- 1Department of General Medicine, Third Hospital of Shanxi Medical University, Shanxi Bethune Hospital, Shanxi Academy of Medical Sciences, Tongji Shanxi Hospital, Taiyuan, China
- 2Department of Infectious Diseases, Jingzhou First People’s Hospital, Jingzhou, China
- 3Department of Orthopedics, Third Hospital of Shanxi Medical University, Shanxi Bethune Hospital, Shanxi Academy of Medical Sciences, Tongji Shanxi Hospital, Taiyuan, China
- 4Department of Orthopedics, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
Natural Killer (NK) cells play a crucial role as effector cells within the tumor immune microenvironment, capable of identifying and eliminating tumor cells through the expression of diverse activating and inhibitory receptors that recognize tumor-related ligands. Therefore, harnessing NK cells for therapeutic purposes represents a significant adjunct to T cell-based tumor immunotherapy strategies. Presently, NK cell-based tumor immunotherapy strategies encompass various approaches, including adoptive NK cell therapy, cytokine therapy, antibody-based NK cell therapy (enhancing ADCC mediated by NK cells, NK cell engagers, immune checkpoint blockade therapy) and the utilization of nanoparticles and small molecules to modulate NK cell anti-tumor functionality. This article presents a comprehensive overview of the latest advances in NK cell-based anti-tumor immunotherapy, with the aim of offering insights and methodologies for the clinical treatment of cancer patients.
1 Introduction
The occurrence of tumors results from abnormal cell proliferation induced by imbalanced homeostasis of cells in the body under the influence of genetic and environmental factors (1). Tumor cells can inhibit the anti-tumor immune response of immune cells in the tumor microenvironment through immune escape mechanisms, thereby promoting the occurrence and development of tumors (2). Tumor immunotherapy involves modifying the inhibitory tumor microenvironment, restoring immune system activity, and ultimately clearing tumor cells.
Immunotherapy, as an emerging cancer treatment strategy, has rapidly developed in recent years. Currently, tumor immunotherapy based on T cells is widely used, including chimeric antigen receptor (CAR) T cell therapy and immune checkpoint blockade (ICB) therapy, among others. Although significant success has been achieved, there are also some limitations and drawbacks. For instance, it is limited by the expression of major histocompatibility complex (MHC) molecules, infusing a large number of CAR-T cells may induce cytokine storms or produce non-target effects due to their persistence, causing damage to other cells (3, 4). Therefore, there is an urgent need to develop more effective and less toxic treatment methods.
Recent studies have shown that NK cells play a crucial role in controlling the occurrence and development of tumors (5, 6). NK cells are cytotoxic lymphocytes in the natural immune system that exert a direct killing effect. Their anti-tumor effects do not require antigen sensitization (7, 8) and do not rely on MHC-I molecules. Compared with CD8+ T cells, their recognition mechanism is more flexible, and they have the ability to quickly kill tumor cells. They are currently the most promising tumor-killing cells besides T cells. This non-specific recognition mechanism and efficient anti-tumor activity may supplement the shortcomings of anti-tumor T cell therapy.
Based on this, NK cells have become an important research object in tumor therapy. Immunotherapy strategies that enhance the anti-tumor response of NK cells have rapidly developed, including adoptive NK cell therapy, cytokine therapy, antibody-based NK cell therapy (enhancing antibody-dependent cellular cytotoxicity (ADCC) mediated by NK cells, NK cell engagers, ICB therapy), and the use of nanoparticles and small molecules to regulate the anti-tumor function of NK cells. These methods are expected to open up new immunotherapy pathways and bring better therapeutic effects to cancer patients. In this review, we provide an overview of the latest advances in tumor immunotherapy strategies based on NK cells.
2 Biological characteristics of NK cells
NK cells originate from bone marrow hematopoietic stem cells and belong to the innate lymphocyte group. They rank as the third largest lymphocyte group after B cells and T cells, accounting for approximately 5% to 15% of the total number of peripheral blood lymphocytes (9–13). They play a vital role in resisting tumor formation and combating pathogenic microbial infections in innate immunity. However, for NK cells to fully exert their optimal cytotoxicity and immune regulatory effects, they need to undergo a series of cell signaling molecules and transcription factors to transition from an immature state to a mature state.
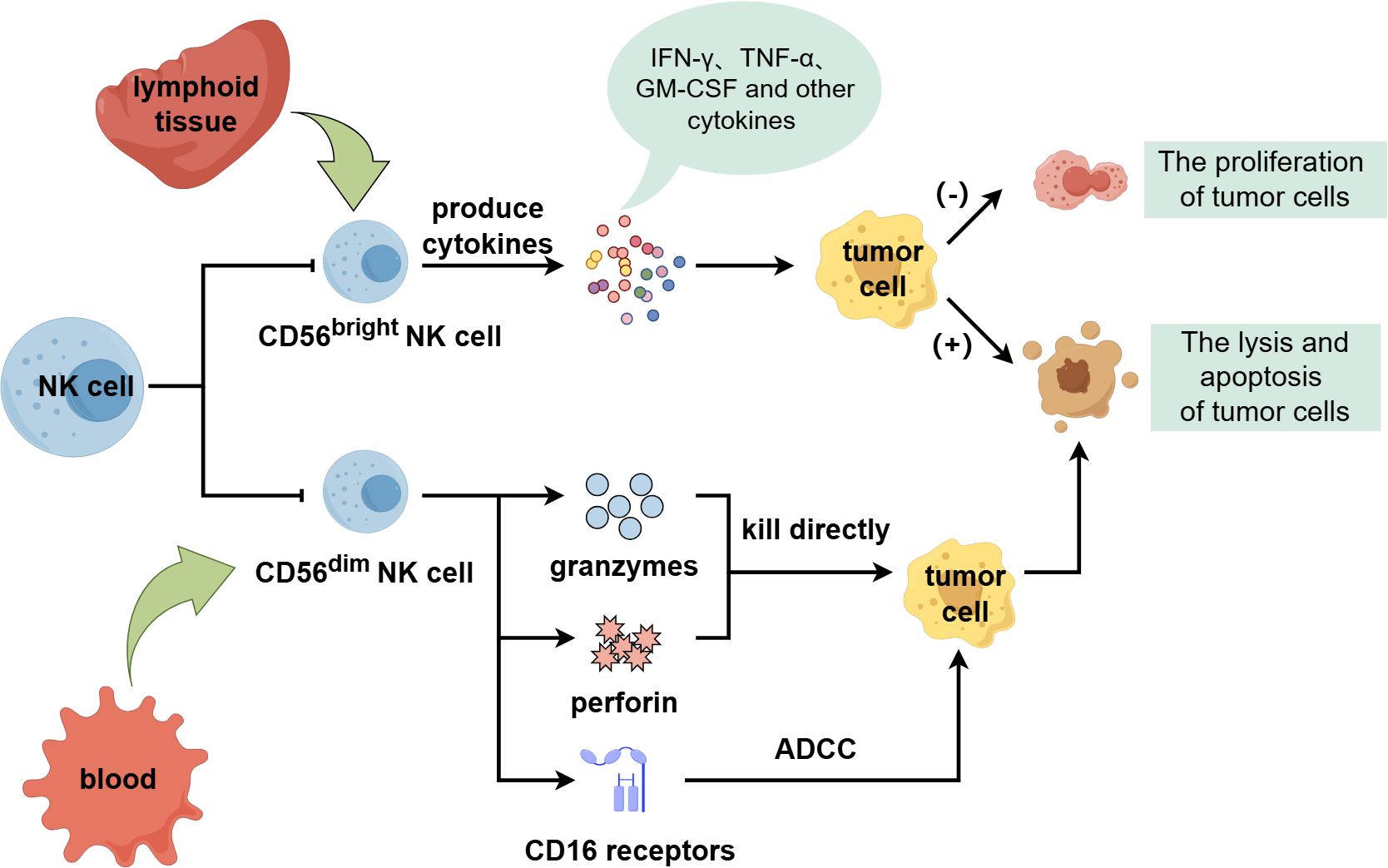
Human NK cells can be categorized into two subgroups with distinct functions and phenotypes, CD56bright and CD56dim, based on differences in surface molecule CD56 expression abundance (11, 14). The CD56 bright subgroup is immature and primarily exists in lymphoid tissue, which can secrete interferon γ (IFN-γ), tumor necrosis factor α (TNF-α), granulocyte-macrophage colony-stimulating factor (GM-CSF) and other cytokines and chemokines. It has important immunomodulatory function, can promote tumor cell apoptosis and inhibit its proliferation, but its cytotoxicity is weak (15, 16). The CD 56 dim subgroup is a mature cytotoxic population, accounting for the majority of circulating NK cells and mainly exists in blood. It can express granzymes and perforin to directly kill tumor cells. And it can also express CD16 receptors to bind to tumor cells and mediate ADCC to exert anti-tumor effects (Figure 1) (6, 17–22).
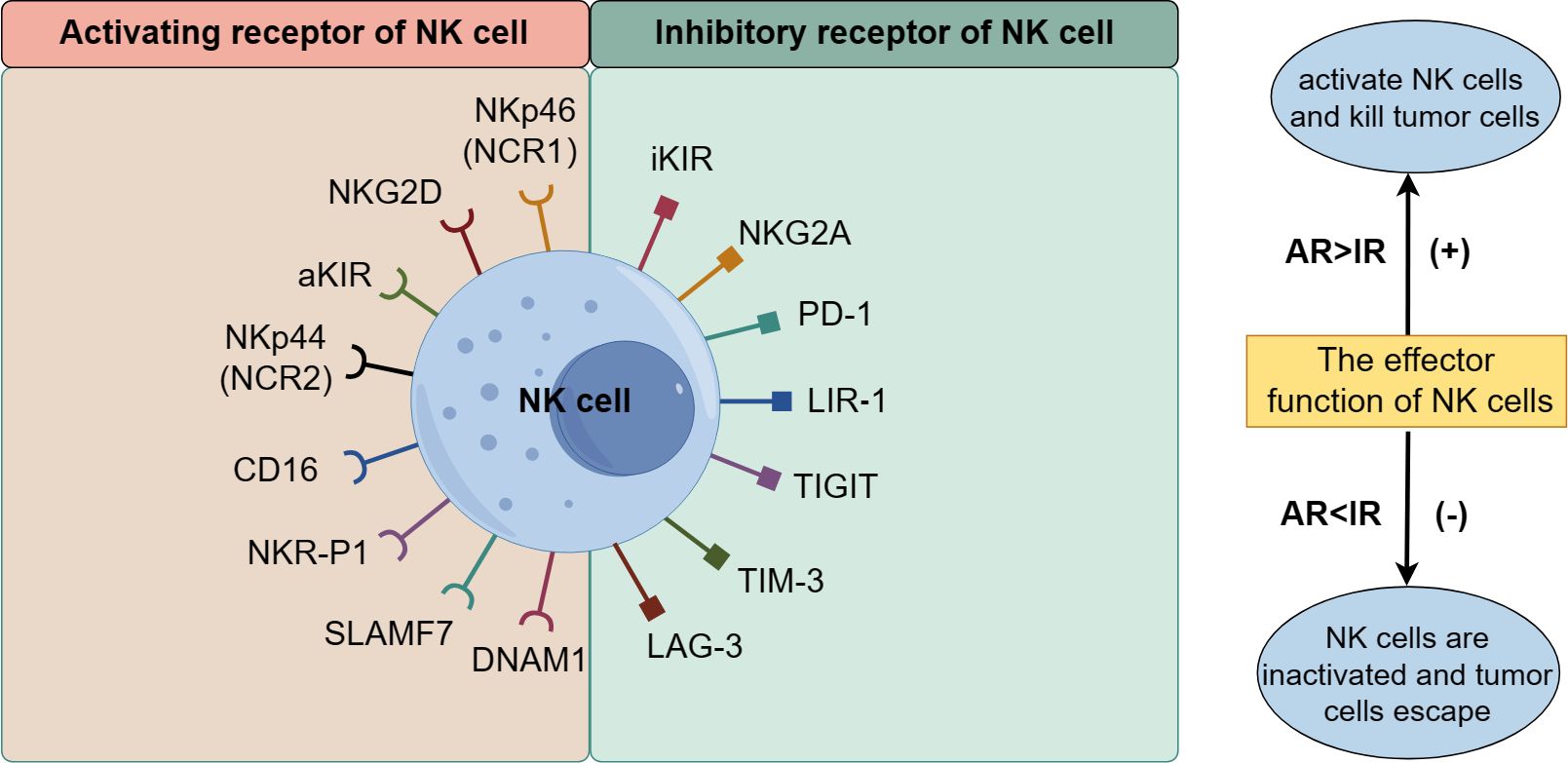
NK cells recognize normal and abnormal tissue cells by expressing regulatory receptors related to killing function, selectively targeting tumor cells while sparing normal cells in the body. Their killing effect depends on the coordination and balance of multiple receptors on their membrane surface, mainly divided into activated receptors (AR) and inhibitory receptors (IR). AR includes the natural cytotoxic receptor (NCR) family, signaling lymphocyte-activation molecule family (SLAMF) receptor, activated killer cell immunoglobulin-like receptor (aKIR), NK cell receptor protein 1 (NKR-P1), and NK cell group 2D receptor (NKG2D), NKG2C, NKG2E, CD16, DNAX accessory molecule 1 (DNAM1), etc. IR includes inhibitory KIR (iKIR), CD94/NKG2A, programmed cell death receptor 1 (PD-1), and leukocyte immunoglobulin-like receptor 1 (LIR-1), T cell immunoreceptor with Ig and ITIM domains (TIGIT), T-cell immunoglobulin and mucin domain 3 (TIM-3), lymphocyte activation gene 3 (LAG3), etc. (Figure 2) (23–31). The recognition and functional regulation of NK cells require the detection of ligands on the surface of target cells through inhibitory receptors, as well as the activation of NK cells through activating receptors. The dynamic balance between activated receptors and inhibitory receptors is crucial for ensuring the regulation of NK cell effector function. When the activated receptor dominates over the inhibitory receptor, NK cells exert strong killing effects on tumor cells; otherwise, the killing effect of NK cells is inhibited, allowing tumor cells to evade destruction.
