- 1Department of Ophthalmology and Otolaryngology, Jingmen Centra Hospital, Jingmen Central Hospital Affiliated to Jingchu University of Technology, Jingmen, Hubei, China
- 2Department of Infectious Disease, Jingmen Central Hospital, Jingmen Central Hospital Affiliated to Jingchu University of Technology, Jingmen, Hubei, China
- 3Department of Pediatrics, Zhongnan Hospital of Wuhan University, Wuhan, Hubei, China
- 4Department of Pediatrics, Jingmen Central Hospital, Jingmen Central Hospital affiliated to Jingchu University of Technology, Jingmen, Hubei, China
The prevalence of allergic rhinitis (AR) in children is steadily increasing, and its onset is closely associated with genetic factors, living environment, and exposure to allergens. In recent years, an increasing number of diagnostic methods have been employed to assist in diagnosing AR. In addition to pharmaceutical treatments, personalized approaches such as environmental control and allergen-specific immunotherapy are gradually gaining popularity. In this article, we reviewed recent research on the etiology, diagnostic classification, treatment methods, and health management of AR in children. These insights will benefit the implementation of personalized diagnosis and treatment for children with AR, promoting health management strategies that improve symptoms and quality of life.
1 Introduction
Allergic rhinitis (AR) is a chronic non-infectious inflammatory disease of the nasal mucus mainly mediated by IgE, triggered by exposure to allergens (1). AR is among the most prevalent chronic diseases globally and is the leading chronic disease in children in the United States (2, 3). It is estimated that approximately 500 million people globally suffer from AR symptoms, leading to substantial economic burden and health impacts. The primary clinical manifestations of AR include rhinorrhea, nasal congestion, nasal pruritus, and sneezing (4). Although AR symptoms may appear mild, their impact should not be underestimated in children. Approximately 20% of children experience AR symptoms by ages 2 to 3, about 40% by age 6, and roughly 30% during adolescence. AR can significantly affect sleep, emotional well-being, cognitive function, and productivity in both work and study environments (5). In children, the impact of AR on quality of life is often more subtle compared to adults, frequently leading to fatigue, reduced attention span, impaired learning, and memory, which are sometimes overlooked or misinterpreted by parents as behavioral issues (6).
Currently, the awareness of AR in children among healthcare providers and the families of affected children remains insufficient. The quality of life of children with AR continues to be impacted by delayed or improper treatment. Treatment methods such as environmental control measures and allergen-specific immunotherapy (AIT) have garnered significant attention. Additionally, health management strategies utilizing mobile communication technology to collect data and guide treatment offer new perspectives for managing AR in children. This review addresses the epidemiology, Etiology, diagnosis, classification, treatment, and management of AR in children. It aims to enhance the understanding of healthcare providers and families, improve diagnostic accuracy and timeliness, and promote appropriate treatment and adherence.
2 Epidemiology
Data from the International Study of Asthma and Allergies in Childhood (ISAAC), which includes multi-center data from over 300 countries worldwide, showed that AR often begins in early life, with a prevalence of over 5% at the age of 3, 8.5% at 6-7 years, and increasing to 14.6% at 13-14 years (7). In the 13-14 age group, the incidence of AR is 9.2% in Northern and Eastern Europe, 18% in Africa, 17.3% in Latin America, and as high as 51% in the United Arab Emirates (8). These data suggest that the prevalence of AR in children increases with age and varies significantly across different regions.
It is noteworthy that due to the limited self-reporting ability of infants and young children, AR in children is prone to be overlooked by parents or mistakenly treated as non-AR, especially in lower-income areas, where this phenomenon may be more pronounced (9, 10). A recent meta-analysis indicates an approximately 22% increase in the prevalence of AR among Chinese children in recent years, with significant regional variations and higher incidence rates in industrially developed cities (11). Furthermore, the reported prevalence of childhood AR by family members may be higher than the actual level (12). A survey conducted in Wuhan, China, revealed a self-reported AR prevalence of 28.6% among 6-12-year-old children, whereas the doctor-diagnosed prevalence of AR in children aged 0-17 was 14.4% (13). Therefore, strengthening health education on AR within families may serve as a measure to reduce treatment delays resulting from inadequate awareness and to mitigate overtreatment due to an overestimation of symptoms.
3 Etiology
The primary cause of AR in children is allergen exposure. Outdoor allergens include pollen and mold, while indoor allergens comprise dust mites, animal dander, insects, and mold. Additionally, genetic susceptibility, family history of allergies (such as AR, asthma, and atopic dermatitis), antibiotic use, and passive smoking are factors associated with the risk of developing AR in children (14, 15). The occurrence and progression of AR depend on the interaction between genetic predisposition and environmental factors.
In 1989, British scholar Strachan first reported a negative correlation between the prevalence of hay fever in children and both family size and the number of older siblings in the household. This observation led to the formulation of the hygiene hypothesis which posits that fewer infections in early childhood increase the likelihood of developing allergic diseases later in life (16). The underlying mechanism of the hygiene hypothesis is that microbial antigens promote Th1 responses and inhibit Th2 responses. This aligns with the pathogenesis of AR, where an imbalance between Th1/Th2 immune responses leads to the release of inflammatory mediators by effector cells such as mast cells, basophils, and eosinophils, causing inflammation of the nasal mucosa.
Recent studies explored the impact of the hygiene hypothesis on the development of pediatric AR by investigating the diversity of “microbial burden” in early life and its relationship with allergic predisposition (17). For example, a Polish study found that children who started kindergarten at age two had double the risk of developing AR compared to those who started at age one (18). Similarly, Han et al. identified that not having pneumonia in early childhood and shorter playtime were risk factors for AR in Korean children (19). A recent meta-analysis involving over two million subjects also reported that higher birth order and a greater number of siblings were associated with a lower risk of AR (20). Early kindergarten attendance, larger family size, higher birth order, and longer playtime may indicate earlier or more frequent exposure to pathogens. Additionally, some studies have shown that reduced gut microbiota diversity in newborns, due to factors such as antibiotic use, is related to the development of AR in children (21). Although these studies support the hygiene hypothesis, these factors only roughly reflect early microbial exposure in children and lack more direct and robust evidence.
The hygiene hypothesis, while influential, falls short in comprehensively explaining the pathogenesis of AR. It attributes the development of allergic diseases primarily to a lack of pathogen exposure, overlooking other critical factors such as genetics, environmental influences, and dietary habits. Family history-based studies underscore the significance of genetics in AR, with genome-wide association studies (GWAS) in recent years identifying numerous genetic susceptibility loci and candidate genes (22, 23). For instance, the interleukin-4 receptor α (IL-4Rα) gene is recognized as a candidate gene for AR, encoding a receptor subunit shared by IL-4R and IL-13R, and involving several polymorphisms (24). Additionally, researchers have identified various single nucleotide polymorphisms (SNPs) associated with AR across different populations (25). Andiappan et al. discovered SNPs in the MRPL4 and BCAP genes, as well as associations within the HLA-DQ and NPSR1 loci, in a Singapore Chinese population. Similarly, studies in a Han Chinese population identified SNPs in the MRPL4 and TNF-α genes linked to AR (26). In European populations, studies reported associations of AR with variants in HLA, C11orf30, LRRC32, and rs2155219 (27). A study in a Korean population also found SNPs associated with AR: rs7275360, an intron variant on chromosome 21q21 linked to NCAM2, and rs698195 on 7q31.1, a region linked to chronic rhinosinusitis susceptibility (28). Additionally, research in ethnically diverse North American populations linked a locus on chromosome 7p21.1 near the FERD3L gene to AR (22). While these studies have identified various genetic variants associated with AR, they only partially explain the heritability of the condition. Emerging evidence suggests that environmental exposures may interact with epigenetic modifications, such as DNA methylation and histone modifications, influencing gene expression and contributing to both the development and severity of AR (29).
In recent years, the concept of trained immunity has provided new insights into the etiology of AR. Netea et al. first defined trained immunity as a form of long-term functional reprogramming of innate immune cells induced by exogenous or endogenous stimuli, leading to enhanced or diminished responses to subsequent non-specific stimuli (30). Trained immunity demonstrates that both microbial pathogens and non-microbial antigens, including dietary and other environmental factors, can influence innate immune regulation at both central and peripheral levels. The mechanisms primarily involve cellular epigenetic reprogramming and immune metabolic pathways. Different stimuli, such as β-glucans, LPS, or the Bacillus Calmette-Guérin vaccine, can induce distinct trained immunity programs, manifesting as either immune enhancement or tolerance. Specifically, after antigen stimulation, innate immune cells undergo reprogramming of pro-inflammatory and anti-inflammatory gene transcription through epigenetic modifications like histone H3K27ac, H3K4m3, and H3K4m1, as well as metabolic pathways involving glycolysis, glutamine, and cholesterol metabolism, altering their inflammatory and anti-inflammatory phenotypes upon subsequent stimulation (31–33).
Unlike the concept of a single microbial antigen in the hygiene hypothesis, the training immunity theory involves the effects of multiple endogenous and exogenous antigens on the immune system. This theory emphasizes the interplay of environmental, genetic, and metabolic factors, and how the type and timing of antigen stimulation affect immune tolerance or enhancement. Current research indicates that training immunity mechanisms play a significant role in the development and progression of infectious diseases, asthma, coronary atherosclerosis, neurodegenerative diseases, and tumor growth and metastasis. For example, a Western diet induces pro-inflammatory transcription and epigenetic reprogramming in mice prone to atherosclerosis, with these effects persisting even after diet modification to a standard diet (34). Familial hypercholesterolemia and severe coronary atherosclerosis patients exhibit significant levels of histone methylation modifications in monocytes and altered glycolytic metabolism, leading to an enhanced pro-inflammatory phenotype (35, 36). Recently, Machiels et al. demonstrated that trained alveolar macrophages, which retain the memory of prior viral infections, can offer protection against asthma induced by allergens (37). This study provides experimental evidence that training immunity not only supports but also extends the original hygiene hypothesis by showing how past infections can modulate immune responses to allergens.
