- 1Department of Thyroid and Parathyroid Surgery, West China Hospital, Sichuan University, Chengdu, China
- 2West China School of Medicine, Sichuan University, Chengdu, China
Papillary thyroid cancer (PTC) is the most common type of primary thyroid cancer. Despite the low malignancy and relatively good prognosis, some PTC cases are highly aggressive and even develop refractory cancer in the thyroid. Growing evidence suggested that microenvironment in tumor affected PTC biological behavior due to different immune states. Different interconnected components in the immune system influence and participate in tumor invasion, and are closely related to PTC metastasis. Immune cells and molecules are widely distributed in PTC tissues. Their quantity and proportion vary with the host’s immune status, which suggests that immunotherapy may be a very promising therapeutic modality for PTC. In this paper, we review the role of immune cells and immune checkpoints in PTC immune microenvironment based on the characteristics of the PTC tumor microenvironment.
1 Introduction
Thyroid cancer (TC) represents a prevalent malignancy within the endocrine system, demonstrating a higher incidence in women compared to men and predominantly affecting individuals aged 40 to 50 (1, 2). The biological properties of various thyroid cancer subtypes span a broad spectrum. Based on their histological characteristics and cellular origins, thyroid cancers are classified into papillary, medullary, and follicular carcinomas (3). Papillary thyroid carcinoma (PTC) is a differentiated cancer subtype in the thyroid, constituting most form of primary thyroid malignancy (4). Over recent decades, the incidence of PTC has exhibited an increasing trend and a shift towards younger age groups (5, 6). For PTC, the current traditional therapies include surgical resection, radiotherapy, chemotherapy, endocrine inhibition and other therapeutic means, but the efficacy of various treatment methods has different degrees of limitations (7, 8). Despite their slow tumor growth, low malignancy, and overall favorable prognosis, over 10% of patients had tumor recurrence or metastasized to other sites after surgery (9). Some cases appear highly aggressive and may even progress to refractory thyroid cancer (10). Immune cell infiltration is frequently observed in the vicinity or within primary PTC tissue. The prognosis of PTC might be associated with the surrounding inflammatory response (11). Increasing evidence suggests that the immune microenvironment influences tumor biological behavior.
2 Immune cells In PTC tumor microenvironment
In 2002, Dunn proposed the immune editing hypothesis, which categorized the reciprocity between the tumors and immune system into “elimination”, “equilibrium”, and “escape”. The “elimination” phase, also called “surveillance”, involves the immune system clearing tumor cells before diagnosis. During the “equilibrium” phase, Tumor cells vary in the direction of low immunogenicity, which makes themselves not easily detected by the body’s immune surveillance mechanism (12). Studies (13, 14) have shown that tumor cells can “camouflage” themselves by reducing MHC I expression, thus evading immune system surveillance. Another study (15) analyzed the influence of the immune environment on the clinical manifestations of patients and found that immune cells in PTC patients’ thyroids differed from healthy ones. Specifically, the proportions of B cells, T cells (mainly CD8+ T cells) and M1 macrophages showed obvious reduction. The larger the difference between these immune cells and healthy thyroid tissue, the greater the likelihood of PTC progression and recurrence, and the lower the patients’ overall survival rate.
The tumor microenvironment (TME) contains tumor cells and their living environment (including immune cells, stromal cells and blood vessels), which cooperate with each other (16). Each component in TME plays a crucial role in tumor initiation and progression. Their quantity as well as proportion vary with the host’s immune status (17). In most cancers, a high proportion of M2/M1 macrophages is strongly associated with poor clinical prognosis (18). In thyroid cancer, tumor-related macrophages (Tumor-associated macrophages, TAMs) are dominated by M2 polarized macrophages, providing a good tumor microenvironment for tumor growth, survival and angiogenesis. Experimental results of various tumors, including thyroid cancer, show that high-density TAMs are associated with poor prognosis of tumors (19, 20). At present, many cytokines, chemokines and their signaling pathways also have been found in PTC. For example, activation of IL-6/JAK2/STAT3 pathway could promote PTC cell proliferation and migration, and IL-34 promotes PTC cell proliferation (21), epithelial-stromal transition and extracellular regulatory kinase signaling pathway and inhibits apoptosis (22). In PTC tumor microenvironment, overexpression of IL-6 promotes the growth of PTC (23). The infiltration of plasma cells in the DTC microenvironment was positively correlated with a favorable prognosis (24). Immature Dendritic cells in the PTC microenvironment can secrete immunosuppressive cytokines, such as IL-10 and TGF- β, so as to inhibit the immune response and result in the development of PTC, while CD8+ T cells recognize tumor cells to express antigen and thus participate in the killing of tumor cells, exhibiting protective effects on PTC (25, 26).
Xie Z et al. (27) investigated immune-related cells in TME, focusing on the relationship between PTC and chronic inflammation. The study included 799 PTC patients and 194 healthy ones. It was found that compared with normal thyroids, the overall immune level of PTC tissues was stronger, and many cells in TME such as Tregs and M0 macrophages were elevated. Furthermore, the more advanced the tumor, the greater the proportion and abundance above normal levels. Higher immune group had a later stage than the lower one, with a larger tumor size, increased metastasis of lymph node, and a higher frequency of BRAF mutations. This suggests that changes in immune status within the TME are closely related to tumor progression, and that various immune cells can either promote or inhibit PTC metastasis and recurrence to different extents.
2.1 Natural killer cells
NK cells are essential components of inherent immunity that express various regulatory receptors associated with activation or inhibition. These receptors facilitate the distinction between “self” and “non-self,” enabling them to selectively “eliminate” (28). NK cell infiltration in tumors is often linked to the initiation or progression of cancer of early and metastatic stages of tumor development, and is generally predictive of a favorable prognosis (29).
In PTC, NK cells in TME are elevated in comparison to normal thyroid tissue, but not in peripheral blood (30). The abundance of NK cells in TME is significantly negatively associated with tumor progression. NK cells are able to kill cancer cells directly, and also responsible for the immune surveillance (31, 32). They may provide new ideas for PTC diagnosis and therapy. However, their efficacy is somewhat limited during the anti-tumor process due to the secretion of immunosuppressive factors by tumor cells, which reduce the activation receptors on NK cells while upregulating inhibitory receptors, making NK cell activation difficult. Tumor cells can also evade immune surveillance by reducing MHC I molecule expression, which blocks tumor antigen presentation (33). Additionally, the number and functionality of NK cells in the TME typically decline with tumor progression (34), and NK cells may be rendered dysfunctional due to metabolic disorders (35). These limitations of NK cells within the TME should be considered when utilizing them for PTC diagnosis, staging, and treatment.
2.2 T lymphocytes
T lymphocytes can be classified into helper T cells (Th), cytotoxic T cells (CTL), and regulatory T cells (Treg) according to their various functions. They mature from lymphoid progenitor cells in the thymus and are central to cellular immunity. CD4 is expressed in all Th cells. Naive CD4+T cells, known as Th0 cells, can differentiate into Th1, Th2, and Th17 lineages that have distinct immune roles through antigen stimulation and cytokine regulation. Th1 cells enhance and amplify cellular responses by secreting regulatory molecule, including interleukin (IL)-2 and IFN-γ, and induce other immune cells to exhibit antitumor activity (36). In contrast, Th2 cells inhibit the antitumor effects of cellular immunity by secreting IL-4 and suppressing NK cell activation (37). The Th1/Th2 ratio serves as a useful indicator of dynamic changes in the antitumor immune process. Moreover, Th17 levels in PTC tissue samples are higher than in healthy thyroid tissue, with this difference also observed in patients’ peripheral blood. More Th17 in peripheral blood tend to predict larger tumor volume (38).
The primary function of CTLs is to specifically recognize endogenous antigen peptide-MHC I molecular complexes and subsequently kill tumor cells. This has become an essential marker for evaluating tumor prognosis (39–41). PTC patients with a higher expression of CD8+ CTLs show lower tumor stages and higher survival rates, while the reduction of CD8+ T cells weakens the immune system’s ability to eliminate tumor cells, making tumors more aggressive (42). In the study by Modi J et al (43). PTC patients with CD8+ T cell infiltration experienced slower tumor progression, reduced tumor growth, and fewer recurrences.
Tregs, commonly referred to as CD4+CD25+Foxp3+ T cells, primarily weaken immune level through direct contact to target cells and cytokine secretion. High Tregs expression in cancer tissue is typically related to poor prognosis. Tregs are highly aggregated in the tumor site and peripheral blood of cancer patients (44, 45), and their inhibitory effect on the immune function of cancer patients is stronger than in healthy individuals (46). Tregs in PTC patients’ peripheral blood are significantly increased compared to normal thyroid tissue and thyroid adenoma patients (47, 48). In the TME, Tregs can weaken the body’s immune response to tumors through various mechanisms, including affecting cytokine secretion (49, 50), increasing cAMP-mediated immunosuppression via adenosine and prostaglandin (51, 52), regulating signal transduction through receptor-ligand binding (53, 54), and mediating immunosuppression through the exosome pathway (55). French JD et al. (42) using immunohistochemical analysis, quantitatively counted lymphocytes in the TME of PTC tissues, and found that T cells in the PTC tissues of patients were mainly composed of CD4 + T cells. The quantity of Foxp3+ regulatory T cells was related to lymph node metastasis (r = 0.858; P = 0.002), and the ratio of CD8 to Treg was strongly negatively associated with tumor size.
In the future, the frequency of Treg cells in TME is likely to become an important factor in predicting, diagnosing, and evaluating the prognosis of PTC. Furthermore, the suppressive effect of Treg cells should be taken into account when designing immunotherapy for PTC. Overall, a better understanding of the complex interactions between various immune cell types in the TME is significant for the exploration of more effective diagnostic and therapeutic strategies for PTC and other cancers.
