- 1Dermatology and leprosy department, Hunan Provincial Center for Disease Control and Prevention, Changsha, Hunan, China
- 2Department of Mycobacterium, Jiangsu Key Laboratory of Molecular Biology for Skin Diseases and STIs, Institute of Dermatology & Hospital for Skin Diseases, Chinese Academy of Medical Sciences & Peking Union Medical College, Nanjing, Jiangsu, China
Leprosy is a chronic infectious disease that mainly affects the skin and peripheral nerves, it can also invade deeper tissues and organs, including mucous membranes, lymph nodes, testes, eyes, and internal organs. Severe cases can result in deformities and disabilities. We encountered the case of a 39-year-old male with unexplained fever, headache and rash. The patient’s lesions were taken for histopathological examination and slit skin smear analysis. Further, the patient was detected of Mycobacterium leprae (M.leprae) nucleic acid sequences in the cerebrospinal fluid (CSF) and plasma, and M.leprae gene targets in the skin lesion tissue and blood. The patient was eventually diagnosed with multibacillary leprosy and type II leprosy reaction. These results suggest the possibility of bacteremia in patients with leprosy to some extent, and observation implies the potential invasion of CSF by M.leprae or its genetic material.
Introduction
Leprosy is a chronic infectious disease caused by Mycobacterium leprae (M.leprae) and Mycobacterium lepromatosis (M.lepromatosis) complex. Leprosy mainly affects the skin and peripheral nerves, it can also invade deeper tissues and organs, such as mucous membranes, eyes, and internal organs. Severe cases can lead to deformities and disabilities (1, 2). Clinically, the manifestations of leprosy are extensive and non-specific due to the complex immune response to pathogens, while slit skin smear (SSS) and histopathological examination have limited sensitivity to leprosy, making diagnosis difficult. The literature shows that the sensitivity of SSS varies between 10~50% while its specificity is nearly 100%, 37.4% accuracy (3).The histopathological conformation rate varies from 29% to 61% (4, 5). Moreover, they are also difficult to detect abnormalities in other types of specimens, such as blood and cerebrospinal fluid (CSF) (6, 7). This ultimately leads to treatment delays and more severe complications. Therefore, early diagnosis and timely initiation of treatment are crucial for preventing irreversible damage and disability.
Molecular assays, like PCR are powerful tools for diagnosing pure neural and paucibacillary (PB) leprosy. PCR is used to identify possible sources of M.leprae dissemination (8). Nested PCR is at least 100 times more sensitive than traditional PCR and microscopy, crucial for early diagnosis in patients with negative microscopy or inconclusive histopathology (9). Its sensitivity is highest for skin biopsies, detecting M. leprae DNA in over 80% of clinically multibacillary (MB) cases and 30%~40% of BI negative PB cases. Key gene targets for diagnostic applications include RLEP, 16srRNA, folP, gyrA, and rpoB. These genes are used to develop assays for diagnosing critical leprosy cases (10). For PB specimens, 95% positivity was achieved by three tested genes (folP, rpoB and gyrA) in nested PCR (11). Metagenomic Next-Generation Sequencing (mNGS) is a novel high-throughput assay based on a second-generation sequencing (NGS) platform that can simultaneously detect hundreds of pathogens, and many studies have demonstrated the great potential of mNGS in infectious disease diagnosis (12).
Leprosy reactions are acute hypersensitivity response to M.leprae antigen caused by immune imbalance disruption. They are classified into three types: type I, type II (Erythema Nodosum Leprosum, ENL), and type III (Lucio’s phenomenon). Type II leprosy reaction is typically characterized by fever, headache, erythema nodosum or acute iridocyclitis (13). However, in a few cases, the clinical manifestations are not specific.
