- Jiangxi Provincial Key Laboratory of Hematological Diseases, Department of Hematology, The 2nd Affiliated Hospital, Jiangxi Medical College, Nanchang University, Nanchang, Jiangxi, China
Background: Chimeric antigen receptor (CAR) T-cell therapy (CAR-T therapy) has demonstrated significant efficacy in the ZUMA-2 study. After regulatory approvals, several clinical trials and real-world studies on CAR-T therapy for relapsed or refractory mantle cell lymphoma (R/R MCL) were conducted. However, data on clinical safety and efficacy are inconsistent. In this study, we aimed to conduct a systematic analysis of the effectiveness and safety of CAR-T therapy across a wider and more representative cohort of patients with R/R MCL.
Methods: We performed a systematic review and meta-analysis of studies on patients with R/R MCL who received CAR-T cell therapy. Data were extracted and consolidated, with primary focus on the evaluation of safety and efficacy outcome measures. This study has not been registered with PROSPERO.
Results: This meta-analysis identified and included 16 studies with 984 patients. The pooled estimate for overall response rate (ORR) was 89%; complete remission (CR) rate was 74%. The 6-month and 12-month progression-free survival (PFS) rates were 69% and 53%, respectively, while the overall survival (OS) rates were 80% and 69%, respectively. Cytokine release syndrome (CRS) of grade 3 or higher was observed in 8% of patients, whereas neurotoxicity of grade 3 or higher was observed in 22% of patients. The risk of bias was assessed as low in 9 studies and moderate in 7 studies.
Conclusion: CAR-T therapy exhibited promising efficacy and manageable adverse reactions in patients with R/R MCL.
Introduction
Mantle cell lymphoma (MCL) is a distinct, rare subtype of B-cell non-Hodgkin lymphoma (representing approximately 2.5%–6% of total cases) (1). The clinical course of the disease is heterogeneous, ranging from indolent forms that may not require treatment for years to highly aggressive variants that carry a grave prognosis despite intensive therapeutic regimens (2). Particularly for patients with MCL with high-risk disease profiles, including those with blastoid variants, elevated Ki-67 proliferation indices, TP53 gene mutations or increased protein expression, and disease progression within 24 months of initial diagnosis, the prognosis is generally poor (3).
Although Bruton’s tyrosine kinase inhibitors (BTKis) have significantly improved clinical outcomes for patients with MCL, the average progression-free survival (PFS) after BTK treatment remains unsatisfactory at 16.4 months. This suggested that most patients exhibit early disease progression through second line treatment. For individuals who experience disease after BTKi treatment failure, the prognosis is particularly severe, with a median overall survival (OS) of only 2.9 months. Treatment of patients with R/R MCLwho are resistant to BTKi therapy remains challenging (4–6).
Recent years have witnessed a significant transformation in the therapeutic landscape for R/R MCL (7, 8). A shift from traditional chemotherapy and immunotherapy to advanced targeted and cellular therapies, particularly CAR-T therapy, marks a new era in treatment modalities (9, 10). Groundbreaking results from the ZUMA-2 and TRANSCEND NHL 001 trials highlight the efficacy of CAR-T therapy in relapsed and drug-resistant patients with MCL (11–13). This therapy exhibits notable response rates in subsets of patients characterized by advanced age, blastoid phenotypes, elevated Ki-67 proliferation indices, high MIPI scores, and TP53 mutations, as well as those with central nervous system involvement (12). Though CAR-T therapy demonstrates strong antitumor efficacy in relapsed or refractory B-cell hematologic malignancies, it is imperative to acknowledge its adverse effects including neurotoxicity, cytokine release syndrome (CRS), on-target/off-tumor recognition, and insertional oncogenesis (14, 15).
Approval by FDA in July 2020 and subsequent endorsement by European medical authorities in January 2021 for the use of CD19 CAR-T cells in patients with R/R MCL signifies a crucial step forward. As CAR-T therapy gains momentum in clinical application and long-term follow-up data are accumulated, it is imperative to study safety and efficacy evidence. In this study, we aimed to meticulously review the existing trials of CAR-T therapy for MCL, to evaluate the clinical outcomes and toxicity profiles rigorously, and to determine the factors that influence divergent treatment responses.
Methods
Search strategy and selection criteria
We conducted literature searches across multiple databases including PubMed, Embase, Cochrane Systematic Reviews, and ClinicalTrials.gov. Data were collected up to July 4, 2024. The search strategy employed the terms “CAR-T” and “mantle cell lymphoma,” with the detailed methodology outlined in the Supplementary File 1. The inclusion criteria were studies with patients with MCL undergoing CAR-T therapy, without any restrictions on date or study design. Exclusion criteria included studies without complete data, basic research, case series with fewer than 10 patients, and studies on dual targets, which were discussed separately.
In this meta-analysis, key data including demographic information, treatment history, and clinical outcomes of CAR-T therapy in patients were compiled. This included number of enrolled patients, median age, sex ratio, prior treatment rounds, specific CAR-T therapy targets, use of combination therapies, and BTKi application. Genetic and cellular markers such as TP53 mutations and Ki67 proliferation index were noted, along with central nervous system (CNS) involvement and past transplantation procedures. The primary outcome measures of concern were safety and efficacy.
