- 1Department of Obstetrics and Gynecology, West China Second University Hospital, Sichuan University, Chengdu, China
- 2Key Laboratory of Birth Defects and Related Diseases of Women and Children (Sichuan University), Ministry of Education, West China Second University Hospital, Sichuan University, Chengdu, China
Immunotherapy stands as a critical and auspicious therapeutic approach in the fight against cancer nowadays. Immune checkpoint inhibitors, in particular, have garnered widespread employment and delivered groundbreaking therapeutic outcomes across various malignancies. However, the efficacy is unsatisfactory in the ovarian cancer. The pressing concerns of the substantial non-response rate require immediate attention. The pursuit of novel targets and the formulation of synergistic combination therapy approaches are imperative for addressing this challenge. B7-H4, a member of the B7 family of co-inhibitory molecules, exhibits high expression levels in ovarian cancer, correlating closely with tumor progression, drug resistance, and unfavorable prognosis. B7-H4 has the potential to serve as a valuable biomarker for evaluating the immune response of patients. Recent investigations and preclinical trials focusing on B7-H4 in the context of ovarian cancer immunotherapy highlight its emergence as a promising immunotherapeutic target. This review aims to discuss these findings and anticipate the future prospects of leveraging B7-H4 in ovarian cancer immunotherapy and targeted therapy.
1 Introduction
According to the latest global cancer statistics for 2022, it is estimated that there will be over 320,000 new cases of ovarian cancer (OC) worldwide, with approximately 200,000 associated deaths (1). Both the incidence and mortality rates of ovarian cancer (OC) are increasing, with a trend towards affecting younger individuals (2–4). Early symptoms in OC patients often manifest subtly, leading to a low diagnosis rate, and consequently, a prevalence of advanced and metastatic cases. This scenario imposes a significant healthcare burden on women.
In the current treatment approach for OC, an initial evaluation is essential. For patients without contraindications for surgery and where satisfactory Primary cytoreductive surgery (PCS) can be achieved, comprehensive staging surgery should be performed. For patients unable to undergo satisfactory debulking surgery, neoadjuvant chemotherapy followed by interval cytoreductive surgery (ICS) is recommended. The majority of patients require adjuvant chemotherapy postoperatively, with paclitaxel and carboplatin being the standard first-line chemotherapy regimen for advanced ovarian cancer. Depending on the stage and pathological subtype, the addition of bevacizumab to the TC regimen may be considered. Maintenance therapy with PARP inhibitors and/or bevacizumab should be selected based on BRCA and homologous recombination deficiency (HRD) gene test results. Nevertheless, a considerable proportion of women experience relapse, develop drug resistance and ultimately die of the disease.
Over the past two decades, immunotherapy has undergone rapid advancement and reshaped the management landscape of numerous cancers. The most extensively studied and commonly employed immunotherapy for solid tumor is immune checkpoint inhibitor (ICI) therapy, including anti-programmed death-1 (PD-1), anti-PD-L1, and anti-cytotoxic T lymphocyte-associated antigen-4 (CTLA-4), which has demonstrated efficacy in multiple clinical settings. Mounting evidence suggests that OC possesses the capacity to elicit endogenous anti-tumor immune responses, thereby indicating the potential benefits of immunotherapy for patients. Clinical trials reports indicated that the objective response rates to ICI in patients with recurrent or refractory ovarian cancer range from 6% to 15%, with some studies reporting rates as high as 45% (5–8), suggesting an already encouraging outcome. Currently, immunotherapy is recommended by the NCCN guidelines for the treatment of recurrent epithelial ovarian cancer, fallopian tube cancer, and primary peritoneal cancer with MSI-H/dMMR or TMB-H status, including patients sensitive or resistant to platinum-based therapy. Moreover, some combination therapy with ICI exhibits higher response rates compared to monotherapy (5). However, challenges such as low response rates and recurrence persist. Therefore, further investigation into the mechanisms underlying non-response, identification of precise and appropriate targets, and exploration of safer and more effective combination therapy regimens is imperative.
Research increasingly highlights the role of immunosuppression in the development and prognosis of OC. The tumor microenvironment (TME), a complex structure around tumor cells, includes blood vessels, immune cells, fibroblasts, adipocytes, and the extracellular matrix (9, 10). Tumors employ various suppressive mechanisms within the TME to evade host immune surveillance actively (11). Most solid tumors, including OC, harbor infiltrates of immune cells from myeloid and lymphoid lineages, orchestrating the construction of the TME during tumor progression, a phenomenon referred to as Tumor Infiltrating Lymphocytes (TILs) (12). TILs encompass a spectrum of immune cell types, including CD4+ and CD8+ T cells, B lymphocytes, Natural Killer (NK) cells, macrophages, and dendritic cells (DCs) (12, 13). While some TILs eliminate tumor cells, others, like regulatory T cells (Tregs), suppress immune responses, aiding tumor evasion. TILs enhance the expression of interferon-gamma(IFN), interleukin-2(IL-2), and lymphocyte-attracting chemokines in OC, associating with a good prognosis in patients with OC (14–18).
B7-H4, a transmembrane glycoprotein belonging to the B7 family of co-inhibitory molecules, exhibits widespread expression in various malignancies, particularly OC (19, 20). It is implicated in multiple processes including tumor development, immune evasion, and immune resistance (21–23). Several monoclonal antibodies, bispecific antibodies, and antibody-drug conjugates (ADCs) targeting B7-H4 have progressed to preclinical or clinical trials, demonstrating promising initial results. This review consolidates the research advancements concerning B7-H4 in OC immunotherapy and presents future perspectives.
2 B7 family and B7-H4
B7-H4, also called B7x、B7h.5、B7-Homolog 4 (B7-H4、v-set domain containing T cell activation inhibitor 1 (VTCN1), or B7 superfamily member 1 (B7S1), was first identified in 2003 (24, 25). It belongs to the type I transmembrane glycoprotein class and is a constituent of the B7/CD28 superfamily, situated on chromosome 1p12/13.1. Encoded by the VTCN1 gene, B7-H4 negatively regulates T-cell function (26–29). The B7 family plays a pivotal role in modulating the immune response, preventing excessive activation. The B7 family, which includes co-stimulatory and co-inhibitory molecules, is one of the most crucial second-signaling pathways in T-signaling activation. B7-H4 emerges as a novel member of the B7 family of co-inhibitory molecules, functioning as an immune checkpoint modulator involved in regulating anti-inflammatory and immune responses. Structurally, B7-H4 comprises two immunoglobulin (Ig)-like domains and a large hydrophobic trans-membrane domain followed by two intracellular amino acids. It shares variable levels of amino acid sequence identity with other B7 family members: B7-1 (12%), B7-2 (13%), PD-L1 (18%), PD-L2 (18%), and B7-H3 (24%) (30). B7-H4 generally expresses on antigen-presenting cells (APCs) and is induced by local cytokine production such as Il-6, IL-10, and hypoxia (31, 32). The receptor for B7-H4, distinct from other members of the CD28 receptor family, is expressed on activated T cells (30), suggesting a potential role for B7-H4 in the regulation of T-cell activation and exhaustion.
B7-H4 exhibits widespread expression across various malignancies and is inversely associated with patient prognosis and T cell infiltration within tumors. Moreover, it is implicated in potential associations with drug resistance (31, 33–35). Currently, the role of B7-H4 in tumor immunity has been partially elucidated in solid tumors such as liver cancer, breast cancer and OC, yet further research is needed to fully uncover its complete and potential mechanisms. Additionally, there may be more than one receptor for B7-H4 on the surface of T cells, and the precise molecular structures of these receptors remain undetermined.
3 Expression and clinical significance of B7-H4 in OC
B7-H4 expression is notably minimal, if not entirely absent, in normal ovarian tissue as well as other healthy tissues such as lung, liver, pancreas, spleen, thymus, and kidney (36). Nonetheless, elevated levels of B7-H4 mRNA have been detected in these tissues, indicating potential translational or post-transcriptional regulatory mechanisms governing its expression. Of note, B7-H4 exhibits significant upregulation in tumor tissues of OC patients (19–21, 37–40), a trend widely associated with advanced cancer stage and heightened aggressiveness according to the majority of studies. This suggests that B7-H4 exhibits a distinct expression pattern compared to PD-L1 in human cancers, characterized by heightened sensitivity and specificity. B7-H4 predominantly localizes on the membrane surface of antigen-presenting cells (APCs) and both intracellular and membrane surfaces of OC cells. Notably, tumor-associated macrophages (TAMs) expressing membrane-bound B7-H4 rather than tumor cells, exert an inhibitory influence on T cell immunity (27, 31, 41, 42), suggesting the importance of elucidating the mechanism and functional implications of B7-H4’s subcellular localization in OC. In OC cells, TAMs induce regulatory T cells (Tregs) to secrete IL-6 and IL-10, thereby promoting B7-H4 expression on APCs. B7-H4 overexpression has been documented in 48%, 55%, and 67% of patients at stages I, II, and advanced stages of OC, respectively (37). Additionally, B7-H4 is highly expressed in primary and metastatic serous, endometrioid, clear cell, and epithelial ovarian cancers, while its expression is lower in mucinous and non-epithelial ovarian cancers (37, 43–45). Zang et al. collected tumor tissue samples from 103 patients with epithelial ovarian cancer and constructed a tissue microarray, demonstrated 100% expression of B7-H4 in ovarian junction tumors and OC. While the intensity of B7-H4 expression varied among different pathological subtypes, these differences did not reach statistical significance (21, 46). Further studies incorporating a larger sample size are needed for more comprehensive investigation. Recent studies have consistently demonstrated that B7-H4 is upregulated in 92% of high-grade serous ovarian carcinoma (HGSOC) cases at diagnosis (n = 12) and maintains stable or increased expression following standard-of-care chemotherapy. Moreover, B7-H4 remains consistently overexpressed or more highly expressed across metastatic sites even after the development of multidrug resistance (47).
