- 1The Department of Pharmacy Administration, School of Pharmacy, Xi’an Jiaotong University, Xi’an, Shaanxi, China
- 2The Center for Drug Safety and Policy Research, Xi’ an Jiaotong University, Xi’ an, Shaanxi, China
Background: On April 18, 2024, the U.S. Food and Drug Administration officially required updating of the “boxed warning” for T cell malignancies for all chimeric antigen receptor T cell (CAR-T) therapies. Given the clinical significance of these therapies, a rigorous safety assessment is paramount. However, comprehensive real-world safety studies have been lacking for the newly marketed CAR-T products idecabtagene vicleucel (ide-cel) and ciltacabtagene autoleucel (cilta-cel), which target B cell maturation antigen, especially regarding the risk of secondary malignancies. Therefore, we aimed to thoroughly analyze the adverse events (AEs) information in the FDA Adverse Event Reporting System (FAERS) database to comprehensively understand the safety risks of ide-cel and cilta-cel.
Methods: We extracted AE reports related to ide-cel and cilta-cel from the FAERS database (https://fis.fda.gov/extensions/FPD-QDE-FAERS/FPD-QDE-FAERS.html.) from January 1, 2019 to December 31, 2023. Disproportionality analysis and Bayesian analysis were used to identify risk signals across subgroups and specific cases (including for death and secondary malignancies). Weibull distribution analysis was employed to determine the time to AE onset.
Results: A total of 695 AE reports for ide-cel and 848 for cilta-cel were included in the FAERS database. This analysis identified 81 positive signals for ide-cel and 74 for cilta-cel. Notably, comparisons with the drug labels revealed “unexpected signals,” including febrile bone marrow aplasia (reporting odds ratio=69.10; confidence interval 39.12–122.03) and plasma cell myeloma (12.45; 8.18–18.95) for ide-cel, and increased serum ferritin (24.98; 8.0–77.58) and large intestine perforation (18.57; 5.98–57.69) for cilta-cel. Both drugs showed a higher AE incidence among male recipients and patients aged ≥65 years, although female recipients faced a greater risk. Most AEs occurred at the early stage of administration. However, secondary malignancies were detected for both drugs, primarily occurring one-year post-administration.
Conclusion: This study provides a foundation for understanding the safety profile of CAR-T cell therapy, particularly in relation to the emergence of secondary malignancies. Such insights are helpful for clinical decision-making and the safe and effective utilization of these therapeutic agents.
1 Introduction
As a novel treatment modality, chimeric antigen receptor T (CAR-T) cell therapies are a breakthrough therapy for a variety of hematological malignancies (1). CAR-T cell therapy targeting B cell maturation antigen (BCMA) relies on the specific recognition of BCMA by a chimeric antigen receptor (CAR) that is introduced into T cells via genetic engineering technology; this therapy makes full use of the immune activity of T cells to accurately attack tumor cells expressing BCMA (2, 3).
The US Food and Drug Administration (FDA) approved idedecabtagene vicleucel (ide-cel) in March 2021, relying on a Phase II (KarMMa) trial for adult patients with relapsed or refractory multiple myeloma (RRMM) (4). This trial demonstrated a 72% overall response rate (ORR), with 28% achieving a stringent complete response (CR) and a median duration of response (DoR) of 11 months (4). Subsequently, in February 2022, the FDA approved ciltacabtagene autoleucel (cilta-cel) based on the Phase II (CARTITUDE-1) trial for adult patients with RRMM, showcasing an ORR of 97.9%, a stringent CR rate of 78.4%, and a median DoR of 21.8 months (5, 6). Both therapies incorporate the same second-generation design of the domain (CD3ζand 4-1BB) targeting BCMA on target cells (7). However, their ectodomains differ, with ide-cel containing a mouse-derived binding domain specific to one BCMA epitope, while cilta-cel with two camelid VH binding domains (8, 9). Studies have indicated that ide-cel and cilta-cel exhibit favorable risk–benefit ratios during treatment, outperforming standard therapy with hazard ratios of 0.49 and 0.26, respectively (10, 11). thereby markedly improving the treatment and prognosis of patients with RRMM (10, 12).
Although CAR-T cell therapies have achieved notable therapeutic efficacy in the realm of hematological oncology, the FDA has recently required updating of the “boxed warning” for T cell malignancies for all CAR-T cell therapies, which are rare but extremely serious adverse reactions (13). In addition, similar to other innovative therapies, CAR-T cell therapy is prone to inducing immune-related serious adverse events (AEs), such as cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS) (14). Recent studies indicate that the prevalence of AEs is escalating in tandem with increasing clinical utilization, and, alarmingly, the incidence of secondary malignancies stands at a substantial 6% (15). However, for the novel anti-BCMA CAR-T products, ide-cel and cilta-cel, the current research landscape is limited to case reports and lacks a comprehensive safety analysis, especially for secondary malignancies (16, 17). At present, the safety data for the two drugs—primarily from clinical trials—are constrained by limited durations, stringent selection criteria, and a single experimental design, impeding the timely identification of rare AEs (18). Given the crucial role of anti-BCMA CAR-T therapy for the treatment of hematological malignancies, there is still a lack of comprehensive pharmacovigilance studies with large sample sizes or utilizing extensive databases, particularly for AEs not addressed in clinical trials or product inserts.
The FDA Adverse Event Reporting System (FAERS), a database designed to support the FDA’s post-market surveillance of drugs and therapeutic biologic products, gathers a large amount of AE information from actual clinical application, and is currently the largest pharmacovigilance database (19, 20). Disproportionality analysis can identify potential risk signals from the huge amount of AE information in the FAERS database (21). The aim of this study was to comprehensively evaluate the real-world safety profile of anti-BCMA CAR-T cell therapy by analyzing the AEs recorded in the FAERS database, particularly focusing on secondary malignancies. We also sought to examine the uniformity and differences between anti-BCMA CAR-T product-related AEs to provide a more reliable and comprehensive basis for clinical decision-making and to promote the safe, effective use of the drugs.
2 Methods
2.1 Data source
This study comprises a retrospective pharmacovigilance analysis utilizing the FAERS database to identify potential associations between ide-cel or cilta-cel and AEs. FAERS(https://fis.fda.gov/extensions/FPD-QDE-FAERS/FPD-QDE-FAERS.html.), a vast spontaneous reporting system, encompasses diverse AE reports from healthcare professionals, consumers, and clinical studies. It comprises seven data tables, which include patient demographics, drug information, and AE details (22). Adhering to the International Safety Reporting Guidelines (ICH E2B), FAERS utilizes the International Dictionary of Medical Terms (MedDRA) to standardize AE terminology, ensuring data accuracy (23). This study extracted AE reports involving ide-cel and cilta-cel as “primary suspects” (PS) or “secondary suspects” (SS) from the FAERS database from January 1, 2019 to December 31, 2023. AEs were described and classified according to the preferred term (PT) and system organ class (SOC) in MedDRA terms.
To ensure the accuracy, consistency, and reliability of the data, we cleaned and deduplicated the adverse event data according to the method recommended by the FDA. In accordance with the FDA’s guidance, we identified the most recent FDA_DT as the temporal marker for instances where the PRIMARY_ID was identical. In scenarios where both FDA_DT and CASE_ID were identical, we prioritized selecting the record with the higher PRIMARY_ID in order to discard redundant reports originating from various sources (24, 25).
2.2 Statistical analysis
To detect suspicious signals, we used disproportionality analyses, which compare the incidence of an AE among persons who were taking a target drug with those who were not. If AE frequency in the drug population significantly exceeded that in the non-drug population, surpassing a set threshold, a potential association was considered (26). As there is no definitive gold standard for risk signal detection, each method has its merits and limitations (27). The reporting odds ratio (ROR) may show better early signal detection, but its monitoring ability decreases with an increase in reporting time (28). Conversely, the Bayesian confidence propagation neural network, represented by the information component (IC), has a strong ability to detect unique signals even when the number of AEs related to the target drug is small (29). In order to improve the reliability of the results and avoid the interference of false positive signals, we selected ROR and IC analyses to mine suspicious signals. The formulas are provided in Supplementary Table 1.
We used descriptive statistics to analyze demographic information related to ide-cel and cilta-cel. To enhance the reliability of the findings, we conducted separate disproportionality analyses based on AE signals. Then, we performed in-depth subgroup analyses, stratifying patients by sex (male and female) and age groups (18-64 years and ≥65 years, choosing 65 as the boundary. Patients aged below18 years were exclude because of insufficient reports) (30). Signals not listed in the drug instructions were labeled “unexpected signals.” Additionally, we extracted AE reports with death outcomes to assess the safety of BCMA products.