2.3 Mast cells
Mast cells are tissue-resident component ubiquitously distributed across nearly all tissues. Their regulatory role in the tumor microenvironment (TME) is often multifaceted, exhibiting both pro-tumorigenic and anti-tumorigenic effects (56). The tumor-promoting effects primarily involve the secretion of vascular endothelial growth factors (VEGF) to promote neovascularization, the secretion of matrix metalloproteinases (MMPs) to enhance cancer progression, and the release of regulatory molecules to facilitate immune tolerance. Conversely, their anticancer effects include direct inhibition of tumor growth, immune stimulation, and reduction of cell motility (57). Mast cells situated within or surrounding tumors may exhibit different roles. While mast cells generally play a pro-carcinogenic role in most tumors (58, 59), their contributions to cancer progression can vary depending on which stage the tumors are at and where they are in tumor tissue (60).
Limited studies (61) have assessed the correlation between mast cells and PTC. One study reported that mast cell accumulation was observed in 95% of PTC samples, with the density positively correlated with cancer aggressiveness. Other studies demonstrated that mast cell derivatives, such as histamine and chemokines, accelerated the progression of PTC as well as distant metastasis in vitro. But this phenomenon will be exactly the opposite when inhibitors of mast cells are applied (62), potentially providing novel therapeutic strategies for PTC treatment.
2.4 Tumor-associated macrophages
Tumor-associated macrophages (TAMs) are the most abundant in the tumor microenvironment (TME). They can differentiate into two subpopulations that exert opposing effects on the host’s immune response to tumors. M1 macrophages predominantly suppress tumor growth and angiogenesis by producing cytokines like IL-1. In contrast, M2 macrophages generate IL-13, IL-10, and other factors that foster tumor development and enhance the invasive capabilities of tumor cells (63). Within the TME, cancer cells secrete signaling factors, mediated by exosomes, that induce mononuclear macrophages to differentiate into the M2 subtype (64), resulting in an imbalance between M1 and M2 populations and ultimately promoting cancer progression (65).
Elevated TAM in PTC is closely related with biological behavior of the tumors (66). Studies (67, 68) have revealed the macrophage infiltration rate in PTC is significantly higher than that in benign tumors, with the extent of infiltration positively correlating with lymph node metastasis. The underlying mechanism remains incompletely understood; however, it may involve TAMs promoting tumor cells of PTC metastasis through the cytokine CXCL8 and its paracrine interaction with CXCR1/2 (69). Consequently, a comprehensive understanding of the functional differences between distinct TAM subtypes in the thyroid gland may potentially establish TAMs as new idea for thyroid tumor therapy.
2.5 Dendritic cells
Dendritic cells (DCs) are the most functionally specialized APCs in immune system. They serve as initiators of the adaptive immune response and act as a “bridge” connecting innate and adaptive immunity.
Normally, DCs are scarcely present in thyroid tissue. However, their prevalence increases in human papillary thyroid carcinoma (PTC) tissue (70). Immature DCs possess robust antigen-processing capabilities but are less effective in promoting immune responses. Interestingly, they may even weaken immune responses by secreting inhibitory cytokines including IL-10 and TGF-β (71).
Moreover, Tregs and DCs can interact and collaboratively involve in immune regulation in TME. In PTC tissues, Tregs can inhibit DC function, co-stimulatory ligands expression, CD8+ T cells activation (72). DCs are able to restore their function by blocking PD-1 pathways, IL-10 secretion, and production of lactic acid (73). Therefore, disrupting the interaction between Tregs and DCs in PTC may shed new light on immune therapy.
2.6 Neutrophils
Neutrophils have long been recognized for their pivotal role in acute phase of inflammatory. Recently, they’ve emerged as a new subject of investigation in the field of oncology. Accumulating experimental evidence suggests that neutrophils may exert both antitumor and protumor effects by releasing various regulatory molecules within the tumor microenvironment (74). Neutrophils exhibit a dual role in PTC development and progression. On one hand, they promote genetic instability, proliferation, invasion (75), and vascular remodeling of cancer cells by releasing neutrophil elastase (76). Conversely, neutrophils have demonstrated antitumor properties, possessing the capacity to “eliminate” through antibody-dependent cellular cytotoxicity (ADCC) (77). Maria et al. found that PTC tissue extended the survival of human neutrophils and enhances its activity and reactive oxygen species (ROS) generation, suggesting that neutrophils can acquire a cytotoxic antitumor phenotype under the influence of thyroid tumor microenvironment. Notably, during tumor progression, the neutrophil population increases, and their phenotype undergoes alterations. Several subsets of circulating neutrophils with distinct maturity and immunological properties can be identified in advanced cancer, each playing a unique role in tumor immunity (78).
In PTC tissues, tumor cells recruit neutrophils by releasing CXCL8/IL-8 and reduce apoptosis rate of neutrophils through secretion of granulocyte colony-stimulating factor (GM-CSF) (79). The ratio of neutrophil count to lymphocyte count (neutrophil to lymphocyte ratio; NLR) in peripheral blood is associated with tumor development and progression (80), and higher NLR is associated with larger tumor volume and higher risk of recurrence in thyroid cancer (81).
3 Immune checkpoints of PTC
Lymphocyte activation primarily relies on the specific recognition of antigens by antigen receptors, with the strength, duration, and nature of the activation signal often regulated by cell surface receptor molecules. Immune checkpoints act as regulatory components, controlling timing and intensity of immune responses, maintaining self-tolerance, and preventing immune hyperactivity. In TME, these regulators inhibits immune responses, rendering the body incapable of mounting an efficient immune response against cancer, thus facilitating immune evasion (82). Common immune checkpoints in PTC include programmed cell death protein 1 (PD-1), programmed cell death ligand 1 (PD-L1), cytotoxic T lymphocyte antigen 4 (CTLA-4), and indoleamine 2,3-dioxygenase (IDO) (83).
A recent study (15) revealed that several key immune checkpoints, including LAG3, PD-1, and IDO1, are inhibited in early PTC compared to normal thyroid tissue, potentially associated to the prevention of immune cell-mediated damage to healthy thyroid tissue. Interestingly, during the pathological stage, most of the immune checkpoints were upregulated, particularly the N stage, advanced. Likewise, the BRAFV600E mutation has been associated with the elevation of most checkpoints (84, 85).
3.1 Programmed cell death protein 1/Programmed cell death ligand 1
The PD-1/PD-L1 pathway has emerged as a vital suppressive regulator in cancer. The overexpression of PD-L1 suggests that PD-L1 undermines immune surveillance of tumor in TME (86). Due to the cell and tissue-specific distribution of PD-L1, PD-1 play its part in at distinct stages of T cell activation, altering T cell function under antigen-specific stimulation, inhibiting CTLs, and enhancing tumor proliferation and invasion (87, 88). When T cells are recognized with PD-L1-positive tumor cells, tumor cells can cause programmed T cell death. In addition, tumor cells can produce cytokines including IL-10, allowing tumor cells to escape the clearance of CTL (47).. These mechanisms facilitate immune evasion by thyroid cancer cells and play a critical role in the transformation of normal cells into tumor cells (89).
PD-1 is widely expressed on lymphocytes capable of receiving antigen stimulation, acting as a “rheostat” for immune responses and regulating lymphocyte reactions to antigens. During antigen recognition, PD-1 binds to its ligands, recruiting tyrosine phosphatase (SHP-2), which can dephosphorylate and inactivate proximal effector molecules of antigen receptors on lymphocyte surfaces (87), such as inactivating Zap70 in T lymphocytes to inhibit TCR signaling (90) or inactivating Syk in B lymphocytes to inhibit BCR signaling (91).
PD-1’s effects on biochemical signaling pathways also promote T cell conversion of naive into inducible Treg (iTreg) cell through various mechanisms. Firstly, PD-1 enhances Foxp3 expression by inhibiting Akt activation (92). Secondly, by inhibiting cyclin-dependent kinase 2 (Cdk2), PD-1 amplifies Smad3-mediated transactivation by transforming growth factor β (TGF-β) (93, 94), promoting Foxp3 transcription (95). Thirdly, through metabolic reprogramming of activated T cells, PD-1 inhibits glucose metabolism (96) and promotes fatty acid β-oxidation (97), specifically activating metabolic programs that support Treg cell generation while inhibiting Th0 cell differentiation into Th1 or Th17 cells (98, 99). Therefore, targeting PD-1 and its downstream signaling pathways is an effective means of improving immunity in cancers. The PD-1 pathway represents one of the primary factor in immune escape. Given their specificity and significance, PD-1-blocking agents have shown considerable promise in cancer immunotherapy. Currently, these agents are widely employed in diagnosing and treating clinical diseases, exhibiting high clinical value for advanced cancers. They hold the potential to control other immune diseases through PD-1 signaling as well (100) (Figure 1).