Two authors (HX Wan and SQ Weng) independently conducted the literature screening based on a predefined search strategy to identify preliminary reports potentially relevant to the topic of study. The screening process strictly adhered to established inclusion and exclusion criteria, ensuring that only studies meeting all criteria were considered for final analysis. The researchers conducted an in-depth full-text review of all initially selected citations and compiled a list of studies that met the eligibility requirements. Any discrepancies in the screening results were resolved through mutual discussion between the researchers, and persistent disagreements were refereed by an external panel of experts.
Quality assessment
To assess the quality of the literature, the MINORS tool was used, tailored specifically for assessing the methodological quality of nonrandomized studies. The methodological quality of each study was evaluated by categorizing the risk of bias as low or high; scores below 10 indicated a low risk, whereas scores of 10 or greater denoted a high risk.
Statistical analysis
The cumulative incidence (event rate) and 95% confidence intervals (CIs) were calculated for each specified outcome. The distribution of outcome proportions was validated for normality using the Shapiro–Wilk test, implemented through the “shapiro. test” function in R, aligning with the assumptions of our chosen meta-analytic model. Depending on the I² statistic, a random-effects model was used for I² values > 50% and a fixed-effects model for values ≤ 50%. Subgroup analyses were conducted to investigate the sources of heterogeneity and impact of various factors on treatment efficacy. In addition, we grouped and discussed prospective and retrospective studies to clarify the differences between them. To explore the effects of studies with a high risk of bias, subgroup analyses were performed for studies classified with low or high risks of bias.
This meta-analysis was conducted using the R software (R Foundation for Statistical Computing, Vienna, Austria, version 4.3.3). The “meta” package was used for statistical analysis.
Results
Characteristics of included literature
From an initial screening of 1,317 potentially relevant studies, we used EndNote software to preliminarily exclude 174 review articles, 28 case reports, and 131 duplicates. Based on their abstracts and titles, we further excluded 984 studies due to reasons such as basic research, irrelevance to the study topic, duplication, or lack of quantitative data. We conducted a full-text review of 22 studies, excluding those dual-target CAR-T, and case series with fewer than 10 patients. Ultimately, 16 studies (16–30) encompassing 984 patients were included in the analysis (Supplementary Figure 1).
