- 1Department Transfusion Medicine, Division Blood Bank, Sanquin Blood Supply Foundation, Amsterdam, Netherlands
- 2Department Hematology, Erasmus Medical Centre, Rotterdam, Netherlands
- 3Graduate Institute of Biomedical Materials and Tissue Engineering, College of Biomedical Engineering, Taipei Medical University, Taipei, Taiwan
- 4International PhD Program in Biomedical Engineering, College of Biomedical Engineering, Taipei Medical University, Taipei, Taiwan
- 5Department of Hematology, Sultan Qaboos University Hospital, Muscat, Oman
- 6Department of Pathology, Johns Hopkins University School of Medicine, Baltimore, MD, United States
- 7Radcliffe Department of Medicine, University of Oxford and National Health Service (NHS) Blood and Transplant, Oxford, United Kingdom
- 8Division of Hematology/Oncology, Simmons Cancer Institute at Southern Illinois University (SIU) School of Medicine, Springfield, IL, United States
- 9Dept Corporate Medical Affairs, Vitalant Corporate Medical Affairs, Scottsdale, AZ, United States
- 10Etablissement Français du Sang, La Plaine-St-Denis and Université de Franche-Comté, Besançon, France
- 11Department of Transfusion Medicine and Technical Services, The South African National Blood Service, Roodepoort, South Africa
- 12Dept Transfusion Medicine, Hospital Sírio-Libanês Blood Bank, São Paulo, Brazil
- 13Transfusion Research Unit, School of Public Health and Preventive Medicine, Monash University, Melbourne, VIC, Australia
- 14Department of Clinical Haematology, Monash Health, Melbourne, VIC, Australia
Introduction: When Coronavirus Disease-19 (COVID-19) struck the world in December 2019, initiatives started to investigate the efficacy of convalescent plasma, a readily available source of passive antibodies, collected from recovered patients as a therapeutic option. This was based on historical observational data from previous virus outbreaks.
Methods: A scoping review was conducted on the efficacy and safety of convalescent plasma and hyperimmune immunoglobulins for COVID-19 treatment. This review included the latest Cochrane systematic review update on 30-day mortality and safety. We also covered use in pediatric and immunocompromised patients, as well as the logistic challenges faced in donor recruitment and plasma collection in general. Challenges for low resource countries were specifically highlighted.
Results: A major challenge is the high donation frequency required from first-time donors to ensure a safe product, which minimizes the risk of transfusion-transmitted infectious. This is particularly difficult in low- and middle- income countries due to inadequate infrastructure and insufficient blood product supplies. High-certainty evidence indicates that convalescent plasma does not reduce mortality or significantly improve clinical outcomes in patients with moderate to severe COVID-19 infection. However, CCP may provide a viable treatment for patients unable to mount an endogenous immune response to SARS-CoV-2, based on mostly observational studies and subgroup data of published and ongoing randomized trials. Convalescent plasma has been shown to be safe in adults and children with COVID-19 infection. However, the efficacy in pediatric patients remains unclear.
Discussion: Data on efficacy and safety of CCP are still underway in ongoing (randomized) studies and by reporting the challenges, limitations and successes encountered to-date, research gaps were identified to be addressed for the future.
Conclusion: This experience serves as a valuable example for future pandemic preparedness, particularly when therapeutic options are limited, and vaccines are either being developed or ineffective due to underlying immunosuppression.
Introduction
In December 2019, a new strain of coronavirus, named Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) emerged in Wuhan, China, spurring a global health crisis (1). As convalescent plasma (CP) had been used in other infectious outbreaks (2), collection and transfusion of Coronavirus Disease-19 (COVID-19) convalescent plasma (CCP) was rapidly deployed globally to treat patients with COVID-19.
Use of CP to prevent and/or treat infectious diseases dates back to the mid-1880s, when serum therapies were employed to control diphtheria and tetanus (3, 4). CP is advantageous in infectious disease outbreaks when there is insufficient time to produce and disseminate other directed therapies, such as vaccines or hyperimmune immunoglobulins, extracted from convalescent plasma. CP can be mobilized rapidly, and historical accounts supported its safety and —potentially— its effectiveness against viral diseases like influenza, poliomyelitis, measles, and mumps (5–9). The Spanish influenza A (H1N1) of 1918 was the first viral pandemic in which a potential benefit of CP was reported (10–13). CP had since been used without notable adverse events or complications during other outbreaks such as West African Ebola epidemic (2013-2016) (14), avian influenza A (H5N1) and the influenza A (H1N1) pandemic in 2009 (15). In a prospective study, administration of CP to patients with severe H1N1 infection was associated with a significantly lower mortality and viral load as compared to controls (16). Pertinent to COVID-19, CP was used during the SARS-CoV outbreak in 2003 (17–21). In a study of patients with SARS-CoV in Hong Kong, the transfusion of CP with an antibody titer ≥1:160 before day 14 of illness was reported to improve outcomes (17). In a clinical trial, patients with confirmed Junin virus (the cause of Argentine Hemorrhagic Fever) who were transfused with high-titer CP within 8 days of symptom onset, had a significantly lower case-fatality rate compared to controls [1.1% vs 16.5%, respectively; (p<0.01)] (22). Both historic accounts of use, coupled with favorable reports of CCP early in the COVID-19 pandemic (23), provided a scientific and clinical rationale for passive polyclonal immune therapy to treat COVID-19.
Nonetheless, despite numerous reports of the use of CP, studies attesting to its benefit were overwhelmingly observational in design, limited by small sample sizes, and hindered by methodological limitations due to need of use on emergency grounds with the absence of other therapies. Moreover, the variability in how CP was qualified with regards to antibody titers, likely contributed to inconsistent dosing. Another limitation was the lack of uniformity in timing of administration. Despite consistent observations that CP needed to be administered early in relation to the onset of the infection for optimal benefit (22–24); several studies were conducted, highlighting, not unexpectedly, that CP was ineffective in unselected patients with advanced disease, such as influenza (25). Furthermore, the collective heterogeneity spanning the type of pathogen, study design and patient population accounted for uncertainty as to the therapeutic role of CP in the antimicrobial armamentarium. This review aims to provide an overview of the use of CCP and hyperimmune immunoglobulins for the treatment of SARS-CoV2 infected patients (Figure 1).

Figure 1. Topic overview of the use of convalescent plasma for COVID-19 (CCP) and Hyperimmune immunoglobulins (HIG).
SARS-CoV-2 characteristics
The SARS-CoV-2 virus is an enveloped, positive-sense, single-stranded RNA virus of the genus Betacoronavirus (26). It is a respiratory virus that relies on the receptor for angiotensin-converting enzyme 2 (ACE2) for entry into cells. The coronavirus virion is made up of a nucleocapsid (N) protein, membrane (M), envelope (E), and spike (S) proteins, which are structural proteins. The S protein of the viral particle is assembled as a homotrimer (three identical subunits) and is inserted in multiple copies into the membrane of the virion, giving it its crown-like appearance. The S protein of SARS-CoV-2 is cleaved into S1 and S2 subunits during its biosynthesis in the infected cells (27, 28). The S1 subunit binds ACE2 and the S2 subunit anchors the S protein to the membrane.
Transmission from animals to humans led SARS-CoV-2 to acquire a furin-cleavage site at the boundary of the S1 and S2 domains (27). This cleavage site was retained throughout the pandemic and acquired a D614G mutation, detected in Europe in February 2021 that compensated for the S-protein instability (29). Studies showed that the D614G mutation is associated with higher viral loads, enhancing binding of the virus spike to the ACE2 receptor, and increased infectivity (30).
In general, viruses escape immunity by mutating so that they avoid recognition by neutralizing antibodies (nAbs). As immune escape is necessary only in the presence of immune pressure, few escape mutations leading to variants of concern appeared in the early days of the pandemic, with lower dissemination and in the absence of vaccination. However, as the number of infected or vaccinated people increased, SARS-CoV-2 evolved to acquire S protein mutations as an escape route from nAbs, also potentially increasing the risks for immunocompromised patients (31). A number of variants of concern (VOC) have now been identified globally; the Alpha, Beta and Gamma VOC all emerged around the same time period between September and December 2020 in the UK, South Africa and Brazil, respectively, and had similar mutations in the receptor-binding domain (RBD) (29). These mutations were associated with higher viral loads leading to enhanced binding to the ACE2 receptor and increased infectivity (32–34). All variants exhibit decreased sensitivity to neutralization by immune plasma derived from convalescent patients with COVID-19 or vaccinated individuals in vitro (35–37). Later, the Delta variant and the Epsilon variant (California lineage) were identified (29). Studies using models to estimate population-level immune escape showed that the Beta and Delta VOC had limited reinfection rates in the population (38). However, this changed when the Omicron VOC was identified in South Africa in November 2021. Omicron, with 32 mutations in the spike protein, has rapidly spread globally. There have now been numerous subvariants of the Omicron variant with the currently predominant subvariant JN.1 and the latest identified subvariant BA.2.87.1 (39). Since BA.1, the virus has shown significant immune evasion from vaccines and the serum of patients who have recovered (40, 41). Thus, the capacity of SARS-CoV-2 to generate variants is a potential impediment to an optimal efficacy of CCP collected from donors who recovered from infection with previous lineages of the virus (42–44).
