
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol., 20 August 2024
Sec. Autoimmune and Autoinflammatory Disorders : Autoimmune Disorders
Volume 15 - 2024 | https://doi.org/10.3389/fimmu.2024.1452849
This article is part of the Research TopicNext Generation Therapeutic Modality to Cure Autoimmune DiseasesView all 11 articles
Background: High interleukin-6 levels correlate with diseases like cancer, autoimmune disorders, and infections. IL-6 receptor inhibitors (IL-6Ri), used for rheumatoid arthritis and COVID-19, may have wider uses. We apply drug-target Mendelian Randomization (MR) to study IL-6Ri’s effects.
Method: To simulate the effects of genetically blocking the IL-6R, we selected single nucleotide polymorphisms (SNPs) within or near the IL6R gene that show significant genome-wide associations with C-reactive protein. Using rheumatoid arthritis and COVID-19 as positive controls, our primary research outcomes included the risk of asthma, asthmatic pneumonia, cor pulmonale, non-small cell lung cancer, small cell lung cancer, Parkinson’s disease, Alzheimer’s disease, ulcerative colitis, Crohn’s disease, systemic lupus erythematosus, type 1 diabetes, and type 2 diabetes. The Inverse Variance Weighted (IVW) method served as our principal analytical approach, with the hypotheses of MR being evaluated through sensitivity and colocalization analyses. Additionally, we conducted Bayesian Mendelian Randomization analyses to minimize confounding and reverse causation biases to the greatest extent possible.
Results: IL-6 inhibitors significantly reduced the risk of idiopathic pulmonary fibrosis (OR= 0.278, 95% [CI], 0.138–0.558; P <0.001), Parkinson’s disease (OR = 0.354, 95% CI, 0.215–0.582; P <0.001), and positively influenced the causal relationship with Type 2 diabetes (OR = 0.759, 95% CI, 0.637–0.905; P = 0.002). However, these inhibitors increased the risk for asthma (OR = 1.327, 95% CI, 1.118–1.576; P = 0.001) and asthmatic pneumonia (OR = 1.823, 95% CI, 1.246–2.666; P = 0.002). The causal effect estimates obtained via the BWMR method are consistent with those based on the IVW approach. Similarly, sIL-6R also exerts a significant influence on these diseases.Diseases such as Alzheimer’s disease, Crohn’s disease, pulmonary heart disease, systemic lupus erythematosus, Type 1 diabetes, Non-small cell lung cancer and ulcerative colitis showed non-significant associations (p > 0.05) and were excluded from further analysis. Similarly, Small cell lung cancer were excluded due to inconsistent results. Notably, the colocalization evidence for asthmatic pneumonia (coloc.abf-PPH4 = 0.811) robustly supports its association with CRP. The colocalization evidence for Parkinson’s disease (coloc.abf-PPH4 = 0.725) moderately supports its association with CRP.
Conclusion: IL-6Ri may represent a promising therapeutic avenue for idiopathic pulmonary fibrosis, Parkinson’s disease, and Type 2 diabetes.
Excessive production of IL-6 is characteristic of many rheumatic diseases, including Rheumatoid Arthritis, Juvenile Idiopathic Arthritis, and Adult-Onset Still’s Disease (1). IL-6Ri are increasingly used when traditional treatments with DMARDs, corticosteroids, and non-steroidal anti-inflammatory drugs prove ineffective, particularly in conditions such as RA, systemic Juvenile Idiopathic Arthritis, and Castleman’s disease (2). Recent advances in understanding the pathogenesis of rheumatic diseases have expanded the use of IL-6Ri to other rheumatic conditions, including AOSD, Giant Cell Arteritis, Behçet’s Disease, and Polymyalgia Rheumatica, thus positioning IL-6 or its receptor blockade as a novel strategy for managing certain rheumatic ailments. IL-6 inhibitors include Tocilizumab, Sarilumab, Sirukumab, Clazakizumab, Olokizumab, MEDI-5117, and ALX-0061 (3). Currently, only Tocilizumab and Sarilumab are commercially available, with Tocilizumab being the most widely used internationally, while other inhibitors are either in development or under research (4).
IL-6 is produced by a variety of cells, including hepatocytes, T cells, B cells, fibroblasts, monocytes, mesangial cells, keratinocytes, endothelial cells, and many tumor cells (5). The IL-6R is an 80 kDa type I transmembrane protein primarily expressed on immune effector cells such as T cells, B cells, monocytes, neutrophils, and macrophages, as well as some non-immune effector cells, including pancreatic and hepatic cells. IL-6R exists in two forms: a membrane-bound version (mIL-6R) and a soluble version (sIL-6R) (6). The binding of IL-6 to mIL-6R can induce homodimerization of gp130, forming a high-affinity IL-6/IL-6R/gp130 complex. The sIL-6Rs are produced either through the cleavage of mIL-6R by the protease ADAM-17 or via selective mRNA splicing. IL-6 can also bind to circulating sIL-6R, forming complexes with gp130 (7). The binding of IL-6 to mIL-6R activates the classical signaling pathway, while its interaction with sIL-6R initiates the trans-signaling pathway, both of which engage with membrane-bound glycoprotein 130 (mgp130) to trigger cascades involving the Janus kinase/signal transducer and activator of transcription (JAK/STAT) and the mitogen-activated protein kinase (MAPK) pathways (8). These complexes induce tyrosine phosphorylation in the cytoplasmic domain of gp130, which promotes the recruitment of STAT-3, leading to the expression of pro-inflammatory genes and suppressor of cytokine signaling proteins (SOCS) (9). The activation of RAS/mitogen-activated protein kinases (MAPKs) mediates the phosphorylation and activation of nuclear factor (NF)-IL-6, which binds to the IL-6 response elements in the promoter regions of acute-phase gene, inducing the production of acute-phase proteins. The IL-6/IL-6R/gp130 complex also activates JAK-1 and JAK-2 kinases, as well as downstream transcription factors such as STAT1, STAT3, and phosphoinositide 3-kinase (PI3K). The activated STATs translocate to the nucleus, regulating several genes, while the activated PI3K in turn activates the serine/threonine-protein kinase (B/AKT). The activation of JAK/STATS/PI3K and MAPK/ERK pathways mediated by IL-6 can induce a broad spectrum of immune responses (10).
MR is an instrumental variable analysis method that employs SNPs from genome-wide association studies (GWAS) as genetic tools to estimate the causal effects of exposures on outcomes. Compared to observational studies, MR’s advantage lies in leveraging the random allocation of alleles to circumvent biases from unobserved confounders, such as lifestyle factors and other environmental impacts, as well as issues related to reverse causality (11). This study utilizes drug-target Mendelian Randomization analysis, which employs genetic variants of simulated pharmacological inhibitions as instrumental variables. By conducting regression analysis, this method elucidates the long-term effects of medications and enhances causal inferences about these drug targets’ potential impact on diseases. Evidence suggests that drug-target MR effectively identifies targets with up to 70% efficiency (12). We have gathered recently published GWAS summary-level statistics to explore the causal relationships between genetically predicted IL6R inhibition and 15 diseases, including COVID-19, rheumatoid arthritis, asthma, asthmatic pneumonia, cor pulmonale, non-small cell lung cancer, small cell lung cancer, Parkinson’s, Alzheimer’s disease, ulcerative colitis, Crohn’s disease, systemic lupus erythematosus, and types 1 and 2 diabetes through drug-target MR analysis.
Figure 1 summarizes the design of this study. C-reactive protein (CRP) GWAS data was derived from a study encompassing 204,402 Europeans(GWAS ID:ieu-b-35). CRP was selected as a biomarker because it is well-documented that pharmacological inhibition of IL-6R can reduce CRP levels, a finding substantiated by clinical trials (13, 14). By acquiring instrumental variables that target IL6R to diminish CRP levels, we can simulate the effects of IL6Ri (15). These instrumental variables are SNPs located within ±100kb of the IL6R gene locus, which are associated with CRP levels and meet the genome-wide significance threshold set at p<5×10^-8. To mitigate the impact of strong linkage disequilibrium (LD) on the results, an LD threshold (r^2<0.3) was established. Ultimately, 10 significant IL6R SNPs were retained (Supplementary Material 1: Supplementary Table S2). To verify the absence of weak instrument bias in our selected instrumental variables, we employed the F-statistic, where an F > 10 indicates the absence of weak instrument bias, further validating the associative hypotheses. The formula for the F-statistic is F = [(N - K - 1)/K] × [R^2/(1 - R^2)], where N is the sample size of the exposure, K is the number of instrumental variables, and R^2 is the proportion of exposure variance explained by the instrumental variables.