This case aimed to describe a patient presenting with unexplained fever, headache and rash, who was detected of M.leprae nucleic acid sequences in the CSF and plasma by mNGS, and M.leprae gene targets in the skin lesion tissue and blood by nested PCR. This suggests the possibility of bacteremia in patients with leprosy to some extent, and observation implies the potential invasion of CSF by M. leprae or its genetic material. We aim to explore the diagnostic challenges of leprosy in non-endemic areas and highlight the value of molecular diagnostic techniques in managing complex cases.
Case report
A 39-year-old male from Hunan Province, China, was admitted to the hospital due to recurrent fever lasting for five days and systemic rash persisting for four days. Prior to the onset of his illness, he had been exposed to the rain which resulted in a subsequent fever reaching up to 40.6°C accompanied by headache and weakness. Within one day, rashes began appearing on his trunk and both upper limbs, gradually spreading downwards towards both lower limbs. The patient had previously undergone surgery for right common peroneal nerve release with no significant postoperative improvement; however, the cause of his illness remains unknown. Physical examination revealed diffuse cutaneous infiltration throughout the body (particularly on the limbs), along with symmetrically distributed brown patches on both buttocks, lower limbs, and palms (Figures 1A–C).
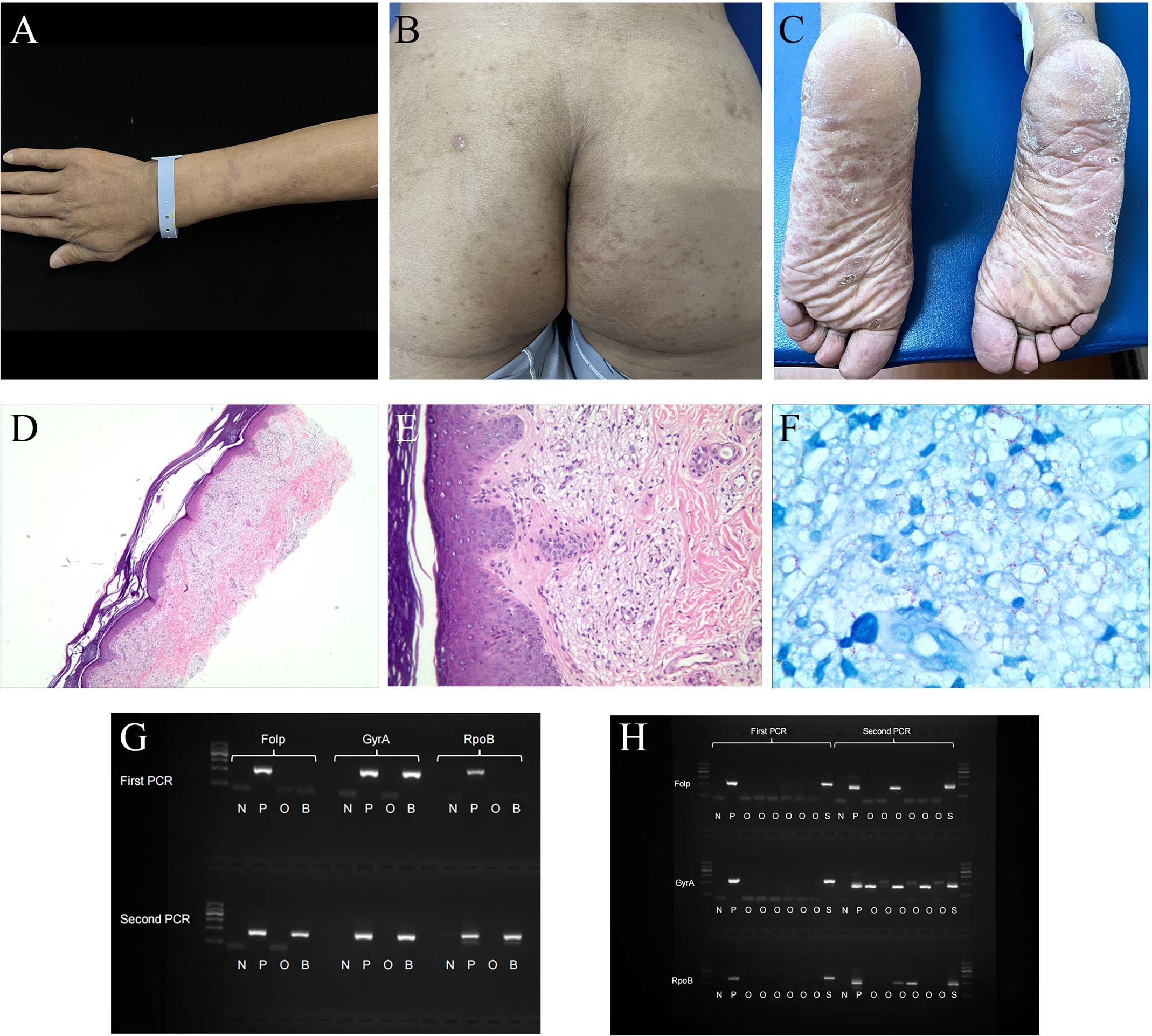
Figure 1. Patient clinical photos and related examination results. (A–C) Mycobacterium leprae infection in a 39-year-old man (before treatment, China). (A) invasive erythema, papule could be seen on the upper extremities, with unclear boundaries. (B, C) erythema was symmetrically distributed on the hip and pelma. (D–F) Hematoxylin and eosin staining and acid-fast staining of the pelma. (D, E) Excessive keratosis, a large number of virchow cells in the dermis were banded and clumpy infiltrated, accompanied by a varying number of lymphocytes. (F) A large number of acid-fast bacilli could be seen. (G) Nested PCR result of the patient’s blood sample. (H) Nested PCR result of the patient’s skin lesion sample. N, negative control; P, positive control; O, other people’s samples, not relevant to this case report; B, blood sample; S, skin lesion sample. DNA Marker: 100, 300, 500, 700, 900, 1200 bp, with 700 bp as reference; folp, gyrA and rpoB were around 300bp in the gel.
