- 1Department of Translational Medical Sciences, University of Naples Federico II, Naples, Italy
- 2Department of Oncology and Molecular Medicine, Istituto Superiore di Sanità (ISS), Rome, Italy
- 3World Allergy Organization (WAO) Center of Excellence, Naples, Italy
- 4Center for Basic and Clinical Immunology Research (CISI), University of Naples Federico II, Naples, Italy
- 5Department of Woman, Child and General and Specialistic Surgery, University of Campania “Luigi Vanvitelli”, Naples, Italy
- 6Institute of Experimental Endocrinology and Oncology (IEOS), National Research Council, Naples, Italy
Introduction: The Janus kinase (JAK) family includes four cytoplasmic tyrosine kinases (JAK1, JAK2, JAK3, and TYK2) constitutively bound to several cytokine receptors. JAKs phosphorylate downstream signal transducers and activators of transcription (STAT). JAK-STAT5 pathways play a critical role in basophil and mast cell activation. Previous studies have demonstrated that inhibitors of JAK-STAT pathway blocked the activation of mast cells and basophils.
Methods: In this study, we investigated the in vitro effects of ruxolitinib, a JAK1/2 inhibitor, on IgE- and IL-3-mediated release of mediators from human basophils, as well as substance P-induced mediator release from skin mast cells (HSMCs).
Results: Ruxolitinib concentration-dependently inhibited IgE-mediated release of preformed (histamine) and de novo synthesized mediators (leukotriene C4) from human basophils. Ruxolitinib also inhibited anti-IgE- and IL-3-mediated cytokine (IL-4 and IL-13) release from basophils, as well as the secretion of preformed mediators (histamine, tryptase, and chymase) from substance P-activated HSMCs.
Discussion: These results indicate that ruxolitinib, inhibiting the release of several mediators from human basophils and mast cells, is a potential candidate for the treatment of inflammatory disorders.
1 Introduction
The Janus kinase (JAK) family includes four cytoplasmic tyrosine kinases: JAK1, JAK2, JAK3, and tyrosine-protein kinase 2 (TYK2) (1, 2). JAKs are constitutively bound to several cytokine receptors and upon ligand binding to its receptor, JAKs phosphorylate downstream signal transducers and activators of transcription (STAT) (3, 4). The STAT family has seven members (STAT1, STAT2, STAT3, STAT4, STAT5A, STAT5B, and STAT6) (5), which have a major role in the regulation of hematopoietic and immune cells (2). The tyrosine kinase domain of JAKs is the site of catalytic activity and is blocked by first- and second-generation JAK inhibitors (6).
The JAK2-STAT5 signaling pathway is crucial for the activation growth and survival of mast cells (7, 8) and basophils (9–12). STAT5 also plays a role in IgE-mediated mast cell degranulation, making the JAK2-STAT5 pathway an appealing target for the inhibition of mast cell activation (8). Ruxolitinib, a JAK1/JAK2 inhibitor (13), has shown clinical benefits in polycythemia vera (PV) patients which carry an activity mutation of JAK2 gene (i.e., V617F) (14–16). Ruxolitinib inhibits anaphylaxis in mice, and two studies reported a decrease in mast cell mediator-related symptoms in patients with systemic mastocytosis treated with ruxolitinib (17, 18). Several JAK1/2 and STAT5 inhibitors suppress the activation of mastocytoma cell lines (19, 20) and human basophils (21–23).
Human peripheral blood basophils share some similarities with tissue-resident mast cells (24, 25). Both cell types express high-affinity immunoglobulin (Ig)E receptors (FcϵRI), contain basophilic granules in the cytoplasm, and release histamine and other inflammatory mediators (24). Several studies have demonstrated the distinct roles of basophils in allergic inflammation in both mice (26, 27) and humans (28–30). Atopic dermatitis and chronic spontaneous urticaria are characterized by chronic pruritus (31) and basophils are involved in their pathobiology (32) and likely contribute to itch (33). Itching is also a common symptom in PV, affecting more than 50% of patients (34, 35). Pruritus can be an initial symptom or precede the development of hematologic manifestations (35–38). Importantly, Pieri and collaborators first demonstrated that basophils from JAK2 V617F PV patients overexpressed CD63, a marker of basophil activation (39), compared to controls when challenged with IL-3 plus fMLP (21). Moreover, the JAK2 inhibitor compound AZD1480 reduced CD63 expression in basophils of PV patients in response to IL-3 plus fMLP.
Mast cells are in close anatomical association with myelinated and unmyelinated neural structures and blood vessels (40), forming an important functional unit that maintains homeostasis and responds to insults (41–44). A critical aspect of this multicellular crosstalk includes the interaction between mast cells and sensory nerves (45). Sensory nerves express neuropeptides (e.g., substance P, VIP) and neurotransmitters that facilitate neural-immune communication, leading to mast cell mediator release which subsequently activates sensory neurons via different receptors (33). Mast cell density in the skin was increased in JAK2 V617F transgenic mice compared to controls (46).
Ruxolitinib inhibits the catalytic activity of wild-type JAK2 as well as mutant JAK2 (6). This drug was approved for the treatment of MF by the US Food and Drug Administration (FDA) in 2011 and by the European Medicines Agency (EMA) in 2012, followed by the approval for the treatment of hydroxyurea-resistant or -intolerant PV in 2014. Recent evidence demonstrates that ruxolitinib inhibits the release of hexosaminidase and TNF-α from mast cell lines (47) and the expression on human basophils of CD300f induced by IL-3 (48). In this study, we have evaluated the in vitro effects of pharmacologic concentrations of ruxolitinib on IgE-mediated release of proinflammatory mediators (histamine and LTC4) and cytokines (IL-4 and IL-13) from highly purified human basophils. Additionally, we have examined the effects of ruxolitinib on IL-3-mediated release of cytokines (IL-4 and IL-13) from basophils and on substance P-induced secretion of several preformed mediators (histamine, tryptase, and chymase) from human skin mast cells.
2 Materials and methods
2.1 Reagents
Bovine serum albumin, human serum albumin, piperazine-N, N’-bis (2-ethanesulfonic acid) (Pipes), hyaluronidase, chymopapain, elastase type I, substance P, LTC4 (Sigma Chemical Co., St. Louis, MO, USA), and ruxolitinib (Cambridge Bioscience, Cambridge, UK) were commercially obtained. Ruxolitinib was dissolved in ethanol at the concentration of 13 mg/ml. Collagenase (Worthington Biochemical Co., Freehold, NJ, USA), Hanks’ balanced salt solution and fetal calf serum (FCS), Iscove modified Dulbecco medium (IMDM) (GIBCO, Grand Island, NY, USA), human recombinant IL-3 (R & D System, Minneapolis, MN, USA), deoxyribonuclease I and pronase (Calbiochem, La Jolla, CA, USA), Percoll® (Pharmacia Fine Chemicals, Uppsala, Sweden), HClO4 (Baker Chemical Co., Deventer, The Netherlands), (3H)-LTC4 (New England Nuclear, Boston, MA, USA) were commercially purchased. Basophil Isolation Kit II and CD117 MicroBead kit were obtained from Miltenyi, Biotec (Bologna, Italy). Anti-IgE produced by rabbit immunization with the Fc fragment of a human IgE myeloma (patient PS) and then absorbed with the IgE Fab (49) was a gift of Drs. Teruko and Kimishige Ishizaka (La Jolla Institute for Allergy and Immunology, La Jolla, CA, USA). Rabbit anti-LTC4 antibody was donated by Dr. Lawrence M. Lichtenstein (The Johns Hopkins University, Baltimore, MD, USA). Tryptase fluoroenzyme immunoassay (Phadia Diagnostic AB, Uppsala, Sweden) was kindly donated by Kabi Pharmacia (Milan, Italy).
2.2 Buffers
The Pipes buffer was made by 25 mM Pipes, 110 mM NaCl, 5 mM KCl, pH 7.4 and referred to as P buffer. P2CG contains, in addition to P buffer, 2 mM CaCl2 and 1 g/l dextrose (32) and was used for short-term (45 min) incubations of basophils and skin mast cells. PGMD contains 1 mM MgCl2, 10 mg/l DNase, and 1 g/l gelatin in addition to P buffer, pH 7.37 and was used to wash skin mast cells during the isolation. IMDM was used for long-term incubation of human basophils (4 hours for IL-4 and 16 hours for IL-13).