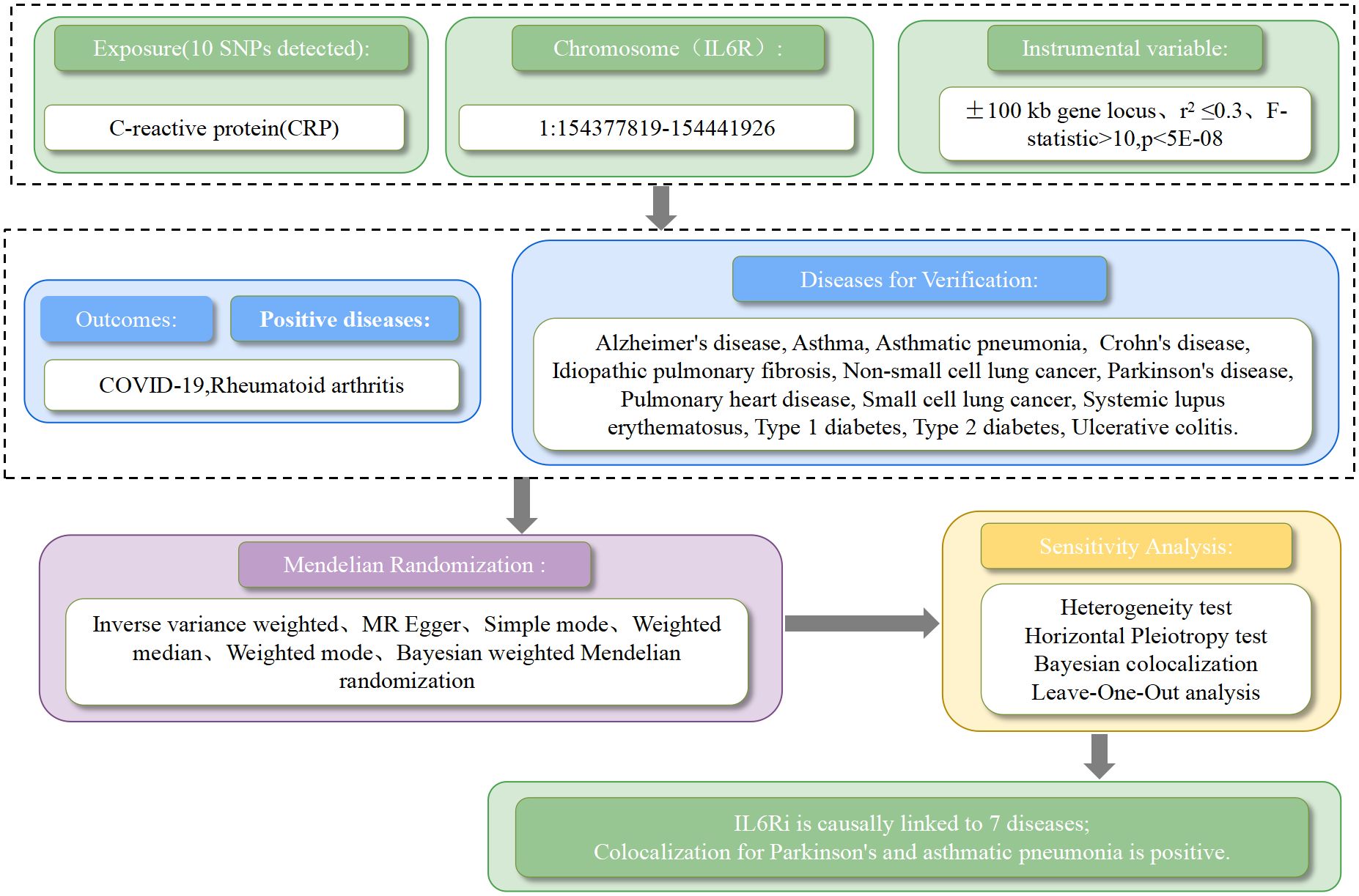
Figure 1. The workflow of drug target mendelian randomization study that exhibits causality between IL6 receptor inhibitors and multiple diseases.
We utilized fifteen diseases for our drug-target Mendelian Randomization analysis, with rheumatoid arthritis(GWAS ID:finn-b-RHEUMA_NOS,n=217134) and COVID-19(GWAS ID:ebi-a-GCST011077,n=1059456) serving as two positive control datasets, all sourced from European populations. Additionally, we compiled GWAS summary data sets for idiopathic pulmonary fibrosis (GWAS ID:finn-b-IPF,n=198014), asthma (GWAS ID:finn-b-J10_ASTHMA,n=156078),asthmatic pneumonia (GWAS ID:finn-b-ASTHMA_PNEUMONIA_AND_SEPSIS,n=140994), cor pulmonale (GWAS ID:finn-b-FG_PULMHEART,n=218792), non-small cell lung cancer (GWAS ID:finn-b-C3_LUNG_NONSMALL_EXALLC,n=175633), small cell lung cancer (GWAS ID:finn-b-C3_SCLC,n=218792),Parkinson’s disease(GWAS ID:finn-b-PDSTRICT,n=218473),Alzheimer’s disease (GWAS ID:finn-b-G6_ALZHEIMER,n=156078),ulcerative colitis (GWAS ID:finn-b-K11_ULCER,n=214620),Crohn’s disease (GWAS ID:finn-b-K11_CROHN,n=212356),systemic lupus erythematosus (GWAS ID:finn-b-M13_SLE,n=213683), type 1 diabetes (GWAS ID:finn-b-E4_DM1,n=189113), and type 2 diabetes (GWAS ID:finn-b-E4_DM2,n=215654)(Supplementary Material 1: Supplementary Table S1). These principal outcome datasets originated from the Finnish database, accessible via the MR-Base platform (https://www.mrbase.org/). There were no overlapping samples between the exposure and outcome GWAS, and all participants were of European descent.This research complies with the Strengthening the Reporting of Observational Studies in Epidemiology Using Mendelian Randomization(STROBE-MR) guidelines (16), and the corresponding checklist can be found in the Supporting Information (Supplementary Material 1: STROBE-MR checklist).
IL-6Ri have been extensively utilized in the treatment of rheumatoid arthritis and COVID-19. Consequently, we employed GWAS summary data from these diseases as positive controls to validate the efficacy of our instrumental variables. Initially, we harmonized drug-target instrumental variables related to exposure with the outcome datasets. We implemented five MR methods to assess the causal association between these drug-target instrumental variables and the outcome datasets, including MR-Egger regression, Weighted Median Estimator (WME), Inverse Variance Weighted (IVW), Simple Mode, and Weighted Mode. The IVW method was chosen as the primary analytical approach because it provides the most precise estimates by analyzing each Wald ratio under the ideal condition that all instrumental variables are valid (17). Given that this represents an ideal scenario, it is crucial to corroborate these findings with other methods to ensure robustness and validity across various analytical conditions. Additionally, a Bayesian weighting strategy was used to simultaneously address measurement errors and pleiotropy, thus enhancing the robustness of our analysis (18).
Heterogeneity tests were conducted using MR Egger and IVW methods. Cochran’s Q value was employed to evaluate the heterogeneity of genetic instruments, with p > 0.05 indicating no significant heterogeneity. MR Egger regression and MR PRESSO were used to assess pleiotropy of the genetic instruments, with p > 0.05 indicating no pleiotropy (19, 20). These steps help ensure the reliability of our instrumental variables in assessing causal relationships.
