- Department of Oncology, The Affiliated Hospital of Qingdao University, Qingdao, Shandong, China
Transformation from non-small cell lung cancer (NSCLC) to small cell lung cancer (SCLC) is rare and is associated with poor prognosis. However, the standard treatment protocols for patients with SCLC transformation remain unknown. Here, we report the case of a patient with advanced EGFR exon 19 deletion (19del) NSCLC who underwent SCLC transformation during targeted therapy. Biopsies and genetic testing were performed to adjust treatment regimens accordingly. The patient responded favorably to a combined treatment regimen comprising etoposide plus cisplatin chemotherapy and adebrelimab plus osimertinib. This case highlights the critical importance of acknowledging tumor heterogeneity in clinical decision-making and identifying potentially effective treatment options for patients with SCLC transformation. Additionally, we reviewed cases of the transformation of NSCLC to SCLC from 2017 to 2023.
Introduction
The management of non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC) is a critical area of investigation in the field of oncology. NSCLC, which accounts for 80-85% of all lung cancers, plays a significant role in targeted therapy (1, 2). EGFR exon 19 deletion (19del) is a common genetic alteration observed in patients with advanced NSCLC (3). When treated with EGFR-tyrosine kinase inhibitor (TKI), some EGFR-mutated NSCLC patients may undergo rare pathological transformations to SCLC (4), which is an important mechanism for resistance to EGFR-TKI treatment. Several studies have reported that NSCLC-derived SCLCs exhibit clinical features similar to primary SCLCs (5). However, for patients who undergo transformation from NSCLC to SCLC, chemotherapy provides only short-term effectiveness and leads to poor prognosis, with a median overall survival (OS) of less than 1 year (6). Therefore, the timely identification and development of effective treatment strategies are crucial. Although SCLC transformation in NSCLC patients has been documented in the literature (Table 1), there is no clear consensus on the optimal treatment regimen for these patients.
Table 1. Summary of cases of small cell lung cancer transformed from non-small cell lung cancer (2017 to 2023).
Here, we describe the case of a patient with advanced NSCLC with EGFR 19del who underwent pathological transformation from NSCLC to SCLC. Repeated biopsies and next-generation sequencing (NGS) tests, along with clinical disease evolution, have underscored tumor heterogeneity. These findings indicate that multimodal treatment, including chemotherapy, targeted therapy, and immunotherapy, may be a viable therapeutic strategy for this specific patient group.
Case presentation
Diagnosis and initial treatment response
A 68-year-old female was admitted to the hospital on July 12, 2021, because of cough and expectoration for 2 months. The patient had no history of smoking or cancer history. Contrast-enhanced chest computed tomography (CT) revealed a mass in the upper lobe of the left lung, along with multiple small nodules in both lower lobes and enlarged mediastinal and hilar lymph nodes. Moreover, pleural thickening and pleural effusion were observed (Figure 1A). Biopsy of the enlarged lesion in the left upper lobe (LUL) revealed poorly differentiated adenocarcinoma of the lung (Figure 2A). 14-gene panel testing identified an EGFR 19del mutation (Table 2). The patient was diagnosed with stage IV lung adenocarcinoma with EGFR 19del. The patient achieved partial response (PR) after first-line treatment with osimertinib (Figure 1B). Progression-free survival (PFS) after the first-line treatment was 24 months.
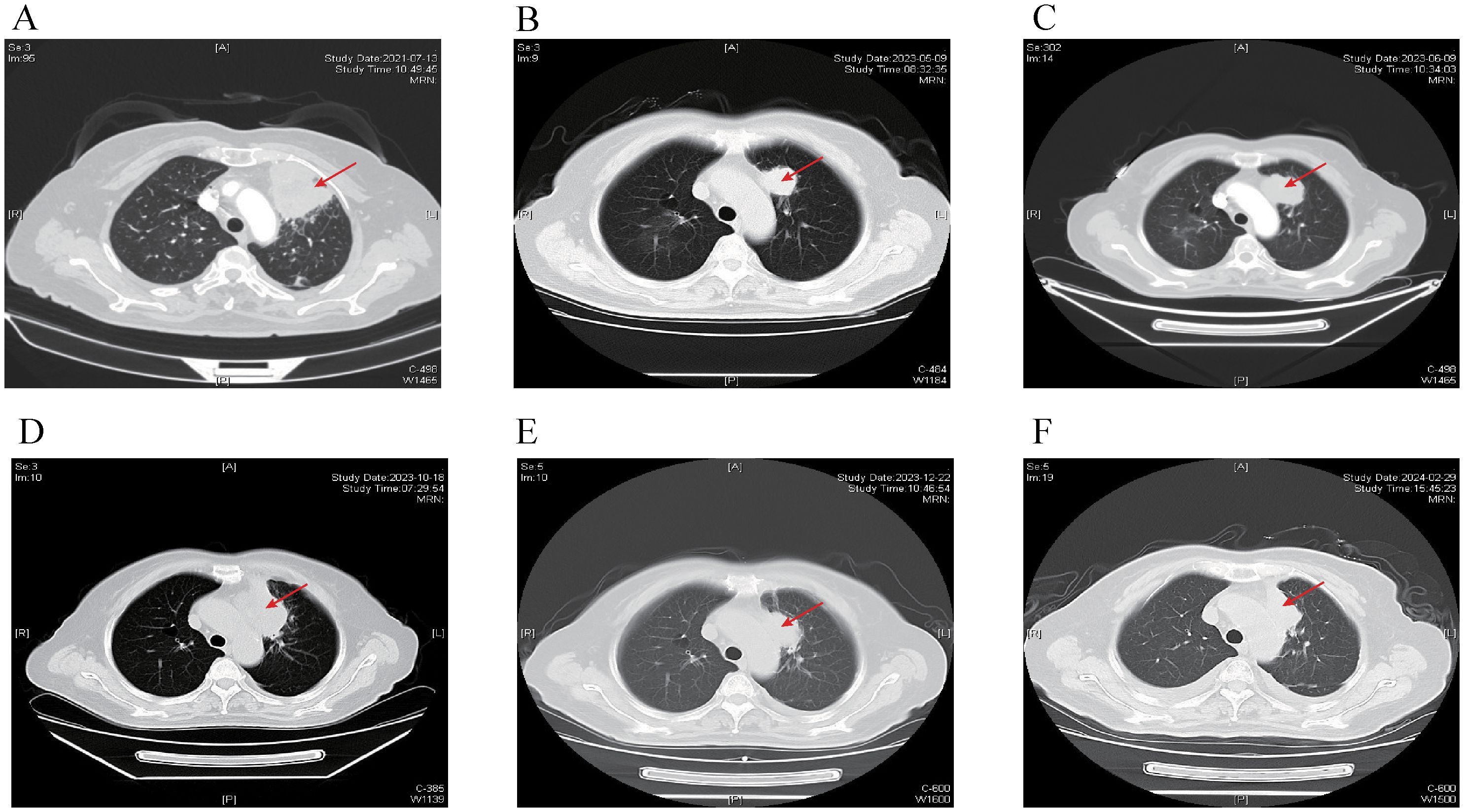
Figure 1. Chest CT scans at different time points. The red arrow indicates primary lesion in the left upper lobe of the lung. (A) Chest CT scan of baseline. (B) Chest CT scan of best response PR after first-line treatment with Osimertinib. (C) Chest CT scan showing progression after 24 months of Osimertinib. (D) Chest CT scan showing progression after 4 months of Anlotinib and Aumolertinib. (E) Chest CT scan showing reduction in the LUL lesion after 2 cycles of EP chemotherapy plus Adebrelimab. (F) Chest CT scan showing regression in the LUL lesion after fourth-line treatment with Osimertinib in addition to the existing chemotherapy and immunotherapy regimen. CT, computed tomography; PR, partial response; LUL, left upper lobe; EP, etoposide plus cisplatin.