2.3 Time-to-onset analysis
To understand the timings of ide-cel– and cilta-cel– induced AEs and identify the AEs requiring long-term monitoring, we statistically analyzed the time to onset (TTO) for ide-cel and cilta-cel. In addition, Weibull distribution analysis has the best test performance for assessing AE occurrence period after treatment (31). Therefore, we applied the Weibull distribution to model the TTO of AEs, deriving the shape parameter (β). If β<1, the AE is mainly concentrated in the early stage of administration of the medication; if β=1, the AE occurs randomly with no obvious time aggregation; and β>1 is thought to indicate an increase with time (32). Enhanced monitoring during periods of increased AE incidence may reduce the risk to patients.
3 Results
3.1 Descriptive analysis
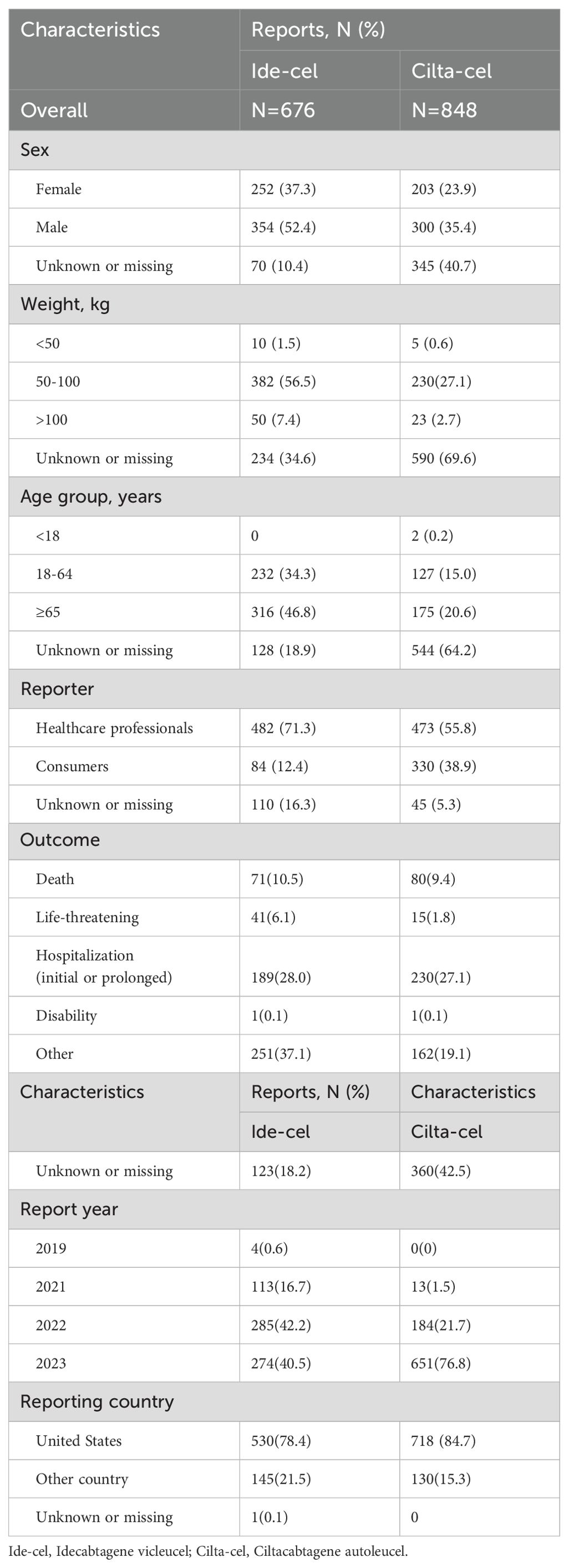
This study extracted 11,900,484 reports from the FAERS database. After data preprocessing, 695 AE reports related to ide-cel and 848 AE reports related to cilta-cel were screened. Table 1 summarizes the demographic characteristics of these AE reports. Among ide-cel AE reports, we found that over half of patients were male (52.4%), patients aged ≥65 years accounted for the largest proportion (46.6%), and most patients reported weights ranging from 50 to 100 kg (56.5%). Furthermore, the outcome of death accounted for 10.5% of all reports. For cilta-cel, the proportion of male patients was higher than female (35.4% versus 23.9%), patients aged ≥65 years accounted for the largest proportion (20.6%), and the outcome of death accounted for 9.4%. Notably, there were many missing values for “Characteristics” for reports related to cilta-cel, possibly owing to over one-third of the reports coming from consumers (38.9%). In terms of report sources, whether for ide-cel (71.3%) or cilta-cel (55.8%), most reports were provided by healthcare professionals. Additionally, the majority of reports originated from the United States, accounting for 78.4% of ide-cel reports and 84.7% of cilta-cel reports.
3.2 Disproportionality signals at the SOC level
Utilizing the criteria of ROR or IC, we identified significant associations of SOCs affected by AEs linked with ide-cel and cilta-cel. Statistical analysis revealed that 23 SOCs were impacted by AEs related to ide-cel, with significant SOCs for ide-cel including immune system disorders, blood and lymphatic system disorders, nervous system disorders, investigations, metabolism and nutrition disorders, and vascular disorders. Notably, immune system disorders and blood and lymphatic system disorders met both ROR and IC criteria (Supplementary Table 2). For cilta-cel, 23 SOCs were involved, with immune system disorders being the only SOC that satisfied both ROR and IC standards. Importantly, signals meeting only the ROR criteria, such as nervous system disorders, infections and infestations, blood and lymphatic system disorders, surgical and medical procedures, and product issues, may also indicate important and frequently occurring AEs (Supplementary Table 3). These findings highlight the most common SOCs associated with AEs induced by ide-cel and cilta-cel, thereby pinpointing areas that merit further clinical scrutiny and research.
3.3 Disproportionality signals at the PT level and for subgroups
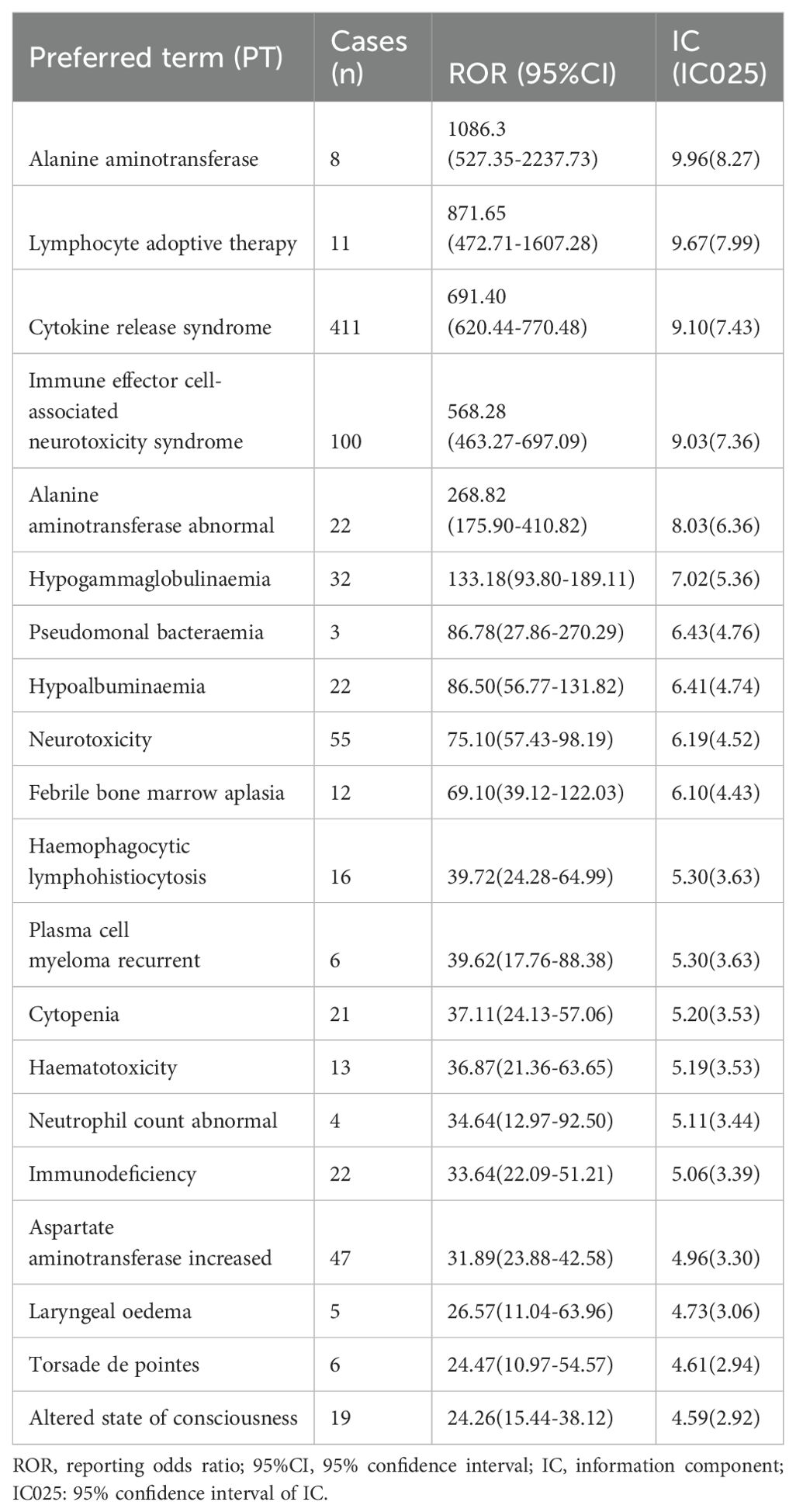
We identified 81 positive signals associated with ide-cel and 74 signals with cilta-cel. Tables 2, 3 present the top 20 signals ranked by ROR values for ide-cel and cilta-cel, and indicate the strongest associations with the target drugs. The most common AEs associated with ide-cel include CRS (n=411), fatigue (n=151), and ICANS (n=100). The signal with the highest ROR value was alanine aminotransferase (ROR=1086.3; confidence interval [CI] 527.35–2237.73). Notably, comparison with the drug instructions (33) revealed “unexpected signals” for ide-cel, such as febrile bone marrow aplasia (ROR=69.1; CI 39.12–122.03), myelodysplastic syndrome (ROR=12.93; CI 5.80–28.83), and hepatotoxicity (ROR=13.86; CI 5.76–33.34) (complete information in Supplementary Table 4).
