- 1Department of Medical Oncology, Cancer Center, West China Hospital, Sichuan University, Chengdu, China
- 2Division of Abdominal Tumor Multimodality Treatment, Cancer Center, West China Hospital, Sichuan University, Chengdu, China
- 3Jiangsu Hengrui Pharmaceuticals Co., Ltd, Shanghai, China
Background: The third-line treatment for refractory colorectal cancer (CRC) has limited efficacy. This study aimed to evaluate the safety and efficacy of SHR-8068 (an anti-CTLA-4 antibody), combined with adebrelimab (an anti-PD-L1 antibody), and bevacizumab in refractory non-microsatellite instability-high (MSI-H) or proficient mismatch repair (pMMR) CRC.
Method: This study is a prospective, open-label, single-center phase Ib/II clinical trial. Patients with pathologically confirmed pMMR/non-MSI-H metastatic colorectal adenocarcinoma who have failed ≥2 lines prior standard systemic treatments will be enrolled (n=36). The Ib phase will evaluate two dosing regimens of SHR-8068 in combination therapy (n=9 each dosage): SHR-8068 (1 mg per kilogram, every six weeks, intravenously) or SHR-8068 (4 mg per kilogram, every twelve weeks, intravenously) combined with adebrelimab (1200 mg, every three weeks, intravenously) and bevacizumab (7.5 mg per kilogram, every three weeks, intravenously). The efficacy and adverse events (AEs) of these regimens will be assessed to determine the recommended phase II dose (RP2D) of SHR-8068. Those of RP2D group from the phase Ib will be included in the phase II. The study will go to include 18 additional patients according to the one-sample log-rank test design in the phase II. The primary endpoint of the Ib phase is safety, with secondary endpoints including the objective response rate (ORR), progression-free survival (PFS), overall survival (OS), disease control rate (DCR), and quality of life (QOL). The primary endpoint for phase II was PFS, with secondary endpoints including ORR, OS, DCR, safety, and QOL. Identifying biomarkers to predict the efficacy of this regimen is the exploratory study endpoint.
Discussion: This proof-of-concept study would provide safety and efficacy signals of this novel combination treatment for the MSS CRCs in the late-line setting. And it may offer new insights on the clinical application of dual immunotherapy combined with anti-angiogenic therapy in the MSS CRC.
1 Background
Colorectal cancer (CRC) ranks third in incidence and second in mortality among malignancies worldwide, with approximately 30-40% of patients eventually progressing to unresectable advanced stages (1). Current guidelines recommend chemotherapy with or without targeted agents as standard first- and second-line treatments for microsatellite stable (MSS) or proficient mismatch repair (pMMR) advanced CRC. For patients who fail second-line therapy, regorafenib, fruquintinib, or TAS-102 have shown positive results compared to placebo in clinical trials (2–4), but their efficacy is limited, with objective response rates (ORRs) less than 5% and a median overall survival (OS) of only 1.4-2.8 months, coupled with high adverse event rates. Thus, new treatment strategies are urgently needed to improve the prognosis of refractory CRC patients.
Recent evidence has shown that immunotherapy is highly effective in patients with advanced dMMR/MSI-H CRC. However, dMMR/MSI-H CRC accounts for only approximately 5% of advanced cases, limiting the application of immune checkpoint inhibitors. In patients with advanced-stage HCC or RCC, ICI doublet [typically, anti-PD-1 plus anti-CTLA4 antibodies (5, 6)] or triplet combinations [ICI doublet plus an antiangiogenic agent, such as ipilimumab plus nivolumab and cabozantinib (7)] tend to provide a more prolonged duration of response and a higher likelihood of a prolonged OS relative to antiangiogenic-ICI combinations. Anti-CTLA-4, and anti-PD-(L)1 antibodies act differentially on the immune cells (8). Programmed death-ligand 1 (PD-L1, B7-H1, CD274) expressed by tumors interacts with PD-1 on T cells to suppress T-cell effector function. CTLA-4 is a receptor on activated T lymphocytes that contains the natural ligands B7.1 and B7.2. These ligands bind to CTLA-4, releasing inhibitory signals to activated T lymphocytes and limiting their proliferation. Combination of anti-CTLA-4 and anti-PD-(L)1 therapy may prompt a stronger anti-tumor immune response leading tumor growth inhibition. Preclinical study has shown that dual CTLA-4 and PD-L1 blockade inhibits tumor growth in the microsatellite stable highly aggressive orthotopic mouse model of colon cancer due to anti-tumorigenic T cell responses mediated by CTLA-4 inhibition and M1 macrophage polarization predominantly induced by PD-L1 blockade (9). CCTG CO.26 trial of durvalumab (D) in combination with tremelimumab (T) did not show good responses in patients with advanced refractory colorectal cancer MSS type, while OS was prolonged by 2.5 months in the D+T group relative to the best-support group (10).
MSS tumors typically experience a hypoxic state, which would upregulate VEGF gene transcription and mRNA stability by stabilizing and activating hypoxia-inducible factors (11). Abnormal vasculature and elevated vascular endothelial growth factor levels lead to immunosuppression, inhibit cytotoxic T-lymphocyte (CTL) function and antigen presentation, and promote aggregation of immunosuppressive cells (12–14). Combining antiangiogenic agents with immune checkpoint inhibitors (ICIs) can potentially normalize the TME, enhancing T-cell infiltration and activity. A study combining nivolumab, plus the CTLA-4 inhibitor ipilimumab, and regorafenib, in refractory MSS CRC showed promising results in chemotherapy-refractory MSS CRC patients, with an ORR of 27.6%, a DCR of 68.2%, and a median PFS and OS of 4 and 20 months, respectively, in 29 patients (15).
SHR-8068 is an anti-CTLA-4 antibody in the clinical research phase. Adebrelimab is a high-affinity, humanized IgG4 monoclonal antibody against PD-L1.Bevacizumab (Bev) is the first approved anti-angiogenic drug and has shown synergistic effects with immune checkpoint inhibitors in the treatment of melanoma and metastatic renal cell carcinoma in previous preclinical studies (16, 17). Herein, we plan to conduct a prospective study to evaluate the safety and efficacy of SHR-8068 combined with adebrelimab and bevacizumab in advanced CRC patients to inform clinical practice.
In this study, the administration and dose selection of SHR-8068, adebrelimab, and bevacizumab were based on the previous clinical trial and authoritative guidelines. The doses for the combination of SHR-8068 (1 mg/kg every six weeks or 4 mg/kg every twelve weeks) and adebrelimab (1200 mg every three weeks) refer to the clinical trial in liver cancer (NCT05444088, data not yet public). The dose of bevacizumab (7.5 mg/kg every three weeks) was according to the 2024 NCCN Colorectal Cancer Treatment Guidelines, which endorse this regimen as safe, controlled, and effective in colorectal cancer treatment (18).
