- 1Guangdong Key Laboratory of Regional Immunity and Diseases, Department of Pathogen Biology, Shenzhen University School of Medicine, Shenzhen, China
- 2Guangdong Key Laboratory for Biomedical Measurements and Ultrasound Imaging, National-Regional Key Technology Engineering Laboratory for Medical Ultrasound, School of Biomedical Engineering, Shenzhen University Medical School, Shenzhen, China
- 3National Clinical Research Center for Infectious Disease, The Third People's Hospital of Shenzhen, Southern University of Science and Technology, Shenzhen, China
- 4Department of Preventive Medicine, School of Public Health, Shenzhen University, Shenzhen, China
- 5Guangdong Key Lab for Diagnosis & Treatment of Emerging Infectious Diseases, Shenzhen Third People's Hospital, Shenzhen, China
This review explores the evolving landscape of blood biomarkers in the diagnosis of tuberculosis (TB), focusing on biomarkers derived both from the pathogen and the host. These biomarkers provide critical insights that can improve diagnostic accuracy and timeliness, essential for effective TB management. The document highlights recent advancements in molecular techniques that have enhanced the detection and characterization of specific biomarkers. It also discusses the integration of these biomarkers into clinical practice, emphasizing their potential to revolutionize TB diagnostics by enabling more precise detection and monitoring of the disease progression. Challenges such as variability in biomarker expression and the need for standardized validation processes are addressed to ensure reliability across different populations and settings. The review calls for further research to refine these biomarkers and fully harness their potential in the fight against TB, suggesting a multidisciplinary approach to overcome existing barriers and optimize diagnostic strategies. This comprehensive analysis underscores the significance of blood biomarkers as invaluable tools in the global effort to control and eliminate TB.
Introduction
Tuberculosis (TB), caused by Mycobacterium tuberculosis (Mtb), remains a formidable public health challenge globally. In 2022, it was responsible for 1.3 million deaths worldwide, underscoring its persistent threat despite medical and public health advancements (1). The situation is further aggravated by the emergence of multidrug-resistant TB (MDR-TB), which poses severe hurdles to eradication efforts, particularly in regions with dense populations and limited healthcare infrastructure (2–5). To address these challenges, the World Health Organization (WHO) launched its End Tuberculosis 2035 strategy, aiming to reduce TB’s prevalence and burden (6). A critical aspect of this strategy, outlined in the WHO’s 2014 consensus report, is the development of a triage test with high negative predictive value (NPV) to confidently rule out disease in negative cases and reduce unnecessary testing (7). Furthermore, systematic screening is required, with the test having at least 90% sensitivity and 70% specificity, usable by first-contact clinicians to identify patients needing further testing (7).
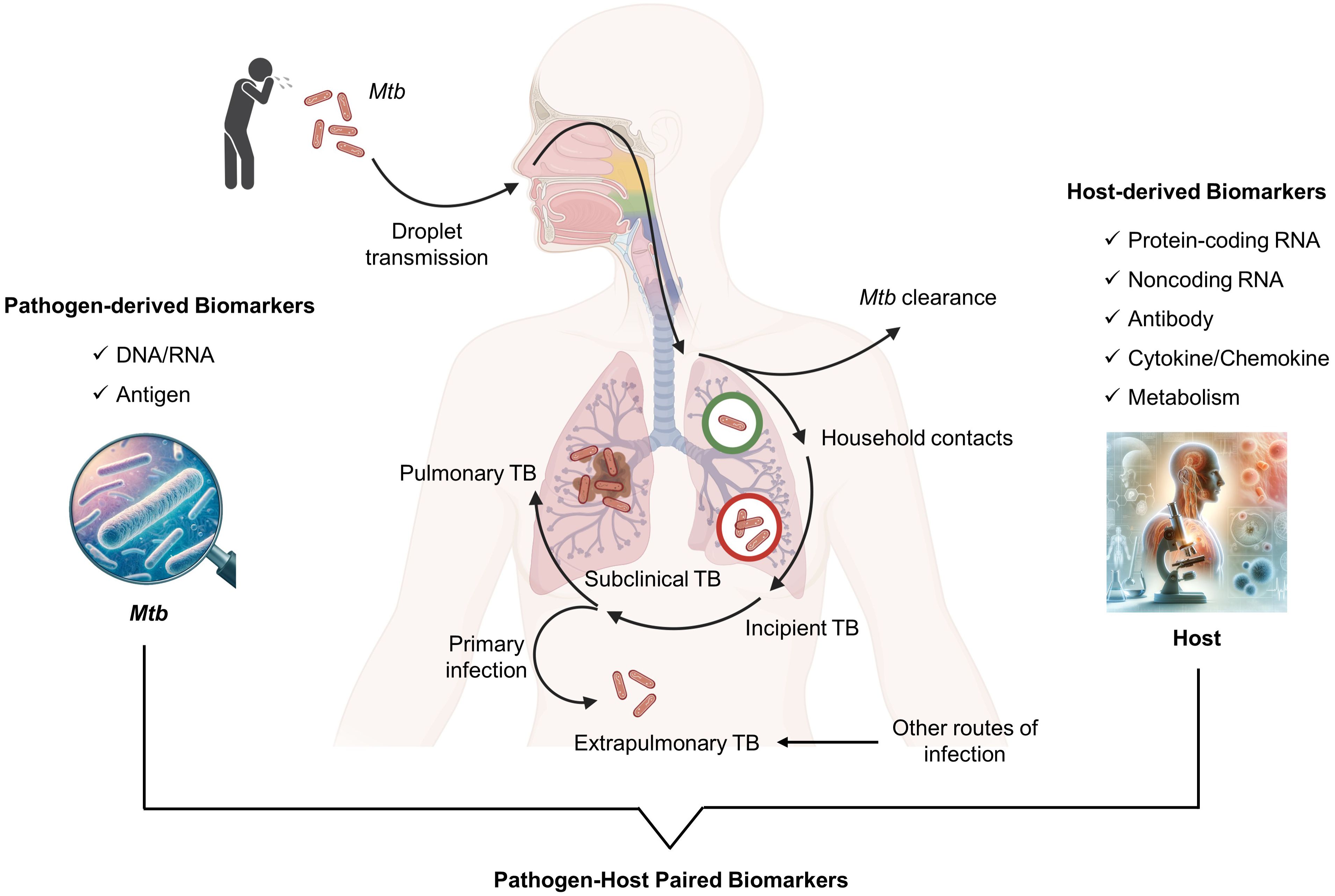
The dynamics of TB infection result from a balance between the host’s immune system and Mtb. In addition to the established states of disease and infection, there are two distinct intermediate clinical phases: incipient TB and subclinical TB (Figure 1). Incipient TB involves bacterial replication within affected tissues and a partial breakdown of the immune response, without any clinical, radiological, or microbiological evidence of disease. It’s believed that without therapeutic intervention, this incipient stage of the disease could evolve into active TB disease (8, 9). Subclinical TB, while symptom-free, shows increased Mtb replication and tissue damage detectable through microbiological assays and radiological imaging (8, 10). However, some individuals can control the infection at the incipient and subclinical phases by inducing robust immune responses, preventing progression to active TB (11). This highlights the critical role of the host’s immune system in managing TB infection and the potential for early intervention strategies to prevent disease progression to active TB. Accurate diagnosis of TB infection is vital for preventing disease progression and providing preventive therapy, a key strategy in global TB control (12).
TB diagnostics has evolved significantly over the years, featuring a variety of methods each developed to address specific needs and challenges associated with the disease’s detection. For example, solid medium Mtb culture, which is highly specific but with variable sensitivity, is less practical for quick decision-making as it takes up to 8 weeks to confirm the result. Liquid medium Mtb culture using Mycobacteria Growth Indicator Tube system, although reducing processing time to 2–3 weeks and improving sensitivity to 80–90%, demands high setup costs and advanced lab facilities (13–16). Sputum-smear microscopy is quick and cost-effective but less sensitive and specific, particularly in low-bacterial-load cases (17–19). Tuberculin skin test (TST) is inexpensive and simple but less specific in BCG-vaccinated individuals and requires a follow-up visit (20–22). Interferon gamma release assays (IGRA) provide a more specific alternative to TST with results in 24 hours, though costs are higher, limiting use in constrained budgets (23). GeneXpert MTB/RIF, detecting Mtb DNA and rifampicin resistance in two hours, suits point-of-care use with its minimal lab needs, but its high cost may restrict widespread adoption (24). Additionally, Line Probe Assays and Whole Genome Sequencing (WGS) can offer precise genetic data for diagnosing and guiding treatment but are expensive and require sophisticated labs, limiting their use to well-resourced healthcare settings or complex cases (25).
In summary, limitations of these methods primarily involve around their accessibility, cost, and operational requirements, which can be prohibitive in regions most affected by TB. Furthermore, the need for specialized training for accurate operation and interpretation of results adds another layer of complexity, restricting their utility in lower-resource settings. Therefore, it is in great need to develop more accessible, cost-effective, and rapid diagnostics, such as novel blood biomarkers, which could potentially transform TB detection globally. Here, we review the current findings and diagnostic potential of blood biomarkers, and explore their implications for improving TB detection globally.
Overview of blood biomarkers of tuberculosis
In the current landscape of TB diagnosis, blood biomarkers are categorized into those derived from the pathogen and the host (Figure 1). Pathogen-derived biomarkers include specific mycobacterial DNA or RNA sequences and antigens detectable in blood, such as microbial cell-free DNA (cfDNA), IS6110, IS1081, lipoarabinomannan (LAM), early secreted antigenic target 6 (ESAT-6), and CFP-10. These are primarily used for diagnosing TB disease. On the other hand, host-derived markers typically involve components of the immune response, such as antibodies (IgG and IgA against specific Mtb antigens), cytokines (IFN-gamma, VEGF, TNF-alpha), metabolites, and transcriptional profiles indicative of Mtb exposure or infection. For instance, recent advances have identified host RNA signatures that can differentiate between TB and control individuals with excellent diagnostic efficacy, boasting AUC values over 0.900 (26, 27). These findings highlight their potential as diagnostic biomarkers for detecting TB disease. Thus, the blood biomarkers, both pathogen and host-derived, could be useful for a more tailored approach to manage TB.
Pathogen-derived biomarkers
Nucleic acid biomarkers
Application of Polymerase Chain Reaction (PCR) and real-time PCR in nucleic acid amplification tests (NAATs) are typical methods for directly detecting TB by targeting specific DNA or RNA sequences unique to Mtb (28, 29). However, the sensitivity of PCR for directly detecting Mtb DNA in blood samples is notably low, ranging from 0.304 to 0.490 (30, 31). Developments such as the GeneXpert MTB/RIF and GeneXpert MTB/RIF Ultra have been introduced to diagnose TB rapidly with high specificity but have shown limited sensitivity, particularly in immunocompromised patients (32–34). Additionally, these tests are typically used on non-blood samples from individuals suspected of having TB, indicating that they are not directly comparable in the context of blood-based TB biomarkers. To address these gaps, there is a critical need to innovate and enhance PCR technologies to improve the accuracy of TB diagnosis using blood samples. Emerging techniques such as digital PCR (dPCR) and droplet digital PCR (ddPCR) show promise for achieving this goal (27, 35–37).
The dPCR testing method, which combines PCR with microfluidic technology, accurately measures low levels of nucleic acid targets. A previous study has exhibited the ability of dPCR quantifying targets IS6110 (dPCR-IS6110) and IS1081 (dPCR-IS1081) in discriminating TB individuals from healthy volunteers with AUCs of 0.790 and 0.720, respectively, and they had a promising specificity (0.934) but diminished sensitivity (<0.450) (36), which was further proved by another publication (38). Additionally, dPCR was used to determine Mtb complex DNA in PBMCs, revealing that 156 out of 197 (79%) PBMCs were detectable (39). Droplet Digital PCR (ddPCR), developed based on dPCR, subdivides the sample into thousands or even millions of tiny, independent droplets, each containing a small fraction of the total sample (35). Characterized by high sensitivity, precision, and robustness against inhibitors, this method allows for absolute quantification without the need for standard curves, making it particularly effective for analyzing complex clinical samples (40). By quantifying IS6100 copy numbers using ddPCR, Yang et al. reported that Mtb DNA can be detected Mtb in all examined full blood samples from both pulmonary TB cases (28/28) and extrapulmonary TB patients (28/28) (40). Besides IS6110, CFP-10 also serves as an amplification target for ddPCR, showing promising performance in TB diagnosis with 100% sensitivity and specificity (41). Besides TB diagnosis, ddPCR has also been applied to vaccine efficacy evaluation (41).
