- 1Department of Neurology, Brain Research Institute, Niigata University, Niigata, Japan
- 2Department of Translational Research, Brain Research Institute, Niigata University, Niigata, Japan
- 3Department of Pathology, Brain Research Institute, Niigata University, Niigata, Japan
- 4Department of Virology 1, National Institute of Infectious Diseases, Tokyo, Japan
- 5Department of Neurosurgery, Brain Research Institute, Niigata University, Niigata, Japan
Progressive multifocal leukoencephalopathy (PML) is a rare central nervous system disease caused by JC virus (JCV) infection. Human immunodeficiency virus (HIV) infection is the greatest risk factor for PML. Other immunological diseases, including systemic sarcoidosis, have also been reported as risk factors for PML. Herein, we report a case of PML co-occurring with neurosarcoidosis. Early diagnosis using brain biopsy and appropriate therapeutic interventions achieved favorable outcomes. PML in patients with active intracranial neurosarcoidosis is extremely rare. We believe that it is important to perform brain biopsy at an early stage to allow diagnosis, even for central nervous system involvement with a progressive parenchymal lesion in patients with sarcoidosis, if PML is possible.
1 Introduction
Progressive multifocal leukoencephalopathy (PML) is an opportunistic and rare central nervous system infection caused by infection with the JC virus (JCV). Human immunodeficiency virus (HIV) infection is the greatest risk factor for PML; however, other immunological diseases, including systemic sarcoidosis, have also been reported as risk factors (1). Herein, we report a case of PML co-occurring with neurosarcoidosis in a patient with systemic sarcoidosis. PML is extremely rare in patients with active intracranial neurosarcoidosis. Early diagnosis using brain biopsy and appropriate therapeutic interventions resulted in favorable outcomes.
2 Case description
2.1 History
A 65-year-old female presented with difficulties in speech, reading, and writing in November. She had medical histories of breast cancer and renal cancer 15 and 14 years earlier, respectively. She also had cutaneous, ocular, and pulmonary sarcoidosis 3 years before, which was diagnosed by skin biopsy. Due to the low disease activity of sarcoidosis, the patient did not receive immunosuppressive therapy. The patient was bothered by difficulties in speech, reading, and writing in January of following year. Brain magnetic resonance imaging (MRI) revealed a hyperintense