
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 13 September 2024
Sec. Cancer Immunity and Immunotherapy
Volume 15 - 2024 | https://doi.org/10.3389/fimmu.2024.1426024
Objective: Nivolumab, recently proven in a phase 3 clinical trial (CheckMate 901) to enhance survival when combined with gemcitabine-cisplatin for advanced urothelial carcinoma. This study aimed to assess its cost-effectiveness against gemcitabine-cisplatin alone, from US and Chinese payers’ perspectives.
Methods: A partitioned survival model was established to assess the life-years, quality-adjusted life-years (QALYs), lifetime costs, and incremental cost-effectiveness ratios (ICERs) of nivolumab plus gemcitabine-cisplatin versus gemcitabine-cisplatin alone as first-line treatment for advanced urothelial carcinoma. Univariate, two-way, and probabilistic sensitivity analyses were conducted to assess the model’s robustness. Additionally, subgroup analyses were performed.
Results: Nivolumab plus gemcitabine-cisplatin and gemcitabine-cisplatin achieved survival benefits of 4.238 life-years and 2.979 life-years for patients with advanced urothelial carcinoma, respectively. Compared with gemcitabine-cisplatin, nivolumab plus gemcitabine-cisplatin resulted in ICERs of $116,856/QALY in the US and $51,997/QALY in China. The probabilities of achieving cost-effectiveness at the current willingness-to-pay thresholds were 77.5% in the US and 16.5% in China. Cost-effectiveness could be reached if the price of nivolumab were reduced to $920.87/100mg in China. Subgroup analyses indicated that the combination had the highest probability of cost-effectiveness in patients under 65 or with an Eastern Cooperative Oncology Group (ECOG) performance-status score of 0 in the US and China.
Conclusion: Nivolumab plus gemcitabine-cisplatin first-line treatment for advanced urothelial carcinoma results in longer life expectancy than gemcitabine-cisplatin, but is not cost-effective in China at current price. However, cost-effectiveness is likely to be achieved in most patient subgroups in the US.
Cancer represents a significant public health challenge worldwide. Among these, bladder cancer stands as the world’s tenth most prevalent cancer and ranks sixth among males (1, 2). Urothelial carcinoma is the predominant histological type of bladder cancer and the most frequent malignancy within the urinary tract (3). Chemotherapy, primarily cisplatin-based, has served as the cornerstone of first-line treatment for unresectable or metastatic urothelial carcinoma over the past four decades (4, 5). In recent years, studies have shown that immune checkpoint inhibitors can markedly enhance survival outcomes for patients with urothelial carcinoma (6, 7), thus offering innovative therapeutic alternatives for managing this advanced-stage malignancy.
Platinum-based drugs can induce immunomodulatory effects and thus thereby enhancing the efficacy of immune checkpoint blockade, which provides theoretical support for the combination therapy of programmed death-1/ligand-1 (PD-1/L1) inhibitors with platinum-based chemotherapy in treating advanced urothelial carcinoma (5, 8). Nivolumab is a humanized immunoglobulin G4 monoclonal antibody that targets PD-1 (9). The phase 3 randomized trial, CheckMate 901, assessed the therapeutic efficacy and safety of nivolumab plus gemcitabine-cisplatin versus gemcitabine-cisplatin alone in patients with untreated, unresectable, or metastatic urothelial carcinoma (9). The results demonstrated that adding nivolumab to gemcitabine-cisplatin significantly improved overall survival (OS, 21.7 months vs 18.9 months; hazard ratio [HR], 0.78; 95% confidence interval [CI], 0.63-0.96) and progression-free survival (PFS, 7.9 months vs 7.6 months; HR, 0.72; 95% CI, 0.59-0.88) in patients with urothelial carcinoma. However, it also increased the incidence of grade 3 or higher adverse events when combined with chemotherapy (61.8% versus 51.7%) (9).
While the addition of nivolumab to chemotherapy improved treatment outcomes for patients with advanced urothelial carcinoma, it concurrently elevated the incidence of adverse events. Moreover, the significantly higher cost of nivolumab compared to gemcitabine-cisplatin substantially increases the treatment cost per cycle for patients. This introduces considerable economic uncertainty for those suffering from advanced urothelial carcinoma. This study aimed to evaluate the cost-effectiveness of nivolumab plus gemcitabine-cisplatin as a first-line treatment for advanced urothelial carcinoma, from the perspectives of payers in the US and the healthcare system in China.
Partitioned survival models are among the most commonly utilized modeling approaches in pharmacoeconomic evaluations, especially for the economic evaluation of oncology therapies (10). We developed a partitioned survival model using Microsoft Excel 2019 (Redmond, Washington, US) that includes three health states: PFS, progressive disease (PD), and death. Patients entered the model in the PFS state and transitioned to the PD state or death after treatment with nivolumab and/or gemcitabine-cisplatin, with these state transitions being irreversible (Figure 1). The model cycle length was set at three weeks, simulating a lifetime horizon. Health outcomes for the nivolumab group and the chemotherapy group were measured in quality-adjusted life-years (QALYs) and life-years, with the incremental cost-effectiveness ratio (ICER) as the primary measure for assessing the cost-effectiveness of the two treatment options. Based on the recommendations of the Institute for Clinical and Economic Review and published literature, this study set the willingness-to-pay threshold for the US at $150,000 per QALY (11–13). For China, the willingness-to-pay threshold was determined according to the WHO-CHOICE guidelines and the China Guidelines for Pharmacoeconomic Evaluations, set at three times the per capita gross domestic product, or $38,043 per QALY (14–16). An annual discount rate of 3% for the US perspective and 5% for China was applied to both costs and health outcomes (17, 18). This research was conducted following the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) reporting guideline (19) (Supplementary Table 1).
This study simulated a hypothetical patient cohort with baseline characteristics and treatment regimens consistent with the CheckMate 901 trial (9). A total of 608 adult patients with unresectable or metastatic urothelial carcinoma (median age 65 years) were randomly assigned in a 1:1 ratio (304:304) to either the nivolumab group or the chemotherapy group. The chemotherapy group received gemcitabine (1000 mg/m2, days 1 and 8 of each cycle) plus cisplatin (70 mg/m2, day 1 of each cycle) every three weeks for up to six cycles. The nivolumab group received nivolumab (360 mg, day 1 of each cycle) plus gemcitabine and cisplatin for up to six cycles every three weeks, followed by nivolumab treatment at a dose of 480 mg every four weeks until disease progression, unacceptable toxic effects, withdrawal of consent, death, or up to a maximum of 24 months. According to the CheckMate 901 trial, the median duration of treatment in the nivolumab and chemotherapy groups was 7.4 and 3.7 months, respectively (9). After disease progression, patients received second-line treatment, followed by best supportive care until death, with the proportion of second-line treatment drugs shown in Supplementary Table 2. Given that pembrolizumab is not approved by the NMPA for urothelial carcinoma and avelumab is not available in China, this study assumes that patients in China would participate in clinical trials as an alternative to using pembrolizumab and avelumab in subsequent treatments (20).