A soluble form of B7-H4 exists, and soluble B7-H4(sB7-H4) protein can be detected in the serum or plasma of OC patients. Simon et al. showed that the sensitivity of OC detection increases from 52% for CA-125 alone to 65% with 97% specificity when used in combination with B7-H4. Simon et al. also demonstrated that serum B7-H4 levels are more stable compared to CA-125 and do not fluctuate in patients with inflammatory diseases or during pregnancy (45, 48). Gyllensten et al. employed high-precision proteomics to identify 1,463 plasma proteins and validated their findings using two cohorts of previously untreated patients with benign or malignant ovarian tumors (n=111 and n=37, respectively). They found that the positivity rate of sB7-H4 was significantly higher in patients with malignant OC compared to those with benign ovarian lesions, suggesting that B7-H4 may serve as a potential adjunctive plasma biomarker (49). The meta-analysis conducted by Zhu et al. indicated that overall diagnostic sensitivity and specificity of serum B7-H4 in OC were 0.782 (95% confidence interval [CI]: 0.732–0.825) and 0.870 (95% CI: 0.804–0.916), respectively. The Summary Receiver Operating Characteristic Curve(SROC) analysis revealed that the combined detection of B7-H4 and CA-125 for OC had a higher Area Under Curve(AUC) than B7-H4 alone (0.94 vs. 0.86) (50). The combination of B7-H4 and CA-125 may enhance the early and effective screening of OC. However, further validation through additional multicenter and randomized controlled trials is needed to more precisely determine the clinical utility of B7-H4 (19, 44, 45, 51, 52). Mach et al. detected soluble B7-H4 (sB7-H4) in 12 out of 85 patients with advanced epithelial ovarian cancer (EOC) and also collected circulating tumor cells (CTCs). They indicated that positivity for sB7-H4 in EOC patients is associated with poorer overall survival (OS) and platinum resistance (53).
The majority of studies have linked B7-H4 with unfavorable prognostic outcomes and a heightened recurrence rate in OC (22, 23, 54). However, Liang et al. demonstrated that heightened B7-H4 expression in tumor tissue of patients with ovarian plasmacytoid carcinoma does not correlate with OS or disease-free survival, but it is associated with advanced tumor stage (43). Overall, confounding factors such as different B7-H4 assays and sample sizes may contribute to different results. Further investigations are warranted to elucidate the expression profiles of B7-H4 across various pathological subtypes of OC and its correlation with tumor grading, staging, and prognosis.
In summary, B7-H4 emerges as a promising biomarker for early cancer detection, prediction of immunotherapy response, and evaluation of patient prognosis in OC (21, 23, 36–38, 53, 55). Further exploration is warranted into the regulatory mechanisms driving B7-H4 overexpression in ovarian carcinogenesis, along with delineating the specific impacts of its various subcellular localizations within tumor cells, particularly the role of intracellularly expressed B7-H4, which necessitates deeper investigation.
4 The role of B7-H4 in tumor immunity of OC
4.1 Immune regulation by B7-H4 in the TME of OC
Tolerance in the TME is a dynamic and intricate process characterized by a network of interactions among diverse cell types (56). The immunosuppressive TME of OC predominantly comprises T and B lymphocytes, T regs, NK cells, tumor-associated macrophages (TAMs), and myeloid-derived suppressor cells (MDSCs). Antigen-presenting cells (APCs) play a pivotal role in initiating and sustaining tumor-associated antigen (TAA)-specific T-cell immunity, which significantly impacts survival and recurrence rates in OC (31, 57). TAMs, the most abundant APCs within the OC TME, prominently express B7-H4, with levels surpassing 70% (52). The intensity of B7-H4 expression with TAM in OC positively correlates with the intratumoral Treg population. Moreover, the presence of more Tregs further releases more IL-6 and IL-10, which continue to induce B7-H4 expression on APCs, including TAM and M2 macrophages (27, 31, 34, 52, 58, 59). This cascade establishes a positive feedback loop that fosters an immunosuppressive TME. The interaction between B7-H4 and its receptor on T cells leads to diminished T cell proliferation by limiting the entry of CD4+ and CD8+ T cells into the cell cycle and decreasing their division rate. Furthermore, it inhibits the secretion of cytokines by T cells, such as IL-2, and IFN-γ (24, 25, 30, 60). Additionally, B7-H4 overexpression can protect tumor cell from apoptosis and facilitates their growth in OC (19). Cai et al. found that B7-H4 expression on APC negatively correlates with infiltration and cytolytic function of CD8+ TILs, but no significant correlation was found between B7-H4 expression and tumor grade or stage (40). These observations may partly stem from incongruent findings attributable to variances in B7-H4 assay methodologies and the limited scale of sampled populations.
Some studies have presented alternative perspectives. An investigation focusing on ovarian serous carcinoma indicated a positive correlation between B7-H4 expression and TILs, while noting that B7-H4 expression was not inducible by interleukin-4 (IL-4), IL-6, or IL-10. This suggests that B7-H4 might not actively participate in immune evasion mechanisms, although it does affirm that heightened B7-H4 expression correlates with higher tumor grade and lower overall survival (61). Pagnotti G M et al. found no association between B7-H4 protein level and the infiltration degree of CD3+, CD4+, CD8+, and CD14+ lymphocytes in serous or endometrioid OC, but observed an inverse correlation in clear cell OC (62). MacGregor et al. reported no discernible relationship between B7-H4 expression levels and the abundance or phenotype of T and B cells, nor any interaction between B7-H4 and other inhibitory ligands such as PD-1, Tim-3, or LAG3 (26).
It has been reported in other tumors that B7-H4 participates in modulating intracellular oncogenic signaling pathways, with intracellular localization of B7-H4 facilitating signals conducive to tumor cell proliferation. As mentioned before, a high intracellular B7-H4 expression state has been found in OC tumor cells. Difference in subcellular localization of B7-H4 suggests potential differential roles in tumor development, a facet yet to be comprehensively elucidated in the context of OC. In conclusion, B7-H4 in OC TME inhibits the activation and function of effector T cells, promotes the suppression of immune responses by immunosuppressive cells, and protects tumor cells from apoptosis. These contributes to tumor immune evasion and tumor progression (Figure 1).
