
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Immunol., 09 September 2024
Sec. Microbial Immunology
Volume 15 - 2024 | https://doi.org/10.3389/fimmu.2024.1430179
Adult-onset immunodeficiency (AOID) mediated by anti-interferon-γ autoantibodies (AIGA) is a rare condition, particularly prevalent in Southeast Asia and southern China. We present a case study of a 62-year-old female with AOID who developed a severe pulmonary infection caused by Talaromyces marneffei (TM), leading to acute respiratory failure, generalized rash, multiple lymphadenopathies, bone destruction, and a mediastinal mass. Treatment included mechanical ventilation, antifungal medication, and corticosteroids, resulting in complete recovery and discharge. This case underscores the challenges of managing complex infections in AOID patients and highlights the importance of early diagnosis through metagenomic next-generation sequencing (mNGS) and appropriate intervention to improve clinical outcomes.
Adult-onset immunodeficiency (AOID) is a rare condition mediated by autoantibodies against interferon-γ (anti-interferon-γ autoantibodies, AIGA). Initially, AIGAs were considered a sporadic late-onset immunodeficiency observed in patients with mycobacterial infections (1). It is more prevalent in populations from Southeast Asia and southern China, with an estimated incidence rate of 0.5-1.0 per million people (1–4). The earliest documented cases internationally were reported around the year 2004 (5–7). Since 2012, several cohorts of patients with AIGAs have been reported in Thailand, Taiwan, Japan, and South China. Additionally, sporadic cases have been documented throughout Southeast Asia, including the Philippines, Vietnam, Cambodia, Singapore, and Malaysia (2, 8–10). To date, over 600 patients with AIGAs have been diagnosed, predominantly among adults of Asian descent, with more than two-thirds of the cases reported in Thailand and Taiwan (2–4, 8–12). Currently, reported cases of AOID in China are primarily concentrated in southern provinces such as Guangxi, Guangdong, and Taiwan (3, 4, 8, 10, 13–16). AIGAs have been detected in patients across a wide age range, from 10 to 87 years, with most cases occurring in adults around 50 years old (2, 3, 9, 10, 12). Although autoimmune diseases generally display a female preponderance, there is no obvious sex bias in patients with AIGAs (male/female ratio = 0.82) (2, 3, 9, 10, 12).
The initial clinical signs and symptoms of AIGA-related disease predominantly result from disseminated non-tuberculous mycobacterial (NTM) infections (8, 9). These often include bilateral cervical or generalized lymphadenitis and associated reactive skin lesions. Additionally, non-specific symptoms such as fever, malaise, cough, bone pain, and weight loss may be observed, similar to those seen in various other diseases (8–10). Mycobacteria are the most frequently observed pathogens, affecting 85.5% of patients with AIGAs and often causing severe, disseminated infections (2, 3, 9). Invasive non-typhoid salmonellosis is the most common bacterial infection observed in patients with AIGAs. Cohort studies have reported that it affects 29–40% of these patients (2, 3, 12). Shingles, caused by the varicella–zoster virus (VZV), is the most common viral infection observed in patients with AIGAs (2, 3, 12). A cohort study reported that up to 62.2% of these patients had a history of shingles, which is notably higher than the 32% lifetime risk observed in the general population (3). Additionally, VZV, herpes simplex virus (HSV), and cytomegalovirus (CMV) infections have been documented in patients with Mendelian susceptibility to mycobacterial disease (MSMD), particularly those with interferon-γ (IFN-γ) receptor 1 (IFN-γR1) deficiency (17). Fungal infections, such as cryptococcosis, aspergillosis, and candidiasis, have been observed in patients with AIGAs (2, 3, 12, 18). Moreover, AIGAs have been shown to be associated with severe Talaromyces marneffei (TM) infections in human immunodeficiency virus (HIV)-negative patients (10). TM is the second most common pathogen in these cases, likely due to its high prevalence in Southeast Asia (19). Additionally, AIGA can lead to severe opportunistic infections that are difficult to control, particularly in patients with recurrent infections (19). Therefore, this condition requires significant clinical attention.
TM is a significant pathogenic thermally dimorphic fungus that causes systemic mycosis in Southeast Asia (20–22). In 1956, TM was initially isolated from the liver of a bamboo rat (23, 24). The first documented natural human infection was reported in 1973, involving an American minister with Hodgkin’s disease who lived in Southeast Asia (25). Historically, TM infection in humans has been considered exclusively associated with acquired immunodeficiency syndrome (AIDS) caused by HIV infection (20, 26). However, in recent years, there have been increasing reports of Talaromyces infections in non-HIV-infected individuals (22). Most of these cases involve other immunosuppressive conditions, including primary immunodeficiencies, autoimmune diseases, malignancies, and iatrogenic immunosuppression (27). Currently, bamboo rats and various other rodents are frequently identified as reservoirs for TM infections (20, 28). TM has the ability to enter the body’s reticuloendothelial system, travel through the bloodstream, and infect other organs. This can occur through the respiratory and digestive tracts, potentially leading to severe systemic infections and presenting life-threatening risks, especially in individuals with weakened immune systems (27, 29).
To improve empirical treatment decisions and enhance patient survival, it is crucial to rapidly identify pathogens in patients infected with TM. Metagenomic next-generation sequencing (mNGS) tests, an advanced nucleic acid detection technology for pathogen identification, have the capability to detect a broad spectrum of pathogens, predict antibiotic resistance, and deliver results within 24 hours (30, 31). Cohort studies focused on respiratory tract infections have demonstrated that the positive rate of mNGS (>60%) for detecting respiratory pathogens is significantly higher than that of traditional microbial detection methods (30–50%) (32, 33). Notably, mNGS has a significant advantage in diagnosing unexplained infections and co-infections (34).
Cases of AOID infection with TM are relatively rare. We report a case of a diagnosed AOID patient who was infected with TM identified through mNGS, presenting with severe pulmonary infection, acute respiratory failure, generalized rash, multiple lymphadenopathies, bone destruction, and a mediastinal mass. The patient was successfully treated with mechanical ventilation, antifungal medication, management of shock, and corticosteroids, leading to complete recovery and discharge from the hospital.
A 62-year-old female patient, a farmer by trade, with no history of chronic diseases such as diabetes, no infectious disease history, no history of trauma or surgery, and no significant family history, was admitted to the Oncology Department of the First Affiliated Hospital of Zhejiang University School of Medicine on January 5, 2024, due to “headache and neck pain for 2 weeks.”