lesion on diffusion-weighted imaging (DWI) and fluid-attenuated inversion recovery (FLAIR) imaging in the white matter of the left temporo-occipital region as depicted in Figure 1. The patient was initially suspected of cerebral infarction at a local hospital. However, her symptoms gradually worsened, and she was unable to understand conversations. The white matter lesion enlarged (Figure 1). In early February, the patient was referred to our hospital (Day 1).
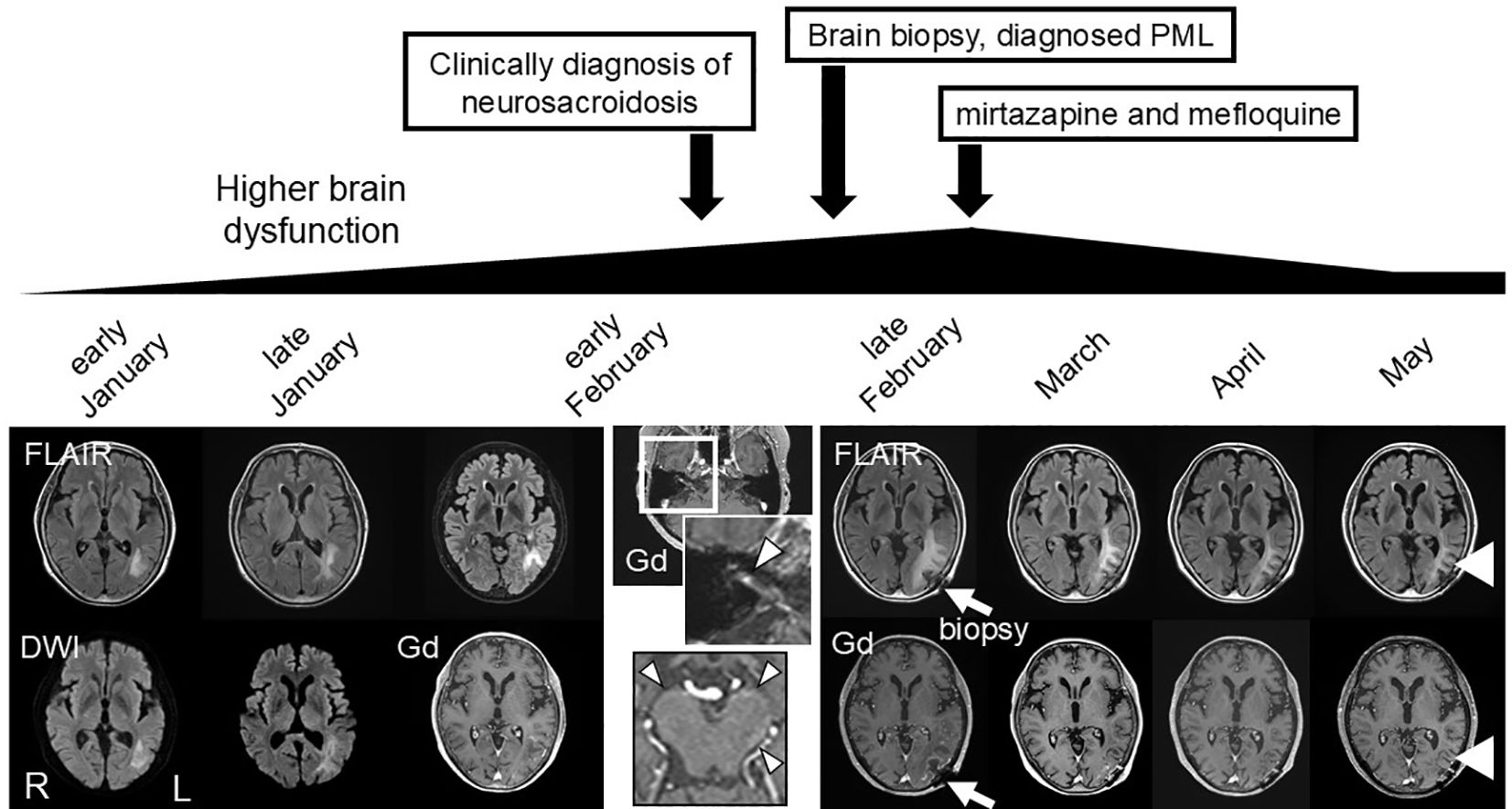
Figure 1. Clinical course and MRI findings. Magnetic resonance imaging (MRI) findings. Diffusion-weighted imaging (DWI) and fluid attenuated inversion recovery (FLAIR) revealed an enlarging hyperintense lesion in the left temporal, occipital, and parietal lobes before treatment. Gadolinium-enhanced T1-weighted images revealed an enhancing area in the lesion, and enhancement of the right cranial nerve VII (arrowhead), and leptomeninges in the brainstem (arrowhead). Brain biopsy was performed for the enhancing brain parenchymal lesion in late February (arrow), and mirtazapine and mefloquine treatment was started in March. Higher brain dysfunction and the MRI lesion gradually improved following the treatment (arrowhead).
2.2 Examination
Her general and physical findings at admission were normal. Neurological examination revealed higher brain dysfunction, disorientation, aphasia, right apraxia, alexia, agraphia, acalculia, and right-left disorientation. Laboratory examination revealed elevated soluble interleukin-2 receptor (sIL-2R) 2350 U/mL (normal range 122–496 U/mL), and angiotensin I-converting enzyme (ACE) 45.8 U/L (normal range 8.3–21.4 U/L). Hepatic, renal, and coagulation functions were normal. Cerebrospinal fluid (CSF) analysis yielded the following normal results: total cells, 4/mm3 (all mononuclear cells); albumin, 29 mg/dL; glucose, 52 mg/dL (blood glucose, 87 mg/dL); and CSF-ACE, 0.78 U/L. However, in the CSF, the values of protein, 59 mg/dL, β2-microglobulin 5.51 μg/mL, sIL-2R 195 U/mL, and the cluster of differentiation (CD) 4/8 ratio 10.4 were elevated. Oligoclonal bands were positive. CSF cultures were negative for both bacterial and acid-fast bacillus infections. Right facial nerve palsy developed on the second day of hospitalization. On day 3, gadolinium-enhanced MRI revealed enhancement of the leptomeninges at the brainstem and the right cranial nerve VII, characteristic MRI findings for neurosarcoidosis, with a left temporo-occipital parenchymal lesion (Figure 1).
