
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 27 November 2024
Sec. Cancer Immunity and Immunotherapy
Volume 15 - 2024 | https://doi.org/10.3389/fimmu.2024.1419544
 Luigi Liguori1,2
Luigi Liguori1,2 Gabriele Giorgio1
Gabriele Giorgio1 Giovanna Polcaro1
Giovanna Polcaro1 Valentina Pagliara1
Valentina Pagliara1 Domenico Malandrino1
Domenico Malandrino1 Francesco Perri3
Francesco Perri3 Marco Cascella1
Marco Cascella1 Alessandro Ottaiano4
Alessandro Ottaiano4 Valeria Conti1
Valeria Conti1 Alberto Servetto2
Alberto Servetto2 Roberto Bianco2
Roberto Bianco2 Stefano Pepe1
Stefano Pepe1 Francesco Sabbatino1*
Francesco Sabbatino1*Introduction: Immune checkpoint inhibitor (ICI)-based immunotherapy targeting programmed cell death 1 (PD-1) or its ligand 1 (PD-L1) has radically changed the management of many types of solid tumors including non-small cell lung cancer (NSCLC). Many clinical trials have demonstrated that ICIs improve the survival and the quality of life of patients with advanced non oncogene NSCLC as compared to standard therapies. However, not all patients achieve a clinical benefit from this immunotherapeutic approach. As a result, real-word validation of the efficacy and safety of ICIs can be useful for defining potential predictive biomarkers as well as for overcoming limitations linked to clinical trial restrictions.
Methods: We retrospectively retrieved the clinical data of patients with advanced non oncogene NSCLC treated with ICIs (anti-PD-1 or anti-PD-L1) as single agent or in combination with chemotherapy at “San Giovanni di Dio e Ruggi D’Aragona” University Hospital from January 2016 to December 2023. Potential correlations between clinical-pathological characteristics and safety or survival outcomes were investigated employing the Fisher’s exact test, Mann-Whitney U test, the Kruskal-Wallis method and log-rank test, as applicable. Multivariate survival analyses were performed using the Cox proportional hazards model.
Results: Clinical data of 129 patients were retrieved. At a median follow-up of 29.70 months, progression-free survival (PFS) and overall survival (OS) were 5.27 months and 8.43 months, respectively. At the multivariate analyses, smoking status, presence of bone metastases and the occurrence of immune-related adverse events (irAEs) were correlated with both PFS and OS. Moreover, patients treated with anti-PD-1-based therapy achieved an increased clinical benefit than those treated with anti-PD-L1.
Discussion: In this study we described our real-world experience of ICIs for the treatment of patients with advanced non oncogene NSCLC. A decreased OS in our study population was reported as compared to that of patients included in the clinical trials. Noteworthy, correlations between clinical-pathological characteristics and survival outcomes emerged. Nevertheless, the potential integration of clinical-pathological characteristics as predictive biomarkers in more accurate therapeutic algorithms as well as the underlying biological mechanisms should be further validated in ad hoc studies.
Lung cancer, mainly represented by non-small cell lung cancer (NSCLC), is the leading cause of cancer-related death in USA, with about 120.000 deaths per year (1). In the past few years, immune checkpoint inhibitors (ICIs) targeting programmed cell death 1 (PD-1), its ligand 1 (PD-L1) and cytotoxic T-lymphocyte antigen-4 (CTLA-4) have revolutionized the management of many types of solid tumors including NSCLC (2). This novel immunotherapeutic approach has demonstrated to improve the survival and the quality of life of the patients with advanced non oncogene NSCLC as compared to standard chemotherapy (2). Based on the results of many clinical trials, ICIs as monotherapy or in combination with chemotherapy represent the standard-of-care for the treatment of patients with advanced non oncogene NSCLC, so far (2). However, not all treated patients achieved a sustained clinical benefit. Indeed, the efficacy of this therapy is limited to an half of treated patients, and only a small portion of them (10-15%) achieves long-term tumor response (3–9, 11). Moreover, about 10-15% of treated patients develops severe immune-related adverse events (irAEs), potentially causing prolonged sequelae or even fatal consequences (3–9, 11). As a result, there is the urgent need to identify biomarkers of tumor response as well as patients at higher risk to develop severe irAEs. In the last decade, several pathological biomarkers including PD-L1 tumor proportion score (TPS), tumor mutational burden (TMB), human leucocyte antigen (HLA) class I and II expression, β2-microglobulin (β2m) mutations, tumor microenvironment (TME) composition, and gene expression profiles (GEPs) have been investigated with various results (12–16). PD-L1 TPS, the most widely investigated, is integrated in therapeutic algorithm currently utilized in clinical practice for the treatment of advanced non oncogene NSCLC patients. Indeed, increased levels of PD-L1 TPS are correlated to a higher likelihood of tumor response (6, 11, 13, 17). However, not all patients with high PD-L1 TPS achieve a clinical benefit. In addition, even patients with low or negative PD-L1 TPS may also benefit from this therapy. Consequently, PD-L1 TPS is not efficient in predicting tumor response, being considered a “surrogate biomarker” (13, 17–19). On the same line, no other biomarker has demonstrated to efficiently predict either tumor response and/or development of irAEs (12–15).
Beyond pathological biomarkers, many clinical characteristics including gender, Eastern Cooperative Oncology Group (ECOG) Performance Status (PS), Body Mass Index (BMI), specific sites of metastases, concomitant medications (i.e. antibiotics and corticosteroids) and occurrence of irAEs have also been investigated for their potential predictive role with various results (20). Here by analyzing real-world data we aim to further validate the efficacy and safety of ICIs in study populations by defining potential predictive biomarkers as well as by overcoming limitations linked to clinical trial restrictions.
Clinical data of Caucasian patients with confirmed advanced (stage IV) NSCLC treated with ICIs from January 2016 to December 2023 at “San Giovanni di Dio e Ruggi D’Aragona” University Hospital, was retrieved. The study was performed without interfering with clinical practice. Selection of patients to be included in the study was performed based on: (i) signed informed consent for clinical-pathological data acquisition; (ii) age >18 years; (iii) treatment with ICIs as monotherapy or in combination with chemotherapy; (iv) absence of active autoimmune disease. Patients with Epidermal Growth Factor Receptor (EGFR), Anaplastic Lymphoma Kinase (ALK), c-ros oncogene 1 (ROS1), V-Raf Murine Sarcoma Viral Oncogene Homolog B (BRAF), Mesenchymal-epithelial transition factor (MET), REarranged during Transfection (RET) and Neurotrophic Tropomyosin Receptor Kinases (NTRK) tumor alterations were excluded from the study. Evaluation of ALK, BRAF, EGFR, MET, NTRK, RET and ROS1 tumor alterations was performed on tumor samples (when available) or liquid biopsy according to national pathology guidelines. Clinical-pathological characteristics including age, sex, ECOG PS, smoking status, alcohol abuse, comorbidities, previous cancer, concomitant medications, baseline prednisone equivalent dose, PD-L1 TPS, histologic subtypes, specific sites of metastasis, type of immunotherapy and previous chemotherapy and/or targeted therapy and/or radiotherapy were retrospectively collected. Patient privacy and personal data were preserved by assigning a progressive anonymous identification number. PD-L1 was evaluated on tumor samples as clinically indicated when tumor tissue was available and reported as TPS (21) according to European Society for Medical Oncology (ESMO) guidelines. Patients received one of the following ICI-based immunotherapy according to Italian guidelines: i) atezolizumab or nivolumab after the failure of platinum-based chemotherapy, regardless PD-L1 TPS; ii) pembrolizumab after the failure of platinum-based chemotherapy for PD-L1 TPS ≥1%; iii) the combination of chemotherapy and pembrolizumab or the combination of chemotherapy and nivolumab and ipilimumab as first-line for PD-L1 TPS <50%; iv) atezolizumab or pembrolizumab as first-line for PD-L1 TPS ≥50%;. irAEs were defined as adverse events displaying a certain, likely or possible correlation with ICIs according to Common Terminology Criteria for Adverse Events (CTCAE) v 4.0 (22). Radiographic imaging was performed every two months, according to clinical practice. Response rate was determined according to Response Evaluation Criteria in Solid Tumours version 1.1 (RECIST v1.1) (23) and reported as complete response (CR), partial response (PR), stable disease (SD) and progression disease (PD). Objective response rate (ORR) was defined as the proportion of patients with a CR or PR whereas disease control rate (DCR) as the proportion of patients with CR or PR or SD. Progression-free survival (PFS) was defined as the time from the start of the treatment to the first documented PD or death by any cause. Overall survival (OS) was defined from the start of the treatment to death by any cause or last follow-up date. Patients dead from COVID-19 were excluded. The study was approved by the local ethics committee (prot./SCCE n.85275), in accordance with the Declaration of Helsinki and its amendments.
