- 1Department of Anesthesiology, State Key Laboratory of Oncology in South China, Guangdong Provincial Clinical Research Center for Cancer, Sun Yat-sen University Cancer Center, Guangzhou, Guangdong, China
- 2Department of Anatomy and Neurobiology, Zhongshan School of Medicine, Sun Yat-sen University, Guangzhou, Guangdong, China
Background: Opioid anesthesia can modulate the impaired immune response and opioid-sparing anesthesia may preserve immune functions. This study was performed to assess the effects of opioid-free anesthesia (OFA) and opioid-based anesthesia (OA) on perioperative macrophages differentiation, cytokine changes, and perioperative complications in locally advanced GC (LAGC) patients.
Methods: We used quality of recovery-15 (QoR-15) questionnaire scores and visual analog scale (VAS) scores to compare postoperative quality of recovery and pain level. In addition, the adverse reactions of patients in the two groups were compared. The perioperative serum level of inflammatory cytokines and the ratio of macrophage subtypes were detected.
Results: The OFA group had significantly longer extubation time and PACU stay, whereas the OA group had significantly higher rate of hypotension, higher doses of norepinephrine, higher PONV and dizziness rate, and delayed flatus passage time. The QoR-15 score on postoperative 24 h was significantly higher in OFA group than in OA group. At the end of or after the surgery, the OFA group had higher levels of interleukin (IL)-12, IL-1β, tumor necrosis factor (TNF)-α, CD68+CD163− macrophage rate, but lower levels of IL-10, transforming growth factor (TGF)-β, and CD68+CD163+ macrophage rate, indicating OFA attenuated perioperative immunosuppression by diminishing M2 and promoting M1 macrophage polarization. And the reversal tendency is more obvious in LAGC patients with neoadjuvant PD-1 inhibitor.
Conclusions: The OFA may attenuate perioperative immunosuppression by diminishing M2 and promoting M1 macrophage polarization in LAGC patients with neoadjuvant PD-1 inhibitor.
Clinical trial registration: http://gcpgl.sysucc.org.cn, identifier 2022-FXY-001.
Introduction
Gastric cancer (GC) ranks as the fifth most prevalent malignancy and the fourth most lethal neoplasm globally, with scarce therapeutic alternatives and dismal patient prognosis. It is noteworthy that locally advanced GC (LAGC) constitutes the bulk of newly detected gastric malignancies annually (1, 2). Currently, the principal therapeutic options for LAGC comprise surgical resection, chemotherapy, and immunotherapy (3–5). Immune checkpoint inhibitors (ICIs) targeting programmed cell death 1 (PD-1) can increase pathological response rate (pCR) (6–8) and improve overall survival (OS) in GC (9–11). Efficacy of PD-1 inhibitor can be influenced by tumor microenvironment (TME), while immunosuppressive TME correlates with poor prognosis and resistants to chemotherapy (12–14) and PD-1 inhibitor (15, 16). Evidence shows anesthesia may induce immunosuppression in patients during the perioperative period, thus adjusting anesthetic schemes to attenuate perioperative immunosuppression may be beneficial to GC patients.
Opioids are the most potent analgesics in anesthetic regimens to induce perioperative analgesia, sedation and suppression of sympathetic nervous system (17). The adverse effects related to opioid administration encompass excessive analgesia (18), persistent postoperative pain (18), respiratory depression (19), postoperative nausea and vomiting (PONV) (20) and postoperative delirium (21). More importantly, considerable evidences showed that endogenous and exogenous opioids regulate immune function by changing biochemical pathways and proliferative characteristics of cell components of immune system (22, 23). Previous research have indicated that opioid-sparing anesthesia may preserve immune functions in esophageal cancer (24) and breast cancer (25).
Macrophages infiltrate the TME majority in neoplasms and exert a crucial role in tumor immunity. During oncogenesis, the tumor associated monocytes/macrophages (TAMMs) in circulating blood are chemoattracted into tumor focus and differentiate into tumor associated macrophages (TAMs) (26, 27). Macrophages exhibit plasticity under various kinds of stimuli and can be polarized into distinct functional phenotypes, classical activation (M1, marked as CD68+CD163−) or alternative activation (M2, marked as CD68+CD163+) (28–30). M1 phenotype can eliminate invading microbes and cancer cells by producing pro-inflammatory cytokines, such as interleukin (IL)-12, IL-1β, and tumor necrosis factor-α (TNF-α) (28–30). On the other hand, M2 phenotype can foster immunosuppression and immune escape in neoplasms progression by secreting anti-inflammatory cytokines, such as IL-10 and transforming growth factor-β (TGF-β) (28–30). In GC, the presence and density of M2 macrophages correlate with poor prognosis and resistant to chemotherapy (12–14, 31) and PD-1 inhibitor (15, 16).
Nevertheless, scant studies have elucidated the influence of opioids on the TME of GC patients, especially in LAGC patients with neoadjuvant PD-1 inhibitor. Hence, this research aimed to compare the opioid-free anesthesia (OFA) and opioid-based anesthesia (OA) on perioperative macrophages differentiation, cytokine alterations, and perioperative complications of LAGC patients.
Methods
Patients selection and exclusion
The single-center, prospective and randomized trial was approved by the Ethics Committee of Sun Yat-Sen University Cancer Center (SYSUCC) (SL-B2022-299-02), and registered in the SYSUCC Clinical Trial Register (http://gcpgl.sysucc.org.cn, identifier: 2022-FXY-001).After obtaining the written consent from GC participants, we enrolled LAGC patients aged 18–60 years with American Society of Anesthesiologists (ASA) I-III who had laparoscopic radical resection of LAGC after neoadjuvant therapy in SYSUCC from July 2022 to December 2023. We excluded patients who were: (1) pregnant, (2) allergic to any experimental drug or its ingredients, (3) suffering from central nervous system disorders (such as epilepsy, cerebral infarction, or cerebral hemorrhage history), (4) having a history of chronic pain, alcohol, or drug abuse, (5) converted to open surgery, (6) transfused perioperatively, or (7) having severe cardiac, pulmonary, hepatic, renal, or endocrine diseases. The eligible patient gave their written informed consent after agreeing to participate.
Sample size calculation and masking method
We measured the inflammatory response, the main outcome of the study, by the level of IL-10. Previous research on IL-10 showed that its in vivo standard deviation (SD) is around 3.8 pg/ml. We calculated that we needed twenty patients to detect a decrease of 3.4 pg/ml deviation with an α-value of 0.05 and a power of 0.8. We enrolled twenty-five patients to account for potential dropouts. Moreover, we divided LAGC patients based on the type of neoadjuvant therapy (chemotherapy or PD-1 inhibitor plus chemotherapy), and we doubled the sample size accordingly.
At the beginning of the study, patients were randomly assigned to two groups (group OA and group OFA) using computer-generated codes. The doctors who performed postoperative evaluation and biological testing did not know the group assignments until the end of the follow-up and the final analysis.
