- Department of Dermatology, State Key Laboratory of Complex Severe and Rare Diseases, National Clinical Research Center for Dermatologic and Immunologic Diseases, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
Macrophages, as specialized, long-lasting phagocytic cells of the innate immune system, have garnered increasing attention due to their wide distribution and various functions. The skin, being the largest immune organ in the human body, presents an intriguing landscape for macrophage research, particularly regarding their roles in inflammatory skin diseases and skin tumors. In this review, we compile the latest research on macrophages in conditions such as atopic dermatitis, psoriasis, systemic sclerosis, systemic lupus erythematosus, rosacea, bullous pemphigoid, melanoma and cutaneous T-cell lymphoma. We aim to contribute to illustrating the pathogenesis and potential new therapies for inflammatory skin diseases and skin tumors from the perspective of macrophages.
1 Introduction
Macrophages are present in all tissues of adult animals (1). They have crucial roles in an organism’s biology, including development, maintaining homeostasis, facilitating repair, and reacting to immunological assaults from pathogens. M0 macrophages, as the unmature and inactive form, polarize in different directions depending on the surrounding microenvironment, and form distinguished macrophage subtypes, such as M1 and M2 phenotype (2).
M1 macrophages, also known as classically activated macrophages, can be polarized by lipopolysaccharide (LPS) either alone or in synergism with interferon (IFN)-γ. M1 macrophages are characterized by an enhanced capacity to secrete pro-inflammatory cytokines such as interleukin (IL)-1β, IL-6, and IL-12. Phenotypically, M1 macrophages exhibit significant levels of cluster of differentiation (CD)68, CD80 and CD86. M1 macrophages play an essential role in promoting inflammation, and display anti-infection and anti-tumoral activity. However, they can also mediate reactive oxygen species (ROS)-triggered tissue impairment, affecting tissue regeneration and wound recovery.
M2 macrophages, also known as alternatively activated macrophages, are polarized by IL-4 and IL-13. They display an anti-inflammatory cytokine profile with elevated levels of IL-10 and transforming growth factor (TGF)-β. Based on the stimuli, M2 macrophages can be categorized into four subgroups, and they vary in terms of surface markers, released molecules, and biological roles. However, it is important to note that all M2 macrophages share the characteristic of co-express IL-10. M2 macrophages are crucial for clearing parasites, modifying tissues, promoting angiogenesis, and contributing to allergy disorders (3, 4).
Inflammatory skin diseases are a group of diseases resulting from immune system disorders and cause damage to skin tissue, including atopic dermatitis, psoriasis, systemic sclerosis, systemic lupus erythematosus, rosacea, bullous pemphigoid. Macrophages are recognized as significant cellular contributors to persistent inflammation across diverse tissues and illnesses (5). Concurrently, skin tumors, comprising both benign and malignant neoplasms, develop from the skin simultaneously. Nevus and hemangiomas are the most common benign skin tumors, and they are not life-threatening but impact aesthetics. Skin malignancies, including malignant melanoma, basal cell carcinoma, and cutaneous T-cell lymphoma, can be deadly and demand urgent attention. The function of macrophages in the tumor microenvironment (TME) has been extensively researched in many types of tumors, including skin malignancies. Macrophages are crucial in controlling the body’s immunological response and metabolism, perhaps contributing to the development of many diseases (4, 6). This review seeks to outline recent discoveries about the role of macrophages in different inflammatory skin diseases and skin tumors.
2 Atopic dermatitis
The symptoms of atopic dermatitis (AD), a chronic inflammatory skin condition, include intense itching and recurrent superficial and spongiotic inflammation (7). A complicated interplay between genetic and environmental variables, including immunological response, skin barrier failure, and pruritus, may be instrumental in the pathogenesis of AD (8). Numerous investigations have revealed a robust correlation between AD and macrophages.
2.1 The characteristic of macrophage in AD
Using molecular imaging approaches, 2,4-dinitrofluorobenzene (DNCB) induced AD-like skin lesions have been observed to exhibit infiltrated-macrophage profile (9). The difference in macrophage polarization between skin samples from AD and psoriasis is evident. M2 macrophages were almost exclusively detected in AD samples. While traditionally regarded as an anti-inflammatory phenotype, recent study suggested that M2 macrophages contribute to the pathogenesis of AD through the secretion of CCL18, thus promoting the continued recruitment of Th2 cells and maintaining inflammation (10).
AD macrophages have lower toll-like receptor (TLR)-2 expression and less release of pro-inflammatory cytokines in response to TLR-2 ligand stimulation when compared to healthy controls. This may be a factor in AD patients’ increased vulnerability to Staphylococcus aureus skin infections (11). Notably, psoriasis patients also exhibit colonization with Staphylococcus aureus. When exposed to Staphylococcus aureus α-toxin, macrophages from AD patients generated less C-X-C Motif Chemokine Ligand (CXCL)10 than those from psoriasis patients. Decreased secretion of CXCL10 results in reduced Th1 polarization (12).
A distinct cluster of macrophages expressing C-C Motif Chemokine Ligand (CCL)13 and CCL18 was discovered with single-cell RNA-sequencing in the leukocyte-infiltrated region of the lesional skin in AD. Analysis of ligand-receptor interactions revealed interactions between T cells, dendritic cell (DC)s, fibroblasts, and M2 macrophages that expressed CCL13 and CCL18. This provides a thorough understanding of the immunological milieu in AD (13).
2.2 The pathogenic roles of the macrophages in AD
Macrophages contribute to the development of AD through a variety of processes. An important factor in human AD is CLDN1, a component of epidermal tight junctions. The association between human AD patients’ CLDN1 levels and macrophage recruitment has been elucidated by recent research. Mice with reduced CLDN1 expression levels displayed AD-like morphological traits and attracted more macrophages to the skin lesion (14). YKL-40 is a crucial inflammatory marker in type II inflammation. Compared to normal persons, AD patients’ skin had a greater level of YKL-40. Subsequent research indicated that the primary source of YKL-40 was dermal macrophages, indicating that macrophages may be involved in the pathophysiology of AD (15).
Macrophages participate in the mechanism of AD itch as well. IL-31 is a type II cytokine linked to pruritus in many dermatologic diseases. For instance, it has been reported that CD206+ M2-like macrophages are the primary producers of IL-31 in recessive dystrophic epidermolysis bullosa (16). M2 macrophages are dominant sources of IL-31 in AD as well. Moreover, AD itch is caused by a sophisticated network of periostin, basophils, thymic stromal lymphopoietin, and IL-31-expressing macrophages (17).
Autophagy of macrophages is essential for immunological regulation and has been linked to the onset of AD. Compared to wild-type mice, autophagy-related gene 5 cKO mice display deficient autophagy activity, lower cutaneous inflammation and decreased M2 macrophage infiltration. Mechanistically, deficiency of autophagy causes CCAAT enhancer binding protein beta to accumulate, which in turn stimulates the production of suppressor of cytokine signaling 1/3, ultimately suppresses the expression of the M2 marker (18).
One hallmark of AD is inflammation-mediated lymphangiogenesis, which is intimately related to macrophage recruitment. Strong macrophage chemoattractant monocyte chemoattractant protein-1 is expressed at high levels by IL-4-stimulated keratinocyte cells. Furthermore, a notable rise in dermal macrophages expressing vascular endothelial growth factor-C, a pro-lymphangiogenic factor, is observed in the AD mice model (19).
Research has also been conducted regarding the role of chemokines related to macrophages in the etiology of AD, particularly macrophage migration inhibitory factor (MIF). The stratum corneum MIF levels in the skin lesions were found substantially higher compared to unaffected regions in the same patient. MIF provides a helpful gauge to measure the degree of AD locally (20). There is a link between the MIF promoter 173G/C polymorphism and a higher risk of AD (21). MIF promoter polymorphisms, namely the C-173 allele and the C/5-CATT and C/7-CATT haplotypes, were found to be substantially linked to a higher risk of AD in Korean patients (22). The characteristics and pathogenetic roles of the macrophages in AD are summarized in Figure 1.
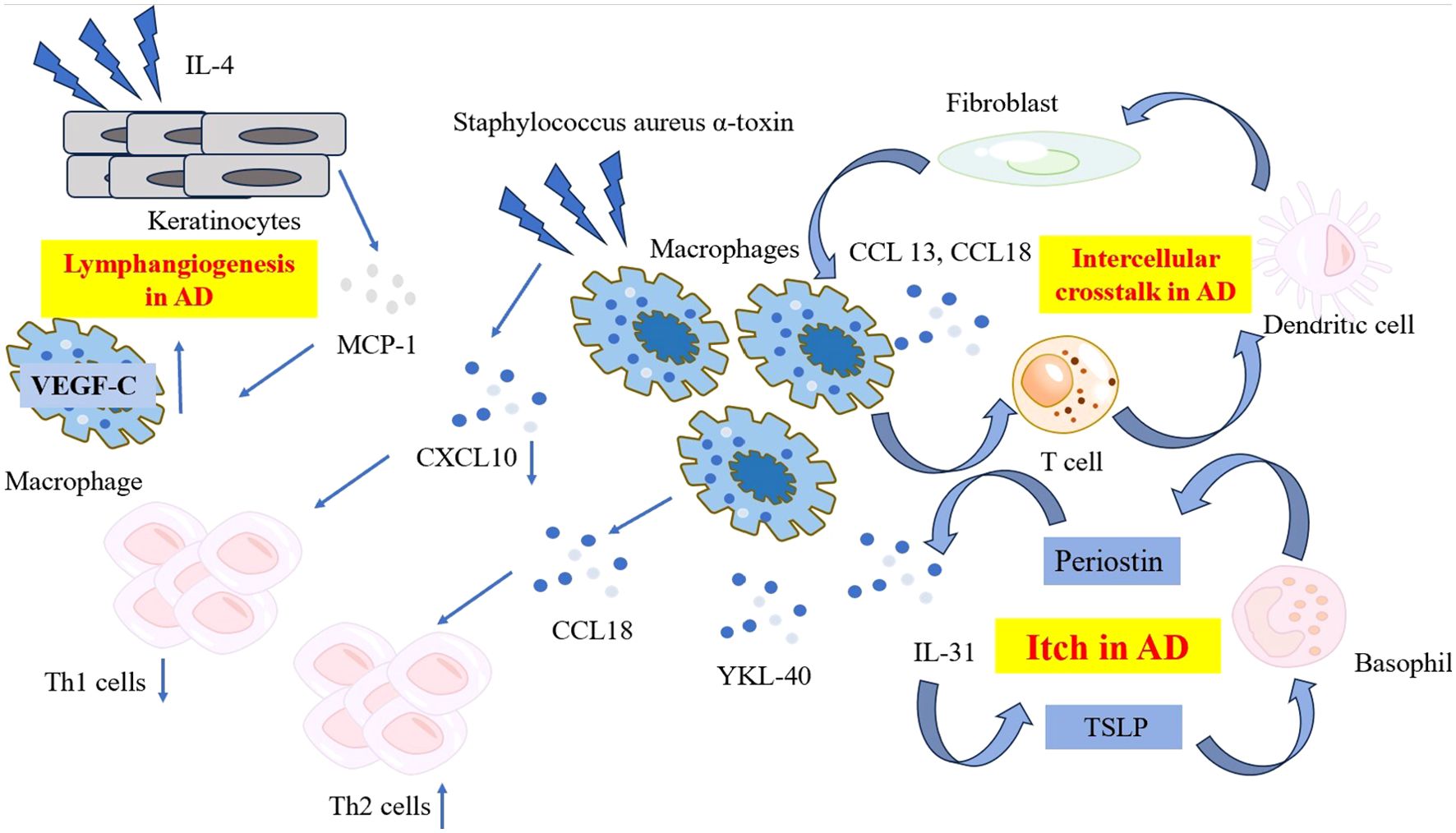
Figure 1. The characteristic and pathogenetic roles of the macrophages in AD. Compared to psoriasis, macrophages in AD produce lower level of CXCL10 when exposed to Staphylococcus aureus α-toxin, resulting in reduced Th1 polarization. Instead, macrophages in AD produce high levels of CCL18, recruiting more Th2 cells to affected skin and release YKL-40, an important Th2 marker. A network comprising periostin, TSLP, basophils and macrophage-derived IL-31 contribute to the mechanism of itch in AD. Ligand-receptor interactions data revealed the intracellular crosstalk between CCL13, CCL18-macrohages, T cells, DCs and fibroblasts. Macrophages also get involved in the lymphangiogenesis in AD by expressing significant level of VEGF-C. AD, atopic dermatitis; CCL, C-C Motif Chemokine Ligand; CXCL, C-X-C Motif Chemokine Ligand; DC, dendritic cell; IL, interleukin; MCP-1, monocyte chemoattractant protein-1; TSLP, thymic stromal lymphopoietin; VEGF-C, vascular endothelial growth factor-C; YKL-40, Chitinase 3-like 1.