Among the included studies, 10 were published in peer-reviewed journals, and 6 were presented at conferences.13 studies employed the Brexu-cel CAR-T cell product (16–25, 28–30), and CTL019 CAR-T cell product (27), Liso-cel CAR-T cell product and mixed CAR-T cell product (26) (including Brexu-cel, Tisa-cel, and Liso-cel) were used in the remain three studies, respectively. The median age were 66.5 years (ranged from 38 to 89) of included patients, and male patients account for 73% of the total population. Most patients were those who had relapsed after multiple lines (ranged from 1 to 12) of therapy, including BTKi treatment. 8 studies also included patients with secondary central nervous system infiltration (19–21, 24, 28–30). Baseline patient characteristics are provided in Table 1.
In our meta-analysis, we included 16 studies with varied standards for assessing adverse events and efficacy. Specifically, 12 studies utilized the American Society for Transplantation and Cellular Therapy (ASTCT) consensus guidelines for grading CRS and ICANS (17–20, 22–29), 3 studies used the Lee criteria(2014) (16, 21), and 1 study did not clearly specify the adverse event assessment criteria (30). For efficacy assessment, all studies that reported efficacy used the Lugano classification (2014) to determine the response to CAR-T therapy.
Efficacy
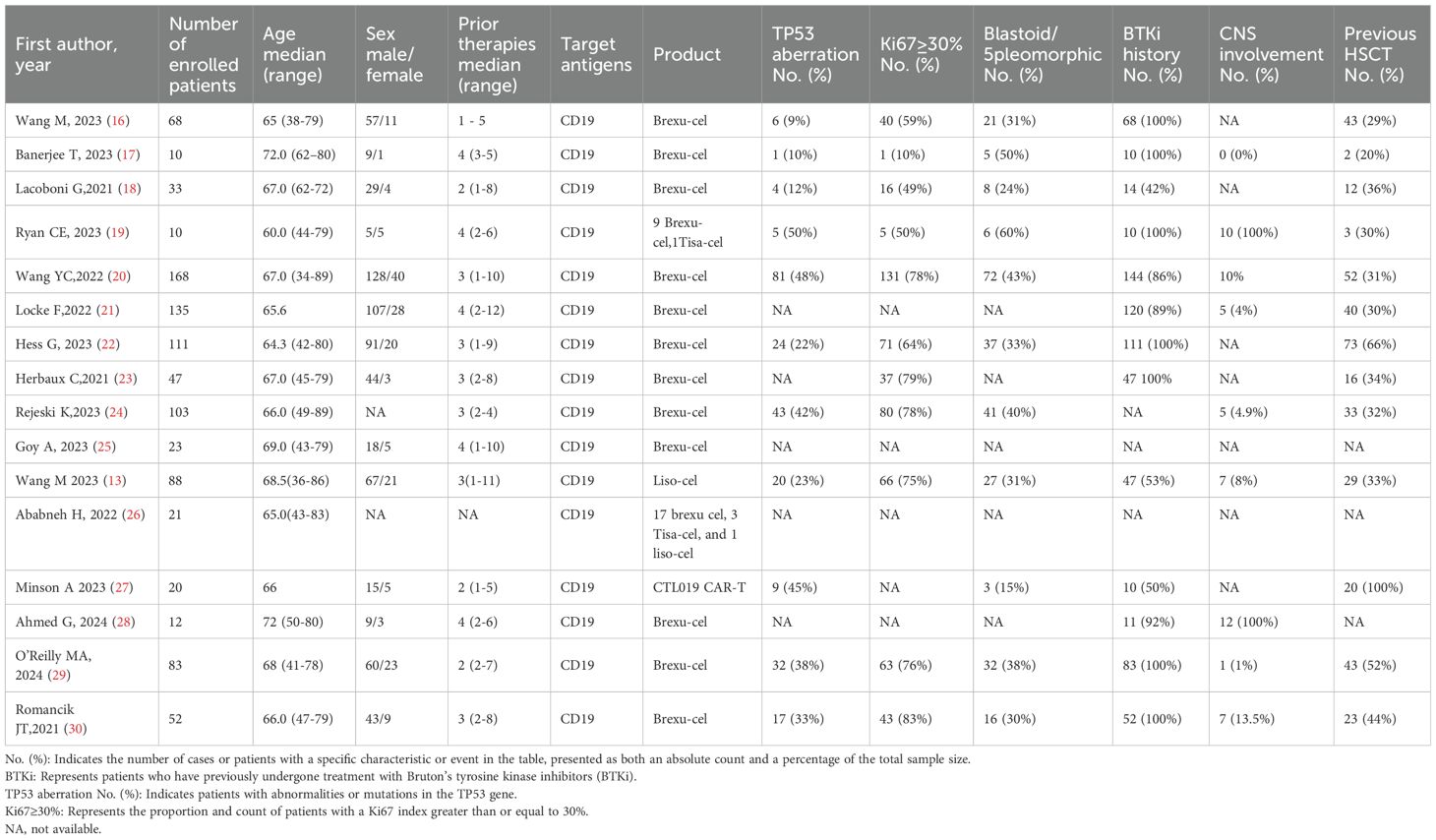
All studies provided clinical remission data (Supplementary Table 1). Among the 984 analyzed patients who received CAR-T therapy for R/R MCL, an ORR of 89% (95% CI: 87%–91%, I²: 13%) was observed (Figure 1A). Of these, 74% (95% CI: 69%–79%, I²: 60%) patients attained complete remission (Figure 1B). In total, the 6-month PFS rate was 68% (95% CI: 59%–76%, I²: 74%) (Figure 1C), the 12-month PFS rate was 51% (95% CI: 42%–60%, I²:70%) (Figure 1D). For OS, the 6-month OS rate was 80% (95% CI: 72%–87%, I²: 75%) (Figure 1E), whereas the 12-month OS rate remained at 69% (95% CI: 54%–82%, I²: 86%) (Figure 1F).
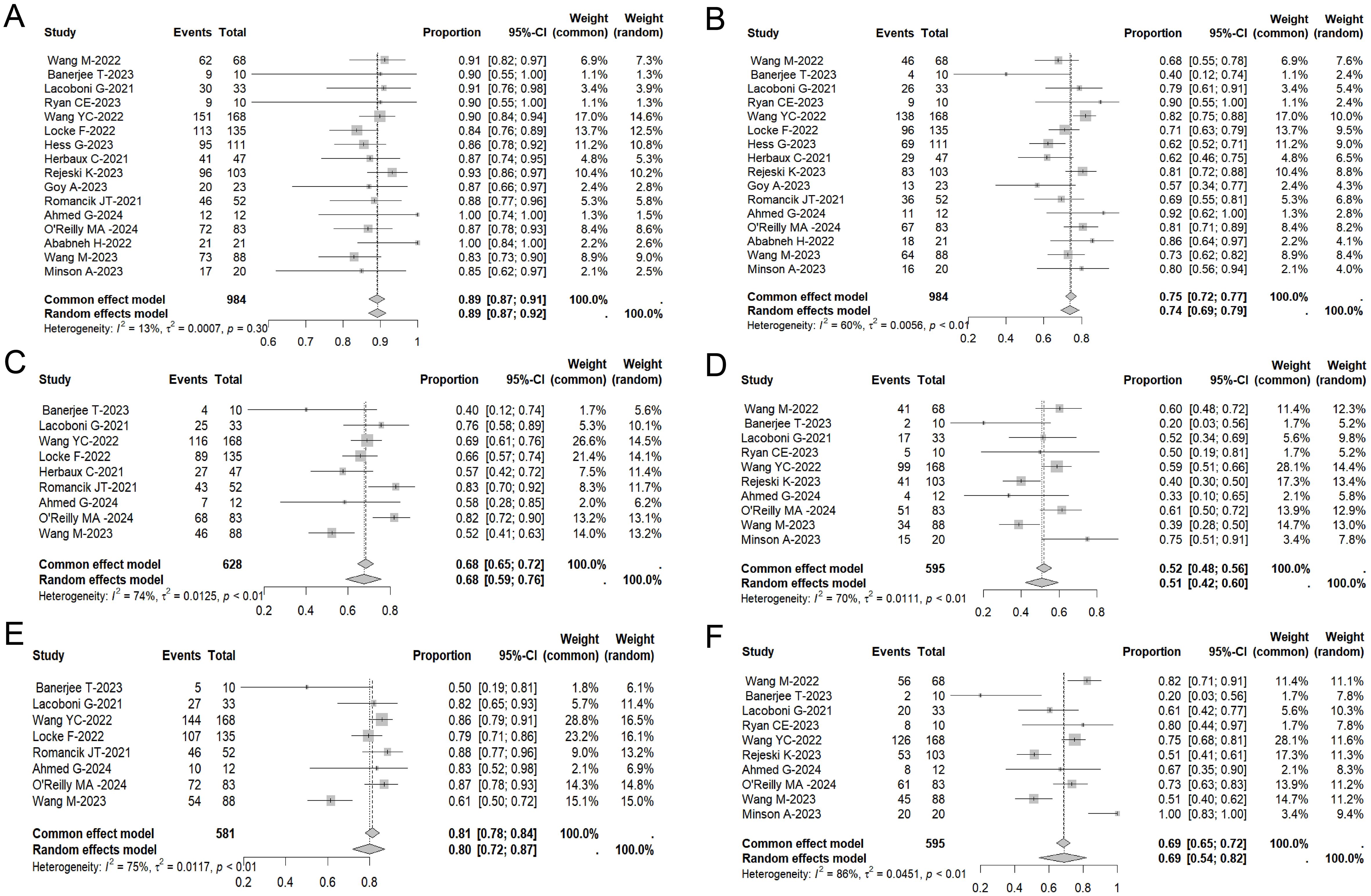
Figure 1. Forest plot of ORR (A), CR rates (B), 6-month-PFS (C), 6-month-OS (D), 1-year PFS rate (E) and 1-year OS rate (F) in patients with MCL treated with CAR-T therapy across multiple studies.
