- 1Shandong Academy of Occupational Health and Occupational Medicine, Shandong First Medical University & Shandong Academy of Medical Sciences, Ji’nan, Shandong, China
- 2School of Basic Medicine and Clinical Pharmacy, China Pharmaceutical University, Nanjing, Jiangsu, China
- 3Public Health Monitoring and Evaluation Institute of Shandong Provincial Center for Disease Control and Prevention, Ji’nan, Shandong, China
As a small molecule, hydrogen is colorless, odorless and lightest. Many studies conducted that hydrogen can protect almost every organ, including the brain, heart muscle, liver, small intestine, and lungs. To verify whether high concentrations of hydrogen (HCH) has anti-inflammatory and antioxidant activities on respiratory system, we product a systematic review and meta-analysis. We investigated MEDLINE-PubMed, Cochrane Library, ScienceDirect, Wiley and SpringerLink database and selected in vivo studies related to the anti-inflammatory or antioxidant effects of HCH in the lung diseases which were published until September 2023. We firstly identified 437 studies and only 12 met the inclusion criteria. They all conducted in rodents. The results showed that HCH had a positive effect on the reduction of tumor necrosis factor alpha (TNF-α), interleukin (IL)-1β, IL-4, IL-8, malondialdehyde (MDA), superoxide dismutase (SOD) and reactive oxygen species (ROS); but there is no effect on IL-6, we speculated that may contribute to the test results for different body fluids and at different points in time. This meta-analysis discovered the protective effects on inflammation and oxidative stress, but whether there exists more effects on reduction of inflammatory and oxidant mediators needs to be further elucidated.
Background
By now, the incidence of respiratory diseases is increasing, and its mortality rate is among the top three in the world. It also imposes a huge economic burden. In addition, it has an emotional impact on patients (1).. Therefore, there needs a more efficient and economical method to save patients’ survival and quality of life. Many researchers believe that pulmonary inhalation may be a more direct and effective way with fewer side effects. Many similar studies are under way. Hydrogen, for example, is a treatment that is inhaled directly into lungs.
Hydrogen (H2), a diatomic gas composed of two hydrogen atoms connected by covalent bonds, is produced by the intestinal bacteria of mammals; H2 is colorless and odorless and is a stable neutral molecule (2). In 2007, Ohsawa et al (3) reported that H2 can react with cytotoxic oxygen free radicals by reacting with hydroxyl free radicals (•OH) in cultured cells. H2 does not react with •O2-, H2O2 or NO. Due to its potential ability to anti oxidative stress, inflammation, and apoptosis, H2 is emerging as the fourth gas signaling molecule in the body (4). Generally, hydrogen concentrations between 4% and 75% will not increase, and this paper defines hydrogen concentrations above 4% as high-concentration hydrogen (HCH). A systematic review by Yuan et al. (5) reported its potential protective effects on ischemia/reperfusion injury in multiple organs, neurodegenerative diseases, bone and joint diseases, and respiratory diseases.
The commonly used hydrogen administration methods include direct inhalation of hydrogen, injection of hydrogen-rich water and oral hydrogen-rich water (6). This paper mainly explored the therapeutic effect of hydrogen inhalation on respiratory diseases. In 1975, American scholar Dole et al. (7) reported in Science that inhaling hydrogen at 8 atmospheres for 14 consecutive days could significantly reduce the size of skin cancer tumors in mice; this was the first study in human history to determine the medical effect of hydrogen. In 2007, Wood et al (8) evaluated hydrogen as a cytoprotective therapy for ischemia-reperfusion injury and stroke, calling it a selective antioxidant with explosive potential, and this effect has also been confirmed in human experiments (9). At first, most experiments explored the therapeutic effects of low concentrations of hydrogen, but considering the actual concentration of hydrogen inhaled in the body, a higher concentration of hydrogen was derived.
Clinically, Chen et al. (10) reported that inhaling 67% hydrogen can alleviate the disease progression of non-small cell lung cancer; Zheng et al. (11) found that hydrogen therapy can treat acute episodes of chronic obstructive pulmonary disease (COPD); Akagi et al. (12) found that hydrogen can improve the prognosis of advanced colorectal cancer patients; Zeng et al. (13) reported that in the treatment of COVID-19, a mixture of hydrogen and oxygen can improve patients’ percutaneous arterial oxygen saturation (SpO2) and shorten the length of hospital stay. Some animal experiments have shown that a high concentration of hydrogen can reduce the secretion of inflammatory factors, possibly through a variety of signaling pathways, such as nuclear factor-kappa B (NF-κb), and can reduce the content of reactive oxygen species (ROS) and some oxidation products.
These studies verified the therapeutic effects of HCH. In order to further evaluate its anti-inflammatory and antioxidant capacity in respiratory diseases, we demonstrated this through this systematic review and meta-analysis.
Methods
We conducted this study following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (14) and Cochrane Manual.
Eligibility criteria
Our criteria for inclusion are: (a) the experimental model was an animal model with lung disease; (b) the intervention was treatment inhalation with high concentration hydrogen alone; (c) the results are an indicator of anti-inflammatory or antioxidant outcomes in the treatment of lung disease; and (d) the type of study was experimental.
All the retrieved titles, abstracts, and full texts were read and screened independently by at least two researchers. If a disagreement arises, it is discussed with reference to the inclusion exclusion criteria. The inclusion criteria were as follows: animal studies; suffers from respiratory problems; high concentration hydrogen inhalation was used alone; and anti-inflammatory or antioxidant outcome measures were used. The exclusion criteria were as follows: articles that do not meet the inclusion criteria, review articles, meta-analyses, abstracts, conference proceedings, editorials/letters, and case report.
Search strategy
Five databases were used to search for papers that met the criteria of the study: the National Library of Medicine (MEDLINE-PubMed), Cochrane Library, ScienceDirect, Wiley and SpringerLink databases. Different combinations of the following keywords were used: “hydrogen,” “respiratory tract diseases,” “respiratory system,” “lung injury,” “pulmonary” and “trachea”.
The search strategy is as follows: (hydrogen gas) AND (respiratory system disease or lung disease or pulmonary disease or trachea disease). In addition, we checked the references in the article to make sure there were no potential missing articles.
The databases were searched for studies published until September 2023. This retrieval strategy was used to search for the anti-inflammatory and antioxidant effects of high concentration hydrogen in animal models of respiratory diseases. After reading the retrieved literature, we investigated the relevant references and included the relevant articles in the study. We did not contact the original author when there was data in the article that was not available, nor did we cite data from unpublished articles.