Two weeks before admission, the patient developed headaches accompanied by neck pain, numbness in both hands, and fever (highest temperature approximately 37.6°C), and sought medical attention at a local hospital (First People’s Hospital of Lin’an District, Hangzhou). Blood tests showed a white blood cell count (WBC) of 19.5 x10E9/L, C-reactive protein (CRP) of 223.1mg/L, total protein of 90.3 g/L, globulin of 54.8 g/L, and an erythrocyte sedimentation rate (ESR) of 120 mm/h. Chest CT revealed irregular mass in the left upper mediastinum, soft tissue nodules and masses adjacent to the left side of the aortic arch in the mediastinum, suggestive of a tumorous lesion, with a C6 spinous process fracture.
On December 29, 2023, a biopsy of the mass above the left clavicle was performed, showing lymphocyte proliferation in fibrous tissue, predominantly T lymphocytes, suggesting the need for further lymph node excision to exclude vascular immunoblastic lymphoma. Lumbar puncture and routine cerebrospinal fluid analysis and general bacterial culture were unremarkable. The local hospital diagnosed a mediastinal mass, lymph node enlargement, and cervical spine fracture, and treated the patient with piperacillin-tazobactam for infection, celecoxib for fever and pain relief, among other treatments. However, the patient’s condition worsened, leading to transfer to our hospital and admission to the Oncology Department.
Upon admission, the patient’s physical examination revealed a body temperature of 36.5°C, a pulse rate of 104 beats per minute, a respiratory rate of 19 breaths per minute, and a blood pressure of 153/84 mmHg, with a pain score (visual analogue scale, VAS) of 1 point. The patient was alert and oriented, and the neurological examination showed no abnormalities. Scattered reddish-brown maculopapular lesions were observed throughout the body, with a higher concentration on the trunk, some exhibiting a ring-shaped appearance but without significant infiltration (Figure 1A). A palpable mass, approximately 3 × 4 cm in size, with a tough texture and limited mobility, was detected above the left collarbone, with no tenderness noted. Additionally, coarse breath sounds were heard in both lungs, with no noticeable crackles or wheezes.
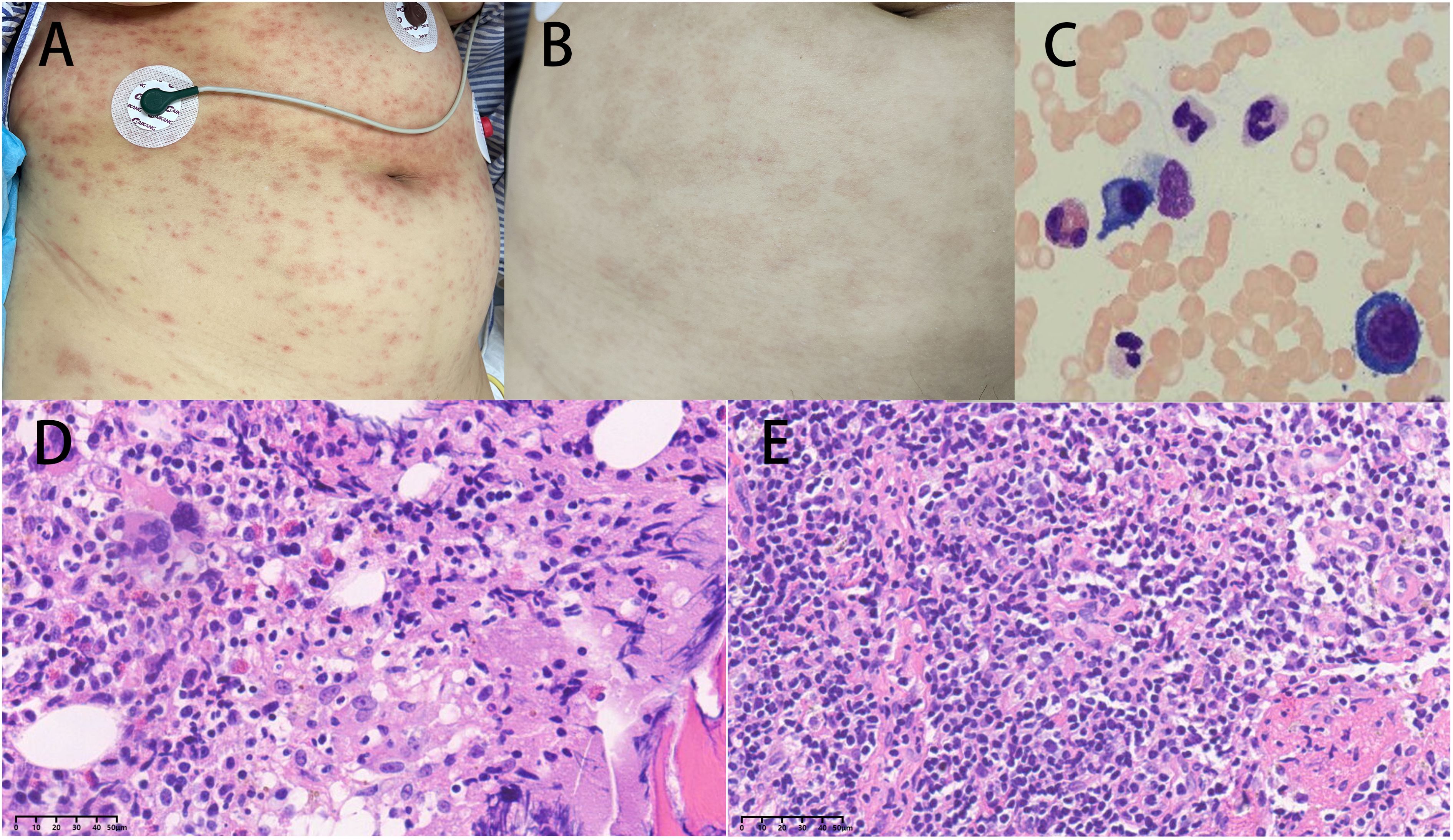
Figure 1. (A) Upon admission, the patient had scattered reddish-brown papules on the whole body. (B) After treatment, the rash significantly subsided. (C) The bone marrow showed an increased proportion of plasma cells. (D) The hematopoietic tissue in the bone marrow was highly active (70-80% cellularity), accompanied by active proliferation of T lymphoid tissue and increased plasma cells (magnification × 40). (E) (Histopathology from lymph node puncture above the left clavicle) showed active proliferation of lymphoid tissue with a predominance of neutrophils, plasma cell infiltration, and tendencies toward inflammatory changes (magnification × 40).
