- 1Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, United States
- 2Department of Medicine, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA, United States
- 3Office of the Clinical Director, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, United States
- 4National Center for Advancing Translational Sciences, National Institutes of Health, Bethesda, MD, United States
- 5Dermatology Branch, National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Institutes of Health, Bethesda, MD, United States
- 6Laboratory of Viral Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, United States
- 7Department of Immunobiology, College of Medicine, BIO5 Institute, Cancer Biology Graduate Interdisciplinary Program, Genetics Graduate Interdisciplinary Program, University of Arizona Cancer Center, University of Arizona, Tucson, AZ, United States
- 8Office of the Clinical Director, National Human Genome Research Institute, National Institutes of Health, Bethesda, MD, United States
- 9Gynecology/Obstetrics Unit, Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) San Raffaele Scientific Institute, Vita-Salute San Raffaele University, Milan, Italy
- 10Rehabilitation Medicine Department, Clinical Center, National Institutes of Health, Bethesda, MD, United States
- 11Department of Surgery, Eastern Virginia Medical School, Norfolk, VA, United States
- 12Liver Diseases Branch, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD, United States
- 13Retired, Bethesda, MD, United States
- 14Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD, United States
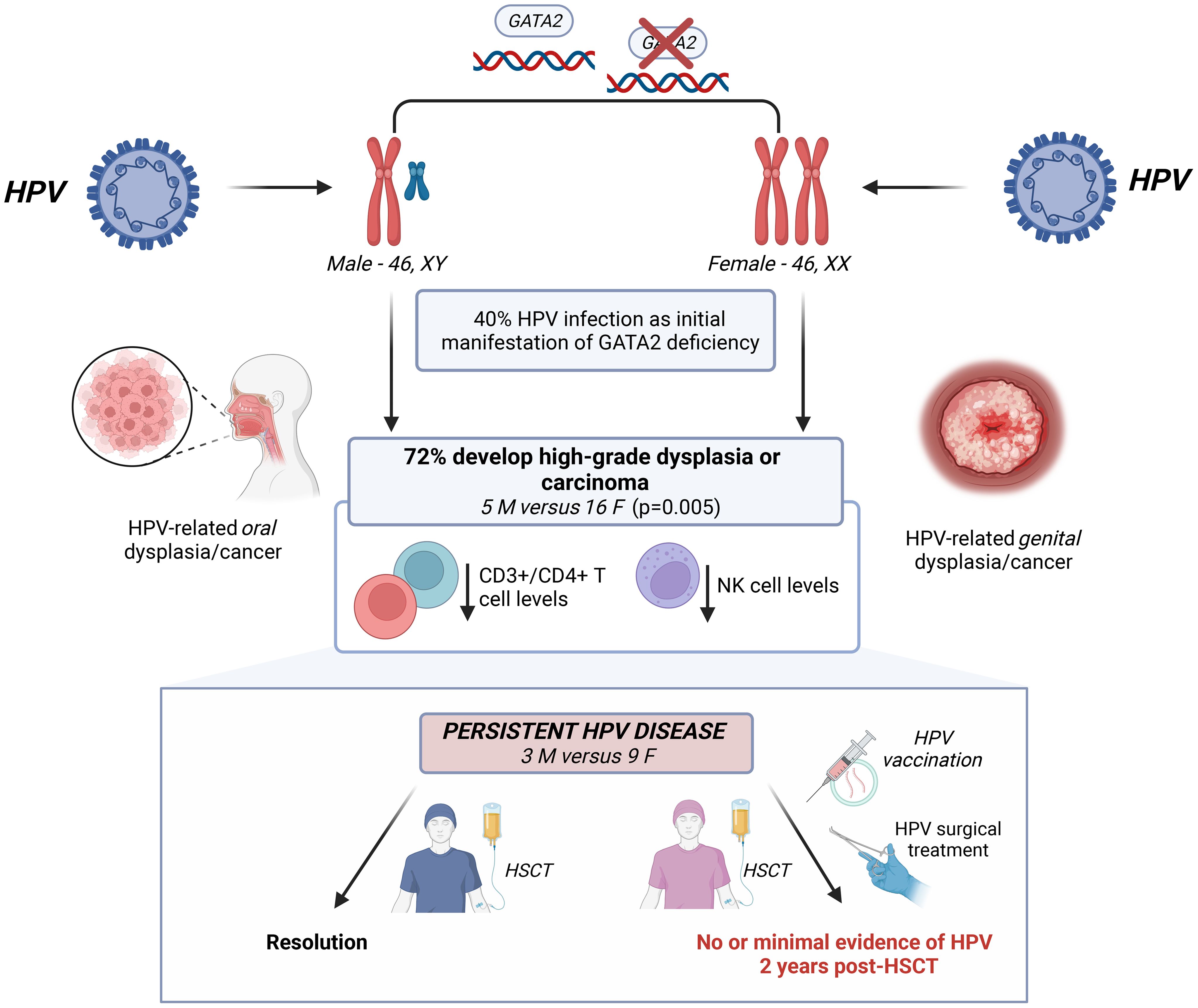
Objective: Patients with pathogenic variants in the GATA Binding Protein 2 (GATA2), a hematopoietic transcription factor, are at risk for human papillomavirus-related (HPV) anogenital cancer at younger than expected ages. A female cohort with GATA2 haploinsufficiency was systematically assessed by two gynecologists to characterize the extent and severity of anogenital HPV disease, which was also compared with affected males.
Methods: A 17-year retrospective review of medical records, including laboratory, histopathology and cytopathology records was performed for patients diagnosed with GATA2 haploinsufficiency followed at the National Institutes of Health. Student’s t-test and Mann-Whitney U test or Fisher’s exact test were used to compare differences in continuous or categorical variables, respectively. Spearman’s rho coefficient was employed for correlations.
