A Commentary on
Gut dysbiosis in patients with chronic pain: a systematic review and meta-analysis
By Goudman L, Demuyser T, Pilitsis JG, Billot M, Roulaud M, Rigoard P and Moens M (2024). Front. Immunol. 15:1342833. doi: .10.3389/fimmu.2024.1342833
Introduction
Chronic pain has been demonstrated to significantly reduce an individual’s quality of life. Various treatment modalities have been employed to address this condition, including pharmacotherapy, cognitive-behavioral therapy, and transcranial magnetic stimulation therapy (1). However, there is currently no established treatment for chronic pain due to the incomplete understanding of its pathogenesis. It has recently been proposed that the gut microbiota may play a role in the etiology of these conditions (2). I found the article by Goudman et al. on the meta-analysis of the gut microbiota in patients with chronic pain to be of great interest. The study demonstrated that the gut microbiota of individuals with chronic pain exhibited dysbiosis, which resulted in a reduction in alpha diversity and a relative increase in the genus Eggerthella (3). The reduction in alpha diversity is associated with a decline in bacteria that produce short-chain fatty acids, such as butyrate (4). This weakens the barrier function of the intestinal epithelium, making it easier for information about changes in the intestinal environment, such as an increase in Eggerthella spp., to be transmitted directly to the brain. The purpose of this paper is to examine the impact of Eggerthella spp. on the development of chronic pain.
Chronic pain and dopamine
The genus Eggerthella are involved in dopamine metabolism. For instance, Eggerthella lenta has the capacity to dehydroxylate dopamine (5). In the intestines of individuals diagnosed with Parkinson’s disease, this bacterium has been demonstrated to degrade dopamine to m-tyramine (5). It is important to note that dopamine does not cross the blood-brain barrier, and therefore, dopamine in the digestive tract does not migrate to the central nervous system. Nevertheless, the observed increase in genera containing bacteria that affect dopamine metabolism indicates that dopamine metabolism is altered in the central nervous system. This is likely due to the fact that the brain and gut are connected via the brain-gut axis. However, it is currently unclear whether this phenomenon is a cause or an effect. Dopamine in the central nervous system exerts an analgesic effect by stimulating dopamine D2 receptors in the dorsal striatum and nucleus accumbens during persistent pain. Increased dopaminergic output from the striatal dopamine loop stimulates the reward system and the periaqueductal gray, the origin of the descending pain inhibition pathway (6). Consequently, the diminished activity of striatal D2 receptors and diminished output of the striatal dopamine loop are associated with chronic pain (7). Chronic pain is associated with decreased dopamine function, which renders it susceptible to coexistence with other conditions, such as depression and Parkinson’s disease, which are also associated with decreased dopaminergic neurotransmission (8). In addition, reduced function of the dopamine loop in the basal ganglia promotes pain at the level of the spinal cord. Parkinson’s model mice in which dopamine cells were reduced by administration of a small amount of 6-hydroxydopamine to the midbrain exhibited chronic pain, the mechanism of which is spontaneous excitatory postsynaptic currents in the superficial dorsal horn. The reduced pain threshold of these Parkinson’s model mice was improved by stimulating the dopamine D2 receptor with a dopamine agonist (9).
Nevertheless, pharmacotherapy for chronic pain that regulates monoamines primarily targets serotonin, as evidenced by the administration of antidepressants (10). This is because serotonin is the primary neurotransmitter in the descending pain inhibitory pathway. Research on the gut microbiota of chronic pain patients has focused on tryptophan-producing bacteria. It has been demonstrated that excessive activation of microglial cells, which use tryptophan as a raw material, is caused by a decrease in serotonin and melatonin in the brain. This leads to increased pain and the coexistence of depression and insomnia (11). However, treatment with tricyclic antidepressants for chronic pain can be efficacious in certain instances but less so in others (12). Moreover, drugs that only enhance serotonin, such as selective serotonin reuptake inhibitors, can cause chronic pain, as is known as the serotonin paradox (13). In light of the findings by Goudman et al. indicating a potential association between gut microbiota characteristics and central dopamine dysfunction in chronic pain patients, it is recommended that the benefits of combination therapies, which aim to maintain the function of the striatal dopamine loop, be considered alongside conventional serotonin-targeting therapies in the pharmacological treatment of chronic pain (Figure 1).
