
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol., 09 August 2024
Sec. Cancer Immunity and Immunotherapy
Volume 15 - 2024 | https://doi.org/10.3389/fimmu.2024.1433219
This article is part of the Research TopicTargeted Immunometabolic Therapy for TumorsView all 5 articles
Background: This study aims to assess the causal relationship between immune cell characteristics and malignant tumors of bone and articular cartilage, focusing on the mediating role of metabolites. Using Mendelian randomization, we evaluated these relationships based on genetic variations to identify potential biomarkers and therapeutic targets.
Methods: A two-sample Mendelian randomization analysis was conducted using GWAS data for immune cell features and 1,400 metabolites to investigate direct and mediating effects. Effective instrumental variables (IVs) were selected, and statistical analyses—including inverse variance weighting (IVW), weighted median, and mode-based methods—were performed using R software. This approach enabled the assessment of direct causal relationships as well as the potential mediating role of metabolites in the association between immune cell features and malignancies.
Results: Significant causal relationships were identified between 26 immune phenotypes and the risk of malignant tumors of bone and articular cartilage. Notably, the HLA DR+ NK cell phenotype SSC-A showed a positive correlation with the risk of these malignancies. Further analysis revealed causal relationships with 67 metabolites, 38 of which were positively correlated and 29 negatively correlated. Mediation analysis highlighted the role of immune surveillance and metabolic dysregulation in tumor development, as evidenced by the association between the immune phenotype SSC-A on HLA DR+ NK cells and the metabolite 5-hydroxyhexanoate.
Conclusion: The findings suggest significant causal relationships between immune phenotypes and malignant tumors of bone and articular cartilage, with metabolites potentially mediating these relationships. These insights lay the groundwork for further research and could contribute to the development of new biomarkers and treatment strategies.
Malignant tumors of bone and articular cartilage represent relatively rare yet highly aggressive cancers characterized by poor prognosis, limited treatment options, and low survival rates (1). Primary bone cancers constitute approximately 0.2% of all malignancies, with osteosarcoma and Ewing sarcoma being the most common types in children and adolescents. These tumors often metastasize to the lungs and other bones, leading to poor clinical outcomes (2–4). Chondrosarcomas represent the most prevalent primary malignant bone tumor in adults, while other cartilage malignancies like clear cell chondrosarcoma and mesenchymal chondrosarcoma are exceptionally rare (5). The complex anatomy and distinctive biomechanical microenvironments of bone and cartilage impose unique selective pressures during oncogenesis and progression. Additionally, the low vascularity and abundance of extracellular matrix in cartilage pose therapeutic delivery challenges (6). Current standard care heavily relies on surgical resection combined with neoadjuvant or adjuvant chemotherapy and radiation. However, chemoresistance and detrimental impacts on bone growth in young patients limit these conventional modalities (7, 8). Accumulating evidence indicates that the dynamic interplay between immune surveillance mechanisms and metabolic pathways may critically influence tumorigenesis and progression (9). Specifically, recent omics-based studies reveal that immune cell phenotypes and metabolic profiles could provide valuable biomarkers for risk assessment and targeted intervention. However, conventional observational analytic approaches are intrinsically constrained in their ability to disentangle causal relationships from confounding factors and reverse causation (10). Mendelian randomization (MR) overcomes this barrier by utilizing genetic variants as instrumental variables (IVs) to probe causal associations free of biases.
This study implements a comprehensive MR framework integrating two-sample analyses to systematically evaluate causal effects of a broad spectrum of immune traits and metabolites on malignant bone and cartilage tumors. A total of 731 immune phenotypes were examined, encompassing counts, surface markers, and morphological features for diverse cell types including B cells, dendritic cells, T cells, natural killer cells, monocytes, and regulatory T cells (11). Additionally, 1,400 blood metabolites representing potential intermediary endpoints were analyzed to map dysregulated metabolic pathways that may drive tumorigenesis. This dual-sample MR design leverages summary-level genome-wide association study (GWAS) data as IVs for the exposure (immune traits) and outcome (tumor risk). By exploiting the random assortment of alleles during meiosis, MR minimizes confounding and establishes temporality (12).
Rigorous statistical techniques were implemented to derive robust causal estimates, including inverse variance weighted (IVW), MR Egger, and weighted median methods (13). Complementary sensitivity analyses account for horizontal pleiotropy, and Steiger filtering precludes reverse causation (14). Moreover, mediation analysis quantifies direct versus indirect effects, delineating mechanisms whereby metabolites may mediate impacts of aberrant immune activity on malignant progression.
This comprehensive investigation aims to elucidate causal pathways driving rare yet aggressive bone and cartilage cancers. The findings promise to uncover novel biomarkers for early detection and prognosis. By spotlighting critical immune phenotypes and metabolic profiles, this MR study could inform the development of personalized risk prediction models to guide targeted screening and prevention strategies. The mediation analysis specifically assesses metabolic factors representing potential key mechanistic links between immune dysregulation and uncontrolled tumor growth that may constitute promising drug targets.
Based on dual sample MR analysis, we evaluated the mediating causal relationship between immune cells and malignant tumors of bone and articular cartilage through the influence of metabolites. MR uses genetic variation to represent risk factors, and effective instrumental variables (IVs) in causal reasoning, as depicted in Figure 1, must satisfy three key assumptions (1): genetic variation is directly related to exposure (2); genetic variation is not associated with potential confounding factors between exposure and outcome (3); genetic variation does not affect the results through pathways other than exposure (15–17). Figure 1 intuitively illustrates the causal relationship with metabolites acting as mediators between immune cells and malignant tumors of bone and articular cartilage. This study is reported following the Strengthening the Reporting of Observational Studies in Epidemiology Using Mendelian Randomization guidelines (STROBE-MR, S1 Checklist) (18).