Routine laboratory tests showed the following results: white blood cell count (WBC) 21.04 × 109/L, neutrophils 19.36 × 109/L, lymphocytes 1.03 × 109/L, hemoglobin 100 g/L, red blood cell count 4.05 × 1012/L, platelet count 192 × 109/L, B-type natriuretic peptide precursor quantification 1171 pg/mL, activated partial thromboplastin time 46.0 seconds, prothrombin time 14.0 seconds, D-dimer 6830 μg/L, creatinine 138 μmol/L, total bilirubin (TBIL) 33.5 μmol/L, direct bilirubin (DBIL) 26.2 μmol/L, globulin 55.2 g/L, albumin 23.1 g/L, C-reactive protein (CRP) 351.30 mg/L, procalcitonin 0.75 ng/mL, glycated hemoglobin A1c 6.7%, immunoglobulin G 2450.0 mg/dL, cancer antigen 125 101.9 U/mL, ferritin 2331.4 ng/mL. Serum protein electrophoresis (M protein) showed: albumin 34.2%, α1 6.3%, β 8.2%, γ 40.3%. Thyroid function tests revealed: tetraiodothyronine 47.44 nmol/L, triiodothyronine 0.31 nmol/L, thyroid-stimulating hormone 0.218 mIU/L, free triiodothyronine 1.96 pmol/L. Immunoglobulin free light chain assay showed: kappa free light chain 89.0 mg/L, lambda free light chain 111.0 mg/L, free light chain ratio 0.80. HIV negative. Epstein-Barr virus antibody (IgM) negative. The complete laboratory results throughout the course of the illness are summarized in Table 1.
The special examination results revealed several significant findings. Neck ultrasound showed bilateral supraclavicular lymphadenopathy and a hypoechoic mass in the left supraclavicular region (Figure 2G). Chest CT scan displayed diffuse infiltrates with consolidation in both lungs, along with local cavitation (Figures 2A, B). Enhanced CT examination of the chest revealed an irregular mass in the left upper mediastinum (Figure 2I). Neck CT scan indicated bone structure destruction at various sites, including the slope, left occipital condyle, C6 vertebral body, and spinous process, accompanied by soft tissue mass formation (Figure 2C). Head CT scan showed localized low-density shadows in the frontal bone and soft tissue density shadows with bone destruction at the slope (Figure 2F). Bone marrow cytology indicated an increased ratio of plasma cells (Figure 1C), while histology showed highly active hematopoietic tissue with active proliferation of T lymphoid tissue and increased plasma cells (Figure 1D). Pathological examination of a lymph node biopsy from the left supraclavicular region revealed minimal lymphoid tissue proliferation with specific immunohistochemical staining patterns (Figure 1E). Additionally, ultrasound-guided aspiration of a left neck mass yielded purulent fluid for microbial culture.
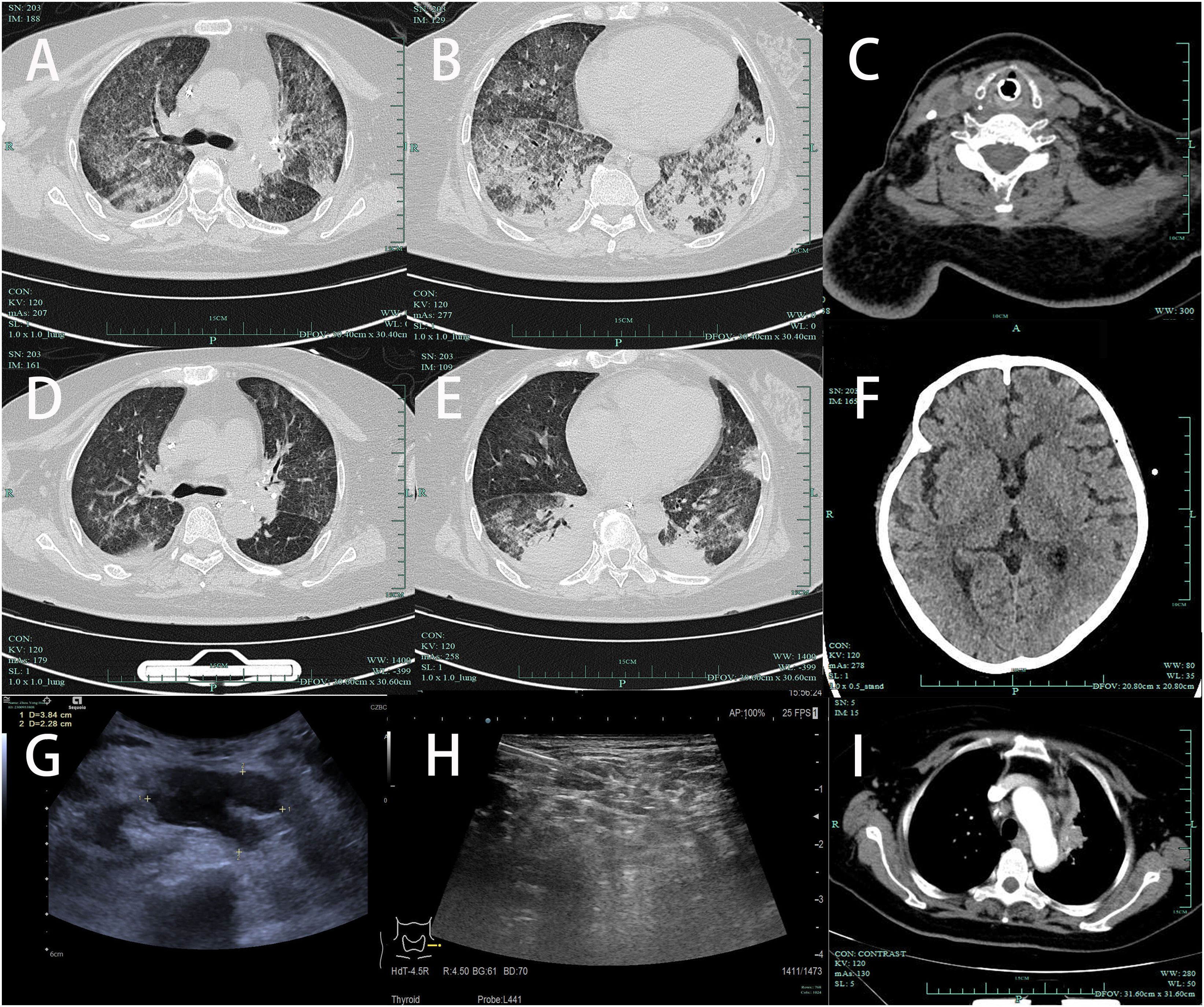
Figure 2. (A, B) Chest CT (2024-01-11): Diffuse infiltration with consolidation in both lungs, accompanied by local cavitation. (C) Neck CT (2024-01-11): Destruction of the C6 vertebral body and spinous process with soft tissue mass formation. (D, E) After treatment, follow-up chest CT on 2024-01-16 showed significant absorption of lung patchy shadows compared to before, with a reduction in local cavities. (F) Head CT (2024-01-16): Localized low-density shadow in the frontal bone, suggesting bone destruction. (G) Neck ultrasound: Bilateral supraclavicular multiple lymph node enlargement, hypoechoic mass in the left supraclavicular region, measuring 3.84cm × 2.28cm; (H) Post-treatment on 2024.1.16, reduction in lymph nodes in the supraclavicular region. (I) On admission, enhanced CT examination of the chest revealed an irregular mass in the left upper mediastinum.