Data was collected using Microsoft Excel. Statistical analyses were performed using STATA v13 software released by StataCorp LP (College Station, TX, USA). Continuous variables were expressed as medians and ranges, whereas categorical variables were expressed as frequencies and percentages. PFS and OS were calculated using the Kaplan-Meier method. Median follow-up was calculated using the inverse Kaplan-Meier method. Correlations between clinical-pathological characteristics and irAE rates were performed using the Fisher’s exact test, Mann–Whitney U test and the Kruskal-Wallis method, as appropriate. Correlation between clinical-pathological characteristics and survival outcomes (PFS and OS) was performed using log-rank test. Multivariate survival analyses were performed using the Cox proportional hazards model. The difference between groups was considered significant when the P value was <0.05.
Clinical-pathological characteristics of 129 Caucasian patients with stage IV non oncogene NSCLC at the “San Giovanni di Dio e Ruggi D’Aragona” University Hospital, treated with ICIs from January 2016 to December 2023 were retrieved. Baseline characteristics of patients are summarized in Table 1.
The median age was 68 years (range, 45-83 years). One hundred patients (77.52%) were male. Forty-seven (36.43%), 58 (44.96%), 19 (14.73%), and 5 (3.88%) had ECOG PS of 0, 1, 2 and 3, respectively. Fourteen patients (10.85%) were never smokers, while 77 (59.69%) and 38 (29.46%) were previous and current smokers, respectively. Alcohol abuse was also reported in ten patients (7.75%). Relevant comorbidities included hypertension (53.49%), dyslipidemia (27.90%), diabetes (20.16%), chronic obstructive pulmonary disease (COPD) (17.05%), hearth failure (7.75%), depressive disorder (3.10%) and chronic renal failure (CRF) (0.77%). Thirteen and one patients reported a previous prostate cancer and breast cancer, respectively. Relevant concomitant medications included antihypertensive (53.49%), low dose aspirin (31.78%), opioids (25.78%), statins (24.81%), anticoagulants (18.60%), antiplatelet drugs (18.60%), oral hypoglycemic drugs (14.73%), and antidepressants (selective serotonin reuptake inhibitors (SSRIs)) (8.53%). In addition, 16, 28 and 20 patients received equivalent prednisone dose of ≤10 mg/die, >10 mg/die and ≤20 mg/die, and >20 mg/die, respectively. Eighty tumors (62.02%) were classified as adenocarcinomas, 44 (34.11%) as squamous cell carcinoma, 4 (3.10%) as large cell carcinoma and 1 (0.77%) as sarcomatoid carcinoma. PD-L1 TPS was available for 98 patients (75.97%). A PD-L1 TPS <1%, ≥1% and <50%, and ≥50% were reported in 37.76%, 31.63% and 30.61%, respectively, of the available tumors. Main sites of metastasis included lymph nodes (87.50%), lung (65.63%), bone (29.69%), central nervous system (CNS) (21.88%), adrenal gland (17.97%), liver (11.72%) and skin (4.69%). Sixty patients (46.51%), 4 (3.10%), and 39 (30.23%) of patients had previously received chemotherapy, anti-Vascular Endothelial Growth Factor Receptor (VEGFR)-targeted therapy and radiotherapy, respectively. Forty-four (34.11%), 36 (27.91%), 38 (29.46%), and 11 (8.53%) patients received ICI as first, second, third, and fourth or subsequent lines of treatment, respectively. More in detail, i) forty-five (34.87%) patients received nivolumab; ii) twenty-five (19.37%) patients received pembrolizumab; iii) nine (6.98%) patients received atezolizumab; iv) thirty-eight (28.68%) patients received carboplatin-pemetrexed-pembrolizumab; v) seven (5.43%) patients received carboplatin-nab-paclitaxel-pembrolizumab, and vi) five (3.88%) patients received carboplatin-pemetrexed-ipilimumab-nivolumab. The median cycle of ICI received was 15 (1–123). ORR was 20.19% while DCR was 60.57%. CRs, PRs, SDs, and PDs were reported in 1 (0.96%), 20 (19.23%), 42 (40.38%), and 41 (39.43%) of treated patients, respectively. In addition, pseudo-progression was also reported in two patients (1.96%). At a median follow-up of 15.00 months, median PFS and OS were 5.27 months (range, 0.30-93.23 months) and 8.43 months (range, 0.30-93.23 months), respectively (Figure 1).
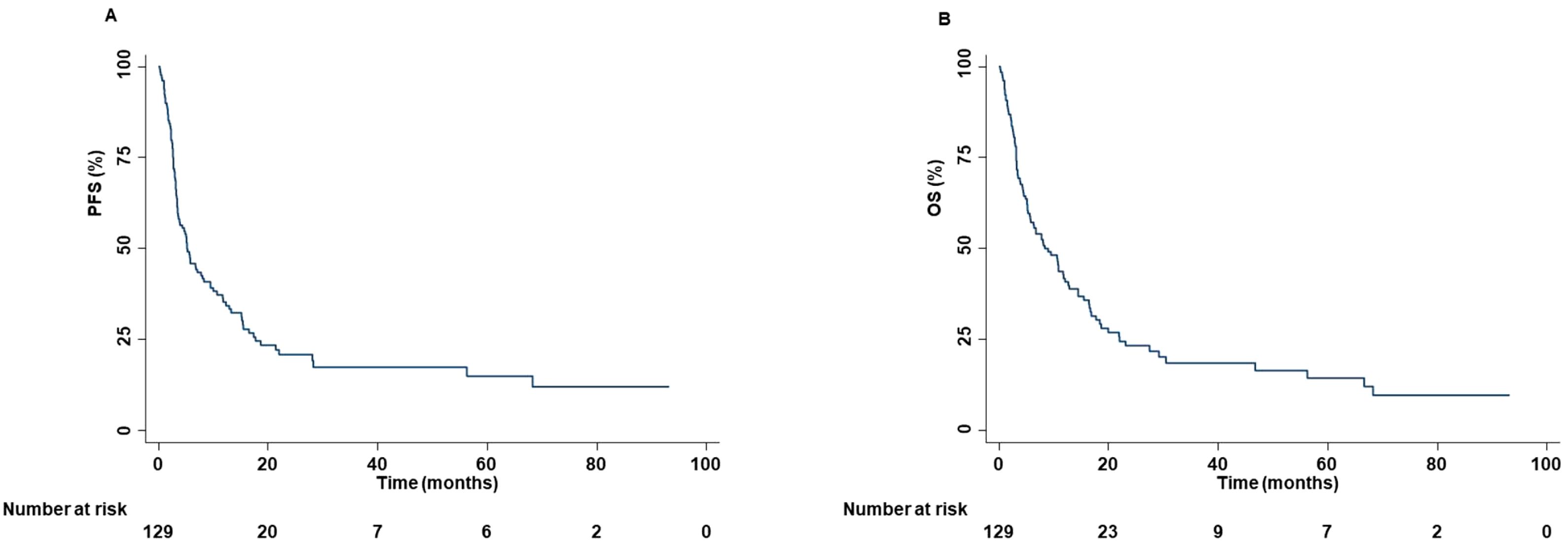
Figure 1. PFS and OS of advanced non oncogene NSCLC patients treated with ICI-based immunotherapy. At a median follow-up of 15.00 months, median PFS and OS were 5.27 months (range, 0.30-93.23 months) (A) and 8.43 months (range, 0.30-93.23 months) (B), respectively. PFS and OS analysis was performed using the Kaplan-Meier method.