Laboratory tests showed that the total leukocyte count was 11.21 × 109/L, and the absolute neutrophil count was 9.38 × 109/L. Inflammatory markers, including C-reactive protein, erythrocyte sedimentation rate, ferritin, and procalcitonin, are all elevated (Table 1). No abnormalities were detected in blood culture, viral testing, rheumatism screening, lupus evaluation, idiopathic inflammatory myopathy spectrum investigation, vasculitis assessment, brucella antibody examination, Widal test, Weil-Felix test and T-spot test. CSF analysis showed no significant abnormalities (Table 2). Head MRI and chest and abdomen CT scans showed no abnormalities either. Although the exact pathogen could not be identified, the patient was diagnosed with infectious fever and treated with a broad-spectrum antibiotic regimen consisting of piperacillin tazobactam and moxifloxacin for five days. While his body temperature temporarily returned to normal during treatment period it subsequently increased again.
To confirm the diagnosis clearly, the patient’s plantar skin lesions were taken for histopathological examination. The epidermis exhibited slight hypertrophy, and a virchow cells infiltration surrounded the vessels in the superficial dermis. Additionally, lymphocytes were observed in the dermis, while acid-fast staining revealed a significant presence of bacilli (Figures 1D–F). The mean Bacteriological Index obtained from SSS analysis was 5.7. Furthermore, plasma and CSF samples underwent mNGS testing. In order to prevent the introduction of M.leprae from skin lesions into the bloodstream and CSF during puncture, a non-damaged area was selected for the procedure. The analysis revealed three unique sequence reads of M.leprae in the plasma sample and fourteen sequence reads in the CSF sample (Table 3). To ensure accurate results, negative controls (ddH2O) were included during metagenomic sequencing. In addition, M.leprae gene targets (folp, gyrA, rpoB) were identified in the blood and skin lesion tissue through nested PCR, the results showed that all three targets were positive (Figures 1G, H). Primer sequences for three target genes are shown in Supplementary Table 4. We used negative control (ddH2O) and positive control (M.leprae) to ensure the results were more reliable and accurate. To avoid cross-contamination, we use these three targets(folp, gyrA, rpoB) in nested PCR to eliminate the false-positive results (11).