After admission, the patient received treatment including levofloxacin for infection, acetaminophen for fever reduction, sustained-release tramadol for pain relief, and cervical collar immobilization.
On the 5th day after admission, the patient’s respiratory distress significantly worsened, with a further decrease in percutaneous oxygen saturation and audible crackles in both lungs on auscultation. Despite oxygen mask ventilation with a reservoir bag, oxygen saturation was maintained around 95%. Consequently, the patient was transferred to the intensive care unit (ICU). Treatment in the ICU included oral endotracheal intubation and mechanical ventilation, antimicrobial therapy with meropenem 1.0 q8h plus voriconazole 0.2 q12h, continuous intravenous infusion of norepinephrine to maintain blood pressure, intravenous methylprednisolone sodium succinate 40mg Q12H for anti-inflammatory purposes, fentanyl for pain management, midazolam for sedation, enteral nutrition support, low molecular weight heparin for venous thrombosis prophylaxis, and enhanced sputum suction management. On the second day in the ICU, a bronchoalveolar lavage fluid (BALF) sample was collected for metagenomic next-generation sequencing (mNGS). Two days later (2024-01-12), the NGS results from the BALF showed TM (Figure 3G). Consequently, the antifungal regimen was modified, discontinuing voriconazole and meropenem in favor of amphotericin B, gradually titrated up to a therapeutic dose of 0.5mg/kg/day. Additionally, on the 5th day in the ICU (2024-01-15), culture results from the aspirated fluid of the left neck mass also confirmed TM (Figures 3A, B, D-F). Subsequent blood culture results from the patient also confirmed TM (Figure 3C).
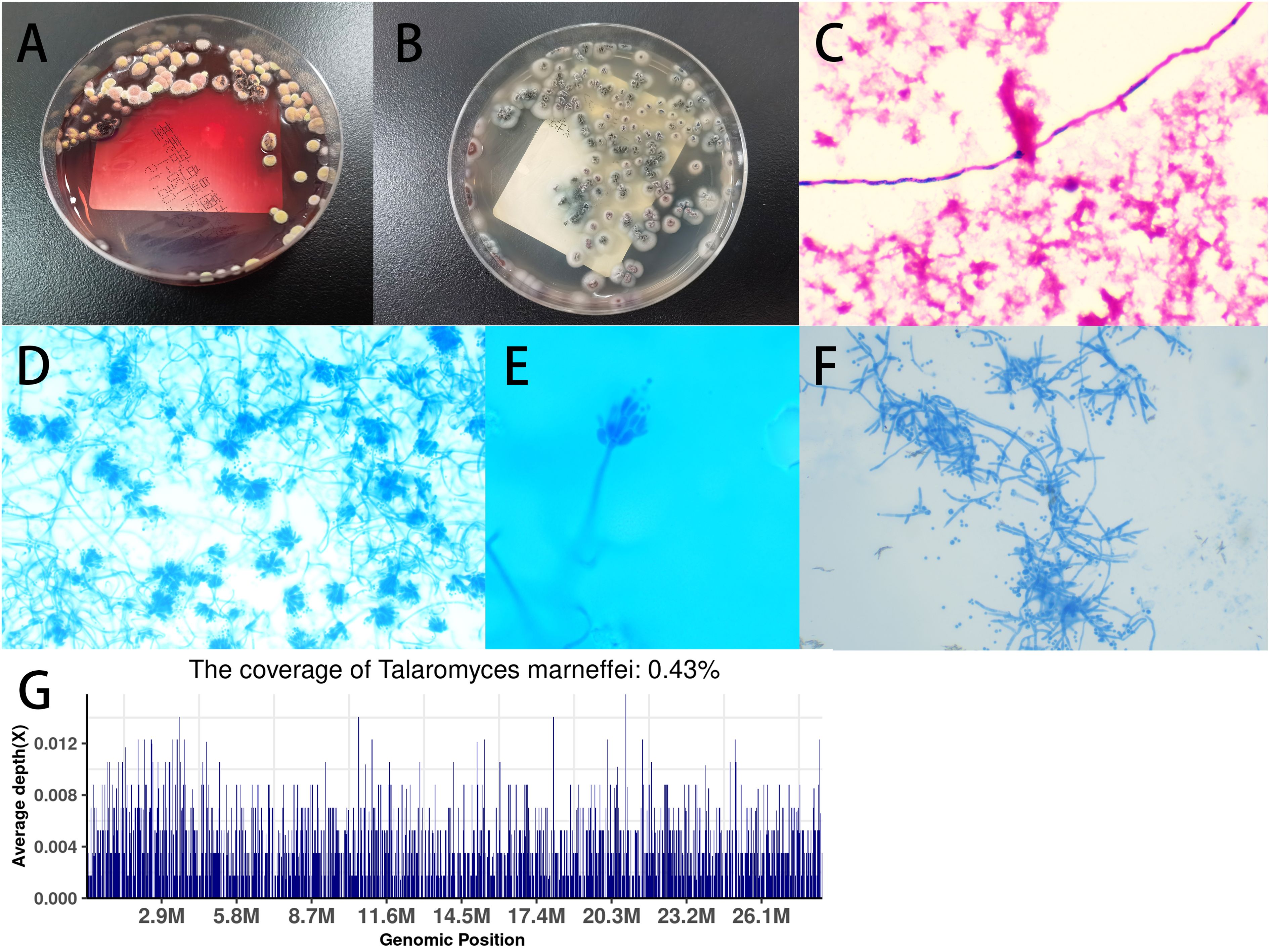
Figure 3. (A) Blood culture showed Talaromyces marneffei (TM) in the mold phase at 25°C, with characteristic red pigment production observed; (B) Culture of pus sample from left neck mass on Sabouraud’s agar medium plate at 37°C showed yeast phase; (C) Blood culture sample showed TM under microscopy (magnification × 400); (D) Microscopic analysis of colonies grown on Sabouraud’s agar medium plate at 25°C using lactophenol cotton blue staining revealed scattered, slightly asymmetric, typical double-helical broom-like fungal hyphae with short, smooth-walled, ellipsoidal, monoverticillate Penicillium conidiophores (magnification × 400); (E) Microscopic analysis at magnification × 1000; (F) Microscopic analysis of colonies grown on Sabouraud’s agar medium plate at 37°C by lactophenol cotton blue staining (magnification × 400); (G) Examination of BALF using metagenomic next-generation sequencing (mNGS) revealed TM.
