- 1Neurology Unit, Department of Neuroscience (DNS), Università degli Studi di Padova, Padua, Italy
- 2Laboratory of Hematology and Immunology, Department of Medicine (DIMED), Università degli Studi di Padova, Padua, Italy
- 3Multiple Sclerosis Centre, Neurology Unit, Azienda Ospedaliera di Padova, Padua, Italy
Blood-brain barrier dysfunction might be driven by peripheral inflammation. TNFα inhibitors (TNF-αi) are occasionally associated with a wide spectrum of neurological immuno-mediated disorders. However, patients with systemic autoimmune disorders, including rheumatoid arthritis (RA), might be prone to develop further organ-specific, including central nervous system (CNS), autoimmunity. Here we report the case of a patient, affected by RA and treated with etanercept, who suddenly developed focal neurological symptoms. Cerebrospinal fluid, magnetic resonance imaging (MRI), and positron emission tomography (PET)/MRI findings are reported and support the diagnosis of TNF-αi -associated aseptic meningitis.
Introduction
Peripheral inflammation might induce blood-brain barrier (BBB) dysfunction, breaking the central nervous system (CNS) immune privilege and inducing local inflammation (1). Indeed, TNFα inhibitors (TNF-αi) are occasionally associated with a wide spectrum of neurological immuno-mediated disorders (2) that are commonly divided into demyelinating (3–8) and non-demyelinating (9–15). Multiple sclerosis (MS), radiologically isolated syndrome (16), transverse myelitis, and neuromyelitis optica spectrum disorder (NMOSD) are commonly included in demyelinating disorders, while neurosarcoidosis, CNS vasculitis, leptomeningitis, or meningoencephalitis define the non-demyelinating CNS events.
Conversely, patients with systemic autoimmune disorders, including rheumatoid arthritis (RA), might be prone to develop further organ-specific, including CNS and meningeal, autoimmunity (15). Therefore, the presence of CNS involvement in a systemic autoimmune disorder treated with TNF-αi constitutes a diagnostic challenge for physicians. Here we report the case of a patient, affected by RA and treated with etanercept, who developed aseptic meningitis.
Case report
A 68-year-old woman was evaluated for a sudden onset transient paresis of the left lower limb lasting for 15 minutes. She reported a few similar spells before, always very brief, occasionally involving the contralateral limb. She was diagnosed with RA 2 years before, without any evidence of either peripheral nervous system (PNS) or CNS involvement. She was temporarily treated with acetylsalicylic acid for the neuroradiologic evidence of microvascular ischemic disease, which was discontinued after spontaneous ecchymosis. Ongoing disease-modifying drugs included low-dose prednisone, etanercept (28 infusions, the last 1 week before the onset of symptoms), and methotrexate. Moreover, she took alendronate for osteoporosis, venlafaxine for depression, and beta-blocker for tachycardia. Neurological examination was normal, while a brain CT scan revealed a slight disappearance of parafalcial cortical sulci bilaterally, confirmed by a high field (3T) magnetic resonance imaging (MRI) scan (Figure 1). In addition, a high field (3T) positron emission tomography (PET)/MRI confirmed the cortical hypermetabolism along the falx cerebri (Figure 1E). Altogether, brain imaging revealed an inflammation along pachy- and leptomeningeal sheets.