Anesthetic management
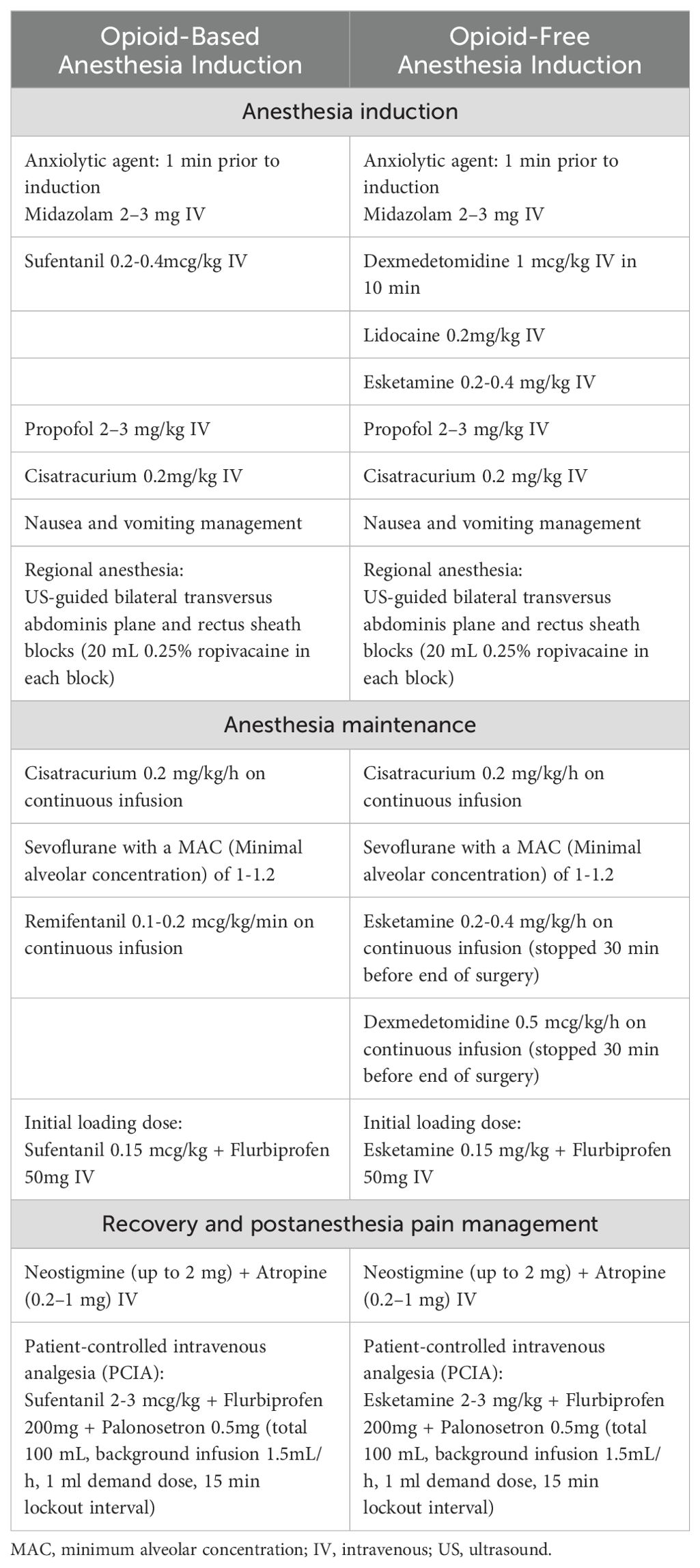
All patients routinely fasted for 8 h and abstained from drinking for 2 h before surgery. After entering the operating room, electrocardiography (ECG), pulse oximetry, invasive blood pressure, and temperature measurements were routinely monitored. The IV bolus medications ready to use were: Atropine 0.1 mg/mL and Norepinephrine 40 mcg/mL. Pre-oxygenation was administered for 2 min before anesthesia induction. The anesthetic management administration regimen differed between the groups (Table 1). Anesthetic depth was monitored using the bispectral index sensor (BIS), and the sevoflurane concentration and anesthetic drug doses (remifentanil or esketamine) were adjusted to keep BIS values between 45 and 60 during the anesthesia maintenance. Hypotension was defined as a decrease in mean blood pressure [MBP] > 30% of baseline or MBP < 65 mmHg, and Hypertension was defined as an increase in MBP > 30% of baseline or MBP > 90 mmHg. Norepinephrine was administrated as a continuous infusion at the discretion of the attending physician to keep MBP within the target blood pressure (within ±20% of the baseline MAP). Bradycardia (defined as HR < 50 beats/min) will be treated with intravenous atropine 0.3–0.5 mg. Tachycardia (defined as HR > 100 beats/min) will be treated with intravenous esmolol 20 mg.
Cytokine and macrophages surface markers quantification by enzyme-linked immunosorbent assay and flow cytometry
We collected 10 ml of whole blood from each patient in the OFA and OA group at four time points: before anesthesia, at the end of the surgery, and on postoperative 24 hours and postoperative 48 hours.
We transferred 4 ml of whole blood into separate tubes with blood clot activating gel for serum extraction (cytokine assay). We measured the serum level of IL-10, IL-12, IL-1β, TGF-β, and TNF-α with ELISA kits (Abcam) following the manufacturer’s protocol.
We collected 6 ml of whole blood into heparin tubes for peripheral blood mononuclear cells (PBMCs) isolation using Ficoll-Paque (GE Health-care) density gradient centrifugation as standard procedure. Then we labeled PBMCs samples with FITC-conjugated anti-human CD68 (BD Biosciences) and PE-conjugated anti-human CD163 (BD Biosciences). We washed labeled cells three times in flow cytometry buffer before flow cytometric analysis on flow cytometer (Beckman–Coulter) according to the manufacturer’s instructions.
Quality of recovery-15 questionnaire scores, visual analog scale scores, and postoperative complications
The QoR-15 is a survey consisting of 15 questions used to measure a patient’s QoR, including pain, nausea, sleep and well being. Each question is rated on a Likert scale from 0 to 10, with a maximum score of 150, indicating ideal health status (32)(Supplementary Figure S1). A anesthesiologist who was unaware of the group allocation evaluated QoR-15 questionnaire scores before surgery, on postoperative hours 24 and 48, respectively. And we also record postoperative pain intensity by the visual analogue scale (VAS) at 6, 24, and 48 hours after surgery. Other information was recorded from the electronic medical record including gender, age, body mass index (BMI), ASA grade, tumor and node stage, perioperative white blood cell (WBC), neutrophil, and hemoglobin, vasoactive drugs dose, hypotension, hypertension, anesthesia and surgery duration, fluid infusion, blood loss, urinary volume, extubation time, duration in PACU, time of the first flatus passage, remove drainage tube time, and some postoperative complications (nausea and vomiting, dizziness, infection).
Statistical analysis
We performed all statistical analyses with SPSS 26.0. We reported continuous data as mean (standard deviation, SD) or median (interquartile range, IQR) based on the normality of distribution. We used the chi-squared test to compare categorical variables and presented them as number (percentage). We considered P<0.05 as statistically significant.
