- 1Department of Intensive Care, Erasmus University Medical Center, Rotterdam, Netherlands
- 2Department of Viroscience, Erasmus University Medical Center, Rotterdam, Netherlands
- 3Department of Immunology, Erasmus University Medical Center, Rotterdam, Netherlands
- 4Laboratory Medical Immunology, Department of Immunology, Erasmus University Medical Center, Rotterdam, Netherlands
- 5Department of Internal Medicine, Division of Allergy & Clinical Immunology, Erasmus University Medical Center, Rotterdam, Netherlands
- 6Department of Internal Medicine, Erasmus University Medical Center, Rotterdam, Netherlands
Introduction: Severe COVID-19 is associated with reduced absolute lymphocyte counts, suggesting that lymphocyte subsets may serve as predictors of clinical outcomes in affected patients. Early identification of patients at risk for severe disease is crucial for optimizing care, accurately informing patients and their families, guiding therapeutic interventions, and improving patient flow in the ED. Given that immunosuppressive drugs significantly impact lymphocyte profiles, we aimed to determine the association between prior use of immunosuppressive drugs, lymphocyte subsets, and COVID-19 severity in our population with a high prevalence of immunosuppression.
Methods: In 2021, suspected COVID-19 patients were included in the ED. Lymphocyte subsets were determined in peripheral blood within 24 hours after presentation and comparative analyses was performed between SARS-CoV-2 negative and positive patients, mild versus severe disease and patients with and without prior immunosuppressive drug use. Mild cases were patients discharged home or admitted to a general ward, severe cases were patients with COVID-19-related mortality or necessitating ICU admission. Logistic regression analysis was performed to assess the association between lymphocyte subsets and COVID-19 severity, and between prior immunosuppressive drug use and COVID-19 severity.
Results: Twenty-five SARS-CoV-2 negative and 77 SARS-CoV-2 positive patients were included, whereof 57 (74%) had mild and 20 (26%) severe COVID-19. No significant differences were observed in the absolute counts of CD3+, CD4+, and CD8+ T-lymphocytes, B-lymphocytes, and NK-cells between SARS-CoV-2 negative and positive patients or between mild and severe cases. The 36 patients with prior use of immunosuppressive drugs had significantly lower CD4+ T-lymphocytes (p<0.01). Prior use of immunosuppressive drugs was not associated with COVID-19 severity (adjusted OR 1.074, 0.355-3.194).
Conclusion: Lymphocyte subsets were not significantly different between SARS-CoV-2 negative and positive patients and between mild versus severe cases. Neither lymphocyte subsets nor prior immunosuppressive drug use were associated with COVID-19 severity.
1 Introduction
The emergence of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in Wuhan, China, causing the Coronavirus disease 2019 (COVID-19), led to a global pandemic. Initially, COVID-19 was associated with a high risk of morbidity and mortality (1). Although the risk of severe disease has decreased in the general population due to acquired immunity through natural infection or vaccination, specific patient groups remain vulnerable, including immunocompromised patients (2–5). Upon hospital presentation, COVID-19 patients show a broad clinical spectrum, ranging from mild respiratory symptoms to life-threatening acute respiratory distress syndrome (ARDS) (6–9). Anticipating the course of their disease in an early state remains challenging and requires further understanding.
Early identification of patients at risk for severe disease is crucial for optimizing care, accurately informing patients and their families, guiding therapeutic interventions, and improving patient flow in the ED (10). Furthermore, in times of limited hospital admission capacity it is imperative to identify patients at low risk of severe outcomes, so home-based monitoring or treatment can be considered (11).
One approach that might identify patients at risk for severe outcome is lymphocyte subset analysis (12–15). Lymphocytes play a pivotal role in coordinating innate and adaptive immune response, and serve as key players in the recognition and elimination of viral pathogens, including SARS-CoV-2. Patients with severe COVID-19 often show reduced absolute lymphocyte counts, particularly CD4+ T- lymphocytes and CD8+ T-lymphocytes (16, 17). Therefore, exploring the association between lymphocyte subsets and severe outcomes in patients presenting to the ED holds interest.
Although the exact impact of the use of immunosuppressive drugs on outcome in COVID-19 remains incompletely understood, it is generally found that immunosuppressed individuals have an increased risk of severe outcome (2, 4, 18, 19). The increased susceptibility to severe COVID-19 might be attributable to an absolute decrease in lymphocyte subsets or to other factors such as functional impairment of immune cells, disturbed response to vaccination, or specific patient characteristics like comorbidities (5, 20). When treating patients with immunosuppressive drugs, a delicate balance needs to be found between suppressing the immune system and maintaining its ability to defend against pathogens. Early testing of lymphocyte subsets might provide diagnostic and therapeutic guidance in these patients.
In this study, conducted at our tertiary academic medical center with a high prevalence of immunosuppressive drug use, we hypothesize that differences in lymphocyte subsets are associated with severe or fatal COVID-19 disease. Our aims are to compare lymphocyte subsets between SARS-CoV-2 negative and positive patients, as well as in mild versus severe COVID-19 disease, and to evaluate the association between lymphocyte subsets and the severity of COVID-19. Additionally, we study the association between prior immunosuppressive drug use on lymphocyte subsets and COVID-19 severity.
2 Methods
2.1 Study participants and design
This single-center cohort study was conducted at the Erasmus MC in Rotterdam, a tertiary academic medical center in the Netherlands. Patients were enrolled between March 10, 2021 and June 4, 2021 at the ED. Patients were eligible for inclusion if they presented at the ED with suspected COVID-19, were at least 18 years of age, and provided informed consent. A suspected SARS-CoV-2 infection was defined as a patient having complaints that could fit the diagnosis COVID-19 including: dry cough, fever, headache, diarrhea, dyspnea, rhinitis and/or lack of taste or scent. SARS-CoV-2 positivity was diagnosed by a positive nasopharyngeal swab for SARS-CoV-2 detected through reverse- transcriptase polymerase chain reaction (RT-PCR). Exclusion criteria for participation were insufficient knowledge of the Dutch language and disabilities unrelated to COVID-19. Follow-up was defined as date of ED presentation until 30 days after discharge. Patients directly transferred from the ED to the ICU could not be included due to logistical reasons. If providing informed consent was not possible, e.g. in case of sedation for invasive mechanical ventilation and/or lack of consciousness, informed consent could be obtained from a legal representative. Withdrawal of participation was possible at all times.