2.3 Purification and activation of human basophils
The study was approved by the Ethics Committee of the University of Naples Federico II (198/18), and written informed consent was obtained from all subjects involved in the study according to the recommendations from the Declaration of Helsinki. Basophils were isolated from peripheral blood of healthy volunteers (26% females), aged 19-44 years, undergoing hemapheresis at the University of Naples Federico II. Buffy coats were subjected to double-Percoll density centrifugation, which produced basophil-depleted cell and basophil-enriched cell suspensions (50, 51). Basophils were purified from the basophil-enriched cell suspensions using the Basophil Isolation Kit II (Miltenyi, Biotec, Bologna, Italy). Duplicate basophil aliquots, with a purity of ≥ 98% assessed by Alcian blue staining (52) were resuspended in P2CG and the cell suspension were placed in 12 x 75 mm polyethylene tubes and warmed to 37°C; anti-IgE (10-1 μg/ml) was added, and incubation was continued for 45 min at 37°C (53). At the end of incubations, cells were centrifuged (1000 g, 22°C, 2 min) and the supernatants were stored at -20°C for subsequent assay of histamine and LTC4 (54). Histamine was expressed as percent of the total content assessed in samples lysed with the addition of 2% HClO4, minus the spontaneous release (53, 55). LTC4 was analyzed by radioimmunoassay. Individual histamine and LTC4 release values were the means of duplicate determinations, replicates differing from each other by < 5%. In experiments evaluating the release of cytokines, basophils with purity ≥ 99% were incubated at 37°C for 4 hours (IL-4) or 16 hours (IL-13) (56) in IMDM in the presence of anti-IgE (10-1 μg/ml) or IL-3 (10 ng/ml). At the end of incubations, the cell-free supernatants were harvested and stored at -20°C for subsequent assay of IL-4 and IL-13 by ELISA (56).
2.4 Purification and activation of human skin mast cells
Skin samples were obtained from female patients, aged 20-58 years, undergoing either elective cosmetic surgery or mastectomy for breast cancer (54). The subcutaneous fat was eliminated by blunt dissection and skin tissue was cut into 1-2 mm fragments and dispersed into single cell suspension as previously described (54). Yields with this technique ranged between 0.1 and 0.8 x 106 skin mast cells/g of wet tissue. At the end of this procedure, skin mast cell (HSMC) purities were between 4% and 8%. HSMCs were purified using a CD117 MicroBead Kit cell sorting system (Miltenyi Biotech, Bologna, Italy) according to the manufacturer’s instructions, reaching purities between 91% and 96% (54). Duplicate aliquots of purified HSMCs were suspended in P2CG and 0.3 ml of the cell suspensions were placed in 12 x 75 mm polyethylene tubes at 30°C; 0.2 ml of each prewarmed stimulus (substance P) was added, and incubation was continued at 30°C for 45 min (57). Mediator release from HSMCs is optimal at 30°C (54, 58). At the end of incubations, cells were centrifuged (1000 g, 22°C, 2 min) and the supernatants were stored at -20°C for subsequent assay of histamine, tryptase, and chymase.
2.5 Assay of histamine and LTC4
Histamine concentrations in supernatants of basophils and HSMCs were measured in duplicate samples with an automated fluorometric technique (32, 59). LTC4 was assayed in duplicate samples as previously described (60). The anti-LTC4 antibody is highly specific, with less than 1% cross-reactivity to other eicosanoids (60, 61). All determinations were run from duplicate samples against a standard curve also in duplicate. In calculating net LTC4 release, spontaneous release of LTC4 from basophils was always subtracted.
2.6 Assay of tryptase and chymase
Tryptase concentrations were measured in duplicate samples by fluoroenzyme immunoassay (FEIA) using Uni-CAP100 (Phadia Diagnostics AB, Uppsala, Sweden) as previously described (62). Chymase concentrations in supernatants of HSMCs were measured by DuoSet™ ELISA (R&D Systems, Minneapolis, MN, USA). The ELISA detection range was 100-8,000 pg/ml).
2.7 Assay of IL-4 and IL-13
IL-4 and IL-13 concentrations were assessed in duplicate samples using ELISA kits according to manifacturer’s instructions (Quantikine Elisa Kit) (R & D Systems, Minneapolis, MN, USA). The ELISA detection range was 31-2,000 pg/ml (IL-4) and 125-4,000 pg/ml (IL-13).
2.8 Assay of lactate hydrogenase
Lactate hydrogenase (LDH) concentrations were assessed in duplicate samples using LDH activity assay kit according to manufacturer instructions (Thermo Fischer Scientific, Monza, Italy).
2.9 Statistical analysis
Data were analyzed with the GraphPad Prism 9 software package (GraphPad Software, La Jolla, CA, USA). Values are expressed as mean ± SD (standard deviation of the mean). Normality tests (Shapiro-Wilk and Kolmogorov-Smirnov tests) were performed through GraphPad Prism 9 software. Since the normal distribution of the results was demonstrated, we performed one-way analysis of variance (ANOVA) (63). Correlations between two variables were assessed by Spearman’s rank correlation analysis and reported as coefficient of correlation (r). Values of p ≤ 0.05 were considered significant. A log concentration-inhibition curve for mediator release (histamine, LTC4, IL-4, IL-13, tryptase, and chymase) was constructed by plotting the log concentration of ruxolitinib against percent inhibition of release. IC50 values were assessed by interpolation.
3 Results
3.1 Effects of ruxolitinib on IgE-mediated release of mediators from human basophils
In a first series of experiments, we evaluated the effects of ruxolitinib on IgE-mediated release of preformed (histamine) and de novo synthesized mediators (leukotriene C4: LTC4) from basophils purified from healthy donors. Basophils were preincubated (30 min, 37°C) with increasing concentrations of ruxolitinib (3 - 30 μM) and then challenged with an optimal concentration of anti-IgE (10-1 μg/ml). The concentrations of ruxolitinib used in these experiments reflect those achieved in vivo during treatment (64, 65) and are known to inhibit JAK1/JAK2 in human blood cells (13). These ruxolitinib concentrations did not affect the spontaneous release of LDH and histamine from basophils. Moreover, the vehicle (ethanol) corresponding to the highest concentrations of ruxolitinib (30 μM) did not affect the spontaneous or anti-IgE-mediated release of mediators (LDH, histamine, and IL-13) from basophils (data not shown). Ruxolitinib caused a concentration-dependent inhibition of histamine release from basophils activated by anti-IgE (Figure 1A). The inhibition ranged from approximately 4% at 3 μM to 80% at 30 μM, with an IC50 of 13.60 ± 3.93 μM.
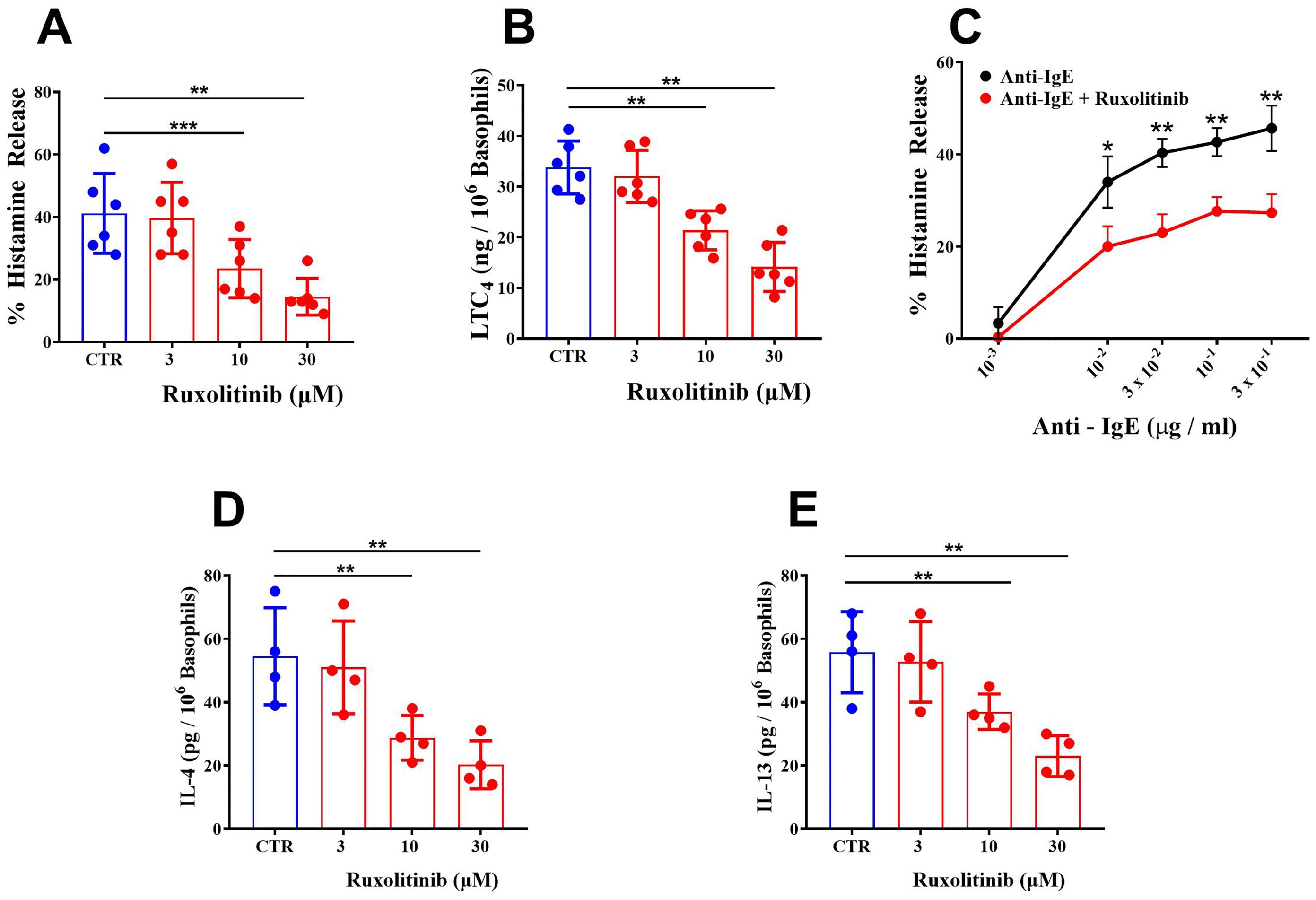
Figure 1. (A) Effects of increasing concentrations of ruxolitinib on anti-IgE-mediated histamine release from human basophils. Cells were preincubated (30 min, 37°C) with or without the indicated concentrations of ruxolitinib and then challenged (45 min, 37°C) with anti-IgE (10-1 μg/ml). Each bar represents the mean ± SD of six experiments with different preparations of basophils. **p < 0.01; ***p < 0.001 compared with histamine release in the absence of ruxolitinib (CTR). (B) Effects of increasing concentrations of ruxolitinib on anti-IgE-mediated LTC4 release from human basophils. Cells were preincubated (30 min, 37°C) with or without the indicated concentrations of ruxolitinib and then challenged (45 min, 37°C) with anti-IgE (10-1 μg/ml). Each bar represents the mean ± SD from six experiments with different preparations of basophils. **p < 0.01; compared with histamine release in the absence of ruxolitinib (CTR); (C) Effects of increasing concentrations of anti-IgE, alone or preincubated (30 min, 37°C) with ruxolitinib (10 μΜ) on histamine release from basophils. Cells were preincubated (30 min, 37°C) with or without ruxolitinib (10 μΜ) and then challenged (45 min, 37°C) with increasing concentrations of anti-IgE (10-3 – 3 x 10-1 μg/ml). Each point represents the mean ± SD from three experiments from different preparations of basophils. *p < 0.05; **p < 0.01. Effects of increasing concentrations of ruxolitinib on anti-IgE-mediated IL-4 (D) and IL-13 (E) release from human basophils. Cells were incubated with or without (CTR) the indicated concentrations of ruxolitinib and then challenged (4 hours for IL-4 and 16 hours for IL-13) with anti-IgE (10-1 μg/ml). Each bar represents the mean ± SD from four experiments with different preparations of basophils. **p < 0.01 compared with IL-4/IL-13 release in the absence of ruxolitinib (CTR).