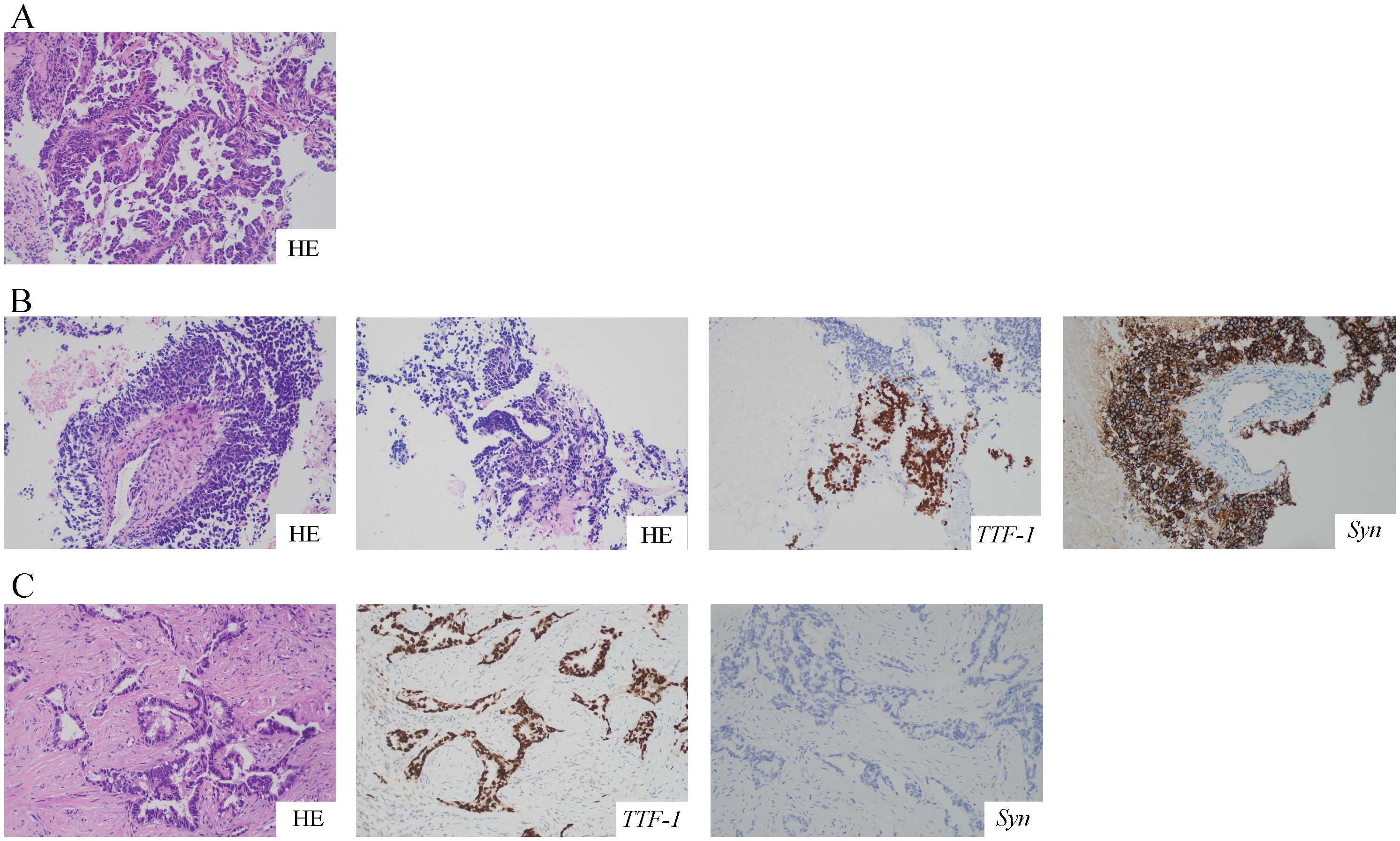
Figure 2. HE and IHC staining of the tumor at different time points. All pictures were taken at a 200-fold magnification using a light microscope. (A) Biopsy specimen of LUL revealed poorly differentiated lung adenocarcinoma with HE staining. (B) The second biopsy of LUL revealed mixed histology of adenocarcinoma and SCLC with HE and IHC staining for TTF-1 and Syn. (C) The third biopsy of the right cervical lymph node revealed poorly differentiated adenocarcinoma with HE and IHC staining for TTF-1 and Syn. HE, hematoxylin and eosin; IHC, immunohistochemistry; LUL, left upper lobe; SCLC, small cell lung cancer; TTF-1, thyroid transcription factor-1; Syn, synaptophysin.
