- 1Affiliated Hospital of Nanjing University of Chinese Medicine, Nanjing, Jiangsu, China
- 2Department of Rheumatology and Immunology, Guangdong Second Provincial General Hospital, Guangzhou, China
- 3Department of Rheumatology and Immunology, Affiliated Hospital of Nanjing University of Chinese Medicine, Nanjing, Jiangsu, China
This study aims to report a case of progressive pseudorheumatoid dysplasia (PPRD) with two kinds of cellular communication network factor 6 (CCN6) gene mutation. In this paper, the clinical profile and the process of diagnosis were analyzed, and the related literature was reviewed. A 15-year-old boy, who developed progressive ankle and hip joint pain and enlargement with spine involvement, was diagnosed with PPRD. The erythrocyte sedimentation rate and C-reactive protein (CRP) were in the normal range; rheumatoid factor and anti-cyclic citrullinated peptide antibody (ACPA) were all negative. Human leukocyte antigen 27 (HLA-B27) was also negative. Cene study discovered two kinds of mutations in CCN6 gene: c. 802T>C and c.624dup. Radiographic studies revealed spine platyspondyly and shaped beaked, osteoporosis, and bilateral symmetric bony enlargements of the interphalangeal joints. Hip shows bilateral acetabulum and femoral head bone marrow edema, which revealed hip arthritis. Gene