This proof-of-concept study aims to provide signals and generate preliminary evidence whether the dual immunotherapy with antiangiogenic therapy (SHR-8068 combined with adebrelimab and bevacizumab) is efficacious and well-tolerated for patients with MSS mCRC in the late-line setting.
2 Methods and analysis
This study was approved by the Biomedical Ethics Committee of West China Hospital, Sichuan University. The trial is registered on the National Clinical Trial Registry (NCT06373133). The study will be conducted in West China Hospital, Sichuan University. All participants will provide written informed consent. The trial protocol and this manuscript were developed in accordance with the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) guidelines (Figure 1).
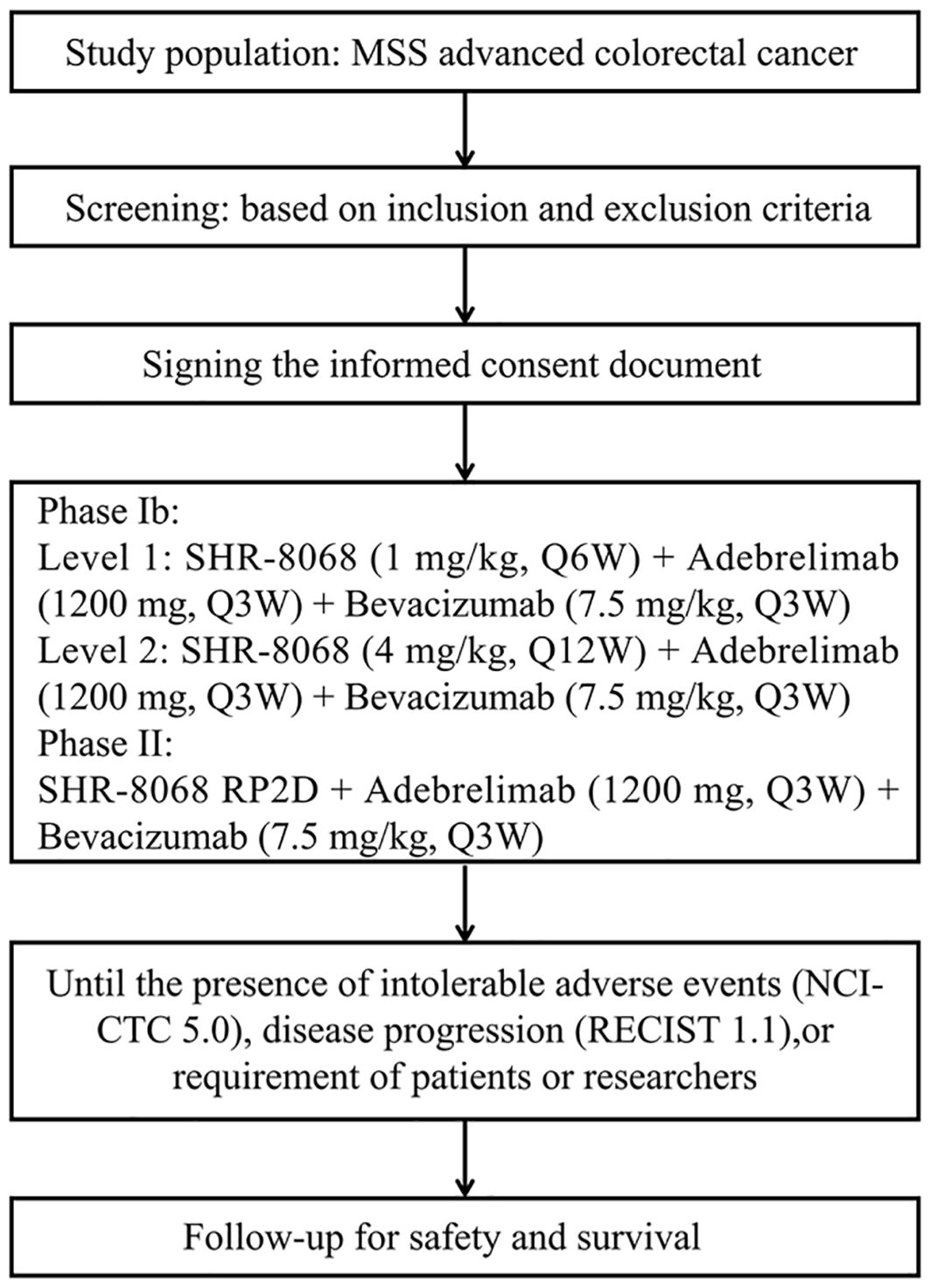
Figure 1. The SPIRIT flow diagram of this trial. MSS, microsatellite stable; RECIST, Response Evaluation Criteria in Solid Tumors; NCI-CTC, National Cancer Institute Common Toxicity Criteria.
2.1 Study objectives
This study is a prospective, open-label, single-center, Ib/II phase clinical trial aimed at evaluating the safety and efficacy of the anti-CTLA-4 antibody SHR-8068 combined with the anti-PD-L1 antibodies adebrelimab and bevacizumab in patients with advanced refractory CRC.
This study consisted of two phases. Phase Ib focused on assessing the tolerability and safety of SHR-8068 combined with adebrelimab and bevacizumab in patients with advanced refractory CRC. Phase II evaluated the efficacy and safety of combination therapy in patients with advanced refractory CRC.
Phase Ib
• Primary endpoints:
Incidence of DLTs, TRAEs, and various grades of AEs (based on NCI-CTCAE v5.0)
• Secondary endpoints:
ORR based on RECIST 1.1, PFS, OS, DCR, and QOL scores
Phase II
• Primary endpoint:
PFS
• Secondary endpoints:
ORR based on RECIST 1.1, OS, DCR, safety, and QOL scores
2.2 Study design
This study includes two phases (Figure 2). The phase Ib of the study aims to determine the safety of the triple-combination therapy and identify the RP2D of SHR-8068. Initially, 9 participants will receive adebrelimab (1200 mg, every three weeks, intravenously) plus SHR-8068 (1 mg/kg, every six weeks, intravenously) plus bevacizumab (7.5 mg/kg, every three weeks, intravenously). The tolerability observation period was 21 ± 3 days. If the regimen is intolerable (dose-limiting toxicity (DLT) incidence ≥ 33.3%), the researchers will assess whether to adjust the dosage, or frequency of administration, or terminate the study. If tolerable (DLT incidence less than 33.3%), another nine participants will receive adebrelimab (1200 mg, every three weeks, intravenously) plus SHR-8068 (4 mg per kilogram, every twelve weeks, intravenously) plus bevacizumab (7.5 mg per kilogram, every three weeks, intravenously). After completing the tolerability observation (21 ± 3 days), if this dose is intolerable (DLT incidence ≥ 33.3%), the RP2D of SHR-8068 will be determined to be 1 mg/kg, Q6W. If tolerable (DLT incidence < 33.3%), the efficacy and adverse event (AE) profiles of both dose groups will be comprehensively evaluated to determine the RP2D of SHR-8068 for continuation into phase II.