Moreover, among patients treated with ICI as first-line of treatment, at a median follow-up of 21.30 months, the median PFS and OS were 5.87 months (range, 0.30-75.73 months) and 11.80 months (range, 0.30-75.73 months), respectively (Supplementary Figure S1). On the other hand, among patients treated with ICI as second or subsequent lines of treatment, at a median follow-up of 69.80 months, the median PFS and OS were 3.90 months (range, 0.47-93.23 months) and 8.12 months (range, 0.80-93.23 months), respectively (Supplementary Figure S2). We also evaluated the survival outcomes of patients treated with the combination of chemotherapy and ICI as well as of those treated with ICI as monotherapy. Specifically, in the former, at a median follow-up of 23.40 months, median PFS and median OS were 8.37 months (range, 0.30-35.00 months) and 11.80 months (range, 0.30-35.00 months), respectively (Supplementary Figure S3). Whereas, in the latter, at a median follow-up of 69.80 months, median PFS and median OS were 4.43 months (range, 0.33-93.23 months) and 8.17 months (range, 0.33-93.23 months), respectively (Supplementary Figure S4).
Lastly, the safety profile was also described. All grade (grade 1-2 and/or grade 3-4), grade 1-2 and grade 3-4 irAEs were reported in 81 (62.79%), 80 (62.02%) and 12 (9.38%) of treated patients, respectively. All irAEs were reported in detail in Table 2.
Age was significantly correlated with the type of therapy. Specifically, older patients received ICI as monotherapy more frequently than the combination of chemotherapy and ICI (P=0.0480) (Figure 2A). However, the sample size of our study population was not enough to demonstrate this association (effect size: 0.0473; power: 0.0832). In addition, older patients received anti-PD-L1 therapy more frequently than anti-PD-1 therapy (P=0.0700) (Figure 2B).
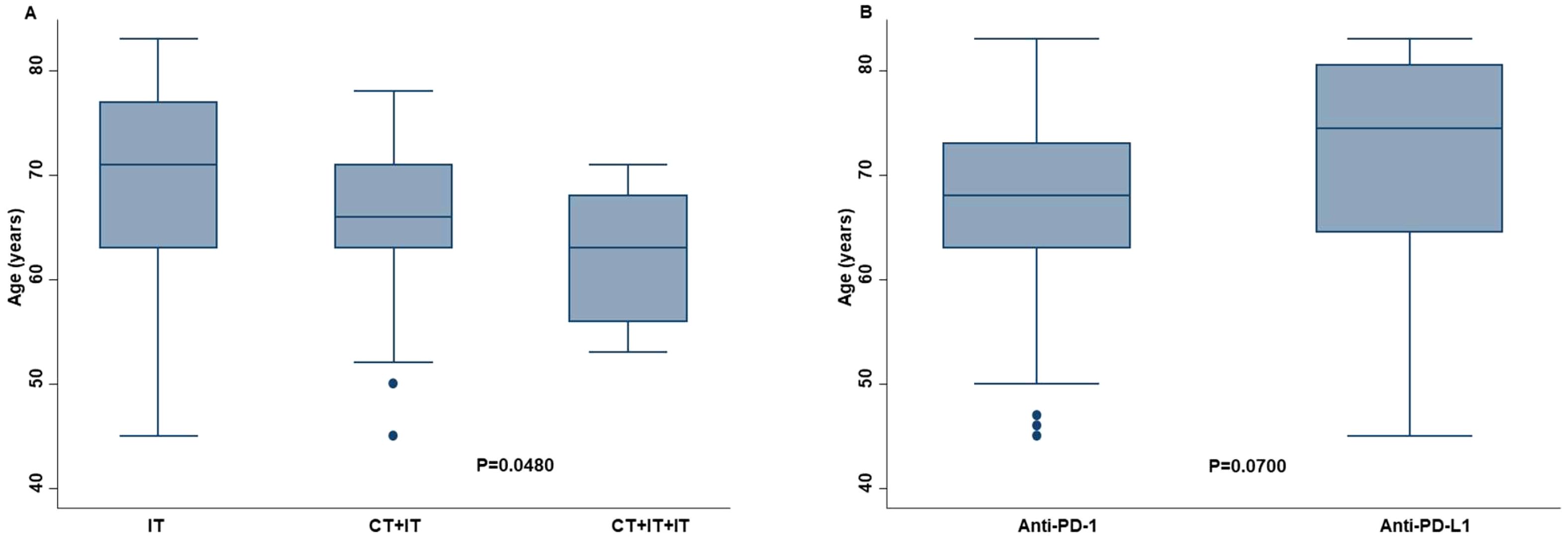
Figure 2. Correlation between age of the advanced non oncogene NSCLC patients and the chosen type of ICI-based immunotherapy. Older patients received more ICI as monotherapy (IT) than younger patients (A). The former received less frequently the combination of chemotherapy and mono (CT+IT) or double (CT+IT+IT) ICI. Older patients also received more frequently anti-PD-L1 therapy than anti-PD-1 therapy (B). Differences between groups were correlated by Kruskal-Wallis method. P <0.05 was considered statistically significant.
Significant correlations between clinical-pathological characteristics and survival outcomes were found. PFS and OS were significantly correlated with ORR (P=0.0000 and P=0.0000) and DCR (P=0.0000 and P=0.0000). In addition, survival outcomes were also correlated with the number of ICI cycles received by the patients. Indeed, patients with longer PFS and OS received a higher number of ICI cycles (P=0.0000 and P=0.0000) than those who received a lower number. Smoking status, concomitant medications (antidepressants or opioids) and ECOG PS, were correlated with survival outcomes. Smoking status correlated with PFS (P=0.0283) and OS (P=0.0470). Specifically, patients who were never smoked or current smokers displayed an increased PFS, OS than those who were previously smokers (Figure 3).

Figure 3. Association between smoking status and clinical outcomes in advanced non oncogene NSCLC patients treated with ICI-based immunotherapy. PFS (A) and OS (B) of advanced NSCLC patients treated with ICI-based immunotherapy were stratified based on smoking status. PFS and OS were compared using the Kaplan-Meier method. Differences in patients’ survival were analyzed using a log-rang test. P <0.05 was considered statistically significant.
In addition, smoker patients achieved an increased DCR than patients who had stopped smoking. Concomitant administration of antidepressants was significantly correlated with increased PFS (P=0.0340) and OS (P=0.0220) (Figure 4).