Safety
In the pooled analysis of safety data from all treated patients, 86% (95% CI: 81%–91%, I2: 71%) of individuals experienced varying grades of CRS. 8% (95% CI: 5%–11%, I2: 60%) of patients had CRS of grade 3 or higher (Figure 2A). Additionally, immune effector cell-associated neurotoxicity syndrome (ICANS) was observed in 52% (95% CI: 43%–61%, I2: 77%) of 805 assessed patients, with 22% (95% CI: 14%–30%, I2: 79%) experiencing ICANS of grade 3 or higher (Figure 2B).
Figure 2. Forest plot of the incidence of ≥ Grade 3 CRS (A) and the incidence of ≥ Grade 3 ICANS (B) across multiple studies involving CAR-T therapy in MCL patients.
Subgroup analysis
The subgroup analysis of prognostic high-risk factors for MCL indicated varying CR rates among different patient categories. Patients with Ki-67 index below 30% had ORR rate of 87% and CR rate of 65%, whereas those with Ki-67 index of 30% or more had ORR rate of 88% and CR rate of 74%. Patients without and with TP53 mutations had ORR rate of 95% and 89%, and CR rate of 81% and 70%, respectively. The absence and presence of CNS involvement was associated with ORR rate of 88% and 89%, and CR rate of 72% and 82%, respectively. Nonblastoid/pleomorphic patients and blastoid/pleomorphic patients had ORR rate of 91% and 85%, and CR rate of 75% and 75%, respectively. Patients who had not received prior BTKi treatment exhibited ORR rate of 93% and CR rate of 83%, whereas those with a history of such treatment exhibited ORR rate of 83%, and CR rate of 70%. Patients without a history of hematopoietic stem cell transplantation (HSCT) achieved a CR rate of 70%, while those with a prior HSCT history had a notably higher CR rate of 83%. When stratified by the number of prior lines of therapy, patients who received three or fewer treatments showed a CR rate of 75%, whereas those who had more than three lines of therapy had a slightly lower CR rate of 71% (Table 2).
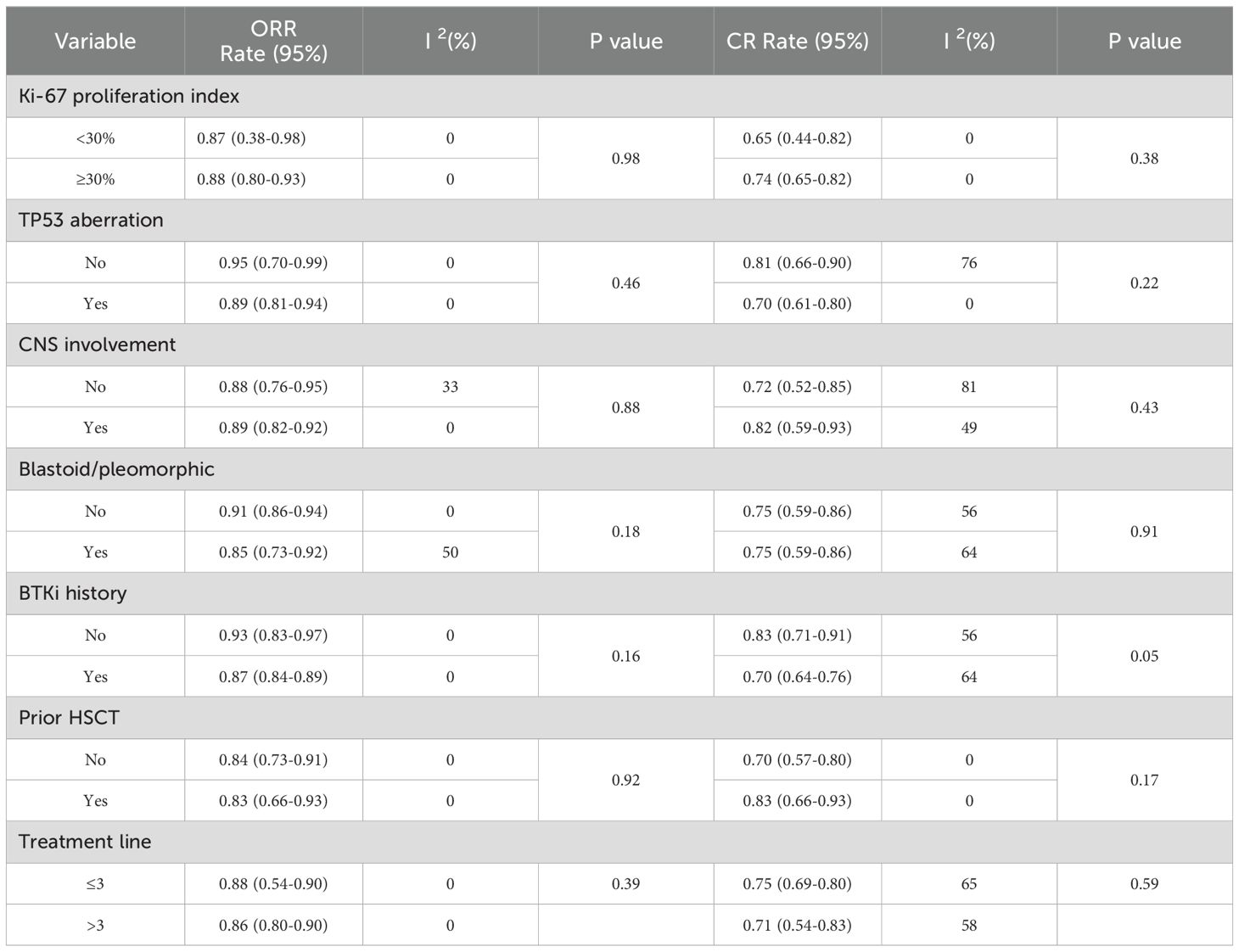
Table 2. Subgroup analysis of the impact of biomarkers and clinical features on remission rates in meta-analysis.