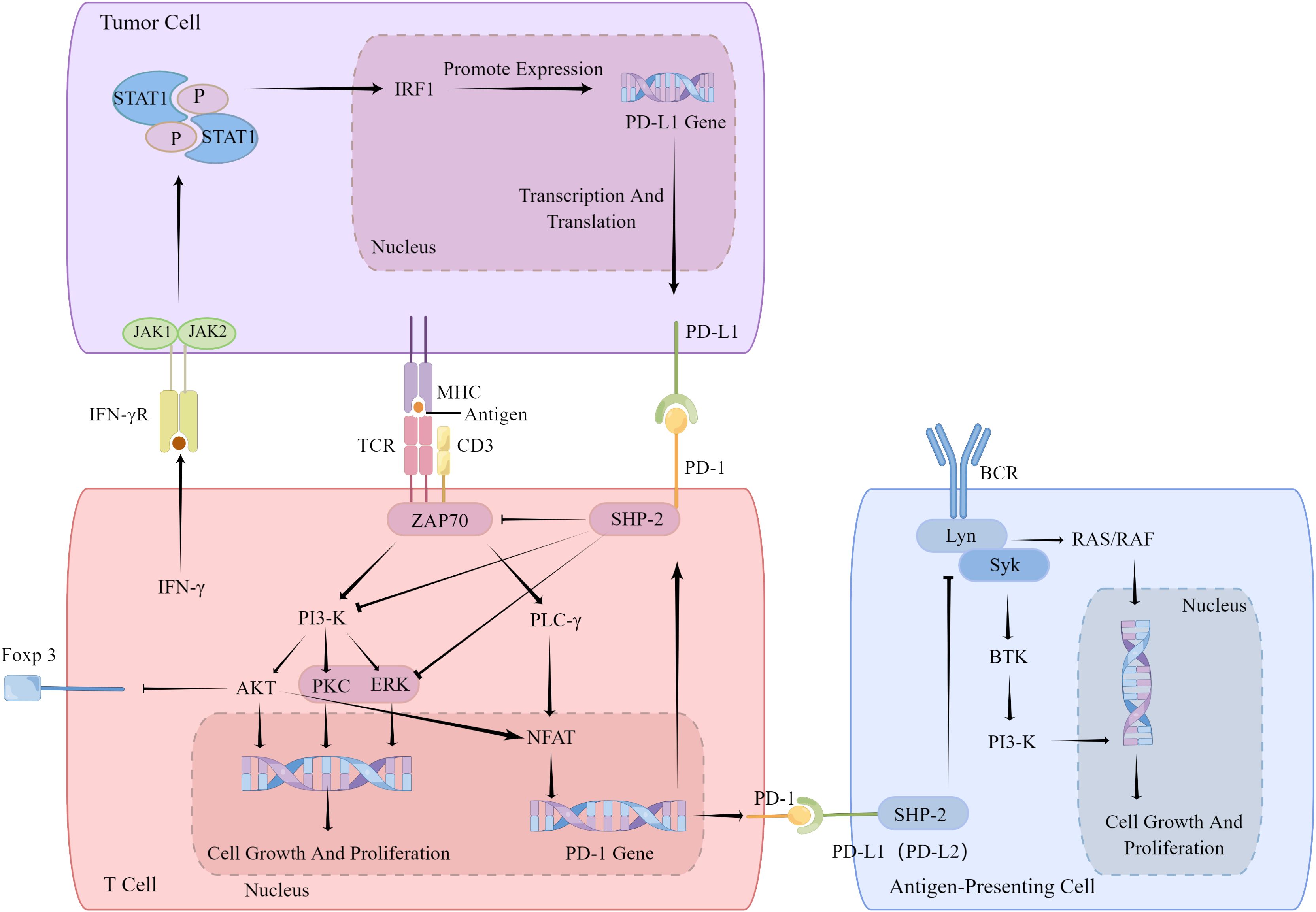
Figure 1. PD-1 inhibit TCR and BCR signaling. PD-1 inhibits the co-stimulatory signal of T cell activation by raising SHP-2,so that T cells cannot be activated normally and lead to increases Foxp3 expression. IFN-γ secreted by T cells will induce tumor cells to express PD-1 receptor PD-L1. PD-1 inhibits B cell activation by inhibiting downstream signal of BCR. IFN-γ, interferon-γ; IRF1, Interferon regulatory factor 1; CD3, coreceptor; PI3-K, SHP-2, ZAP70, JAK1 and JAK2, kinases; PLC-γ, phospholipase C-γ; AKT, kinase; PKC, Protein kinase C; ERK, extracellular regulated protein kinases; NFAT, activating T nuclear factor; NF-κB, transcription factor; Lyn, Syk, BTK, kinases.
3.2 Cytotoxic T lymphocyte antigen-4
CTLA-4 is a transmembrane protein implicated in immune regulation, typically occur on activated T cells. It attenuates T cell activation primarily by inhibiting the CD28 costimulatory signal (Figure 2). This is partially due to its competition with CD28 for recognition to CD80 and CD86 on APCs, which obstructs costimulatory signals essential for T cell activation and prevents downstream signal transduction promoting T cell activation and proliferation (101, 102). Consequently, CTLA-4 makes it difficult for T cells to activate. Upon CTLA-4 activation, T cell activation and IL-2 secretion are diminished, exerting a negative regulatory effect on tumor immunity (Figure 3). Recent studies have also demonstrated that PD-1+Tim-3+CD8+ T lymphocytes exhibit varying degrees of functional impairment in patients with regional metastatic PTC (103).
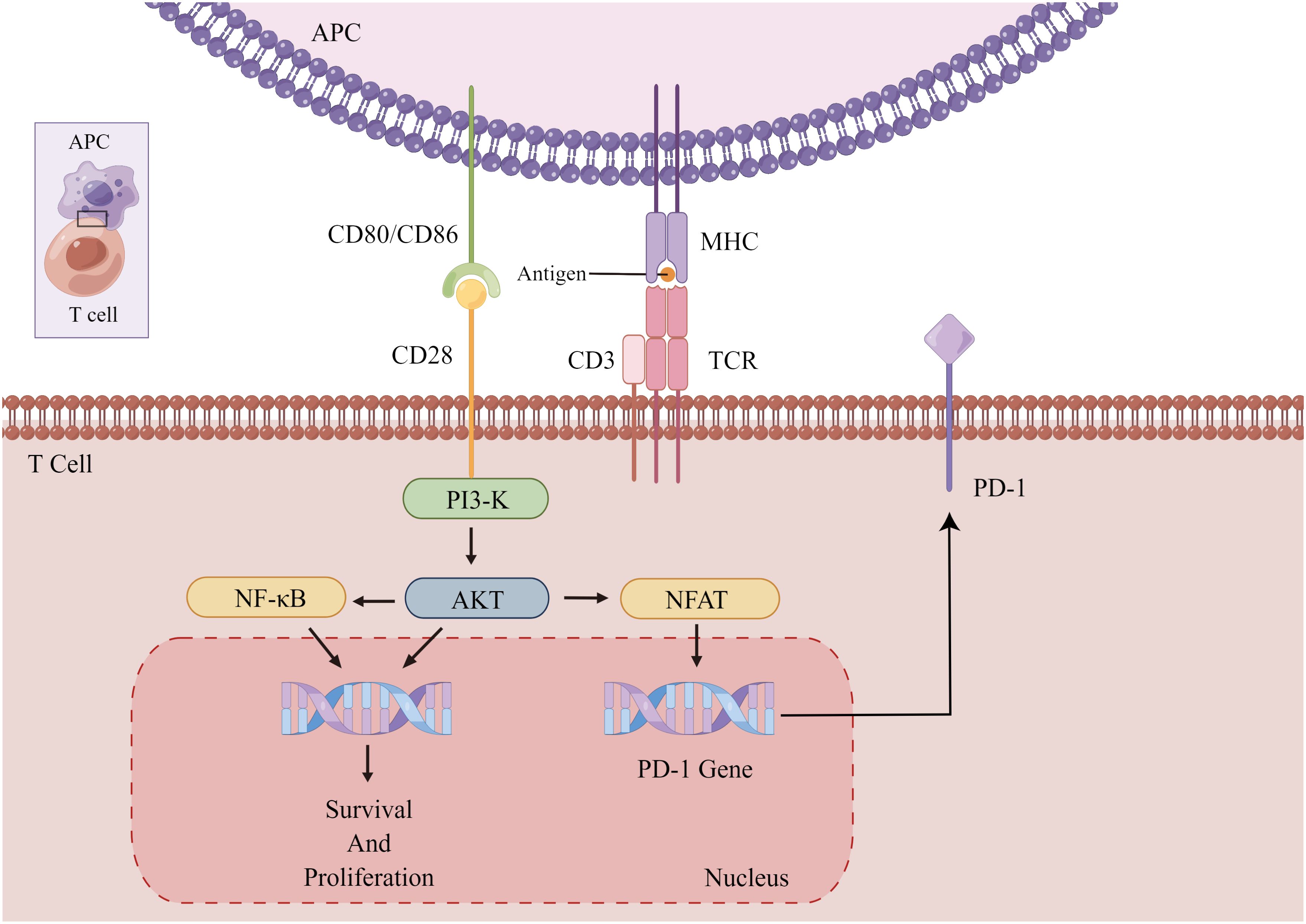
Figure 2. Activation and proliferation of normal T cells. The binding of CD28 and CD80/86 provides a co-stimulatory signal for T cell activation, causing T cell activation and proliferation. Chronically activated T cells increase the expression of PD-1 to prevent immune overshoot. APC, antigen-presenting cells; PI3-K, AKT, kinase; NF-κB, transcription factor; CD3, coreceptor; NFAT, activating T nuclear factor.