IgE-mediated activation of basophils induces the de novo synthesis of LTC4 (66), a proinflammatory and vasoactive mediator implicated in several inflammatory disorders (67, 68) and angiogenesis (60, 69). The pharmacologic modulation of de novo synthesized mediators from basophils and mast cells does not always parallel that of preformed mediators (e.g., histamine). Figure 1B shows that in the same experiments illustrated in Figure 1A, ruxolitinib (3 - 30 μM) induced a concentration-dependent inhibition (5 to 58%) of LTC4 release from anti-IgE-activated basophils. In these experiments, the IC50 for the inhibition of LTC4 release from basophils was 21.70 ± 6.73 μM.
We also evaluated the effects of ruxolitinib on histamine release induced by suboptimal (10-3 to 3 x 10-2 μg/ml) and supraoptimal concentrations of anti-IgE (3 x 10-1 μg/ml). Figure 1C shows that increasing the concentrations of anti-IgE (10-3 to 3 x 10-1 μg/ml) induced a progressive increase in the percentage of histamine release from basophils. When basophils were preincubated (30 min, 37°C) with a suboptimal concentration (10 μM) of ruxolitinib, there was a significant inhibition of histamine release from basophils activated by all tested concentrations of anti-IgE.
3.2 Effects of ruxolitinib on IgE-mediated release of cytokines from human basophils
IgE-mediated activation of basophils results in the release of Type (T)-2 cytokines (IL-4 and IL-13) (70–73). The release of IL-4 from basophils is optimal after 4 hours of incubations, whereas IL-13 release is optimal after 16-18 hours of incubation (56, 71). To evaluate the effect of ruxolitinib on anti-IgE-induced IL-4 release, experiments were performed using purified (> 90%) basophils from healthy donors. As shown in Figure 1D, ruxolitinib (3 - 30 μM) caused a concentration-dependent inhibition of IL-4 release from basophils incubated (4 hours) with anti-IgE. The inhibition ranged from approximately 7% at 3 μΜ to 71% at 30 μΜ, with an IC50 of 13.20 ± 2.58 μΜ.
In parallel experiments, we evaluated the effects of graded concentrations of ruxolitinib (3 - 30 μM) on IL-13 release from anti-IgE-activated human basophils. Based on previous findings (56, 71), basophils were preincubated with ruxolitinib (30 min, 37°C) and then incubated for 16 hours at 37°C. Figure 1E shows that ruxolitinib concentration-dependently inhibited IL-13 release from anti-IgE-activated basophils. The inhibition ranged from 5% at 3 μΜ to approximately 59% at 30 μΜ, with an IC50 21.60 ± 4.47 μΜ.
3.3 Effects of ruxolitinib on IL-3-induced cytokine release from human basophils
IL-3 induces the release of T2 high cytokines (IL-4 and IL-13) from basophils (10, 50, 56, 71, 74, 75) through the activation of the IL-3 receptor (76). We evaluated the effects of increasing concentrations (3 - 30 μM) of ruxolitinib on the release of IL-4 and IL-13 from basophils challenged with IL-3 (10 ng/ml). Figure 2A shows that ruxolinitib caused a concentration-dependent inhibition of IL-4 from IL-3-activated basophils. The inhibition ranged from approximately 8% at 3 μΜ to 61% at 30 μM, with an IC50 of 21.03 ± 5.55. The inhibition of IL-3-induced IL-13 release from basophils caused by ruxolitinib varied from 4% at 3 μΜ to 67% at 30 μΜ, with an IC50 of 18.60 ± 8.86 μΜ (Figure 2B).

Figure 2. Effects of increasing concentrations of ruxolitinib on IL-3-mediated release of IL-4 (A) and IL-13 (B) from human basophils. Cells were incubated with the indicated concentrations of ruxolitinib and then challenged (4 hours for IL-4 and 16 hours for IL-13) with IL-3 (10 ng/ml). Each bar represents the mean ± SD from four different preparations of basophils. *p < 0.05; **p < 0.01 compared with IL-4/IL-13 release in the absence of ruxolitinib (CTR).
3.4 Effects of ruxolitinib on substance P- mediated release of mediators from human skin mast cells
Mast cells are widely distributed in almost all human tissues (40, 53). The secretory granules of mast cells contain performed mediators, including histamine, tryptase and chymase (77, 78). Mast cells containing tryptase and chymase (MCTC) are predominant in human skin (HSMCs) (77, 78) and can be activated by substance P through the engagement of MAS-related G protein-coupled receptor-X2 (MRGPRX2) receptor (79). Substance P, a neuropeptide (80) which induces only the release of preformed mediators from HSMCs (54), is a potent endogenous pruritogen in mice and humans (81, 82).
In a series of five experiments, we evaluated the parallel release of histamine, tryptase and chymase from highly purified (> 90%) HSMCs challenged in vitro with increasing concentrations of substance P. Substance P (5 x 10-7 – 5 x 10-6 M) induced the concentration-dependent release of histamine (Figure 3A), tryptase (Figure 3B), and chymase (Figure 3C) from HSMCs. There was a linear correlation (r = 0.81; p < 0.001) between the release of histamine and tryptase from substance P-activated HSMCs (Figure 3D). Similarly, there was a linear correlation (r = 0.77; p < 0.001) between histamine and chymase release from HSMCs (Figure 3E). No significant correlation (r = 0.48; NS) was found between tryptase and chymase release from HSMCs induced by substance P (Figure 3F).