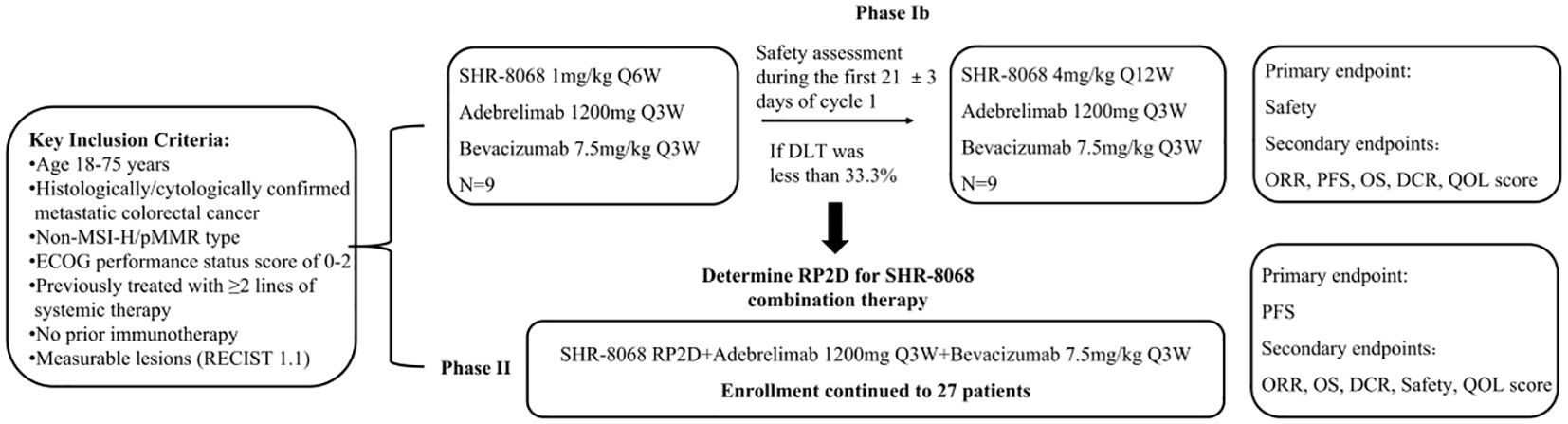
Figure 2. A technical roadmap for clinical trials. MSI-H, microsatellite instability-high; pMMR, proficient mismatch repair; ECOG, Eastern Cooperative Oncology Group; DLT, dose-limiting toxicity; RP2D, recommended phase 2 dose; ORR, objective response rate; PFS, progression-free survival; OS, overall survival; DCR, disease control rate; QOL, quality of life.
The phase II will assess the efficacy of the combination with RP2D on 27 patients (details in 2.5 sample size), including those of RP2D group from the phase Ib.
The treatment will continue until disease progression (PD), intolerable adverse events (AEs), as required by patients and researchers, or up to 24 months.
2.3 Eligibility and enrollment
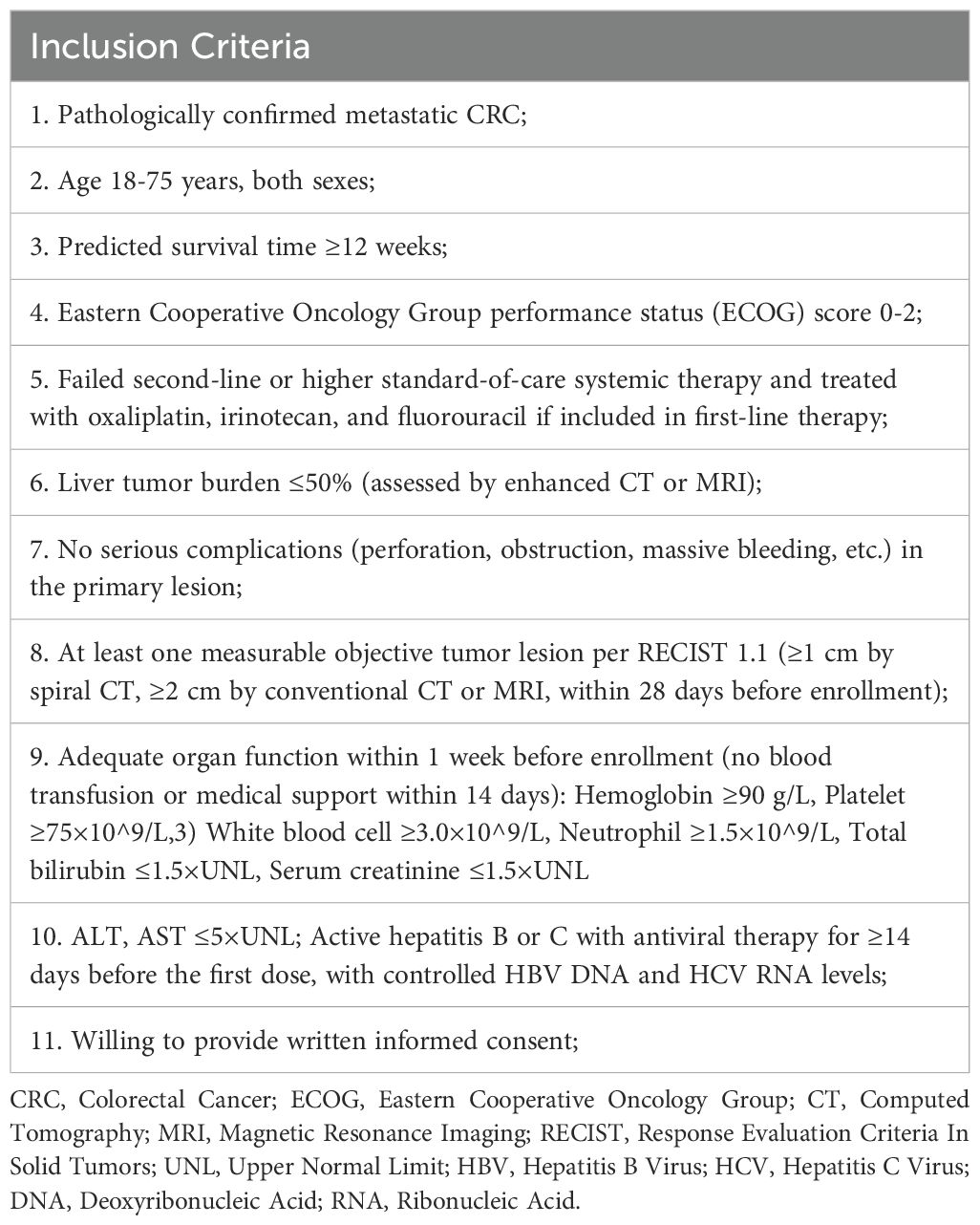
Patients diagnosed with non-MSI-H/pMMR metastatic colorectal cancer, and failure of at least two lines of standard systemic therapy will be enrolled in this study. Further key inclusion criteria are 1) 18 to 75 years old; 2) an ECOG performance status of 0-2; 3) a predicted survival of at least 12 weeks; 4) at least one measurable tumor lesion. Major exclusion criteria include 1) a history of other malignancies within the past five years; 2) known CNS metastases; 3) severe cardiovascular conditions; 4) the presence of uncontrolled complications; 5) significant autoimmune diseases or immunodeficiency syndrome. Details of inclusion and exclusion criteria are listed in Table 1 and Table 2.