3 Crosstalk between NK cells and other immune cells in the tumor microenvironment
Natural killer (NK) cells play a crucial role in anti-tumor immunity, but their proliferation, maturation, secretion of effector molecules, and overall function are influenced by the tumor microenvironment. The TME contains a diverse group of immune cells, including T cells, dendritic cells, neutrophils, and macrophages, among others. The interaction between NK cells and these immune cells significantly impacts the anti-tumor response.
3.1 Crosstalk between NK cells and T cells
Within the TME, the T cell population primarily consists of CD8+ T cells and CD4+ T cells. Activated CD8+ T cells express homing and chemokine receptors and kill tumor cells by producing high levels of IFN-γ and TNF-α (32). CD4+ T cells indirectly promote anti-tumor responses by regulating the composition and activity of infiltrating immune cells in the TME (33).
In this complex immune microenvironment, NK cells and T cells collaborate in regulating anti-tumor immunity and complement each other in MHC-induced immune evasion. Cancer cells can evade recognition by cytotoxic CD8+ T cells through down-regulating MHC-I expression. This down-regulation also triggers NK cells to initiate a “self-deletion” mechanism to target cancer cells. Thus, NK cells and T cells work together to prevent cancer cells from escaping immune surveillance (34).
Research has demonstrated that NK cells can enhance or impair T cell responses both directly and indirectly. NK cells can induce the proliferation of autologous T cells (35), provide IFN-γ for naïve T cells, and induce the polarization of T helper cell type 1 (Th1) (36). Activated NK cells can also mediate IFN-γ secretion, stimulate dendritic cells to produce IL-12, and subsequently initiate CD8+ T cell anti-tumor responses (37, 38). However, cooperation between DNAM-1 and NKG2D can negatively impact T cell responses (39). There is bidirectional crosstalk between T cells and NK cells, and T cells can also affect NK cells in turn. Cytokines released by T cells, such as IL-2 and IL-15, have been shown to activate NK cell cytotoxicity and enhance anti-tumor responses (40–42).
3.2 Crosstalk between NK cells and regulatory T cells
It has been confirmed that regulatory T cells (Tregs) can inhibit NK cell function and induce immune suppression within tumors (43). In 2004, Trzonkowski et al. observed that human NK cell activity was suppressed in the presence of Tregs (44). In 2005, Ghiringhelli et al. first reported the mechanism of Treg-NK interaction, noting that Tregs directly inhibited NK cell responses through membrane-bound TGF-β. The deletion of Tregs restored NK cells’ ability to mediate the lysis of human cancer cells (45). Similarly, Liu et al. found that Tregs in patients with non-small cell lung cancer effectively inhibited the anti-tumor ability of autologous NK cells, and treatment with anti-TGF-β antibody restored the damaged cytotoxic activity of NK cells in tumor tissue (46).
Tregs can interfere with NK cells through various mechanisms, including the downregulation of NKG2D expression induced by TGF-β (47), and by consuming large amounts of IL-2. Littwitz-Salomon et al. found in a transgenic mouse model that the selective removal of Tregs can improve the proliferation, maturation, and effector cell differentiation of NK cells. The inhibition of NK cell function depends on the consumption of IL-2 by Tregs, which can be overcome by stimulating specific NK cells with an IL-2/anti-IL-2 mAb complex (48). Additionally, it has been found that the stimulation of IL-12 and IL-18 can eliminate Treg inhibition and enhance the cytotoxicity of NK cells (49). These findings may provide a new therapeutic strategy for tumor immunotherapy.
3.3 Crosstalk between DCs and NK cells
Dendritic cells (DCs) are key players in the adaptive immune response, including classical DCs (cDCs), plasmacytoid DCs (pDCs), and monocyte-derived DCs (MoDCs). Among these, cDCs are particularly associated with anti-tumor functions, presenting tumor antigens by phagocytosing dead tumor cells or fragments, thereby exerting an anti-tumor effect (50).
Recent studies have revealed bidirectional crosstalk between DCs and NK cells. DCs can activate NK cells and enhance their anti-tumor activity (51, 52), while NK cells can influence the recruitment and maturation of DCs (53, 54). Cazzetta et al. found that DCs can release cytokines and chemokines, such as IL-12, IL-15, and IFN-γ, to promote the activation of NK cells. In turn, activated NK cells can promote the recruitment and maturation of DCs by producing IFN-γ and TNF-α (55). Similarly, Bottcher et al. discovered that NK cells can facilitate the migration of cDC1 to tumors, inducing their accumulation in the TME to enhance tumor immune control. Conversely, they also found that NK cell activity can reduce the accumulation of cDCs by impairing their viability, thereby leading to tumor immune escape (54). Additionally, studies have shown that the up-regulation of CTLA-4 expression on NK cells negatively regulates the maturation of DCs in human non-small cell lung cancer (NSCLC) (56). Moreover, DCs may also impair the function of NK cells (57). In summary, the crosstalk between NK cells and DCs is crucial for regulating anti-tumor immunity and could become a promising target for anti-tumor therapy (58).
3.4 Crosstalk between neutrophils and NK cells
Neutrophils play a key role in regulating both innate and adaptive immunity (59). Traditionally, neutrophils have dual functions in primary tumors, exhibiting both promotional and inhibitory effects (60, 61). Li et al. found that in a breast cancer (BC) mouse model, neutrophils showed an inhibitory effect on tumor metastasis in the absence of NK cells, while they exhibited a promotional effect on tumor metastasis in the presence of NK cells (62). Similarly, Ogura et al. discovered that NK cells regulate the tumor-promoting activities of neutrophils, with neutrophils showing tumor-promoting effects when NK cells are absent (63). The dual role of neutrophils may be related to their heterogeneity and function in different environments.
Characterizing the crosstalk between neutrophils and NK cells is challenging. Increasing evidence suggests that soluble mediators, intercellular interactions, and extracellular vesicles (EVs) facilitate the crosstalk between neutrophils and NK cells (64). In a mouse model of colorectal cancer, neutrophils have been shown to reduce NK cell infiltration by down-regulating CCR1, while simultaneously inhibiting the activity of NK cell activation receptors NKp46 and NKG2D (65). In a mouse model injected with 4T1 breast cancer cells, neutrophils inhibit NK cell activity, thereby weakening the NK cell-mediated clearance of cancer cells (66). Additionally, it has been reported that NK cells regulate neutrophil function through an interferon-γ mediated pathway. When NK cells are exhausted, neutrophils produce high levels of VEGFA and adopt a tumor-promoting phenotype (63). Neutrophils can also influence NK cells by producing IL-12.
3.5 Crosstalk between macrophages and NK cells
Macrophages exist at all stages of tumor development and play an important role in tumor immunomodulation. Macrophages can be divided into M1 and M2 types, with M1 macrophages being associated with anti-tumor activity and M2 macrophages with tumor-promoting activity (67). Macrophages and NK cells engage in crosstalk through various mechanisms. In the TME, macrophages promote the anti-tumor cytotoxicity of NK cells by releasing activating cytokines (such as IL-12, IL-15, IL-18, and TNF-α) and inhibit the expression of NK cell activation receptors while promoting the expression of inhibitory receptors by releasing inhibitory cytokines (such as TGF-β) (68, 69).
Studies have shown that in the early stages of tumor development, macrophages are predominantly M1-type, and NK cells exhibit strong tumor-killing activity. M1-type macrophages can activate NK cells by secreting soluble mediators and establishing intercellular interactions, thereby enhancing the cytotoxicity of NK cells against various target cells (70–72). As the tumor progresses, M1-type macrophages in the TME are stimulated by Th2-type cytokines to transform into M2-type macrophages, resulting in a significant decrease in the M1-to-M2 ratio in advanced tumors. IL-10 and TGF-β secreted by M2 macrophages have dual functions: they promote tumor invasion, angiogenesis, and metastasis while also inhibiting the proportion of NK cells and T cells. Additionally, they suppress the effector function of NK cells by inhibiting the secretion of effector molecules such as IFN-γ (72). Studies have found that IL-10 can increase the secretion of IFN-γ by NK cells and enhance their cytotoxicity. IL-10 induces metabolic reprogramming by upregulating glycolysis and oxidative phosphorylation (OXPHOS) in NK cells, thereby enhancing their effector functions. In this process, IL-10 stimulation triggers the activation of the Mammalian Target of Rapamycin Complex 1 (MTORC1) signaling pathway, which is crucial for IL-10-induced metabolic reprogramming and the enhancement of NK cell effector functions (73). Additionally, IL-10/Fc can promote the metabolic reprogramming of T cells, dependent on pyruvate and Mitochondrial Pyruvate Carrier (MPC), and induce the reactivation of terminally exhausted T cells, thereby enhancing anti-tumor immunity (74).
The crosstalk between macrophages and NK cells in the TME is extremely complex. Studying this crosstalk network may provide new insights for developing immunotherapy strategies.
4 Tumor immunotherapy based on NK cells
4.1 Adoptive NK cell therapy
Adoptive NK cell therapy involves the infusion of autologous or allogeneic NK cells that have been expanded or genetically modified in vitro into tumor patients, with the aim of increasing the number and anti-tumor activity of NK cells in the patient’s body. The sources of NK cells utilized in this therapy are diverse, including peripheral blood, umbilical cord blood, NK cells differentiated from induced pluripotent stem cells (iPSCs), and NK cell lines cultured in vitro (75, 76). NK cells derived from peripheral blood are readily obtained from patients or donors and can be swiftly activated and expanded in vitro through cytokine stimulation before being administered to tumor patients, demonstrating potent anti-tumor effects. Umbilical cord blood, rich in NK cells and containing a proportion of NK cell precursors with the potential to differentiate into mature NK cells, has emerged as an important NK cell source. Nonetheless, it presents some drawbacks, such as delayed collection, heterogeneity of white blood cells in donor blood, and high costs. NK cells differentiated from iPSCs serve as another vital source. These NK cells, obtained as “ready-to-use” products for any patient, are amenable to genetic manipulation. Additionally, NK cell lines derived from in vitro culture, standardized and irradiated before being injected into the patient’s body, exert anti-tumor effects.
4.1.1 NK cells
One method of adoptive NK cell therapy is NK cell infusion, which includes autologous NK cell infusion and allogeneic NK cell infusion. Autologous NK cell infusion uses the patient’s own blood as the cell source, offering convenience and avoiding the risk of graft-versus-host disease (GVHD), making it a promising anti-tumor immunotherapy. However, it has been found that although the infused cells can expand in vivo, they show a poor response to blood cancers or solid tumors. This poor response may be partly due to the inhibitory effect of interactions between autologous NK cells and MHC I molecules (34). Additionally, extensive pretreatment before cell collection and treatment may negatively impact the expansion and function of NK cells (77). As a result, many researchers have begun to explore new directions, shifting focus from autologous NK cell infusion to allogeneic NK cell infusion. Lin et al. (78) found that injecting pembrolizumab into patients with advanced non-small cell lung cancer, together with allogeneic NK cells, can effectively prolong the survival time of patients to 18.5 months. With the continuous improvement of NK cell purification and amplification technologies, NK cell infusion is expected to become an important component of adoptive immunotherapy.
4.1.2 Cytokine induced killer cells
Cytokine induced killer (CIK) cells are a group of heterogeneous cells induced by various cytokines (such as IL-2 and IL-1) and anti-CD3 antibodies. These cells express both T cell markers (CD3+) and NK cell surface markers (CD56+), possessing the anti-tumor activity of T lymphocytes and the non-MHC-restricted anti-tumor properties of NK cells. CIK cells can kill tumor cells through multiple mechanisms, including the release of perforin and granzyme to directly lyse tumor cells, direct inhibition of tumor cells, or indirect regulation of immune function by secreting cytokines such as IL-2, IL-6, IFN-γ, and GM-CSF. They can also promote tumor cell apoptosis by activating or up-regulating the expression of tumor cell apoptosis genes and death receptors.
Numerous clinical trials worldwide have confirmed that CIK cells have significant therapeutic effects on lung cancer, ovarian cancer, lymphoma, gastric cancer, melanoma, and other malignant tumors (79, 80). CIK cells can be used for adoptive cell therapy in both hematological and solid tumors, with good clinical responses. However, a notable issue with CIK cell therapy is the lack of specificity for tumor antigens.
In recent years, combination strategies involving CIK cells with traditional chemotherapy, cytokines, dendritic cells (DC), immune checkpoint inhibitors (ICI), and genetic engineering methods have been extensively studied. These combination therapies have shown better clinical responses compared to the use of CIK cells alone (81). Therefore, CIK cells combined with other therapies may represent a promising approach for future tumor immunotherapy.