Disease progression and SCLC transformation
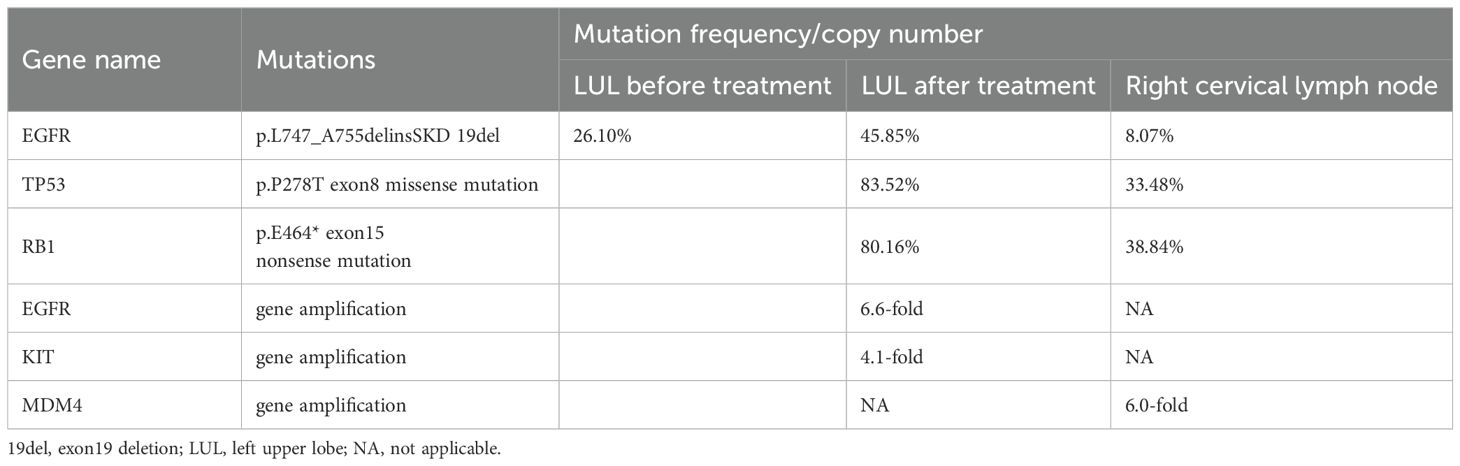
Subsequently, the patient experienced progressive disease (PD), with an increase in the size of the LUL lesion (Figure 1C) and emergence of cervical lymph node metastasis (Figure 3A). In June 2023, a second LUL biopsy was performed. Unexpectedly, hematoxylin and eosin (HE) staining showed mixed histology of adenocarcinoma and SCLC. Immunohistochemical (IHC) staining confirmed the presence of thyroid transcription factor-1 (TTF-1) (weakly +), napsin A (+), synaptophysin (+), CD56 (+), and CgA (+) (Figure 2B). In addition to EGFR 19del, 1012-gene panel testing further demonstrated a TP53 missense mutation, RB1 truncating mutation, EGFR amplification, KIT amplification, and tumor mutational burden (TMB) of 11 mutations per megabase (mt/Mb) (Table 2).
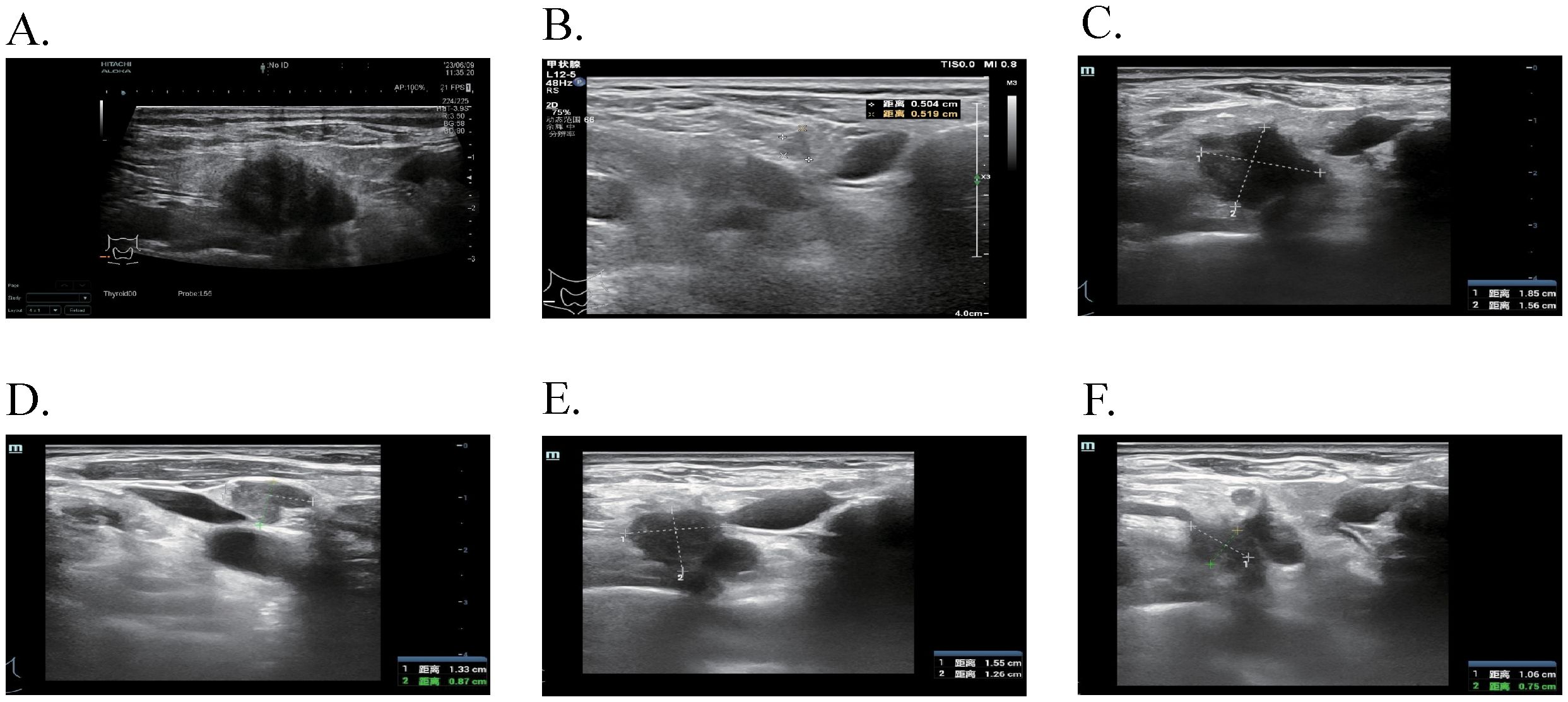
Figure 3. The ultrasound features of cervical lymph nodes at different time points. (A) After 24 months of Osimertinib, enlarged lymph nodes were observed in the IV region of the right neck, with a maximum size of 2.3 × 1.7 cm. (B) Following dual-targeted therapy, a previously enlarged lymph node in the IV region of the right neck reduced to 0.5 × 0.5 cm. (C, D) After 2 cycles of EP chemotherapy plus Adebrelimab, increased and enlarged lymph nodes were detected in the right neck IV area, with the largest measuring 1.9 × 1.6 cm and 1.3 × 0.9 cm. (E, F) With the addition of Osimertinib to the existing chemotherapy and immunotherapy regimen, the enlarged lymph nodes in the right neck IV area measured 1.6 × 1.3 cm and 1.1 × 0.8 cm.
