- 1Shandong University Cancer Center, Shandong University, Jinan, Shandong, China
- 2Department of Radiation Oncology, Shandong Cancer Hospital and Institute, Shandong First Medical University, and Shandong Academy of Medical Sciences, Jinan, Shandong, China
- 3Department of Graduate, Shandong First Medical University and Shandong Academy of Medical Sciences, Jinan, Shandong, China
- 4Department of Nuclear Medicine, Shandong Cancer Hospital and Institute, Shandong First Medical University and Shandong Academy of Medical Sciences, Jinan, Shandong, China
- 5Department of Hematology, Shandong Cancer Hospital and Institute, Shandong First Medical University and Shandong Academy of Medical Sciences, Jinan, Shandong, China
Background: High-risk double-expressor diffuse large B-cell lymphoma has an inferior prognosis following standard first-line therapy. After failure of second-line therapy, treatment options are limited if accompanied by localized compressive symptoms. Chimeric Antigen Receptor T cell (CAR-T) therapy preceded by bridging radiotherapy may be an effective emerging therapy.
Case presentation: We report a 66-year-old female patient diagnosed with stage IV double-expressor diffuse large B-cell lymphoma. The patient achieved progressive disease after two cycles of rituximab, cyclophosphamide, liposomal doxorubicin, vincristine, and prednisone and continued to develop cervical lymph node recurrence after second-line therapy. The patient was infused with CAR-T cells after receiving focal bridging radiotherapy and remained in complete response more than 9 months after treatment. In addition, the patients did not experience serious adverse reactions related to radiotherapy as well as CAR-T cell therapy.
Conclusions: In this article, we describe a patient with double-expressor diffuse large B-cell lymphoma with localized compression symptoms after second-line treatment failure who benefited from CAR-T combined with focal bridging radiotherapy.
1 Introduction
Diffuse large B-cell lymphoma is an aggressive tumor derived from mature B cells, accounting for approximately 30% to 40% of all non-Hodgkin lymphomas (1). After treatment with first-line rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone regimen, 45% to 50% of patients will experience relapse or refractory treatment (2, 3). Asthe number of treatment lines increase, about 73% of patients with relapsed/refractory diffuse large B-cell lymphoma still cannot obtain remission after receiving second-line and later-line treatments (4–6). Among them, double-expressor diffuse large B-cell lymphoma, as a high-grade lymphoma, is more aggressive and has a worse prognosis (7). A consensus on double-expressor diffuse large B-cell lymphoma treatment is still lacking. Chimeric Antigen Receptor T cell (CAR-T) targeting CD19 has been approved by the United States Food and Drug Administration for the treatment of relapsed/refractory diffuse large B-cell lymphoma, with a 1-year recurrence-free survival of approximately 44% to 65% (8–10). Bridging radiotherapy is expected to further improve the recurrence-free survival (11), which double-expressor diffuse large B-cell lymphoma patients bring a therapeutic ray of hope. Here, we report a 68-year-old woman with dual-expression type relapsed refractory lymphoma who successfully transitioned to CAR-T cell therapy with local bridging radiotherapy and has achieved a 9-month complete remission to date. To our knowledge, this is the first report of CAR-T cell infusion using localized radiotherapy as a transitional therapy for the treatment of relapsed/refractory dual-expression lymphoma.
2 Case presentation
The patient received a diagnosis of double-expressor diffuse large B-cell lymphoma in November 2021, at the age of 66 years, after presenting with fatigue. She received chemoimmunotherapy with 2 cycles of rituximab, cyclophosphamide, liposomal doxorubicin, vincristine, and prednisone which resulted in progressive disease in the central nervous system (as reported by the patient, no report available). Subsequent treatment included regimens incorporating rituximab, methotrexate, lenalidomide, and orelabrutinib (Figure 1). On February 1, 2023, a biopsy of the left cervical mass diagnosed high-grade B-cell lymphoma. Immunohistochemical staining showed: CD3 (-), CD5 (+), CD20 (+), PAX5 (+), CD10 (-), BCL-2 (+), BCL-6 (50%+) MUM-1 (-), C-MYC (40%+), CyclinD1(-), Ki-67 (90%+), EBER (-). Upon admission to our hospital, the International Prognostic Index (IPI) score was 3.