Data collection process and study risk of bias assessment
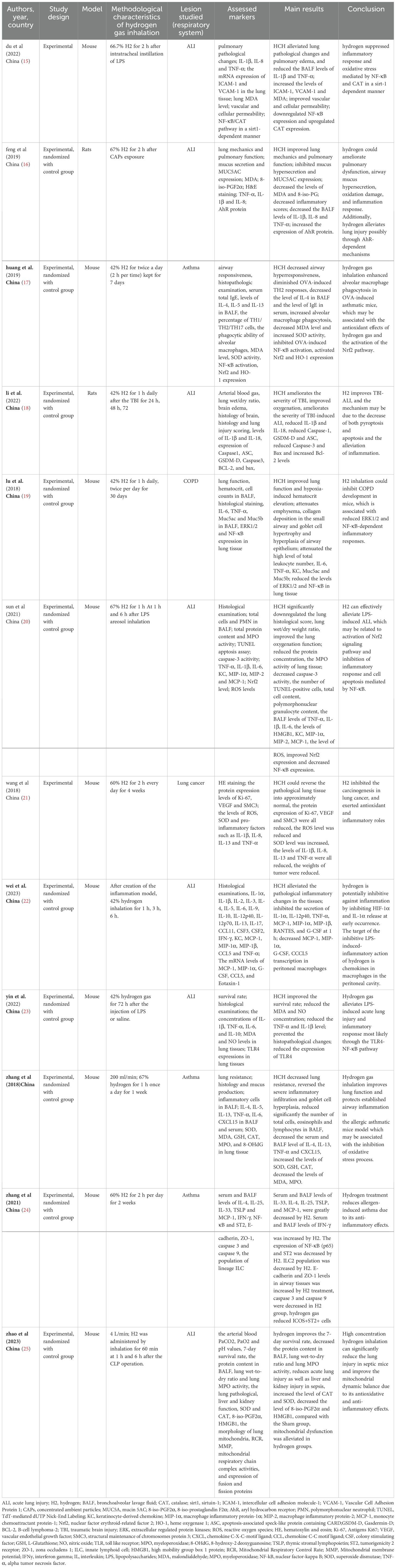
The data were extracted by one researcher according to Table 1 and examined by another researcher. The data to be extracted were as follows: study design, animal model studied, methodological characteristics of high concentrations of hydrogen, respiratory injury studied, markers evaluated, main results, conclusions.
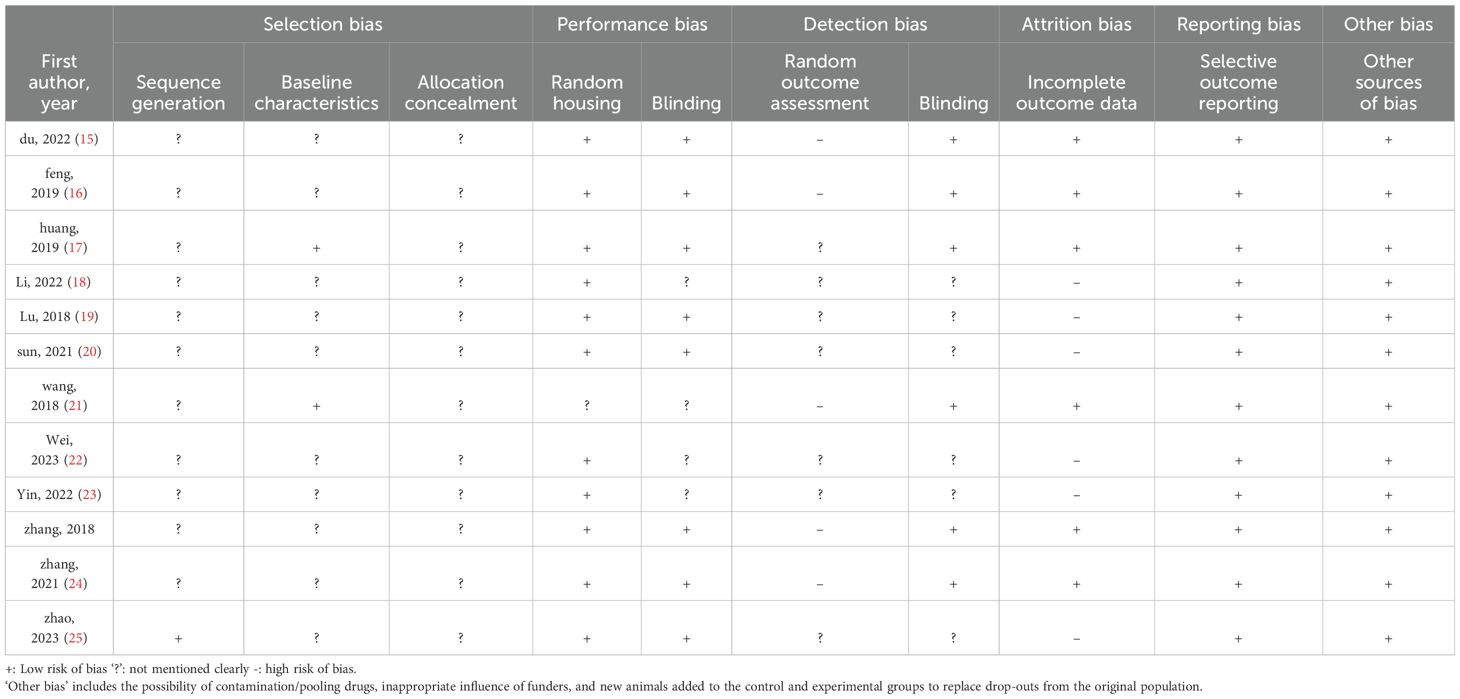
According to the Cochrane Manual, since we analyzed fewer than 10 articles in each group, we used SYRCLE’s risk of bias (RoB) tool for animal studies to assess the study risk of bias. Each article was evaluated by two different researchers, and if there was any disagreement, it was resolved through negotiation. The risk of bias was rated as low, uncertain, or high. The contents include Selection bias, Performance bias, Detection bias, Attrition bias, Reporting bias and Other bias.
Synthesis methods
We used standardized mean difference (SMDs) of 95% confidence intervals (CI) to evaluate the treatment effect. If SMD = 0, it indicates no difference, SMD > 0 indicates more occurrence in the experimental group, and SMD < 0 indicates less occurrence in the experimental group. The mean and standard deviation (SD) of the control and treatment groups are obtained by extracting graphs in the article, and the effect size of the target outcome will be calculated. The negative effect size indicated that HCH could effectively reduce inflammatory mediators and markers of oxidative stress, and the positive effect size indicated that HCH could effectively reduce oxidative stress response for superoxide dismutase (SOD).
We used forest maps to graphically represent the effect size and 95% CI. We used Z test to evaluate the overall effect. If P < 0.05, it indicates that there was a significant difference. We used Chi2 test to evaluate the heterogeneity of the literature. If I2 < 50% or P > 0.1, the heterogeneity is small, and the fixed-effect model is used. If I2 ≥ 50% or P ≤ 0.1, it indicates that there is large heterogeneity in the study, and a random-effects model is used. We performed subgroup analyses based on the markers analyzed. If the results of the study did not include numerical values for the target results, we used the software GetData Graph Digitizer to evaluate their result graph to get an average and SD. We used Review Manager 5.3 (RevMan), 2014) for all of our analyses.