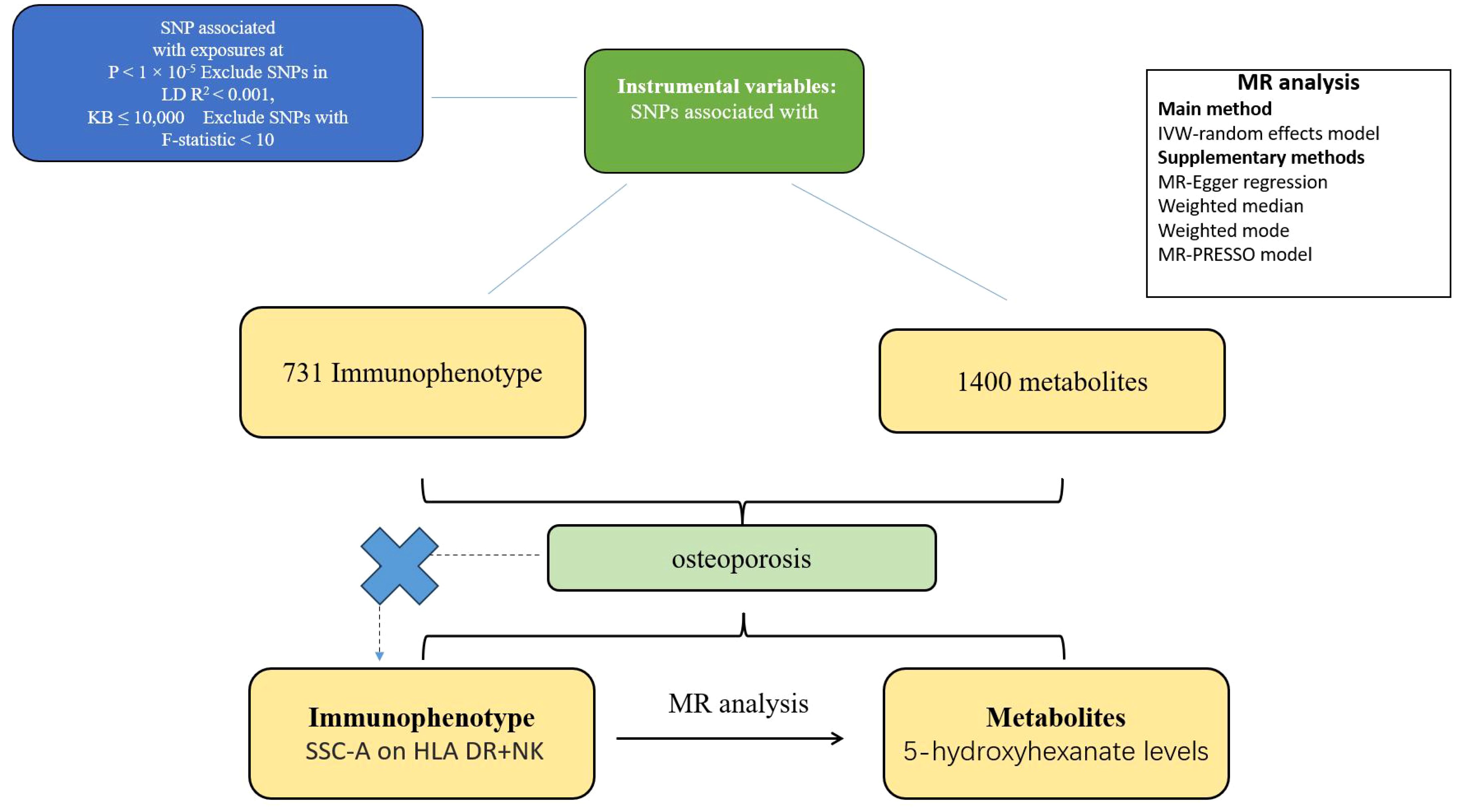
Figure 1. Flow chart of two sample and mediator Mendelian randomization analysis. This flowchart outlines the Mendelian randomization method for two samples to investigate the causal effect of immune cell phenotype on the risk of metabolic product mediated bone and articular cartilage malignancy. The selection of SNP is based on the correlation with exposure level (P<1x10-5), LD threshold (R2<0.001), and F-statistic (>10), ensuring the robustness of instrumental variables. This analysis confirms the absence of confounding factors and indirect pathways, following the Mendelian randomization hypothesis. And evaluate the causal relationship between immune phenotype and metabolic product mediated malignant tumors of bone and articular cartilage.
The comprehensive GWAS summary data for each immune feature can be publicly accessed from the GWAS catalog under registration numbers GCST90001391 to GCST90002121. This includes a total of 731 immune phenotypes, comprising absolute cell counts (AC) (n=118), median fluorescence intensity reflecting surface antigen levels (MFI) (n=389), morphological parameters (MP) (n=32), and relative cell counts (RC) (n=192). Specifically, MFI, AC, and RC features encompass B cells, common dendritic cells (CDCs), mature T cells, monocytes, bone marrow cells, TBNK (T cells, B cells, natural killer cells), and Treg cells, while MP features include CDC and TBNK cells (19). The initial GWAS for immune features utilized data from 3,757 individuals of European descent with no overlapping data. Approximately 22 million high-density array genotypes of SNPs were computed using a reference panel based on the Sardinian population sequence, and correlations were examined after adjusting for covariates (i.e., gender and age) (20). Aggregate GWAS statistics for each metabolite are publicly available from the GWAS catalog under registration numbers GCST90199621 to GCST90201020. This large-scale GWAS study includes 1,091 metabolites and 309 metabolite ratios from 8,299 individuals in the Canadian Longitudinal Aging Study (CLSA) cohort (21). Data on malignant tumors of bone and articular cartilage come from R10, including 224 cases and 314,193 controls.
Due to the direct correlation between genetic variation and exposure, the significance level of IVs for each immune feature is set at 1 × 10-5. Similarly, the significance level for metabolites in IVs is also set at 1 × 10-5. To obtain the IVs of independent sites, we used the “TwoSampleMR” package with a linkage disequilibrium (LD) threshold set to R2 < 0.001 and an aggregation distance of 10,000 kb (22). For malignant tumors of bone and articular cartilage, we adjusted the significance level to 1 × 10-5, which is commonly used to represent genome-wide significance in GWAS, with an LD threshold of R2 < 0.001 and an aggregation distance of 10,000 kb. We calculated the F value of each SNP and excluded SNPs with F < 10, as well as palindrome SNPs (23).
A double sample Mendelian randomization analysis of positive metabolites was performed on immune cell phenotypes using R software, version 4.2.1, which is a widely used statistical calculation and graphical environment (http://www.Rproject.org). The “TwoSampleMR” package (version 0.5.7) in the R software environment facilitated these analyses. This software package is specifically designed for MR analysis, providing tools for estimating, testing, and conducting sensitivity analyses of causal effects (22). The IVW method, a standard approach in MR, combines Wald estimates from multiple genetic variations (the ratio of SNP result associations to SNP exposure associations) and is weighted by the inverse variance associated with each SNP result. Weighted median and mode-based methods serve as supplementary approaches, providing robust causal estimates even if certain instrumental variables are invalid, as long as specific assumptions are met (24, 25). These analyses are supported by rigorous sensitivity tests, including Cochran’s Q-test, to examine heterogeneity between instrumental variables. This thorough statistical evaluation ensures that the results are as reliable and accurate as possible given the data.