4.1.3 Chimeric antigen receptor modified NK cells
Chimeric antigen receptor (CAR) is an artificial receptor molecule engineered via genetic engineering technology, designed to enhance the ability of immune cells to recognize antigens specifically and augment their activation function (82). Currently, CAR-T cell therapy has been widely used in cell immunotherapy for various tumors, however, it presents certain challenges, such as cytokine release syndrome (CRS), graft versus host disease (GVHD), and immune effector cell-associated neurotoxicity syndrome (ICANS) (83, 84). Consequently, attention has shifted towards engineering modifications of NK cells.
Similar to CAR-T cells, CAR-NK cells can recognize tumor antigens through the single-chain fragment variable of the CAR structure, enhancing the specificity of NK cells. Additionally, CAR-NK cells have the inherent ability to recognize and target tumor cells through activating receptors such as NKG2D, NKp46, and DNAM-1, as well as the capability to kill cancer cells through CD16-mediated ADCC. Therefore, CAR-NK cells can recognize and kill tumor cells using both CAR-dependent and CAR-independent mechanisms. They have the ability to efficiently eradicate tumor cells even in cases of MHC-I downregulation, loss, or mutation of tumor antigens. This dual mechanism helps overcome the resistance to CAR-T cell therapy caused by antigen loss and reduces the risk of recurrence due to the loss of specific tumor antigens targeted by CARs. Compared to CAR-T cells, CAR-NK cells offer more stable sources with fewer side effects (85–87). CAR-NK cells have a lower risk of cytokine release syndrome (CRS). Frey et al. suggested that this could be attributed to the fact that activated NK cells do not release cytokine IL-6, thereby circumventing CRS (88). Additionally, NK cells can target tumor cells without antigen stimulation or human leukocyte antigen (HLA) matching, thereby avoiding graft-versus-host disease (GVHD) reactions (89).
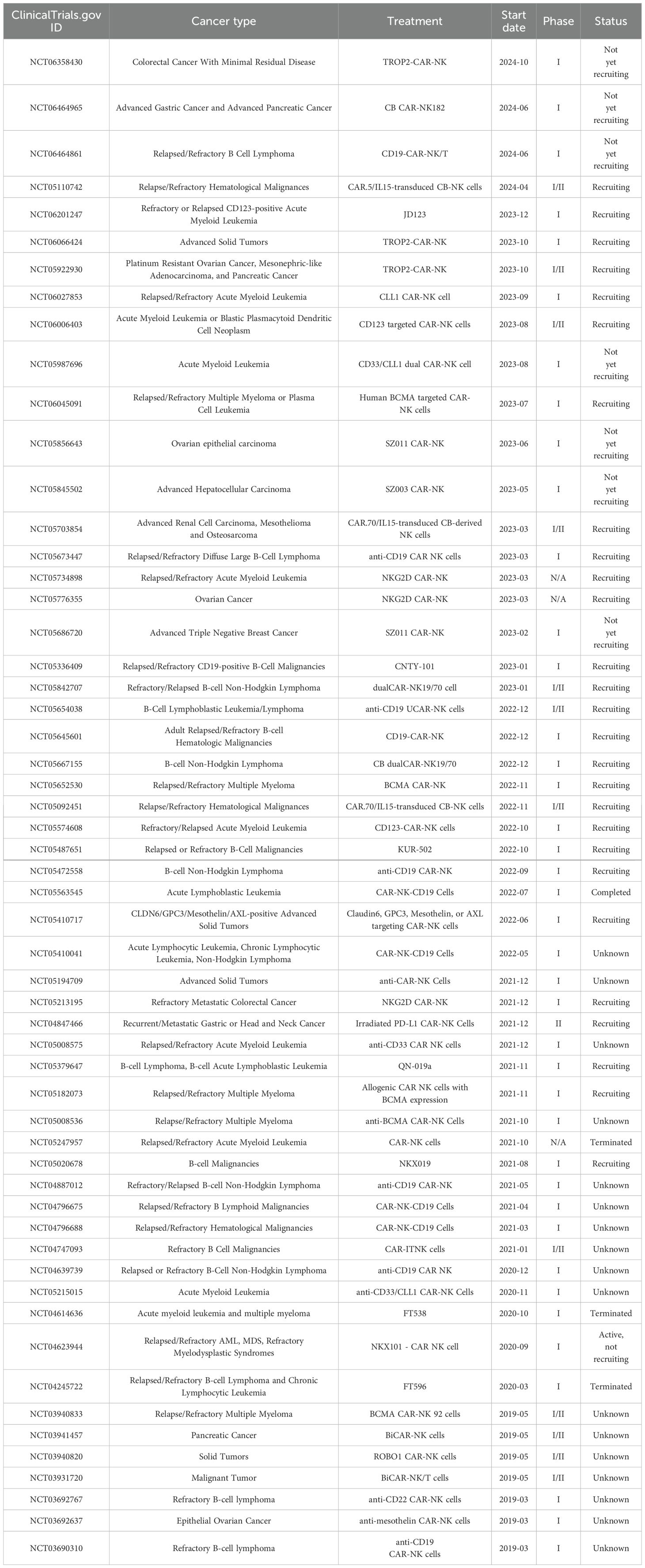
Current research primarily focuses on improving the activity, targeting, and persistence of NK cells. CAR-NK cells have emerged as the most promising candidate for immunotherapy, reinvigorating adoptive NK cell therapy. Presently, CAR-NK cells have demonstrated promising efficacy in preclinical studies for hematological and solid tumors. Boissel et al. (90) injected CD19/CD20-CAR-NK cells into a chronic lymphocytic leukemia model in immunodeficient mice, observing effective clearance of tumor cells. Romanski et al. (91)constructed NK-92-scFv (CD19)-CD3 ζ cells for B-cell malignant tumors, enhancing tumor cell killing. Similarly, Han et al. (92) applied CAR-NK92 cells to glioma treatment, achieving robust killing effects. Furthermore, ErBb2-CAR modified NK92 cells exhibited strong killing effects on HER2-positive breast cancer and ovarian cancer cell lines, along with tumor growth inhibition in vivo (93). Numerous similar research findings, such as those by Liu et al. (94), applying CAR-NK-92 cells to small cell lung cancer (SCLC) overexpressing delta-like ligand 3 (DLL3), have demonstrated good anti-tumor activity. In other studies, CAR-NK cells from allogeneic sources targeting prostate stem cell antigen (PSCA) were employed in human metastatic pancreatic cancer models, showing significant tumor inhibitory effects (95). These results underscore the promising prospects of CAR-NK cells in the treatment of hematological and solid tumors. Given the safety and effectiveness of CAR-NK cell therapy, numerous clinical trials are currently being conducted for hematological cancers and solid tumors. Table 1 presents recent 5-year clinical trials in tumor immunotherapy based on CAR-NK cells.
Although CAR-NK cell therapy has powerful therapeutic advantages, there are also several challenges and obstacles. One of the biggest obstacles to clinical application is the difficulty in obtaining a large number of high-purity NK cells, as the number of NK cells from a single donor is insufficient for treatment, and it takes time to culture NK cells (96). Another obstacle is the transduction of CAR into NK cells (34). The transfection efficiency of lentiviral vectors to peripheral blood NK cells is very low; while retroviral vectors have high transfection efficiency, they may cause insertional mutations and carcinogenesis. Additionally, the anti-tumor effect of CAR-NK cells transfected with mRNA through electroporation is transient (34), necessitating the search for a more suitable method to transduce CAR into NK cells. Furthermore, CAR-NK therapy also faces challenges related to the influence of the tumor microenvironment (TME). If these issues can be resolved, CAR-NK therapy will reach a new level of efficacy.
4.2 Cytokine therapy
Cytokine therapy entails the use of cytokines to promote the mobilization of endogenous NK cells and subsequently regulate the anti-tumor immune response. It has been observed that cytokines such as IL-2, IL-12, IL-15, IL-18, and TGF-β can modulate the anti-tumor immune response mediated by NK cells (97–103), offering new strategies and choices for immunotherapy by regulating the function and activity of NK cells.
IL-2 serves to activate NK cell cytotoxicity and is currently widely employed as a cytokine in clinical cancer treatment. In multiple myeloma, IL-2 has the potential to dissolve tumor cells and enhance the killing activity of CD16+ NK cells by promoting the perforin effect mechanism of activating NK cells through the NKG2D pathway (104). Furthermore, in vitro or in vivo studies have demonstrated that injection of high-dose IL-2 can stimulate the production of lymphokine-activated killer (LAK) cells, primarily composed of NK cells (105). These cells exhibit potent anti-tumor activity and have been utilized in the treatment of various malignant diseases, including metastatic kidney cancer and metastatic melanoma (106, 107). Nonetheless, its application is limited due to serious side effects. Multiple strategies have been devised to enhance the efficacy of IL-2 treatment and reduce toxicity. One such strategy involves the use of “super IL-2,” screened through molecular modification, which significantly enhances binding affinity with IL-2Rβ, exhibits robust activity in stimulating CD8+ T cells and NK cells, and reduces Treg cell accumulation and toxicity. Additionally, fusing IL-2 or its mutants with the Fc region of albumin or antibodies can markedly prolong their half-life in vivo. Fusion with antibodies targeting fibroblast activation protein (FAP) and carcinoembryonic antigen (CEA) can enhance their tumor-targeting capabilities and mitigate cytotoxicity caused by IL-2 retention in peripheral blood (108, 109).
IL-15 can activate and amplify NK cells and CD8+ T cells. In vitro studies have demonstrated that IL-15 can restore depleted NK cell mitochondria integrity in the tumor microenvironment, enhance cytotoxicity, and promote IFN-γ generation. Studies also suggest that IL-15 upregulates the expression of the activating receptor NKG2D on NK cell surfaces. Following tumor exposure, overnight IL-15 treatment leads to increased expression of NKG2D and IFN-γ, partially restoring production (110). In addition, recombinant IL-15 (rIL-15) has been found to promote regression of melanoma, colorectal cancer, and lymphoma tumors and reduce metastasis in transplanted tumor mouse models. HetIL-15, a fusion protein composed of IL-15 and IL-15Rα, has demonstrated efficacy in preclinical studies, slowing tumor growth, increasing tumor infiltration of NK cells and CD8+ T cells, and promoting IFN-γ production, cytotoxic particles, and anti-apoptosis protein B-cell lymphoma-2(BCL-2) expression (111).
Pro-inflammatory cytokines IL-12 and IL-18 stimulate NK cell activation and enhance their IFN-γ production and cytotoxicity. Researchers have fused IL-12 with tumor-targeted antibody domains or delivered recombinant IL-12 through intratumoral injection of DNA or RNA encoding IL-12. Alternative drug delivery methods, such as nanoparticles and exosomes, have been explored to localize cytokines at the injection site, significantly reducing systemic toxicity (112). This approach increases tumor-infiltrating NK cells and CD8+ T cells, enhancing anti-tumor immune responses. IL-18 has also exhibited therapeutic effects in tumor treatment. Researchers have screened and obtained mutant decoy-resistant IL-18 (DR-18), which binds to IL-18Rα but not IL-18 binding protein (IL-18BP). DR-18 treatment increases the number of CD8+ T cells and NK cells, promotes IFN-γ production, enhances cytotoxic activity, and effectively inhibits tumor growth (113).
Anti-inflammatory cytokine TGF-β inhibits the anti-tumor immune effect mediated by NK cells through various mechanisms. Inhibiting TGF-β can restore NK cell function and inhibit tumor growth. Galunisertib, a small molecule inhibitor of exogenous TGF-β Type I receptor, upregulates the expression of activated receptors DNAM-1, NKp30, and NKG2D on the surface of activated NK (aNK) cells in vitro, as well as the TNF-related apoptosis-inducing ligand (TRAIL), enhancing cytotoxicity and ADCC effects on neuroblastoma cells and improving therapeutic outcomes for neuroblastoma (114). Moreover, galunisertib treatment improves overall survival rates in patients with liver cancer and pancreatic cancer.
4.3 Antibody-based NK cell therapy
4.3.1 Enhancing NK cell-mediated ADCC effect
In the tumor microenvironment, NK cells efficiently eliminate tumors mainly through the mechanism of “missing self” (34, 115–118). This mechanism recognizes tumor cells with downregulated expression of MHC class I molecules and responds to cells with this phenotype, ultimately leading to target cell lysis. However, the “missing self” mechanism alone cannot achieve specific killing of tumor cells (119). ADCC emerges as a pivotal mechanism by which NK cells specifically target and kill tumor cells. Leveraging ADCC mediated by NK cells to specifically eliminate tumor cells represents a significant strategy for NK cell-based tumor immunotherapy.
Currently, relevant clinical studies have demonstrated the enhancement of NK cell-mediated ADCC effect in anti-tumor treatment, proving its efficacy. Examples include the treatment of follicular lymphoma with rituximab (120), human epidermal growth factor receptor-2 (HER2) positive breast cancer with trastuzumab (121), non-small cell lung cancer with cetuximab and avelumab (122), and multiple myeloma cases with daratumab and all-trans retinoic acid (123), among others. These monoclonal antibodies augment the killing activity of NK cells against tumors by enhancing the ADCC effect, resulting in favorable therapeutic outcomes. These findings underscore the significant potential of enhancing NK cell-mediated ADCC killing of tumor cells in tumor immunotherapy.
4.3.2 NK cell engagers
The interaction between NK cells and tumor cells is constrained by various immune escape mechanisms. To direct NK cells towards tumor cells and activate NK cell receptors to elicit an anti-tumor response, NK cell engagers (NKCEs) have been developed to facilitate specific contact between tumor-infiltrating NK cells and tumor cells. Initially, NKCEs were designed as bispecific killer engagers (BiKEs), comprising a single-chain variable fragment (scFv) of an anti-NK cell activating receptor antibody and another scFv targeting a specific tumor antigen (124). Building upon this concept, additional scFvs or cytokines have been incorporated to create trispecific or tetraspecific killer cell engagers (TriKEs and TetraKEs), further augmenting NK cell proliferation and survival (125).