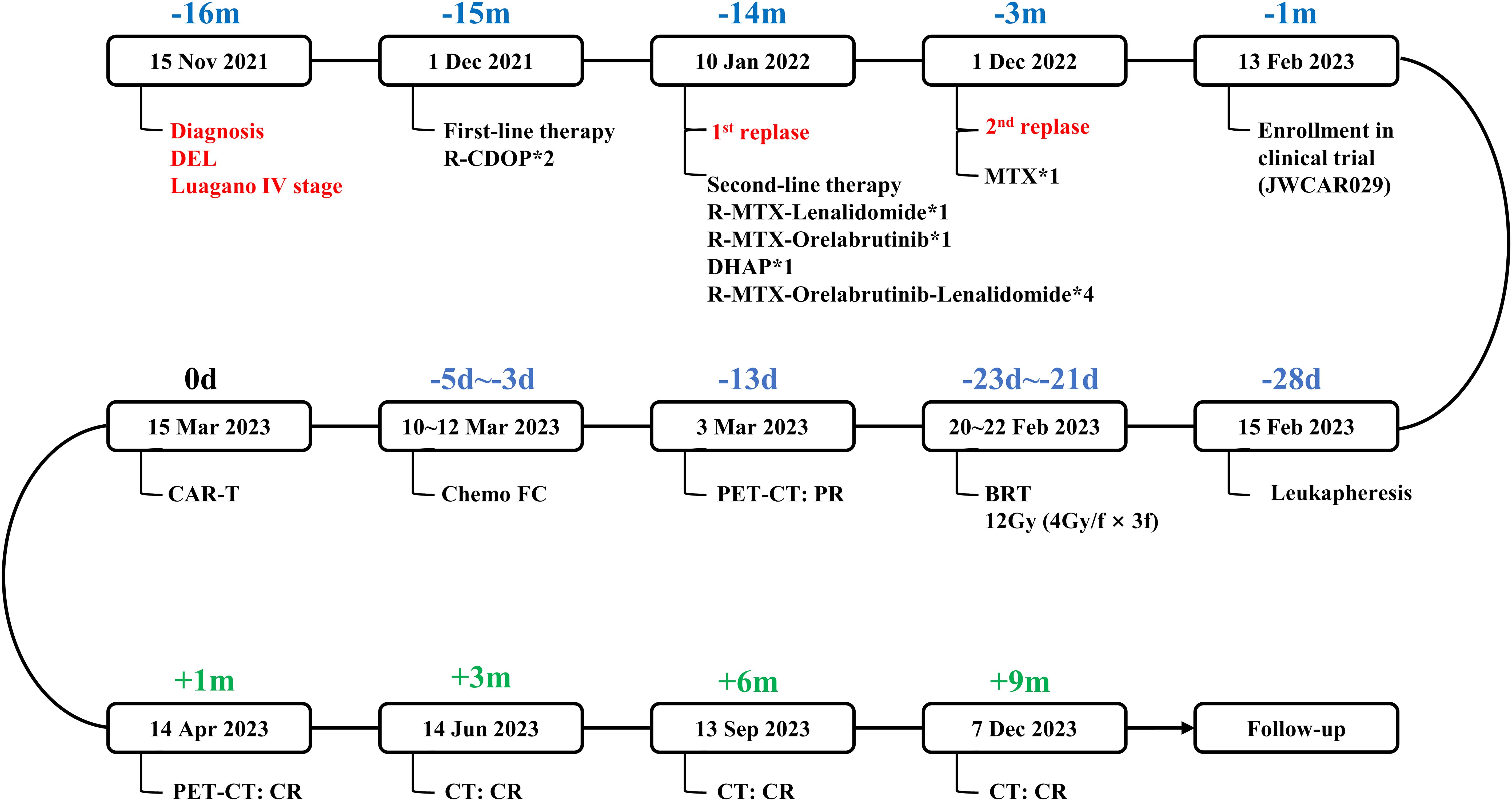
Figure 1. Flow chart of the disease process and therapeutic modalities. DEL, double-expressor diffuse large B-cell lymphoma. R-CDOP, rituximab, cyclophosphamide, liposomal doxorubicin, vincristine, and prednisone. methotrexate, methotrexate. BRT, bridging radiotherapy. FC, fludarabine and cyclophosphamide. PR, partial response. CR, complete response. R-CDOP*2, two cycles of R-CDOP treatment. R-MTX-Lenalidomide*1, one cycle of R-MTX-Lenalidomide treatment. R-MTX-Orelabrutinib*1, one cycle of R-MTX-Orelabrutinib treatment. DHAP*1, one cycle of DHAP treatment. R-MTX-Orelabrutinib-Lenalidomide*4, four cycle of R-MTX-Orelabrutinib-Lenalidomide treatment. MTX*1, one cycle of MTX treatment.