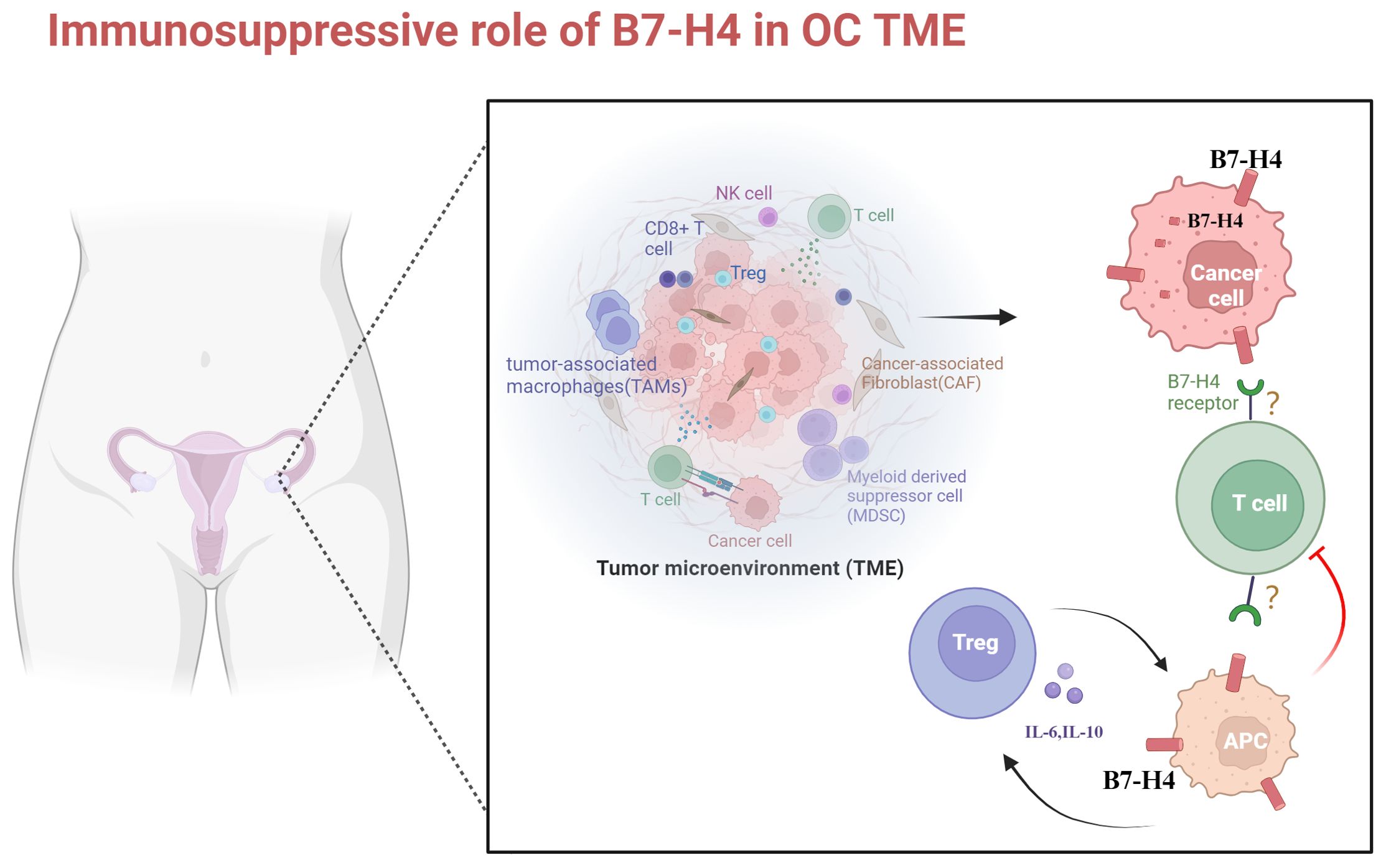
Figure 1. The role of B7-H4 in microenvironmental immunosuppression in ovarian cancer. OC is a cold tumor. The immunosuppressive TME of OC predominantly comprises T and B lymphocytes, T regs, NK cells, TAMs, and MDSCs. B7-H4 is highly expressed in OC cells and APCs. Its elevated expression in OC cells inhibits apoptosis. Upon binding to unidentified receptors on T cells, B7-H4 inhibits T cell proliferation and cytokine secretion. The expression intensity of B7-H4 on APCs positively correlates with the number of Tregs, leading to increased release of IL-6 and IL-10. This further induces overexpression of B7-H4 on APCs, fostering an immunosuppressive TME in OC. TME, tumor microenvironment; NK cell, natural killer cell; TAMs, tumor-associated macrophages; MDSCs, myeloid-derived suppressor cells; APC, antigen-presenting cell. Created with BioRender.com.
4.2 B7-H4 is involved in the antitumor immunity of OC and interacts synergistically with other immune checkpoints
In recent years, it has emerged that heightened expression of B7-H4 within tumor-infiltrating antigen-presenting cells in the microenvironment of hepatocellular carcinoma suppresses the proliferation and cytotoxicity of CD8+ TIL cells by inducing up-regulation of the transcription factor eomesodermin. This phenomenon promotes TIL depletion, thereby facilitating tumor progression and inhibiting antitumor immune responses (27). They also revealed that an intricate interplay exists between B7-H4 signaling and other pivotal pathways, such as immunomodulatory signaling. B7-H4 knockout notably impacts cell cycle and NF-kB pathways, resulting in up-regulation of co-stimulatory ligands or receptors (e.g., ICOSL, CD27, CD28), while concurrently down-regulating certain co-inhibitory receptors (e.g., LAG3, CTLA4) and up-regulating others (e.g., LILRB4, PDCD1).
However, in OC, the co-expression of B7-H4 with other immune checkpoints and the specific mechanisms of their interactions have not yet been fully elucidated. Cai et al. collected fresh tumor tissue from 32 chemotherapy-naive patients with newly diagnosed OC and found a notable positive correlation between the expression of B7-H4 and several immune co-inhibitory checkpoints, including CTLA4, HAVR2, LAG3, TIGIT, and C10orf54 (40).
Previous researches have indicated a negative or non- correlation between B7-H4 and PD-L1 in various tumors, characterized by low rates of double-positive expression (63–67). The same expression profile has been observed in OC. However, elevated levels of co-expression of B7-H4 and PD-L1 have been found in ovarian clear cell carcinoma, suggesting a potential suitability for combinatorial therapeutic approaches (62, 68), although further validation through trials is needed.
Cai et al. also demonstrated that B7-H4 was mainly involved in diverse OC anti-tumor immune responses and signaling pathways, including but not limited to IL-2/signal transducer and activator of transcription (STAT)5 signaling, the p53 pathway, mammalian target of rapamycin complex 1 (mTORC1) signaling, and apoptosis (40). Although the specific mechanisms remain incompletely understood, the important role of B7-H4 in antitumor immunity in OC has been emphasized, suggesting that it is a potential target for immunotherapy and combinatorial immunotherapy.
5 Preclinical or clinical trials of B7-H4 in immunotherapy of OC
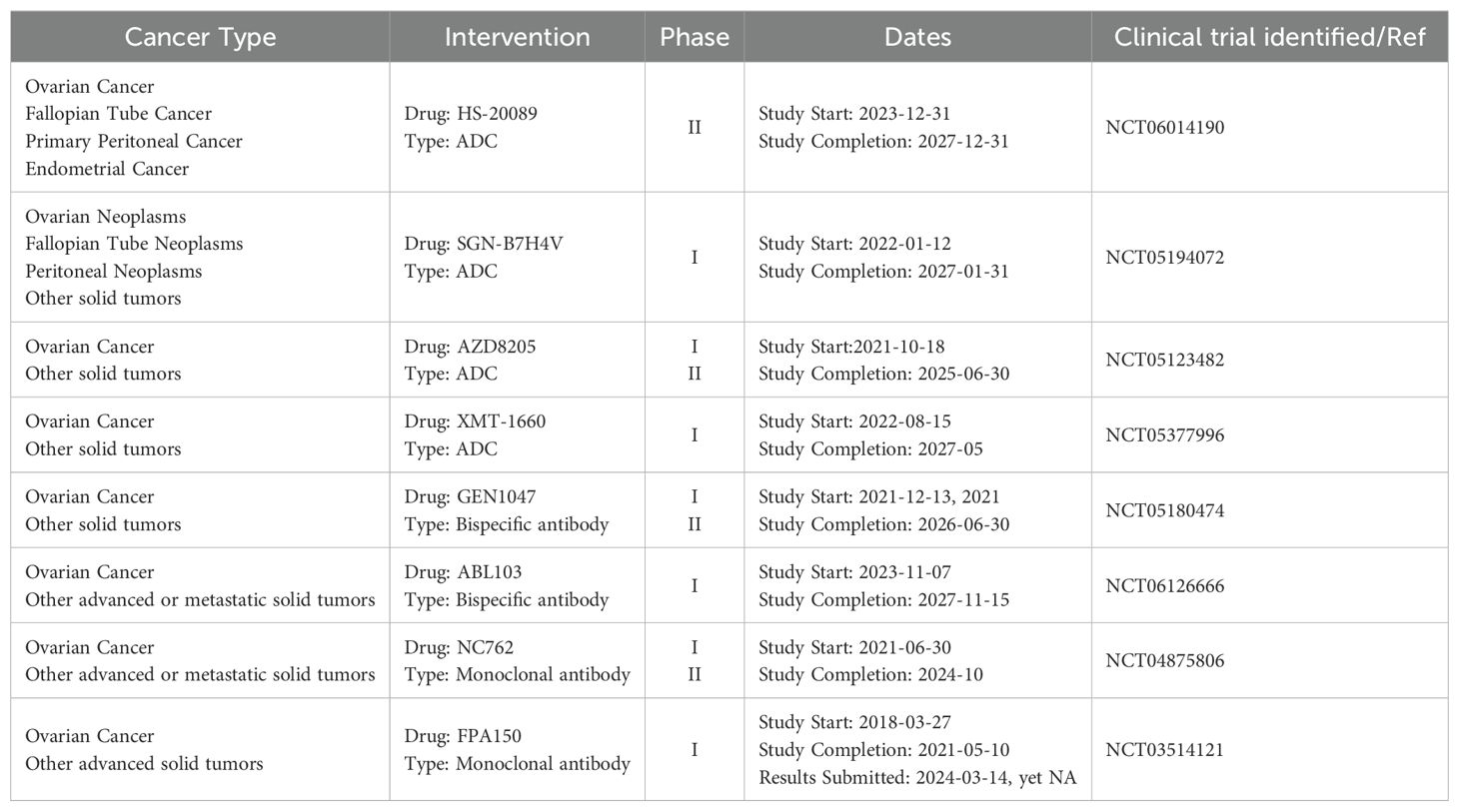
Currently, the focus of immunotherapies targeting B7-H4 in OC revolves around ICI, adoptive cell therapy(ACT), and antibody-drug conjugates (ADC) (69). However, the efficacy of ICI monotherapy in OC is suboptimal, necessitating further investigation into novel immune targets and combination therapeutic modalities, which are still in the exploratory and clinical trial phases and require additional validation through rigorous research. B7-H4 presents an attractive target for cancer immunotherapy, with potential blockade via various mechanisms, including monoclonal antibodies (mAbs), single chain fragment variables(scFv), antibody-drug conjugates (ADCs), CD3 bispecific antibodies (BiTEs), and chimeric antigen receptor T cells (CAR-Ts) in OC (70–73). We provide a comprehensive summary of both preclinical and clinical trials investigating B7-H4 in the context of OC immunotherapy in recent years (Tables 1, 2).