Results: Of 68 patients with GATA2 haploinsufficiency, HPV disease was the initial manifestation in 27 (40%). HPV occurred at median 18.9 (15.2-26.2) years in females, and 25.6 (23.4-26.9) years in males. Fifty-two (76%), 27 females and 25 males, developed HPV-related squamous intraepithelial lesions (SIL) including two males with oral cancer. Twenty-one patients developed anogenital high-grade SIL (HSIL) or carcinoma (16 females versus 5 males, (59% versus 20%, respectively, p=0.005) at median 27 (18.6-59.3) years for females and 33 (16.5-40.1) years for males. Females were more likely than males to require >2 surgeries to treat recurrent HSIL (p=0.0009). Of 30 patients undergoing hematopoietic stem cell transplant (HSCT) to manage disease arising from GATA2 haploinsufficiency, 12 (nine females, three males) had persistent HSIL/HPV disease. Of these nine females, eight underwent peri-transplant surgical treatment of HSIL. Five of seven who survived post-HSCT received HPV vaccination and had no or minimal evidence of HPV disease 2 years post-HSCT. HPV disease persisted in two receiving immunosuppression. HPV disease/low SIL (LSIL) resolved in all three males.
Conclusion: Females with GATA2 haploinsufficiency exhibit a heightened risk of recurrent, multifocal anogenital HSIL requiring frequent surveillance and multiple treatments. GATA2 haploinsufficiency must be considered in a female with extensive, multifocal genital HSIL unresponsive to multiple surgeries. This population may benefit from early intervention like HSCT accompanied by continued, enhanced surveillance and treatment by gynecologic oncologists and gynecologists in those with anogenital HPV disease.
1 Introduction
GATA Binding Protein 2 (GATA2) haploinsufficiency is an autosomal dominant or sporadically inherited primary immunodeficiency first genetically described in 2011. Nonsense, regulatory, intronic, and missense mutations in GATA2 lead to clinical manifestations refractory to medical and surgical therapy including human papillomavirus (HPV) leading to high grade squamous intraepithelial lesions (HSIL), hematologic malignancy, and other associated conditions (1, 2). GATA2 is a transcription factor predominately expressed in early hematopoietic progenitor cells, adult stem cells, and mast cells (3, 4). GATA2 haploinsufficiency occurs due to inactivation or loss of one copy of GATA2, and has been associated with: monocytopenia and nontuberculous mycobacterial (NTM) infections (MonoMac Syndrome), dendritic/monocyte/B/Natural Killer (NK)-cell lymphoid deficiencies (DCML), familial myelodysplastic syndrome, Emberger syndrome, and classical NK cell deficiency (5). Allogeneic hematopoietic stem cell transplantation (HSCT) is curative of the hematologic and immunologic manifestations of GATA2 haploinsufficiency (6, 7).
HPV disease has been reported in 79% of patients with GATA2 haploinsufficiency (8). Cutaneous common warts, and oropharyngeal and anogenital disease are prevalent with 35% of patients developing HSIL caused by HPV (5, 9). In patients followed at the National Institutes of Health (NIH) Clinical Center, most GATA2 haploinsufficient patients had marked monocytopenia, B lymphocytopenia or NK lymphocytopenia, which developed and worsened over time (5, 10).
Patients with primary immunodeficiency often present with phenotypical features typical to each immune dysfunction including HPV infection which underscores the important role the immune system plays in control of HPV disease and enables clinicians to identify particular primary immunodeficiencies. As described by Leiding and Holland, HPV infection that becomes severe and recalcitrant to treatment is common to primary immunodeficiencies such as warts, hypogammaglobulinemia, infections, myelokathexis (WHIM), idiopathic CD4 lymphopenia, dedicator of cytokinesis 8 (DOCK8) deficiency and Emberger syndrome to name a few (11). Although the frequency of severe, genital HPV disease in these immunodeficiencies is not well-established given the rarity overall of these disorders, GATA2 haploinsufficiency is unique in that HPV disease may be the first or the earliest phenotypic presentation of immune dysfunction.
HPV is a small non-enveloped DNA virus; persistent HPV infection with high-risk types is a risk factor for anogenital and oral malignancy (12–14). HPV is one of the most common sexually transmitted infections among women and men in the United States, with a 22.0% prevalence of disease-associated HPV infection in the sexually active population (15, 16). High risk oncogenic HPV types are associated with >93% of cervical cancers, >80% of vulvar HSIL, >92% of vaginal HSIL, 50% of penile cancers, and 20% of oropharyngeal cancers (17). In the general population, the latency from HPV infection to development of HSIL varies depending on high-risk HPV type (18).
The risk factors for developing persistent HPV disease are influenced by viral (genetic and possibly epigenetic), host, and behavioral factors such as smoking, multiparity, and long-term use of hormonal contraceptives (15); immune competence is critical, especially NK cytotoxicity (19, 20). We hypothesized that individuals with GATA2 haploinsufficiency might exhibit more pronounced clinical manifestations of HPV disease than the general population, with sex differences between males and females potentially accounting for some variations in clinical manifestations and disease severity.
2 Materials and methods
2.1 Study participant details
A retrospective review of medical records from May 2000 to February 2017 at the National Institutes of Health (NIH) was performed to identify patients with known GATA2 mutations, including clinical notes, patient questionnaires, laboratory, histopathology, and cytopathology records. All patients provided written informed consent and were enrolled on protocols approved by an NIH IRB in which the natural history of GATA2 mutations was studied (ClinicalTrials.gov Identifier: NCT01905826, NCT00018044, NCT00404560).