Due to TM being an opportunistic infection and the patient testing HIV-negative, further examination revealed positive AIGA (209.40 AU/mL, detected by chemiluminescence immunoassay, >20 AU/mL considered positive), which remained positive upon retesting (213.00 AU/mL). Consequently, the patient was diagnosed with AOID mediated by AIGA. Treatment continued with intravenous methylprednisolone sodium succinate.
On 2024-01-16, a chest CT scan revealed patchy opacities in both lungs, with partial absorption compared to the scan on 2024-01-11 (Figures 2D, E). Additionally, irregular consolidations with cavitation formation were observed in the left subclavian region and left upper mediastinum, accompanied by reduced lymphadenopathy in the hilum and mediastinum compared to previous imaging.
On the 14th day of admission (2024-01-19), the patient’s condition significantly improved with stable circulation, improved lung function, and an oxygenation index of 300, leading to the removal of the endotracheal tube and discontinuation of mechanical ventilation. Concurrently, the widespread rash on the patient’s body markedly improved (Figure 1B), and a follow-up examination showed reduced lymphadenopathy (Figure 2H).
On the 19th day of admission (2024-01-24), the patient was discharged from the hospital. The treatment was changed from amphotericin B to oral itraconazole capsules, and outpatient follow-up was scheduled with recommendations for regular follow-ups for up to 6 months after discharge.
Talaromycosis, a fungal infection, is common in tropical and subtropical parts of Asia. It’s caused by the fungus TM. Each year, approximately 17,300 cases are diagnosed, with about one-third of those cases resulting in death (35). Despite its high mortality rate, this disease has not received much attention globally (35). TM is an opportunistic pathogen, primarily affecting individuals with compromised immune systems, such as those with HIV. Due to the lack of specific clinical manifestations, the disease often presents with atypical symptoms or is masked by other infection symptoms, making diagnosis challenging and leading to a high rate of misdiagnosis.
The patient we reported not only had severe lung infection and acute respiratory failure but also presented with widespread rash, enlarged lymph nodes in multiple areas, mediastinal mass, and cervical vertebral destruction, along with pain behind the sternum, symptoms that could easily lead to a misdiagnosis of lymphoma. We ruled out the possibility of lymphoma through lymph node biopsy, bone marrow cytology, and bone marrow histopathology examinations. Additionally, by conducting mNGS on the pulmonary lavage fluid, we promptly detected the presence of TM. Ultimately, confirmation of TM infection was obtained through fungal culture of the aspirate from the neck mass.
Despite the patient testing negative for HIV, she was infected with TM, raising our suspicions about an underlying condition. It is increasingly recognized that certain conditions can predispose individuals to disseminated infections caused by opportunistic pathogens, including NTM, non-typhoidal Salmonella, Burkholderia pseudomallei, Cryptococcus, Histoplasma capsulatum, and TM (4). Subsequent tests for AIGA were positive twice, confirming our suspicions. The patient was diagnosed with AOID associated with neutralizing AIGA (4).
The detection of antibodies is crucial for diagnosing the disease. However, the median diagnostic time was 12 months, and several cases have reported delays in diagnosis leading to missed treatment opportunities (19). As an emerging infectious disease, timely diagnosis of AOID can be challenging due to limited capabilities at some medical centers. Some authors have suggested using the QuantiFERON Gold In-tube assay to screen for autoantibodies (16). Improving detection methods for AOID is essential to enhance early diagnosis and treatment outcomes. Given the disease’s prevalence in Southeast Asia, clinicians should remain vigilant for AOID in patients from these regions who present with opportunistic infections.
IFN-γ, the sole type II interferon, plays a crucial role in immunity against intracellular bacteria. Genetic susceptibility in its signaling pathway can lead to opportunistic infections, such as those caused by mycobacteria, TM, and Salmonella. Doffinger et al. (6)reported a 47-year-old Filipino male with multiple disseminated infections who had negative results for known Mendelian defects in the interleukin (IL) -12/IFN-γ pathway. While his serum showed an intact response to the purified-protein derivative, it had defective secretion of IFN-γ in vitro. They identified a high titer of IgG antibody to IFN-γ, showing it was capable of inhibiting the IFN-γ-dependent augmentation of lipopolysaccharide (LPS)-induced tumor necrosis factor (TNF)-α production (6). Functional studies have shown that the antibody targets a major epitope on free IFN-γ, which is essential for the activation of the IFN-γ receptor (IFN-γR) (36). This interaction inhibits IFN-γ-induced phosphorylation of signal transducer and activator of transcription 1 (STAT1) and subsequent cytokine production (37). Furthermore, the autoantibodies also impair CD4+ Th1 and CD8+ T cell responses (38). Consequently, patients exhibit impaired intracellular immunity despite having normal lymphocyte counts and cellular responses (2, 6). These studies suggest that in AOID patients, the presence of AIGA is a central pathogenic mechanism, but the development of AIGA remains not fully understood. A study that investigated serial serum samples from a single patient over a 7-year period found that these antibodies appeared 18 months before the clinical onset of symptoms, suggesting their acquisition prior to the disease manifestation (39). Therefore, AIGA is considered pathogenic antibodies rather than antibodies secondary to infection. On the other hand, this disease has been mostly reported in Southeast Asia; even in the USA, most patients (91%) are Asian immigrants, suggesting a genetic propensity for the disease (40). Genetic studies have found a high LD association between several type II HLA alleles with AIGA, including DRB116:02, DRB115:02, DQB105:02, and DQB105:01 (8, 41), and these risk alleles have synergistic effects in contributing to the disease (41). In cases of severe infection, the concentration of IFN should typically increase accordingly. However, in this patient, the serum IFN-γ levels were found to be within the normal range during two separate tests, indicating that some IFN-γ was neutralized by AIGA.