Donor recruitment and screening, and CP requirements
Recruiting CP donors during a pandemic is very challenging. First, a host of logistical considerations requires strategies differing from those typically applied to routine whole blood or plasma donors. Second, a specific set of donor eligibility and screening procedures has to be approved and implemented to optimize the safety of the collection procedure for the donor and to the operators of the collection center, as well as that of the CP product for the recipient (45). In that regard, early availability of guidelines from international transfusion organizations and health authorities, and early sharing of experiences, were vital in providing clear recommendations worldwide on optimal collection and testing procedures of CCP (46–48). Third, early in the pandemic, the factors motivating convalescent patients to become CP donors (altruism, relief, gratitude for having survived, etc.) while anticipated, had yet to be determined in this specific setting. Notwithstanding, donor trust and attention to donor safety and privacy concerns are of paramount importance in a successful CCP program (49).
Once a CP donor has been effectively recruited, repeated donations, particularly at the early stage after convalescence, when nAbs are still at their peak levels (50), should be encouraged. However, with new VOCs described with recognized immune evasion capacity, periodic revisions are needed to reflect the predominant infectious variant and whether previous collected CCPs (mainly from the first and second COVID-19 surge) are still capable of adequate neutralization. Current advice recommends that newly CCP collections from recent infected and/or vaccinated donors should replace old CCP units remaining in stock (43).
CP donors are different from typical community blood donors in that they have recently recovered (in some cases have been recently hospitalized) and, may be donating for the first time. First-time donors usually present more risk factors than repeated donors (51), leading to higher deferral rates to decrease the risk of transfusion-transmitted infectious diseases (TTID). Although there is no evidence that SARS-CoV-2 is transfusion-transmitted (52), all CCP donors must meet the same eligibility criteria as regular plasma donors and be screened for TTID, according to current national or international guidelines. CCP can also undergo pathogen-reduction, with no impact on neutralizing antibody (nAb) activity (53), and should be considered specifically in settings where infectious testing and quality systems are suboptimal.
What constitutes a minimum level of nAbs suitable for CCP donation remains controversial, as there is great variability both in total IgG and nAb levels in each CCP donation (54). According to regulatory guidances (55), only very high titer, selected super-immunized CCP donors, mainly from those who had recovered from previous infection, should be accepted for transfusion (56, 57). Vaccines are known to increase IgG avidity against wild SARS-CoV-2 type in previously infected donors (58). A hybrid immunity (naturally infected and/or vaccinated CP donors) is also associated with increased binding and cross-reactive neutralizing antibodies against the newest Omicron SARS-CoV-2 variant and subvariants (44, 57–59). Molecular SARS-CoV-2 tests (by reverse transcriptase polymerase chain reaction methods; RT-PCR) are not required for CCP selection. There is still important variation between SARS-CoV-2 sero-neutralization assays (e.g. targeting live vs pseudo-virus; use of wild type vs currently dominant variants of concern; viral neutralization vs plaque reduction tests), with testing for nAbs serving as a gold standard. In addition, there is a correlation between the level of binding (anti-spike or nucleocapsid) with neutralizing antibody titers (60–62). Finally, if CCP donors donate more frequently or have their donor eligibility criteria modified (e.g., lower hemoglobin cut-off requirements) additional medical measures should be in place to ensure donor safety during and after CCP donation.
Hyperimmune immunoglobulins
Although CCP has by far been the major human polyclonal immune therapy, polyclonal human hyperimmune immunoglobulin (HIG) against SARS-CoV-2 can be fractionated from CCP plasma. CCP units used for fractionation into HIG should meet all general quality requirements of plasma for fractionation set by plasma fractionators and their regulatory authorities. Fractionators may have additional specifications for donor selection compared to that for CCP (such as specific exclusion criteria related to travel restrictions or other safety criteria for blood-borne pathogens) and minimum requirements for anti-SARS-CoV-2 antibody titer in each donation (63).
Plasma for fractionation into HIG is most often collected by apheresis. The collected plasma is pooled prior to manufacture of HIG to ensure batch-to-batch standardization; these plasma pools may reach 4000 liters or more, representing approximately 5000 donations, unless a pilot-scale production is initially implemented (when feasible). The fractionation process is similar to that used for licensed immune globulins (IGs), which includes pathogen reduction measures, and concentration of the SARS-CoV-2 antibodies at least five to ten-fold (63–65). Each batch undergoes a range of mandatory tests to ensure consistency, quality and safety (64). Advantages of HIG over CCP include the diversity and concentration of Abs, the consistency and small volume of the final product; however, a major disadvantage is the relatively long delay (6 to 9 months) required for its production when using current industrial fractionation practices (63). General points to consider in the production and quality requirements of human polyclonal HIG against SARS-CoV-2 have been published recently (63).
Efficacy and safety of CCP and HIG
Efficacy and safety of CCP and HIG have been evaluated in large cohorts and analyzed in systematic reviews, performing meta-analyses of high-level evidence from randomized clinical trials. Living systematic reviews (LSRs) have been performed by the Cochrane Hematology group on convalescent plasma, HIG and monoclonal antibodies (66, 67). The latest LSR on CCP and HIG evaluated clinical studies up to November 8th 2023, and March 31st, 2022, respectively. For both LSRs, the search strategy to identify completed and ongoing studies was performed using the World Health Organization (WHO) COVID-19 Global literature on coronavirus disease Research Database, MEDLINE, Embase, the Cochrane COVID-19 Study Register, and the Epistemonikos COVID-19 L*OVE Platform.
For the CCP LSR, only randomized controlled trials (RCTs) evaluating CCP were included, irrespective of disease severity, age, gender, or ethnicity. Excluded were studies encompassing populations with other coronavirus diseases [SARS or Middle East respiratory syndrome (MERS)]. In this review, 46 completed studies with 25,469 participants were included, of whom 12,218 received CCP. Most participants included within the trials were affected by wild type or alpha variants of COVID-19, if reported. A further 45 ongoing studies were identified evaluating CCP. This review concluded, with high certainty, that for individuals with moderate to severe disease, CCP did not reduce mortality and had little to no impact on clinical improvement or disease progression. However, a subgroup analysis of a total of 1999 inpatients without antibodies at baseline showed significantly less risk of progression to mechanical ventilation or death after receiving CCP compared to 1657 controls with antibodies receiving standard care or placebo (risk ratio (RR) 0.91; 95% CI 0.84-0.98), with a statistically significant subgroup effect (p=0.02; 2 studies; I² = 82.8%). For individuals with mild disease, CCP may reduce the risk of hospitalization or death in unvaccinated individuals. This was seen for trials that compared CCP with standard plasma (reduction from 73 per 1000 to 36 per 1000, 95% CI 23 to 55; 2 trials, 1595 participants; moderate certainty), but not for trials that compared CCP to standard care or placebo. The potential benefit for immunocompromised patients is addressed in more detail in the next section on patient subgroups.
There was no clear difference in serious adverse events (SAEs); however, consistency in reporting of clinical outcomes and adverse events (for both the intervention and control arm) in future trials would help comparisons. In Table 1, a gap analysis of the clinical evidence on efficacy and safety of CCP is summarized. The large majority of published studies have reported no evidence of an antibody-dependent enhancement (ADE) associated with CCP. However, infrequent occurrence of early transient pulmonary worsening after transfusion of CP has been reported in two studies (78, 79). In vitro, antibody-dependent Fc receptor-mediated SARS-CoV-2 infection of macrophages is associated with inflammation while ultimately inhibiting viral replication (80, 81). Recently, such early pulmonary worsening followed by reduced virus replication in lungs and improved infection outcome has been reported in SARS-CoV-2–infected hamsters treated with high-titer CCP (82).
Regarding the LSR of HIG studies, five published studies were identified for hospitalized patients with moderate-to-severe disease, three were human-derived HIG products. The largest RCT (579 participants: ITAC study) compared the effectiveness of high-dose HIG 0.4g/kg (up to 40g) to saline placebo (83). These few studies resulted in limited evidence to know whether HIG affects death from any cause. No data are available for asymptomatic people with COVID-19 or people with mild COVID-19. Regarding safety, the ITAC study showed that HIG has little to no impact on adverse events of any grade on day 1 (RR 0.98; 95% CI 0.81-1.18; low-certainty evidence) compared to placebo. However, patients receiving HIG may, for still unclear reasons, experience more grade 3 and grade 4 adverse events compared to placebo (RR 4.09; 95% CI 1.39-12.01; moderate-certainty evidence).
Immunocompromised patients
Passive polyclonal immunotherapies may offer particular benefit to patients with preexisting immunosuppression; this patient group is not only at high risk of complications of COVID-19, but they may also lack robust responses to SARS-CoV-2 vaccines (84). Furthermore, the emergence of SARS-CoV-2 variants and their associated resistance to monoclonal antibodies put immunosuppressed patients at risk for severe COVID-19.
The Cochrane group has published LSRs and a rapid review of the literature on the use of CCP and HIG (66, 85–87). Among these, five RCTs have assessed CCP efficacy among hospitalized patients with preexisting immunosuppression (i.e. malignancy, solid organ transplant, chronic steroid use, use of B-cell depleting therapies) (71, 88). Of these, the REMAP-CAP trial had a pre-specified outcome of evaluating CCP efficacy in immunosuppressed patients (71). A meta-analysis of the subgroup of patients who were immunosuppressed at baseline suggested that CCP decreased mortality compared to standard of care or placebo. Based on these analyses, the Association for the Advancement of Blood and Biotherapies (AABB) has suggested “CCP transfusion in addition to the usual standard of care for hospitalized patients with pre-existing immunosuppression, as a weak recommendation with moderate certainty of evidence” (89).