2.3 Treatments for AD involving macrophages
Traditional Chinese medicine exhibited great potential in treating AD, including Periploca forrestii Schltr saponin and Stellariae Radix. Periploca forrestii Schltr saponin, which was traditionally used to treat rheumatoid arthritis, exhibits substantial potential for therapy in AD by suppressing the expression of both M1 and M2 macrophage markers (23). Stellariae Radix, which was previously used to treat fever and insomnia, successfully inhibited M1 macrophage infiltration in a DNCB-induced AD mouse model. Mechanistically, Stellariae Radix suppressed the production of tumor necrosis factor (TNF)-α, CXC-10, IL-12, and IL-1β and reduced the expression of NOD-like receptor thermal protein domain associated protein 3 (NLRP3) in M1 macrophages (24). A new topical medication for AD, called Nuclear Transport Checkpoint Inhibitor, inhibited the invasion of macrophages, and decreased the proliferation of Ki-67-positive cells (a subset of cells within the basal layer of the epidermis) (25). Naringenin, a flavonoid derived from plants, can reduce AD symptoms by inhibiting the M1-like macrophage phenotype, high mobility group box-1 (HMGB1) cascade, and levels of inflammatory cytokines. Moreover, naringenin can induce anti-inflammatory gene expression through the transformation of the M1 to M2 phenotype, resulting in increased levels of CD36 and IL-10 (26). Dictamnine, a natural alkaloid isolated from the root of Dictamnus albus, hinders DNCB-triggered AD skin inflammation by blocking M1 macrophage differentiation and enhancing macrophage autophagy at inflammation sites. Furthermore, dictamnine decreases the secretion and suppresses the genetic expression of inflammatory molecules (27). However, the curative effect of these potential therapies was evaluated in AD-mouse models and bone marrow-derived macrophages. In the future, we anticipate more large-scale clinical trials to verify these outcomes.
Nemolizumab, a humanized monoclonal antibody against IL-31 receptor A, holds great promise for alleviating pruritus and inflammation in AD patients in many clinical trials (28, 29). Dupilumab is another humanized monoclonal antibody that has gained approval for the treatment treating moderate-to-severe AD. Dupilumab can specifically bind to the IL-4Rα subunit, thereby inhibiting the signal transduction of IL-4 and IL-13, and blocking the Th2 inflammatory response. Both IL-4/13 and IL-31 pathway contributes to AD itch. Recent findings suggest that IL-31 can induce itching independently of IL-4 and IL-13 in vivo (30). M2 macrophages are implicated in the pathogenesis of AD pruritus and inflammation through the secretion of IL-31 and Th2 cytokines. However, there is a lack of direct studies addressing the impact of nemolizumab and dupilumab on immune cells, particularly the phenotype and number of macrophages. The janus kinase (JAK) pathway is activated in the signaling transduction of many cytokines relevant to AD. A network meta-analysis has demonstrated that many JAK inhibitors can ameliorate the signs and symptoms of AD, with upadacitinib showing particular efficacy (31). It has been documented that JAK inhibitor can reduce the infiltration of macrophages in lesional sites in allergic contact dermatitis mouse models (32). However, no analogous experiments have been conducted in AD mouse models.
3 Psoriasis
Psoriasis is a prevalent chronic inflammatory skin disorder distinguished by a significant inflammatory presence along with enlarged and distorted blood vessels. Infiltrated macrophages in psoriatic skin lesions are crucial in the advancement of this unregulated skin inflammation.
3.1 The characteristic of macrophage in psoriasis
Analyzed data from the GEO database showed a notable rise in the level of expression of macrophage markers and inflammatory cytokines in lesional tissues as compared to normal tissues in 58 patients with psoriasis (33). Significant variations in the composition of innate immune cells were found between psoriatic plaques and normal skin. There is a notable increase in the quantity of M0 and M1 macrophages in psoriatic skin. Both the count and proportion of macrophages underwent alterations. The abundance of M0 macrophages was linked to the psoriasis severity degree (34, 35). Psoriatic patients had a greater ratio of M1 to M2a macrophage polarization compared to controls (36). The proportion of C-C Motif Chemokine Receptor (CCR) 1+ macrophages increase in psoriasis-affected skin compared to healthy skin, as determined by single-cell RNA sequencing and flow cytometry data. CCR1+ macrophages exhibited elevated expression of genes associated with inflammatory cytokines and chemokines, such as CXCL-8, CXCL-2, and IL-1B (37).
Immune cell composition varies between the early and late stages of psoriatic skin lesions. Neutrophils infiltrated the epidermis in the early phase, but monocytes and monocyte-derived DCs were mostly present in the dermis. During the late phase, there was a temporary rise in the number of macrophages in the dermis (38).
3.2 The pathogenic roles of the macrophages in psoriasis
Several efforts have been undertaken to determine the function of macrophages in the development of psoriasis. The IL-23/IL-17 immunological axis plays an important role in the initiation and progression of psoriasis. A novel pathogenic macrophage subpopulation, triggered by IL-23 and characterized by a unique gene expression profile, has been discovered recently. M (IL-23) produce significant quantities of IL-17A, IL-22, and IFN-γ, contributing to the development of psoriasis-like dermatitis in a mouse model (39). Additionally, the IL-23/IL-17 immunological axis is proposed to play a role in the development of psoriasis by initiating ACT1/TRAF6/TAK1/NF-κB pathway in macrophages (40). Two important autoantigens in psoriasis are LL-37 and ADAMTS-Like Protein 5. It has been observed that ADAMTS-Like Protein 5+ and LL-37+ cells are co-expressed with CD163+ macrophages in both the superficial and deep dermis (41).
Interactions between macrophages and keratinocytes play a significant role in the development of psoriasis. Keratinocytes can interact with macrophages via HMGB1, promoting macrophage inflammatory polarization (42). The interaction between macrophages and exosomes generated from vitamin D receptor-deficient keratinocytes is crucial for the advancement of psoriasis. Exosomes-sh vitamin D receptor markedly enhanced macrophage proliferation and directed their polarization toward the M1 phenotype, while suppressing macrophage apoptosis (43).
Psoriasis is more prevalent and severe in men than in women. A recent investigation has shown that the root cause is linked to estrogen. Estradiol can inhibit the production of IL-1β by macrophages, and IL-1β is necessary for the generation of IL-17A in the psoriasis model. This perspective may clarify the disparity in both the occurrence and seriousness of psoriasis between genders (44).
Macrophages and psoriasis-related comorbidities have also been studied. Psoriasis patients with comorbidities have elevated levels of chitotriosidase compared to those without comorbidities. Chitotriosidase is primarily produced by activated macrophages in reaction to pro-inflammatory signals (45).
Macrophage-related cytokines are also linked to the development of psoriasis. The levels of macrophage inflammatory protein (MIP)-1α, MIP-1β, and monocyte chemoattractant protein-1 were considerably elevated in patients with psoriasis vulgaris and positively associated with psoriasis area and severity index score (46). While MIF levels were elevated in the blood, MIF-positive staining in the psoriatic epidermis was notably reduced. MIF mRNA level decreased simultaneously in the psoriatic lesions, supporting this discovery (47). Further investigation is required to understand the disparity in MIF levels between the psoriatic epidermis and the circulation. The -173 GC genotype and the 6C haplotype of MIF polymorphisms are linked to an increased risk of plaque psoriasis in the Mexican population (48). Patients with psoriasis showed significantly lower frequencies of genotypes -794*CATT 5/7 and 7/7, while the CATT*5/MIF-173*C haplotype was more common (49).
3.3 Treatments for psoriasis involving macrophages
Shikonin is an organic matter extracted from the roots of Lithospermum erythrorhizon. Combining Shikonin with methotrexate has been demonstrated to hinder the advancement of psoriasis by controlling the polarization of macrophages. Administration of Shikonin and methotrexate in an imiquimod (IMQ)-induced psoriasis mice model can reduce the expression of F4/80 positive cells and decrease the mRNA levels of M1 macrophage markers (50). The PSORI-CM02 formula, a novel Chinese medicine, has been proven to have an anti-psoriatic effect. It can decrease macrophage infiltration, diminish M1 but increase M2 markers in IMQ-induced psoriasis mice (51). Etanercept, the first anti-TNF inhibitor, blocks the JAK/STAT3 pathway, decreasing the ratio of Th17/Treg and promoting M2 polarization, ultimately relieving psoriasis in mice (52). Application of Mung bean-derived nanoparticles topically can facilitate maintaining the balance of polarized macrophages and inhibit the activation of the NF-κB signaling pathway, leading to a reduction in skin inflammation (53). The pathogenetic roles of the macrophages in psoriasis and treatments involving the M1 phenotype are summarized in Figure 2.

Figure 2. The pathogenetic roles of macrophages in psoriasis and treatments involving M1 phenotype. Macrophages co-express with two important psoriasis autoantigens LL-37 and ADAMTSL5. Macrophages triggered by IL-23 produce significant quantities of IL-7A, IL-22 and IFN-γ. Chitotriosidase secreted by activated macrophages is related to psoriasis-related comorbidities. Estrogen suppresses the production of IL-1β, furthermore reducing the level of IL-17A. Keratinocytes interact with macrophages via HMGB1, and exosomes derived from VDR-deficient keratinocytes polarize macrophages toward M1 phenotype, exaggerating the inflammation condition. Shikonin combined with methotrexate, PSORI-CM02 formula, and Mung bean-derived nanoparticles exhibit anti-psoriatic properties by hindering M1 polarization. ADAMTSL5, ADAMTS-Like Protein 5; CD, cluster of differentiation; CCL, C-C Motif Chemokine Ligand; CXCL, C-X-C Motif Chemokine Ligand; GM-CSF, granulocyte-macrophage colony-stimulating factor; HMGB1, High Mobility Group Box-1; IFN, interferon; IL, interleukin; LL-37, Cathelicidin; LPS, lipopolysaccharide; MHC, major histocompatibility complex; TNF, tumor necrosis factor; VDR, vitamin D receptor.
4 Systemic sclerosis
Systemic sclerosis (SSc) is a paradigmatic rheumatic disease characterized by immune dysfunction-driven inflammation affecting multiple organs, finally leads to fibrosis. Skin involvement is among the most prominent manifestations of SSc. Raynaud’s phenomenon is the most prevalent skin lesion observed in SSc patients. Other skin lesions of SSc encompass puffy fingers, skin thickening and induration, digital ulcers, and hyperpigmentation. The exact cause of SSc is not well understood yet.