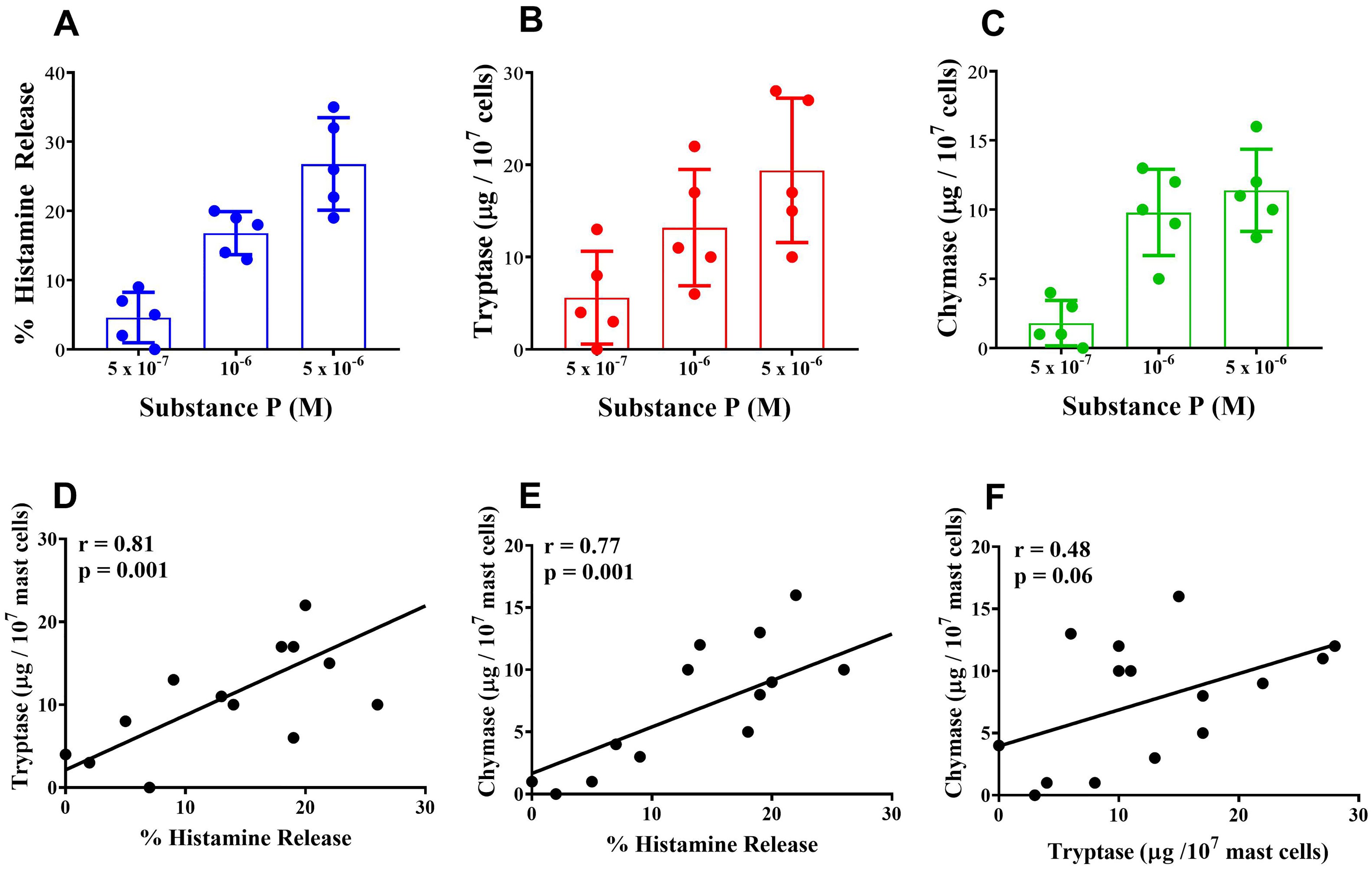
Figure 3. Effects of increasing concentration of substance P on the release of histamine (A), tryptase (B) and chymase (C) from human skin mast cells (HSMCs). Cells were incubated (30 min, 30°C) with the indicated concentrations of substance P. Each bar represents the mean ± SD from five experiments with different preparations of HSMCs. Correlation between the release of histamine and tryptase (D), histamine and chymase (E), and tryptase and chymase (F) induced by the individual concentrations of substance P used in the five experiments.
In a next group of experiments, we compared the effects of increasing concentrations of ruxolitinib (3 - 30 μΜ) on the release of histamine, tryptase, and chymase from purified HSMCs activated by substance P (5 x 10-6 M). Figure 4A shows that ruxolitinib (3 - 30 μΜ) caused a concentration-dependent inhibition of histamine release from substance P-activated HSMCs. Similarly, in the same experiments, ruxolitinib inhibited the release of both tryptase (Figure 4B) and chymase (Figure 4C) from substance P-activated HSMCs. The IC50 for histamine (13.5 ± 2.29 μΜ), tryptase (17.7 ± 6.82 μΜ), and chymase (13.87 ± 2.60 μΜ) did not differ significantly.
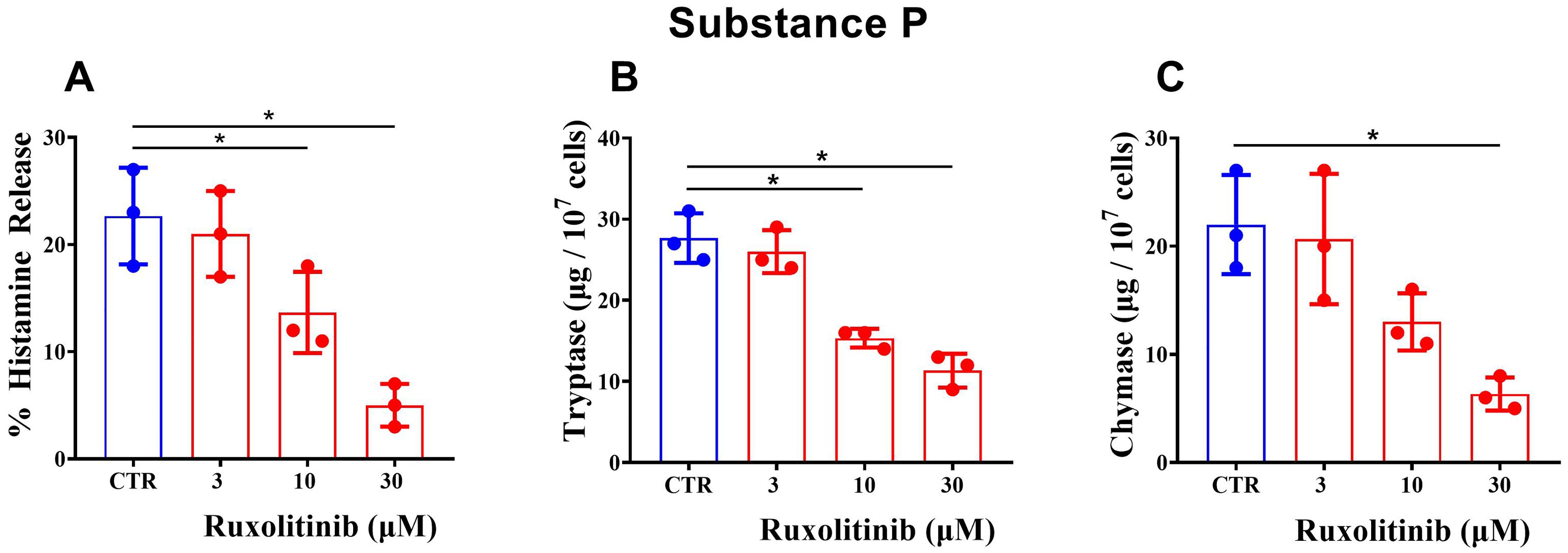
Figure 4. Effects of increasing concentrations of ruxolitinib on substance P-mediated release of histamine (A), tryptase (B), and chymase (C) from human skin mast cells (HSMCs). Cells were preincubated (30 min, 30°C) with the indicated concentrations of ruxolitinib and then challenged with buffer alone (CTR) or with substance P (5 x 10-6 M) (30 min, 30°C). Each bar represents the mean ± SD from three experiments with different preparations of HSMCs. *p < 0.05 compared with histamine/tryptase/chymase release in the absence of ruxolitinib (CTR).
4 Discussion
This study demonstrates that ruxolitinib inhibits the IgE-mediated release of preformed (histamine) and de novo synthesized proinflammatory mediators (LTC4) from highly purified human basophils. Furthermore, ruxolitinib inhibits the IgE- and IL-3-mediated release of cytokines (IL-4 and IL-13) from human basophils. Finally, ruxolitinib inhibits the release of several preformed mediators (histamine, tryptase, and chymase) from HSMCs activated by substance P.
Pharmacologic concentrations of ruxolitinib (64, 65), known to inhibit JAK1/2 in human blood cells (13), inhibited the release of histamine and cytokines induced by IgE cross-linking and IL-3, which activate distinct membrane receptors on basophils. Anti-IgE cross-links IgE bound to FcϵRI (83) and the JAK2-STAT5 signaling pathways play a critical role in IgE-mediated activation of basophils (9–12). IL-3 activates the heterodimeric receptor comprising the βc receptor and a cytokine-specific α chain (IL-3Rα) (76). The βc chain is the primary signaling component of the IL-3 receptor, while the specificity of IL-3 is determined by IL-3Rα. The cytoplasmic tail of βc chain binds mainly to JAK2, which phosphorylates and activates STAT5 (76). While JAK2 plays a central role in phosphorylating the βc (84, 85), JAK1 is also involved in mediating some βc chain signaling (86, 87). Collectively, these findings explain the inhibitory effects of ruxolitinib, a JAK1/2 inhibitor 6 on the anti-IgE- and IL-3-mediated release of cytokines from basophils.
Hermans and collaborators demonstrated that ruxolitinib inhibited the release of β-hexosaminidase from the human mast cell line LAD2 activated by substance P (47). Moreover, they found that ruxolitinib inhibited the release of TNF-α induced by the Ca2+ ionophore A23187 and MCP-1 production caused by substance P from the mast cell line HMC-1. We have extended their findings showing that ruxolitinib inhibited the IgE- and substance P-induced release of mediators from human basophils and HSMCs, respectively. These findings may have translational relevance in different inflammatory disorders in which basophils, mast cells, and their mediators play a pathogenic role.