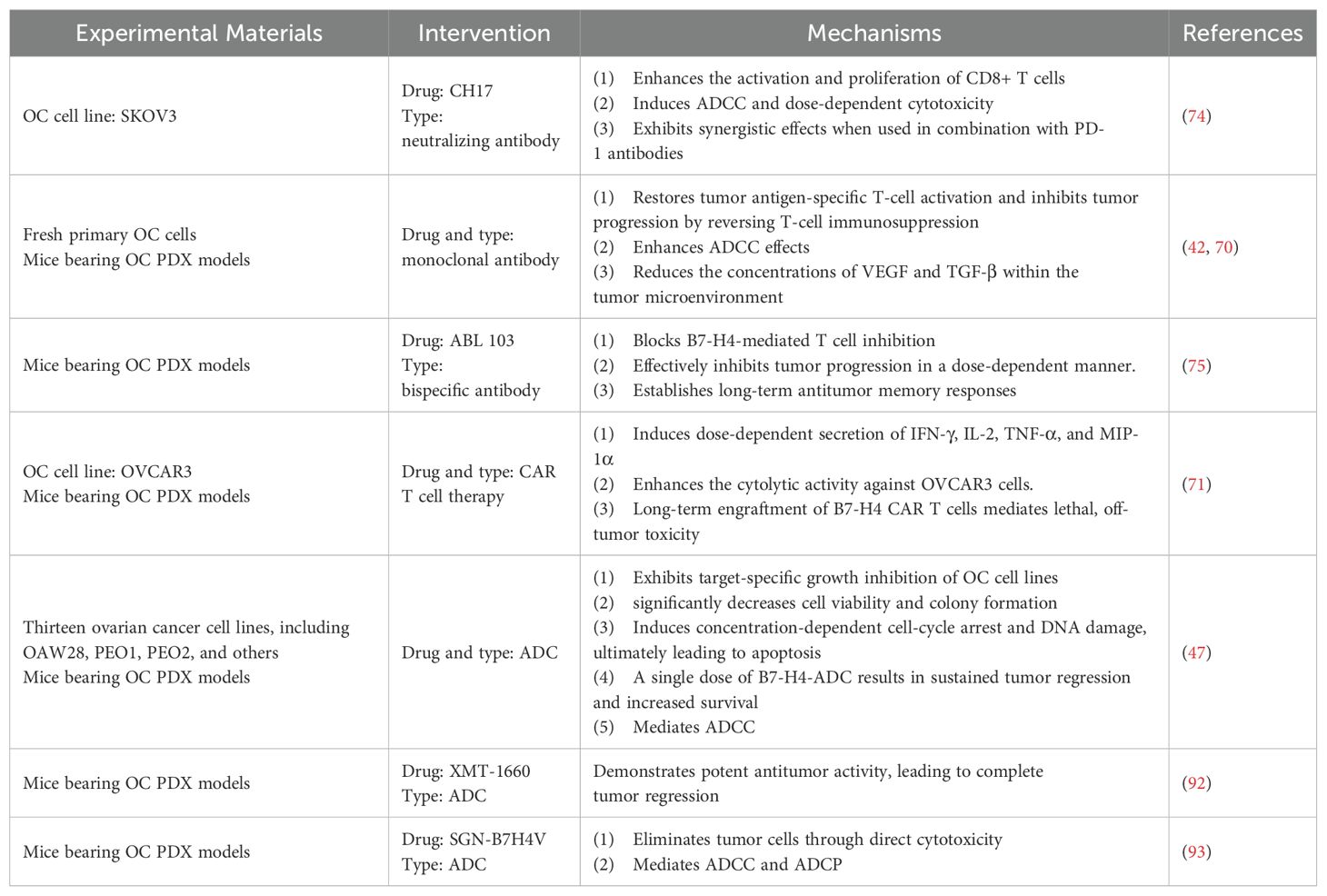
Table 1. Preclinical trials of B7-H4 in immunotherapy for ovarian cancer (PDX, patient-derived xenograft; CDX: cell line-derived xenograft; ADCC, antibody-dependent cellular cytotoxicity; ADCP, Antibody dependent cell-mediated phagocytosis).
5.1 Preclinical studies of B7-H4 in OC
5.1.1 B7-H4 and ICI
Enhancing the tumor-killing capability of immune cells by reinstating T cell functionality through the blockade of B7-H4 inhibitory immune checkpoint signaling is a very competitive option. Miao et al, developed a neutralizing antibody against B7-H4 named CH17, can enhance antigen specific T cell responses in OC cell line (SKOV3). Notably, the B7-H4 antibody(CH17) exhibited robust in vivo anti-tumor efficacy in a human T-cell transplantation xenograft model and synergistic effects when combined with anti-PD-1 antibody (74). Monoclonal antibody directed against B7-H4 have shown promising outcomes in inhibits tumor progression by blocking T-cell immunosuppression and augmenting antibody-dependent cellular cytotoxicity(ADCC) effects in OC, concurrently reducing the concentrations of VEGF and TGF-β within the TME (42, 70).
On the other hand, development of drugs featuring dual inhibitory action represents a particularly promising avenue. In recent research, ABL103, a novel T-cell engaging bispecific antibody designed to target both B7-H4 and 4-1BB, was developed. ABL103 operates through a dual mechanism, enhancing T cell functionality by inhibiting B7-H4 while simultaneously activating 4-1BB. This novel approach has yielded robust in vitro and vivo anti-tumor activity, coupled with a favorable safety profile, achieved through B7-H4-dependent 4-1BB activation in the TME of OC. Moreover, long-term anti-tumor response memory can be established in OC model mice (75). Chang et al, engineered a novel anti-B7-H4/IL15 fusion antibody, which enhances the immunogenicity of the TME by fostering the proliferation of CD8+ T cell and facilitating the elimination of B7H4-expressing tumor cells by activated immune cells, including ADCC dependent cellular cytotoxicity (76).These studies strongly suggest bispecific antibodies targeting B7-H4 is a promising therapeutic agent.
Of note, it is possible that the combination of PD-1 and B7-H4 blockade may offer enhanced efficacy and safety compared to the combination of PD-1 and CTLA-4 blockade. There exists the potential for synergistic blockade by concurrently targeting B7-H4 and PD-1 in OC, though this necessitates further validation. In addition, the safety and clinical efficacy of B7-H4 in combination with other ICIs in OC need to be further explored. In conclusion, given OC’s potential for mediating immunosuppression through multiple immunosuppressive checkpoints, and the likelihood of complementary effects among different inhibitory checkpoints, monotherapy with a single immune checkpoint inhibitor is expected to be insufficient. Therefore, combination therapy with ICIs is imperative in the future, especially in the treatment of advanced or recurrent OC, emphasizing the importance of combination drug regimens.
5.1.2 B7-H4 and chimeric antigen receptor T cells
Chimeric antigen receptors (CARs) are synthetic receptors engineered to endow T cells with the capability to recognize tumor-associated antigens (TAAs) in a manner independent of major histocompatibility complex (MHC) presentation (77–80). CAR-T therapy stands as the pioneering genetically modified cell-based therapeutic endorsed by the US Food and Drug Administration (81–84). However, obstacles to the use of CAR-T in solid tumors such as OC, primarily attributed to the heterogeneity of TAA, limited transport and infiltration within solid tumors owing to their substantial dimensions, and the ubiquitous expression of target antigens across vital healthy tissues.
The expression of B7-H4 on tumor cells surfaces provides an avenue for targeted therapy utilizing T cells expressing CARs. Notably, in a mouse ovarian tumor xenograft model, a CAR targeting both human and mouse B7-H4 exhibited efficacy in inducing tumor regression, marking the inaugural instance of CAR T cell therapy targeting B7-H4. However, this therapeutic approach precipitated delayed and ultimately fatal lung tumor toxicity after 6-8 weeks, thereby constraining further applications of CAR-T (71). Analysis of post-mortem mice revealed that multi-organ lymphocytic infiltration was predominantly associated with membranous B7-H4-positive tissue expression, with extensive histological lesions observed in associated with B7-H4(+) expression. Notably, lesions were also evident in tissues lacking B7-H4 expression. Furthermore, while robust expression of B7-H4 protein was observed in ovarian cancer tissues, varying degrees of expression, ranging from weak to strong, were also detected in human mammary glands, kidneys, pancreatic islet cells, esophagus, salivary glands, and liver, thus updating prior assumptions regarding the spectrum of B7-H4 expression in healthy human tissues. Consequently, the clinical application of CAR-T targeting B7-H4 in OC warrants careful consideration, particularly concerning the potential for fatal non-tumor toxicity associated with prolonged use.
Efforts to enhance the efficacy of B7-H4 CAR-T therapy include strategies for reducing dosing duration, such as utilizing transient CAR expression through inducible suicide genes (85) or RNA electroporation (77, 86), as well as investigating combination therapies to augment anti-tumor efficacy. Additionally, exploring the withdrawal of B7-H4 CAR-T prior to onset of lethal toxicity represents a prospective approach. Despite the potential for a broad spectrum of toxic effects, the excellent targeting effect and precise anti-tumor efficacy of B7-H4 CAR-T still underscore its status as a promising therapeutic avenue in OC, deserving more research in the future.