We initially extracted time and survival rate data from the OS and PFS survival curves of the CheckMate 901 trial using the WebPlotDigitizer program (version 4.6, https://automeris.io/WebPlotDigitizer/). Following the method of Guyot et al. (21), we generated pseudo-individual patient data using R software (version 4.3.0, http://www.r-project.org) to reconstruct the survival curves. We then fitted exponential, Weibull, gamma, Gompertz, log-logistic, lognormal, and generalized gamma parametric models. Model fit was assessed based on the Akaike information criterion (AIC) and Bayesian information criterion (BIC), with smaller values indicating a better fit (AIC and BIC results are shown in Supplementary Table 3). The best-fitting parametric model for the OS survival curves of both the nivolumab group and the chemotherapy group, as well as the PFS curve of the chemotherapy group, was the log-logistic distribution. The best-fitting parametric model for the PFS curve of the nivolumab group was the generalized gamma distribution (Table 1). Survival curves were extrapolated to the point where 99% of patients had died, resulting in a time horizon of 30 years. In this study, to minimize the error in survival data of the simulated cohort in the partitioned survival model, survival rate data within 60.2 months for both treatment groups were derived from the survival curves of the CheckMate 901 trial. The fitted survival curves using the parametric models are shown in Supplementary Figure 1.
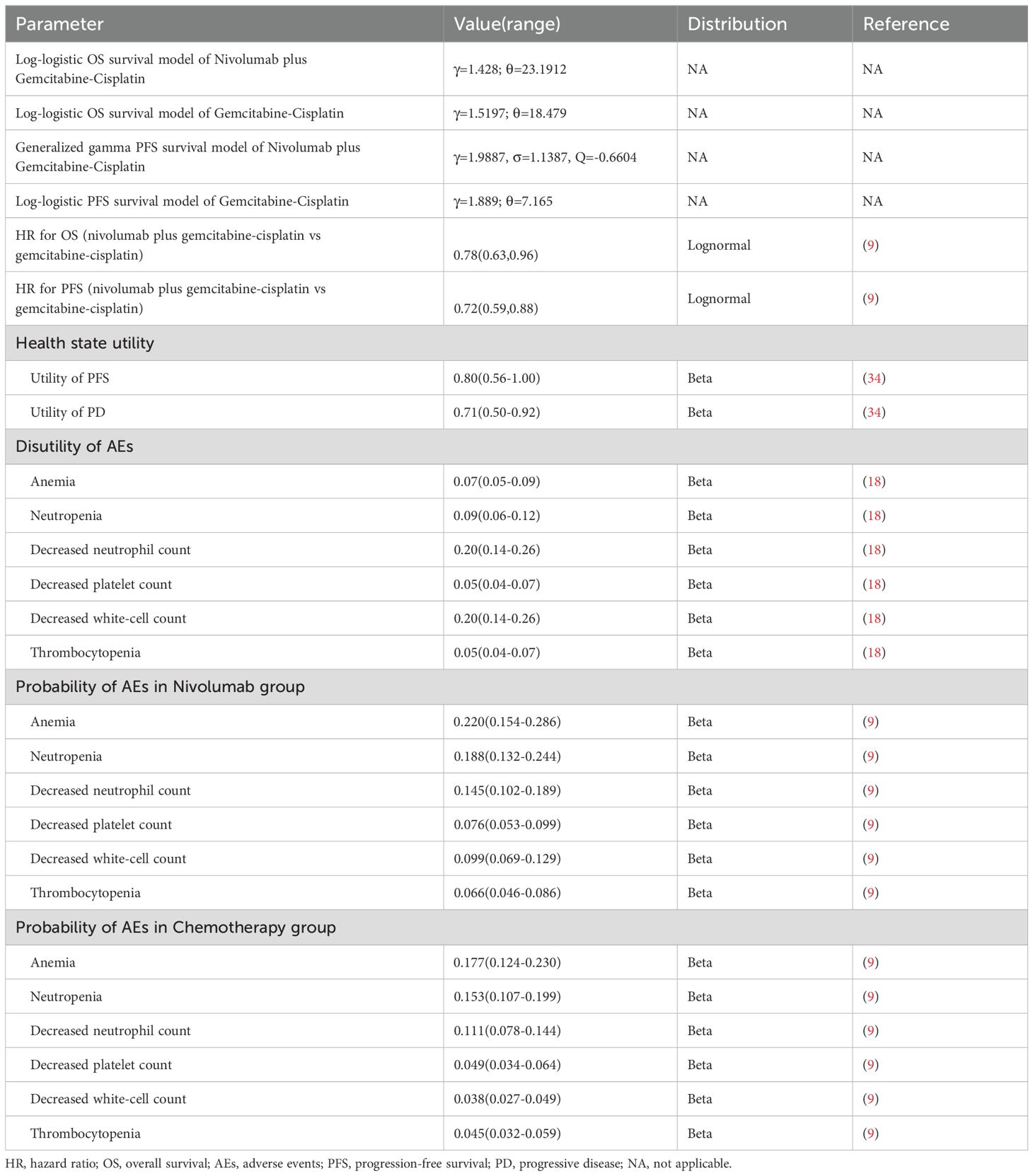
Table 1. Basic clinical and health parameters: baseline values, ranges, and distributions for sensitivity analysis.
This study estimated the lifetime treatment costs for patients, including costs related to drugs, laboratory tests, computer tomography (CT) scans, drug administration, supportive care, terminal care, and management of serious adverse events (Table 2). Drug price data were sourced from publicly available price databases in the US or China (22, 23). Laboratory test costs included those for immunohistochemical tests, blood tests, urinalysis, liver function blood test panels, and thyroid function tests. CT costs covered head, chest, and abdominal CT scans. This study considered adverse events of grade 3 or higher with an incidence rate of ≥5%, including anemia, neutropenia, decreased neutrophil count, decreased platelet count, decreased white-cell count, and thrombocytopenia (9) (Table 1). Costs for laboratory tests, CT scans, disease management, adverse event management, and supportive care were derived from publicly available databases and published literature (13, 18, 24–33). To calculate the costs of cisplatin and gemcitabine, the average body surface areas for patients in the US and China were set at 1.86 m2 and 1.72 m2, respectively (18, 29). All costs were adjusted to 2023 values using the consumer price index in the US or China and reported in US dollars, with the exchange rate set at the 2023 average rate of 1 USD = 7.05 Chinese Yuan.