Ruxolitinib is effective for the treatment of PV (88), a myeloproliferative neoplasm frequently associated with refractory and severe pruritus (89). Histamine and tryptase released from basophils and skin mast cells are involved in the pathophysiology of pruritus in atopic dermatitis (90, 91). Consistent with our findings, ruxolitinib is emerging as an effective therapy for the treatment of pruritus not only for patients with PV but also in human and experimental dermatitis (92).
It is known that de novo synthesized (LTC4) and preformed (histamine, tryptase, chymase) proinflammatory mediators play a role in skin inflammatory disorders (93). Moreover, T2-high cytokines, IL-4 and IL-13, play a key role in the pathophysiology of skin inflammation (94), such as atopic dermatitis. It has been recently demonstrated that ruxolitinib cream is effective in the treatment of adults and adolescents with atopic dermatitis (95–97). Activation of both resident skin mast cells and infiltrating basophils plays a key role in atopic dermatitis pathobiology (98, 99). In this study, we found that ruxolitinib inhibits the release of several preformed mediators such as histamine, tryptase, and chymase from substance P-activated HSMCs. There was a linear correlation between the release of histamine and both tryptase and chymase from HSMCs activated by substance P. These results are consistent with the notion that these preformed mediators are stored in cytoplasmatic compartments of HSMCs (100). Our findings showing an inhibitory effect of ruxolitinib on the release of proinflammatory mediators and T2-high cytokines from basophils and mast cells may explain, at least in part, the efficacy of this drug in the treatment of atopic dermatitis (95–97).
LTC4 and histamine are involved in lung inflammatory disorders (101). Furthermore, IL-4 and IL-13 play a critical role in asthma pathobiology (102). Recent evidences indicate that ruxolitinib reduces airway inflammation and airway hyperresponsiveness in different murine models of asthma (103, 104). The inhibitory effects of ruxolitinib on the in vitro release of histamine, LTC4, and T2-high cytokines (IL-4 and IL-13) from human basophils suggest that future studies should investigate the safety and efficacy of systemic or topical ruxolitinib in the treatment of the upper and lower airway inflammation.
Several studies have recently demonstrated that ruxolitinib inhibits in vitro and in vivo the release of different cytokines and chemokines from immune and structural cells involved in airway inflammation. In particular, ruxolitinib inhibits the release of IL-6 from human fibroblasts in vivo (105) and the production of IL-6, TNF-α and CXCL8 from monocyte-derived macrophages (MDM) in vitro (106–108), as well as IL-6 and TNF-α from human lung macrophages (109) and LAD2 cells (47). Ruxolitinib also inhibits the release of CCL5, a chemokine involved in asthma exacerbations, from bronchial epithelial cells in vitro (110). Our results extend previous findings showing for the first time that pharmacologic concentrations of ruxolitinib inhibit the release of T2 cytokines (IL-4 and IL-13) from human basophils.
Systemic mastocytosis is a rare clonal myeloproliferative neoplasm characterized by the proliferation and activation of mast cells (62, 111). Mast cell activation leads to the release of cytokines, histamine, and tryptase causing pruritus, flushing, hypotension and even shock (62, 111). Preliminary findings reported that ruxolitinib improved symptoms and quality of life in patients with systemic mastocytosis (17, 18). Our findings indicating that ruxolitinib inhibits mediator release from skin mast cells suggest that the potential properties of this drug require further exploration in mastocytosis.
Ruxolitinib has been approved by FDA and EMA for the treatment of myelofibrosis in patients with PV. Several preclinical studies have demonstrated the efficacy of systemic or topical JAK inhibitors in different animal models of lung inflammation (112). The modulation of a wide spectrum of inflammatory and immunomodulatory cytokines released by human mast cells, basophils, macrophages, and fibroblasts by ruxolitinib suggests that this drug is a potential candidate for the treatment of several inflammatory diseases beyond PV.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The study involving the use of human blood cells was approved by the Ethics Committee of the University of Naples Federico II (198/18), and written informed consent was obtained from all subjects involved in the study according to the recommendations from the Declaration of Helsinki.
Author contributions
RP: Conceptualization, Data curation, Formal analysis, Investigation, Writing – original draft, Writing – review & editing. LC: Data curation, Formal analysis, Investigation, Software, Writing – original draft. GC: Data curation, Formal analysis, Project administration, Software, Writing – original draft. CS: Data curation, Formal analysis, Investigation, Writing – original draft. FP: Data curation, Formal analysis, Software, Validation, Writing – original draft. GL: Data curation, Investigation, Methodology, Writing – original draft. ADS: Data curation, Investigation, Methodology, Validation, Writing – original draft. GM: Conceptualization, Investigation, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. GS: Conceptualization, Methodology, Supervision, Validation, Writing – original draft, Writing – review & editing. SL: Conceptualization, Data curation, Formal analysis, Methodology, Supervision, Writing – original draft, Writing – review & editing. GV: Conceptualization, Funding acquisition, Investigation, Methodology, Resources, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported in part by grants from the CISI-Lab Project (University of Naples Federico II), TIMING Project and Campania Bioscience (Regione Campania) to GM and GV.
Acknowledgments
The authors thank the administrative staff (Dr. Roberto Bifulco, Dr. Anna Ferraro and Dr. Maria Cristina Fucci), without whom it would not be possible to work as a team.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ANOVA, Analysis of variance; CTR, control; EMA, European medicines agency; ET, essential thrombocythemia; FcϵRI, high-affinity immunoglobulin (Ig)E receptors; FDA, food and drug administration; HSMCs, skin mast cells; IC50, half-maximal inhibitory concentration; IMDM, Iscove modified Dulbecco medium; JAK, Janus kinase; LDH, lactate hydrogenase; LTC4, leukotriene C4; MCTC, mast cell expressing tryptase and chymase; MDM, monocyte-derived macrophage; MF, myelofibrosis; MPN, Philadelphia (Ph)-negative myeloproliferative neoplasms; MRGPRX2, MAS-related G protein-coupled receptor-X2; PGMD, poly-glycerol-malic acid-dodecanedioic acid; Pipes, piperazine-N, N’-bis (2-ethanesulfonic acid); PV, polycythemia vera; STAT, signal transducer and activator of transcription; SD, standard deviation; TYK2, tyrosine-protein kinase 2; r, coefficient of correlation; VIP,vasoactive intestinal peptide
References
1. Meyer SC, Levine RL. Molecular pathways: molecular basis for sensitivity and resistance to JAK kinase inhibitors. Clin Cancer Res. (2014) 20:2051–9. doi: 10.1158/1078-0432.CCR-13-0279