Numerous preclinical studies have utilized NK cell engagers for the treatment of hematological and solid tumors, demonstrating robust anti-tumor effects. For instance, the CD16 bispecific antibody AFM13, targeting CD30, achieved an objective remission rate of nearly 100% when combined with NK cells derived from umbilical cord blood for the treatment of patients with relapsed/refractory Hodgkin lymphoma (RR-HL) (126). Another example is AFM24 (CD16A-NKCEs), a bispecific IgG1-scFv fusion antibody targeting CD16A on innate immune NK cells and epidermal growth factor receptor (EGFR) on tumor cells, effectively targeting tumors expressing human epidermal growth factor receptor at similar levels (127). Furthermore, NKG2D-NKCEs, targeting HER2 on tumor cells and NKG2D on NK cells, were found to induce cytotoxicity in vitro through unstimulated NK cells (128). Presently, an increasing number of NKCEs are under development, offering a promising strategy for tumor treatment.
4.3.3 Immune checkpoint blockade therapy
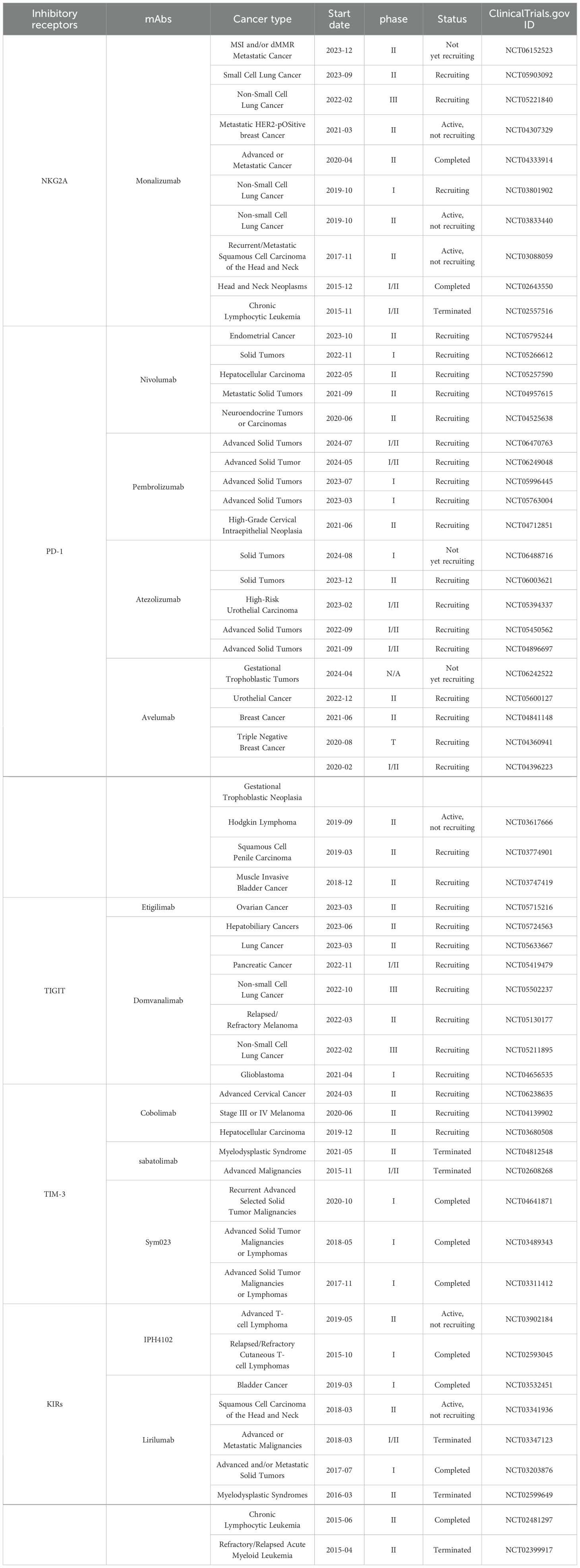
Immune checkpoints can hinder NK cell function by recognizing specific ligands on tumor cells and engaging with them, resulting in NK cell depletion and facilitating tumor immune evasion (119, 129). Inhibiting immune checkpoints aids in reinstating NK cell anti-tumor activity. The identified NK cell immune checkpoints encompass NKG2A, PD-1, TIGIT, TIM-3, KIRs, LIRs, CD96, cytotoxic T lymphocyte-associated antigen-4(CTLA-4), B7-H3(CD276), LAG-3, Siglec-7/9, SIRPα, CD200R, and CD47, among others. Based on this discovery, a variety of monoclonal antibodies targeting various immune checkpoints have been continuously developed for clinical tumor treatment. They have proven to be safe and effective both in vitro and in vivo. Currently, numerous clinical trials on immune checkpoint inhibitors are underway, with some trials reporting exciting results (Table 2).
4.3.3.1 NKG2A
NKG2A belongs to the inhibitory receptor family 2 in NK cells (130) and recognizes the non-classical MHC-I molecule HLA-E as its ligand. NKG2A is expressed in nearly 50% of NK cells in peripheral blood, while HLA-E expression is low in normal tissue cells but elevated in tumor-infiltrating NK cells, CD8+ T cells, and tumor cells (131). André et al. (132) noted that blocking the interaction between NKG2A on CD8+ T cells and NK cells and HLA-E on cancer cells can stimulate anti-tumor immunity. Currently, a humanized antibody, monalizumab, has been developed for NKG2A (133). In vitro and in vivo research findings demonstrate the safety and efficacy of humanized anti-NKG2A antibody treatment for malignant hematological diseases (134, 135). In vitro studies have shown that monalizumab can ameliorate NK cell dysfunction in patients with chronic lymphocytic leukemia (136). Monalizumab monotherapy is well tolerated in the treatment of gynecological malignant tumors (137). Additionally, monalizumab significantly enhances NK cytotoxicity in head and neck squamous cell carcinoma (HNSCC) cell lines with high expression of HLA-E (138).
4.3.3.2 PD-1
PD-1 is a crucial immunosuppressive molecule expressed in CD4+, CD8+ T cells, NK cells, NKT cells, B cells, and other innate lymphocytes (139–144). Upregulation of PD-1 expression has been observed in peripheral blood and tumor-infiltrating NK cells of various cancer patients (145), resulting in weakened NK cell responses. Blocking the PD-1/PD-L1 interaction can alleviate NK cell inhibition, thereby enhancing their anti-tumor immune function (146). Targeted PD-1/PD-L1 inhibitors have been increasingly utilized in the treatment of hematological and solid tumors, demonstrating effectiveness (147, 148). Examples include pembrolizumab, durvalumab, and Avelumab. Studies have shown that pembrolizumab and durvalumab can inhibit PD-1/PD-L1 in human non-small cell lung cancer, subsequently activating NK cells and exerting effective anti-tumor immune responses (149, 150). In another study, Avelumab facilitated the killing of breast cancer cells by inducing cytokine production in NK cells (151).
4.3.3.3 TIGIT
TIGIT is an immunoglobulin superfamily receptor expressed on the surface of NK cells and T cells. It belongs to the immunosuppressive receptor family and is highly expressed in tumor-infiltrating NK cells (31), where it directly inhibits NK cell function. Blocking TIGIT can reverse NK cell depletion and stimulate anti-tumor immunity (31). Research has demonstrated that the TIGIT inhibitor tiragolumab activates the anti-tumor activity of T cells and NK cells by inhibiting the binding of TIGIT to poliovirus receptors. Moreover, its objective response rate, when combined with the PD-1 inhibitor atezolizumab for the treatment of recurrent or metastatic non-small cell lung cancer, is significantly improved compared to single therapy (152). In another study, Chauvin et al. (153) pointed out that combining TIGIT inhibitors with IL-15 enhances NK cell cytotoxicity against melanoma and reduces tumor metastasis in a mouse melanoma model.
4.3.3.4 TIM-3
TIM-3, a co-inhibitory receptor, recognizes galectin-9 as its ligand. The combination of TIM-3 and its ligand induces immune tolerance by depleting T cells and NK cells. TIM-3 is expressed in the resting CD56bright NK cell population, and its upregulation is observed in many cancers and chronic infections (154–160), leading to NK cell depletion. As tumors progress, the level of TIM-3 in NK cells increases, suggesting that TIM-3 expression may serve as a prognostic biomarker [122-126] (161–163). Studies have shown that TIM-3 blockade in vitro can reverse NK cell depletion in patients with metastatic melanoma, promoting NK cell proliferation, increasing IFN-γ production, and enhancing cytotoxicity (164). Additionally, TIM-3 blockade enhances the functional capacity of peripheral NK cells in patients with bladder cancer (163). Currently, TIM-3 inhibitors such as Cobolimab, Sabatolimab, and Sym 023 are undergoing clinical studies to assess their effects on various cancers (135).
4.3.3.5 KIRs
There are two types of KIRs: activating KIRs (aKIRs) and inhibitory KIRs (iKIRs). Studies have revealed that aKIRs are down-regulated in many tumors, while iKIRs are up-regulated, including in breast cancer, lymphoma, and non-small cell lung cancer (165–167). This places NK cells in a low-reactive state, rendering them unable to effectively clear the tumor, thereby allowing tumor cells to evade destruction. Currently, monoclonal antibodies targeting KIRs, such as lirilumab, have been developed, showing potential for anti-tumor therapy in preclinical studies. However, satisfactory results have not been achieved in many clinical trials as monotherapy (168–170). Increasingly, clinical trials of combined blocking strategies are underway. An ongoing phase I clinical study demonstrates that lirilumab enhances the cell-killing effect mediated by elotuzumab (171).
4.3.3.6 Other immune checkpoints
In addition to the aforementioned immune checkpoints, more immune checkpoints have been continuously discovered, including LIRs, CD96, CTLA-4, B7-H3, LAG-3, Siglec-7/9, SIRPα, CD200R, and CD47, among others (167). These represent potential immunotherapy targets, expanding the possibilities for NK cell immunotherapy.
4.4 Nanoparticles and small molecules
Studies have demonstrated the significant potential of nanoparticles in enhancing NK cell-mediated anti-tumor immunity (172–175). Various nanoparticle strategies have been devised to enhance the homing and infiltration capabilities of NK cells. These include the utilization of liposomes loaded with TUSC2 or nano-composite microspheres coated with IFN-γ, resulting in a notable increase in NK cell infiltration (176–178). Additionally, nano-carriers have been employed to silence NK cell inhibitory signals, thereby activating NK cell activity, while multi-target nano-connection platforms have been developed to promote NK cell recruitment and activation (179, 180).Xu et al. (181)employed lipid calcium phosphate nanoparticles and liposome protamine hyaluronic acid nanoparticles to modulate TGF-β signal transduction, resulting in a roughly 50% downregulation of TGF-β in the tumor microenvironment (TME) and an increase in NK cell infiltration in a melanoma model. Similarly, Liu et al. (182)developed a nanoemulsion containing selenocysteine and TGF-β antagonists, effectively inhibiting TGF-β/TGF-βRI/Smad2/3 signal transduction and enhancing the upregulation of NKG2DL on cancer cells. Au et al. (179)fabricated tri-specific NK cell engagers based on PLGA nanoparticles, successfully combining NK cells with tumor cells to activate NK cells against EGFR-positive colorectal adenocarcinoma, triple-negative breast cancer, epidermoid cancer, and melanoma, respectively. Another emerging strategy involves the use of cationic nanoparticles to enhance NK cell activation. Treatment of NK cells with polyethyleneimine-coated cationic iron oxide nanoparticles demonstrated more than a twofold increase in cytotoxicity against triple-negative breast cancer and improved interaction with breast cancer cells (183).
Furthermore, studies have revealed that small molecules, due to their ability to penetrate cell membranes and target intracellular components, hold promise in overcoming the challenges posed by the TME. Indeed, numerous small molecules have been identified to regulate the anti-tumor function of NK cells by modulating the balance of activation and inhibition signals or participating in the expansion, activation, differentiation, and maturation of NK cells, including Src/Bcr-Abl, GSK3, Smad3, Cbl-b, etc. (184). Thus, small molecules represent a promising avenue in NK cell-mediated tumor immunotherapy.
5 Conclusion
NK cells are an important part of innate immunity and play a key role in anti-tumor responses. Immunotherapy based on NK cells, along with T cell therapy, complements each other and has become a promising field of tumor immunotherapy, making significant progress. By understanding the biological characteristics of NK cells and regulating their functions and activities, various effective NK cell immunotherapies have been developed. These include infusing autologous or allogeneic NK cells expanded in vitro or genetically engineered into tumor patients to increase the number and anti-tumor activity of NK cells; using cytokines to promote the mobilization of endogenous NK cells to regulate the anti-tumor immune response; targeting NK cell inhibitory receptors with antibodies to enhance NK cell response; employing ADCC-mediated specific killing of tumor cells by NK cells; developing NK cell engagers to direct NK cells to tumor cells and trigger their anti-tumor immune response; and using nanoparticles and small molecules to regulate the anti-tumor function of NK cells.