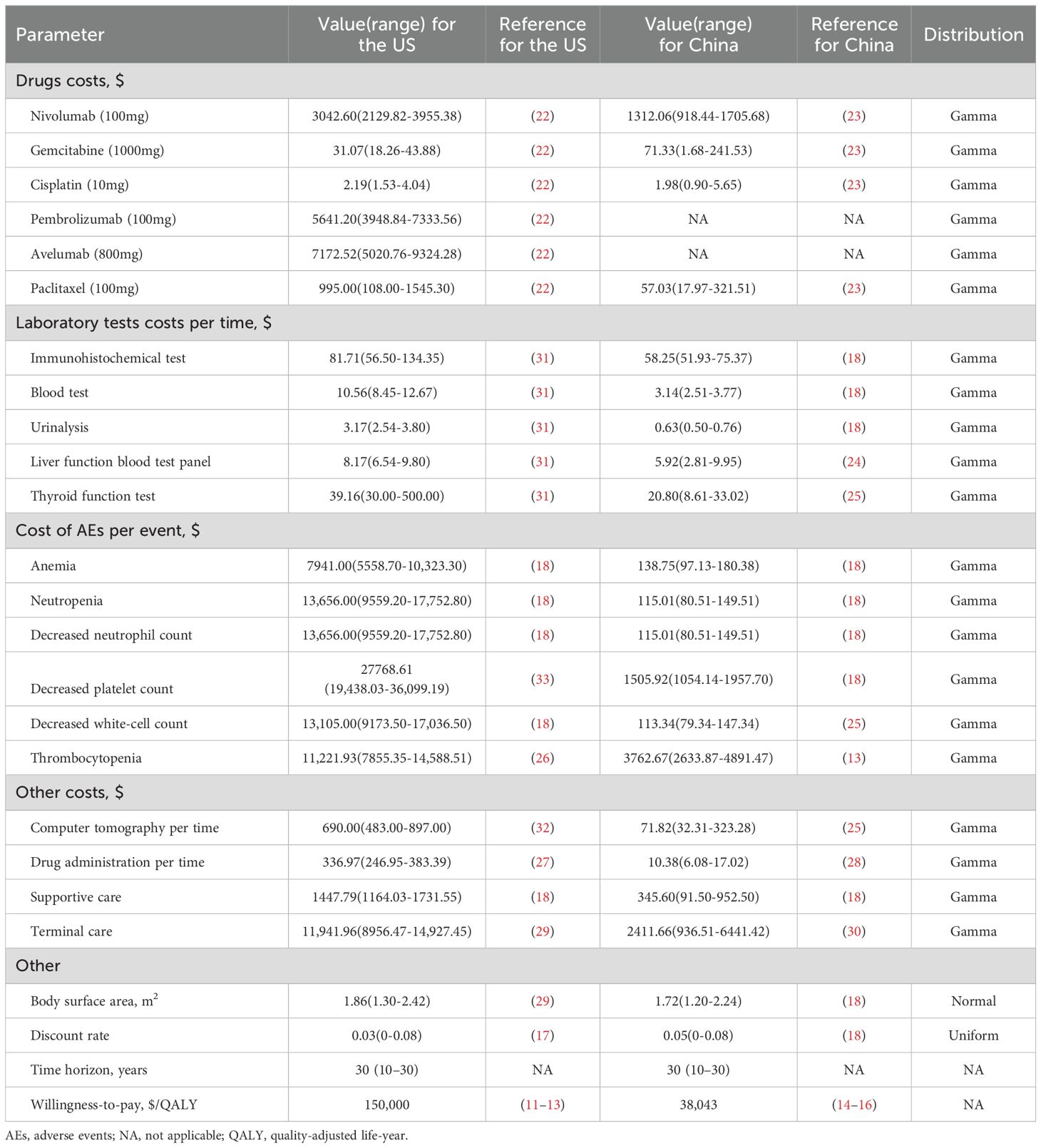
Table 2. Basic cost and other parameters: baseline values, ranges, and distributions for sensitivity analysis.
In calculating patient health outcomes, this study considered the quality of life of patients, where the utility value for the PFS state was set at 0.80, the PD state at 0.71, and death at 0. Disutility values for grade 3 or higher adverse events with an incidence rate of ≥5% were also considered, with data extracted from published literature (18, 34) (Table 1).
We conducted sensitivity analyses to examine the robustness of the model results when parameters changed. Univariate sensitivity analyses were performed to explore the impact of individual parameter variations on the base case results and the price of nivolumab at which the nivolumab group becomes cost-effective. Parameters varied within their 95% CIs or ±30% of their baseline values, while the annual discount rate varied between 0-8%, and the time horizon ranged from 10 to 30 years (Tables 1, 2). In addition, two-way sensitivity analyses were conducted on the utilities of PFS and PD states, as well as the HRs for OS and PFS. In the probabilistic sensitivity analysis, we performed 1000 Monte Carlo simulations to investigate the uncertainty of the model results when all parameters varied simultaneously. Parameters varied according to specific distributions, with the ranges and distributions of the parameters detailed in Tables 1 and 2.
To explore the economic outcomes of nivolumab plus gemcitabine-cisplatin in different patient subgroups with urothelial carcinoma in the US or China, we analyzed the cost-effectiveness of the subgroups reported in the CheckMate 901 trial by varying the subgroup-specific HRs for OS and PFS. These subgroups included variations in gender, age, Eastern Cooperative Oncology Group (ECOG) performance-status score, tumor cell PD-L1 expression level, presence of liver metastases, and previous systemic cancer therapy. The HRs for OS and PFS in different subgroups are presented in Supplementary Table 4.
The base-case analysis found that first-line treatment with nivolumab in combination with gemcitabine-cisplatin (combination therapy) and gemcitabine-cisplatin (chemotherapy) resulted in survival benefits of 4.238 life-years and 2.979 life-years, respectively, in patients with advanced urothelial carcinoma. Notably, with patients in the nivolumab group gaining an additional 1.259 life-years compared to those in the chemotherapy group (Table 3). After considering quality of life, nivolumab plus gemcitabine-cisplatin provided an additional 0.931 QALYs and 0.923 QALYs for the US and Chinese populations, respectively, while also increasing the total cost by $108,838 and $48,001, respectively. This resulted in ICERs for nivolumab plus gemcitabine-cisplatin compared to gemcitabine-cisplatin alone of $116,856/QALY in the US and $51,997/QALY in China, which were below the willingness-to-pay threshold in the US and above the threshold in China, respectively.
Univariate sensitivity analysis results revealed that HR for OS, time horizon, and the cost of nivolumab had the greatest impact on ICER (Figure 2). In China, the ICER values exceeded the willingness-to-pay threshold when most parameters varied within the set range (Figure 2B). In contrast, the ICER remained below the willingness-to-pay threshold for all parameters, except when varying HR for OS and the time horizon in the US (Figure 2A). ICER was highly sensitive to the time horizon; at a 5-year horizon, the ICERs for the US and China reached $329,258/QALY and $157,297/QALY, respectively, and gradually decreased with the extension of the time horizon (Supplementary Figure 2). Simulation results for the price of nivolumab are shown in Figure 3 and Supplementary Table 5. The ICERs for both the US and Chinese populations decreased with the reduction in the price of nivolumab. When the price of nivolumab in China dropped from the current $1312.06/100mg to $920.87/100mg, nivolumab plus gemcitabine-cisplatin became cost-effective, with price reduction of 29.81% (Figure 3, Supplementary Table 5). Additionally, the model showed good robustness to changes in other factors such as laboratory test costs and adverse event-related disutilities (Figure 2). Univariate sensitivity analysis results for all parameters are presented in Supplementary Figures 3 and 4.