Taking into account the inclusion of different types of CAR-T products, we conducted a subgroup analysis comparing Brexu-cel CAR-T with other CAR-T cell therapies, including Tisa-cel, Liso-cel, and CTL019 CAR-T. Brexu-cel and other CAR-T therapies demonstrated similar ORR and CR rates. Specifically, the ORR was 88% (95% CI: 86%-90%) for Brexu-cel and 87% (95% CI: 62%-96%) for other CAR-T products. The CR rates were 73% (95% CI: 67%-78%) for Brexu-cel and 76% (95% CI: 68%-83%) for others. However, Brexu-cel had a higher incidence of CRS and ICANS, particularly grade 3 ICANS, but also showed better short-term efficacy with higher 6-month PFS and 6-month OS rates (Supplementary Table 2).
Due to differences in study design and underlying patient populations, a subset analysis was conducted to compare data from prospective clinical trials with retrospective/case series to identify if differences exist between real-world data and clinical trial data. In the subgroup analysis, retrospective (a total of 456 patients) and prospective (a total of 528 patients) studies were compared. Retrospective studies demonstrated slightly higher ORR and CR rates. Specifically, the ORR was 91% (95% CI: 88%-93%) in retrospective studies and 86% (95% CI: 82%-88%) in prospective studies. The CR rate was 77% (95% CI: 69%-83%) in retrospective studies and 70% (95% CI: 65%-75%) in prospective studies. However, 12-month PFS and 12-month OS were significantly better in prospective studies, with 12-month PFS at 57% (95% CI: 44%-69%) compared to 46% (95% CI: 36%-56%) in retrospective studies, and 12-month OS at 80% (95% CI:54%-94%) compared to 61% (95% CI: 47%-73%) in retrospective studies. The incidence of CRS was similar between the two groups, but Grade 3 CRS was more frequent in prospective studies. ICANS rates were comparable between retrospective and prospective studies (Supplementary Table 3).
Among the included studies, 9 were considered to have a low risk of bias and 7 had a moderate risk of bias (Supplementary Tables 4, 5). There was no significant correlation between bias risk and outcomes such as disease remission, PFS at 12 months, or adverse events like CRS or ICANS.
Discussion
The results of this meta-analysis demonstrate that CAR-T cell therapy is an effective treatment for R/R MCL with manageable safety. In the 16 studies included, a total of 984 patients received CAR-T therapy, achieving an ORR of 89% and a CR rate of 74%. Additionally, the 6-month and 12-month PFS rates were 69% and 53%, respectively, while the OS rates were 80% and 69%, respectively. Although 8% of patients experienced grade 3 or higher CRS and 22% experienced grade 3 or higher ICANS, overall, CAR-T therapy demonstrated promising efficacy and manageable adverse effects in patients with R/R MCL.
Over the past 10 years, BTKis have greatly improved the treatment outcome of patients with R/R MCL. However, a considerable number of patients still experience relapse and poor outcomes after BTKi therapy. Therefore, it is important to explore alternative treatment options for patients with MCL that are resistant to BTKis. A study compared the OS of patients with R/R MCL with failed covalent BTKi treatment. The patients were either treated with standard of care or Brexu-cel (31, 32). The results indicated that patients who received Brexu-cel had a significantly higher OS rate compared with those who received standard of care treatment.
We conducted a detailed search of the database, which currently encompassing numerous clinical trials of CAR-T cell products for MCL. Due to the limited number of studies on bispecific and other targets of CAR-T therapy for MCL, and differences in targets and manufacturing processes compared to CD19 single-target CAR-T, this study excluded dual-target and case series with fewer than 10 patients to reduce heterogeneity and bias. Therefore, our article mainly represents the efficacy of CD19 CAR-T therapy for R/R MCL, primarily Brexu-cel. This may not be representative of other CAR-T products. In a subgroup analysis of different CD19 CAR-T therapies, the ORR for the Brexu-cel product was 88% with a CR rate of 73%, while other CAR-T products had an ORR of 87% and a CR rate of 76%. Brexu-cel product and other CAR-T products exhibited similar effectiveness. However, the literature of other CAR-T is limited, these results should be taken with caution.