All patients with known GATA2 haploinsufficiency enrolled in these natural history studies were included. Their charts from the NIH Clinical Center’s electronic medical record were retrospectively reviewed for symptoms commonly observed with GATA2 haploinsufficiency. Information about time of onset and disease progression for common cutaneous and anogenital warts, hematologic abnormalities, infections (including nontuberculous mycobacterial, fungal and non-HPV viral infections), myelodysplastic syndrome, and allogeneic HSCT and post-HSCT HPV disease were summarized. Values for NK cells, CD3+/CD4+ T-helper cells and monocytes from the most recent visit or immediately prior to HSCT for those who underwent HSCT, were abstracted. In general, the female patients over age 18 were systematically examined and assessed by one of 2 gynecologists (PS and MM) and included review of outside gynecology records. Male patients with anogenital HPV disease and female patients with perianal and anal HPV disease were assessed by a single general surgeon (MH) and gastroenterologist (TH). This report includes a subset of a larger published cohort (8) and unpublished data narrowed to the time period in which female subjects were evaluated based on the standard examination by the aforementioned gynecological team. For subjects for whom only the year was provided as the date of diagnosis, the month and date were assigned as June 15th.
2.2 HPV infection
HPV infection was identified based on clinical, pathologic or laboratory evidence and its location. Extensive oropharyngeal or anogenital HPV disease was defined as multifocal disease that, in the lower genital tract spanned multiple sites such as vulvar and vaginal, vulvar and cervical, or scrotal and inguinal. Severity of HPV disease was categorized as follows: mild HPV disease (localized genital or common cutaneous in the absence of histologically confirmed severe dysplasia; responsive to local treatments); severe HPV disease (multifocal anogenital or oropharyngeal HPV disease, histologically confirmed severe dysplasia to carcinoma in situ; high-grade lesions difficult to treat and non-responsive to multiple local treatments). Pathological grade was determined by evidence of low grade squamous intraepithelial lesions (LSIL), HSIL or malignancy on histopathology. Laboratory confirmation of HPV infection was determined by HPV DNA detection. HPV typing from cervical or anal swabs was performed commercially using hybrid capture pooled for high and low risk types (Quest Diagnostics Nichols Institute, Chantilly, VA).
2.3 HPV genotyping from lesional tissue
Clinical biopsies obtained from some subjects receiving care at the NIH Clinical Center were genotyped for HPV as previously described (21). One µl of extracted DNA was amplified using the SPF10 primer set (22). PCR amplicons were cloned into the TOPO-TA cloning kit (ThermoFisher). Ten to 22 clones were isolated and sequenced using the M13F and M13R primers. Primer sequences used are shown in Supplementary Table 1.
2.4 HPV post-HSCT
Resolution and persistence of HPV disease were described at 2 years posttransplant based on pretransplant history. Peri-HSCT and post-HSCT management and clinical examination findings at the most recent post-HSCT follow-up are described. Some subjects underwent treatment of pre-malignant anogenital lesions in the six months prior to transplant and others had treatment delayed until soon after HSCT.
2.5 Statistical analysis
Shapiro-Wilk test was employed to assess the normality of the data distribution. The Student’s t-test was used to compare differences in continuous normally distributed variables, which are presented as mean ± standard deviation (range). The Mann-Whitney U test was used to compare differences in continuous not normally distributed variables, which are presented as median (interquartile range, IQR). Fisher’s exact test was employed to compare differences in categorical variables, which are reported as absolute values (%). Comparisons between groups were based on biological sex. Immunologic values were stratified into quartiles given the nonparametric distribution of immunologic laboratory values. Spearman’s rho coefficient was employed as a nonparametric measure of the correlation between immunologic laboratory values and the incidence and severity of HPV disease. Correlation coefficients were interpreted as follows: 0.00-0.19 as very weak, 0.20-0.39 as weak, 0.40-0.59 as moderate, 0.60-0.79 as strong, and 0.80-1.00 as very strong (23). Positive and negative values were interpreted as positive or negative correlations, respectively. STATA version 18 software (Stata Corp LLC, 2021, College Station, TX, USA) was used for all statistical analyses. p values <0.05 were considered significant.
3 Results
We studied 68 patients with GATA2 haploinsufficiency followed at the NIH. The median age of onset of GATA2 haploinsufficiency signs and symptoms in this cohort was 15.9 years in females (9.9 – 23.7 years), and 13.8 years in males (11.2 – 22.9 years). HPV disease was the initial manifestation of GATA2 haploinsufficiency in 27 of 68 (40%) patients. Age at first HPV manifestation was 18.9 in females (15.2-26.2 years), and 25.6 years in males (23.4-26.9 years). Thirty-four of 68 (50%) patients were infected with a nontuberculous mycobacteria infection and 39 of 68 (56%) progressed to myelodysplastic syndrome.
3.1 HPV infection and disease manifestations
HPV disease was reported in a total of 52 of 68 patients, 27 of 36 (75%) females and 25 of 32 (78%) males (Table 1, Figure 1). Twenty-one patients developed anogenital HSIL or carcinoma (16 females versus 5 males, (59% versus 20% of those affected by HPV, respectively, p=0.005). The mean time from initial HPV diagnosis to first HSIL in patients with GATA2 haploinsufficiency was 9.36 ± 7.8 years (range: at first clinical evaluation to 23 years), 8.96 ± 7.68 years (range: 0 – 23.9) in females compared to 10.65 ± 9.26 years (range: 2.5 – 23) for males (p = 0.717). The majority of patients developed HPV-associated HSIL by their early 30s, regardless of biological sex (median age in females: 27 years (range: 18.6-59.3), in males: 33 years (range: 16.5-40.1), Figure 1). In the 10 patients under age 20 (5 males, 5 females), only one male had evidence of genital HPV disease. Seventeen patients developed oral (2 males) or anogenital carcinoma or carcinoma in situ (2 males; 13 females). The two cases with oropharyngeal cancer were successfully treated with medical and surgical treatment. Sixteen female patients had severe, multifocal anogenital HPV with the most frequent areas of involvement as vulvar (n=15) and cervical (n=13) sites. Surgical management for HPV/HSIL/cancer was reported in 24 patients. Females were more likely than males to undergo medical or surgical treatment of HPV/HSIL (19/20 females versus 5/12 males p=0.0006). In addition, a greater number of females were significantly more likely than males to need more than 2 surgical treatments to treat recurrent disease (range 3 to 20+ procedures, 12/19 females versus 1/5 males p=0.0009). Surgical treatment varied depending on anatomic location. Cervical HSIL was treated by cone biopsy, loop electrocautery excision procedure, or laser ablation of the cervix. Vaginal HSIL was treated by surgical laser ablation of lesions. Vulvar and other external anogenital HSIL was treated with surgical excision and laser or plasma-jet ablation of lesions. Five female patients underwent vulvectomies, of which two required skin grafting. One vulvectomy patient required a temporary diverting colostomy and one required co-incident total hysterectomy.