While adaptive Th2-type immune responses form the basis of specific IgE responses in AR, innate immune cells involved in training immunity, such as monocytes, macrophages, and dendritic cells, also play a crucial role in the sensitization and reactivation processes of AR (38). Some studies have suggested that trained immunity may either promote or protect against AR. Jin et al. found through bidirectional two-sample Mendelian randomization analysis that Coriobacteriia and its subcategories (Coriobacteriales and Coriobacteriaceae) in the gut microbiota have a protective effect against AR, whereas Victivallaceae is a risk factor (39). Additionally, a 13-year follow-up study in a Finnish probiotic intervention cohort found that early-life gut microbiota is associated with the development of AR (40). These findings suggest that microorganisms may influence AR by modulating immune cells in the gut through trained immunity mechanism. Additionally, the severity of AR is influenced by the frequency and timing of allergen exposure, suggesting a role for training immunity. A cross-sectional survey in India showed that the severity and type of AR are related to allergen exposure, with healthcare workers exposed to dust mites and farmers exposed to pollen showing higher rates of moderate to severe AR (41). Tulic et al. found that the timing of endotoxin exposure after sensitization also affects IgE responses (42). Moreover, AR in children is more influenced by genetic factors and has greater plasticity in immune responses, making it more susceptible to regulation by training immunity. Therefore, introducing the concept of training immunity may help address the limitations of the hygiene hypothesis in explaining the onset and development of AR.
In addition, researchers have been exploring effective measures to prevent the onset of AR, with breastfeeding strategies being a key focus. Some earlier studies suggested a protective role of breastfeeding in preventing AR. For example, Codispoti et al. reported that prolonged breastfeeding among African American participants was associated with a reduced risk of AR at age three (43). Breastfeeding influences the gut microbiota, which in turn regulates immune homeostasis (44). In breastfed infants, the gut microbiota is dominated by Bifidobacterium, whereas in formula-fed infants, Bacteroides, Clostridium, and Enterobacteriaceae are more prevalent (45). Several clinical studies have reported that long-term supplementation with probiotics, such as Lactobacillus casei, Lactobacillus rhamnosus, and Lactobacillus gasseri, may alleviate AR symptoms in preschool children and help prevent IgE-mediated allergies and other allergic conditions (46–48). However, an increasing body of long-term cohort studies and cluster RCT evidence indicates that breastfeeding, along with probiotic supplementation, has no lasting effect on the prevention of AR (49–52). Notably, a Finnish cohort study involving 3,781 consecutively born children followed for five years, and the GINIplus study tracking 4,058 individuals until 20 years of age, found no significant association between breastfeeding and reduced AR risk (49, 53). Thus, while breastfeeding plays an important role in early immune modulation, there is currently insufficient evidence to support its preventive effect on AR. As a result, the 2022 German S3 Guideline for Allergy Prevention recommends breastfeeding, but this recommendation is not based on evidence related to AR prevention (54).
4 Diagnosis
The diagnosis of AR is typically based on a detailed medical history, physical examination, and supported by specific allergen testing (Figure 1). To differentiate AR from other forms of rhinitis, physicians may employ additional tests, including nasal allergen challenge, CT scans, nasal nitric oxide measurements, nasal cytology, nasal culture, and nasal fluid β-transferrin analysis. However, due to the limited availability of allergen testing in infants and young children, along with inconsistent guidance from physicians across various disciplines, many diagnoses of AR in children rely solely on chief complaints, symptoms, or symptom scores.
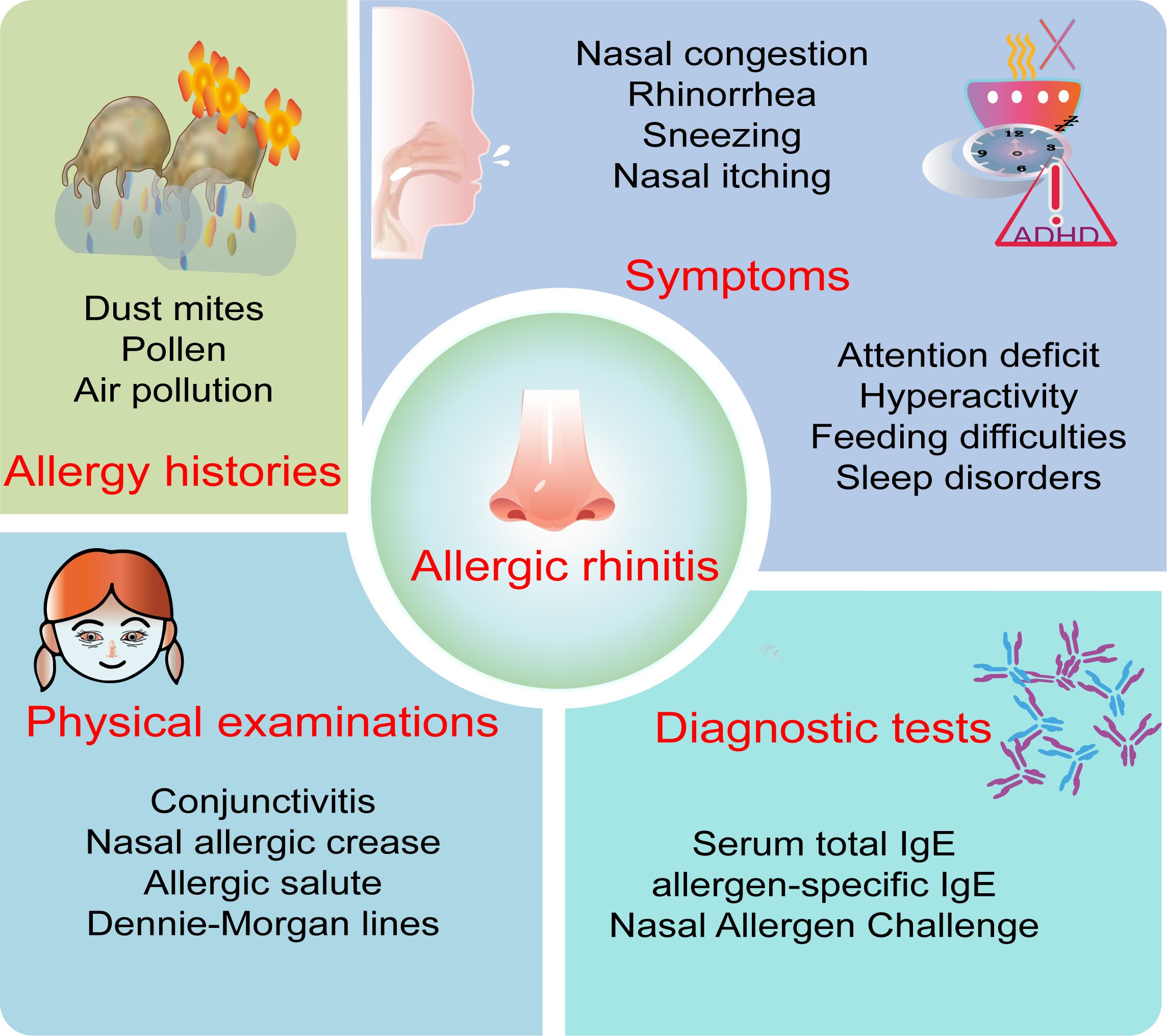
Figure 1. Key diagnostic points of allergic rhinitis in children. The key points for diagnosing allergic rhinitis in children include allergy history, symptoms, physical examination, and diagnostic test.
4.1 Symptoms and signs
Caregivers’ descriptions of AR symptoms in children may not accurately reflect the severity of the condition compared to reports from adult patients. Typical symptoms of AR include sneezing, itching, rhinorrhea, and nasal congestion, with nasal congestion often being more pronounced at night and presenting bilaterally, unilaterally, or alternating between sides (55). Children may also exhibit mouth breathing and nighttime snoring, which, if chronic, can lead to facial developmental abnormalities and malocclusion. Ocular symptoms are more common in polysensitized patients and are correlated with the severity of nasal symptoms (3). Other symptoms may include itching of the palate, postnasal drip, cough, nosebleeds, hyperactivity, and attention deficit, while younger children may exhibit decreased appetite and feeding difficulties. Special signs such as conjunctivitis, nasal allergic crease, allergic salute, and Dennie-Morgan lines are also critical for the detection and diagnosis of pediatric AR. Interestingly, a study evaluating the value of history impression and physical examination in diagnosing AR found that the average sensitivity, specificity, positive predictive value, and negative predictive value of history impression were all higher than those of physical examination (56). Therefore, physicians must first obtain a detailed history of the patient’s symptoms and allergens and conduct a comprehensive physical examination.
4.2 Diagnostic tests
However, diagnosing AR in children is challenging, as its symptoms often overlap with those of upper respiratory tract infections, non-allergic rhinitis, and other conditions, which can mislead both families and healthcare providers. Therefore, appropriate diagnostic methods should be selected based on the specific needs for differential diagnosis or classification, including specific allergy tests, nonspecific allergy tests, and others.
Commonly used specific allergen detection methods include skin prick tests, serum specific IgE tests, and nasal provocation tests. While infants or young children may have difficulty cooperating with skin prick tests, blood tests can usually be performed. Among these methods, skin prick testing demonstrates high sensitivity and specificity (exceeding 80%) (57, 58). While skin prick testing can be used in children of all ages without contraindications (such as uncontrolled or severe asthma), it is important to note that infants may exhibit small wheals, false positives may occur in control groups, and the results are susceptible to influences such as the child’s immune status and procedural factors, requiring cautious interpretation (59). In cases where skin prick testing yields negative results but clinical suspicion for specific allergen sensitization remains high, intradermal testing may be considered. Compared to skin prick testing, serum allergen-specific IgE testing has advantages of being free from adverse reactions and less susceptible to interference from medication and skin conditions. Although some studies suggest that skin prick testing is generally more sensitive than serum allergen-specific IgE testing, comprehensive decision-making is still necessary, considering factors such as the child’s medication regimen, comorbidities, skin condition, and family preferences.
Although specific IgE measurement is crucial in allergy diagnosis, nasal allergen challenge (NAC) remains the preferred method when the clinical relevance of an allergen needs confirmation (60). NAC, also known as Nasal Allergen Provocation Test, is increasingly utilized in clinical practice for diagnosing local AR, identifying allergen components, and assessing AR treatment efficacy. Under standardized and controlled conditions, NAC can accurately reproduce nasal allergic responses. Studies have demonstrated that NAC is highly safe and reproducible in both adults and children, with minimal risk of systemic allergic reactions or bronchospasm (61). In a study by Eguiluz-Gracia et al. involving 518 children and 5830 adults undergoing NAC, only 4 adverse events were reported, with repeatability, positive predictive value, and negative predictive value of 97.32%, 100%, and 92.91%, respectively (61, 62).