Approximately 11 months later, the disease progressed again and a physical examination revealed a mass about 5cm in diameter in the neck. 18F-Fluorodeoxyglucose positron emission tomography/computed tomography showed multiple enlarged lymph nodes in the left neck and left supraclavicular region with a maximum diameter of 4.7 cm and a standard uptake volume of 24.0 (Figure 2A). Give methotrexate treatment for 1 cycle. Considering the reported anti-lmphoma effects of anti-CD19 CAR-T cells and the failure of second-line therapy in this patient, we encouraged her to enroll in a clinical trial of anti-CD19 CAR-T treatment to which she agreed (Ethics NO. SDZLEC2022-164-01). At the same time, the multidisciplinary team recommended local bridging radiotherapy to relieve the compression symptoms of neck mass, and the patient agreed.
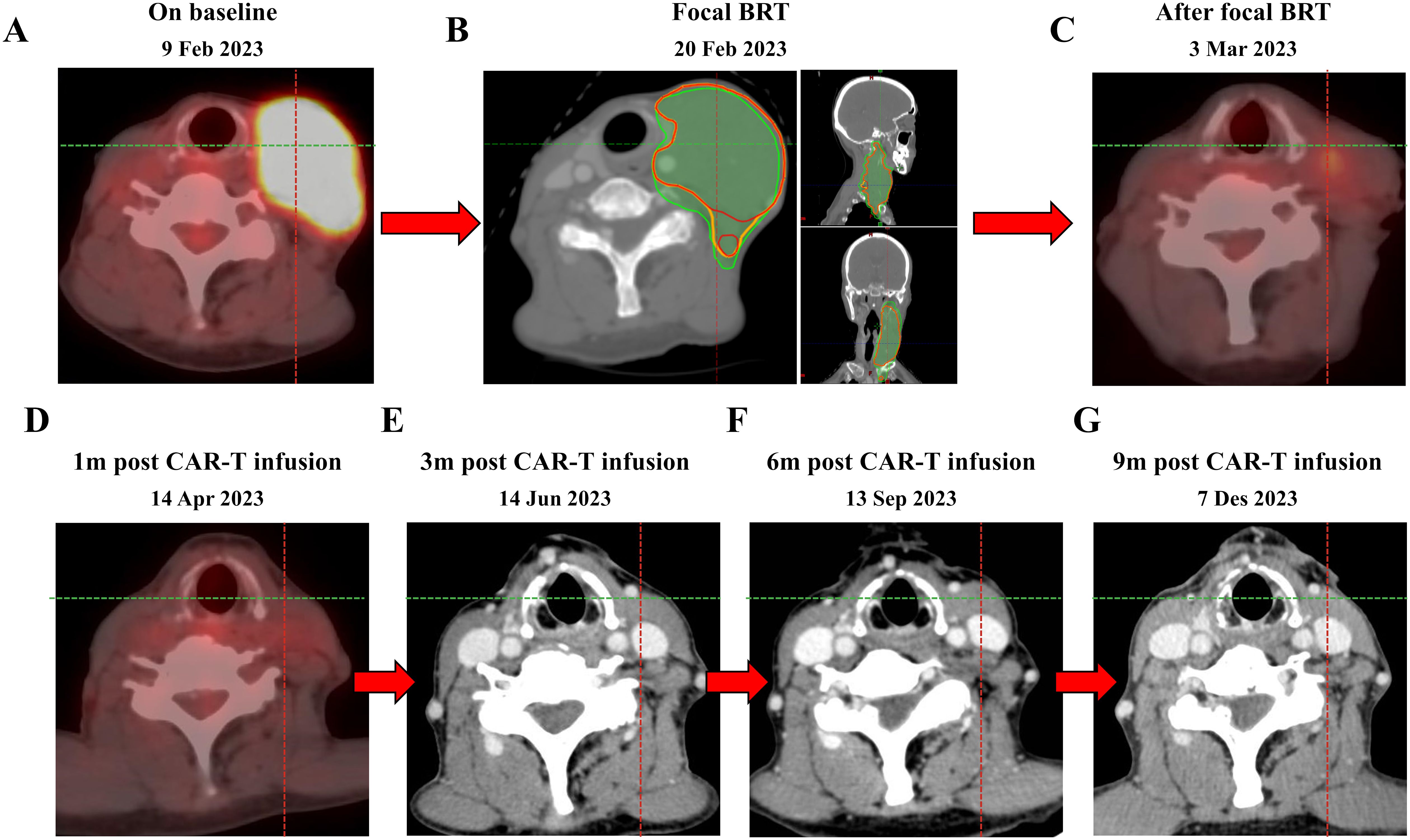
Figure 2. PET-CT scan before bridging radiotherapy (9 Feb 2023) (A), bridging radiotherapy treatment (20 Feb 2023) (B) and after bridging radiotherapy treatment (3 Mar 2023) (C). The patient remains in CR with CAR-T therapy to date (D–G). bridging radiotherapy, bridging radiotherapy. GTV, gross tumor volume. CTV, clinical tumor volume. PTV, planning target volume. The red line represents GTV, the yellow line represents CTV, and the green line represents PTV.
A large-aperture CT was performed for supine positioning, a large mask was immobilized, and a laser light system was used to identify body surface marker points. The left supraclavicular and left cervical lymph nodes were outlined as GTV, with 3 mm of externals as CTV and 5 mm of externals