Figure 2. Tornado diagrams of univariable sensitivity analyses. (A) US setting; (B) China setting. QALY, quality-adjusted life-year; HR, hazard ratio; OS, overall survival; PFS, progression-free survival; PD, progressive disease.
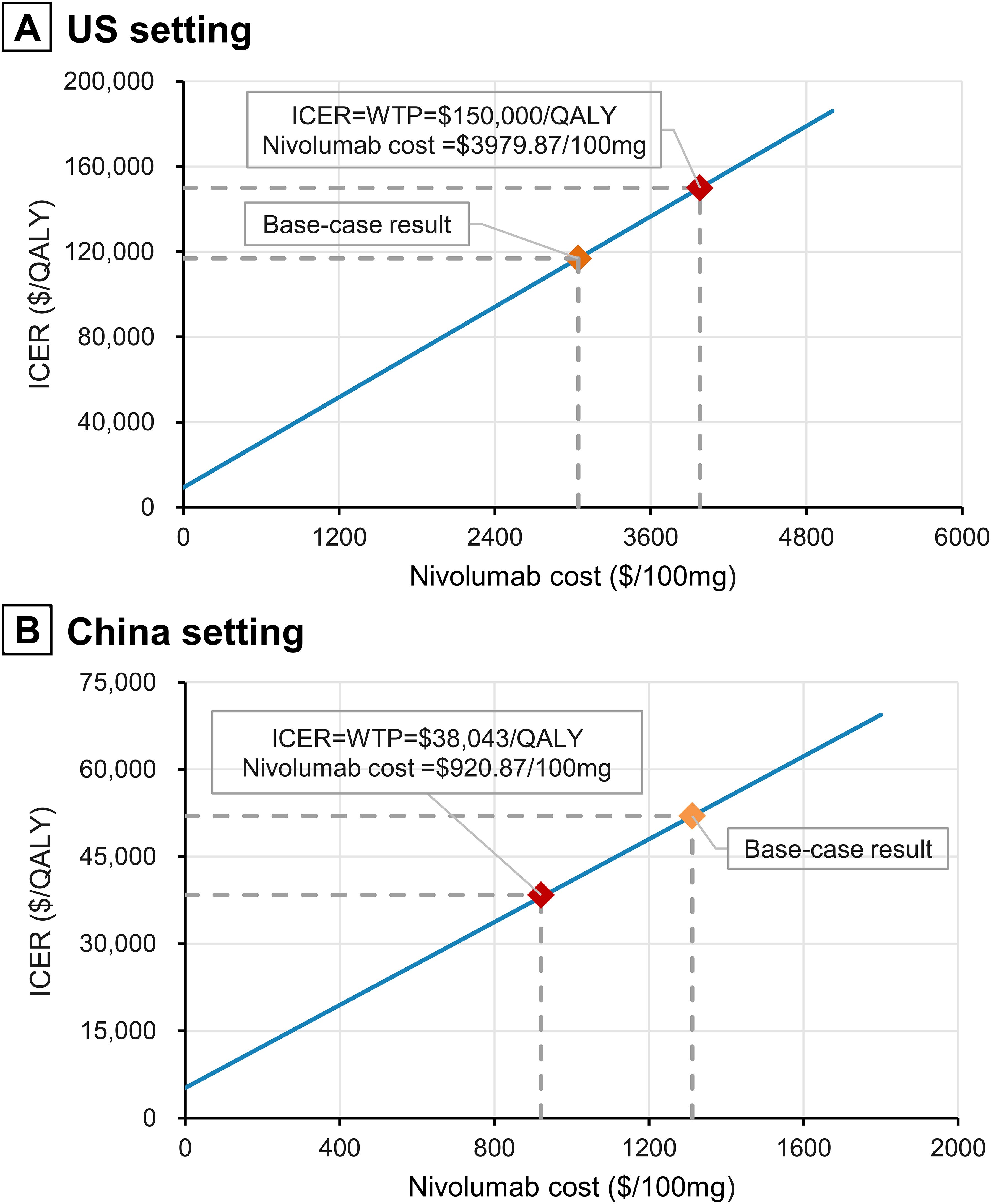
Figure 3. Impact of nivolumab prices on ICERs. (A) US setting; (B) China setting. QALY, quality-adjusted life-year; ICER, incremental cost-effectiveness ratio.
Two-way sensitivity analysis results showed that when the utilities of PFS and PD simultaneously increased within the set range, the ICER would decrease but remain above the willingness-to-pay for Chinese populations. When the utilities of PFS and PD fell below 0.63 and 0.57, respectively, the ICER for nivolumab plus gemcitabine-cisplatin exceeded the willingness-to-pay threshold in the US (Supplementary Figure 5). However, when the HR for OS and HR for PFS were less than 0.85 and 0.88 respectively, regardless of how the HRs changed, nivolumab plus gemcitabine-cisplatin was cost-effective in the US (Supplementary Figure 6). In China, however, the HR for OS and HR for PFS would need to be controlled below 0.74 and 0.88, respectively, for nivolumab plus gemcitabine-cisplatin to achieve cost-effectiveness compared to gemcitabine-cisplatin.
Probabilistic sensitivity analysis results indicated that, with willingness-to-pay thresholds set at $150,000 per QALY in the US and $38,043 per QALY in China, the probabilities that the combination of nivolumab and gemcitabine-cisplatin being cost-effective compared to gemcitabine-cisplatin alone were 77.5% and 16.5%, respectively (Figures 4, 5). When the willingness-to-pay threshold for China increased to $51,997/QALY, the probability of nivolumab combined with chemotherapy being cost-effective exceeded that of chemotherapy alone.