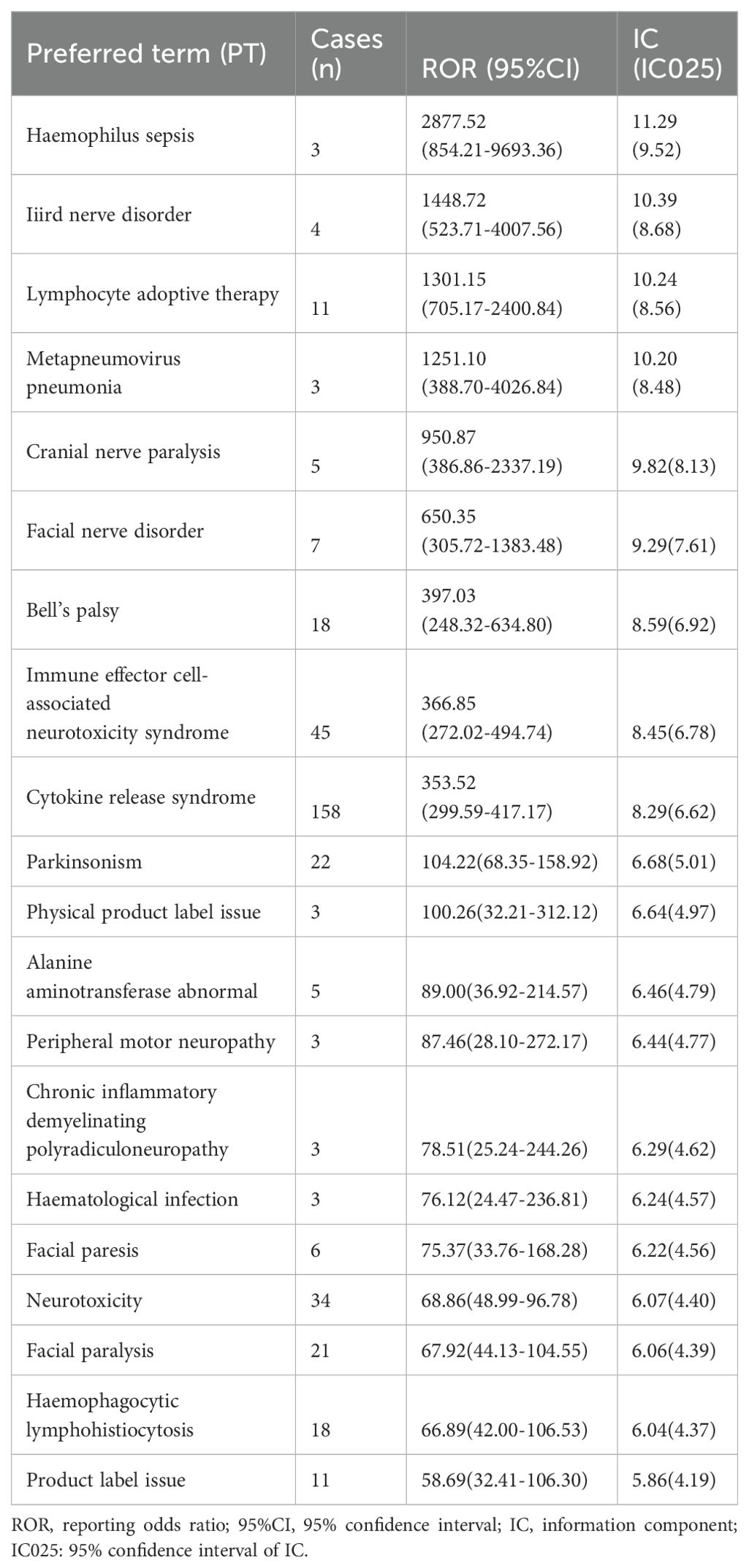
For cilta-cel, frequently occurring AEs include CRS (n=158), pyrexia (n=58), and ICANS (n=45). Additionally, strong associations were observed with Haemophilus sepsis (ROR=2877.52; CI 854.21–9693.36). We also detected AE signals not mentioned in the drug instructions (34), including physical product label issue (ROR=100.26; CI 32.21–312.12), large intestine perforation (ROR=18.57; CI 5.98–57.69), and blood lactate dehydrogenase increased (ROR=16.27; CI 6.76–39.16) (complete information in Supplementary Table 5).
To mitigate the potential confounding of signal mining outcomes by demographic factors, we conducted a disproportionality analysis for various subgroups and calculated the ROR of AE exposure based on sex and age groups (18-64 and ≥65 years). For ide-cel, CRS, fatigue, and ICANS were the most frequently reported in all age groups. Among the top 15 AEs reported across age groups, acute kidney injury, parkinsonism, and sepsis were more prevalent in patients aged 18-64 years whereas alanine aminotransferase abnormal, hemophagocytic lymphohistiocytosis, and headache were more common in older patients. Notably, signals for parkinsonism and transaminases increased were detected exclusively in male patients aged 18-64 years, whereas torsade de pointes was observed only in female patients aged ≥65 years. (Supplementary Figures 1, 2).
A similar subgroup analysis was conducted for cilta-cel. CRS, pyrexia, and ICANS were the most reported AEs across age groups. Pneumonia, febrile neutropenia, and acute kidney injury were more prevalent in patients aged 18-64 years, while parkinsonism, septic shock, and third nerve disorder were more common in patients aged ≥65 years. Additionally, Guillain-Barre syndrome and parkinsonism were detected as signals only in male patients (Supplementary Figures 3, 4).
3.4 In-depth analysis of secondary malignancies
Furthermore, given the severity and rarity of secondary malignancies associated with CAR-T cell therapy during clinical trials and practical application, we conducted a thorough analysis. Owing to the limited number of reports on secondary malignancy AEs, we employed IC analysis, which is effective for detecting important signals even with limited AE data, to monitor risk signals for secondary malignancies.