Subsequent treatment regimen and treatment response
The patient declined the therapeutic option of chemotherapy and instead opted for second-line treatment with a combination of anlotinib and aumolertinib. However, 4 months later, follow-up enhanced CT and neck ultrasonography revealed PD of the LUL lesion (Figure 1D) and shrinkage of the cervical lymph nodes (Figure 3B). Therefore, the regimen was changed to etoposide plus cisplatin (EP) chemotherapy plus adebrelimab. Following two cycles of EP chemotherapy combined with immunotherapy, the primary lesion located in the LUL exhibited a significant reduction in size (Figure 1E), while enlargement of the right cervical lymph node was observed (Figures 3C, D). Fine-needle aspiration biopsy of the right cervical lymph node was performed to determine the underlying reasons for the inconsistent response in distinct lesions. Pathological examination revealed poorly differentiated adenocarcinoma originating in the lung (Figure 2C). IHC staining demonstrated TTF-1 (+), napsin A (+), CK7 (+), synaptophysin (-), CD56 (-), and CgA (-). 1012-gene panel testing revealed multiple gene mutations, including EGFR 19del, TP53 missense mutation, RB1 truncating mutation, NDM4 amplification, and a TMB of 11 mt/Mb (Table 2). Considering the heterogeneity of lung cancer, we introduced osimertinib in addition to the existing chemotherapy and immunotherapy regimens from the third cycle onward. After two cycles of combined treatment, both the primary LUL lesion and metastatic lesion in the cervical lymph nodes showed a notable decrease (Figures 1F, 3E, F). Until the last follow-up in February 2024, no deaths occurred and the follow-up time was 32 months. The flowchart of the treatment process is shown in Figure 4.
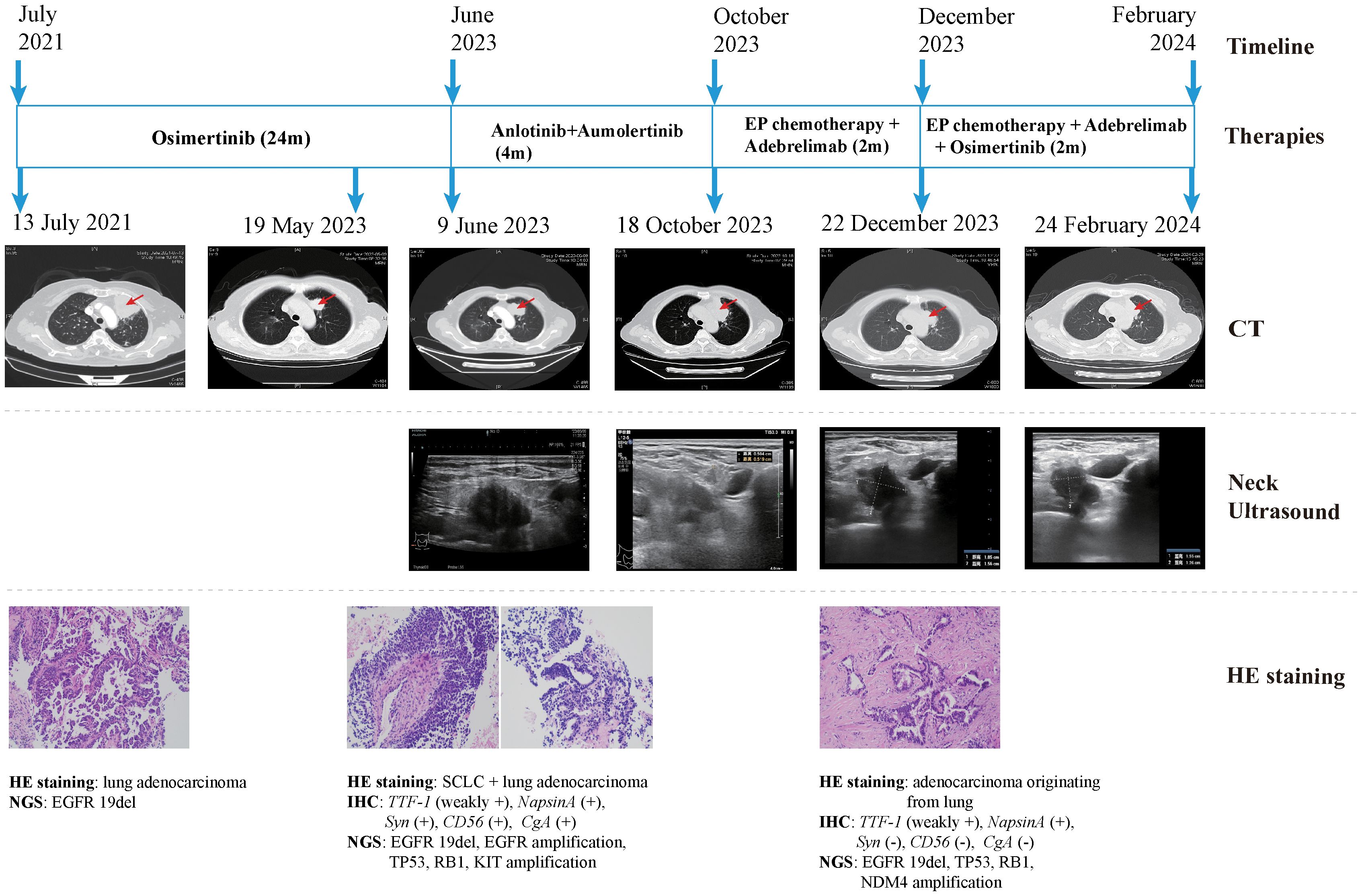
Figure 4. Case presentation of the 68-year-old female patient of SCLC transformation, including timeline, treatment details, chest CT images, neck ultrasound images, HE staining images and NGS findings. CT, computed tomography; HE, hematoxylin and eosin; SCLC, small cell lung cancer; NGS, next-generation sequencing; 19del, exon19 deletion; IHC, immunohistochemistry; TTF-1, thyroid transcription factor-1; Syn, synaptophysin.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of the case report and accompanying images. A copy of the written consent form is available for review by the journal’s editorial office.