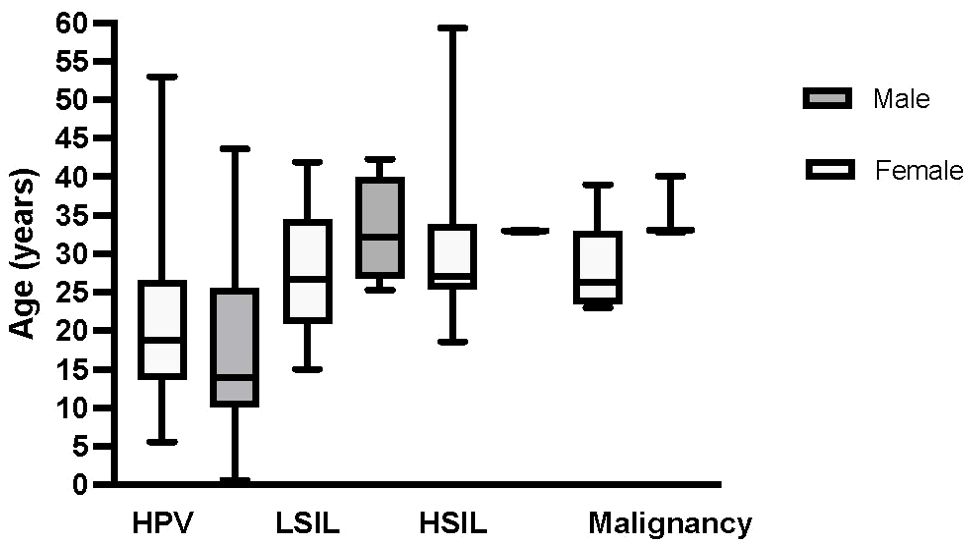
Figure 1. Median age of HPV disease diagnosis in female and male patients with GATA2 haploinsufficiency. The y-axis represents the age, and the x-axis shows HPV disease progression to cancer to include initial HPV disease diagnosis, low-grade squamous intraepithelial lesion (LSIL), high-grade squamous intraepithelial lesion (HSIL), and HPV-related malignancy that includes either carcinoma in situ or carcinoma. Box plots depict females in light grey and males in dark grey. Median age and range by sex for each HPV disease category is as follows: HPV at 19 (6 – 53) years and 14 (3 – 40) years in females and males, respectively, LSIL at 27 (15 – 42) years and 32 (25 – 33) years in females and males, respectively, HSIL at 27 (18 – 59) years and 33 (33 – 40) years in females and males, respectively and malignancy at 33 (23 – 59) years and 36 (33 – 40) years in females and males, respectively.
HPV genotype was identified by PCR on 9 histological specimens obtained at NIH of which 8 were vulvar and 1 was cervical (Table 2). HPV-16 and -18 were identified in 6 of 9 lesions. Two HSIL vulvar specimens only expressed HPV-66, a weakly carcinogenic HPV type and one LSIL cervical specimen expressed HPV-90 (23, 24). Three different HPV types were identified in two separate samples.
We then sought to analyze immunologic subtype values and HPV disease incidence and severity in the overall cohort and in sex-specific cohorts (Supplementary Table 2). Prior to transplant across the whole cohort, there were positive correlations among the various immune cell lineages, specifically among NK cell, CD3+/CD4+ T cell, and monocyte levels. Notably, higher CD3+/CD4+ T cell levels were negatively correlated with HPV incidence (rs=-0.339, p=0.008), while higher NK cell levels were negatively correlated with both HPV incidence (rs=-0.377, p=0.002) and severity (rs=-0.3151, p=0.0136). In sex-specific quartiles, higher NK cell levels in males were negatively correlated with HPV presence (rs=-0.397, p=0.033), while higher CD3+/CD4+ T cell levels were negatively associated with both HPV incidence (rs=-0.383, p=0.039) and severity (rs=-0.415, p=0.024). No significant correlations were observed between female-specific immune cell quartiles and HPV presence or severity.
3.2 HPV and HSCT
Thirty patients (18 females and 12 males) underwent HSCT. Nine of 18 females had anogenital HSIL prior to transplant with seven having undergone more than four surgical treatments. Five of nine females underwent surgical treatment prior to and three others after HSCT.
All females underwent anogenital assessment post-HSCT for evidence of HPV disease. Of the seven females who survived 2 years post-HSCT, five had no or minimal evidence of persistent/recurrent HPV disease. These five females all received HPV vaccination as part of routine post-transplant care. The two others who continued immunosuppression did not undergo HPV vaccination. Three of the 12 males who underwent HSCT had HPV disease prior to transplant involving penis, anal, or oral cavity. All three males experienced resolution of their LSIL HPV disease after HSCT without surgical intervention.
4 Discussion
4.1 Summary of main results
GATA2 haploinsufficiency includes early-onset multifocal, recurrent, HPV-related genital squamous intraepithelial neoplasia and carcinoma, disproportionately affecting females and frequently involving both the vulva and cervix. Multilineage cytopenias involving NK cells, B cells and monocytes are common and characteristic of GATA2 haploinsufficiency, as is progression to myelodysplastic syndrome and acute myeloid leukemia, which suggests early HSCT would be beneficial (5–7). Importantly, in 70% of our patients, severe infection, including HPV, was the initial presentation of GATA2 haploinsufficiency (2). We cannot determine whether NK or T cells are the critical elements in the development of severe, chronic HPV disease in GATA2 haploinsufficiency, as both were more depleted in HPV-affected than non-affected individuals; the lack of association with monocyte numbers suggest that monocytes are not the critical elements in HPV disease. A report of a patient with a pathogenic germline mutation in the interleukin-2 receptor subunit gamma gene demonstrated that restoring natural killer cell function with HSCT facilitated treatment of relapsing HPV disease (20).