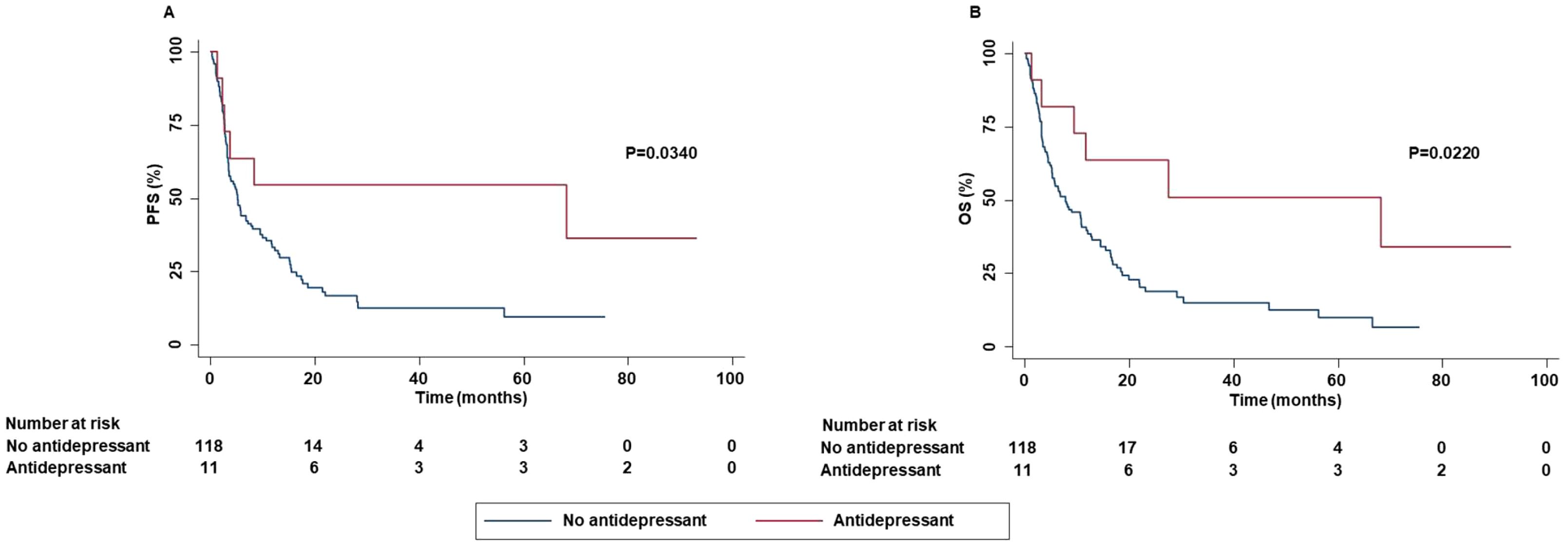
Figure 4. Association between concomitant administration of antidepressant and clinical outcomes in advanced NSCLC patients treated with ICI-based immunotherapy. PFS (A) and OS (B) of advanced NSCLC patients treated with ICI-based immunotherapy were stratified based on concomitant administration of antidepressant. PFS and OS were compared using the Kaplan-Meier method. Differences in patients’ survival were analyzed using a log-rang test. P <0.05 was considered statistically significant.
In contrast, concomitant administration of opioids was significantly correlated with decreased PFS (P=0.0461), OS (P=0.0340) (Figure 5) while no statistically significant correlation between concomitant administration of opioids and ORR (P=0.0500) and DCR (P=0.0910) was found.
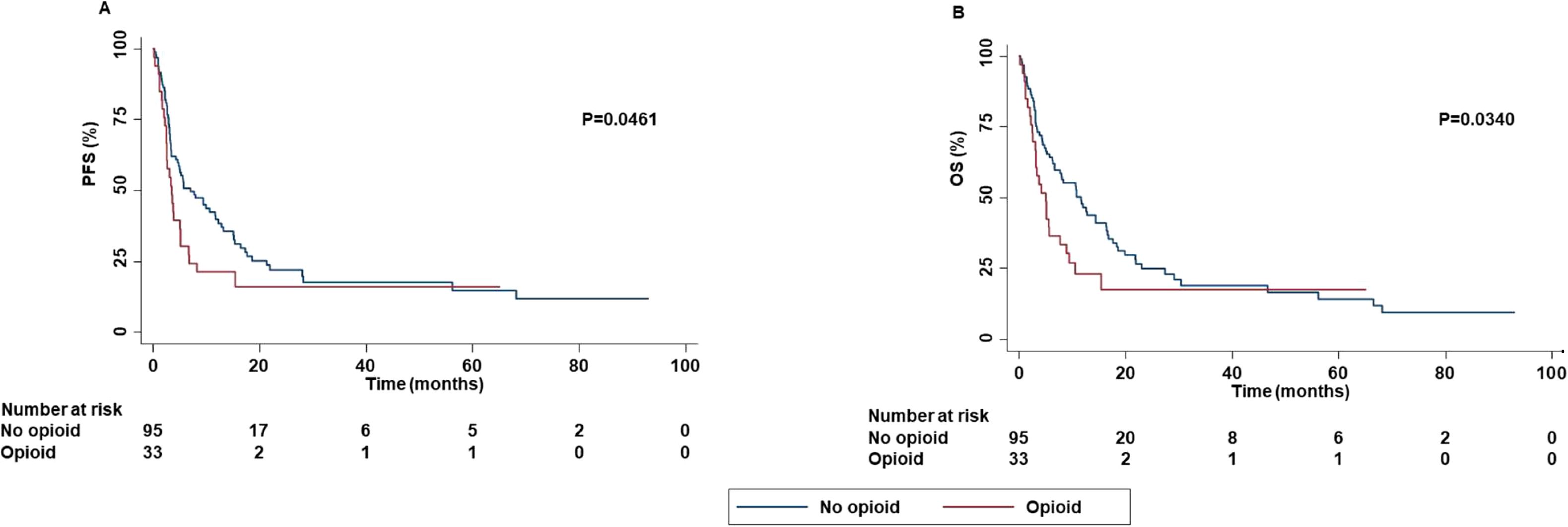
Figure 5. Association between concomitant administration of opioid and clinical outcomes in advanced non oncogene NSCLC patients treated with ICI-based immunotherapy. PFS (A) and OS (B) of advanced NSCLC patients treated with ICI-based immunotherapy were stratified based on concomitant administration of opioid. PFS and OS were compared using the Kaplan-Meier method. Differences in patients’ survival were analyzed using a log-rang test. P <0.05 was considered statistically significant.
Patients with ECOG PS 0-1 were strongly associated with increased PFS (P=0.0000), OS (P=0.0000), ORR (P=0.0481) and DCR (P=0.0100) than those with ECOG PS 2-3 (Figure 6).