as PTV. The prescribed dose of 400 cGy/dose × 3 sessions was drawn up and the TPS developed an IMRT plan. Evaluating the radiotherapy plan at a total of 1,200 cGy, the isodose curve of 1,200 cGy encompassed 95% of the PTV, with a mean dose to the right parotid gland of 103.8 cGy, a mean dose to the left parotid gland of 627.7 cGy, and a maximal point dose to the spinal cord of 533.8 cGy, with the remaining critical organs in the tolerable range. There was no discomfort during radiotherapy, and the cervical lymph nodes on examination after radiotherapy were significantly smaller and softer than before (Figure 2B). Post-radiotherapy PET-CT showed (March 3, 2023) left supraclavicular and left cervical lymph node involvement was reduced after treatment compared to before (February 9, 2023), metabolism was reduced (Figure 2C).
Chemotherapy (fludarabine 36mg qd and cyclophosphamide 360mg qd) was administered 5, 4, and 3 days before the first infusion of CAR-T cells (March 15, 2023) which was followed by one infusion administered. The effective anti-CD19 CAR-T cells totaled 100 * 106. The patient developed fever once on March 20, 2023, with a maximum body temperature of 38.0°C, which dropped to normal on its own. A grade 1 cytokine release syndrome reaction occurred on March 24, 2023, and granulocyte colony stimulating factor was given to increase white blood cells. Furthermore, the patient did not develop grade 3-4 cytokine release syndrome or immune effector cell-associated neurotoxic syndrome. The patient remained complete response for more than 9 months after anti-CD19 CAR-T reinfusion (Figures 2D–G).