HPV morbidity in GATA2 haploinsufficiency is high. Female patients undergo extensive, disfiguring, surgical excisions at young ages including hysterectomy and vulvectomy for HSIL or malignant disease. These procedures, especially vulvectomy, can have harmful effects on mental health and sexual function (25). Undergoing complex surgery increases the risk of complications including surgical diversions related to anogenital malignancies. Female patients with multifocal anogenital sites of HPV disease or those requiring several surgical interventions for HPV must warrant evaluation for GATA2 haploinsufficiency.
Characterizations of HPV types responsible for dysplasia or carcinoma in immunodeficiency are limited to case reports (26–30). HPV typing performed in 9 of 27 affected females identified high risk HPV types, HPV-16 and HPV-18 in female genital samples. This finding and the observation that only one subject under age 20 had genital HPV disease underscores the potential to boost immune response to HPV infection in order to potentially prevent severe disease by routine HPV vaccination during adolescence, prior to initiation of sexual activity (31).
In the thirty patients who underwent HSCT, twelve patients had HPV disease: nine females with HPV-associated anogenital HSIL and three males with anogenital LSIL. These female patients underwent systematic, comprehensive examinations of the genital and anal sites for HSIL/HPV disease followed by treatment of HSIL. All but two females with a history of recurrent vulvar and cervical HSIL experienced resolution of HPV disease after HSCT by two years with peritransplant surveillance and management. In a recent paper describing outcome of HPV infection in GATA2 haploinsufficiency post-HSCT, post-HSCT monocyte or NK cell numbers were not correlated with HPV outcome, suggesting that active management (comprised of surveillance and surgery by a gynecologic and gastrointestinal team) likely contributed to lesion resolution (8). Additionally, HPV vaccination after transplant appears to be safe and may be effective in generating immunity (32). As HPV vaccination is not therapeutic, it is unlikely that HPV vaccination after HSCT contributed to resolution of disease in these 5 females.
4.2 Results in the context of published literature
Other primary immunodeficiencies, such as Dedicator Of Cytokinesis 8 (DOCK8) deficiency, Warts, Hypogammaglobulinemia, Infections, and Myelokathexis (WHIM) syndrome, and Epidermodysplasia Verruciformis are associated with HPV disease, implying that multiple mechanisms are involved in the control of HPV infection (11). Studies on HPV disease in HIV suggest that low CD4 T-cell counts alone do not account for refractory HPV disease (19, 33, 34). The importance of NK cells in viral immunity, including against HPV infection, is well established (35). Type I interferons activate NK cells, which directly kill infected cells and produce pro-inflammatory cytokines.
Patients with GATA2 haploinsufficiency have dysfunctional as well as low numbers of NK cells (36). We found a significant negative correlation between NK cell and CD3+/CD4+ T cell levels and both the incidence and severity of HPV disease for the whole cohort that persisted in males when stratified by sex-specific quartiles. This association was not observed in females, likely due to the small number of females without severe HPV disease. The reduction in NK number and function likely contributes to the recalcitrant nature of HPV infection in GATA2 haploinsufficiency, similar to the pattern that has been described for severe EBV infection (37).
4.3 Strengths and weaknesses
Importantly, when immune reconstitution in GATA2 haploinsufficient patients was examined over time in an NIH HSCT cohort from just before through survival after HSCT, HPV disease was controlled and resolved (8). In this study, the systematic collection of immune features at one time point minimized ascertainment and recall bias. However, examination of the interplay between immunological parameters and HPV disease over the life course was not possible and is a limitation. Detailed immunological profiles obtained within this study were not measured as part of routine gynecologic care prior to study participation. Longitudinal analysis is further complicated by the individual variability inherent in the unpredictable time between occurrence of HPV infection, disease and diagnosis as disease onset is generally asymptomatic, except when lesions become extensive. This variability in disease presentation undoubtedly contributes to ascertainment and recall bias regarding the timing of development of disease. The need for multiple surgeries that are unsuccessful in controlling HSIL may lead to underreporting of surgical procedures and recall bias. Despite these limitations, the ability to document the severity and recurrence of HPV disease in affected females and the lack of HPV disease recurrence in males suggests these findings may be generalizable to other cohorts of GATA2 affected patients.
At the time of this study, we systematically collected information on HPV vaccination in female patients but did not routinely ask male patients. Not collecting it on male patients is a study limitation. As a result of this work on HPV, we have changed clinical practice within our studies of GATA2 haploinsufficiency. We now ask all study participants about their HPV vaccination status. Additionally, we vaccinate anyone who has not received HPV vaccination, regardless of their biological sex.
4.4 Implications for practice and future research
The extent and morbidity of genital HPV disease in this large cohort, especially among female patients, identifies another genetic predisposition to HPV-associated malignancy. This heightened risk is like that observed in females with other conditions with compromised immune response (38). GATA2 haploinsufficient women affected by HPV should undergo more frequent cervical cancer screening as recommended for these other populations (38).
The occurrence of severe, recurrent HPV disease beyond the cervix, across the lower genital tract, underscores some unique aspects of gynecologic screening in GATA2 haploinsufficient females. In particular, the heightened risk of vulva cancer we observed among females in their 20s in this cohort study underscores the importance of comprehensive lower genital tract assessment undertaken as part of routine care and continuing across their reproductive life. Colposcopy of the vulva, vagina, or cervix should be performed when any abnormal areas are seen on visual inspection or if a cervical cytology test is abnormal. Lesions identified during colposcopy or routine examination should be biopsied promptly to identify HSIL needing treatment prior to development of cancer. Among those identified with HSIL, continued frequent assessment every six months or so, is critically important to preventing or identifying cancer at its earliest stages.