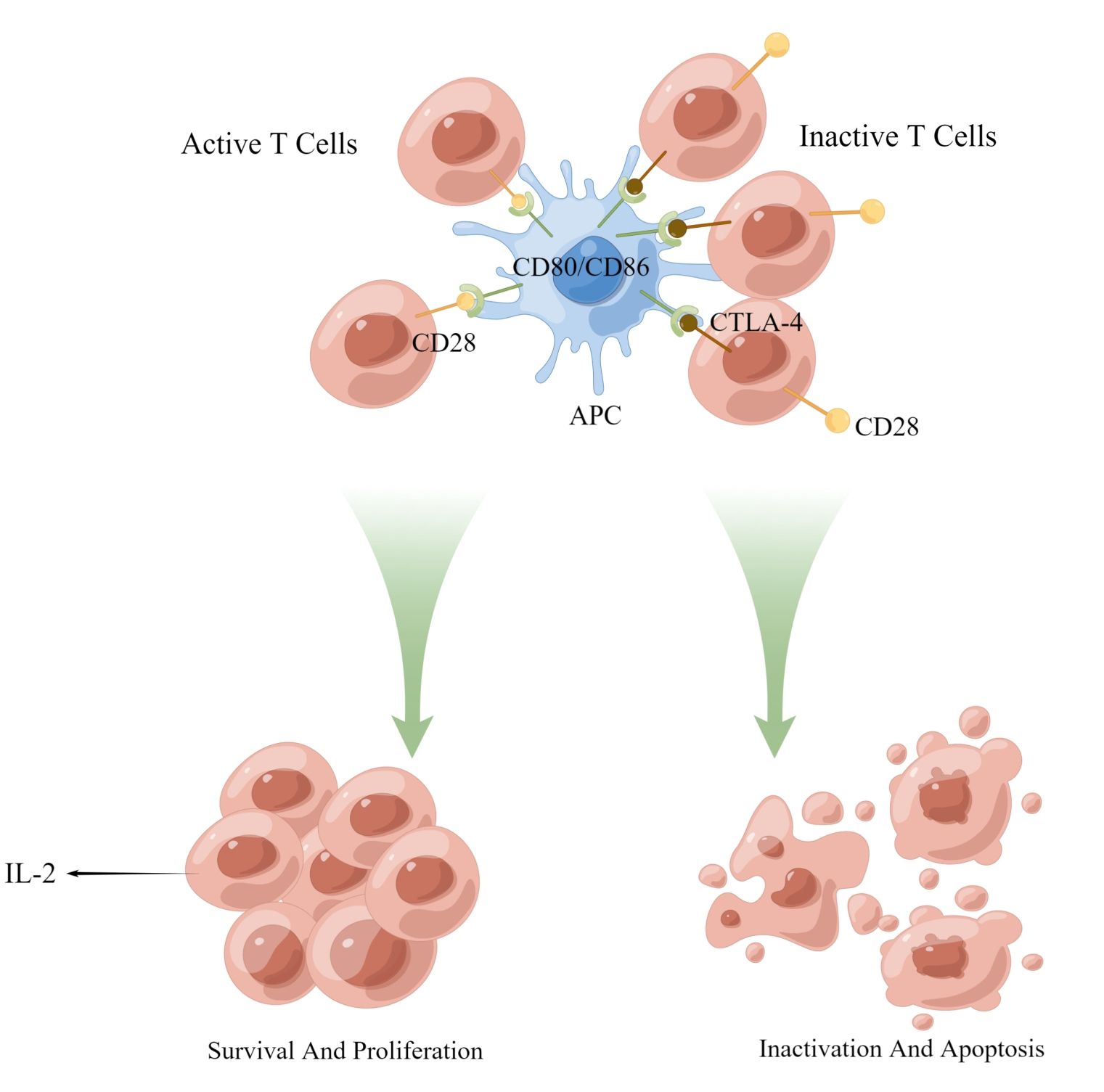
Figure 3. Activation and proliferation of normal T cells. The binding of CD28 and CD80/86 provides a co-stimulatory signal for T cell activation, causing T cell activation and proliferation. Chronically activated T cells increase the expression of PD-1 to prevent immune overshoot. APC, antigen-presenting cells; PI3-K, AKT, kinase; NF-κB, transcription factor; CD3, coreceptor; NFAT, activating T nuclear factor.
In comparison, PD-1 indirectly hinders TCR or BCR responses to antigens via intracellular signaling, while CTLA-4 entirely obstructs CD28 costimulation through competitive inhibition, acting more comprehensively and rapidly (87).
3.3 Indoleamine 2, 3-dioxygenase 1
Indoleamine 2,3-dioxygenase 1 (IDO1) is a oxidoreductase responsible for catalyzing. In papillary thyroid microcarcinoma (PTMC), 31% of the cells were positive for IDO, which may be associated with tumor metastasis (104). In cancer, IDO1 can exert an immunosuppressive function, and its expression is significantly correlated with FoxP3. This relationship promotes tumor immune evasion by inducing FoxP3 phenotype regulation, consequently suppressing the immune microenvironment (105).
4 Regulatory effect of BRAF V600E mutation
BRAF is an activator of the RAS-regulated serine-threonine kinase and the MAPK signaling cascade. This pathway mediates the regulation of cell proliferation, differentiation, and survival in response to extracellular signals. The BRAFV600E mutation simulates phosphorylation in the activating fragment of BRAF, resulting in the dysregulation of cell proliferation (106).
The BRAFV600E gene mutation is closely related to elevated quantity of immunosuppressive regulators in PTC cells. Studies have reported (24) that CTLA-4 and PD-L1 expression levels are inversely associated with thyroid differentiation score (TDS) in PTC, a relationship more pronounced in tumors harboring the BRAFV600E mutation. BRAFV600E tumors expressed higher levels of PD-1 compared to BRAF wild-type tumors (53% vs. 12.5%). BRAFV600E promotes thyroid cancer development by increasing myeloid-derived suppressor cells (MDSCs) (107). As a heterogeneous population of immature myeloid cells, MDSCs are the primary coordinator of the immunosuppressive environment in cancer. MDSCs, primarily through CXCR2, show ligand recruitment to the TME (108). MDSCs are amplified during cancer progression and has the remarkable ability to inhibit T cell function in the tumor microenvironment (109), which is able to produce mediators necessary for neoangiogenesis and tissue invasion (110). In the peripheral circulation, MDSCs promote PTC progression. By inhibiting miR-486-3p, MDSCs promoted the activity of the NF-κB2 signaling pathway, leading to the accelerated invasion (111).
In addition, BRAFV600E upregulated T-box transcription factor 3 (TBX3) induced MAPK pathway activation. Therefore, TBX3 could be associated with BRAFV600E-related tumor genesis (112). TBX3 belongs to the T-box transcription factors family, associated with tumor progression and metastasis (113). Analysis of PTC patient specimens revealed that TBX3 is highly expressed in cancerous thyroid cells, indicating down regulation of TBX3 could delay the G1/S phase transition, decreased cell growth in vitro and inhibited tumor formation in vivo (114).
Considering the strong correlation between BRAF and the pathological characteristic of PTC, BRAF mutation status has the potential to serve as a risk assessment indicator and prognostic marker for PTC. However, similar prediction models are challenging to adapt to multivariate factors, such as patient age and gender, which may increase the cost and complexity of evaluation. These limitations necessitate further exploration (115). Beyond risk assessment and prognosis, the BRAF mutation may play a crucial role as a therapeutic target for PTC. Currently, BRAF kinase inhibitors have been utilized in non-small cell lung cancer and melanoma, while research on PTC treatment remains in its early stages (116).
5 Immunotherapy strategies for PTC
For patients with advanced PTC or distant metastases, conventional therapies, including chemotherapy and radiotherapy, are prone to developing tolerance (117), thereby limiting their effectiveness. Consequently, treatment options for patients with advanced disease or distant metastases are restricted. Harnessing the immune system appears to be a highly promising strategy for addressing these challenges.
5.1 Adoptive cell therapy
Adoptive cell therapy (ACT) involves the extraction of precursor cells from autologous or allogeneic anti-tumor effector cells, followed by their in vitro induction, activation, and expansion using activators such as IL-2 and specific peptides. Proliferating cells are then transfused back into cancer patients and enhance their anti-tumor immunity, aiming to achieve therapeutic effects and prevent recurrence (118, 119).
Phase I clinical trial results have demonstrated that dendritic cells stimulated with autologous PTC tumor lysates can effectively control tumor progression without significant adverse effects (120). In this study, patients with refractory PTC and distant metastases were selected, and some experienced stabilization after treatment, confirming the feasibility of ACT for advanced PTC management.
Apart from DCs, chimeric antigen receptor T (CAR-T) cell immunotherapy has also undergone modifications and been applied in clinical practice in recent years. Genetic engineering techniques enable the addition of chimeric antibodies to T cells, allowing T cells to recognize and simultaneously activate tumor cell killing. There has been preclinical validation on the therapy for intercellular adhesion molecule (ICAM)-1 in thyroid cancer. Based on previous study findings (121), some investigators (122) have verified the feasibility of ICAM-1 as a CAR-targeting antigen by examining its relationship with tumor malignancy in patients with recurrent advanced PTC lacking other treatment options. Other studies (123, 124) have also reported a favorable safety profile for this therapy, suggesting the potential of ICAM-1 as a target for treatment of advanced recurrent thyroid tumors.
Since T cells upregulate ICAM-1 expression upon activation, ICAM-1 CAR-T cells may engage in mutual attacks, potentially reducing T cell infiltration into PTC tissues and causing collateral tissue damage (125). Therefore, further refinement is necessary before this therapy can be widely adopted in clinical practice.
5.2 Immune checkpoint inhibitors
Immune checkpoint inhibitors (ICIs) are monoclonal antibody (mAb) drugs developed to target specific immune checkpoints. Tumor cells cannot interact with immune cells through immune checkpoints above when ICIs are applied, which can block immune checkpoint-mediated immune escape. There has been monoclonal antibodies against PD-1/PD-L1 and CTLA-4, such as pembrolizumab and ipilimumab (126).
Existing trials have demonstrated that ICIs exhibit good efficacy and safety in PTC treatment (126, 127). The potential of combining ICIs with currently available drugs for advanced thyroid cancer has garnered interest. Animal studies have confirmed that combinations of BRAF inhibitors and checkpoint inhibitor immunotherapies synergistically reduce tumor volume in mouse models of carcinoma (128). However, mAbs can sometimes cause immune-related adverse events resembling autoimmune reactions (129), prompting consideration of small molecule inhibitors as alternative therapeutic strategies. Unlike mAbs, small molecule inhibitors can interact with both receptor on the surface and intracellular molecular targets (26), making them a promising therapeutic approach.
The efficacy of ICIs is influenced by the host’s immune status, as they target immune checkpoints and the function of immune cells and molecules in TME changes accordingly. Intrinsic microorganisms contribute to the body’s overall and local immunological regulation and can significantly impact the efficacy of ICIs (130). In PTC, VEGF can inhibit DC antigen presentation, enhance Treg amplification, and mediate the upregulation of PD-1 on T cells in TME. Combining VEGF inhibitors with ICIs can synergistically promote immune checkpoint blockade effect (131–133). Given the unique influence of the immune microenvironment on tumor progression, the combination of anti-inflammatory drugs and ICIs is also common. For instance, aspirin is widely used in cancer treatment and can reduce the mortality rate of various adenocarcinomas (134). Metformin and phenformin affect angiogenesis (135), regulate immune responses (136), and can be used in combination with ICIs. Consequently, to widely apply ICIs in the clinical treatment of PTC, a comprehensive assessment of the patient’s immune status is necessary.