Bayesian colocalization analysis is employed to assess the probability that two traits share the same causal variant, facilitated by the ‘coloc’ package using default parameters (https://github.com/chr1swallace/coloc) (21). Bayesian colocalization offers posterior probabilities for five hypotheses, determining whether two traits share a single variant. This analysis evaluates support for five exclusive hypotheses: 1) associated with neither phenotype; 2) associated only with phenotype 1; 3) associated only with phenotype 2; 4) both phenotypes are associated, but with different causal variants; and 5) both phenotypes are associated with the same causal variant. Strong colocalization support is considered when the posterior probability of a shared causal variant (PH4) exceeds 0.8. Moderate colocalization is defined as a PH4 value between 0.5 and 0.8.
The statistical efficacy of the research was assessed using an online power calculator developed by Burgess (https://sb452.shinyapps.io/power/) (22). This comprehensive evaluation incorporated the total sample size, a significance level of 0.05, the variance explained by instrumental variables concerning exposure, and the ratio of exposure between the case and control groups, as well as the OR from the MR analysis. We established an objective to achieve a statistical power threshold of at least 0.8 to ensure that when the p-value is less than 0.05, the findings achieve statistical significance, thereby validating the scientific rigor of the results.
The causal effects of IL6Ri on fifteen diseases were analyzed using Mendelian Randomization (MR), setting a strict p-value threshold of 5 × 10^−8. Through rigorous criteria (r^2 < 0.3, kb = 100 KB), ten significant IL6Ri SNPs were identified. The F-statistics for these instrumental variables were all above 10, indicating the absence of weak instrumental bias and confirming the reliability of the results (Supplementary Material 1: Supplementary Table S2). Genetic variations in the IL6R target associated with inhibitors were linked to a reduced risk of rheumatoid arthritis and COVID-19. Specifically, there was a negative causal relationship between IL6Ri and rheumatoid arthritis (OR = 0.567, 95% CI, 0.329–0.977; P< 0.041) and COVID-19 (OR = 0.653, 95% CI, 0.449–0.950; P= 0.026). The causal effect estimates from the BWMR method were consistent with those based on the IVW method (Figure 2, Supplementary Material 1: Supplementary Tables S3, S4).
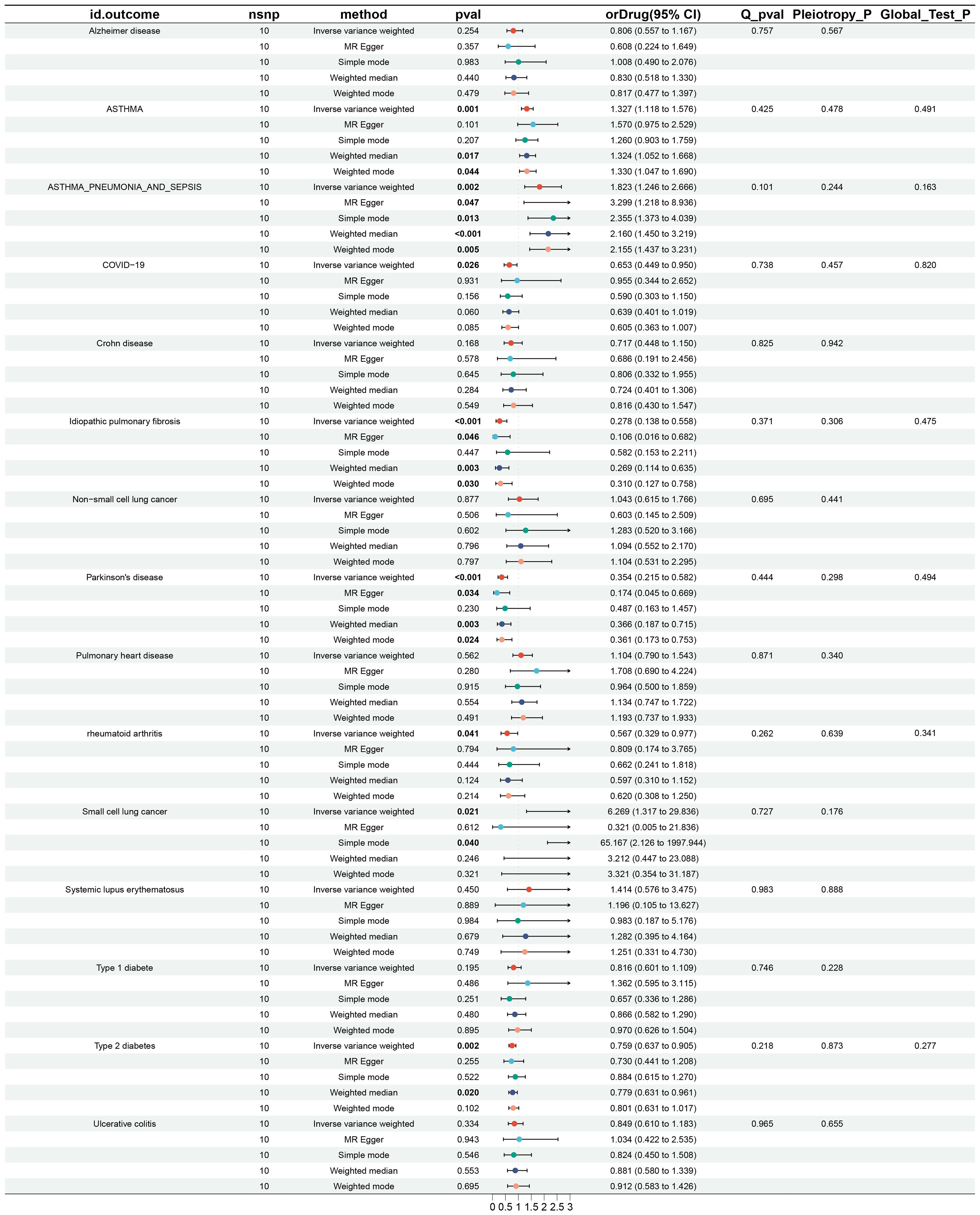
Figure 2. Results and forest plot of MR analysis of IL-6Ri’s causal relationship with 15 diseases.nsnp: The final number of SNPs used in the analysis. orDrug: The estimated effect of IL6Ri on diseases. Q_pval: The P value of the Cochran Q test. Pleiotropy_P: The P value of the MR-Egger regression intercept hypothesis test. Global_Test_P: The P value of the MR-PRESSO global test.
MR analysis revealed associations between IL6Ri and diseases such as idiopathic pulmonary fibrosis, Parkinson’s disease, type 2 diabetes, asthma, and asthmatic pneumonia. Specifically, there was a negative causal relationship between IL6Ri and idiopathic pulmonary fibrosis (OR = 0.278, 95% CI, 0.138–0.558; P <0.001), Parkinson’s disease (OR = 0.354, 95% CI, 0.215–0.582; P <0.001), and type 2 diabetes (OR = 0.759, 95% CI, 0.637–0.905; P = 0.002). Conversely, a positive causal relationship was found between IL6Ri and asthma (OR = 1.327, 95% CI, 1.118–1.576; P = 0.001), and asthmatic pneumonia (OR = 1.823, 95% CI, 1.246–2.666; P = 0.002 (Figure 2 and Supplementary Material 1: Supplementary Table S3).The BWMR method were consistent with those based on the IVW method (Figure 3A and Supplementary Material 1: Supplementary Table S4). And the effect of each SNP locus on these diseases is shown in Figures 4, 5. Moreover, the robust statistical power of 100% for all these IVW results emphasizes the findings’ reliability (Supplementary File 1: Supplementary Table S4). Diseases such as ulcerative colitis, crohn’s disease, systemic lupus erythematosus, and cor pulmonale had p-values greater than 0.05 and were excluded from further analysis. Moreover, non-small cell and small cell lung cancers were excluded due to inconsistent effect directions across the six methods.

Figure 3. (A) Forest plot of BWMR analysis of IL-6Ri’s causal relationship with 15 diseases; (B) The results of colocalization analysis.