This cohort study suggests that routine HPV vaccination should be implemented early, prior to acquiring HPV infection and to take advantage of intact immune function early in life. The effectiveness of HSCT on immune reconstitution for the resolution of HSIL suggests that anogenital HPV disease itself may be an important consideration in the decision to undergo HSCT. Comprehensive evaluation for anogenital HPV-related disease is warranted before HSCT and should continue post-HSCT so that surgical and other therapeutic measures can be undertaken in those with new HSIL or persistent disease. Most importantly, these data confirm that recurrent or multifocal HSIL should prompt consideration of GATA2 haploinsufficiency as the underlying cause.
The therapeutic challenges in treatment of severe HPV disease using surveillance and standard surgical approaches illustrate important research opportunities in GATA2 haploinsufficient females. Identification of genetic mutations causative in GATA2 haploinsufficiency across familial generations could identify those at risk for developing HPV disease and prompt early intervention and surveillance by gynecologists. Such identification could then allow examination of whether prophylactic HPV vaccination alone or in combination with HSCT are effective in preventing HSIL or persistent disease in GATA2. Females with GATA2 haploinsufficiency are a potential cohort for study of therapeutic HPV vaccination once available.
4.5 Conclusion
Female patients with GATA2 haploinsufficiency exhibit a heightened risk for severe, extensive, difficult to treat HPV disease and develop anogenital cancer at younger than expected ages compared to the general population. Accordingly, in females with extensive, multifocal genital HSIL unresponsive to multiple surgeries, as demonstrated by this study, GATA2 haploinsufficiency must be considered. Therefore, gynecologic oncologists and other women’s health practitioners could and should play an important role in early identification of rare immunodeficiencies when treating severe, multifocal, refractory anogenital HPV disease. Subsequent ability to effectively manage recurrent vulvar and cervical HSIL with surgery in patients with GATA2 haploinsufficiency who underwent HSCT suggests that restoration of normal immune function alongside anogenital surveillance and treatment together embody the successful approach to controlling HPV disease in this population.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the NIH Institutional Review Board at the National Institute of Allergy and Infectious Diseases. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants.
Author contributions
ED: Writing – original draft, Formal analysis, Data curation, Conceptualization, Methodology. PS: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. DP: Writing – review & editing, Methodology, Investigation, Data curation. BM: Writing – review & editing, Supervision, Methodology, Formal analysis, Conceptualization. EC: Writing – review & editing, Supervision, Investigation, Data curation. AM: Writing – review & editing, Validation, Supervision, Resources, Project administration, Investigation, Funding acquisition, Methodology. KV: Writing – review & editing, Visualization, Validation, Methodology, Investigation, Formal analysis, Data curation. MM: Writing – review & editing, Visualization, Methodology, Investigation, Data curation, Conceptualization. NS: Writing – review & editing, Writing – original draft, Validation, Methodology, Formal analysis, Data curation, Conceptualization. MH: Writing – review & editing, Methodology, Investigation, Data curation, Visualization. TH: Writing – review & editing, Visualization, Resources, Methodology, Investigation, Data curation. MP: Writing – review & editing, Visualization, Methodology, Investigation, Data curation, Conceptualization. DH: Writing – review & editing, Supervision, Resources, Project administration, Methodology, Investigation, Funding acquisition, Data curation. HK: Writing – review & editing, Visualization, Supervision, Resources, Methodology, Investigation, Data curation. SH: Writing – review & editing, Supervision, Resources, Project administration, Methodology, Investigation, Funding acquisition, Data curation, Conceptualization. CZ: Writing – review & editing, Writing – original draft, Supervision, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was funded by the Intramural Research Programs of the National Institute of Allergy and Infectious Diseases, National Institute of Neurological Disorders and Stroke, National Institutes of Health Clinical Center, National Cancer Institute, National Center for Advancing Translational Sciences, National Human Genome Research Institute, National Institute of Diabetes and Digestive and Kidney Diseases, and the National Institute of Arthritis and Musculoskeletal and Skin Diseases.
Acknowledgments
This work was presented in part at the ACOG annual meeting in Washington, DC in May 14-17, 2016 and the Society for Gynecologic Oncology Annual Meeting in San Diego, California in March 19-22 2016. We would like to acknowledge Janine Daub, Amy Hsu, Cindy Palmer, Victoria Anderson, Jennifer Cuellar-Rodriguez, and Juan Gea-Banacloche who provided invaluable contributions in the care of our patients and as members of our research team.
Conflict of interest
PS, also add participated in an Endometriosis Research Day at the Open Endoscopy Forum Cambridge, Massachusetts, and reviewed a book proposal on endometriosis for Elsevier.