At this juncture, we harbored suspicions that the patient’s previously reported common peroneal nerve entrapment, which occurred seven years ago, could be attributed to leprosy. Subsequent neurological physical examinations revealed bilateral thickening of the ulnar and common peroneal nerves, diminished sensation in both lower extremities, loss of plantar sensation, as well as reduced dorsiflexion and valgus function in the right foot. Color Doppler ultrasound examination indicated edema and thickening of the right common peroneal nerve at the upper edge of the popliteal fossa (Supplementary Figure 1). Electromyography results showed abnormal conduction in the motor and sensory nerves of all four limbs, with a predominant abnormality in the motor conduction of the right common peroneal nerve (Supplementary Table 1 and Supplementary Table 2). No significant abnormalities were observed in the muscle examinations of the four limbs (Supplementary Table 3).The patient gave informed consent.
Based on the combined evidence, a final diagnosis of multibacillary leprosy was established. The patient’s fever was attributed to a type II leprosy reaction and subsided after administering 10 mg dexamethasone for one day. After three days, oral prednisone at a dosage of 40mg/d was substituted for dexamethasone, after which prednisone was gradually reduced and discontinued after 6 months. Standard multi-drug therapy (MDT) was administered concurrently, namely Rifampicin 600mg once a month, Dapsone 100mg daily, Clofazimine 300mg once a month, for a duration of 12 months. After 13 months, the patient’s symptoms resolved and the lesions subsided (Figures 2A–C). The timeline of the patient’s illness is shown in the Figure 2D.