Results
Studies selection
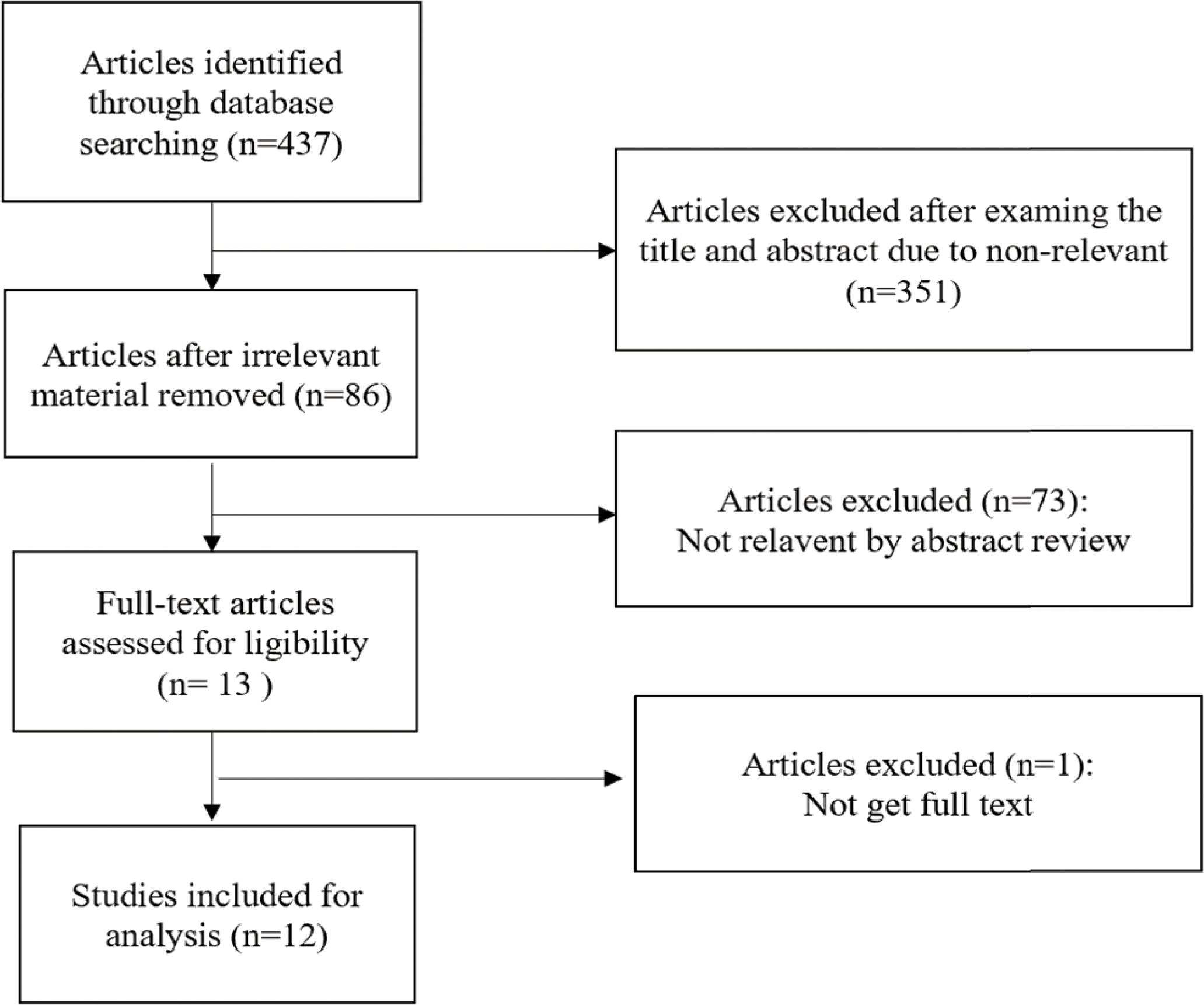
The steps of article retrieval filtering are shown in Figure 1. We identified 437 articles from the five databases and the bibliographies of relevant articles. By reading the title and abstract of the article, we got 86 articles after eliminating irrelevant articles. After excluding papers not shown in full, duplicates, letters, case studies and those whose themes did not match the criteria of this study, 12 articles remained (Figure 1). The two researchers who screened the articles had a high degree of agreement on inclusion and exclusion (Kappa index >96%).
Included studies characteristics
We selected 12 studies conducted in China. These studies were published between 2018 and 2023 (Table 1).
The levels of inflammatory markers, such as TNF-α, decreased in all the studies in which TNF-α was analyzed (15, 16, 19–21, 23, 26). IL-8 levels decreased in three studies (15, 16, 21). In three studies, there were no differences in IL-6 compared to that in the control group (22, 23, 26), but in two other studies (19, 20), there was an improvement in this marker. IL-4 decreased in three studies in which it was analyzed (17, 24, 26), but there were no differences in one study (Wei et al, 2023) (22). Oxidative stress, shown by the MDA levels, was lower in the high-concentration hydrogen group in every study in which it was analyzed (15–17, 23, 26). SOD levels were greater in the high-concentration hydrogen group in every study in which it was (17, 21, 25). ROS decreased in all the studies in which ROS were analyzed (20, 21).
Data synthesis
In the literature we searched, most of the studies assessed the expression levels of different markers, which made it impossible to conduct a uniform meta-analysis of all the literature, so we conducted a subgroup analysis of the consistent results in some of the literature. Among them, the anti-inflammatory effect of high concentration hydrogen was evaluated using IL-1β, IL-4, IL-8, IL-6 and TNF-α as inflammatory mediators, and the antioxidant effect was evaluated using MDA, SOD and ROS markers.
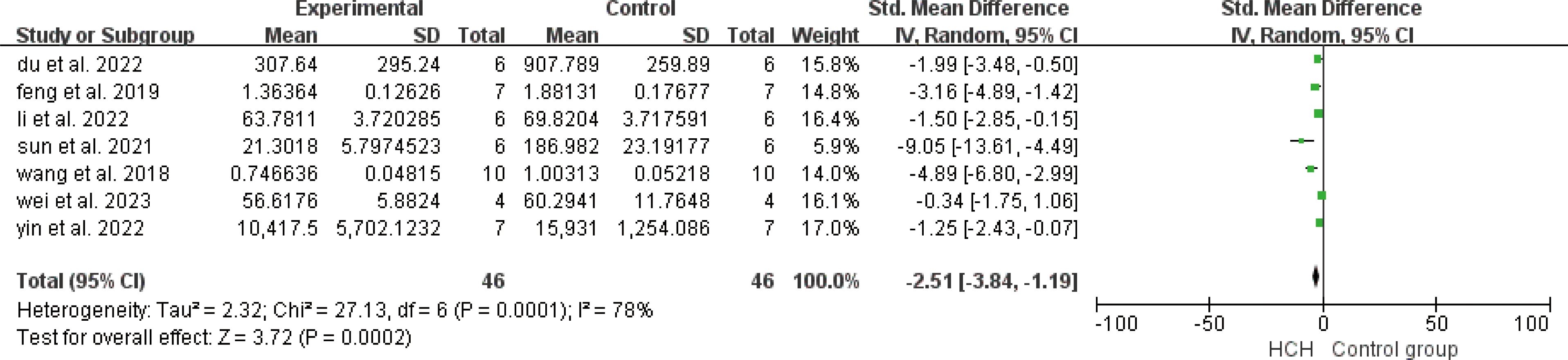
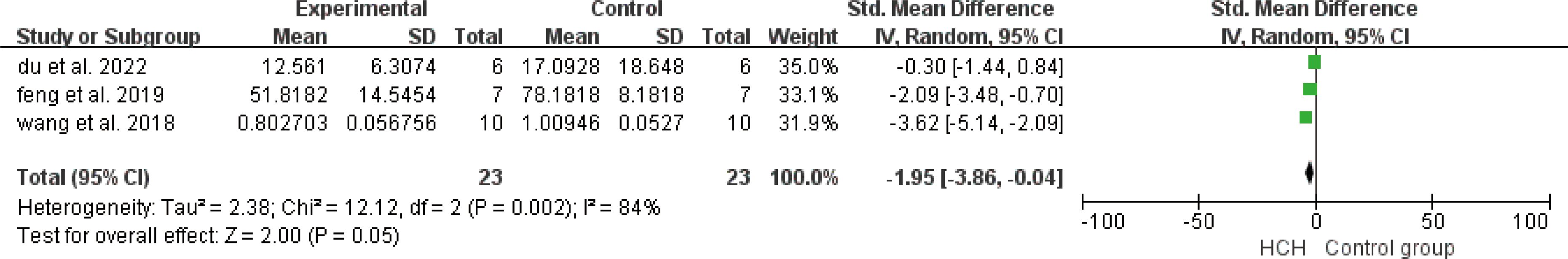
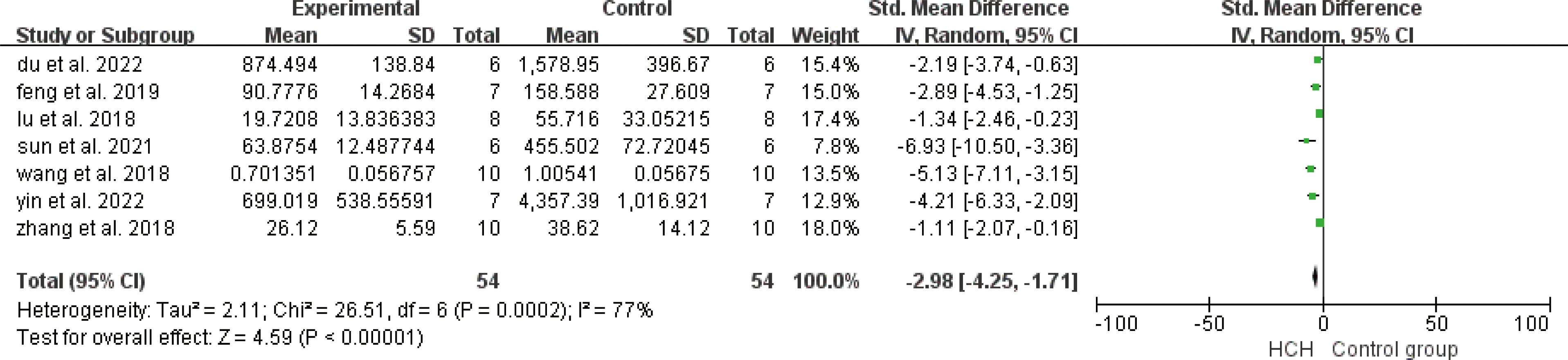
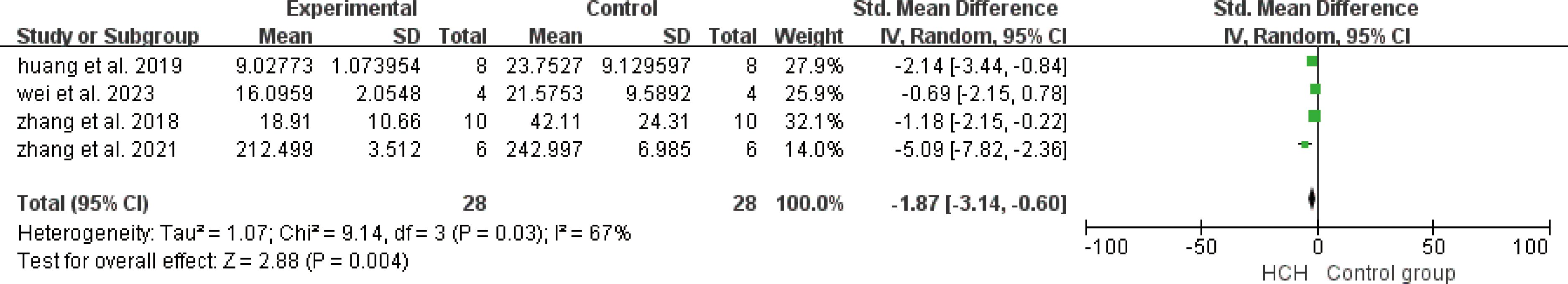
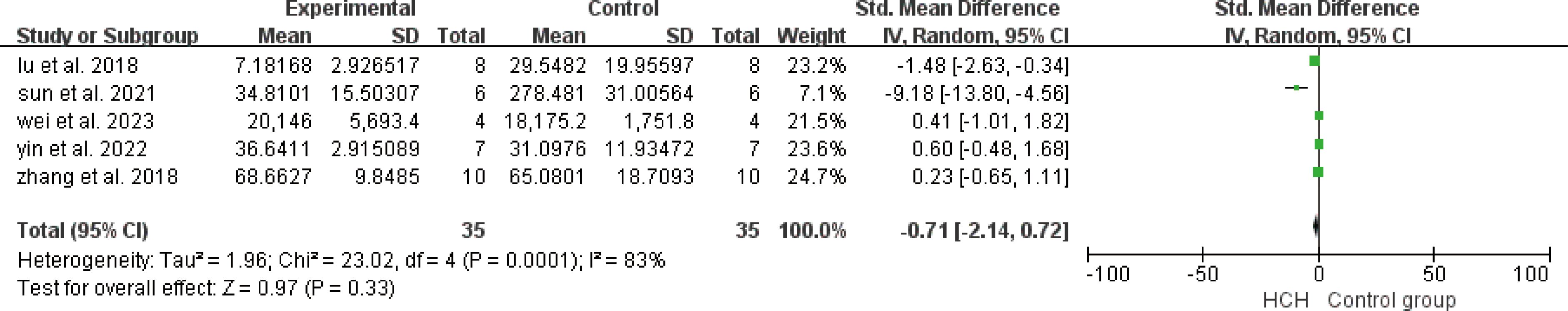