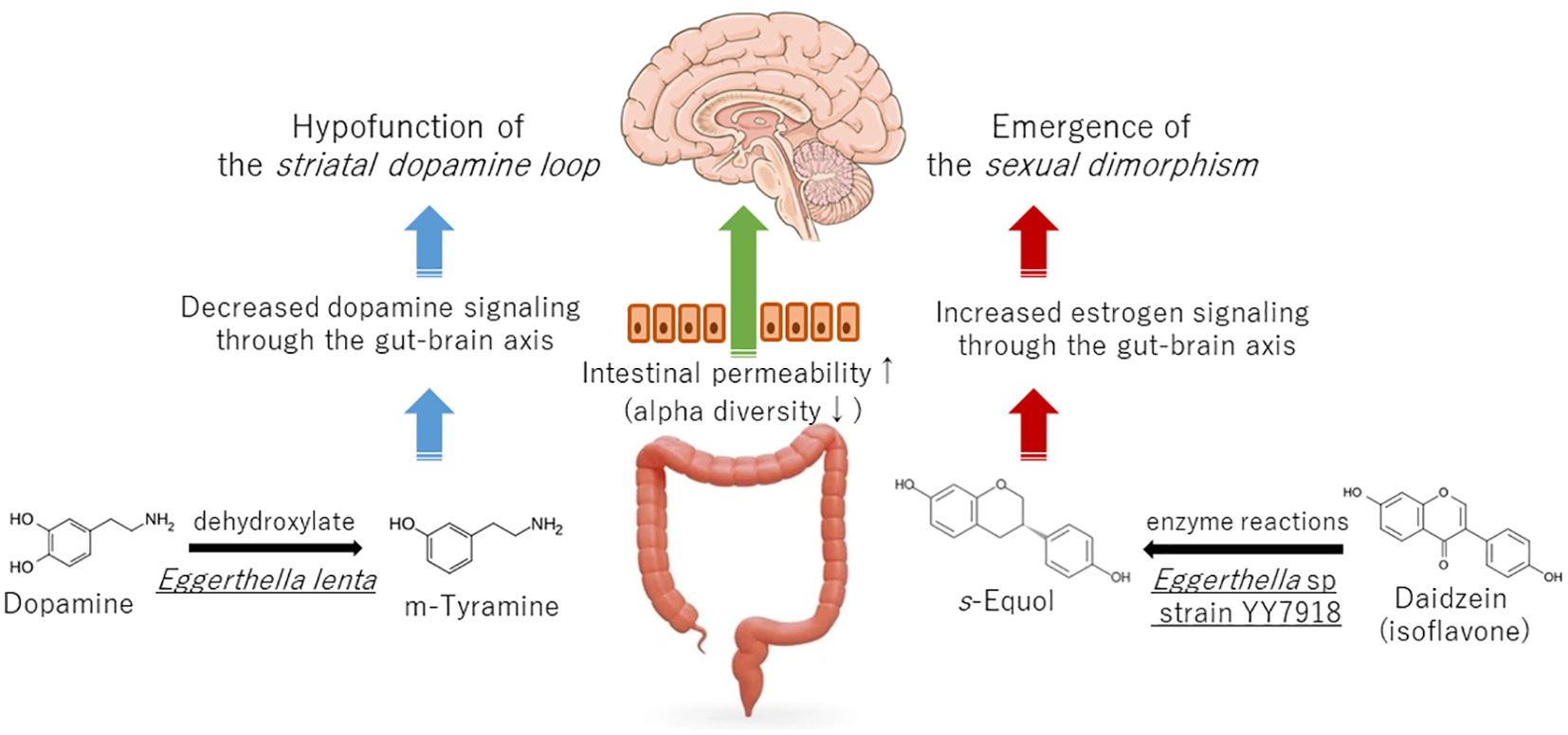
Figure 1. Effect of Eggerthella spp. on the development of chronic pain via dopamine and estrogen signaling.