3 Discussion and conclusions
Our work demonstrated that receiving anti-CD19 CAR-T cell infusion after focal bridging radiotherapy had a significant effect in patients with double-expressor diffuse large B-cell lymphoma. The double-expressor diffuse large B-cell lymphoma case we described had a rapidly that showed poor response to conventional therapy. She responded favorably to anti-CD19 CAR-T cells therapy. To the best of our knowledge, this is the first case report of second-line relapsed double-expressor diffuse large B-cell lymphoma treatment with anti-CD19 CAR-T cell with bridging radiotherapy. Complete response was still observed 9 months after CAR-T infusion, and no treatment is expected to prolong complete response duration. In addition, the case did not experience serious adverse events.
Refractory/relapsed lymphomas are highly heterogeneous and require constant experimentation with new modes of diagnosis and evaluation. CAR-T cell therapy is the treatment of choice for patients with refractory relapsed, post-transplant relapsed, and salvage treatment failures/relapses, with the number of lines of therapy constantly moving forward. Currently, in the field of double-expressor diffuse large B-cell lymphoma, CAR-T cells have demonstrated favorable efficacy in treating high-risk diffuse large B-cell lymphoma patients (12–14). The ZUMA-12 study included 40 patients with high-risk diffuse large B-cell lymphoma treated with CD19 CAR-T cells and showed an objective response rate of 89% and a complete response rate of 78% in 37 evaluable patients (15). Still about 30% fail to obtain a CR before waiting for the CAR-T cells to be manufactured. Therefore, the “bridging therapy” from cell harvesting to clearing chemotherapy and successful CAR-T infusion may be a key factor in controlling the progression of the disease and influencing the clinical outcome (11).
Multiple retrospective studies have found that in patients with diffuse large B-cell lymphoma, overall survival is significantly prolonged in patients who receive bridging therapy compared to those who do not (16–18). Comparison of different bridging modalities revealed that bridging radiotherapy was superior to other bridging treatment modalities (17–21), which may be related to the unique biological effects of radiotherapy (11). Radiotherapy can directly inhibit the ability of tumor cells to divide and proliferate by damaging their DNA, while also promoting the migration and activation of pro-inflammatory immune cells toward irradiated areas of the tumor (22–24). The above retrospective clinical studies confirm that CAR-T combined with bridging radiotherapy may be a safer and more effective treatment modality for diffuse large B-cell lymphoma, but the optimal timing, site, dose, and modality of radiotherapy still need to be explored (25–27).
Currently, many international clinical trials of CAR-T combined with bridging radiotherapy have been conducted, and these clinical trials are mainly in phase I (28–34), with the timing of radiotherapy focusing on the period between single-treatment and clear lymphoma (28, 29, 32–35), and there are also a few salvage therapies after the failure of CAR-T treatment (36). Comprehensive radiotherapy, i.e., radiotherapy to all high uptake sites shown by PET-CT, is the mainstay of radiotherapy, and there are also clinical trials related to Focal radiotherapy. Radiotherapy doses were based on conventional fractionated radiotherapy (28, 31, 32, 35) and hypofractionated radiotherapy (28, 29, 33). Compared with conventional fractionated radiotherapy, hypofractionated radiotherapy can better mobilize local and systemic immune responses and remodel the tumor microenvironment by inducing immune cell infiltration (37). Local radiotherapy also removes tumor-resident lymphocytes, providing space for CAR-T cells to infiltrate (11, 38). There are no investigational trials demonstrating the safety and efficacy of CAR-T in combination with hypofractionated radiotherapy-bridged radiotherapy in patients with double-expressor diffuse large B-cell lymphoma who have failed second-line therapy with large localized masses, and new clinical trials are needed to investigate the safety and efficacy of this treatment option.
Although our case found that bridging radiotherapy before CAR-T infusion may be an effective treatment for patients with localized compressive symptoms, in actual clinical practice, the choice between bridging radiotherapy and early CAR-T cell therapy needs to balance multiple factors. Radiotherapy can control locally progressive disease, alleviate symptomatic disease, and clear high metabolic tumors without increasing the toxicity of subsequent CAR-T infusion (39). However, early CAR-T cell therapy is also a potential option, especially when patients respond poorly to conventional treatment regimens and the disease progresses rapidly. Direct CAR-T cell therapy can reduce treatment delays, rapidly intervene to control tumor burden, and potentially avoid acute and chronic toxicities associated with radiotherapy. Therefore, an individualized bridging strategy should be developed based on the patient’s condition (whether the disease is stable, whether there are large masses or symptomatic lesions).