In addition, this study thoroughly examined various factors that impact CAR-T therapy for MCL. The findings suggested that CAR-T therapy can be effective even in high-risk patients with MCL with characteristics such as TP53 lesions (17p13 deletions or TP53 mutations), blastoid/pleomorphic histology, CNS involvement, high Ki-67 index, and complex karyotype. These indicating CAR-T have a wider applicability in MCL and suggesting CAR-T maybe a promising therapeutic approach for MCL patients. Notably, results showed prior exposure to BTKis may be linked to lower CR rates. In some way, it indicated early adoption of CAR-T therapy may result in better treatment outcomes.
Patients with MCL that has spread to the CNS typically have a poor prognosis and limited treatment options, with a median OS of less than 5 months (33, 34). Recent studies have reported that CAR T-cell therapy may be a promising treatment option for MCL with CNS involvement (35). In our meta-analysis, 8 studies encompassed patients with CNS involvement. The aggregated results from our meta-analysis demonstrated a remarkable ORR of 89% and a CR rate of 82% for R/RMCL with CNS involvement, indicating significant efficacy of CAR-T cells in treating R/R MCL. However, further prospective clinical trials are needed to confirm these encouraging results.
There are certain differences among various adverse event grading standards (36). Among the 16 articles in our study, 13 used the ASTCT criteria. We compared the different grades of CRS and ICANS between the two standards and found minimal differences in grade 3 or higher adverse events (36, 37). Therefore, we discussed the incidence of adverse events across all articles together. Additionally, a stratified analysis of articles using different assessment criteria showed consistent evaluation results (data not shown). In conclusion, different grading standards have little impact on the safety assessment in this study.
There were some limitations in this study. Firstly, the CAR-T cells used in included articles were mainly Brexu-cel, indicating our article mainly represents the efficacy of Brexu-cel for R/R MCL, and may not be representative of other CAR-T products. Secondly, due to the median PFS and OS not being reached in some clinical studies, coupled with the likely missing follow-up data from many real-world, non-clinical trial studies, there may be biases in the analysis of follow-up data. This may lead to systematic biases in the follow-up data, affecting the accuracy and reliability of the results. Nonetheless, to the best of our knowledge, this is the first comprehensive analysis of the efficacy and safety of CAR-T therapy in treating MCL, as well as exploration of high-risk factors. This study with largest sample size would provide more reliable information for CAR-T cell in MCL.
In conclusion, CAR-T therapy is highly effective as a salvage treatment for R/R MCL, even in patients with high-risk features. However, due to the lack of longer follow-up, the long-term efficacy remains to be determined. The variability in patient responses underscores the importance of personalized treatment approaches. Future research should focus on long-term efficacy assessments to optimize treatment strategies and improve overall patient outcomes.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
HXW: Data curation, Formal analysis, Software, Visualization, Writing – original draft. SQW: Data Curation, Investigation, Resources, Writing – original draft. SMS: Methodology, Validation, Writing – original draft. ZLK: Software, Validation, Writing – original draft. QMW: Conceptualization, Investigation, Supervision, Writing – review & editing. LHH: Conceptualization, Project administration, Methodology, Software, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study is supported by the Jiangxi Province Key Science and Technology Cooperation Project (20212BDH80014).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1435127/full#supplementary-material
Supplementary Figure 1 | The flow chart of literature screening.
Abbreviations
MCL, Mantle cell lymphoma; CAR-T, Chimeric antigen receptor T; R/R MCL, Relapsed or refractory mantle cell lymphoma; CRS, Cytokine release syndrome; ORR, Overall response rate; BTKi, Bruton’s tyrosine kinase inhibitor; PFS, Progression-free survival; OS, Overall survival; 95% CI, 95% Confidence interval; CR, Complete response; CNS, Central nervous system; HSCT, Hematopoietic stem cell transplantation; CNS, Central nervous system; ICANS, Immune effector cell-associated neurotoxicity syndrome.