NAC results can be evaluated by assessing clinical symptoms through total nasal symptom score (TNSS) and Visual Analogue Scale (VAS), or by measuring nasal patency using peak nasal inspiratory flow (PNIF), rhinomanometry, and acoustic rhinometry (63). Currently, worsening subjective symptoms and increased objective nasal resistance are both considered as criteria for NAC positivity, either separately or in combination. For instance, Sasiwimon et al. reported that NAC is considered positive if any one of the following three criteria is met: nasal airway resistance increases by at least 20% from baseline, TNSS changes by at least 3 points; PNIF decreases by at least 20% from baseline, TNSS changes by at least 3 points; or nasal airway resistance increases by at least 40% from baseline, regardless of TNSS change (64).
Since children may be less accurate in describing subjective symptoms or may not cooperate well with nasal resistance tests, the European Academy of Allergy and Clinical Immunology (EAACI) recommends NAC for children over five years old (65). However, age is not an absolute contraindication; the child’s ability to understand and cooperate with the procedure should be considered. Additionally, potential false negatives due to medications (e.g., antihistamines, corticosteroids) or false positives due to environmental allergens should be ruled out before conducting and interpreting NAC results (66).
Additionally, although total serum IgE levels and eosinophilia are often used in adults as screening tests for allergies, their relatively low sensitivity precludes their routine use in diagnosing AR. Given that serum total IgE levels are frequently normal in AR children, and interference from other allergic factors is likely, the diagnostic value of serum total IgE in AR is generally considered limited (67). In recent years, biomarkers such as nasal nitric oxide (nNO) measurement and eosinophil detection in nasal smears have also been commonly used in AR. A large-scale study involving 173 patients who underwent nasal sinus CT scans and 46 normal controls revealed that patients with AR exhibited significantly higher levels of nNO compared to those with non-allergic rhinitis. The determination of nNO levels proved to be an effective means of distinguishing between these two phenotypes of rhinitis (68). Dynamic monitoring of nNO may contribute to disease assessment and monitoring (69). Measuring nNO has advantages such as being non-invasive and convenient. However, due to the lack of a normal reference value standard for exhaled NO in the nasal cavity, its clinical diagnostic value cannot be determined at present. Other emerging biomarkers for AR, such as nasal mucosal osteoprotegerin, can provide additional information on inflammation and remodeling. The expression of CD203c on the surface of eosinophils may be associated with the temporal characteristics of AR symptoms (70). Furthermore, nasal CT scans have certain value in exclusive diagnosis. However, due to the presence of ionizing radiation, the indications for its use in children should be carefully considered.
4.3 Ongoing clinical trials in AR diagnostics
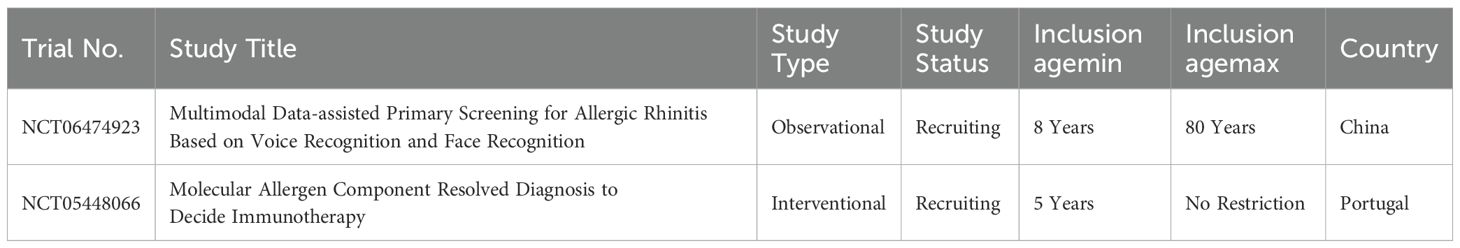
Currently, few ongoing clinical trials on AR diagnostics include pediatric populations, as cataloged in ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (Table 1). One trial investigates the application of multimodal data, integrating voice and facial recognition, to enhance early screening efficiency via artificial intelligence. Another focuses on molecular allergen component-resolved diagnostics to refine personalized immunotherapy. These studies hold promise for advancing diagnostic accuracy and optimizing treatment outcomes in AR.
4.4 Comorbidities of AR
Beyond its primary symptoms and clinical manifestations, AR is frequently associated with other allergic conditions, including asthma, atopic, conjunctivitis, and dermatitis. Over 80% of asthma patients are also affected by AR, while approximately 10-40% of AR patients have asthma (71). The inflammatory processes in the nasal mucosa of AR and the bronchial mucosa of asthma share similar characteristics, such as pro-inflammatory mediators, T-helper cell type 2 (Th2) cytokines, chemokines, and adhesion molecules, supporting the concept of “one airway, one disease” (72). Evidence from cohort studies indicates that early childhood AR is a significant predictor of the persistence of asthma in children (73). The comorbidity of AR with asthma or atopic dermatitis may be attributed to shared genetic polymorphisms and allergen-triggered pathogenic mechanisms. Recent research involving integrated transcriptomic analysis of over 1,200 participants has identified a specific gene signature linked to the multimorbidity of AR, asthma, and atopic dermatitis. The study consistently found eight overexpressed genes (e.g., CLC, IL5RA, SIGLEC8) across these conditions, indicating a shared biological foundation for their frequent co-occurrence (74). Additionally, a meta-analysis has revealed a significant association between AR and symptoms of attention-deficit hyperactivity disorder in children, including total symptom scores, hyperactivity/impulsivity, and inattention (75). AR is also the most common non-rheumatic comorbidity in Juvenile Idiopathic Arthritis (76). The multimorbidity associated with AR exacerbates the overall disease burden in children and has long-term adverse effects on their health. Therefore, it is essential to assess comorbidities in children with AR and consider combined treatment strategies when necessary.
5 Classification
Effective management of AR requires dynamic decision-making, particularly in children, where classification is often based on the onset, timing, and severity of symptoms (77). Traditionally, AR was classified into perennial, seasonal, and occupational forms, depending on allergen exposure (78). Seasonal AR is typically triggered by outdoor allergens like pollen, while perennial AR is associated with indoor allergens such as dust mites and pet dander (79, 80). However, due to the year-round presence of certain plant pollens, variations in pollination seasons across different regions, the existence of patients sensitive to perennial allergens but exhibiting only short-term symptoms, the high prevalence of polysensitized patients, and asymptomatic allergic individuals, the ARIA (Allergic Rhinitis and its Impact on Asthma) guidelines proposed a new classification system for AR (71). This classification subdivides AR based on symptom frequency into ‘intermittent’ and ‘persistent’ forms, with persistence defined as symptoms occurring more than four days per week for at least four weeks. Additionally, ARIA classifies AR into mild, moderate, or severe categories based on the severity of symptoms and their impact on quality of life.
The ARIA classification system is currently recognized and widely adopted by most countries (81, 82). However, there is still no unified standard for assessing the severity of AR. Clinically, severity is often evaluated using TNSS, VAS, nasal obstruction measurements, and olfactory assessments (71). Evidence suggests that using VAS to assess AR severity is not influenced by treatment or allergy diagnostic tests (83). The ARIA classification based on frequency and severity aids in AR management, with moderate to severe persistent AR showing a stronger association with respiratory comorbidities and sensitization compared to mild AR (84). Therefore, treatment strategies based on the ARIA classification have practical significance. For instance, the 2022 Chinese expert consensus on the stepwise treatment of pediatric AR employs a straightforward and quantifiable VAS to score AR, distinguishing between mild (VAS < 5) and moderate-severe (VAS ≥ 5) cases (85). This scoring system further categorizes AR into sneezing/rhinorrhea-predominant and nasal obstruction-predominant types, each with corresponding stepwise treatment plans and assessment methods.
Recent studies have identified a subset of AR patients who test positive in NAC but show no sensitization in skin prick tests or serum-specific IgE assays, a condition known as local allergic rhinitis (LAR) (86). Evidence suggests that while LAR in children is less likely to progress to systemic allergic diseases, it may worsen over time and serves as a risk factor for asthma (87). The diagnosis of LAR relies on a positive NAC response to one or more allergens; however, both adult and pediatric LAR remain underdiagnosed.
6 Treatment and health management
The management of AR in children emphasizes a comprehensive step-by-step strategy, which includes environmental control, medication therapy, immunotherapy, and health management (88). Strategies may involve allergen avoidance, patient education, antihistamine treatment, saline nasal irrigation, and specific immunotherapy. In moderate to severe cases, combination therapy using corticosteroids and leukotriene receptor antagonists (LTRA) might be necessary. In situations where mild AR is not well-controlled, an escalation of the treatment regimen is warranted, while for adequately managed moderate to severe cases, a gradual reduction in treatment intensity can be considered.
6.1 Environmental control
Clinical epidemiological studies have shown that environmental air pollution, dust mites, and pets can promote the development of AR. A study in Changchun, China, demonstrated that for each standard deviation increase in PM2.5 pollutants, the number of visits by AR patients increased by 10.2% (89). Some studies support that environmental control can effectively reduce allergen exposure and improve the health of children with AR. For example, for AR caused by dust mites, acaricides and high-efficiency particulate air filters have specific therapeutic effects, while the removal of pets also shows some effectiveness for certain patients with AR (1). However, some studies indicate that although environmental control measures reduce allergen exposure levels, their effect on alleviating symptoms or improving the quality of life of AR patients is limited (90).
Another environmentally focused strategy that has gained attention in recent years is allergen barrier agents, primarily including nasal sprays and nasal ointments. These formulations create a mechanical barrier to avoid allergen contact with the nasal mucosa, thereby alleviating allergy symptoms. Multiple studies have shown that allergen barrier agents, whether used as monotherapy or in combination with other medications for pediatric AR, significantly reduce symptom scores, improve quality of life, and do not increase the incidence of adverse reactions (91–93). Although the use of allergen barrier agents in infants and toddlers may be influenced by nasal medication compliance, if this form of physical barrier treatment can mitigate the side effects of drug therapy, it is likely to be more accepted by children and parents. However, existing studies have small sample sizes and short follow-up periods, so the long-term efficacy of allergen barrier agents requires further validation through large-sample, long-term clinical studies.
6.2 Medication treatment
Considering factors such as efficacy and treatment duration, pharmacotherapy remains the most widely accepted treatment approach currently. Pharmacological interventions are primarily selected based on the frequency and severity of symptoms in AR, encompassing oral or intranasal H1 antihistamines, intranasal corticosteroids (INCS), and fixed combinations of intranasal H1 antihistamines and corticosteroids (94).