detection, laboratory examination, and typical radiographic features are helpful for the diagnosis of PPRD. This is the first report of c. 802T>C and c.624dup mutations in patients with PPRD in our country.
1 Introduction
Progressive pseudorheumatoid dysplasia (PPRD) is a rare—autosomal recessive noninflammatory arthropathy. It is associated with WNT1-inducible signaling pathway 3 (WISP3) gene mutations, first reported by Wynne Davies (1), which the current name of this gene is now CCN6. As there is no diagnostic standard for it, according to the reported cases, it is characterized by progressive degeneration of articular cartilage that leads to a significant disability with pain, stiffness, and joint deformities (2). The patient’s physical signs are common finger joint and knee joint enlargement, an abnormal front-to-back curvature of the spine (kyphosis) and a short torso, normal level of serological inflammation (3). Imaging in a small number of cases reported showed inflammatory changes, joint space narrowing, and osteophyte formation. Common coracoid changes or platyspondyly of vertebral body. To date, more than 64 different CCN6 mutations have been reported in more than 200 PPRD patients (4).
PPRD is often misdiagnosed as juvenile rheumatoid arthritis (JIA), spondyloarthritis (SpA), and other inflammatory diseases clinically. In order to avoid misdiagnosis or delayed diagnosis, imaging and gene detection play an important role in the differential diagnosis of the two diseases. Herein, we describe a case of PPRD with rarely reported CCN6 mutations, c.802T>C and c.624dup. Additionally, the patient had bone marrow edema, which has not been previously reported.
2 Case presentation
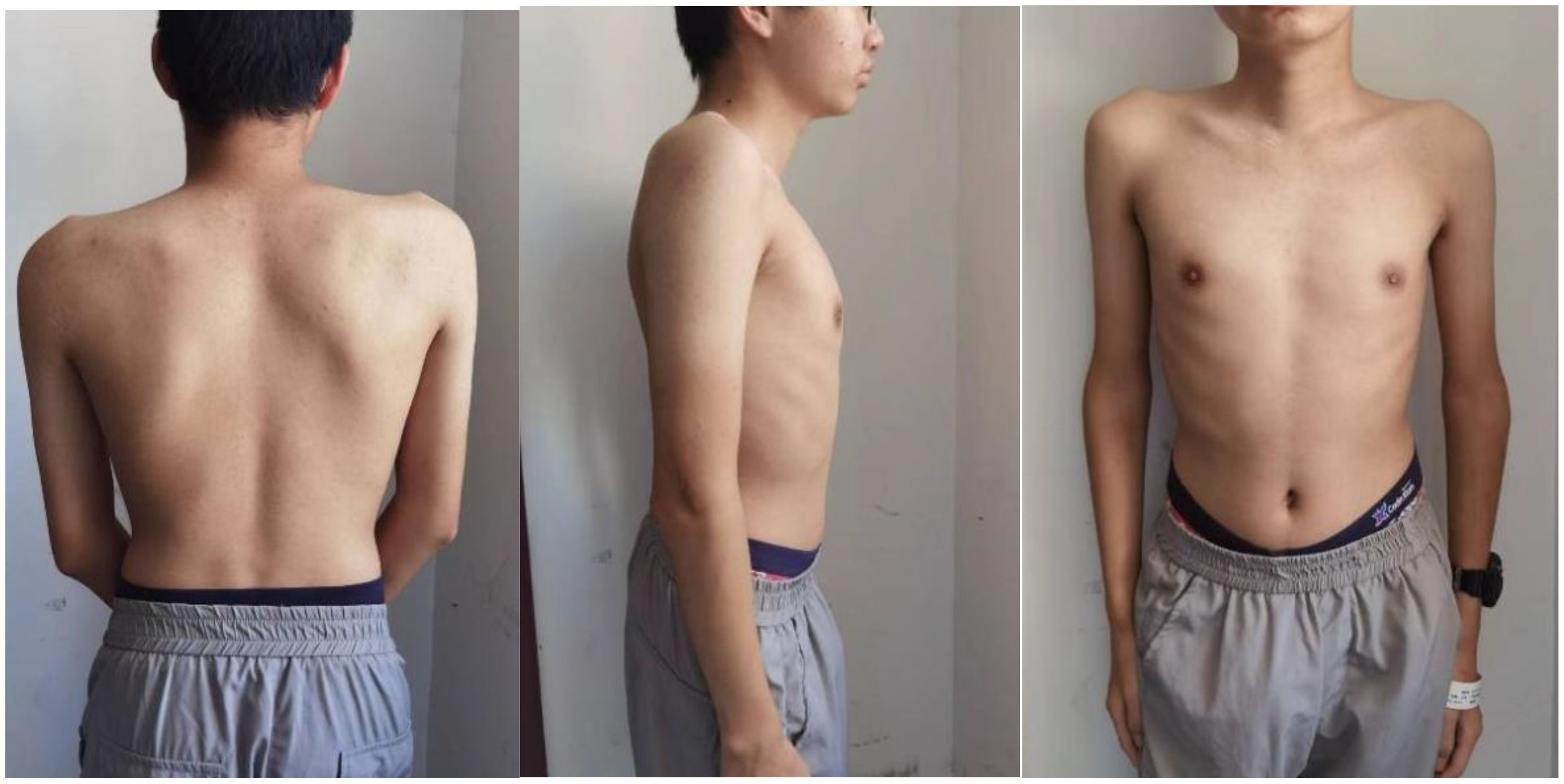
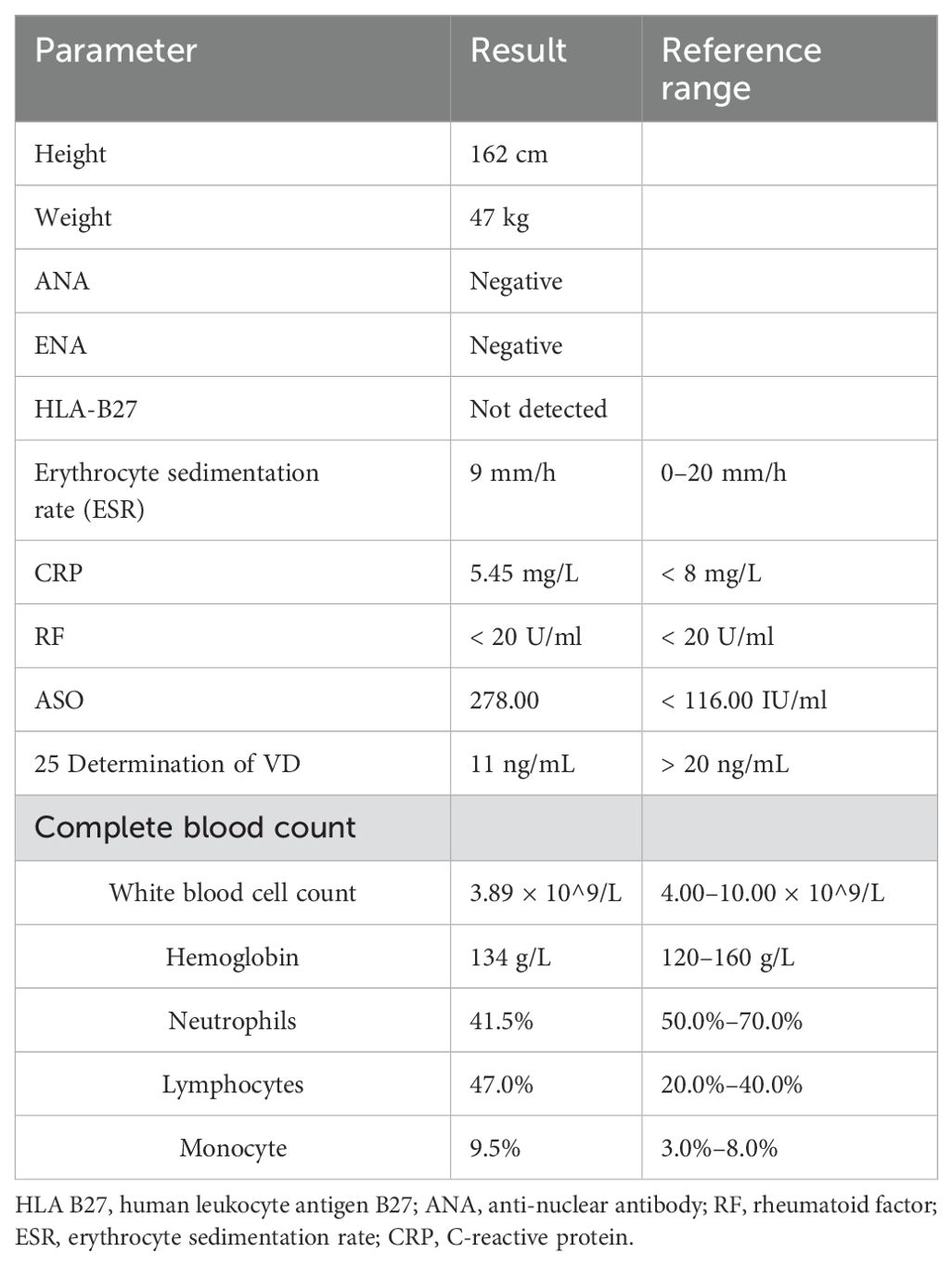
A 15-year-old boy (height: 1.62 m; weight: 47kg) was admitted with pain in ankle joints that had lasted for 5 years, who presented with scoliosis (Figure 1). In 2018, the patient experienced no apparent trigger for ankle joint pain and did not give it significant consideration. Until March 2019, when his ankle pain and swelling got worse so as to walk hard, he went to the hospital to check Biological markers, which contained complete blood count, ACPA, rheumatoid factor (RF), antinuclear antibody (ANA), extractable nuclear antigen (ENA), mlli—neutrophil cytoplasmic antibodies (ANCA), anti–double-stranded DNA antibody(anti–ds-DNA), enzyme-linked immunospot (ELISPOT), Hepatitis B virus-DNA (HBV-DNA), and Epstein-Barr virus-DNA (EB-DNA), and HLA-B27 quantification, which were all negative; and the antistreptolysin “O”(ASO) level was increased