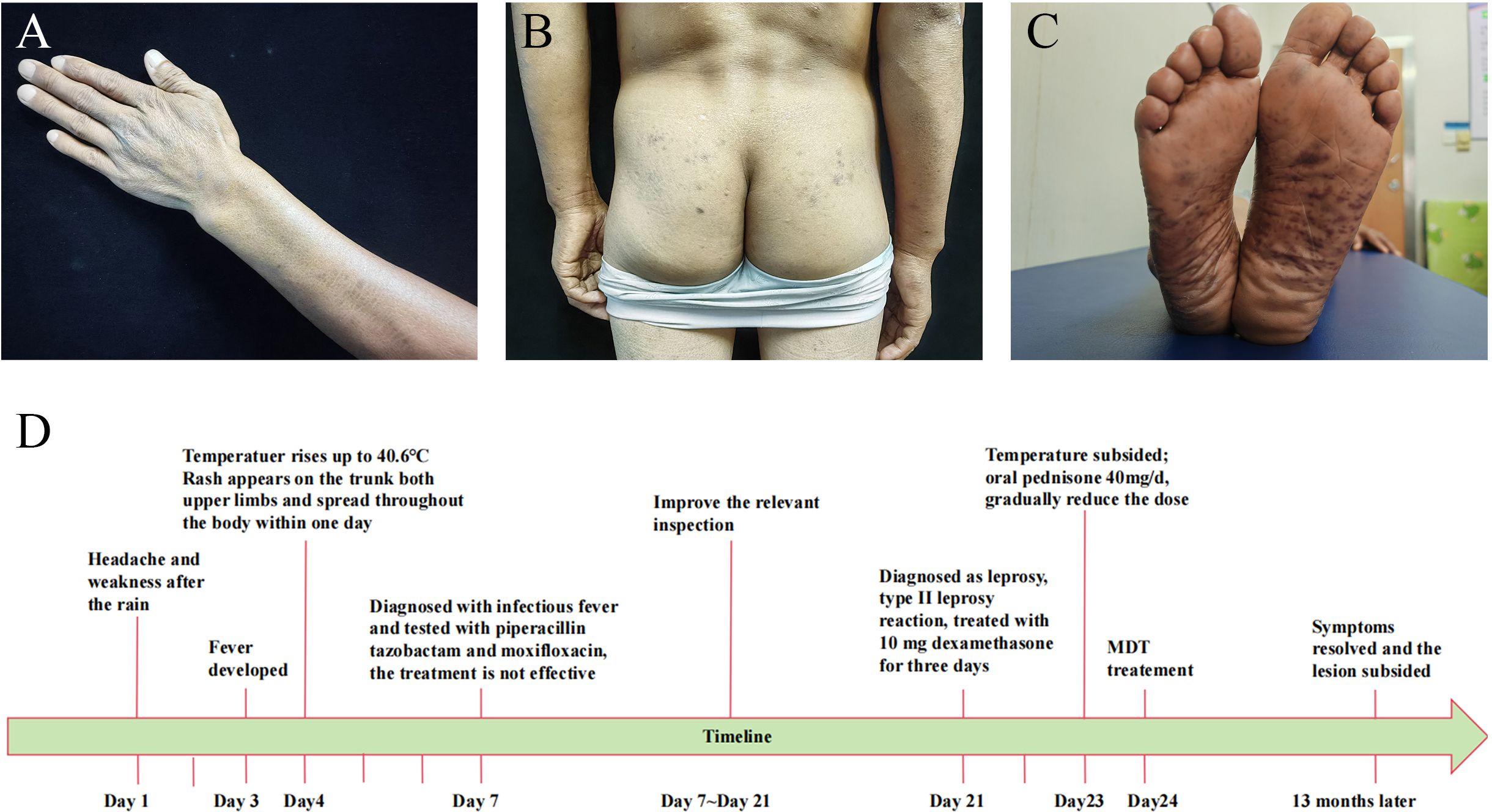
Figure 2. Patient clinical photos after treatment and disease timeline. (A–C) The recovery of skin lesions after treatment. (D) Patient’s illness timeline.
Discussion
According to the relevant literature, M.leprae has been found in the liver, spleen and bone marrow of patients with poor resistance to leprosy (14), which is usually thought to have developed into bacteremia, but there is little direct evidence (15, 16). Lane JE et al. confirmed M.leprae bacteremia in untreated patients, and support the contention that blood‐borne M.leprae is viable and theoretically infectious. This is the first time that we confirmed the presence of M.leprae DNA in the patient’s blood by both mNGS and nested PCR. These results suggest the possibility of bacteremia in patients with leprosy to some extent. In view of Scollard et al. had demonstrated the presence of M.leprae in the endothelium of blood vessels (17), we speculated M. leprae entered the bloodstream through the broken endothelial cells of blood vessels. However, this was also possibly just a disintegrating fragment of leprosy DNA, as we did not find leprosy bacteria in the blood smear, which warrants further study. The patient’s neurological symptoms have persisted for approximately seven years, indicating a long disease duration, and the patient is classified as multibacillary. We hypothesize that these factors may be related to the occurrence of bacteremia in the patient, but this requires further investigation.