References
1. Arora R, John L, Saba NS. Evolving mantle cell lymphoma trends: update from 2000 to 2020 SEER data. Am J Med Sci. (2024) 367:S351–2. doi: 10.1016/S0002-9629(24)00634-7
2. Desai K, Iqbal S, Choi E, Thar YY, Rather M, Thirumaran R. A retrospective analysis of the trends in mantle cell lymphomas in the united states. Blood. (2023) 142:6165. doi: 10.1182/blood-2023-174398
3. Lymphoma Research Foundation, L.R.F. Twenty years of advancing discoveries and treatment of mantle cell lymphoma. Oncol (Williston Park). (2024) 38(2):51–67. doi: 10.46883/2024.25921013
4. Hanel W, Epperla N. Emerging therapies in mantle cell lymphoma. J Hematol Oncol. (2020) 13(1):79. doi: 10.1186/s13045-020-00914-1
5. Lee YP, Jung YJ, Cho J, Ko YH, Kim WS, Kim SJ, et al. A retrospective analysis of ibrutinib outcomes in relapsed or refractory mantle cell lymphoma. Blood Res. (2023) 58(4):208–20. doi: 10.5045/br.2023.2023208
6. Rai S, Tanizawa Y, Cai Z, Huang YJ, Taipale K, Tajimi M. Outcomes for recurrent mantle cell lymphoma post-ibrutinib therapy: A retrospective cohort study from a japanese administrative database. Adv Ther. (2022) 39(10):4792–807. doi: 10.1007/s12325-022-02258-3
7. Amin R, Darwin R, Chakraborty S, Dey A, Dhama K, Emran TB. Advances in CAR T-cell therapy for treating patients with mantle cell lymphoma: a critical appraisal. Int J Surg (London England). (2023) 109(12):3742–4. doi: 10.1097/js9.0000000000000691
8. Anderson MK, Torosyan A, Halford Z. Brexucabtagene autoleucel: A novel chimeric antigen receptor T-cell therapy for the treatment of mantle cell lymphoma. Ann Pharmacother. (2022) 56(5):609–19. doi: 10.1177/10600280211026338
9. Eyre TA, Cheah CY, Wang ML. Therapeutic options for relapsed/refractory mantle cell lymphoma. Blood. (2022) 139(5):666–77. doi: 10.1182/blood.2021013326
10. Arun Kumar S, Gao J, Patel SA. The shifting therapeutic paradigm for relapsed/refractory mantle cell lymphoma. Leuk Res. (2023) 134:107385. doi: 10.1016/j.leukres.2023.107385
11. Wang M, Munoz J, Goy A, Locke FL, Jacobson CA, Hill BT, et al. KTE-X19 CAR T-cell therapy in relapsed or refractory mantle-cell lymphoma. N Engl J Med. (2020) 382(14):1331–42. doi: 10.1056/NEJMoa1914347
12. Jain P, Wang ML. Mantle cell lymphoma in 2022-a comprehensive update on molecular pathogenesis, risk stratification, clinical approach, and current and novel treatments. Am J Hematol. (2022) 97(5):638–56. doi: 10.1002/ajh.26523
13. Wang M, Siddiqi T, Gordon LI, Kamdar M, Lunning M, Hirayama AV, et al. Lisocabtagene maraleucel in Relapsed/Refractory mantle cell lymphoma: Primary analysis of the mantle cell lymphoma cohort from TRANSCEND NHL 001, a phase i multicenter seamless design study. J Clin Oncol. (2024) 42(10):1146–57. doi: 10.1200/jco.23.02214
14. Shaikh S, Shaikh H. CART cell therapy toxicity. In: StatPearls. Treasure Island (FL: StatPearls Publishing LLC (2024). StatPearls Publishing Copyright © 2024.
15. Sterner RC, Sterner RM. CAR-t cell therapy: current limitations and potential strategies. Blood Cancer J. (2021) 11(4):69. doi: 10.1038/s41408-021-00459-7
16. Wang M, Munoz J, Goy A, Locke FL, Jacobson CA, Hill BT, et al. Three-year follow-up of KTE-X19 in patients with Relapsed/Refractory mantle cell lymphoma, including high-risk subgroups, in the ZUMA-2 study. J Clin Oncol. (2023) 41(3):555–67. doi: 10.1200/jco.21.02370
17. Banerjee T, Newman M, Chen A, Maziarz RT, Schachter L, Spurgeon SE, et al. A retrospective single-center analysis of CD-19 directed CAR T-cell therapy in relapsed/refractory mantle cell lymphoma. Leuk Lymphoma. (2023) 64(8):1472–5. doi: 10.1080/10428194.2023.2212098
18. Iacoboni G, Rejeski K, Villacampa G, van Doesum JA, Chiappella A, Bonifazi F, et al. Real-world evidence of brexucabtagene autoleucel for the treatment of relapsed or refractory mantle cell lymphoma. Blood Adv. (2022) 6(12):3606–10. doi: 10.1182/bloodadvances.2021006922
19. Ryan CE, Zon RL, Redd R, Fisher DC, Shouval R, Kumar A, et al. Clinical efficacy and safety of chimeric antigen receptor T-cell therapy for mantle cell lymphoma with secondary central nervous system involvement. Br J Haematol. (2023) 203(5):774–80. doi: 10.1111/bjh.19037
20. Wang Y, Jain P, Locke FL, Maurer MJ, Frank MJ, Munoz JL, et al. Brexucabtagene autoleucel for relapsed or refractory mantle cell lymphoma in standard-of-Care practice: Results from the US lymphoma CAR T consortium. J Clin Oncol. (2023) 41(14):2594–606. doi: 10.1200/jco.22.01797
21. Locke F, Hu ZH, Gerson J, Frank MJ, Budde LE, Wang M, et al. REAL-WORLD OUTCOMES OF BREXUCABTAGENE AUTOLEUCEL (BREXU-CEL) FOR THE TREATMENT OF RELAPSED OR REFRACTORY (R/R) MANTLE CELL LYMPHOMA (MCL) IN THE UNITED STATES (US). HemaSphere. (2022) 6:2548–9. doi: 10.1097/01.HS9.0000852292.38263.b8
22. Hess G, Vucinic V, Rejeski K, Aydilek E, Simon L, Penack O, et al. Real world results of brexucabtagene autoleucel for patients with Relapsed/Refractory mantle cell lymphoma - first German/Swiss analysis. Blood. (2023) 142:4394. doi: 10.1182/blood-2023-182415
23. Herbaux C, Bret C, Di Blasi R, Bachy E, Beauvais D, Gat E, et al. Kte-x19 in relapsed or refractory mantle-cell lymphoma, a 'real-life' study from the DESCAR-T registry and lysa group. Blood. (2021) 138(SUPPL 1):743. doi: 10.1182/blood-2021-148626
24. Rejeski K, Wang Y, Albanyan O, Munoz J, Sesques P, Iacoboni G, et al. Infectious complications, and poor treatment outcomes following brexucabtagene autoleucel for relapsed or refractory MCL. Am J Hematol. (2023) 98(11):1699–710. doi: 10.1002/ajh.27056
25. Goy A, Jacobson CA, Flinn IW, Hill BT, Weng WK, Mountjoy L, et al. Outcomes of patients with Relapsed/Refractory mantle cell lymphoma (R/R MCL) treated with brexucabtagene autoleucel (Brexu-cel) in ZUMA-2 and ZUMA-18, an expanded access study. Blood. (2023) 142:106. doi: 10.1182/blood-2023-174273
26. Ababneh H, Frigault M, Patel CG. Outcomes of bridging and salvage radiotherapy in relapsed or refractory mantle cell lymphoma patients undergoing CD19-targeted CAR T-cell therapy. Int J Radiat Oncol Biol Phys. (2023) 117(2):E456–6.