2. Banerjee S, Biehl A, Gadina M, Hasni S, Schwartz DM. JAK-STAT signaling as a target for inflammatory and autoimmune diseases: current and future prospects. Drugs. (2017) 77:521–46. doi: 10.1007/s40265-017-0701-9
3. Mertens C, Darnell JE Jr. SnapShot: JAK-STAT signaling. Cell. (2007) 131:612. doi: 10.1016/j.cell.2007.10.033
4. Luo Y, Alexander M, Gadina M, O'Shea JJ, Meylan F, Schwartz DM. JAK-STAT signaling in human disease: From genetic syndromes to clinical inhibition. J Allergy Clin Immunol. (2021) 148:911–25. doi: 10.1016/j.jaci.2021.08.004
5. Schwartz DM, Kanno Y, Villarino A, Ward M, Gadina M, O'Shea JJ. JAK inhibition as a therapeutic strategy for immune and inflammatory diseases. Nat Rev Drug Discovery. (2017) 16:843–62. doi: 10.1038/nrd.2017.201
6. Helbig G. Classical Philadelphia-negative myeloproliferative neoplasms: focus on mutations and JAK2 inhibitors. Med Oncol. (2018) 35:119. doi: 10.1007/s12032-018-1187-3
7. Morales JK, Falanga YT, Depcrynski A, Fernando J, Ryan JJ. Mast cell homeostasis and the JAK-STAT pathway. Genes Immun. (2010) 11:599–608. doi: 10.1038/gene.2010.35
8. Pullen NA, Falanga YT, Morales JK, Ryan JJ. The fyn-STAT5 pathway: A new frontier in igE- and igG-mediated mast cell signaling. Front Immunol. (2012) 3:117. doi: 10.3389/fimmu.2012.00117
9. Li Y, Qi X, Liu B, Huang H. The STAT5-GATA2 pathway is critical in basophil and mast cell differentiation and maintenance. J Immunol. (2015) 194:4328–38. doi: 10.4049/jimmunol.1500018
10. Salabert-Le Guen N, Hemont C, Delbove A, Poli C, Braudeau C, Fantou A. Thymic stromal lymphopoietin does not activate human basophils. J Allergy Clin Immunol. (2018) 141:1476–1479 e6. doi: 10.1016/j.jaci.2017.11.012
11. Pecaric-Petkovic T, Didichenko SA, Kaempfer S, Kaempfer S, Spiegl N, Dahinden CA. Human basophils and eosinophils are the direct target leukocytes of the novel IL-1 family member IL-33. Blood. (2009) 113:1526–34. doi: 10.1182/blood-2008-05-157818
12. Verweij MM, Sabato V, Nullens S, Bridts CH, De Clerck LS, Stevens WJ, et al. STAT5 in human basophils: IL-3 is required for its FcepsilonRI-mediated phosphorylation. Cytometry B Clin Cytom. (2012) 82:101–6. doi: 10.1002/cyto.b.20629
13. Clark JD, Flanagan ME, Telliez JB. Discovery and development of Janus kinase (JAK) inhibitors for inflammatory diseases. J Med Chem. (2014) 57:5023–38. doi: 10.1021/jm401490p
14. Passamonti F, Maffioli M. The role of JAK2 inhibitors in MPNs 7 years after approval. Blood. (2018) 131:2426–35. doi: 10.1182/blood-2018-01-791491
15. Harrison CN, Vannucchi AM, Kiladjian JJ, Al-Ali HK, Gisslinger H, Knoops L, et al. Long-term findings from COMFORT-II, a phase 3 study of ruxolitinib vs best available therapy for myelofibrosis. Leukemia. (2017) 31:775. doi: 10.1038/leu.2016.323
16. Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. (2016) 127:2391–405. doi: 10.1182/blood-2016-03-643544
17. Yacoub A, Prochaska L. Ruxolitinib improves symptoms and quality of life in a patient with systemic mastocytosis. biomark Res. (2016) 4:2. doi: 10.1186/s40364-016-0056-5
18. Dowse R, Ibrahim M, McLornan DP, Moonim MT, Harrison CN, Radia DH. Beneficial effects of JAK inhibitor therapy in Systemic Mastocytosis. Br J Haematol. (2017) 176:324–7. doi: 10.1111/bjh.13951
19. Yamaki K, Yoshino S. Remission of food allergy by the Janus kinase inhibitor ruxolitinib in mice. Int Immunopharmacol. (2014) 18:217–24. doi: 10.1016/j.intimp.2013.11.029
20. Keller A, Wingelhofer B, Peter B, Bauer K, Berger D, Gamperl S, et al. The JAK2/STAT5 signaling pathway as a potential therapeutic target in canine mastocytoma. Vet Comp Oncol. (2018) 16:55–68. doi: 10.1111/vco.12311
21. Pieri L, Bogani C, Guglielmelli P, Zingariello M, Rana RA, Bartalucci N, et al. The JAK2V617 mutation induces constitutive activation and agonist hypersensitivity in basophils from patients with polycythemia vera. Haematologica. (2009) 94:1537–45. doi: 10.3324/haematol.2009.007047
22. Ramsey N, Kazmi W, Phelan M, Lozano-Ojalvo D, Berin MC. JAK1 inhibition with abrocitinib decreases allergen-specific basophil and T-cell activation in pediatric peanut allergy. J Allergy Clin Immunol Glob. (2023) 2:100103. doi: 10.1016/j.jacig.2023.100103
23. Peng W, Benfadal S, Yu C, Wenzel J, Oldenburg J, Novak N. JAK1/2 inhibitor but not IL-4 receptor alpha antibody suppresses allergen-mediated activation of human basophils. vitro Allergy. (2022) 77:2253–6. doi: 10.1111/all.15322
24. Miyake K, Karasuyama H. Emerging roles of basophils in allergic inflammation. Allergol Int. (2017) 66:382–91. doi: 10.1016/j.alit.2017.04.007
25. Varricchi G, Raap U, Rivellese F, Marone G, Gibbs BF. Human mast cells and basophils-How are they similar how are they different? Immunol Rev. (2018) 282:8–34. doi: 10.1111/imr.12627
26. Mukai K, Matsuoka K, Taya C, Suzuki H, Yokozeki H, Nishioka K, et al. Basophils play a critical role in the development of IgE-mediated chronic allergic inflammation independently of T cells and mast cells. Immunity. (2005) 23:191–202. doi: 10.1016/j.immuni.2005.06.011
27. Obata K, Mukai K, Tsujimura Y, Ishiwata K, Kawano Y, Minegishi Y, et al. Basophils are essential initiators of a novel type of chronic allergic inflammation. Blood. (2007) 110:913–20. doi: 10.1182/blood-2007-01-068718
28. Ito Y, Satoh T, Takayama K, Miyagishi C, Walls AF, Yokozeki H. Basophil recruitment and activation in inflammatory skin diseases. Allergy. (2011) 66:1107–13. doi: 10.1111/all.2011.66.issue-8
29. Karasuyama H, Miyake K, Yoshikawa S, Yamanishi Y. Multifaceted roles of basophils in health and disease. J Allergy Clin Immunol. (2018) 142:370–80. doi: 10.1016/j.jaci.2017.10.042
30. Poto R, Gambardella AR, Marone G, Schroeder JT, Mattei F, Schiavoni G, et al. Basophils from allergy to cancer. Front Immunol. (2022) 13:1056838. doi: 10.3389/fimmu.2022.1056838
31. Hashimoto T, Yosipovitch G. Itching as a systemic disease. J Allergy Clin Immunol. (2019) 144:375–80. doi: 10.1016/j.jaci.2019.04.005
32. Poto R, Quinti I, Marone G, Taglialatela M, de Paulis A, Casolaro V, et al. IgG autoantibodies against igE from atopic dermatitis can induce the release of cytokines and proinflammatory mediators from basophils and mast cells. Front Immunol. (2022) 13:880412. doi: 10.3389/fimmu.2022.880412
33. Misery L, Pierre O, Le Gall-Ianotto C, Lebonvallet N, Chernyshov PV, Le Garrec R, et al. Basic mechanisms of itch. J Allergy Clin Immunol. (2023) 152:11–23. doi: 10.1016/j.jaci.2023.05.004
34. Mesa RA, Niblack J, Wadleigh M, Verstovsek S, Camoriano J, Barnes S, et al. The burden of fatigue and quality of life in myeloproliferative disorders (MPDs): an international Internet-based survey of 1179 MPD patients. Cancer. (2007) 109:68–76. doi: 10.1002/cncr.22365
35. Diehn F, Tefferi A. Pruritus in polycythaemia vera: prevalence, laboratory correlates and management. Br J Haematol. (2001) 115:619–21. doi: 10.1046/j.1365-2141.2001.03161.x
36. Abdel-Naser MB, Gollnick H, Orfanos CE. Aquagenic pruritus as a presenting symptom of polycythemia vera. Dermatology. (1993) 187:130–3. doi: 10.1159/000247223
37. Gerlini G, Prignano F, Pimpinelli N. Acute leucocytoclastic vasculitis and aquagenic pruritus long preceding polycythemia rubra vera. Eur J Dermatol. (2002) 12:270–1.