4.1 The characteristic of macrophage in SSc
In the skin of patients with SSc, there is a notable increase in the quantity of CD163+ cells located among collagen fibers when compared to the skin of healthy individuals (54). Macrophage signatures were found to be upregulated in early SSc patients compared to healthy controls. M2 and M1 macrophage signatures were present in 96% and 94% of patients, respectively. Furthermore, M2 and M1 signatures were associated with a higher extent of skin involvement, but also skin thickness progression rate prior to biopsy, an independent predictor of mortality (55). Dual phenotypic macrophages were recently identified in SSc disease. SSc patients exhibited elevated proportions of peripheral cells displaying M1, M2, and a combination of M1/M2 phenotypes in comparison to the control group (56). The transcriptome profile of macrophages in SSc shows increased activity in glycolysis, hypoxia, and mTOR signaling, while exhibiting decreased activity in IFN-γ response pathways (57). Single-cell transcriptome data have revealed three specific myeloid cell clusters in diffuse cutaneous SSc, including one macrophage cluster. This cluster expresses Fcγ receptor IIIA at high level, indicating a transition from normal CCR1+ and MARCO+ macrophages (58).
4.2 The pathogenic roles of the macrophages in SSc
Macrophages in SSc exhibit a profibrotic activation profile, meanwhile emit signaling molecules and have surface indicators linked to both M1 and M2 macrophage activation (59). M1 macrophage is associated with the beginning of fibrosis and accelerates its advancement in SSc. Research has shown that LPS-induced M1 macrophage pyroptosis contributes to fibrosis in SSc via the Cathepsin B/NLRP3/GSDMD pathway (60). In addition, ferroptosis presents in the bleomycin (BLM)-induced SSc mice model, where the M1 macrophage upregulates the expression of the ferroptosis driver Acyl-CoA synthetase long chain family member 4 and enhances its susceptibility to ferroptosis (61). Besides M1 macrophage, periostin contributes to the inflammation and fibrosis of SSc by potentially influencing M2 macrophages. Periostin-stimulated macrophages from healthy controls showed a substantial decrease in the proportion of M2 macrophages compared to those from SSc patients. Periostin stimulation led to a considerable upregulation of pro-fibrotic cytokines, chemokines, and extracellular matrix proteins in macrophages at the mRNA level (62).
Macrophages and fibroblasts contribute to the development of SSc by reciprocally activating each other. Macrophages show enhanced secretion of proinflammatory cytokines when stimulated with exosomes generated from fibroblasts of SSc patients. Collagen and fibronectin synthesis is greatly activated in fibroblasts when receiving signals from SSc exosome-stimulated macrophages (63). Co-culture investigations in Transwell experiments also demonstrated that SSc macrophages induce fibroblast activation (59). A self-assembled skin equivalent system was created to investigate the communication between macrophages and fibroblasts in SSc. The outcome provides more evidence supporting the mutual activation that relies partially on TGF-β (64). Depleting B cells has been suggested as a novel strategy for treating SSc, given that B cells can inhibit the differentiation of profibrotic macrophages. The extent of profibrotic macrophage activation induced by B cells is correlated with the fibrosis severity (65).
SSc-interstitial lung disease (ILD) is a complication associated with high morbidity and mortality. Immunohistochemistry analysis showed an accumulation of CD68+ and mannose-R+ macrophages in the lungs of SSc patients. Furthermore, single-cell RNA sequencing investigation of tissue-resident CD14+ pulmonary macrophages in SSc-ILD patients has shown an active profibrotic signature and increased Fibronectin 1 expression (66). Elevated levels of mixed M1/M2 macrophages in the circulation are linked to SSc-ILD, systolic pulmonary artery pressure, and the presence of anti-topoisomerase antibodies, which are established predictors of lung involvement in SSc (67). The upregulation of CCL18 and CD163 in the lungs of patients with SSc-ILD strongly implicates the pathogenetic roles of activated macrophages in this complication. Levels of CCL18 and CD163 are positively correlated with FibMax, an indicator for accessing lung fibrosis progression (68).
Levels of Serum MIF were considerably higher in both limited and diffuse SSc groups compared to healthy controls (69, 70). Microvascular endothelial cells and fibroblasts showed increased production of MIF when exposed to SSc serum, indicating the cellular source of MIF (70). MIF has the potential to serve as biomarkers and prognostic variables for pulmonary arterial hypertension (PAH) secondary to SSc. Patients with PAH related to SSc had elevated levels of MIF in their circulation compared to SSc patients without PAH. Patients with a higher New York Heart Association class exhibited higher levels of MIF (71). The MIF 7C haplotype is linked to an increased risk of SSc in the southern Mexican population and is correlated with increased MIF mRNA levels. MIF is associated with a proinflammatory response in SSc, as it correlates positively with the Th1 and Th17 cytokine profile (72). Except for MIF, Citrullinated vimentin, a biomarker of macrophage activation, was elevated in early diffuse-SSc compared to late diffuse-SSc (73). The characteristic and pathogenetic roles of the macrophages in SSc are summarized in Figure 3.

Figure 3. The characteristic and pathogenetic roles of the macrophages in SSc. Macrophages and fibroblasts mutually activate each other and contribute to the pathology in SSc. B cells promote the differentiation of profibrotic macrophages, and is indispensable for the progression of SSc. Periostin induces higher ratio of M2 macrophage and upregulates the mRNA level of pro-fibrotic cytokines, chemokines, and ECM proteins. M1 macrophage facilitates fibrosis by pyroptosis and ferroptosis. CD14+ tissue resident pulmonary macrophages in SSc-ILD patients’ lungs show an active profibrotic signature. Elevated levels of mixed M1/M2 phenotype macrophages are observed in the circulation of SSc-ILD patients. ACSL4, Acyl-CoA synthetase long chain family member4; CD, cluster of differentiation; ECM, extracellular matrix; GSDMD, Gasdermin D; LPS, lipopolysaccharide; NLRP3, NOD-like receptor thermal protein domain associated protein 3; SSc-ILD, systemic sclerosis-interstitial lung disease.
4.3 Treatments for SSc involving macrophages
Imatinib is a tyrosine kinase inhibitor typically used in the treatment of chronic myeloid leukemia. Notably, imatinib-loaded gold nanoparticles have demonstrated great efficacy in reducing IL-8 secretion, cell viability, and M2 polarization in alveolar macrophages (74). Nintedanib, another tyrosine kinase inhibitor, has shown promising antifibrotic effects in a SSc animal model. The underlying mechanism is associated with impaired M2 polarization of monocytes and reduced numbers of M2 macrophages (75). As for pulmonary fibrosis, an intractable problem in SSc patients, Zhang et al. proposed methyl-CpG-binding domain 2 (MBD2) as a novel therapeutic target. Depletion of MBD2 has been shown to prevent pulmonary fibrosis in a BLM-treated mouse model and to reduce the infiltration of M2 macrophage in the lungs of BLM-treated mice. MBD2 suppresses the SHIP expression and enhances PI3K/Akt signaling, thereby promoting the macrophage M2 phenotype (76). Ruxolitinib, a JAK inhibitor, exhibited anti-fibrosis properties in a BLM-SSc mouse model. In vitro experiments have revealed that ruxolitinib enhances macrophage efferocytosis when exposed to IFN, and reduced TGF-β- activated marker in fibroblasts derived from SSc-related pulmonary fibrosis tissues (77).
5 Systemic lupus erythematosus
Systemic lupus erythematosus (SLE) is a prototypical autoimmune disease characterized by complex pathophysiology and genetic susceptibility. The disease is defined by the involvement of multiple systems and organs, recurring flare-ups and remissions, and the emergence of various autoantibodies in the body. Untreated SLE can lead to permanent harm to organs and finally lead to death. Skin lesions are frequently observed in the majority of SLE patients. Nearly half of SLE presents with acute cutaneous lupus erythematosus, characterized by a butterfly-shaped rash over the cheeks and nose. Additionally, SLE patients may exhibit subacute and chronic cutaneous lupus erythematosus. Photosensitivity, alopecia, and oral mucosal ulcers are also frequently observed in SLE patients.
5.1 The pathogenic roles of the macrophages in SLE
Some scientists have suggested that M1 and M2 macrophages have distinct functions in the development of SLE. M1 macrophages exacerbate SLE, whereas M2 macrophages seem to alleviate its effects (78). The involvement of M2 macrophages in SLE is still a topic of debate. Other researchers observed a rise in the presence of CD163+ M2 macrophages in SLE skin and elevated soluble (s)CD163 levels in SLE patient blood specimens. Increased systemic and local CD163 expression indicates that M2 macrophages may contribute to the development of SLE as well (79). Furthermore, M2 macrophages have been suggested to play a role in the development of lupus nephritis. Urine sCD163 is highly associated with the current activity index of renal pathology and several particular pathological characteristics. M2 macrophages are a significant source of increased urine sCD163 levels, indicating its potential for predicting renal pathology (80).
Macrophages release ROS and inflammatory cytokines, which aggravate the inflammatory condition and tissue damage in SLE. Anti- dsDNA antibodies are crucial in the advancement of SLE. Anti-dsDNA antibodies can trigger NLRP3 inflammasome activation by binding to TLR-4 on macrophages, resulting in elevated mitochondrial ROS generation (81). Myeloid-derived suppressor cells may aggravate the IMQ-induced lupus model by enhancing TLR-7 pathway activation in macrophages. Mechanically, Myeloid-derived suppressor cells derived S100 Calcium Binding Protein A 8/9 increased IFN-γ secretion by macrophages, which then stimulated TLR-7 pathway activation in an autocrine manner (82). Activated lymphocyte-derived DNA induces macrophages to polarize toward M2b. M2b macrophages are distinguished by their production of inflammatory cytokines and their role in promoting inflammation condition, which is crucial in the progression of SLE (83). Activated lymphocyte-derived DNA-stimulated macrophages exhibit heightened glycolysis, reduced pentose phosphate pathway activity, and increased glycogenesis in glucose metabolism. The reduced pentose phosphate pathway activity ultimately resulted in increased levels of ROS (84).
Macrophages also play a role in the development of SLE by efferocytosis. Efferocytosis is the phagocytic elimination of apoptotic cells, and individuals with SLE show impairments in this process (85). The diminished efferocytosis is not an inherent defect but rather dependent on serum, linked to lower levels of C1q, C4, and C3 (86). Genes related to inflammation, autophagy, and signaling are upregulated in macrophages engulfing apoptotic cells from SLE patients (87). Efferocytosis capability differs between male and female mice. Female mice had a more pronounced impairment in macrophage efferocytosis compared to male mice, which could be reversed by administering male microbiota (85). SLE patients have been shown to exhibit elevated levels of urokinase-type plasminogen activator receptor expression. TLR-7 controls urokinase-type plasminogen activator receptor expression through ERK/c-JNK signaling and hinders macrophage efferocytosis (88). Tyro3 is a receptor that plays a role in identifying apoptotic cells in the process of efferocytosis. Autoantibodies targeting Tyro3 have been linked to increased disease activity in SLE and can hinder the ability of macrophage efferocytosis (89). Efferocytosis activity can be restored by co-culturing with human umbilical cord-derived mesenchymal stem cells. This reversal effect has been observed in vitro experiments and in SLE patients who underwent umbilical cord-derived mesenchymal stem cells transplantation (90). Bone marrow-derived mesenchymal stem cells release exosomes including miR-16 and miR-21, subsequently stimulate the anti-inflammatory transformation of macrophages. Furthermore, these macrophages exhibit enhanced efferocytosis ability and can be used to alleviate lupus nephritis (91).
5.2 Treatments for SLE involving macrophages
Azithromycin, a macrolide antibiotic, has emerged as a novel medication for SLE. In vitro experiments using macrophages that mimic the SLE phenotype have shown a reduction in M1 markers and an increase in M2 markers after azithromycin application, and this effect is dependent on Akt phosphorylation (92). Diffuse alveolar hemorrhage (DAH) is a potentially fatal complication of SLE. Serp-1, a rabbit myxomavirus-encoded serpin, has been shown to prevent the occurrence of SLE-associated DAH in a mouse model by modulating macrophage function. According to Zhuang et al., Serp-1 inhibits DAH by enhancing LXR-regulated M2 macrophage polarization and IL-10 production by KLH4 regulation (93). Additionally, PAM3, a TLR2/1 agonist, has shown promise in the treatment of SLE. It not only induces the differentiation of monocytes into an immunosuppressive M2 phenotype in vitro but also reduces disease severity in a lupus-prone mouse model (94).