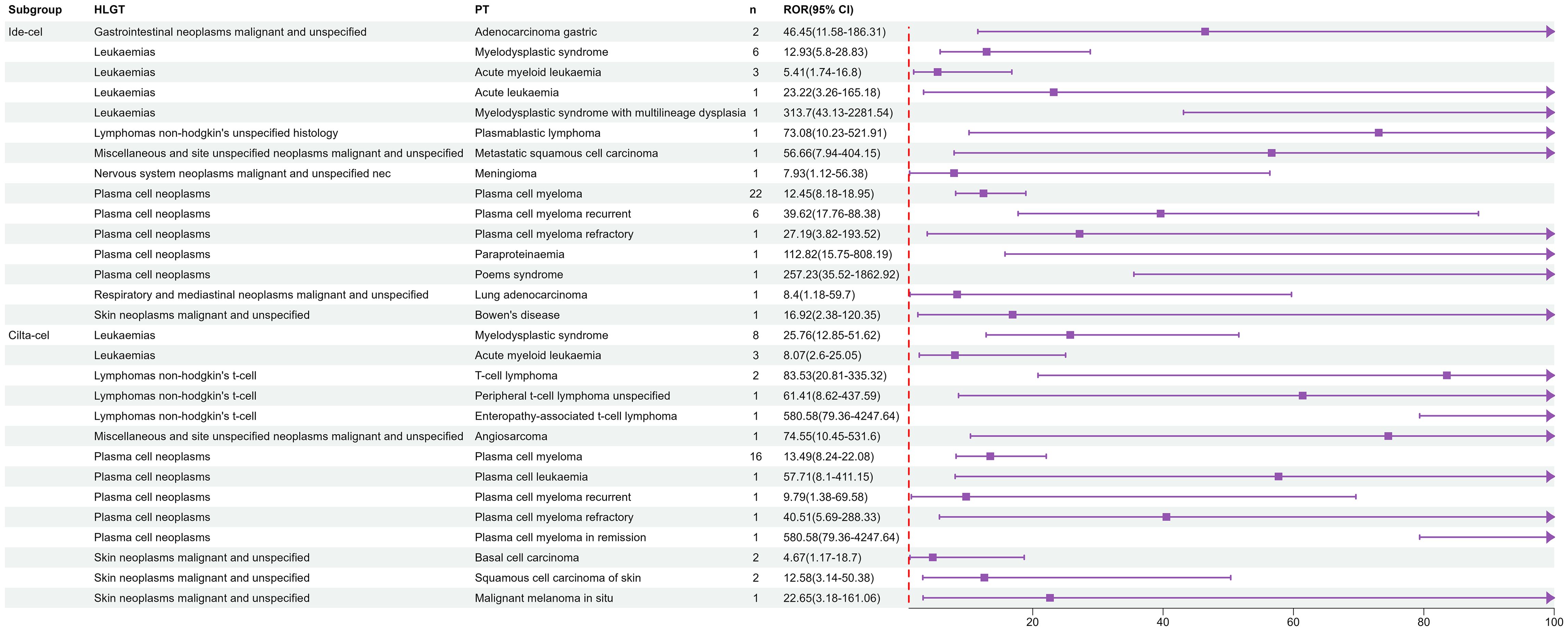
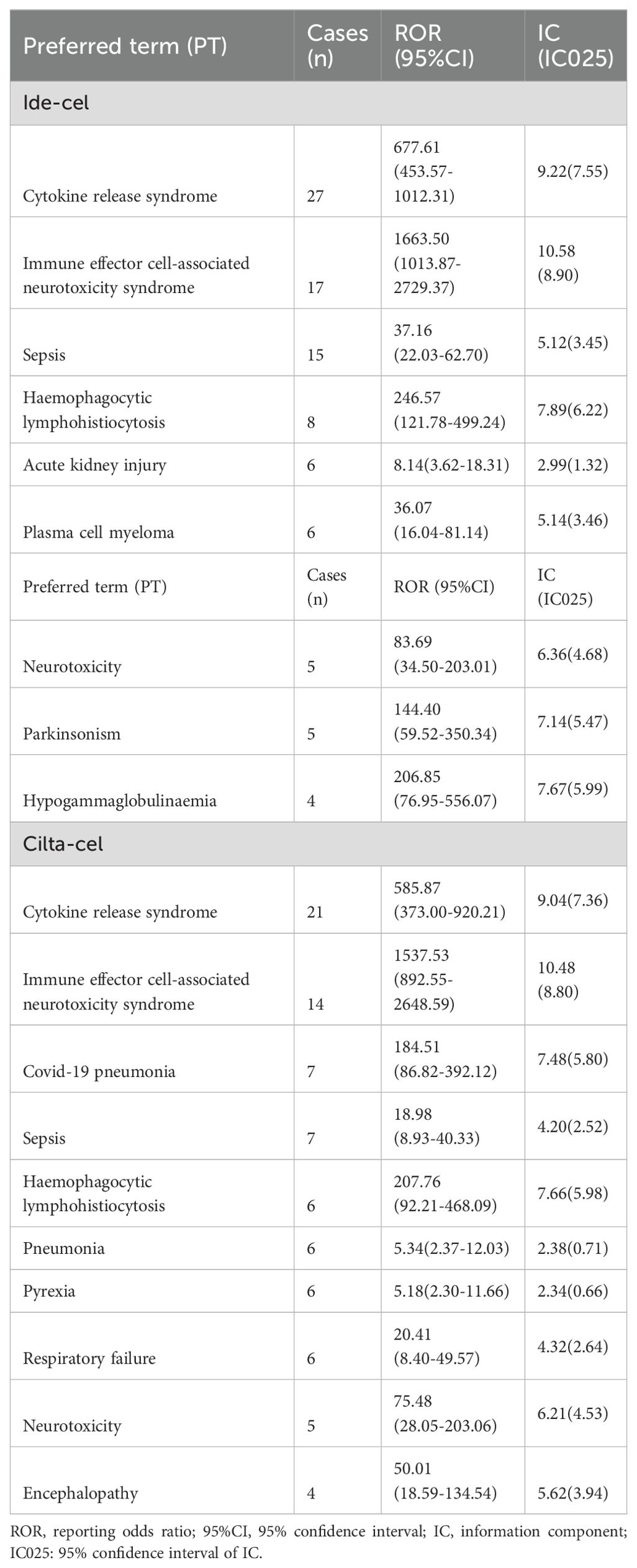
At the high-level group term level, significant signals for ide-cel included gastrointestinal neoplasms malignant and unspecified, leukemias, and lymphomas non-hodgkin’s unspecified histology. For cilta-cel, significant AE signals included those for leukemias, lymphomas non-hodgkin’s t-cell, and plasma cell neoplasms. As shown in Figure 1, leukemias, plasma cell neoplasms, skin neoplasms malignant and unspecified, and miscellaneous and site unspecified neoplasms malignant and unspecified were common signals for both drugs. Notably, we only detected signals for T cell malignancies for cilta-cel.
Additionally, TTO analysis of secondary malignancies revealed that the mean time to malignancy onset for ide-cel was 498.9 days with a median of 205.5 days, whereas for cilta-cel, the mean time was 390 days with a median of 311 days (Figure 2B). The identified malignancy risk signals and their temporal patterns provide deeper insights into the clinical safety profiles of these two drugs.