The most recent version of this LSR, which is not yet published, includes additional evidence from the CORIPLASM study (78). In a pre-specified subgroup analysis from this trial, CCP was associated with reduced mortality compared to standard of care only in hospitalized COVID-19 patients with underlying immunosuppression, while not reaching statistical significance (hazard ratio 0.39; 95% CI 0.14- 1.10). Of note, two independent case-control studies with propensity score matching in COVID-19 patients with underlying immunosuppression also reported reduced mortality with CCP treatment (90, 91).
CCP has demonstrated persistent ability to sero-neutralize variants (92, 93), as well as scalability and versatility. CCP may therefore provide a reliable treatment option for patients unable to mount an endogenous immune response to SARS-CoV-2 and its variants.
Pediatric patients
While children usually develop milder COVID-19 disease, some groups of children with COVID-19 may have a higher risk of disease progression in association with immunosuppression, lung disease and/or cardiovascular disease. A passive polyclonal therapy could also be considered in immune-deficient children, who are unable to adequately respond to vaccinations, or in situations or countries where COVID-19 vaccines in children, especially the youngest ones who represent a particularly vulnerable group, have not yet been approved (94, 95).
Case reports early in the pandemic suggested that CCP transfusion was safe in children (96). A case-series of 13 children with severe COVID-19 and underlying chronic disease reported CCP as a safe intervention and showing clinical improvement when used within a median of seven days from symptom onset (97). One published protocol (98) and three clinical trials included children and neonates (99–101). One trial evaluated the safety and pharmacokinetics of high-titer CCP (≥1:320) at a dose of five mL/kg (maximum volume of 500 mL) in high-risk children (one month-18 years) who were either exposed or infected with SARS-CoV-2 (100). Although no adverse events were reported with CCP, this trial, which enrolled 14 treated children, showed that CCP use in high-risk children achieved neutralizing capacity and may protect against severe disease, but it was unlikely to provide lasting protection (100). The RECOVERY trial, a randomized open-label trial that randomized patients to standard of care with or without high titer CCP, included 26 children (<18 years), demonstrating no significant difference in 28-day mortality or hospital discharge between the two groups (99). In Canada, the CONCOR-KIDS trial, a randomized, multi-center, open-label phase two clinical trial of the safety and efficacy of CCP for treatment of COVID-19 disease in hospitalized children withdrew its registration without enrolment (101). Another single-center prospective, open-label trial evaluated CCP safety, neutralizing antibody kinetics, and outcomes in 46 children and young adults with moderate/severe COVID-19 (April 2020-March 2021). CCP showed significant improvement in COVID-19 severity score and neutralizing antibody kinetics suggesting CCP is well tolerated in children and young adults, providing rapid and robust increased neutralizing antibodies. Most recently, a multi-institute experience of 95 children receiving CCP in the USA was published. Median total plasma dose administered and transfusion rates were 5.0 ml/kg and 2.6 ml/kg/h, respectively (102). No serious adverse events were reported. Severity scores decreased significantly 7 days after CCP transfusion or at discharge, and 94.4% children survived to hospital discharge (103).
In summary, the use of CCP in children with COVID-19 disease appears safe, yet the evidence to conclude its efficacy remains limited. While multi-institutional collaborative trials will be ideal to study CCP efficacy in children, given the significant challenges in protocol approvals and enrolment, the role of national and international registries should be stressed for future pandemics.
Preparation and use of CCP in low-resource settings
High-income countries (HICs) have an established infrastructure of (nationally coordinated) blood establishments to screen donors, collect and test donations, and implement pathogen-reduction technology. Such an infrastructure ensures that plasma products, including CP, have a high safety profile. The major challenges in low- and middle- income countries (LMICs) are access to and affordability of safe and effective treatments. CCP can be collected in low-resource settings, drawing on existing infrastructure. However, the blood system in most LMICs is less advanced than in HICs, and both safety and sufficiency of the blood supply are lacking in many LMICs.
Passive human polyclonal therapies using locally-collected plasma is attractive to LMICs in times of outbreak (104), pending the potential availability of low-cost treatment and/or preventive strategies, such as direct acting antivirals, monoclonal therapies, and vaccines. Manufacture of polyclonal therapies, such as CP, relies on the blood collection system for collection and testing of blood and plasma donations. Areas of need span donor selection (which is challenged by a typically high proportion of first-time, paid and family replacement donors, which, in the absence of advanced testing strategies, confer higher TTID risk than volunteer non-remunerated donors), infrastructure (e.g. equipment and facilities) to collect, process and store blood, human capacity (i.e. lack of skilled phlebotomists and technologists), availability of laboratory-based donor testing, quality management systems and regulatory oversight (104).
Two elements deserve specific attention regarding production of passive immunotherapies in LMICs. First, infectious risk is substantially higher than in HICs given the described challenges. If plasma is to be transfused, improved measures are needed to ensure low risk of TTIDs. These include rigorous risk-based assessment of potential donors, with a view to defer those with socio-behavioral and/or medical risk factors for TTIDs, and robust laboratory-based testing for the major TTIDs (e.g. HIV, HBV, and HCV) (104). Second is the mode of collection: the majority of CCP in HICs has been collected using apheresis, which is high-cost and requires skilled trained nursing personnel. CCP can also be produced from whole blood collections, which are low-cost and readily available, even in austere settings (105). However, its production is limited by the frequency of donations possible.
There is already a blood deficit in LMICs (106, 107). Therefore, production of CCP in LMICs risks the unintended consequences of diverting resources away from routine blood collections (107). Specifically, there is already an inability to respond to transfusion clinical demand, largely due to suboptimal donor recruitment (108). Demand for, and efforts to supply, CCP could exacerbate that deficit, thus adversely affecting clinical care for a wider group of patients.
Donor recruitment in LMICs often relies on replacement or even paid donors, both of which are potential risk factors for TTID in the absence of stringent donation testing (109). In large part the infectious risk is ascribed to over-representation of first-time donors (108, 110). Coupled with a high background prevalence for the major TTIDs (e.g., HIV) in many LMICs, along with deficient testing practices (e.g., limited quality oversight, exclusive serological testing in high incidence areas), render blood transfusion in LMICs to be a much higher risk medical procedure than that in HICs. In short, while CCP has been well tolerated in HICs (111), its risk-benefit profile needs to be re-evaluated in a low-income setting where infectious risk is pervasive.
Optimal effect of CCP is contingent upon the early timing of its administration relative to symptom onset —ideally in an outpatient setting (68, 69) as well as the antibody content of the product (112). Both these elements are challenging to ensure in a low-resource setting. Given limited access to care in LMICs, patients are more likely to present late in their disease process. Even in HICs, outpatient transfusion has been difficult to implement given a host of logistical, regulatory and administrative considerations (113). Further, given that high titers of antibodies are necessary for optimal effect, there is a need to qualify CCP donors and units accordingly. This requires testing, which in turn relies on laboratory capacity, which is frequently lacking in LMICs (114). Supply chain and procurement of assays, reagent costs, equipment (if run on automated platforms), and skilled personnel with the necessary technical expertise are needed to enable timely validation and implementation. Point-of-care tests have been developed that could facilitate qualification of CCP in a low-resource setting (115).
What we have learned and implications for future pandemics?
It is now clear that we need international standards for the collection, testing, provision, and use of CCP. These standards should include estimates of the nAb titer since that is the most relevant functional component of CCP. Standardized protocols, including for donor screening and selection, would help ensure that products are comparable across different practices and regulatory systems. Key stakeholders included the International Society for Blood Transfusion (ISBT), the AABB, and international plasma collection and fractionation organizations including the CoVIg-19 plasma alliance. A host of other groups, spanning the clinical trial consortia, regulatory agencies, and international organizations (e.g., WHO) that are also involved in oversight of blood transfusion practices and/or provide technical assistance are critical to these efforts.