Microbial cell-free DNA (cfDNA) circulating in the bloodstream has garnered substantial attention due to its potential as a promising biomarker for detecting infections (42, 43). The microbial CRISPR system is an essential tool for detecting cfDNA. It first targets specific DNA or RNA sequences by designing particular guide RNAs and then cuts these targets with CRISPR effector enzymes such as Cas9, Cas12a, and Cas13, followed by the amplification and identification of the cut fragments. Therefore, combination of CRISPR system and isothermal amplification has established a CRISPR-based diagnostic (CRISPR-Dx) technology, providing rapid DNA or RNA detection. For example, Gootenberg JS, et al. have developed a Cas13a-based molecular detection platform named Specific High-Sensitivity Enzymatic Reporter UnLOCKing (SHERLOCK), which can detect single specific RNA and DNA targets to identify particular strains of Zika and Dengue virus, distinguish pathogenic bacteria, and detect human genotype DNA, but it was not initially applied to detecting Mtb (44). Subsequently, Gootenberg JS, et al. optimized and upgraded SHERLOCK (termed SHERLOCK-V2), adding Cas12a and the CRISPR type III effector nuclease Csm6 to the original single-channel detection to expand it to four channels, enhancing the signal sensitivity by 3.5 times (45). SHERLOCK-V2 holds a promising diagnostic platform with optimal accuracy for TB detection, although it has not been available for TB.
In 2023, Thakku SG, et al. evaluated the effectiveness of SHERLOCK in detecting Mtb, showing a detection limit of 0.5–5 fg of fragmented DNA and a compromised positive detection rate of 55% (6/11) (43). To improve the sensitivity of SHERLOCK, Thakku SG, et al. applied the SHERLOCK to conduct pooled amplification and subsequently simultaneously identified multiple target molecules tiled across the pathogen genome based on CRISPR-Cas13 detection, which was a novel method called Wholegenome Assay using Tiled Surveillance Of Nucleic acids (WATSON). WATSON improves the detection limit by 10- to 100-fold (0.05–0.5 fg) and achieves a 91% positive identification rate (10/11) in the TB cohort (43). Additionally, Huang Z, et al. developed an ultra-sensitive fluorescence CRISPR-Cas12a detection technique (CRISPR-TB) for identifying Mtb-cfDNA in blood, facilitating TB diagnosis (42). In an HIV-negative cohort, CRISPR-TB demonstrated a sensitivity of 0.960 and a specificity of 0.940 for detecting TB in adults, and a sensitivity of 0.830 and specificity of 0.950 in children. In HIV-positive cohorts, it achieved an overall TB detection rate of 85%, with a 100% detection rate in pediatric cases (42). Beyond TB, cfDNA/CRISPR-Dx holds potential for broader applications in detecting various infectious diseases and monitoring conditions.
Antigen biomarkers
Mtb antigens can trigger detectable immune responses in human hosts. The most commonly utilized antigen for TB diagnostics is lipoarabinomannan (LAM) found in urine, especially among HIV-positive patients, and in pleural fluid (46, 47). Additionally, Mtb LAM in blood also serves as a biomarker for diagnosing TB. For instance, Brock M and colleagues have developed a method using the Single Molecule Array (Simoa) to detect serum LAM, achieving a sensitivity of 37% with 100% specificity in HIV-positive TB patients. The sensitivity can be 60% in HIV-positive/smear-positive TB patients (48). This method offers perfect specificity but poor sensitivity, insufficient to meet WHO standards, highlighting the need for innovative diagnostic techniques that leverage the unique properties of LAM to improve its sensitivity in blood sample. To this end, Zhang W, et al. developed a nanoparticle-enhanced immunoassay read by dark-field microscopy that detects LAM and its carrier protein on the surface of circulating extracellular vesicles, through which, 74% of HIV-positive children, 73% in cases missed by microbial tests, and 80% in undiagnosed TB cases were positive for LAM test (49).
Another significant antigen-based method is the use of Mtb culture filtrate proteins, such as the early secreted antigenic target 6 (ESAT-6), a major virulence factor of Mycobacterium pathogenic species, secreted in the blood and sputum of infected individuals. ESAT-6 enabled a rapid, specific, and accurate detection at all Mtb infection stages (50). In earlier years, Poulakis N, et al. applied flow cytometry to assess Mtb ESAT-6 levels in the blood of TB patients, accurately diagnosing 90% of TB cases, with sputum culture-positive patients consistently showing ESAT-6 expression (50). More recently, the growing interest in electrochemical sensors for diagnosing Mtb has been noteworthy. For instance, an anti-ESAT-6 monoclonal antibody as a bio-receptor is first covalently immobilized on the surface of a gold-plated screen-printed electrode (SPE) in a label-free electrochemical immunosensor, which has a detection limit of 7ng/ml (51). However, this sensor has not been tested for stability and reusability. Moreover, the cost of SPE sensors for TB diagnosis is prohibitive, especially in developing countries where Mtb is prevalent. Therefore, Omar RA, et al. found a cost-effective electrode substitute (Ni-rGO-PANI) and conducted qualitative and quantitative measurement of ESAT-6 using cyclic voltammetry. This substitute, made from Ni, metal-reduced graphene oxide (rGO), and polyaniline (PANI), not only reduces diagnostic costs but also lowers the detection limit for EAST-6 to 1ng/ml (52). Recently, a new electrochemical aptasensor with MXene/C60NPs/Au@Pt for signal generation and amplification was developed to detect and quantify EAST-6 in blood, with detection limits of 2.88 fg/ml (DPV measurement) and 13.50 fg/ml (IT measurement), respectively (53). It shows optimal potential in distinguishing TB patients from healthy donors and other lung disease patients, with 97.5% sensitivity and 96.7% specificity, suggesting a promising significant diagnostic application in clinic (53).
Additionally, immune-PCR (I-PCR), which is equipped with the characteristics of antigen–antibody interaction in ELISA and the capability of exponential amplification in PCR (54). It has been demonstrated that the sensitivity of I-PCR is much higher than that of ELISA (55). With CFP-10 as the target protein, I-PCR exhibited a sensitivity range of 0.762–0.837 in detecting TB patients, with a specificity of 0.935–0.938 (56). Singh N, et al. had previously stated that in addition to CFP-10, Mtb ESAT-6, CFP-10, MPT64, Ag85B, PstS1, and LAM can also serve as target proteins for I-PCR (57), indicating I-PCR’s potential as a promising tool for Mtb infection. Subsequently, real-time I-PCR has an advantage over conventional I-PCR in minimizing carry-over contamination, and thus it can monitor the dynamic alterations of Mtb secreted proteins in clinical samples, highlighting its potential for monitoring TB progression and treatment responses (58).
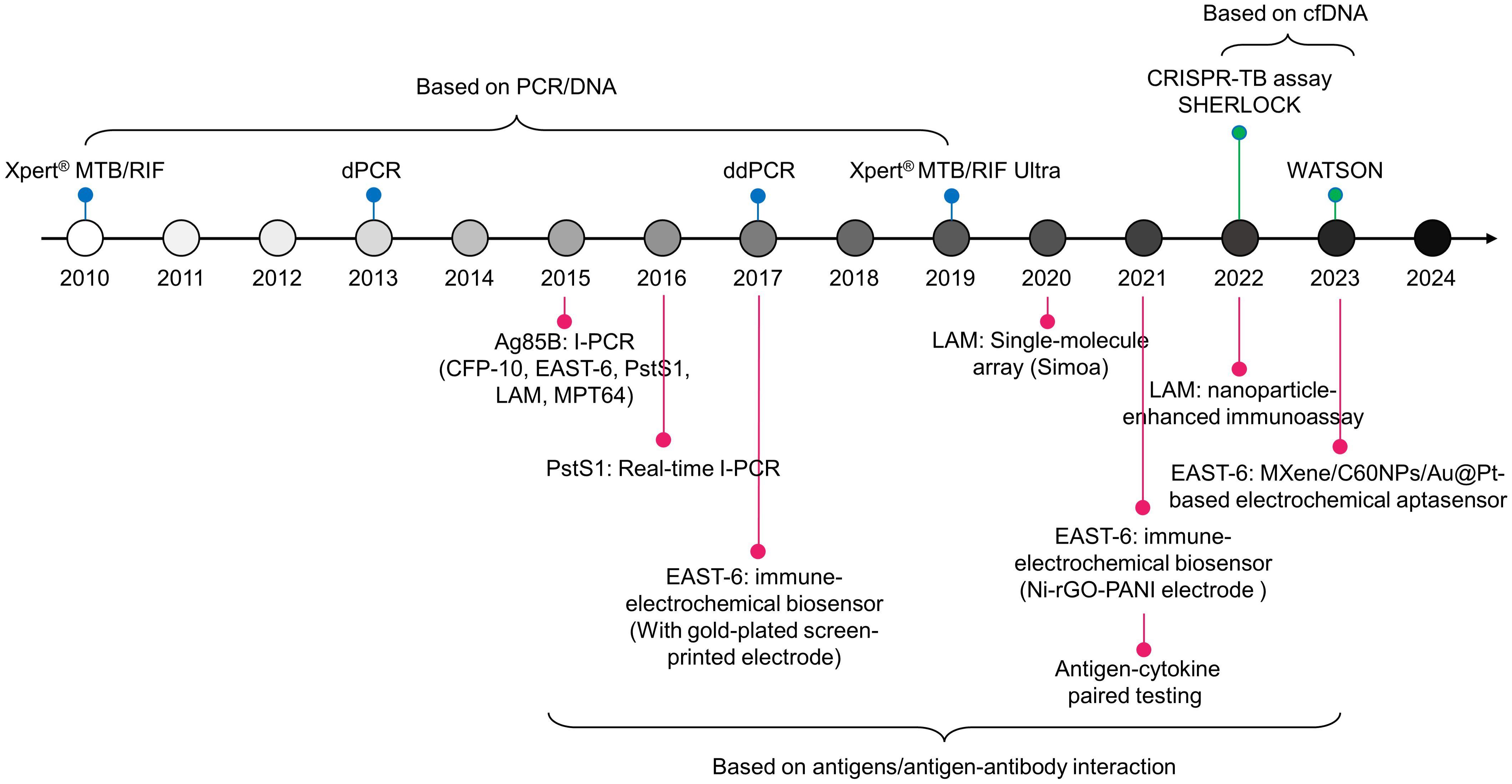
Recent research suggests that incorporating host cytokines into Mtb antigens will enhance the performance of TB detection (59). Meier NR, et al. identified ten antigen-cytokine pairs among 154 combinations, where the biomarker consisting of these ten pairs exhibited an AUC value of 0.920 in discriminating TB from controls. The most discriminative classifiers included Rv2346/47c-IP-10, Rv3614/15c-IP-10, Rv2031c-GM-CSF, and ESAT-6/CFP-10-TNF-alpha, which were the top four combinations (60). Additionally, Rv2431c-IP-10, Rv2031c-TNF-alpha, Rv2346/47c-TNF-alpha, and Rv2431c-TNF-alpha showed significant differences between TB individuals and control volunteers, with corresponding AUCs of 0.929, 0.953, 0.938, and 0.921, respectively (61). The goal of these combined biomarkers is to enhance the diagnostic performance of TB, especially in cases with atypical clinical presentations or complex infection scenarios, such as in children and HIV-positive populations (60, 61). In practice, development and validation of these combined biomarkers require extensive clinical trials and scientific verification to ensure their accuracy and practicality. To date, the new technologies discussed in this paper for developing markers for Mtb have been summarized in Figure 2.