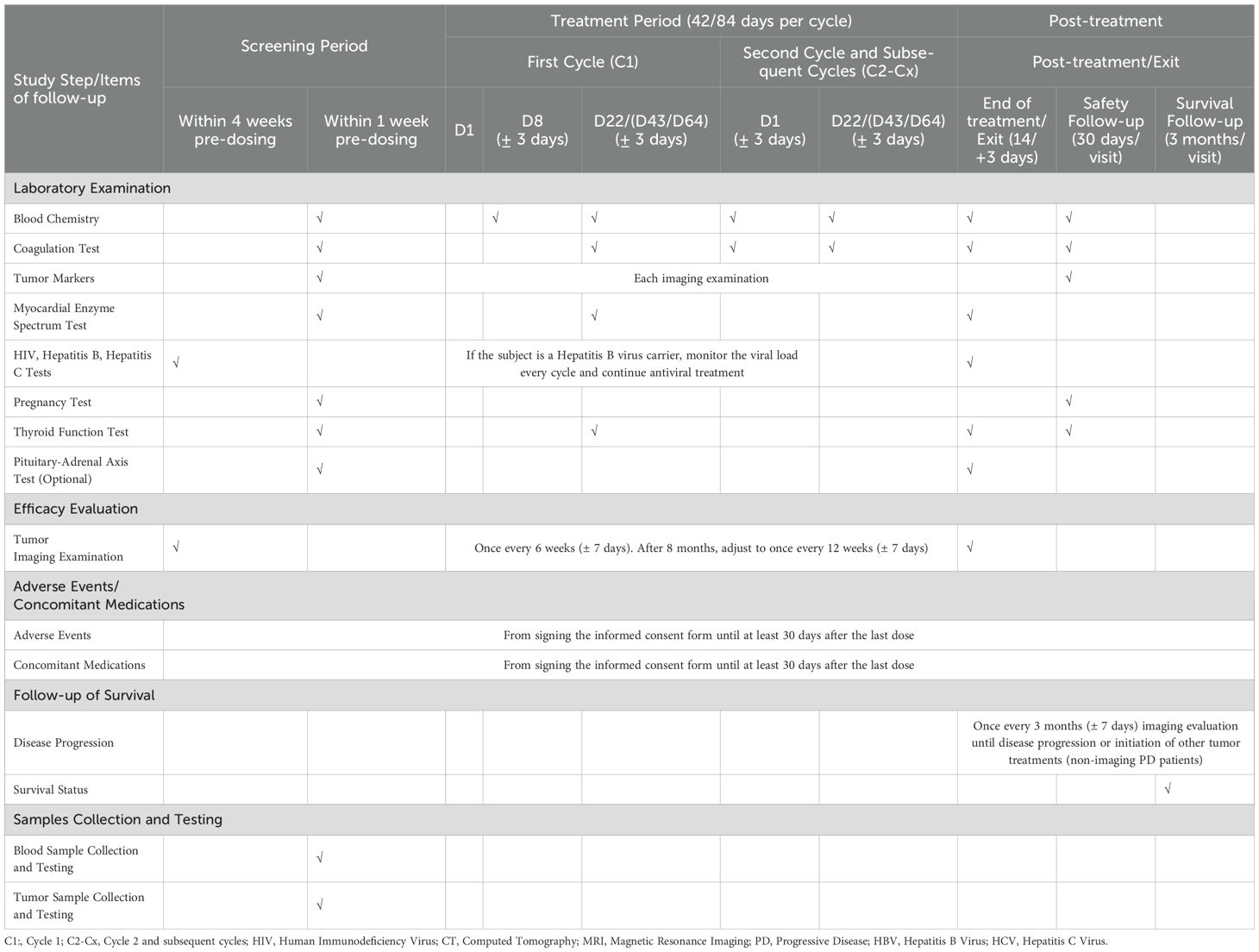
Information collection and medical examination including demographic data, tumor history, medical history, quality of life scores, tumor imaging, echocardiography, HIV/HBV/HCV tests, drug allergy history, ECOG score, physical examination, vital signs, ECG, complete blood count, urinalysis, stool analysis, blood biochemistry, coagulation function, cardiac enzyme profile, pregnancy test for female patients, and thyroid function test are required to undergo during the screening period (Table 3).
Patients are included after giving written informed consent and finally enrolled in the clinical trial when the inclusion and exclusion criteria are met. Qualified study personnel will provide patients with comprehensive information regarding the study’s purpose, procedures, potential risks, benefits, and the rights of patients. The informed consent process will comply with IRB guidelines and local regulations to protect patient rights and privacy.
2.4 Analysis and evaluation
2.4.1 MSI/MMR analysis criteria
Tumor MSI/MMR was assessed per polymerase chain reaction (PCR) and immunohistochemistry (IHC), respectively, prior to screening.
According to the recommendations of National Cancer Institute (19), MSI was identified by the panel of five microsatellites markers: BAT-25, BAT-26, D2S123, D5S346 and D17S250. MSI-H is reported if two or more markers show instability (i.e., have insertion/deletion mutations), MSI-L is reported if only one marker shows instability, and MSS indicates that no markers were positive.
IHC for four MMR proteins (MLH1, PMS2, MSH2 and MSH6) was performed to identify MMR status. pMMR (proficient MMR) was defined as all four MMR proteins present. And if one or more of four proteins are absent, it is classified as dMMR (deficient MMR). The processed IHC slides were evaluated by two pathologists.
The microsatellites status assessed with PCR-based testing and MMR status evaluated by IHC are generally highly consistent (20).
2.4.2 Efficacy and toxicity assessment procedures
Evaluations of tumor response to treatment utilize images from MRI or contrast-enhanced (preferred) CT will be performed every 6 weeks (adjusted to every 12 weeks after 8 months) according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 until disease progression or intolerable toxicity. The first tumor evaluation should be conducted at least 6 weeks after the first dose. For patients showing initial radiological PD requires confirmation with repeat imaging after ≥4 weeks if patients are clinically stable to account for the possibility of pseudo-progression.