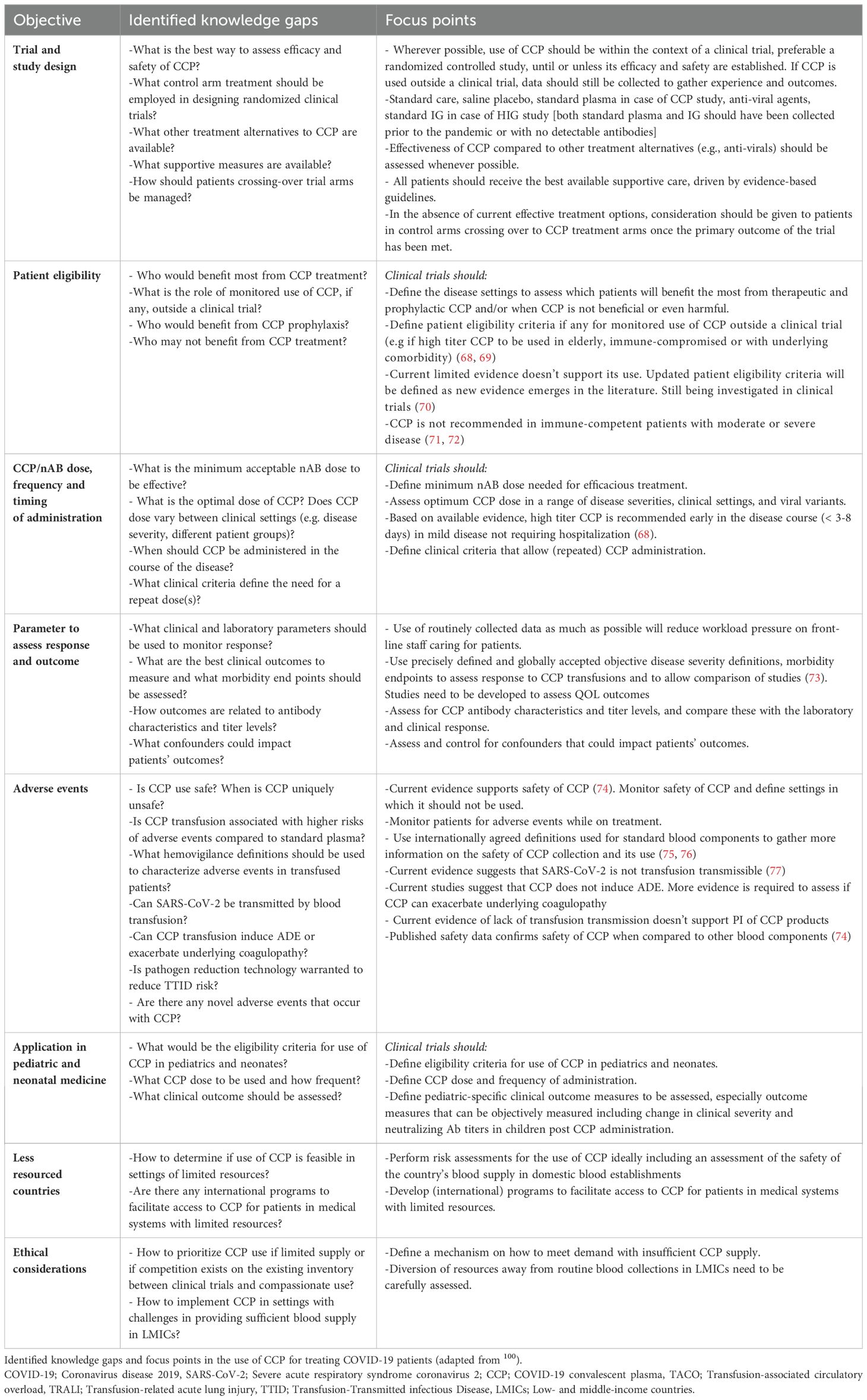
On the patient side, uniformity of clinical study criteria in terms of patient selection and outcomes evaluation is also needed. By introducing a common clinical scoring system to define the severity of illness, the effect of the interventions could be more easily assessed and compared (116). Next to the gap analysis on the clinical use of CCP (Table 1), a summary of key messages (Table 2) is provided.
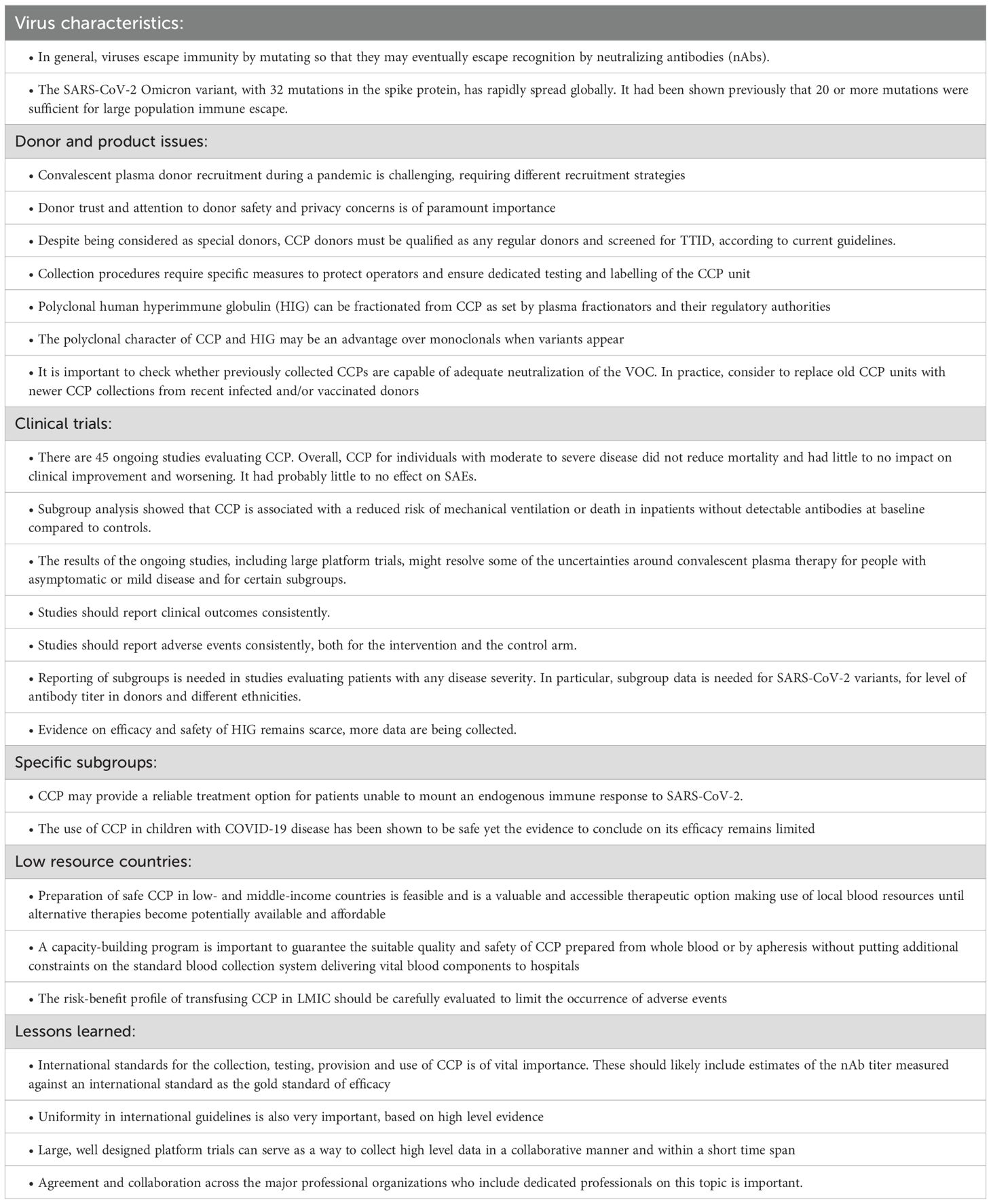
It became clear with SARS-CoV-2 that, when a new highly infectious virus is emerging, it is essential to gain knowledge as quickly as possible on accumulating scientific evidence as well as practical (operational) experiences and possible limits in clinical studies (117). Whichever a new pandemic afflicts mankind, it is essential in the future to keep up with three main principles of immunotherapy: a) presence of specific antibody; b) its presence in sufficient amounts and c) used early in the course of the disease (118). Again, collaboration is essential and can be achieved by sharing donor selection and testing approaches, as well as study trial concepts and protocols. Clinical study groups can collaborate by sharing data from ongoing and/or completed clinical trials as pre-prints, on a shared database (119), as meta-analyses (120), or by joining forces on collaborative studies. The international platform trials RECOVERY (99) and REMAP-CAP (71) are good examples of this approach. Their established platform designs facilitated rapid inclusion of new domains, their broad inclusion criteria were pragmatic and permitted greater generalizability of findings, and their multicenter approach enabled rapid recruitment. Together with the randomized study designs, they were able to rapidly achieve high-quality results which were implemented in national and international guidelines as soon as available (121). The Cochrane LSRs and meta-analyses are also very valuable in summarizing available data in a systematic way and on a regular basis (66).
How can professional organizations help? For CP, the worldwide collaboration of the key stakeholders mentioned above, together with regional and national societies, to share information and experiences has been essential. In 2020, at the start of the pandemic, the ISBT scientific working group on CCP performed an analysis to identify the gaps in scientific knowledge on how CP could be used most efficiently (122). However, four years after the start of the SARS-CoV-2 pandemic, many gaps still need to be addressed. Recently, a new collaborative action has been launched aiming to expedite clinical trials, ethical approval, reliable testing infrastructure to identify safe and efficacious CP, and donation pipelines during similar future crises (123). We can do even better in the future as we reflect on the lessons of the CCP trials, and we will be ready for the next challenge (124).
Conclusions
There have been many global questions, challenges, and successes of CCP and, to a lesser extent, HIG, regarding efficacy and safety during the course of the COVID-19 pandemic. CCP appears safe, but efficacy of this therapeutic was not demonstrated in unselected patients with moderate to severe disease. However, it is of interest that vulnerable, immunocompromised, patients, lacking antibody responses, may benefit from this readily available human resource. The evolutive polyclonal character of CCP makes it likely more beneficial compared to mAbs preparations, as evidenced by the failure of mAbs to withstand the pace of mutation, resulting in diminished efficacy against variants overtime. Additionally, due to their high costs, mAbs are of limited interest in LMICs. It also remains to be seen whether CCP is confirmed to be effective when transfused early in the disease course. The lessons learned from use of these passive human polyclonal immune therapies can serve as examples for future pandemic preparedness, when therapeutic options are lacking, and while vaccinations are not yet available or are ineffective because of underlying immunosuppression.