Results
Patient characteristics
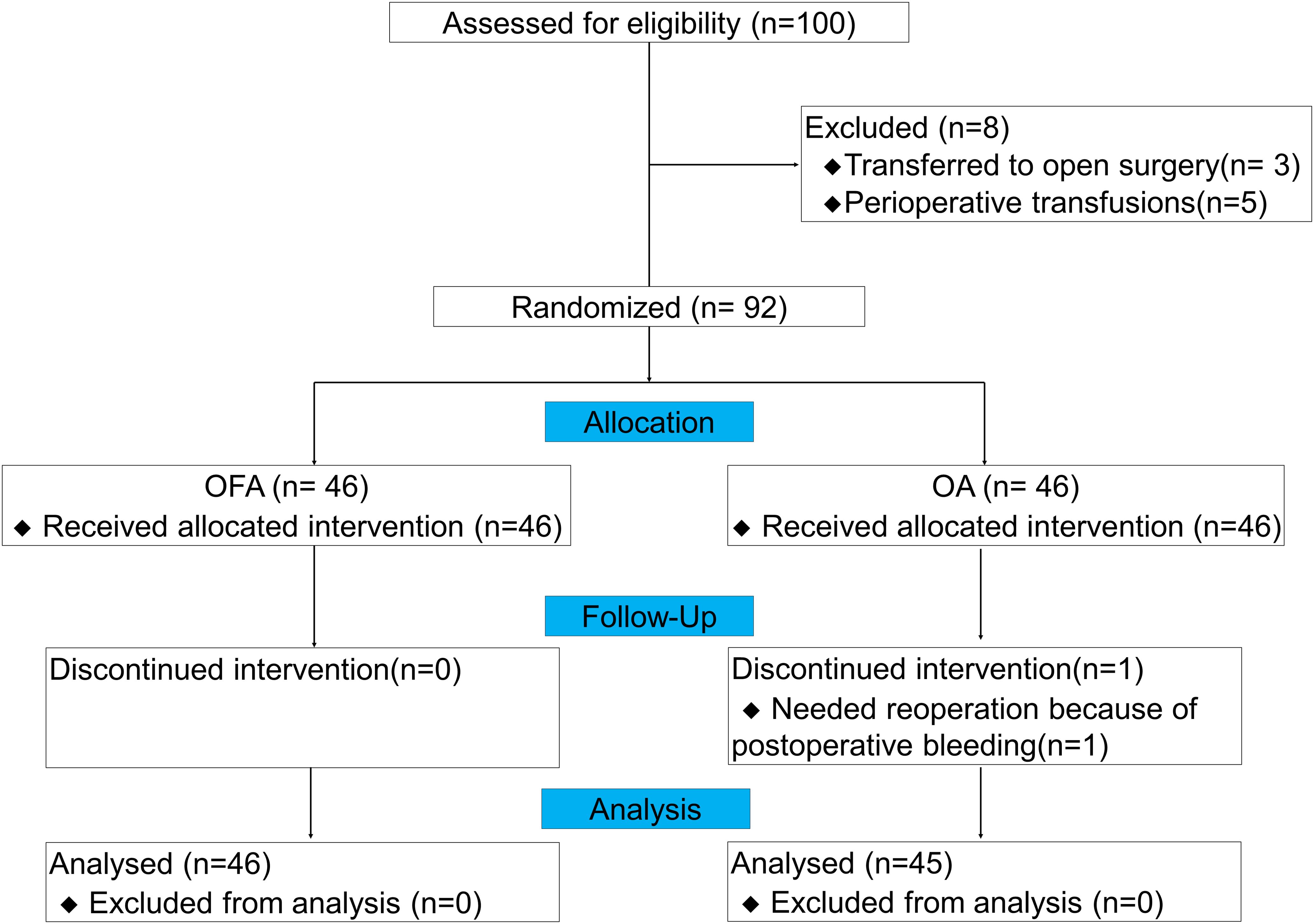
From July 2022 to December 2023, we assessed 100 patients for this study, and 92 patients were finally included (3 patients transferred to open surgery, and 5 patients with perioperative transfusions). During the follow-up period, 1 patient received reoperation due to postoperative bleeding in OA group, none of the patients were transferred to the ICU, developed respiratory failure, needed reintubation, or died. Therefore, 46 patients in the OFA group and 45 in the OA group were finally enrolled in the analysis (Figure 1). Among all LAGC patients in the two groups, there were no significant differences in gender, age, BMI, ASA grade, clinical tumor stage and node stage, preoperative white blood cell (WBC), preoperative neutrophil, preoperative hemoglobin, neoadjuvant therapy regimens, anesthesia duration, surgery duration, fluid infusion volumes, blood loss volume, urinary volume, drainage tube removal time, or postoperative infections (defined as any clinical-related infection after gastrectomy and before first discharge) (all P > 0.05) (Table 2).
The OFA group had significantly longer extubation time and PACU stay than the OA group, whereas the OA group had significantly higher rate of hypotension, higher doses of norepinephrine, higher PONV and dizziness rate, and delayed flatus passage time, compared to the OFA group (P < 0.05) (Table 2).
QoR-15 scores and VAS scores
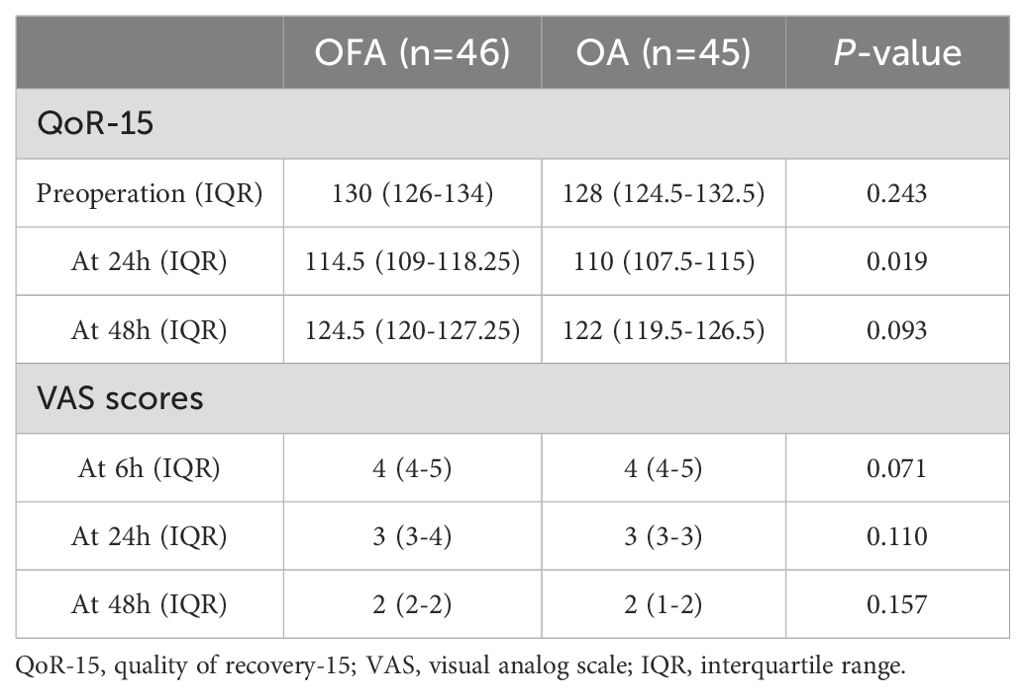
All patients filled out the QoR-15 questionnaire without any problems. The QoR-15 score before surgery and postoperative 48 h were not significantly different between the OFA group and OA group, P > 0.05, Table 3). However, the QoR-15 score on postoperative 24 h was significantly higher in OFA group than in OA group (114.5 [109-118.25] in OFA group vs 110 [107.5-115] in OA group, P < 0.05, Table 3). Likewise, the VAS score at 6, 24, or 48 hours postoperatively did not differ significantly between the two groups (P > 0.05).
Cytokine concentrations and the ratio of macrophage subsets
At the end of the surgery, the OA group had higher levels of IL-10 and CD68+CD163+ macrophages than the OFA group (P < 0.05). In contrast, the OA group had lower levels of IL-12 and CD68+CD163− macrophages than the OFA group (P < 0.05). As depicted in Figure 2 and Supplementary Figure S2.