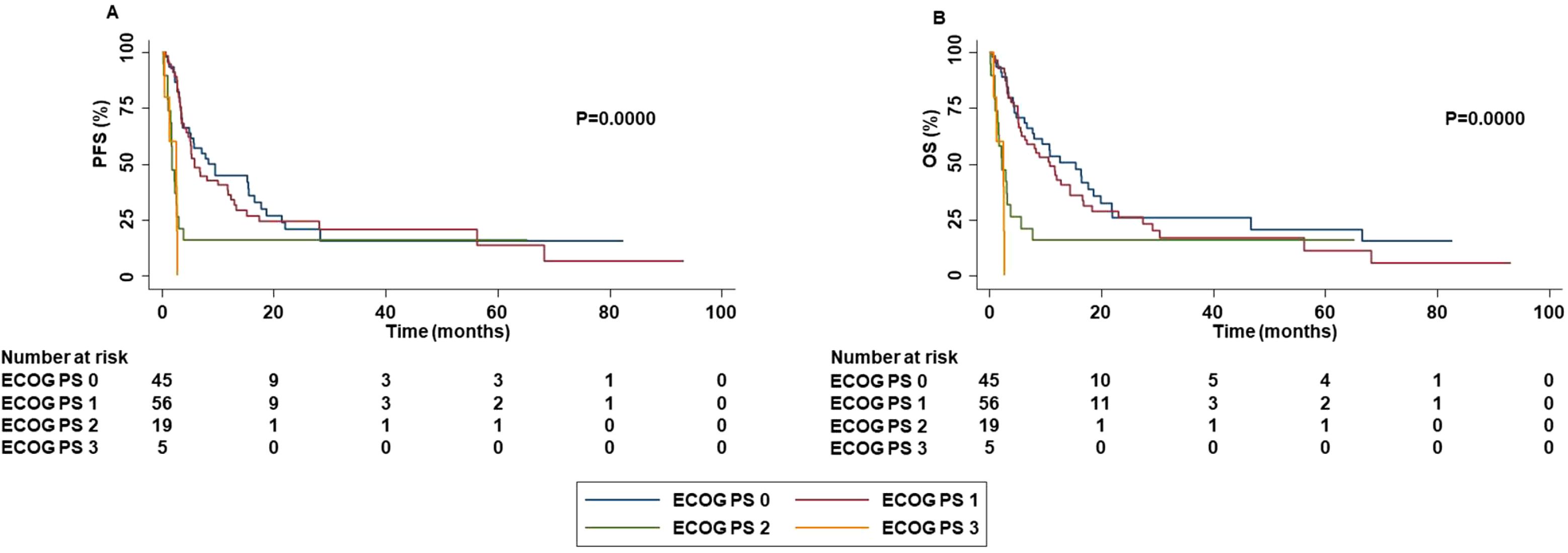
Figure 6. Association between ECOG PS and clinical outcomes in advanced non oncogene NSCLC patients treated with ICI-based immunotherapy. PFS (A) and OS (B) of advanced NSCLC patients treated with ICI-based immunotherapy were stratified based ECOG PS. PFS and OS were compared using the Kaplan-Meier method. Differences in patients’ survival were analyzed using a log-rang test. P <0.05 was considered statistically significant.
Patients with bone metastases displayed a decreased PFS (P=0.0020), OS (P=0.0010) and DCR (P=0.0240) than those without bone metastases (Figure 7).
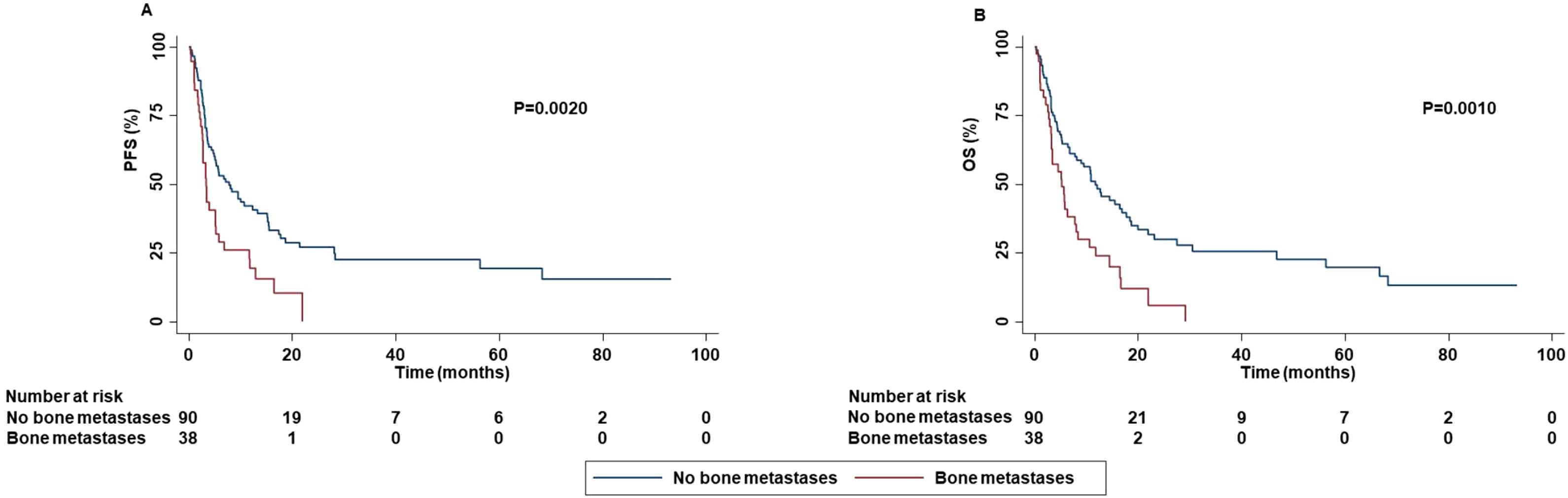
Figure 7. Association between bone metastases and clinical outcomes in advanced non oncogene NSCLC patients treated with ICI-based immunotherapy. PFS (A) and OS (B) of advanced NSCLC patients treated with ICI-based immunotherapy were stratified based on the presence of bone metastases. PFS and OS were compared using the Kaplan-Meier method. Differences in patients’ survival were analyzed using a log-rang test. P <0.05 was considered statistically significant.
In contrast in patients with skin metastases an increased PFS (P=0.0410) and OS (P=0.0473) as well as a higher ORR (P=0.0500) were reported as compared to that of patients without skin metastases (Figure 8).
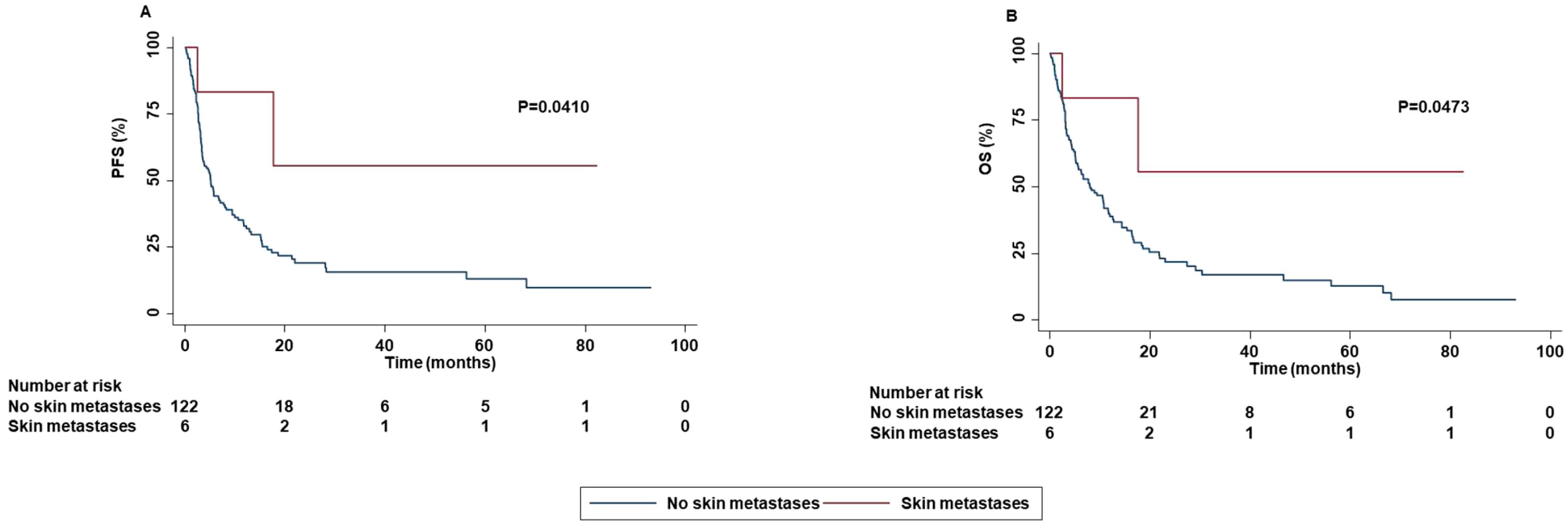
Figure 8. Association between skin metastases and clinical outcomes in advanced oncogene NSCLC patients treated with ICI-based immunotherapy. PFS (A) and OS (B) of advanced NSCLC patients treated with ICI-based immunotherapy were stratified based on the presence of skin metastases. PFS and OS were compared using the Kaplan-Meier method. Differences in patients’ survival were analyzed using a log-rang test. P <0.05 was considered statistically significant.