Author contributions
CS-O: Conceptualization, Methodology, Supervision, Writing – original draft, Writing – review & editing. TB: Writing – original draft, Writing – review & editing, Conceptualization, Methodology. AA-R: Writing – original draft, Writing – review & editing, Conceptualization, Methodology. EB: Writing – original draft, Writing – review & editing, Conceptualization, Methodology. LE: Writing – original draft, Writing – review & editing, Conceptualization, Methodology. RG: Writing – original draft, Writing – review & editing, Conceptualization, Methodology. PT: Writing – original draft, Writing – review & editing, Conceptualization, Methodology. MV: Writing – original draft, Writing – review & editing, Conceptualization, Methodology. SW: Writing – original draft, Writing – review & editing, Conceptualization, Methodology. EW: Writing – original draft, Writing – review & editing, Conceptualization, Methodology.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We would like to acknowledge the members of the passive immune therapy subgroup of the International Society of Blood Transfusion Clinical Transfusion Working Party. This paper has been written on behalf of this expert subgroup.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Zhou P, Yang X-L, Wang X-G, Hu B, Zhang L, Zhang W, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. nature. (2020) 579:270–3. doi: 10.1038/s41586-020-2012-7
2. Marano G, Vaglio S, Pupella S, Facco G, Catalano L, Liumbruno GM, et al. Convalescent plasma: new evidence for an old therapeutic tool? Blood Transfusion. (2016) 14:152. doi: 10.2450/2015.0131-15
3. von Behring E, Kitasato S. The mechanism of diphtheria immunity and tetanus immunity in animals. 1890. Mol Immunol. (1991) 28:1317, 1319–20.
4. Simon J. Emil Behring’s medical culture: from disinfection to serotherapy. Med history. (2007) 51:201–18. doi: 10.1017/S0025727300001198
5. Park WH. Therapeutic use of antipoliomyelitis serum in preparalytic cases of poliomyelitis. J Am Med Assoc. (1932) 99:1050–3. doi: 10.1001/jama.1932.02740650008003
6. Park WH, Freeman RG. The prophylactic use of measles convalescent serum. J Am Med Assoc. (1926) 87:556–8. doi: 10.1001/jama.1926.02680080022009
7. Gallagher JR. Use of convalescent measles serum to control measles in a preparatory school. Am J Public Health Nations Health. (1935) 25:595–8. doi: 10.2105/AJPH.25.5.595
8. Rambar AC. Mumps: use of convalescent serum in the treatment and prophylaxis of orchitis. Am J Dis Children. (1946) 71:1–13. doi: 10.1001/archpedi.1946.02020240008001
9. Luke TC, Casadevall A, Watowich SJ, Hoffman SL, Beigel JH, Burgess TH. Hark back: passive immunotherapy for influenza and other serious infections. Crit Care Med. (2010) 38:e66–73. doi: 10.1097/CCM.0b013e3181d44c1e
10. Francis F, Hall M, Gaines A. Early use of convalescent serum in influenza. Mil Surg. (1920) 47:177–9.
12. Miller O, McConnell W. Report of influenza treated with serum from recovered cases. Ky Med J. (1919) 17:218–9.
13. Redden WR. Treatment of influenza-pneumonia by use of convalescent human serum. Boston Med Surg J. (1919) 181:688–91. doi: 10.1056/NEJM191912111812406
14. Dodd LE, Follmann D, Proschan M, Wang J, Malvy D, van Griensven J, et al. A meta-analysis of clinical studies conducted during the West Africa Ebola virus disease outbreak confirms the need for randomized control groups. Sci Trans Med. (2019) 11:1–10. doi: 10.1126/scitranslmed.aaw1049
15. Mair-Jenkins J, Saavedra-Campos M, Baillie JK, Cleary P, Khaw F-M, Lim WS, et al. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis. J Infect Dis. (2015) 211:80–90. doi: 10.1093/infdis/jiu396
16. Hung IF, To KK, Lee C-K, Lee K-L, Chan K, Yan W-W, et al. Convalescent plasma treatment reduced mortality in patients with severe pandemic influenza A (H1N1) 2009 virus infection. Clin Infect Dis. (2011) 52:447–56. doi: 10.1093/cid/ciq106
17. Cheng Y, Wong R, Soo Y, Wong W, Lee C, Ng M, et al. Use of convalescent plasma therapy in SARS patients in Hong Kong. Eur J Clin Microbiol Infect Dis. (2005) 24:44–6. doi: 10.1007/s10096-004-1271-9
18. Soo Y, Cheng Y, Wong R, Hui D, Lee C, Tsang K, et al. Retrospective comparison of convalescent plasma with continuing high-dose methylprednisolone treatment in SARS patients. Clin Microbiol infection. (2004) 10:676–8. doi: 10.1111/j.1469-0691.2004.00956.x
19. Wong V, Dai D, Wu A, Sung J. Treatment of severe acute respiratory syndrome with convalescent plasma. Hong Kong Med J. (2003) 9:199–201.
20. Yeh K-M, Chiueh T-S, Siu L, Lin J-C, Chan PK, Peng M-Y, et al. Experience of using convalescent plasma for severe acute respiratory syndrome among healthcare workers in a Taiwan hospital. J Antimicrobial Chemother. (2005) 56:919–22. doi: 10.1093/jac/dki346
21. Ortiz JR, Rudd KE, Clark DV, Jacob ST, West TE. Clinical research during a public health emergency: a systematic review of severe pandemic influenza management. Crit Care Med. (2013) 41:1345–52. doi: 10.1097/CCM.0b013e3182771386
22. Maiztegui JI, Fernandez NJ, de Damilano AJ. Efficacy of immune plasma in treatment of Argentine haemorrhagic fever and association between treatment and a late neurological syndrome. Lancet. (1979) 2:1216–7. doi: 10.1016/s0140-6736(79)92335-3
23. Roback JD, Guarner J. Convalescent plasma to treat COVID-19: possibilities and challenges. JAMA. (2020) 323:1561–2. doi: 10.1001/jama.2020.4940
24. Luke TC, Kilbane EM, Jackson JL, Hoffman SL. Meta-analysis: convalescent blood products for Spanish influenza pneumonia: a future H5N1 treatment? Ann Intern Med. (2006) 145:599–609. doi: 10.7326/0003-4819-145-8-200610170-00139
25. Beigel JH, Tebas P, Elie-Turenne MC, Bajwa E, Bell TE, Cairns CB, et al. Immune plasma for the treatment of severe influenza: an open-label, multicentre, phase 2 randomised study. Lancet Respir Med. (2017) 5:500–11. doi: 10.1016/s2213-2600(17)30174-1
26. Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. (2020) 395:565–74. doi: 10.1016/S0140-6736(20)30251-8
27. Hoffmann M, Kleine-Weber H, Pohlmann S. A multibasic cleavage site in the spike protein of SARS-coV-2 is essential for infection of human lung cells. Mol Cell. (2020) 78:779–784 e5. doi: 10.1016/j.molcel.2020.04.022
28. Hoffmann M, Kleine-Weber H, Schroeder S, Kruger N, Herrler T, Erichsen S, et al. SARS-coV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. (2020) 181(2):271–80. doi: 10.1016/j.cell.2020.02.052
29. Choi JY, Smith DM. SARS-coV-2 variants of concern. Yonsei Med J. (2021) 62:961–8. doi: 10.3349/ymj.2021.62.11.961
30. Groves DC, Rowland-Jones SL, Angyal A. The D614G mutations in the SARS-CoV-2 spike protein: Implications for viral infectivity, disease severity and vaccine design. Biochem Biophys Res Commun. (2021) 538:104–7. doi: 10.1016/j.bbrc.2020.10.109
31. Scherer EM, Babiker A, Adelman MW, Allman B, Key A, Kleinhenz JM, et al. SARS-coV-2 evolution and immune escape in immunocompromised patients. N Engl J Med. (2022) 386:2436–8. doi: 10.1056/NEJMc2202861
32. Davies NG, Abbott S, Barnard RC, Jarvis CI, Kucharski AJ, Munday JD, et al. Estimated transmissibility and impact of SARS-CoV-2 lineage B.1.1.7 in England. Science. (2021) 372:1–9. doi: 10.1126/science.abg3055
33. Sabino EC, Buss LF, Carvalho MPS, Prete CA Jr., Crispim MAE, Fraiji NA, et al. Resurgence of COVID-19 in Manaus, Brazil, despite high seroprevalence. Lancet. (2021) 397:452–5. doi: 10.1016/S0140-6736(21)00183-5
34. Starr TN, Greaney AJ, Hilton SK, Ellis D, Crawford KHD, Dingens AS, et al. Deep mutational scanning of SARS-coV-2 receptor binding domain reveals constraints on folding and ACE2 binding. Cell. (2020) 182:1295–1310 e20. doi: 10.1016/j.cell.2020.08.012
35. Wibmer CK, Ayres F, Hermanus T, Madzivhandila M, Kgagudi P, Oosthuysen B, et al. SARS-CoV-2 501Y.V2 escapes neutralization by South African COVID-19 donor plasma. Nat Med. (2021) 27:622–5. doi: 10.1038/s41591-021-01285-x
36. Cele S, Gazy I, Jackson L, Hwa SH, Tegally H, Lustig G, et al. Escape of SARS-CoV-2 501Y.V2 from neutralization by convalescent plasma. Nature. (2021) 593:142–6. doi: 10.1038/s41586-021-03471-w
37. Planas D, Veyer D, Baidaliuk A, Staropoli I, Guivel-Benhassine F, Rajah MM, et al. Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature. (2021) 596:276–80. doi: 10.1038/s41586-021-03777-9
38. Pulliam JRC, van Schalkwyk C, Govender N, von Gottberg A, Cohen C, Groome MJ, et al. Increased risk of SARS-CoV-2 reinfection associated with emergence of Omicron in South Africa. Science. (2022) 376:eabn4947. doi: 10.1126/science.abn4947
39. CDC tracks new SARS-coV-2 variant, BA.2.87.1 (2024). Available online at: https://www.cdc.gov/ncird/whats-new/covid-19-variant-update-2024-02-09.html (Accessed February 24, 2024).