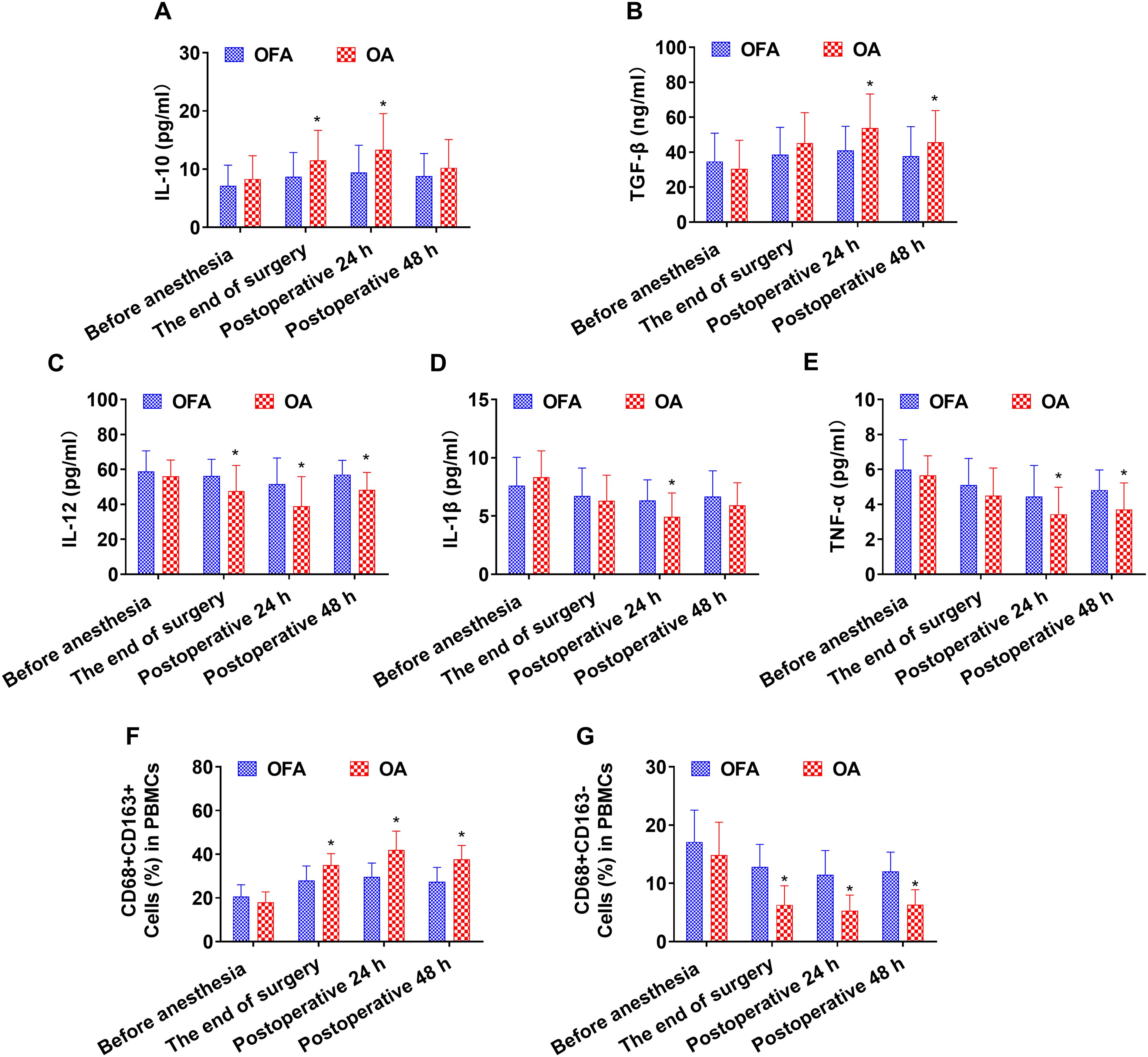
Figure 2. Cytokine concentrations and the ratio of macrophage subsets in total patients at different intraoperative times. (A–E). Serum level of IL-10, TGF-β, IL-12, IL-1β, TNF-α measured by ELISA. (F–G). Quantification analysis of CD68+CD163+ and CD68+CD163− macrophages in PBMCs. Data were shown as the mean (SD). *P < 0.05.
At postoperative 24 h, the OA group had higher levels of IL-10, TGF-β, and CD68+CD163+ macrophages than the OFA group (P < 0.05). On the other hand, the OA group had lower levels of IL-12, IL-1β, TNF-α, and CD68+CD163− macrophages than the OFA group (P < 0.05). As depicted in Figure 2 and Supplementary Figure S2.
At postoperative 48 h, the OA group had higher levels of TGF-β and CD68+CD163+ macrophages than the OFA group (P < 0.05). Conversely, the OA group had lower levels of IL-12, TNF-α, and CD68+CD163− macrophages than the OFA group (P < 0.05). As depicted in Figure 2 and Supplementary Figure S2.
Subgroup analysis for cytokine concentrations and the ratio of macrophage subsets
We performed subgroup analysis of cytokine levels and macrophage subset ratios in LAGC patients who received neoadjuvant chemotherapy (chemo subgroup) or neoadjuvant PD-1 inhibitor + chemotherapy (PD-1 inhibitor subgroup).
At the end of the surgery in the chemo subgroup, the OA group had lower levels of IL-12 and CD68+CD163− macrophages than the OFA group (P < 0.05) (Figure 3). At the end of the surgery in the PD-1 inhibitor subgroup, the IL-10 and TGF-β concentrations, and the proportion of CD68+CD163+ macrophages in the OA group were significantly higher than those in the OFA group (P < 0.05); the IL-12, IL-1β, and TNF-α concentrations, and the proportion of CD68+CD163− macrophages in the OA group were significantly lower than those in the OFA group (P < 0.05). As depicted in Figure 4.
