chemoimmunotherapy in locally advanced esophageal squamous cell cancer-A phase II study. Front Immunol. (2021) 12:772450. doi: 10.3389/fimmu.2021.772450
33. Wu Z, Zheng Q, Chen H, Xiang J, Hu H, Li H, et al. Efficacy and safety of neoadjuvant chemotherapy and immunotherapy in locally resectable advanced esophageal squamous cell carcinoma. J Thorac Dis. (2021) 13:3518–28. doi: 10.21037/jtd-21-340
34. He W, Leng X, Mao T, Luo X, Zhou L, Yan J, et al. Toripalimab plus paclitaxel and carboplatin as neoadjuvant therapy in locally advanced resectable esophageal squamous cell carcinoma. Oncologist. (2022) 27:e18–28. doi: 10.1093/oncolo/oyab011
35. Lu Z, Wang J, Shu Y, Liu L, Kong L, Yang L, et al. Sintilimab versus placebo in combination with chemotherapy as first line treatment for locally advanced or metastatic oesophageal squamous cell carcinoma (ORIENT-15): multicentre, randomised, double blind, phase 3 trial. Bmj. (2022) 377:e068714. doi: 10.1136/bmj-2021-068714
36. Jing SW, Zhai C, Zhang W, He M, Liu QY, Yao JF, et al. Comparison of neoadjuvant immunotherapy plus chemotherapy versus chemotherapy alone for patients with locally advanced esophageal squamous cell carcinoma: A propensity score matching. Front Immunol. (2022) 13:970534. doi: 10.3389/fimmu.2022.970534
37. Doki Y, Ajani JA, Kato K, Xu J, Wyrwicz L, Motoyama S, et al. Nivolumab combination therapy in advanced esophageal squamous-cell carcinoma. N Engl J Med. (2022) 386:449–62. doi: 10.1056/NEJMoa2111380
38. van den Ende T, de Clercq NC, van Berge HM, Gisbertz SS, Geijsen ED, Verhoeven R, et al. Neoadjuvant chemoradiotherapy combined with atezolizumab for resectable esophageal adenocarcinoma: A single-arm phase II feasibility trial (PERFECT). Clin Cancer Res. (2021) 27:3351–59. doi: 10.1158/1078-0432.CCR-20-4443
39. Yang G, Su X, Huang Y, Luo G, Wang Z, Cai P, et al. Intensive cycles of neoadjuvant camrelizumab combined with chemotherapy in locally advanced esophageal squamous cell carcinoma: a single-arm, phase II trial. J Transl Med. (2023) 21:411. doi: 10.1186/s12967-023-04273-6
Keywords: Neoadjuvant, sintilimab, esophageal squamous cell carcinoma, Immunotherapy, overall survival
Citation: Cai H, Chen L, Huang J, Ma H, Zhang S, Zhong K, Yang D, Sun J, Liu H and Song R (2024) Mid-term follow-up results of neoadjuvant sintilimab combined with chemotherapy for locally advanced resectable esophageal squamous cell carcinoma. Front. Immunol. 15:1453176. doi: 10.3389/fimmu.2024.1453176
Received: 22 June 2024; Accepted: 07 November 2024;
Published: 04 December 2024.
Edited by:
Hai Fang, Shanghai Jiao Tong University, ChinaReviewed by:
Alessio Vagliasindi, Oncological Center of Basilicata (IRCCS), ItalyMasaichi Ohira, Osaka City University, Japan
Copyright © 2024 Cai, Chen, Huang, Ma, Zhang, Zhong, Yang, Sun, Liu and Song. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haibo Cai, MTM1MTg2NzA4MDFAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Haibo Cai
Haibo Cai Liji Chen
Liji Chen Junjun Huang4†
Junjun Huang4†