This study was approved by the local Medical Ethics Review Committee of the Erasmus University Medical Center (Erasmus MC) under protocol number MEC- 2020-0337 and conducted according to the principles of the World Medical Association Declaration of Helsinki. All participants or their legal representatives provided written informed consent before enrollment.
2.2 Data collection
Peripheral blood samples were drawn at the ED within 24 hours after presentation from all patients in three Na-heparin tubes and one serum separating tube. To determine lymphocyte subsets, flow cytometric analysis was performed on the collected peripheral blood samples. This analysis was conducted in an ISO15189 certified laboratory, utilizing a FACS CantoII instrument manufactured by Becton Dickinson (14). Lymphocyte subsets, including CD3+ T-lymphocytes, CD4+ T-lymphocytes, CD8+ T-lymphocytes, B-lymphocytes, and NK-cells were defined as follows: CD3+ T-lymphocytes as CD45+CD3+CD4-CD8-, CD4 T-lymphocytes as CD45+CD3+CD4+CD8-, CD8 T-lymphocytes as CD45+CD3+CD8+CD4-, B-lymphocytes as CD45+CD3-CD16/56-CD19+, and NK-cells as CD45+CD3-CD19-CD16/56+.
Demographic data, pre-existing comorbidities, medication use, laboratory parameters, vital parameters, use of respiratory support, treatment and outcome data were obtained from electronic medical records. Information on COVID-19 complaints and COVID-19 vaccination status was collected at inclusion. The following laboratory parameters were included in this study: C-reactive protein (CRP), procalcitonin, ferritin, lactate dehydrogenase (LDH), white blood cell count, red blood cell count, platelets and the lymphocyte subsets as described. Respiratory support was defined as oxygen therapy by means of a nasal cannula, non- rebreathing mask, high-flow oxygen therapy or invasive mechanical ventilation. Immunosuppressive drug use was defined as the use of systemic corticosteroids >7,5 mg prednisone per day or equivalent, TNF-a inhibitors, mycophenolate mofetil, calcineurin blockers, azathioprine, methotrexate, hydroxychloroquine, interleukin antagonists or any other type of immunosuppressive drug use.
2.3 Study outcome
The study population was divided into two groups: SARS-CoV-2 negative and SARS-CoV-2 positive patients, confirmed by RT-PCR. Within the group of SARS-CoV-2 positive patients, the primary outcome was categorization into either mild or severe COVID-19 disease. Mild COVID-19 was defined as admission to the general ward or no hospitalization requirement, while severe COVID-19 was defined as requiring ICU admission or COVID-19 related mortality, as assessed by the treating physician’s clinical judgment.
2.4 Statistical analysis
Baseline characteristics were described as frequencies with percentages for categorical variables and, continuous variables were described by using either median and interquartile ranges (IQR) or mean and standard deviations (SD). White blood cell counts and all lymphocyte subsets were compared between SARS-CoV-2 negative and positive patients, between mild and severe COVID-19 cases and between SARS-CoV-2 positive patients with and without prior immunosuppressive drug use by using the Pearson’s Chi-square test or Fisher’s exact test for categorical data, and the Student’s t-test and Mann-Whitney U test (non-normal distributions) for continuous variables. Standardized mean differences (SMD) with confidence intervals were calculated to quantify the magnitude of differences between groups. Furthermore, we calculated the ORs of the lymphocyte subsets for mild versus severe COVID-19 using multivariate logistic regression analysis adjusting for age, sex and BMI. Additionally, we examined the association between prior immunosuppressive drug use and COVID-19 severity, adjusting for age, sex, and white blood cell counts. Missing data were imputed using multiple imputation when more than 2.5% of a variable was missing. The imputation model included all available clinical characteristics, and five imputed datasets were generated for statistical analysisA p-value of less than 0.05 was considered statistically significant. For statistical analysis IBM SPSS Statistics version 25 was used.
3 Results
From March 10, 2021, to June 4, 2021, a total of 240 suspected COVID-19 patients were eligible for inclusion and 103 patients were included in the study. Reasons for exclusion included a language barrier, blood collection failure, lack of informed consent, and other miscellaneous reasons. Within the ‘no informed consent’ group, 17 patients were excluded because they were directly admitted to the ICU from the ED, which prevented obtaining informed consent. One patient was included twice, so only data from the first inclusion was considered for analysis. Of the included patients, 25 patients tested negative and 77 patients tested positive for SARS-CoV-2. Fifty-seven patients were classified as having mild COVID-19, and 20 patients were classified as having severe COVID-19 (Figure 1).