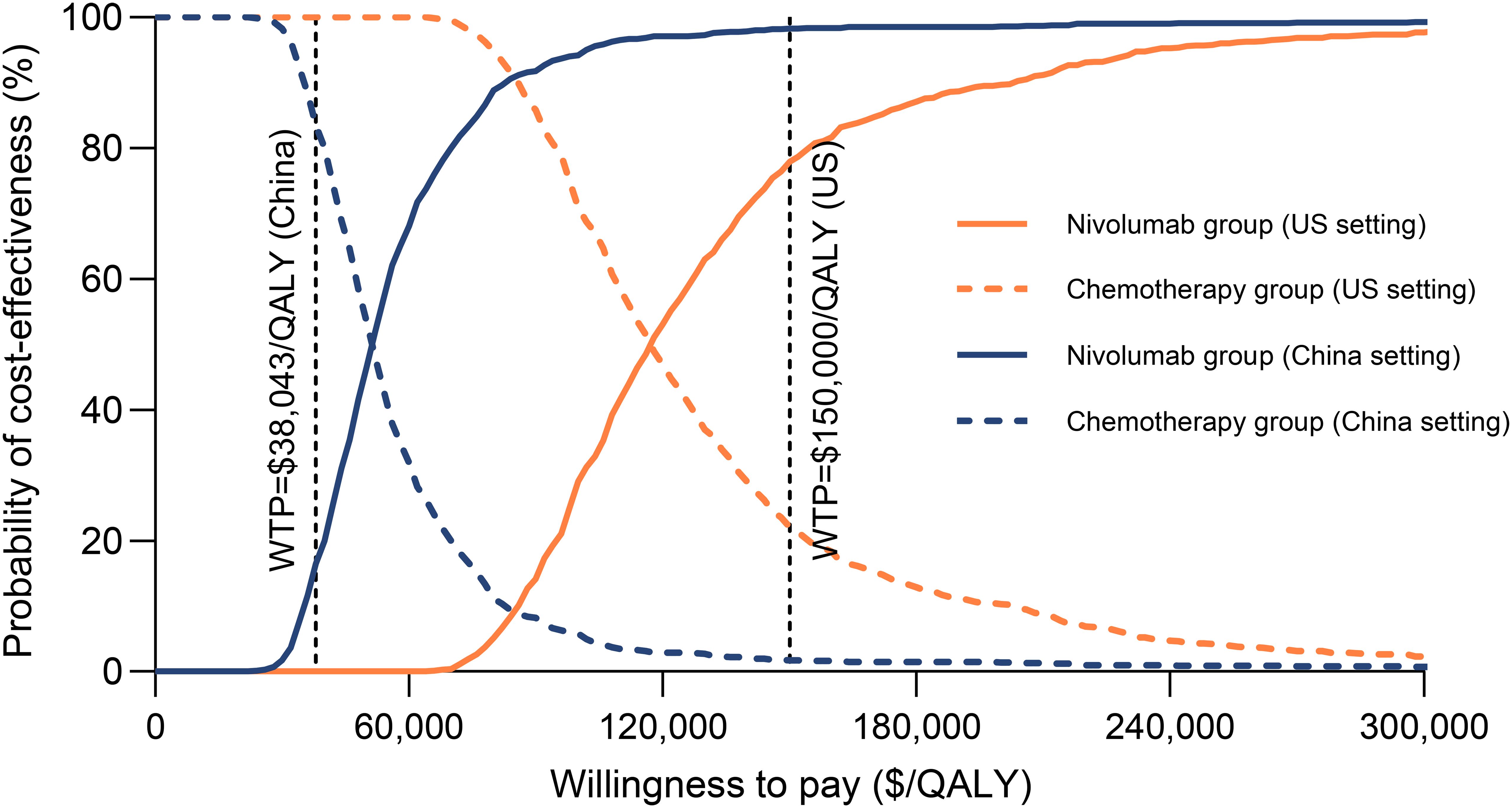
Figure 4. Cost-effectiveness acceptability curves for nivolumab plus gemcitabine-cisplatin vs gemcitabine-cisplatin. QALY, quality-adjusted life-year; WTP, willingness-to-pay.
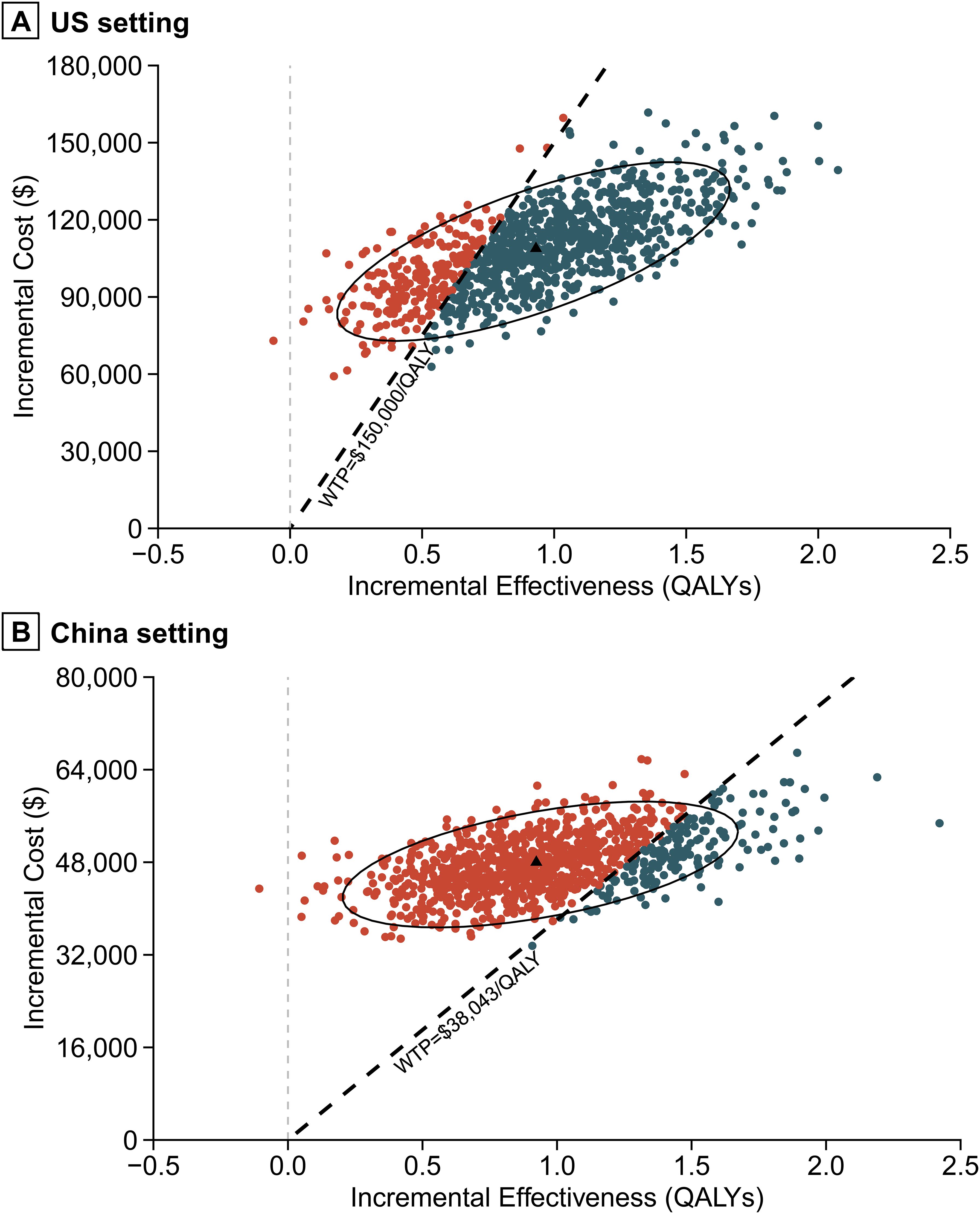
Figure 5. Incremental cost-effectiveness scatter plot. (A) US setting. (B) China setting. QALY, quality-adjusted life-year.
In the US, the ICERs for nivolumab plus gemcitabine-cisplatin compared to gemcitabine-cisplatin alone fell below the willingness-to-pay threshold for patients under 65 years old, those over 75, and those with an ECOG performance-status score of 0, and those who were previously untreated. The probability of being cost-effective exceeding 50% in these subgroups. This indicated that nivolumab combined with chemotherapy could be considered cost-effective in these patient subgroups. For the Chinese population, the combination of nivolumab and chemotherapy had the highest cost-effectiveness probability in patients with an ECOG performance-status score of 0, under 65 years old, with ICERs for these two subgroups falling below the willingness-to-pay threshold. Additionally, the combination therapy showed a relatively high probability of cost-effectiveness in the subgroup with tumor cell PD-L1 expression ≥1%, but its ICER exceeded the willingness-to-pay threshold (Table 4).