38. Vannucchi AM, Antonioli E, Guglielmelli P, Rambaldi A, Barosi G, Marchioli R, et al. Clinical profile of homozygous JAK2 617V>F mutation in patients with polycythemia vera or essential thrombocythemia. Blood. (2007) 110:840–6. doi: 10.1182/blood-2006-12-064287
39. MacGlashan D Jr. Expression of CD203c and CD63 in human basophils: relationship to differential regulation of piecemeal and anaphylactic degranulation processes. Clin Exp Allergy. (2010) 40:1365–77. doi: 10.1111/j.1365-2222.2010.03572.x
40. Varricchi G, Marone G, Kovanen PT. Cardiac mast cells: underappreciated immune cells in cardiovascular homeostasis and disease. Trends Immunol. (2020) 41:734–46. doi: 10.1016/j.it.2020.06.006
41. Steinhoff M, Vergnolle N, Young SH, Tognetto M, Amadesi S, Ennes HS, et al. Agonists of proteinase-activated receptor 2 induce inflammation by a neurogenic mechanism. Nat Med. (2000) 6:151–8. doi: 10.1038/72247
42. Olsson Y. Mast cells in human peripheral nerve. Acta Neurol Scand. (1971) 47:357–68. doi: 10.1111/ane.1971.47.issue-3
43. Kleij HP, Bienenstock J. Significance of conversation between mast cells and nerves. Allergy Asthma Clin Immunol. (2005) 1:65–80. doi: 10.1186/1710-1492-1-2-65
44. Riol-Blanco L, Ordovas-Montanes J, Perro M, Naval E, Thiriot A, Alvarez D, et al. Nociceptive sensory neurons drive interleukin-23-mediated psoriasiform skin inflammation. Nature. (2014) 510:157–61. doi: 10.1038/nature13199
45. Forsythe P, Bienenstock J. The mast cell-nerve functional unit: a key component of physiologic and pathophysiologic responses. Chem Immunol Allergy. (2012) 98:196–221. doi: 10.1159/000336523
46. Jin X, Zhao W, Kirabo A, Park SO, Ho WT, Sayeski PP, et al. Elevated levels of mast cells are involved in pruritus associated with polycythemia vera in JAK2V617F transgenic mice. J Immunol. (2014) 193:477–84. doi: 10.4049/jimmunol.1301946
47. Hermans MAW, Schrijver B. The JAK1/JAK2- inhibitor ruxolitinib inhibits mast cell degranulation and cytokine release. Clin Exp Allergy. (2018) 48:1412–20. doi: 10.1111/cea.13217
48. Zenarruzabeitia O, Vitalle J, Terren I, et al. CD300c costimulates IgE-mediated basophil activation, and its expression is increased in patients with cow's milk allergy. J Allergy Clin Immunol. (2019) 143:700–711 e5. doi: 10.1016/j.jaci.2018.05.022
49. Ishizaka K, Ishizaka T, Lee EH. Biologic function of the Fc fragments of E myeloma protein. Immunochemistry. (1970) 7:687–702. doi: 10.1016/0019-2791(70)90175-8
50. Schroeder JT, Bieneman AP. Activation of human basophils by A549 lung epithelial cells reveals a novel igE-dependent response independent of allergen. J Immunol. (2017) 199:855–65. doi: 10.4049/jimmunol.1700055
51. Schroeder JT, Bieneman AP. Isolation of human basophils. Curr Protoc Immunol. (2016) 112:7 24 1–8. doi: 10.1002/0471142735.im0724s112
52. Gilbert HS, Ornstein L. Basophil counting with a new staining method using alcian blue. Blood. (1975) 46:279–86. doi: 10.1182/blood.V46.2.279.279
53. Varricchi G, Loffredo S, Borriello F, et al. Superantigenic activation of human cardiac mast cells. Int J Mol Sci. (2019) 20:1828. doi: 10.3390/ijms20081828
54. Varricchi G, Pecoraro A, Loffredo S, et al. Heterogeneity of human mast cells with respect to MRGPRX2 receptor expression and function. Front Cell Neurosci. (2019) 13:299. doi: 10.3389/fncel.2019.00299
55. Spadaro G, Giurato G, Stellato C, et al. Basophil degranulation in response to IgE ligation is controlled by a distinctive circadian clock in asthma. Allergy. (2020) 75:158–68. doi: 10.1111/all.14002
56. Gambardella AR, Poto R, Tirelli V, et al. Differential effects of alarmins on human and mouse basophils. Front Immunol. (2022) 13:894163. doi: 10.3389/fimmu.2022.894163
57. Varricchi G, Pecoraro A, Marone G, et al. Thymic stromal lymphopoietin isoforms, inflammatory disorders, and cancer. Front Immunol. (2018) 9:1595. doi: 10.3389/fimmu.2018.01595
58. Lawrence ID, Warner JA, Cohan VL, et al. Purification and characterization of human skin mast cells. Evidence for human mast cell heterogeneity. J Immunol. (1987) 139:3062–9. doi: 10.4049/jimmunol.139.9.3062
59. Siraganian RP. An automated continuous-flow system for the extraction and fluorometric analysis of histamine. Anal Biochem. (1974) 57:383–94. doi: 10.1016/0003-2697(74)90093-1
60. Cristinziano L, Poto R, Criscuolo G, et al. IL-33 and superantigenic activation of human lung mast cells induce the release of angiogenic and lymphangiogenic factors. Cells. (2021) 10:145. doi: 10.3390/cells10010145
61. Patella V, Casolaro V, Bjorck L, Protein L. A bacterial Ig-binding protein that activates human basophils and mast cells. J Immunol. (1990) 145:3054–61. doi: 10.4049/jimmunol.145.9.3054
62. Marcella S, Petraroli A, Cane L, et al. Thymic stromal lymphopoietin (TSLP) is a substrate for tryptase in patients with mastocytosis. Eur J Intern Med. (2023) 117:111–8. doi: 10.1016/j.ejim.2023.07.026
63. Taracanova A, Alevizos M, Karagkouni A, et al. SP and IL-33 together markedly enhance TNF synthesis and secretion from human mast cells mediated by the interaction of their receptors. Proc Natl Acad Sci U.S.A. (2017) 114:E4002–9. doi: 10.1073/pnas.1524845114
64. Appeldoorn TYJ, Munnink THO, Morsink LM, et al. Pharmacokinetics and pharmacodynamics of ruxolitinib: A review. Clin Pharmacokinet. (2023) 62:559–71. doi: 10.1007/s40262-023-01225-7
65. Li Z, Sun N, Zhang Q, et al. Development and application of an LC-MS/MS method for pharmacokinetic study of ruxolitinib in children with hemophagocytic lymphohistiocytosis. Anal Methods. (2022) 14:2293–303. doi: 10.1039/D2AY00533F
66. Casolaro V, Meliota S, Marino O, et al. Nimesulide, a sulfonanilide nonsteroidal anti-inflammatory drug, inhibits mediator release from human basophils and mast cells. J Pharmacol Exp Ther. (1993) 267:1375–85.
67. Kanaoka Y, Austen KF. Roles of cysteinyl leukotrienes and their receptors in immune cell-related functions. Adv Immunol. (2019) 142:65–84. doi: 10.1016/bs.ai.2019.04.002
68. Serezani CH, Divangahi M, Peters-Golden M. Leukotrienes in innate immunity: still underappreciated after all these years? J Immunol. (2023) 210:221–7. doi: 10.4049/jimmunol.2200599
69. Loffredo S, Bova M, Suffritti C, et al. Elevated plasma levels of vascular permeability factors in C1 inhibitor-deficient hereditary angioedema. Allergy. (2016) 71:989–96. doi: 10.1111/all.12862
70. Silver MR, Margulis A, Wood N, et al. IL-33 synergizes with IgE-dependent and IgE-independent agents to promote mast cell and basophil activation. Inflammation Res. (2010) 59:207–18. doi: 10.1007/s00011-009-0088-5
71. Redrup AC, Howard BP, MacGlashan DW Jr., et al. Differential regulation of IL-4 and IL-13 secretion by human basophils: their relationship to histamine release in mixed leukocyte cultures. J Immunol. (1998) 160:1957–64. doi: 10.4049/jimmunol.160.4.1957
72. Gibbs BF, Haas H, Falcone FH, et al. Purified human peripheral blood basophils release interleukin-13 and preformed interleukin-4 following immunological activation. Eur J Immunol. (1996) 26:2493–8. doi: 10.1002/eji.1830261033
73. Genovese A, Borgia G, Bjorck L, et al. Immunoglobulin superantigen protein L induces IL-4 and IL-13 secretion from human Fc epsilon RI+ cells through interaction with the kappa light chains of IgE. J Immunol. (2003) 170:1854–61. doi: 10.4049/jimmunol.170.4.1854
74. Smithgall MD, Comeau MR, Yoon BR, et al. IL-33 amplifies both Th1- and Th2-type responses through its activity on human basophils, allergen-reactive Th2 cells, iNKT and NK cells. Int Immunol. (2008) 20:1019–30. doi: 10.1093/intimm/dxn060
75. Rivellese F, Suurmond J, de Paulis A, et al. IgE and IL-33-mediated triggering of human basophils inhibits TLR4-induced monocyte activation. Eur J Immunol. (2014) 44:3045–55. doi: 10.1002/eji.201444731
76. Varricchi G, Poto R, Marone G, et al. IL-3 in the development and function of basophils. Semin Immunol. (2021) 54:101510. doi: 10.1016/j.smim.2021.101510
77. Wernersson S, Pejler G. Mast cell secretory granules: armed for battle. Nat Rev Immunol. (2014) 14:478–94. doi: 10.1038/nri3690
78. Irani AM, Schwartz LB. Human mast cell heterogeneity. Allergy Proc. (1994) 15:303–8. doi: 10.2500/108854194778816472
79. Babina M, Guhl S, Artuc M, et al. Allergic FcepsilonRI- and pseudo-allergic MRGPRX2-triggered mast cell activation routes are independent and inversely regulated by SCF. Allergy. (2018) 73:256–60. doi: 10.1111/all.13301