Host-derived biomarkers
Messenger RNA biomarkers
One prominent example involves the use of gene signatures, which are combinations of multiple genes whose collective expression levels provide a robust diagnostic indicator. A notable case is a study by Kaforou M, et al., which developed a 27-transcript signature to discriminate TB from LTBI and a 44-transcript signature to distinguish TB from other diseases, with an excellent sensitivity and specificity regardless of HIV status (62). However, large number of transcripts signatures makes them unfeasible as biomarkers for clinical TB diagnosis, especially in point-of-care and resource-limited settings. Sweeney et al. addressed this issue by further refining the diagnostic gene set based on a multicohort study (26). They developed a three-gene signature (Risk3), comprising GBP5, DUSP3, and KLF2, to effectively separate TB individuals from healthy controls (AUC, 0.900), LTBI subjects (AUC, 0.880), and ODs patients (AUC, 0.840). Subsequently, Wu X, et al. quantified the mRNA expression levels of Risk3 in the Shanghai Pulmonary Hospital Cohort to evaluate the signature’s performance in TB detection, which showed a classification ability in distinguishing TB from LTBI, ODs, and healthy volunteers with AUCs of 0.739 (sensitivity 0.597, specificity 0.781), 0.825 (sensitivity 0.821, specificity 0.656), and 0.892 (sensitivity 0.761, specificity 0.880), respectively (63). To some extent, these results suggest that Risk3 has potential as a rapid, blood-based screening and triage test for TB. Another transcript signature, comprising GBP1, IFITM3, P2RY14, and ID3, was developed based on forest models and confidence interval decision trees, discriminating between TB and healthy controls with an AUC of 0.910 in the HIV-negative cohort (64). However, HIV co-infection could decrease the performance of the signature with an AUC of 0.840 (64), which contrasts with the viewpoints of Kaforou, M, et al. and Sweeney et al. (26, 62). The contrasting viewpoints may be due to cohort heterogeneity, model-building methods, and sample processing techniques.
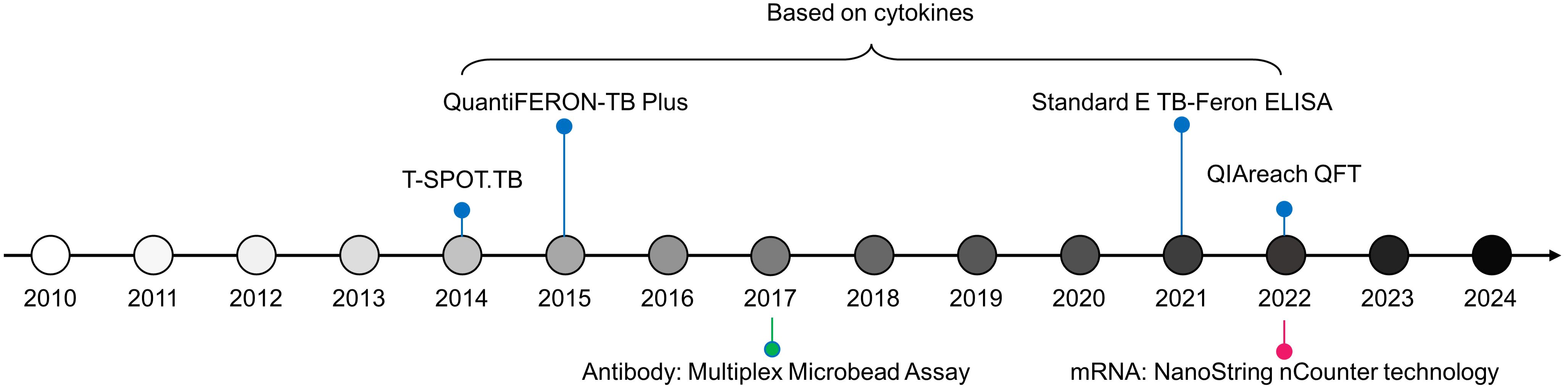
Based on the signatures from Karorou M, et al., Gliddon HD, et al. reduced the number of transcripts using feature selection algorithms and further quantified the expression levels of these characteristics using dPCR (27). Therefore, a 4-transcript signature (GBP6, TMCC1, PRDM1, and ARG1), derived from the 44-transcript signature (62), distinguishes TB from other diseases with an AUC of 0.938; a 3-gene transcript (FCGR1A, ZNF296, and C1QB), derived from the 27-transcript signature (62), separates TB from LTBI with an AUC of 0.973 (27). Additionally, using NanoString nCounter technology, Kaipilyawar V, et al. developed a NanoString 6-gene set (NANO6), comprising CCR6, BLK, DARS2, EXOC2, ATP1A1, ANKRD22, which accurately distinguishes TB from LTBI with an AUC of 0.987, sensitivity of 1.000, and specificity of 0.932 (65), which is a powerful method providing robust and precise quantification of mRNA based on color-coded barcode probes to directly measure gene expression levels without the need for amplification. Besides, this tool offers optimal accuracy and ability to simultaneously analyze multiple targets, holding promising prospects in clinical applications. The novel technologies for developing markers for host discussed in this paper are summarized in Figure 3.
In recent years, Ho J, et al. established two early diagnostic models for tuberculosis (TB) (11). Initially, they identified patients who were Xpert MTB positive and MTB sputum culture positive and/or had a chest x-ray (CXR) but lacked symptoms typical of active pulmonary TB, as early-stage TB patients. They then developed a 7-gene signature and an 8-gene signature, the former to distinguish TB from uninfected individuals (AUC=0.856), and the latter to differentiate TB from latent TB (AUC=0.892) (11). Establishing an early diagnosis model for TB is crucial because it enables timely initiation of treatment, reducing transmission and improving patient outcomes. Additionally, Sivakumaran D, et al. classified the TB disease process into four stages: household contacts, incipient TB, subclinical TB, and active TB (8). They developed a TB diagnostic biomarker based on 11 immune-related genes (11-gene signature) to distinguish between these stages (active TB vs. subclinical TB, AUC=1.000; active TB vs. household contacts, AUC=0.860; subclinical TB vs. household contacts, AUC=0.990), and it can differentiate between progressive TB and incipient TB patients (AUC=0.820) (8). This differentiation aids in tailoring interventions, monitoring disease progression, and implementing preventive strategies effectively, thereby reducing the overall burden of TB and preventing outbreaks within communities.
Non-coding RNA biomarkers
It’s reported that 80% of DNA is transcribed into RNA, and only 1.5% of this RNA is translated into protein, suggesting cellular RNA consists of two distinct forms: non-coding RNA (ncRNA) and protein-coding RNA (66). The potential of host-derived non-coding RNAs, specifically microRNAs (miRNAs), as biomarkers for TB diagnosis has drawn considerable attention. One key miRNA identified is miR-155, which was recognized as early as 2012 for its potential as a TB diagnostic marker (67). Both Mycobacterium bovis BCG infection and ROS are known to upregulate miR-155 expression (68), and it is involved in immune responses mediated by macrophages and T-cells (69). Daniel EA, et al. reviewed 21 studies to perform a meta-analysis evaluating miRNAs’ potential as TB biomarkers, showing the performance of miR-155 in TB diagnosis with a sensitivity of 0.898 and specificity of 0.809 (70). Diagnostic performance of miR-31, an inflammation-related factor, surpasses that of miR-155, exhibiting a sensitivity of 0.960 and a specificity of 0.890 (70, 71). Additionally, Shepelkova GS, et al. utilized miR-155 to characterize the pathological states of TB (72), demonstrating that miR-155 distinguishes TB patients without tissue lesions from healthy controls with a sensitivity of 0.730 and specificity of 0.999; and distinguishes TB individuals with tissue lesions from those without, with a sensitivity of 0.909 and specificity of 0.929, offering new insights for TB diagnosis and treatment. However, the small number of cases used in this study means that the accuracy and stability of the biomarkers could not be thoroughly evaluated.
Recently, Gunasekaran H, et al. conducted a meta-analysis derived from seven studies, revealing that both miR-197 and miR-144 exhibited higher sensitivity and specificity in differentiating TB from healthy controls, with sensitivities of 0.935 and 0.953, and specificities of 1.000 and 0.991, respectively (73), highlighting two potentially valuable miRNA candidates. Additionally, miR-29a-3p and miR-361–5p showed higher sensitivity in distinguishing TB from LTBI, with sensitivities of 0.905 and 0.887, but with compromised specificities of only 0.622 and 0.439, respectively (73). A 3-miRNA signature, comprising miR-197–3p, miR-let-7e-5p, and miR-223–3p, was developed to diagnose DR-TB with a sensitivity of 1.000 and specificity of 0.750 (73). The 3 candidate miRNAs identified by Gunasekaran H, et al. were inconsistent with another 3-miRNA signature developed by Liang Q, et al. (74). Liang Q, et al. combined miR-506–3p with miR-543 and miR-195–5p and developed a new 3-miRNA signature to distinguish spinal TB (STB) from healthy controls with a better AUC of 0.902 (74). Particularly, the miRNA signature had significantly higher risk scores in patients with STB compared to those with pulmonary TB and other spinal diseases, demonstrating the miRNA signature’s potential to differentiate STB from other conditions (74).
Circular RNA (circRNA), known for its stability and tissue specificity, is involved in many pathological and physiological processes in individuals with tuberculosis (TB), offering a promising avenue for biomarkers in TB diagnosis and assessing the risk of TB progression. Both hsa_circ_0001953 and hsa_circ_0009024 are significantly increased in individuals with TB compared to healthy controls and are positively associated with radiological scores, suggesting that both circRNAs may be involved in TB severity (75). Hsa_circ_0001953 has good performance in TB diagnosis with an AUC of 0.856, sensitivity of 0.740, and specificity of 0.900, followed by hsa_circ_0009024 with an AUC of 0.808, sensitivity of 0.700, and specificity of 0.800 (75). Moreover, the combination of hsa_circ_0001953 and hsa_circ_0009024, a 2-circRNA signature, achieves an AUC of 0.915. Meanwhile, this 2-circRNA signature shows promising potential to distinguish TB from other diseases (75). The circRNAs biomarkers for TB diagnosis by Huang Z, et al. are completely different from the characteristic circRNAs identified by Qian Z, et al. (76). Qian Z, et al. developed a 7-circRNA signature to detect TB with an improved AUC of 0.946. Meanwhile, Fu Y, et al. consider circRNA_103017 (AUC=0.870) to be a key candidate circRNA for TB diagnosis, followed by circRNA_059914 (AUC=0.821) and circRNA_101128 (AUC=0.817) (77). Additionally, the corresponding AUCs for circRNA_051239, circRNA_029965, and circRNA_404022 were 0.974, 0.944, and 0.968, respectively, in distinguishing TB from healthy controls (78). The reasons for these inconsistencies might due to the following reasons. First, the differences sample used, which mainly include whole blood, peripheral blood, serum, PBMCs, and plasma; Second, variations in detection methods, primarily comprising whole-genome sequencing, RNA sequencing, microarray, qRT-PCR, and dPCR. Third, the differences in the selection methods and criteria for candidate core genes, mainly reflected in the thresholds set for logFC and p-value (or adjusted p-value).