5.1.3 B7-H4 and ADCs
B7-H4 presents a viable target for cytotoxic therapy or immune-mediated killing (87). Monoclonal antibodies against B7-H4 can induce cell killing through ADCC, as previously mentioned (42). However, a more direct cytotoxic strategy involves the use of antibody-drug conjugates (ADCs) (88), a well-established class of targeted therapies across various cancers, including OC (89). Following recent FDA approvals of mirvetuximab soravtansinegynx(Elahere) for OC, ADCs, often regarded as “smart chemotherapy”, deserve more investment and greater application prospects (90, 91). ADCs comprise an antibody, a cytotoxic payload, and a linker connecting them. The tumor-specific antigenic properties of the B7-H4 protein, characterized by its heightened expression in malignant tumors while low or no expression in normal tissues, make it one of the promising targets for ADCs.
A recent study has yielded promising outcomes regarding ADC agents targeting B7-H4 in patient-derived xenograft (PDX) models of OC. Notably, in PDX models of PARPi and platinum-resistant HGSOC, scheduled administration of B7-H4-ADC demonstrated sustained tumor regression and increased overall survival (OS) (47). Moreover, this study elucidated that B7-H4-ADC induces concentration-dependent cell-cycle arrest and DNA damage. In addition, B7-H4-ADC has bystander killing activity, thereby augmenting its efficacy and potentially targeting OC cases with low or moderate B7-H4 expression levels. Toader et al, reported XMT-1660, an ADC targeting B7-H4, showing potent anti-tumor activity in a PDX model of OC. It revealed ADCs may be effective in patients refractory or resistant to immune checkpoint inhibitors (92). Gray et al, have pioneered a novel investigational vedotin ADC named SGN-B7H4V. They validated its potent antitumor activity in the OC PDX model and observed enhanced effectiveness when combined with anti-PD-1 agents (93). Kinneer et al, developed AZD8205, a B7-H4-directed ADC, and preliminary data indicate that its combination with PARPi can sensitize triple negative breast cancer(TNBC) PDX tumors expressing low levels of B7-H4 (39).
Overall, the current studies reinforce the notion of B7-H4 as an attractive target for ADCs, indicating potential for enhanced efficacy when utilized in combination with immunosuppressants or PARP inhibitors (Figure 2).
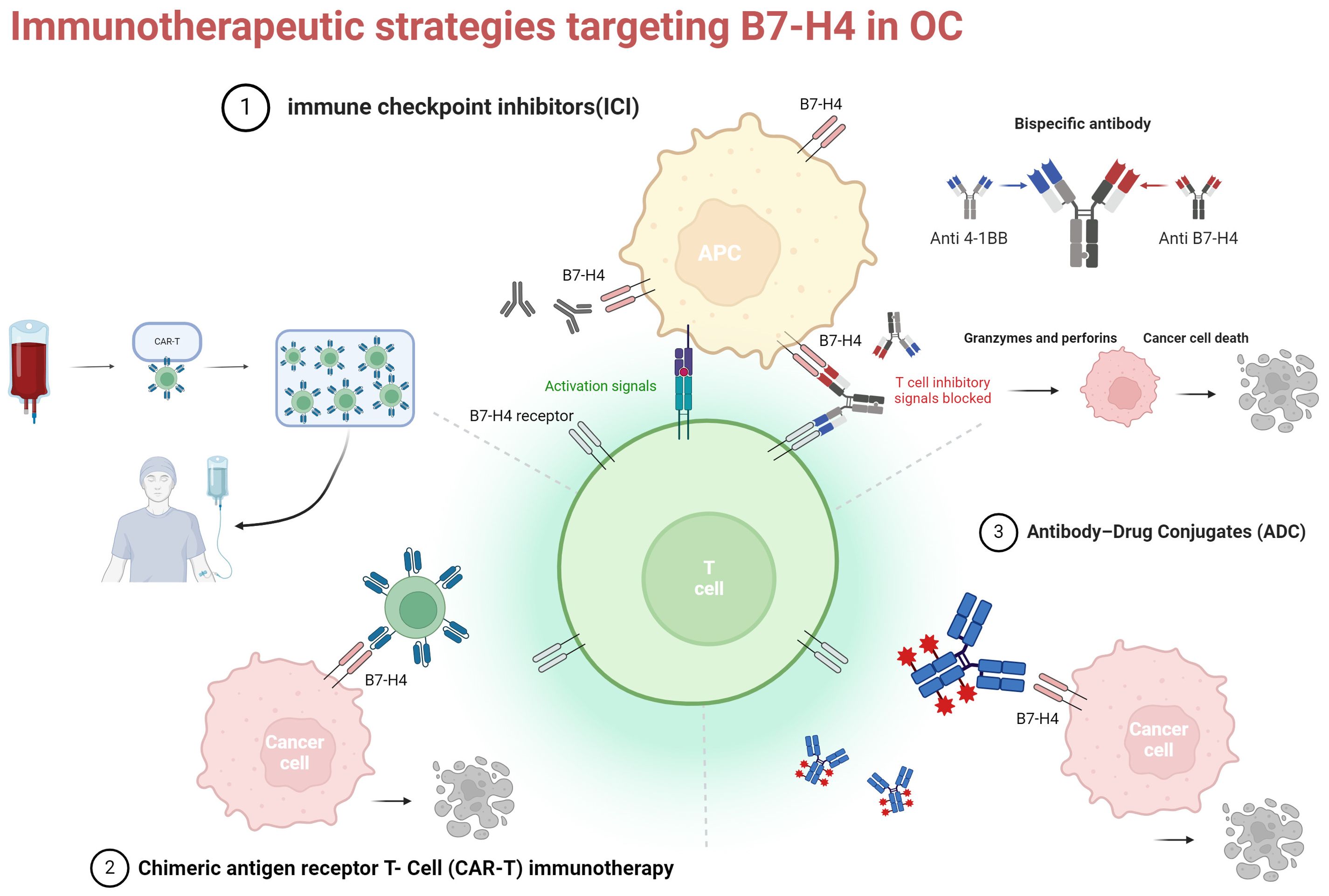
Figure 2. Three current immunotherapy strategies for targeting B7-H4 in OC. ICI, immune checkpoint inhibitors: monoclonal antibodies and bispecific antibodies (example illustration with bispecific antibodies targeting B7-H4 and 4-1BB); CAR-T, chimeric antigen receptor t-cell; ADC, antibody-drug conjugates. Created with BioRender.com.
5.2 Clinical trials of B7-H4 in OCA
Phase 1a/1b clinical trial evaluating the anti-B7-H4 antibody(FPA150) commenced in patients with advanced solid tumors, including OC, aiming to assess the safety, tolerability, and preliminary efficacy of FPA150 either as monotherapy or in combination with anti-PD1 therapy (38, 94). Initial findings reported in 2019 showed a favorable safety profile for FPA150; however, subsequent data regarding its efficacy as monotherapy in OC patients have not been disclosed. The clinical Phase I and Phase II trials of the monoclonal antibody NC762 and the bispecific antibody GEN1047 are currently underway. Another Phase I clinical trial of the bispecific antibody ABL-103 is also currently underway.
Several B7-H4-ADCs are being explored in patients afflicted with metastatic or recurrent OC. The clinical trials of SGN-B7H4V, AZD8205 and XMT-1660 in Phase I or Phase II hold promise, and we eagerly await forthcoming data to clarify their efficacy in OC. Recently, initial results have been published from a Phase I investigation of HS-20089, a novel B7-H4-targeted ADC. HS-20089 showed great anti-tumor activity in OC, yielding an objective response rate (ORR) of 66.7% and a disease control rate (DCR) of 100% in platinum-resistant ovarian cancer(PROC) (95). Subsequent research data are eagerly anticipated (Table 1).
6 Conclusion
We summarize the expression and clinical significance of B7-H4 in OC, discuss the current landscape of immunotherapy research in OC, recent advancements, and delineate future research directions aimed at deeper elucidation of B7-H4’s role in OC. The expression pattern of B7-H4 distinguishes it from PD-1 and CTLA-4, with high mRNA expression and low protein expression in normal tissues, while demonstrating widespread expression in malignant tumors, particularly in OC. This distinct expression pattern suggests that B7-H4 holds greater tumor specificity and sensitivity compared to PD-1 and CTLA-4, rendering it a promising emerging target for tumor therapy. Compared with the studies of B7-H4 in other types of tumors, there are still a lot of mist unknown in OC, underscoring the imperative for in-depth exploration of B7-H4 in the context of OC.
Despite the fact that much remains unknown about B7-H4 in OC, evidence has already substantiated its status as a highly promising and emerging immunotherapeutic target for OC. In summary, it is imperative to elucidate the immunoregulatory pathways and expression patterns of B7-H4 in ovarian cancer, identify its receptor(s), investigate downstream mechanisms of B7-H4 with effector T cells and other APC surface receptors, examine its role within the ovarian cancer microenvironment, including potential variances across different histological subtypes. Explore predictive biomarkers for B7-H4 immunotherapy specificity, mechanisms of drug resistance, devise combination therapies with different immune checkpoints, and develop multi-strategy immunotherapeutic drugs targeting B7-H4. These endeavors hold the promise of expanding the repertoire of immunotherapeutic options and improving the prognosis of OC patients.