At present, no suitable medium for M. leprae cultivation has been found, although some researchers have found models for maintaining ex-vivo culture of human skin (18), there is almost no literature on the potential of M.leprae to invade human central nervous system (CNS) (19, 20). Patil SA et al. used a monoclonal-antibody-based sandwich immunoradiometric assay (SIRMA) to detect M.leprae antigens in the CSF of leprosy patients (21). This study may suggest the presence of M. leprae antigens in the CSF of leprosy patients and M.leprae may invade the CNS. Although the blood–brain barrier, which is one of the tightest barriers in the body, protects the brain from infections, Coureuil, M et al. pointed out that there are two blood–CNS barriers that can potentially be circumvented by bacterial pathogens: the blood–brain barrier (BBB) and the blood–cerebrospinal fluid barrier (BCSFB) (22). When bacterial pathogens cross from parenchymal arteries, they are transported to the subarachnoid area via the glymphatic pathway. Regardless of the site of crossing, CNS invasion requires the crossing of two cellular barriers: an endothelial monolayer followed by an epithelial monolayer. It should be noted that regardless of the mechanisms that are used to invade the meninges from the bloodstream, the level of bacteremia plays an important role. Intracellular microbes, like Mycobacterium tuberculosis, can also cause meningitis (23). However, the CNS inflammation generated by these pathogens is not limited to the meninges and can potentially affect the brain parenchyma (24). Furthermore, these bacteria, which spread through macrophages and dendritic cells, may enter the brain via host peripheral immune cells instead of directly interacting with CNS barriers. This is an important finding that we detected M. leprae nucleic acid sequences in the patient’s CSF through mNGS. This is consistent with recent findings of Zhao, et al (25). This observation implies the potential invasion of CSF by M. leprae or its genetic material.
mNGS is a hypothesis-free method that has been developed and optimized for the clinical detection of various pathogens and aids in the differential diagnosis of low-incidence infectious diseases. In our case, the advantages of mNGS were confirmed. Additionally, nested PCR, with its high sensitivity and specificity, greatly assisted us in diagnosing leprosy and detecting bacteremia.
We emphasize the critical need for investigating leprosy in cases showing neurological impairments, irrespective of whether they occur in endemic or non-endemic areas. In evaluating leprosy patients with neurological complications, testing CSF can provide important diagnostic information. Recognizing leprosy as a potential diagnosis across diverse geographical locations ensures timely and appropriate treatment, preventing further damage. The patient exhibited lower limb sensory abnormalities seven years ago and underwent common peroneal nerve release surgery. However, due to the failure of the doctors at that time to make an accurate diagnosis, more severe clinical manifestations subsequently developed, serving as a cautionary reminder. In addition, Although leprosy is a universal imitator, it often occurs on the cooler surfaces of the body. The symmetrical distribution of dark erythema on both feet as in this case is extremely rare, which provides us an interesting suggestion to avoid misdiagnosis of leprosy.
In conclusion, we report a rare leprosy case of fever of unknown origin, blood and CSF Involvement. This case suggests that leprosy patients may develop bacteremia, and M. leprae or its genetic material may invade the CSF. Our objective is to raise awareness among physicians about leprosy and emphasize the value of molecular diagnostic techniques, such as nested PCR and mNGS, in complex cases.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Hunan Provincial Center for Disease Control and Prevention. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
Author contributions
HC: Funding acquisition, Writing – original draft, Writing – review & editing. YJ: Writing – original draft, Writing – review & editing. YS: Writing – review & editing. WZ: Writing – review & editing. HJ: Writing – review & editing. ZW: Writing – review & editing. RZ: Writing – review & editing. HW: Funding acquisition, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by grants from the National Key R&D Program of China (2019YFE0113300), National Natural Science Foundation of China (Grant 81972950, 82173431, 82103748), Jiangsu Provincial Medical Key Laboratory, Jiangsu Province Capability Improvement Project through Science, Technology and Education (ZDXYS202204), the Nanjing Incubation Program for National Clinical Research Center (2019060001), Natural Science Foundation of Hunan Province, China. (No.2018JJ6010).