Discussion
For advanced NSCLC patients with EGFR mutation, the first-line treatment option is EGFR-TKIs, including gefitinib, erlotinib, afatinib, osimertinib, anlotinib, and aumolertinib (34). However, single-agent targeted therapies for NSCLC frequently fail because of the development of acquired drug resistance. Transformation into SCLC represents a rare mechanism of resistance to EGFR-TKIs in advanced lung adenocarcinoma harboring EGFR mutations, accounting for approximately 5-15% of resistance etiologies (35, 36). However, the precise mechanisms underlying this transformation remain unknown. The potential mechanisms of SCLC transformation include epithelial-to-mesenchymal transition (EMT); mutations that affect TP53, RB1, and PIK3CA; and acquired EGFR mutations (35, 37, 38). Patients with a triple-positive mutation profile of EGFR, TP53, and RB1 exhibited a 6-fold augmented susceptibility to SCLC conversion compared with patients without mutations in TP53 and RB1 (39, 40). Few cases of SCLC transformation have been reported in patients receiving immunotherapy, such as programmed death-1 inhibitors (41).
Patients with EGFR-mutated NSCLC who underwent transformation to SCLC exhibited a significantly unfavorable prognosis in terms of survival. A study involving 39 patients reported an average survival duration of merely 6 months after SCLC conversion (42). An analysis of 67 patients revealed a median OS of 10.9 months after SCLC transformation (43). These data imply that timely recognition and efficient intervention play crucial roles in the management of patients undergoing SCLC transformation.
Due to the lack of established treatment guidelines for patients undergoing SCLC transformation, current therapeutic approaches are based on retrospective studies and case reports (6). Platinum and etoposide-based chemotherapy remains the standard treatment for patients with SCLC transformation, with the median disease control time of approximately 3 months. A real-world study included 29 patients who developed SCLC transformation following EGFR-targeted therapy. The analysis indicated that compared to chemotherapy alone, the combination of chemotherapy and targeted therapy improved objective response rates and PFS, although it did not significantly extend OS. Anti-angiogenic therapy and local radiotherapy can prolong OS after transformation (44). A multicenter study involving 32 patients with EGFR-mutant NSCLC who experienced SCLC transformation after targeted therapy revealed that the most commonly used chemotherapy regimen post-transformation was etoposide combined with platinum (n=27), with a median PFS of 3.5 months. Additionally, 3 patients received irinotecan combined with platinum, achieving a median PFS of 7.6 months. Five patients were treated with anlotinib, and the anlotinib group showed a median PFS of 6.2 months (45). Although data suggest that irinotecan combined with platinum and anlotinib may yield better survival outcomes, the limited sample size makes this conclusion less convincing. Furthermore, a case report compared the outcomes of two patients with EGFR-mutant NSCLC who underwent SCLC transformation and received different treatment regimens. One patient received the EP regimen alone post-transformation, achieving a PFS of only 3 months. The other patient received erlotinib combined with the EP regimen, followed by long-term maintenance therapy with erlotinib and oral etoposide, ultimately achieving a PFS of 8 months (46). However, to date, there have been no reports on combined use of chemotherapy, targeted therapy, and immunotherapy for patients with SCLC transformation. In this case, the patient developed PD that transformed into SCLC after 24 months of osimertinib treatment. Further PD occurred following the dual-targeted therapy. Subsequent EP chemotherapy and immunotherapy led to a reduction in the size of the primary lesion and enlargement of cervical lymph nodes. The addition of osimertinib for two cycles resulted in a reduction in both the LUL and cervical lymph node lesions. This finding suggests that EGFR-TKIs only inhibit the EGFR-mutant NSCLC component, allowing the SCLC component to rapidly proliferate and reach PD. EP chemotherapy combined with adebrelimab is the standard treatment for SCLC; thus, simple inhibition of SCLC may lead to rapid regrowth of the NSCLC component. The combination of targeted therapy, chemotherapy, and immunotherapy resulted in a reduction in both primary and metastatic lesions, indicating that mixed histological components of SCLC and NSCLC should be considered. This suggests that for patients experiencing SCLC transformation who still harbor EGFR mutations, a combination of chemotherapy, immunotherapy, and targeted therapy may be an effective treatment approach. However, additional randomized controlled trials are required for further validation. Moreover, recognizing tumor heterogeneity and performing timely biopsies and genetic testing during changes in a patient’s condition are pivotal for facilitating the rapid detection of pathological transformations, tailoring individualized treatment strategies, and enhancing the prognoses of patients.
EGFR-mutated lung adenocarcinoma accompanied by RB1 and TP53 mutations represents the highest-risk group for SCLC transformation during targeted therapy, with a transformation probability of up to 18%. Patients harboring EGFR, RB1, and TP53 mutations exhibit the poorest treatment outcomes, with median time to treatment discontinuation and OS of 9.5 months and 29.1 months, respectively (40). In our case, re-biopsy following disease progression on EGFR-TKIs revealed concurrent EGFR, RB1, and TP53 mutations. Unfortunately, due to the lack of comprehensive genetic analysis at the initial NSCLC diagnosis, only a 14-gene panel was performed, missing critical baseline information on TP53 and RB1 gene status. This underscores the importance of re-biopsy in EGFR/RB1/TP53-mutant lung adenocarcinoma, particularly in patients with poor response to EGFR-TKIs.