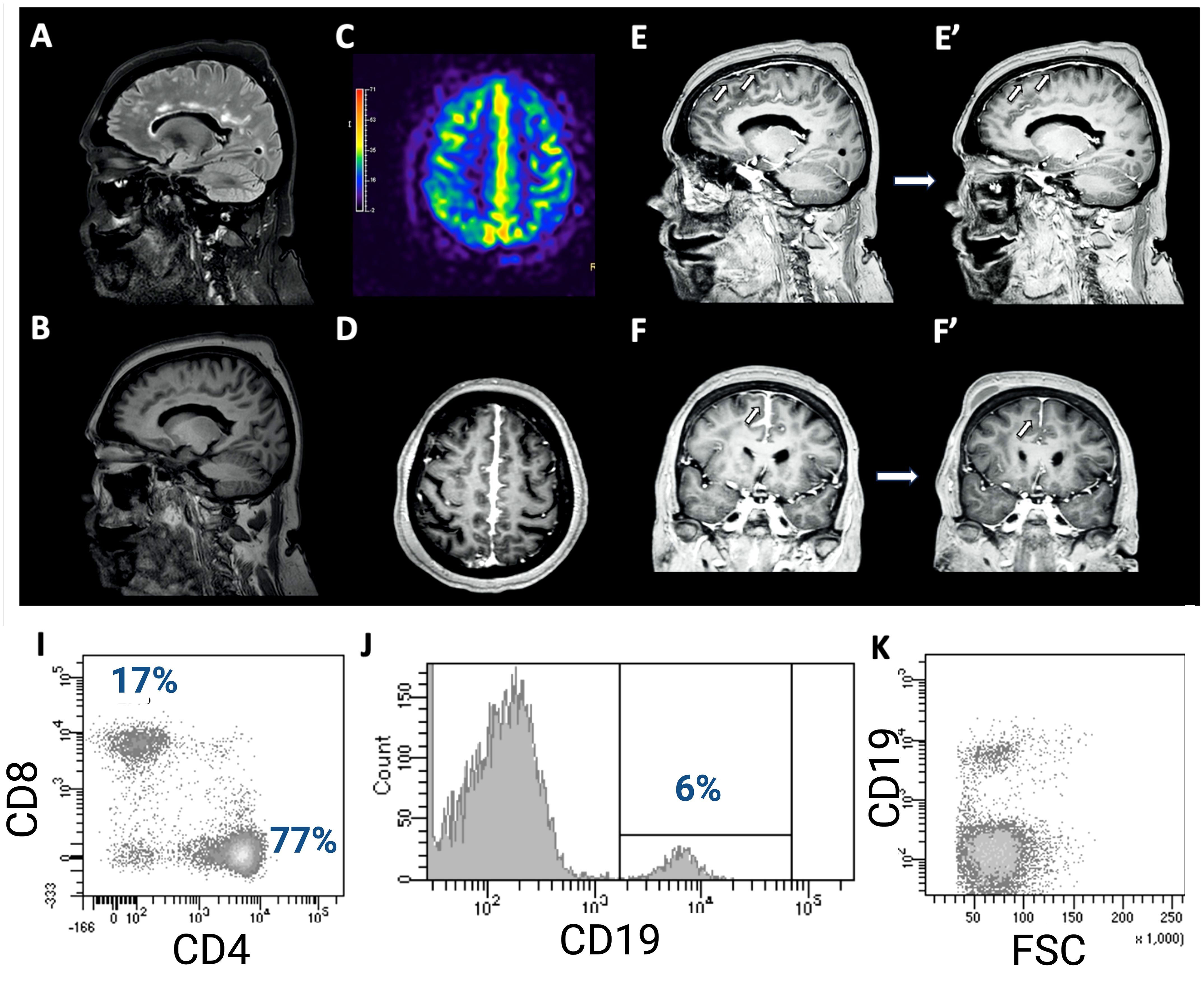
Figure 1. (A) Brain MRI showing a slight T2-FLAIR hyperintensity of the cortical gyri in the fronto-parietal area at the vertex, in the parasagittal area, with obliteration of the sulci. Comparing T1 sequences (B) and contrast-enhanced T1 sequences (D–F), there is a noticeable pathological pachymeningeal and leptomeningeal enhancement along the profile of the cerebral falx, especially in the posterior region, further confirmed by PET/MRI (C). In addition, a diffuse slight dural meningeal thickening was also reported. Follow-up brain MRI 4 weeks later shows significant improvement of the meningeal enhancement (E’, F’). The total body PET/MRI confirmed the cortical hypermetabolism along the falx cerebri, showing also a slightly hypometabolic left parietal cortex (E). Flowcytometry analysis of CSF-infiltrating cells revealed both CD4+ and CD8+ T-cells (I) with an increased ratio (4.5), as well as a small percentage of B cells (6%, (J)), with normal physical parameters (K).