40. Liu L, Iketani S, Guo Y, Chan JF, Wang M, Liu L, et al. Striking antibody evasion manifested by the Omicron variant of SARS-CoV-2. Nature. (2022) 602:676–81. doi: 10.1038/s41586-021-04388-0
41. Zhang L, Li Q, Liang Z, Li T, Liu S, Cui Q, et al. The significant immune escape of pseudotyped SARS-CoV-2 variant Omicron. Emerg Microbes Infect. (2022) 11:1–5. doi: 10.1080/22221751.2021.2017757
42. Yang J, Hong W, Lei H, He C, Lei W, Zhou Y, et al. Low levels of neutralizing antibodies against XBB Omicron subvariants after BA.5 infection. Signal Transduct Target Ther. (2023) 8:252. doi: 10.1038/s41392-023-01495-4
43. Gallian P, Brisbarre N, Nurtop E, Le Cam S, Franck T, Isnard C, et al. Low neutralization capacity against SARS-CoV-2 Omicron BQ.1.1 of convalescent plasma collected during circulation of Omicron BA.1. Vox Sang. (2023) 118:407–8. doi: 10.1111/vox.13418
44. Lin YJ, Evans DH, Robbins NF, Orjuela G, Abe KT, Rathod B, et al. Diminished neutralization capacity of SARS-coV-2 omicron BA.1 in donor plasma collected from January to March 2021. Microbiol Spectr. (2023) 11:e0525622. doi: 10.1128/spectrum.05256-22
45. Al-Riyami AZ, Burnouf T, Wood EM, Devine DV, Oreh A, Apelseth TO, et al. International Society of Blood Transfusion survey of experiences of blood banks and transfusion services during the COVID-19 pandemic. Vox Sang. (2022) 117:822–30. doi: 10.1111/vox.13256
46. Epstein J, Burnouf T. Points to consider in the preparation and transfusion of COVID-19 convalescent plasma. Vox Sang. (2020) 115:485–7. doi: 10.1111/vox.12939
47. Budhai A, Wu AA, Hall L, Strauss D, Paradiso S, Alberigo J, et al. How did we rapidly implement a convalescent plasma program? Transfusion. (2020) 60:1348–55. doi: 10.1111/trf.15910
48. An EU programme of COVID-19 convalescent plasma collection and transfusion Guidance on collection, testing, processing, storage, distribution and monitored use (2021). Available online at: https://health.ec.europa.eu/system/files/2021-03/guidance_plasma_covid19_en_0.pdf (Accessed June 3, 2022).
49. Wendel S, Land K, Devine DV, Daly J, Bazin R, Tiberghien P, et al. Lessons learned in the collection of convalescent plasma during the COVID-19 pandemic. Vox Sang. (2021) 116(8):872–9. doi: 10.1111/vox.13096
50. Robbiani DF, Gaebler C, Muecksch F, Lorenzi JCC, Wang Z, Cho A, et al. Convergent antibody responses to SARS-CoV-2 in convalescent individuals. Nature. (2020) 584:437–42. doi: 10.1038/s41586-020-2456-9
51. Dodd RY, t. Notari EP, Stramer SL. Current prevalence and incidence of infectious disease markers and estimated window-period risk in the American Red Cross blood donor population. Transfusion. (2002) 42:975–9. doi: 10.1046/j.1537-2995.2002.00174.x
52. Cappy P, Candotti D, Sauvage V, Lucas Q, Boizeau L, Gomez J, et al. No evidence of SARS-CoV-2 transfusion transmission despite RNA detection in blood donors showing symptoms after donation. Blood. (2020) 136:1888–91. doi: 10.1182/blood.2020008230
53. Hindawi S, Elgemmezi T, El-Kafrawy SA, Samadani H, Tilmisani M, Assiri O, et al. Assessment of the impact of pathogen reduction technologies on the neutralizing activity of COVID-19 convalescent plasma. Transfus Apher Sci. (2023) 62:103688. doi: 10.1016/j.transci.2023.103688
54. Sullivan D, Casadevall A. COVID-19 serology data provide guidance for future deployments of convalescent plasma. mBio. (2023) 14:e0042823. doi: 10.1128/mbio.00428-23
55. FDA. FDA Guidance of Tests Acceptable for Use in the Manufacture of COVID-19 Convalescent Plasma with High Titers of Anti-SARS-CoV-2 Antibodies. (2021). Available online at: https://www.fda.gov/search?s=FDA.+FDA+Guidance+of+Tests+Acceptable+for+Use+in+the+Manufacture+of+COVID-19+Convalescent+Plasma+with+High+Titers+of+Anti-SARS-CoV-2+Antibodies.+2021 (Accessed March 23, 2022).
56. Klein SL, Pekosz A, Park HS, Ursin RL, Shapiro JR, Benner SE, et al. Sex, age, and hospitalization drive antibody responses in a COVID-19 convalescent plasma donor population. J Clin Invest. (2020) 130:6141–50. doi: 10.1172/JCI142004
57. Seidel A, Hoffmann S, Jahrsdorfer B, Korper S, Ludwig C, Vieweg C, et al. SARS-CoV-2 vaccination of convalescents boosts neutralization capacity against Omicron subvariants BA.1, BA.2 and BA.5 and can be predicted by anti-S antibody concentrations in serological assays. Front Immunol. (2023) 14:1170759. doi: 10.3389/fimmu.2023.1170759
58. Nurmi V, Knight C, Estcourt L, Hepojoki J, Lamikanra AA, Tsang HP, et al. The relationship between SARS-coV-2 neutralizing antibody titers and avidity in plasma collected from convalescent nonvaccinated and vaccinated blood donors. J Infect Dis. (2023) 228:245–50. doi: 10.1093/infdis/jiad070
59. Planas D, Peng L, Zheng L, Guivel-Benhassine F, Staropoli I, Porrot F, et al. Beta-variant recombinant booster vaccine elicits broad cross-reactive neutralization of SARS-CoV-2 including Omicron variants. Heliyon. (2024) 10:e27033. doi: 10.1016/j.heliyon.2024.e27033
60. Goodhue Meyer E, Simmons G, Grebe E, Gannett M, Franz S, Darst O, et al. Selecting COVID-19 convalescent plasma for neutralizing antibody potency using a high-capacity SARS-CoV-2 antibody assay. Transfusion. (2021) 61(4):1160–70. doi: 10.1111/trf.16321
61. Wendel S, Fachini R, Fontao-Wendel RCL, Mello R, Velasquez CV, MaChado RRG, et al. Surrogate test performance for SARS-CoV-2 neutralizing antibodies (nAbs) for convalescent plasma (CCP): How useful could they be? Transfusion. (2021) 61:3455–67. doi: 10.1111/trf.16714
62. Zhang S, Ma P, Orzechowski M, Lemmer A, Rzasa K, Bagnall J, et al. High-throughput neutralization and serology assays reveal correlated but highly variable humoral immune responses in a large population of individuals infected with SARS-coV-2 in the US between March and August 2020. mBio. (2023) 14:e0352322. doi: 10.1128/mbio.03523-22
63. Burnouf T, Gathof B, Bloch EM, Bazin R, de Angelis V, Patidar GK, et al. Production and quality assurance of human polyclonal hyperimmune immunoglobulins against SARS-coV-2. Transfusion Med Rev. (2022) 36(3):125–32. doi: 10.1016/j.tmrv.2022.06.001
64. Radosevich M, Burnouf T. Intravenous immunoglobulin G: trends in production methods, quality control and quality assurance. Vox Sang. (2010) 98:12–28. doi: 10.1111/j.1423-0410.2009.01226.x
65. Vandeberg P, Cruz M, Diez JM, Merritt WK, Santos B, Trukawinski S, et al. Production of anti-SARS-CoV-2 hyperimmune globulin from convalescent plasma. Transfusion. (2021) 61:1705–9. doi: 10.1111/trf.16378
66. Iannizzi C, Chai KL, Piechotta V, Valk SJ, Kimber C, Monsef I, et al. Convalescent plasma for people with COVID-19: a living systematic review. Cochrane Database Systematic Rev. (2023). doi: 10.1002/14651858.CD013600.pub6
67. Kreuzberger N, Hirsch C, Chai KL, Tomlinson E, Khosravi Z, Popp M, et al. SARS-CoV-2-neutralising monoclonal antibodies for treatment of COVID-19. Cochrane Database Systematic Rev. (2021) 9). doi: 10.1002/14651858.CD013825.pub2
68. Libster R, Pérez Marc G, Wappner D, Coviello S, Bianchi A, Braem V, et al. Early high-titer plasma therapy to prevent severe covid-19 in older adults. N Engl J Med. (2021) 384:610–8. doi: 10.1056/NEJMoa2033700
69. Sullivan DJ, Gebo KA, Shoham S, Bloch EM, Lau B, Shenoy AG, et al. Early outpatient treatment for covid-19 with convalescent plasma. N Engl J Med. (2022) 386:1700–11. doi: 10.1056/NEJMoa2119657