6 Conclusion
In summary, immune cells and molecules in TME are of vital importance in papillary thyroid carcinoma (PTC) progression by modulating immune response against cancer. Immune checkpoints are regulatory molecules in the immune system, with the PD-1/PD-L1 and CTLA-4 pathways emerging as significant contributors to tumor immunosuppression. Furthermore, the BRAFV600E mutation is intimately linked to PTC development and progression, potentially leading to aberrant cell proliferation and subsequent PTC onset. BRAFV600E also exerts a regulatory effect on immune checkpoints. CTLA-4 and PD-L1 levels are inversely associated with TDS, particularly in tumors harboring the BRAFV600E mutation. Consequently, BRAFV600E may serve as a critical target and prognostic marker for PTC treatment.
Patients with advanced disease or distant metastases face limited treatment options, making the utilization of the immune system a particularly promising approach. Adoptive cell therapy, utilizing dendritic cells (DC) and chimeric antigen receptor T (CAR-T) cells, has proven effective for patients with advanced PTC. Employing immune checkpoint inhibitors (ICIs) to modulate PD-1 targets and their downstream signaling pathways effectively enhances the host’s immunity to cancer; however, ICIs can sometimes result in immune-related adverse events, warranting consideration of small molecule inhibitors as an alternative. Moreover, ICI efficacy is easily influenced by gut microorganisms and the body’s immune levels, necessitating the assessment of the host’s immune status during treatment. Combination of ICIs with vascular endothelial growth factor (VEGF) inhibitors or anti-inflammatory drugs has demonstrated improved efficacy and is expected to offer potential therapeutic value for PTC management.
Author contributions
XZ: Funding acquisition, Investigation, Methodology, Supervision, Writing – review & editing. RS: Formal Analysis, Investigation, Methodology, Writing – original draft. TW: Methodology, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Sichuan University West China Hospital Discipline Excellence Development 1 · 3 · 5 Project (No. ZYJC21033).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Brito JP, Kim HJ, Han SJ, Lee YS, Ahn HS. Geographic distribution and evolution of thyroid cancer epidemic in South Korea. Thyroid. (2016) 26:864–5. doi: 10.1089/thy.2016.0057
2. Sipos JA, Mazzaferri EL. Thyroid cancer epidemiology and prognostic variables. Clin Oncol (R Coll Radiol). (2010) 22:395–404. doi: 10.1016/j.clon.2010.05.004
3. Prete A, Borges de Souza P, Censi S, Muzza M, Nucci N, Sponziello M. Update on fundamental mechanisms of thyroid cancer. Front Endocrinol (Lausanne). (2020) 11:102. doi: 10.3389/fendo.2020.00102
4. Vasileiadis I, Boutzios G, Karalaki M, Misiakos E, Karatzas T. Papillary thyroid carcinoma of the isthmus: Total thyroidectomy or isthmusectomy? Am J Surg. (2018) 216:135–9. doi: 10.1016/j.amjsurg.2017.09.008
5. Lim H, Devesa SS, Sosa JA, Check D, Kitahara CM. Trends in thyroid cancer incidence and mortality in the United States, 1974-2013. JAMA. (2017) 317:1338–48. doi: 10.1001/jama.2017.2719
6. Kitahara CM, Sosa JA. The changing incidence of thyroid cancer. Nat Rev Endocrinol. (2016) 12:646–53. doi: 10.1038/nrendo.2016.110
7. Albero A, Lopéz JE, Torres A, de la Cruz L, Martín T. Effectiveness of chemotherapy in advanced differentiated thyroid cancer: a systematic review. Endocr Relat Cancer. (2016) 23:R71–84. doi: 10.1530/ERC-15-0194
8. Agrawal AK, Noronha V, Patil V, Menon N, Kapoor A, Chougule A, et al. Systemic therapy in thyroid cancer. Indian J Surg Oncol. (2022) 13:68–80. doi: 10.1007/s13193-021-01398-2
9. Bai Y, Kakudo K, Li Y, Liu Z, Ozaki T, Ito Y, et al. Subclassification of non-solid-type papillary thyroid carcinoma identification of high-risk group in common type. Cancer Sci. (2008) 99:1908–15. doi: 10.1111/j.1349-7006.2008.00908.x
10. Lin R, Fogarty CE, Ma B, Li H, Ni G, Liu X, et al. Identification of ferroptosis genes in immune infiltration and prognosis in thyroid papillary carcinoma using network analysis. BMC Genomics. (2021) 22:576. doi: 10.1186/s12864-021-07895-6
11. Lundgren CI, Hall P, Dickman PW, Zedenius J. Clinically significant prognostic factors for differentiated thyroid carcinoma: a population-based, nested case-control study. Cancer. (2006) 106:524–31. doi: 10.1002/cncr.21653
12. Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. (2002) 3:991–8. doi: 10.1038/ni1102-991
13. del Campo AB, Carretero J, Aptsiauri N, Garrido F. Targeting HLA class I expression to increase tumor immunogenicity. Tissue Antigens. (2012) 79:147–54. doi: 10.1111/j.1399-0039.2011.01831.x
14. DhatChinamoorthy K, Colbert JD, Rock KL. Cancer immune evasion through loss of MHC class I antigen presentation. Front Immunol. (2021) 12:636568. doi: 10.3389/fimmu.2021.636568
15. Yang Z, Wei X, Pan Y, Xu J, Si Y, Min Z, et al. A new risk factor indicator for papillary thyroid cancer based on immune infiltration. Cell Death Dis. (2021) 12:51. doi: 10.1038/s41419-020-03294-z
16. Bilotta MT, Antignani A, Fitzgerald DJ. Managing the TME to improve the efficacy of cancer therapy. Front Immunol. (2022) 13:954992. doi: 10.3389/fimmu.2022.954992
17. Alzubi MA, Turner TH, Olex AL, Sohal SS, Tobin NP, Recio SG, et al. Separation of breast cancer and organ microenvironment transcriptomes in metastases. Breast Cancer Res. (2019) 21:36. doi: 10.1186/s13058-019-1123-2
18. Shapouri-Moghaddam A, Mohammadian S, Vazini H, Taghadosi M, Esmaeili SA, Mardani F, et al. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol. (2018) 233:6425–40. doi: 10.1002/jcp.26429
19. Kim DI, Kim E, Kim YA, Cho SW, Lim JA, Park YJ. Macrophage densities correlated with CXC chemokine receptor 4 expression and related with poor survival in anaplastic thyroid cancer. Endocrinol Metab (Seoul). (2016) 31:469–75. doi: 10.3803/EnM.2016.31.3.469
20. Gulubova MV, Ivanova KV. The expression of tumor-associated macrophages and multinucleated giant cells in papillary thyroid carcinoma. Open Access Maced J Med Sci. (2019) 7:3944–9. doi: 10.3889/oamjms.2019.715
21. Zhao X, Ma W, Li X, Li H, Li J, Li H, et al. ANXA1 enhances tumor proliferation and migration by regulating epithelial-mesenchymal transition and IL-6/JAK2/STAT3 pathway in papillary thyroid carcinoma. J Cancer. (2021) 12:1295–306. doi: 10.7150/jca.52171
22. Zhang P, Zhang H, Dong W, Wang Z, Qin Y, Wu C, et al. IL-34 is a potential biomarker for the treatment of papillary thyroid cancer. J Clin Lab Anal. (2020) 34:e23335. doi: 10.1002/jcla.23335
23. Zhang X, Li S, Wang J, Liu F, Zhao Y. Relationship between serum inflammatory factor levels and differentiated thyroid carcinoma. Technol Cancer Res Treat. (2021) 20:1533033821990055. doi: 10.1177/1533033821990055
24. Na KJ, Choi H. Immune landscape of papillary thyroid cancer and immunotherapeutic implications. Endocr Relat Cancer. (2018) 25:523–31. doi: 10.1530/ERC-17-0532