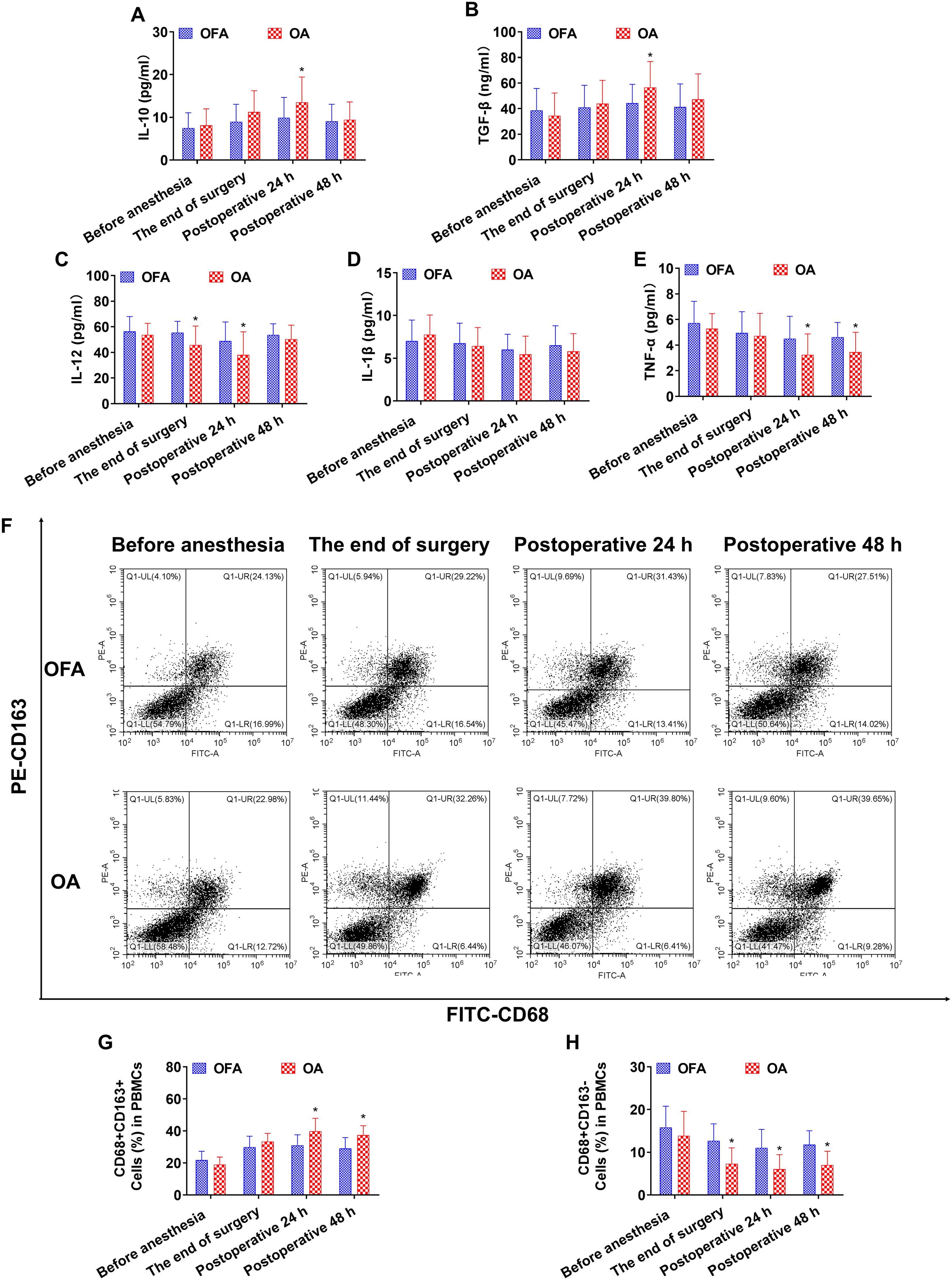
Figure 3. Cytokine concentrations and the ratio of macrophage subsets in chemo subgroup at different intraoperative times. (A–E). Serum level of IL-10, TGF-β, IL-12, IL-1β, TNF-α measured by ELISA. (F). Proportion of CD68+CD163+ and CD68+CD163− macrophages in PBMCs measured by flow cytometry. (G, H). Quantification analysis of CD68+CD163+ and CD68+CD163− macrophages in PBMCs. Data were shown as the mean (SD). *P < 0.05.
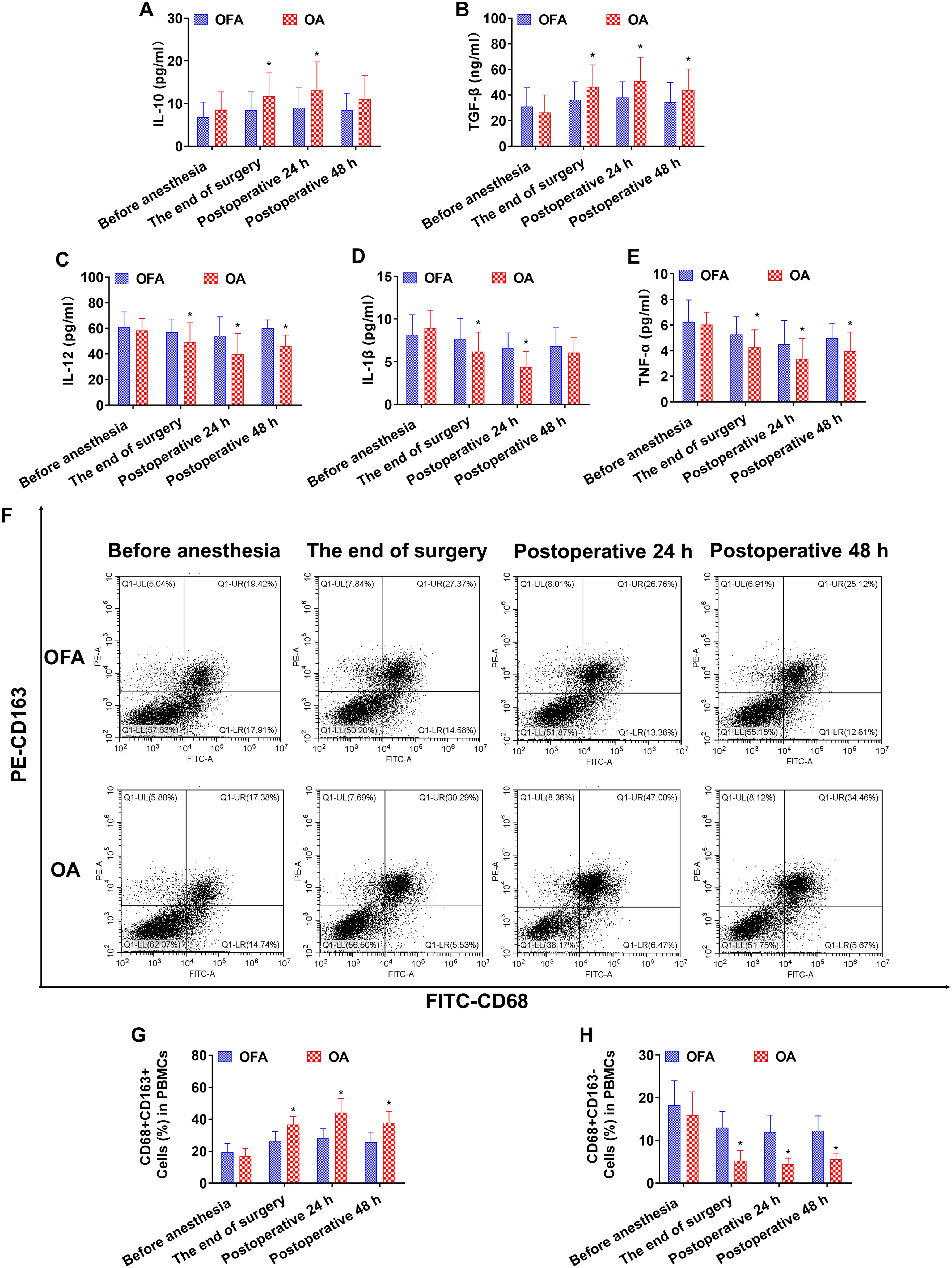
Figure 4. Cytokine concentrations and the ratio of macrophage subsets in PD-1 inhibitor subgroup at different intraoperative times. (A–E). Serum level of IL-10, TGF-β, IL-12, IL-1β, TNF-α measured by ELISA. (F). Proportion of CD68+CD163+ and CD68+CD163− macrophages in PBMCs measured by flow cytometry. (G, H). Quantification analysis of CD68+CD163+ and CD68+CD163− macrophages in PBMCs. Data were shown as the mean (SD). *P < 0.05.