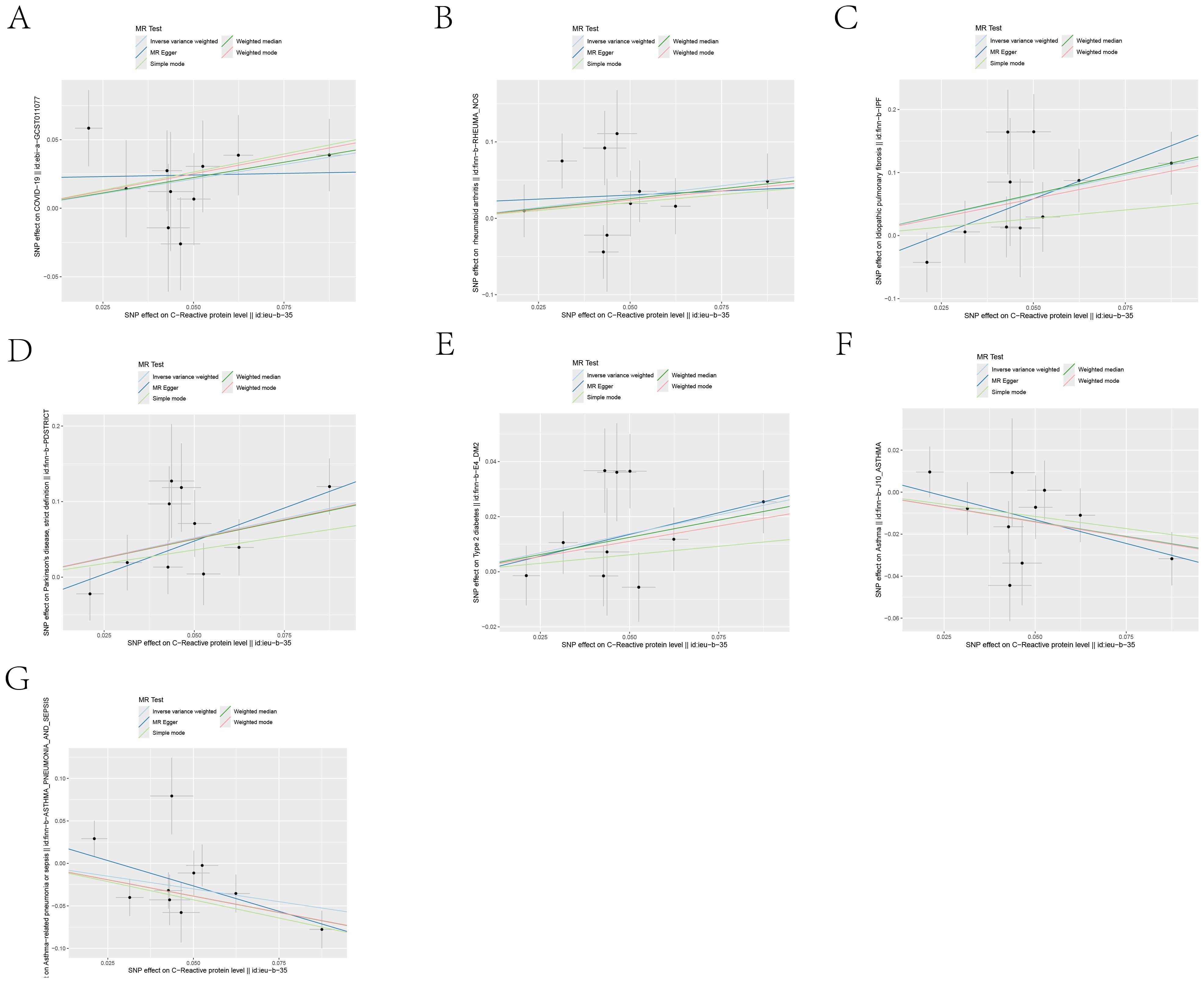
Figure 4. Scatter plots of the 5 MR analysis methods. The causal effect of IL-6R inhibitors on seven types of diseases, including: (A) COVID-19, (B) Rheumatoid arthritis, (C) Idiopathic pulmonary fibrosis, (D) Parkinson’s disease, (E) Type 2 diabetes, (F) Asthma, (G) Asthmatic pneumonia.The vertical axis denotes the influence of single nucleotide polymorphisms on exposure variables,the horizontal axis delineates their impact on outcome variables.
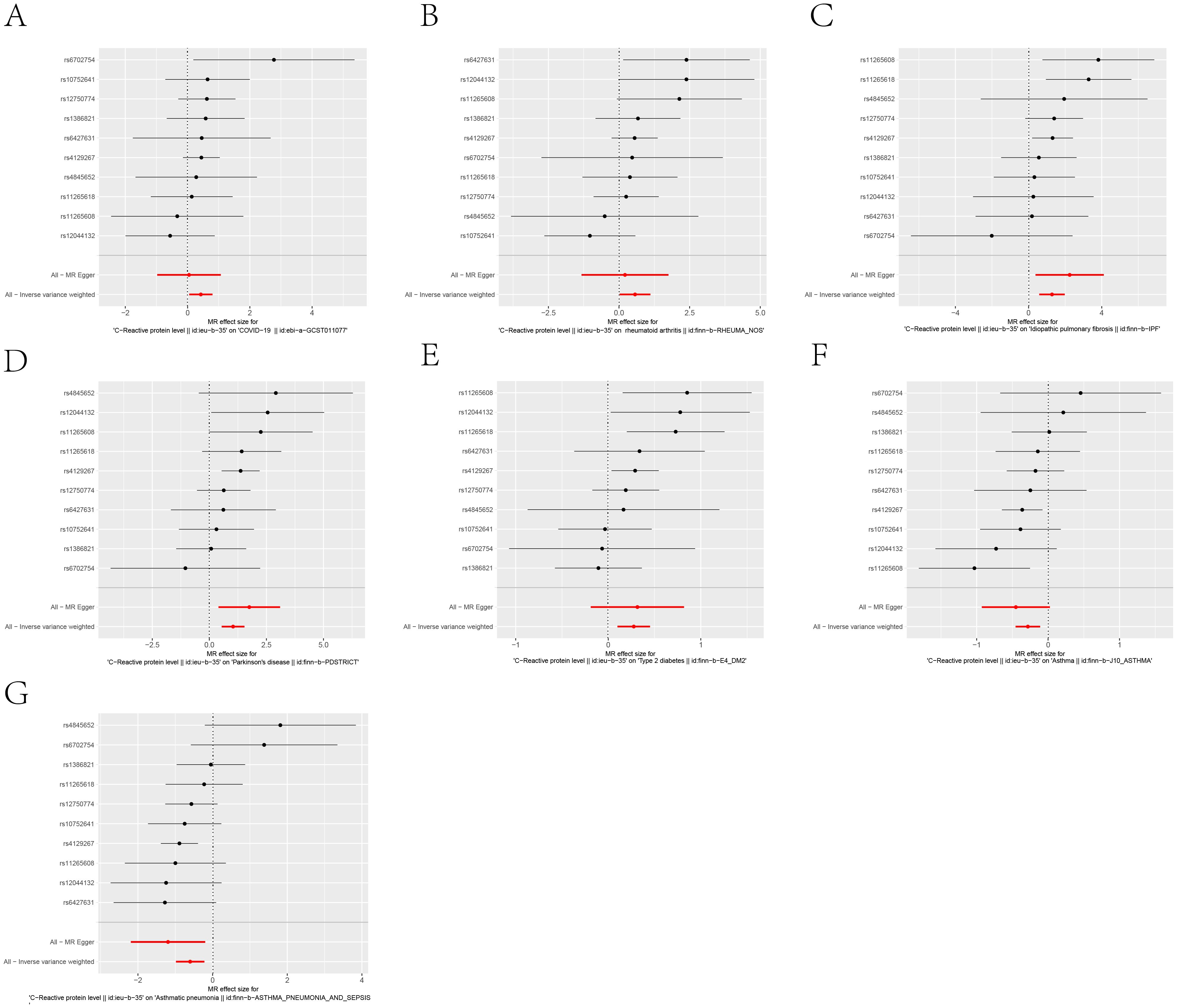
Figure 5. Forest plot of each SNP’s causal relationship with seven diseases, including: (A) COVID-19, (B) Rheumatoid arthritis, (C) Idiopathic pulmonary fibrosis, (D) Parkinson’s disease, (E) Type 2 diabetes, (F) Asthma, (G) Asthmatic pneumonia.The black dots represent the mean values,All-MR Egger and All-Inverse variance weighted represent the overall effect of all instrumental variables.