Correlation between PD-L1 TPS and survival outcomes showed that the risk of progression was significantly decreased for the patients with PD-L1 TPS ≥ 50% as compared to those with PD-L1 TPS < 50% (P=0.0430) (Figure 9).
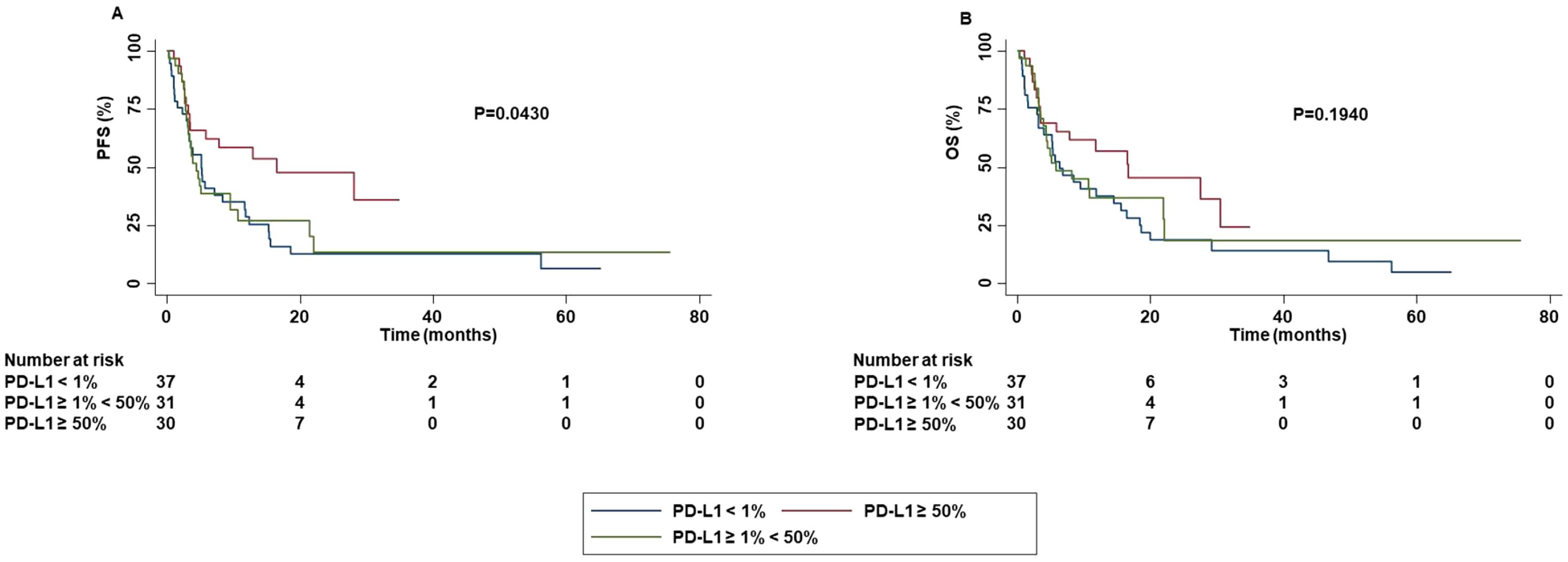
Figure 9. Association between PD-L1 TPS and clinical outcomes in advanced non oncogene NSCLC patients treated with ICI-based immunotherapy. PFS (A) and OS (B) of advanced NSCLC patients treated with ICI-based immunotherapy were stratified based PD-L1 TPS. PFS and OS were compared using the Kaplan-Meier method. Differences in patients’ survival were analyzed using a log-rang test. P <0.05 was considered statistically significant.
No significant difference in OS was detected based on PD-L1 TPS. In addition, a significantly higher ORR (P=0.0210) and DCR (P=0.0030) was obtained in patients with PD-L1 TPS > 1% or < 50% as compared to those with TPS ≥ 50%. Both patients with PD-L1 TPS < 1% or ≥ 50% achieved a lower rate of complete or partial response than those with TPS ≥ 1% and < 50%. Stratification of patients based on their negative (PD-L1 TPS < 1%) or positive (PD-L1 TPS ≥ 1%) value showed that patients with PD-L1 TPS ≥ 1% achieved a significant higher percentage of survival at 60 months as compared to those with PD-L1 TPS < 1% in both PFS and OS. Differences in median PFS and OS were not significant (PFS: P=0.1010; OS:P=0.1500) (Figure 10).
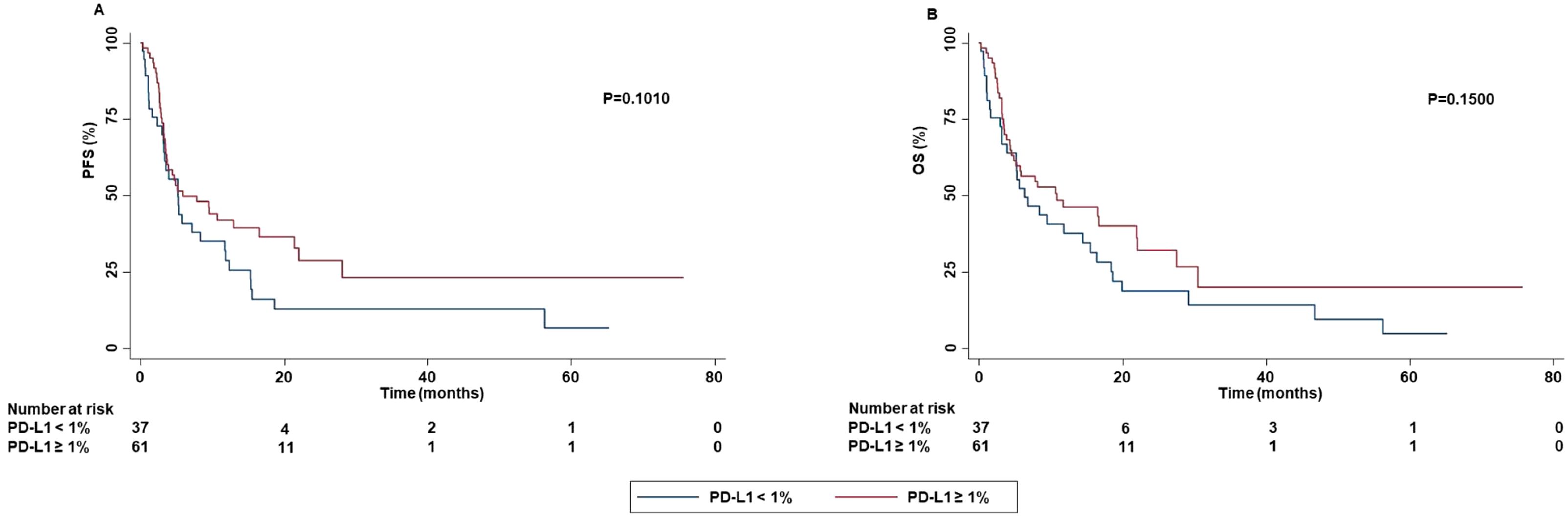
Figure 10. Association between PD-L1 TPS and clinical outcomes in advanced non oncogene NSCLC patients treated with ICI-based immunotherapy. PFS (A) and OS (B) of advanced NSCLC patients treated with ICI-based immunotherapy were stratified based on the absence (PD-L1 TPS < 1%) or the presence (PD-L1 TPS ≥ 1%) of PD-L1 TPS. PFS and OS were compared using the Kaplan-Meier method. Differences in patients’ survival were analyzed using a log-rang test. P <0.05 was considered statistically significant.