3 Diagnostic assessment
Based on the MRI findings and results of the CSF analysis, neurosarcoidosis was strongly suggested initially. However, the white matter lesion rapidly extended to the right temporo-parieto-occipital lesion, which seemed atypical as a sarcoid lesion. Open biopsy was performed through craniotomy for the left temporo-occipital lesion to explore the possibility of lymphoma or PML (day 7). Histopathological examination revealed multiple small demyelinating foci with well-preserved white-matter axons (Figure 2). Oligodendroglial enlarged nuclei were scattered mainly in the periphery of the demyelinating foci, some of which were labelled with anti-JCV antibody. Although inflammatory cell infiltration was observed in the perivascular area, non-caseating granulomas, a characteristic histological feature of sarcoidosis, were not evident. Based on these findings, the diagnosis of PML was confirmed. Then mirtazapine and mefloquine were administered. One month later, PCR for JCV performed at National Institute of Infectious Diseases on the formalin-fixed paraffin-embedded (FFPE) brain tissue (1170 copies/cell) and CSF (205 copies/ml) revealed positive results, supporting the diagnosis of PML. After starting therapy for PML, the patient’s higher brain dysfunction and the MRI findings gradually improved (Figure 1). The patient was discharged in April, and her clinical course after discharge was uneventful. She could walk by herself, and could perform daily activities with her family’s support.
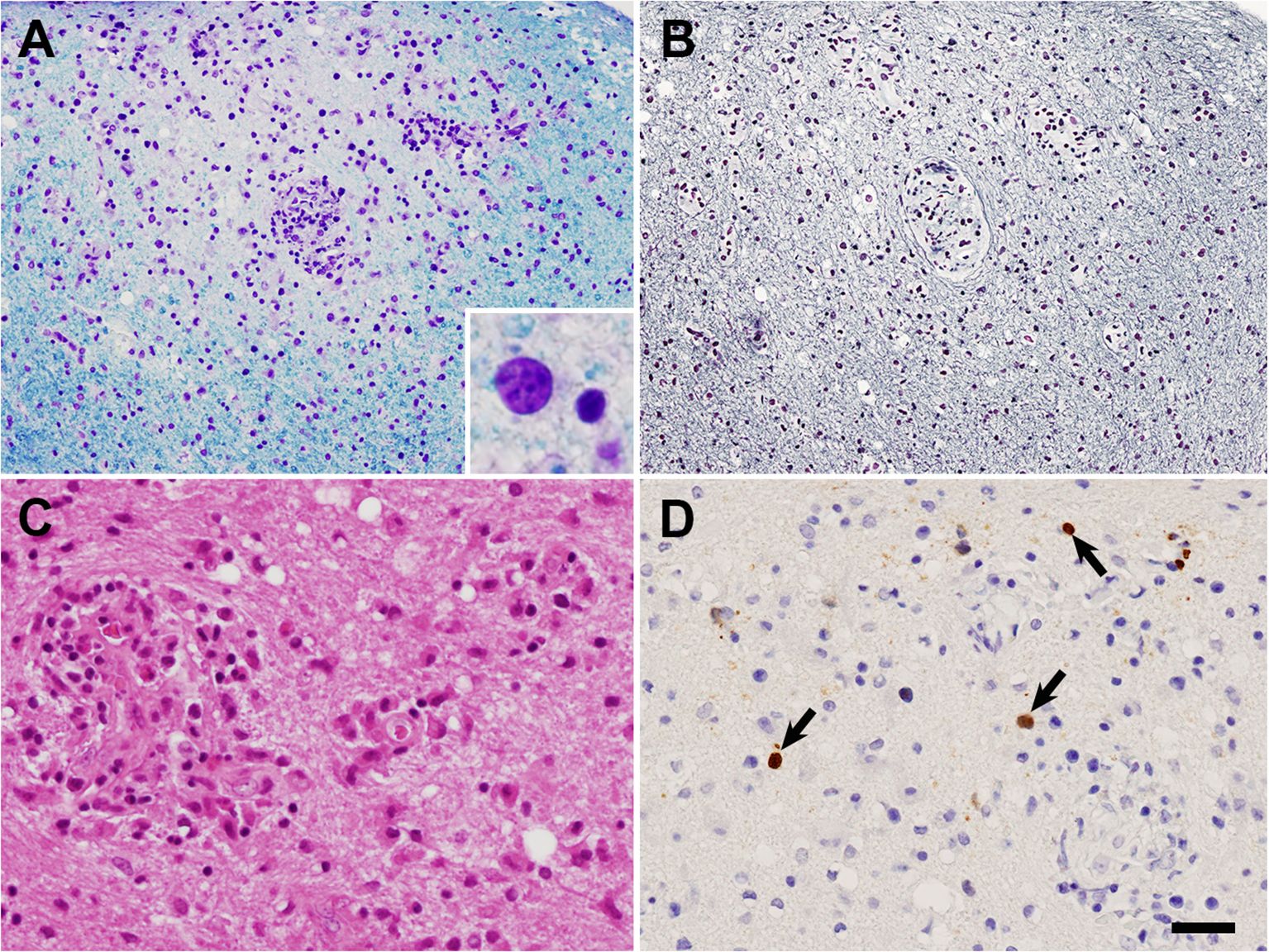
Figure 2. Histological findings of brain biopsy. (A, B) Adjacent sections of the white matter. A small ill-defined demyelinating lesion with well-preserved axons can be observed. Oligodendroglial enlarged nuclei [inset in (A)] were scattered mainly in the periphery of the lesion, on both (A), Klüver-Barrera staining; and (B), Bodian staining. (C) Reactive astrocytes and perivascular inflammatory cell infiltration in the demyelinating lesion on Hematoxylin-eosin staining. (D) Oligodendroglial JCV-positive nuclei (arrows) in the demyelinating lesion observed following JCV VP1 immunohistochemistry. Bar = 50 μm in (A, B), 25 μm in (C, D), 7 μm in inset in (A).
4 Discussion
Herein, we report a case of PML complicates sarcoidosis with CNS involvement. Avoiding inappropriate steroid therapy and treatment with mirtazapine and mefloquine for PML may have resulted in a favorable prognosis in this patient. Although HIV infection is the largest risk factor for PML (1), it is crucial to recognize that other immunosuppressive or immunological diseases, such as multiple sclerosis and systemic sarcoidosis, also increase the risk of PML. Systemic sarcoidosis is known to be complicated by PML, even in the absence of therapeutic immune suppression (2); however, PML complicates neurosarcoidosis has rarely been