From Figure 2, we can see the protective effect of high concentration of hydrogen on the reduction in IL-1β (SMD = −2.51, 95% CI −3.84 to −1.19, P < 0.005). The I2 was 78% and P = 0.0001, indicating that there is high heterogeneity in all studies of IL-1β (Figure 2). In order to reduce heterogeneity, we excluded low-quality literature and left two high-quality literature (du et al, 2022; feng et al, 2019) (15, 16). I2 < 50%, the fixed-effect model was used to analyze the results, which showed little difference from the original results and that means good stability. From Figure 3, we can see the positive effect of high concentration of hydrogen on the reduction in IL-8 (SMD = −1.95, 95% CI −3.86 to −0.04, P = 0.05). The I2 was 84% and P = 0.002, indicating that there is high heterogeneity in all studies of IL-8 (Figure 3). In order to reduce the heterogeneity, the group with the smallest sample size was excluded (15), and the heterogeneity was reduced to 52%, which still had a significant difference. However, the heterogeneity of retained high-quality literature is still high, and the results are not significant and the results are poor in stability. From Figure 4, we can see the protective effect of HCH on the reduction in TNF-α (SMD = −2.98, 95% CI −4.25 to −1.71, P < 0.005). The I2 = 77% and P = 0.0002, indicating that there is high heterogeneity in all studies of TNF-α (Figure 4). In order to reduce heterogeneity, we retained high-quality literature for analysis (15, 16, 26), and the heterogeneity became smaller, I2 < 50%, and the results still had significant differences and they have good stability. From Figure 5, we can see the positive effect on the reduction in IL-4 (SMD = −1.87, 95% CI −3.14 to -0.6, P < 0.05. The I2 = 67% and P = 0.03, indicating that there is high heterogeneity in all studies of IL- 4 (Figure 5). In order to reduce heterogeneity, studies with a small sample size were eliminated (22, 24), and the heterogeneity became smaller with I2 < 50%, indicating little difference in results and have good stability. From Figure 6, we can see there was no effect on IL-6 (SMD = −0.71, 95% CI −2.14 to 0.72, P = 0.33). The I2 = 83% and P = 0.0001, indicating that there is high heterogeneity in all studies of IL-6 (Figure 6). To reduce heterogeneity, we assessed the quality of the literature, but there was only one high-quality literature (zhang et al, 2018) (26), and the heterogeneity was high regardless of the group, and there was no significant difference in the results.