This study investigated the cost-effectiveness of adding nivolumab to gemcitabine-cisplatin versus gemcitabine-cisplatin alone in advanced urothelial carcinoma, based on results from the CheckMate 901 trial. Our analysis found that combining nivolumab with gemcitabine-cisplatin extended the life expectancy of patients with unresectable or metastatic urothelial carcinoma by 1.259 years, at an additional total cost of $ 108,838 and $ 48,001 for patients in the US and China, respectively. Consequently, ICERs exceeded the willingness-to-pay threshold of $38,043/QALY in China, but below was lower than the cost-effectiveness threshold of $150,000/QALY in the US, suggesting that the addition of nivolumab to chemotherapy was cost-effective for advanced urothelial carcinoma in the US but not in China.
Univariate sensitivity analysis demonstrated that the ICER decreased as the time horizon extended, with the ICER at a 30-year time horizon falling to about one-third of that at the 5-year time horizon, which was the endpoint of the CheckMate 901 trial follow-up. This suggested that nivolumab in combination with gemcitabine-cisplatin could yield more favorable economic outcomes in patients with a longer life expectancy. The results of the price sensitivity analysis indicated that applying price discounts to nivolumab might be the most viable strategy for making the combination treatment cost-effective across all patient populations. Specifically, discount of at least 29.81% in China were required to achieve cost-effectiveness. These findings can inform reimbursement and pricing decisions by public health insurance agencies and private health insurance companies. Moreover, two-way sensitivity analysis revealed that improving the quality of life during treatment for patients with advanced urothelial carcinoma could also enhance the cost-effectiveness of nivolumab combination therapy.
Subgroup analysis validated the results of the sensitivity analysis for HRs related to OS and PFS. Within the context of precision medicine, individualized cancer treatment must consider not only the patient’s physical condition and disease status but also their financial capacity to bear the costs. The subgroup analysis revealed that the combination of nivolumab and chemotherapy exhibited lower ICERs among patients under 65 years of age, males, those with an ECOG performance-status score of 0, tumor cell PD-L1 expression ≥1%, and those who had not received previous systemic therapy for advanced urothelial carcinoma. Furthermore, the combination therapy showed the highest probability of being cost-effective in patients younger than 65 years and those with an ECOG performance-status score of 0. It potentially offers a cost-effective option for patients who are younger than 65 or have an ECOG score of 0 in the US and China. This could inform decision-making for the selection of first-line treatment with nivolumab plus gemcitabine-cisplatin in advanced urothelial carcinoma. Moreover, our study indicated that the cost-effectiveness of nivolumab plus gemcitabine-cisplatin was superior in patients with PD-L1 expression ≥1% compared to those with PD-L1 expression <1%, consistent with previous studies (35, 36). Given the varying association between PD-L1 expression and the efficacy of immune checkpoint inhibitors in urothelial carcinoma patients (37), it is increasingly important to explore the relationship between PD-L1 expression and the cost-effectiveness of these therapies. We recommend increasing patient stratification in clinical trials based on PD-L1 expression levels to provide more detailed information for economic evaluations and clinical individualized drug treatment.
Current cost-effectiveness analyses of first-line immunotherapy treatments for advanced urothelial carcinoma present mixed results. Qin et al. assessed the cost-effectiveness of atezolizumab combined with gemcitabine and platinum-based chemotherapy from the perspective of US payers, based on the IMvigor130 trial, finding it not cost-effective with an ICER of $434,317/QALY (34). Similarly, Hale et al. evaluated the cost-effectiveness of pembrolizumab plus gemcitabine-carboplatin in the US, showing an ICER of $78,925/QALY, which is considered cost-effective under the willingness-to-pay threshold of $100,000/QALY (38). However, Hale et al.’s study was based on the phase 2 clinical trial KEYNOTE-052, and its conclusions require further validation. Furthermore, phase 3 clinical trials KEYNOTE-361 and IMvigor130 failed to demonstrate a significant OS benefit of first-line treatment with pembrolizumab or atezolizumab combined with chemotherapy compared to chemotherapy alone (39, 40). Due to the absence of head-to-head trial data, an analysis comparing the cost-effectiveness of nivolumab against atezolizumab or pembrolizumab has not been conducted. Future research is necessary to explore the cost-effectiveness of different first-line immunotherapies for advanced urothelial carcinoma.
Although this study evaluated the cost-effectiveness of nivolumab plus gemcitabine-cisplatin as first-line treatment for advanced urothelial carcinoma from the U.S. perspective, our findings might still hold relevance for certain European countries, which are also developed nations. Contieri et al. calculated the cost-effectiveness thresholds for five populous European countries (Italy, Spain, Germany, the United Kingdom, and France), which were $106,980, $92,100, $153,600, $144,240, and $130,980, respectively (41). Assuming no differences in the price of cancer drugs or other treatment costs, the probabilities of nivolumab plus gemcitabine-cisplatin being cost-effective in Italy, Spain, Germany, the United Kingdom, and France were 37.5%, 18.8%, 81.3%, 76.1%, and 66.1%, respectively. Moreover, the study by Vokinger et al. showed that the median monthly treatment cost of cancer drugs in the U.S. was 2.31 times higher than in the evaluated European countries (42). When accounting for differences in drug prices but no other cost variations, the ICER for nivolumab plus gemcitabine-cisplatin as first-line treatment for advanced urothelial carcinoma in Europe was $65,474 per QALY, which was below the cost-effectiveness thresholds of the aforementioned European countries. This preliminary analysis suggested that nivolumab plus gemcitabine-cisplatin may be cost-effective as first-line treatment for advanced urothelial carcinoma in some European countries. However, this was an idealized estimate based on price differences with other costs held constant, and actual results would require future analysis using real-world cost data from each country.
This study has several limitations. First, it was based on mathematical modeling using data from the CheckMate 901 phase 3 clinical trial. Given that the longest follow-up period reported in the CheckMate 901 trial was 60.2 months, the extrapolation of OS and PFS data beyond this period employed common methods in economic evaluation, which might diverge from actual outcomes. Second, this study assumed that patients receiving subsequent treatments in the Chinese setting participated in clinical trials as a substitute for pembrolizumab and avelumab therapy. This assumption may have underestimated the drug costs in both groups, potentially leading to biased ICER estimates. Third, the CheckMate 901 trial did not report quality-of-life data; hence, the utility values used in this study were derived from literature and did not account for the disutility of grade 1/2 adverse events, potentially leading to overestimation or underestimation of patient benefits. Additionally, since there are currently no reported utility values for urothelial carcinoma based on the Chinese population, we used utility values from US patients in the China setting. This may result in discrepancies between the ICER for Chinese patients and the actual values. If future clinical studies report health-related quality of life outcomes for the Chinese population, using more reliable utility data could optimize our study’s findings. Lastly, due to the unavailability of raw data, we estimated the costs and effectiveness for each subgroup using subgroup-specific constant HRs for OS and PFS, a common approach in the economic evaluation of oncology drugs. This may introduce bias into the economic results, and caution is advised when interpreting the findings of the subgroup analysis.