H1-antihistamines are commonly used for patients with mild symptoms or those who are averse to INCS therapy. This includes second-generation oral H1-antihistamines with lower sedation levels (such as desloratadine, loratadine, cetirizine, levocetirizine, and rupatadine) and non-sedating H1-antihistamines (such as fexofenadine and bilastine). Oral H1-antihistamines, administered once daily, are rapidly effective and can be used as a single agent intermittently or continuously, effectively controlling symptoms in many pediatric patients while also offering the advantage of lower cost. However, the potential systemic side effects, including sedation, dry eye syndrome, and urinary retention, should not be overlooked. Previous research has confirmed the safety of desloratadine, levocetirizine, and levocetirizine in children aged 6 months and older, as well as the safety of loratadine in children aged 2 years and older. The latest evidence also affirms the efficacy and safety of rupatadine (with dual affinity for H1 receptors and platelet-activating factor receptors) in children aged 2 years and older (95). For children aged 6 years and older with seasonal or perennial AR, intranasal antihistamines are also a viable option. Currently, intranasal antihistamines approved by the U.S. FDA, including azelastine and olopatadine, are more effective for nasal congestion and have a faster onset of action than oral antihistamines, but they may increase the risk of local side effects, such as epistaxis (96).
INCS remains the most effective monotherapy for treating AR and are commonly employed as the first-line treatment option for patients with persistent or moderate-to-severe symptoms. Frequently used medications include beclomethasone, budesonide, ciclesonide, fluticasone propionate, fluticasone furoate, mometasone furoate, and triamcinolone acetonide. INCS not only effectively controls nasal symptoms in AR patients but also demonstrates efficacy in managing allergic ocular symptoms (57). The therapeutic effectiveness of regularly used INCS surpasses that of oral antihistamines, especially in alleviating nasal congestion. Additionally, the addition of oral antihistamines to INCS treatment does not typically enhance therapeutic outcomes. Recent evidence from randomized controlled trials suggests that, for patients with moderate-to-severe AR, both as-needed and regular use of INCS yield similar improvements in nasal symptom scores and Rhinitis Life Quality-36 questionnaire scores, with the exposure dose for as-needed use being only half of that for regular use (97). Systematic reviews and meta-analyses further support the comparable efficacy of as-needed INCS use to regular use (98). Given the absence of systemic absorption and concerns about systemic adverse reactions, the most common local adverse reactions associated with INCS include nasal irritation, stinging, and nosebleeds, which can be prevented by directing the spray away from the nasal septum.
When the efficacy of monotherapy is suboptimal, consideration may be given to the fixed combination of intranasal antihistamines and INCS), such as fluticasone propionate-azelastine and mometasone-olopatadine. Studies have demonstrated that the fixed combination of INCS and intranasal H1-antihistamines is more effective than individual drug administration and is well-tolerated (99). In the context of combination therapy, caution should be exercised to avoid the use of combinations without additional benefits, with careful consideration of potential side effects and interactions associated with drug co-administration.
In addition, montelukast, a LTRA, is commonly used to treat pediatric AR. The U.S. Food and Drug Administration (FDA) has approved its use for seasonal AR in children aged two and above and perennial AR in children aged six months and above. Children using montelukast generally exhibit good tolerability, but occasional neurobehavioral events may occur. A study from Korea showed an increased risk of neurobehavioral events in adolescents (12-18 years) and young adults (19-30 years) using LTRA in patients with asthma or AR, while no such increase was observed in children (3-11 years) (100). Although some children with concomitant asthma may benefit, overall, its efficacy is not superior to oral H1-antihistamines or INCS. Therefore, there is currently no evidence supporting the routine use of LTRA for the treatment of pediatric AR.
6.3 AIT
Due to concerns about long-term medication and drug side effects, there is a growing preference for AIT that gradually introduces allergens to enhance tolerance in pediatric patients, thereby reducing or eliminating allergic reactions. The goal of AIT is to alleviate allergy symptoms, improve quality of life, modify the natural course of the disease, and provide lasting relief from allergies over the long term. Evidence suggests that AIT can also prevent new sensitizations and reduce the risk of asthma development in AR patients (101). AIT treatment can be considered in cases with allergen-specific IgE positivity.
The typical duration of AIT is 3-5 years, including an induction phase and a maintenance phase. During the induction phase, allergen doses are gradually increased to establish tolerance. After reaching the maintenance phase, regular administration of maintenance doses of allergens is required to sustain tolerance. Currently, AIT mainly involves subcutaneous immunotherapy and sublingual immunotherapy. Real-world evidence confirms the effectiveness of subcutaneous or sublingual immunotherapy in pediatric AR, with outcomes possibly superior in children compared to adults, as demonstrated by a significant reduction in prescription medication use for AR in children evaluated over 3-9 years post-treatment (102). Sublingual immunotherapy, in comparison to subcutaneous immunotherapy, exhibits higher compliance and fewer, milder adverse reactions (103). Studies have shown that increasing the immunization dose within a certain range can enhance the effectiveness of sublingual immunotherapy (104).
In recent years, researchers have explored some relatively short-duration alternatives, such as intralymphatic immunotherapy, epicutaneous immunotherapy, and intradermal immunotherapy, showing some effectiveness and relative safety. However, there is still insufficient evidence to support their superiority over subcutaneous or sublingual immunotherapy in children (103). Nevertheless, AIT may lead to serious adverse reactions, such as systemic allergic reactions, and should be conducted under the guidance of a physician. Additionally, for children in the developmental stage, careful consideration is needed due to the potential adverse impact of AIT on delaying symptom control.
6.4 Other treatment options
Researchers have been continuously exploring more convenient and less side-effect treatment methods for AR. Saline irrigation can remove some allergens and inflammatory mediators, offering advantages such as safety and convenience, and it is easily accepted by families of older children and infants. A Cochrane systematic review showed that isotonic or hypertonic saline irrigation effectively reduces the severity of AR symptoms and enhances the effectiveness of pharmacotherapy (105).
Moreover, increasing evidence in recent years supports the regulatory role of traditional Chinese medicine in treating AR. For instance, several studies revealed the efficacy and safety of Xiao Qing Long Tang in treating AR (92, 106). However, more robust evidence is needed for the use of traditional Chinese medicine in children with AR. Additionally, careful consideration is required regarding children’s tolerance of the unpleasant taste of herbal medicine and the potential for drug-induced liver and kidney damage. Some studies suggest that acupuncture and moxibustion can improve AR symptoms (107, 108). However, their application in children is rare.
6.5 Health management
Ensuring the optimal effectiveness of various AR treatment measures relies on long-term adherence and the standardization of treatment. Research indicates that the compliance of patients undergoing AIT during a 12-month follow-up period is significantly higher than those with a 3-month or 6-month follow-up period (65). Therefore, effective health management is crucial for improving treatment compliance and ensuring the efficacy of AR therapy in children.
With the widespread adoption of smartphones globally and breakthroughs in artificial intelligence technology, there is a promising prospect for the development and promotion of mobile health (mHealth) applications that enable real-time collection, analysis, and feedback of patient data (109). More health management measures are anticipated to enhance treatment adherence, improve quality of life, and disease prognosis for children with AR through behavior change strategies such as reminders, consultations, reinforcement, or education (Figure 2).
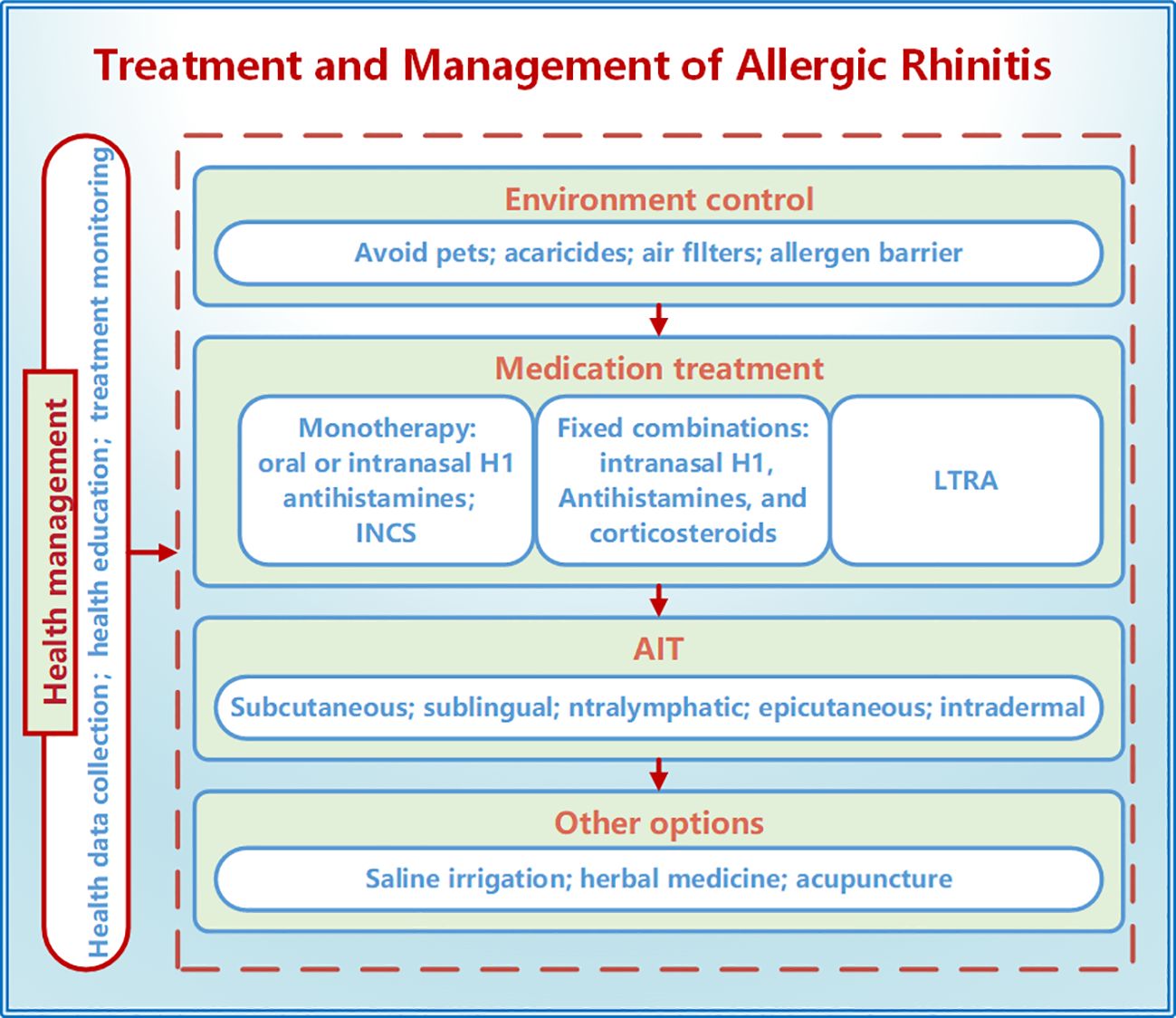
Figure 2. Treatment and management of allergic rhinitis in children. The treatment methods for allergic rhinitis in children include environmental control, medication treatment, AIT, saline irrigation, traditional Chinese medicine, and acupuncture. Health management can improve treatment adherence and efficacy through measures such as data collection, health education, and treatment management. INCS, intranasal corticosteroids; LTRA, leukotriene receptor antagonists; AIT, allergen-specific immunotherapy.