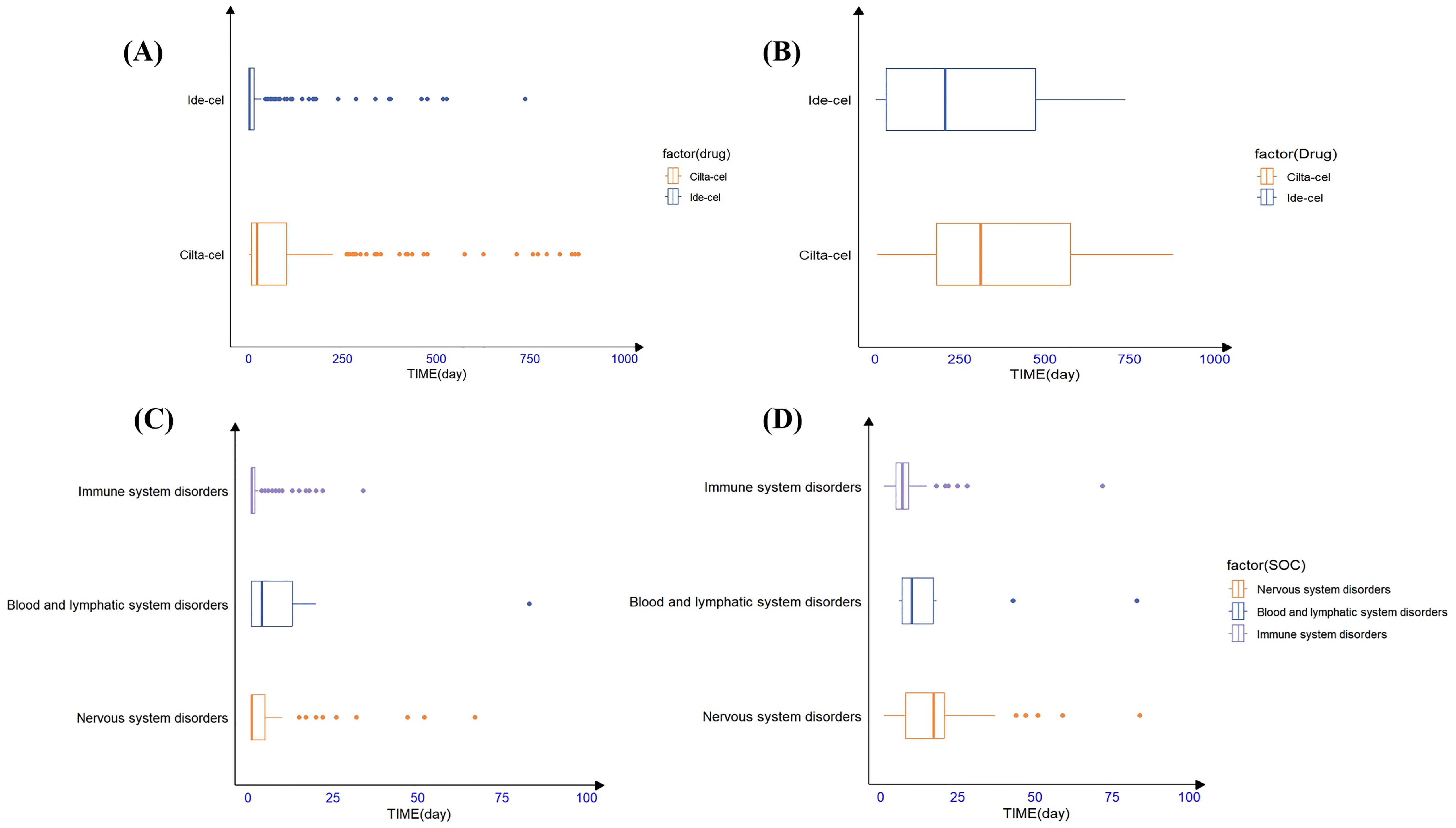
Figure 2. The timing of AE occurrence for ide-cel and cilta-cel; (A) The time to onset of all of AEs for ide-cel and cilta-cel. (B) The time to onset of secondary malignancies for ide-cel and cilta-cel. (C) The time to onset of AEs occurred at the SOC level for ide-cel. (D) The time to onset of AEs occurred at the SOC level for cilta-cel.
3.5 Gender difference risk signals
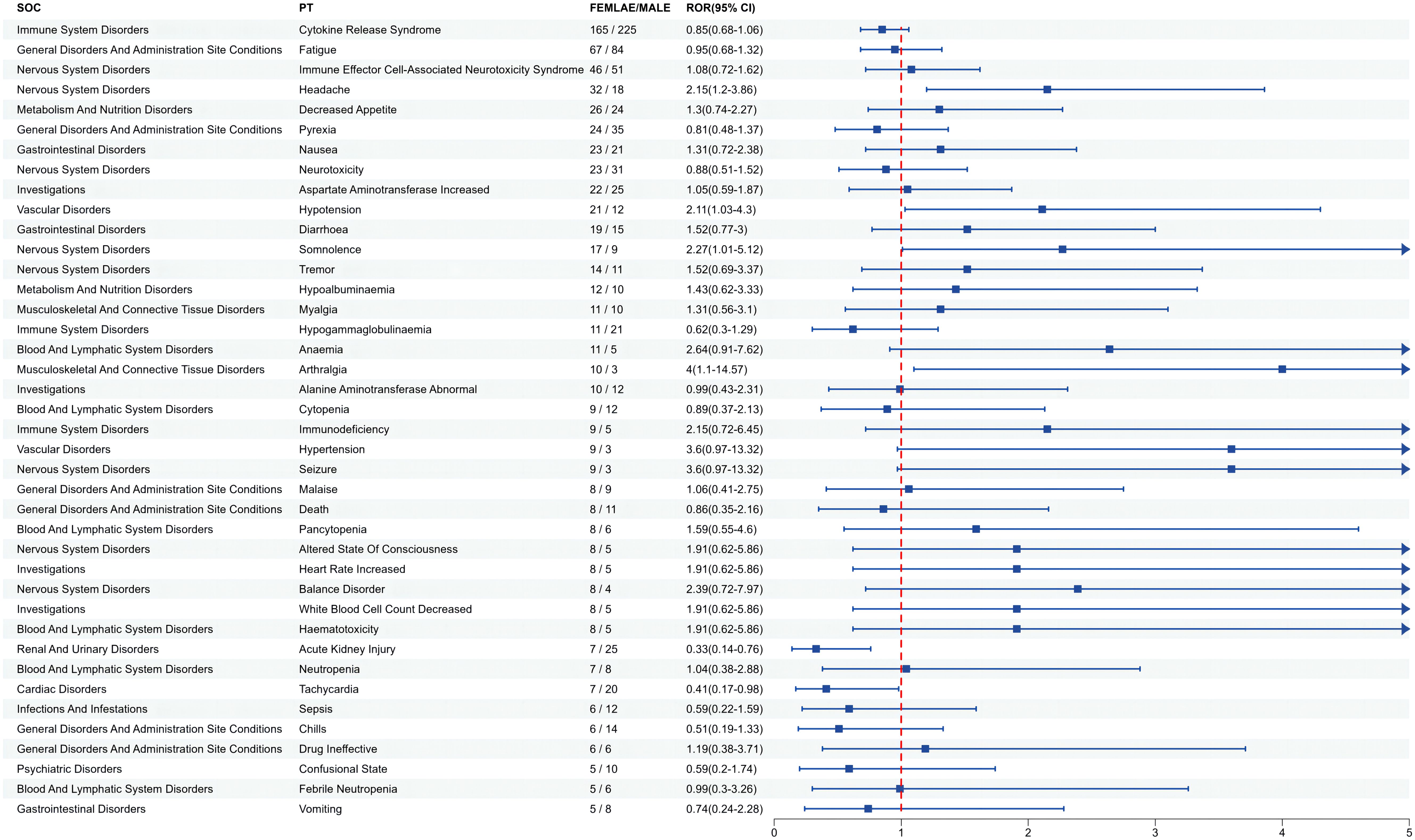
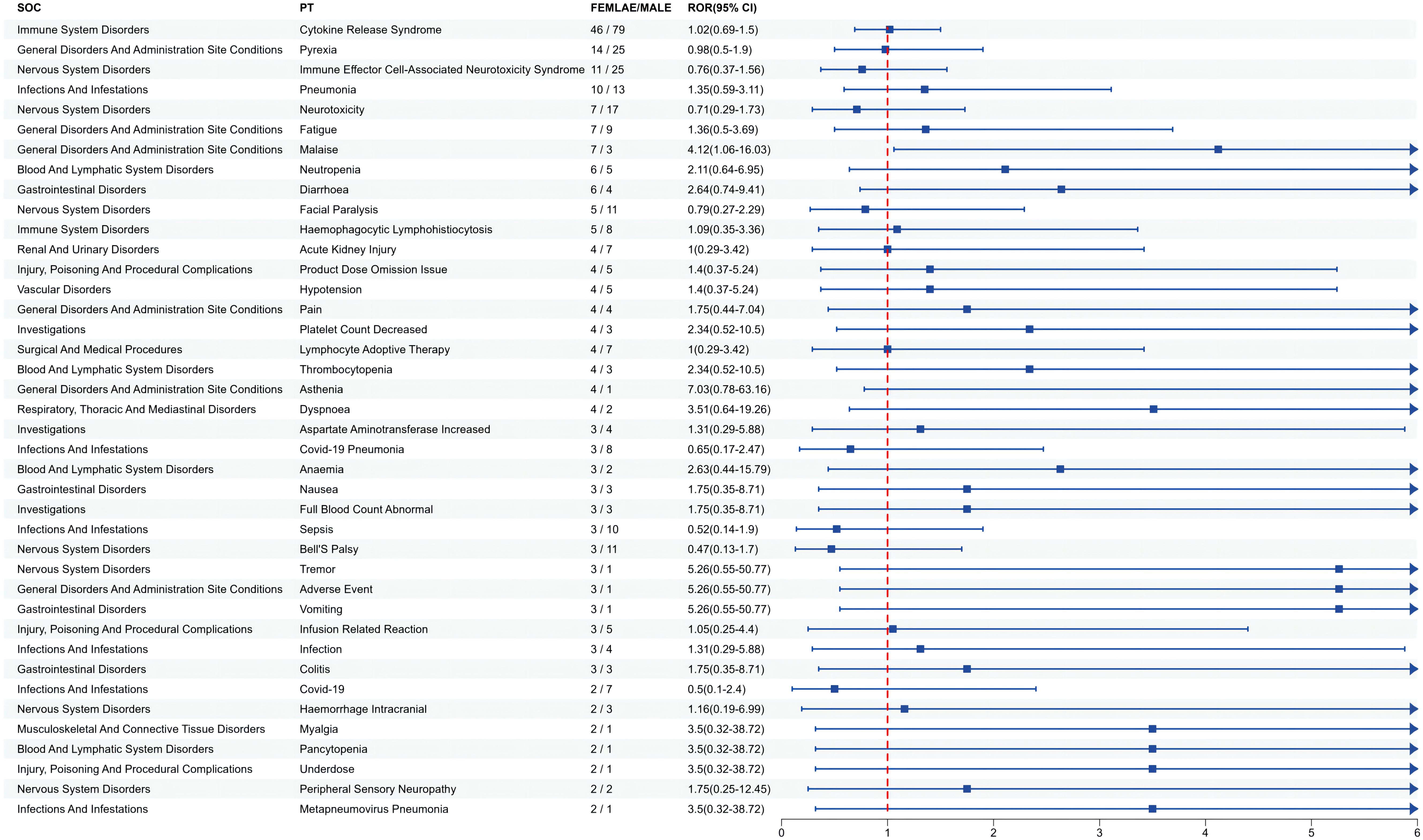
Since gender differences were present at the overall AE reporting level, we further analyzed sex differences in the mined AE results. Figure 3 shows that for ide-cel, female patients were more likely than male patients to experience most of the AEs. The most likely AEs in women included arthralgia, hypertension, seizure, anemia, and balance disorder. Men were most likely to develop sepsis, acute kidney injury, chills, tachycardia and hypogammaglobulinemia. Similarly, Figure 4 shows that, among all the AEs for cilta-cel, women were more prone than men to experience most kinds of AEs, including asthenia, tremor, vomiting, and dyspnea. Men were most likely to suffer from confusion, coronavirus disease 2019 (COVID-19), sepsis, Bell’s palsy, and neurotoxicity.