6 Rosacea
Rosacea is a long-lasting inflammatory skin disorder identified by erythema and pustules. Macrophage infiltration is considered a frequently overlooked characteristic present in all kinds of rosacea (95). A large amount of CD68+ macrophages have been found to infiltrate the rosacea lesions (95, 96). Immune infiltration analysis also suggests that M1 macrophages play a significant role in rosacea (97).
6.1 The pathogenic roles of the macrophages in rosacea
Macrophages have been documented as participants in the deterioration mechanisms of rosacea. Guanylate Binding Protein 5 has been recognized as a crucial regulator of rosacea by promoting M1 macrophage polarization through the NF-κB signaling pathways (98). Elevated levels of the antimicrobial peptide LL-37 are commonly linked to the development of rosacea. LL-37 can enter macrophages’ cytoplasm via P2X7 receptor-mediated endocytosis and enhance NLRP3-mediated inflammasome activation in macrophages (99). ADAM-like metalloprotease Decysin-1 is considered to be associated with inflammation. Recent studies show that ADAM-like metalloprotease Decysin-1 may contribute to inflammation in rosacea by influencing the M1 polarization of macrophages (100).
6.2 Treatments for rosacea involving macrophages
Carvedilol, a nonselective beta-adrenoceptor antagonist, is an effective treatment for rosacea. In vitro studies have shown that carvedilol can reduce TLR-2 expression in macrophages, leading to decreased kallikrein related peptidase 5 secretion and LL-37 expression (101). Paeoniflorin, a monoterpenoid glycoside with various pharmacological activities, can alleviate rosacea-like inflammatory response by inducing suppressor of cytokine signaling 3 expression and suppressing the LPS-induced upregulation of TLR-2 and LL-37 via the ASK1-p38 cascade in macrophages (96). Artemisinin, the most effective antimalarial drug, decreases the presence of macrophages and immune cells in mice rosacea lesions, furthermore suppresses the production of chemokines associated with immune cells (102).
7 Bullous pemphigoid
Bullous pemphigoid (BP) is a deadly autoimmune dermatological disorder marked by initial red lesions and the subsequent formation of subepidermal blisters. The pathology of BP is linked to autoantibodies that target two hemidesmosomal proteins: BP180 and BP230.
7.1 The pathogenic roles of the macrophages in BP
There is a significant occurrence of CD163+ tissue-associated macrophages in BP. The increased levels of sCD163 in the serum of patients with BP compared to healthy individuals confirmed the activation of CD163+ tissue-associated macrophages. Chen et al. demonstrated that mice with macrophage deficiency were resistant to blister formation induced by pathogenic antibodies. In contrast, mice lacking T cells or B cells did not exhibit this resistance, indicating that macrophages, rather than T and B lymphocytes, play a pivotal role in the development of subepidermal blisters in experimental BP. Macrophages can facilitate the infiltration of neutrophils, a key step of experimental BP formation, and this mechanism relies on the activation or degranulation of mast cells (103). BP M2 macrophages showed a notable increase in both mRNA expression and production of CCL18 when exposed to IL-4 or IL-13 (104). Nuclear receptor related 1 belongs to the orphan nuclear receptor family and can regulate inflammation in both directions. Nuclear receptor related 1 is highly expressed in a specific group of cutaneous macrophages in patients with BP. This particular subgroup of macrophages in skin lesions is distinguished by elevated TNF levels and reduced expression of the anti-inflammatory marker CD163L1 (105).
7.2 Treatments for BP involving macrophages
Minocycline, a conventional medication for BP, has been shown to reduce the production of Th2 chemokines by M2 macrophages, thereby preventing the recruitment of Th2 cells and eosinophils to lesional skin in BP. While both CCL18 and CCL22 are Th2 chemokines implicated in BP, minocycline selectively suppresses the production of CCL18. The precise mechanism behind this selective effect remains to be elucidated (106). Dipeptidyl peptidase-4 inhibitors are associated with a higher incidence of BP. However, the concurrent use of lisinopril, a medication used to treat hypertension and heart failure, may counteract this risk. Lisinopril is capable of inhibiting the upregulation of matrix metalloproteinase and angiotensin-converting enzyme-2 in macrophages, thus exerting a mitigating effect on dipeptidyl peptidase-4 inhibitor-induced BP (107). T-cell immunoglobulin and mucin domain-3 is a well-recognized immune checkpoint molecule. Elevated levels of T-cell immunoglobulin and mucin domain 3 in macrophages within the affected skin of BP patients suggest its potential as a target for future immunotherapeutic interventions (108).
8 Melanoma
Melanomas are malignant tumors originating from melanocytes that can appear on any part of the body. Tumor-associated macrophages (TAMs) and other innate immune cells are crucial in chronic inflammatory processes that support tumor growth and advancement. M1 macrophages have immunostimulatory, anti-tumorigenic, and anti-angiogenic properties, while M2 macrophages support tumor growth and angiogenesis.
8.1 The characteristic of macrophage in melanoma
Studies have shown that invasive melanomas have a greater quantity of CD68+ and CD163+ TAMs in comparison to benign nevi (109). TAMs in melanoma are a diverse and constantly changing group, with a subset of unpolarized CD68+/CD163–/iNOS– macrophages consistently existing (110). Different stages of melanomas display distinct macrophage constituents. During the initial phase of malignant melanoma, the number of M1 intratumoral macrophages is lower than that of the M2 population. As the disease advanced, M1 macrophage recruitment was quickly and increasingly surpassed by an upsurge in M2 TAMs (111).Macrophages’ function differs based on their location. Stromal macrophages have a unique transcriptional profile compared to those found in tumor nests, as they are reprogrammed to take on DC activity (112). The quantity and composition of macrophages are associated with the outcome of melanoma. High numbers of CD68+ macrophages inside tumor cell nests are linked to recurrence, while a low proportion of CD163+ macrophages in the tumor stroma is related to recurrence and, in initial melanomas, also with poor overall survival (109). The state of macrophage polarization is linked to the level of lymphocytic infiltration in melanoma, which also impacts the prognosis (110).
8.2 The pathogenic roles of the macrophages in melanoma
Increasing evidence has revealed that macrophages are implicated in melanoma migration. CD163+ macrophages found within the tumors are associated with the development of metastases (113). Angiogenesis is a crucial step in the preparation of lymph nodes for melanoma metastasis. Exosomes from melanoma cells stimulate the generation of granulocyte-macrophage colony stimulating factor in pre-metastatic lymph nodes. Granulocyte-macrophage colony stimulating factor could activate hypoxia-inducible factor (HIF)-1α in M1 macrophages and HIF-2α in M2 macrophages. HIF-1α stimulates new blood vessel formation, whereas HIF-2α contributes to the structural normalization of newly formed blood vessels (114). TAMs promoted endothelial cell movement, tube creation, and tumor development through TAM-derived adrenomedullin. Adrenomedullin possess endocrine and paracrine activities simultaneously. The paracrine effect is mediated by the endothelial NOS signaling pathway, while the autocrine effect induces macrophages to polarize toward the M2 phenotype (115).
Tumor cells and TAMs interactions are crucial for initiating tumor cell motility. TAMs can transmit cytoplasmic modules to tumor cells, enhancing tumor cell motility and dissemination (116). Another hypothesis for metastasis mentions the fusion of macrophages with tumor cells (MTFs). After being injected subcutaneously into nude mice, cultivated MTFs spread and formed metastatic tumors at remote locations. The cultivated MTFs consistently displayed pan-macrophage markers, M2 polarization markers, and melanocyte-specific markers (117). HMGB1 has a significant role in the growth and spread of murine melanoma. HMGB1 is secreted by melanoma tumor cells as a consequence of hypoxia, and could increase M2-like TAMs accumulation and an create an IL-10-rich TME (118). CD34- melanoma-initiating cells rely on M2 macrophages for their survival and growth. This discovery provides additional confirmation that macrophages play a role in the distant spread of melanoma (119).
8.3 Treatments for melanoma involving macrophages
Transitioning the polarization state of TAMs from the tumor-favoring M2 phenotype to the anti-tumor M1 phenotype is a promising strategy in oncotherapy. Chemotherapy occupies an important component position in combination treatments of melanoma. Doxorubicin-loaded polysaccharide hydrogels have demonstrated effective polarization of TAMs toward the M1 phenotype (120).
In addition to traditional chemotherapy drugs, researchers are now exploring new methods by regulating macrophage polarization to treat melanoma. TLR-7/8 agonists, such as resiquimod (RES) and telratolimod, can induce the polarization of macrophages toward the M1 phenotype. Bexarotene (BEX), a highly affinity selective retinoid X receptor, can reduce M2 polarization. A dual macrophage polarizer was created by mixing BEX with RES to enhance the M1 phenotype while inhibit the M2 phenotype. This combination exhibited incomparable inhibitory effects on B16F10 cells (121). Tumor-associated adipocyte exhibits a transformed pro-tumorigenic characteristic which can attract monocytes and stimulate their transformation into the M2 phenotype. Telratolimod is encapsulated within the lipid droplets of adipocytes and is intended to be discharged at the tumor site. Injecting drug-loaded adipocytes boosted tumor-inhibiting M1 macrophages in primary and distant tumors, halting tumor growth in a melanoma model (122). These innovative treatments have demonstrated anti-tumor effects in animal and cell models, but they have not yet been implemented in clinical practice.
9 Cutaneous T-cell lymphoma
Cutaneous T-cell lymphoma (CTCL) is a rare kind of lymphoma originating in the skin, and consists of a collection of subtypes with different clinical manifestations, histological features, and prognosis. Mycosis fungoides (MF) and Sézary syndrome (SS) are the two main types of CTCL (123). While CTCL may progress slowly in its initial stages, it can result in considerable morbidity and mortality as it proceeds (124).
9.1 The characteristic of macrophage in CTCL
A prominent subtype of M2 TAM expressing PD-1 has been found in CTCL TME, and playing an immunosuppressive role. Lenalidomide is an immunomodulatory drug typically used in treating hematological malignancies. Anti–PD-L1 combined with lenalidomide induces functional changes in TAMs, thereby enhancing phagocytic activity and impairing migration of M2-like TAMs and augmenting T cell proliferation to improve antitumor immunity. Combining anti-PD-L1 and lenalidomide treatment induces a functional transition from a PD-1+ M2 phenotype toward a proinflammatory M1 phenotype in vitro. Meanwhile, this transformation enhances phagocytic activity by blocking NF-κB and JAK/STAT (125).
The polarization state of macrophages in CTCL TME is not static. Granulomatous MF shows a transition of macrophage polarization from M1 in the initial phases to M2 in the later stages (126). The quantity of macrophages varies depending on the tumor stage, with a notably greater amount of CD68+ macrophages in the tumor-stage compared to early-stage folliculotropic MF (127).
Granulomatous slack skin is a very uncommon type of CTCL distinguished by a high quantity of macrophages. Macrophages in granulomatous slack skin are divided into three distinct subpopulations with unique transcript characteristics (128):
● The CD163+/CD206+ cluster displays a TAM M2-like phenotype and expresses markers involved in T-cell interaction and tumor progression.
● The apolipoprotein C1+/APOE+ cluster has a non-M1 or -M2 phenotype and may be associated with lipid metabolism.
● The CD11c+/lysozyme+ cluster demonstrates an M1-like phenotype and expresses matrix metalloproteinase-9 strongly.
9.2 The pathogenic roles of the macrophages in CTCL
The interaction between malignant T cells and macrophages is extensively studied in CTCL TME. A subtyping system has been created using the genetic characteristics of malignant T cells and the surrounding TME that promotes tumor growth (129). The interaction between malignant CTCL cells and CCL13+ macrophages has been demonstrated to promote tumor growth by increasing S100 Calcium Binding Protein A9 levels and activating NF-κB (130). Similar intercellular communications have been observed in the transformed CTCL tumor ecosystem. Malignant T cells that express MIF interact with macrophages, and B cells that express CD74 are also involved in this interaction (131).