Dysregulation of long non-coding RNAs (lncRNAs) has been demonstrated to be closely involved in various pathological and physiological processes by regulating innate and adaptive responses. In recent years, lncRNAs in host blood have gained prominence as potential biomarkers for TB diagnosis. For example, a diagnostic lncRNA signature consisting of four lncRNAs (NR_038221, NR_003142, ENST0000057036, and ENST00000422183) was reported to discriminate TB from healthy controls with an AUC of 0.845, a sensitivity of 79.2%, and specificity of 75% (79). LOC152742 was also regarded as a biomarker for TB diagnosis with a better AUC value of 0.923 in discriminating active TB from healthy individuals, and an AUC of 0.852 among active TB and obsolete TB patients (80), which was the only publication for a biomarker used to distinguish between active and obsolete TB patients. Differing from the above in sample source, Huang S, et al. directly used neutrophils to screen for lncRNAs as TB diagnostic biomarkers, namely lncRNA ZNF100–6:2, with a good AUC value of 0.980 (81). However, this study did not specify which kind of individuals were used to distinguish TB for achieving the reported AUC (81). In addition to comparisons between TB and healthy controls, lncRNAs as diagnostic biomarkers for cured TB have been reported, such as a combined lncRNA signature comprising uc.48+ and NR_105053 with an AUC of 0.945 (sensitivity 0.900, specificity 0.864) (82). Due to limitations in sample size, different populations, and lack of external validation, it is challenging to ensure the consistency and validity of lncRNA biomarkers in TB diagnostic outcomes. Perhaps the following method could address this issue.
lncRNAs can also be combined with electronic health record (EHR) metrics to form lncRNA-EHR biomarkers (83–85). The diagnostic AUC of lncRNA-EHR biomarkers for TB ranges from 0.850 to 0.900. EHR data can significantly enhance the diagnostic efficiency and clinical applicability of disease diagnostic models (86). These EHR metrics primarily include age, hemoglobin, cough, weight loss, low-grade fever, calcification detected by computed tomography [CT calcification], and the interferon gamma release assay for tuberculosis [TB-IGRA]. Besides lncRNAs, circRNAs, miRNAs, transcripts, and proteins can all be integrated with EHR for diagnosing TB and other diseases, which is increasingly becoming a new focus of research in disease diagnostics.
Antibody-based methods for TB diagnosis
An ideal TB diagnostic biomarker should be simple, able to be measured on-site, capable of rapid testing, and discern across different stages of TB (87). A growing body of research suggests that antibodies fulfill these criteria due to their low cost, ease of use, and versatile functionality throughout the various stages of TB infection (87, 88). Currently, three main types of antibody-based tests are used in TB diagnosis: monoclonal antibodies, polyclonal antibodies, and combined antibodies targeting single and/or multiple Mtb antigens (87). Antibody-based tests targeting a single Mtb antigen typically show variable and sometimes suboptimal sensitivity (ranging from 0.290 to 0.940), but they maintain high specificity (ranging from 0.844 to 1.000) in distinguishing TB from non-TB controls (89, 90). Examples of such Mtb antigens include mammalian cell entry protein 1A (Mce1A), PstS1, proline-proline-glutamic acid protein 17 (PPE17), A60, Ag85, HspX, CFP-10, CFP-21, ESAT-6, and MPT-64 (89–92).
To address the issue of low sensitivity associated with antibody-based tests, innovative testing tools like the Multiplex Microbead Assay and Protein Chip Array have been developed for TB identification. These tools have demonstrated optimal sensitivity (0.900–0.940), though their specificity remains suboptimal (0.770–0.890) (93). Moreover, combinations of Mtb antibodies have been shown to achieve optimal sensitivity and specificity in TB diagnosis. Awoniyi DO and colleagues utilized Anti-Tpx+L16 IgG, anti-Tpx IgG, and anti-MPT64 IgA to develop a multi-antibody-based test that distinguished TB individuals from control volunteers with a sensitivity of 0.952 and a specificity of 0.976 (94). Another study reported a 7-antibody-associated test including IgA antibodies against Rv3019c, PstS, Apa, NarL, MPT64, Tpx, and the 19 kDa antigen, along with an IgM antibody against LAM. This test differentiated TB from other respiratory diseases with an AUC of 0.800, a sensitivity of 0.654, and specificity of 0.769 (95). Furthermore, the application of antibodies in the clinical diagnosis of TB has been well summarized (40, 87, 96).
Cytokine biomarkers
The importance of IFN-gamma as the first biomarker used for TB diagnosis cannot be overstated. Its role in detecting Mtb infection has paved the way for the development of various diagnostic tests, such as the QuantiFERON-TB Gold In-Tube test (QFT-GIT) (97, 98) and T-SPOT.TB assay (99), which utilize IFN-gamma release assays (IGRAs) to measure serum levels of Mtb-specific interferon-gamma (IFN-gamma) release (100). Meanwhile, IFN-gamma quantification remains the gold standard for detecting Mtb infection (101).
In 2015, QIAGEN upgraded the QFT-GIT to QuantiFERON-TB Plus (QFT-plus). Based on a meta-analysis involving 12 publications, Sotgiu G, et al. demonstrated that the corresponding values of AUC, sensitivity, and specificity were 0.990, 0.940, and 0.960, respectively, for QFT-plus in discriminating between TB and healthy controls (102). Compared to QFT-GIT (sensitivity 0.910), the sensitivity of QFT-plus was higher in detecting TB (102), which was also demonstrated by Chien JY, et al. (QFT-plus vs. QFT-GIT, 1.000 vs. 0.894) (103). Additionally, among the cured TB cases, the sensitivity of QFT-plus in TB diagnosis was also higher than QFT-GIT (0.820 vs. 0.730) (104). The promising sensitivity of QFT-plus mainly resulted from the TB2 responses. QFT-GIT only had a TB1 tube containing ESAT-6 and CFP-10 antigens to quantify the IFN-gamma from CD4+ T-cells (105). However, QFT-plus included another antigen tube, TB2, and was designed to measure IFN-gamma production by both CD4+ and CD8+ T-cells, improving sensitivity in diagnosing TB particularly among individuals with compromised immunity (106).
The T-SPOT.TB assay, which utilizes the enzyme-linked immunospot (ELISPOT) technique, quantifies IFN-γ-producing T-cells in response to Mtb-specific antigens (ESAT-6 and CFP-10) to detect Mtb infection (107). This assay demonstrated sensitivity values of 0.910 for detecting TB peritonitis and 0.780 for meningitis, with specificities of 0.780 and 0.680, respectively (108, 109). Additionally, it showed excellent performance in diagnosing TB among rheumatic patients, with a sensitivity of 0.930 and specificity of 0.940 (110), highlighting its potential in clinical practice. However, similar to QFT-Plus, the T-SPOT.TB assay requires advanced laboratory equipment and is more costly compared to the tuberculin skin test (TST).
On the other hand, IGRAs have various limitations, such as higher false-positive and false-negative rates (111), incomplete separation between TB and LTBI (112), and a lack of appropriate cut-off criteria for positive cases (113). It is urgent to develop novel testing tools based on new Mtb-specific antigens and new immunodiagnostic biomarkers to overcome the problems associated with IGRAs, such as Standard E TB-Feron ELISA and QIAreach QFT. Both of the IFN-gamma-testing tools have demonstrated excellent diagnostic efficacy and cost-effectiveness in detecting TB disease (114).
In addition to IFN-gamma, TNF-alpha, IL-2, and IL-17A have been identified as biomarkers for TB detection (115). Kumar NP and colleagues found that a combination of these three cytokines (TNF-alpha, IL-2, and IL-17A) formed a signature that was more effective in distinguishing TB from non-TB individuals than any individual marker or any combination of two markers (115). Furthermore, a current publication developed a support vector machine (SVM) model by combining IFN-gamma with TNF-alpha, IL-6, and IL-10, measuring serum levels of these four cytokines, which held a potential as biomarker for diagnosing TB with an AUC of 0.850, a sensitivity of 0.850 and specificity of 0.780 (101). Notably, this model outperformed the IGRA among HIV-infected volunteers (101). As an Mtb-specific biomarker, VEGF showed significantly different expression levels between TB and LTBI patients, underscoring its diagnostic capabilities (116–118). Additionally, chemokines such as CXCL9/MIG and CXCL10/IP-10 were reported to be significantly upregulated in TB patients compared to uninfected volunteers (119, 120). Meanwhile, a diagnostic model based on MIG and IP-10 demonstrated promising performance in differentiating TB from healthy individuals, achieving a sensitivity of 1.000 and a specificity of 0.950 (120).
Metabolic biomarkers
Upon Mtb infection, the metabolite dynamics resulting from the interaction between Mtb and the host play crucial roles in the innate immune response and in regulating the host’s defense system (121), indicating their potential as biomarkers for TB diagnosis. A recent publication identified 8 candidate metabolites for detecting TB based on a random forest algorithm, of which the most powerful classifying efficacy was LysoPE (18:1(11Z)/0:0) with an AUC of 0.995, a sensitivity of 1.000, and specificity of 0.963, followed by (2R)-O-Phospho-3-sulfolactate (AUC, 0.990; sensitivity, 0.933; specificity, 0.963) and 8-Demethyl-8-formylriboflavin 5′-phosphate (AUC, 0.986; sensitivity, 1.000; specificity, 0.889) (122). However, the study did not assess the overall classifying performance of the 8 candidates. Additionally, aside from active TB, Li YX, et al. have also used LC-MS/MS to identify Inosine, 16, 16-dimethyl-6-keto Prostaglandin E1, Theophylline, and Cotinine as potential serum indicators for Mtb latent infection and the metabolites were involved in amino acid metabolism, the endocrine system, the immune system, and lipid metabolism (123). Meanwhile, Wang X, et al. also identified seven metabolites as biomarkers to distinguish LTBI from healthy controls (124). Unlike Li YX, et al. who screened for metabolic biomarkers, Wang X, et al. not only assessed the diagnostic efficacy of each metabolite but also combined the 7 markers into a signature with an AUC of 0.979 (124).
Future perspectives and research directions
One of the main challenges of using host biomarkers for disease diagnostics is their lack of specificity. Host biomarkers often represent general immune responses rather than responses specific to a particular pathogen, leading to false positives and difficulties in distinguishing between different infections or inflammatory conditions. For instance, biomarkers such as C-reactive protein (CRP) and various cytokines (e.g., IL-6, TNF-α) are elevated in many infectious and inflammatory diseases, not just tuberculosis (TB) (125, 126). This lack of specificity can result in misdiagnosis or delayed diagnosis, as elevated levels of these biomarkers do not unequivocally indicate TB infection. Conditions such as autoimmune diseases, other bacterial, viral, and fungal infections, as well as chronic inflammatory conditions, can also cause similar changes in host biomarker levels, necessitating the use of a combination of biomarkers and additional diagnostic tests to improve accuracy (100, 127, 128). Recent research has focused on identifying more specific host biomarkers for TB, such as IP-10 and certain TB-specific T-cell responses (129, 130); however, these markers still require further validation and testing in diverse populations to ensure their reliability and specificity. In summary, while host biomarkers hold great promise for non-invasive and rapid diagnostics, their lack of specificity remains a significant hurdle. Ongoing research and the development of more specific biomarkers, along with multi-modal diagnostic approaches, are essential to overcome these challenges and improve the accuracy of TB diagnostics.
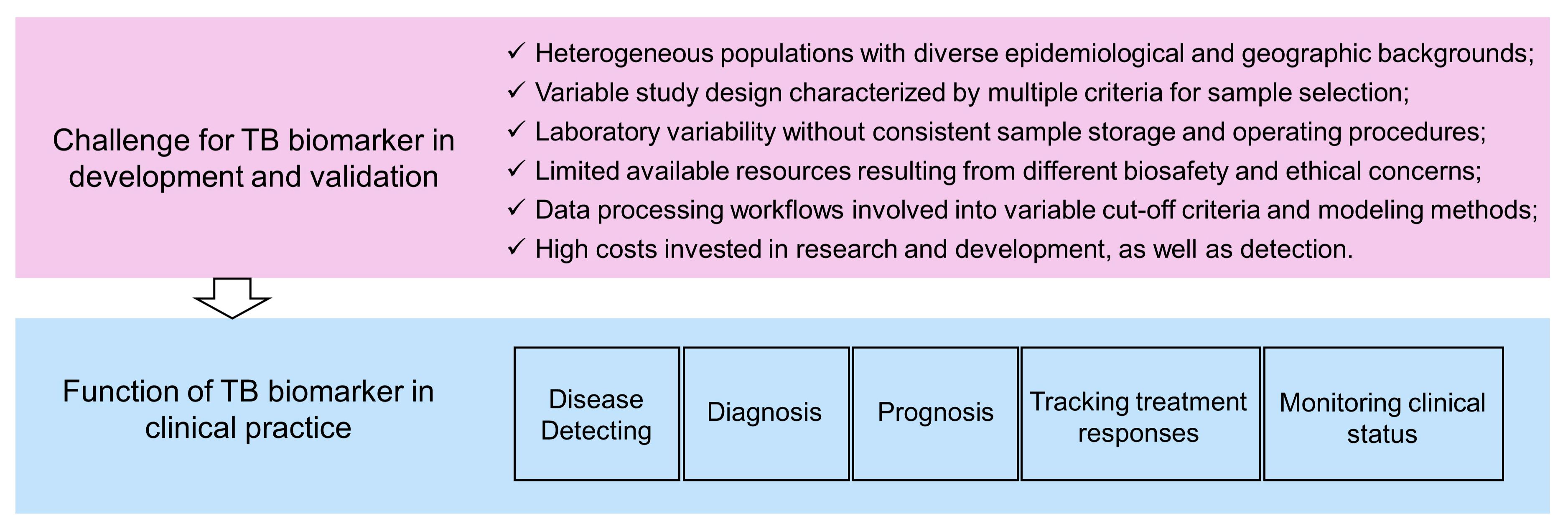
An ideal biomarker for TB detection should meet at least the following two requirements. First, it has excellent sensitivity and specificity, using either binary classification or quantified data. Second, it should be suitable for routine clinical tests with the properties of a short detection cycle, ease of operation, easily accessible samples, and low testing costs (131). Host blood diagnostic testing is undoubtedly a very convenient method, among which genetic testing is most common. Current molecular biomarkers applied in blood tests for TB patients face a general challenge: they have ideal sensitivity and specificity in internal validation cohorts (training cohort and testing cohort), but show diminished diagnostic efficacy in external validation cohorts (independent dataset). For this reason, Nogueira BMF, et al. have suggested that heterogeneous populations, variable study design, laboratory variability, limited available resource, and the different data processing workflow contribute to inconsistent results (Figure 4) (133. Particularly, they have proposed solutions for the corresponding limitations (132).