Although these therapies have achieved some success, several challenges and dilemmas remain. These include obtaining a large number of NK cells, expanding them to clinical scale in vitro, and maintaining the survival and activity of infused NK cells in vivo. In CAR-NK therapy, finding a suitable method to transduce CAR into NK cells that is both efficient and safe is necessary. In ICB therapy, a single checkpoint receptor blockade may not suffice to fully rescue NK cells expressing multiple immune checkpoint ligands in the TME. Additionally, challenges from the TME itself persist, and there is a lack of clinically relevant animal models that can encapsulate the complexity of interactions in the TME to evaluate its impact. Currently, various immune combination therapies are being tried. Combination therapy centered on NK cells should provide the next wave of clinical progress by finding an appropriate treatment balance, improving targeted activation, and effectively overcoming inherent inhibitory mechanisms. Multiple immune combination therapies based on NK cells represent a strategy to further improve the anti-tumor effect and warrant further exploration. Additionally, further studies on the anti-tumor mechanisms of NK cells in basic research and clinical trials are necessary to develop new immunotherapies, offering more and better treatment strategies for tumor patients in the future.
Author contributions
PJ: Writing – original draft. SJ: Writing – original draft. GS: Writing – review & editing. FJ: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
Thanks to Figdraw, and all figures are created with Figdraw.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Reviewer PL declared a shared parent affiliation with the author GS to the handling editor at the time of review.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ADCC, antibody-dependent cell-mediated cytotoxicity; CAR, chimeric antigen receptor; ICB, immune checkpoint blockade; MHC, major histocompatibility complex; NKCEs, NK cell engagers; IFN-γ, interferon γ; TNF-β, tumor necrosis factor β; GM-CSF, granulocyte-macrophage colony-stimulating factor; AR, activated receptors; IR, inhibitory receptors; NCR, natural cytotoxic receptor; SLAMF, signaling lymphocyte-activation molecule family; aKIR, activated killer cell immunoglobulin-like receptor; NKR-P1, NK cell receptor protein 1; NKG2D, NK cell group 2D receptor; DNAM1, DNAX accessory molecule 1; iKIR, Inhibitory killer cell immunoglobulin-like receptor; PD-1, programmed cell death receptor 1; LIR-1, leukocyte immunoglobulin-like receptor 1; TIGIT, T cell immunoreceptor with Ig and ITIM domains; TIM-3, T-cell immunoglobulin and mucin domain 3; LAG3, lymphocyte activation gene 3; HER2, human epidermal growth factor receptor-2; iPSCs, induced pluripotent stem cells; CRS, cytokine release syndrome; GVHD, graft versus host disease; ICANS, immune effector cell-associated neurotoxicity syndrome; Tregs, regulatory Tcells; CIK, cytokine induced killer.
References
1. Hanahan D. Hallmarks of cancer: new dimensions. Cancer Discovery. (2022) 12:31–46. doi: 10.1158/2159-8290.Cd-21-1059
2. Liu Y, Wang Y, Yang Y, Weng L, Wu Q, Zhang J, et al. Emerging phagocytosis checkpoints in cancer immunotherapy. Signal Transduct Target Ther. (2023) 8:104. doi: 10.1038/s41392-023-01365-z
3. Iyer JG, Afanasiev OK, McClurkan C, Paulson K, Nagase K, Jing L, et al. Merkel cell polyomavirus-specific CD8+ and CD4+ T-cell responses identified in Merkel cell carcinomas and blood. Clin Cancer Res. (2011) 17:6671–80. doi: 10.1158/1078-0432.Ccr-11-1513
4. Zhang C, Oberoi P, Oelsner S, Waldmann A, Lindner A, Tonn T, et al. Chimeric antigen receptor-engineered NK-92 cells: an off-the-shelf cellular therapeutic for targeted elimination of cancer cells and induction of protective antitumor immunity. Front Immunol. (2017) 8:533. doi: 10.3389/fimmu.2017.00533
5. Valipour B, Velaei K, Abedelahi A, Karimipour M, Darabi M, Charoudeh HN. NK cells: An attractive candidate for cancer therapy. J Cell Physiol. (2019) 234:19352–65. doi: 10.1002/jcp.28657
6. Bald T, Krummel MF, Smyth MJ, Barry KC. The NK cell-cancer cycle: advances and new challenges in NK cell-based immunotherapies. Nat Immunol. (2020) 21:835–47. doi: 10.1038/s41590-020-0728-z
7. Herberman RB, Nunn ME, Lavrin DH. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic acid allogeneic tumors. I. Distribution of reactivity and specificity. Int J Cancer. (1975) 16:216–29. doi: 10.1002/ijc.2910160204
8. Terrén I, Orrantia A, Vitallé J, Zenarruzabeitia O, Borrego F. NK cell metabolism and tumor microenvironment. Front Immunol. (2019) 10:2278. doi: 10.3389/fimmu.2019.02278
9. Guillerey C, Huntington ND, Smyth MJ. Targeting natural killer cells in cancer immunotherapy. Nat Immunol. (2016) 17:1025–36. doi: 10.1038/ni.3518
10. Chiossone L, Dumas PY, Vienne M, Vivier E. Natural killer cells and other innate lymphoid cells in cancer. Nat Rev Immunol. (2018) 18:671–88. doi: 10.1038/s41577-018-0061-z
11. Vivier E, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, et al. Innate lymphoid cells: 10 years on. Cell. (2018) 174:1054–66. doi: 10.1016/j.cell.2018.07.017
12. Huntington ND, Cursons J, Rautela J. The cancer-natural killer cell immunity cycle. Nat Rev Cancer. (2020) 20:437–54. doi: 10.1038/s41568-020-0272-z
13. Reina-Ortiz C, Giraldos D, Azaceta G, Palomera L, Marzo I, Naval J, et al. Harnessing the potential of NK cell-based immunotherapies against multiple myeloma. Cells. (2022) 11:392. doi: 10.3390/cells11030392
14. Cong J, Wei H. Natural killer cells in the lungs. Front Immunol. (2019) 10:1416. doi: 10.3389/fimmu.2019.01416
15. Campbell KS, Hasegawa J. Natural killer cell biology: an update and future directions. J Allergy Clin Immunol. (2013) 132:536–44. doi: 10.1016/j.jaci.2013.07.006
16. Cózar B, Greppi M, Carpentier S, Narni-Mancinelli E, Chiossone L, Vivier E. Tumor-infiltrating natural killer cells. Cancer Discovery. (2021) 11:34–44. doi: 10.1158/2159-8290.Cd-20-0655
17. Caligiuri MA. Human natural killer cells. Blood. (2008) 112:461–9. doi: 10.1182/blood-2007-09-077438
18. Fu B, Tian Z, Wei H. Subsets of human natural killer cells and their regulatory effects. Immunology. (2014) 141:483–9. doi: 10.1111/imm.12224
19. Zhang J, Marotel M, Fauteux-Daniel S, Mathieu AL, Viel S, Marçais A, et al. T-bet and Eomes govern differentiation and function of mouse and human NK cells and ILC1. Eur J Immunol. (2018) 48:738–50. doi: 10.1002/eji.201747299
20. Duan S, Guo W, Xu Z, He Y, Liang C, Mo Y, et al. Natural killer group 2D receptor and its ligands in cancer immune escape. Mol Cancer. (2019) 18:29. doi: 10.1186/s12943-019-0956-8
21. Widowati W, Jasaputra DK, Sumitro SB, Widodo MA, Mozef T, Rizal R, et al. Effect of interleukins (IL-2, IL-15, IL-18) on receptors activation and cytotoxic activity of natural killer cells in breast cancer cell. Afr Health Sci. (2020) 20:822–32. doi: 10.4314/ahs.v20i2.36
22. Ramírez-Labrada A, Pesini C, Santiago L, Hidalgo S, Calvo-Pérez A, Oñate C, et al. All about (NK cell-mediated) death in two acts and an unexpected encore: initiation, execution and activation of adaptive immunity. Front Immunol. (2022) 13:896228. doi: 10.3389/fimmu.2022.896228
23. Bauer S, Groh V, Wu J, Steinle A, Phillips JH, Lanier LL, et al. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. (1999) 285:727–9. doi: 10.1126/science.285.5428.727
24. Chan CJ, Andrews DM, McLaughlin NM, Yagita H, Gilfillan S, Colonna M, et al. DNAM-1/CD155 interactions promote cytokine and NK cell-mediated suppression of poorly immunogenic melanoma metastases. J Immunol. (2010) 184:902–11. doi: 10.4049/jimmunol.0903225
25. Fauriat C, Ivarsson MA, Ljunggren HG, Malmberg KJ, Michaëlsson J. Education of human natural killer cells by activating killer cell immunoglobulin-like receptors. Blood. (2010) 115:1166–74. doi: 10.1182/blood-2009-09-245746
26. Grier JT, Forbes LR, Monaco-Shawver L, Oshinsky J, Atkinson TP, Moody C, et al. Human immunodeficiency-causing mutation defines CD16 in spontaneous NK cell cytotoxicity. J Clin Invest. (2012) 122:3769–80. doi: 10.1172/jci64837
27. Venstrom JM, Pittari G, Gooley TA, Chewning JH, Spellman S, Haagenson M, et al. HLA-C-dependent prevention of leukemia relapse by donor activating KIR2DS1. N Engl J Med. (2012) 367:805–16. doi: 10.1056/NEJMoa1200503
28. Boudreau JE, Giglio F, Gooley TA, Stevenson PA, Le Luduec JB, Shaffer BC, et al. KIR3DL1/HLA-B subtypes govern acute myelogenous leukemia relapse after hematopoietic cell transplantation. J Clin Oncol. (2017) 35:2268–78. doi: 10.1200/jco.2016.70.7059
29. Narni-Mancinelli E, Gauthier L, Baratin M, Guia S, Fenis A, Deghmane AE, et al. Complement factor P is a ligand for the natural killer cell-activating receptor NKp46. Sci Immunol. (2017) 2:eaam9628. doi: 10.1126/sciimmunol.aam9628
30. Sun C, Xu J, Huang Q, Huang M, Wen H, Zhang C, et al. High NKG2A expression contributes to NK cell exhaustion and predicts a poor prognosis of patients with liver cancer. Oncoimmunology. (2017) 6:e1264562. doi: 10.1080/2162402x.2016.1264562
31. Zhang Q, Bi J, Zheng X, Chen Y, Wang H, Wu W, et al. Blockade of the checkpoint receptor TIGIT prevents NK cell exhaustion and elicits potent anti-tumor immunity. Nat Immunol. (2018) 19:723–32. doi: 10.1038/s41590-018-0132-0
32. Balta E, Wabnitz GH, Samstag Y. Hijacked immune cells in the tumor microenvironment: molecular mechanisms of immunosuppression and cues to improve T cell-based immunotherapy of solid tumors. Int J Mol Sci. (2021) 22:5736. doi: 10.3390/ijms22115736
33. Melssen M, Slingluff CL Jr. Vaccines targeting helper T cells for cancer immunotherapy. Curr Opin Immunol. (2017) 47:85–92. doi: 10.1016/j.coi.2017.07.004
34. Myers JA, Miller JS. Exploring the NK cell platform for cancer immunotherapy. Nat Rev Clin Oncol. (2021) 18:85–100. doi: 10.1038/s41571-020-0426-7
35. Zingoni A, Sornasse T, Cocks BG, Tanaka Y, Santoni A, Lanier LL. Cross-talk between activated human NK cells and CD4+ T cells via OX40-OX40 ligand interactions. J Immunol. (2004) 173:3716–24. doi: 10.4049/jimmunol.173.6.3716
36. Agaugué S, Marcenaro E, Ferranti B, Moretta L, Moretta A. Human natural killer cells exposed to IL-2, IL-12, IL-18, or IL-4 differently modulate priming of naive T cells by monocyte-derived dendritic cells. Blood. (2008) 112:1776–83. doi: 10.1182/blood-2008-02-135871
37. Mocikat R, Braumüller H, Gumy A, Egeter O, Ziegler H, Reusch U, et al. Natural killer cells activated by MHC class I(low) targets prime dendritic cells to induce protective CD8 T cell responses. Immunity. (2003) 19:561–9. doi: 10.1016/s1074-7613(03)00264-4
38. Adam C, King S, Allgeier T, Braumüller H, Lüking C, Mysliwietz J, et al. DC-NK cell cross talk as a novel CD4+ T-cell-independent pathway for antitumor CTL induction. Blood. (2005) 106:338–44. doi: 10.1182/blood-2004-09-3775
39. Ardolino M, Zingoni A, Cerboni C, Cecere F, Soriani A, Iannitto ML, et al. DNAM-1 ligand expression on Ag-stimulated T lymphocytes is mediated by ROS-dependent activation of DNA-damage response: relevance for NK-T cell interaction. Blood. (2011) 117:4778–86. doi: 10.1182/blood-2010-08-300954
40. Fehniger TA, Cooper MA, Nuovo GJ, Cella M, Facchetti F, Colonna M, et al. CD56bright natural killer cells are present in human lymph nodes and are activated by T cell-derived IL-2: a potential new link between adaptive and innate immunity. Blood. (2003) 101:3052–7. doi: 10.1182/blood-2002-09-2876
41. Horowitz A, Newman KC, Evans JH, Korbel DS, Davis DM, Riley EM. Cross-talk between T cells and NK cells generates rapid effector responses to Plasmodium falciparum-infected erythrocytes. J Immunol. (2010) 184:6043–52. doi: 10.4049/jimmunol.1000106