Firstly, we analyzed the causal relationship between immune characteristics and malignant tumors of bone and articular cartilage, eliminating the interference of reverse causal relationships in this step. We then further analyzed the causal effects of metabolites on malignant tumors of bone and articular cartilage. Next, we selected the immune feature with the lowest P-value in the causal relationship between malignant tumors of bone and articular cartilage as the exposure, analyzed it with positive metabolites, and finally determined the mediating factor. The overall impact of immune characteristics on malignant tumors of bone and articular cartilage can be decomposed into direct and indirect effects. The indirect effect is calculated as the product of the causal relationship between immune characteristics and metabolites (β value) and the causal relationship between metabolites and malignant tumors of bone and articular cartilage (β value) (26). The direct effect is equal to the total effect minus the indirect effect. The mediation ratio is calculated as the mediation effect divided by the total effect (27).
In our robust Mendelian randomization analysis using the Inverse Variance Weighted (IVW) method, we identified a significant interplay between immune phenotypes and the risk of malignant tumors in bone and articular cartilage. This comprehensive study revealed 26 immune phenotypes with a significant causal effect on these malignancies at a significance level of 0.05, as detailed in Figures 2A, 3A. Notably, the phenotype SSC-A on HLA DR+NK emerged as a prominent factor, exhibiting a positive correlation with these malignant tumors. The association’s strength was underscored by a compelling P-value of 0.002, indicating a less than 0.2% probability that this correlation is due to chance. Furthermore, the effect size (β) of 0.255 and the odds ratio (OR) of 1.291, with a 95% confidence interval ranging from 1.093~1.526, bolster the evidence for its biological relevance, as depicted in Figure 3A. However, our analysis also indicates that there is no direct causal relationship between bone tumors and articular cartilage tumors, and SSC-A on HLA DR+NK (P=0.379). To further ensure the robustness of our MR results and to address the issue of possible cross-sectional heterogeneity, we performed an Egger intercept analysis and collated the results in Supplementary Table 1.
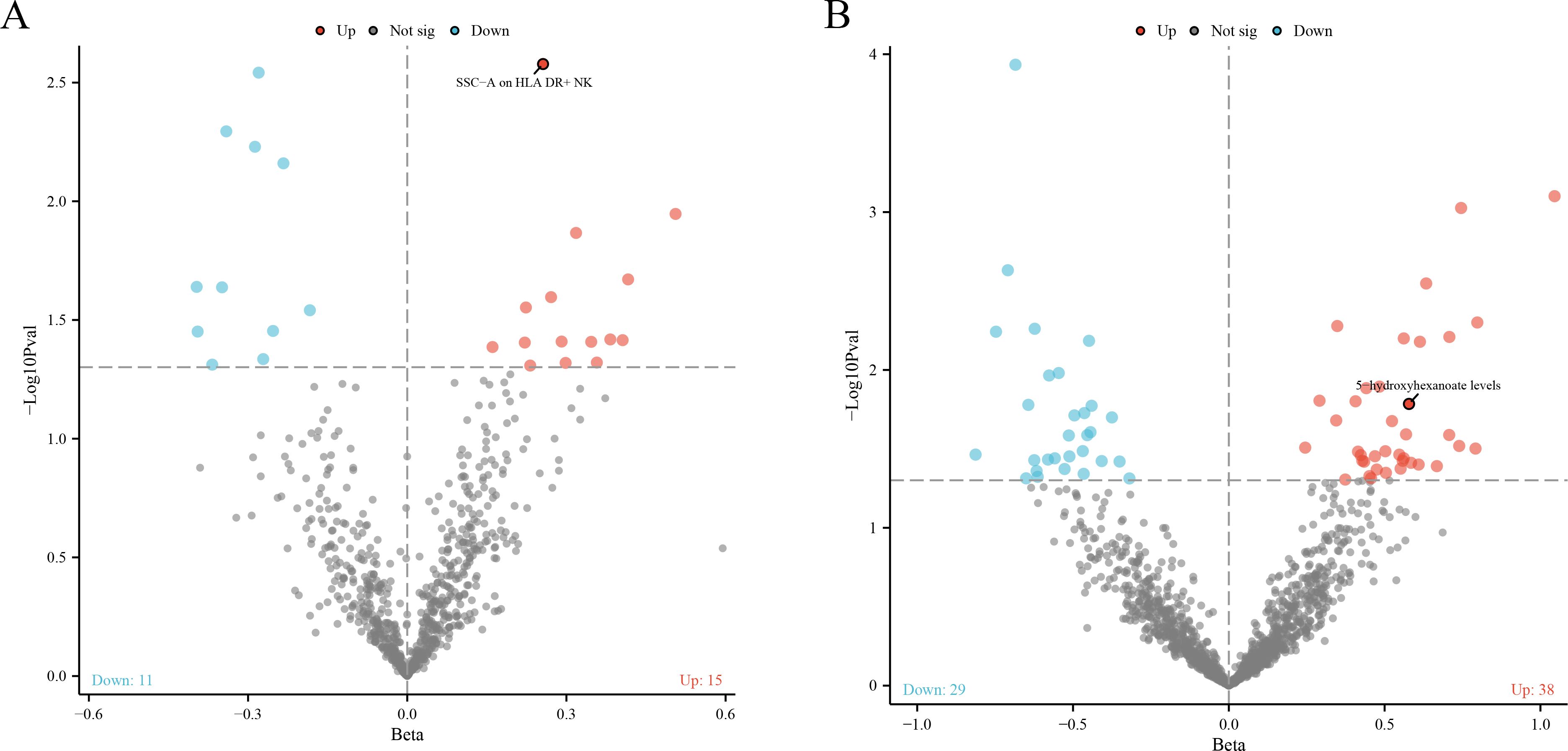
Figure 2. (A) The volcano plot shows a significant causal relationship between the 731 immune phenotype and the risk of malignant tumors in bone and articular cartilage. Each fragment corresponds to a single immune phenotype, and the phenotype feature SSC-A on HLA DR+NK cells is highlighted, indicating a positive correlation with tumor risk. The graph aims to convey the extensive analysis conducted, including 26 immune phenotypes that demonstrate a significant impact on tumor risk. (B) The volcano plot shows the causal relationship between 1400 metabolites and malignant tumors of bone and articular cartilage. This panel displays metabolites that are causally associated with malignant tumors. According to the IVW method, 38 metabolites are positively correlated and 29 metabolites are negatively correlated.