Author contributions
LZ: Writing – original draft, Conceptualization. YD: Writing – review & editing. KF: Writing – original draft. MZ: Writing – review & editing. KL: Writing – review & editing. RY: Writing – review & editing, Funding acquisition.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Key Project of Sichuan Provincial Department of Science and Technology(2019YFS0532)and Study on the key factors affecting the diagnosis and treatment of major diseases in obstetrics and gynecology. (Ethical Lot Number:20220129). Horizontal Science and Technology Project of Sichuan University (23H1223) and Real-world study on targeted immunotherapy for gynecological malignant tumors. (Ethical Lot Number:20230423).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin. (2024) 74:229–263. doi: 10.3322/caac.21834
2. Han B, Zheng R, Zeng H, Wang S, Sun K, Chen R, et al. Cancer incidence and mortality in China, 2022. J Natl Cancer Center. (2024) 4:47–53. doi: 10.1016/j.jncc.2024.01.006
3. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA: Cancer J Clin. (2020) 70:7–30. doi: 10.3322/caac.21590
4. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
5. Hinchcliff E, Hong D, Le H, Chisholm G, Iyer R, Naing A, et al. Characteristics and outcomes of patients with recurrent ovarian cancer undergoing early phase immune checkpoint inhibitor clinical trials. Gynecologic Oncol. (2018) 151:407–13. doi: 10.1016/j.ygyno.2018.10.008
6. Hamanishi J, Mandai M, Ikeda T, Minami M, Kawaguchi A, Murayama T, et al. Safety and antitumor activity of anti-PD-1 antibody, nivolumab, in patients with platinum-resistant ovarian cancer. J Clin Oncol. (2015) 33:4015–22. doi: 10.1200/JCO.2015.62.3397
7. Varga A, Piha-Paul SA, Ott PA, Mehnert JM, Berton-Rigaud D, Johnson EA, et al. Antitumor activity and safety of pembrolizumab in patients (pts) with PD-L1 positive advanced ovarian cancer: Interim results from a phase Ib study. J Clin Oncol. (2015) 33:5510–0. doi: 10.1200/jco.2015.33.15_suppl.5510
8. Disis ML, Patel MR, Pant S, Hamilton EP, Lockhart AC, Kelly K, et al. Avelumab (MSB0010718C; anti-PD-L1) in patients with recurrent/refractory ovarian cancer from the JAVELIN Solid Tumor phase Ib trial: Safety and clinical activity. J Clin Oncol. (2016) 34:5533–3. doi: 10.1200/JCO.2016.34.15_suppl.5533
9. Antonio MJ, Zhang C, Le A. Different tumor microenvironments lead to different metabolic phenotypes. Adv Exp Med Biol. (2021) 1311:137–47. doi: 10.1007/978-3-030-65768-0_10
10. Kao KC, Vilbois S, Tsai CH, Ho PC. Metabolic communication in the tumour-immune microenvironment. Nat Cell Biol. (2022) 24:1574–83. doi: 10.1038/s41556-022-01002-x
11. Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol. (2008) 8:467–77. doi: 10.1038/nri2326
12. Santoiemma PP, Powell DJ. Tumor infiltrating lymphocytes in ovarian cancer. Cancer Biol Ther. (2015) 16:807–20. doi: 10.1080/15384047.2015.1040960
13. Kazemi MH, Sadri M, Najafi A, Rahimi A, Baghernejadan Z, Khorramdelazad H, et al. Tumor-infiltrating lymphocytes for treatment of solid tumors: It takes two to tango? Front Immunol. (2022) 13:1018962. doi: 10.3389/fimmu.2022.1018962
14. Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. New Engl J Med. (2003) 348:203–13. doi: 10.1056/NEJMoa020177
15. Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci United States America. (2005) 102:18538–43. doi: 10.1073/pnas.0509182102
16. de Jong RA, Leffers N, Boezen HM, ten Hoor KA, van der Zee AGJ, Hollema H, et al. Presence of tumor-infiltrating lymphocytes is an independent prognostic factor in type I and II endometrial cancer. Gynecologic Oncol. (2009) 114:105–10. doi: 10.1016/j.ygyno.2009.03.022
17. Piersma SJ, Jordanova ES, van Poelgeest MIE, Kwappenberg KMC, van der Hulst JM, Drijfhout JW, et al. High number of intraepithelial CD8+ tumor-infiltrating lymphocytes is associated with the absence of lymph node metastases in patients with large early-stage cervical cancer. Cancer Res. (2007) 67:354–61. doi: 10.1158/0008-5472.CAN-06-3388
18. Shah W, Yan X, Jing L, Zhou Y, Chen H, Wang Y. A reversed CD4/CD8 ratio of tumor-infiltrating lymphocytes and a high percentage of CD4+FOXP3+ regulatory T cells are significantly associated with clinical outcome in squamous cell carcinoma of the cervix. Cell Mol Immunol. (2011) 8:59–66. doi: 10.1038/cmi.2010.56
19. Salceda S, Tang T, Kmet M, Munteanu A, Ghosh M, Macina R, et al. The immunomodulatory protein B7-H4 is overexpressed in breast and ovarian cancers and promotes epithelial cell transformation. Exp Cell Res. (2005) 306:128–41. doi: 10.1016/j.yexcr.2005.01.018
20. Tringler B, Liu W, Corral L, Torkko KC, Enomoto T, Davidson S, et al. B7-H4 overexpression in ovarian tumors. Gynecologic Oncol. (2006) 100:44–52. doi: 10.1016/j.ygyno.2005.08.060
21. Zang X, Sullivan PS, Soslow RA, Waitz R, Reuter VE, Wilton A, et al. Tumor associated endothelial expression of B7-H3 predicts survival in ovarian carcinomas. Modern pathology. (2010) 23:1104–12. doi: 10.1038/modpathol.2010.95
22. Song X, Shao Y, Gu W, Xu C, Mao H, Pei H, et al. Prognostic role of high B7-H4 expression in patients with solid tumors: a meta-analysis. Oncotarget. (2016) 7:76523–33. doi: 10.18632/oncotarget.8598
23. Niu N, Shen W, Zhong Y, Bast RC, Jazaeri A, Sood AK, et al. Expression of B7-H4 and IDO1 is associated with drug resistance and poor prognosis in high-grade serous ovarian carcinomas. Hum Pathology. (2021) 113:20–7. doi: 10.1016/j.humpath.2021.04.003
24. Sica GL, Choi IH, Zhu G, Tamada K, Wang SD, Tamura H, et al. B7-H4, a molecule of the B7 family, negatively regulates T cell immunity. Immunity. (2003) 18:849–61. doi: 10.1016/S1074-7613(03)00152-3
25. Prasad DVR, Richards S, Mai XM, Dong C. B7S1, a novel B7 family member that negatively regulates T cell activation. Immunity. (2003) 18:863–73. doi: 10.1016/s1074-7613(03)00147-x
26. MacGregor HL, Garcia-Batres C, Sayad A, Elia A, Berman HK, Toker A, et al. Tumor cell expression of B7-H4 correlates with higher frequencies of tumor-infiltrating APCs and higher CXCL17 expression in human epithelial ovarian cancer. Oncoimmunology. (2019) 8:e1665460. doi: 10.1080/2162402X.2019.1665460
27. Li J, Lee Y, Li Y, Jiang Y, Lu H, Zang W, et al. Co-inhibitory molecule B7 superfamily member 1 expressed by tumor-infiltrating myeloid cells induces dysfunction of anti-tumor CD8+ T cells. Immunity. (2018) 48:773–786.e5. doi: 10.1016/j.immuni.2018.03.018
28. Rodriguez-Garcia A, Minutolo NG, Robinson JM, Powell DJ. T-cell target antigens across major gynecologic cancers. Gynecologic Oncol. (2017) 145:426–35. doi: 10.1016/j.ygyno.2017.03.510
29. Bregar A, Deshpande A, Grange C, Zi T, Stall J, Hirsch H, et al. Characterization of immune regulatory molecules B7-H4 and PD-L1 in low and high grade endometrial tumors. Gynecologic Oncol. (2017) 145:446–52. doi: 10.1016/j.ygyno.2017.03.006
30. Zang X, Loke P, Kim J, Murphy K, Waitz R, Allison JP. B7x: a widely expressed B7 family member that inhibits T cell activation. Proc Natl Acad Sci United States America. (2003) 100:10388–92. doi: 10.1073/pnas.1434299100
31. Kryczek I, Wei S, Zhu G, Myers L, Mottram P, Cheng P, et al. Relationship between B7-H4, regulatory T cells, and patient outcome in human ovarian carcinoma. Cancer Res. (2007) 67:8900–5. doi: 10.1158/0008-5472.CAN-07-1866
32. Terry S, Engelsen AST, Buart S, Elsayed WS, Venkatesh GH, Chouaib S. Hypoxia-driven intratumor heterogeneity and immune evasion. Cancer Letters. (2020) 492:1–10. doi: 10.1016/j.canlet.2020.07.004
33. Xu H, Chen X, Tao M, Chen K, Chen C, Xu G, et al. B7-H3 and B7-H4 are independent predictors of a poor prognosis in patients with pancreatic cancer. Oncol Letters. (2016) 11:1841–6. doi: 10.3892/ol.2016.4128
34. Kryczek I, Wei S, Zou L, Zhu G, Mottram P, Xu H, et al. Cutting edge: induction of B7-H4 on APCs through IL-10: novel suppressive mode for regulatory T cells1. J Immunol. (2006) 177:40–4. doi: 10.4049/jimmunol.177.1.40
35. Bolandi N, Derakhshani A, Hemmat N, Baghbanzadeh A, Asadzadeh Z, Afrashteh Nour M, et al. The positive and negative immunoregulatory role of B7 family: promising novel targets in gastric cancer treatment. Int J Mol Sci. (2021) 22:10719. doi: 10.3390/ijms221910719
36. Zheng C, Yang R. RCD24, B7-H4 and PCNA expression and clinical significance in ovarian cancer. J B.U.ON. (2019) 24:715–9.