reported (3), so PML and neurosarcoidosis likely co-occurred. We initially diagnosed the patient with neurosarcoidosis and planned to administer steroids for following reasons: First, the patient presented with facial nerve palsy, and gadolinium-enhanced MRI revealed enhancement of the leptomeninges and the right cranial nerve VII (4). Second, CSF analysis revealed increased CSF-CD4/CD8 ratio. CSF-CD4/CD8 ratio >5 is highly suggestive of active neurosarcoidosis (5). These findings strongly suggested a clinical diagnosis of neurosarcoidosis, although this was not pathologically proven. However the extending white matter lesion was atypical as a neurosarcoid lesion, a brain biopsy was performed. Finally, the patient was diagnosed PML co-occurring with neurosarcoidosis. Steroid administration has been shown to worsen PML (6); therefore, we avoided steroid administration for neurosarcoidosis, and initiated PML therapy (7). While there is very little data on the effect of mirtazapine and mefloquine on the sarcoidosis-associated PML disease course (3), we administered them. Therefore, it is unclear whether the improvement in symptoms is due to the effectiveness of mirtazapine and mefloquine or occurs naturally. Anti-TNF-α therapy has been reported to be effective (8). In our case, anti-TNF-α therapy may have been a therapeutic candidate. Non-HIV-associated PML has a poor prognosis, with an overall median survival of only 3 months (9). However, in our patient, prioritizing PML therapy led to a favorable clinical course.
5 Conclusion
Brain biopsy is crucial for the accurate and rapid diagnosis of PML, even in systemic sarcoidosis with active CNS involvement. When PML coexists with neurosarcoidosis, prioritizing PML therapy and avoiding immunosuppressive therapy can lead to a favorable prognosis.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical approval was not required for the studies on humans in accordance with the local legislation and institutional requirements because only commercially available established cell lines were used. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
QW: Writing – original draft, Data curation. ST: Writing – review & editing, Conceptualization. KO: Writing – review & editing. MT: Writing – review & editing, Investigation. AK: Writing – review & editing, Investigation. KN: Writing – review & editing, Investigation. MO: Writing – review & editing, Investigation. MK: Writing – review & editing, Conceptualization. OO: Writing – review & editing, Supervision.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was partly supported by the Research Committee of Prion Disease and Slow Virus Infection, Research on Policy Planning and Evaluation for Rare and Intractable Diseases, Health and Labor Sciences Research Grants, Ministry of Health, Labor and Welfare, Japan, and JSPS KAKENHI (grant no. 21K07450).