In a comprehensive systematic review by Roca et al., 39 patients who underwent SCLC transformation between 2006 and 2016 were systematically evaluated (42). To delve deep into the demographic characteristics, therapeutic interventions, and prognoses of patients experiencing SCLC transformation, we reviewed 33 cases of SCLC transformation from 2017 to 2023 and summarized their genetic mutations, treatment modalities, and patient outcomes in Table 1. Among the 33 reported cases, the majority were of Asian ethnicity and demonstrated a pronounced association with poor prognoses, frequently accompanied by central nervous system metastases. Notably, 13 out of 33 patients (39%) presented with central nervous system metastasis. Observational data suggest that male patients (66%) may be more likely to undergo SCLC transformation. What’s more, among the 33 cases, the majority of patients had either an unmentioned family history or no family history, and the patient presented in this case had no history of cancer. It was worth noting that 63% were smokers and 18% were non-smokers, suggesting that smoking may have a potential impact on transformation to SCLC. Disparities in the implementation of personalized medicine across different countries and regions underscore variations in treatment standards and medication accessibility, potentially impacting treatment efficacy and patient survival rates. For instance, Asian populations may prioritize the utilization of the EGFR-TKIs, while Western countries may prioritize the utilization of immunotherapy. EGFR, ALK, and TP53 mutations are commonly observed in patients undergoing SCLC transformation. Among them, EGFR mutations were reported in 13 cases (39%), including 8 cases with EGFR 19 del (62%) and 3 case with EGFR exon 21 L858R (23%). Therefore, we speculate that SCLC transformation is more likely to occur in patients with EGFR mutation and subsequent resistance to targeted therapy.
Surgical specimens were unattainable in patients with unresectable NSCLC at the initial diagnosis. The presence of two histological components could not be definitively excluded because of the inherent limitations of the existing examination methods and techniques. This highlights the importance of obtaining an ample number of tissue specimens from patients with advanced lung cancer to mitigate misdiagnoses resulting from limited sampling.
Despite multiple reported cases of SCLC transformation, treatment strategies remain inadequately explored. In our case report, we document the successful use of EP chemotherapy in combination with adebrelimab and osimertinib for the first time in the management of advanced SCLC transformation. Encouragingly, imaging results indicate a favorable therapeutic response. Nevertheless, the precise molecular mechanism underlying this transformation remains elusive, and consensus treatment guidelines are lacking. Future work should focus on unraveling the molecular mechanisms of this transformation and conducting prospective studies to establish evidence-based treatment protocols.
Conclusions
SCLC transformation is a rare but crucial cause of acquired EGFR-TKI resistance. It is essential to conduct repeated biopsies and employ NGS and IHC tests to identify alterations in histological types. We found that the combination of EP chemotherapy plus adebrelimab and osimertinib had a significant therapeutic effect in patients with NSCLC pathological transformed to SCLC. The multimodal treatment approach involving chemotherapy, targeted therapy and immunotherapy may be a promising strategy for this distinct patient cohort.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
XXL: Writing – original draft, Writing – review & editing. XCL: Writing – original draft, Writing – review & editing. MZ: Writing – original draft. RW: Writing – review & editing. JG: Methodology, Writing – review & editing. JL: Investigation, Writing – review & editing. WQ: Supervision, Writing – review & editing. SZ: Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We would like to thank patients and their families for their support of our work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Glossary
amp: amplification
AMR: Ceritinib, alectinib, amrubicin
CNS: central nervous system
CT: computed tomography
EMT: epithelial-to-mesenchymal transition
EC: etoposide plus carboplatin
EP: etoposide plus cisplatin
GP: gemcitabine plus cisplatin
HE: hematoxylin and eosin
IC: irinotecan plus carboplatin
IHC: immunohistochemistry
IP: irinotecan plus cisplatin
LUL: left upper lobe
MSS: microsatellite stability
mt/Mb: mutations per megabase
NA: not applicable
NGS: next-generation sequencing
NM: Not mentioned
NSCLC: non-small cell lung cancer
OS: overall survival
PC: pemetrexed plus carboplatin
PP: pemetrexed plus cisplatin
PD: progressive disease
PFS: progression-free survival
PR: partial response
SCLC: small cell lung cancer
Syn: synaptophysin
TC: paclitaxel plus carboplatin
TKI: tyrosine kinase inhibitors
TMB: tumor mutational burden
TTF-1: thyroid transcription factor-1
19del: exon 19 deletion.
References
1. Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA: Cancer J Clin. (2024) 74:12–49. doi: 10.3322/caac.21820
2. Lingling X, Maoxi C, Wei Y, Jieting Z, Yuanyuan Y, Ning X. Transformation of NSCLC to SCLC harboring EML4-ALK fusion with V1180L mutation after alectinib resistance and response to lorlatinib: A case report and literature review. Lung Cancer. (2023) 186:107415. doi: 10.1016/j.lungcan.2023.107415
3. Qiao M, Li D, He Y, Zhang C, Chi H, Li X, et al. Detection and significance of cell-free DNA mutation in pleural effusion in patients with advanced NSCLC. Emergency Med Int. (2022) 2022:3112281. doi: 10.1155/2022/3112281
4. Ohmori T, Yamaoka T, Ando K, Kusumoto S, Kishino Y, Manabe R, et al. Molecular and clinical features of EGFR-TKI-associated lung injury. Int J Mol Sci. (2021) 22. doi: 10.3390/ijms22020792