From Figure 7, we can see the protective effect of HCH on SOD (SMD = 3.22, 95% CI 0.43 to 6.01, P < 0.05). The I2 = 87% and P = 0.0004, indicating that there is high heterogeneity in all studies of SOD (Figure 7). In order to reduce the heterogeneity, the minimum SMD was removed for analysis (huang et al, 2019) (17), and the heterogeneity was reduced with I2 < 50%. The fixed-effect model was used for analysis, and the results showed no significant difference and have good stability. From Figure 8, we can see the positive effect on the reduction in ROS (SMD = −2.71, 95% CI −4.84 to −0.59, P < 0.05). The I2 = 82% and P = 0.01, indicating that there is high heterogeneity in all studies of ROS (Figure 8). However, there were only two literatures in this group, which could not narrow the heterogeneity for subgroup analysis. From Figure 9, we can see the positive effect on the reduction in MDA (SMD = −1.65, 95% CI −2.60 to -0.71, P < 0.05). The I2 = 52% and P = 0.10, indicating that there is high heterogeneity in all studies of MDA (Figure 9). In order to reduce the heterogeneity, we conducted subgroup analysis of the experimental animal with mouse, the group whose experimental animals were rats was excluded (feng et al, 2019) and the results showed that the heterogeneity was reduced (16), I2 < 50%, but the results were not different and the stability was good.
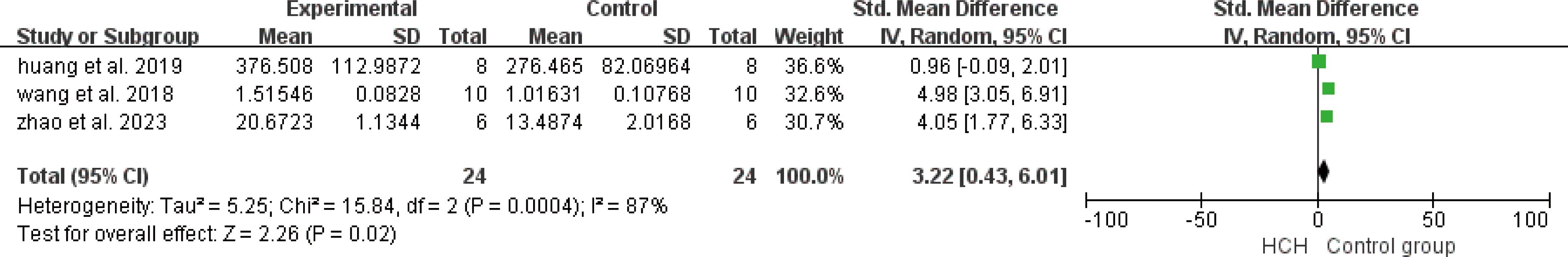
Figure 7. Meta-analysis of superoxide dismutase (SOD) differences—in the HCH group versus the control group.
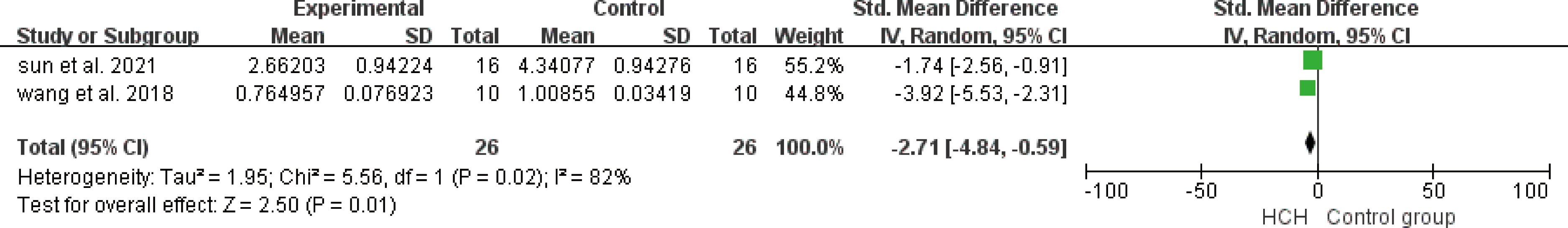
Figure 8. Meta-analysis of reactive oxygen species (ROS) differences—the HCH group versus the control group.
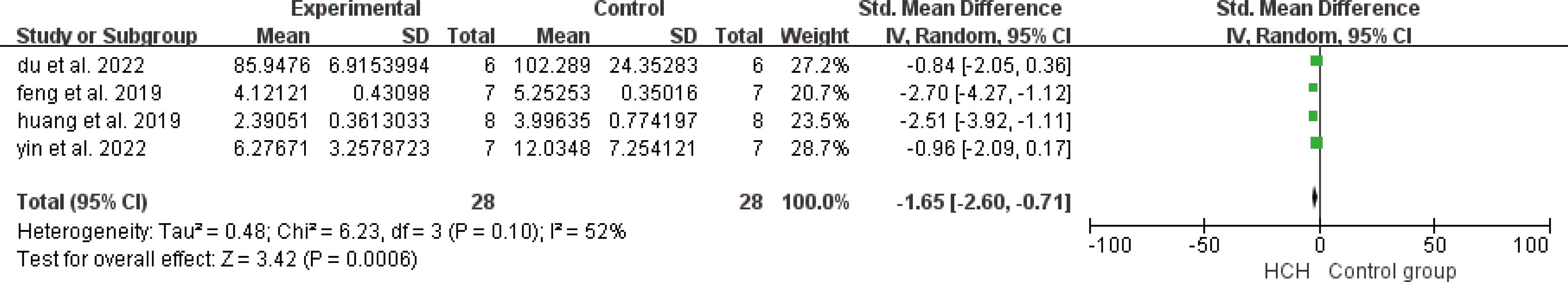
Figure 9. Meta-analysis of malondialdehyde (MDA) differences—in the HCH group versus the control group.
Risk of bias
Table 2 summarizes the risk of bias of the 12 studies based on the Systematic Review Centre for Laboratory Animal Experimentation (SYRCLES) risk of bias tool. In Sequence generation, only one study had a low risk (25), they described using a random number table in the literature, while the other studies had unclear risk, there is no evidence how random sequence produced. For baseline characteristics, two studies had low risk (17, 21), they conducted baseline measurement in the article, while others did not describe and had unknown risk. The risk of allocation concealment in all studies is unknown, they all not specify whether there is allocation concealment. Almost all studies showed a low risk in performance bias, they described the same feeding environment and the same administration conditions. For the random outcome assessment, there were five studies with high risk (15, 16, 21, 24, 26), because that did not describe using a random number table to choose experimental animals and the remaining risks were unclear. Meanwhile, six studies showed low risk in detection bias of blinding (15–17, 21, 24, 26), because almost all study used all animal’s results in the outcome that indicate there is no detection bias. There were six studies with low risk in incomplete outcome data (15–17, 21, 24, 26), because all animals were absorbed in the outcome. The rest with high risk (18–20, 22, 23, 25), because they did not describe how to deal with missing data. In reporting bias and other bias, all studies showed low risk. All the data described in method has been reported in results and there is no drug sharing and undue influence from funders. So, they all in low risk.