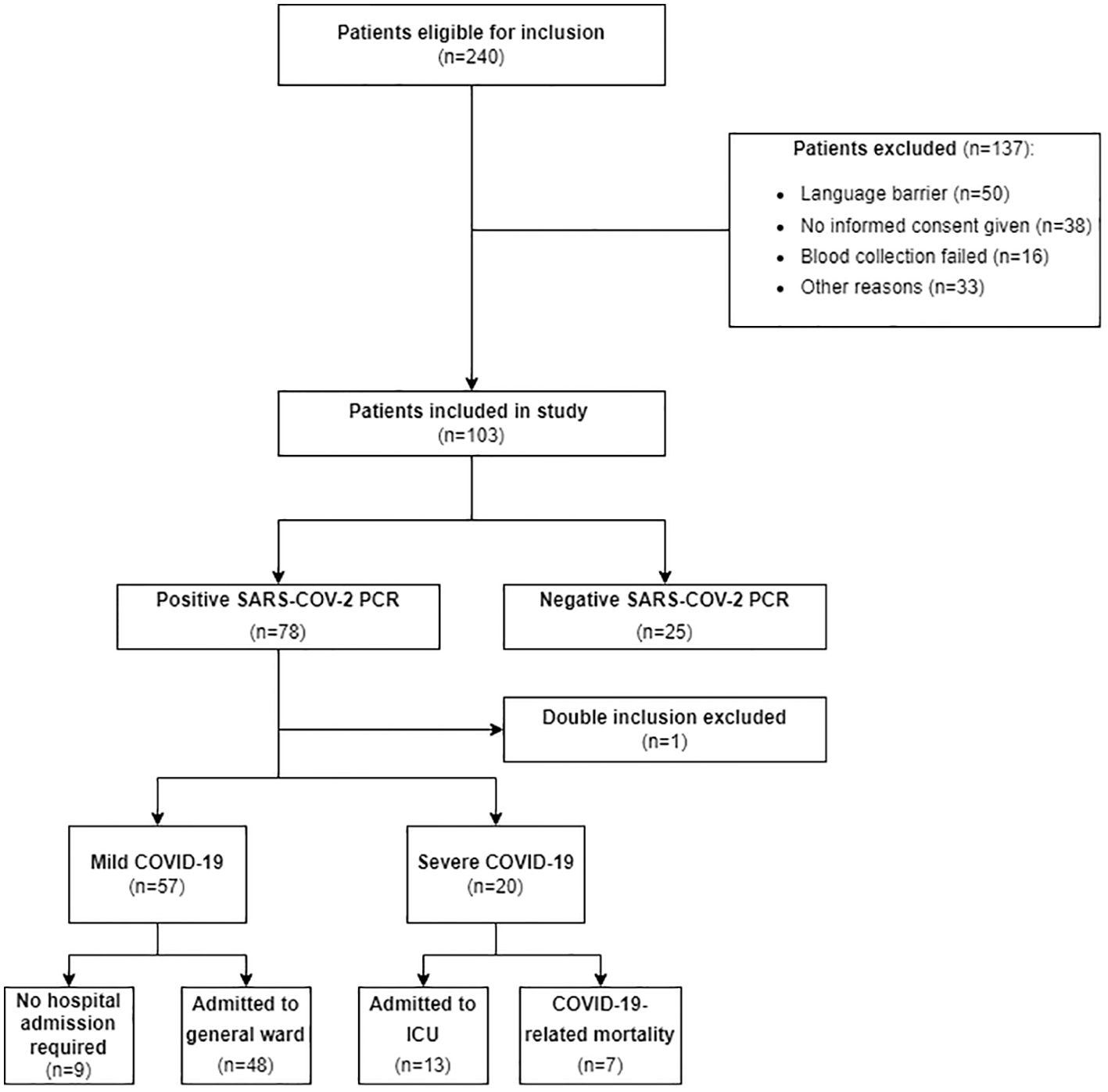
Figure 1. Flow diagram of patient selection. This figure shows the flow diagram detailing the patient selection process for our study, indicating the number of patients included and excluded, along with the reasons for exclusion. Mild COVID-19 was defined as patients admitted to a general ward or discharged home and severe COVID-19 was defined as patients requiring ICU admission or experiencing COVID-19-related mortality. SARS-CoV-2, Severe Acute Respiratory Syndrome Coronavirus 2; PCR, transcriptase polymerase chain reaction; ICU, Intensive Care Unit.
3.1 Clinical characteristics of SARS-CoV-2 positive patients
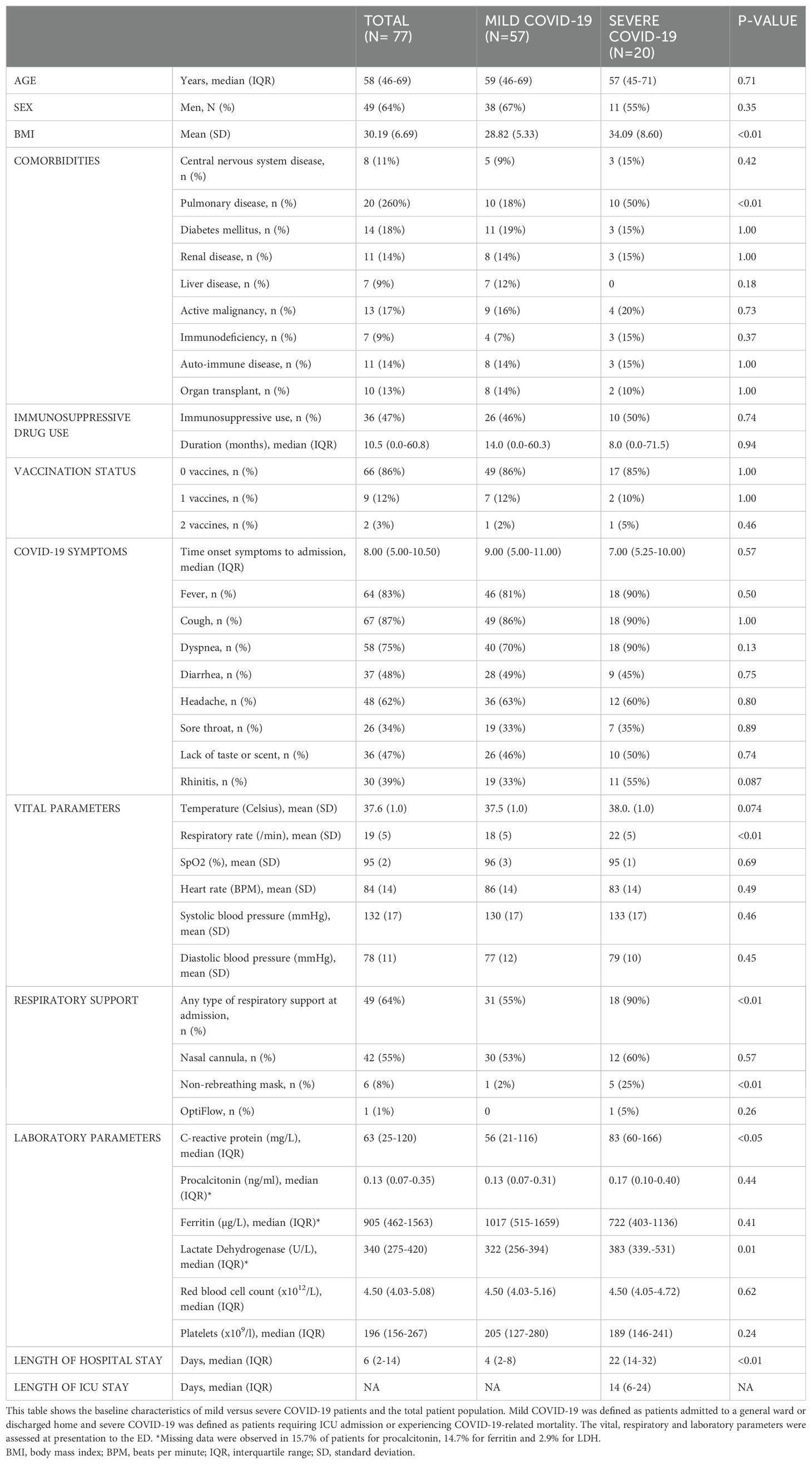
The patient characteristics at baseline of the SARS-CoV-2 positive patients, categorized into mild and severe COVID-19, are presented in Table 1. No differences were found in age between the mild COVID-19 patients [mean 59 years (IQR 46.0-69.0)] and severe COVID-19 patients [mean 57 years (IQR 45-71)]. In both groups the majority of patients were male and BMI was significantly higher in the severe COVID-19 group (p<0.01). From all the reported comorbidities, cardiovascular comorbidities were most common in both groups. Pulmonary disease was the only comorbidity with a significantly higher prevalence in the severe COVID-19 group (p<0.01). The interval between initial onset of clinical symptoms and presentation at the ED ranged from 0 to 21 days, with a median of 8 days (IQR 5.0-10.5). There was no significant difference