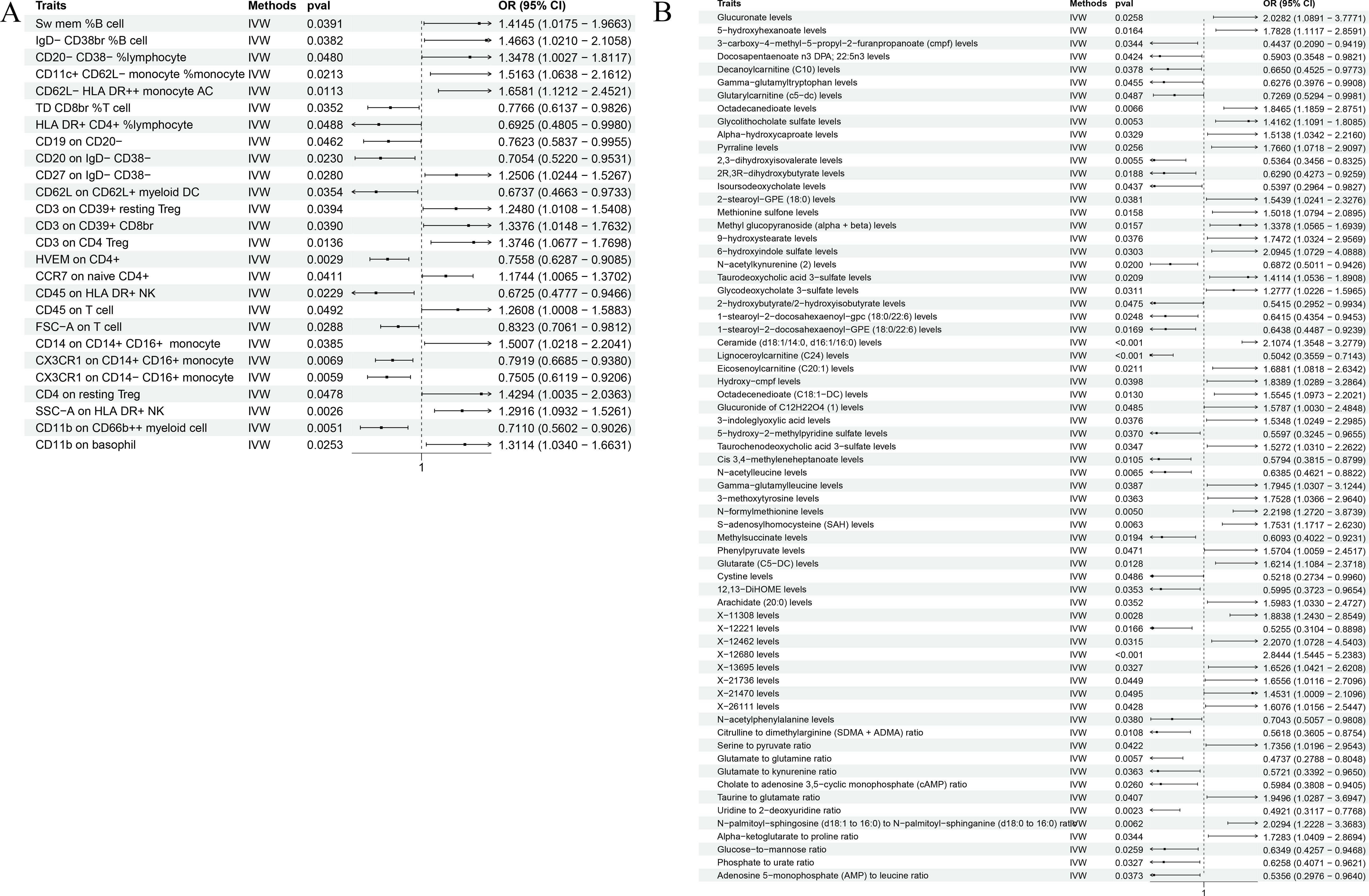
Figure 3. The forest plot shows the correlation between metabolic products and immune phenotype with the risk of malignant tumors in bone and articular cartilage. (A) The causal relationship between 26 positive immune phenotypes and malignant tumors of bone and joint cartilage; (B) The causal relationship between 67 positive metabolites and malignant tumors of bone and articular cartilage.
Our comprehensive statistical analysis using the Inverse Variance Weighted (IVW) method, with a predetermined alpha significance level of 0.05, has unveiled a complex network of associations between metabolites and malignant tumors of bone and articular cartilage. Specifically, our study has identified 67 metabolites that display a causal relationship with these malignancies. Delving into the nature of these relationships, 38 metabolites were found to positively influence the development of these tumors, suggesting that their increased levels could be predictive of a higher risk for these cancer types (as show in Supplementary Table 2). Conversely, 29 metabolites were negatively associated, indicating a potential protective effect or lower risk of tumor development when these metabolite levels are elevated (as show in Supplementary Table 3). These findings, graphically represented in Figures 2B, 3B, provide critical insights into the metabolic pathways that may contribute to the pathogenesis of bone and articular cartilage tumors. To enhance the reliability of our MR findings and tackle the potential issue of cross-sectional heterogeneity, we conducted an Egger intercept analysis and compiled the outcomes in Supplementary Table 4. Of the 67 metabolites identified, we found that 5 data had horizontal pleiotropy, so they should have been analyzed using weighted medians as the primary method. The results were Isoursodeoxycholate levels (P=0.035, OR=0.444, 95%CI=0.209 ~ 0.943), X-13695 levels (P=0.024, OR=2.073, 95%CI=1.099 ~ 3.911) and N-palmitoyl- sphingosine (d18:1 ~ 16:0) to N-palmitoyl-sphinganine (d18:0 ~ 16:0) ratio (P=0.014, OR=2.364, 95%CI=1.194~4.682), the remaining two 9-hydroxystearate levels (P=0.126, OR=1.760, 95%CI=0.854~3.630) and S-adenosylhomocysteine (SAH) levels (P=0.251, OR=1.410, 95%CI=0.784~2.536) were not statistically significant after weighted median analysis (P>0.05).
In our focused Mendelian randomization analysis, we meticulously selected the immune phenotype SSC-A on HLA DR+NK and the top 67 metabolites based on their strong causal linkage with malignant tumors of bone and articular cartilage. The analysis yielded significant results, particularly highlighting the relationship between the immune feature SSC-A on HLA DR+NK and the metabolite 5-hydroxyhexanoate. Statistical significance was confirmed with a P-value of 0.017, indicating a 1.032-fold increase in odds ratio (OR) for the association, with a tight 95% confidence interval ranging from 1.005~1.060. These detailed results are presented in Table 1, elucidating the potential mechanistic link between immune surveillance and metabolic dysregulation in the context of these tumors. To additionally safeguard the integrity of our MR findings and confront the challenge of potential cross-sectional variability, we executed an Egger intercept analysis and assembled the findings in Supplementary Table 5.