ARIA proposed the use of mobile communication technology to develop and validate information technology tools that strengthen self-medication management for AR patients and facilitate shared decision-making with healthcare professionals (110). Currently, the Mobile Airways Sentinel Network (MASK) stands as the most influential AR mHealth tool. MASK is a patient-centered information and communication technology system that includes a treatment list, which contains country-specific medications, and a visual analogue scale to assess AR control, sleep, work efficiency, etc. The study, conducted among over 9,000 users from 22 countries/regions as part of the MASK initiative, reveals poor treatment adherence among patients with AR. This includes self-medication, on-demand treatment when symptoms are not optimally controlled, switching medications to gain control when symptoms are unmanageable, and non-compliance with medical guidelines or physician prescriptions (111–113).
The Control of Allergic Rhinitis/Asthma Test (CARAT) is a powerful tool for screening AR diagnosis and assessing disease control (114). CARAT consists of a questionnaire regarding symptoms, sleep, activity, medication usage, and other aspects over the past four weeks. It summarizes the patient’s clinical condition and supports shared decision-making among patients, doctors, and the healthcare team. These tools have been used in clinical research and practice, enabling personalized and real-time assessment for patients. A study involving 643 patients revealed a significantly worse RHINASTHMA questionnaire total and subdomain scores and symptom control in patients with AR + asthma than in patients with AR alone (115). Therefore, the importance of implementing health management and comprehensive management through mHealth to achieve optimal care and ensure the effectiveness of AR treatment cannot be overstated.
6.6 Ongoing clinical trials in AR treatment and management
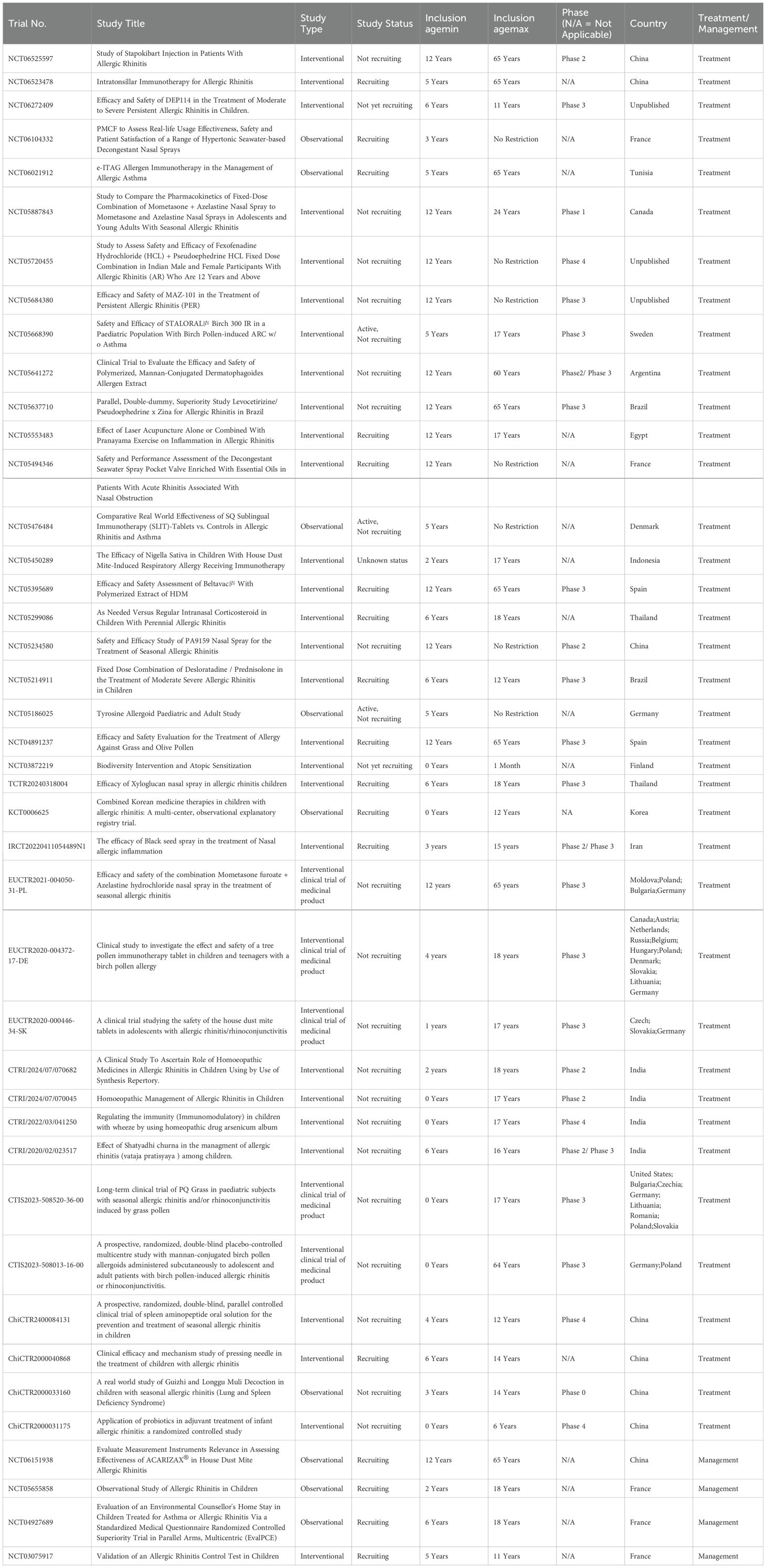
Data from ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (Table 2) reveal that current clinical trials on pediatric AR predominantly target immunotherapy, combination therapies, and alternative treatments. For instance, a fixed-dose nasal spray combining mometasone and azelastine is being tested for its enhanced anti-inflammatory and decongestant effects. Natural therapies, such as black seed spray and laser acupuncture, are also under investigation, offering patients additional treatment options and aiming to improve quality of life.
In the management of AR, there is a growing emphasis on refining personalized treatment strategies for children. One trial is evaluating the efficacy of ACARIZAX® in children with house dust mite-induced AR, aiming to optimize efficacy assessment metrics to enhance therapeutic outcomes. A French study is analyzing real-world management of pediatric AR, while the Evaluation of an Environmental Counsellor’s Home Stay trial explores the role of environmental counseling in AR caring. Furthermore, a novel allergic rhinitis control test is undergoing validation, aiming to enhance disease monitoring precision.
Notably, an increasing number of clinical trials are incorporating real-world data to evaluate the effectiveness and patient satisfaction of these new therapies in practical settings. In pediatric AR, the trend toward personalized and precision-based treatment strategies is gaining momentum, with the potential to significantly improve both short- and long-term patient outcomes.
7 Conclusion
The rising prevalence of pediatric AR highlights the complex interaction between genetics and environmental factors. The concept of training immunity helps explain how environmental exposures influence immune responses and AR development. Combining environmental data with multi-omics can lead to better prevention and early identification of high-risk pediatric AR cases.
Despite advances in allergen testing and molecular diagnostics, choosing the right diagnostic methods remains challenging for clinicians. Optimizing diagnostic protocols for different ages and symptoms, and improving family education to prevent self-diagnosis, are essential.
For treatment, a stepwise approach tailored to age, AR type, and severity is recommended. Environmental control, saline irrigation, pharmacotherapy, immunotherapy, and traditional Chinese medicine can enhance quality of life. Developing long-term management strategies and personalized treatments is crucial. mHealth offers a promising tool for refining and optimizing AR management based on real-time feedback.