in initial symptoms between the mild COVID-19 group and the severe COVID-19 group (p=0.571). The only vital parameter that showed a statistically significant difference between patients with mild and severe COVID-19 was the respiratory rate (p<0.005). Severe COVID-19 patients were more often in need of respiratory support at presentation at the ED (p<0.01) and had a significantly longer hospital stay (p<0.01). Of the 20 patients with severe COVID-19, the median time to ICU admission following ED presentation was 3 days (IQR: 1–8 days). COVID-19-related mortality was observed in 7 of these patients.
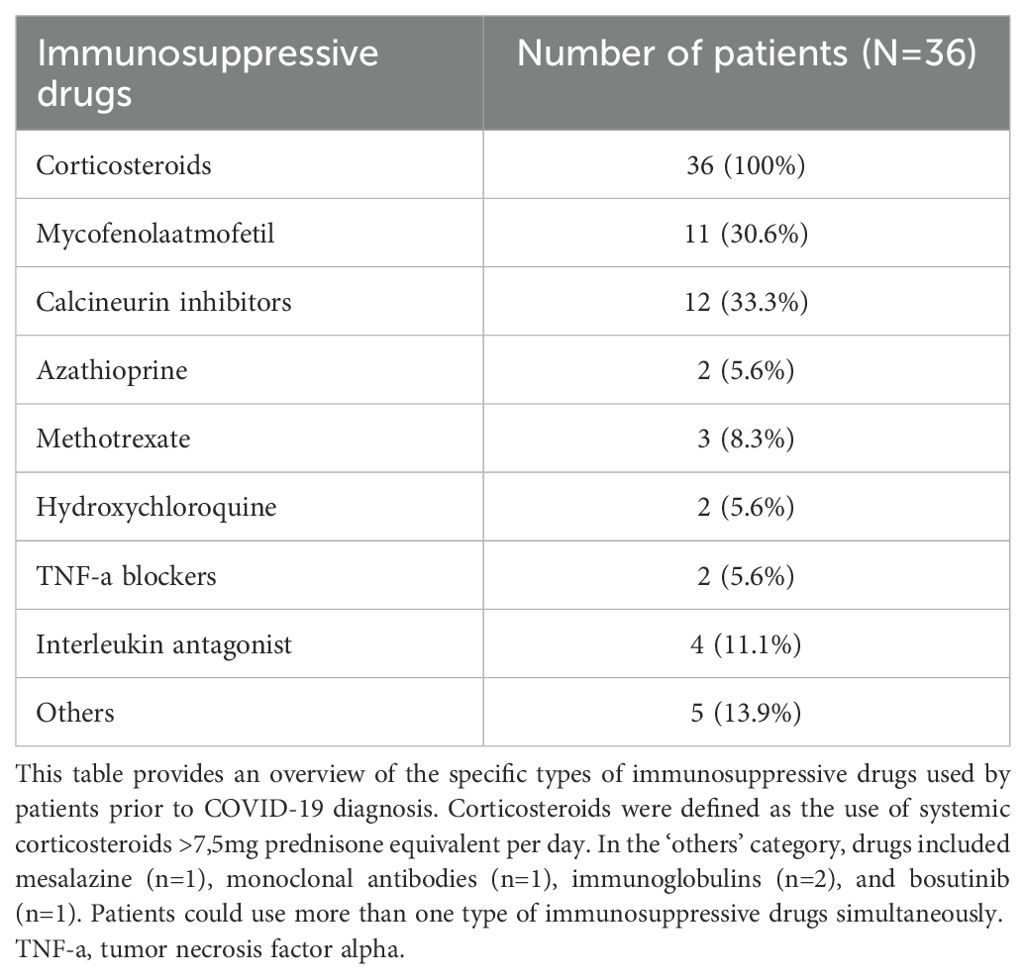
Forty-seven percent (N=36) of all SARS-CoV-2 positive patients were using any immunosuppressive drugs prior to infection. In the severe COVID-19 group this was 50.0% (N=10), and in the mild COVID-19 group this was 45.6% (N=26). Among SARS-CoV-2 positive patients discharged home from the ED, only 1 patient (11.1%) was on immunosuppressive drugs prior to presentation, whereas there were 25 patients (52.1%) in the hospitalized group that were on immunosuppressive drugs. In total, eight distinct types of immunosuppressive drugs were used, and the remaining medications were grouped in the ‘others’ category. Corticosteroids were the most frequently prescribed immunosuppressive drugs (n=36). Calcineurin inhibitors were used by twelve patients, while eleven patients used Mycophenolate mofetil. Twenty-seven patients were using more than one type of immunosuppressive drugs at the same