37. Simon I, Katsaros D, Rigault de la Longrais I, Massobrio M, Scorilas A, Kim NW, et al. B7-H4 is over-expressed in early-stage ovarian cancer and is independent of CA125 expression. Gynecologic Oncol. (2007) 106:334–41. doi: 10.1016/j.ygyno.2007.03.035
38. Sachdev JC, Bauer TM, Chawla SP, Pant S, Patnaik A, Wainberg ZA, et al. Phase 1a/1b study of first-in-class B7-H4 antibody, FPA150, as monotherapy in patients with advanced solid tumors. J Clin Oncol. (2019) 37:2529–9. doi: 10.1200/JCO.2019.37.15_suppl.2529
39. Kinneer K, Wortmann P, Cooper ZA, Dickinson NJ, Masterson L, Cailleau T, et al. Design and preclinical evaluation of a novel B7-H4-directed antibody-drug conjugate, AZD8205, alone and in combination with the PARP1-selective inhibitor AZD5305. Clin Cancer Res. (2023) 29:1086–101. doi: 10.1158/1078-0432.CCR-22-2630
40. Cai D, Wang F, Wang C, Jin L. Phenotypic and functional analyses of B7S1 in ovarian cancer. Front Mol biosciences. (2021) 8:686803. doi: 10.3389/fmolb.2021.686803
41. Zhang L, Wu H, Lu D, Li G, Sun C, Song H, et al. The costimulatory molecule B7-H4 promote tumor progression and cell proliferation through translocating into nucleus. Oncogene. (2013) 32:5347–58. doi: 10.1038/onc.2012.600
42. Jeon H, Vigdorovich V, Garrett-Thomson SC, Janakiram M, Ramagopal UA, Abadi YM, et al. Structure and cancer immunotherapy of the B7 family member B7x. Cell Rep. (2014) 9:1089–98. doi: 10.1016/j.celrep.2014.09.053
43. Liang L, Jiang Y, Chen JS, Niu N, Piao J, Ning J, et al. B7-H4 expression in ovarian serous carcinoma: a study of 306 cases. Hum Pathology. (2016) 57:1–6. doi: 10.1016/j.humpath.2016.06.011
44. Bignotti E, Tassi RA, Calza S, Ravaggi A, Romani C, Rossi E, et al. Differential gene expression profiles between tumor biopsies and short-term primary cultures of ovarian serous carcinomas: identification of novel molecular biomarkers for early diagnosis and therapy. Gynecologic Oncol. (2006) 103:405–16. doi: 10.1016/j.ygyno.2006.03.056
45. Simon I, Zhuo S, Corral L, Diamandis EP, Sarno MJ, Wolfert RL, et al. B7-h4 is a novel membrane-bound protein and a candidate serum and tissue biomarker for ovarian cancer. Cancer Res. (2006) 66:1570–5. doi: 10.1158/0008-5472.CAN-04-3550
46. Chen GM, Kannan L, Geistlinger L, Kofia V, Safikhani Z, Gendoo DMA, et al. Consensus on molecular subtypes of high-grade serous ovarian carcinoma. Clin Cancer Research: Off J Am Assoc Cancer Res. (2018) 24:5037–47. doi: 10.1158/1078-0432.CCR-18-0784
47. Gitto SB, Whicker M, Davies G, Kumar S, Kinneer K, Xu H, et al. A B7-H4-targeting antibody-drug conjugate shows antitumor activity in PARPi and platinumresistant cancers with B7-H4 expression. Clin Cancer research: an Off J Am Assoc Cancer Res. (2024) 30:1567–81. doi: 10.1158/1078-0432.CCR-23-1079
48. Simon I, Liu Y, Krall KL, Urban N, Wolfert RL, Kim NW, et al. Evaluation of the novel serum markers B7-H4, Spondin 2, and DcR3 for diagnosis and early detection of ovarian cancer. Gynecologic Oncol. (2007) 106:112–8. doi: 10.1016/j.ygyno.2007.03.007
49. Gyllensten U, Hedlund-Lindberg J, Svensson J, Manninen J, Öst T, Ramsell J, et al. Next generation plasma proteomics identifies high-precision biomarker candidates for ovarian cancer. Cancers. (2022) 14:1757. doi: 10.3390/cancers14071757
50. Lan Z, Fu D, Xi M. Serum B7 homologous body 4 for the diagnosis of ovarian cancer in Chinese Han women: A meta-analysis. J Cancer Res Ther. (2018) 14:S433–6. doi: 10.4103/0973-1482.177216
51. Choi IH, Zhu G, Sica GL, Strome SE, Cheville JC, Lau JS, et al. Genomic organization and expression analysis of B7-H4, an immune inhibitory molecule of the B7 family. J Immunol (Baltimore Md.: 1950). (2003) 171:4650–4. doi: 10.4049/jimmunol.171.9.4650
52. Kryczek I, Zou L, Rodriguez P, Zhu G, Wei S, Mottram P, et al. B7-H4 expression identifies a novel suppressive macrophage population in human ovarian carcinoma. J Exp Med. (2006) 203:871–81. doi: 10.1084/jem.20050930
53. Mach P, Kimmig R, Kasimir-Bauer S, Buderath P. Association of soluble B7-H4 and circulating tumor cells in blood of advanced epithelial ovarian cancer patients. Front Oncol. (2021) 11:721067. doi: 10.3389/fonc.2021.721067
54. Ye Y, Wang JJ, Li SL, Wang SY, Jing FH. Does B7-H4 expression correlate with clinicopathologic characteristics and survival in ovarian cancer?: A systematic review and PRISMA-compliant meta-analysis. Medicine. (2018) 97:e11821. doi: 10.1097/MD.0000000000011821
55. Xu M, Zhang B, Zhang M, Liu Y, Yin FL, Liu X, et al. Clinical relevance of expression of B7-H1 and B7-H4 in ovarian cancer. Oncol Letters. (2016) 11:2815–9. doi: 10.3892/ol.2016.4301
56. Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. (2005) 5:263–74. doi: 10.1038/nrc1586
57. Yang C, Xia BR, Zhang ZC, Zhang YJ, Lou G, Jin WL. Immunotherapy for ovarian cancer: adjuvant, combination, and neoadjuvant. Front Immunol. (2020) 11:577869. doi: 10.3389/fimmu.2020.577869
58. Zou W, Machelon V, Coulomb-L’Hermin A, Borvak J, Nome F, Isaeva T, et al. Stromal-derived factor-1 in human tumors recruits and alters the function of plasmacytoid precursor dendritic cells. Nat Med. (2001) 7:1339–46. doi: 10.1038/nm1201-1339
59. Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. (2004) 10:942–9. doi: 10.1038/nm1093
60. Podojil JR, Chiang MY, Ifergan I, Copeland R, Liu LN, Maloveste S, et al. B7-H4 modulates regulatory CD4+ T cell induction and function via ligation of a semaphorin 3a/plexin A4/neuropilin-1 complex. J Immunol. (2018) 201:897–907. doi: 10.4049/jimmunol.1700811
61. Hwang C, Lee HJ, Na JY, Kim KH, Song YJ, Kim JY, et al. The stromal tumor-infiltrating lymphocytes, cancer stemness, epithelial-mesenchymal transition, and B7-H4 expression in ovarian serous carcinoma. J Ovarian Res. (2023) 16:3. doi: 10.1186/s13048-022-01076-z
62. Pagnotti GM, Atkinson RM, Romeiser J, Akalin A, Korman MB, Shroyer KR. B7-H4 is inversely correlated with T-cell infiltration in clear cell but not serous or endometrioid ovarian cancer. Appl immunohistochemistry Mol morphology. (2019) 27:515–22. doi: 10.1097/PAI.0000000000000608
63. Cheng H, Borczuk A, Janakiram M, Ren X, Lin J, Assal A, et al. Wide expression and significance of alternative immune checkpoint molecules, B7x and HHLA2, in PD-L1-negative human lung cancers. Clin Cancer Research: Off J Am Assoc Cancer Res. (2018) 24:1954–64. doi: 10.1158/1078-0432.CCR-17-2924
64. Chen D, Li G, Ji C, Lu Q, Qi Y, Tang C, et al. Enhanced B7-H4 expression in gliomas with low PD-L1 expression identifies super-cold tumors. J Immunotherapy Cancer. (2020) 8:e000154. doi: 10.1136/jitc-2019-000154
65. Lu Y, Wu F, Cao Q, Sun Y, Huang M, Xiao J, et al. B7-H4 is increased in lung adenocarcinoma harboring EGFR-activating mutations and contributes to immunosuppression. Oncogene. (2022) 41:704–17. doi: 10.1038/s41388-021-02124-6