Acknowledgments
Real-time PCR for JCV in brain tissue was performed by Dr. Kenta Takahashi and Dr. Tadaki Suzuki in the Department of Pathology of National Institute of Infectious Diseases.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Tan CS, Koralnik IJ. Progressive multifocal leukoencephalopathy and other disorders caused by JC virus: clinical features and pathogenesis. Lancet Neurol. (2010) 9:425–37. doi: 10.1016/S1474-4422(10)70040-5
2. McEntire CRS, Fletcher A, Toledano M, Epstein S, White E, Tan CS, et al. Characteristics of progressive multifocal leukoencephalopathy associated with sarcoidosis without therapeutic immune suppression. JAMA Neurol. (2023) 80:624–33. doi: 10.1001/jamaneurol.2023.0841
3. Jamilloux Y, Néel A, Lecouffe-Desprets M, Fèvre A, Kerever S, Guillon B, et al. Progressive multifocal leukoencephalopathy in patients with sarcoidosis. Neurology. (2014) 82:1307–13. doi: 10.1212/WNL.0000000000000318
4. Nwebube CO, Bou GA, Castilho AJ, Hutto SK. Facial nerve palsy in neurosarcoidosis: clinical course, neuroinflammatory accompaniments, ancillary investigations, and response to treatment. J Neurol. (2022) 269:5328–36. doi: 10.1007/s00415-022-11189-6
5. Chazal T, Costopoulos M, Maillart E, Fleury C, Psimaras D, Legendre P, et al. The cerebrospinal fluid CD 4/CD 8 ratio and interleukin-6 and-10 levels in neurosarcoidosis: a multicenter, pragmatic, comparative study. Eur J Neurol. (2019) 26:1274–80. doi: 10.1111/ene.13975
6. Tawara T, Kai H, Kageyama M, Akiyama T, Matsunaga T, Sakuma A, et al. A case report of progressive multifocal leukoencephalopathy during steroid treatment for ANCA-associated renal vasculitis. CEN Case Rep. (2020) 9:354–8. doi: 10.1007/s13730-020-00482-w
7. Kizaki T, Kanazawa M, Ishiguro T, Natsumeda M, Tada M, Shimizu H, et al. Indications for a brain biopsy in neurological diseases of unknown etiology: The role of magnetic resonance imaging findings and liquid biopsy in yielding definitive pathological diagnoses. J Neurol Sci. (2024) 463:123150. doi: 10.1016/j.jns.2024.123150
8. Rosenkranz SC, Häußler V, Kolster M, Willing A, Matschke J, Röcken C, et al. Treating sarcoidosis-associated progressive multifocal leukoencephalopathy with infliximab. Brain Commun. (2022) 4:fcab292. doi: 10.1093/braincomms/fcab292
Keywords: PML, neurosarcoidosis, brain biopsy, avoiding immunosuppressive therapy, favorable prognosis
Citation: Wang Q, Tsuboguchi S, Okamoto K, Tada M, Kakita A, Nakamichi K, Oishi M, Kanazawa M and Onodera O (2024) Case report: Progressive multifocal leukoencephalopathy co-occurring with neurosarcoidosis: early brain biopsy and appropriate therapy for PML resulted in a favorable prognosis. Front. Immunol. 15:1447992. doi: 10.3389/fimmu.2024.1447992
Received: 12 June 2024; Accepted: 26 September 2024;
Published: 11 October 2024.
Edited by:
Zhenlong Liu, McGill University, CanadaReviewed by:
Elena Grebenciucova, Northwestern University, United StatesCaleb McEntire, Massachusetts General Hospital and Harvard Medical School, United States
Copyright © 2024 Wang, Tsuboguchi, Okamoto, Tada, Kakita, Nakamichi, Oishi, Kanazawa and Onodera. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shintaro Tsuboguchi, dHN1Ym9ndWNoaUBicmkubmlpZ2F0YS11LmFjLmpw
 Qiannan Wang1
Qiannan Wang1 Shintaro Tsuboguchi
Shintaro Tsuboguchi Kouichirou Okamoto
Kouichirou Okamoto Mari Tada
Mari Tada Kazuo Nakamichi
Kazuo Nakamichi Makoto Oishi
Makoto Oishi Masato Kanazawa
Masato Kanazawa Osamu Onodera
Osamu Onodera