3.6 Death outcome in-depth analysis
In AE reports with the outcomes of death for ide-cel, male patients accounted for a higher proportion (52.1%) than female patients (36.6%), and there was a slightly higher proportion of patients aged ≥65 years than those aged 18–64 years. For cilta-cel, male patients also comprised a larger share (50.0%) than female patients (16.3%), with over one-third aged ≥65 years (33.8%). Most of the reports for both drugs originated from the United States (63.4% and 81.3%) (Supplementary Table 6).
Table 4 shows the top 10 AEs with the highest number of correlations with ide-cel and cilta-cel death outcomes at the PT level. The statistical results showed that CRS and ICANS were the most common AEs associated with death outcomes for ide-cel and cilta-cel, and had the strongest correlations with death outcomes. We also found that other AEs with strong correlations with ide-cel–related death were hemophagocytic lymphohistiocytosis, hypogammaglobulinemia, and parkinsonism. Hemophagocytic lymphohistiocytosis, COVID-19 pneumonia, and neurotoxicity were also strongly correlated with cilta-cel–related death.
3.7 AE TTO
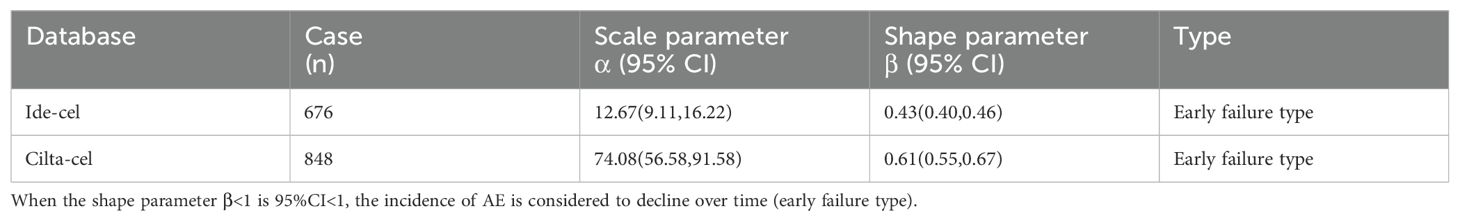
Statistical analysis revealed that the majority of AEs associated with ide-cel (83.2%) and cilta-cel (56.25%) occurred within the first 30 days following treatment. For ide-cel, the median onset time for AEs was 2 days, with a noteworthy proportion (47.7%) occurring on the day of administration. For cilta-cel, the median onset time for AEs was 21.50 days. To further understand the timing of AE occurrence at the SOC level for both drugs, we conducted an analysis based on common positive SOC signals. Figure 2 visually presents the statistical results in the form of box plots, providing insights into the distribution of the data and outliers. The results indicated that, at the SOC level, the onset of corresponding SOC AEs occurred within 30 days of treatment (Figure 2A). Additionally, the Weibull distribution analysis revealed that β<1 for both drugs, indicating an early failure pattern, in which the majority of AEs occurred during the initial stages of treatment. (Table 5).
Notably, we found many AEs that emerged one year after drug treatment (Supplementary Table 7). Among those associated with ide-cel were myelodysplastic syndrome, Bowen’s disease, bladder cancer, metastatic squamous cell carcinoma, and squamous cell carcinoma of the skin. For cilta-cel, AEs that occurred one year after treatment included myelodysplastic syndrome, basal cell carcinoma, prostate cancer, malignant melanoma, and transient ischemic attack.
4 Discussion
We conducted a retrospective pharmacovigilance study utilizing the FAERS database to evaluate BCMA-targeted CAR-T cell therapies, focusing on novel potential safety signals, the safety profiles for secondary malignancy, subgroup signal variations, and the time distribution of AE occurrence. These findings are intended to inform and shape clinical safety practices.
Our study found that CRS and ICANS were the most frequently occurring AEs associated with ide-cel and cilta-cel (Figures 2C, D), exhibiting significant signals and the strongest correlations with death. These findings were consistent with the drug instructions and clinical trial results, thereby validating the reliability of our research outcomes (11, 15, 35–37). At the SOC level, the incidence of Nervous system disorders associated with cilta-cel (n=316, 46.74%) and ide-cel (n=381, 44.9%) was substantial, each approximating half of their respective AE reports. This highlights a critical clinical concern and emphasizes the vital need for rigorous monitoring of neurological symptoms in patients treated with either drug.
Notably, performing a disproportionality analysis and comparing the results with the drug instructions, we uncovered “unexpected signals” for ide-cel, such as plasma cell myeloma and hepatotoxicity, and for cilta-cel, including large intestine perforation and blood lactate dehydrogenase increased. These findings indicate new potential safety risks associated with these drugs in real-world settings. Additionally, we discovered that the only AEs significantly associated with cilta-cel but not with ide-cel were Guillain-Barre syndrome and COVID-19 pneumonia. It has been reported that patients receiving cilta-cel treatment may have an increased risk of COVID-19 pneumonia (11). Furthermore, the median TTO for ide-cel–related AEs was 2 days, whereas that for cilta-cel–related AEs was 21.50 days. This difference may be attributable to the varying dosages used for the two drugs, as cilta-cel is generally administered at a dosage that is 10%–15% of that for ide-cel, potentially resulting in the induction of distinct AEs (37).
In particular, we observed a risk of secondary malignancies associated with both ide-cel and cilta-cel. In particular, cilta-cel has a risk signal for T cell malignancies, a rare yet severe AE associated with CAR-T cell therapy. The FDA has issued a boxed warning for T cell malignancies across CAR-T cell therapies. Although these reports constitute a minority of cases, our signal mining results indicate an increasing risk of malignancy. This concurs with prior studies documenting disproportionately high frequencies of myeloid and T cell tumors associated with CAR-T therapies (38). Based on the TTO analysis of AEs, we found that secondary malignancies typically occur after one year of treatment. Prior studies have shown that anti-BCMA CAR-T products can induce protracted adverse reactions, even leading to relapse in patients who had initially responded well. This has been attributed to factors such as the characteristics of myeloma tumor cells, CAR-T cell properties, and the tumor microenvironment (38). Some studies have proposed that since both ide-cel and cilta-cel target only one antigen, BCMA, CAR-T cells may experience persistent insufficiency, resulting in decreased antigen-targeting ability and subsequent tumor escape (39). Researchers are actively exploring methods to improve the safety of CAR-T products (39, 40). In clinical practice, this finding serves as a reminder of the need for long-term, continuous monitoring for malignancy occurrence.