time. Comprehensive information on the specific types of immunosuppressive drugs is shown in Table 2. In SARS-CoV-2 positive patients, nine patients (11.7%) had received one vaccination before they tested positive for SARS-CoV-2. Two patients got infected with SARS-CoV-2 despite being fully vaccinated with the Moderna vaccine (2 vaccinations), of which one had to be admitted to the ICU due to respiratory failure. Both patients had a history of solid organ transplant, for which they used immunosuppressive drugs. Ten of the patients that were on immunosuppressive drugs prior to presentation were vaccinated (27.8%), of whom five had received one vaccination before they tested positive for SARS-CoV-2 and five were fully vaccinated.
3.2 Lymphocyte subsets in SARS-CoV-2 negative and SARS-CoV-2 positive patients
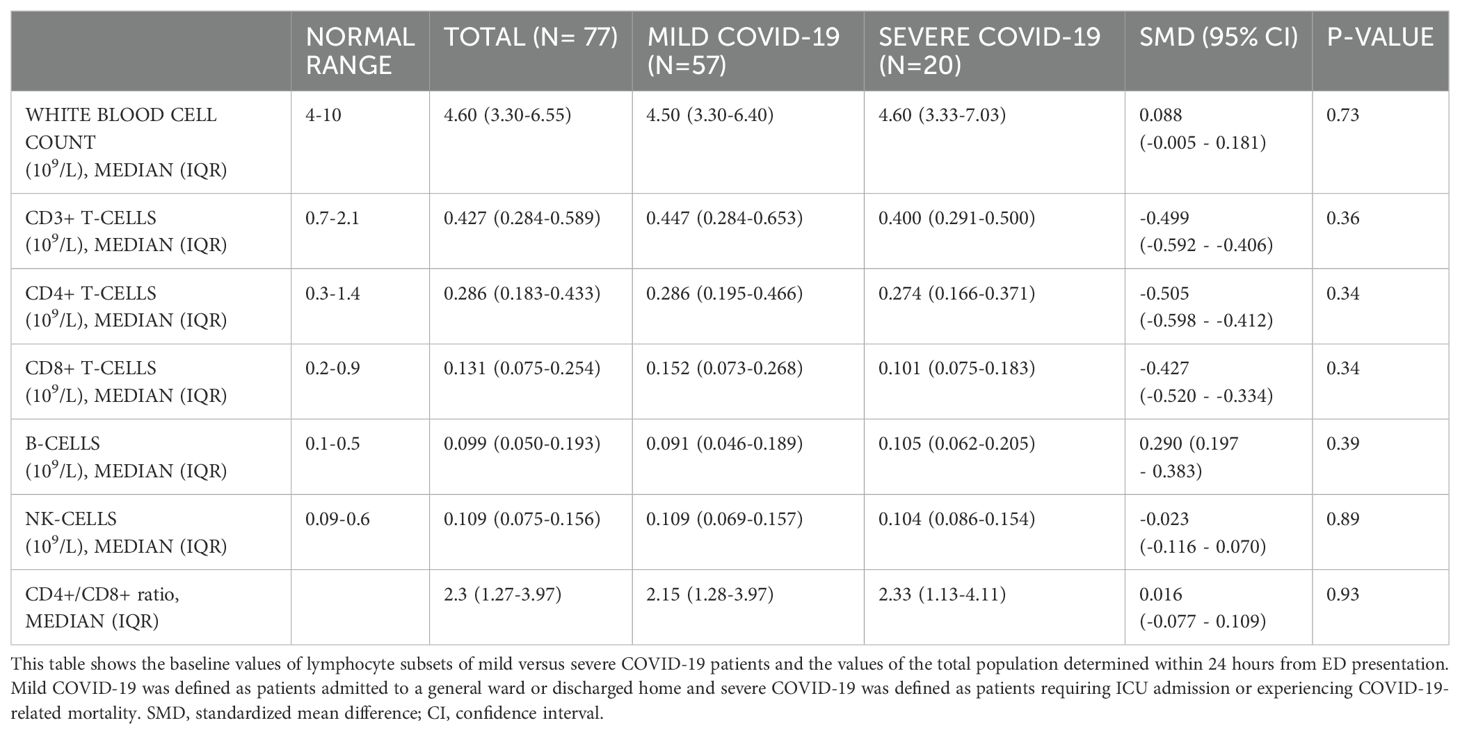
Lymphocyte subsets were compared between SARS-CoV-2 negative and SARS-CoV-2 positive patients (Table 3). The absolute count of CD3+ T-lymphocytes was significantly lower in the SARS-CoV-2 positive group (median 0.427, IQR 0.284-0.589 versus median 0.689, IQR 0.321-1.066, p<0.05). No significant differences were found in white blood cell count between SARS-CoV-2 negative patients (median 6.10, IQR 3.10-11.10) and SARS-CoV-2 positive patients (median 4.60, IQR 3.30-6.55)0, nor did absolute CD4+ T-lymphocyte levels differ significantly between SARS-CoV-2 negative patients (median 0.389, IQR 0.243-0.617) and SARS-CoV-2 positive patients (median 0.286, IQR 0.183-0.433).