The etiology of this process was investigated with cerebrospinal fluid (CSF) analysis, which disclosed a moderate pleocytosis (125 cells/μL, all mononuclear cells) with a mild increase in protein concentration (54 mg/dL) and CSF/serum quotient of albumin (QALB) (11.2 x10-3, QALB/QLIM: 1.31, mild BBB damage) (1). Moreover, while isoelectric focusing did not disclose any IgG oligoclonal band (IgGOB), quantitative parameters of intrathecal IgG synthesis were increased (IgG Index 1.2, nv <0.7; IgGLOC 21.0 mg/L). CSF microbiological screening (Streptococcus agalactiae, Streptococcus pneumoniae, Cryptococcus neoformans, cytomegalovirus, enterovirus, herpes simplex virus 1, herpes simplex virus 2, herpes simplex virus 6, human parechovirus, varicella zoster virus, M. tuberculosis cDNA, E. coli K1, Haemophilus influenzae, Lysteria monocytogenes, Neisseria meningitidis) was negative, while the immunophenotype of CSF-infiltrating leucocyte revealed that CSF-infiltrating cells were all lymphocytes, namely both B (6%) and T cells (94%, 77% CD4+, 17% CD8+) (Supplementary Figure). No T cell had increased HLA-DR expression. Physical parameters and the lack of HLA-DR expression suggested the recruitment of inactivated B cells. Immunological screening in serum revealed an elevated rheuma-test (153 kU/L, nv <30), while C-reactive protein, angiotensin-converting enzyme (also in CSF), complement factors, and erythrocyte sedimentation rate were normal. Moreover, anti-nuclear, anti-neutrophilic cytoplasmic, anti-double-stranded DNA, anti-extractable nuclear antigen, anti-anticardiolipin, and β2-glycoproteinI antibodies were negative, as well as neoplastic and paraneoplastic markers. Therefore, these findings were strongly consistent with an immuno-mediated CNS-restricted inflammation. Since there were neither systemic symptoms nor serological tests suggestive for RA worsening or progression, TNF-αi was considered as the trigger of this picture. Therefore, said therapy was discontinued, and the patient was treated with methylprednisolone 1 g daily for 5 days, then slowly tapered with oral prednisone. The 1-month follow-up MRI showed radiological improvement with significant resolution of the abnormal meningeal enhancement. The patient did not experience any further spells in the following 6 months.
Discussion
Our patient reported transitory neurological deficit, characterized by left limb weakness. To evaluate the ischemic etiology, brain imaging was acquired, revealing a severe and diffuse inflammatory meningeal involvement, further confirmed by standard CSF analysis (moderate pleocytosis). Although the clinical symptom could not be considered as a typical or atypical clinically isolated syndrome (17, 18), the MRI picture also disclosed white matter lesion around ventricles, which, especially in presence of an ongoing TNF-αi, called for a carefully exclusion of any demyelinating disorder. Since white matter lesion etiology is wide, and in presence of a putative radiologically isolated syndrome, the evaluation of their characteristic is strongly recommended to increase the specificity of criteria for MS diagnosis (19). In our case, the patient presented periventricular lesions, which were not suggestive for MS. Indeed, callosal lesions touched the top of the corpus callosum, not the bottom, suggesting an arteriolar pathology rather than a perivenular inflammation (20, 21). In addition, the radiological scenario was characterized by diffuse meningeal inflammation, which is not suggestive of MS. Finally, the dissemination in space criterion was not met in the absence of any cortical-juxtacortical or infratentorial or spinal cord lesion. In addition, the absence of IgGOB or any contrast-enhancing lesions did not allow us to fulfill the dissemination in time criterion. Therefore, a better explanation was more convincing (18).
CSF microbiological screening excluded an infective origin of the severe meningeal inflammation, while total body PET/MRI did not identify any malignancy, ruling out meningeal carcinomatosis.
Therefore, the inflammatory etiology seemed to be more plausible. To distinguish TNF-αi aseptic meningitis from rheumatoid meningitis the evaluation of serological markers, as well as of clinical symptoms, is mandatory.
In our patient, the lack of any clinical or biochemical sign of disease worsening (i.e., the absence of systemic involvement and the normal immunological screening, except for a mild increase of rheumatoid factor), together with the absence of relevant BBB damage suggested an aseptic meningeal involvement.
Indeed, meningeal involvement as an expression of RA (rheumatoid meningitis) is a rare condition that normally presents with an increase in acute phase reactants and in anticitrullinated protein antibodies, that we did not find in this case (22–29). Notably, two case of rheumatoid meningitis were treated with etanercept (24, 26). However, a few cases of RA developed aseptic meningitis when treated with infliximab (14, 30), etanercept (31), and adalimumab (32, 33), suggesting that it is an extremely rare TNF-αi -induced adverse event (15).