Outside of this work, PS has received royalties from UpToDate for a section about acute pelvic pain, from Frontiers in Reproductive Health as Specialty Chief Editor, Gynecology, and participated in an AbbVie advisory board. PS also participated in an Endometriosis Research Day at the Open Endoscopy Forum Cambridge, Massachusetts, and reviewed a book proposal on endometriosis for Elsevier.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Author disclaimer
The opinions expressed within the content are solely the author’s and do not reflect the opinions of the National Institute of Allergy and Infectious Diseases, National Institute of Neurological Disorders and Stroke, National Institutes of Health Clinical Center, National Cancer Institute, National Center for Advancing Translational Sciences, National Human Genome Research Institute, National Institute of Diabetes and Digestive and Kidney Diseases, and the National Institute of Arthritis and Musculoskeletal and Skin Diseases at the National Institutes of Health of the United States Government.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1445711/full#supplementary-material
References
1. Dickinson RE, Griffin H, Bigley V, Reynard LN, Hussain R, Haniffa M, et al. Exome sequencing identifies GATA-2 mutation as the cause of dendritic cell, monocyte, B and NK lymphoid deficiency. Blood. (2011) 118:2656–8. doi: 10.1182/blood-2011-06-360313
2. Hsu AP, Sampaio EP, Khan J, Calvo KR, Lemieux JE, Patel SY, et al. Mutations in GATA2 are associated with the autosomal dominant and sporadic monocytopenia and mycobacterial infection (MonoMAC) syndrome. Blood. (2011) 118:2653–5. doi: 10.1182/blood-2011-05-356352
3. Rodrigues NP, Janzen V, Forkert R, Dombkowski DM, Boyd AS, Orkin SH, et al. Haploinsufficiency of GATA-2 perturbs adult hematopoietic stem-cell homeostasis. Blood. (2005) 106:477–84. doi: 10.1182/blood-2004-08-2989
4. Kitajima K, Tanaka M, Zheng J, Yen H, Sato A, Sugiyama D, et al. Redirecting differentiation of hematopoietic progenitors by a transcription factor, GATA-2. Blood. (2006) 107:1857–63. doi: 10.1182/blood-2005-06-2527
5. Spinner MA, Sanchez LA, Hsu AP, Shaw PA, Zerbe CS, Calvo KR, et al. GATA2 deficiency: a protean disorder of hematopoiesis, lymphatics, and immunity. Blood. (2014) 123:809–21. doi: 10.1182/blood-2013-07-515528
6. Grossman J, Cuellar-Rodriguez J, Gea-Banacloche J, Zerbe C, Calvo K, Hughes T, et al. Nonmyeloablative allogeneic hematopoietic stem cell transplantation for GATA2 deficiency. Biol Blood Marrow Transplant. (2014) 20:1940–8. doi: 10.1016/j.bbmt.2014.08.004
7. Parta M, Shah NN, Baird K, Rafei H, Calvo KR, Hughes T, et al. Allogeneic hematopoietic stem cell transplantation for GATA2 deficiency using a busulfan-based regimen. Biol Blood Marrow Transplant. (2018) 24:1250–9. doi: 10.1016/j.bbmt.2018.01.030
8. Parta M, Cole K, Avila D, Duncan L, Baird K, Schuver BB, et al. Hematopoietic cell transplantation and outcomes related to human papillomavirus disease in GATA2 deficiency. Transplant Cell Ther. (2021) 27:435 e1–435.e11. doi: 10.1016/j.jtct.2020.12.028
9. Vinh DC, Patel SY, Uzel G, Anderson VL, Freeman AF, Olivier KN, et al. Autosomal dominant and sporadic monocytopenia with susceptibility to mycobacteria, fungi, papillomaviruses, and myelodysplasia. Blood. (2010) 115:1519–29. doi: 10.1182/blood-2009-03-208629
10. Dickinson RE, Milne P, Jardine L, Zandi S, Swierczek SI, McGovern N, et al. The evolution of cellular deficiency in GATA2 mutation. Blood. (2014) 123:863–74. doi: 10.1182/blood-2013-07-517151
11. Leiding JW, Holland SM. Warts and all: human papillomavirus in primary immunodeficiencies. J Allergy Clin Immunol. (2012) 130:1030–48. doi: 10.1016/j.jaci.2012.07.049
12. Tan SC, Ankathil R. Genetic susceptibility to cervical cancer: role of common polymorphisms in apoptosis-related genes. Tumour Biol. (2015) 36:6633–44. doi: 10.1007/s13277-015-3868-2
13. Forman D, de Martel C, Lacey CJ, Soerjomataram I, Lortet-Tieulent J, Bruni L, et al. Global burden of human papillomavirus and related diseases. Vaccine. (2012) 30 Suppl 5:F12–23. doi: 10.1016/j.vaccine.2012.07.055
14. Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet. (2007) 370:890–907. doi: 10.1016/S0140-6736(07)61416-0
15. Schiffman M, Doorbar J, Wentzensen N, de Sanjose S, Fakhry C, Monk BJ, et al. Carcinogenic human papillomavirus infection. Nat Rev Dis Primers. (2016) 2:16086. doi: 10.1038/nrdp.2016.86
16. Lewis RM, Laprise JF, Gargano JW, Unger ER, Querec TD, Chesson HW, et al. Estimated prevalence and incidence of disease-associated human papillomavirus types among 15- to 59-year-olds in the United States. Sex Transm Dis. (2021) 48:273–7. doi: 10.1097/OLQ.0000000000001356
17. de Martel C, Plummer M, Vignat J, Franceschi S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int J Cancer. (2017) 141:664–70. doi: 10.1002/ijc.30716
18. Sand FL, Munk C, Frederiksen K, Junge J, Iftner T, Dehlendorff C, et al. Risk of CIN3 or worse with persistence of 13 individual oncogenic HPV types. Int J Cancer. (2019) 144:1975–82. doi: 10.1002/ijc.31883
19. Reusser NM, Downing C, Guidry J, Tyring SK. HPV carcinomas in immunocompromised patients. J Clin Med. (2015) 4:260–81. doi: 10.3390/jcm4020260
20. Lisco A, Hsu AP, Dimitrova D, Proctor DM, Mace EM, Ye P, et al. Treatment of relapsing HPV diseases by restored function of natural killer cells. N Engl J Med. (2021) 385:921–9. doi: 10.1056/NEJMoa2102715
21. Van Doorslaer K, Chen Z, McBride AA. Detection and genotyping of human papillomaviruses from archival formalin-fixed tissue samples. Curr Protoc Microbiol. (2016) 43:14B 9 1–14B 9 20. doi: 10.1002/cpmc.16
22. Quint WG, Scholte G, van Doorn LJ, Kleter B, Smits PH, Lindeman J. Comparative analysis of human papillomavirus infections in cervical scrapes and biopsy specimens by general SPF(10) PCR and HPV genotyping. J Pathol. (2001) 194:51–8. doi: 10.1002/path.855
23. Hinkle DE, Wiersma W, Jurs SG. Applied Statistics for the Behavioral Sciences. 5th ed. Boston, Mass: Houghton Mifflin (2003).