3.3 Lymphocyte subsets and laboratory parameters in severe and mild COVID-19 patients
The analysis of absolute counts of lymphocyte subsets in severe versus mild COVID-19 patients is shown in Table 4, and no significant differences were observed. In the overall cohort of SARS-CoV-2 positive patients, the medians of CD3+, CD4+, and CD8+ lymphocyte counts were below the normal range, while the medians of B-lymphocytes and NK cell counts were within the normal range. In univariate logistic regression analysis, none of the lymphocyte subsets were significantly associated with severe COVID-19 (Supplementary Table S1). Only levels of CRP and LDH at baseline were significantly higher in the severe COVID-19 group compared to the mild COVID-19 (p ≤ 0.01).
3.4 Lymphocyte subsets in COVID-19 patients with and without immunosuppressive drugs prior to COVID-19
We analyzed if the lymphocyte subset values were different in COVID-19 patients with and without using immunosuppressive drugs before inclusion in the study (Supplementary Table S2). The absolute number of CD4+ T lymphocytes was significantly lower in the group using immunosuppressive drugs (p<0.01), while no significant differences were observed for the other lymphocyte subsets (Figure 2). We found no significant association between the use of immunosuppressive drug and COVID-19 severity (adjusted OR 1.07, 95% CI 0.36 - 3.19, p= 0.90, Supplementary Table S3). Notably, 40% of the SARS-CoV-2 negative patients were using immunosuppression prior to admission, and lymphocyte subset counts were all at the bottom of or below reference values.

Figure 2. Lymphocyte subsets in patients with and without prior immunosuppressive drug use. This figure shows the lymphocyte subset values of COVID-19 patients with and without immunosuppressive drugs prior to SARS-CoV-2 infection.
4 Discussion
No significant differences were found in lymphocyte subsets between the SARS-CoV-2 negative and SARS-CoV-2 positive patients presenting to the emergency department of our tertiary academic medical center, except for a lower CD3+ lymphocyte count in SARS-CoV-2 positive patients. In SARS-CoV-2 positive patients, patients using immunosuppressive drugs prior to COVID-19 had significantly lower CD4+ T-lymphocyte counts, but no differences in lymphocyte subsets were found between mild and severe cases. Our study found no association between lymphocyte subsets or prior immunosuppressive drug use and COVID-19 severity.
To our knowledge, CD3+ T-lymphocyte counts are not a reliable diagnostic marker to identify SARS-CoV-2 infection, but can be used as indicators of severe COVID-19 (13, 21). Previous studies, including several meta-analyses, also consistently report significantly lower counts of lymphocyte subsets CD4+ and CD8+ T-lymphocytes, NK-cells, and B-lymphocytes, along with a reduced total lymphocyte count in severely ill COVID-19 patients (12, 14). However, in our study we found no association between lymphocyte subsets and severe COVID-19. One possible explanation might be the high proportion of patients on immunosuppressive drug use prior to SARS-CoV-2 infection in our tertiary academic medical center, namely 47%. Given the dynamic nature of the host response over time, the timing of determination of lymphocytes counts, whether at ED admission, later during hospitalization, or during treatment, can be crucial, making a direct comparison with other studies challenging. Multiple previously published studies lack information on the use of immunosuppressive drugs prior to SARS-CoV-2 infection and study inclusion. Generally, immunosuppressive drug use is associated with reduced absolute lymphocytes counts (22). In our study, absolute CD4+ T-lymphocyte counts were indeed significantly lower in patients who used immunosuppressive drugs prior to SARS-CoV-2 infection. However, these lower counts were not found to be associated with severe COVID-19 outcomes. This might suggest that reduced lymphocyte subsets may only be linked to severe outcomes when caused by the infection itself. This could propose a limitation in relying solely on immunological parameters for predicting COVID-19 severity in patient populations with a high proportion of patients on immunosuppressive drugs. Alternative predictors, like organ failure-based scores like the SOFA score, may offer more reliable predictive value in settings with a high prevalence of immunosuppression (23). Interestingly, in the group of COVID-19 patients discharged home from the ED, only one patient was using immunosuppressive drugs, whereas this accounted for half of the patients in the hospitalized group. Since the use of immunosuppressive drugs prior to SARS-CoV-2 infection was not associated with severe COVID-19 in our cohort, it could be considered that the higher admission rate in this group might be attributed to the medical team’s caution rather than disease severity alone.
One strength of our study is that all patients were included within 24 hours of presentation at the ED. Additionally, the majority of our cohort had not received any COVID-19-related treatment prior to inclusion, thereby reducing the potential influence on immunological parameters at the time of sampling. Another strength of our study is the inclusion of patients with suspected COVID-19, without a confirmed diagnosis yet. This real-world approach without preselection, reflects daily clinical practice, where clinicians often face uncertainty regarding the cause of disease. A limitation of this study is the exclusion of patients admitted directly from the ED to the ICU due to logistical constraints. This exclusion potentially introduces bias, as the initially most critically ill patients have been omitted and therefore are not included in the severe COVID-19 group. However, this subset was relatively small, comprising only 17 patients, and we believe that identifying a predictive biomarker in the ED is particularly relevant for patients initially admitted to a regular ward, as these patients may benefit more from early risk stratification compared to those who already require immediate ICU care and intensive monitoring. Although this study was designed as an exploratory investigation, the limited sample size might have resulted in insufficient statistical power to detect differences within specific subgroups or to conduct sensitivity analyses. Nevertheless, we believe our findings—including the significant differences observed in CD4+ populations—are robust and contribute meaningfully to the current body of knowledge. Furthermore, in line with the FAIR data use policy, our results can be shared and utilized in future pooled analyses, enhancing their contribution to broader meta-analyses and supporting further investigation.