From a clinical point of view, the epileptic etiology of the transient symptoms was excluded, since the electroencephalography showed short sequences of slow bitemporal waves that were not consistent with either atonic seizures (high voltage slow wave) or focal negative motor seizures (involving the motor area). Ultimately, the most reliable cause of these stroke-like episodes seems to be the involvement of cortical vessel walls by the leptomeningeal inflammation. From an immunological point of view, the absence of activated B and T cells in CSF, as well as the lack of CSF-restricted IgGOB and of any parenchymal lesion, in the presence of the BBB damage (increased QALB) that we observed in our patient seems to drive a bystander recruitment of lymphocytes, questioning the presence of a specific CNS-derived-antigen and suggesting a TNF-αi-induced innate-immunity disorder. The link between TNF-αi and BBB function warrants further investigation.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
BK: Conceptualization, Data curation, Formal analysis, Writing – original draft. MF: Formal analysis, Writing – original draft, Methodology. AM: Methodology, Writing – original draft, Conceptualization, Visualization. SC: Visualization, Writing – original draft, Data curation. FR: Visualization, Writing – original draft, Conceptualization, Methodology. PG: Conceptualization, Visualization, Writing – original draft, Validation. MP: Conceptualization, Visualization, Writing – original draft, Data curation, Formal analysis, Methodology.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Open Access funding provided by Università degli Studi di Padova | University of Padua, Open Science Committee.
Conflict of interest
AM received a travel grant from Novartis, Sanofi-Genzyme, and Biogen. MP received a travel grant from Novartis, Genzyme, Biogen, Teva, Almirall and Sanofi-Genzyme; he has been a consultant for Sanofi-Genzyme, Novartis., Biogen. FR serves as an advisory board member of Biogen-Idec and has received funding for travel and speaker honoraria from Merck Serono, Biogen Idec, Sanofi-Aventis, Teva and Bayer Schering Pharma. PG reports grants and personal fees from Novartis, grants and personal fees from Almirall, grants and personal fees from Biogen Idec, grants and personal fees from Sanofi Genzyme, grants and personal fees from Teva, grants and personal fees from Merck Serono, grants from University of Padova, grants from Italian Ministry of Public Health, grants from Veneto Region of Italy, grants from Italian Association for Multiple Sclerosis, outside the submitted work.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1432360/full#supplementary-material
References
1. Puthenparampil M, Tomas-Ojer P, Hornemann T, Lutterotti A, Jelcic I, Ziegler M, et al. Altered CSF albumin quotient links peripheral inflammation and brain damage in MS. Neurology(R) neuroimmunology Neuroinflamm. (2021) 8(2):e951. doi: 10.1212/NXI.0000000000000951
2. Kunchok A, Aksamit AJ, Davis JM, Kantarci OH, Keegan BM, Pittock SJ, et al. Association between tumor necrosis factor inhibitor exposure and inflammatory central nervous system events. JAMA Neurol. (2020) 77:937–46. doi: 10.1001/jamaneurol.2020.1162
3. Mohan N, Edwards ET, Cupps TR, Oliverio PJ, Sandberg G, Crayton H, et al. Demyelination occurring during anti-tumor necrosis factor α therapy for inflammatory arthritides. Arthritis Rheum. (2001) 44:2862–9. doi: 10.1002/1529-0131(200112)44:12<2862::AID-ART474>3.0.CO;2-W
4. Chey SY, Kermode AG. Central nervous system demyelination related to tumour necrosis factor alpha inhibitor. Mult Scler J Exp Transl Clin. (2022) 8(1):20552173211070750. doi: 10.1177/20552173211070750