Discussion
According to Matei et al. (27), the therapeutic potential of hydrogen has received much attention, and researchers have reported that hydrogen has a beneficial effect on a variety of diseases, including lung diseases such as COPD and ALI.
Other studies have shown that HCH has many pharmacological properties, such as antioxidant and anti-inflammatory effects. The anti-inflammatory effect of HCH may be mediated by the regulation of NF-κB (28). The forest plot (Figure 4) shows that HCH has a positive effect on reducing TNF-α, and all of the analyzed studies included showed that HCH was able to reduce this inflammatory mediator. According to Gardam (29), TNF-α is a key mediator of the activation and recruitment of inflammatory cells, including polymorphonuclear neutrophils (PMNs) and macrophages. In addition, it can also induce the release of proinflammatory markers and oxidative and nitrosation stress in the lung endothelium (30, 31). According to Carvalho (1), the primary action of IL-8 is to stimulate the migration of immune system cells, mainly neutrophils, to increase the expression of adhesion molecules by endothelial cells. This relationship between IL-8 and neutrophilic stimulation was also observed in studies by Hamahata et al. (32) and Qiu et al. (33). According to Zwahlen et al. (34), IL-8 can also activate polymorphonuclear neutrophils and increase oxidative metabolism. The forest plot (Figure 3) shows that HCH has a positive effect on reducing IL-8. Hamahata et al. (32), Laffon et al. (35) and Qiu et al. (33) reported that inflammatory cytokines such as IL-1β play an important role in the occurrence and development of lung injury. In this paper, the effects of HCH on IL-1β were analyzed by making forest plot, and it can be concluded that IL-1β is not only crucial for lung injury, but also can regulate the disease process of lung injury by regulating IL-1β (Figure 2). The forest plot shows that for IL-4, HCH can reduce its secretion. According to Kianmehr et al (36), IL-4 has been detected in the BALF and airway biopsies of patients with mild or asymptomatic asthma and COPD.
In addition, according to Lu et al. (19) and Sun et al. (20) reported that HCH can regulate the secretion of TNF-α, IL-6 and HMGB1 to affect inflammation; but our results didn’t support this conclusion. Among the five studies that evaluated the effects of HCH on IL-6 levels, the studies by Wei et al. (22), Yin et al. (23) and Zhang et al. (26) did not discovered significantly change in the level of IL-6, which make it no significant difference in the result of meta-analysis. We hypothesize that the non-significant difference in results may be due to the following reasons: first, the sources of IL-6 measured in literature are different. When detecting IL-6 in BALF in zhang et al (26), there is no significant difference in hydrogen group, but there is a significant difference in serum, but the data processing software cannot obtain this result. Therefore, only IL-6 levels in BALF were analyzed. In addition, yin et al (23) analyzed IL-6 levels in different time periods, each time point showed different therapeutic effects, but in this study, we only analyzed one of the time points, so IL-6 levels are constantly changing in the course of disease and treatment. This time point we chose is not representative of the therapeutic level of hydrogen in IL-6 over the course of treatment.
De Carvalho et al. (37) reported that smoke inhalation can cause lung and systemic lesions, mainly involving inflammatory processes and oxidative stress, in which the oxidative stress mediators include MDA and so on. MDA is an important marker of oxidative stress, and HCH can significantly reduce its production (Figure 9). In addition, in a population study, it was found that HCH can significantly reduce the level of MDA in lung disease (38). This study confirms the conclusion that HCH can downregulate the level of oxidative stress in the body in lung disease. The quantification of MDA in biological systems is an important parameter for evaluating cellular oxidative stress and is used to estimate lipid peroxidation in the lung (37, 39). Many studies with hydrogen, which uses this marker to analyze the progression of the inflammatory process, have shown that it has positive effects (40, 41).
ROS include free radicals, such as ·OH, superoxide anion radicals (O2· −), and nonfree radical species, such as singlet oxygen (1O2) and H2O2 (42). They are generated inside the body by aerobic organisms as a byproduct of energy metabolism through oxidative phosphorylation (43). Normally, there are antioxidant defense systems in cells that protect biological systems from free radical toxicity, such as SOD, catalase (CAT), glutathione peroxidase (GSH-Px), and heme oxygenase-1 (HO-1) (44, 45). The effects of HCH on ROS and SOD were studied in this paper. Positive effects were observed in the included studies.
Hancock et al. (46) states that hydrogen, a well-known antioxidant, has possible positive effects on lung diseases. Its oxidative stress-reducing parameters have been widely studied in several pathologies (47–49).
However, this study has certain limitations. All the included studies were from China, and the application of high concentrations of hydrogen needs to be confirmed by more researchers. In addition, for cases with heterogeneous sources or no significant difference, we analyzed that the therapeutic effect of hydrogen in the indicators of inflammation and oxidative stress changes with time, so there should be studies to explore which stage hydrogen plays the most important role in the occurrence of inflammatory factors or oxidative stress. Or explore whether hydrogen mainly works by acting directly on the lungs or after entering the blood. Finally, most of the published clinical studies on hydrogen in the respiratory system are on HCH, and the detection indicators are limited to the relief of clinical symptoms. More in-depth clinical studies need to be carried out.
Conclusion
Our results suggest that high concentrations of hydrogen have anti-inflammatory and antioxidant effects in certain inflammatory or oxidative stress mediators. However, at present, there are problems such as small sample size of animal studies or small number of human experiments. A more targeted experimental design would make it possible to more clearly elucidate the relationship between high concentrations of hydrogen and mediators of inflammation and oxidative stress. For now, more high-quality studies are needed to validate these findings. Whether there are more effects on reducing inflammation and oxidation mediators remains to be further elucidated.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
KX: Methodology, Writing – original draft. JL: Conceptualization, Writing – review & editing. YS: Investigation, Writing – original draft. SC: Software, Writing – original draft. JM: Data curation, Writing – original draft. MC: Visualization, Writing – review & editing. YY: Validation, Writing – review & editing. ZP: Resources, Writing – review & editing. PL: Funding acquisition, Writing – review & editing. ZD: Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Key Research and Development Program of China [grant number 2022YFC2503202], National Natural Science Foundation of China (NSFC) [grant numbers 81602893, 81872575], Natural Science Foundation of Shandong Province [grant numbers ZR2015YL049 ZR2021MH218 and ZR2022MH184]; Shandong Province Medical and Health Technology Development Plan [grant number 202104020224, 202212040403, 202312010854]; Shandong Province Traditional Chinese Medicine Science and Technology Plan [grant numbers, 2021M151, Z-2023114], and Jinan Science and Technology Plan [grant number 202328074] and The innovation Project of Shandong Academy of Medical Science.