to 762 IU/L. Magnetic resonance imaging (MRI) revealed bilateral sacroiliac joint surface is not smooth with effusion, and multiple abnormal signals of bilateral heel talus and left foot scaphoid with calcaneal talus joint effusion. In order to make a clear diagnosis, the hospital completed bone marrow puncture that revealed granulocyte proliferation was active, erythroid and megakaryocytic proliferation was active, and platelet clusters were visible. The diagnosis made at the external hospital was JIA and streptococcal infection, for which the patient received intramuscular penicillin injections, disease-modifying antirheumatic drugs (DMARDs), nonsteroidal anti-inflammatory drugs (NSAIDs), calcium supplements, and stomach protection, which without any improvement so that he stopped the drug after 1 month. According to the patient’s past history, we are highly skeptical of his diagnosis of JIA, so that we focused on genetic testing and observations of the spine and hip joints. He did the biological markers and complete blood count (Table 1), and Radiological assessment after he was admitted to our hospital.
2.1 Radiological assessment
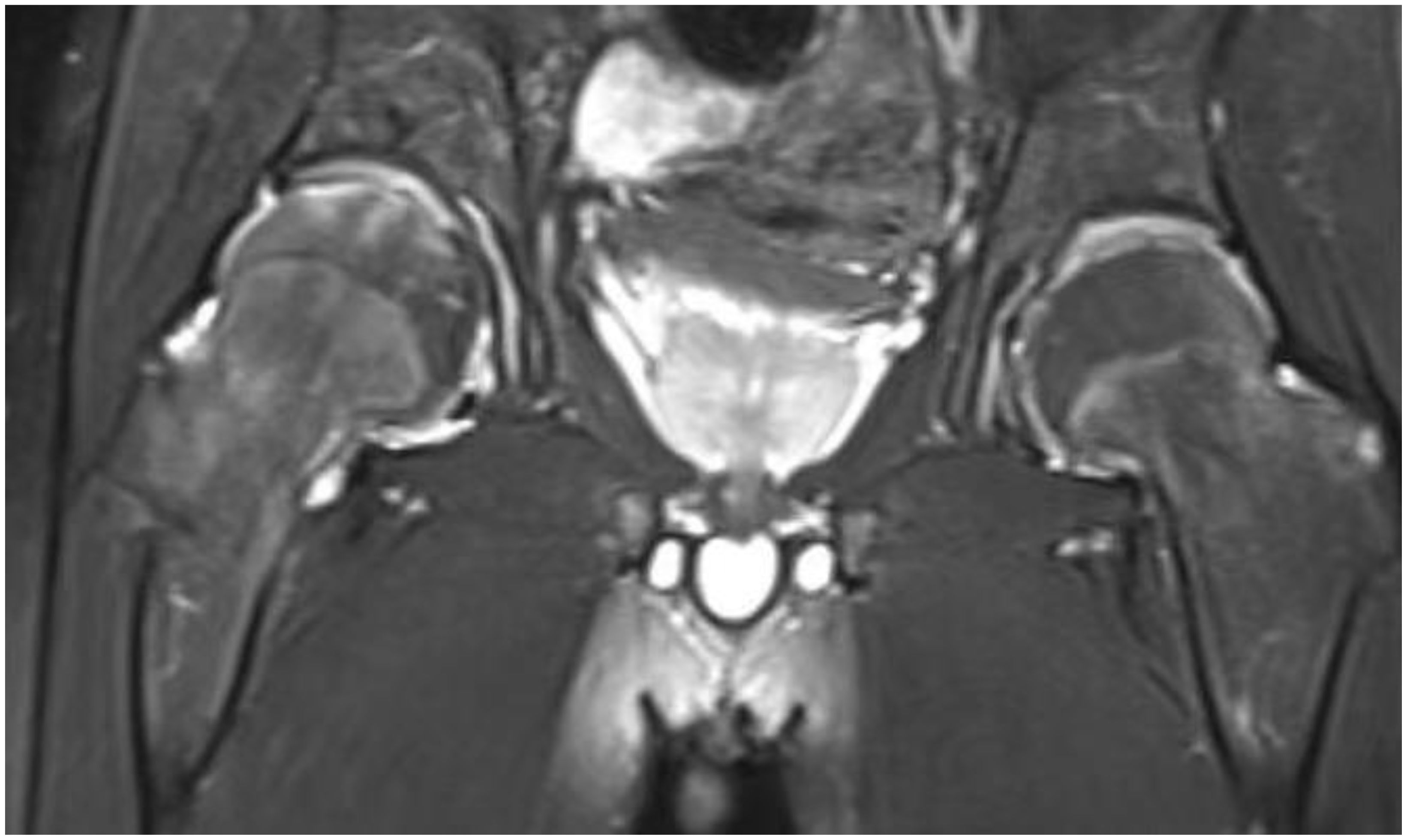