Furthermore, we observed a higher proportion of male patients receiving both drugs, yet it was female patients who exhibited a greater risk of AEs. Research has shown significant individual differences in the pharmacokinetics of ide-cel and cilta-cel (41). However, analysis of death as an outcome revealed that male patients had a higher mortality rate than female patients. This observation could potentially be attributed to unhealthy habits, such as smoking and alcohol consumption, among male patients, which increase the likelihood of complications and result in a poorer prognosis for male patients receiving CAR-T cell therapy (30). Furthermore, we analyzed AEs at the PT level in each subgroup and identified both similarities and differences in signals across subgroups. As the clinical application of CAR-T cell therapy continues to expand, this information is crucial for more refined management based on specific patient characteristics.
Our analysis revealed that the majority of AEs associated with ide-cel and cilta-cel occurred during the initial phase of treatment, with the highest risk occurring within the first 15 days. The Weibull distribution analysis confirmed an early failure pattern, corroborating our statistical AE TTO findings. Consistent with the findings of Vigibase research, we observed that common AEs like CRS and ICANS typically emerged within the first week of CAR-T cell administration (42). Additionally, both drugs had outlier values, indicating delayed AEs. In particular, secondary malignancies emerged, on average, more than a year after treatment. Hence, close monitoring for AEs during the early stages of CAR-T therapies and long-term surveillance for delayed AEs are crucial in clinical practice.
This study has limitations. Firstly, the FAERS database is a self-reporting system, with the inherent limitation of the underreporting of AEs (25). Secondly, while FAERS reports are informative, the reporting rate is influenced by various factors, including the severity of an AE and public perception, rendering the drug–AE causal relationship uncertain (43–45). To enhance reliability, we integrated clinical trial and analogous study results for comparative analysis. Thirdly, the disproportionality analysis can only provide preliminary evidence indicating that there may be an association between drugs and AEs, but it may be affected by confounding factors and it cannot determine whether the association is causal (27). Therefore, when applying the method, we performed subgroup analysis at the level of demographic characteristics to ensure the accuracy of the results. Although not definitive, this study’s comprehensive safety analysis of AE signals associated with BCMA-targeted CAR-T cell therapies could lay the foundation for safe clinical use in the future.
This study validated clinical trial findings on AEs related to ide-cel and cilta-cel administration using the FAERS database, and identified previously unknown or underestimated risks associated with both drugs, thereby complementing existing research. The results of the disproportionality analysis of secondary malignancies indicated that the risk of secondary malignancy is increasing. In addition, the comparison of the signals between the subgroups provides key information for more refined clinical management in the future. In conclusion, this study highlights the importance of long-term ongoing safety studies to identify emerging AEs and potential risk signals associated with the use of ide-cel and cilta-cel, with the potential to promote safe and rational use in clinical settings.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: https://fis.fda.gov/extensions/FPD-QDE-FAERS/FPD-QDE-FAERS.html.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributions
WL: Software, Writing – original draft, Writing – review & editing, Formal Analysis. SLi: Supervision, Writing – review & editing. XZ: Data curation, Writing – original draft. LY: Software, Writing – review & editing. QL: Data curation, Writing – review & editing. SLe: Writing – review & editing, Formal Analysis. BF: Writing – review & editing, Supervision, Conceptualization.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We extend our sincere appreciation to all those who have contributed to this study. We thank Amanda Holland, PhD, from Liwen Bianji (Edanz) (www.liwenbianji.cn) for editing the English text of a draft of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1433075/full#supplementary-material
References
1. Killock D. CAR T cells show superiority over standard therapies for RRMM. Nat Rev Clin Oncol. (2023) 20:210. doi: 10.1038/s41571-023-00749-y
2. Atrash S, Ali SA, Usmani SZ. Chimeric antigen receptor T-cell therapy for multiple myeloma. Clin Lymphoma Myeloma Leuk. (2021) 21:21–34. doi: 10.1016/j.clml.2020.08.027
3. Sadek NL, Costa BA, Nath K, Mailankody S. CAR T-cell therapy for multiple myeloma: A clinical practice-oriented review. Clin Pharmacol Ther. (2023) 114:1184–95. doi: 10.1002/cpt.3057
4. Sharma P, Kanapuru B, George B, Lin X, Xu Z, Bryan WW, et al. FDA approval summary: idecabtagene vicleucel for relapsed or refractory multiple myeloma. Clin Cancer Res. (2022) 28:1759–64. doi: 10.1158/1078-0432.Ccr-21-3803
5. Martin T, Usmani SZ, Berdeja JG, Agha M, Cohen AD, Hari P, et al. Ciltacabtagene autoleucel, an anti-B-cell maturation antigen chimeric antigen receptor T-cell therapy, for relapsed/refractory multiple myeloma: CARTITUDE-1 2-year follow-up. J Clin Oncol. (2023) 41:1265–74. doi: 10.1200/jco.22.00842
6. Natrajan K, Kaushal M, George B, Kanapuru B, Theoret MR. FDA approval summary: ciltacabtagene autoleucel for relapsed or refractory multiple myeloma. Clin Cancer Res. (2024) 30(14):2865–71. doi: 10.1158/1078-0432.Ccr-24-0378
7. Davis J, McGann M, Shockley A, Hashmi H. Idecabtagene vicleucel versus ciltacabtagene autoleucel: a Sophie's choice for patients with relapsed refractory multiple myeloma. Expert Rev Hematol. (2022) 15:473–5. doi: 10.1080/17474086.2022.2081147
8. Fang J, Zhou F. BCMA-targeting chimeric antigen receptor T cell therapy for relapsed and/or refractory multiple myeloma. Ann Hematol. (2024) 103:1069–83. doi: 10.1007/s00277-023-05444-7
9. Chekol Abebe E, Yibeltal Shiferaw M, Tadele Admasu F, Asmamaw Dejenie T. Ciltacabtagene autoleucel: The second anti-BCMA CAR T-cell therapeutic armamentarium of relapsed or refractory multiple myeloma. Front Immunol. (2022) 13:991092. doi: 10.3389/fimmu.2022.991092
10. Rodriguez-Otero P, Ailawadhi S, Arnulf B, Patel K, Cavo M, Nooka AK, et al. Ide-cel or standard regimens in relapsed and refractory multiple myeloma. N Engl J Med. (2023) 388:1002–14. doi: 10.1056/NEJMoa2213614
11. San-Miguel J, Dhakal B, Yong K, Spencer A, Anguille S, Mateos MV, et al. Cilta-cel or standard care in lenalidomide-refractory multiple myeloma. N Engl J Med. (2023) 389:335–47. doi: 10.1056/NEJMoa2303379
12. Yang J, Zhou W, Li D, Niu T, Wang W. BCMA-targeting chimeric antigen receptor T-cell therapy for multiple myeloma. Cancer Lett. (2023) 553:215949. doi: 10.1016/j.canlet.2022.215949
13. (FDA) USFaDA. FDA Requires Boxed Warning for T cell Malignancies Following Treatment with BCMA-Directed or CD19-Directed Autologous Chimeric Antigen Receptor (CAR) T cell Immunotherapies 2024. Available online at: https://www.fda.gov/vaccines-blood-biologics/safety-availability-biologics/fda-requires-boxed-warning-t-cell-malignancies-following-treatment-bcma-directed-or-cd19-directed. (accessed [April 19, 2024])