42. Malhotra A, Shanker A. NK cells: immune cross-talk and therapeutic implications. Immunotherapy. (2011) 3:1143–66. doi: 10.2217/imt.11.102
43. Plitas G, Rudensky AY. Regulatory T cells: differentiation and function. Cancer Immunol Res. (2016) 4:721–5. doi: 10.1158/2326-6066.Cir-16-0193
44. Trzonkowski P, Szmit E, Myśliwska J, Dobyszuk A, Myśliwski A. CD4+CD25+ T regulatory cells inhibit cytotoxic activity of T CD8+ and NK lymphocytes in the direct cell-to-cell interaction. Clin Immunol. (2004) 112:258–67. doi: 10.1016/j.clim.2004.04.003
45. Ghiringhelli F, Ménard C, Terme M, Flament C, Taieb J, Chaput N, et al. CD4+CD25+ regulatory T cells inhibit natural killer cell functions in a transforming growth factor-beta-dependent manner. J Exp Med. (2005) 202:1075–85. doi: 10.1084/jem.20051511
46. Liu W, Wei X, Li L, Wu X, Yan J, Yang H, et al. CCR4 mediated chemotaxis of regulatory T cells suppress the activation of T cells and NK cells via TGF-β pathway in human non-small cell lung cancer. Biochem Biophys Res Commun. (2017) 488:196–203. doi: 10.1016/j.bbrc.2017.05.034
47. Smyth MJ, Teng MW, Swann J, Kyparissoudis K, Godfrey DI, Hayakawa Y. CD4+CD25+ T regulatory cells suppress NK cell-mediated immunotherapy of cancer. J Immunol. (2006) 176:1582–7. doi: 10.4049/jimmunol.176.3.1582
48. Littwitz-Salomon E, Akhmetzyanova I, Vallet C, Francois S, Dittmer U, Gibbert K. Activated regulatory T cells suppress effector NK cell responses by an IL-2-mediated mechanism during an acute retroviral infection. Retrovirology. (2015) 12:66. doi: 10.1186/s12977-015-0191-3
49. Dean JW, Peters LD, Fuhrman CA, Seay HR, Posgai AL, Stimpson SE, et al. Innate inflammation drives NK cell activation to impair Treg activity. J Autoimmun. (2020) 108:102417. doi: 10.1016/j.jaut.2020.102417
50. Mildner A, Jung S. Development and function of dendritic cell subsets. Immunity. (2014) 40:642–56. doi: 10.1016/j.immuni.2014.04.016
51. Sköld AE, Mathan TSM, van Beek JJP, Flórez-Grau G, van den Beukel MD, Sittig SP, et al. Naturally produced type I IFNs enhance human myeloid dendritic cell maturation and IL-12p70 production and mediate elevated effector functions in innate and adaptive immune cells. Cancer Immunol Immunother. (2018) 67:1425–36. doi: 10.1007/s00262-018-2204-2
52. Bosch NC, Martin LM, Voskens CJ, Berking C, Seliger B, Schuler G, et al. A chimeric IL-15/IL-15Rα Molecule expressed on NFκB-activated dendritic cells supports their capability to activate natural killer cells. Int J Mol Sci. (2021) 22:10227. doi: 10.3390/ijms221910227
53. Allen F, Bobanga ID, Rauhe P, Barkauskas D, Teich N, Tong C, et al. CCL3 augments tumor rejection and enhances CD8(+) T cell infiltration through NK and CD103(+) dendritic cell recruitment via IFNγ. Oncoimmunology. (2018) 7:e1393598. doi: 10.1080/2162402x.2017.1393598
54. Böttcher JP, Bonavita E, Chakravarty P, Blees H, Cabeza-Cabrerizo M, Sammicheli S, et al. NK Cells Stimulate Recruitment of cDC1 into the Tumor Microenvironment Promoting Cancer Immune Control. Cell. (2018) 172:1022–1037.e1014. doi: 10.1016/j.cell.2018.01.004
55. Cazzetta V, Franzese S, Carenza C, Della Bella S, Mikulak J, Mavilio D. Natural killer-dendritic cell interactions in liver cancer: implications for immunotherapy. Cancers (Basel). (2021) 13:2184. doi: 10.3390/cancers13092184
56. Russick J, Joubert PE, Gillard-Bocquet M, Torset C, Meylan M, Petitprez F, et al. Natural killer cells in the human lung tumor microenvironment display immune inhibitory functions. J Immunother Cancer. (2020) 8:e001054. doi: 10.1136/jitc-2020-001054
57. Perez-Martinez A, Iyengar R, Gan K, Chotsampancharoen T, Rooney B, Holladay M, et al. Blood dendritic cells suppress NK cell function and increase the risk of leukemia relapse after hematopoietic cell transplantation. Biol Blood Marrow Transplant. (2011) 17:598–607. doi: 10.1016/j.bbmt.2010.10.019
58. Cancel JC, Crozat K, Dalod M, Mattiuz R. Are conventional type 1 dendritic cells critical for protective antitumor immunity and how? Front Immunol. (2019) 10:9. doi: 10.3389/fimmu.2019.00009
59. Jaillon S, Ponzetta A, Di Mitri D, Santoni A, Bonecchi R, Mantovani A. Neutrophil diversity and plasticity in tumour progression and therapy. Nat Rev Cancer. (2020) 20:485–503. doi: 10.1038/s41568-020-0281-y
60. Wculek SK, Malanchi I. Neutrophils support lung colonization of metastasis-initiating breast cancer cells. Nature. (2015) 528:413–7. doi: 10.1038/nature16140
61. Cui C, Chakraborty K, Tang XA, Zhou G, Schoenfelt KQ, Becker KM, et al. Neutrophil elastase selectively kills cancer cells and attenuates tumorigenesis. Cell. (2021) 184:3163–3177.e3121. doi: 10.1016/j.cell.2021.04.016
62. Li P, Lu M, Shi J, Hua L, Gong Z, Li Q, et al. Dual roles of neutrophils in metastatic colonization are governed by the host NK cell status. Nat Commun. (2020) 11:4387. doi: 10.1038/s41467-020-18125-0
63. Ogura K, Sato-Matsushita M, Yamamoto S, Hori T, Sasahara M, Iwakura Y, et al. NK cells control tumor-promoting function of neutrophils in mice. Cancer Immunol Res. (2018) 6:348–57. doi: 10.1158/2326-6066.Cir-17-0204
64. Scapini P, Cassatella MA. Social networking of human neutrophils within the immune system. Blood. (2014) 124:710–9. doi: 10.1182/blood-2014-03-453217
65. Sun R, Xiong Y, Liu H, Gao C, Su L, Weng J, et al. Tumor-associated neutrophils suppress antitumor immunity of NK cells through the PD-L1/PD-1 axis. Transl Oncol. (2020) 13:100825. doi: 10.1016/j.tranon.2020.100825
66. Spiegel A, Brooks MW, Houshyar S, Reinhardt F, Ardolino M, Fessler E, et al. Neutrophils suppress intraluminal NK cell-mediated tumor cell clearance and enhance extravasation of disseminated carcinoma cells. Cancer Discovery. (2016) 6:630–49. doi: 10.1158/2159-8290.Cd-15-1157
67. Locati M, Curtale G, Mantovani A. Diversity, mechanisms, and significance of macrophage plasticity. Annu Rev Pathol. (2020) 15:123–47. doi: 10.1146/annurev-pathmechdis-012418-012718
68. Castriconi R, Cantoni C, Della Chiesa M, Vitale M, Marcenaro E, Conte R, et al. Transforming growth factor beta 1 inhibits expression of NKp30 and NKG2D receptors: consequences for the NK-mediated killing of dendritic cells. Proc Natl Acad Sci U.S.A. (2003) 100:4120–5. doi: 10.1073/pnas.0730640100
69. Nuñez SY, Ziblat A, Secchiari F, Torres NI, Sierra JM, Raffo Iraolagoitia XL, et al. Human M2 macrophages limit NK cell effector functions through secretion of TGF-β and engagement of CD85j. J Immunol. (2018) 200:1008–15. doi: 10.4049/jimmunol.1700737
70. Alter G, Malenfant JM, Altfeld M. CD107a as a functional marker for the identification of natural killer cell activity. J Immunol Methods. (2004) 294:15–22. doi: 10.1016/j.jim.2004.08.008
71. Brunetta E, Fogli M, Varchetta S, Bozzo L, Hudspeth KL, Marcenaro E, et al. The decreased expression of Siglec-7 represents an early marker of dysfunctional natural killer-cell subsets associated with high levels of HIV-1 viremia. Blood. (2009) 114:3822–30. doi: 10.1182/blood-2009-06-226332
72. He Y, Du J, Dong Z. Myeloid deletion of phosphoinositide-dependent kinase-1 enhances NK cell-mediated antitumor immunity by mediating macrophage polarization. Oncoimmunology. (2020) 9:1774281. doi: 10.1080/2162402x.2020.1774281
73. Wang Z, Guan D, Huo J, Biswas SK, Huang Y, Yang Y, et al. IL-10 Enhances Human Natural Killer Cell Effector Functions via Metabolic Reprogramming Regulated by mTORC1 Signaling. Front Immunol. (2021) 12:619195. doi: 10.3389/fimmu.2021.619195
74. Guo Y, Xie YQ, Gao M, Zhao Y, Franco F, Wenes M, et al. Metabolic reprogramming of terminally exhausted CD8(+) T cells by IL-10 enhances anti-tumor immunity. Nat Immunol. (2021) 22:746–56. doi: 10.1038/s41590-021-00940-2
75. Morandi F, Yazdanifar M, Cocco C, Bertaina A, Airoldi I. Engineering the bridge between innate and adaptive immunity for cancer immunotherapy: focus on γδ T and NK cells. Cells. (2020) 9:1757. doi: 10.3390/cells9081757
76. Laskowski TJ, Biederstädt A, Rezvani K. Natural killer cells in antitumour adoptive cell immunotherapy. Nat Rev Cancer. (2022) 22:557–75. doi: 10.1038/s41568-022-00491-0
77. Veluchamy JP, Kok N, van der Vliet HJ, Verheul HMW, de Gruijl TD, Spanholtz J. The rise of allogeneic natural killer cells as a platform for cancer immunotherapy: recent innovations and future developments. Front Immunol. (2017) 8:631. doi: 10.3389/fimmu.2017.00631
78. Lin M, Luo H, Liang S, Chen J, Liu A, Niu L, et al. Pembrolizumab plus allogeneic NK cells in advanced non-small cell lung cancer patients. J Clin Invest. (2020) 130:2560–9. doi: 10.1172/jci132712
79. Kim JS, Kim YG, Pyo M, Lee HK, Hong JT, Kim Y, et al. Adoptive cell therapy of melanoma with cytokine-induced killer cells. Immune Netw. (2015) 15:58–65. doi: 10.4110/in.2015.15.2.58
80. Lee JH, Lee JH, Lim YS, Yeon JE, Song TJ, Yu SJ, et al. Adjuvant immunotherapy with autologous cytokine-induced killer cells for hepatocellular carcinoma. Gastroenterology. (2015) 148:1383–1391.e1386. doi: 10.1053/j.gastro.2015.02.055
81. Shirjang S, Alizadeh N, Mansoori B, Mahmoodpoor A, Kafil HS, Hojjat-Farsangi M, et al. Promising immunotherapy: Highlighting cytokine-induced killer cells. J Cell Biochem. (2019) 120:8863–83. doi: 10.1002/jcb.28250
82. Sadelain M, Brentjens R, Rivière I. The promise and potential pitfalls of chimeric antigen receptors. Curr Opin Immunol. (2009) 21:215–23. doi: 10.1016/j.coi.2009.02.009
83. Chow VA, Gopal AK, Maloney DG, Turtle CJ, Smith SD, Ujjani CS, et al. Outcomes of patients with large B-cell lymphomas and progressive disease following CD19-specific CAR T-cell therapy. Am J Hematol. (2019) 94:E209–e213. doi: 10.1002/ajh.25505
84. Pang Z, Wang Z, Li F, Feng C, Mu X. Current progress of CAR-NK therapy in cancer treatment. Cancers (Basel). (2022) 14:4318. doi: 10.3390/cancers14174318
85. Wang W, Jiang J, Wu C. CAR-NK for tumor immunotherapy: Clinical transformation and future prospects. Cancer Lett. (2020) 472:175–80. doi: 10.1016/j.canlet.2019.11.033
86. Xie G, Dong H, Liang Y, Ham JD, Rizwan R, Chen J. CAR-NK cells: A promising cellular immunotherapy for cancer. EBioMedicine. (2020) 59:102975. doi: 10.1016/j.ebiom.2020.102975
87. Elahi R, Heidary AH, Hadiloo K, Esmaeilzadeh A. Chimeric antigen receptor-engineered natural killer (CAR NK) cells in cancer treatment; recent advances and future prospects. Stem Cell Rev Rep. (2021) 17:2081–106. doi: 10.1007/s12015-021-10246-3
88. Frey N, Porter D. Cytokine release syndrome with chimeric antigen receptor T cell therapy. Biol Blood Marrow Transplant. (2019) 25:e123–7. doi: 10.1016/j.bbmt.2018.12.756
89. Siegler EL, Zhu Y, Wang P, Yang L. Off-the-shelf CAR-NK cells for cancer immunotherapy. Cell Stem Cell. (2018) 23:160–1. doi: 10.1016/j.stem.2018.07.007
90. Boissel L, Betancur-Boissel M, Lu W, Krause DS, Van Etten RA, Wels WS, et al. Retargeting NK-92 cells by means of CD19- and CD20-specific chimeric antigen receptors compares favorably with antibody-dependent cellular cytotoxicity. Oncoimmunology. (2013) 2:e26527. doi: 10.4161/onci.26527
91. Romanski A, Uherek C, Bug G, Seifried E, Klingemann H, Wels WS, et al. CD19-CAR engineered NK-92 cells are sufficient to overcome NK cell resistance in B-cell Malignancies. J Cell Mol Med. (2016) 20:1287–94. doi: 10.1111/jcmm.12810
92. Han J, Chu J, Keung Chan W, Zhang J, Wang Y, Cohen JB, et al. CAR-engineered NK cells targeting wild-type EGFR and EGFRvIII enhance killing of glioblastoma and patient-derived glioblastoma stem cells. Sci Rep. (2015) 5:11483. doi: 10.1038/srep11483
93. Uherek C, Tonn T, Uherek B, Becker S, Schnierle B, Klingemann HG, et al. Retargeting of natural killer-cell cytolytic activity to ErbB2-expressing cancer cells results in efficient and selective tumor cell destruction. Blood. (2002) 100:1265–73.