Due to the patient’s confirmed diagnosis of AOID, we believe that solely providing anti-infective treatment may not effectively clear the pathogen, as immune modulation is a crucial part of treatment for such patients. In terms of immunotherapy, the efficacy of corticosteroids, subcutaneous IFN-γ injections, and intravenous immunoglobulin is limited. However, combination therapy with cyclophosphamide and steroids has shown promising results in some patients (42). Rituximab, an anti-CD20 monoclonal antibody, is one of the most extensively studied biologics in patients with adult-onset immunodeficiency diseases. This medication can eliminate circulating B cells, reduce AIGA titers, restore IFN-γ signal transduction, and improve clinical conditions (37). In a Thai study involving 17 patients, 6 of whom received rituximab and 11 received cyclophosphamide due to progressive infections, the effectiveness rates of rituximab and cyclophosphamide were comparable (4/6 and 8/11, respectively) (43). Bortezomib, a proteasome inhibitor targeting plasma cells, has demonstrated additional suppression of autoantibodies following rituximab failure (44). Combining rituximab with bortezomib is likely necessary to prevent generation of new autoantibody-producing plasma cells (45). Daratumumab, an antibody targeting CD38+ plasmablasts and plasma cells, has further reduced tissue plasma cells, total IgG levels, AIGA titers, and disease progression (46).
However, when using immunosuppressants or biologics to reduce antibody levels, caution must be exercised to avoid drug-related immunosuppression, which may lead to secondary opportunistic infections. In developing the treatment plan for this patient, we initially recommended rituximab. However, the patient’s family, fully considering the side effects and potential secondary immunosuppression, declined our recommendation. Our multidisciplinary team, including ICU and infectious disease specialists, thoroughly discussed the situation and, taking into account the family’s concerns and the need to avoid drug-related immunosuppression, decided to use corticosteroids alone. This approach aimed to balance reducing antibody titers and avoiding secondary opportunistic infections.
Simultaneously, we implemented a comprehensive treatment plan including amphotericin B, shock management, mechanical ventilation, and nutritional support. Subsequently, the patient’s temperature and inflammatory markers rapidly decreased, and the rash and pulmonary infection significantly improved within a few days. This indicated that the infection was effectively controlled, thereby reinforcing our confidence in the chosen treatment strategy.
In conclusion, our case highlights the importance of a tailored treatment approach for managing AOID patients, balancing the reduction of pathogenic antibodies with the minimization of secondary infection risks. Although AOID is relatively rare and infrequently encountered in clinical practice, patients with this condition are prone to recurrent and complex infections, such as those caused by TM. Therefore, it is crucial to raise awareness about AOID. For suspected cases, timely antibody screening, early diagnosis, and the use of mNGS to rapidly identify infections can facilitate early treatment and improve patient outcomes.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
The studies involving humans were approved by Clinical Research Ethics Committee of the First Affiliated Hospital of Zhejiang University School of Medicine. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
KL: Writing – review & editing, Funding acquisition, Data curation, Writing – original draft, Methodology. YZ: Writing – original draft, Data curation. DZ: Writing – original draft, Funding acquisition, Data curation. QC: Writing – original draft, Data curation. XF: Writing – review & editing, Supervision, Conceptualization.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research received external funding from the National Natural Science Foundation of China (82300007), the General scientific research project of the Zhejiang Provincial Department of Education (Y201941857), and the Zhejiang Province Medical and Health Science and Technology Program (2019RC170). The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
The study was supported by various colleagues from the hospital’s Department of Critical Care Medicine.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Shih HP, Ding JY, Yeh CF, Chi CY, Ku CL. Anti-interferon-gamma autoantibody-associated immunodeficiency. Curr Opin Immunol. (2021) 72:206–14. doi: 10.1016/j.coi.2021.05.007
2. Browne SK, Burbelo PD, Chetchotisakd P, Suputtamongkol Y, Kiertiburanakul S, Shaw PA, et al. Adult-onset immunodeficiency in Thailand and Taiwan. N Engl J Med. (2012) 367(8):725–34. doi: 10.1056/NEJMoa1111160
3. Chi CY, Lin CH, Ho MW, Ding JY, Huang WC, Shih HP, et al. Clinical manifestations, course, and outcome of patients with neutralizing anti-interferon-gamma autoantibodies and disseminated nontuberculous mycobacterial infections. Med (Baltimore). (2016) 95:e3927. doi: 10.1097/MD.0000000000003927