In comparison to gemcitabine-cisplatin, the first-line treatment of unresectable or metastatic urothelial carcinoma with nivolumab plus gemcitabine-cisplatin resulted in ICERs of $116,856/QALY in the US and $51,997/QALY in China. The ICER was below the willingness-to-pay threshold of $150,000 per QALY in the US, indicating that the combination therapy was cost-effective, while in China, the ICER exceeded the threshold of $38,043 per QALY, suggesting that nivolumab plus gemcitabine-cisplatin was not cost-effective. However, this combination therapy may represent a cost-effective option for specific subgroups in China, specifically patients under the age of 65 or those with an ECOG performance-status score of 0. Additionally, a price reduction of at least 29.81% for nivolumab is likely to achieve cost-effectiveness across all patient populations with advanced urothelial carcinoma in China.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
GX: Conceptualization, Data curation, Formal analysis, Methodology, Software, Visualization, Writing – original draft. YH: Data curation, Formal analysis, Writing – review & editing. LG: Data curation, Writing – review & editing. LW: Data curation, Methodology, Writing – review & editing. YD: Data curation, Investigation, Writing – review & editing. YW: Data curation, Writing – review & editing. HX: Writing – review & editing. YL: Conceptualization, Funding acquisition, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Chongqing Clinical Pharmacy Key Specialties Construction Project.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1426024/full#supplementary-material
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. (2023) 73:17–48. doi: 10.3322/caac.21763
3. Lobo N, Afferi L, Moschini M, Mostafid H, Porten S, Psutka SP, et al. Epidemiology, screening, and prevention of bladder cancer. Eur Urol Oncol. (2022) 5:628–39. doi: 10.1016/j.euo.2022.10.003
4. Loriot Y, Matsubara N, Park SH, Huddart RA, Burgess EF, Houede N, et al. Erdafitinib or chemotherapy in advanced or metastatic urothelial carcinoma. N Engl J Med. (2023) 389:1961–71. doi: 10.1056/NEJMoa2308849
5. Bamias A, Davis ID, Galsky MD, Arranz J, Kikuchi E, Grande E, et al. Atezolizumab monotherapy versus chemotherapy in untreated locally advanced or metastatic urothelial carcinoma (Imvigor130): final overall survival analysis from a randomised, controlled, phase 3 study. Lancet Oncol. (2024) 25:46–61. doi: 10.1016/s1470-2045(23)00539-9
6. Powles T, Park SH, Voog E, Caserta C, Valderrama BP, Gurney H, et al. Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. N Engl J Med. (2020) 383:1218–30. doi: 10.1056/NEJMoa2002788
7. Powles TB, Perez Valderrama B, Gupta S, Bedke J, Kikuchi E, Hoffman-Censits S, et al. Lba6 ev-302/keynote-A39: open-label, randomized phase iii study of enfortumab vedotin in combination with pembrolizumab (Ev+P) vs chemotherapy (Chemo) in previously untreated locally advanced metastatic urothelial carcinoma (La/muc). Ann Oncol. (2023) 34:S1340. doi: 10.1016/j.annonc.2023.10.106
8. Hato SV, Khong A, de Vries IJ, Lesterhuis WJ. Molecular pathways: the immunogenic effects of platinum-based chemotherapeutics. Clin Cancer Res. (2014) 20:2831–7. doi: 10.1158/1078-0432.Ccr-13-3141
9. van der Heijden MS, Sonpavde G, Powles T, Necchi A, Burotto M, Schenker M, et al. Nivolumab plus gemcitabine-cisplatin in advanced urothelial carcinoma. N Engl J Med. (2023) 389:1778–89. doi: 10.1056/NEJMoa2309863
10. Verma V, Sprave T, Haque W, Simone CB 2nd, Chang JY, Welsh JW, et al. A systematic review of the cost and cost-effectiveness studies of immune checkpoint inhibitors. J Immunother Cancer. (2018) 6:128. doi: 10.1186/s40425-018-0442-7
11. Institute for Clinical and Economic Review. The 2023 Value Assessment Framework (2023). Available online at: https://icer.org/wp-content/uploads/2023/10/ICER_2023_VAF_For-Publication_101723.pdf (accessed August 5, 2024).
12. Ding D, Hu H, Li S, Zhu Y, Shi Y, Liao M, et al. Cost-effectiveness analysis of durvalumab plus chemotherapy in the first-line treatment of extensive-stage small cell lung cancer. J Natl Compr Canc Netw. (2021) 19:1141–7. doi: 10.6004/jnccn.2020.7796
13. Wu B, Zhang Q, Sun J. Cost-effectiveness of nivolumab plus ipilimumab as first-line therapy in advanced renal-cell carcinoma. J Immunother Cancer. (2018) 6:124. doi: 10.1186/s40425-018-0440-9
14. World Health Organization. Making Choices in Health: Who Guide to Cost-Effectiveness Analysis (2003). Available online at: https://iris.who.int/bitstream/handle/10665/42699/9241546018.pdf (accessed August 5, 2024).
15. Chinese Pharmaceutical Association. China Guidelines for Pharmacoeconomic Evaluations (2020). Available online at: https://www.cpa.org.cn/cpadmn/attached/file/20201203/1606977380634185.pdf (accessed August 5, 2024).
16. The Central People’s Government of the People’s Republic of China. China’s Gdp Grows by 5.2% Year-on-Year in 2023. Available online at: https://data.stats.gov.cn/easyquery.htm?cn=C01&zb=A0201&sj=2023 (accessed accessed January 17, 2024).
17. Sanders GD, Neumann PJ, Basu A, Brock DW, Feeny D, Krahn M, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. Jama. (2016) 316:1093–103. doi: 10.1001/jama.2016.12195
18. Shao T, Zhao M, Liang L, Tang W. Serplulimab plus chemotherapy vs chemotherapy for treatment of us and Chinese patients with extensive-stage small-cell lung cancer: A cost-effectiveness analysis to inform drug pricing. BioDrugs. (2023) 37:421–32. doi: 10.1007/s40259-023-00586-6
19. Husereau D, Drummond M, Augustovski F, de Bekker-Grob E, Briggs AH, Carswell C, et al. Consolidated health economic evaluation reporting standards 2022 (Cheers 2022) statement: updated reporting guidance for health economic evaluations. Bmj. (2022) 376:e067975. doi: 10.1136/bmj-2021-067975
20. Chinese Society of Clinical Oncology. Urothelial Cancer Guidelines 2023. Beijing: People’s Medical Publishing House (2023).
21. Guyot P, Ades AE, Ouwens MJ, Welton NJ. Enhanced secondary analysis of survival data: reconstructing the data from published kaplan-meier survival curves. BMC Med Res Methodol. (2012) 12:9. doi: 10.1186/1471-2288-12-9
22. Centers for Medicare & Medicaid Services. 2024 Asp Drug Pricing Files . Available online at: https://www.cms.gov/medicare/payment/all-fee-service-providers/medicare-part-b-drug-average-sales-price/asp-pricing-files (accessed December 26, 2023).