Radiography and computerized tomography (CT) showed no significant cardiopulmonary abnormalities. Radiography of spine showed abnormal changes in the shape of the lumbar spine presented platyspondyly and beaked which consistent with typical spinal manifestations of PPRD (Figure 2B). Spindle-shaped enlargement of proximal and distal finger joints of both hands (Figure 3). CT of sacroiliac joint revealed bilateral sacroiliac arthritis possibly. Coxae MRI revealed bilateral acetabulum and femoral head bone marrow edema (Figure 4), joint space narrowing (Figure 2A), and periarticular effusion which was similar to the imaging findings of ankylosing spondylitis. Spindle-shaped enlargement of proximal and distal finger joints of both hands.
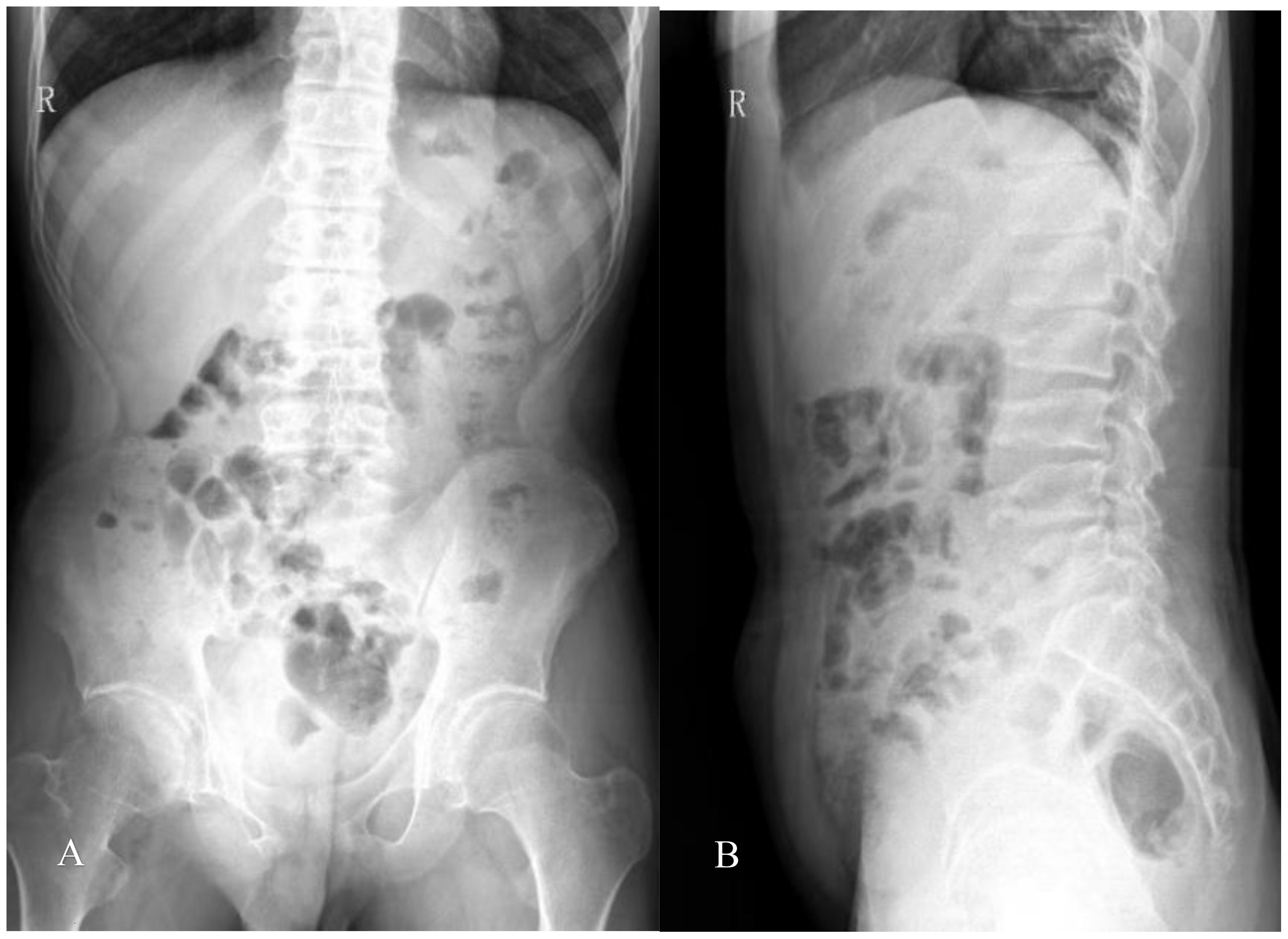
Figure 2. (A) Slight scoliosis of spine and narrowing of coxae space; (B) flat vertebra of cervical, thoracic, and lumbar segments, and the upper and lower edges of the vertebral body are concave, which showed coracoid changes; Irregular vertebral endplate and shortened pedicle.
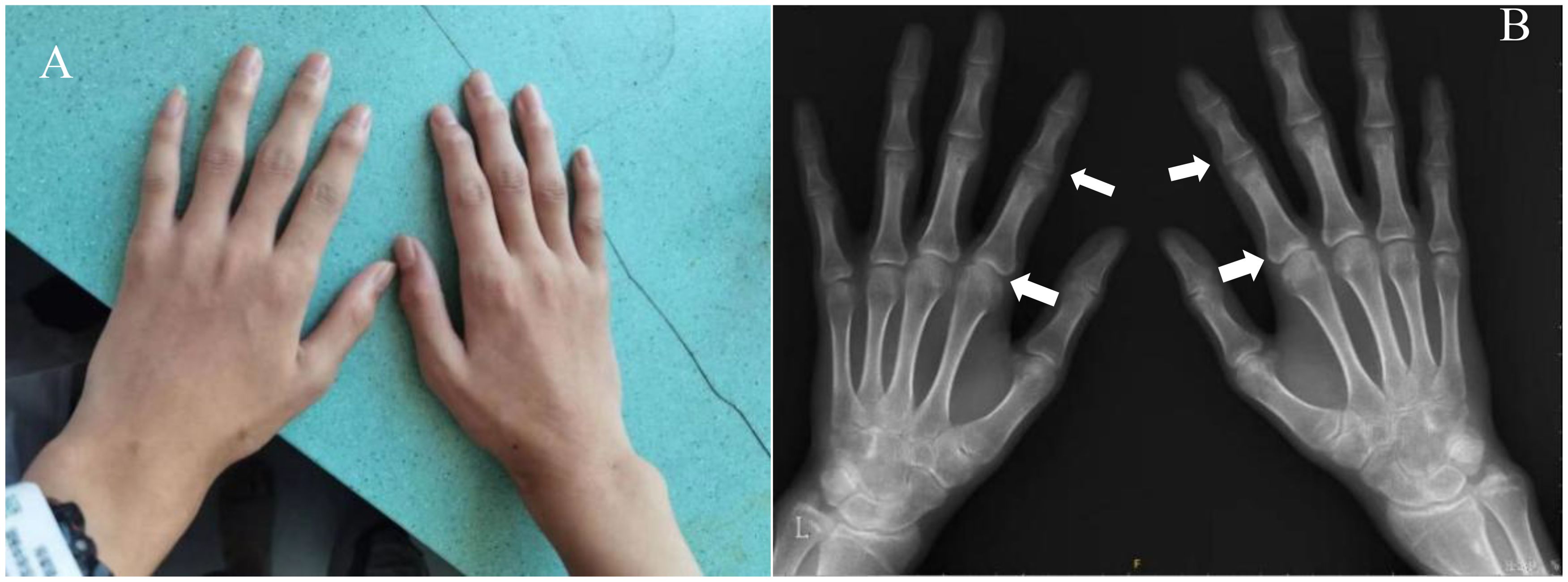
Figure 3. (A) Spindle-shaped enlargement of proximal and distal finger joints of both hands; (B) hands x-ray showing enlarged epiphyses and metaphyses of the metacarpals and phalanges, with joint spaces narrowing and osteophytic formations (arrows). The bone age of the patient was 16 years old. No significant inflammatory changes were observed.