Regarding to the safety assessments, treatment toxicity will be evaluated at each study visit and AEs will be reported according to the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0. In the first medication cycle, ECOG score, physical examination, vital signs, ECG, complete blood count, urinalysis, blood biochemistry will be taken on D8, D22(for 6-week regimen), D43, and D64 (for 12-week regimen). From the second cycle onward, routine examinations (vital signs, ECG, complete blood count, urinalysis, stool analysis, blood biochemistry, coagulation function) will be conducted on D1, D22(for 6-week regimen), D43, and D64 (for 12-week regimen). Upon treatment termination, a comprehensive assessment will be performed, and the reasons for discontinuation will be recorded. Serious AEs (SAEs) occurring during treatment or until 90 days after the last dose of treatment should be recorded and reported immediately and not exceeding 24h after the knowledge of SAEs.
For patients with disease progression, survival status should be assessed every 3 months after treatment discontinuation. Survival data will be calculated.
A more detailed study schedule is shown in Table 3.
2.4.3 Biomarker exploration
Patients are asked to consent to allow access to any available archival tumor tissue previously obtained. And blood samples will be collected at baseline (pre-treatment). Tumor tissue and blood sample will undergo next-generation sequencing (NGS) to explore potential predictive markers for response to the therapy.
2.5 Sample size
This study consists of two phases. In phase Ib, two dose groups will enroll 9 participants each to determine the RP2D of SHR-8068 in combination therapy. Participants in the RP2D dose group from phase Ib will continue into phase II.
Phase II employs a one-sample log-rank test design. Based on the results of three phase III trials of third-line standard treatments for colorectal cancer (CORRECT, FRESCO, and TERRA studies), the null hypothesis is the median PFS of 2 months. And the expected median PFS is assumed to be 4 months. With M0 = 2, M1 = 4, a power of 0.9, and a one-sided α of 0.05, the sample size is estimated using the one-sample log-rank test in PASS. With an enrollment period of 12 months and a follow-up period of 12 months, a total of 24 participants were needed. Considering a 10% dropout rate, the total number of participants enrolled should be 27.
The study will last a total of 2.5 years, with participant enrollment occurring in the first year.
2.6 Dose modifications
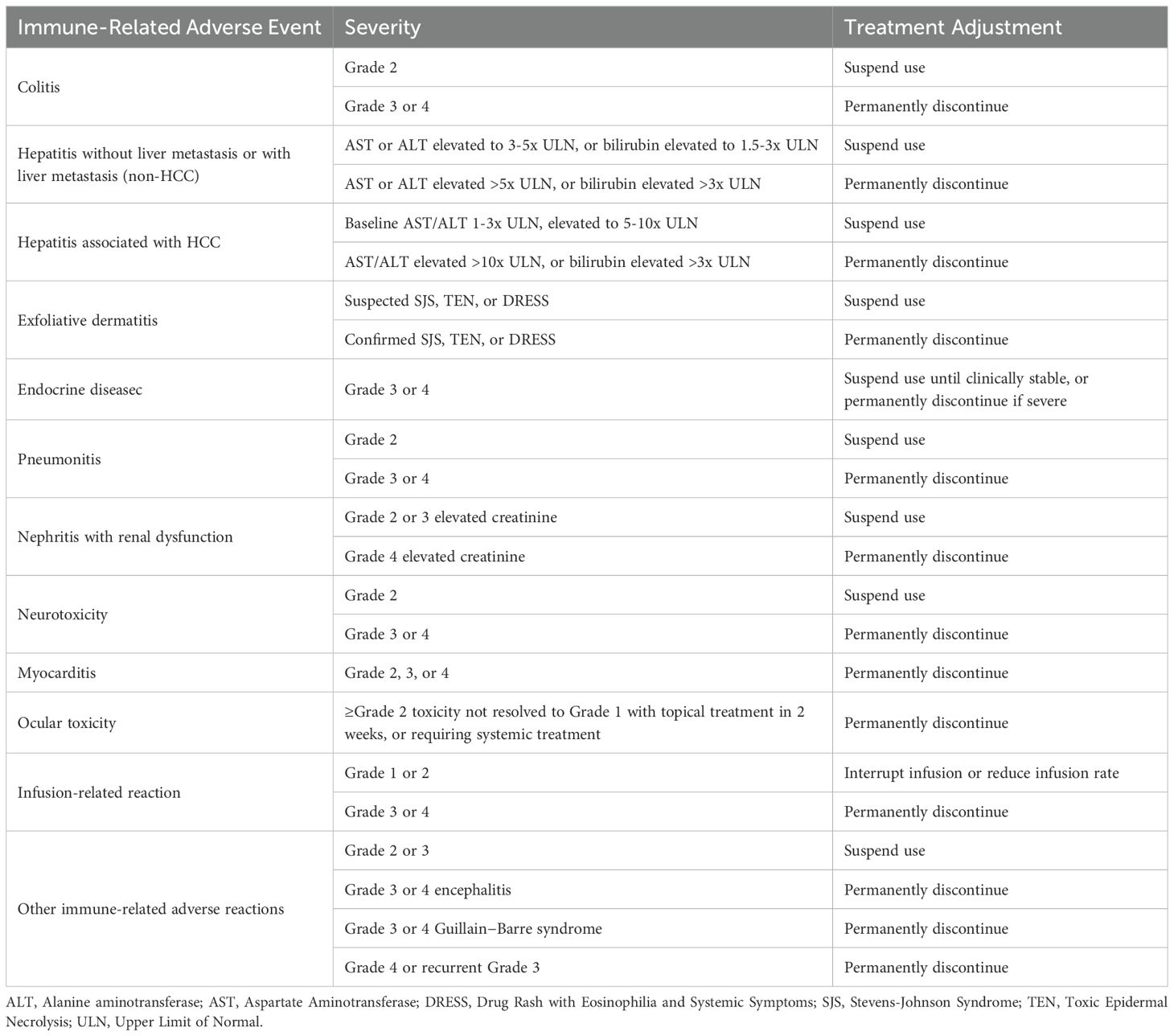
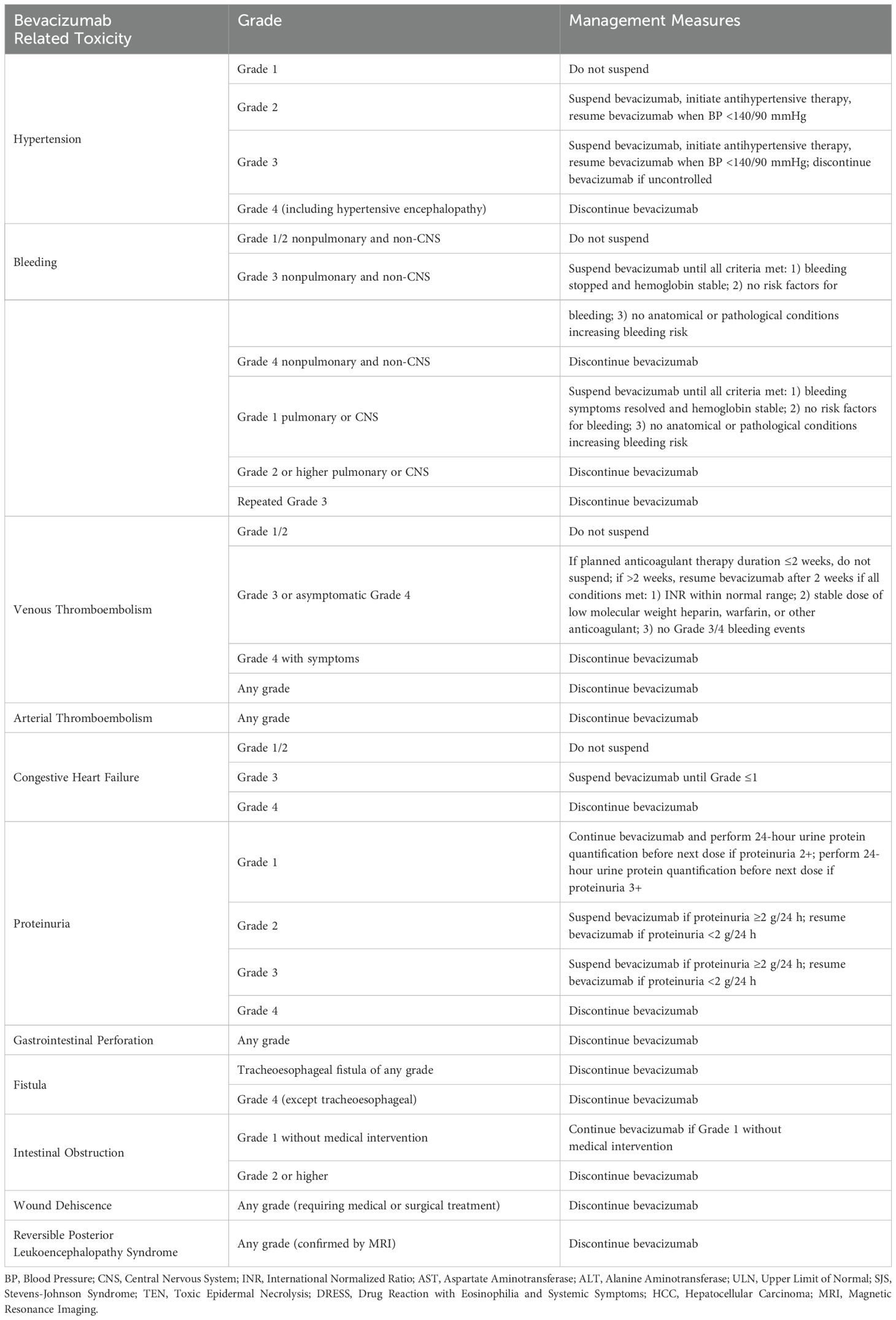
During the study, dose adjustments for adebrelimab and SHR-8068 were not allowed, and only interruptions were permitted, with a maximum of 12 weeks (Table 4). If a serious adverse event or grade 3/4 nonserious adverse event related to bevacizumab occurs, it should be suspended. Bevacizumab can be resumed if the event resolves to grade 1 and is deemed beneficial to the patient. If toxicity delays treatment for more than 6 weeks, bevacizumab should be discontinued (Table 5).