25. Ferrari SM, Fallahi P, Galdiero MR, Ruffilli I, Elia G, Ragusa F, et al. Immune and inflammatory cells in thyroid cancer microenvironment. Int J Mol Sci. (2019) 20(18):4413. doi: 10.3390/ijms20184413
26. Liotti F, Prevete N, Vecchio G, Melillo RM. Recent advances in understanding immune phenotypes of thyroid carcinomas: prognostication and emerging therapies. F1000Res. (2019) 8:F1000 Faculty Rev-227. doi: 10.12688/f1000research
27. Xie Z, Li X, He Y, Wu S, Wang S, Sun J, et al. Immune cell confrontation in the papillary thyroid carcinoma microenvironment. Front Endocrinol (Lausanne). (2020) 11:570604. doi: 10.3389/fendo.2020.570604
28. Arianfar E, Khandoozi SR, Mohammadi S, Memarian A. Suppression of CD56(bright) NK cells in breast cancer patients is associated with the PD-1 and TGF-βRII expression. Clin Transl Oncol. (2023) 25:841–51. doi: 10.1007/s12094-022-02997-3
29. Ishigami S, Natsugoe S, Tokuda K, Nakajo A, Che X, Iwashige H, et al. Prognostic value of intratumoral natural killer cells in gastric carcinoma. Cancer. (2000) 88:577–83. doi: 10.1002/(ISSN)1097-0142
30. Xu X, Rao G, Gaffud MJ, Ding HG, Maki G, Klingemann HG, et al. Clinicopathological significance of major histocompatibility complex class I-related chain a and B expression in thyroid cancer. J Clin Endocrinol Metab. (2006) 91:2704–12. doi: 10.1210/jc.2006-0492
31. Gogali F, Paterakis G, Rassidakis GZ, Kaltsas G, Liakou CI, Gousis P, et al. Phenotypical analysis of lymphocytes with suppressive and regulatory properties (Tregs) and NK cells in the papillary carcinoma of thyroid. J Clin Endocrinol Metab. (2012) 97:1474–82. doi: 10.1210/jc.2011-1838
32. Gogali F, Paterakis G, Rassidakis GZ, Liakou CI, Liapi C. CD3(-)CD16(-)CD56(bright) immunoregulatory NK cells are increased in the tumor microenvironment and inversely correlate with advanced stages in patients with papillary thyroid cancer. Thyroid. (2013) 23:1561–8. doi: 10.1089/thy.2012.0560
33. Zhang Q, Bi J, Zheng X, Chen Y, Wang H, Wu W, et al. Blockade of the checkpoint receptor TIGIT prevents NK cell exhaustion and elicits potent anti-tumor immunity. Nat Immunol. (2018) 19:723–32. doi: 10.1038/s41590-018-0132-0
34. Guillerey C, Ferrari de Andrade L, Vuckovic S, Miles K, Ngiow SF, Yong MC, et al. Immunosurveillance and therapy of multiple myeloma are CD226 dependent. J Clin Invest. (2015) 125:2077–89. doi: 10.1172/JCI77181
35. Cong J, Wang X, Zheng X, Wang D, Fu B, Sun R, et al. Dysfunction of natural killer cells by FBP1-induced inhibition of glycolysis during lung cancer progression. Cell Metab. (2018) 28:243–55.e5. doi: 10.1016/j.cmet.2018.06.021
36. Xu HM. Th1 cytokine-based immunotherapy for cancer. Hepatobiliary Pancreat Dis Int. (2014) 13:482–94. doi: 10.1016/S1499-3872(14)60305-2
37. Schreiber S, Hammers CM, Kaasch AJ, Schraven B, Dudeck A, Kahlfuss S. Metabolic interdependency of th2 cell-mediated type 2 immunity and the tumor microenvironment. Front Immunol. (2021) 12:632581. doi: 10.3389/fimmu.2021.632581
38. Jiang G, Ma S, Wei Y, Wu Y, Yu X, Liu H. The prevalence and distribution of Th17 and Tc17 cells in patients with thyroid tumor. Immunol Lett. (2014) 162:68–73. doi: 10.1016/j.imlet.2014.07.005
39. Chen Z, Guo ML, Li YY, Yan K, Li L, Shen F, et al. Immune profiling identifies CD8(+) T-cell subset signatures as prognostic markers for recurrence in papillary thyroid cancer. Front Immunol. (2022) 13:894919. doi: 10.3389/fimmu.2022.894919
40. Farhood B, Najafi M, Mortezaee K. CD8(+) cytotoxic T lymphocytes in cancer immunotherapy: A review. J Cell Physiol. (2019) 234:8509–21. doi: 10.1002/jcp.27782
41. Calik I, Calik M, Turken G, Ozercan IH, Dagli AF, Artas G, et al. Intratumoral cytotoxic T-lymphocyte density and PD-L1 expression are prognostic biomarkers for patients with colorectal cancer. Medicina (Kaunas). (2019) 55(11):723. doi: 10.3390/medicina55110723
42. French JD, Weber ZJ, Fretwell DL, Said S, Klopper JP, Haugen BR. Tumor-associated lymphocytes and increased FoxP3+ regulatory T cell frequency correlate with more aggressive papillary thyroid cancer. J Clin Endocrinol Metab. (2010) 95:2325–33. doi: 10.1210/jc.2009-2564
43. Modi J, Patel A, Terrell R, Tuttle RM, Francis GL. Papillary thyroid carcinomas from young adults and children contain a mixture of lymphocytes. J Clin Endocrinol Metab. (2003) 88:4418–25. doi: 10.1210/jc.2003-030342
44. Whiteside TL. FOXP3+ Treg as a therapeutic target for promoting anti-tumor immunity. Expert Opin Ther Targets. (2018) 22:353–63. doi: 10.1080/14728222.2018.1451514
45. Ondondo B, Jones E, Godkin A, Gallimore A. Home sweet home: the tumor microenvironment as a haven for regulatory T cells. Front Immunol. (2013) 4:197. doi: 10.3389/fimmu.2013.00197
46. Whiteside TL. What are regulatory T cells (Treg) regulating in cancer and why? Semin Cancer Biol. (2012) 22:327–34. doi: 10.1016/j.semcancer.2012.03.004
47. Cheah CY, Fowler NH, Neelapu SS. Targeting the programmed death-1/programmed death-ligand 1 axis in lymphoma. Curr Opin Oncol. (2015) 27:384–91. doi: 10.1097/CCO.0000000000000212
48. Shi J, Zhou J. [Role of CD4+ CD25+ regulatory T cells in peripheral blood from patients with papillary thyroid carcinoma]. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. (2012) 26:965–9, 72.
49. Sawant DV, Hamilton K, Vignali DA. Interleukin-35: expanding its job profile. J Interferon Cytokine Res. (2015) 35:499–512. doi: 10.1089/jir.2015.0015
50. Strauss L, Bergmann C, Szczepanski M, Gooding W, Johnson JT, Whiteside TL. A unique subset of CD4+CD25highFoxp3+ T cells secreting interleukin-10 and transforming growth factor-beta1 mediates suppression in the tumor microenvironment. Clin Cancer Res. (2007) 13:4345–54. doi: 10.1158/1078-0432.CCR-07-0472
51. Czystowska M, Strauss L, Bergmann C, Szajnik M, Rabinowich H, Whiteside TL. Reciprocal granzyme/perforin-mediated death of human regulatory and responder T cells is regulated by interleukin-2 (IL-2). J Mol Med (Berl). (2010) 88:577–88. doi: 10.1007/s00109-010-0602-9
52. Xie F, Ling L, van Dam H, Zhou F, Zhang L. TGF-β signaling in cancer metastasis. Acta Biochim Biophys Sin (Shanghai). (2018) 50:121–32. doi: 10.1093/abbs/gmx123
53. Whiteside TL, Mandapathil M, Schuler P. The role of the adenosinergic pathway in immunosuppression mediated by human regulatory T cells (Treg). Curr Med Chem. (2011) 18:5217–23. doi: 10.2174/092986711798184334
54. Mandapathil M, Szczepanski MJ, Szajnik M, Ren J, Jackson EK, Johnson JT, et al. Adenosine and prostaglandin E2 cooperate in the suppression of immune responses mediated by adaptive regulatory T cells. J Biol Chem. (2010) 285:27571–80. doi: 10.1074/jbc.M110.127100
55. Agarwal A, Fanelli G, Letizia M, Tung SL, Boardman D, Lechler R, et al. Regulatory T cell-derived exosomes: possible therapeutic and diagnostic tools in transplantation. Front Immunol. (2014) 5:555. doi: 10.3389/fimmu.2014.00555
56. Varricchi G, Galdiero MR, Loffredo S, Marone G, Iannone R, Marone G, et al. Are mast cells MASTers in cancer? Front Immunol. (2017) 8:424. doi: 10.3389/fimmu.2017.00424