No abnormality was found in the inflammatory indicators of the patient, and no obvious JIA characteristic expression was found in the imaging, but it was consistent with the imaging performance reported by PPRD. As a result, a whole exome sequencing test for genetic diseases was performed. The findings revealed two mutations within the CCN6 gene, which included previously unreported PPRD-related mutation sites. These mutations were identified as follows: NM_003880.4 (CCN6) c.8T>C, classified with partial pathogenicity as VUS (variant of uncertain significance), and NM_003880.4 (CCN6) c.624dup, classified with suspected pathogenicity (Figure 5). This variation may be helpful to explain the clinic. Parents refused to take the test, so they were not evaluated.
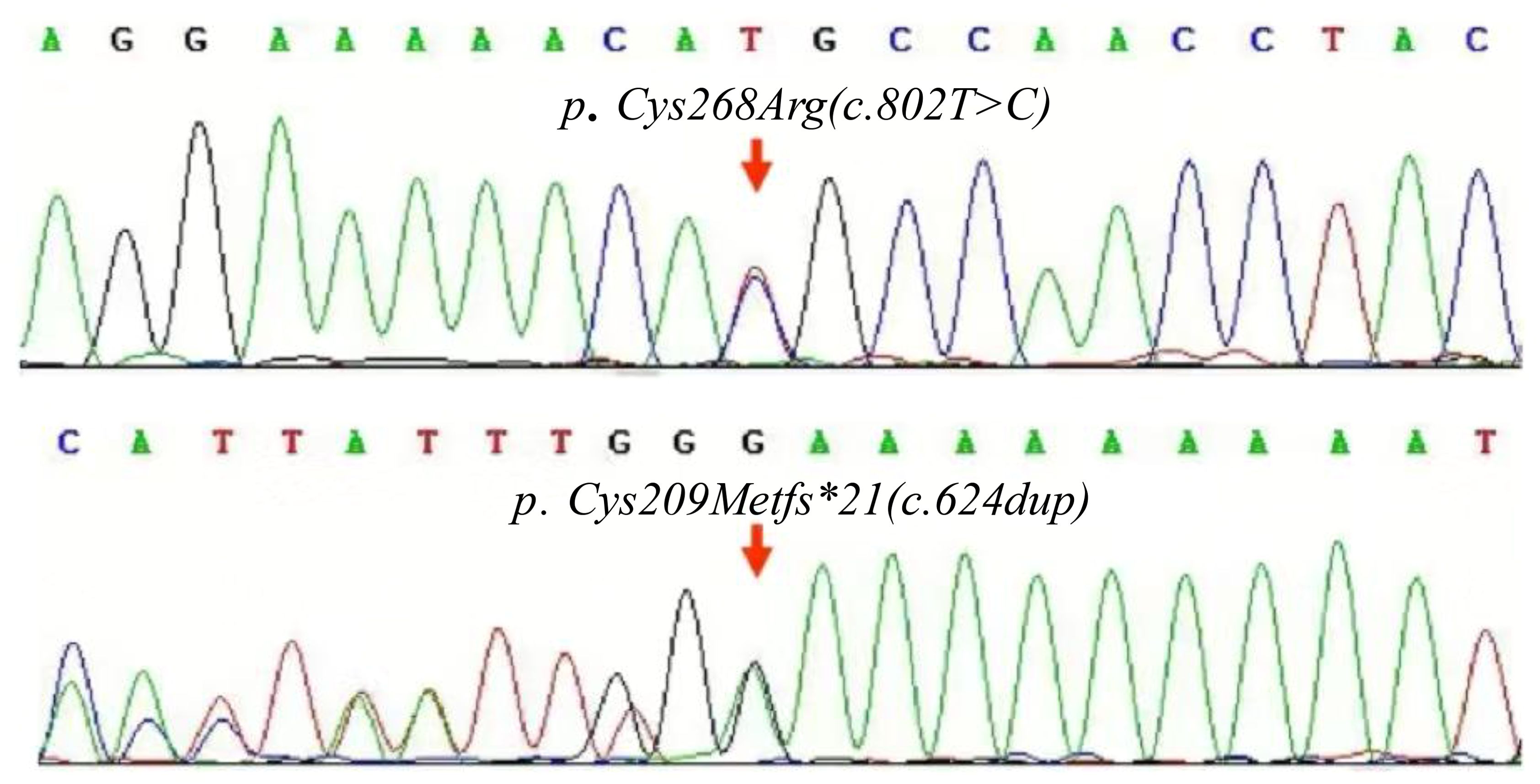
Figure 5. Maps of the CCN6 gene mutation in the patient. The compound heterozygous mutations, c.802T>C and c.624dup carried by the patient.