5. Gharib MH, AlKahlout MA, Canibano BG, Deleu DT, AlEssa HM, AlEmadi S. Demyelinating neurological adverse events following the use of anti-TNF-α Agents: A double-edged sword. Case Rep Neurol Med. (2022) 2022:3784938. doi: 10.1155/2022/3784938
6. Taylor TRP, Galloway J, Davies R, Hyrich K, Dobson R. Demyelinating events following initiation of anti-TNFα Therapy in the british society for rheumatology biologics registry in rheumatoid arthritis. Neurol Neuroimmunol Neuroinflamm. (2021) 8(3):e992. doi: 10.1212/NXI.0000000000000992
7. Kucharz EJ, Kotulska-Kucharz A. Tumor necrosis factor alpha inhibitors and demyelinating disease: what is behind it? Reumatologia. (2021) 59:65. doi: 10.5114/REUM.2021.105438
8. Seror R, Richez C, Sordet C, Rist S, Gossec L, Direz G, et al. Pattern of demyelination occurring during anti-TNF-α therapy: A french national survey. Rheumatol (United Kingdom). (2013) 52:868–74. doi: 10.1093/RHEUMATOLOGY/KES375
9. Winthrop KL, Chen L, Fraunfelder FW, Ku JH, Varley CD, Suhler E, et al. Initiation of anti-TNF therapy and the risk of optic neuritis: From the safety assessment of biologic ThERapy (SABER) study. Am J Ophthalmol. (2013) 155:183–189.e1. doi: 10.1016/j.ajo.2012.06.023
10. Grau RG. Drug-induced vasculitis: new insights and a changing lineup of suspects. Curr Rheumatol Rep. (2015) 17(12):71. doi: 10.1007/S11926-015-0545-9
11. Kaltsonoudis E, Zikou AK, Voulgari PV, Konitsiotis S, Argyropoulou MI, Drosos AA. Neurological adverse events in patients receiving anti-TNF therapy: A prospective imaging and electrophysiological study. Arthritis Res Ther. (2014) 16(3):R125. doi: 10.1186/AR4582
12. Berrios I, Jun-O’Connell A, Ghiran S, Ionete C. Case Report: A case of neurosarcoidosis secondary to treatment of etanercept and review of the literature. BMJ Case Rep. (2015) 2015:bcr2014208188. doi: 10.1136/BCR-2014-208188
13. Hegde N, Gayomali C, Rich MW. Infliximab-induced headache and infliximab-induced meningitis: two ends of the same spectrum? South Med J. (2005) 98:564–6. doi: 10.1097/01.SMJ.0000155499.21189.75
14. Marotte H, Charrin JE, Miossec P. Infliximab-induced aseptic meningitis. Lancet. (2001) 358:1784. doi: 10.1016/S0140-6736(01)06810-6
15. Rodriguez FF, Minkyung K, Jinna S, Farshad S, Davila F. Rheumatoid meningoencephalitis: A feared condition in the era of TNF blockers. Case Rep Rheumatol. (2018) 2018:1–4. doi: 10.1155/2018/4610260
16. Ali F, Laughlin RS. Asymptomatic CNS demyelination related to TNF-α inhibitor therapy. Neurol Neuroimmunol Neuroinflamm. (2017) 4(1):e298. doi: 10.1212/NXI.0000000000000298/ASSET/E8CC82E7-EFF5-4072-8C34-436E20EABB05/ASSETS/GRAPHIC/2FF1.JPEG
17. Miller DH, Chard DT, Ciccarelli O. Clinically isolated syndromes. Lancet Neurol. (2012) 11:157–69. doi: 10.1016/S1474-4422(11)70274-5
18. Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, Comi G, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. (2017) 17:162–73. doi: 10.1016/S1474-4422(17)30470-2
19. Filippi M, Preziosa P, Banwell BL, Barkhof F, Ciccarelli O, De Stefano N, et al. Assessment of lesions on magnetic resonance imaging in multiple sclerosis: practical guidelines. Brain. (2019) 142:1858. doi: 10.1093/BRAIN/AWZ144
20. Renard D, Castelnovo G, Campello C, Bouly S, Le Floch A, Thouvenot E, et al. An MRI review of acquired corpus callosum lesions. J Neurol Neurosurg Psychiatry. (2014) 85:1041–8. doi: 10.1136/JNNP-2013-307072
21. Garg N, Reddel SW, Miller DH, Chataway J, Riminton DS, Barnett Y, et al. The corpus callosum in the diagnosis of multiple sclerosis and other CNS demyelinating and inflammatory diseases. J Neurol Neurosurg Psychiatry. (2015) 86:1374–82. doi: 10.1136/jnnp-2014-309649