57. Dyduch G, Kaczmarczyk K, Okoń K. Mast cells and cancer: enemies or allies? Pol J Pathol. (2012) 63:1–7.
58. Thio M, Groot Kormelink T, Fischer MJ, Blokhuis BR, Nijkamp FP, Redegeld FA. Antigen binding characteristics of immunoglobulin free light chains: crosslinking by antigen is essential to induce allergic inflammation. PloS One. (2012) 7:e40986. doi: 10.1371/journal.pone.0040986
59. Siiskonen H, Poukka M, Bykachev A, Tyynelä-Korhonen K, Sironen R, Pasonen-Seppänen S, et al. Low numbers of tryptase+ and chymase+ mast cells associated with reduced survival and advanced tumor stage in melanoma. Melanoma Res. (2015) 25:479–85. doi: 10.1097/CMR.0000000000000192
60. Hölzel M, Landsberg J, Glodde N, Bald T, Rogava M, Riesenberg S, et al. A preclinical model of Malignant peripheral nerve sheath tumor-like melanoma is characterized by infiltrating mast cells. Cancer Res. (2016) 76:251–63. doi: 10.1158/0008-5472.CAN-15-1090
61. Melillo RM, Guarino V, Avilla E, Galdiero MR, Liotti F, Prevete N, et al. Mast cells have a protumorigenic role in human thyroid cancer. Oncogene. (2010) 29:6203–15. doi: 10.1038/onc.2010.348
62. Visciano C, Liotti F, Prevete N, Cali G, Franco R, Collina F, et al. Mast cells induce epithelial-to-mesenchymal transition and stem cell features in human thyroid cancer cells through an IL-8-Akt-Slug pathway. Oncogene. (2015) 34:5175–86. doi: 10.1038/onc.2014.441
63. Pan Y, Yu Y, Wang X, Zhang T. Tumor-associated macrophages in tumor immunity. Front Immunol. (2020) 11:583084. doi: 10.3389/fimmu.2020.583084
64. Zhao H, Achreja A, Iessi E, Logozzi M, Mizzoni D, Di Raimo R, et al. The key role of extracellular vesicles in the metastatic process. Biochim Biophys Acta Rev Cancer. (2018) 1869:64–77. doi: 10.1016/j.bbcan.2017.11.005
65. Braun DA, Street K, Burke KP, Cookmeyer DL, Denize T, Pedersen CB, et al. Progressive immune dysfunction with advancing disease stage in renal cell carcinoma. Cancer Cell. (2021) 39:632–48.e8. doi: 10.1016/j.ccell.2021.02.013
66. Jung KY, Cho SW, Kim YA, Kim D, Oh BC, Park DJ, et al. Cancers with higher density of tumor-associated macrophages were associated with poor survival rates. J Pathol Transl Med. (2015) 49:318–24. doi: 10.4132/jptm.2015.06.01
67. Qing W, Fang WY, Ye L, Shen LY, Zhang XF, Fei XC, et al. Density of tumor-associated macrophages correlates with lymph node metastasis in papillary thyroid carcinoma. Thyroid. (2012) 22:905–10. doi: 10.1089/thy.2011.0452
68. Ryder M, Ghossein RA, Ricarte-Filho JC, Knauf JA, Fagin JA. Increased density of tumor-associated macrophages is associated with decreased survival in advanced thyroid cancer. Endocr Relat Cancer. (2008) 15:1069–74. doi: 10.1677/ERC-08-0036
69. Fang W, Ye L, Shen L, Cai J, Huang F, Wei Q, et al. Tumor-associated macrophages promote the metastatic potential of thyroid papillary cancer by releasing CXCL8. Carcinogenesis. (2014) 35:1780–7. doi: 10.1093/carcin/bgu060
70. Hilly O, Koren R, Raz R, Rath-Wolfson L, Mizrachi A, Hamzany Y, et al. The role of s100-positive dendritic cells in the prognosis of papillary thyroid carcinoma. Am J Clin Pathol. (2013) 139:87–92. doi: 10.1309/AJCPAKYDO56NKMYZ
71. Scouten WT, Francis GL. Thyroid cancer and the immune system: a model for effective immune surveillance. Expert Rev Endocrinol Metab. (2006) 1:353–66. doi: 10.1586/17446651.1.3.353
72. Jang JE, Hajdu CH, Liot C, Miller G, Dustin ML, Bar-Sagi D. Crosstalk between regulatory T cells and tumor-associated dendritic cells negates anti-tumor immunity in pancreatic cancer. Cell Rep. (2017) 20:558–71. doi: 10.1016/j.celrep.2017.06.062
73. Mould RC, van Vloten JP, AuYeung AWK, Karimi K, Bridle BW. Immune responses in the thyroid cancer microenvironment: making immunotherapy a possible mission. Endocr Relat Cancer. (2017) 24:T311–t29. doi: 10.1530/ERC-17-0316
74. Tecchio C, Scapini P, Pizzolo G, Cassatella MA. On the cytokines produced by human neutrophils in tumors. Semin Cancer Biol. (2013) 23:159–70. doi: 10.1016/j.semcancer.2013.02.004
75. Scapini P, Cassatella MA. Social networking of human neutrophils within the immune system. Blood. (2014) 124:710–9. doi: 10.1182/blood-2014-03-453217
76. Scapini P, Morini M, Tecchio C, Minghelli S, Di Carlo E, Tanghetti E, et al. CXCL1/macrophage inflammatory protein-2-induced angiogenesis in vivo is mediated by neutrophil-derived vascular endothelial growth factor-A. J Immunol. (2004) 172:5034–40. doi: 10.4049/jimmunol.172.8.5034
77. van Egmond M, Bakema JE. Neutrophils as effector cells for antibody-based immunotherapy of cancer. Semin Cancer Biol. (2013) 23:190–9. doi: 10.1016/j.semcancer.2012.12.002
78. Mishalian I, Granot Z, Fridlender ZG. The diversity of circulating neutrophils in cancer. Immunobiology. (2017) 222:82–8. doi: 10.1016/j.imbio.2016.02.001
79. Galdiero MR, Varricchi G, Loffredo S, Bellevicine C, Lansione T, Ferrara AL, et al. Potential involvement of neutrophils in human thyroid cancer. PloS One. (2018) 13:e0199740. doi: 10.1371/journal.pone.0199740
80. Bhatti I, Peacock O, Lloyd G, Larvin M, Hall RI. Preoperative hematologic markers as independent predictors of prognosis in resected pancreatic ductal adenocarcinoma: neutrophil-lymphocyte versus platelet-lymphocyte ratio. Am J Surg. (2010) 200:197–203. doi: 10.1016/j.amjsurg.2009.08.041
81. Liu CL, Lee JJ, Liu TP, Chang YC, Hsu YC, Cheng SP. Blood neutrophil-to-lymphocyte ratio correlates with tumor size in patients with differentiated thyroid cancer. J Surg Oncol. (2013) 107:493–7. doi: 10.1002/jso.23270
82. Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. (2013) 39:1–10. doi: 10.1016/j.immuni.2013.07.012
83. Ferrari SM, Fallahi P, Galetta F, Citi E, Benvenga S, Antonelli A. Thyroid disorders induced by checkpoint inhibitors. Rev Endocr Metab Disord. (2018) 19:325–33. doi: 10.1007/s11154-018-9463-2
84. Angell TE, Lechner MG, Jang JK, Correa AJ, LoPresti JS, Epstein AL. BRAF V600E in papillary thyroid carcinoma is associated with increased programmed death ligand 1 expression and suppressive immune cell infiltration. Thyroid. (2014) 24:1385–93. doi: 10.1089/thy.2014.0134
85. Bai Y, Guo T, Huang X, Wu Q, Niu D, Ji X, et al. In papillary thyroid carcinoma, expression by immunohistochemistry of BRAF V600E, PD-L1, and PD-1 is closely related. Virchows Arch. (2018) 472:779–87. doi: 10.1007/s00428-018-2357-6
86. Mahoney KM, Freeman GJ, McDermott DF. The next immune-checkpoint inhibitors: PD-1/PD-L1 blockade in melanoma. Clin Ther. (2015) 37:764–82. doi: 10.1016/j.clinthera.2015.02.018
87. Okazaki T, Chikuma S, Iwai Y, Fagarasan S, Honjo T. A rheostat for immune responses: the unique properties of PD-1 and their advantages for clinical application. Nat Immunol. (2013) 14:1212–8. doi: 10.1038/ni.2762
88. Cunha LL, Marcello MA, Morari EC, Nonogaki S, Conte FF, Gerhard R, et al. Differentiated thyroid carcinomas may elude the immune system by B7H1 upregulation. Endocr Relat Cancer. (2013) 20:103–10. doi: 10.1530/ERC-12-0313
89. Shi X, Yu PC, Lei BW, Li CW, Zhang Y, Tan LC, et al. Association between programmed death-ligand 1 expression and clinicopathological characteristics, structural recurrence, and biochemical recurrence/persistent disease in medullary thyroid carcinoma. Thyroid. (2019) 29:1269–78. doi: 10.1089/thy.2019.0079
90. Yokosuka T, Takamatsu M, Kobayashi-Imanishi W, Hashimoto-Tane A, Azuma M, Saito T. Programmed cell death 1 forms negative costimulatory microclusters that directly inhibit T cell receptor signaling by recruiting phosphatase SHP2. J Exp Med. (2012) 209:1201–17. doi: 10.1084/jem.20112741
91. Good-Jacobson KL, Szumilas CG, Chen L, Sharpe AH, Tomayko MM, Shlomchik MJ. PD-1 regulates germinal center B cell survival and the formation and affinity of long-lived plasma cells. Nat Immunol. (2010) 11:535–42. doi: 10.1038/ni.1877
92. Francisco LM, Salinas VH, Brown KE, Vanguri VK, Freeman GJ, Kuchroo VK, et al. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. (2009) 206:3015–29. doi: 10.1084/jem.20090847
93. Appleman LJ, van Puijenbroek AA, Shu KM, Nadler LM, Boussiotis VA. CD28 costimulation mediates down-regulation of p27kip1 and cell cycle progression by activation of the PI3K/PKB signaling pathway in primary human T cells. J Immunol. (2002) 168:2729–36. doi: 10.4049/jimmunol.168.6.2729
94. Sherr CJ, Roberts JM. Living with or without cyclins and cyclin-dependent kinases. Genes Dev. (2004) 18:2699–711. doi: 10.1101/gad.1256504
95. Matsuura I, Denissova NG, Wang G, He D, Long J, Liu F. Cyclin-dependent kinases regulate the antiproliferative function of Smads. Nature. (2004) 430:226–31. doi: 10.1038/nature02650
96. Boussiotis VA, Chatterjee P, Li L. Biochemical signaling of PD-1 on T cells and its functional implications. Cancer J. (2014) 20:265–71. doi: 10.1097/PPO.0000000000000059
97. Pearce EL, Walsh MC, Cejas PJ, Harms GM, Shen H, Wang LS, et al. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature. (2009) 460:103–7. doi: 10.1038/nature08097
98. Frauwirth KA, Riley JL, Harris MH, Parry RV, Rathmell JC, Plas DR, et al. The CD28 signaling pathway regulates glucose metabolism. Immunity. (2002) 16:769–77. doi: 10.1016/S1074-7613(02)00323-0