5. Herzog BH, Devarakonda S, Govindan R. Overcoming chemotherapy resistance in SCLC. J Thorac oncology: Off Publ Int Assoc Study Lung Cancer. (2021) 16:2002–15. doi: 10.1016/j.jtho.2021.07.018
6. Yin X, Li Y, Wang H, Jia T, Wang E, Luo Y, et al. Small cell lung cancer transformation: From pathogenesis to treatment. Semin Cancer Biol. (2022) 86:595–606. doi: 10.1016/j.semcancer.2022.03.006
7. Imakita T, Fujita K, Kanai O, Terashima T, Mio T. Small cell lung cancer transformation during immunotherapy with nivolumab: A case report. Respir Med Case Rep. (2017) 21:52–5. doi: 10.1016/j.rmcr.2017.03.019
8. Oya Y, Yoshida T, Uemura T, Murakami Y, Inaba Y, Hida T. Serum ProGRP and NSE levels predicting small cell lung cancer transformation in a patient with ALK rearrangement-positive non-small cell lung cancer: A case report. Oncol Lett. (2018) 16:4219–22. doi: 10.3892/ol
9. Abdallah N, Nagasaka M, Abdulfatah E, Shi D, Wozniak AJ, Sukari A. Non-small cell to small cell lung cancer on PD-1 inhibitors: two cases on potential histologic transformation. Lung Cancer (Auckland NZ). (2018) 9:85–90. doi: 10.2147/LCTT
10. Liu Y. Small cell lung cancer transformation from EGFR-mutated lung adenocarcinoma: A case report and literatures review. Cancer Biol Ther. (2018) 19:445–9. doi: 10.1080/15384047.2018.1435222
11. Nishioka N, Yamada T, Harita S, Hirai S, Katayama Y, Nakano T, et al. Successful sequential treatment of refractory tumors caused by small cell carcinoma transformation and EGFR-T790M mutation diagnosed by repeated genetic testing in a patient with lung adenocarcinoma harboring epidermal growth factor receptor mutations: A case report. Respir Med Case Rep. (2018) 25:261–3. doi: 10.1016/j.rmcr.2018.10.004
12. Iams WT, Beckermann KE, Almodovar K, Hernandez J, Vnencak-Jones C, Lim LP, et al. Small cell lung cancer transformation as a mechanism of resistance to PD-1 therapy in KRAS-mutant lung adenocarcinoma: A report of two cases. J Thorac oncology: Off Publ Int Assoc Study Lung Cancer. (2019) 14:e45–e8. doi: 10.1016/j.jtho.2018.11.031
13. Okeya K, Kawagishi Y, Muranaka E, Izumida T, Tsuji H, Takeda S. Hyperprogressive disease in lung cancer with transformation of adenocarcinoma to small-cell carcinoma during pembrolizumab therapy. Internal Med. (2019) 58:3295–8. doi: 10.2169/internalmedicine.2892-19
14. Bar J, Ofek E, Barshack I, Gottfried T, Zadok O, Kamer I, et al. Transformation to small cell lung cancer as a mechanism of resistance to immunotherapy in non-small cell lung cancer. Lung Cancer. (2019) 138:109–15. doi: 10.1016/j.lungcan.2019.09.025
15. Miura N, Matsubara T, Takamori S, Haratake N, Toyozawa R, Yamaguchi M, et al. Histological conversion from adenocarcinoma to small cell carcinoma of the lung after treatment with an immune checkpoint inhibitor: a case report. Oxford Med Case Rep. (2020) 2020:omaa026. doi: 10.1093/omcr/omaa026
16. Si X, You Y, Zhang X, Wang H, Wang M, Zhang L. Histologic transformation of lung cancer during pembrolizumab therapy: A case report. Thorac Cancer. (2020) 11:793–6. doi: 10.1111/1759-7714.13312
17. Sehgal K, Varkaris A, Viray H, VanderLaan PA, Rangachari D, Costa DB. Small cell transformation of non-small cell lung cancer on immune checkpoint inhibitors: uncommon or under-recognized? J immunotherapy Cancer. (2020) 8. doi: 10.1136/jitc-2020-000697
18. Arakawa S, Yoshida T, Shirasawa M, Takayanagi D, Yagishita S, Motoi N, et al. RB1 loss induced small cell lung cancer transformation as acquired resistance to pembrolizumab in an advanced NSCLC patient. Lung Cancer. (2021) 151:101–3. doi: 10.1016/j.lungcan.2020.11.016
19. Zhang C, Lin L, Guo X, Chen P. Significance of genetic sequencing in patients with lung adenocarcinoma with transformation to small cell lung cancer: a case report and systematic review. Trans Cancer Res. (2020) 9:3725–33. doi: 10.21037/tcr
20. Otoshi R, Sekine A, Okudela K, Asaoka M, Sato Y, Ikeda S, et al. Small-cell lung carcinoma transformation of lung adenocarcinoma diagnosed by pericardial effusion: A case report. Mol Clin Oncol. (2020) 13:129–32. doi: 10.3892/mco
21. Leonetti A, Minari R, Mazzaschi G, Gnetti L, La Monica S, Alfieri R, et al. Small cell lung cancer transformation as a resistance mechanism to osimertinib in epidermal growth factor receptor-mutated lung adenocarcinoma: case report and literature review. Front Oncol. (2021) 11:642190. doi: 10.3389/fonc.2021.642190
22. Imakita T, Fujita K, Kanai O, Okamura M, Hashimoto M, Nakatani K, et al. Small cell transformation of non-small cell lung cancer under immunotherapy: Case series and literature review. Thorac Cancer. (2021) 12:3062–7. doi: 10.1111/1759-7714.14180
23. Zhai X, Liu J, Liang Z, Li Z, Liu Y, Huang L, et al. Case report: re-sensitization to gefitinib in lung adenocarcinoma harboring EGFR mutation and high PD-L1 expression after immunotherapy resistance, which finally transform into small cell carcinoma. Front Oncol. (2021) 11:661034. doi: 10.3389/fonc.2021.661034
24. Yang Z, Lin Y, Wang H. Transformation of non-small cell lung cancer into small cell lung cancer in a patient with advanced lung cancer: a case report. J Int Med Res. (2021) 49:3000605211035005. doi: 10.1177/03000605211035005
25. Li YC. Durable response to durvalumab-based immunochemotherapy in small-cell lung carcinoma transformation from EGFR-mutant non-small cell lung cancer: A case report. Thorac Cancer. (2022) 13:775–9. doi: 10.1111/1759-7714.14325
26. Liu H, Chen LH, Zhang ZH, Wang N, Zhuang SH, Chen H, et al. Histomorphological transformation from non-small cell lung carcinoma to small cell lung carcinoma after targeted therapy or immunotherapy: A report of two cases. Front Oncol. (2022) 12:1022705. doi: 10.3389/fonc.2022.1022705