22. Fan S, Zhao J, Hou B, Liu M, Niu J, Zhou Y, et al. Rheumatoid meningitis: a rare neurological complication of rheumatoid arthritis. Front Immunol. (2023) 14:1065650/BIBTEX. doi: 10.3389/FIMMU.2023.1065650/BIBTEX
23. Choi SJ, Ho Park Y, JA K, JH H, Choe G, Kim S. Pearls & Oy-sters: Asymmetric meningeal involvement is a common feature of rheumatoid meningitis. Neurology. (2017) 88:e108–10. doi: 10.1212/WNL.0000000000003744/SUPPL_FILE/TABLE-E-1.DOCX
24. Gherghel N, Stan A, Stan H. Pearls & Oy-sters: Rheumatoid meningitis occurring during treatment with etanercept. Neurology. (2018) 91:806–8. doi: 10.1212/WNL.0000000000006397/ASSET/8A83D575-4E1B-4636-831A-90093B1AFEA7/ASSETS/GRAPHIC/21FF1.JPEG
25. Khan O, Aslam S, Mohammadrezaei F, Moncayo Wilches RD, Mehrabi J, Yehounatan M, et al. New-onset seizures: an unusual neurologic manifestation of rheumatoid arthritis. Oxf Med Case Rep. (2024) 2024(2):omad159. doi: 10.1093/OMCR/OMAD159
26. Tsuzaki K, Nakamura T, Okumura H, Tachibana N, Hamano T. Rheumatoid meningitis occurring during etanercept treatment. Case Rep Neurol Med. (2017) 2017:1–5. doi: 10.1155/2017/7638539
27. Huys ACML, Guerne PA, Horvath J. Rheumatoid meningitis occurring during adalimumab and methotrexate treatment. Joint Bone Spine. (2012) 79:90–2. doi: 10.1016/J.JBSPIN.2011.07.008
28. Ahmed M, Luggen M, Herman JH, Weiss KL, Decourten-Myers G, Quinlan JG, et al. Hypertrophic pachymeningitis in rheumatoid arthritis after adalimumab administration. J Rheumatol. (2006) 33(11):2344-6.
29. Nakamura M, Haryu S, Kameyama M, Sato Ki, Sasaki T, Nakashima I. A case of rheumatoid meningitis with elevated antibody titer indices of anti-cyclic citrullinated peptide antibody and anti-galactose-deficient IgG antibody. Neurol Clin Neurosci. (2021) 9:381–3. doi: 10.1111/NCN3.12527
30. Cavazzana I, Taraborelli M, Fredi M, Tincani A, Franceschini F. Aseptic meningitis occurring during anti-TNF-alpha therapy in rheumatoid arthritis and ankylosing spondylitis. Clin Exp Rheumatol. (2014) 32:732–4. https://www.clinexprheumatol.org/abstract.asp?a=8056.
31. Booker MJ, Flint J, Saravana S. Aseptic meningitis in a patient taking etanercept for rheumatoid arthritis: a case report. cases J. (2008) 1:364. doi: 10.1186/1757-1626-1-364
32. Jazeron A, Lallier JC, Rihn B, Thiercelin MC. Aseptic meningitis possibly induced by adalimumab. Joint Bone Spine. (2010) 77:618–9. doi: 10.1016/J.JBSPIN.2010.06.001
Keywords: rheumatoid arteritis, etanercept, aseptic meningitis, blood-brain barrier, cerebrospinal fluid, PET-MRI
Citation: Kassabian B, Facco M, Miscioscia A, Carraro S, Rinaldi F, Gallo P and Puthenparampil M (2024) Case report: Breaking CNS immuno-privilege: TNFα-inhibitor triggers aseptic meningitis in a patient with rheumatoid arthritis. Front. Immunol. 15:1432360. doi: 10.3389/fimmu.2024.1432360
Received: 13 May 2024; Accepted: 25 July 2024;
Published: 10 September 2024.
Edited by:
Mattia Bellan, University of Eastern Piedmont, ItalyReviewed by:
Narjes Saheb Sharif-Askari, University of Sharjah, United Arab EmiratesYasuhiro Shimojima, Shinshu University, Japan
Copyright © 2024 Kassabian, Facco, Miscioscia, Carraro, Rinaldi, Gallo and Puthenparampil. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marco Puthenparampil, bWFyY28ucHV0aGVucGFyQU1waWxAdW5pcGQuaXQ=
 Benedetta Kassabian
Benedetta Kassabian Monica Facco
Monica Facco Alessandro Miscioscia
Alessandro Miscioscia Samuela Carraro2
Samuela Carraro2 Paolo Gallo
Paolo Gallo Marco Puthenparampil
Marco Puthenparampil