Complex pathophysiological mechanisms involving multiple factors in conditions such as cor pulmonale, non-small cell lung cancer, Alzheimer’s disease, type 1 diabetes, and inflammatory bowel disease, which includes both ulcerative colitis and Crohn’s disease, potentially obscure the efficacy of IL6Ri. In cor pulmonale, factors like chronic obstructive pulmonary disease and pulmonary embolism might interact with or independently influence the disease progression, complicating the role of IL6Ri. Non-small cell lung cancer, driven by mutations in numerous oncogenes and tumor suppressor genes, may diminish the impact of IL6Ri, overshadowed by other predominant genetic factors. The complex etiology of Alzheimer’s disease involving amyloid-beta deposition and neuroinflammation suggests that while IL6Ri may participate in controlling inflammation, its overall role in disease progression is limited. Similarly, in type 1 diabetes and inflammatory bowel disease, despite the role of IL6R in immune modulation, the effects of IL6Ri are likely moderated by other significant genetic or environmental factors, reducing its singular impact. Thus, even though IL6Ri demonstrates potential therapeutic promise, its actual effectiveness in these complex diseases may be limited by interference from other pathophysiological mechanisms.
Extensive literature suggests that the IL-6 trans-signaling pathway plays a pivotal role in the IL-6 signaling cascade within coronary artery disease (23–26). MR analysis, utilizing rs2228145 as the sole instrumental variable, has demonstrated that attenuated IL-6 signaling can mitigate the risk of coronary artery disease (27). The rs2228145 variant, situated on the IL6R gene, modulates the proteolytic cleavage of membrane-bound IL6R into its soluble form (sIL-6R) (28). Consequently, we selected rs2228145 as an instrumental variable for sIL-6R to investigate the impact of the IL-6 trans-signaling pathway on the disease.
The influence of sIL-6R on Type 2 diabetes (OR: 1.298, 95% CI: 1.025-1.643; P=0.030), Parkinson’s disease (OR: 3.570, 95% CI: 1.653-7.709; P= 0.001), idiopathic pulmonary fibrosis (OR: 3.308, 95% CI: 1.178-9.292; P= 0.023), asthma (OR: 0.713, 95% CI: 0.548-0.927; P= 0.011), and asthmatic pneumonia(OR: 0.438, 95% CI: 0.277-0.693; P<0.001), suggests that IL-6 receptor blockade may modulate these five diseases by inhibiting the IL-6 trans-signaling pathway(Supplementary Material 1: Supplementary Table S5).
Leave-one-out analysis demonstrated consistent risk estimates for idiopathic pulmonary fibrosis, Parkinson’s disease, type 2 diabetes, asthma, and asthmatic pneumonia after sequentially removing each SNP (Figure 6), and the funnel plots appeared approximately symmetrical(Figure 7).Cochran’s Q test did not indicate any signs of heterogeneity. Furthermore, neither MR-Egger regression nor MR-PRESSO detected the presence of horizontal pleiotropy (Figure 2 and Supplementary Material 1: Supplementary Table S3).We further investigated the colocalization of idiopathic pulmonary fibrosis, asthma, asthmatic pneumonia, Parkinson’s disease, and type 2 diabetes with CRP. The colocalization evidence for asthmatic pneumonia (coloc.abf-PPH4 = 0.811) strongly supports its association with CRP. For Parkinson’s disease, the colocalization evidence (coloc.abf-PPH4 = 0.725) moderately supports a link with CRP. However, the evidence for idiopathic pulmonary fibrosis (coloc.abf-PPH4 = 0.222), asthma (coloc.abf-PPH4 = 0.123), and type 2 diabetes (coloc.abf-PPH4 = 0.062) shows limited colocalization (Figure 3B and Supplementary Material 1: Supplementary Table S6).
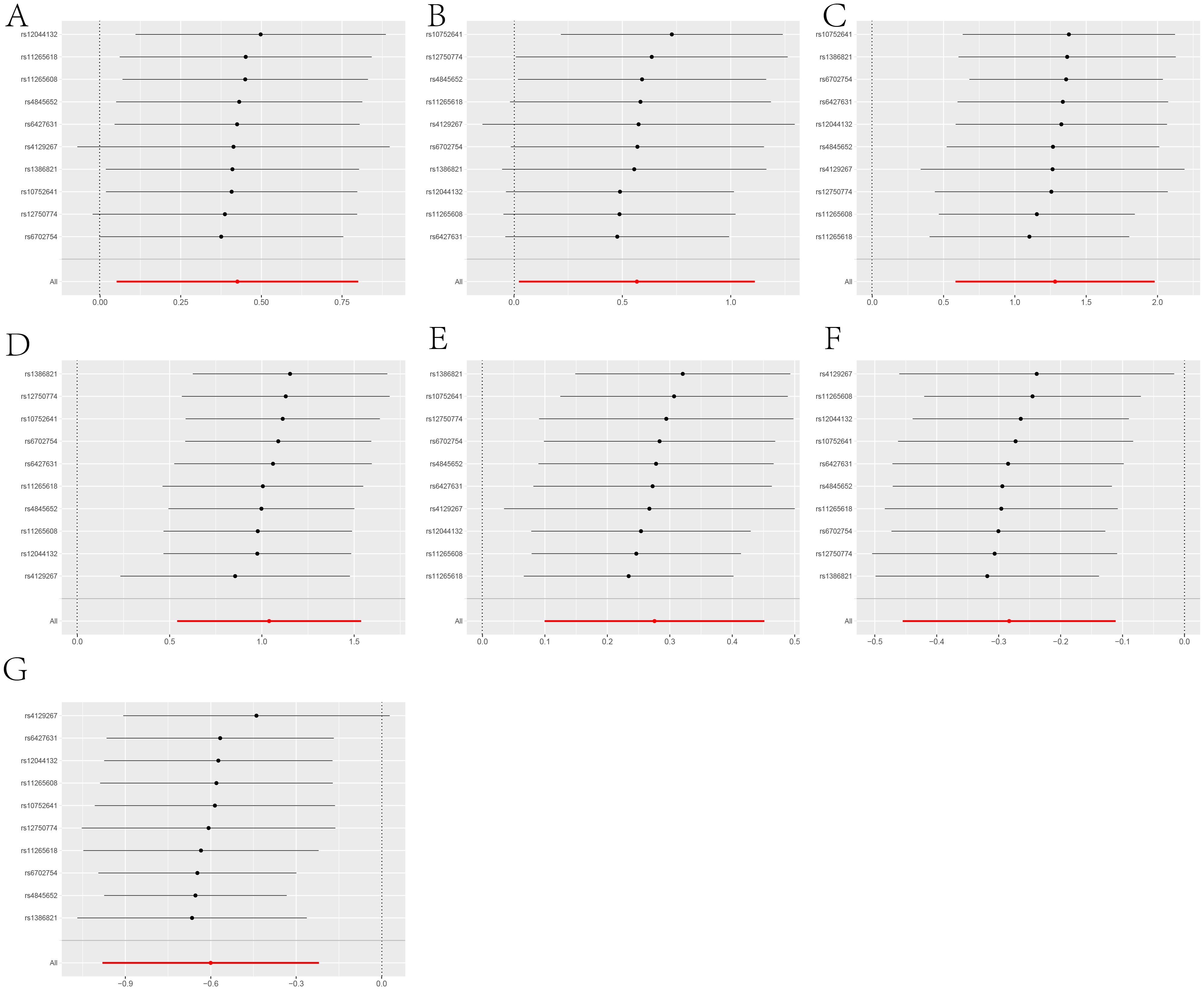
Figure 6. A leave-one-out analysis of statistically significant differences in the causal effects of IL-6R inhibitors on seven types of diseases, including: (A) COVID-19, (B) Rheumatoid arthritis, (C) Idiopathic pulmonary fibrosis, (D) Parkinson’s disease, (E) Type 2 diabetes, (F) Asthma, (G) Asthmatic pneumonia.The Y-axis corresponds to each excluded rsid number and the ‘all’ condition not excluded by the IVW method. The X-axis corresponds to specific IVW values, with black and red dots representing beta effect values, and the lines indicating the confidence intervals of these beta values.