We also explored whether different treatment regimens influenced the survival outcomes. Patients treated with ICIs as first-line achieved numerically longer PFS and OS as compared to those treated with ICIs as second or subsequent lines. However, these differences are not statistically significant (PFS: P=0.1500 and OS: P=0.2300). In contrast, the differences in terms of survival outcomes were statistically significant when we considered the specific lines of treatment (first, second, third…) (PFS: P=0.039 and OS: P=0.0330). Lastly, patients treated with the combination of chemotherapy and ICIs achieved a numerically longer PFS and OS as compared to those treated with ICIs as monotherapy. However, these differences are not statistically significant (PFS: P=0.2800 and OS: P=0.5200).
Concomitant administration of antiplatelet drugs, statin or low dose of aspirin was significantly correlated with the occurrence of irAEs. Specifically, patients who assumed antiplatelet drugs (P=0.0489) or statin (P=0.0400) had an increased risk to develop grade 3-4 irAEs as well as those who assumed low dose of aspirin had an increased risk to develop all grade irAEs (P=0.0016). In addition, the line of treatment was also associated with the occurrence of irAEs. Indeed, patients treated with ICI as first-line reported a lower rate of all grade (P=0.0348) and grade 1-2 (P=0.0329) irAEs than those treated with ICIs as second or subsequent-line of treatment. The occurrence of irAEs was significantly associated with survival outcomes. Specifically, the occurrence of all grade irAEs was significantly correlated with increased PFS (P=0.0017) and OS (P=0.0023) (Figure 11).
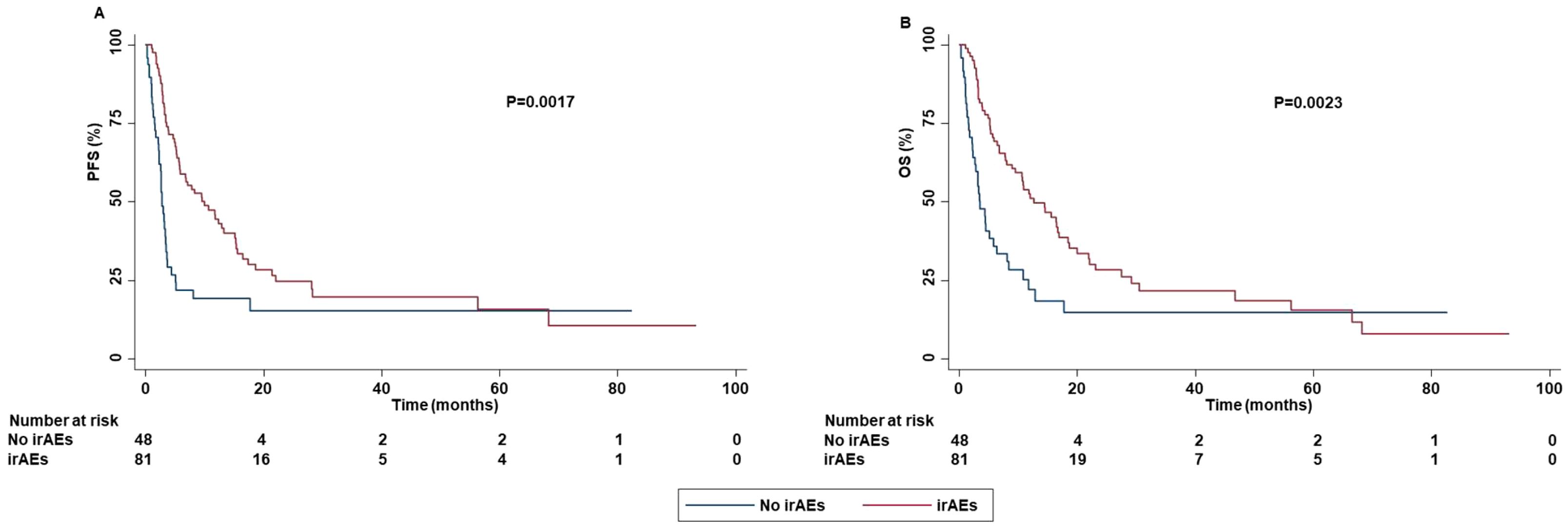
Figure 11. Association between the occurrence of irAEs and clinical outcomes in advanced non oncogene NSCLC patients treated with ICI-based immunotherapy. PFS (A) and OS (B) of advanced NSCLC patients treated with ICI-based immunotherapy were stratified based on the presence or absence of irAEs. PFS and OS were compared using the Kaplan-Meier method. Differences in patients’ survival were analyzed using a log-rang test. P <0.05 was considered statistically significant.
In addition, the occurrence of grade 1-2 irAEs was strongly correlated with an increased PFS (P=0.0046) and OS (P=0.0038) (Figure 12).
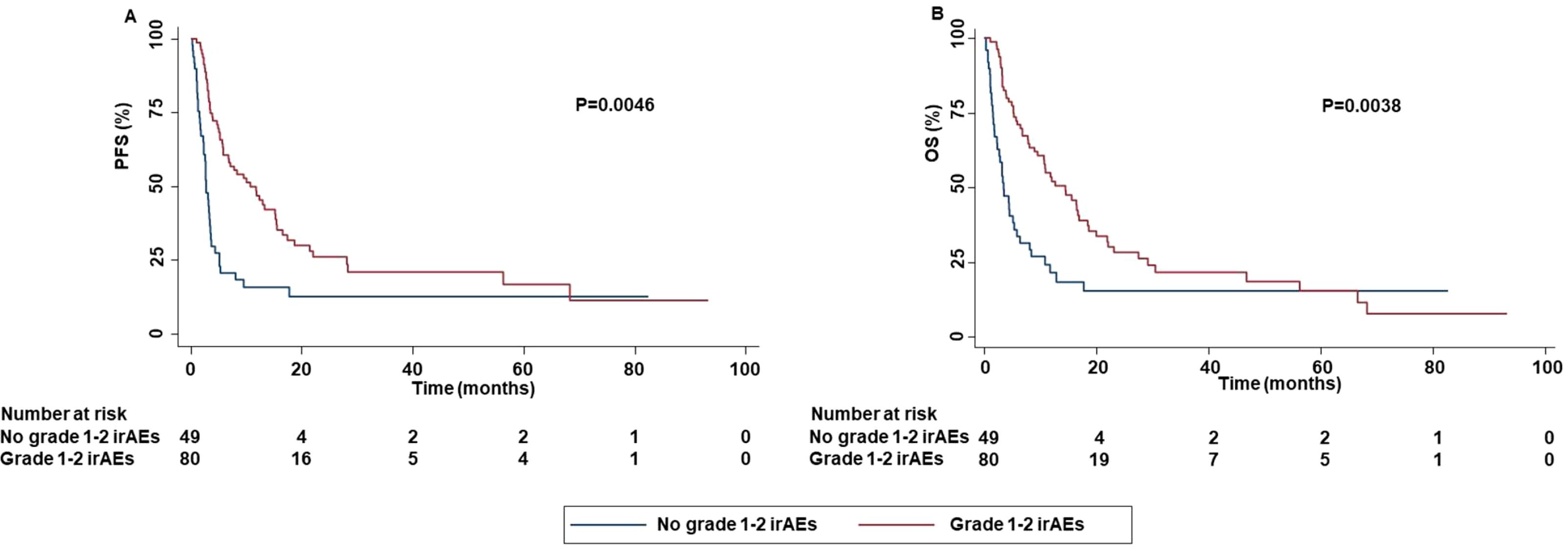
Figure 12. Association between grade 1-2 irAEs and clinical outcomes in advanced non oncogene NSCLC patients treated with ICI-based immunotherapy. PFS (A) and OS (B) of advanced NSCLC patients treated with ICI-based immunotherapy were stratified based on the occurrence of irAEs. PFS and OS were compared using the Kaplan-Meier method. Differences in patients’ survival were analyzed using a log-rang test. P <0.05 was considered statistically significant.