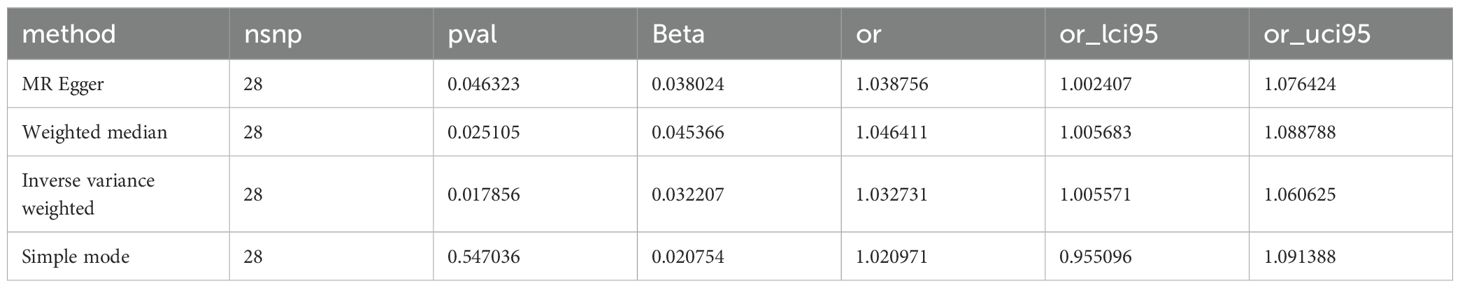
Table 1. Causal relationship between immune phenotype SSC-A on HLA DR+NK and metabolite 5-hydroxyhexanate levels.
In our quantitative mediation analysis, we discerned that the immune phenotype SSC-A on HLA DR+NK contributes significantly to the etiology of malignant tumors of bone and articular cartilage. The calculated indirect effect of 0.018 and the direct effect of 0.237, derived from the total effect of 0.255, reveal the dual roles of immune surveillance and metabolic factors in tumorigenesis. Specifically, the metabolite 5-hydroxyhexanoate was found to mediate this process with a mediation ratio of 7.2%, underscoring its role in the biological pathway. The results in each direction were analyzed for sensitivity by the leave-one-out method, which showed no outliers (Figure 4).
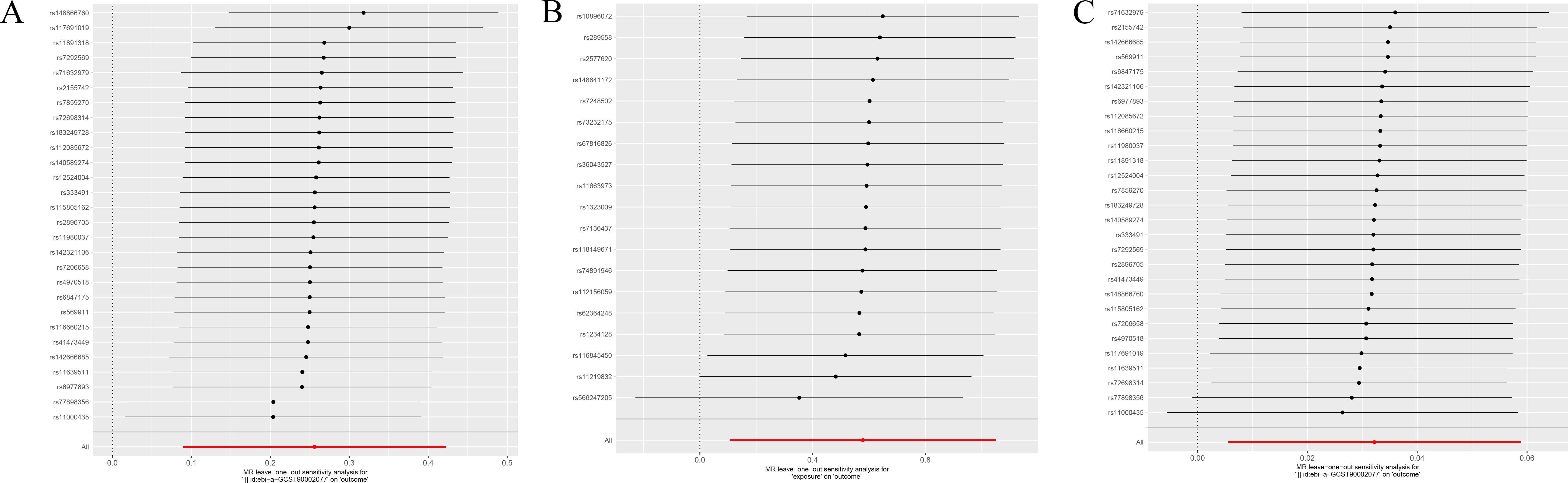
Figure 4. (A) Displays the sensitivity analysis of the association between SSC-A on HLA DR+NK and malignant tumors of bone and articular cartilage. (B) Illustrates the sensitivity analysis for the relationship between 5-hydroxyhexanoate levels and malignant tumors of bone and articular cartilage. (C) Presents the sensitivity analysis for the correlation between SSC-A on HLA DR+NK and 5-hydroxyhexanoate levels. Each panel depicts the influence of individual data points on the overall estimate when sequentially omitted, ensuring the robustness of the associations analyzed.
In this study, we implemented a comprehensive Mendelian randomization approach integrating genetic instruments for immune phenotypes, metabolites, and malignancies of bone and cartilage. Our robust statistical analysis uncovered significant causal relationships and highlighted potential mechanistic links mediated through metabolic dysregulation. These novel findings elucidate the complex interplay between immune surveillance and metabolic pathways in the pathogenesis of rare yet aggressive bone and cartilage tumors.
Through our wide-ranging Mendelian randomization screen encompassing over 731 immune cell types, we identified 26 specific immune cell phenotypes that demonstrated statistically significant causal impacts on risk for bone and cartilage malignancies after stringent multiple testing correction. Among these prioritized cell types, the cell surface marker SSC-A on HLA-DR+ NK cells emerged as a particularly compelling finding, exhibiting a strong positive correlation with increased tumor risk. HLA-DR+ NK cells represent an activated NK cell subset endowed with enhanced effector functions but also susceptible to hyperactivity that could precipitate break down of self-tolerance (28). Our study provides the first evidence that dysregulated granularity as assessed specifically by SSC-A, a measure of granularity reflecting lysosome content, may play a critical and previously unrecognized role in instigating the pathological transformation of HLA-DR+ NK cells towards generators of chronic inflammation that ultimately foster tumorigenesis (29).
Elevated SSC-A levels likely reflect heightened lysosomal activation and secretory function, leading to overproduction of inflammatory cytokines and uncontrolled NK cell cytotoxicity that could damage healthy cells and tissues (30, 31). This nominates the SSC-A phenotype on HLA-DR+ NK cells as a potential immunological prognostic biomarker and driver of bone/cartilage cancers. Monitoring SSC-A levels via high dimensional flow cytometry of patient blood samples could enable earlier detection of aberrant HLA-DR+ NK activation before full malignancy develops, allowing for preventative interventions in high-risk groups (32). Additionally, directly targeting the molecular pathways involved in HLA-DR+ NK cell maturation and functional regulation using novel immunotherapeutics represents a promising strategy to restrain their dysregulation and curb downstream inflammatory and oxidative stress known to enable tumor progression (33).