Macrophage enrichment has a role in creating an immunosuppressive TME. Elimination of M2-like TAMs using liposomes containing clodronate (the first-generation bisphosphonate treating osteoporosis) has been demonstrated to postpone the progression of CTCL (132). Furthermore, the expressions of vascular markers also decrease by macrophage exhaustion, suggesting macrophages are implicated in both the advancement of CTCL and neoangiogenesis. CCR2 inhibitor, which hinders the movement of monocytes through CCR2, can lead to the reduction of macrophages. Mice treated with CCR2 inhibitor showed significantly reduced tumor sizes and weights compared to the control group, providing more evidence of the adverse impact of macrophages in CTCL (133).
Macrophages play a predictive role in the progression of CTCL, with the quantities of CD163+ cells in affected skin and serum sCD163 levels correlating with disease advancement (134). Another study suggests that the CD163/CD68 ratio should be used to evaluate TAMs instead of focusing on the total TAM count. A high ratio of CD163/CD68 in tumor stage MF and SS suggests M2 polarization of TAMs, which is associated with tumor advancement. Serum levels of sCD163 and CCL22 can indicate M2 load and may serve as indicators for evaluating disease progression (135). However, there is still no consensus on the relationship between CD163+ cells with tumor progression. Some researchers suggest that the proportion of CD206+ cells, as opposed to CD163+ cells, increases in correlation with tumor advancement (136).
9.3 Treatments for CTCL involving macrophages
BEX has been authorized for the treatment of relapsed CTCL after at least one prior systemic therapy. BEX’s clinical benefits are partly attributable to its ability to decrease the synthesis of CCL22 by M2 TAMs (137). IFNs are efficacious in treating advanced-stage MF, potentially by influencing M2 TAMs as well. Mechanistically, IFN-α2a and IFN-γ reduce CCL17 and CCL18 expression and synthesis, while raising CXCL10 and CXCL11 levels in M2 macrophages (138).
10 Conclusion and prospect
Numerous immune cells get involved in the pathogenesis of inflammatory skin diseases and skin tumors. In this review, we aim to understand the pathogenesis from the perspective of macrophages. Due to their complex functions and dynamic polarization states, macrophages are extensively implicated in the occurrence of AD, psoriasis, SSc, SLE, rosacea, BP, melanoma and CTCL. The mechanism of macrophages in these conditions is multifaceted, including intercellular interactions (macrophages and B cells, T cells, keratinocytes, basophils and fibroblasts), cell death (ferroptosis and pyroptosis), and cell functions (autophagy and efferocytosis). Additionally, multiple signaling pathways and molecules, such as exosomes, ILs, CCLs, CXCLs, are also involved.
In the future, we anticipate that more macrophage-related indicators can be developed to assess the disease severity, prognosis and complication occurrence and to guide more precise treatment. Furthermore, targeting the number and polarization state of the macrophages holds promise for the exploration of new therapeutic approaches. For example, M2 macrophages are considered to play immunosuppressive roles in the TME. Research may focus on depleting M2 macrophages or converting them to an anti-tumoral M1 phenotype within the TME with safe medications. The investigation of macrophages in inflammatory skin diseases and skin tumors remains a vibrant research area and we are confident that patients will benefit from these advancements in the future.
Author contributions
S-HL: Writing – original draft, Writing – review & editing. JZ: Writing – review & editing. Y-GZ: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by grants from Beijing Natural Science Foundation, grant numbers 7232118; National High Level Hospital Clinical Research Funding, grant number 2022-PUMCH-B-092, and National Key Clinical Specialty Project of China.
Acknowledgments
We sincerely thank the supports from the Beijing Natural Science Foundation, National High Level Hospital Clinical Research Funding and National Key Clinical Specialty Project of China.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. (2005) 5:953–64. doi: 10.1038/nri1733
2. Atri C, Guerfali FZ, Laouini D. Role of human macrophage polarization in inflammation during infectious diseases. Int J Mol Sci. (2018) 19:1801. doi: 10.3390/ijms19061801
3. Kloc M, Ghobrial RM, Wosik J, Lewicka A, Lewicki S, Kubiak JZ. Macrophage functions in wound healing. J Tissue Eng regenerative Med. (2019) 13:99–109. doi: 10.1002/term.2772
4. Shapouri-Moghaddam A, Mohammadian S, Vazini H, Taghadosi M, Esmaeili SA, Mardani F, et al. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol. (2018) 233:6425–40. doi: 10.1002/jcp.v233.9
5. Netea MG, Balkwill F, Chonchol M, Cominelli F, Donath MY, Giamarellos-Bourboulis EJ, et al. A guiding map for inflammation. Nat Immunol. (2017) 18:826–31. doi: 10.1038/ni.3790
6. Chen S, Saeed A, Liu Q, Jiang Q, Xu H, Xiao GG, et al. Macrophages in immunoregulation and therapeutics. Signal transduct targeted Ther. (2023) 8:207. doi: 10.1038/s41392-023-01452-1
8. Nattkemper LA, Tey HL, Valdes-Rodriguez R, Lee H, Mollanazar NK, Albornoz C, et al. The genetics of chronic itch: gene expression in the skin of patients with atopic dermatitis and psoriasis with severe itch. J Invest Dermatol. (2018) 138:1311–7. doi: 10.1016/j.jid.2017.12.029
9. Lee SB, Park H, Lee JE, Kim KS, Jeon YH. In vivo optical reporter-gene-based imaging of macrophage infiltration of DNCB-induced atopic dermatitis. Int J Mol Sci. (2020) 21:6205. doi: 10.3390/ijms21176205
10. Zhang B, Roesner LM, Traidl S, Koeken V, Xu CJ, Werfel T, et al. Single-cell profiles reveal distinctive immune response in atopic dermatitis in contrast to psoriasis. Allergy. (2023) 78:439–53. doi: 10.1111/all.15486
11. Niebuhr M, Lutat C, Sigel S, Werfel T. Impaired TLR-2 expression and TLR-2-mediated cytokine secretion in macrophages from patients with atopic dermatitis. Allergy. (2009) 64:1580–7. doi: 10.1111/j.1398-9995.2009.02050.x
12. Kasraie S, Niebuhr M, Kopfnagel V, Dittrich-Breiholz O, Kracht M, Werfel T. Macrophages from patients with atopic dermatitis show a reduced CXCL10 expression in response to staphylococcal α-toxin. Allergy. (2012) 67:41–9. doi: 10.1111/j.1398-9995.2011.02710.x
13. Mitamura Y, Reiger M, Kim J, Xiao Y, Zhakparov D, Tan G, et al. Spatial transcriptomics combined with single-cell RNA-sequencing unravels the complex inflammatory cell network in atopic dermatitis. Allergy. (2023) 78:2215–31. doi: 10.1111/all.15781
14. Tokumasu R, Yamaga K, Yamazaki Y, Murota H, Suzuki K, Tamura A, et al. Dose-dependent role of claudin-1 in vivo in orchestrating features of atopic dermatitis. Proc Natl Acad Sci United States America. (2016) 113:E4061–8. doi: 10.1073/pnas.1525474113
15. Kwak EJ, Hong JY, Kim MN, Kim SY, Kim SH, Park CO, et al. Chitinase 3-like 1 drives allergic skin inflammation via Th2 immunity and M2 macrophage activation. Clin Exp Allergy. (2019) 49:1464–74. doi: 10.1111/cea.v49.11
16. Lee SG, Kim SE, Jeong IH, Lee SE. Mechanism underlying pruritus in recessive dystrophic epidermolysis bullosa: Role of interleukin-31 from mast cells and macrophages. J Eur Acad Dermatol Venereol: JEADV. (2023) 38:895–903. doi: 10.1111/jdv.19738
17. Hashimoto T, Yokozeki H, Karasuyama H, Satoh T. IL-31-generating network in atopic dermatitis comprising macrophages, basophils, thymic stromal lymphopoietin, and periostin. J Allergy Clin Immunol. (2023) 151:737–46.e6. doi: 10.1016/j.jaci.2022.11.009
18. Zhu Y, Liu Y, Ma Y, Chen L, Huang H, Huang S, et al. Macrophage autophagy deficiency-induced CEBPB accumulation alleviates atopic dermatitis via impairing M2 polarization. Cell Rep. (2023) 42:113430. doi: 10.1016/j.celrep.2023.113430
19. Shi VY, Bao L, Chan LS. Inflammation-driven dermal lymphangiogenesis in atopic dermatitis is associated with CD11b+ macrophage recruitment and VEGF-C up-regulation in the IL-4-transgenic mouse model. Microcirculation. (2012) 19:567–79. doi: 10.1111/j.1549-8719.2012.00189.x
20. Yasuda C, Enomoto A, Ishiwatari S, Mori N, Kagoyama K, Matsunaga K, et al. Macrophage migration inhibitory factor (MIF) in the stratum corneum: a marker of the local severity of atopic dermatitis. Exp Dermatol. (2014) 23:764–6. doi: 10.1111/exd.2014.23.issue-10
21. Ma L, Xue HB, Guan XH, Qi RQ, Liu YB. Macrophage migration inhibitory factor promoter 173G/C polymorphism is associated with atopic dermatitis risk. Int J Dermatol. (2014) 53:e75–7. doi: 10.1111/j.1365-4632.2012.05597.x
22. Kim JS, Choi J, Hahn HJ, Lee YB, Yu DS, Kim JW. Association of macrophage migration inhibitory factor polymorphisms with total plasma igE levels in patients with atopic dermatitis in Korea. PloS One. (2016) 11:e0162477. doi: 10.1371/journal.pone.0162477
23. Zeng L, Liu Y, Xing C, Huang Y, Sun X, Sun G. Saponin from Periploca forrestii Schltr Mitigates Oxazolone-Induced Atopic Dermatitis via Modulating Macrophage Activation. Mediators inflamm. (2020) 2020:4346367. doi: 10.1155/2020/4346367
24. Wu W, Song L, Wang H, Feng L, Li Z, Li Y, et al. Supercritical CO(2) fluid extract from Stellariae Radix ameliorates 2,4-dinitrochlorobenzene-induced atopic dermatitis by inhibit M1 macrophages polarization via AMPK activation. Environ toxicol. (2024) 39:3188–97. doi: 10.1002/tox.24145
25. Liu Y, Zienkiewicz J, Qiao H, Gibson-Corley KN, Boyd KL, Veach RA, et al. Genomic control of inflammation in experimental atopic dermatitis. Sci Rep. (2022) 12:18891. doi: 10.1038/s41598-022-23042-x
26. Karuppagounder V, Arumugam S, Thandavarayan RA, Sreedhar R, Giridharan VV, Pitchaimani V, et al. Naringenin ameliorates skin inflammation and accelerates phenotypic reprogramming from M1 to M2 macrophage polarization in atopic dermatitis NC/Nga mouse model. Exp Dermatol. (2016) 25:404–7. doi: 10.1111/exd.2016.25.issue-5