Chronic pain and estrogen
The genus Eggerthella may also be associated with the regulation of female hormones. For instance, Eggerthella sp. YY7918, which is found in human feces, metabolizes isoflavonoids to produce S-equol, which has been demonstrated to possess strong estrogenic activity (14). The majority of chronic pain disorders are more prevalent in women than in men, with sex ratios varying considerably by disease (15). For instance, the prevalence of the burning mouth syndrome, a chronic orofacial pain, is three to five times higher in women than in men, with a higher incidence observed in peri- and postmenopausal women. One potential explanation for the heightened prevalence of chronic pain among menopausal women is the involvement of estrogen in the pathogenesis of chronic pain at two distinct stages: during puberty and menopause. This phenomenon is known as the two-hit theory by estrogen (16). The expression of the transient receptor potential vanilloid 1 (TRPV1), a pain receptor, is increased by estrogenic effects and is associated with chronic facial pain (17). As a first hit by estrogen, increased estrogen during puberty increases TRPV1 expression, making at-risk individuals more sensitive to pain. On the other hand, estrogen alleviates pain by downregulating nerve growth factor (NGF), which can translocate TRPV1 to the cell surface membrane. As a second hit by estrogen, when estrogen decreases during menopause, NGF increases, TRPV1 increases at the cell surface, pain sensitivity increases, neuroinflammation also occurs, and chronic pain develops in at-risk individuals (16). In fact, TRPV1 expression is increased in the oral mucosa of menopause chronic orofacial pain patients (18). First of all, the intestinal flora is influenced by estrogen, and its composition differs according to sex, known as the “estrobolome”. During puberty, estrogen induces a female-type intestinal flora (19), and during menopause, the intestinal flora becomes more similar to that of male (20). Both of these stages of change may be involved in the expression of pain.
Moreover, studies employing animal models have demonstrated that the expression of monoamine receptors in the descending pain inhibitory pathway is influenced by estradiol administration (21). It is evident that sexual dimorphism exists in various components of the pain circuitry such as the descending pain inhibitory pathway and the bed nucleus of the stria terminalis (22). The gut microbiota of chronic pain patients may transmit estrogen stimulation to the brain, and one of these may be Eggerthella sp. (Figure 1).
Conclusion
In conclusion, further investigation is required to determine whether the gut microbiota of chronic pain patients reduces central dopamine function or has an estrogen-like stimulating effect. The gut microbiota may represent a potential therapeutic target for chronic pain patients. Probiotics that produce short-chain fatty acids including butyrate have been demonstrated to be efficacious in restoring dopamine function in Parkinson’s disease (23). Therefore, it would be beneficial to investigate whether probiotics that produce short-chain fatty acids can also improve chronic pain. The mounting evidence indicating the pivotal role of the gut microbiota in regulating chronic pain has opened up new avenues in the field of pain management.
Author contributions
TN: Conceptualization, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author declares that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Rahman S, Kidwai A, Rakhamimova E, Elias M, Caldwell W, Bergese SD. Clinical diagnosis and treatment of chronic pain. Diagnostics (Basel). (2023) 13:3689. doi: 10.3390/diagnostics13243689
2. Ustianowska K, Ustianowski Ł, Machaj F, Gorący A, Rosik J, Szostak B, et al. The role of the human microbiome in the pathogenesis of pain. Int J Mol Sci. (2022) 23:13267. doi: 10.3390/ijms232113267
3. Goudman L, Demuyser T, Pilitsis JG, Billot M, Roulaud M, Rigoard P, et al. Gut dysbiosis in patients with chronic pain: a systematic review and meta-analysis. Front Immunol. (2024) 15:1342833. doi: 10.3389/fimmu.2024.1342833
4. Rashidah NH, Lim SM, Neoh CF, Majeed ABA, Tan MP, Khor HM, et al. Differential gut microbiota and intestinal permeability between frail and healthy older adults: A systematic review. Ageing Res Rev. (2022) 82:101744. doi: 10.1016/j.arr.2022.101744