Author contributions
MC: Conceptualization, Data curation, Investigation, Resources, Writing – original draft. QD: Data curation, Resources, Writing – original draft, Project administration, Validation, Formal analysis. ZL: Data curation, Formal analysis, Validation, Visualization, Writing – original draft. YW: Conceptualization, Investigation, Methodology, Supervision, Writing – review & editing. CZ: Conceptualization, Supervision, Writing – review & editing, Project administration, Validation.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This paper is supported by the Jingmen City Guiding Research Project (2023YDKY083) without financial funding.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Wise SK, Damask C, Roland LT, Ebert C, Levy JM, Lin S, et al. International consensus statement on allergy and rhinology: Allergic rhinitis - 2023. Int Forum Allergy Rhinol. (2023) 13:293–859. doi: 10.1002/alr.23090
2. Seidman MD, Gurgel RK, Lin SY, Schwartz SR, Baroody FM, Bonner JR, et al. Clinical practice guideline: Allergic rhinitis. Otolaryngol Head Neck Surg. (2015) 152:S1–S43. doi: 10.1177/0194599814561600
3. Bousquet J, Anto JM, Bachert C, Baiardini I, Bosnic-Anticevich S, Walter CG, et al. Allergic rhinitis. Nat Rev Dis Primers. (2020) 6:95. doi: 10.1038/s41572-020-00227-0
4. Skoner DP. Allergic rhinitis: definition, epidemiology, pathophysiology, detection, and diagnosis. J Allergy Clin Immunol. (2001) 108:S2–8. doi: 10.1067/mai.2001.115569
5. Meltzer EO, Blaiss MS, Derebery MJ, Mahr TA, Gordon BR, Sheth KK, et al. Burden of allergic rhinitis: results from the Pediatric Allergies in America survey. J Allergy Clin Immunol. (2009) 124:S43–70. doi: 10.1016/j.jaci.2009.05.013
6. Schuler IC, Montejo JM. Allergic rhinitis in children and adolescents. Pediatr Clin North Am. (2019) 66:981–93. doi: 10.1016/j.pcl.2019.06.004
7. Asher MI, Montefort S, Björkstén B, Lai CK, Strachan DP, Weiland SK, et al. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet. (2006) 368:733–43. doi: 10.1016/S0140-6736(06)69283-0
8. Aït-Khaled N, Pearce N, Anderson HR, Ellwood P, Montefort S, Shah J. Global map of the prevalence of symptoms of rhinoconjunctivitis in children: The International Study of Asthma and Allergies in Childhood (ISAAC) Phase Three. Allergy. (2009) 64:123–48. doi: 10.1111/j.1398-9995.2008.01884.x
9. Veskitkul J, Vichyanond P, Visitsunthorn N, Jirapongsananuruk O. The development of allergic rhinitis in children previously diagnosed as nonallergic rhinitis. Am J Rhinol Allergy. (2013) 27:43–7. doi: 10.2500/ajra.2013.27.3839
10. Sinha B, Singla R, Chowdhury R. Allergic Rhinitis: A neglected disease - A community based assessment among adults in Delhi. J Postgrad Med. (2015) 61:169–75. doi: 10.4103/0022-3859.159418
11. Pang K, Li G, Li M, Zhang L, Fu Q, Liu K, et al. Prevalence and risk factors for allergic rhinitis in China: A systematic review and meta-analysis. Evidence-Based Complementary Altern Med. (2022) 2022:7165627. doi: 10.1155/2022/7165627
12. Kim DH, Lim DH, Samra M, Kim EH, Kim JH. How accurate are the ISAAC questions for diagnosis of allergic rhinitis in Korean children? Int J Environ Res Public Health. (2018) 15(7):1527. doi: 10.3390/ijerph15071527
13. Tong H, Gao L, Deng Y, Kong Y, Xiang R, Tan L, et al. Prevalence of allergic rhinitis and associated risk factors in 6 to 12 years schoolchildren from Wuhan in Central China: A cross-sectional study. Am J Rhinol Allergy. (2020) 34:632–41. doi: 10.1177/1945892420920499
14. Deng SZ, Jalaludin BB, Antó JM, Hess JJ, Huang CR. Climate change, air pollution, and allergic respiratory diseases: a call to action for health professionals. Chin Med J (Engl). (2020) 133:1552–60. doi: 10.1097/CM9.0000000000000861
15. Zhang Y, Lan F, Zhang L. Advances and highlights in allergic rhinitis. Allergy. (2021) 76:3383–9. doi: 10.1111/all.15044
16. Strachan DP. Hay fever, hygiene, and household size. BMJ. (1989) 299:1259–60. doi: 10.1136/bmj.299.6710.1259
17. Prescott SL. Allergy: the price we pay for cleaner living? Ann Allergy Asthma Immunol. (2003) 90:64–70. doi: 10.1016/s1081-1206(10)61663-8
18. Krzych-Fałta E, Wojas O, Furmańczyk K, Dziewa-Dawidczyk D, Piekarska B, Samoliński B, et al. Evaluation of selected aspects of the hygiene hypothesis and their effect on the incidence of allergy. Int J Occup Med Environ Health. (2023) 36:69–83. doi: 10.13075/ijomeh.1896.01880
19. Han DH, Ahn JC, Mun SJ, Park SK, Oh SY, Rhee CS. Novel risk factors for allergic rhinitis in Korean elementary school children: ARCO-kids phase II in a community. Allergy Asthma Immunol Res. (2015) 7:234–40. doi: 10.4168/aair.2015.7.3.234
20. Lisik D, Ermis S, Ioannidou A, Milani GP, Nyassi S, Spolidoro G, et al. Siblings and risk of allergic rhinitis: A systematic review and meta-analysis. Pediatr Allergy Immunol. (2023) 34:e13991. doi: 10.1111/pai.13991
21. Alm B, Goksör E, Pettersson R, Möllborg P, Erdes L, Loid P, et al. Antibiotics in the first week of life is a risk factor for allergic rhinitis at school age. Pediatr Allergy Immunol. (2014) 25:468–72. doi: 10.1111/pai.12244
22. Bunyavanich S, SChadt EE, Himes BE, Lasky-Su J, Qiu W, Lazarus R, et al. Integrated genome-wide association, coexpression network, and expression single nucleotide polymorphism analysis identifies novel pathway in allergic rhinitis. BMC Med Genomics. (2014) 7:48. doi: 10.1186/1755-8794-7-48
23. Wise SK, Lin SY, Toskala E. International consensus statement on allergy and rhinology: allergic rhinitis-executive summary. Int Forum Allergy Rhinol. (2018) 8:85–107. doi: 10.1002/alr.22070
24. Hershey GK, Friedrich MF, Esswein LA, Thomas ML, Chatila TA. The association of atopy with a gain-of-function mutation in the alpha subunit of the interleukin-4 receptor. N Engl J Med. (1997) 337:1720–5. doi: 10.1056/NEJM199712113372403
25. Andiappan AK, Wang DY, Anantharaman R, Parate PN, Suri BK, Low HQ, et al. Genome-wide association study for atopy and allergic rhinitis in a Singapore Chinese population. PloS One. (2011) 6:e19719. doi: 10.1371/journal.pone.0019719
26. Wei X, Zhang Y, Fu Z, Zhang L. The association between polymorphisms in the MRPL4 and TNF-α genes and susceptibility to allergic rhinitis. PloS One. (2013) 8:e57981. doi: 10.1371/journal.pone.0057981
27. Ramasamy A, Curjuric I, Coin LJ, Kumar A, McArdle WL, Imboden M, et al. A genome-wide meta-analysis of genetic variants associated with allergic rhinitis and grass sensitization and their interaction with birth order. J Allergy Clin Immunol. (2011) 128:996–1005. doi: 10.1016/j.jaci.2011.08.030
28. Kim JW, Kim MJ, Paik K, Kim BR, Choi CW, Na JI. Genome-wide association study of susceptibility loci for self-reported atopic dermatitis and allergic rhinitis in the Korean population. Ann Dermatol. (2024) 36:74–80. doi: 10.5021/ad.22.160
29. Long A, Bunning B, Sampath V, DeKruyff RH, Nadeau KC. Epigenetics and the environment in airway disease: asthma and allergic rhinitis. Adv Exp Med Biol. (2020) 1253:153–81. doi: 10.1007/978-981-15-3449-2_6
30. Netea MG, Domínguez-Andrés J, Barreiro LB, Chavakis T, Divangahi M, Fuchs E, et al. Defining trained immunity and its role in health and disease. Nat Rev Immunol. (2020) 20:375–88. doi: 10.1038/s41577-020-0285-6
31. van der Heijden C, Noz MP, Joosten L, Riksen NP, Keating ST. Epigenetics and trained immunity. Antioxid Redox Signal. (2018) 29:1023–40. doi: 10.1089/ars.2017.7310
32. Ghisletti S, Barozzi I, Mietton F, Polletti S, De Santa F, Venturini E, et al. Identification and characterization of enhancers controlling the inflammatory gene expression program in macrophages. Immunity. (2010) 32:317–28. doi: 10.1016/j.immuni.2010.02.008
33. Riksen NP, Netea MG. Immunometabolic control of trained immunity. Mol Aspects Med. (2021) 77:100897. doi: 10.1016/j.mam.2020.100897
34. Christ A, Günther P, Lauterbach M, Duewell P, Biswas D, Pelka K, et al. Western diet triggers NLRP3-dependent innate immune reprogramming. Cell. (2018) 172:162–75. doi: 10.1016/j.cell.2017.12.013
35. Bekkering S, van den Munckhof I, Nielen T, Lamfers E, Dinarello C, Rutten J, et al. Innate immune cell activation and epigenetic remodeling in symptomatic and asymptomatic atherosclerosis in humans in vivo. Atherosclerosis. (2016) 254:228–36. doi: 10.1016/j.atherosclerosis.2016.10.019
36. Shirai T, Nazarewicz RR, Wallis BB, Yanes RE, Watanabe R, Hilhorst M, et al. The glycolytic enzyme PKM2 bridges metabolic and inflammatory dysfunction in coronary artery disease. J Exp Med. (2016) 213:337–54. doi: 10.1084/jem.20150900
37. Machiels B, Dourcy M, Xiao X, Javaux J, Mesnil C, Sabatel C, et al. A gammaherpesvirus provides protection against allergic asthma by inducing the replacement of resident alveolar macrophages with regulatory monocytes. Nat Immunol. (2017) 18:1310–20. doi: 10.1038/ni.3857
38. Noel JC, Berin MC. Role of innate immunity and myeloid cells in susceptibility to allergic disease. Ann N Y Acad Sci. (2021) 1499:42–53. doi: 10.1111/nyas.14654
39. Jin Q, Ren F, Dai D, Sun N, Qian Y, Song P. The causality between intestinal flora and allergic diseases: Insights from a bi-directional two-sample Mendelian randomization analysis. Front Immunol. (2023) 14:1121273. doi: 10.3389/fimmu.2023.1121273
40. Kallio S, Jian C, Korpela K, Kukkonen AK, Salonen A, Savilahti E, et al. Early-life gut microbiota associates with allergic rhinitis during 13-year follow-up in a Finnish probiotic intervention cohort. Microbiol Spectr. (2024) 12:e413523. doi: 10.1128/spectrum.04135-23
41. Sandbhor A, Jain S, Deshmukh P, Gaurkar S, Murali M, Hande V, et al. Pattern and severity of allergic rhinitis correlated with patient characteristics: A rural hospital-based cross-sectional study. Indian J Otolaryngol Head Neck Surg. (2024) 76:514–22. doi: 10.1007/s12070-023-04198-y