70. Shoham S, Bloch EM, Casadevall A, Hanley D, Lau B, Gebo K, et al. Transfusing convalescent plasma as post-exposure prophylaxis against severe acute respiratory syndrome coronavirus 2 (SARS-coV-2) infection: A double-blinded, phase 2 randomized, controlled trial. Clin Infect Dis. (2023) 76:e477–86. doi: 10.1093/cid/ciac372
71. Writing Committee for the REMAP-CAP Investigators, Estcourt LJ, Turgeon AF, McQuilten ZK, McVerry BJ, Al-Beidh F, et al. Effect of convalescent plasma on organ support-free days in critically ill patients with COVID-19: A randomized clinical trial. JAMA. (2021) 326:1690–702. doi: 10.1001/jama.2021.18178
72. Alemany A, Millat-Martinez P, Corbacho-Monne M, Malchair P, Ouchi D, Ruiz-Comellas A, et al. High-titre methylene blue-treated convalescent plasma as an early treatment for outpatients with COVID-19: a randomised, placebo-controlled trial. Lancet Respir Med. (2022) 10:278–88. doi: 10.1016/S2213-2600(21)00545-2
73. Tong A, Baumgart A, Evangelidis N, Viecelli AK, Carter SA, Azevedo LC, et al. Core outcome measures for trials in people with coronavirus disease 2019: respiratory failure, multiorgan failure, shortness of breath, and recovery. Crit Care Med. (2021) 49:503–16. doi: 10.1097/CCM.0000000000004817
74. Joyner MJ, Bruno KA, Klassen SA, Kunze KL, Johnson PW, Lesser ER, et al. Safety update: COVID-19 convalescent plasma in 20,000 hospitalized patients. Mayo Clin Proc. (2020) 95:1888–97. doi: 10.1016/j.mayocp.2020.06.028
75. ISBT Proposed standard definitions for surveillance of non-infectious adverse transfusion reactions (2011). Available online at: https://www.isbtweb.org/resource/en-2011-isbt-proposed-standard-definitions-for-surveillance-of-non-infectious-adverse-transfusion-reactions.html (Accessed June 7, 2024).
76. AABB-quick-reference-guide-nhsn-hemovigilance-module. Available online at: https://www.aabb.org/docs/default-source/default-document-library/resources/aabb-quick-reference-guide-nhsn-hemovigilance-module.pdf?sfvrsn=30f1600b_4 (Accessed June 7, 2024).
77. Leblanc JF, Germain M, Delage G, O’Brien S, Drews SJ, Lewin A. Risk of transmission of severe acute respiratory syndrome coronavirus 2 by transfusion: A literature review. Transfusion. (2020) 60:3046–54. doi: 10.1111/trf.16056
78. Lacombe K, Hueso T, Porcher R, Mekinian A, Chiarabini T, Georgin-Lavialle S, et al. Use of covid-19 convalescent plasma to treat patients admitted to hospital for covid-19 with or without underlying immunodeficiency: open label, randomized clinical trial. BMJ Med. (2023) 2:e000427. doi: 10.1136/bmjmed-2022-00042
79. Avendaño-Solá C, Ramos-Martínez A, Muñez-Rubio E, Ruiz-Antorán B, de Molina RM, Torres F, et al. A multicenter randomized open-label clinical trial for convalescent plasma in patients hospitalized with COVID-19 pneumonia. J Clin Invest. (2021) 131:p1–10. doi: 10.1172/JCI152740
80. Junqueira C, Crespo A, Ranjbar S, de Lacerda LB, Lewandrowski M, Ingber J, et al. FcgammaR-mediated SARS-CoV-2 infection of monocytes activates inflammation. Nature. (2022) 606:576–84. doi: 10.1038/s41586-022-04702-4
81. Sefik E, Qu R, Junqueira C, Kaffe E, Mirza H, Zhao J, et al. Inflammasome activation in infected macrophages drives COVID-19 pathology. Nature. (2022) 606:585–93. doi: 10.1038/s41586-022-04802-1
82. Carroll TD, Wong T, Morris MK, Di Germanio C, Ma ZM, Stone M, et al. Vaccine-boosted CCP decreases virus replication and hastens resolution of infection despite transiently enhancing disease in SARS-coV-2-infected hamsters. J Infect Dis. (2024) 229(6):1702–10. doi: 10.1093/infdis/jiad568
83. Group IS. Hyperimmune immunoglobulin for hospitalised patients with COVID-19 (ITAC): a double-blind, placebo-controlled, phase 3, randomised trial. Lancet. (2022) 399:530–40. doi: 10.1016/S0140-6736(22)00101-5
84. Boyarsky BJ, Werbel WA, Avery RK, Tobian AAR, Massie AB, Segev DL, et al. Antibody response to 2-dose SARS-coV-2 mRNA vaccine series in solid organ transplant recipients. JAMA. (2021) 325:2204–6. doi: 10.1001/jama.2021.7489
85. Valk SJ, Piechotta V, Chai KL, Doree C, Monsef I, Wood EM, et al. Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: a rapid review. Cochrane Database Syst Rev. (2020) 5:CD013600. doi: 10.1002/14651858.CD013600
86. Bar KJ, Shaw PA, Choi GH, Aqui N, Fesnak A, Yang JB, et al. A randomized controlled study of convalescent plasma for individuals hospitalized with COVID-19 pneumonia. J Clin Invest. (2021) 131. doi: 10.1172/JCI155114
87. Hirsch C, Park YS, Piechotta V, Chai KL, Estcourt LJ, Monsef I, et al. SARS-CoV-2-neutralising monoclonal antibodies to prevent COVID-19. Cochrane Database Systematic Rev. (2022) 6). doi: 10.1002/14651858.CD014945.pub2
88. Gharbharan A, Jordans CCE, GeurtsvanKessel C, den Hollander JG, Karim F, Mollema FPN, et al. Effects of potent neutralizing antibodies from convalescent plasma in patients hospitalized for severe SARS-CoV-2 infection. Nat Commun. (2021) 12:3189. doi: 10.1038/s41467-021-23469-2
89. Expert panel updates guidelines for clinical CCP use (2022). Available online at: https://www.aabb.org/news-resources/news/article/2022/04/13/aabb-expert-panel-updates-guidelines-for-clinical-ccp-use (Accessed July 27, 2022).
90. Thompson MA, Henderson JP, Shah PK, Rubinstein SM, Joyner MJ, Choueiri TK, et al. Association of convalescent plasma therapy with survival in patients with hematologic cancers and COVID-19. JAMA Oncol. (2021) 7:1167–75. doi: 10.1001/jamaoncol.2021.1799
91. Hueso T, Godron A-S, Lanoy E, Pacanowski J, Levi LI, Gras E, et al. Convalescent plasma improves overall survival in patients with B-cell lymphoid Malignancy and COVID-19: a longitudinal cohort and propensity score analysis. Leukemia. (2022) 36:1025–34. doi: 10.1038/s41375-022-01511-6
92. Stamatatos L, Czartoski J, Wan YH, Homad LJ, Rubin V, Glantz H, et al. mRNA vaccination boosts cross-variant neutralizing antibodies elicited by SARS-CoV-2 infection. Science. (2021) 372(6549):1413–8. doi: 10.1126/science.abg9175
93. Seidel A, Jahrsdörfer B, Körper S, Albers D, von Maltitz P, Müller R, et al. SARS-CoV-2 vaccination of convalescents boosts neutralization capacity against SARS-CoV-2 Delta and Omicron that can be predicted by anti-S antibody concentrations in serological assays. medRxiv. (2022), p1–21. doi: 10.1101/2022.01.17.22269201
94. Parri N, Lenge M, Buonsenso D, G. Coronavirus Infection in Pediatric Emergency Departments Research. Children with covid-19 in pediatric emergency departments in Italy. N Engl J Med. (2020) 383(2):187–90. doi: 10.1056/NEJMc2007617
95. Ma H, Hu J, Tian J, Zhou X, Li H, Laws MT, et al. A single-center, retrospective study of COVID-19 features in children: a descriptive investigation. BMC Med. (2020) 18:123. doi: 10.1186/s12916-020-01596-9
96. Zaffanello M, Piacentini G, Nosetti L, Franchini M. The use of convalescent plasma for pediatric patients with SARS-CoV-2: A systematic literature review. Transfus Apher Sci. (2021) 60:103043. doi: 10.1016/j.transci.2020.103043
97. Małecki P, Faltin K, Mania A, Mazur-Melewska K, Cwalińska A, Zawadzka A, et al. Effects and safety of convalescent plasma administration in a group of polish pediatric patients with COVID-19: A case series. Life (Basel). (2021) 11:p1–10. doi: 10.3390/life11030247
98. National COVID-19 Convalescent Plasma Project; Protocols for Pediatrics (2020). Available online at: https://ccpp19.org/healthcare_providers/component_3/protocols_for_pediatrics.html (Accessed July 27th 2022).