27. Yang MH, Yu J, Cai CL, Li W. Small cell lung cancer transformation and tumor heterogeneity after sequential targeted therapy and immunotherapy in EGFR-mutant non-small cell lung cancer: A case report. Front Oncol. (2022) 12:1029282. doi: 10.3389/fonc.2022.1029282
28. Fang G, Liu W, Shang Y, Huo R, Shi X, Wang Y, et al. Characterization of non-small cell lung cancer transforming to small cell lung cancer and its response to EGFR-TKI: a case report. Ann Trans Med. (2022) 10:115. doi: 10.21037/atm
29. Wang D, Ye W, Chen D, Shi Q, Ma D. Transformation of lung squamous cell carcinoma to small cell lung cancer after immunotherapy resistance: A case report. Cancer Manage Res. (2023) 15:803–8. doi: 10.2147/CMAR.S420485
30. Wang X, Liang J, Li L, Pan Z, Wang L. Reuse of osimertinib after small cell lung cancer transformation in lung adenocarcinoma with de-novo epidermal growth factor receptor T790M mutation: case report. Anti-cancer Drugs. (2023) 34:306–10. doi: 10.1097/CAD.0000000000001403
31. Gazeu A, Aubert M, Pissaloux D, Lantuejoul S, Pérol M, Ikhlef N, et al. Small-cell lung cancer transformation as a mechanism of resistance to pralsetinib in RET-rearranged lung adenocarcinoma: A case report. Clin Lung Cancer. (2023) 24:72–5. doi: 10.1016/j.cllc.2022.10.005
32. Peng Y, Zheng Z, Zewen W, Yanan L, Mingyan Z, Meili S. Whole-exome sequencing explored mechanism of selpercatinib resistance in RET-rearranged lung adenocarcinoma transformation into small-cell lung cancer: a case report. BMC pulmonary Med. (2023) 23:492. doi: 10.1186/s12890-023-02799-5
33. Xia G, Huang J, Ni J, Song M, Zhang J, Hofman P, et al. Transformation of ALK-positive NSCLC to SCLC after alectinib resistance and response to combined atezolizumab: a case report. Trans Lung Cancer Res. (2023) 12:637–46. doi: 10.21037/tlcr
34. Oshima Y, Tanimoto T, Yuji K, Tojo A. EGFR-TKI-associated interstitial pneumonitis in nivolumab-treated patients with non-small cell lung cancer. JAMA Oncol. (2018) 4:1112–5. doi: 10.1001/jamaoncol.2017.4526
35. Lee JK, Lee J, Kim S, Kim S, Youk J, Park S, et al. Clonal history and genetic predictors of transformation into small-cell carcinomas from lung adenocarcinomas. J Clin oncology: Off J Am Soc Clin Oncol. (2017) 35:3065–74. doi: 10.1200/JCO.2016.71.9096
36. Oser MG, Niederst MJ, Sequist LV, Engelman JA. Transformation from non-small-cell lung cancer to small-cell lung cancer: molecular drivers and cells of origin. Lancet Oncol. (2015) 16:e165–72. doi: 10.1016/S1470-2045(14)71180-5
37. Sequist LV, Waltman BA, Dias-Santagata D, Digumarthy S, Turke AB, Fidias P, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Trans Med. (2011) 3:75ra26. doi: 10.1126/scitranslmed.3002003
38. Yu HA, Arcila ME, Rekhtman N, Sima CS, Zakowski MF, Pao W, et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer research: an Off J Am Assoc Cancer Res. (2013) 19:2240–7. doi: 10.1158/1078-0432.CCR-12-2246
39. Shaurova T, Zhang L, Goodrich DW, Hershberger PA. Understanding lineage plasticity as a path to targeted therapy failure in EGFR-mutant non-small cell lung cancer. Front Genet. (2020) 11:281. doi: 10.3389/fgene.2020.00281
40. Offin M, Chan JM, Tenet M, Rizvi HA, Shen R, Riely GJ, et al. Concurrent RB1 and TP53 Alterations Define a Subset of EGFR-Mutant Lung Cancers at risk for Histologic Transformation and Inferior Clinical Outcomes. J Thorac oncology: Off Publ Int Assoc Study Lung Cancer. (2019) 14:1784–93. doi: 10.1016/j.jtho.2019.06.002
41. Hsu PC, Jablons DM, Yang CT, You L. Epidermal growth factor receptor (EGFR) pathway, yes-associated protein (YAP) and the regulation of programmed death-ligand 1 (PD-L1) in non-small cell lung cancer (NSCLC). Int J Mol Sci. (2019) 20. doi: 10.3390/ijms20153821
42. Roca E, Gurizzan C, Amoroso V, Vermi W, Ferrari V, Berruti A. Outcome of patients with lung adenocarcinoma with transformation to small-cell lung cancer following tyrosine kinase inhibitors treatment: A systematic review and pooled analysis. Cancer Treat Rev. (2017) 59:117–22. doi: 10.1016/j.ctrv.2017.07.007
43. Marcoux N, Gettinger SN, O’Kane G, Arbour KC, Neal JW, Husain H, et al. EGFR-mutant adenocarcinomas that transform to small-cell lung cancer and other neuroendocrine carcinomas: clinical outcomes. J Clin oncology: Off J Am Soc Clin Oncol. (2019) 37:278–85. doi: 10.1200/JCO.18.01585
44. Wang S, Xie T, Hao X, Wang Y, Hu X, Wang L, et al. Comprehensive analysis of treatment modes and clinical outcomes of small cell lung cancer transformed from epidermal growth factor receptor mutant lung adenocarcinoma. Thorac Cancer. (2021) 12:2585–93. doi: 10.1111/1759-7714.14144
45. Wang W, Xu C, Chen H, Jia J, Wang L, Feng H, et al. Genomic alterations and clinical outcomes in patients with lung adenocarcinoma with transformation to small cell lung cancer after treatment with EGFR tyrosine kinase inhibitors: A multicenter retrospective study. Lung Cancer. (2021) 155:20–7. doi: 10.1016/j.lungcan.2021.03.006
Keywords: non-small cell lung cancer (NSCLC), small cell lung cancer (SCLC), pathological transformation, EGFR exon 19 deletion (19 del), combination therapy, case report
Citation: Li X, Luan X, Zhang M, Wang R, Guo J, Lv J, Qiu W and Zhao S (2024) Potential therapeutic option for EGFR-mutant small cell lung cancer transformation: a case report and literature review. Front. Immunol. 15:1439033. doi: 10.3389/fimmu.2024.1439033
Received: 27 May 2024; Accepted: 05 August 2024;
Published: 21 August 2024.
Edited by:
Qinglin Shen, Jiangxi Provincial People’s Hospital, ChinaReviewed by:
Zhen Guan, Beijing Cancer Hospital, ChinaXinglu Zhang, Capital Medical University, China
Copyright © 2024 Li, Luan, Zhang, Wang, Guo, Lv, Qiu and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wensheng Qiu, d3NxaXVxZGZ5QHFkdS5lZHUuY24=; Shufen Zhao, emhhb3NmNzlAMTI2LmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Xiaoxuan Li
Xiaoxuan Li Xinchi Luan
Xinchi Luan Mengqi Zhang
Mengqi Zhang Rui Wang
Rui Wang Jing Guo
Jing Guo Wensheng Qiu
Wensheng Qiu