4. Wu UI, Wang JT, Sheng WH, Sun HY, Cheng A, Hsu LY, et al. Incorrect diagnoses in patients with neutralizing anti-interferon-gamma-autoantibodies. Clin Microbiol Infect. (2020) 26:1684 e1–1684.e6. doi: 10.1016/j.cmi.2020.02.030
5. Hoflich C, Sabat R, Rosseau S, Temmesfeld B, Slevogt H, Docke WD, et al. Naturally occurring anti-IFN-gamma autoantibody and severe infections with Mycobacterium cheloneae and Burkholderia cocovenenans. Blood. (2004) 103:673–5. doi: 10.1182/blood-2003-04-1065
6. Doffinger R, Helbert MR, Barcenas-Morales G, Yang K, Dupuis S, Ceron-Gutierrez L, et al. Autoantibodies to interferon-gamma in a patient with selective susceptibility to mycobacterial infection and organ-specific autoimmunity. Clin Infect Dis. (2004) 38:e10–4. doi: 10.1086/380453
7. Kampmann B, Hemingway C, Stephens A, Davidson R, Goodsall A, Anderson S, et al. Acquired predisposition to mycobacterial disease due to autoantibodies to IFN-gamma. J Clin Invest. (2005) 115:2480–8. doi: 10.1172/JCI19316
8. Chi CY, Chu CC, Liu JP, Lin CH, Ho MW, Lo WJ, et al. Anti-IFN-gamma autoantibodies in adults with disseminated nontuberculous mycobacterial infections are associated with HLA-DRB1*16:02 and HLA-DQB1*05:02 and the reactivation of latent varicella-zoster virus infection. Blood. (2013) 121:1357–66. doi: 10.1182/blood-2012-08-452482
9. Aoki A, Sakagami T, Yoshizawa K, Shima K, Toyama M, Tanabe Y, et al. Clinical significance of interferon-γ Neutralizing autoantibodies against disseminated nontuberculous mycobacterial disease. Clin Infect Dis. (2018) 66:1239–45. doi: 10.1093/cid/cix996
10. Guo J, Ning XQ, Ding JY, Zheng YQ, Shi NN, Wu FY, et al. Anti-IFN-gamma autoantibodies underlie disseminated Talaromyces marneffei infections. J Exp Med. (2020) 217(12). doi: 10.1084/jem.20190502
11. Patel SY, Ding L, Brown MR, Lantz L, Gay T, Cohen S, et al. Anti-IFN-γ Autoantibodies in disseminated nontuberculous mycobacterial infections. J Immunol. (2005) 175:4769–76. doi: 10.4049/jimmunol.175.7.4769
12. Rottenberg ME, Wongkulab P, Wipasa J, Chaiwarith R, Supparatpinyo K. Autoantibody to interferon-gamma associated with adult-onset immunodeficiency in non-HIV individuals in northern Thailand. PloS One. (2013) 8(9):e76371. doi: 10.1371/journal.pone.0076371
13. Tang M, Huang J, Zeng W, Huang Y, Lei Y, Qiu Y, et al. Retrospective analysis of 10 cases of disseminated nontuberculous mycobacterial disease with osteolytic lesions. Infect Drug Resist. (2021) 14:4667–79. doi: 10.2147/IDR.S337956
14. Chen ZM, Li ZT, Li SQ, Guan WJ, Qiu Y, Lei ZY, et al. Clinical findings of Talaromyces marneffei infection among patients with anti-interferon-gamma immunodeficiency: a prospective cohort study. BMC Infect Dis. (2021) 21:587. doi: 10.1186/s12879-021-06255-9
15. Qiu Y, Tang M, Zeng W, Feng X, Pan M, Li W, et al. Clinical findings and predictive factors for positive anti-interferon-gamma autoantibodies in patients suffering from a non-tuberculosis mycobacteria or Talaromyces marneffei infection: a multicenter prospective cohort study. Sci Rep. (2022) 12:9069. doi: 10.1038/s41598-022-13160-x
16. Wu UI, Chuang YC, Sheng WH, Sun HY, Jhong YT, Wang JY, et al. Use of QuantiFERON-TB Gold In-tube assay in screening for neutralizing anti-interferon-gamma autoantibodies in patients with disseminated nontuberculous mycobacterial infection. Clin Microbiol Infect. (2018) 24:159–65. doi: 10.1016/j.cmi.2017.06.029
17. Bustamante J, Boisson-Dupuis S, Abel L, Casanova J-L. Mendelian susceptibility to mycobacterial disease: Genetic, immunological, and clinical features of inborn errors of IFN-γ immunity. Semin Immunol. (2014) 26:454–70. doi: 10.1016/j.smim.2014.09.008
18. Tang BS-F, Chan JF-W, Chen M, Tsang OT, Mok MY, Lai RW, et al. Disseminated penicilliosis, recurrent bacteremic nontyphoidal salmonellosis, and burkholderiosis associated with acquired immunodeficiency due to autoantibody against gamma interferon. Clin Vaccine Immunol. (2010) 17:1132–8. doi: 10.1128/cvi.00053-10
19. Zhang B, Fan J, Huang C, Fan H, Chen J, Huang X, et al. Characteristics and outcomes of anti-interferon gamma antibody-associated adult onset immunodeficiency. J Clin Immunol. (2023) 43:1660–70. doi: 10.1007/s10875-023-01537-0
20. Vanittanakom N, Cooper CR Jr., Fisher MC, Sirisanthana T. Penicillium marneffei infection and recent advances in the epidemiology and molecular biology aspects. Clin Microbiol Rev. (2006) 19:95–110. doi: 10.1128/CMR.19.1.95-110.2006
21. Wong SSY, Siau H, Yuen KY. Penicilliosis marneffei–West meets East. J Med Microbiol. (1999) 48:973–5. doi: 10.1099/00222615-48-11-973
22. Hu Y, Zhang J, Li X, Yang Y, Zhang Y, Ma J, et al. Penicillium marneffei infection: an emerging disease in mainland China. Mycopathologia. (2013) 175:57–67. doi: 10.1007/s11046-012-9577-0
23. Capponi M, Segretain G, Sureau P. Penicillosis from rhizomys sinensis. Bull Soc Pathol Exot Filiales. (1956) 49:418–21.