80. Mashaghi A, Marmalidou A, Tehrani M, et al. Neuropeptide substance P and the immune response. Cell Mol Life Sci. (2016) 73:4249–64. doi: 10.1007/s00018-016-2293-z
81. Azimi E, Reddy VB, Pereira PJS, et al. Substance P activates Mas-related G protein-coupled receptors to induce itch. J Allergy Clin Immunol. (2017) 140:447–453 e3. doi: 10.1016/j.jaci.2016.12.980
82. Gupta K, Harvima IT. Mast cell-neural interactions contribute to pain and itch. Immunol Rev. (2018) 282:168–87. doi: 10.1111/imr.12622
83. MacGlashan D Jr., Saini S, Schroeder JT. Response of peripheral blood basophils in subjects with chronic spontaneous urticaria during treatment with omalizumab. J Allergy Clin Immunol. (2021) 147:2295–2304 e12. doi: 10.1016/j.jaci.2021.02.039
84. Guthridge MA, Stomski FC, Barry EF, et al. Site-specific serine phosphorylation of the IL-3 receptor is required for hemopoietic cell survival. Mol Cell. (2000) 6:99–108. doi: 10.1016/S1097-2765(05)00002-X
85. Silvennoinen O, Witthuhn BA, Quelle FW, et al. Structure of the murine Jak2 protein-tyrosine kinase and its role in interleukin 3 signal transduction. Proc Natl Acad Sci U.S.A. (1993) 90:8429–33. doi: 10.1073/pnas.90.18.8429
86. Parganas E, Wang D, Stravopodis D, et al. Jak2 is essential for signaling through a variety of cytokine receptors. Cell. (1998) 93:385–95. doi: 10.1016/S0092-8674(00)81167-8
87. Rodig SJ, Meraz MA, White JM, et al. Disruption of the Jak1 gene demonstrates obligatory and nonredundant roles of the Jaks in cytokine-induced biologic responses. Cell. (1998) 93:373–83. doi: 10.1016/S0092-8674(00)81166-6
88. Vannucchi AM, Kiladjian JJ, Griesshammer M, et al. Ruxolitinib versus standard therapy for the treatment of polycythemia vera. N Engl J Med. (2015) 372:426–35. doi: 10.1056/NEJMoa1409002
89. Al-Mashdali AF, Kashgary WR, Yassin MA. Ruxolitinib (a JAK2 inhibitor) as an emerging therapy for refractory pruritis in a patient with low-risk polycythemia vera: A case report. Med (Baltimore). (2021) 100:e27722. doi: 10.1097/MD.0000000000027722
90. Yamanishi Y, Mogi K, Takahashi K, et al. Skin-infiltrating basophils promote atopic dermatitis-like inflammation via IL-4 production in mice. Allergy. (2020) 75:2613–22. doi: 10.1111/all.14362
91. Wang F, Trier AM, Li F, et al. A basophil-neuronal axis promotes itch. Cell. (2021) 184:422–440 e17. doi: 10.1016/j.cell.2020.12.033
92. Scuron MD, Fay BL, Connell AJ, et al. Ruxolitinib cream has dual efficacy on pruritus and inflammation in experimental dermatitis. Front Immunol. (2020) 11:620098. doi: 10.3389/fimmu.2020.620098
93. Poto R, Loffredo S, Marone G, et al. Basophils beyond allergic and parasitic diseases. Front Immunol. (2023) 14:1190034. doi: 10.3389/fimmu.2023.1190034
95. Papp K, Szepietowski JC, Kircik L, et al. Long-term safety and disease control with ruxolitinib cream in atopic dermatitis: Results from two phase 3 studies. J Am Acad Dermatol. (2023) 88:1008–16. doi: 10.1016/j.jaad.2022.09.060
96. Simpson EL, Kircik L, Blauvelt A, et al. Clinically relevant improvements in adults and adolescents with atopic dermatitis who did not achieve Investigator's Global Assessment treatment success following 8 weeks of ruxolitinib cream monotherapy. J Dermatol. (2023) 50:1523–30. doi: 10.1111/1346-8138.16975
97. Eichenfield LF, Liu J, Marwaha S, et al. Satisfaction with control of mild to moderate atopic dermatitis with ruxolitinib cream: US physician and patient perspectives. Dermatol Ther (Heidelb). (2024) 14:685–96. doi: 10.1007/s13555-024-01116-0
98. Marone G, Borriello F, Varricchi G, et al. Basophils: historical reflections and perspectives. Chem Immunol Allergy. (2014) 100:172–92. doi: 10.1159/000358734
99. Varricchi G, Granata F, Loffredo S, et al. Angiogenesis and lymphangiogenesis in inflammatory skin disorders. J Am Acad Dermatol. (2015) 73:144–53. doi: 10.1016/j.jaad.2015.03.041
100. Atiakshin D, Buchwalow I, Samoilova V, et al. Tryptase as a polyfunctional component of mast cells. Histochem Cell Biol. (2018) 149:461–77. doi: 10.1007/s00418-018-1659-8
101. Poto R, Criscuolo G, Marone G, et al. Human lung mast cells: therapeutic implications in asthma. Int J Mol Sci. (2022) 23:14466. doi: 10.3390/ijms232214466
102. Varricchi G, Ferri S, Pepys J, et al. Biologics and airway remodeling in severe asthma. Allergy. (2022) 77:3538–52. doi: 10.1111/all.15473
103. Li RF, Wang GF. JAK/STAT5 signaling pathway inhibitor ruxolitinib reduces airway inflammation of neutrophilic asthma in mice model. Eur Rev Med Pharmacol Sci. (2018) 22:835–43. doi: 10.26355/eurrev_201802_14320
104. Subramanian H, Hashem T, Bahal D, et al. Ruxolitinib ameliorates airway hyperresponsiveness and lung inflammation in a corticosteroid-resistant murine model of severe asthma. Front Immunol. (2021) 12:786238. doi: 10.3389/fimmu.2021.786238
105. Baghdassarian H, Blackstone SA, Clay OS, et al. Variant STAT4 and response to ruxolitinib in an autoinflammatory syndrome. N Engl J Med. (2023) 388:2241–52. doi: 10.1056/NEJMoa2202318
106. Verres Y, da Silva CO, Aljebawi B, et al. Impact of JAK/STAT inhibitors on human monocyte-derived-macrophages stimulated by cigarette smoke extract and lipopolysaccharide. Clin Exp Pharmacol Physiol. (2022) 49:1187–96. doi: 10.1111/1440-1681.13705
107. Febvre-James M, Lecureur V, Augagneur Y, et al. Repression of interferon beta-regulated cytokines by the JAK1/2 inhibitor ruxolitinib in inflammatory human macrophages. Int Immunopharmacol. (2018) 54:354–65. doi: 10.1016/j.intimp.2017.11.032
108. Lescoat A, Lelong M, Jeljeli M, et al. Combined anti-fibrotic and anti-inflammatory properties of JAK-inhibitors on macrophages in vitro and in vivo: Perspectives for scleroderma-associated interstitial lung disease. Biochem Pharmacol. (2020) 178:114103. doi: 10.1016/j.bcp.2020.114103
109. Mantov N, Zrounba M, Brollo M, et al. Ruxolitinib inhibits cytokine production by human lung macrophages without impairing phagocytic ability. Front Pharmacol. (2022) 13:896167. doi: 10.3389/fphar.2022.896167
110. Sada M, Watanabe M, Inui T, et al. Ruxolitinib inhibits poly(I:C) and type 2 cytokines-induced CCL5 production in bronchial epithelial cells: A potential therapeutic agent for severe eosinophilic asthma. Immun Inflammation Dis. (2021) 9:363–73. doi: 10.1002/iid3.397
111. Valent P, Akin C, Sperr WR, et al. New insights into the pathogenesis of mastocytosis: emerging concepts in diagnosis and therapy. Annu Rev Pathol. (2023) 18:361–86. doi: 10.1146/annurev-pathmechdis-031521-042618
Keywords: asthma, basophil, histamine, IL-4, IL-13, mast cell, polycythemia vera, ruxolitinib
Citation: Poto R, Cristinziano L, Criscuolo G, Strisciuglio C, Palestra F, Lagnese G, Di Salvatore A, Marone G, Spadaro G, Loffredo S and Varricchi G (2024) The JAK1/JAK2 inhibitor ruxolitinib inhibits mediator release from human basophils and mast cells. Front. Immunol. 15:1443704. doi: 10.3389/fimmu.2024.1443704
Received: 04 June 2024; Accepted: 29 July 2024;
Published: 12 August 2024.
Edited by:
Jennifer Vandooren, KU Leuven, BelgiumReviewed by:
Joakim Dahlin, Karolinska Institutet (KI), SwedenChristophe Pellefigues, CNRS EMR8252 Centre de Recherche sur l’Inflammation, France
Copyright © 2024 Poto, Cristinziano, Criscuolo, Strisciuglio, Palestra, Lagnese, Di Salvatore, Marone, Spadaro, Loffredo and Varricchi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gilda Varricchi, Z2lsZGFuZXRAZ21haWwuY29t
†These authors share first authorship
‡ORCID: Remo Poto, orcid.org/0000-0002-4723-0167
Leonardo Cristinziano, orcid.org/0000-0002-7835-2212
Gjada Criscuolo, orcid.org/0000-0002-5928-1869
Caterina Strisciuglio, orcid.org/0000-0002-9005-6571
Francesco Palestra, orcid.org/0000-0001-6145-7475
Gianluca Lagnese, orcid.org/0000-0001-9504-429X
Antonio Di Salvatore, orcid.org/0000-0002-6434-3112
Gianni Marone, orcid.org/0000-0002-9849-4701
Giuseppe Spadaro, orcid.org/0000-0001-7889-425X
Stefania Loffredo, orcid.org/0000-0002-5871-1898
Gilda Varricchi, orcid.org/0000-0002-9285-4657
 Remo Poto
Remo Poto Leonardo Cristinziano1,3,4†‡
Leonardo Cristinziano1,3,4†‡ Caterina Strisciuglio
Caterina Strisciuglio Francesco Palestra
Francesco Palestra Gianluca Lagnese
Gianluca Lagnese Antonio Di Salvatore
Antonio Di Salvatore Gianni Marone
Gianni Marone Giuseppe Spadaro
Giuseppe Spadaro Stefania Loffredo
Stefania Loffredo Gilda Varricchi
Gilda Varricchi