99. Goldberg MV, Maris CH, Hipkiss EL, Flies AS, Zhen L, Tuder RM, et al. Role of PD-1 and its ligand, B7-H1, in early fate decisions of CD8 T cells. Blood. (2007) 110:186–92. doi: 10.1182/blood-2006-12-062422
100. Chamoto K, Al-Habsi M, Honjo T. Role of PD-1 in immunity and diseases. Curr Top Microbiol Immunol. (2017) 410:75–97. doi: 10.1007/82_2017_67
101. Linsley PS, Greene JL, Brady W, Bajorath J, Ledbetter JA, Peach R. Human B7-1 (CD80) and B7-2 (CD86) bind with similar avidities but distinct kinetics to CD28 and CTLA-4 receptors. Immunity. (1994) 1:793–801. doi: 10.1016/S1074-7613(94)80021-9
102. Fife BT, Bluestone JA. Control of peripheral T-cell tolerance and autoimmunity via the CTLA-4 and PD-1 pathways. Immunol Rev. (2008) 224:166–82. doi: 10.1111/j.1600-065X.2008.00662.x
103. Severson JJ, Serracino HS, Mateescu V, Raeburn CD, McIntyre RC Jr., Sams SB, et al. PD-1+Tim-3+ CD8+ T lymphocytes display varied degrees of functional exhaustion in patients with regionally metastatic differentiated thyroid cancer. Cancer Immunol Res. (2015) 3:620–30. doi: 10.1158/2326-6066.CIR-14-0201
104. Ryu HS, Park YS, Park HJ, Chung YR, Yom CK, Ahn SH, et al. Expression of indoleamine 2,3-dioxygenase and infiltration of FOXP3+ regulatory T cells are associated with aggressive features of papillary thyroid microcarcinoma. Thyroid. (2014) 24:1232–40. doi: 10.1089/thy.2013.0423
105. Moretti S, Menicali E, Voce P, Morelli S, Cantarelli S, Sponziello M, et al. Indoleamine 2,3-dioxygenase 1 (IDO1) is up-regulated in thyroid carcinoma and drives the development of an immunosuppressant tumor microenvironment. J Clin Endocrinol Metab. (2014) 99:E832–40. doi: 10.1210/jc.2013-3351
106. Zaman A, Wu W, Bivona TG. Targeting oncogenic BRAF: past, present, and future. Cancers (Basel). (2019) 11(8): 1197. doi: 10.3390/cancers11081197
107. Zhang P, Guan H, Yuan S, Cheng H, Zheng J, Zhang Z, et al. Targeting myeloid derived suppressor cells reverts immune suppression and sensitizes BRAF-mutant papillary thyroid cancer to MAPK inhibitors. Nat Commun. (2022) 13:1588. doi: 10.1038/s41467-022-29000-5
108. Taki M, Abiko K, Baba T, Hamanishi J, Yamaguchi K, Murakami R, et al. Snail promotes ovarian cancer progression by recruiting myeloid-derived suppressor cells via CXCR2 ligand upregulation. Nat Commun. (2018) 9:1685. doi: 10.1038/s41467-018-03966-7
109. Marvel D, Gabrilovich DI. Myeloid-derived suppressor cells in the tumor microenvironment: expect the unexpected. J Clin Invest. (2015) 125:3356–64. doi: 10.1172/JCI80005
110. Sevko A, Umansky V. Myeloid-derived suppressor cells interact with tumors in terms of myelopoiesis, tumorigenesis and immunosuppression: thick as thieves. J Cancer. (2013) 4:3–11. doi: 10.7150/jca.5047
111. Chen L, Xiong L, Hong S, Li J, Huo Z, Li Y, et al. Circulating myeloid-derived suppressor cells facilitate invasion of thyroid cancer cells by repressing miR-486-3p. J Clin Endocrinol Metab. (2020) 105(8):dgaa344. doi: 10.1210/clinem/dgaa344
112. Boyd SC, Mijatov B, Pupo GM, Tran SL, Gowrishankar K, Shaw HM, et al. Oncogenic B-RAF(V600E) signaling induces the T-Box3 transcriptional repressor to repress E-cadherin and enhance melanoma cell invasion. J Invest Dermatol. (2013) 133:1269–77. doi: 10.1038/jid.2012.421
113. Willmer T, Cooper A, Peres J, Omar R, Prince S. The T-Box transcription factor 3 in development and cancer. Biosci Trends. (2017) 11:254–66. doi: 10.5582/bst.2017.01043
114. Li X, Ruan X, Zhang P, Yu Y, Gao M, Yuan S, et al. TBX3 promotes proliferation of papillary thyroid carcinoma cells through facilitating PRC2-mediated p57(KIP2) repression. Oncogene. (2018) 37:2773–92. doi: 10.1038/s41388-017-0090-2
115. Xing M, Alzahrani AS, Carson KA, Viola D, Elisei R, Bendlova B, et al. Association between BRAF V600E mutation and mortality in patients with papillary thyroid cancer. Jama. (2013) 309:1493–501. doi: 10.1001/jama.2013.3190
116. Scheffel RS, Dora JM, Maia AL. BRAF mutations in thyroid cancer. Curr Opin Oncol. (2022) 34:9–18. doi: 10.1097/CCO.0000000000000797
117. Subbiah V, Kreitman RJ, Wainberg ZA, Cho JY, Schellens JHM, Soria JC, et al. Dabrafenib and trametinib treatment in patients with locally advanced or metastatic BRAF V600-mutant anaplastic thyroid cancer. J Clin Oncol. (2018) 36:7–13. doi: 10.1200/JCO.2017.73.6785
118. Yin H, Tang Y, Guo Y, Wen S. Immune microenvironment of thyroid cancer. J Cancer. (2020) 11:4884–96. doi: 10.7150/jca.44506
119. Rosenberg SA, Restifo NP. Adoptive cell transfer as personalized immunotherapy for human cancer. Science. (2015) 348:62–8. doi: 10.1126/science.aaa4967
120. Kuwabara K, Nishishita T, Morishita M, Oyaizu N, Yamashita S, Kanematsu T, et al. Results of a phase I clinical study using dendritic cell vaccinations for thyroid cancer. Thyroid. (2007) 17:53–8. doi: 10.1089/thy.2006.0178
121. Vedvyas Y, Shevlin E, Zaman M, Min IM, Amor-Coarasa A, Park S, et al. Longitudinal PET imaging demonstrates biphasic CAR T cell responses in survivors. JCI Insight. (2016) 1:e90064. doi: 10.1172/jci.insight.90064
122. Min IM, Shevlin E, Vedvyas Y, Zaman M, Wyrwas B, Scognamiglio T, et al. CAR T therapy targeting ICAM-1 eliminates advanced human thyroid tumors. Clin Cancer Res. (2017) 23:7569–83. doi: 10.1158/1078-0432.CCR-17-2008
123. Hansson M, Gimsing P, Badros A, Niskanen TM, Nahi H, Offner F, et al. A phase I dose-escalation study of antibody BI-505 in relapsed/refractory multiple myeloma. Clin Cancer Res. (2015) 21:2730–6. doi: 10.1158/1078-0432.CCR-14-3090
124. Kavanaugh AF, Davis LS, Jain RI, Nichols LA, Norris SH, Lipsky PE. A phase I/II open label study of the safety and efficacy of an anti-ICAM-1 (intercellular adhesion molecule-1; CD54) monoclonal antibody in early rheumatoid arthritis. J Rheumatol. (1996) 23:1338–44.
125. French JD, Haugen BR. Thyroid cancer: CAR T cell therapy - potential in advanced thyroid cancer? Nat Rev Endocrinol. (2018) 14:10–1. doi: 10.1038/nrendo.2017.160
126. Menicali E, Guzzetti M, Morelli S, Moretti S, Puxeddu E. Immune landscape of thyroid cancers: new insights. Front Endocrinol (Lausanne). (2020) 11:637826. doi: 10.3389/fendo.2020.637826
127. Görgün G, Samur MK, Cowens KB, Paula S, Bianchi G, Anderson JE, et al. Lenalidomide enhances immune checkpoint blockade-induced immune response in multiple myeloma. Clin Cancer Res. (2015) 21:4607–18. doi: 10.1158/1078-0432.CCR-15-0200
128. Gunda V, Gigliotti B, Ndishabandi D, Ashry T, McCarthy M, Zhou Z, et al. Combinations of BRAF inhibitor and anti-PD-1/PD-L1 antibody improve survival and tumour immunity in an immunocompetent model of orthotopic murine anaplastic thyroid cancer. Br J Cancer. (2018) 119:1223–32. doi: 10.1038/s41416-018-0296-2
129. Zhou X, Yao Z, Yang H, Liang N, Zhang X, Zhang F. Are immune-related adverse events associated with the efficacy of immune checkpoint inhibitors in patients with cancer? A systematic review and meta-analysis. BMC Med. (2020) 18:87. doi: 10.1186/s12916-020-01549-2
130. Gopalakrishnan V, Spencer CN, Nezi L, Reuben A, Andrews MC, Karpinets TV, et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. (2018) 359:97–103. doi: 10.1126/science.aan4236
131. Voron T, Marcheteau E, Pernot S, Colussi O, Tartour E, Taieb J, et al. Control of the immune response by pro-angiogenic factors. Front Oncol. (2014) 4:70. doi: 10.3389/fonc.2014.00070
132. Mortara L, Benest AV, Bates DO, Noonan DM. Can the co-dependence of the immune system and angiogenesis facilitate pharmacological targeting of tumours? Curr Opin Pharmacol. (2017) 35:66–74. doi: 10.1016/j.coph.2017.05.009
133. Schmittnaegel M, Rigamonti N, Kadioglu E, Cassará A, Wyser Rmili C, Kiialainen A, et al. Dual angiopoietin-2 and VEGFA inhibition elicits antitumor immunity that is enhanced by PD-1 checkpoint blockade. Sci Transl Med. (2017) 9(385):eaak9670. doi: 10.1126/scitranslmed.aak9670
134. Cuzick J, Thorat MA, Bosetti C, Brown PH, Burn J, Cook NR, et al. Estimates of benefits and harms of prophylactic use of aspirin in the general population. Ann Oncol. (2015) 26:47–57. doi: 10.1093/annonc/mdu225
135. Orecchioni S, Reggiani F, Talarico G, Mancuso P, Calleri A, Gregato G, et al. The biguanides metformin and phenformin inhibit angiogenesis, local and metastatic growth of breast cancer by targeting both neoplastic and microenvironment cells. Int J Cancer. (2015) 136:E534–44. doi: 10.1002/ijc.29193
Keywords: papillary thyroid cancer, immune microenvironment, immunization therapy, immune checkpoints, immune cells
Citation: Zheng X, Sun R and Wei T (2024) Immune microenvironment in papillary thyroid carcinoma: roles of immune cells and checkpoints in disease progression and therapeutic implications. Front. Immunol. 15:1438235. doi: 10.3389/fimmu.2024.1438235
Received: 25 May 2024; Accepted: 14 August 2024;
Published: 03 September 2024.
Edited by:
Lei Cai, Chongqing General Hospital, ChinaReviewed by:
S. Peter Goedegebuure, Washington University in St. Louis, United StatesMingjian Zhao, Dalian Medical University, China
Copyright © 2024 Zheng, Sun and Wei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tao Wei, c3VyZ2VvbndlaTU3NzZAMTI2LmNvbQ==
†These authors have contributed equally to this work
 Xun Zheng
Xun Zheng Ruonan Sun
Ruonan Sun Tao Wei
Tao Wei