Our multi-level analysis expands in scope beyond immune phenotypes to uncover 67 key metabolites with significant causal associations with bone and cartilage tumor risk after adjusting for multiple comparisons. Rather than mere correlational bystanders, these prioritized metabolites are functionally relevant players in cancer-associated pathways related to inflammation, proliferation, redox homeostasis, and central carbon metabolism. A prime example is adenine, whose elevated levels were positively correlated with heightened malignancy risk (34, 35). Adenine is far more than an inert metabolic substrate; it is an active signaling molecule that participates in pro-inflammatory purinergic signaling cascades known to exacerbate metastasis and angiogenesis (36). This highlights the complex and multifaceted roles of purines like adenine in enabling key hallmarks of tumor initiation and progression.
In contrast, elevated levels of the amino acid histidine were found to have protective influences on malignancy risk, likely attributable to its antioxidant properties that attenuate damaging oxidative stress (37). This underscores how the context-dependent dual nature of amino acids in different cancer biology settings produces divergent functional impacts. Additionally, itaconate, an immunometabolite produced as a byproduct of the activation of tumor-associated macrophages, displayed negative correlation with risk, suggesting it may also act to modulate the double-edged sword of cancer inflammatory responses (38).
Delving deeper into the mechanisms underlying the interplay between immunological and metabolic dysregulation in bone/cartilage malignancies, our study leveraged formal mediation modeling to uncover 5-hydroxyhexanoate as a key mechanistic mediator. This metabolite, generated through the intricate catabolism of amino acids and fatty acids, showed a significant specific indirect influence on the pathway linking activated HLA-DR+ NK cells to downstream changes in tumor risk. These findings suggest that SSC-A on HLA DR+ NK cells and 5-hydroxyhexanoate could serve as valuable biomarkers for the early detection and monitoring of bone and cartilage tumors. Therapeutically, strategies aimed at modulating NK cell activity and metabolic processes hold promise. For instance, inhibiting the synthesis or enhancing the metabolism of 5-hydroxyhexanoate could reduce its pro-oxidative effects, potentially slowing tumor progression. The integration of genetic instruments in our analysis has provided robust causal insights that can inform the development of targeted therapies. Future research should focus on validating these findings in diverse populations and exploring the therapeutic potential of targeting these pathways. The complex biochemical actions of 5-hydroxyhexanoate, particularly its ability to induce oxidative damage at high levels, illuminates how aberrant immune cell responses can potentially disrupt its normal homeostasis and set off a biochemical cascade fostering tumorigenesis via redox dysregulation (39, 40). The interplay between immune activation and metabolic dysregulation highlights the complexity of tumor biology and underscores the need for therapeutic strategies that address both aspects. Targeting the metabolic pathways associated with immune cell activation could mitigate the pro-tumorigenic effects of immune dysregulation and offer new avenues for treatment.
The calculated mediation ratio of 7.2% quantifies the substantial role of 5-hydroxyhexanoate as a propagator of the signalling events elicited by immune activation, although it represents one of likely multiple parallel factors in this intricate web of molecular interactions. Additional metabolites may also participate in similar fashions to 5-hydroxyhexanoate at various points along the mechanistic chain. Nevertheless, the identification of 5-hydroxyhexanoate as a contributor to this pathway provides tangible proof-of-concept of utilizing mediation modeling to dissect complex, multistep biological processes implicated in rare tumor types.
These causal findings are not merely academic; they offer tangible and actionable targets for therapeutic intervention and treatment strategies. For example, agents that modulate 5-hydroxyhexanoate availability by either inhibiting its synthesis or enhancing its further metabolism present promising approaches to mitigate its harmful pro-oxidative effects in the context of immune dysfunction (41). Furthermore, circulating 5-hydroxyhexanoate levels emerge as a potential clinically useful biomarker for non-invasive, longitudinal monitoring of personalized cancer risk, early detection of recurrence, and treatment efficacy in bone/cartilage cancer patients (42). More broadly, the approach of integrating genetic instruments to strengthen causal inference exemplified in our study should be incorporated more widely in cancer biology research to unravel mechanisms of diverse malignancies.
Some limitations of this work warrant consideration. The study population comprised predominantly individuals of European descent, necessitating additional analyses across more diverse ethnicities and ancestries (43). We were restricted to examining easily assessed circulating factors in the blood, providing an incomplete picture lacking potentially critical tissue-level and solid tumor microenvironmental data. Our genetic association findings require further experimental validation through cell line and animal model studies to definitively confirm the functional impacts of prioritized immune cell types and metabolites on bone/cartilage tumor biology. Nevertheless, our innovative methodology overcomes key confounds in conventional observational studies to elucidate causal immunometabolic pathways implicated in rare yet aggressive malignancies where traditional mechanistic studies are hampered by limited patient samples (44).
In summary, this Mendelian randomization study sheds new light on key immune cell subsets and metabolic pathways that influence the pathogenesis of rare but clinically challenging bone and cartilage cancers. The findings nominate concrete prognostic biomarkers and therapeutic candidates at both immunological and metabolic levels that could translate to improved early detection, management, and clinical outcomes for patients with these malignancies that currently lack sufficient treatment options. Moreover, the formal mediation modeling provides an analytical framework to systematically unravel step-wise causal mechanisms that can be applied to diverse tumor types beyond the scope of this study. This work exemplifies the power of capitalizing on large-scale genome-wide association study resources through genetic instrumental variable techniques to derive biologically relevant insights that can drive innovation in oncology precision medicine.
Looking ahead, follow-up investigations should expand the mediation modeling to map a more comprehensive network and identify additional players in the mechanistic chain linking immune activity to metabolic dysregulation to bone/cartilage malignancy outcomes. It will also be critical to delineate which specific stages of tumor initiation versus progression are most impacted by the prioritized cell types and metabolites. From a clinical translation perspective, development of rapid assays to monitor circulating SSC-A levels and 5-hydroxyhexanoate abundance could enable convenient liquid biopsies for early detection and treatment monitoring. Additionally, high-throughput drug screens should be undertaken to uncover agents that can selectively normalize the phenotypes of overactivated HLA-DR+ NK cells and modulate 5-hydroxyhexanoate availability. Cancer immunotherapies targeting the HLA-DR+ NK axis and drugs that interact with 5-hydroxyhexanoate-associated pathways represent particularly promising avenues for future drug development and repurposing efforts. With additional research building on the blueprint provided by our study, the long-term goal is to translate these insights into new targeted diagnostics and treatments that will tangibly improve the prognosis and quality of life for patients afflicted with rare cancers of the bone and cartilage.