Acknowledgments
We would like to give sincerely thank the patient and her family for their understanding and supporting the publication of this case report.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1450490/full#supplementary-material
References
1. Sharma R, Singh P, McCoy RC, Lenz SM, Donovan K, Ochoa MT, et al. Isolation of Mycobacterium lepromatosis and development of molecular diagnostic assays to distinguish Mycobacterium leprae and M. lepromatosis. Clin Infect Dis. (2020) 71:e262–9. doi: 10.1093/cid/ciz1121
2. Benjak A, Avanzi C, Singh P, Loiseau C, Girma S, Busso P, et al. Phylogenomics and antimicrobial resistance of the leprosy bacillus Mycobacterium leprae. Nat Commun. (2018) 9:352. doi: 10.1038/s41467-017-02576-z
3. Lima FR, de Paula NA, Simões MMR, Manso GMDC, Albertino GS, Felisbino GC, et al. Bacilloscopy and polymerase chain reaction of slit-skin smears and anti-phenolic glycolipid-I serology for Hansen's disease diagnosis. Front Med (Lausanne). (2022) 9:972244. doi: 10.3389/fmed.2022.972244
4. Reja AH, Biswas N, Biswas S, Dasgupta S, Chowdhury IH, Banerjee S, et al. Fite-Faraco staining in combination with multiplex polymerase chain reaction: a new approach to leprosy diagnosis. Indian J Dermatol Venereol. Leprol. (2013) 79:693–700. doi: 10.4103/0378-6323.116740
5. Lockwood DN, Nicholls P, Smith WC, Das L, Barkataki P, van Brakel W, et al. Comparing the clinical and histological diagnosis of leprosy and leprosy reactions in the INFIR cohort of Indian patients with multibacillary leprosy. PLoS Negl Trop Dis. (2012) 6:e1702. doi: 10.1371/journal.pntd.0001702
6. Alemu Belachew W, Naafs B. Position statement: LEPROSY: diagnosis, treatment and follow-up. J Eur Acad Dermatol Venereol. (2019) 33:1205–13. doi: 10.1111/jdv.15569
7. Kundakci N, Erdem C. Leprosy: A great imitator. Clin Dermatol. (2019) 37:200–12. doi: 10.1016/j.clindermatol.2019.01.002
8. Chokkakula S, Shui T, Jiang H, Yang J, Li X, He J, et al. Genotyping of Mycobacterium leprae for understanding the distribution and transmission of leprosy in endemic provinces of China. Int J Infect Dis. (2020) 98:6–13. doi: 10.1016/j.ijid.2020.06.032
9. Araujo S, Freitas LO, Goulart LR, Goulart IM. Molecular evidence for the aerial route of infection of Mycobacterium leprae and the role of asymptomatic carriers in the persistence of leprosy. Clin Infect Dis. (2016) 63:1412–20. doi: 10.1093/cid/ciw570
10. Beissner M, Woestemeier A, Saar M, Badziklou K, Maman I, Amedifou C, et al. Development of a combined RLEP/16S rRNA (RT) qPCR assay for the detection of viable M. leprae nasal swab samples. BMC Infect Dis. (2019) 19:753. doi: 10.1186/s12879-019-4349-9
11. Jiang H, Shi Y, Chokkakula S, Zhang W, Long S, Wang Z, et al. Utility of multi-target nested PCR and ELISPOT assays for the detection of paucibacillary leprosy: A possible conclusion of clinical laboratory misdiagnosis. Front Cell Infect Microbiol. (2022) 12:814413. doi: 10.3389/fcimb.2022.814413
12. Chiu CY, Miller SA. Clinical metagenomics. Nat Rev Genet. (2019) 20:341–55. doi: 10.1038/s41576-019-0113-7
13. Adhe V, Dongre A, Khopkar U. A retrospective analysis of histopathology of 64 cases of lepra reactions. Indian J Dermatol. (2012) 57:114–7. doi: 10.4103/0019-5154.94278