27. Huang Y, Zhao C, Zheng G, Yuan Y, Gong L, Liu R, et al. Dictamnine ameliorates DNFB-induced atopic dermatitis like skin lesions in mice by inhibiting M1 macrophage polarization and promoting autophagy. Biol Pharm bullet. (2024) 47:175–86. doi: 10.1248/bpb.b23-00436
28. Kabashima K, Matsumura T, Komazaki H, Kawashima M. Nemolizumab-JP01 study group. Trial of nemolizumab and topical agents for atopic dermatitis with pruritus. New Engl J Med. (2020) 383:141–50. doi: 10.1056/NEJMoa1917006
29. Silverberg JI, Wollenberg A, Reich A, Thaçi D, Legat FJ, Papp KA, et al. Nemolizumab with concomitant topical therapy in adolescents and adults with moderate-to-severe atopic dermatitis (ARCADIA 1 and ARCADIA 2): results from two replicate, double-blind, randomised controlled phase 3 trials. Lancet (London England). (2024) 404:445–60. doi: 10.1016/S0140-6736(24)01203-0
30. Kawai R, Ichimasu N, Katagiri K. IL-4 and IL-13 are not involved in IL-31-induced itch-associated scratching behaviour in mice. Exp Dermatol. (2024) 33:e15115. doi: 10.1111/exd.15115
31. Silverberg JI, Hong HC, Thyssen JP, Calimlim BM, Joshi A, Teixeira HD, et al. Comparative efficacy of targeted systemic therapies for moderate to severe atopic dermatitis without topical corticosteroids: systematic review and network meta-analysis. Dermatol Ther. (2022) 12:1181–96. doi: 10.1007/s13555-022-00721-1
32. Okamoto M, Omori-Miyake M, Kuwahara M, Okabe M, Eguchi M, Yamashita M. The inhibition of glycolysis in T cells by a jak inhibitor ameliorates the pathogenesis of allergic contact dermatitis in mice. J Invest Dermatol. (2023) 143:1973–1982.e5. doi: 10.1016/j.jid.2023.03.1667
33. Lu CH, Lai CY, Yeh DW, Liu YL, Su YW, Hsu LC, et al. Involvement of M1 macrophage polarization in endosomal toll-like receptors activated psoriatic inflammation. Mediators inflamm. (2018) 2018:3523642. doi: 10.1155/2018/3523642
34. Gong X, Wang W. Profiles of innate immune cell infiltration and related core genes in psoriasis. BioMed Res Int. (2021) 2021:6656622. doi: 10.1155/2021/6656622
35. Su W, Wei Y, Huang B, Ji J. Identification of hub genes and immune infiltration in psoriasis by bioinformatics method. Front Genet. (2021) 12:606065. doi: 10.3389/fgene.2021.606065
36. Lin SH, Chuang HY, Ho JC, Lee CH, Hsiao CC. Treatment with TNF-α inhibitor rectifies M1 macrophage polarization from blood CD14+ monocytes in patients with psoriasis independent of STAT1 and IRF-1 activation. J Dermatol science. (2018) 91:276–84. doi: 10.1016/j.jdermsci.2018.05.009
37. Nakamizo S, Dutertre CA, Khalilnezhad A, Zhang XM, Lim S, Lum J, et al. Single-cell analysis of human skin identifies CD14+ type 3 dendritic cells co-producing IL1B and IL23A in psoriasis. J Exp Med. (2021) 218:e20202345. doi: 10.1084/jem.20202345
38. Terhorst D, Chelbi R, Wohn C, Malosse C, Tamoutounour S, Jorquera A, et al. Dynamics and transcriptomics of skin dendritic cells and macrophages in an imiquimod-induced, biphasic mouse model of psoriasis. J Immunol. (2015) 195:4953–61. doi: 10.4049/jimmunol.1500551
39. Hou Y, Zhu L, Tian H, Sun HX, Wang R, Zhang L, et al. IL-23-induced macrophage polarization and its pathological roles in mice with imiquimod-induced psoriasis. Protein Cell. (2018) 9:1027–38. doi: 10.1007/s13238-018-0505-z
40. Chen WC, Wen CH, Wang M, Xiao ZD, Zhang ZZ, Wu CL, et al. IL-23/IL-17 immune axis mediates the imiquimod-induced psoriatic inflammation by activating ACT1/TRAF6/TAK1/NF-κB pathway in macrophages and keratinocytes. Kaohsiung J Med Sci. (2023) 39:789–800. doi: 10.1002/kjm2.12683
41. Fuentes-Duculan J, Bonifacio KM, Hawkes JE, Kunjravia N, Cueto I, Li X, et al. Autoantigens ADAMTSL5 and LL37 are significantly upregulated in active Psoriasis and localized with keratinocytes, dendritic cells and other leukocytes. Exp Dermatol. (2017) 26:1075–82. doi: 10.1111/exd.13378
42. Chen J, Fu Y, Xiong S. Keratinocyte derived HMGB1 aggravates psoriasis dermatitis via facilitating inflammatory polarization of macrophages and hyperproliferation of keratinocyte. Mol Immunol. (2023) 163:1–12. doi: 10.1016/j.molimm.2023.09.004
43. Sun W, Chen J, Li J, She X, Ma H, Wang S, et al. Vitamin D receptor-deficient keratinocytes-derived exosomal miR-4505 promotes the macrophage polarization towards the M1 phenotype. PeerJ. (2023) 11:e15798. doi: 10.7717/peerj.15798
44. Adachi A, Honda T, Egawa G, Kanameishi S, Takimoto R, Miyake T, et al. Estradiol suppresses psoriatic inflammation in mice by regulating neutrophil and macrophage functions. J Allergy Clin Immunol. (2022) 150:909–19.e8. doi: 10.1016/j.jaci.2022.03.028
45. İlanbey B, Elmas ÖF, Sözmen EY, Günay Ü, Demirbaş A, Atasoy M, et al. A novel marker of systemic inflammation in psoriasis and related comorbidities: chitotriosidase. Turkish J Med Sci. (2021) 51:2318–23. doi: 10.3906/sag-2101-137
46. Dai YJ, Li YY, Zeng HM, Liang XA, Xie ZJ, Zheng ZA, et al. Effect of pharmacological intervention on MIP-1α, MIP-1β and MCP-1 expression in patients with psoriasis vulgaris. Asian Pacific J Trop Med. (2014) 7:582–4. doi: 10.1016/S1995-7645(14)60098-5
47. Shimizu T, Nishihira J, Mizue Y, Nakamura H, Abe R, Watanabe H, et al. Histochemical analysis of macrophage migration inhibitory factor in psoriasis vulgaris. Histochem Cell Biol. (2002) 118:251–7. doi: 10.1007/s00418-002-0435-x
48. Hernández-Bello J, Rodríguez-Puente M, Gutiérrez-Cuevas J, García-Arellano S, Muñoz-Valle JF, Fafutis-Morris M, et al. Macrophage migration inhibitory factor gene polymorphisms (SNP -173 G>C and STR-794 CATT5-8) confer risk of plaque psoriasis: A case-control study. J Clin Lab anal. (2021) 35:e23999. doi: 10.1002/jcla.23999
49. Chhabra S, Banerjee N, Narang T, Sood S, Bishnoi A, Goel S, et al. Single-nucleotide polymorphism and haplotype analysis of macrophage migration inhibitory factor gene and its correlation with serum macrophage migration inhibitory factor levels in North Indian psoriatic patients with moderate disease severity: A cross-sectional study. Indian J dermatol venereol leprol. (2023) 89:247–53. doi: 10.25259/IJDVL_988_19
50. Tao T, Chen Y, Lai B, Wang J, Wang W, Xiao W, et al. Shikonin combined with methotrexate regulate macrophage polarization to treat psoriasis. Bioengineered. (2022) 13:11146–55. doi: 10.1080/21655979.2022.2062090
51. Li L, Zhang HY, Zhong XQ, Lu Y, Wei J, Li L, et al. PSORI-CM02 formula alleviates imiquimod-induced psoriasis via affecting macrophage infiltration and polarization. Life Sci. (2020) 243:117231. doi: 10.1016/j.lfs.2019.117231
52. Li X, Jiang M, Chen X, Sun W. Etanercept alleviates psoriasis by reducing the Th17/Treg ratio and promoting M2 polarization of macrophages. Immun Inflammation disease. (2022) 10:e734. doi: 10.1002/iid3.v10.12
53. Sun H, Zhao Y, Zhang P, Zhai S, Li W, Cui J. Transcutaneous delivery of mung bean-derived nanoparticles for amelioration of psoriasis-like skin inflammation. Nanoscale. (2022) 14:3040–8. doi: 10.1039/D1NR08229A
54. Higashi-Kuwata N, Jinnin M, Makino T, Fukushima S, Inoue Y, Muchemwa FC, et al. Characterization of monocyte/macrophage subsets in the skin and peripheral blood derived from patients with systemic sclerosis. Arthritis Res Ther. (2010) 12:R128. doi: 10.1186/ar3066
55. Skaug B, Khanna D, Swindell WR, Hinchcliff ME, Frech TM, Steen VD, et al. Global skin gene expression analysis of early diffuse cutaneous systemic sclerosis shows a prominent innate and adaptive inflammatory profile. Ann rheumatic diseases. (2020) 79:379–86. doi: 10.1136/annrheumdis-2019-215894
56. Mohamed ME, Gamal RM, El-Mokhtar MA, Hassan AT, Abozaid HSM, Ghandour AM, et al. Peripheral cells from patients with systemic sclerosis disease co-expressing M1 and M2 monocyte/macrophage surface markers: Relation to the degree of skin involvement. Hum Immunol. (2021) 82:634–9. doi: 10.1016/j.humimm.2021.03.009
57. Moreno-Moral A, Bagnati M, Koturan S, Ko JH, Fonseca C, Harmston N, et al. Changes in macrophage transcriptome associate with systemic sclerosis and mediate GSDMA contribution to disease risk. Ann rheumatic diseases. (2018) 77:596–601. doi: 10.1136/annrheumdis-2017-212454
58. Xue D, Tabib T, Morse C, Yang Y, Domsic RT, Khanna D, et al. Expansion of fcγ Receptor IIIa-positive macrophages, ficolin 1-positive monocyte-derived dendritic cells, and plasmacytoid dendritic cells associated with severe skin disease in systemic sclerosis. Arthritis Rheumatol (Hoboken NJ). (2022) 74:329–41. doi: 10.1002/art.41813
59. Bhandari R, Ball MS, Martyanov V, Popovich D, Schaafsma E, Han S, et al. Profibrotic activation of human macrophages in systemic sclerosis. Arthritis Rheumatol (Hoboken NJ). (2020) 72:1160–9. doi: 10.1002/art.41243
60. Liu C, Tang J, Liu S, Shen C, Zhou X, Lu J, et al. Cathepsin B/NLRP3/GSDMD axis-mediated macrophage pyroptosis induces inflammation and fibrosis in systemic sclerosis. J Dermatol science. (2022) 108:127–37. doi: 10.1016/j.jdermsci.2022.12.006
61. Cao D, Zheng J, Li Z, Yu Y, Chen Z, Wang Q. ACSL4 inhibition prevents macrophage ferroptosis and alleviates fibrosis in bleomycin-induced systemic sclerosis model. Arthritis Res Ther. (2023) 25:212. doi: 10.1186/s13075-023-03190-9
62. Suzuki M, Ototake Y, Akita A, Asami M, Ikeda N, Watanabe T, et al. Periostin-An inducer of pro-fibrotic phenotype in monocytes and monocyte-derived macrophages in systemic sclerosis. PloS One. (2023) 18:e0281881. doi: 10.1371/journal.pone.0281881
63. Bhandari R, Yang H, Kosarek NN, Smith AE, Garlick JA, Hinchcliff M, et al. Human dermal fibroblast-derived exosomes induce macrophage activation in systemic sclerosis. Rheumatology. (2023) 62:Si114–si24. doi: 10.1093/rheumatology/keac453
64. Huang M, Smith A, Watson M, Bhandari R, Baugh LM, Ivanovska I, et al. Self-assembled human skin equivalents model macrophage activation of cutaneous fibrogenesis in systemic sclerosis. Arthritis Rheumatol (Hoboken NJ). (2022) 74:1245–56. doi: 10.1002/art.42097