After a total of 35 doses of adebrelimab, and/or after 18 doses of SR-8068 (1mg/kg, Q6W)/9 doses of SHR-8068(4mg/kg, Q12W), treatment should be terminated. If SHR-8068 is terminated prematurely due to toxic intolerance, adebrelimab alone or in combination with bevacizumab may be considered. However, if adebrelimab is discontinued due to toxic intolerance or other reasons, SHR-8068 and bevacizumab therapy should be terminated.
2.7 DLT determination
During the tolerability observation period (21 ± 3 days), any event judged by the investigator to be related to the study drug (grading criteria based on NCI-CTCAE 5.0) was considered as a DLT if it met any of the following criteria:
1. Grade 4 hematologic toxicity (excluding grade 4 lymphocyte count reduction unless accompanied by opportunistic infection).
2. Grade ≥3 thrombocytopenia with bleeding.
3. Grade ≥3 neutropenia with fever (defined as absolute neutrophil count [ANC]<1000/mm³ with a single temperature of 38.3°C or a sustained temperature of 38°C for more than 1 hour).
4. Grade ≥3 nonhematologic toxicity (excluding abnormal laboratory test results), with the following exceptions: fatigue/asthenia improving to≤grade 2 within 3 days; untreated grade ≥3 diarrhea, nausea, or vomiting; untreated grade ≥3 rash not managed with corticosteroids or anti-inflammatory drugs.
5. Grade ≥3 nonhematologic laboratory abnormalities requiring medical intervention, leading to hospitalization, or persisting for ≥7 days.
6. Drug-related toxicity causing a delay in the administration of adebrelimab or SHR-8068 for >7 days.
7. Drug-related toxicity leading to the discontinuation of treatment during the DLT observation period.
2.8 Statistics
This study will follow the ITT principle, analyzing all participants who signed informed consent. The analysis populations included the FAS, PPS, and SAS. Efficacy analyses will be performed in the FAS and PPS populations, while safety analyses will be performed in SAS.
Baseline characteristics will be described using the mean ± SD for continuous variables and frequency (percentage) for categorical variables. Survival data will be analyzed using the Kaplan−Meier method to estimate PFS and OS curves, with medians and 95% CIs calculated. The ORR and DCR will be descriptively analyzed, with 95% CIs calculated using the Clopper–Pearson method. The incidence of AEs, treatment-related AEs, SAEs, and AEs leading to study withdrawal will be summarized.
Post-hoc analysis of objective response, PFS, and OS across different groups will be performed. Univariate and Cox multivariate analyses will be used to assess the impact of baseline characteristics on PFS and OS.
All the statistical analyses will be conducted using SPSS version 22 or above.
3 Discussion
Considering that the current therapies and trialed regiments for patient with mCRC obtained unsatisfactory survival outcomes (12, 21, 22), seeking further effective treatment strategy to extend life is the urgent requirement. Herein, we have a study design to evaluate the safety and efficacy of the dual-immunology therapy (CTLA-4 inhibitor, SHR-8068 and anti-PD-L1 antibody, adebrelimab) combined with antiangiogenic agent (bevacizumab) in late-line line for advanced CRC patients.