14. Han XY, Jessurun J. Severe leprosy reactions due to Mycobacterium lepromatosis. Am J Med Sci. (2013) 345:65–9. doi: 10.1097/MAJ.0b013e31826af5fb
15. Drutz DJ, Chen TS, Lu WH. The continuous bacteremia of lepromatous leprosy. N Engl J Med. (1972) 287:159–64. doi: 10.1056/NEJM197207272870402
16. Lane JE, Balagon MV, Dela Cruz EC, Abalos RM, Tan EV, Cellona RV, et al. Mycobacterium leprae in untreated lepromatous leprosy: more than skin deep. Clin Exp Dermatol. (2006) 31:469–70. doi: 10.1111/j.1365-2230.2006.02075.x
17. Scollard DM, McCormick G, Allen JL. Localization of Mycobacterium leprae to endothelial cells of epineurial and perineurial blood vessels and lymphatics. Am J Pathol. (1999) 154:1611–20. doi: 10.1016/S0002-9440(10)65414-4
18. de Paula NA, Leite MN, de Faria Bertoluci DF, Soares CT, Rosa PS, Frade MAC. Human skin as an ex vivo model for maintaining Mycobacterium leprae and leprosy studies. Trop Med Infect Dis. (2024) 9:135. doi: 10.3390/tropicalmed9060135
19. Aung T, Kitajima S, Nomoto M, En J, Yonezawa S, Arikawa I, et al. Mycobacterium leprae in neurons of the medulla oblongata and spinal cord in leprosy. J Neuropathol. Exp Neurol. (2007) 66:284–94. doi: 10.1097/nen.0b013e31803d597e
20. Lee KH, Moon KS, Yun SJ, Won YH, Lee JH, Lee MC, et al. Brain involvement by leprosy presenting as a frontal cystic lesion. J Neurosurg. (2014) 121:184–8. doi: 10.3171/2014.1.JNS131429
21. Patil SA, Tyagi P, Katoch K, Sreevatsa, Sengupta U. Antigens of Mycobacterium leprae in the cerebrospinal fluid of leprosy patients: detection by monoclonal-antibody-based sandwich immunoradiometric assay and avidin/biotin immunoblotting. Clin Exp Immunol. (1991) 84:515–21.
22. Coureuil M, Lécuyer H, Bourdoulous S, Nassif X. A journey into the brain: insight into how bacterial pathogens cross blood-brain barriers. Nat Rev Microbiol. (2017) 15:149–59. doi: 10.1038/nrmicro.2016.178
23. Thigpen MC, Whitney CG, Messonnier NE, Zell ER, Lynfield R, Hadler J, et al. Bacterial meningitis in the United States, 1998–2007. N Engl J Med. (2011) 364:2016–25. doi: 10.1056/NEJMoa1005384
24. Be NA, Kim KS, Bishai WR, Jain SK. Pathogenesis of central nervous system tuberculosis. Curr Mol Med. (2009) 9:94–9. doi: 10.2174/156652409787581655
Keywords: leprosy, fever, cerebrospinal fluid, nested PCR, next-generation sequencing
Citation: Chen H, Jiang Y, Shi Y, Zhang W, Jiang H, Wang Z, Zeng R and Wang H (2024) Fever of unknown origin, blood and cerebrospinal fluid involvement: a leprosy case report. Front. Immunol. 15:1450490. doi: 10.3389/fimmu.2024.1450490
Received: 17 June 2024; Accepted: 07 August 2024;
Published: 27 August 2024.
Edited by:
Angélica Rita Gobbo, Federal University of Pará, BrazilReviewed by:
Thabatta Leal Silveira Andrezo Rosa, Oswaldo Cruz Foundation (Fiocruz), BrazilFilipe Rocha Lima, University of São Paulo, Brazil
Natália Aparecida De Paula, University of Sao Paulo, Brazil
Copyright © 2024 Chen, Jiang, Shi, Zhang, Jiang, Wang, Zeng and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongsheng Wang, d2hzMzNAdmlwLnNpbmEuY29t
†These authors have contributed equally to this work and share first authorship
‡ORCID: Hongsheng Wang, orcid.org/0000-0001-5725-5053
 Huan Chen1†
Huan Chen1† Yumeng Jiang
Yumeng Jiang Ying Shi
Ying Shi Wenyue Zhang
Wenyue Zhang Haiqin Jiang
Haiqin Jiang Zhenzhen Wang
Zhenzhen Wang Hongsheng Wang
Hongsheng Wang