The PPRD diagnosis of the patient was clear based on the patient’s symptoms, auxiliary examination, gene, and other results. Because PPRD does not an immune disease, we are giving it to him NSAIDs, prevention and treatment of osteoporosis, and traditional Chinese medicine. He is on follow-up, and there has been a marked reduction in the pain, although the deformity persists.
3 Discussion and conclusion
We report a novel case of PPRD with involving a novel CCN6 mutation and sacroiliac and hip arthritis, including bone marrow edema and joint effusion, which contradicts the previous assumption that PPRD is a noninflammatory disease.
The gene of c.802T>C mutations has been detected in the genes of Indian patients with PPRD (5), Chinese patients with epilepsy (6), and Czech patients with diabetes (7). The gene of c.624dup mutations has never been reported. This is the first report of c. 802T>C and c.624dup mutations in patients with PPRD in our country.
Previous reports indicate that patients with PPRD typically do not exhibit the characteristic bone erosion or bone marrow edema seen in JIA. However, the patient in this case presented with typical hip arthritis, along with bone marrow edema. This uncommon manifestation contributed to the misdiagnosis of JIA. These results suggest that PPRD patients have local inflammatory changes, such as bone marrow edema, which may be different from local joint inflammatory changes seen in the Enthesitis of SpA and the synovitis of JIA. In addition to that, heterozygous COL2A1 variants cause a wide spectrum of skeletal dysplasia-termed type II collagenopathies, which include a wide variety of skeletal dysplasia ranging from lethal disorders, such as achondrogenesis, type II or hypochondrogenesis (MIM#200610), to adult early onset osteoarthritis at the milder end of the spectrum (8). Spondyloepiphyseal dysplasia type Stanescu (SED-S, MIM 616583) is also the frequent forms of type II collagenopathies, characterized by COL2A1 gene mutation. One of the main differential diagnoses of SED-S is PPRD, which has overlapping clinical and radiologic manifestations of these two entities overlap (9). Both of these disorders require genetic testing to distinguish them from PPRD. Notably, some experiments have shown that WISP3, encoded by a gene mutated in PPRD, is located in the intermediate region of chondrocytes and regulates the expression of type II collagen (10). These data link SED-S to PPRD and may explain the overlapping phenotypes of the two entities.
Through searching the web of science database, a total of 55 cases of PPRD were reported, having flattened bones in the spine platyspondyly for 7%, abnormally shaped beaked for 32% (11–16), and bilateral symmetric bony enlargements of the interphalangeal joints for 100%.
The diagnosis of PPRD is specific imaging findings revealed spine platyspondyly and shaped beaked, osteoporosis, bilateral symmetric bony enlargements of the interphalangeal joints, and the characteristic pathogenic gene in CCN6. Therefore, early detection and diagnosis of PPRD can avoid unnecessary anti-inflammatory and immunosuppressive treatment, and it is very important to choose anti-osteoporosis treatment as early as possible, or choose corrective surgery or joint replacement surgery to help alleviate pain and disability related to PPRD. Although we can distinguish PPRD from SpA and JIA on typical spinal alterations and genetic tests, more cases and experiments are needed to demonstrate the relationship between specific clinical manifestations of PPRD and CCN6 gene variants, and to demonstrate the specific relationship between PPRD and SpA, JIA, and COL2A1 mutation.
Data availability statement
The data presented in the study are deposited in the Gene Bank repository, accession number SAMN44260485.