At postoperative 24 h in subgroup of chemo, the IL-10 and TGF-β concentrations, and the proportion of CD68+CD163+ macrophages in the OA group were significantly higher than those in the OFA group (P < 0.05); the IL-12 and TNF-α concentrations, and the proportion of CD68+CD163− macrophages in the OA group were significantly lower than those in the OFA group (P < 0.05) (Figures 3). At postoperative 24 h in the PD-1 inhibitor subgroup, the IL-10 and TGF-β concentrations, and the proportion of CD68+CD163+ macrophages in the OA group were significantly higher than those in the OFA group (P < 0.05); the IL-12, IL-1β, and TNF-α concentrations, and the proportion of CD68+CD163− macrophages in the OA group were significantly lower than those in the OFA group (P < 0.05) (Figure 4).
At postoperative 48 h in subgroup of chemo, only the proportion of CD68+CD163+ macrophages in the OA group was significantly higher than that in the OFA group (P < 0.05); the TNF-α concentration and the proportion of CD68+CD163− macrophages in the OA group were significantly lower than those in the OFA group (P < 0.05) (Figure 3). At postoperative 48 h in subgroup of PD-1 inhibitor, the TGF-β concentration and the proportion of CD68+CD163+ macrophages in the OA group were significantly higher than those in the OFA group (P < 0.05); the IL-12 and TNF-α concentrations, and the proportion of CD68+CD163− macrophages in the OA group were significantly lower than those in the OFA group (P < 0.05) (Figure 4).
Discussion
This prospective randomized study showed that the OFA regimen basing on continuous infusion of esketamine and dexmedetomidine improved the quality of recovery, decreases the incidence of PONV and dizziness, and reduced the time to first flatus compared with OA anesthesia in LAGC patients. However, patients receiving OFA had delayed extubation and longer stay in the PACU.
It was reported that esketamine improved the quality of postoperative recovery after surgery on postoperative days 1 and 3 in breast cancer patients (33). Other clinical studies proved that intravenous dexmedetomidine reduced PONV rate, improved quality of postoperative recovery, and alleviated the intensity of postoperative pain (34–36). In this study, we revealed higher QoR-15 scores, lower PONV and dizziness rates, and quicker first flatus in OFA groups than those in OA group. Intravenous dexmedetomidine associate with longer awaken and extubation times (37, 38), and esketamine infusion can prolong recovery time (39). Consistently, we found that the extubation time and PACU stay were longer in OFA group than those in OA group, indicating that dexmedetomidine plus esketamine resulted in better sedation.
By measuring the cytokine concentrations in serum, we found that LAGC patients in the OA group had increased levels of anti-inflammatory cytokines (IL-10 and TGF-β) and decreased levels of pro-inflammatory cytokines (IL-12, IL-1β, and TNF-α) than those in the OFA group. Consistent with the results of cytokine, LAGC patients in the OA group had higher proportion of CD68+CD163+ and lower proportion of CD68+CD163−macrophages than those in the OFA group. Subgroup analysis shows that OA caused a faster shift from M1 to M2 phenotype in the PD-1 inhibitor subgroup than that in the chemo subgroup.
In the TME, programmed death ligand-1(PD-L1) expressed on the surface of tumor cells can bind to PD-1 on T cells, which resist the killing effect of T cells and eventually cause tumor immune escape. The utilization of PD-1 inhibitor to block the PD-1/PD-L1 signal has demonstrated remarkable anti-cancer effectiveness across a diverse range of solid tumors (40). The infiltration of T cells in TME is a prerequisite for anti-tumor immunity, while the infiltration of immunosuppressive cells is a prerequisite for tumor immune escape. In the process of anti-PD-1/PD-L1 immunotherapy, M2 macrophages are immunosuppressive cells which can induce drug resistance to PD-1/PD-L1 therapy by inhibiting T-cell activity and enhancing the expression of PD-L1 in the TME (41, 42).
The immunomodulatory effects of opioids may be mediated by opioid receptors expressed in immune cells such as neutrophils, NK cells, T cells and equally in macrophages (43). And opioid receptors are the main target receptors for potent opioids such as morphine and fentanyl (44). Gong et al. investigated the effects of opioids on regulatory T cells frequencies and found that fentanyl and sufentanil could exacerbate immunosuppression via expansion of CD4+CD25+Foxp3+ regulatory T cells population in vitro (45). Khabbazi et al. revealed that morphine can regulate the production of macrophage proteases and M2 polarization via preventing MMP-9 increase and arginase-1 induction in TME of breast cancer, and morphine antagonists (naloxone and methylnaltrexone) can reverse this process (46).
It is also revealed that opioid receptor agonists or antagonists regulate macrophage function, thereby affecting tumor progression. Leu-enkephalin, an opioid receptor agonist, was predicted to have anti-survival effects in clear cell renal cell carcinoma (ccRCC), mainly through Th2 immunity and NRF2-dependent macrophage networks (47). Conversely, the opioid receptor antagonist, naloxone, was predicted to have pro-survival effects in ccRCC, primarily through angiogenesis, fatty acid metabolism, and hemopoiesis pathways (47). Low-dose naltrexone inhibits colorectal cancer progression and promotes tumor apoptosis by increasing M1 macrophages via the Bax/Bcl-2/caspase-3/PARP pathway (48).
Botticelli et al (49) conducted an observational, multicenter, retrospective study to explore the relationship between the administration of concomitant to ICIs (such as opioids) and the prognosis in order to evaluate a possible negative drug interaction able to impair the ICIs. Results showed that opioids use during immunotherapy is associated with early progression, potentially representing a predictive factor for PFS and negatively influencing OS as well.
Consistent with the previous researches, our study also found that the use of OA may cause more shift from M1 to M2 phenotype, and subgroup analysis shows that OA caused a faster shift from M1 to M2 phenotype in the PD-1 inhibitor subgroup than that in the chemo subgroup, suggesting that OFA may be more appropriate for LAGC patients receiving neoadjuvant PD-1 inhibitor. Our findings suggest the urgent need to further explore the impact of opioids on immune system modulation and their negative role in the response to immunotherapy treatment.
Our study had some limitations. First, we only assessed macrophage subsets and cytokines. The immune system is a complicated system. There are many other immune cells, such as NK cells, and other immune factors such as chemokines, which could be explored in the future. Second, we employed a combination of lidocaine, esketamine, and dexmedetomidine to replace opioid drugs, making it difficult to distinguish which specific drugs or methods directly affect inflammatory factors and macrophages, thereby complicating the identification of a precise causal relationship. According to this concept, other non-opioid drugs can be added arbitrarily to enhance the anesthetic effect, suppress intraoperative stress, and ensure the quality of postoperative recovery. Last but not least, we could not obtain the long-term outcomes of these patients, such as long-term survival, relapse and metastasis rates, at this time. Further studies are needed to confirm the findings here in more detail.
In summary, our results showed that OFA attenuated perioperative immunosuppression by diminishing M2 and promoting M1 macrophage polarization in LAGC patients, especially in LAGC patients treated with neoadjuvant PD-1 inhibitor.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Ethics Committee of Sun Yat-Sen University Cancer Center. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
WL: Data curation, Formal analysis, Investigation, Writing – original draft, Writing – review & editing. CO: Data curation, Formal analysis, Investigation, Writing – original draft. RX: Formal analysis, Investigation, Writing – original draft. XY: Formal analysis, Writing – original draft. YY: Methodology, Writing – original draft. JX: Funding acquisition, Supervision, Visualization, Writing – original draft, Writing – review & editing. XW: Data curation, Formal analysis, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1438859/full#supplementary-material
Supplementary Figure 1 | The quality of recovery (QoR)-15 score questionnaire.