Figure 7. Funnel plots showing the symmetry of the causal effect of IL-6R inhibitors on seven cancers, including: (A) COVID-19, (B) Rheumatoid arthritis, (C) Idiopathic pulmonary fibrosis, (D) Parkinson’s disease, (E) Type 2 diabetes, (F) Asthma, (G) Asthmatic pneumonia.The black dots in the figure represent single nucleotide polymorphisms, with the horizontal axis displaying the β values of these polymorphisms, and the vertical axis showing their standard errors.
Positive results from the colocalization analysis have revealed shared genetic signals within the genetic locus linking CRP with Parkinson’s disease and asthmatic pneumonia. These genetic signals are located in the IL6R gene region. These findings suggest that genetic variations in the IL6R region may influence the development of Parkinson’s disease and asthmatic pneumonia by modulating inflammation-related biological pathways.
In this study, we conducted a systematic analysis of the causal relationships between IL-6Ri and various diseases. To our knowledge, this represents the first large-scale genetic consortium-based MR analysis to establish causal links between IL-6Ri and multiple diseases. Utilizing drug-target Mendelian Randomization and colocalization analyses, we concluded that IL-6Ri significantly reduce the incidence risk of Parkinson’s disease, idiopathic pulmonary fibrosis, and type 2 diabetes, while potentially increasing the risk of asthma and asthmatic pneumonia.
Parkinson’s disease is the most common severe motor disorder and the second most prevalent neurodegenerative disease after Alzheimer’s, affecting 400 to 1,900 individuals per 100,000 globally (29). With the aging population, the incidence of Parkinson’s disease is expected to double by 2040 (30). In research conducted by Chen et al. (31), an analysis of 84 Parkinson’s patients and 165 control samples revealed that plasma inflammation markers—including C-reactive protein, fibrinogen, tumor necrosis factor-alpha, and IL-6—are critical for predicting and diagnosing Parkinson’s disease, particularly as elevated IL-6 significantly increases the risk of developing the disease. Studies indicate that IL-6 activates the JAK-STAT pathway by increasing pSTAT3 expression (32). QingQin et al. confirmed that overexpression of α-SYN in a Parkinson’s disease model activates the JAK/STAT pathway. Inhibition of the JAK/STAT pathway, demonstrated for the first time, disrupts the circuits of neuroinflammation and neurodegenerative changes, thereby mitigating the pathogenesis of Parkinson’s disease (33). In a Parkinson’s disease rat model, inhibitors of JAK1 and JAK2, such as AZD1480, reduced microglial proliferation and macrophage infiltration and decreased MHC class II expression. Additionally, treatment with AZD1480 inhibited the activation of STAT1/3/4 and blocked the differentiation of Th1 and Th17 cells, jointly promoting an immune response in the Parkinson’s disease model. In this study, we are excited to report that IL6Ri can reduce the risk of Parkinson’s disease, and colocalization showed moderate strength. The results of colocalization analysis further confirm that the role of IL6R in the nervous system may be related to its anti-inflammatory properties, providing significant insights into the specific mechanisms of IL6R in disease. Our research underscores the potential value of targeting the IL6R pathway in the prevention and treatment of Parkinson’s disease.
Idiopathic Pulmonary Fibrosis is a chronic fibrosing interstitial pneumonia of unknown origin that predominantly affects the elderly, characterized by progressive respiratory difficulty and continual decline in lung function. Prognosis for patients is poor, with an average life expectancy of about three to five years post-diagnosis (34). According to the Global Burden of Disease study, as of 2019, the incidence rate of interstitial lung diseases and pulmonary sarcoidosis among Chinese men was approximately 65 cases per 100,000 people, while globally, the incidence rates for men and women were 68 and 59 per 100,000 respectively. With the increasing trend of an aging population, the number of individuals with Idiopathic Pulmonary Fibrosis in China is expected to reach at least 500,000. In developed regions like the European Union, the number of Idiopathic Pulmonary Fibrosis patients increases by about 3,500 annually, posing a significant socioeconomic burden and an urgent health care challenge worldwide (35). The JAK/STAT signaling pathway is one of the classic inflammatory pathways closely associated with the development of Idiopathic Pulmonary Fibrosis (36). In this pathway, when the JAK1/STAT1 pathway is activated, JAK1 becomes phosphorylated, which in turn induces the phosphorylation of STAT1. The phosphorylated STAT1 forms heterodimers and translocates to the nucleus, promoting the release of chemokines and pro-inflammatory cytokines such as TNF-α, IL-6, ICAM1, and MCP1 (37). These inflammatory mediators have been identified as potential therapeutic targets for various fibrotic diseases. Furthermore, the SOCS family proteins serve as important negative regulators within the JAK1/STAT1 pathway. As negative regulators of cytokine receptor signaling, they inhibit the overactivation of the JAK/STAT pathway. This feedback inhibition mechanism plays a crucial role in regulating inflammatory responses and preventing excessive tissue fibrosis (38). Therefore, the potential of IL6Ri to reduce the risk of developing Idiopathic Pulmonary Fibrosis may be attributed to their ability to inhibit the JAK-STAT pathway, though further in vivo and in vitro studies are necessary to validate this effect.
Diabetes is an endocrine and metabolic disorder characterized by defects in insulin secretion and/or action and chronic hyperglycemia, caused by various factors (39). Epidemiological studies indicate that approximately 537 million people worldwide suffer from diabetes, with projections suggesting this number will rise to 693 million by 2045 (40). The global economic burden of adult diabetes is substantial, with costs estimated at 1.3 trillion USD in 2015, expected to increase to between 2.1 and 2.8 trillion USD by 2030 (41). Since 1999, research has progressively revealed a close association between type 2 diabetes mellitus and inflammation, recognizing the disease as an inflammatory condition mediated by inflammatory cells, their secretory factors, and acute-phase reactants, constituting an innate immune response. Particularly, chronic inflammation of visceral adipose tissue plays a pivotal role in the pathogenesis of type 2 diabetes. Obesity triggers adipocyte hypertrophy and hyperplasia, reduces the anti-inflammatory factor APN, and increases pro-inflammatory cytokines such as IL-6, IL-1β, TNF-α, and chemokines (CCL2, CCL3, and CXCL8), leading to the infiltration of immune cells (M1-type macrophages, CD8+ and CD4+ T lymphocytes, and B lymphocytes) and consequently promoting adipose inflammation (42). Our studies suggest that IL-6 inhibitors can significantly reduce type 2 diabetes, potentially through the inhibition of the JAK-STAT signaling pathway, thereby alleviating insulin resistance.
Common side effects of IL-6Ri include nasopharyngitis, headaches, upper respiratory tract infections, and gastritis. Infections are the most frequently occurring serious side effect, which may lead to gastrointestinal perforations. Typical laboratory abnormalities include neutropenia and elevated liver enzymes (43). Local reactions to injections and infusion reactions are common, but systemic allergic reactions are rare (44, 45). In rheumatoid arthritis patients, the development of resistance antibodies is possible, though they do not affect the efficacy of the treatment. Clinical trials indicate that IL-6Ri increase the risk of infections, consistent with other similar medications, including serious infections such as bacterial pneumonia and atypical infections (46). Interestingly, our research suggests a potential increase in the risk of asthma and asthmatic pneumonia, providing crucial information for clinicians in making therapeutic decisions, especially when considering pre-existing respiratory conditions in patients. Therefore, it is recommended that patients using these inhibitors be closely monitored for potential respiratory complications.