14. Freyer CW, Porter DL. Cytokine release syndrome and neurotoxicity following CAR T-cell therapy for hematologic Malignancies. J Allergy Clin Immunol. (2020) 146:940–8. doi: 10.1016/j.jaci.2020.07.025
15. Cordas dos Santos DM, Tix T, Rejeski K. Infections drive non-relapse mortality following CAR-T therapy across disease entities and CAR products - a meta-analysis of clinical trials and real-world studies. Blood. (2023) 142:1064–. doi: 10.1182/blood-2023-187516
16. Lipe DN, Rajha E, Wechsler AH, Gaeta S, Palaskas NL, Alhajji Z, et al. Cardiotoxicity associated with immune checkpoint inhibitors and CAR T-cell therapy. Am J Emerg Med. (2021) 50:51–8. doi: 10.1016/j.ajem.2021.07.014
17. Singh V, Master S. Incidence of Parkinsonism as a complication of anti-BCMA CAR-T cell therapy in multiple myeloma. Blood. (2023) 142:6937–. doi: 10.1182/blood-2023-188090
18. Friedman KM, Garrett TE, Evans JW, Horton HM, Latimer HJ, Seidel SL, et al. Effective targeting of multiple B-cell maturation antigen-expressing hematological Malignances by anti-B-cell maturation antigen chimeric antigen receptor T cells. Hum Gene Ther. (2018) 29:585–601. doi: 10.1089/hum.2018.001
19. U.S. Food and Drug Administration (FDA). Available online at: https://www.fda.gov/drugs/surveillance/questions-and-answers-fdas-adverse-event-reporting-system-faers. (accessed [April 15, 2024])
20. Rodriguez EM, Staffa JA, Graham DJ. The role of databases in drug postmarketing surveillance. Pharmacoepidemiol Drug Saf. (2001) 10:407–10. doi: 10.1002/pds.615
21. Sakaeda T, Kadoyama K, Minami K, Okuno Y. Commonality of drug-associated adverse events detected by 4 commonly used data mining algorithms. Int J Med Sci. (2014) 11:461–5. doi: 10.7150/ijms.7967
22. Sakaeda T, Tamon A, Kadoyama K, Okuno Y. Data mining of the public version of the FDA Adverse Event Reporting System. Int J Med Sci. (2013) 10:796–803. doi: 10.7150/ijms.6048
23. Brown EG. Using MedDRA: implications for risk management. Drug Saf. (2004) 27:591–602. doi: 10.2165/00002018-200427080-00010
24. Hu Y, Bai Z, Tang Y, Liu R, Zhao B, Gong J, et al. Fournier gangrene associated with sodium-glucose cotransporter-2 inhibitors: A pharmacovigilance study with data from the U.S. FDA adverse event reporting system. J Diabetes Res. (2020) 2020:3695101. doi: 10.1155/2020/3695101
25. Shu Y, He X, Liu Y, Wu P, Zhang Q. A real-world disproportionality analysis of olaparib: data mining of the public version of FDA adverse event reporting system. Clin Epidemiol. (2022) 14:789–802. doi: 10.2147/clep.S365513
26. Bate A, Evans SJ. Quantitative signal detection using spontaneous ADR reporting. Pharmacoepidemiol Drug Saf. (2009) 18:427–36. doi: 10.1002/pds.1742
27. van Puijenbroek EP, Bate A, Leufkens HG, Lindquist M, Orre R, Egberts AC. A comparison of measures of disproportionality for signal detection in spontaneous reporting systems for adverse drug reactions. Pharmacoepidemiol Drug Saf. (2002) 11:3–10. doi: 10.1002/pds.668
28. Rothman KJ, Lanes S, Sacks ST. The reporting odds ratio and its advantages over the proportional reporting ratio. Pharmacoepidemiol Drug Saf. (2004) 13:519–23. doi: 10.1002/pds.1001
29. Lindquist M, Ståhl M, Bate A, Edwards IR, Meyboom RH. A retrospective evaluation of a data mining approach to aid finding new adverse drug reaction signals in the WHO international database. Drug Saf. (2000) 23:533–42. doi: 10.2165/00002018-200023060-00004
30. Zou F, Zhu C, Lou S, Cui Z, Wang D, Ou Y, et al. A real-world pharmacovigilance study of mepolizumab in the FDA adverse event reporting system (FAERS) database. Front Pharmacol. (2023) 14:1320458. doi: 10.3389/fphar.2023.1320458
31. Sauzet O, Cornelius V. Generalised weibull model-based approaches to detect non-constant hazard to signal adverse drug reactions in longitudinal data. Front Pharmacol. (2022) 13:889088. doi: 10.3389/fphar.2022.889088
32. Sauzet O, Carvajal A, Escudero A, Molokhia M, Cornelius VR. Illustration of the weibull shape parameter signal detection tool using electronic healthcare record data. Drug Saf. (2013) 36:995–1006. doi: 10.1007/s40264-013-0061-7
33. (FDA) USFaDA. The Abecma Prescribing Information and Medication Guide at DailyMed(2024). Available online at: https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm?event=IndvRemsDetails.page&REMS=406. (accessed [April 15, 2024])
34. (FDA) USFaDA. The Carvykti Prescribing Information at DailyMed 2024. Available online at: https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm?event=IndvRemsDetails.page&REMS=412. (accessed [April 15, 2024])
35. Jakubowiak A, Usmani SZ, Berdeja JG, Agha M, Cohen AD, Hari P, et al. Efficacy and safety of ciltacabtagene autoleucel in patients with relapsed/refractory multiple myeloma: CARTITUDE-1 subgroup analysis. Blood. (2021) 138:3938–. doi: 10.1182/blood-2021-146069
36. Raje N, Berdeja J, Lin Y, Siegel D, Jagannath S, Madduri D, et al. Anti-BCMA CAR T-cell therapy bb2121 in relapsed or refractory multiple myeloma. N Engl J Med. (2019) 380:1726–37. doi: 10.1056/NEJMoa1817226
37. Berdeja JG, Madduri D, Usmani SZ, Jakubowiak A, Agha M, Cohen AD, et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): a phase 1b/2 open-label study. Lancet. (2021) 398:314–24. doi: 10.1016/s0140-6736(21)00933-8
38. Elsallab M, Ellithi M, Lunning MA, D'Angelo C, Ma J, Perales MA, et al. Second primary Malignancies after commercial CAR T cell therapy: analysis of FDA adverse events reporting system (FAERS). Blood. (2024) 143(20):2099–105. doi: 10.1182/blood.2024024166
39. Majzner RG, Mackall CL. Tumor antigen escape from CAR T-cell therapy. Cancer Discovery. (2018) 8:1219–26. doi: 10.1158/2159-8290.Cd-18-0442
40. van de Donk N, Usmani SZ, Yong K. CAR T-cell therapy for multiple myeloma: state of the art and prospects. Lancet Haematol. (2021) 8:e446–e61. doi: 10.1016/s2352-3026(21)00057-0
41. Zhang L, Shen X, Yu W, Li J, Zhang J, Zhang R, et al. Comprehensive meta-analysis of anti-BCMA chimeric antigen receptor T-cell therapy in relapsed or refractory multiple myeloma. Ann Med. (2021) 53:1547–59. doi: 10.1080/07853890.2021.1970218
42. Dolladille C, Ederhy S, Ezine E, Choquet S, Nguyen LS, Alexandre J, et al. Chimeric antigen receptor T-cells safety: A pharmacovigilance and meta-analysis study. Am J Hematol. (2021) 96:1101–11. doi: 10.1002/ajh.26259
43. Hazell L, Shakir SA. Under-reporting of adverse drug reactions : a systematic review. Drug Saf. (2006) 29:385–96. doi: 10.2165/00002018-200629050-00003
44. Alomar M, Tawfiq AM, Hassan N, Palaian S. Post marketing surveillance of suspected adverse drug reactions through spontaneous reporting: current status, challenges and the future. Ther Adv Drug Saf. (2020) 11:2042098620938595. doi: 10.1177/2042098620938595
Keywords: chimeric antigen receptor T cell therapy, B cell maturation antigen, FDA adverse event reporting system, adverse events, disproportionality analysis
Citation: Liu W, Lin S, Zhu X, Yin L, Liu Q, Lei S and Feng B (2024) Safety assessment of anti-B cell maturation antigen chimeric antigen receptor T cell therapy: a real-world study based on the FDA adverse event reporting system database. Front. Immunol. 15:1433075. doi: 10.3389/fimmu.2024.1433075
Received: 15 May 2024; Accepted: 09 August 2024;
Published: 03 September 2024.
Edited by:
Hong-Ming Hu, Independent Researcher, Shanghai, ChinaReviewed by:
Pouya Safarzadeh Kozani, Tarbiat Modares University, IranKotaro Miyao, Anjo Kosei Hospital, Japan
Copyright © 2024 Liu, Lin, Zhu, Yin, Liu, Lei and Feng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bianling Feng, ZmVuZ2JpYW5saW5nQDE2My5jb20=
 Wei Liu1,2
Wei Liu1,2 Qian Liu
Qian Liu Bianling Feng
Bianling Feng