The journey of a biomarker from discovery to clinical application is complex process, involving multiple phases such as discovery, validation, and eventual integration into clinical practice (131). Advancements in technologies like single-cell next-generation sequencing (133), liquid biopsies for circulating targets (134), and other high-throughput methods (135–137) have significantly accelerated biomarker discovery by generating vast amounts of data quickly and cost-effectively. Challenges remain in the effective analysis and use of this data, particularly in harnessing electronic health records for biomarker-driven healthcare (131, 138). Additionally, when evaluating multiple biomarkers, it’s crucial to control for multiple comparisons, using metrics such as the false discovery rate, to enhance the reliability of the findings. This careful evaluation, which includes assessing associations with disease status and demographic or clinical features, informs the design of validation studies and ultimately aids in establishing robust biomarkers across different stages of disease management (131).
In clinical oncology and medicine, definitions for levels of evidence help assess biomarkers’ clinical utility, but challenges like bias and missing data often complicate validation efforts (139). Bias, particularly, arises from various sources during a study such as patient selection, specimen handling, and analysis, which can skew results away from the truth. Techniques like randomization and blinding are critical for mitigating bias; they ensure unbiased distribution and evaluation of specimens and data across study groups (140, 141). Additionally, it’s crucial to have a robust plan for addressing missing data in studies, which includes identifying why data are missing and implementing strategies to minimize the impact of such missingness on the study results (131). These measures are essential to preserve the integrity and reliability of biomarker research. Additionally, to enhance diagnostic accuracy, leveraging a panel of multiple biomarkers typically outperforms a single biomarker. Using variable selection methods like shrinkage helps minimize overfitting and improves validation likelihood (142). For complex models involving interactions or advanced techniques like machine learning, generating pilot data for simulations is crucial to guide sample size estimations and develop an effective analytical approach (143, 144).
Conclusion
In conclusion, the investigation into blood biomarkers for tuberculosis (TB) diagnosis reveals a promising avenue towards enhancing early detection and accurate monitoring of the disease. The review underscores the significance of both pathogen-derived and host-derived biomarkers in providing critical insights into TB’s pathophysiology and the host’s immune response. These biomarkers are critical for detecting TB at various stages of infection and in different patient populations, including those co-infected with HIV and those suffering from ETB. Pathogen-derived biomarkers offer direct indicators of TB presence, while host-derived markers are essential for understanding the immune dynamics and could guide treatment decisions. Additionally, the work advocates for a more integrated approach in clinical practice, combining these biomarkers with traditional diagnostic methods to develop a more comprehensive and accurate TB diagnostic strategy, offering insights into the host-pathogen interactions and disease pathology. Despite the potential, the review acknowledges challenges such as the need for comprehensive validation and standardization across diverse clinical environments to ensure the biomarkers’ efficacy and reliability. The integration of advanced analytical tools and multidisciplinary approaches is recommended to overcome these barriers, facilitating the transition from traditional methods to more precise and predictive diagnostics. This could significantly impact global TB management, reducing the disease burden by enabling timely and targeted therapeutic interventions.
Author contributions
ZL: Writing – original draft. YH: Writing – original draft. WW: Writing – review & editing. FZ: Writing – original draft. JY: Writing – original draft. WG: Writing – original draft. SF: Writing – original draft. GC: Writing – original draft. CS: Writing – review & editing. YC: Writing – review & editing. GD: Writing – review & editing. XC: Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was funded by the Shenzhen Medical Research Fund (Grant No. A2304001), National Key Research and Development Program (Grant No. 2022YFC2302901), Shenzhen Peacock Team (Grant No. KQTD20210811090219022), Shenzhen Basic Research (Key Project, Grant No. JCYJ20220818095610021), Shenzhen Major Technological Breakthrough Project (Grant No. JSGG20220822095200001), Provincial Natural Science Foundation of Guangdong (Grant No. 2022A1515220034), Shenzhen Science and Technology Innovation Foundation (Grant No. JCYJ20210324094614038/JCYJ20220530160207015).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. World Health Organization. Global Tuberculosis Report 2023. Geneva: World Health Organization (2023).
2. World Health Organization. Global Tuberculosis Report 2016. Geneva: World Health Organization (2023).
3. World Health Organization. Global Tuberculosis Report 2022. Geneva: World Health Organization (2022).
4. Cegielski JP, Chan PC, Lan Z, Udwadia ZF, Viiklepp P, Yim JJ, et al. Aminoglycosides and capreomycin in the treatment of multidrug-resistant tuberculosis: individual patient data meta-analysis of 12 030 patients from 25 countries, 2009–2016. Clin Infect Dis. (2021) 73:e3929–36. doi: 10.1093/cid/ciaa621
5. Chakaya J, Khan M, Ntoumi F, Aklillu E, Fatima R, Mwaba P, et al. Global Tuberculosis Report 2020 - Reflections on the Global TB burden, treatment and prevention efforts. Int J Infect Dis. (2021) 113 Suppl 1:S7–S12. doi: 10.1016/j.ijid.2021.02.107
6. World Health Organization. The End TB Strategy. Global strategy and targets for tuberculosis prevention, care and control after 2015. Geneva: World Health Organization (2015).
7. World Health Organization. High-priority target product profiles for new tuberculosis diagnostics: report of a consensus meeting. Geneva: World Health Organization (2014).
8. Sivakumaran D, Jenum S, Srivastava A, Steen VM, Vaz M, Doherty TM, et al. Host blood-based biosignatures for subclinical TB and incipient TB: A prospective study of adult TB household contacts in Southern India. Front Immunol. (2023) 13:1051963. doi: 10.3389/fimmu.2022.1051963
9. Migliori GB, Ong CWM, Petrone L, D’Ambrosio L, Centis R, Goletti D. The definition of tuberculosis infection based on the spectrum of tuberculosis disease. Breathe (Sheff). (2021) 17:210079. doi: 10.1183/20734735.0079-2021
10. Drain PK, Bajema KL, Dowdy D, Dheda K, Naidoo K, Schumacher SG, et al. Incipient and subclinical tuberculosis: a clinical review of early stages and progression of infection. Clin Microbiol Rev. (2018) 31:e00021–18. doi: 10.1128/CMR.00021-18
11. Zhuang L, Yang L, Li L, Ye Z, Gong W. Mycobacterium tuberculosis: immune response, biomarkers, and therapeutic intervention. MedComm. (2020) . 2024. 5:e419. doi: 10.1002/mco2.419
12. Migliori GB, Tiberi S. WHO drug-resistant TB guidelines 2022: what is new? Int J Tuberc Lung Dis. (2022) 26:590–1. doi: 10.5588/ijtld.22.0263
13. Shah NS, Moodley P, Babaria P, Moodley S, Ramtahal M, Richardson J, et al. Rapid diagnosis of tuberculosis and multidrug resistance by the microscopic-observation drug-susceptibility assay. Am J Respir Crit Care Med. (2011) 183:1427–33. doi: 10.1164/rccm.201009-1449OC
14. Adikaram CP, Perera J, Wijesundera SS. The manual mycobacteria growth indicator tube and the nitrate reductase assay for the rapid detection of rifampicin resistance of M. Tuberculosis low resource settings. BMC Infect Dis. (2012) 12:326. doi: 10.1186/1471-2334-12-326
15. Battaglioli T, Soto A, Agapito J, Acurio V, van der Stuyft P. Manual liquid culture on simple Middlebrook 7H9 or MGIT for the diagnosis of smear-negative pulmonary tuberculosis. Trop Med Int Health. (2014) 19:1500–3. doi: 10.1111/tmi.12384
16. Kim J, Lee KS, Kim EB, Paik S, Chang CL, Park TJ, et al. Early detection of the growth of Mycobacterium tuberculosis using magnetophoretic immunoassay in liquid culture. Biosens Bioelectron. (2017) 96:68–76. doi: 10.1016/j.bios.2017.04.025
17. World Health Organization. Systematic Screening for Active Tuberculosis Principles and Recommendations. Geneva, Switzerland: World Health Organization (2013).
18. Cattamanchi A, Dowdy DW, Davis JL, Worodria W, Yoo S, et al. Sensitivity of direct versus concentrated sputum smear microscopy in HIV-infected patients suspected of having pulmonary tuberculosis. BMC Infect Dis. (2009) 9:53. doi: 10.1186/1471-2334-9-53
19. Kunkel A, Abel Zur Wiesch P, Nathavitharana RR, Marx FM, Jenkins HE, Cohen T. Smear positivity in paediatric and adult tuberculosis: systematic review and meta-analysis. BMC Infect Dis. (2016) 16:282. doi: 10.1186/s12879-016-1617-9
20. World Health Organization. Use of Tuberculosis Interferon-Gamma Release Assays (IGRAs) in Low- and Middle Income Countries. Geneva, Switzerland: World Health Organization (2011).
21. Kik SV, Franken WP, Mensen M, Cobelens FG, Kamphorst M, Arend SM, et al. Predictive value for progression to tuberculosis by IGRA and TST in immigrant contacts. Eur Respir J. (2010) 35:1346–53. doi: 10.1183/09031936.00098509
22. Carranza C, Pedraza-Sanchez S, de Oyarzabal-Mendez E, Torres M. Diagnosis for latent tuberculosis infection: new alternatives. Front Immunol. (2020) 11:2006. doi: 10.3389/fimmu.2020.02006
23. Goletti D, Delogu G, Matteelli A, Migliori GB. The role of IGRA in the diagnosis of tuberculosis infection, differentiating from active tuberculosis, and decision making for initiating treatment or preventive therapy of tuberculosis infection. Int J Infect Dis. (2022) 124 Suppl 1:S12–9. doi: 10.1016/j.ijid.2022.02.047
24. Park M, Kon OM. Use of Xpert MTB/RIF and Xpert Ultra in extrapulmonary tuberculosis. Expert Rev Anti Infect Ther. (2021) 19:65–77. doi: 10.1080/14787210.2020.1810565
25. Acharya B, Acharya A, Gautam S, Ghimire SP, Mishra G, Parajuli N, et al. Advances in diagnosis of Tuberculosis: an update into molecular diagnosis of Mycobacterium tuberculosis. Mol Biol Rep. (2020) 47:4065–75. doi: 10.1007/s11033-020-05413-7
26. Sweeney TE, Braviak L, Tato CM, Khatri P. Genome-wide expression for diagnosis of pulmonary tuberculosis: a multicohort analysis. Lancet Respir Med. (2016) 4:213–24. doi: 10.1016/S2213-2600(16)00048-5
27. Gliddon HD, Kaforou M, Alikian M, Habgood-Coote D, Zhou C, Oni T, et al. Identification of reduced host transcriptomic signatures for tuberculosis disease and digital PCR-based validation and quantification. Front Immunol. (2021) 12:637164. doi: 10.3389/fimmu.2021.637164
28. Lee HJ, Kim NH, Lee EH, Yoon YS, Jeong YJ, Lee BC, et al. Multicenter testing of a simple molecular diagnostic system for the diagnosis of mycobacterium tuberculosis. Biosensors (Basel). (2023) 13:259. doi: 10.3390/bios13020259
29. He G, Chen CY, Zhang X, Ding PP, Hu CZ, Huang XF, et al. Clinical performance of quantitative PCR for the molecular identification of skeletal tuberculosis from formalin-fixed paraffin-embedded tissues. BMC Infect Dis. (2022) 22:651. doi: 10.1186/s12879-022-07641-7
30. Schijman AG, Losso MH, Montoto M, Saez CB, Smayevsky J, Benetucci JA. Prospective evaluation of in-house polymerase chain reaction for diagnosis of mycobacterial diseases in patients with HIV infection and lung infiltrates. Int J Tuberc Lung Dis. (2004) 8:106–13.