Currently available IL6R inhibitors, such as Tocilizumab and Sarilumab, operate by targeting the IL-6 receptor, effectively obstructing the interaction between IL-6 and its receptor, thus inhibiting both the classical and trans-signaling pathways. These inhibitors not only reduce the availability of membrane-bound IL-6R but also decrease the activity post-binding to sIL-6R, showcasing their suppressive effects across both signaling pathways (28). Conceptually, our genetic tools mirror the actions of these anti-IL6R monoclonal antibodies, although they exhibit a comparatively weaker effect in inhibiting IL6R signaling. Nevertheless, they possess a profound conceptual similarity with IL-6 inhibition. Empirical data supports the variation at this site and its impact on subsequent randomized trial data. Particularly when we employ the “canonical” rs2228145 SNP for MR analysis, despite reduced experimental efficacy, the outcomes align with expectations, exhibiting similar effects. Moreover, Tocilizumab, based on the IgG1 subtype, activates a variety of immune responses, such as antibody-dependent cell-mediated cytotoxicity and complement-dependent cytotoxicity. In contrast, Sarilumab, derived from the IgG2 subtype, exhibits lower activity in activating these immune effects. Both Tocilizumab and Sarilumab demonstrate similar therapeutic effects (47); however, Sarilumab presents a reduced risk of side effects (48). Therefore, we remain optimistic about the potential of these inhibitors in treating conditions such as idiopathic pulmonary fibrosis, type 2 diabetes, and Parkinson’s disease, anticipating that Sarilumab’s side effects, particularly in asthma and asthmatic pneumonia, may be significantly lesser than those of Tocilizumab.
The primary limitations of this study stem from the fact that the causal relationships are based on “genetic predispositions,” which can be easily influenced if the instrumental variable data are not properly managed. Due to constraints related to databases and software, this study focused exclusively on Europeans, but the causal conclusions drawn from European populations may not necessarily apply to other groups, such as Asians. Future studies should verify these findings using larger samples across multiple regions and diverse ethnic populations.
In summary, this study assessed the causal relationships between IL-6Ri and various diseases, discovering that IL-6Ri can reduce the risk of three diseases while potentially increasing the risk of asthma and asthmatic pneumonia. This provides preliminary evidence for the potential expansion of therapeutic applications for IL-6Ri.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
CF: Writing – original draft. LW: Data curation, Investigation, Writing – review & editing. WC: Writing – review & editing, Methodology.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study is supported by the funding from the Anhui Provincial Health Commission (No.AHWJ2023BAa20028).
We gratefully acknowledge the authors and participants of all data from which we used in the study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1452849/full#supplementary-material
1. Kaneko Y, Takeuchi T. An update on the pathogenic role of IL-6 in rheumatic diseases. Cytokine. (2021) 146:155645. doi: 10.1016/j.cyto.2021.155645
2. Mihara M, Nishimoto N, Ohsugi Y. The therapy of autoimmune diseases by anti-interleukin-6 receptor antibody. Expert Opin Biol Ther. (2005) 5:683–90. doi: 10.1517/14712598.5.5.683
3. Guo Q, Wang Y, Xu D, Nossent J, Pavlos NJ, Xu J. Rheumatoid arthritis: pathological mechanisms and modern pharmacologic therapies. Bone Res. (2018) 6:15. doi: 10.1038/s41413-018-0016-9
4. Pelechas E, Voulgari PV, Drosos AA. Clinical evaluation of the safety, efficacy and tolerability of sarilumab in the treatment of moderate to severe rheumatoid arthritis. Ther Clin Risk Manage. (2019) 4:1073–9. doi: 10.2147/TCRM
5. Kishimoto T. Interleukin-6: from basic science to medicine—40 years in immunology. Annu Rev Immunol. (2005) 23:1–21. doi: 10.1146/annurev.immunol.23.021704.115806
6. Jones SA, Richards PJ, Scheller J, Rose-John S. IL-6 transsignaling: the in vivo consequences. J Interferon Cytokine Res. (2005) 25:241–53. doi: 10.1089/jir.2005.25.241
7. Rose-John S. Interleukin-6 family cytokines. Cold Spring Harbor Perspect Biol. (2018) 10:a028415. doi: 10.1101/cshperspect.a028415
8. Heinrich PC, Behrmann I, Haan S, Hermanns HM, Müller-Newen G, Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J. (2003) 374:1–20. doi: 10.1042/bj20030407
9. Naka T, Kishimoto T. Joint disease caused by defective gp130-mediated STAT signaling. Arthritis Res Ther. (2002) 4:1–3. doi: 10.1186/ar400
10. Ataie-Kachoie P, Pourgholami MH, Richardson DR, Morris DL. Gene of the month: interleukin 6 (IL-6). J Clin Pathol. (2014) 67:932–7. doi: 10.1136/jclinpath-2014-202493
11. Davies NM, Holmes MV, Smith GD. Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. bmj. (2018) 362:k601. doi: 10.1136/bmj.k601
12. Schmidt AF, Finan C, Gordillo-Marañón M, Asselbergs FW, Freitag DF, Patel RS, et al. Genetic drug target validation using Mendelian randomisation. Nat Commun. (2020). 11:3255. doi: 10.1038/s41467-020-16969-0
13. Ridker PM, Pare G, Parker A, Zee RY, Danik JS, Buring JE, et al. Loci related to metabolic-syndrome pathways including LEPR, HNF1A, IL6R, and GCKR associate with plasma C-reactive protein: the Women's Genome Health Study. Am J Hum Genet. (2008) 82:1185–92. doi: 10.1016/j.ajhg.2008.03.015
14. Qi L, Rifai N, Hu FB. Interleukin-6 receptor gene, plasma C-reactive protein, and diabetes risk in women. Diabetes. (2009) 58:275–8. doi: 10.2337/db08-0968
15. Spencer S, Köstel Bal S, Egner W, Lango Allen H, Raza SI, Ma CA, et al. Loss of the interleukin-6 receptor causes immunodeficiency, atopy, and abnormal inflammatory responses. J Exp Med. (2019) 216:1986–98. doi: 10.1084/jem.20190344
16. Skrivankova VW, Richmond RC, Woolf BA, Yarmolinsky J, Davies NM, Swanson SA, et al. Strengthening the reporting of observational studies in epidemiology using Mendelian randomization: the STROBE-MR statement. Jama. (2021) 326:1614–21. doi: 10.1001/jama.2021.18236
17. Burgess S, Smith GD, Davies NM, Dudbridge F, Gill D, Glymour MM, et al. Guidelines for performing Mendelian randomization investigations: update for summer 2023. Wellcome Open Res. (2019) 4:186. doi: 10.12688/wellcomeopenres
18. Zhao J, Ming J, Hu X, Chen G, Liu J, Yang C. Bayesian weighted Mendelian randomization for causal inference based on summary statistics. Bioinformatics. (2020) 36:1501–8. doi: 10.1093/bioinformatics/btz749
19. Greco M FD, Minelli C, Sheehan NA, Thompson JR. Detecting pleiotropy in Mendelian randomisation studies with summary data and a continuous outcome. Stat Med. (2015) 34:2926–40. doi: 10.1002/sim.6522
20. Verbanck M, Chen C-Y, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. (2018) 50:693–8. doi: 10.1038/s41588-018-0099-7
21. Wang G, Sarkar A, Carbonetto P, Stephens M. A simple new approach to variable selection in regression, with application to genetic fine mapping. J R Stat Soc Ser B: Stat Method. (2020) 82:1273–300. doi: 10.1111/rssb.12388