24. Schiffman M, Clifford G, Buonaguro FM. Classification of weakly carcinogenic human papillomavirus types: addressing the limits of epidemiology at the borderline. Infect Agent Cancer. (2009) 4:8. doi: 10.1186/1750-9378-4-8
25. Jones GL, Jacques RM, Thompson J, Wood HJ, Hughes J, Ledger W, et al. The impact of surgery for vulval cancer upon health-related quality of life and pelvic floor outcomes during the first year of treatment: a longitudinal, mixed methods study. Psychooncology. (2016) 25:656–62. doi: 10.1002/pon.3992
26. Tobin E, Rohwedder A, Holland SM, Philips B, Carlson JA. Recurrent ‘sterile’ verrucous cyst abscesses and epidermodysplasia verruciformis-like eruption associated with idiopathic CD4 lymphopenia. Br J Dermatol. (2003) 149:627–33. doi: 10.1046/j.1365-2133.2003.05543.x
27. Palm MD, Tyring SK, Rady PL, Tharp MD. Human papillomavirus typing of verrucae in a patient with WHIM syndrome. Arch Dermatol. (2010) 146:931–2. doi: 10.1001/archdermatol.2010.184
28. Mendes Araujo F, dos Santos Gon A, Sichero L, Simao Sobrinho J. Human papillomavirus type 57 in generalized verrucosis without immunodeficiency. J Am Acad Dermatol. (2013) 69:e189–90. doi: 10.1016/j.jaad.2013.04.039
29. Horev L, Unger S, Molho-Pessach V, Meir T, Maly A, Stepensky P, et al. Generalized verrucosis and HPV-3 susceptibility associated with CD4 T-cell lymphopenia caused by inherited human interleukin-7 deficiency. J Am Acad Dermatol. (2015) 72:1082–4. doi: 10.1016/j.jaad.2015.02.1118
30. Beaussant Cohen S, Fenneteau O, Plouvier E, Rohrlich PS, Daltroff G, Plantier I, et al. Description and outcome of a cohort of 8 patients with WHIM syndrome from the French Severe Chronic Neutropenia Registry. Orphanet J Rare Dis. (2012) 7:71. doi: 10.1186/1750-1172-7-71
31. Immunization Expert Work Group CoAHC. Committee opinion no. 704: human papillomavirus vaccination. Obstet Gynecol. (2017) 129:e173–8. doi: 10.1097/AOG.0000000000002052
32. Stratton P, Battiwalla M, Tian X, Abdelazim S, Baird K, Barrett AJ, et al. Immune response following quadrivalent human papillomavirus vaccination in women after hematopoietic allogeneic stem cell transplant: A nonrandomized clinical trial. JAMA Oncol. (2020) 6:696–705. doi: 10.1001/jamaoncol.2019.6722
33. Beachler DC, D’Souza G, Sugar EA, Xiao W, Gillison ML. Natural history of anal vs oral HPV infection in HIV-infected men and women. J Infect Dis. (2013) 208:330–9. doi: 10.1093/infdis/jit170
34. Moscicki AB, Ellenberg JH, Farhat S, Xu J. Persistence of human papillomavirus infection in HIV-infected and -uninfected adolescent girls: risk factors and differences, by phylogenetic type. J Infect Dis. (2004) 190:37–45. doi: 10.1086/421467
35. Gutierrez-Hoya A, Soto-Cruz I. NK cell regulation in cervical cancer and strategies for immunotherapy. Cells. (2021) 10(11):3104. doi: 10.3390/cells10113104
36. Mace EM, Hsu AP, Monaco-Shawver L, Makedonas G, Rosen JB, Dropulic L, et al. Mutations in GATA2 cause human NK cell deficiency with specific loss of the CD56(bright) subset. Blood. (2013) 121:2669–77. doi: 10.1182/blood-2012-09-453969
37. Cohen JI, Dropulic L, Hsu AP, Zerbe CS, Krogmann T, Dowdell K, et al. Association of GATA2 deficiency with severe primary epstein-barr virus (EBV) infection and EBV-associated cancers. Clin Infect Dis. (2016) 63:41–7. doi: 10.1093/cid/ciw160
Keywords: GATA2 haploinsufficiency, human papillomavirus, HPV, high-grade squamous epithelial lesion, HSIL, hematopoietic stem cell transplantation, HSCT or HCT, HPV vaccination
Citation: Dancy E, Stratton P, Pichard DC, Marciano BE, Cowen EW, McBride AA, Van Doorslaer K, Merideth MA, Salmeri N, Hughes MS, Heller T, Parta M, Hickstein DD, Kong HH, Holland SM and Zerbe CS (2024) Human papillomavirus disease in GATA2 deficiency: a genetic predisposition to HPV-associated female anogenital malignancy. Front. Immunol. 15:1445711. doi: 10.3389/fimmu.2024.1445711
Received: 07 June 2024; Accepted: 08 August 2024;
Published: 29 August 2024.
Edited by:
Sudhir Gupta, University of California, Irvine, United StatesReviewed by:
Hal M. Hoffman, University of California, San Diego, United StatesPaolo Palma, Bambino Gesù Children’s Hospital (IRCCS), Italy
Copyright © 2024 Dancy, Stratton, Pichard, Marciano, Cowen, McBride, Van Doorslaer, Merideth, Salmeri, Hughes, Heller, Parta, Hickstein, Kong, Holland and Zerbe. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pamela Stratton, c3RyYXR0b3BAbmloLmdvdg==
 Ehren Dancy1,2
Ehren Dancy1,2 Pamela Stratton
Pamela Stratton Dominique C. Pichard
Dominique C. Pichard Beatriz E. Marciano
Beatriz E. Marciano Edward W. Cowen
Edward W. Cowen Theo Heller
Theo Heller Dennis D. Hickstein
Dennis D. Hickstein Steven M. Holland
Steven M. Holland Christa S. Zerbe
Christa S. Zerbe