42. Tulić MK, Wale JL, Holt PG, Sly PD. Modification of the inflammatory response to allergen challenge after exposure to bacterial lipopolysaccharide. Am J Respir Cell Mol Biol. (2000) 22:604–12. doi: 10.1165/ajrcmb.22.5.3710
43. Codispoti CD, Levin L, LeMasters GK, Ryan P, Reponen T, Villareal M, et al. Breast-feeding, aeroallergen sensitization, and environmental exposures during infancy are determinants of childhood allergic rhinitis. J Allergy Clin Immunol. (2010) 125:1054–60. doi: 10.1016/j.jaci.2010.02.004
44. Groer MW, Luciano AA, Dishaw LJ, Ashmeade TL, Miller E, Gilbert JA. Development of the preterm infant gut microbiome: a research priority. Microbiome. (2014) 2:38. doi: 10.1186/2049-2618-2-38
45. Azad MB, Konya T, Maughan H, Guttman DS, Field CJ, Chari RS, et al. Gut microbiota of healthy Canadian infants: profiles by mode of delivery and infant diet at 4 months. CMAJ. (2013) 185:385–94. doi: 10.1503/cmaj.121189
46. Giovannini M, Agostoni C, Riva E, Salvini F, Ruscitto A, Zuccotti GV, et al. A randomized prospective double blind controlled trial on effects of long-term consumption of fermented milk containing Lactobacillus casei in pre-school children with allergic asthma and/or rhinitis. Pediatr Res. (2007) 62:215–20. doi: 10.1203/PDR.0b013e3180a76d94
47. Wickens K, Black P, Stanley TV, Mitchell E, Barthow C, Fitzharris P, et al. A protective effect of Lactobacillus rhamnosus HN001 against eczema in the first 2 years of life persists to age 4 years. Clin Exp Allergy. (2012) 42:1071–9. doi: 10.1111/j.1365-2222.2012.03975.x
48. Kawase M, He F, Kubota A, Hiramatsu M, Saito H, Ishii T, et al. Effect of fermented milk prepared with two probiotic strains on Japanese cedar pollinosis in a double-blind placebo-controlled clinical study. Int J Food Microbiol. (2009) 128:429–34. doi: 10.1016/j.ijfoodmicro.2008.09.017
49. Libuda L, Filipiak-Pittroff B, Standl M, Schikowski T, von Berg A, Koletzko S, et al. Full breastfeeding and allergic diseases-long-term protection or rebound effects? Nutrients. (2023) 15(12):2780. doi: 10.3390/nu15122780
50. Gorlanova O, Appenzeller R, Mahmoud YS, Ramsey KA, Usemann J, Decrue F, et al. Effect of breastfeeding duration on lung function, respiratory symptoms and allergic diseases in school-age children. Pediatr Pulmonol. (2020) 55:1448–55. doi: 10.1002/ppul.24733
51. Hendaus MA, Jomha FA, Ehlayel M. Allergic diseases among children: nutritional prevention and intervention. Ther Clin Risk Manag. (2016) 12:361–72. doi: 10.2147/TCRM.S98100
52. Güngör D, Nadaud P, LaPergola CC, Dreibelbis C, Wong YP, Terry N, et al. Infant milk-feeding practices and food allergies, allergic rhinitis, atopic dermatitis, and asthma throughout the life span: a systematic review. Am J Clin Nutr. (2019) 109:772S–99S. doi: 10.1093/ajcn/nqy283
53. Nwaru BI, Takkinen H, Niemelä O, Kaila M, Erkkola M, Ahonen S, et al. Timing of infant feeding in relation to childhood asthma and allergic diseases. J Allergy Clin Immunol. (2013) 131:78–86. doi: 10.1016/j.jaci.2012.10.028
54. Kopp MV, Muche-Borowski C, Abou-Dakn M, Ahrens B, Beyer K, Blümchen K, et al. S3 guideline allergy prevention. Allergol Select. (2022) 6:61–97. doi: 10.5414/ALX02303E
55. Subspecialty Group of Rhinology, Editorial Board of Chinese Journal of Otorhinolaryngology Head and Neck Surgery Subspecialty Groups of Rhinology and Pediatrics, Society of Otorhinolaryngology Head and Neck Surgery, Chinese Medical Association. Guideline for diagnosis and treatment of pediatric allergic rhinitis (2022, revision). Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. (2022) 57:392–404. doi: 10.3760/cma.j.cn115330-20220303-00092
56. Raza SN, Yousuf K, Small P, Frenkiel S. Diagnosing allergic rhinitis: effectiveness of the physical examination in comparison to conventional skin testing. J Otolaryngol Head Neck Surg. (2011) 40:407–12.
57. Dykewicz MS, Wallace DV, Amrol DJ, Baroody FM, Bernstein JA, Craig TJ, et al. Rhinitis 2020: A practice parameter update. J Allergy Clin Immunol. (2020) 146:721–67. doi: 10.1016/j.jaci.2020.07.007
58. Bielory L, Leonov A. Stereoconfiguration of antiallergic and immunologic drugs. Ann Allergy Asthma Immunol. (2008) 100:1–8, 8-11, 36. doi: 10.1016/S1081-1206(10)60396-1
59. Bousquet J, Heinzerling L, Bachert C, Papadopoulos NG, Bousquet PJ, Burney PG, et al. Practical guide to skin prick tests in allergy to aeroallergens. Allergy. (2012) 67:18–24. doi: 10.1111/j.1398-9995.2011.02728.x
60. Huss-Marp J, Darsow U, Brockow K, Pfab F, Weichenmeier I, Schober W, et al. Can immunoglobulin E-measurement replace challenge tests in allergic rhinoconjunctivits to grass pollen? Clin Exp Allergy. (2011) 41:1116–24. doi: 10.1111/j.1365-2222.2011.03745.x
61. Augé J, Vent J, Agache I, Airaksinen L, Campo MP, Chaker A, et al. EAACI Position paper on the standardization of nasal allergen challenges. Allergy. (2018) 73:1597–608. doi: 10.1111/all.13416
62. Eguiluz-Gracia I, Testera-Montes A, González M, Pérez-Sánchez N, Ariza A, Salas M, et al. Safety and reproducibility of nasal allergen challenge. Allergy. (2019) 74:1125–34. doi: 10.1111/all.13728
63. Eguiluz-Gracia I, Testera-Montes A, Salas M, Perez-Sanchez N, Ariza A, Bogas G, et al. Comparison of diagnostic accuracy of acoustic rhinometry and symptoms score for nasal allergen challenge monitoring. Allergy. (2021) 76:371–5. doi: 10.1111/all.14499
64. Traiyan S, Manuyakorn W, Kanchongkittiphon W, Sasisakulporn C, Jotikasthira W, Kiewngam P, et al. Skin prick test versus phadiatop as a tool for diagnosis of allergic rhinitis in children. Am J Rhinol Allergy. (2021) 35:98–106. doi: 10.1177/1945892420938300
65. Roberts G, Pfaar O, Akdis CA, Ansotegui IJ, Durham SR, Gerth VWR, et al. EAACI guidelines on allergen immunotherapy: allergic rhinoconjunctivitis. Allergy. (2018) 73:765–98. doi: 10.1111/all.13317
66. Gosepath J, Amedee RG, Mann WJ. Nasal provocation testing as an international standard for evaluation of allergic and nonallergic rhinitis. Laryngoscope. (2005) 115:512–6. doi: 10.1097/01.MLG.0000149682.56426.6B
67. Bernstein IL, Li JT, Bernstein DI, Hamilton R, Spector SL, Tan R, et al. Allergy diagnostic testing: an updated practice parameter. Ann Allergy Asthma Immunol. (2008) 100:S1–S148. doi: 10.1016/s1081-1206(10)60305-5
68. Liu C, Zheng K, Liu X, Zheng M, Liu Z, Wang X, et al. Use of nasal nitric oxide in the diagnosis of allergic rhinitis and nonallergic rhinitis in patients with and without sinus inflammation. J Allergy Clin Immunol Pract. (2020) 8:1574–81. doi: 10.1016/j.jaip.2019.12.017
69. Abdullah AA, Zahedi FD, Husain S, Wan HA, Abdullah B. Diagnostic value and clinical application of nasal fractional exhaled nitric oxide in subjects with allergic rhinitis. Am J Rhinol Allergy. (2023) 37:307–12. doi: 10.1177/19458924221145084
70. Breiteneder H, Peng YQ, Agache I, Diamant Z, Eiwegger T, Fokkens WJ, et al. Biomarkers for diagnosis and prediction of therapy responses in allergic diseases and asthma. Allergy. (2020) 75:3039–68. doi: 10.1111/all.14582
71. Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen). Allergy. (2008) 63 Suppl 86:8–160. doi: 10.1111/j.1398-9995.2007.01620.x
72. Bachert C, Vignola AM, Gevaert P, Leynaert B, Van Cauwenberge P, Bousquet J. Allergic rhinitis, rhinosinusitis, and asthma: one airway disease. Immunol Allergy Clin North Am. (2004) 24:19–43. doi: 10.1016/S0889-8561(03)00104-8
73. Peat JK, Salome CM, Woolcock AJ. Longitudinal changes in atopy during a 4-year period: relation to bronchial hyperresponsiveness and respiratory symptoms in a population sample of Australian schoolchildren. J Allergy Clin Immunol. (1990) 85:65–74. doi: 10.1016/0091-6749(90)90223-q
74. Lemonnier N, Melén E, Jiang Y, Joly S, Ménard C, Aguilar D, et al. A novel whole blood gene expression signature for asthma, dermatitis, and rhinitis multimorbidity in children and adolescents. Allergy. (2020) 75:3248–60. doi: 10.1111/all.14314
75. Chuang Y, Wang C, Huang W, Wang L, Kuo H, Chen Y, et al. Two meta-analyses of the association between atopic diseases and core symptoms of attention deficit hyperactivity disorder. Sci Rep. (2022) 12:3377. doi: 10.1038/s41598-022-07232-1
76. Haşlak F, Guliyeva V, Hotaman B, Duman Ç, Yıldız M, Günalp A, et al. Non-rheumatic chronic comorbidities in children with juvenile idiopathic arthritis. Turk Arch Pediatr. (2023) 58:212–9. doi: 10.5152/TurkArchPediatr.2023.22303
77. Otolaryngology Professional Committee P B C M. Clinical practice guidelines for diagnosis and treatment of pediatric allergic rhinitis. Chin J Pract Pediatr. (2019) 34:169–75. doi: 10.19538/j.ek2019030601
78. van Cauwenberge P, Bachert C, Passalacqua G, Bousquet J, Canonica GW, Durham SR, et al. Consensus statement on the treatment of allergic rhinitis. Eur Acad Allergol Clin Immunol Allergy. (2000) 55:116–34. doi: 10.1034/j.1398-9995.2000.00526.x
79. Brożek JL, Bousquet J, Agache I, Agarwal A, Bachert C, Bosnic-Anticevich S, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines-2016 revision. J Allergy Clin Immunol. (2017) 140:950–8. doi: 10.1016/j.jaci.2017.03.050
80. Montoro J, Del CA, Mullol J, Molina X, Bartra J, Dávila I, et al. Validation of the modified allergic rhinitis and its impact on asthma (ARIA) severity classification in allergic rhinitis children: the PEDRIAL study. Allergy. (2012) 67:1437–42. doi: 10.1111/all.12011
81. Pawankar R, Bunnag C, Chen Y, Fukuda T, Kim YY, Le LT, et al. Allergic rhinitis and its impact on asthma update (ARIA 2008)–western and Asian-Pacific perspective. Asian Pac J Allergy Immunol. (2009) 27:237–43.