In conclusion, bridging hypofractionated radiotherapy before CAR-T treatment may be a safe and effective treatment option for patients with double-expressor diffuse large B-cell lymphoma who have failed second-line therapy and have localized compression symptoms.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Ethical Review Committee of Shandong Cancer Hospital (Ethics NO. SDZLEC2022-164-01). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
LY: Conceptualization, Project administration, Writing – original draft, Writing – review & editing. MW: Investigation, Methodology, Software, Writing – original draft. HY: Data curation, Methodology, Writing – original draft. XS: Software, Supervision, Writing – review & editing. LJX: Funding acquisition, Resources, Writing – review & editing. DL: Resources, Writing – review & editing. LGX: Funding acquisition, Supervision, Validation, Writing – review & editing. JY: Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by grants from the National Natural Science Foundation of China (Grant NO. 82200224), the Shandong Provincial Natural Science Foundation (Grant NO. ZR2021MH072), and the Department of Science and Technology of Shandong Province (Grant NO. 2021CXGC011102).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Susanibar-Adaniya S, Barta SK. 2021 update on diffuse large B cell lymphoma: a review of current data and potential applications on risk stratification and management. Am J Hematol. (2021) 96:617–29. doi: 10.1002/ajh.26151
2. Pennings ERA, Durmaz M, Visser O. Treatment and outcomes for patients with relapsed or refractory diffuse large B-cell lymphoma: a contemporary, nationwide, population-based study in the Netherlands. Blood Cancer J. (2024) 14:3. doi: 10.1038/s41408-023-00970-z
3. Fabbri N, Mussetti A, Sureda A. Second-line treatment of diffuse large B-cell lymphoma: evolution of options. Semin Hematol. (2023) 60:305–12. doi: 10.1053/j.seminhematol.2023.12.001
4. Epperla N, Kumar A, Abutalib SA. ASTCT clinical practice recommendations for transplantation and cellular therapies in diffuse large B cell lymphoma. Transplant Cell Ther. (2023) 29:548–55. doi: 10.1016/j.jtct.2023.06.012
5. Abramson JS, Solomon SR, Arnason J. Lisocabtagene maraleucel as second-line therapy for large B-cell lymphoma: primary analysis of the phase 3 TRANSFORM study. Blood. (2023) 141:1675–84. doi: 10.1182/blood.2022018730
6. Maurer MJ, Habermann TM, Shi Q. Progression-free survival at 24 months (PFS24) and subsequent outcome for patients with diffuse large B-cell lymphoma (DLBCL) enrolled on randomized clinical trials. Ann Oncol. (2018) 29:1822–7. doi: 10.1093/annonc/mdy203
7. Tavakkoli M, Barta SK. 2024 Update: Advances in the risk stratification and management of large B-cell lymphoma. Am J Hematol. (2023) 98:1791–805. doi: 10.1002/ajh.27075
8. Abramson JS, Palomba ML, Gordon LI. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. (2020) 396:839–52. doi: 10.1016/S0140-6736(20)31366-0
9. Schuster SJ, Bishop MR, Tam CS. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. (2019) 380:45–56. doi: 10.1056/NEJMoa1804980
10. Neelapu SS, Locke FL, Bartlett NL. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. (2017) 377:2531–44. doi: 10.1056/NEJMoa1707447
11. Hovhannisyan L, Riether C, Aebersold DM. CAR T cell-based immunotherapy and radiation therapy: potential, promises and risks. Mol Cancer. (2023) 22:82. doi: 10.1186/s12943-023-01775-1
12. Locke FL, Ghobadi A, Jacobson CA. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1-2 trial. Lancet Oncol. (2019) 20:31–42. doi: 10.1016/S1470-2045(18)30864-7
13. Locke FL, Miklos DB, Jacobson CA. Axicabtagene ciloleucel as second-line therapy for large B-Cell lymphoma. N Engl J Med. (2022) 386:640–54. doi: 10.1056/NEJMoa2116133
14. Neelapu SS, Dickinson M, Munoz J. Axicabtagene ciloleucel as first-line therapy in high-risk large B-cell lymphoma: the phase 2 ZUMA-12 trial. Nat Med. (2022) 28:735–42. doi: 10.1038/s41591-022-01731-4
15. Helwick C. February 25, 2023 - Supplement: Conference Highlights ASH 2022 - EPOV Julio C. Chavez. The ASCO Post. Available online at: https://ascopost.com/issues/february-25-2023-supplement-conference-highlights-ash-2022/epov-julio-c-chavez/ (Accessed Jan 1, 2024).