Ethics statement
The studies involving humans were approved by Ethics Committee of the Affiliated Hospital of Nanjing University of Chinese Medicine/The Affiliated Hospital of Nanjing University of Chinese Medicine (Jiangsu Provincial Hospital of Traditional Chinese Medicine). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
Author contributions
YL: Writing – original draft, Writing – review & editing. ZH: Writing – review & editing. YY: Writing – original draft. FG: Writing – review & editing. GW: Writing – original draft. YW: Writing – review & editing. YX: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. ZH received funding from the National Natural Science Foundation of China (No. 82302025), Young Talent Support Project of Guangzhou Association for Science and Technology (QT-2024-032) and Guangzhou Science and Technology Plan Projects (2024A03J0773).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Wynne-Davies R, Hall C, Ansell BM. Spondylo-epiphysial dysplasia tarda with progressive arthropathy. A “new” disorder of autosomal recessive inheritance. J Bone Joint Surg Br. (1982) 64:442–5. doi: 10.1302/0301-620X.64B4.6807993
2. Pomeranz CB, Reid JR. Progressive pseudorheumatoid dysplasia: a report of three cases and a review of radiographic and magnetic resonance imaging findings. Skeletal Radiol. (2019) 48:1323–8. doi: 10.1007/s00256-019-3165-z
3. Maatallah K, Boussaa H, Lassoued Ferjani H, Kaffel D, Hamdi W. Progressive pseudorheumatoid dysplasia: A rare entity mimicking juvenile idiopathic arthritis. Clin Case Rep. (2021) 9:e04670. doi: 10.1002/ccr3.4670
4. Montané LS, Marín OR, Rivera-Pedroza CI, Vallespín E, Del Pozo Á, Heath KE. Early severe scoliosis in a patient with atypical progressive pseudorheumatoid dysplasia (PPD): Identification of two WISP3 mutations, one previously unreported. Am J Med Genet A. (2016) 170:1595–9. doi: 10.1002/ajmg.a.37619
5. Dalal A, Bhavani G SL, Togarrati PP, Bierhals T, Nandineni MR, Danda S, et al. Analysis of the WISP3 gene in Indian families with progressive pseudorheumatoid dysplasia. Am J Med Genet A. (2012) 158A:2820–8. doi: 10.1002/ajmg.a.35620
6. Ma C-L, Wu X-Y, Jiao Z, Hong Z, Wu Z-Y, Zhong M-K. SCN1A, ABCC2 and UGT2B7 gene polymorphisms in association with individualized oxcarbazepine therapy. Pharmacogenomics. (2015) 16:347–60. doi: 10.2217/pgs.14.186
7. Bazalová Z, Rypácková B, Broz J, Brunerová L, Polák J, Rusavý Z, et al. Three novel mutations in MODY and its phenotype in three different Czech families. Diabetes Res Clin Pract. (2010) 88:132–8. doi: 10.1016/j.diabres.2010.01.005
8. Barat-Houari M, Dumont B, Fabre A, Them FT, Alembik Y, Alessandri JL, et al. The expanding spectrum of COL2A1 gene variants IN 136 patients with a skeletal dysplasia phenotype. Eur J Hum Genet. (2016) 24:992–1000. doi: 10.1038/ejhg.2015.250
9. Travessa AM, Díaz-González F, Mirco T, Oliveira-Ramos F, Parrón-Pajares M, Heath KE, et al. Spondyloepiphyseal dysplasia type Stanescu: Expanding the clinical and molecular spectrum of a very rare type II collagenopathy. Am J Med Genet Part A. (2020) 182:2715–21. doi: 10.1002/ajmg.a.61817
10. Sen M, Cheng Y-H, Goldring MB, Lotz MK, Carson DA. WISP3-dependent regulation of type II collagen and aggrecan production in chondrocytes. Arthritis Rheum. (2004) 50:488–97. doi: 10.1002/art.20005
11. Clinical and radiological diagnosis of progressive pseudorheumatoid dysplasia in two sisters with severe polyarthropathy. (Accessed August 18, 2024).
12. el-Shanti HE, Omari HZ, Qubain HI. Progressive pseudorheumatoid dysplasia: report of a family and review. J Med Genet. (1997) 34:559–63. doi: 10.1136/jmg.34.7.559
13. Mampaey S, Vanhoenacker F, Boven K, Van Hul W, De Schepper A. Progressive pseudorheumatoid dysplasia. Eur Radiol. (2000) 10:1832–5. doi: 10.1007/s003300000518
14. Marik I, Marikova O, Zemkova D, Kuklik M, Kozlowski K. Dominantly inherited progressive pseudorheumatoid dysplasia with hypoplastic toes. Skeletal Radiol. (2004) 33:157–64. doi: 10.1007/s00256-003-0708-z
15. Chen W, Mo S, Luo G, Wang Y, Deng X, Zhu J, et al. Progressive pseudorheumatoid dysplasia with new-found gene mutation of Wntl inducible signaling pathway protein 3. Pediatr Rheumatol Online J. (2018) 16:55. doi: 10.1186/s12969-018-0272-7
Keywords: communication network factor 6 (CCN6) gene, case report, progressive pseudorheumatoid dysplasia (PPRD), c. 802T>C, c.624dup
Citation: Li Y, Huang Z, Yan Y, Guo F, Wei G, Wang Y and Xie Y (2024) Progressive pseudorheumatoid dysplasia involving a novel CCN6 mutation: a case report. Front. Immunol. 15:1445420. doi: 10.3389/fimmu.2024.1445420
Received: 10 June 2024; Accepted: 30 September 2024;
Published: 30 October 2024.
Edited by:
Sara Manti, University of Messina, ItalyReviewed by:
Abdul Nasir, Zhengzhou University, ChinaEmanuele Micaglio, IRCCS San Donato Polyclinic, Italy
Copyright © 2024 Li, Huang, Yan, Guo, Wei, Wang and Xie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yu Xie, Nzc2MTI4MDJAcXEuY29t
 Yu Li
Yu Li Zhengping Huang
Zhengping Huang Yun Yan
Yun Yan Feng Guo3
Feng Guo3 Yue Wang
Yue Wang Yu Xie
Yu Xie