Our objective in future is to move from the observational insights gleaned from genetic associations to actionable clinical interventions and diagnostics. To this end, we plan to develop a clinical research protocol that involves enrolling patients with diagnosed or suspected malignant tumors of bone and articular cartilage. We will collect plasma samples from these patients to evaluate the HLA DR+NK cell phenotype SSC-A and the levels of the metabolite 5-hydroxyhexanoate. The aims of this proposed clinical study are twofold: Validation of Biomarkers and Prospective Cohort Analysis. Additionally, given the promising role of these biomarkers, we plan to explore their potential as therapeutic targets. The interventional part of the study would involve: 1. Targeted Therapeutic Interventions: If initial validation is successful, we will explore targeted interventions that modulate the levels of these biomarkers and assess the clinical outcomes, which could lead to the development of novel therapeutic strategies. 2. Clinical Trials: Depending on the outcomes of the targeted interventions, we will design phase I/II clinical trials to test the efficacy and safety of new treatment modalities that either target the HLA DR+NK cell phenotype SSC-A directly or modulate the level of 5-hydroxyhexanoate.
This upcoming clinical study will include a diverse patient population to ensure generalizability of the findings. We aim to collaborate with multiple centers to enrich the cohort diversity and strengthen the validity of our results. The potential impact of these biomarkers on patient care and their application in the clinic could be profound, offering a new avenue for early detection, monitoring, and personalized treatment strategies for patients with malignant tumors of bone and articular cartilage.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
KL: Methodology, Validation, Writing – original draft, Writing – review & editing. AG: Formal analysis, Methodology, Software, Writing – original draft. TZ: Data curation, Formal analysis, Investigation, Writing – review & editing. SL: Data curation, Resources, Writing – review & editing. ZY: Data curation, Validation, Writing – review & editing. CX: Funding acquisition, Methodology, Project administration, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by Mianyang Science and Technology Bureau (No. 2022ZYDF087), Health Commission of Mianyang City (No. 202007), and The Third Hospital of Mianyang (No. 202101).
We extend our heartfelt thanks to Cong Xiao, Shao-Yun Zhang, Zhi-Xiang Gao, and Qiang Su for their invaluable contributions to this study from The Third Hospital of Mianyang, Sichuan Mental Health Center.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1433219/full#supplementary-material
MR, Mendelian Randomization; GWAS, Genome-Wide Association Study; IV, Instrumental Variable; IVW, Inverse Variance Weighted; HLA DR+ NK, Human Leukocyte Antigen DR Positive Natural Killer; SSC-A, Side Scatter Area; AC, Absolute Cell Counts; MFI, Median Fluorescence Intensity; MP, Morphological Parameters; RC, Relative Cell Counts; SNP, Single Nucleotide Polymorphism; LD, Linkage Disequilibrium; F-statistic, Fisher Statistic; CLSA, Canadian Longitudinal Aging Study; SAH, S-adenosylhomocysteine; CDCs, Common Dendritic Cells; TBNK, T cells, B cells, Natural Killer cells; Treg cells, Regulatory T cells; OR, Odds Ratio; CI, Confidence Interval.
1. Hu X, Deng K, Ye H, Sun Z, Huang W, Sun Y, et al. Trends in tumor site-specific survival of bone sarcomas from 1980 to 2018: A surveillance, epidemiology and end results-based study. Cancers. (2021) 13(21):5381. doi: 10.3390/cancers13215381
2. Galyfos G, Karantzikos GA, Kavouras N, Sianou A, Palogos K, Filis K. Extraosseous ewing sarcoma: diagnosis, prognosis and optimal management. Indian J surg. (2016) 78:49–53. doi: 10.1007/s12262-015-1399-0
3. Brown HK, Schiavone K, Gouin F, Heymann MF, Heymann D. Biology of bone sarcomas and new therapeutic developments. Calcif Tissue Int. (2018) 102(2):174–95. doi: 10.1007/s00223-017-0372-2
4. Eaton BR, Schwarz R, Vatner R, Yeh B, Claude L, Indelicato DJ, et al. Osteosarcoma. Pediatr Blood cancer. (2021) 68 Suppl 2:e28352. doi: 10.1002/pbc.28352
5. Italiano A, Mir O, Cioffi A, Palmerini E, Piperno-Neumann S, Perrin C, et al. Advanced chondrosarcomas: role of chemotherapy and survival. Ann Oncol. (2013) 24:2916–22. doi: 10.1093/annonc/mdt374
6. Zhou Y, Yang D, Yang Q, Lv X, Huang W, Zhou Z, et al. Single-cell RNA landscape of intratumoral heterogeneity and immunosuppressive microenvironment in advanced osteosarcoma. Nat Commun. (2020) 11:6322. doi: 10.1038/s41467-020-20059-6
7. Isakoff MS, Bielack SS, Meltzer P, Gorlick R. Osteosarcoma: current treatment and a collaborative pathway to success. J Clin Oncol. (2015) 33:3029–35. doi: 10.1200/JCO.2014.59.4895
8. Schwartz CL, Gorlick R, Teot L, Krailo M, Chen Z, Goorin A, et al. Multiple drug resistance in osteogenic sarcoma: INT0133 from the Children's Oncology Group. J Clin Oncol. (2007) 25:2057–62. doi: 10.1200/JCO.2006.07.7776
9. Murata M. Inflammation and cancer. Environ Health Prev Med. (2018) 23:50. doi: 10.1186/s12199-018-0740-1
10. Kyriacou DN, Greenland P, Mansournia MA. Using causal diagrams for biomedical research. Ann Emergency Med. (2023) 81:606–13. doi: 10.1016/j.annemergmed.2022.08.014
11. Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinf (Oxford England). (2010) 26:139–40. doi: 10.1093/bioinformatics/btp616
12. Smith GD, Ebrahim S. 'Mendelian randomization': can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol. (2003) 32:1–22. doi: 10.1093/ije/dyg070
13. Burgess S, Thompson SG. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur J Epidemiol. (2017) 32:377–89. doi: 10.1007/s10654-017-0255-x
14. Hemani G, Bowden J, Davey Smith G. Evaluating the potential role of pleiotropy in Mendelian randomization studies. Hum Mol Genet. (2018) 27:R195–r208. doi: 10.1093/hmg/ddy163
15. Davey Smith G, Hemani G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet. (2014) 23:R89–98. doi: 10.1093/hmg/ddu328
16. Gupta V, Walia GK, Sachdeva MP. 'Mendelian randomization': an approach for exploring causal relations in epidemiology. Public Health. (2017) 145:113–9. doi: 10.1016/j.puhe.2016.12.033
17. Burgess S, Thompson SG. Mendelian randomization: methods for using genetic variants in causal estimation. CRC Press (2015).