31. Kashyap RS, Nayak AR, Gaherwar HM, Bhullar SS, Husain AA, Shekhawat SD, et al. Laboratory investigations on the diagnosis of tuberculosis in the malnourished tribal population of melghat, India. PloS One. (2013) 8:e74652. doi: 10.1371/journal.pone.0074652
32. Osei Sekyere J, Maphalala N, Malinga LA, Mbelle NM, Maningi NE. A comparative evaluation of the new genexpert MTB/RIF ultra and other rapid diagnostic assays for detecting tuberculosis in pulmonary and extra pulmonary specimens. Sci Rep. (2019) 9:16587. doi: 10.1038/s41598-019-53086-5
33. Zar HJ, Workman LJ, Prins M, Bateman LJ, Mbhele SP, Whitman CB, et al. Tuberculosis diagnosis in children using xpert ultra on different respiratory specimens. Am J Respir Crit Care Med. (2019) 200:1531–8. doi: 10.1164/rccm.201904-0772OC
34. Barr DA, Schutz C, Balfour A, Shey M, Kamariza M, Bertozzi CR, et al. Serial measurement of M. tuberculosis in blood from critically-ill patients with HIV-associated tuberculosis. EBioMedicine. (2022) 78:103949. doi: 10.1016/j.ebiom.2022.103949
35. Hindson BJ, Ness KD, Masquelier DA, Belgrader P, Heredia NJ, Makarewicz AJ, et al. High-throughput droplet digital PCR system for absolute quantitation of DNA copy number. Anal Chem. (2011) 83:8604–10. doi: 10.1021/ac202028g
36. Lyu L, Li Z, Pan L, Jia H, Sun Q, Liu Q, et al. Evaluation of digital PCR assay in detection of M.tuberculosis IS6110 and IS1081 in tuberculosis patients plasma. BMC Infect Dis. (2020) 20:657. doi: 10.1186/s12879-020-05375-y
37. Yang J, Han X, Liu A, Bai X, Xu C, Bao F, et al. Use of digital droplet PCR to detect mycobacterium tuberculosis DNA in whole blood-derived DNA samples from patients with pulmonary and extrapulmonary tuberculosis. Front Cell Infect Microbiol. (2017) 7:369. doi: 10.3389/fcimb.2017.00369
38. Li Z, Wang B, Du B, Sun Q, Wang D, Wei R, et al. The incremental value of Mycobacterium tuberculosis trace nucleic acid detection in CT-guided percutaneous biopsy needle rinse solutions for the diagnosis of tuberculosis. Front Microbiol. (2024) 15:1335526. doi: 10.3389/fmicb.2024.1335526
39. Belay M, Tulu B, Younis S, Jolliffe DA, Tayachew D, Manwandu H, et al. Detection of Mycobacterium tuberculosis complex DNA in CD34-positive peripheral blood mononuclear cells of asymptomatic tuberculosis contacts: an observational study. Lancet Microbe. (2021) 2:e267–75. doi: 10.1016/S2666-5247(21)00043-4
40. Alonzi T, Repele F, Goletti D. Research tests for the diagnosis of tuberculosis infection. Expert Rev Mol Diagn. (2023) 23:783–95. doi: 10.1080/14737159.2023.2240230
41. Song N, Tan Y, Zhang L, Luo W, Guan Q, Yan MZ, et al. Detection of circulating Mycobacterium tuberculosis-specific DNA by droplet digital PCR for vaccine evaluation in challenged monkeys and TB diagnosis. Emerg Microbes Infect. (2018) 7:78. doi: 10.1038/s41426-018-0076-3
42. Huang Z, LaCourse SM, Kay AW, Stern J, Escudero JN, Youngquist BM, et al. CRISPR detection of circulating cell-free Mycobacterium tuberculosis DNA in adults and children, including children with HIV: a molecular diagnostics study. Lancet Microbe. (2022) 3:e482–92. doi: 10.1016/S2666-5247(22)00087-8
43. Thakku SG, Lirette J, Murugesan K, Chen J, Theron G, Banaei N, et al. Genome-wide tiled detection of circulating Mycobacterium tuberculosis cell-free DNA using Cas13. Nat Commun. (2023) 14:1803. doi: 10.1038/s41467-023-37183-8
44. Gootenberg JS, Abudayyeh OO, Lee JW, Essletzbichler P, Dy AJ, Joung J, et al. Nucleic acid detection with CRISPR-Cas13a/C2c2. Science. (2017) 356:438–42. doi: 10.1126/science.aam9321
45. Gootenberg JS, Abudayyeh OO, Kellner MJ, Joung J, Collins JJ, Zhang F. Multiplexed and portable nucleic acid detection platform with Cas13, Cas12a, and Csm6. Science. (2018) 360:439–44. doi: 10.1126/science.aaq0179
46. Mohapatra A, Gaikwad U, Ganga RT, Sharma P. Diagnostic accuracy of Lipoarabinomannan detection by lateral flow assay in pleural tuberculosis. BMC Infect Dis. (2024) 24:178. doi: 10.1186/s12879-024-09088-4
47. Huang H, Qu R, Wu K, Xu J, Li J, Lu S, et al. Proteinase K-pretreated ConA-based ELISA assay: a novel urine LAM detection strategy for TB diagnosis. Front Microbiol. (2023) 14:1236599. doi: 10.3389/fmicb.2023.1236599
48. Brock M, Hanlon D, Zhao M, Pollock NR. Detection of mycobacterial lipoarabinomannan in serum for diagnosis of active tuberculosis. Diagn Microbiol Infect Dis. (2020) 96:114937. doi: 10.1016/j.diagmicrobio.2019.114937
49. Yengo L, Vedantam S, Marouli E, Sidorenko J, Bartell E, Sakaue S, et al. A saturated map of common genetic variants associated with human height. Nature. (2022) 610:704–12. doi: 10.1038/s41586-022-05275-y
50. Poulakis N, Gritzapis AD, Ploussi M, Leventopoulos M, Papageorgiou CV, Anastasopoulos A, et al. Intracellular ESAT-6: A new biomarker for Mycobacterium tuberculosis infection. Cytometry B Clin Cytom. (2016) 90:312–4. doi: 10.1002/cyto.b.21220
51. Diouani MF, Ouerghi O, Refai A, Belgacem K, Tlili C, Laouini D, et al. Detection of ESAT-6 by a label free miniature immuno-electrochemical biosensor as a diagnostic tool for tuberculosis. Mater Sci Eng C Mater Biol Appl. (2017) 74:465–70. doi: 10.1016/j.msec.2016.12.051
52. Omar RA, Verma N, Arora PK. Development of ESAT-6 based immunosensor for the detection of mycobacterium tuberculosis. Front Immunol. (2021) 12:653853. doi: 10.3389/fimmu.2021.653853
53. Huang H, Chen Y, Zuo J, Deng C, Fan J, Bai L, et al. MXene-incorporated C60NPs and Au@Pt with dual-electric signal outputs for accurate detection of Mycobacterium tuberculosis ESAT-6 antigen. Biosens Bioelectron. (2023) 242:115734. doi: 10.1016/j.bios.2023.115734
54. Mehta PK, Dahiya B, Sharma S, Singh N, Dharra R, Thakur Z, et al. Immuno-PCR, a new technique for the serodiagnosis of tuberculosis. J Microbiol Methods. (2017) 139:218–29. doi: 10.1016/j.mimet.2017.05.009
55. Mehta PK, Raj A, Singh NP, Khuller GK. Detection of potential microbial antigens by immuno-PCR (PCR-amplified immunoassay). J Med Microbiol. (2014) 63:627–41. doi: 10.1099/jmm.0.070318-0
56. Dahiya B, Sharma S, Khan A, Kamra E, Mor P, Sheoran A, et al. Detection of mycobacterial CFP-10 (Rv3874) protein in tuberculosis patients by gold nanoparticle-based real-time immuno-PCR. Future Microbiol. (2020) 15:601–12. doi: 10.2217/fmb-2019-0347
57. Singh N, Sreenivas V, Sheoran A, Sharma S, Gupta KB, Khuller GK, et al. Serodiagnostic potential of immuno-PCR using a cocktail of mycobacterial antigen 85B, ESAT-6 and cord factor in tuberculosis patients. J Microbiol Methods. (2016) 120:56–64. doi: 10.1016/j.mimet.2015.11.016
58. Sharma S, Sheoran A, Gupta KB, Yadav A, Varma-Basil M, Sreenivas V, et al. Quantitative detection of a cocktail of mycobacterial MPT64 and PstS1 in tuberculosis patients by real-time immuno-PCR. Future Microbiol. (2019) 14:223–33. doi: 10.2217/fmb-2018-0284
59. Meier NR, Jacobsen M, Ottenhoff THM, Ritz N. A systematic review on novel mycobacterium tuberculosis antigens and their discriminatory potential for the diagnosis of latent and active tuberculosis. Front Immunol. (2018) 9:2476. doi: 10.3389/fimmu.2018.02476
60. Meier NR, Sutter TM, Jacobsen M, Ottenhoff THM, Vogt JE, Ritz N. Machine learning algorithms evaluate immune response to novel mycobacterium tuberculosis antigens for diagnosis of tuberculosis. Front Cell Infect Microbiol. (2021) 10:594030. doi: 10.3389/fcimb.2020.594030
61. Meier NR, Battegay M, Ottenhoff THM, Furrer H, Nemeth J, Ritz N. HIV-infected patients developing tuberculosis disease show early changes in the immune response to novel mycobacterium tuberculosis antigens. Front Immunol. (2021) 12:620622. doi: 10.3389/fimmu.2021.620622
62. Kaforou M, Wright VJ, Oni T, French N, Anderson ST, Bangani N, et al. Detection of tuberculosis in HIV-infected and -uninfected African adults using whole blood RNA expression signatures: a case-control study. PloS Med. (2013) 10:e1001538. doi: 10.1371/journal.pmed.1001538
63. Wu X, Tan G, Ma J, Yang J, Guo Y, Lu H, et al. Assessment of the Cepheid 3-gene Host Response Fingerstick Blood Test (MTB-HR) on rapid diagnosis of tuberculosis. Emerg Microbes Infect. (2023) 12:2261561. doi: 10.1080/22221751.2023.2261561
64. Maertzdorf J, McEwen G, Weiner J 3rd, Tian S, Lader E, Schriek U, et al. Concise gene signature for point-of-care classification of tuberculosis. EMBO Mol Med. (2016) 8:86–95. doi: 10.15252/emmm.201505790
65. Kaipilyawar V, Zhao Y, Wang X, Joseph NM, Knudsen S, Prakash Babu S, et al. Development and Validation of a Parsimonious Tuberculosis Gene Signature Using the digital NanoString nCounter Platform. Clin Infect Dis. (2022) 75:1022–30. doi: 10.1093/cid/ciac010
66. Bhatti GK, Khullar N, Sidhu IS, Navik US, Reddy AP, Reddy PH, et al. Emerging role of non-coding RNA in health and disease. Metab Brain Dis. (2021) 36:1119–34. doi: 10.1007/s11011-021-00739-y
67. Wu J, Lu C, Diao N, Zhang S, Wang S, Wang F, et al. Analysis of microRNA expression profiling identifies miR-155 and miR-155* as potential diagnostic markers for active tuberculosis: a preliminary study. Hum Immunol. (2012) 73:31–7. doi: 10.1016/j.humimm.2011.10.003
68. Wang J, Wu M, Wen J, Yang K, Li M, Zhan X, et al. MicroRNA-155 induction by Mycobacterium bovis BCG enhances ROS production through targeting SHIP1. Mol Immunol. (2014) 62:29–36. doi: 10.1016/j.molimm.2014.05.012