65. Numajiri H, Kuzumi A, Fukasawa T, Ebata S, Yoshizaki-Ogawa A, Asano Y, et al. B cell depletion inhibits fibrosis via suppression of profibrotic macrophage differentiation in a mouse model of systemic sclerosis. Arthritis Rheumatol (Hoboken NJ). (2021) 73:2086–95. doi: 10.1002/art.v73.11
66. Rudnik M, Hukara A, Kocherova I, Jordan S, Schniering J, Milleret V, et al. Elevated fibronectin levels in profibrotic CD14(+) monocytes and CD14(+) macrophages in systemic sclerosis. Front Immunol. (2021) 12:642891. doi: 10.3389/fimmu.2021.642891
67. Trombetta AC, Soldano S, Contini P, Tomatis V, Ruaro B, Paolino S, et al. A circulating cell population showing both M1 and M2 monocyte/macrophage surface markers characterizes systemic sclerosis patients with lung involvement. Respir Res. (2018) 19:186. doi: 10.1186/s12931-018-0891-z
68. Christmann RB, Sampaio-Barros P, Stifano G, Borges CL, de Carvalho CR, Kairalla R, et al. Association of Interferon- and transforming growth factor β-regulated genes and macrophage activation with systemic sclerosis-related progressive lung fibrosis. Arthritis Rheumatol (Hoboken NJ). (2014) 66:714–25. doi: 10.1002/art.38288
69. Vincent FB, Lin E, Sahhar J, Ngian GS, Kandane-Rathnayake R, Mende R, et al. Analysis of serum macrophage migration inhibitory factor and D-dopachrome tautomerase in systemic sclerosis. Clin Trans Immunol. (2018) 7:e1042. doi: 10.1002/cti2.2018.7.issue-12
70. Corallo C, Paulesu L, Cutolo M, Ietta F, Carotenuto C, Mannelli C, et al. Serum levels, tissue expression and cellular secretion of macrophage migration inhibitory factor in limited and diffuse systemic sclerosis. Clin Exp Rheumatol. (2015) 33:S98–105. doi: 10.1136/annrheumdis-2015-eular.2062
71. Stefanantoni K, Sciarra I, Vasile M, Badagliacca R, Poscia R, Pendolino M, et al. Elevated serum levels of macrophage migration inhibitory factor and stem cell growth factor β in patients with idiopathic and systemic sclerosis associated pulmonary arterial hypertension. Reumatismo. (2015) 66:270–6. doi: 10.4081/reumatismo.2014.774
72. Baños-Hernández CJ, Navarro-Zarza JE, Bucala R, Hernández-Bello J, Parra-Rojas I, Ramírez-Dueñas MG, et al. Macrophage migration inhibitory factor polymorphisms are a potential susceptibility marker in systemic sclerosis from southern Mexican population: association with MIF mRNA expression and cytokine profile. Clin Rheumatol. (2019) 38:1643–54. doi: 10.1007/s10067-019-04459-8
73. Siebuhr AS, Juhl P, Bay-Jensen AC, Karsdal MA, Franchimont N, Chavez JC. Citrullinated vimentin and biglycan protein fingerprints as candidate serological biomarkers for disease activity in systemic sclerosis: a pilot study. Biomarkers: Biochem Indic exposure response susceptibility to chemicals. (2019) 24:249–54. doi: 10.1080/1354750X.2018.1548032
74. Codullo V, Cova E, Pandolfi L, Breda S, Morosini M, Frangipane V, et al. Imatinib-loaded gold nanoparticles inhibit proliferation of fibroblasts and macrophages from systemic sclerosis patients and ameliorate experimental bleomycin-induced lung fibrosis. J Controlled release. (2019) 310:198–208. doi: 10.1016/j.jconrel.2019.08.015
75. Wang Y, Zhang L, Wu GR, Zhou Q, Yue H, Rao LZ, et al. MBD2 serves as a viable target against pulmonary fibrosis by inhibiting macrophage M2 program. Sci Adv. (2021) 7:eabb6075. doi: 10.1126/sciadv.abb6075
76. Bellamri N, Lelong M, Joannes A, Le Tallec E, Jouneau S, Vernhet L, et al. Effects of Ruxolitinib on fibrosis in preclinical models of systemic sclerosis. Int immunopharmacol. (2023) 116:109723. doi: 10.1016/j.intimp.2023.109723
77. Huang J, Maier C, Zhang Y, Soare A, Dees C, Beyer C, et al. Nintedanib inhibits macrophage activation and ameliorates vascular and fibrotic manifestations in the Fra2 mouse model of systemic sclerosis. Ann rheumatic diseases. (2017) 76:1941–8. doi: 10.1136/annrheumdis-2016-210823
78. Li F, Yang Y, Zhu X, Huang L, Xu J. Macrophage polarization modulates development of systemic lupus erythematosus. Cell Physiol Biochem. (2015) 37:1279–88. doi: 10.1159/000430251
79. Nakayama W, Jinnin M, Makino K, Kajihara I, Makino T, Fukushima S, et al. CD163 expression is increased in the involved skin and sera of patients with systemic lupus erythematosus. Eur J dermatol: EJD. (2012) 22:512–7. doi: 10.1684/ejd.2012.1756
80. Zhang T, Li H, Vanarsa K, Gidley G, Mok CC, Petri M, et al. Association of urine sCD163 with proliferative lupus nephritis, fibrinoid necrosis, cellular crescents and intrarenal M2 macrophages. Front Immunol. (2020) 11:671. doi: 10.3389/fimmu.2020.00671
81. Zhang H, Fu R, Guo C, Huang Y, Wang H, Wang S, et al. Anti-dsDNA antibodies bind to TLR4 and activate NLRP3 inflammasome in lupus monocytes/macrophages. J Trans Med. (2016) 14:156. doi: 10.1186/s12967-016-0911-z
82. Yang Y, Zhang X, Jing L, Xiao Y, Gao Y, Hu Y, et al. MDSC-derived S100A8/9 contributes to lupus pathogenesis by promoting TLR7-mediated activation of macrophages and dendritic cells. Cell Mol Life sciences: CMLS. (2024) 81:110. doi: 10.1007/s00018-024-05155-w
83. Xiao P, Dong C, Yue Y, Xiong S. Dynamic expression of microRNAs in M2b polarized macrophages associated with systemic lupus erythematosus. Gene. (2014) 547:300–9. doi: 10.1016/j.gene.2014.06.065
84. Zhao H, Wen Z, Xiong S. Activated lymphocyte-derived DNA drives glucose metabolic adaptation for inducing macrophage inflammatory response in systemic lupus erythematosus. Cells. (2023) 12:2093. doi: 10.3390/cells12162093
85. Harder JW, Ma J, Alard P, Sokoloski KJ, Mathiowitz E, Furtado S, et al. Male microbiota-associated metabolite restores macrophage efferocytosis in female lupus-prone mice via activation of PPARγ/LXR signaling pathways. J leukocyte Biol. (2023) 113:41–57. doi: 10.1093/jleuko/qiac002
86. Bijl M, Reefman E, Horst G, Limburg PC, Kallenberg CG. Reduced uptake of apoptotic cells by macrophages in systemic lupus erythematosus: correlates with decreased serum levels of complement. Ann rheumatic diseases. (2006) 65:57–63. doi: 10.1136/ard.2005.035733
87. Majai G, Kiss E, Tarr T, Zahuczky G, Hartman Z, Szegedi G, et al. Decreased apopto-phagocytic gene expression in the macrophages of systemic lupus erythematosus patients. Lupus. (2014) 23:133–45. doi: 10.1177/0961203313511557
88. Li J, Pan Y, Li D, Xia X, Jiang Q, Dou H, et al. Urokinase-type plasminogen activator receptor is required for impairing toll-like receptor 7 signaling on macrophage efferocytosis in lupus. Mol Immunol. (2020) 127:38–45. doi: 10.1016/j.molimm.2020.08.018
89. Zhou Z, Xu A, Teng J, Wang F, Tan Y, Liu H, et al. Anti-tyro3 igG associates with disease activity and reduces efferocytosis of macrophages in new-onset systemic lupus erythematosus. J Immunol Res. (2020) 2020:2180708. doi: 10.1155/2020/2180708
90. Deng W, Chen W, Zhang Z, Huang S, Kong W, Sun Y, et al. Mesenchymal stem cells promote CD206 expression and phagocytic activity of macrophages through IL-6 in systemic lupus erythematosus. Clin Immunol (Orlando Fla). (2015) 161:209–16. doi: 10.1016/j.clim.2015.07.011
91. Zhang M, Johnson-Stephenson TK, Wang W, Wang Y, Li J, Li L, et al. Mesenchymal stem cell-derived exosome-educated macrophages alleviate systemic lupus erythematosus by promoting efferocytosis and recruitment of IL-17(+) regulatory T cell. Stem Cell Res Ther. (2022) 13:484. doi: 10.1186/s13287-022-03174-7
92. Wang J, Xie L, Wang S, Lin J, Liang J, Xu J. Azithromycin promotes alternatively activated macrophage phenotype in systematic lupus erythematosus via PI3K/Akt signaling pathway. Cell Death disease. (2018) 9:1080. doi: 10.1038/s41419-018-1097-5
93. Zhuang H, Han S, Lu L, Reeves WH. Myxomavirus serpin alters macrophage function and prevents diffuse alveolar hemorrhage in pristane-induced lupus. Clin Immunol (Orlando Fla). (2021) 229:108764. doi: 10.1016/j.clim.2021.108764
94. Horuluoglu B, Bayik D, Kayraklioglu N, Goguet E, Kaplan MJ, Klinman DM. PAM3 supports the generation of M2-like macrophages from lupus patient monocytes and improves disease outcome in murine lupus. J autoimmunity. (2019) 99:24–32. doi: 10.1016/j.jaut.2019.01.004
95. Buhl T, Sulk M, Nowak P, Buddenkotte J, McDonald I, Aubert J, et al. Molecular and morphological characterization of inflammatory infiltrate in rosacea reveals activation of th1/th17 pathways. J Invest Dermatol. (2015) 135:2198–208. doi: 10.1038/jid.2015.141
96. Liu Z, Zhang J, Jiang P, Yin Z, Liu Y, Liu Y, et al. Paeoniflorin inhibits the macrophage-related rosacea-like inflammatory reaction through the suppressor of cytokine signaling 3-apoptosis signal-regulating kinase 1-p38 pathway. Medicine. (2021) 100:e23986. doi: 10.1097/MD.0000000000023986
97. Liu L, Chen Y, Chen J, Xue Y, Chen T, Li Y, et al. Association between frontal fibrosing Alopecia and Rosacea: Results from clinical observational studies and gene expression profiles. Front Immunol. (2022) 13:985081. doi: 10.3389/fimmu.2022.985081
98. Zhou L, Zhao H, Zhao H, Meng X, Zhao Z, Xie H, et al. GBP5 exacerbates rosacea-like skin inflammation by skewing macrophage polarization towards M1 phenotype through the NF-κB signalling pathway. J Eur Acad Dermatol Venereol: JEADV. (2023) 37:796–809. doi: 10.1111/jdv.18725
99. Yoon SH, Hwang I, Lee E, Cho HJ, Ryu JH, Kim TG, et al. Antimicrobial peptide LL-37 drives rosacea-like skin inflammation in an NLRP3-dependent manner. J Invest Dermatol. (2021) 141:2885–94.e5. doi: 10.1016/j.jid.2021.02.745
100. Liu T, Deng Z, Xie H, Chen M, Xu S, Peng Q, et al. ADAMDEC1 promotes skin inflammation in rosacea via modulating the polarization of M1 macrophages. Biochem Biophys Res Commun. (2020) 521:64–71. doi: 10.1016/j.bbrc.2019.10.073
101. Zhang J, Jiang P, Sheng L, Liu Y, Liu Y, Li M, et al. A novel mechanism of carvedilol efficacy for rosacea treatment: toll-like receptor 2 inhibition in macrophages. Front Immunol. (2021) 12:609615. doi: 10.3389/fimmu.2021.609615
102. Yuan X, Li J, Li Y, Deng Z, Zhou L, Long J, et al. Artemisinin, a potential option to inhibit inflammation and angiogenesis in rosacea. Biomedicine pharmacotherapy = Biomedecine pharmacotherapie. (2019) 117:109181. doi: 10.1016/j.biopha.2019.109181