Supplementary Figure 2 | Perioperative trends of cytokine concentrations and the ratio of macrophage subsets in total patients. (A-E). Serum level of IL-10, TGF-β, IL-12, IL-1β, TNF-α measured by ELISA. (F-G). Quantification analysis of CD68+CD163+ and CD68+CD163− macrophages in PBMCs. Data were shown as the mean (SD). *P < 0.05 in OFA group, #P < 0.05 in OA group. ns, no statistical difference.
References
1. Lordick F, Carneiro F, Cascinu S, Fleitas T, Haustermans K, Piessen G, et al. Gastric cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. (2022) 33:1005–20. doi: 10.1016/j.annonc.2022.07.004
2. Ajani JA, D'Amico TA, Bentrem DJ, Chao J, Cooke D, Corvera C, et al. Gastric cancer, version 2.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2022) 20:167–92. doi: 10.6004/jnccn.2022.0008
3. Li GZ, Doherty GM, Wang J. Surgical management of gastric cancer: A review. JAMA Surg. (2022) 157:446–54. doi: 10.1001/jamasurg.2022.0182
4. Tokunaga M, Sato Y, Nakagawa M, Aburatani T, Matsuyama T, Nakajima Y, et al. Perioperative chemotherapy for locally advanced gastric cancer in Japan: current and future perspectives. Surg Today. (2020) 50:30–7. doi: 10.1007/s00595-019-01896-5
5. Goetze OT, Al-Batran SE, Chevallay M, Monig SP. Multimodal treatment in locally advanced gastric cancer. Updates Surg. (2018) 70:173–9. doi: 10.1007/s13304-018-0539-z
6. Zhang X, Zhang C, Hou H, Zhang Y, Jiang P, Zhou H, et al. Neoadjuvant PD-1 blockade plus chemotherapy versus chemotherapy alone in locally advanced stage II-III gastric cancer: A single-centre retrospective study. Transl Oncol. (2023) 31:101657. doi: 10.1016/j.tranon.2023.101657
7. Guo H, Ding P, Sun C, Yang P, Tian Y, Liu Y, et al. Efficacy and safety of sintilimab plus XELOX as a neoadjuvant regimen in patients with locally advanced gastric cancer: A single-arm, open-label, phase II trial. Front Oncol. (2022) 12:927781. doi: 10.3389/fonc.2022.927781
8. Yuan SQ, Nie RC, Jin Y, Liang CC, Li YF, Jian R, et al. Perioperative toripalimab and chemotherapy in locally advanced gastric or gastro-esophageal junction cancer: a randomized phase 2 trial. Nat Med. (2024) 30(2):552–9. doi: 10.1038/s41591-023-02721-w
9. Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, Shen L, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. (2021) 398:27–40. doi: 10.1016/S0140-6736(21)00797-2
10. Kang YK, Chen LT, Ryu MH, Oh DY, Oh SC, Chung HC, et al. Nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with HER2-negative, untreated, unresectable advanced or recurrent gastric or gastro-oesophageal junction cancer (ATTRACTION-4): a randomised, multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. (2022) 23:234–47. doi: 10.1016/S1470-2045(21)00692-6
11. Liu T, Bai Y, Lin X, Li W, Wang J, Zhang X, et al. First-line nivolumab plus chemotherapy vs chemotherapy in patients with advanced gastric, gastroesophageal junction and esophageal adenocarcinoma: CheckMate 649 Chinese subgroup analysis. Int J Cancer. (2023) 152:749–60. doi: 10.1002/ijc.34296
12. Zhang H, Li R, Cao Y, Gu Y, Lin C, Liu X, et al. Poor clinical outcomes and immunoevasive contexture in intratumoral IL-10-producing macrophages enriched gastric cancer patients. Ann Surg. (2022) 275:e626–35. doi: 10.1097/SLA.0000000000004037
13. Su P, Jiang L, Zhang Y, Yu T, Kang W, Liu Y, et al. Crosstalk between tumor-associated macrophages and tumor cells promotes chemoresistance via CXCL5/PI3K/AKT/mTOR pathway in gastric cancer. Cancer Cell Int. (2022) 22:290. doi: 10.1186/s12935-022-02717-5
14. He Z, Chen D, Wu J, Sui C, Deng X, Zhang P, et al. Yes associated protein 1 promotes resistance to 5-fluorouracil in gastric cancer by regulating GLUT3-dependent glycometabolism reprogramming of tumor-associated macrophages. Arch Biochem Biophys. (2021) 702:108838. doi: 10.1016/j.abb.2021.108838
15. Shi T, Zhang Y, Wang Y, Song X, Wang H, Zhou X, et al. DKK1 promotes tumor immune evasion and impedes anti-PD-1 treatment by inducing immunosuppressive macrophages in gastric cancer. Cancer Immunol Res. (2022) 10:1506–24. doi: 10.1158/2326-6066.CIR-22-0218
16. Zhao R, Wan Q, Wang Y, Wu Y, Xiao S, Li Q, et al. M1-like TAMs are required for the efficacy of PD-L1/PD-1 blockades in gastric cancer. Oncoimmunology. (2020) 10:1862520. doi: 10.1080/2162402X.2020.1862520
17. Kianian S, Bansal J, Lee C, Zhang K, Bergese SD. Perioperative multimodal analgesia: a review of efficacy and safety of the treatment options. Anesthesiology Perioperative Sci. (2024) 2:9. doi: 10.1007/s44254-023-00043-1
18. Fletcher D, Martinez V. How can we prevent opioid induced hyperalgesia in surgical patients? Br J Anaesth. (2016) 116:447–9. doi: 10.1093/bja/aew050
19. Rathmell JP. Practice guidelines for the prevention, detection, and management of respiratory depression associated with neuraxial opioid administration: an updated report by the american society of anesthesiologists task force on neuraxial opioids and the American society of regional anesthesia and pain medicine. Anesthesiology. (2016) 124:535–52. doi: 10.1097/ALN.0000000000000975
20. Smith HS, Laufer A. Opioid induced nausea and vomiting. Eur J Pharmacol. (2014) 722:67–78. doi: 10.1016/j.ejphar.2013.09.074
21. Minkowitz HS, Scranton R, Gruschkus SK, Nipper-Johnson K, Menditto L, Dandappanavar A. Development and validation of a risk score to identify patients at high risk for opioid-related adverse drug events. J Manag Care Spec Pharm. (2014) 20:948–58. doi: 10.18553/jmcp.2014.20.9.948
22. Franchi S, Panerai AE, Sacerdote P. Buprenorphine ameliorates the effect of surgery on hypothalamus-pituitary-adrenal axis, natural killer cell activity and metastatic colonization in rats in comparison with morphine or fentanyl treatment. Brain Behav Immun. (2007) 21:767–74. doi: 10.1016/j.bbi.2007.01.001
23. Wang X, Tao Y, Zhang C, Tian J, Yu W. Expression heterogeneity, tumor immune characteristics and the prognosis effects of OPRL1 in patients with tumors: a pan-cancer study combined with bioinformation analyses and in vitro validation. Anesthesiology Perioperative Sci. (2024) 2:8. doi: 10.1007/s44254-023-00049-9
24. Cong X, Huang Z, Zhang L, Sun M, Chang E, Zhang W, et al. Effect of different anaesthesia methods on perioperative cellular immune function and long-term outcome in patients undergoing radical resection of esophageal cancer: a prospective cohort study. Am J Transl Res. (2021) 13:11427–38.