66. Schalper KA, Carvajal-Hausdorf D, McLaughlin J, Altan M, Velcheti V, Gaule P, et al. Differential expression and significance of PD-L1, IDO-1, and B7-H4 in human lung cancer. Clin Cancer Research: Off J Am Assoc Cancer Res. (2017) 23:370–8. doi: 10.1158/1078-0432.CCR-16-0150
67. Gruosso T, Gigoux M, Manem VSK, Bertos N, Zuo D, Perlitch I, et al. Spatially distinct tumor immune microenvironments stratify triple-negative breast cancers. J Clin Invest. (2019) 129:1785–800. doi: 10.1172/JCI96313
68. MacGregor HL, Sayad A, Elia A, Wang BX, Katz SR, Shaw PA, et al. High expression of B7-H3 on stromal cells defines tumor and stromal compartments in epithelial ovarian cancer and is associated with limited immune activation. J immunotherapy cancer. (2019) 7:357. doi: 10.1186/s40425-019-0816-5
69. Henriksen A, Dyhl-Polk A, Chen I, Nielsen D. Checkpoint inhibitors in pancreatic cancer. Cancer Treat Rev. (2019) 78:17–30. doi: 10.1016/j.ctrv.2019.06.005
70. Dangaj D, Lanitis E, Zhao A, Joshi S, Cheng Y, Sandaltzopoulos R, et al. Novel recombinant human b7-h4 antibodies overcome tumoral immune escape to potentiate T-cell antitumor responses. Cancer Res. (2013) 73:4820–9. doi: 10.1158/0008-5472.CAN-12-3457
71. Smith JB, Lanitis E, Dangaj D, Buza E, Poussin M, Stashwick C, et al. Tumor regression and delayed onset toxicity following B7-H4 CAR T cell therapy. Mol Ther. (2016) 24:1987–99. doi: 10.1038/mt.2016.149
72. Kaur G, Janakiram M. B7x-from bench to bedside. ESMO Open. (2019) 4:e000554. doi: 10.1136/esmoopen-2019-000554
73. Crawford A, Chiu D. Targeting solid tumors using CD3 bispecific antibodies. Mol Cancer Ther. (2021) 20:1350–8. doi: 10.1158/1535-7163.MCT-21-0073
74. Miao G, Sun X. Development of a novel anti-B7-H4 antibody enhances anti-tumor immune response of human T cells. Biomedicine Pharmacotherapy. (2021) 141:111913. doi: 10.1016/j.biopha.2021.111913
75. Abstract 4246: ABL103, A novel T-cell engaging bispecific antibody, exhibits potent in vitro and vivo antitumor activity and low toxicity via B7-H4 dependent 4-1BB activation in tumor microenvironment | Cancer Research | American Association for Cancer Research. Available online at: https://aacrjournals.org/cancerres/article/82/12_Supplement/4246/702867/Abstract-4246-ABL103-A-novel-T-cell-engaging (Accessed 24th March 2024).
76. Chang TP, Polonskaya Z, Luna X, Martomo S, Zhang Z, Ng S, et al. 45P A novel bi-functional IL15 cytokine fusion antibody selected to kill B7-H4 positive tumor cells. Ann Oncol. (2021) 32:S1391. doi: 10.1016/j.annonc.2021.10.061
77. Hong M, Clubb JD, Chen YY. Engineering CAR-T cells for next-generation cancer therapy. Cancer Cell. (2020) 38:473–88. doi: 10.1016/j.ccell.2020.07.005
78. Vincent RL, Gurbatri CR, Li F, Vardoshvili A, Coker C, Im J, et al. Probiotic-guided CAR-T cells for solid tumor targeting. Sci (New York N.Y.). (2023) 382:211–8. doi: 10.1126/science.add7034
79. Shafer P, Kelly LM, Hoyos V. Cancer therapy with TCR-engineered T cells: current strategies, challenges, and prospects. Front Immunol. (2022) 13:835762. doi: 10.3389/fimmu.2022.835762
81. Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. New Engl J Med. (2014) 371:1507–17. doi: 10.1056/NEJMoa1407222
82. Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. New Engl J Med. (2017) 377:2531–44. doi: 10.1056/NEJMoa1707447
83. Bouchkouj N, Kasamon YL, de Claro RA, George B, Lin X, Lee S, et al. FDA approval summary: axicabtagene ciloleucel for relapsed or refractory large B-cell lymphoma. Clin Cancer Research: Off J Am Assoc Cancer Res. (2019) 25:1702–8. doi: 10.1158/1078-0432.CCR-18-2743
84. O’Leary MC, Lu X, Huang Y, Lin X, Mahmood I, Przepiorka D, et al. FDA approval summary: tisagenlecleucel for treatment of patients with relapsed or refractory B-cell precursor acute lymphoblastic leukemia. Clin Cancer Research: Off J Am Assoc Cancer Res. (2019) 25:1142–6. doi: 10.1158/1078-0432.CCR-18-2035
85. Dotti G, Gottschalk S, Savoldo B, Brenner MK. Design and development of therapies using chimeric antigen receptor-expressing T cells. Immunol Rev. (2014) 257:107–26. doi: 10.1111/imr.12131
86. Zhao Y, Moon E, Carpenito C, Paulos CM, Liu X, Brennan AL, et al. Multiple injections of electroporated autologous T cells expressing a chimeric antigen receptor mediate regression of human disseminated tumor. Cancer Res. (2010) 70:9053–61. doi: 10.1158/0008-5472.CAN-10-2880
87. John P, Wei Y, Liu W, Du M, Guan F, Zang X. The B7x immune checkpoint pathway: from discovery to clinical trial. Trends Pharmacol Sci. (2019) 40:883–96. doi: 10.1016/j.tips.2019.09.008
88. Chau CH, Steeg PS, Figg WD. Antibody-drug conjugates for cancer. Lancet (London England). (2019) 394:793–804. doi: 10.1016/S0140-6736(19)31774-X
89. Tolcher A, Hamilton E, Coleman RL. The evolving landscape of antibody-drug conjugates in gynecologic cancers. Cancer Treat Rev. (2023) 116:102546. doi: 10.1016/j.ctrv.2023.102546
90. Tarantino P, Carmagnani Pestana R, Corti C, Modi S, Bardia A, Tolaney SM, et al. Antibody-drug conjugates: Smart chemotherapy delivery across tumor histologies. CA: Cancer J Clin. (2022) 72:165–82. doi: 10.3322/caac.21705
91. Dumontet C, Reichert JM, Senter PD, Lambert JM, Beck A. Antibody-drug conjugates come of age in oncology. Nat Rev Drug Discovery. (2023) 22:641–61. doi: 10.1038/s41573-023-00709-2
92. Toader D, Fessler SP, Collins SD, Conlon PR, Bollu R, Catcott KC, et al. Discovery and preclinical characterization of XMT-1660, an optimized B7-H4-targeted antibody-drug conjugate for the treatment of cancer. Mol Cancer Ther. (2023) 22:999–1012. doi: 10.1158/1535-7163.MCT-22-0786
93. Gray E, Ulrich M, Epp A, Younan P, Sahetya D, Hensley K, et al. SGN-B7H4V, an investigational vedotin ADC directed to the immune checkpoint ligand B7-H4, shows promising activity in preclinical models. J Immunotherapy Cancer. (2023) 11:e007572. doi: 10.1136/jitc-2023-007572
94. Wainberg ZA, Sachdev JC, Bauer T, Pant S, Chawla S, Marina N, et al. 1198P - FPA150 (B7-H4 antibody) phase I update in advanced solid tumours: Monotherapy and in combination with pembrolizumab. Ann Oncol. (2019) 30:v489. doi: 10.1093/annonc/mdz253.024
Keywords: B7-H4, ovarian cancer, tumor microenvironment, immunotherapy, immune checkpoint inhibitors
Citation: Zhou L, Duan Y, Fu K, Zhang M, Li K and Yin R (2024) The role of B7-H4 in ovarian cancer immunotherapy: current status, challenges, and perspectives. Front. Immunol. 15:1426050. doi: 10.3389/fimmu.2024.1426050
Received: 30 April 2024; Accepted: 13 August 2024;
Published: 29 August 2024.
Edited by:
Nikolaos Gavalas, National and Kapodistrian University of Athens, GreeceReviewed by:
Haitao Wang, National Cancer Institute (NIH), United StatesAfsheen Raza, Abu Dhabi University, United Arab Emirates
Copyright © 2024 Zhou, Duan, Fu, Zhang, Li and Yin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rutie Yin, eWlucnV0aWVAc2N1LmVkdS5jbg==
 Lu Zhou
Lu Zhou Yuanqiong Duan
Yuanqiong Duan Kaiyu Fu
Kaiyu Fu Mengpei Zhang
Mengpei Zhang Kemin Li
Kemin Li Rutie Yin
Rutie Yin