24. Deng ZL, Yun M, Ajello L. Human penicilliosis marneffei and its relation to the bamboo rat (Rhizomys pruinosus). J Med Vet Mycol. (1986) 24(5):383–9. doi: 10.1080/02681218680000581
25. DiSalvo AF, Fickling AM, Ajello L. Infection caused by Penicillium marneffei: description of first natural infection in man. Am J Clin Pathol. (1973) 60:259–63. doi: 10.1093/ajcp/60.2.259
26. Duong TA. Infection due to Penicillium marneffei, an emerging pathogen: review of 155 reported cases. Clin Infect Dis. (1996) 23:125–30. doi: 10.1093/clinids/23.1.125
27. Chan JF, Lau SK, Yuen KY, Woo PC. Talaromyces (Penicillium) marneffei infection in non-HIV-infected patients. Emerg Microbes Infect. (2016) 5:e19. doi: 10.1038/emi.2016.18
28. Cao C, Liang L, Wang W, Luo H, Huang S, Liu D, et al. Common reservoirs for Penicillium marneffei infection in humans and rodents, China. Emerg Infect Dis. (2011) 17:209–14. doi: 10.3201/eid1702.100718
29. Lang Q, Pasheed Chughtai A, Kong WF, Yan HY. Case report: successful treatment of pulmonary talaromyces marneffei infection with posaconazole in a renal transplant recipient. Am J Trop Med Hyg. (2020) 104:744–7. doi: 10.4269/ajtmh.20-0909
30. Chiu CY, Miller SA. Clinical metagenomics. Nat Rev Genet. (2019) 20:341–55. doi: 10.1038/s41576-019-0113-7
31. Han D, Yu F, Zhang D, Yang Q, Xie M, Yuan L, et al. The real-world clinical impact of plasma mNGS testing: an observational study. Microbiol Spectr. (2023) 11:e0398322. doi: 10.1128/spectrum.03983-22
32. Graf EH, Simmon KE, Tardif KD, Hymas W, Flygare S, Eilbeck K, et al. Unbiased detection of respiratory viruses by use of RNA sequencing-based metagenomics: a systematic comparison to a commercial PCR panel. J Clin Microbiol. (2016) 54:1000–7. doi: 10.1128/JCM.03060-15
33. Xie Y, Du J, Jin W, Teng X, Cheng R, Huang P, et al. Next generation sequencing for diagnosis of severe pneumonia: China, 2010-2018. J Infect. (2019) 78:158–69. doi: 10.1016/j.jinf.2018.09.004
34. Diao Z, Han D, Zhang R, Li J. Metagenomics next-generation sequencing tests take the stage in the diagnosis of lower respiratory tract infections. J Adv Res. (2022) 38:201–12. doi: 10.1016/j.jare.2021.09.012
35. Wang F, Han R, Chen S. An overlooked and underrated endemic mycosis-talaromycosis and the pathogenic fungus talaromyces marneffei. Clin Microbiol Rev. (2023) 36:e0005122. doi: 10.1128/cmr.00051-22
36. Lin CH, Chi CY, Shih HP, Ding JY, Lo CC, Wang SY, et al. Identification of a major epitope by anti-interferon-gamma autoantibodies in patients with mycobacterial disease. Nat Med. (2016) 22:994–1001. doi: 10.1038/nm.4158
37. Browne SK, Zaman R, Sampaio EP, Jutivorakool K, Rosen LB, Ding L, et al. Anti-CD20 (rituximab) therapy for anti-IFN-gamma autoantibody-associated nontuberculous mycobacterial infection. Blood. (2012) 119:3933–9. doi: 10.1182/blood-2011-12-395707
38. Chen Z-M, Yang X-Y, Li Z-T, Guan WJ, Qiu Y, Li SQ, et al. Anti-interferon-γ Autoantibodies impair T-lymphocyte responses in patients with talaromyces marneffei infections. Infection Drug Resistance. (2022) 15:3381–93. doi: 10.2147/idr.S364388
39. Wu UI, Hung CC, Chang SY, Jhong YT, Sun HY, Wang JT, et al. Neutralizing antiinterferon-γ autoantibodies causing disseminated Mycobacterium avium complex infection in an HIV-infected patient on successful combination antiretroviral therapy. Aids. (2017) 31:2557–9. doi: 10.1097/qad.0000000000001644
40. Hong GH, Ortega-Villa AM, Hunsberger S, Chetchotisakd P, Anunnatsiri S, Mootsikapun P, et al. Natural history and evolution of anti-interferon-gamma autoantibody-associated immunodeficiency syndrome in Thailand and the United States. Clin Infect Dis. (2020) 71:53–62. doi: 10.1093/cid/ciz786
41. Ku CL, Lin CH, Chang SW, Chu CC, Chan JF, Kong XF, et al. Anti-IFN-gamma autoantibodies are strongly associated with HLA-DR*15:02/16:02 and HLA-DQ*05:01/05:02 across Southeast Asia. J Allergy Clin Immunol. (2016) 137:945–8.e8. doi: 10.1016/j.jaci.2015.09.018
42. Chetchotisakd P, Anunnatsiri S, Nanagara R, Nithichanon A, Lertmemongkolchai G. Intravenous cyclophosphamide therapy for anti-IFN-gamma autoantibody-associated mycobacterium abscessus infection. J Immunol Res. (2018) 2018:6473629. doi: 10.1155/2018/6473629
43. Laisuan W, Pisitkun P, Ngamjanyaporn P, Suangtamai T, Rotjanapan P. Prospective pilot study of cyclophosphamide as an adjunct treatment in patients with adult-onset immunodeficiency associated with anti-interferon-gamma autoantibodies. Open Forum Infect Dis. (2020) 7:ofaa035. doi: 10.1093/ofid/ofaa035
44. Rocco JM, Rosen LB, Hong GH, Treat J, Kreuzburg S, Holland SM, et al. Bortezomib treatment for refractory nontuberculous mycobacterial infection in the setting of interferon gamma autoantibodies. J Trans Autoimmun. (2021) 4:100102. doi: 10.1016/j.jtauto.2021.100102
45. Angkasekwinai N, Suputtamongkol Y, Tantibhedhyangkul W, Onlamoon N, Phoompoung P, Pithukpakorn M, et al. Efficacy of bortezomib for treating anti-interferon-gamma autoantibody-associated adult-onset immunodeficiency syndrome. Clin Infect Dis. (2024) 78:1033–42. doi: 10.1093/cid/ciad676
Keywords: Talaromyces marneffei, adult-onset immunodeficiency, respiratory failure, immunodeficiency, interferon-γ
Citation: Li K, Zhang Y, Zhang D, Chen Q and Fang X (2024) Case report: Systemic multi-organ involvement in an adult-onset immunodeficiency patient infected with Talaromyces marneffei. Front. Immunol. 15:1430179. doi: 10.3389/fimmu.2024.1430179
Received: 09 May 2024; Accepted: 22 August 2024;
Published: 09 September 2024.
Edited by:
Theresa Marie Rossouw, University of Pretoria, South AfricaReviewed by:
Saul Oswaldo Lugo Reyes, National Institute of Pediatrics (Mexico), MexicoCopyright © 2024 Li, Zhang, Zhang, Chen and Fang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xueling Fang, eHVlbGluZ2ZhbmdAemp1LmVkdS5jbg==; Kun Li, TGlraWN1QHpqdS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.