27. Minson AG, Hamad N, Cheah CY, Tam CS, Blombery P, Westerman DA, et al. CAR T-cells and time-limited ibrutinib as treatment for Relapsed/Refractory mantle cell lymphoma: Phase II TARMAC study. Blood. (2023). doi: 10.1182/blood.2023021306
28. Ahmed G, Alsouqi A, Szabo A, Samples L, Shadman M, Awan FT, et al. CAR T-cell therapy in mantle cell lymphoma with secondary CNS involvement: a multicenter experience. Blood Adv. (2024) 8(13):3528–31. doi: 10.1182/bloodadvances.2023012255
29. O'Reilly MA, Wilson W, Burns D, Kuhnl A, Seymour F, Uttenthal B, et al. Brexucabtagene autoleucel for relapsed or refractory mantle cell lymphoma in the united kingdom: A real-world intention-to-treat analysis. Hemasphere. (2024) 8(6):e87. doi: 10.1002/hem3.87
30. Romancik JT, Goyal S, Gerson JN, Ballard HJ, Sawalha Y, Bond DA, et al. Analysis of outcomes and predictors of response in patients with relapsed mantle cell lymphoma treated with brexucabtagene autoleucel. Blood. (2021) 138:1756. doi: 10.1182/blood-2021-153277
31. Hess G, Dreyling M, Oberic L, Gine E, Zinzani PL, Linton K, et al. Indirect treatment comparison of brexucabtagene autoleucel (ZUMA-2) versus standard of care (SCHOLAR-2) in relapsed/refractory mantle cell lymphoma. Leuk Lymphoma. (2024) 65(1):14–25. doi: 10.1080/10428194.2023.2268228
32. Hess G, Dreyling M, Oberic L, Gine E, Zinzani PL, Linton KM, et al. A comparison of overall survival with brexucabtagene autoleucel (Brexu-cel) CAR T-cell therapy (ZUMA-2) and standard of care (SCHOLAR-2) in patients with Relapsed/Refractory mantle cell lymphoma (R/R MCL) previously treated with a covalent bruton tyrosine kinase inhibitor (BTKi). Blood. (2022) 140:10296–9. doi: 10.1182/blood-2022-162638
33. McLaughlin N, Wang Y, Witzig T, Villasboas J, Habermann T, Inwards D, et al. Central nervous system involvement by mantle cell lymphoma. Leuk Lymphoma. (2023) 64(2):371–7. doi: 10.1080/10428194.2022.2148211
34. Rusconi C, Cheah CY, Eyre TA, Tucker D, Klener P, Giné E, et al. Ibrutinib improves survival compared with chemotherapy in mantle cell lymphoma with central nervous system relapse. Blood. (2022) 140(17):1907–16. doi: 10.1182/blood.2022015560
35. Minson A, Dickinson M. Combining forces for good: chimeric antigen receptor T-cells and bruton tyrosine kinase inhibitors in mantle cell lymphoma with central nervous system involvement. Leuk Lymphoma. (2024) 65(5):546–7. doi: 10.1080/10428194.2024.2320833
36. Lee DW, Santomasso BD, Locke FL, Ghobadi A, Turtle CJ, Brudno JN, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant. (2019) 25(4):625–38. doi: 10.1016/j.bbmt.2018.12.758
Keywords: CAR-T, relapsed or refractory, mantle cell lymphoma, meta-analysis, therapy
Citation: Wan H, Weng S, Sheng S, Kuang Z, Wang Q and Hu L (2024) Chimeric antigen receptor T-cell therapy in relapsed or refractory mantle cell lymphoma: a systematic review and meta-analysis. Front. Immunol. 15:1435127. doi: 10.3389/fimmu.2024.1435127
Received: 19 May 2024; Accepted: 12 August 2024;
Published: 06 September 2024.
Edited by:
Saad Zafar Usmani, Levine Cancer Institute, United StatesReviewed by:
Walter Hanel, The Ohio State University, United StatesYang Liu, University of Texas MD Anderson Cancer Center, United States
Copyright © 2024 Wan, Weng, Sheng, Kuang, Wang and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Linhui Hu, aHVsaW5odWkxOTkyQDE2My5jb20=; Qingming Wang, bmRlZnk5ODAwMUBuY3UuZWR1LmNu
 Haixiang Wan
Haixiang Wan Songqin Weng
Songqin Weng Linhui Hu
Linhui Hu