94. Liu M, Huang W, Guo Y, Zhou Y, Zhi C, Chen J, et al. CAR NK-92 cells targeting DLL3 kill effectively small cell lung cancer cells in vitro and in vivo. J Leukoc Biol. (2022) 112:901–11. doi: 10.1002/jlb.5ma0122-467r
95. Teng KY, Mansour AG, Zhu Z, Li Z, Tian L, Ma S, et al. Off-the-shelf prostate stem cell antigen-directed chimeric antigen receptor natural killer cell therapy to treat pancreatic cancer. Gastroenterology. (2022) 162:1319–33. doi: 10.1053/j.gastro.2021.12.281
96. Chu J, Gao F, Yan M, Zhao S, Yan Z, Shi B, et al. Natural killer cells: a promising immunotherapy for cancer. J Transl Med. (2022) 20:240. doi: 10.1186/s12967-022-03437-0
97. Malek TR, Castro I. Interleukin-2 receptor signaling: at the interface between tolerance and immunity. Immunity. (2010) 33:153–65. doi: 10.1016/j.immuni.2010.08.004
98. Pasche N, Neri D. Immunocytokines: a novel class of potent armed antibodies. Drug Discovery Today. (2012) 17:583–90. doi: 10.1016/j.drudis.2012.01.007
99. Tugues S, Burkhard SH, Ohs I, Vrohlings M, Nussbaum K, Vom Berg J, et al. New insights into IL-12-mediated tumor suppression. Cell Death Differ. (2015) 22:237–46. doi: 10.1038/cdd.2014.134
100. Almishri W, Santodomingo-Garzon T, Le T, Stack D, Mody CH, Swain MG. TNFα Augments cytokine-induced NK cell IFNγ Production through TNFR2. J Innate Immun. (2016) 8:617–29. doi: 10.1159/000448077
101. Probst P, Kopp J, Oxenius A, Colombo MP, Ritz D, Fugmann T, et al. Sarcoma eradication by doxorubicin and targeted TNF relies upon CD8(+) T-cell recognition of a retroviral antigen. Cancer Res. (2017) 77:3644–54. doi: 10.1158/0008-5472.Can-16-2946
102. Waters RS, Perry JSA, Han S, Bielekova B, Gedeon T. The effects of interleukin-2 on immune response regulation. Math Med Biol. (2018) 35:79–119. doi: 10.1093/imammb/dqw021
103. Gout DY, Groen LS, van Egmond M. The present and future of immunocytokines for cancer treatment. Cell Mol Life Sci. (2022) 79:509. doi: 10.1007/s00018-022-04514-9
104. Konjević G, Mirjačić Martinović K, Vuletić A, Babović N. In-vitro IL-2 or IFN-α-induced NKG2D and CD161 NK cell receptor expression indicates novel aspects of NK cell activation in metastatic melanoma patients. Melanoma Res. (2010) 20:459–67. doi: 10.1097/CMR.0b013e32833e3286
105. Rosenberg SA, Mulé JJ, Spiess PJ, Reichert CM, Schwarz SL. Regression of established pulmonary metastases and subcutaneous tumor mediated by the systemic administration of high-dose recombinant interleukin 2. J Exp Med. (1985) 161:1169–88. doi: 10.1084/jem.161.5.1169
106. Liu S, Galat V, Galat Y, Lee YKA, Wainwright D, Wu J. NK cell-based cancer immunotherapy: from basic biology to clinical development. J Hematol Oncol. (2021) 14:7. doi: 10.1186/s13045-020-01014-w
107. Wolf NK, Kissiov DU, Raulet DH. Roles of natural killer cells in immunity to cancer, and applications to immunotherapy. Nat Rev Immunol. (2023) 23:90–105. doi: 10.1038/s41577-022-00732-1
108. Klein C, Waldhauer I, Nicolini VG, Freimoser-Grundschober A, Nayak T, Vugts DJ, et al. Cergutuzumab amunaleukin (CEA-IL2v), a CEA-targeted IL-2 variant-based immunocytokine for combination cancer immunotherapy: Overcoming limitations of aldesleukin and conventional IL-2-based immunocytokines. Oncoimmunology. (2017) 6:e1277306. doi: 10.1080/2162402x.2016.1277306
109. Waldhauer I, Gonzalez-Nicolini V, Freimoser-Grundschober A, Nayak TK, Fahrni L, Hosse RJ, et al. Simlukafusp alfa (FAP-IL2v) immunocytokine is a versatile combination partner for cancer immunotherapy. MAbs. (2021) 13:1913791. doi: 10.1080/19420862.2021.1913791
110. Easom NJW, Stegmann KA, Swadling L, Pallett LJ, Burton AR, Odera D, et al. IL-15 overcomes hepatocellular carcinoma-induced NK cell dysfunction. Front Immunol. (2018) 9:1009. doi: 10.3389/fimmu.2018.01009
111. Bergamaschi C, Pandit H, Nagy BA, Stellas D, Jensen SM, Bear J, et al. Heterodimeric IL-15 delays tumor growth and promotes intratumoral CTL and dendritic cell accumulation by a cytokine network involving XCL1, IFN-γ, CXCL9 and CXCL10. J Immunother Cancer. (2020) 8:e000599. doi: 10.1136/jitc-2020-000599
112. Nguyen KG, Vrabel MR, Mantooth SM, Hopkins JJ, Wagner ES, Gabaldon TA, et al. Localized interleukin-12 for cancer immunotherapy. Front Immunol. (2020) 11:575597. doi: 10.3389/fimmu.2020.575597
113. Zhou T, Damsky W, Weizman OE, McGeary MK, Hartmann KP, Rosen CE, et al. IL-18BP is a secreted immune checkpoint and barrier to IL-18 immunotherapy. Nature. (2020) 583:609–14. doi: 10.1038/s41586-020-2422-6
114. Tran HC, Wan Z, Sheard MA, Sun J, Jackson JR, Malvar J, et al. TGFβR1 blockade with galunisertib (LY2157299) enhances anti-neuroblastoma activity of the anti-GD2 antibody dinutuximab (ch14.18) with natural killer cells. Clin Cancer Res. (2017) 23:804–13. doi: 10.1158/1078-0432.Ccr-16-1743
115. Kärre K, Ljunggren HG, Piontek G, Kiessling R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature. (1986) 319:675–8. doi: 10.1038/319675a0
116. Kärre K. Natural killer cell recognition of missing self. Nat Immunol. (2008) 9:477–80. doi: 10.1038/ni0508-477
117. Gras Navarro A, Björklund AT, CheKenya M. Therapeutic potential and challenges of natural killer cells in treatment of solid tumors. Front Immunol. (2015) 6:202. doi: 10.3389/fimmu.2015.00202
118. Wensveen FM, Jelenčić V, Polić B. NKG2D: A master regulator of immune cell responsiveness. Front Immunol. (2018) 9:441. doi: 10.3389/fimmu.2018.00441
119. Maskalenko NA, Zhigarev D, Campbell KS. Harnessing natural killer cells for cancer immunotherapy: dispatching the first responders. Nat Rev Drug Discovery. (2022) 21:559–77. doi: 10.1038/s41573-022-00413-7
120. Decaup E, Rossi C, Gravelle P, Laurent C, Bordenave J, Tosolini M, et al. A tridimensional model for NK cell-mediated ADCC of follicular lymphoma. Front Immunol. (2019) 10:1943. doi: 10.3389/fimmu.2019.01943
121. Zheng G, Guo Z, Li W, Xi W, Zuo B, Zhang R, et al. Interaction between HLA-G and NK cell receptor KIR2DL4 orchestrates HER2-positive breast cancer resistance to trastuzumab. Signal Transduct Target Ther. (2021) 6:236. doi: 10.1038/s41392-021-00629-w
122. Della Corte CM, Fasano M, Ciaramella V, Cimmino F, Cardnell R, Gay CM, et al. Anti-tumor activity of cetuximab plus avelumab in non-small cell lung cancer patients involves innate immunity activation: findings from the CAVE-Lung trial. J Exp Clin Cancer Res. (2022) 41:109. doi: 10.1186/s13046-022-02332-2
123. Naeimi Kararoudi M, Nagai Y, Elmas E, de Souza Fernandes Pereira M, Ali SA, Imus PH, et al. CD38 deletion of human primary NK cells eliminates daratumumab-induced fratricide and boosts their effector activity. Blood. (2020) 136:2416–27. doi: 10.1182/blood.2020006200
124. Phung SK, Miller JS, Felices M. Bi-specific and tri-specific NK cell engagers: the new avenue of targeted NK cell immunotherapy. Mol Diagn Ther. (2021) 25:577–92. doi: 10.1007/s40291-021-00550-6
125. Arvindam US, van Hauten PMM, Schirm D, Schaap N, Hobo W, Blazar BR, et al. A trispecific killer engager molecule against CLEC12A effectively induces NK-cell mediated killing of AML cells. Leukemia. (2021) 35:1586–96. doi: 10.1038/s41375-020-01065-5
126. Nieto Y, Banerjee P, Kaur I, Griffin L, Barnett M, Ganesh C, et al. Innate cell engager (ICE®) AFM13 combined with preactivated and expanded (P+E) cord blood (CB)-derived natural killer (NK) cells for patients with refractory CD30-positive lymphomas: final results. Blood. (2023) 142:774. doi: 10.1182/blood-2023-172980
127. Wingert S, Reusch U, Knackmuss S, Kluge M, Damrat M, Pahl J, et al. Preclinical evaluation of AFM24, a novel CD16A-specific innate immune cell engager targeting EGFR-positive tumors. MAbs. (2021) 13:1950264. doi: 10.1080/19420862.2021.1950264
128. Raynaud A, Desrumeaux K, Vidard L, Termine E, Baty D, Chames P, et al. Anti-NKG2D single domain-based antibodies for the modulation of anti-tumor immune response. Oncoimmunology. (2020) 10:1854529. doi: 10.1080/2162402x.2020.1854529
129. Gemelli M, Noonan DM, Carlini V, Pelosi G, Barberis M, Ricotta R, et al. Overcoming resistance to checkpoint inhibitors: natural killer cells in non-small cell lung cancer. Front Oncol. (2022) 12:886440. doi: 10.3389/fonc.2022.886440
130. Yabe T, McSherry C, Bach FH, Fisch P, Schall RP, Sondel PM, et al. A multigene family on human chromosome 12 encodes natural killer-cell lectins. Immunogenetics. (1993) 37:455–60. doi: 10.1007/bf00222470
131. van Montfoort N, Borst L, Korrer MJ, Sluijter M, Marijt KA, Santegoets SJ, et al. NKG2A blockade potentiates CD8 T cell immunity induced by cancer vaccines. Cell. (2018) 175:1744–1755.e1715. doi: 10.1016/j.cell.2018.10.028
132. André P, Denis C, Soulas C, Bourbon-Caillet C, Lopez J, Arnoux T, et al. Anti-NKG2A mAb is a checkpoint inhibitor that promotes anti-tumor immunity by unleashing both T and NK cells. Cell. (2018) 175:1731–1743.e1713. doi: 10.1016/j.cell.2018.10.014
133. Moretta L, Bottino C, Pende D, Vitale M, Mingari MC, Moretta A. Different checkpoints in human NK-cell activation. Trends Immunol. (2004) 25:670–6. doi: 10.1016/j.it.2004.09.008
134. Ruggeri L, Urbani E, André P, Mancusi A, Tosti A, Topini F, et al. Effects of anti-NKG2A antibody administration on leukemia and normal hematopoietic cells. Haematologica. (2016) 101:626–33. doi: 10.3324/haematol.2015.135301
135. Khan M, Arooj S, Wang H. NK cell-based immune checkpoint inhibition. Front Immunol. (2020) 11:167. doi: 10.3389/fimmu.2020.00167
136. McWilliams EM, Mele JM, Cheney C, Timmerman EA, Fiazuddin F, Strattan EJ, et al. Therapeutic CD94/NKG2A blockade improves natural killer cell dysfunction in chronic lymphocytic leukemia. Oncoimmunology. (2016) 5:e1226720. doi: 10.1080/2162402x.2016.1226720
137. Tinker AV, Hirte HW, Provencher D, Butler M, Ritter H, Tu D, et al. Dose-ranging and cohort-expansion study of monalizumab (IPH2201) in patients with advanced gynecologic Malignancies: A trial of the canadian cancer trials group (CCTG): IND221. Clin Cancer Res. (2019) 25:6052–60. doi: 10.1158/1078-0432.Ccr-19-0298
138. Lee J, Keam B, Park HR, Park JE, Kim S, Kim M, et al. Monalizumab efficacy correlates with HLA-E surface expression and NK cell activity in head and neck squamous carcinoma cell lines. J Cancer Res Clin Oncol. (2023) 149:5705–15. doi: 10.1007/s00432-022-04532-x
139. Chang WS, Kim JY, Kim YJ, Kim YS, Lee JM, Azuma M, et al. Cutting edge: Programmed death-1/programmed death ligand 1 interaction regulates the induction and maintenance of invariant NKT cell anergy. J Immunol. (2008) 181:6707–10. doi: 10.4049/jimmunol.181.10.6707