22. Burgess S. Sample size and power calculations in Mendelian randomization with a single instrumental variable and a binary outcome. Int J Epidemiol. (2014) 43:922–9. doi: 10.1093/ije/dyu005
23. Galicia J, Tai H, Komatsu Y, Shimada Y, Akazawa K, Yoshie H. Polymorphisms in the IL-6 receptor (IL-6R) gene: strong evidence that serum levels of soluble IL-6R are genetically influenced. Genes Immun. (2004) 5:513–6. doi: 10.1038/sj.gene.6364120
24. Consortium CD, Deloukas P, Kanoni S, Willenborg C, Farrall M, Assimes TL, et al. Large-scale association analysis identifies new risk loci for coronary artery disease. Nat Genet. (2013) 45:25–33. doi: 10.1038/ng.2480
25. Van Der Harst P, Verweij N. Identification of 64 novel genetic loci provides an expanded view on the genetic architecture of coronary artery disease. Circ Res. (2018) 122:433–43. doi: 10.1161/CIRCRESAHA.117.312086
26. Karcıoğlu Batur L, Savaş S, Girgin E, Hekim N. Association of the IL-6R gene polymorphic variant rs2228145 (C> A) with IL-6 gene polymorphisms in a healthy cohort of Turkish population. Genes Immun. (2022) 23:118–22. doi: 10.1038/s41435-022-00167-7
27. Rosa M, Chignon A, Li Z, Boulanger M-C, Arsenault BJ, Bossé Y, et al. A Mendelian randomization study of IL6 signaling in cardiovascular diseases, immune-related disorders and longevity. NPJ genomic Med. (2019) 4:23. doi: 10.1038/s41525-019-0097-4
28. Sharma S. Interleukin-6 trans-signaling: a pathway with therapeutic potential for diabetic retinopathy. Front Physiol. (2021) 12:689429. doi: 10.3389/fphys.2021.689429
29. Pringsheim T, Jette N, Frolkis A, Steeves TD. The prevalence of Parkinson's disease: a systematic review and meta-analysis. Movement Disord. (2014) 29:1583–90. doi: 10.1002/mds.25945
30. Kowal SL, Dall TM, Chakrabarti R, Storm MV, Jain A. The current and projected economic burden of Parkinson's disease in the United States. Movement Disord. (2013) 28:311–8. doi: 10.1002/mds.25292
31. Chen H, O'Reilly EJ, Schwarzschild MA, Ascherio A. Peripheral inflammatory biomarkers and risk of Parkinson's disease. Am J Epidemiol. (2008) 167:90–5. doi: 10.1093/aje/kwm260
32. Moran DM, Mayes N, Koniaris LG, Cahill PA, McKillop IH. Interleukin-6 inhibits cell proliferation in a rat model of hepatocellular carcinoma. Liver Int. (2005) 25:445–57. doi: 10.1111/j.1478-3231.2005.01083.x
33. Qin H, Buckley JA, Li X, Liu Y, Fox TH, Meares GP, et al. Inhibition of the JAK/STAT pathway protects against α-synuclein-induced neuroinflammation and dopaminergic neurodegeneration. J Neurosci. (2016) 36:5144–59. doi: 10.1523/JNEUROSCI.4658-15.2016
34. Raghu G, Remy-Jardin M, Richeldi L, Thomson CC, Inoue Y, Johkoh T, et al. Idiopathic pulmonary fibrosis (an update) and progressive pulmonary fibrosis in adults: an official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med. (2022) 205:e18–47. doi: 10.1164/rccm.202202-0399ST
35. Zhang Y, Lu P, Qin H, Zhang Y, Sun X, Song X, et al. Traditional Chinese medicine combined with pulmonary drug delivery system and idiopathic pulmonary fibrosis: Rationale and therapeutic potential. Biomedicine Pharmacotherapy. (2021) 133:111072. doi: 10.1016/j.biopha.2020.111072
36. Yang G, Lyu L, Wang X, Bao L, Lyu B, Lin Z. Systemic treatment with resveratrol alleviates adjuvant arthritis-interstitial lung disease in rats via modulation of JAK/STAT/RANKL signaling pathway. Pulmonary Pharmacol Ther. (2019) 56:69–74. doi: 10.1016/j.pupt.2019.03.011
37. Ma Y, Tang T, Sheng L, Wang Z, Tao H, Zhang Q, et al. Aloin suppresses lipopolysaccharide−induced inflammation by inhibiting JAK1−STAT1/3 activation and ROS production in RAW264. 7 cells. Int J Mol Med. (2018) 42:1925–34. doi: 10.3892/ijmm.2018.3796
38. Gao Y, Zhao H, Wang P, Wang J, Zou L. The roles of SOCS 3 and STAT 3 in bacterial infection and inflammatory diseases. Scandinavian J Immunol. (2018) 88:e12727. doi: 10.1111/sji.12727
39. Enyew A, Nigussie K, Mihrete T, Jemal M, Kedir S, Alemu E, et al. Prevalence and associated factors of physical inactivity among adult diabetes mellitus patients in Felege Hiwot Referral Hospital, Bahir Dar, Northwest Ethiopia. Sci Rep. (2023) 13:118. doi: 10.1038/s41598-022-26895-4
40. Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas. Diabetes Res Clin Pract. (2019) 157:107843. doi: 10.1016/j.diabres.2019.107843
41. Bommer C, Sagalova V, Heesemann E, Manne-Goehler J, Atun R, Bärnighausen T, et al. Global economic burden of diabetes in adults: projections from 2015 to 2030. Diabetes Care. (2018) 41:963–70. doi: 10.2337/dc17-1962
42. Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. (2011) 11:98–107. doi: 10.1038/nri2925
43. Campbell L, Chen C, Bhagat SS, Parker RA, Oestoer AJ. Risk of adverse events including serious infections in rheumatoid arthritis patients treated with tocilizumab: a systematic literature review and meta-analysis of randomized controlled trials. Rheumatology. (2011) 50:552–62. doi: 10.1093/rheumatology/keq343
44. Burmester GR, Rubbert-Roth A, Cantagrel A, Hall S, Leszczynski P, Feldman D, et al. A randomised, double-blind, parallel-group study of the safety and efficacy of subcutaneous tocilizumab versus intravenous tocilizumab in combination with traditional disease-modifying antirheumatic drugs in patients with moderate to severe rheumatoid arthritis (SUMMACTA study). Ann rheumatic Dis. (2014) 73:69–74. doi: 10.1136/annrheumdis-2013-203523
45. Park EH, Lee EY, Shin K, Kim H-A. Tocilizumab-induced anaphylaxis in patients with adult-onset Still’s disease and systemic juvenile idiopathic arthritis: a case-based review. Rheumatol Int. (2020) 40:791–8. doi: 10.1007/s00296-019-04456-9
46. Emery P, Keystone E, Tony H, Cantagrel A, Van Vollenhoven R, Sanchez A. IL-6 receptor inhibition with tocilizumab improves treatment outcomes in patients with rheumatoid arthritis refractory to anti-tumour necrosis factor biologicals: results from a 24-week multicentre randomised placebo-controlled trial. Ann rheumatic Dis. (2009) 68:1516-296. doi: 10.1136/ard.2008.092932
47. Sellner J, Sitte HH, Rommer PS. Targeting interleukin-6 to treat neuromyelitis optica spectrum disorders: Implications from immunology, the FcRn pathway and clinical experience. Drug Discovery Today. (2021) 26:1591–601. doi: 10.1016/j.drudis.2021.03.018
48. Swets MC, Moss RJ, Kor F, Hilarius D, Moes DJA, Berkhout WE, et al. A comparison of the effectiveness of different doses of tocilizumab and sarilumab in the treatment of severe COVID-19: a natural experiment due to drug shortages. Int J Infect Dis. (2023) 129:57–62. doi: 10.1016/j.ijid.2023.01.041
Keywords: IL-6 receptor, Mendelian randomization, idiopathic pulmonary fibrosis, Parkinson’s disease, type 2 diabetes
Citation: Fu C, Wang L and Cai W (2024) IL6 receptor inhibitors: exploring the therapeutic potential across multiple diseases through drug target Mendelian randomization. Front. Immunol. 15:1452849. doi: 10.3389/fimmu.2024.1452849
Received: 21 June 2024; Accepted: 05 August 2024;
Published: 20 August 2024.
Edited by:
Ce Wang, AbbVie, United StatesReviewed by:
Siqi Sun, The State University of New Jersey, United StatesCopyright © 2024 Fu, Wang and Cai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenjiao Cai, Y2Fpd2VuamlhbzIwMTBAMTYzLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.