18. Skrivankova VW, Richmond RC, Woolf BAR, Yarmolinsky J, Davies NM, Swanson SA, et al. Strengthening the reporting of observational studies in epidemiology using Mendelian randomization: the STROBE-MR statement. Jama. (2021) 326:1614–21. doi: 10.1001/jama.2021.18236
19. Buniello A, MacArthur JAL, Cerezo M, Harris LW, Hayhurst J, Malangone C, et al. The NHGRI-EBI GWAS Catalog of published genome-wide association studies, targeted arrays and summary statistics 2019. Nucleic Acids Res. (2019) 47:D1005–d12. doi: 10.1093/nar/gky1120
20. Sidore C, Busonero F, Maschio A, Porcu E, Naitza S, Zoledziewska M, et al. Genome sequencing elucidates Sardinian genetic architecture and augments association analyses for lipid and blood inflammatory markers. Nat Genet. (2015) 47:1272–81. doi: 10.1038/ng.3368
21. Shin SY, Fauman EB, Petersen AK, Krumsiek J, Santos R, Huang J, et al. An atlas of genetic influences on human blood metabolites. Nat Genet. (2014) 46:543–50. doi: 10.1038/ng.2982
22. Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, et al. The MR-Base platform supports systematic causal inference across the human phenome. eLife. (2018) 7. doi: 10.7554/eLife.34408
23. Palmer TM, Lawlor DA, Harbord RM, Sheehan NA, Tobias JH, Timpson NJ, et al. Using multiple genetic variants as instrumental variables for modifiable risk factors. Stat Methods Med Res. (2012) 21:223–42. doi: 10.1177/0962280210394459
24. Minelli C, Del Greco MF, van der Plaat DA, Bowden J, Sheehan NA, Thompson J. The use of two-sample methods for Mendelian randomization analyses on single large datasets. Int J Epidemiol. (2021) 50:1651–9. doi: 10.1093/ije/dyab084
25. Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. (2016) 40:304–14. doi: 10.1002/gepi.21965
26. Böhnke JR. Explanation in causal inference: Methods for mediation and interaction. Q J Exp Psychol (2006). (2016) 69:1243–4. doi: 10.1080/17470218.2015.1115884
27. Imai K, Keele L, Tingley D. A general approach to causal mediation analysis. psychol Methods. (2010) 15:309–34. doi: 10.1037/a0020761
28. Poli A, Michel T, Thérésine M, Andrès E, Hentges F, Zimmer J. CD56bright natural killer (NK) cells: an important NK cell subset. Immunology. (2009) 126:458–65. doi: 10.1111/j.1365-2567.2008.03027.x
29. Yu J, Freud AG, Caligiuri MA. Location and cellular stages of natural killer cell development. Trends Immunol. (2013) 34:573–82. doi: 10.1016/j.it.2013.07.005
30. Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL, et al. Innate or adaptive immunity? The example of natural killer cells. Science. (2011) 331:44–9. doi: 10.1126/science.1198687
31. Orange JS. Formation and function of the lytic NK-cell immunological synapse. Nat Rev Immunol. (2008) 8:713–25. doi: 10.1038/nri2381
32. Lanier LL. Evolutionary struggles between NK cells and viruses. Nat Rev Immunol. (2008) 8:259–68. doi: 10.1038/nri2276
33. Long EO, Kim HS, Liu D, Peterson ME, Rajagopalan S. Controlling natural killer cell responses: integration of signals for activation and inhibition. Annu Rev Immunol. (2013) 31:227–58. doi: 10.1146/annurev-immunol-020711-075005
34. Rauf A, Imran M, Butt MS, Nadeem M, Peters DG, Mubarak MS. Resveratrol as an anti-cancer agent: A review. Crit Rev Food Sci Nutr. (2018) 58:1428–47. doi: 10.1080/10408398.2016.1263597
35. Wikoff WR, Grapov D, Fahrmann JF, DeFelice B, Rom WN, Pass HI, et al. Metabolomic markers of altered nucleotide metabolism in early stage adenocarcinoma. Cancer Prev Res (Philadelphia Pa). (2015) 8:410–8. doi: 10.1158/1940-6207.CAPR-14-0329
36. Giuliani AL, Sarti AC, Di Virgilio F. Extracellular nucleotides and nucleosides as signalling molecules. Immunol Lett. (2019) 205:16–24. doi: 10.1016/j.imlet.2018.11.006
37. Di Giacomo C, Malfa GA, Tomasello B, Bianchi S, Acquaviva R. Natural compounds and glutathione: beyond mere antioxidants. Antioxidants. (2023) 12(7):1445. doi: 10.3390/antiox12071445
38. Mills EL, Kelly B, Logan A, Costa ASH, Varma M, Bryant CE, et al. Succinate dehydrogenase supports metabolic repurposing of mitochondria to drive inflammatory macrophages. Cell. (2016) 167:457–70.e13. doi: 10.1016/j.cell.2016.08.064
39. Sies H, Berndt C, Jones DP. Oxidative stress. Annu Rev Biochem. (2017) 86:715–48. doi: 10.1146/annurev-biochem-061516-045037
40. Trachootham D, Alexandre J, Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov. (2009) 8:579–91. doi: 10.1038/nrd2803
41. Harris IS, DeNicola GM. The complex interplay between antioxidants and ROS in cancer. Trends Cell Biol. (2020) 30:440–51. doi: 10.1016/j.tcb.2020.03.002
42. Hanahan D. Hallmarks of cancer: new dimensions. Cancer Discov. (2022) 12:31–46. doi: 10.1158/2159-8290.CD-21-1059
43. Rotimi CN, Jorde LB. Ancestry and disease in the age of genomic medicine. N Engl J Med. (2010) 363:1551–8. doi: 10.1056/NEJMra0911564
Keywords: causal relationships, immune cell features, Mendelian randomization, malignant tumors of bone and articular cartilage, metabolites, mediation analysis
Citation: Long K, Gong A, Zheng T, Liu S, Ying Z and Xiao C (2024) The relationship between metabolite mediated immune regulatory imbalance and the occurrence of malignant tumors of bone and articular cartilage: a Mendelian randomization study. Front. Immunol. 15:1433219. doi: 10.3389/fimmu.2024.1433219
Received: 15 May 2024; Accepted: 29 July 2024;
Published: 09 August 2024.
Edited by:
Alessandro Isidori, AORMN Hospital, ItalyReviewed by:
Junhao Tu, The First Affiliated Hospital of Nanchang University, ChinaCopyright © 2024 Long, Gong, Zheng, Liu, Ying and Xiao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cong Xiao, c3l5Z2VreGNAMTI2LmNvbQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.