99. Convalescent plasma in patients admitted to hospital with COVID-19 (RECOVERY): a randomised controlled, open-label, platform trial. Lancet. (2021) 397:2049–59. doi: 10.1016/s0140-6736(21)00897-7
100. Gordon O, Brosnan MK, Yoon S, Jung D, Littlefield K, Ganesan A, et al. Pharmacokinetics of high-titer anti-SARS-CoV-2 human convalescent plasma in high-risk children. JCI Insight. (2021) 7(2):e151518. doi: 10.1172/jci.insight.151518
101. Bai H, Ji Y, Wang J, Zhang X. Efficacy of human coronavirus immune convalescent plasma for the treatment of corona virus disease -19 disease in hospitalized children: A protocol for systematic review and meta analysis. Medicine (Baltimore) (2020) 99(45):e22017. doi: 10.1097/MD.0000000000022017
102. Jacquot C, Gordon O, Noland D, Donowitz JR, Levy E, Jain S, et al. Multi-institutional experience with COVID-19 convalescent plasma in children. Transfusion. (2023) 63:918–24. doi: 10.1111/trf.17318
103. Arrieta A, Galvis AE, Osborne S, Morphew T, Imfeld K, Enriquez C, et al. Use of COVID-19 convalescent plasma for treatment of symptomatic SARS-coV-2 infection at a children’s hospital: A contribution to a still inadequate body of evidence. Children (Basel). (2023) 10:p1–12. doi: 10.3390/children10020350
104. Epstein J, Smid WM, Wendel S, Somuah D, Burnouf T. Use of COVID-19 convalescent plasma in low- and middle-income countries: a call for ethical principles and the assurance of quality and safety. Vox Sang. (2020) 116(1):13–14. doi: 10.1111/vox.12964
105. Bloch EM, Goel R, Wendel S, Burnouf T, Al-Riyami AZ, Ang AL, et al. Guidance for the procurement of COVID-19 convalescent plasma: differences between high- and low-middle-income countries. Vox Sang. (2020) 116(1):18–35. doi: 10.1111/vox.12970
106. Bloch EM, Goel R, Montemayor C, Cohn C, Tobian AAR. Promoting access to COVID-19 convalescent plasma in low- and middle-income countries. Vox Sang. (2021) 116(1):18–35. doi: 10.1016/j.transci.2020.102957
107. Roberts N, James S, Delaney M, Fitzmaurice C. The global need and availability of blood products: a modelling study. Lancet Haematol. (2019) 6:e606–15. doi: 10.1016/S2352-3026(19)30200-5
108. Weimer A, Tagny CT, Tapko JB, Gouws C, Tobian AAR, Ness PM, et al. Blood transfusion safety in sub-Saharan Africa: A literature review of changes and challenges in the 21st century. Transfusion. (2019) 59:412–27. doi: 10.1111/trf.14949
109. Busch MP, Bloch EM, Kleinman S. Prevention of transfusion-transmitted infections. Blood. (2019) 133:1854–64. doi: 10.1182/blood-2018-11-833996
110. Bloch EM, Vermeulen M, Murphy E. Blood transfusion safety in Africa: a literature review of infectious disease and organizational challenges. Transfus Med Rev. (2012) 26:164–80. doi: 10.1016/j.tmrv.2011.07.006
111. Senefeld JW, Johnson PW, Kunze KL, Bloch EM, van Helmond N, Golafshar MA, et al. Access to and safety of COVID-19 convalescent plasma in the United States Expanded Access Program: A national registry study. PloS Med. (2021) 18:e1003872. doi: 10.1371/journal.pmed.1003872
112. Joyner MJ, Carter RE, Senefeld JW, Klassen SA, Mills JR, Johnson PW, et al. Convalescent plasma antibody levels and the risk of death from covid-19. New Engl J Med. (2021) 384:1015–27. doi: 10.1056/NEJMoa2031893
113. Bloch EM, Tobian AAR, Shoham S, Hanley DF, Gniadek TJ, Cachay ER, et al. How do I implement an outpatient program for the administration of convalescent plasma for COVID-19? Transfusion. (2022) 62:933–41. doi: 10.1111/trf.16871
114. Wilson ML, Fleming KA, Kuti MA, Looi LM, Lago N, Ru K. Access to pathology and laboratory medicine services: a crucial gap. Lancet. (2018) 391:1927–38. doi: 10.1016/S0140-6736(18)30458-6
115. Focosi D, Franchini M, Maggi F. Modified hemagglutination tests for COVID-19 serology in resource-poor settings: ready for prime-time? Vaccines (Basel). (2022) 10:1–6. doi: 10.3390/vaccines10030406
116. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis. (2020) 20:e192–7. doi: 10.1016/s1473-3099(20)30483-7
117. Focosi D, Franchini M, Pirofski LA, Burnouf T, Paneth N, Joyner MJ, et al. COVID-19 convalescent plasma and clinical trials: understanding conflicting outcomes. Clin Microbiol Rev. (2022 35(3)):e0020021. doi: 10.1128/cmr.00200-21
118. Casadevall A, Pirofski LA, Joyner MJ. The principles of antibody therapy for infectious diseases with relevance for COVID-19. mBio. (2021) 12:1–13. doi: 10.1128/mBio.03372-20
119. Pan H, Peto R, Henao-Restrepo AM, Preziosi MP, Sathiyamoorthy V, Abdool Karim Q, et al. Repurposed antiviral drugs for covid-19 - interim WHO solidarity trial results. N Engl J Med. (2021) 384:497–511. doi: 10.1056/NEJMoa2023184
120. Axfors C, Janiaud P, Schmitt AM, Van’t Hooft J, Smith ER, Haber NA, et al. Association between convalescent plasma treatment and mortality in COVID-19: a collaborative systematic review and meta-analysis of randomized clinical trials. BMC Infect Dis. (2021) 21:1170. doi: 10.1186/s12879-021-06829-7
121. FDA. Regulatory Update: FDA Issues Revised CCP Guidance, AABB Updates CCP Toolkit. (2021). Available online at: https://www.aabb.org/news-resources/news/article/2022/01/11/regulatory-update-fda-issuesrevised-ccp-guidance-aabb-updates-ccp-toolkit (Accessed July 27th 2022).
122. Al-Riyami AZ, Schäfer R, van den Berg K, Bloch EM, Estcourt LJ, Goel R, et al. Clinical use of Convalescent Plasma in the COVID-19 pandemic: a transfusion-focussed gap analysis with recommendations for future research priorities. Vox Sang. (2021) 116:88–98. doi: 10.1111/vox.12973
123. Hartmann J, Bloch EM, Burnouf T. Experience with COVID-19 convalescent plasma provides vital guidance to future pandemics. Transfusion. (2022) 62:681–4. doi: 10.1111/trf.16810
Keywords: COVID-19, SARS-CoV-2, convalescent plasma, scoping review, clinical use, plasma collection, adult, pediatric
Citation: So-Osman C, Burnouf T, Al-Riyami AZ, Bloch EM, Estcourt L, Goel R, Tiberghien P, Vermeulen M, Wendel S and Wood EM (2024) The role of convalescent plasma and hyperimmune immunoglobulins in the COVID-19 pandemic, including implications for future preparedness. Front. Immunol. 15:1448720. doi: 10.3389/fimmu.2024.1448720
Received: 13 June 2024; Accepted: 21 August 2024;
Published: 09 September 2024.
Edited by:
Valentina Mazzotta, National Institute for Infectious Diseases Lazzaro Spallanzani (IRCCS), ItalyReviewed by:
Narayanaiah Cheedarla, Emory University, United StatesValeria De Giorgi, National Institutes of Health (NIH), United States
Copyright © 2024 So-Osman, Burnouf, Al-Riyami, Bloch, Estcourt, Goel, Tiberghien, Vermeulen, Wendel and Wood. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cynthia So-Osman, Yy5zb0BzYW5xdWluLm5s
†ORCID: Cynthia So-Osman, orcid.org/0000-0003-4151-2865
Thierry Burnouf, orcid.org/0000-0002-0507-9243
Arwa Z. Al-Riyami, orcid.org/0000-0001-8649-0650
Evan M. Bloch, orcid.org/0000-0001-8181-9517
Lise Estcourt, orcid.org/0000-0003-4309-9162
Ruchika Goel, orcid.org/0000-0001-9653-9905
Pierre Tiberghien, orcid.org/0000-0002-9310-8322
Marion Vermeulen, orcid.org/0000-0003-4383-4526
Silvano Wendel, orcid.org/0000-0002-1941-7733
Erica M. Wood, orcid.org/0000-0001-7527-2340
 Cynthia So-Osman
Cynthia So-Osman Thierry Burnouf
Thierry Burnouf Arwa Z. Al-Riyami5†
Arwa Z. Al-Riyami5† Lise Estcourt
Lise Estcourt Pierre Tiberghien
Pierre Tiberghien Silvano Wendel
Silvano Wendel Erica M. Wood
Erica M. Wood