69. Rothchild AC, Sissons JR, Shafiani S, Plaisier C, Min D, Mai D, et al. MiR-155-regulated molecular network orchestrates cell fate in the innate and adaptive immune response to Mycobacterium tuberculosis. Proc Natl Acad Sci U S A. (2016) 113:E6172–81. doi: 10.1073/pnas.1608255113
70. Daniel EA, Sathiyamani B, Thiruvengadam K, Vivekanandan S, Vembuli H, Hanna LE. MicroRNAs as diagnostic biomarkers for Tuberculosis: A systematic review and meta- analysis. Front Immunol. (2022) 13:954396. doi: 10.3389/fimmu.2022.954396
71. Zhou M, Yu G, Yang X, Zhu C, Zhang Z, Zhan X. Circulating microRNAs as biomarkers for the early diagnosis of childhood tuberculosis infection. Mol Med Rep. (2016) 13:4620–6. doi: 10.3892/mmr.2016.5097
72. Shepelkova GS, Evstifeev VV, Tarasov RV, Ergeshova AE, Bagirov MA, Yeremeev VV. MicroRNAs as biomarkers of active pulmonary TB course. Microorganisms. (2023) 11:626. doi: 10.3390/microorganisms11030626
73. Gunasekaran H, Sampath P, Thiruvengadam K, Malaisamy M, Ramasamy R, Ranganathan UD, et al. A systematic review and meta-analysis of circulating serum and plasma microRNAs in TB diagnosis. BMC Infect Dis. (2024) 24:402. doi: 10.1186/s12879-024-09232-0
74. Liang Q, Jin W, Huang Z, Yin H, Liu S, Liu L, et al. A plasma 3-marker microRNA biosignature distinguishes spinal tuberculosis from other spinal destructive diseases and pulmonary tuberculosis. Front Cell Infect Microbiol. (2023) 13:1125946. doi: 10.3389/fcimb.2023.1125946
75. Huang Z, Su R, Qing C, Peng Y, Luo Q, Li J. Plasma Circular RNAs hsa_circ_0001953 and hsa_circ_0009024 as Diagnostic Biomarkers for Active Tuberculosis. Front Microbiol. (2018) 9:2010. doi: 10.3389/fmicb.2018.02010
76. Qian Z, Liu H, Li M, Shi J, Li N, Zhang Y, et al. Potential diagnostic power of blood circular RNA expression in active pulmonary tuberculosis. EBioMedicine. (2018) 27:18–26. doi: 10.1016/j.ebiom.2017.12.007
77. Fu Y, Wang J, Qiao J, Yi Z. Signature of circular RNAs in peripheral blood mononuclear cells from patients with active tuberculosis. J Cell Mol Med. (2019) 23:1917–25. doi: 10.1111/jcmm.14093
78. Liu H, Lu G, Wang W, Jiang X, Gu S, Wang J, et al. A panel of circRNAs in the serum serves as biomarkers for mycobacterium tuberculosis infection. Front Microbiol. (2020) 11:1215. doi: 10.3389/fmicb.2020.01215
79. Chen ZL, Wei LL, Shi LY, Li M, Jiang TT, Chen J, et al. Screening and identification of lncRNAs as potential biomarkers for pulmonary tuberculosis. Sci Rep. (2017) 7:16751. doi: 10.1038/s41598-017-17146-y
80. Wang L, Xie B, Zhang P, Ge Y, Wang Y, Zhang D. LOC152742 as a biomarker in the diagnosis of pulmonary tuberculosis infection. J Cell Biochem. (2019) 120:8949–55. doi: 10.1002/jcb.27452
81. Huang S, Kang X, Zeng Z, Zhang Q, Huang Z, Luo K, et al. Neutrophil lncRNA ZNF100–6:2 is a potential diagnostic marker for active pulmonary tuberculosis. Eur J Med Res. (2024) 29:162. doi: 10.1186/s40001-024-01755-1
82. Li ZB, Han YS, Wei LL, Shi LY, Yi WJ, Chen J, et al. Screening and identification of plasma lncRNAs uc.48+ and NR_105053 as potential novel biomarkers for cured pulmonary tuberculosis. Int J Infect Dis. (2020) 92:141–50. doi: 10.1016/j.ijid.2020.01.005
83. Kovács T, Mikó E, Ujlaki G, Sári Z, Bai P. The microbiome as a component of the tumor microenvironment. Adv Exp Med Biol. (2020) 1225:137–53. doi: 10.1007/978–3-030–35727-6_10
84. Chen J, Wu L, Lv Y, Liu T, Guo W, Song J, et al. Screening of long non-coding RNAs biomarkers for the diagnosis of tuberculosis and preliminary construction of a clinical diagnosis model. Front Microbiol. (2022) 13:774663. doi: 10.3389/fmicb.2022.774663
85. Meng Z, Wang M, Guo S, Zhou Y, Lyu M, Hu X, et al. Novel long non-coding RNA and LASSO prediction model to better identify pulmonary tuberculosis: A case-control study in China. Front Mol Biosci. (2021) 8:632185. doi: 10.3389/fmolb.2021.632185
86. Taneja I, Reddy B, Damhorst G, Dave Zhao S, Hassan U, Price Z, et al. Combining biomarkers with EMR data to identify patients in different phases of sepsis. Sci Rep. (2017) 7:10800. doi: 10.1038/s41598-017-09766-1
87. McIntyre S, Warner J, Rush C, Vanderven HA. Antibodies as clinical tools for tuberculosis. Front Immunol. (2023) 14:1278947. doi: 10.3389/fimmu.2023.1278947
88. Harries AD, Kumar AMV. Challenges and progress with diagnosing pulmonary tuberculosis in low- and middle-income countries. Diagnostics (Basel). (2018) 8:78. doi: 10.3390/diagnostics8040078
89. Takenami I, de Oliveira CC, Lima FR, Soares J, MaChado A Jr, Riley LW, et al. Immunoglobulin G response to mammalian cell entry 1A (Mce1A) protein as biomarker of active tuberculosis. Tuberculosis (Edinb). (2016) 100:82–8. doi: 10.1016/j.tube.2016.07.012
90. Abraham PR, Devalraju KP, Jha V, Valluri VL, Mukhopadhyay S. PPE17 (Rv1168c) protein of Mycobacterium tuberculosis detects individuals with latent TB infection. PloS One. (2018) 13:e0207787. doi: 10.1371/journal.pone.0207787
91. Karbalaei Zadeh Babaki M, Soleimanpour S, Rezaee SA. Antigen 85 complex as a powerful Mycobacterium tuberculosis immunogene: Biology, immune-pathogenicity, applications in diagnosis, and vaccine design. Microb Pathog. (2017) 112:20–9. doi: 10.1016/j.micpath.2017.08.040
92. Khaliq A, Ravindran R, Hussainy SF, Krishnan VV, Ambreen A, Yusuf NW, et al. Field evaluation of a blood based test for active tuberculosis in endemic settings. PloS One. (2017) 12:e0173359. doi: 10.1371/journal.pone.0173359
93. Shete PB, Ravindran R, Chang E, Worodria W, Chaisson LH, Andama A, et al. Evaluation of antibody responses to panels of M. tuberculosis antigens as a screening tool for active tuberculosis in Uganda. PloS One. (2017) 12:e0180122. doi: 10.1371/journal.pone.0180122
94. Awoniyi DO, Baumann R, Chegou NN, Kriel B, Jacobs R, Kidd M, et al. Detection of a combination of serum IgG and IgA antibodies against selected mycobacterial targets provides promising diagnostic signatures for active TB. Oncotarget. (2017) 8:37525–37. doi: 10.18632/oncotarget.v8i23
95. Jacobs R, Awoniyi DO, Baumann R, Stanley K, McAnda S, Kaempfer S, et al. Concurrent evaluation of cytokines improves the accuracy of antibodies against Mycobacterium tuberculosis antigens in the diagnosis of active tuberculosis. Tuberculosis (Edinb). (2022) 133:102169. doi: 10.1016/j.tube.2022.102169
96. Melkie ST, Arias L, Farroni C, Jankovic Makek M, Goletti D, Vilaplana C. The role of antibodies in tuberculosis diagnosis, prophylaxis and therapy: a review from the ESGMYC study group. Eur Respir Rev. (2022) 31:210218. doi: 10.1183/16000617.0218-2021
97. Kim SH, Jo KW, Shim TS. QuantiFERON-TB Gold PLUS versus QuantiFERON- TB Gold In-Tube test for diagnosing tuberculosis infection. Korean J Intern Med. (2020) 35:383–91. doi: 10.3904/kjim.2019.002
98. Andersen P, Munk ME, Pollock JM, Doherty TM. Specific immune-based diagnosis of tuberculosis. Lancet. (2000) 356:1099–104. doi: 10.1016/S0140-6736(00)02742-2
99. Pai M, Denkinger CM, Kik SV, Rangaka MX, Zwerling A, Oxlade O, et al. Gamma interferon release assays for detection of Mycobacterium tuberculosis infection. Clin Microbiol Rev. (2014) 27:3–20. doi: 10.1128/CMR.00034-13
100. Kobashi Y. Current status and future landscape of diagnosing tuberculosis infection. Respir Investig. (2023) 61:563–78. doi: 10.1016/j.resinv.2023.04.010
101. Zhang H, Li L, Liu Y, Xiao W, Xu R, Lu M, et al. Serum cytokine biosignatures for identification of tuberculosis among HIV-positive inpatients. Thorax. (2024) 79:465–71. doi: 10.1136/thorax-2023-220782
102. Sotgiu G, Saderi L, Petruccioli E, Aliberti S, Piana A, Petrone L, et al. QuantiFERON TB Gold Plus for the diagnosis of tuberculosis: a systematic review and meta-analysis. J Infect. (2019) 79:444–53. doi: 10.1016/j.jinf.2019.08.018
103. Chien JY, Chiang HT, Lu MC, Ko WC, Yu CJ, Chen YH, et al. QuantiFERON-TB Gold Plus Is a More Sensitive Screening Tool than QuantiFERON-TB Gold In-Tube for Latent Tuberculosis Infection among Older Adults in Long-Term Care Facilities. J Clin Microbiol. (2018) 56:e00427–18. doi: 10.1128/JCM.00427-18
104. Petruccioli E, Vanini V, Chiacchio T, Cuzzi G, Cirillo DM, Palmieri F, et al. Analytical evaluation of QuantiFERON- Plus and QuantiFERON- Gold In-tube assays in subjects with or without tuberculosis. Tuberculosis (Edinb). (2017) 106:38–43. doi: 10.1016/j.tube.2017.06.002
105. Petruccioli E, Chiacchio T, Pepponi I, Vanini V, Urso R, Cuzzi G, et al. Characterization of the CD4 and CD8 T-cell response in the QuantiFERON-TB Gold Plus kit. Int J Mycobacteriol. (2016) 5 Suppl 1:S25–6. doi: 10.1016/j.ijmyco.2016.09.063
106. Pourakbari B, Mamishi S, Benvari S, Mahmoudi S. Comparison of the QuantiFERON-TB Gold Plus and QuantiFERON-TB Gold In-Tube interferon-γ release assays: A systematic review and meta-analysis. Adv Med Sci. (2019) 64:437–43. doi: 10.1016/j.advms.2019.09.001
107. Stout JE, Wu Y, Ho CS, Pettit AC, Feng PJ, Katz DJ, et al. Evaluating latent tuberculosis infection diagnostics using latent class analysis. Thorax. (2018) 73:1062–70. doi: 10.1136/thoraxjnl-2018-211715
108. Luo Y, Xue Y, Mao L, Lin Q, Tang G, Song H, et al. Diagnostic value of T-SPOT.TB assay for tuberculous peritonitis: A meta-analysis. Front Med (Lausanne). (2020) 7:585180. doi: 10.3389/fmed.2020.585180