103. Chen R, Fairley JA, Zhao ML, Giudice GJ, Zillikens D, Diaz LA, et al. Macrophages, but not T and B lymphocytes, are critical for subepidermal blister formation in experimental bullous pemphigoid: macrophage-mediated neutrophil infiltration depends on mast cell activation. J Immunol (Baltimore Md: 1950). (2002) 169:3987–92. doi: 10.4049/jimmunol.169.7.3987
104. Furudate S, Fujimura T, Kambayashi Y, Kakizaki A, Aiba S. Comparison of CD163+ CD206+ M2 macrophages in the lesional skin of bullous pemphigoid and pemphigus vulgaris: the possible pathogenesis of bullous pemphigoid. Dermatol (Basel Switzerland). (2014) 229:369–78. doi: 10.1159/000365946
105. Solís-Barbosa MA, Santana E, Muñoz-Torres JR, Segovia-Gamboa NC, Patiño-Martínez E, Meraz-Ríos MA, et al. The nuclear receptor Nurr1 is preferentially expressed in human pro-inflammatory macrophages and limits their inflammatory profile. Int Immunol. (2024) 36:111–28. doi: 10.1093/intimm/dxad048
106. Tanita K, Fujimura T, Sato Y, Lyu C, Aiba S. Minocycline decreases Th2 chemokines from M2 macrophages: Possible mechanisms for the suppression of bullous pemphigoid by traditional bullous disease drugs. Exp Dermatol. (2018) 27:1268–72. doi: 10.1111/exd.2018.27.issue-11
107. Nozawa K, Suzuki T, Kayanuma G, Yamamoto H, Nagayasu K, Shirakawa H, et al. Lisinopril prevents bullous pemphigoid induced by dipeptidyl peptidase 4 inhibitors via the Mas receptor pathway. Front Immunol. (2023) 13:1084960. doi: 10.3389/fimmu.2022.1084960
108. Ernst N, Friedrich M, Bieber K, Kasperkiewicz M, Gross N, Sadik CD, et al. Expression of PD-1 and Tim-3 is increased in skin of patients with bullous pemphigoid and pemphigus vulgaris. J Eur Acad Dermatol Venereol: JEADV. (2021) 35:486–92. doi: 10.1111/jdv.16780
109. Salmi S, Siiskonen H, Sironen R, Tyynelä-Korhonen K, Hirschovits-Gerz B, Valkonen M, et al. The number and localization of CD68+ and CD163+ macrophages in different stages of cutaneous melanoma. Melanoma Res. (2019) 29:237–47. doi: 10.1097/CMR.0000000000000522
110. Scali E, Mignogna C, Di Vito A, Presta I, Camastra C, Donato G, et al. Inflammation and macrophage polarization in cutaneous melanoma: Histopathological and immunohistochemical study. Int J immunopathol Pharmacol. (2016) 29:715–9. doi: 10.1177/0394632016650895
111. Falleni M, Savi F, Tosi D, Agape E, Cerri A, Moneghini L, et al. M1 and M2 macrophages’ clinicopathological significance in cutaneous melanoma. Melanoma Res. (2017) 27:200–10. doi: 10.1097/CMR.0000000000000352
112. Martinek J, Lin J, Kim KI, Wang VG, Wu TC, Chiorazzi M, et al. Transcriptional profiling of macrophages in situ in metastatic melanoma reveals localization-dependent phenotypes and function. Cell Rep Med. (2022) 3:100621. doi: 10.1016/j.xcrm.2022.100621
113. Emri E, Egervari K, Varvolgyi T, Rozsa D, Miko E, Dezso B, et al. Correlation among metallothionein expression, intratumoural macrophage infiltration and the risk of metastasis in human cutaneous Malignant melanoma. J Eur Acad Dermatol Venereol: JEADV. (2013) 27:e320–7. doi: 10.1111/j.1468-3083.2012.04653.x
114. Hood JL. Melanoma exosome induction of endothelial cell GM-CSF in pre-metastatic lymph nodes may result in different M1 and M2 macrophage mediated angiogenic processes. Med hypotheses. (2016) 94:118–22. doi: 10.1016/j.mehy.2016.07.009
115. Chen P, Huang Y, Bong R, Ding Y, Song N, Wang X, et al. Tumor-associated macrophages promote angiogenesis and melanoma growth via adrenomedullin in a paracrine and autocrine manner. Clin Cancer Res. (2011) 17:7230–9. doi: 10.1158/1078-0432.CCR-11-1354
116. Roh-Johnson M, Shah AN, Stonick JA, Poudel KR, Kargl J, Yang GH, et al. Macrophage-dependent cytoplasmic transfer during melanoma invasion in vivo. Dev Cell. (2017) 43:549–62.e6. doi: 10.1016/j.devcel.2017.11.003
117. Clawson GA, Matters GL, Xin P, Imamura-Kawasawa Y, Du Z, Thiboutot DM, et al. Macrophage-tumor cell fusions from peripheral blood of melanoma patients. PloS One. (2015) 10:e0134320. doi: 10.1371/journal.pone.0134320
118. Huber R, Meier B, Otsuka A, Fenini G, Satoh T, Gehrke S, et al. Tumour hypoxia promotes melanoma growth and metastasis via High Mobility Group Box-1 and M2-like macrophages. Sci Rep. (2016) 6:29914. doi: 10.1038/srep29914
119. Tham M, Tan KW, Keeble J, Wang X, Hubert S, Barron L, et al. Melanoma-initiating cells exploit M2 macrophage TGFβ and arginase pathway for survival and proliferation. Oncotarget. (2014) 5:12027–42. doi: 10.18632/oncotarget.v5i23
120. Sun R, Chen Y, Yang Q, Zhang W, Guo L, Feng M. Polysaccharide hydrogels regulate macrophage polarization and enhance the anti-tumor efficacy of melanoma. Int J pharmaceutics. (2022) 613:121390. doi: 10.1016/j.ijpharm.2021.121390
121. Sallam MA, Wyatt Shields Iv C, Prakash S, Kim J, Pan DC, Mitragotri S. A dual macrophage polarizer conjugate for synergistic melanoma therapy. J Controlled release. (2021) 335:333–44. doi: 10.1016/j.jconrel.2021.05.033
122. Wen D, Liang T, Chen G, Li H, Wang Z, Wang J, et al. Adipocytes encapsulating telratolimod recruit and polarize tumor-associated macrophages for cancer immunotherapy. Advanced Sci (Weinheim Baden-Wurttemberg Germany). (2023) 10:e2206001. doi: 10.1002/advs.202206001
123. Ramelyte E, Dummer R, Guenova E. Investigative drugs for the treatment of cutaneous T-cell lymphomas (CTCL): an update. Expert Opin investigational Drugs. (2019) 28:799–809. doi: 10.1080/13543784.2019.1654995
124. Khan S, Sawas A. Antibody-directed therapies: toward a durable and tolerable treatment platform for CTCL. Front Oncol. (2019) 9:645. doi: 10.3389/fonc.2019.00645
125. Han Z, Wu X, Qin H, Yuan YC, Schmolze D, Su C, et al. Reprogramming of PD-1+ M2-like tumor-associated macrophages with anti-PD-L1 and lenalidomide in cutaneous T cell lymphoma. JCI Insight. (2023) 8:e163518. doi: 10.1172/jci.insight.163518
126. Johanny LD, Sokumbi O, Hobbs MM, Jiang L. Polarization of macrophages in granulomatous cutaneous T cell lymphoma granulomatous mycosis fungoides microenvironment. Dermatopathol (Basel Switzerland). (2022) 9:54–9. doi: 10.3390/dermatopathology9010009
127. Atzmony L, Moyal L, Feinmesser M, Gorovitz B, Hirshberg A, Amitay-Laish I, et al. Stage-dependent increase in expression of miR-155 and ki-67 and number of tumour-associated inflammatory cells in folliculotropic mycosis fungoides. Acta dermato-venereol. (2020) 100:adv00230. doi: 10.2340/00015555-3578
128. Feng Y, Wang S, Xie J, Ding B, Wang M, Zhang P, et al. Spatial transcriptomics reveals heterogeneity of macrophages in the tumor microenvironment of granulomatous slack skin. J pathol. (2023) 261:105–19. doi: 10.1002/path.v261.1
129. Liu X, Jin S, Hu S, Li R, Pan H, Liu Y, et al. Single-cell transcriptomics links Malignant T cells to the tumor immune landscape in cutaneous T cell lymphoma. Nat Commun. (2022) 13:1158. doi: 10.1038/s41467-022-28799-3
130. Du Y, Cai Y, Lv Y, Zhang L, Yang H, Liu Q, et al. Single-cell RNA sequencing unveils the communications between Malignant T and myeloid cells contributing to tumor growth and immunosuppression in cutaneous T-cell lymphoma. Cancer letters. (2022) 551:215972. doi: 10.1016/j.canlet.2022.215972
131. Song X, Chang S, Seminario-Vidal L, de Mingo Pulido A, Tordesillas L, Song X, et al. Genomic and single-cell landscape reveals novel drivers and therapeutic vulnerabilities of transformed cutaneous T-cell lymphoma. Cancer discovery. (2022) 12:1294–313. doi: 10.1158/2159-8290.CD-21-1207
132. Wu X, Schulte BC, Zhou Y, Haribhai D, Mackinnon AC, Plaza JA, et al. Depletion of M2-like tumor-associated macrophages delays cutaneous T-cell lymphoma development in vivo. J Invest Dermatol. (2014) 134:2814–22. doi: 10.1038/jid.2014.206
133. Wu X, Singh R, Hsu DK, Zhou Y, Yu S, Han D, et al. A small molecule CCR2 antagonist depletes tumor macrophages and synergizes with anti-PD-1 in a murine model of cutaneous T-cell lymphoma (CTCL). J Invest Dermatol. (2020) 140:1390–400.e4. doi: 10.1016/j.jid.2019.11.018
134. Sugaya M, Miyagaki T, Ohmatsu H, Suga H, Kai H, Kamata M, et al. Association of the numbers of CD163(+) cells in lesional skin and serum levels of soluble CD163 with disease progression of cutaneous T cell lymphoma. J Dermatol science. (2012) 68:45–51. doi: 10.1016/j.jdermsci.2012.07.007
135. El-Guindy DM, Elgarhy LH, Elkholy RA, Ali DA, Helal DS. Potential role of tumor-associated macrophages and CD163/CD68 ratio in mycosis fungoides and Sézary syndrome in correlation with serum sCD163 and CCL22. J cutaneous pathol. (2022) 49:261–73. doi: 10.1111/cup.14155
136. Furudate S, Fujimura T, Kakizaki A, Kambayashi Y, Asano M, Watabe A, et al. The possible interaction between periostin expressed by cancer stroma and tumor-associated macrophages in developing mycosis fungoides. Exp Dermatol. (2016) 25:107–12. doi: 10.1111/exd.2016.25.issue-2
137. Tanita K, Fujimura T, Sato Y, Lyu C, Kambayashi Y, Ogata D, et al. Bexarotene reduces production of CCL22 from tumor-associated macrophages in cutaneous T-cell lymphoma. Front Oncol. (2019) 9:907. doi: 10.3389/fonc.2019.00907
Keywords: macrophage, inflammatory skin diseases, skin tumors, pathogenesis, treatment
Citation: Liu S-H, Zhang J and Zuo Y-G (2024) Macrophages in inflammatory skin diseases and skin tumors. Front. Immunol. 15:1430825. doi: 10.3389/fimmu.2024.1430825
Received: 10 May 2024; Accepted: 18 November 2024;
Published: 05 December 2024.
Edited by:
Judit Danis, University of Szeged, HungaryReviewed by:
Utpal Sengupta, The Leprosy Mission Trust India, IndiaWilliam D. Shipman, Skin & Beauty Center- Board Certified Dermatologist, United States
Whitney M. Longmate, Albany Medical College, United States
Copyright © 2024 Liu, Zhang and Zuo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ya-Gang Zuo, enVveWFnYW5nQDI2My5uZXQ=
 Si-Han Liu
Si-Han Liu Jie Zhang
Jie Zhang Ya-Gang Zuo
Ya-Gang Zuo