140. Fife BT, Bluestone JA. Control of peripheral T-cell tolerance and autoimmunity via the CTLA-4 and PD-1 pathways. Immunol Rev. (2008) 224:166–82. doi: 10.1111/j.1600-065X.2008.00662.x
141. Golden-Mason L, Klarquist J, Wahed AS, Rosen HR. Cutting edge: programmed death-1 expression is increased on immunocytes in chronic hepatitis C virus and predicts failure of response to antiviral therapy: race-dependent differences. J Immunol. (2008) 180:3637–41. doi: 10.4049/jimmunol.180.6.3637
142. Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. (2008) 26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331
143. Buchbinder EI, Desai A. CTLA-4 and PD-1 pathways: similarities, differences, and implications of their inhibition. Am J Clin Oncol. (2016) 39:98–106. doi: 10.1097/coc.0000000000000239
144. Taylor S, Huang Y, Mallett G, Stathopoulou C, Felizardo TC, Sun MA, et al. PD-1 regulates KLRG1(+) group 2 innate lymphoid cells. J Exp Med. (2017) 214:1663–78. doi: 10.1084/jem.20161653
145. Mariotti FR, Petrini S, Ingegnere T, Tumino N, Besi F, Scordamaglia F, et al. PD-1 in human NK cells: evidence of cytoplasmic mRNA and protein expression. Oncoimmunology. (2019) 8:1557030. doi: 10.1080/2162402x.2018.1557030
146. Hsu J, Hodgins JJ, Marathe M, Nicolai CJ, Bourgeois-Daigneault MC, Trevino TN, et al. Contribution of NK cells to immunotherapy mediated by PD-1/PD-L1 blockade. J Clin Invest. (2018) 128:4654–68. doi: 10.1172/jci99317
147. Tang J, Yu JX, Hubbard-Lucey VM, Neftelinov ST, Hodge JP, Lin Y. Trial watch: The clinical trial landscape for PD1/PDL1 immune checkpoint inhibitors. Nat Rev Drug Discovery. (2018) 17:854–5. doi: 10.1038/nrd.2018.210
148. Wilson RAM, Evans TRJ, Fraser AR, Nibbs RJB. Immune checkpoint inhibitors: new strategies to checkmate cancer. Clin Exp Immunol. (2018) 191:133–48. doi: 10.1111/cei.13081
149. Jain P, Jain C, Velcheti V. Role of immune-checkpoint inhibitors in lung cancer. Ther Adv Respir Dis. (2018) 12:1753465817750075. doi: 10.1177/1753465817750075
150. Trefny MP, Kaiser M, Stanczak MA, Herzig P, Savic S, Wiese M, et al. PD-1(+) natural killer cells in human non-small cell lung cancer can be activated by PD-1/PD-L1 blockade. Cancer Immunol Immunother. (2020) 69:1505–17. doi: 10.1007/s00262-020-02558-z
151. Juliá EP, Amante A, Pampena MB, Mordoh J, Levy EM. Avelumab, an igG1 anti-PD-L1 immune checkpoint inhibitor, triggers NK cell-mediated cytotoxicity and cytokine production against triple negative breast cancer cells. Front Immunol. (2018) 9:2140. doi: 10.3389/fimmu.2018.02140
152. Yeo J, Ko M, Lee DH, Park Y, Jin HS. TIGIT/CD226 axis regulates anti-tumor immunity. Pharm (Basel). (2021) 14:200. doi: 10.3390/ph14030200
153. Chauvin JM, Ka M, Pagliano O, Menna C, Ding Q, DeBlasio R, et al. IL15 stimulation with TIGIT blockade reverses CD155-mediated NK-cell dysfunction in melanoma. Clin Cancer Res. (2020) 26:5520–33. doi: 10.1158/1078-0432.Ccr-20-0575
154. Workman CJ, Dugger KJ, Vignali DA. Cutting edge: molecular analysis of the negative regulatory function of lymphocyte activation gene-3. J Immunol. (2002) 169:5392–5. doi: 10.4049/jimmunol.169.10.5392
155. Li H, Wu K, Tao K, Chen L, Zheng Q, Lu X, et al. Tim-3/galectin-9 signaling pathway mediates T-cell dysfunction and predicts poor prognosis in patients with hepatitis B virus-associated hepatocellular carcinoma. Hepatology. (2012) 56:1342–51. doi: 10.1002/hep.25777
156. Nebbia G, Peppa D, Schurich A, Khanna P, Singh HD, Cheng Y, et al. Upregulation of the Tim-3/galectin-9 pathway of T cell exhaustion in chronic hepatitis B virus infection. PloS One. (2012) 7:e47648. doi: 10.1371/journal.pone.0047648
157. Folgiero V, Cifaldi L, Li Pira G, Goffredo BM, Vinti L, Locatelli F. TIM-3/Gal-9 interaction induces IFNγ-dependent IDO1 expression in acute myeloid leukemia blast cells. J Hematol Oncol. (2015) 8:36. doi: 10.1186/s13045-015-0134-4
158. Ji P, Chen D, Bian J, Xia R, Song X, Wen W, et al. [Up-regulation of TIM-3 on CD4+ tumor infiltrating lymphocytes predicts poor prognosis in human non-small-cell lung cancer]. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. (2015) 31:808–11.
159. Du W, Yang M, Turner A, Xu C, Ferris RL, Huang J, et al. TIM-3 as a target for cancer immunotherapy and mechanisms of action. Int J Mol Sci. (2017) 18:645. doi: 10.3390/ijms18030645
160. He Y, Cao J, Zhao C, Li X, Zhou C, Hirsch FR. TIM-3, a promising target for cancer immunotherapy. Onco Targets Ther. (2018) 11:7005–9. doi: 10.2147/ott.S170385
161. Gallois A, Silva I, Osman I, Bhardwaj N. Reversal of natural killer cell exhaustion by TIM-3 blockade. Oncoimmunology. (2014) 3:e946365. doi: 10.4161/21624011.2014.946365
162. Xu L, Huang Y, Tan L, Yu W, Chen D, Lu C, et al. Increased Tim-3 expression in peripheral NK cells predicts a poorer prognosis and Tim-3 blockade improves NK cell-mediated cytotoxicity in human lung adenocarcinoma. Int Immunopharmacol. (2015) 29:635–41. doi: 10.1016/j.intimp.2015.09.017
163. Farkas AM, Audenet F, Anastos H, Galsky MK, Sfakianos JP, Bhardwaj N. Tim-3 and TIGIT mark Natural Killer cells susceptible to effector dysfunction in human bladder cancer. J Immunol. (2018) 200:124.14. doi: 10.4049/jimmunol.200.Supp.124.14
164. da Silva IP, Gallois A, Jimenez-Baranda S, Khan S, Anderson AC, Kuchroo VK, et al. Reversal of NK-cell exhaustion in advanced melanoma by Tim-3 blockade. Cancer Immunol Res. (2014) 2:410–22. doi: 10.1158/2326-6066.Cir-13-0171
165. Cornillet M, Jansson H, Schaffer M, Hertwig L, Berglin L, Zimmer CL, et al. Imbalance of genes encoding natural killer immunoglobulin-like receptors and human leukocyte antigen in patients with biliary cancer. Gastroenterology. (2019) 157:1067–1080.e1069. doi: 10.1053/j.gastro.2019.06.023
166. Sun H, Sun C. The rise of NK cell checkpoints as promising therapeutic targets in cancer immunotherapy. Front Immunol. (2019) 10:2354. doi: 10.3389/fimmu.2019.02354
167. Ghaedrahmati F, Esmaeil N, Abbaspour M. Targeting immune checkpoints: how to use natural killer cells for fighting against solid tumors. Cancer Commun (Lond). (2023) 43:177–213. doi: 10.1002/cac2.12394
168. Romagné F, André P, Spee P, Zahn S, Anfossi N, Gauthier L, et al. Preclinical characterization of 1-7F9, a novel human anti-KIR receptor therapeutic antibody that augments natural killer-mediated killing of tumor cells. Blood. (2009) 114:2667–77. doi: 10.1182/blood-2009-02-206532
169. Kohrt HE, Thielens A, Marabelle A, Sagiv-Barfi I, Sola C, Chanuc F, et al. Anti-KIR antibody enhancement of anti-lymphoma activity of natural killer cells as monotherapy and in combination with anti-CD20 antibodies. Blood. (2014) 123:678–86. doi: 10.1182/blood-2013-08-519199
170. Carlsten M, Korde N, Kotecha R, Reger R, Bor S, Kazandjian D, et al. Checkpoint inhibition of KIR2D with the monoclonal antibody IPH2101 induces contraction and hyporesponsiveness of NK cells in patients with myeloma. Clin Cancer Res. (2016) 22:5211–22. doi: 10.1158/1078-0432.Ccr-16-1108
171. Sola C, Blery M, Bonnafous C, Bonnet E, Fuseri N, Graziano RF, et al. Lirilumab enhances anti-tumor efficacy of elotuzumab. Blood. (2014) 124:4711. doi: 10.1182/blood.V124.21.4711.4711
172. de Lázaro I, Mooney DJ. A nanoparticle’s pathway into tumours. Nat Mater. (2020) 19:486–7. doi: 10.1038/s41563-020-0669-9
173. Irvine DJ, Dane EL. Enhancing cancer immunotherapy with nanomedicine. Nat Rev Immunol. (2020) 20:321–34. doi: 10.1038/s41577-019-0269-6
174. Phung CD, Tran TH, Kim JO. Engineered nanoparticles to enhance natural killer cell activity towards onco-immunotherapy: a review. Arch Pharm Res. (2020) 43:32–45. doi: 10.1007/s12272-020-01218-1
175. Kim KS, Kim DH, Kim DH. Recent advances to augment NK cell cancer immunotherapy using nanoparticles. Pharmaceutics. (2021) 13:525. doi: 10.3390/pharmaceutics13040525
176. Park W, Gordon AC, Cho S, Huang X, Harris KR, Larson AC, et al. Immunomodulatory magnetic microspheres for augmenting tumor-specific infiltration of natural killer (NK) cells. ACS Appl Mater Interfaces. (2017) 9:13819–24. doi: 10.1021/acsami.7b02258
177. Meraz IM, Majidi M, Cao X, Lin H, Li L, Wang J, et al. TUSC2 immunogene therapy synergizes with anti-PD-1 through enhanced proliferation and infiltration of natural killer cells in syngeneic kras-mutant mouse lung cancer models. Cancer Immunol Res. (2018) 6:163–77. doi: 10.1158/2326-6066.Cir-17-0273
178. Zhou Y, Cheng L, Liu L, Li X. NK cells are never alone: crosstalk and communication in tumour microenvironments. Mol Cancer. (2023) 22:34. doi: 10.1186/s12943-023-01737-7
179. Au KM, Park SI, Wang AZ. Trispecific natural killer cell nanoengagers for targeted chemoimmunotherapy. Sci Adv. (2020) 6:eaba8564. doi: 10.1126/sciadv.aba8564
180. Biber G, Sabag B, Raiff A, Ben-Shmuel A, Puthenveetil A, Benichou JIC, et al. Modulation of intrinsic inhibitory checkpoints using nano-carriers to unleash NK cell activity. EMBO Mol Med. (2022) 14:e14073. doi: 10.15252/emmm.202114073
181. Xu Z, Wang Y, Zhang L, Huang L. Nanoparticle-delivered transforming growth factor-β siRNA enhances vaccination against advanced melanoma by modifying tumor microenvironment. ACS Nano. (2014) 8:3636–45. doi: 10.1021/nn500216y
182. Liu C, Lai H, Chen T. Boosting natural killer cell-based cancer immunotherapy with selenocystine/transforming growth factor-beta inhibitor-encapsulated nanoemulsion. ACS Nano. (2020) 14:11067–82. doi: 10.1021/acsnano.9b10103
183. Kim KS, Han JH, Choi SH, Jung HY, Park JD, An HJ, et al. Cationic nanoparticle-mediated activation of natural killer cells for effective cancer immunotherapy. ACS Appl Mater Interfaces. (2020) 12:56731–40. doi: 10.1021/acsami.0c16357
Keywords: NK cells, tumor, biology, immunotherapy, clinical trial
Citation: Jiang P, Jing S, Sheng G and Jia F (2024) The basic biology of NK cells and its application in tumor immunotherapy. Front. Immunol. 15:1420205. doi: 10.3389/fimmu.2024.1420205
Received: 19 April 2024; Accepted: 31 July 2024;
Published: 16 August 2024.
Edited by:
Cai Zhang, Shandong University, ChinaReviewed by:
Nan Lu, Shandong University, ChinaPeixiang Lan, Huazhong University of Science and Technology, China
Massoud Vosough, Royan institute for Stem Cell Biology and Technology (RI-SCBT), Iran
Copyright © 2024 Jiang, Jing, Sheng and Jia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fajing Jia, MTYwNjc5NjU5OUBxcS5jb20=; Gaohong Sheng, Z2FvaG9uZ3NoZW5nQGh1c3QuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
 Pan Jiang
Pan Jiang Shaoze Jing
Shaoze Jing Gaohong Sheng
Gaohong Sheng Fajing Jia
Fajing Jia