109. Luo Y, Xue Y, Guo X, Lin Q, Mao L, Tang G, et al. Diagnostic accuracy of T-SPOT.TB assay for tuberculous meningitis: an updated meta-analysis. Front Neurol. (2020) 11:866. doi: 10.3389/fneur.2020.00866
110. Jiang B, Ding H, Zhou L, Chen X, Chen S, Bao C. Evaluation of interferon-gamma release assay (T-SPOT.TB(™)) for diagnosis of tuberculosis infection in rheumatic disease patients. Int J Rheum Dis. (2016) 19:38–42. doi: 10.1111/1756-185X.12772
111. Li Q, Ren W, Yuan J, Guo H, Shang Y, Wang W, et al. Significant difference in Th1/Th2 paradigm induced by tuberculosis-specific antigens between IGRA-positive and IGRA-negative patients. Front Immunol. (2022) 13:904308. doi: 10.3389/fimmu.2022.904308
112. Kobashi Y, Shimizu H, Ohue Y, Mouri K, Obase Y, Miyashita N, et al. Comparison of T-cell interferon-gamma release assays for Mycobacterium tuberculosis-specific antigens in patients with active and latent tuberculosis. Lung. (2010) 188:283–7. doi: 10.1007/s00408-010-9238-3
113. Uzorka JW, Bossink AWJ, Franken WPJ, Thijsen SFT, Leyten EMS, van Haeften AC, et al. Borderline QuantiFERON results and the distinction between specific responses and test variability. Tuberculosis (Edinb). (2018) 111:102–8. doi: 10.1016/j.tube.2018.06.002
114. Saluzzo F, Mantegani P, Poletti de Chaurand V, Cirillo DM. QIAreach QuantiFERON-TB for the diagnosis of Mycobacterium tuberculosis infection. Eur Respir J. (2022) 59:2102563. doi: 10.1183/13993003.02563-2021
115. Kumar NP, Hissar S, Thiruvengadam K, Banurekha VV, Suresh N, Shankar J, et al. Discovery and validation of a three-cytokine plasma signature as a biomarker for diagnosis of pediatric tuberculosis. Front Immunol. (2021) 12:653898. doi: 10.3389/fimmu.2021.653898
116. Hur YG, Kang YA, Jang SH, Hong JY, Kim A, Lee SA, et al. Adjunctive biomarkers for improving diagnosis of tuberculosis and monitoring therapeutic effects. J Infect. (2015) 70:346–55. doi: 10.1016/j.jinf.2014.10.019
117. Won EJ, Choi JH, Cho YN, Jin HM, Kee HJ, Park YW, et al. Biomarkers for discrimination between latent tuberculosis infection and active tuberculosis disease. J Infect. (2017) 74:281–93. doi: 10.1016/j.jinf.2016.11.010
118. Maenetje P, Baik Y, Schramm DB, Vangu MDW, Wallis RS, Mlotshwa M, et al. Circulating biomarkers, fraction of exhaled nitric oxide, and lung function in patients with human immunodeficiency virus and tuberculosis. J Infect Dis. (2024) 229:824–32. doi: 10.1093/infdis/jiad232
119. La Manna MP, Orlando V, Li Donni P, Sireci G, Di Carlo P, Cascio A, et al. Identification of plasma biomarkers for discrimination between tuberculosis infection/disease and pulmonary non tuberculosis disease. PloS One. (2018) 13:e0192664. doi: 10.1371/journal.pone.0192664
120. Uzorka JW, Bakker JA, van Meijgaarden KE, Leyten EMS, Delfos NM, Hetem DJ, et al. Biomarkers to identify Mycobacterium tuberculosis infection among borderline QuantiFERON results. Eur Respir J. (2022) 60:2102665. doi: 10.1183/13993003.02665-2021
121. Kim JK, Park EJ, Jo EK. Itaconate, arginine, and gamma-aminobutyric acid: A host metabolite triad protective against mycobacterial infection. Front Immunol. (2022) 13:832015. doi: 10.3389/fimmu.2022.832015
122. Wang C, Lou C, Yang Z, Shi J, Niu N. Plasma metabolomic analysis reveals the metabolic characteristics and potential diagnostic biomarkers of spinal tuberculosis. Heliyon. (2024) 10:e27940. doi: 10.1016/j.heliyon.2024.e27940
123. Li YX, Zheng KD, Duan Y, Liu HJ, Tang YQ, Wu J, et al. Mass spectrometry-based identification of new serum biomarkers in patients with latent infection pulmonary tuberculosis. Med (Baltimore). (2022) 101:e32153. doi: 10.1097/MD.0000000000032153
124. Zhang Z, Zhu Y, Wang Q, Chang T, Liu C, Zhu Y, et al. Global trends and research hotspots of exercise for intervening diabetes: A bibliometric analysis. Front Public Health. (2022) 10:902825. doi: 10.3389/fpubh.2022.902825
125. Levinson T, Wasserman A. C-reactive protein velocity (CRPv) as a new biomarker for the early detection of acute infection/inflammation. Int J Mol Sci. (2022) 23:8100. doi: 10.3390/ijms23158100
126. Ferreira LB, Williams KA, Best G, Haydinger CD, Smith JR. Inflammatory cytokines as mediators of retinal endothelial barrier dysfunction in non-infectious uveitis. Clin Transl Immunol. (2023) 12:e1479. doi: 10.1002/cti2.1479
127. Harrington C, Krishnan S, Mack CL, Cravedi P, Assis DN, Levitsky J. Noninvasive biomarkers for the diagnosis and management of autoimmune hepatitis. Hepatology. (2022) 76:1862–79. doi: 10.1002/hep.32591
128. Grunig G, Baghdassarian A, Park SH, Pylawka S, Bleck B, Reibman J, et al. Painful and non-painful diabetic neuropathy, diagnostic challenges and implications for future management. Brain. (2021) 144:1632–45. doi: 10.1093/brain/awab079
129. Blauenfeldt T, Villar-Hernández R, García-García E, Latorre I, Holm LL, Muriel-Moreno B, et al. Diagnostic accuracy of interferon gamma-induced protein 10 mRNA release assay for tuberculosis. J Clin Microbiol. (2020) 58:e00848–20. doi: 10.1128/JCM.00848-20
130. Mpande CAM, Steigler P, Lloyd T, Rozot V, Mosito B, Schreuder C, et al. Mycobacterium tuberculosis-specific T cell functional, memory, and activation profiles in quantiFERON-reverters are consistent with controlled infection. Front Immunol. (2021) 12:712480. doi: 10.3389/fimmu.2021.712480
131. Ou FS, Michiels S, Shyr Y, Adjei AA, Oberg AL. Biomarker discovery and validation: statistical considerations. J Thorac Oncol. (2021) 16:537–45. doi: 10.1016/j.jtho.2021.01.1616
132. Nogueira BMF, Krishnan S, Barreto-Duarte B, Araújo-Pereira M, Queiroz ATL, Ellner JJ, et al. Diagnostic biomarkers for active tuberculosis: progress and challenges. EMBO Mol Med. (2022) 14:e14088. doi: 10.15252/emmm.202114088
133. Kojima M, Harada T, Fukazawa T, Kurihara S, Touge R, Saeki I, et al. Single-cell next-generation sequencing of circulating tumor cells in patients with neuroblastoma. Cancer Sci. (2023) 114:1616–24. doi: 10.1111/cas.15707
134. Ren F, Fei Q, Qiu K, Zhang Y, Zhang H, Sun L. Liquid biopsy techniques and lung cancer: diagnosis, monitoring and evaluation. J Exp Clin Cancer Res. (2024) 43:96. doi: 10.1186/s13046-024-03026-7
135. Morris JA, Sun JS, Sanjana NE. Next-generation forward genetic screens: uniting high-throughput perturbations with single-cell analysis. Trends Genet. (2024) 40:118–33. doi: 10.1016/j.tig.2023.10.012
136. Liu X, Fang Y, Chen X, Shi W, Wang X, He Z, et al. Cascaded nanozyme-based high-throughput microfluidic device integrating with glucometer and smartphone for point-of-care pheochromocytoma diagnosis. Biosens Bioelectron. (2024) 251:116105. doi: 10.1016/j.bios.2024.116105
137. Gu W, Miller S, Chiu CY. Clinical metagenomic next-generation sequencing for pathogen detection. Annu Rev Pathol. (2019) 14:319–38. doi: 10.1146/annurev-pathmechdis-012418-012751
138. Tsang G, Xie X, Zhou SM. Harnessing the power of machine learning in dementia informatics research: issues, opportunities, and challenges. IEEE Rev BioMed Eng. (2020) 13:113–29. doi: 10.1109/RBME.4664312
139. Bruni L, Serrano B, Roura E, Alemany L, Cowan M, Herrero R, et al. Cervical cancer screening programmes and age-specific coverage estimates for 202 countries and territories worldwide: a review and synthetic analysis. Lancet Glob Health. (2022) 10:e1115–27. doi: 10.1016/S2214-109X(22)00241-8
140. Nguyen T, Mitrea C, Tagett R, Draghici S. DANUBE: Data-driven meta-ANalysis using UnBiased Empirical distributions-applied to biological pathway analysis. Proc IEEE Inst Electr Electron Eng. (2017) 105:496–515. doi: 10.1109/JPROC.2015.2507119
141. Paraš S, Janković O, Trišić D, Čolović B, Mitrović-Ajtić O, Dekić R, et al. Influence of nanostructured calcium aluminate and calcium silicate on the liver: histological and unbiased stereological analysis. Int Endod J. (2019) 52:1162–72. doi: 10.1111/iej.13105
142. Kipruto E, Sauerbrei W. Comparison of variable selection procedures and investigation of the role of shrinkage in linear regression-protocol of a simulation study in low-dimensional data. PloS One. (2022) 17:e0271240. doi: 10.1371/journal.pone
143. Maniruzzaman M, Islam MM, Rahman MJ, Hasan MAM, Shin J. Risk prediction of diabetic nephropathy using machine learning techniques: A pilot study with secondary data. Diabetes Metab Syndr. (2021) 15:102263. doi: 10.1016/j.dsx.2021.102263
Keywords: tuberculosis, mycobacterium tuberculosis, biomarker, blood test, molecular diagnosis
Citation: Li Z, Hu Y, Wang W, Zou F, Yang J, Gao W, Feng S, Chen G, Shi C, Cai Y, Deng G and Chen X (2024) Integrating pathogen- and host-derived blood biomarkers for enhanced tuberculosis diagnosis: a comprehensive review. Front. Immunol. 15:1438989. doi: 10.3389/fimmu.2024.1438989
Received: 27 May 2024; Accepted: 24 July 2024;
Published: 09 August 2024.
Edited by:
Ana Maria Gamero, Temple University, United StatesReviewed by:
Marta Alonso-Hearn, Basque Research and Technology Alliance (BRTA), SpainTaru S. Dutt, Colorado State University, United States
Copyright © 2024 Li, Hu, Wang, Zou, Yang, Gao, Feng, Chen, Shi, Cai, Deng and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xinchun Chen, Y2hlbnhpbmNodW5Ac3p1LmVkdS5jbg==; Guofang Deng, anh4azEwMzVAeWVhaC5uZXQ=
 Zhaodong Li
Zhaodong Li Yunlong Hu
Yunlong Hu Wenfei Wang
Wenfei Wang Fa Zou1
Fa Zou1 Jing Yang
Jing Yang Yi Cai
Yi Cai Guofang Deng
Guofang Deng Xinchun Chen
Xinchun Chen