5. Maini Rekdal V, Bess EN, Bisanz JE, Turnbaugh PJ, Balskus EP. Discovery and inhibition of an interspecies gut bacterial pathway for Levodopa metabolism. Science. (2019) 364:eaau6323. doi: 10.1126/science.aau6323
6. Ziółkowska B. The role of mesostriatal dopamine system and corticostriatal glutamatergic transmission in chronic pain. Brain Sci. (2021) 11:1311. doi: 10.3390/brainsci11101311
7. Hagelberg N, Forssell H, Rinne JO, Scheinin H, Taiminen T, Aalto S, et al. Striatal dopamine D1 and D2 receptors in burning mouth syndrome. Pain. (2003) 101:149–54. doi: 10.1016/s0304-3959(02)00323-8
8. Edinoff A, Sathivadivel N, McBride T, Parker A, Okeagu C, Kaye AD, et al. Chronic pain treatment strategies in parkinson’s disease. Neurol Int. (2020) 12:61–76. doi: 10.3390/neurolint12030014
9. Tang DL, Luan YW, Zhou CY, Xiao C. D2 receptor activation relieves pain hypersensitivity by inhibiting superficial dorsal horn neurons in parkinsonian mice. Acta Pharmacol Sin. (2021) 42:189–98. doi: 10.1038/s41401-020-0433-3
10. Birkinshaw H, Friedrich CM, Cole P, Eccleston C, Serfaty M, Stewart G, et al. Antidepressants for pain management in adults with chronic pain: a network meta-analysis. Cochrane Database Syst Rev. (2023) 5:CD014682. doi: 10.1002/14651858.CD014682.pub2
11. Lassmann Ł, Pollis M, Żółtowska A, Manfredini D. Gut bless your pain-roles of the gut microbiota, sleep, and melatonin in chronic orofacial pain and depression. Biomedicines. (2022) 10:1528. doi: 10.3390/biomedicines10071528
12. Nagamine T, Watanabe T, Toyofuku A. QTc shortening on electrocardiogram with amitriptyline may indicate no effect on pain relief in burning mouth syndrome. Clin Neuropharmacol. (2024) 47:33–6. doi: 10.1097/WNF.0000000000000583
13. Nagamine T. Serotonin paradox in burning mouth syndrome. J Clin Psychopharmacol. (2023) 43:188–9. doi: 10.1097/JCP.0000000000001661
14. Yokoyama S, Oshima K, Nomura I, Hattori M, Suzuki T. Complete genomic sequence of the equol-producing bacterium Eggerthella sp. strain YY7918, isolated from adult human intestine. J Bacteriol. (2011) 193:5570–1. doi: 10.1128/JB.05626-11
15. Osborne NR, Davis KD. Sex and gender differences in pain. Int Rev Neurobiol. (2022) 164:277–307. doi: 10.1016/bs.irn.2022.06.013
16. Nagamine T. Two-hit theory by estrogen in burning mouth syndrome. J Dent Sci. (2022) 17:1833–4. doi: 10.1016/j.jds.2022.06.009
17. Seol SH, Chung G. Estrogen-dependent regulation of transient receptor potential vanilloid 1 (TRPV1) and P2X purinoceptor 3 (P2X3): Implication in burning mouth syndrome. J Dent Sci. (2022) 17:8–13. doi: 10.1016/j.jds.2021.06.007
18. Yilmaz Z, Renton T, Yiangou Y, Zakrzewska J, Chessell IP, Bountra C, et al. Burning mouth syndrome as a trigeminal small fibre neuropathy: Increased heat and capsaicin receptor TRPV1 in nerve fibres correlates with pain score. J Clin Neurosci. (2007) 14:864–71. doi: 10.1016/j.jocn.2006.09.002
19. Sisk-Hackworth L, Kelley ST, Thackray VG. Sex, puberty, and the gut microbiome. Reproduction. (2023) 165:R61–74. doi: 10.1530/REP-22-0303
20. Peters BA, Santoro N, Kaplan RC, Qi Q. Spotlight on the gut microbiome in menopause: current insights. Int J Womens Health. (2022) 14:1059–72. doi: 10.2147/IJWH.S340491
21. Horii K, Sawamura T, Onishi A, Yuki N, Naitou K, Shiina T, et al. Contribution of sex hormones to the sexually dimorphic response of colorectal motility to noxious stimuli in rats. Am J Physiol Gastrointest Liver Physiol. (2022) 323:G1–8. doi: 10.1152/ajpgi.00033.2022
22. Uchida K, Otsuka H, Morishita M, Tsukahara S, Sato T, Sakimura K, et al. Female-biased sexual dimorphism of corticotropin-releasing factor neurons in the bed nucleus of the stria terminalis. Biol Sex Differ. (2019) 10:6. doi: 10.1186/s13293-019-0221-2
Keywords: chronic pain, dopamine, dysbiosis, estrogen, gut brain axis
Citation: Nagamine T (2024) Commentary: Gut dysbiosis in patients with chronic pain: a systematic review and meta-analysis. Front. Immunol. 15:1445334. doi: 10.3389/fimmu.2024.1445334
Received: 07 June 2024; Accepted: 26 August 2024;
Published: 26 September 2024.
Edited by:
Magdalena Plebanski, RMIT University, AustraliaReviewed by:
Youcef Shahali, Centre Hospitalier Universitaire de Besançon, FranceHem Chandra Jha, Indian Institute of Technology Indore, India
Kiran Veer Sandhu, University College Cork, Ireland
Copyright © 2024 Nagamine. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Takahiko Nagamine, dG5hZ2FtaW5lQG91dGxvb2suY29t
†ORCID: Takahiko Nagamine, orcid.org/0000-0002-0690-6271
 Takahiko Nagamine
Takahiko Nagamine