16. Roddie C, Neill L, Osborne W. Effective bridging therapy can improve CD19 CAR-T outcomes while maintaining safety in patients with large B-cell lymphoma. Blood Adv. (2023) 7:2872–83. doi: 10.1182/bloodadvances.2022009019
17. Lutfi F, Holtzman NG, Kansagra AJ. The impact of bridging therapy prior to CD19-directed chimeric antigen receptor T-cell therapy in patients with large B-cell lymphoma. Br J Haematol. (2021) 195:405–12. doi: 10.1111/bjh.17738
18. Johnson PC, Jacobson C, Yi A. Association of bridging therapy utilization with clinical outcomes in patients receiving chimeric antigen receptor (CAR) T-cell therapy. J Immunother Cancer. (2022) 10:e004567. doi: 10.1136/jitc-2022-004567
19. Wright CM, LaRiviere MJ, Baron JA. Bridging radiation therapy before commercial chimeric antigen receptor T-cell therapy for relapsed or refractory aggressive B-cell lymphoma. Int J Radiat Oncol Biol Phys. (2020) 108:178–88. doi: 10.1016/j.ijrobp.2020.05.014
20. Saifi O, Breen WG, Lester SC. Does bridging radiation therapy affect the pattern of failure after CAR T-cell therapy in non-Hodgkin lymphoma? Radiother Oncol. (2022) 166:171–9. doi: 10.1016/j.radonc.2021.11.031
21. Ladbury C, Dandapani S, Hao C. Long-term follow-up of bridging therapies prior to CAR T-cell therapy for relapsed/refractory large B cell lymphoma. Cancers (Basel). (2023) 15:1747. doi: 10.3390/cancers15061747
22. Marciscano AE, Haimovitz-Friedman A, Lee P. Immunomodulatory effects of stereotactic body radiation therapy: preclinical insights and clinical opportunities. Int J Radiat Oncol Biol Phys. (2021) 110:35–52. doi: 10.1016/j.ijrobp.2019.02.046
23. Huan T, Li H, Tang B. Radiotherapy plus CAR-T cell therapy to date: A note for cautions optimism? Front Immunol. (2022) 13:1033512. doi: 10.3389/fimmu.2022.1033512
24. Aghajanian H, Rurik JG, Epstein JA. CAR-based therapies: opportunities for immuno-medicine beyond cancer. Nat Metab. (2022) 4:163–9. doi: 10.1038/s42255-022-00537-5
25. Arscott WT, Miller D, Jones JA, Winchell N, Schuster S, Plastaras JP. Tandem induction radiation and chimeric antigen receptor T cell therapy in patients with relapsed or refractory non-Hodgkin lymphoma. Int J Radiat Oncol Biol Phys. (2018) 102:S122. doi: 10.1016/j.ijrobp.2018.06.306
26. Sim AJ, Jain MD, Figura NB. Radiation therapy as a bridging strategy for CAR T cell therapy with axicabtagene ciloleucel in diffuse large B-cell lymphoma. Int J Radiat Oncol Biol Phys. (2019) 105:1012–21. doi: 10.1016/j.ijrobp.2019.05.065
27. Imber BS, Palomba ML, DeSelm C, Batlevi C, Dahi PB, Giralt S, et al. MSKCc early experience using radiotherapy as a bridging strategy for relapsed diffuse large B cell lymphoma before CD19 CAR T therapy. Hematol Oncol. (2019) 134:3238. doi: 10.1002/hon.68_2630
28. City of Hope Medical Center. Pilot phase I study to evaluate CD8 PET imaging as a marker of immune response to stereotactic body radiation therapy (ELIXR) (2023). Available online at: https://clinicaltrials.gov/study/NCT05371132 (Accessed Jan 1, 2024).