Acknowledgments
We appreciate the help from all our contributing authors in writing this review.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Glossary
References
1. de Carvalho FO, Silva ER, Gomes IA, Santana HSR, do Nascimento Santos D, de Oliveira Souza GP, et al. Anti-inflammatory and antioxidant activity of carvacrol in the respiratory system: A systematic review and meta-analysis. Phytother Res. (2020) 34:2214–29. doi: 10.1002/ptr.6688
2. Zhang Y, Tan S, Xu J, Wang T. Hydrogen therapy in cardiovascular and metabolic diseases: from bench to bedside. Cell Physiol Biochem. (2018) 47:1–10. doi: 10.1159/000489737
3. Ohsawa I, Ishikawa M, Takahashi K, Watanabe M, Nishimaki K, Yamagata K, et al. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med. (2007) 13:688–94. doi: 10.1038/nm1577
4. Ma T, Yang L, Zhang B, Lv X, Gong F, Yang W. Hydrogen inhalation enhances autophagy via the ampk/mtor pathway, thereby attenuating doxorubicin-induced cardiac injury. Int Immunopharmacol. (2023) 119:110071. doi: 10.1016/j.intimp.2023.110071
5. Yuan T, Zhao JN, Bao NR. Hydrogen applications: advances in the field of medical therapy. Med Gas Res. (2023) 13:99–107. doi: 10.4103/2045-9912.344978
6. Li H, Luo Y, Yang P, Liu J. Hydrogen as a complementary therapy against ischemic stroke: A review of the evidence. J Neurol Sci. (2019) 396:240–6. doi: 10.1016/j.jns.2018.11.004
7. Dole M, Wilson FR, Fife WP. Hyperbaric hydrogen therapy: A possible treatment for cancer. Science. (1975) 190:152–4. doi: 10.1126/science.1166304
8. Wood KC, Gladwin MT. The hydrogen highway to reperfusion therapy. Nat Med. (2007) 13:673–4. doi: 10.1038/nm0607-673
9. Ono H, Nishijima Y, Ohta S, Sakamoto M, Kinone K, Horikosi T, et al. Hydrogen gas inhalation treatment in acute cerebral infarction: A randomized controlled clinical study on safety and neuroprotection. J Stroke Cerebrovasc Dis. (2017) 26:2587–94. doi: 10.1016/j.jstrokecerebrovasdis.2017.06.012
10. Chen JB, Kong XF, Qian W, Mu F, Lu TY, Lu YY, et al. Two weeks of hydrogen inhalation can significantly reverse adaptive and innate immune system senescence patients with advanced non-small cell lung cancer: A self-controlled study. Med Gas Res. (2020) 10:149–54. doi: 10.4103/2045-9912.304221
11. Zheng ZG, Sun WZ, Hu JY, Jie ZJ, Xu JF, Cao J, et al. Hydrogen/oxygen therapy for the treatment of an acute exacerbation of chronic obstructive pulmonary disease: results of a multicenter, randomized, double-blind, parallel-group controlled trial. Respir Res. (2021) 22:149. doi: 10.1186/s12931-021-01740-w
12. Akagi J, Baba H. Hydrogen gas restores exhausted cd8+ T cells in patients with advanced colorectal cancer to improve prognosis. Oncol Rep. (2019) 41:301–11. doi: 10.3892/or.2018.6841
13. Zeng Y, Guan W, Wang K, Jie Z, Zou X, Tan X, et al. Effect of hydrogen/oxygen therapy for ordinary covid-19 patients: A propensity-score matched case-control study. BMC Infect Dis. (2023) 23:440. doi: 10.1186/s12879-023-08424-4
14. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The prisma 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
15. Du J, Li J, Li R, Yan X. High concentration of hydrogen ameliorates lipopolysaccharide-induced acute lung injury in a sirt1-dependent manner. Respir Physiol Neurobiol. (2022) 296:103808. doi: 10.1016/j.resp.2021.103808
16. Feng S, Duan E, Shi X, Zhang H, Li H, Zhao Y, et al. Hydrogen ameliorates lung injury in a rat model of subacute exposure to concentrated ambient pm2.5 via aryl hydrocarbon receptor. Int Immunopharmacol. (2019) 77:105939. doi: 10.1016/j.intimp.2019.105939
17. Huang P, Wei S, Huang W, Wu P, Chen S, Tao A, et al. Hydrogen gas inhalation enhances alveolar macrophage phagocytosis in an ovalbumin-induced asthma model. Int Immunopharmacol. (2019) 74:105646. doi: 10.1016/j.intimp.2019.05.031
18. Li TT, Sun T, Wang YZ, Wan Q, Li WZ, Yang WC. Molecular hydrogen alleviates lung injury after traumatic brain injury: pyroptosis and apoptosis. Eur J Pharmacol. (2022) 914:174664. doi: 10.1016/j.ejphar.2021.174664
19. Lu W, Li D, Hu J, Mei H, Shu J, Long Z, et al. Hydrogen gas inhalation protects against cigarette smoke-induced copd development in mice. J Thorac Dis. (2018) 10:3232–43. doi: 10.21037/jtd.2018.05.93
20. Sun R, Zhao N, Wang Y, Su Y, Zhang J, Wang Y, et al. High concentration of hydrogen gas alleviates lipopolysaccharide-induced lung injury via activating nrf2 signaling pathway in mice. Int Immunopharmacol. (2021) 101:108198. doi: 10.1016/j.intimp.2021.108198
21. Wang D, Wang L, Zhang Y, Zhao Y, Chen G. Hydrogen gas inhibits lung cancer progression through targeting smc3. BioMed Pharmacother. (2018) 104:788–97. doi: 10.1016/j.biopha.2018.05.055
22. Wei Y, Wang K, Zhang Y, Duan Y, Tian Y, Yin H, et al. Potent anti-inflammatory responses: role of hydrogen in il-1alpha dominated early phase systemic inflammation. Front Pharmacol. (2023) 14:1138762. doi: 10.3389/fphar.2023.1138762
23. Yin H, Feng Y, Duan Y, Ma S, Guo Z, Wei Y. Hydrogen gas alleviates lipopolysaccharide-induced acute lung injury and inflammatory response in mice. J Inflammation (Lond). (2022) 19:16. doi: 10.1186/s12950-022-00314-x
24. Zhang J, Feng X, Fan Y, Zhu G, Bai C. Molecular hydrogen alleviates asthma through inhibiting il-33/ilc2 axis. Inflamm Res. (2021) 70:569–79. doi: 10.1007/s00011-021-01459-w
25. Zhao N, Sun R, Cui Y, Song Y, Ma W, Li Y, et al. High concentration hydrogen mitigates sepsis-induced acute lung injury in mice by alleviating mitochondrial fission and dysfunction. J Pers Med. (2023) 13:244. doi: 10.3390/jpm13020244
26. Zhang N, Deng C, Zhang X, Zhang J, Bai C. Inhalation of hydrogen gas attenuates airway inflammation and oxidative stress in allergic asthmatic mice. Asthma Res Pract. (2018) 4:3. doi: 10.1186/s40733-018-0040-y
27. Matei N, Camara R, Zhang JH. Emerging mechanisms and novel applications of hydrogen gas therapy. Med Gas Res. (2018) 8:98–102. doi: 10.4103/2045-9912.239959