25. Desmond F, McCormack J, Mulligan N, Stokes M, Buggy DJ. Effect of anaesthetic technique on immune cell infiltration in breast cancer: a follow-up pilot analysis of a prospective, randomised, investigator-masked study. Anticancer Res. (2015) 35:1311–9.
26. Guilliams M, Ginhoux F, Jakubzick C, Naik SH, Onai N, Schraml BU, et al. Dendritic cells, monocytes and macrophages: a unified nomenclature based on ontogeny. Nat Rev Immunol. (2014) 14:571–8. doi: 10.1038/nri3712
27. Li B, Ren M, Zhou X, Han Q, Cheng L. Targeting tumor-associated macrophages in head and neck squamous cell carcinoma. Oral Oncol. (2020) 106:104723. doi: 10.1016/j.oraloncology.2020.104723
28. Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. (2008) 8:958–69. doi: 10.1038/nri2448
29. Lawrence T, Natoli G. Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nat Rev Immunol. (2011) 11:750–61. doi: 10.1038/nri3088
30. Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. (2010) 11:889–96. doi: 10.1038/ni.1937
31. Peng LS, Zhang JY, Teng YS, Zhao YL, Wang TT, Mao FY, et al. Tumor-associated monocytes/macrophages impair NK-cell function via TGFbeta1 in human gastric cancer. Cancer Immunol Res. (2017) 5:248–56. doi: 10.1158/2326-6066.CIR-16-0152
32. Stark PA, Myles PS, Burke JA. Development and psychometric evaluation of a postoperative quality of recovery score: the QoR-15. Anesthesiology. (2013) 118:1332–40. doi: 10.1097/ALN.0b013e318289b84b
33. Zhu M, Xu S, Ju X, Wang S, Yu X. Effects of the different doses of esketamine on postoperative quality of recovery in patients undergoing modified radical mastectomy: A randomized, double-blind, controlled trial. Drug Des Devel Ther. (2022) 16:4291–9. doi: 10.2147/DDDT.S392784
34. Shu T, Xu S, Ju X, Hu S, Wang S, Ma L. Effects of systemic lidocaine versus dexmedetomidine on the recovery quality and analgesia after thyroid cancer surgery: A randomized controlled trial. Pain Ther. (2022) 11:1403–14. doi: 10.1007/s40122-022-00442-5
35. Sivaji P, Agrawal S, Kumar A, Bahadur A. Evaluation of lignocaine, dexmedetomidine, lignocaine-dexmedetomidine infusion on pain and quality of recovery for robotic abdominal hysterectomy: a prospective randomized controlled trial. Braz J Anesthesiol. (2022) 72:593–8. doi: 10.1016/j.bjane.2021.10.005
36. Ren B, Cheng M, Liu C, Zheng H, Zhang J, Chen W, et al. Perioperative lidocaine and dexmedetomidine intravenous infusion reduce the serum levels of NETs and biomarkers of tumor metastasis in lung cancer patients: A prospective, single-center, double-blinded, randomized clinical trial. Front Oncol. (2023) 13:1101449. doi: 10.3389/fonc.2023.1101449
37. Gao S, Wang T, Cao L, Li L, Yang S. Clinical effects of remimazolam alone or in combination with dexmedetomidine in patients receiving bronchoscopy and influences on postoperative cognitive function: a randomized-controlled trial. Int J Clin Pharm. (2023) 45:137–45. doi: 10.1007/s11096-022-01487-4
38. Beloeil H, Garot M, Lebuffe G, Gerbaud A, Bila J, Cuvillon P, et al. Balanced opioid-free anesthesia with dexmedetomidine versus balanced anesthesia with remifentanil for major or intermediate noncardiac surgery. Anesthesiology. (2021) 134:541–51. doi: 10.1097/ALN.0000000000003725
39. Zhang C, He J, Shi Q, Bao F, Xu J. Subanaesthetic dose of esketamine during induction delays anaesthesia recovery a randomized, double-blind clinical trial. BMC Anesthesiol. (2022) 22:138. doi: 10.1186/s12871-022-01662-0
40. Gordon SR, Maute RL, Dulken BW, Hutter G, George BM, McCracken MN, et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature. (2017) 545:495–9. doi: 10.1038/nature22396
41. Pu Y, Ji Q. Tumor-associated macrophages regulate PD-1/PD-L1 immunosuppression. Front Immunol. (2022) 13:874589. doi: 10.3389/fimmu.2022.874589
42. Zhang H, Liu L, Liu J, Dang P, Hu S, Yuan W, et al. Roles of tumor-associated macrophages in anti-PD-1/PD-L1 immunotherapy for solid cancers. Mol Cancer. (2023) 22:58. doi: 10.1186/s12943-023-01725-x
44. Sobczak M, Salaga M, Storr MA, Fichna J. Physiology, signaling, and pharmacology of opioid receptors and their ligands in the gastrointestinal tract: current concepts and future perspectives. J Gastroenterol. (2014) 49:24–45. doi: 10.1007/s00535-013-0753-x
45. Gong L, Qin Q, Zhou L, Ouyang W, Li Y, Wu Y, et al. Effects of fentanyl anesthesia and sufentanil anesthesia on regulatory T cells frequencies. Int J Clin Exp Pathol. (2014) 7:7708–16.
46. Khabbazi S, Goumon Y, Parat MO. Morphine modulates interleukin-4- or breast cancer cell-induced pro-metastatic activation of macrophages. Sci Rep. (2015) 5:11389. doi: 10.1038/srep11389
47. Scarpa JR, DiNatale RG, Mano R, Silagy AW, Kuo F, Irie T, et al. Identifying clear cell renal cell carcinoma coexpression networks associated with opioid signaling and survival. Cancer Res. (2021) 81:1101–10. doi: 10.1158/0008-5472.CAN-20-1852
48. Ma M, Wang X, Liu N, Shan F, Feng Y. Low-dose naltrexone inhibits colorectal cancer progression and promotes apoptosis by increasing M1-type macrophages and activating the Bax/Bcl-2/caspase-3/PARP pathway. Int Immunopharmacol. (2020) 83:106388. doi: 10.1016/j.intimp.2020.106388
Keywords: opioid-based anesthesia, opioid-free anesthesia, macrophages polarization, locally advanced gastric cancer, PD-1 inhibitor
Citation: Liu W, Ou C, Xue R, Yang X, Ye Y, Wang X and Xie J (2024) Opioid−free anesthesia attenuates perioperative immunosuppression by regulating macrophages polarization in gastric cancer patients treated with neoadjuvant PD-1 inhibitor. Front. Immunol. 15:1438859. doi: 10.3389/fimmu.2024.1438859
Received: 26 May 2024; Accepted: 04 September 2024;
Published: 23 September 2024.
Edited by:
Ioannis Vathiotis, National and Kapodistrian University of Athens, GreeceReviewed by:
Lan Lan, First Affiliated Hospital of Guangzhou Medical University, ChinaXiaoran Li, University of Texas MD Anderson Cancer Center, United States
Copyright © 2024 Liu, Ou, Xue, Yang, Ye, Wang and Xie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jingdun Xie, eGllamQ2QG1haWwuc3lzdS5lZHUuY24=; Xudong Wang, d2FuZ3hkQHN5c3VjYy5vcmcuY24=
†These authors have contributed equally to this work and share first authorship
 Wenjian Liu
Wenjian Liu Chaopeng Ou
Chaopeng Ou Ruifeng Xue
Ruifeng Xue Xiaohua Yang1,2
Xiaohua Yang1,2 Jingdun Xie
Jingdun Xie