Our study highlights the challenges associated with using immune system-based markers for outcome prediction in a patient population characterized by a high prevalence of immunosuppressive drug use. In the future, particularly in the context of pandemic preparedness, it would be interesting to compare the predictive value of lymphocyte subsets with that of organ failure-based predictors in this patient population, or to incorporate them into a comprehensive predictive model. While measuring absolute lymphocyte subset counts provides valuable information, it does not capture the immune cell functions. Therefore, a holistic approach integrating absolute counts with assessments of immune cell functions could offer a more comprehensive understanding of immune response dynamics and improve accurate prediction in this setting (24, 25).
5 Conclusion
In conclusion, no significant differences were observed in lymphocyte subsets between SARS-CoV-2 negative and positive patients presenting to the emergency department of a tertiary academic medical center, except for a lower CD3+ lymphocyte count, in contrast to the majority of the previous studies. Furthermore, our study found no association between lymphocyte subsets or prior immunosuppressive drug use and COVID-19 severity among COVID-19 patients. Patients using immunosuppressive drugs prior to COVID-19 had significantly lower CD4+ T-lymphocyte counts compared to those who were not.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Medical Ethics Review Committee of the Erasmus University Medical Center. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
KD: Conceptualization, Data curation, Formal analysis, Methodology, Visualization, Writing – original draft, Writing – review & editing. SV: Conceptualization, Data curation, Formal analysis, Writing – original draft, Writing – review & editing. KT-M: Conceptualization, Data curation, Formal analysis, Methodology, Visualization, Writing – original draft, Writing – review & editing. WD: Writing – original draft, Writing – review & editing. PV: Writing – original draft, Writing – review & editing. JH: Supervision, Writing – original draft, Writing – review & editing. DG: Writing – original draft, Writing – review & editing. EG: Writing – original draft, Writing – review & editing. HE: Supervision, Writing – original draft, Writing – review & editing. VD: Conceptualization, Investigation, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1436637/full#supplementary-material
Supplementary Table 1 | Adjusted odds ratios for severe COVID-19.
Supplementary Table 2 | Comparison of Lymphocyte Subsets at Admission between COVID-19 Patients with and without prior immunosuppressive drug use.
Supplementary Table 3 | Multivariate analysis.
Abbreviations
ARDS, acute respiratory distress syndrome; COVID-19, coronavirus disease-2019; CRP, c-reactive protein; ED, emergency department; ICU, intensive care unit; LDH, lactate dehydrogenase; SARS-CoV-2, severe acute respiratory syndrome coronavirus-2; RT-PCR, reverse- transcriptase polymerase chain reaction.
References
1. WHO Coronavirus (COVID-19) Dashboard . Available online at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (Accessed 6 May 2024).
2. Bahremand T, Yao JA, Mill C, Piszczek J, Grant JM, Smolina K. COVID-19 hospitalisations in immunocompromised individuals in the Omicron era: a population-based observational study using surveillance data in British Columbia, Canada. Lancet Reg Health Am. (2023) 20:100461. doi: 10.1016/j.lana.2023.100461
3. Grivas P, Khaki AR, Wise-Draper TM, French B, Hennessy C, Hsu CY, et al. Painter CA et al: Association of clinical factors and recent anticancer therapy with COVID-19 severity among patients with cancer: a report from the COVID-19 and Cancer Consortium. Ann Oncol. (2021) 32:787–800. doi: 10.1016/j.annonc.2021.02.024
4. Kuderer NM, Choueiri TK, Shah DP, Shyr Y, Rubinstein SM, Rivera DR, et al. et al: Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. (2020) 395:1907–18. doi: 10.1016/S0140-6736(20)31187-9
5. Lee A, Wong SY, Chai LYA, Lee SC, Lee MX, Muthiah MD, et al. Chan YH et al: Efficacy of covid-19 vaccines in immunocompromised patients: systematic review and meta-analysis. BMJ. (2022) 376:e068632. doi: 10.1136/bmj-2021-068632
6. Liu K, Fang YY, Deng Y, Liu W, Wang MF, Ma JP, et al. Li CH et al: Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J (Engl). (2020) 133:1025–31. doi: 10.1097/CM9.0000000000000744
7. Russo A, Gentilini Cacciola E, Borrazzo C, Filippi V, Bucci T, Vullo F, et al. Ceccarelli G et al: Clinical Characteristics and Outcome of Patients with Suspected COVID-19 in Emergency Department (RESILIENCY Study II). Diagnostics (Basel). (2021) 11. doi: 10.3390/diagnostics11081368
8. Morgan G, Casalino S, Chowdhary S, Frangione E, Fung CYJ, Haller S, et al. Young J et al: Characterizing Risk Factors for Hospitalization and Clinical Characteristics in a Cohort of COVID-19 Patients Enrolled in the GENCOV Study. Viruses. (2023) 15. doi: 10.3390/v15081764
9. Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Xiong Y et al: Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. (2020) 323:1061–9. doi: 10.1001/jama.2020.1585
10. Sun X, Wang T, Cai D, Hu Z, Chen J, Liao H, et al. Qiu Y et al: Cytokine storm intervention in the early stages of COVID-19 pneumonia. Cytokine Growth Factor Rev. (2020) 53:38–42. doi: 10.1016/j.cytogfr.2020.04.002
11. Leclerc T, Donat N, Donat A, Pasquier P, Libert N, Schaeffer E, et al. Perrigault PF et al: Prioritisation of ICU treatments for critically ill patients in a COVID-19 pandemic with scarce resources. Anaesth Crit Care Pain Med. (2020) 39:333–9. doi: 10.1016/j.accpm.2020.05.008
12. Huang W, Berube J, McNamara M, Saksena S, Hartman M, Arshad T, et al. Lymphocyte subset counts in COVID-19 patients: A meta-analysis. Cytometry A. (2020) 97:772–6. doi: 10.1002/cyto.a.24172
13. Akbari H, Tabrizi R, Lankarani KB, Aria H, Vakili S, Asadian F, et al. The role of cytokine profile and lymphocyte subsets in the severity of coronavirus disease 2019 (COVID-19): A systematic review and meta-analysis. Life Sci. (2020) 258:118167. doi: 10.1016/j.lfs.2020.118167
14. Panda S, Nanda R, Tripathy PK, Mangaraj M. Immuno-inflammatory predictors of disease severity in COVID-19: A systematic review and meta-analysis. J Family Med Prim Care. (2021) 10:1102–16. doi: 10.4103/jfmpc.jfmpc_2196_20
15. Vafadar Moradi E, Teimouri A, Rezaee R, Morovatdar N, Foroughian M, Layegh P, et al. Increased age, neutrophil-to-lymphocyte ratio (NLR) and white blood cells count are associated with higher COVID-19 mortality. Am J Emerg Med. (2021) 40:11–4. doi: 10.1016/j.ajem.2020.12.003
16. Wen XS, Jiang D, Gao L, Zhou JZ, Xiao J, Cheng XC, et al. Tan XW et al: Clinical characteristics and predictive value of lower CD4(+)T cell level in patients with moderate and severe COVID-19: a multicenter retrospective study. BMC Infect Dis. (2021) 21:57. doi: 10.1186/s12879-020-05741-w
17. Mu QS, Li H, Ye H, Liu YD, Bai J, Yuan L, et al. Association of interleukin-6 and CD4+ T cells and two-week prognosis of patients with COVID-19: a predictive role. Eur Rev Med Pharmacol Sci. (2023) 27:4782–91. doi: 10.26355/eurrev_202305_32489
18. Bakouny Z, Labaki C, Grover P, Awosika J, Gulati S, Hsu CY, et al. Bilen MA et al: Interplay of Immunosuppression and Immunotherapy Among Patients With Cancer and COVID-19. JAMA Oncol. (2023) 9:128–34. doi: 10.1001/jamaoncol.2022.5357
19. Bertini CD Jr., Khawaja F, Sheshadri A. Coronavirus disease-2019 in the immunocompromised host. Clin Chest Med. (2023) 44:395–406. doi: 10.1016/j.ccm.2022.11.012
20. Diao B, Wang C, Tan Y, Chen X, Liu Y, Ning L, et al. Wang G et al: Reduction and Functional Exhaustion of T Cells in Patients With Coronavirus Disease 2019 (COVID-19). Front Immunol. (2020) 11:827. doi: 10.3389/fimmu.2020.00827
21. Yan W, Chen D, Bigambo FM, Wei H, Wang X, Xia Y. Differences of blood cells, lymphocyte subsets and cytokines in COVID-19 patients with different clinical stages: a network meta-analysis. BMC Infect Dis. (2021) 21:156. doi: 10.1186/s12879-021-05847-9
22. Mustafa SS. Steroid-induced secondary immune deficiency. Ann Allergy Asthma Immunol. (2023) 130:713–7. doi: 10.1016/j.anai.2023.01.010
23. Guarino M, Perna B, Remelli F, Cuoghi F, Cesaro AE, Spampinato MD, et al. A new early predictor of fatal outcome for COVID-19 in an italian emergency department: the modified quick-SOFA. Microorganisms. (2022) 10. doi: 10.3390/microorganisms10040806
24. Sette A, Crotty S. Adaptive immunity to SARS-cov-2 and COVID-19. Cell. (2021) 184:861–80. doi: 10.1016/j.cell.2021.01.007
Keywords: COVID-19, lymphocyte subsets, infectious diseases, severity, prediction, immunosuppression
Citation: Daenen K, van Hooijdonk S, Tong-Minh K, Dik WA, van Hagen PM, Huijben JA, Gommers D, van Gorp ECM, Endeman H and Dalm VASH (2024) Exploring lymphocyte subsets in COVID-19 patients: insights from a tertiary academic medical center with a high proportion of patients on immunosuppression. Front. Immunol. 15:1436637. doi: 10.3389/fimmu.2024.1436637
Received: 22 May 2024; Accepted: 18 November 2024;
Published: 03 December 2024.
Edited by:
Nazira El-Hage, Florida International University, United StatesReviewed by:
Aidan Mullan, Mayo Clinic, United StatesSyed Nazeer Mahmood, Philadelphia College of Osteopathic Medicine (PCOM), United States
Copyright © 2024 Daenen, van Hooijdonk, Tong-Minh, Dik, van Hagen, Huijben, Gommers, van Gorp, Endeman and Dalm. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Virgil A. S. H. Dalm, di5kYWxtQGVyYXNtdXNtYy5ubA==
 Katrijn Daenen
Katrijn Daenen Samantha van Hooijdonk
Samantha van Hooijdonk Kirby Tong-Minh
Kirby Tong-Minh Willem A. Dik
Willem A. Dik Petrus M. van Hagen
Petrus M. van Hagen Jilske A. Huijben
Jilske A. Huijben Diederik Gommers
Diederik Gommers Eric C. M. van Gorp
Eric C. M. van Gorp Henrik Endeman1
Henrik Endeman1 Virgil A. S. H. Dalm
Virgil A. S. H. Dalm