28. Huang CS, Kawamura T, Peng X, Tochigi N, Shigemura N, Billiar TR, et al. Hydrogen inhalation reduced epithelial apoptosis in ventilator-induced lung injury via a mechanism involving nuclear factor-kappa B activation. Biochem Biophys Res Commun. (2011) 408:253–8. doi: 10.1016/j.bbrc.2011.04.008
29. Gardam MA, Keystone EC, Menzies R, Manners S, Skamene E, Long R, et al. Anti-tumour necrosis factor agents and tuberculosis risk: mechanisms of action and clinical management. Lancet Infect Dis. (2003) 3:148–55. doi: 10.1016/s1473-3099(03)00545-0
30. Frey RS, Gao X, Javaid K, Siddiqui SS, Rahman A, Malik AB. Phosphatidylinositol 3-kinase gamma signaling through protein kinase czeta induces nadph oxidase-mediated oxidant generation and nf-kappab activation in endothelial cells. J Biol Chem. (2006) 281:16128–38. doi: 10.1074/jbc.M508810200
31. Su X, Ao L, Zou N, Song Y, Yang X, Cai GY, et al. Post-transcriptional regulation of tnf-induced expression of icam-1 and il-8 in human lung microvascular endothelial cells: an obligatory role for the P38 mapk-mk2 pathway dissociated with hsp27. Biochim Biophys Acta. (2008) 1783:1623–31. doi: 10.1016/j.bbamcr.2008.04.009
32. Hamahata A, Enkhbaatar P, Lange M, Cox RA, Hawkins HK, Sakurai H, et al. Direct Delivery of Low-Dose 7-Nitroindazole into the Bronchial Artery Attenuates Pulmonary Pathophysiology after Smoke Inhalation and Burn Injury in an Ovine Model. Shock. (2011) 36:575–9. doi: 10.1097/SHK.0b013e3182360f2e
33. Qiu X, Ji S, Wang J, Li H, Xia T, Pan B, et al. The therapeutic efficacy of ulinastatin for rats with smoking inhalation injury. Int Immunopharmacol. (2012) 14:289–95. doi: 10.1016/j.intimp.2012.08.002
34. Zwahlen R, Walz A, Rot A. In vitro and in vivo activity and pathophysiology of human interleukin-8 and related peptides. Int Rev Exp Pathol. (1993) 34 Pt B:27–42. doi: 10.1016/b978-0-12-364935-5.50008-0
35. Laffon M, Pittet JF, Modelska K, Matthay MA, Young DM. Interleukin-8 mediates injury from smoke inhalation to both the lung endothelial and the alveolar epithelial barriers in rabbits. Am J Respir Crit Care Med. (1999) 160:1443–9. doi: 10.1164/ajrccm.160.5.9901097
36. Kianmehr M, Rezaei A, Boskabady MH. Effect of carvacrol on various cytokines genes expression in splenocytes of asthmatic mice. Iran J Basic Med Sci. (2016) 19:402–10. doi: 10.22038/ijbms.2016.6812
37. de Carvalho FO, Felipe FA, de Melo Costa AC, Teixeira LG, Silva ER, Nunes PS, et al. Inflammatory mediators and oxidative stress in animals subjected to smoke inhalation: A systematic review. Lung. (2016) 194:487–99. doi: 10.1007/s00408-016-9879-y
38. Gong ZJ, Guan JT, Ren XZ, Meng DY, Zhang HR, Wang BL, et al. [Protective effect of hydrogen on the lung of sanitation workers exposed to haze]. Zhonghua Jie He He Hu Xi Za Zhi. (2016) 39:916–23. doi: 10.3760/cma.j.issn.1001-0939.2016.12.003
39. Bagis S, Tamer L, Sahin G, Bilgin R, Guler H, Ercan B, et al. Free radicals and antioxidants in primary fibromyalgia: an oxidative stress disorder? Rheumatol Int. (2005) 25:188–90. doi: 10.1007/s00296-003-0427-8
40. Diao M, Zhang S, Wu L, Huan L, Huang F, Cui Y, et al. Hydrogen gas inhalation attenuates seawater instillation-induced acute lung injury via the nrf2 pathway in rabbits. Inflammation. (2016) 39:2029–39. doi: 10.1007/s10753-016-0440-1
41. Yu Y, Yang Y, Yang M, Wang C, Xie K, Yu Y. Hydrogen gas reduces hmgb1 release in lung tissues of septic mice in an nrf2/ho-1-dependent pathway. Int Immunopharmacol. (2019) 69:11–8. doi: 10.1016/j.intimp.2019.01.022
42. Zhang Y, Zhang J, Fu Z. Molecular hydrogen is a potential protective agent in the management of acute lung injury. Mol Med. (2022) 28:27. doi: 10.1186/s10020-022-00455-y
43. Sies H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. (2015) 4:180–3. doi: 10.1016/j.redox.2015.01.002
44. Birben E, Sahiner UM, Sackesen C, Erzurum S, Kalayci O. Oxidative stress and antioxidant defense. World Allergy Organ J. (2012) 5:9–19. doi: 10.1097/WOX.0b013e3182439613
45. Kellner M, NooNepalle S, Lu Q, Srivastava A, Zemskov E, Black SM. Ros signaling in the pathogenesis of acute lung injury (Ali) and acute respiratory distress syndrome (Ards). Adv Exp Med Biol. (2017) 967:105–37. doi: 10.1007/978-3-319-63245-2_8
46. Hancock JT, Russell G. Downstream signalling from molecular hydrogen. Plants (Basel). (2021) 10:367. doi: 10.3390/plants10020367
47. Matsuoka H, Miyata S, Okumura N, Watanabe T, Hashimoto K, Nagahara M, et al. Hydrogen gas improves left ventricular hypertrophy in dahl rat of salt-sensitive hypertension. Clin Exp Hypertens. (2019) 41:307–11. doi: 10.1080/10641963.2018.1481419
48. Yu Y, Feng J, Lian N, Yang M, Xie K, Wang G, et al. Hydrogen gas alleviates blood-brain barrier impairment and cognitive dysfunction of septic mice in an nrf2-dependent pathway. Int Immunopharmacol. (2020) 85:106585. doi: 10.1016/j.intimp.2020.106585
Keywords: anti-inflammatory, antioxidant, high-concentration hydrogen, meta-analysis, respiratory system, systematic review
Citation: Xiao K, Liu J, Sun Y, Chen S, Ma J, Cao M, Yang Y, Pan Z, Li P and Du Z (2024) Anti-inflammatory and antioxidant activity of high concentrations of hydrogen in the lung diseases: a systematic review and meta-analysis. Front. Immunol. 15:1444958. doi: 10.3389/fimmu.2024.1444958
Received: 06 June 2024; Accepted: 30 July 2024;
Published: 15 August 2024.
Edited by:
Ramcés Falfán-Valencia, National Institute of Respiratory Diseases-Mexico (INER), MexicoReviewed by:
Furkan Ayaz, Biruni University, TürkiyeIfora Ifora, School of Pharmaceutical Science Padang (STIFARM Padang), Indonesia
Copyright © 2024 Xiao, Liu, Sun, Chen, Ma, Cao, Yang, Pan, Li and Du. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhifeng Pan, MTg2NTMxOTkxNzJAMTYzLmNvbQ==; Peng Li, c2R6ZnkyMDA1QDEyNi5jb20=; Zhongjun Du, ZHV6ajE5ODFAMTYzLmNvbQ==; ZHV6aG9uZ2p1bkBzZGZtdS5lZHUsY24=
 Kang Xiao1,2
Kang Xiao1,2 Yuxin Sun
Yuxin Sun Zhongjun Du
Zhongjun Du