23. MENET. Menet Database. Available online at: https://db.menet.com.cn/#/bid?nav=3 (accessed December 21, 2023).
24. Meng R, Cao Y, Zhou T, Hu H, Qiu Y. The cost effectiveness of donafenib compared with sorafenib for the first-line treatment of unresectable or metastatic hepatocellular carcinoma in China. Front Public Health. (2022) 10:794131. doi: 10.3389/fpubh.2022.794131
25. Qiu Y, Zha J, Ma A, Zhou T. Cost-effectiveness analysis of niraparib maintenance therapy in Chinese patients with platinum-sensitive recurrent ovarian cancer. Gynecol Oncol. (2023) 174:175–81. doi: 10.1016/j.ygyno.2023.05.010
26. Wan X, Zhang Y, Tan C, Zeng X, Peng L. First-line nivolumab plus ipilimumab vs sunitinib for metastatic renal cell carcinoma: A cost-effectiveness analysis. JAMA Oncol. (2019) 5:491–6. doi: 10.1001/jamaoncol.2018.7086
27. Su D, Wu B, Shi L. Cost-effectiveness of atezolizumab plus bevacizumab vs sorafenib as first-line treatment of unresectable hepatocellular carcinoma. JAMA Netw Open. (2021) 4:e210037. doi: 10.1001/jamanetworkopen.2021.0037
28. Zhou T, Wang X, Cao Y, Yang L, Wang Z, Ma A, et al. Cost-effectiveness analysis of sintilimab plus bevacizumab biosimilar compared with lenvatinib as the first-line treatment of unresectable or metastatic hepatocellular carcinoma. BMC Health Serv Res. (2022) 22:1367. doi: 10.1186/s12913-022-08661-4
29. Pei R, Shi Y, Lv S, Dai T, Zhang F, Liu S, et al. Nivolumab vs pembrolizumab for treatment of us patients with platinum-refractory recurrent or metastatic head and neck squamous cell carcinoma: A network meta-analysis and cost-effectiveness analysis. JAMA Netw Open. (2021) 4:e218065. doi: 10.1001/jamanetworkopen.2021.8065
30. Wu B, Gu X, Zhang Q. Cost-effectiveness of osimertinib for egfr mutation-positive non-small cell lung cancer after progression following first-line egfr tki therapy. J Thorac Oncol. (2018) 13:184–93. doi: 10.1016/j.jtho.2017.10.012
31. Centers for Medicare & Medicaid Services. Physician Fee Schedule . Available online at: https://www.cms.gov/medicare/physician-fee-schedule/search (accessed December 19, 2023).
32. Medicare. Procedure Price Lookup . Available online at: https://www.medicare.gov/procedure-price-lookup/ (accessed December 25, 2023).
33. Mudumba R, Chan H-H, Cheng Y-Y, Wang C-C, Correia L, Ballreich J, et al. Cost-effectiveness analysis of trastuzumab deruxtecan versus trastuzumab emtansine for patients with human epidermal growth factor receptor 2 positive metastatic breast cancer in the United States. Value Health. (2024) 27:153–63. doi: 10.1016/j.jval.2023.11.004
34. Qin S, Yi L, Li S, Tan C, Zeng X, Wang L, et al. Cost-effectiveness of atezolizumab plus chemotherapy as first-line therapy for metastatic urothelial cancer. Adv Ther. (2021) 38:3399–408. doi: 10.1007/s12325-021-01785-9
35. Aguiar P, De Mello R, Tadokoro H, Santoro I, Babiker H, Avancha K, et al. Cost effectiveness of immune checkpoint inhibitors in non-small cell lung cancer relative to pd-L1 expression. J OF Thorac Oncol. (2016) 11:S169–S70. doi: 10.1016/j.jtho.2016.08.006
36. She L, Hu H, Liao M, Xia X, Shi Y, Yao L, et al. Cost-effectiveness analysis of pembrolizumab versus chemotherapy as first-line treatment in locally advanced or metastatic non-small cell lung cancer with pd-L1 tumor proportion score 1% or greater. Lung Cancer. (2019) 138:88–94. doi: 10.1016/j.lungcan.2019.10.017
37. Galsky MD, Bajorin DF, Witjes JA, Gschwend JE, Tomita Y, Nasroulah F, et al. Disease-free survival analysis for patients with high-risk muscle-invasive urothelial carcinoma from the randomized checkmate 274 trial by pd-L1 combined positive score and tumor cell score. Eur Urol. (2023) 83:432–40. doi: 10.1016/j.eururo.2023.01.016
38. Hale O, Patterson K, Lai Y, Meng Y, Li H, Godwin JL, et al. Cost-effectiveness of pembrolizumab versus carboplatin-based chemotherapy as first-line treatment of pd-L1-positive locally advanced or metastatic urothelial carcinoma ineligible for cisplatin-based therapy in the United States. Clin Genitourin Cancer. (2021) 19:e17–30. doi: 10.1016/j.clgc.2020.07.006
39. Powles T, Csőszi T, Özgüroğlu M, Matsubara N, Géczi L, Cheng SY, et al. Pembrolizumab alone or combined with chemotherapy versus chemotherapy as first-line therapy for advanced urothelial carcinoma (Keynote-361): A randomised, open-label, phase 3 trial. Lancet Oncol. (2021) 22:931–45. doi: 10.1016/s1470-2045(21)00152-2
40. Galsky MD, Arija JÁA, Bamias A, Davis ID, De Santis M, Kikuchi E, et al. Atezolizumab with or without chemotherapy in metastatic urothelial cancer (Imvigor130): A multicentre, randomised, placebo-controlled phase 3 trial. Lancet. (2020) 395:1547–57. doi: 10.1016/s0140-6736(20)30230-0
41. Contieri R, Martini A, Mertens LS, Giannatempo P, Hurle R, Witjes JA, et al. Financial burden of guideline-recommended cancer medications for metastatic urothelial carcinoma. Eur Urol Focus. (2024), 2405–4569. doi: 10.1016/j.euf.2023.12.002
Keywords: nivolumab, urothelial carcinoma, cost-effectiveness, gemcitabine, cisplatin, chemotherapy, partitioned survival model
Citation: Xiang G, Huang Y, Gan L, Wang L, Ding Y, Wu Y, Xing H and Liu Y (2024) Cost-effectiveness of nivolumab plus gemcitabine-cisplatin as first-line treatment for advanced urothelial carcinoma in China and the United States. Front. Immunol. 15:1426024. doi: 10.3389/fimmu.2024.1426024
Received: 30 April 2024; Accepted: 27 August 2024;
Published: 13 September 2024.
Edited by:
Anand Rotte, Arcellx Inc, United StatesReviewed by:
Roberto Contieri, Humanitas University, ItalyCopyright © 2024 Xiang, Huang, Gan, Wang, Ding, Wu, Xing and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yao Liu, bGl1eWFvQHRtbXUuZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.