82. Mullol J, Valero A, Alobid I, Bartra J, Navarro AM, Chivato T, et al. Allergic Rhinitis and its Impact on Asthma update (ARIA 2008). The perspective from Spain. J Investig Allergol Clin Immunol. (2008) 18:327–34.
83. Bousquet PJ, Combescure C, Neukirch F, Klossek JM, Méchin H, Daures JP, et al. Visual analog scales can assess the severity of rhinitis graded according to ARIA guidelines. Allergy. (2007) 62:367–72. doi: 10.1111/j.1398-9995.2006.01276.x
84. Jung S, Lee SY, Yoon J, Cho HJ, Kim YH, Suh DI, et al. Risk factors and comorbidities associated with the allergic rhinitis phenotype in children according to the ARIA classification. Allergy Asthma Immunol Res. (2020) 12:72–85. doi: 10.4168/aair.2020.12.1.72
85. Wu J, Lu AD, Zhang LP, Zuo YX, Jia YP. Chinese expert consensus on stepwise treatment of pediatric allergic rhinitis. Zhonghua Yu Fang Yi Xue Za Zhi. (2022) 56:1182–9. doi: 10.3760/cma.j.cn112150-20220613-00602
86. Papadopoulos NG, Aggelides X, Stamataki S, Prokopakis E, Katotomichelakis M, Xepapadaki P. New concepts in pediatric rhinitis. Pediatr Allergy Immunol. (2021) 32:635–46. doi: 10.1111/pai.13454
87. Rondon C, Campo P, Eguiluz-Gracia I, Plaza C, Bogas G, Galindo P, et al. Local allergic rhinitis is an independent rhinitis phenotype: The results of a 10-year follow-up study. Allergy. (2018) 73:470–8. doi: 10.1111/all.13272
88. Wise SK, Damask C, Greenhawt M, Oppenheimer J, Roland LT, Shaker MS, et al. A synopsis of guidance for allergic rhinitis diagnosis and management from ICAR 2023. J Allergy Clin Immunol Pract. (2023) 11:773–96. doi: 10.1016/j.jaip.2023.01.007
89. Teng B, Zhang X, Yi C, Zhang Y, Ye S, Wang Y, et al. The association between ambient air pollution and allergic rhinitis: further epidemiological evidence from Changchun, Northeastern China. Int J Environ Res Public Health. (2017) 14(3):226. doi: 10.3390/ijerph14030226
90. Krouse HJ. Environmental controls and avoidance measures. Int Forum Allergy Rhinol. (2014) 4 Suppl 2:S32–4. doi: 10.1002/alr.21383
91. Wu XM, Feng YJ, Zeng CR, Wang MJ. Clinical observation of nasal spray allergen blocker combined with antihistamines in treatment of allergic rhinitis children. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. (2020) 34:60–3. doi: 10.13201/j.issn.1001-1781.2020.01.015
92. Li H, Kreiner JM, Wong AR, Li M, Sun Y, Lu L, et al. Oral application of Chinese herbal medicine for allergic rhinitis: A systematic review and meta-analysis of randomized controlled trials. Phytother Res. (2021) 35:3113–29. doi: 10.1002/ptr.7037
93. Schwetz S, Olze H, Melchisedech S, Grigorov A, Latza R. Efficacy of pollen blocker cream in the treatment of allergic rhinitis. Arch Otolaryngol Head Neck Surg. (2004) 130:979–84. doi: 10.1001/archotol.130.8.979
94. Bernstein JA, Bernstein JS, Makol R, Ward S. Allergic rhinitis: A review. JAMA. (2024) 331:866–77. doi: 10.1001/jama.2024.0530
95. Nieto A, Nieto M, Mazón Á. The clinical evidence of second-generation H1-antihistamines in the treatment of allergic rhinitis and urticaria in children over 2 years with a special focus on rupatadine. Expert Opin Pharmacother. (2021) 22:511–9. doi: 10.1080/14656566.2020.1830970
96. Velentza L, Maridaki Z, Blana E, Miligkos M. Antihistamines in the management of pediatric allergic rhinitis: A systematic review. Paediatr Drugs. (2020) 22:673–83. doi: 10.1007/s40272-020-00419-x
97. Thongngarm T, Wongsa C, Phinyo P, Assanasen P, Tantilipikorn P, Sompornrattanaphan M. As-needed versus regular use of fluticasone furoate nasal spray in patients with moderate to severe, persistent, perennial allergic rhinitis: A randomized controlled trial. J Allergy Clin Immunol Pract. (2021) 9:1365–73. doi: 10.1016/j.jaip.2020.09.057
98. Hoang MP, Chitsuthipakorn W, Seresirikachorn K, Snidvongs K. As-needed intranasal corticosteroid spray for allergic rhinitis: a systematic review and meta-analysis. Rhinology. (2022) 60:242–51. doi: 10.4193/Rhin21.355
99. Debbaneh PM, Bareiss AK, Wise SK, McCoul ED. Intranasal azelastine and fluticasone as combination therapy for allergic rhinitis: systematic review and meta-analysis. Otolaryngol Head Neck Surg. (2019) 161:412–8. doi: 10.1177/0194599819841883
100. Park JS, Cho YJ, Yun JY, Lee HJ, Yu J, Yang HJ, et al. Leukotriene receptor antagonists and risk of neuropsychiatric events in children, adolescents and young adults: a self-controlled case series. Eur Respir J. (2022) 60(5):2102467. doi: 10.1183/13993003.02467-2021
101. Jutel M, Agache I, Bonini S, Burks AW, Calderon M, Canonica W, et al. International consensus on allergy immunotherapy. J Allergy Clin Immunol. (2015) 136:556–68. doi: 10.1016/j.jaci.2015.04.047
102. Vogelberg C, Klimek L, Brüggenjürgen B, Jutel M. Real-world evidence for the long-term effect of allergen immunotherapy: Current status on database-derived European studies. Allergy. (2022) 77:3584–92. doi: 10.1111/all.15506
103. Li H, Chen S, Cheng L, Guo Y, Lai H, Li Y, et al. Chinese guideline on sublingual immunotherapy for allergic rhinitis and asthma. J Thorac Dis. (2019) 11:4936–50. doi: 10.21037/jtd.2019.12.37
104. Gao Y, Lin X, Ma J, Wei X, Wang Q. Enhanced Efficacy of Dust Mite Sublingual Immunotherapy in Low-Response Allergic Rhinitis Patients after Dose Increment at 6 Months: A Prospective Study. Int Arch Allergy Immunol. (2020) 181:311–9. doi: 10.1159/000505746
105. Head K, Snidvongs K, Glew S, Scadding G, Schilder AG, Philpott C, et al. Saline irrigation for allergic rhinitis. Cochrane Database Syst Rev. (2018) 6:D12597. doi: 10.1002/14651858.CD012597.pub2
106. Yan Y, Zhang J, Liu H, Lin Z, Luo Q, Li Y, et al. Efficacy and safety of the Chinese herbal medicine Xiao-qing-long-tang for allergic rhinitis: A systematic review and meta-analysis of randomized controlled trials. J Ethnopharmacol. (2022) 297:115169. doi: 10.1016/j.jep.2022.115169
107. He M, Qin W, Qin Z, Zhao C. Acupuncture for allergic rhinitis: a systematic review and meta-analysis. Eur J Med Res. (2022) 27:58. doi: 10.1186/s40001-022-00682-3
108. Du SH, Chen S, Wang SZ, Wang GQ, Du S, Guo W, et al. Clinical practice guideline for acupuncture and moxibustion: Allergic rhinitis. J Integr Med. (2024) 22(3):245–57. doi: 10.1016/j.joim.2024.03.009
109. Aguiar M, Trujillo M, Chaves D, Álvarez R, Epelde G. mHealth apps using behavior change techniques to self-report data: systematic review. JMIR Mhealth Uhealth. (2022) 10:e33247. doi: 10.2196/33247
110. Bousquet J, Hellings PW, Agache I, Amat F, Annesi-Maesano I, Ansotegui IJ, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) Phase 4 (2018): Change management in allergic rhinitis and asthma multimorbidity using mobile technology. J Allergy Clin Immunol. (2019) 143:864–79. doi: 10.1016/j.jaci.2018.08.049
111. Bousquet J, Devillier P, Arnavielhe S, Bedbrook A, Alexis-Alexandre G, van Eerd M, et al. Treatment of allergic rhinitis using mobile technology with real-world data: The MASK observational pilot study. Allergy. (2018) 73:1763–74. doi: 10.1111/all.13406
112. Bédard A, Basagaña X, Anto JM, Garcia-Aymerich J, Devillier P, Arnavielhe S, et al. Mobile technology offers novel insights into the control and treatment of allergic rhinitis: The MASK study. J Allergy Clin Immunol. (2019) 144:135–43. doi: 10.1016/j.jaci.2019.01.053
113. Menditto E, Costa E, Midão L, Bosnic-Anticevich S, Novellino E, Bialek S, et al. Adherence to treatment in allergic rhinitis using mobile technology. MASK Study Clin Exp Allergy. (2019) 49:442–60. doi: 10.1111/cea.13333
114. Bousquet J, Anto JM, Bachert C, Haahtela T, Zuberbier T, Czarlewski W, et al. ARIA digital anamorphosis: Digital transformation of health and care in airway diseases from research to practice. Allergy. (2021) 76:168–90. doi: 10.1111/all.14422
Keywords: allergic rhinitis, children, allergen immunotherapy, health management, trained immunity
Citation: Cheng M, Dai Q, Liu Z, Wang Y and Zhou C (2024) New progress in pediatric allergic rhinitis. Front. Immunol. 15:1452410. doi: 10.3389/fimmu.2024.1452410
Received: 20 June 2024; Accepted: 29 August 2024;
Published: 16 September 2024.
Edited by:
Panagiotis Skendros, Democritus University of Thrace, GreeceReviewed by:
Yashwant Kumar, Post Graduate Institute of Medical Education and Research (PGIMER), IndiaHontian Wang, Capital Medical University, China
Copyright © 2024 Cheng, Dai, Liu, Wang and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cuiyun Zhou, am15eXpob3VjeUAxNjMuY29t; Yulin Wang, MTY2NDY0NzNAcXEuY29t
†These authors have contributed equally to this work and share first authorship
 Miao Cheng
Miao Cheng Qianqian Dai
Qianqian Dai Zhi Liu
Zhi Liu Yulin Wang
Yulin Wang Cuiyun Zhou
Cuiyun Zhou