Previously, the REGONIVO study conducted by Japanese research team has demonstrated the potential of combining anti-angiogenic drug(regorafenib) with PD-1 inhibitor (nivolumab) for the treatment of MSS CRC, which achieved an ORR of 36%, and a median PFS of 7.9 months (12). However, efficacy of this combination in the North American population did not reproduce findings in the Japanese population, obtaining an ORR of 7.1% (23). It is consistent with other similar combination regiment and some real-world studies showing disappointing results (13, 14, 24). These findings put a mist over the treatment of immunotherapy with anti-angiogenic agent for MSS CRCs. The RIN trial took the first step to adopt a combination of dual-immunotherapy (nivolumab plus ipilimumab) with regorafenib in refractory CRCs (15). And it shows promising results with an ORR of 27.6% and a median PFS of 4 months in the total population. However, like previous similar studies mentioned above, the regiment brings limited benefit to patients with liver metastasis. Regrettably, due to the data of the baseline liver tumor burden not released, whether the combination of immunotherapy with anti-angiogenic therapy is only effective for CRCs without liver metastasis is unclear. In the current study, we limit the liver tumor burden in the enrolled patients to less than 50% (assessed by enhanced CT or MRI). This specific inclusion criterion is in the consideration of avoiding enrolling patients with large hepatic tumor loads, who are highly unlikely to derive clinical benefit from the study treatment based on data from previous studies, and to further identify whether this dual-immunotherapy plus anti-angiogenic agent could bring clinical benefit in the CRC patients with low hepatic tumor loads.
Besides the efficacy, toxicity is the most important factor to consider for the third- or later-line therapy choice. Immune-related adverse events typically involve the skin, gastrointestinal, hepatic, and endocrine systems (25). A meta-analysis has reported that the overall AE rate of monotherapy with PD-1 antibody in pMMR CRCs were 54% (26). While all patients enrolled in the CCTG CO.26 trial occurred therapeutic toxicity after receiving durvalumab with tremelimumab, and 64% of patients experienced ≥ Grade 3 AEs. The most common toxicities above 3 degrees were fatigue and abdominal pain (10). Increases in CTLA-4 inhibitor dose/exposure may lead to higher incidence of Grade 3/4 TRAEs, hepatic irAEs, and gastrointestinal irAEs (27). As for the anti-angiogenic agent, common adverse events associated with bevacizumab includes hypertension, thromboembolism, hemorrhage, and proteinuria (28). A meta-analysis shows that the relative risks for bevacizumab were relatively low in comparison to regorafenib, in the case of diarrhea and febrile neutropenia (29). While it should be noticed that bevacizumab may increase the risk of upper gastrointestinal bleeding and colitis when combined with more than one ICI (30). Individuals who are more vulnerable to the risks of the treatment should be excluded per the protocol. Meanwhile, a comprehensive safety monitoring would be conducted in this study to ensure the safety of patients and receive timely treatment.
In order to ensure the safety and maximize the benefits of the combinations for patients, we implemented a safety lead-in phase for two doses. We will take both safety and efficacy data into account to select the RP2D for Phase II expansion, rather than exploring only a fixed dose. Moreover, to identify the survival benefit signal of the treatment, the phase II employs a one-sample log-rank test design based on the primary endpoint of PFS. It was used to determine whether the novel combination of SHR-8068 plus adebrelimab and bevacizumab should be considered for further testing. The one-sample log-rank test is the method of choice for single-arm Phase II trials with time-to-event endpoint. It allows to compare the survival of the patients to a reference survival curve that typically represents the expected survival under standard of care (31). Further larger randomized clinical trial would be needed (dual-immunotherapy + Bev vs. PD-L1 antibody + Bev vs. BSC) in the future if the primary endpoint of this study was met.
This study would offer more compelling evidence on the clinical application of dual immunotherapy combined with anti-angiogenic therapy in the MSS CRC with or without liver metastasis. Additionally, biomarker exploration in the study would give deeper understanding of the dual-immunotherapy combined with anti-angiogenic drug in CRCs, and provide better insights in predictive markers for response to the therapy.
Author contributions
PZ: Writing – original draft, Writing – review & editing. XL: Writing – original draft, Writing – review & editing. XW: Writing – original draft, Writing – review & editing. YY: Writing – original draft. JW: Writing – review & editing. DC: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was sponsored by the 1·3·5 project for disciplines of excellence-Clinical Research Incubation Project, West China Hospital, Sichuan University (ZYJC21016).
Conflict of interest
Author JW was employed by Jiangsu Hengrui Pharmaceuticals Co., Ltd. JW has no role in the study design and interpretation of results.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Mayer RJ, Van Cutsem E, Falcone A, Yoshino T, Garcia-Carbonero R, Mizunuma N, et al. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med. (2015) 372:1909–19. doi: 10.1056/NEJMoa1414325
3. Li J, Qin S, Xu RH, Shen L, Xu J, Bai Y, et al. Effect of fruquintinib vs placebo on overall survival in patients with previously treated metastatic colorectal cancer: the FRESCO randomized clinical trial. Jama. (2018) 319:2486–96. doi: 10.1001/jama.2018.7855
4. Tabernero J, Lenz HJ, Siena S, Sobrero A, Falcone A, Ychou M, et al. Analysis of circulating DNA and protein biomarkers to predict the clinical activity of regorafenib and assess prognosis in patients with metastatic colorectal cancer: a retrospective, exploratory analysis of the CORRECT trial. Lancet Oncol. (2015) 16:937–48. doi: 10.1016/S1470-2045(15)00138-2
5. Yau T, Kang YK, Kim TY, El-Khoueiry AB, Santoro A, Sangro B, et al. Efficacy and safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib: the checkMate 040 randomized clinical trial. JAMA Oncol. (2020) 6:e204564. doi: 10.1001/jamaoncol.2020.4564
6. Albiges L, Tannir NM, Burotto M, McDermott D, Plimack ER, Barthélémy P, et al. Nivolumab plus ipilimumab versus sunitinib for first-line treatment of advanced renal cell carcinoma: extended 4-year follow-up of the phase III CheckMate 214 trial. ESMO Open. (2020) 5:e001079. doi: 10.1136/esmoopen-2020-001079