29. Patel C. Adaptive bridging radiation therapy (ABRT) for relapsed/refractory B-cell lymphoma prior to CAR T-cell therapy (2023). Available online at: https://clinicaltrials.gov/study/NCT06004167 (Accessed Jan 1, 2024).
30. Patel CG. Radiation therapy to enhance CAR T efficacy early in post-CAR T cell therapy refractory lymphoma: a pilot study (2021). Available online at: https://clinicaltrials.gov/study/NCT04473937 (Accessed Jan 1, 2024).
31. City of Hope Medical Center. A feasibility study of bridging radiation to all sites of FDG-avid disease for commercial CAR T-cell infusion in patients with large B-cell lymphoma (2023). Available online at: https://clinicaltrials.gov/study/NCT05800405 (Accessed Jan 1, 2024).
32. University of Nebraska. Feasibility of low dose radiation as bridging therapy for lisocabtagene maraleucel in relapsed B-cell non-hodgkin lymphoma (2023). Available online at: https://clinicaltrials.gov/study/NCT05621096 (Accessed Jan 1, 2024).
33. Memorial Sloan Kettering Cancer Center. A phase I study of split-course bridging radiotherapy (SC-BRT) prior to commercial CD19 CAR T-cell therapies for patients with relapsed or refractory B-cell lymphomas (2023). Available online at: https://clinicaltrials.gov/study/NCT05574114 (Accessed Jan 1, 2024).
34. Ruanjing. The safety and efficacy of ultra-fraction radiotherapy bridging CAR T cell therapy in relapsed/refractory diffuse large B cell lymphoma (2024). Available online at: https://clinicaltrials.gov/study/NCT05514327 (Accessed Jan 1, 2024).
35. University College, London. RadiothErapy priMIng for CAR-T (2023). Available online at: https://clinicaltrials.gov/study/NCT04726787 (Accessed Jan 1, 2024).
36. Ababneh HS, Ng AK, Frigault MJ. Salvage radiotherapy in relapsed/refractory large B-cell lymphoma after failure of CAR T-cell therapy. Haematologica. (2023) 108:2972–81. doi: 10.3324/haematol.2023.282804
37. Sugita M, Yamazaki T, Alhomoud M. Radiation therapy improves CAR T cell activity in acute lymphoblastic leukemia. Cell Death Dis. (2023) 14:305. doi: 10.1038/s41419-023-05829-6
38. Zhang Z, Liu X, Chen D. Radiotherapy combined with immunotherapy: the dawn of cancer treatment. Signal Transduct Target Ther. (2022) 7:258. doi: 10.1038/s41392-022-01102-y
Keywords: lymphoma, double-expressor diffuse large B-cell lymphoma, chimeric antigen receptor T-cell immunotherapy, radiotherapy, bridging therapy
Citation: Yang L, Wu M, Yang H, Sun X, Xing L, Liu D, Xing L and Yu J (2024) Case report: Bridging radiation therapy before chimeric antigen receptor T-cell therapy induces sustained remission in patients with relapsed/refractory double-expressor diffuse large B-cell lymphoma with localized compressive symptoms. Front. Immunol. 15:1441404. doi: 10.3389/fimmu.2024.1441404
Received: 31 May 2024; Accepted: 19 August 2024;
Published: 03 September 2024.
Edited by:
Saad Zafar Usmani, Levine Cancer Institute, United StatesReviewed by:
Ryan Urak, City of Hope National Medical Center, United StatesMin Xiao, Huazhong University of Science and Technology, China
Copyright © 2024 Yang, Wu, Yang, Sun, Xing, Liu, Xing and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ligang Xing, eGluZ2xnQG1lZG1haWwuY29tLmNu
 Liying Yang
Liying Yang Mengdi Wu2,3
Mengdi Wu2,3 Lijie Xing
Lijie Xing Dan Liu
Dan Liu Ligang Xing
Ligang Xing Jinming Yu
Jinming Yu