7. Yau T, Zagonel V, Santoro A, Acosta-Rivera M, Choo SP, Matilla A, et al. Nivolumab plus cabozantinib with or without ipilimumab for advanced hepatocellular carcinoma: results from cohort 6 of the checkMate 040 trial. J Clin Oncol. (2023) 41:1747–57. doi: 10.1200/JCO.22.00972
8. Twyman-Saint Victor C, Rech AJ, Maity A, Rengan R, Pauken KE, Stelekati E, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. (2015) 520:373–7. doi: 10.1038/nature14292
9. Fiegle E, Doleschel D, Koletnik S, Rix A, Weiskirchen R, Borkham-Kamphorst E, et al. Dual CTLA-4 and PD-L1 blockade inhibits tumor growth and liver metastasis in a highly aggressive orthotopic mouse model of colon cancer. Neoplasia. (2019) 21:932–44. doi: 10.1016/j.neo.2019.07.006
10. Chen EX, Jonker DJ, Loree JM, Kennecke HF, Berry SR, Couture F, et al. Effect of combined immune checkpoint inhibition vs best supportive care alone in patients with advanced colorectal cancer: the Canadian Cancer Trials Group CO. 26 Study. JAMA Oncol. (2020) 6:831–8. doi: 10.1001/jamaoncol.2020.0910
11. Wang F, Xu P, Xie KC, Chen XF, Li CY, Huang Q. Effects of tumor microenviromental factors on VEGF expression. Biomed Rep. (2013) 1:539–44. doi: 10.3892/br.2013.115
12. Fukuoka S, Hara H, Takahashi N, Kojima T, Kawazoe A, Asayama M, et al. Regorafenib plus nivolumab in patients with advanced gastric or colorectal cancer: an open-label, dose-escalation, and dose-expansion phase ib trial (REGONIVO, EPOC1603). J Clin Oncol. (2020) 38:2053–61. doi: 10.1200/JCO.19.03296
13. Fakih M, Raghav KPS, Chang DZ, Larson T, Cohn AL, Huyck TK, et al. Regorafenib plus nivolumab in patients with mismatch repair-proficient/microsatellite stable metastatic colorectal cancer: a single-arm, open-label, multicentre phase 2 study. EClinicalMedicine. (2023) 58:101917. doi: 10.1016/j.eclinm.2023.101917
14. Kim RD, Kovari BP, Martinez M, Xie H, Sahin IH, Mehta R, et al. A phase I/Ib study of regorafenib and nivolumab in mismatch repair proficient advanced refractory colorectal cancer. Eur J Cancer. (2022) 169:93–102. doi: 10.1016/j.ejca.2022.03.026
15. Fakih M, Sandhu J, Lim D, Li X, Li S, Wang C. Regorafenib, ipilimumab, and nivolumab for patients with microsatellite stable colorectal cancer and disease progression with prior chemotherapy: A phase 1 nonrandomized clinical trial. JAMA Oncol. (2023) 9:627–34. doi: 10.1001/jamaoncol.2022.7845
16. Wu X, Giobbie-Hurder A, Liao X, Lawrence D, McDermott D, Zhou J, et al. VEGF neutralization plus CTLA-4 blockade alters soluble and cellular factors associated with enhancing lymphocyte infiltration and humoral recognition in melanoma. Cancer Immunol Res. (2016) 4:858–68. doi: 10.1158/2326-6066.CIR-16-0084
17. Wallin JJ, Bendell JC, Funke R, Sznol M, Korski K, Jones S, et al. Atezolizumab in combination with bevacizumab enhances antigen-specific T-cell migration in metastatic renal cell carcinoma. Nat Commun. (2016) 7:12624. doi: 10.1038/ncomms12624
19. Boland CR, Thibodeau SN, Hamilton SR, Sidransky D, Eshleman JR, Burt RW, et al. A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. (1998) 58:5248–57.
20. Shimozaki K, Hayashi H, Tanishima S, Horie S, Chida A, Tsugaru K, et al. Concordance analysis of microsatellite instability status between polymerase chain reaction based testing and next generation sequencing for solid tumors. Sci Rep. (2021) 11:20003. doi: 10.1038/s41598-021-99364-z
21. Gomez-Roca CA, Yanez E, Im S-A, Alvarez EC, Senellart H, Doherty M, et al. LEAP-005: A phase 2 multicohort study of lenvatinib plus pembrolizumab in patients with previously treated selected solid tumors—Results from the colorectal cancer cohort. J Clin Oncol. (2021) 39:3564. doi: 10.1200/JCO.2021.39.15_suppl.3564
22. Wang F, He MM, Yao YC, Zhao X, Wang ZQ, Jin Y, et al. Regorafenib plus toripalimab in patients with metastatic colorectal cancer: a phase Ib/II clinical trial and gut microbiome analysis. Cell Rep Med. (2021) 2:100383. doi: 10.1016/j.xcrm.2021.100383
23. Fakih M, Raghav KPS, Chang DZ, Bendell JC, Larson T, Cohn AL, et al. Single-arm, phase 2 study of regorafenib plus nivolumab in patients with mismatch repair-proficient (pMMR)/microsatellite stable (MSS) colorectal cancer (CRC). J Clin Oncol. (2021) 39:3560. doi: 10.1200/JCO.2021.39.15_suppl.3560
24. Kawazoe A, Xu R, Passhak M, Teng H, Shergill A, Gumus M, et al. LBA-5 lenvatinib plus pembrolizumab versus standard of care for previously treated metastatic colorectal cancer (mCRC): the phase 3 LEAP-017 study. Ann Oncol. (2023) 34:S179. doi: 10.1016/j.annonc.2023.04.015
25. Postow MA. Managing immune checkpoint-blocking antibody side effects. Am Soc Clin Oncol Educ Book. (2015) 76–83. doi: 10.14694/EdBook_AM.2015.35.76
26. Jin C, Zhu X, Huang X, Gong T, Wei Z, You J. Efficacy and safety of PD-1/PD-L1 and CTLA-4 immune checkpoint inhibitors in colorectal cancer: a meta-analysis. J Comp Eff Res. (2022) 11:203–12. doi: 10.2217/cer-2021-0134
27. Shulgin B, Kosinsky Y, Omelchenko A, Chu L, Mugundu G, Aksenov S, et al. Dose dependence of treatment-related adverse events for immune checkpoint inhibitor therapies: a model-based meta-analysis. Oncoimmunology. (2020) 9:1748982. doi: 10.1080/2162402X.2020.1748982
28. Fuloria J. Safety profiles of current antiangiogenic therapies for metastatic colorectal cancer. Onco Targets Ther. (2012) 5:133–42. doi: 10.2147/OTT.S31412
29. Xie X, Zhang J, Hu H, Cai Y, Wu Z, Ling J, et al. Efficacy and safety of regorafenib in combination with chemotherapy as second-line treatment in patients with metastatic colorectal cancer: A network meta-analysis and systematic literature review. Adv Ther. (2020) 37:4233–48. doi: 10.1007/s12325-020-01447-2
30. Gu T, Jiang A, Zhou C, Lin A, Cheng Q, Liu Z, et al. Adverse reactions associated with immune checkpoint inhibitors and bevacizumab: A pharmacovigilance analysis. Int J Cancer. (2023) 152:480–95. doi: 10.1002/ijc.v152.3
Keywords: colorectal cancer, PD-L1, CTLA-4, bevacizumab, MSS
Citation: Zhang P, Li X, Wang X, Yang Y, Wang J and Cao D (2024) SHR-8068 combined with adebrelimab and bevacizumab in the treatment of refractory advanced colorectal cancer: study protocol for a single-arm, phase Ib/II study. Front. Immunol. 15:1450533. doi: 10.3389/fimmu.2024.1450533
Received: 17 June 2024; Accepted: 13 September 2024;
Published: 09 October 2024.
Edited by:
Jack Feehan, Victoria University, AustraliaReviewed by:
Md Ataur Rahman, University of Michigan, United StatesTitto Augustine, Purdue University Indianapolis, United States
Copyright © 2024 Zhang, Li, Wang, Yang, Wang and Cao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dan Cao, Y2FvZGFuQHNjdS5lZHUuY24=
†These authors have contributed equally to this work
 Pei Zhang1,2†
Pei Zhang1,2† Xiaofen Li
Xiaofen Li Dan Cao
Dan Cao