These results were corroborated by the significant association between the occurrence of all grade and grade 1-2 irAEs with DCR (P=0.0021 and P=0.0017). No significant association between grade 3-4 irAEs and survival (PFS (P=0.6510) or OS (0.4971) (Supplementary Figure S5) or response outcomes ((ORR (P=0.8510) and DCR (0.2230)) was found. Analysis of potential correlation between type of ICI-based immunotherapy and survival outcomes showed that patients treated with anti-PD-1-based therapy achieved an increased PFS (P=0.0248) and OS (P=0.0490) than those treated with anti-PD-L1-based therapy (Figure 13).
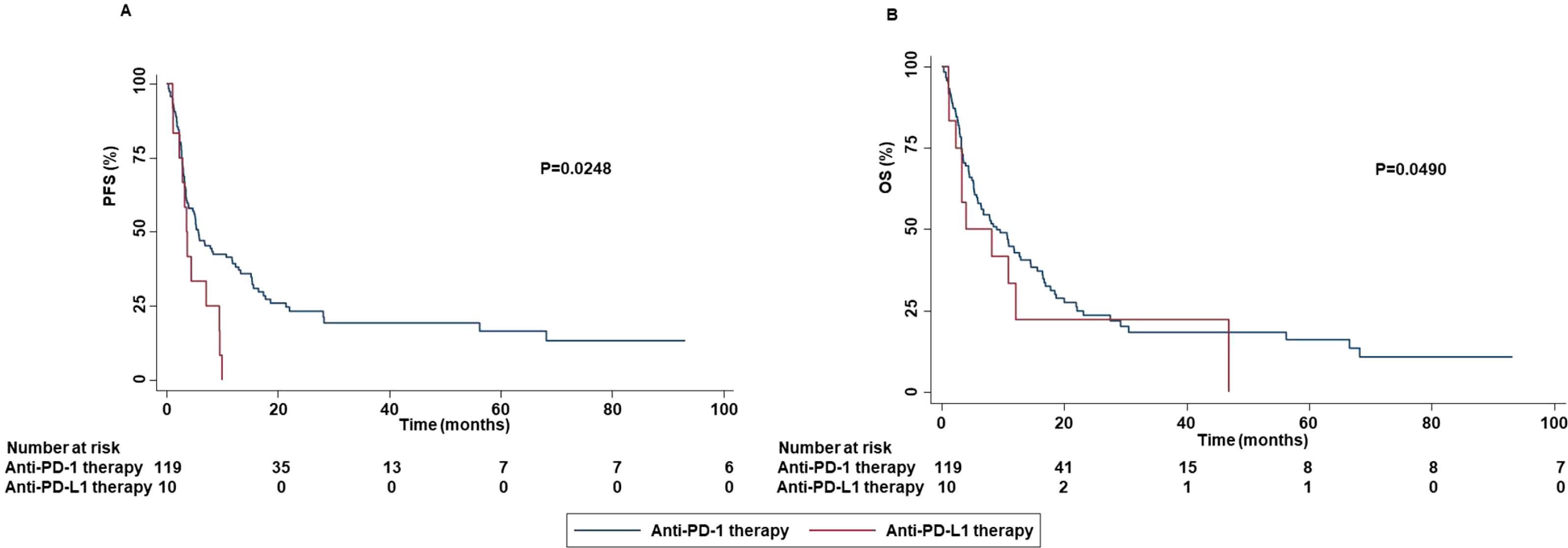
Figure 13. Association between type of ICI-based immunotherapy and clinical outcomes in advanced non oncogene NSCLC patients treated with ICI-based immunotherapy. PFS (A) and OS (B) of advanced NSCLC patients treated with ICI-based immunotherapy were stratified based on type of ICIs. Specifically, anti-PD-1-based immunotherapy versus anti-PD-L1-based immunotherapy. PFS and OS were compared using the Kaplan-Meier method. Differences in patients’ survival were analyzed using a log-rang test. P <0.05 was considered statistically significant.
Nevertheless, no significant difference in terms of DCR (P=0.1530) or ORR (P=0.2100) based on the type of ICI-based immunotherapy (anti-PD-1-based therapy vs anti-PD-L1-based therapy) was found. Validation of the results obtained in univariate analysis by a multivariate analysis demonstrated that smoking status (P=0.0020), ECOG PS (P=0.0020), presence of bone metastases (P=0.0080), PD-L1 TPS (P=0.0050) and occurrence of all grade irAEs (P=0.0010)) significantly correlated with PFS (Figure 14A) while smoking status (P=0.0010), concomitant assumption of antidepressant (P=0.0180), ECOG PS (P=0.0010), presence of skin (P=0.0350) or bone (P=0.0350) metastases, the occurrence of all grade irAEs (P=0.0010) and the specific lines of treatment (P=0.020) were significantly correlated with OS (Figure 14B). No significant difference when we included the specific lines of treatment in the multivariate analyses for PFS was found (data not shown).

Figure 14. Multivariate analysis testing the correlation between clinical-pathological characteristics and PFS (A) or OS (B) in advanced non oncogene NSCLC patients treated with ICI-based immunotherapy. Multivariate survival analyses were performed using the Cox proportional hazards model. Symbols *,**,*** indicate P value < 0.05, 0.005, 0.001.
In the past few years, the efficacy of ICI-based immunotherapy for the treatment of advanced non oncogene NSCLC was demonstrated in many clinical trials (3–11). This evidence was validated in various real-world studies (24–27). In the present work, we reported a real-world experience of 129 patients with advanced non oncogene NSCLC treated with ICI-based immunotherapy. Median PFS and OS were 5.27 months and 8.43 months, respectively. These results are in line with those from other real-world experiences which included patients treated with ICI as second or subsequent-line of treatment (24, 26). In contrast, higher median PFS and OS were reported in other studies which only included patients treated with ICI as first-line of treatment (25, 28). Here, the study population included 100 male patients (77.52%) with a median age of 68 years. These results confirm a high prevalence of advanced non oncogene NSCLC in male and older patients (1). The latter are frequently affected by multiple comorbidities requiring appropriate concomitant medications. As a result, our study population allowed us to investigate whether comorbidities and concomitant medications may impact on the survival outcomes. At least one comorbidity was reported in 94 of the patients (75.78%). The high prevalence of comorbidities is consistent with that of a real-world population of NSCLC patients with a median age of 68 years. For instance, hypertension and COPD were reported in 53.49% and 17.05% of treated patients, respectively. Various studies have already investigated the potential predictive role of comorbidities in this subgroup of patients without unique conclusions (29–32). In our study no significant association between comorbidities and survival outcomes emerged in our study (Supplementary Table S1).
Survival analysis of subpopulation of patients treated with ICI as first-line yielded a median PFS and OS of 5.87 months and 11.80 months, respectively. Noteworthy, while median PFS is in line with data provided in clinical trials and other real-world studies, median OS resulted significantly decreased (25, 28, 33–36). This discrepancy might be explained by the i) the high percentage of comorbidities in our study population; ii) presence of poor prognosis-related clinical characteristics including ECOG PS 2-3 and bone/brain metastases.
On the other hand, among patients treated with ICI as second or subsequent lines of treatment, the median PFS and OS were 3.90 months and 8.12 months, respectively. These results are in line with those reported in the main clinical trials as well as other real-world experiences (4, 5, 37–39).
Survival analyses of the two subgroups of patients treated with the combination of chemotherapy and ICI or ICI as monotherapy were also performed. In the former, at a median follow-up of 23.40 months, median PFS and OS were 8.37 months and 11.80 months, respectively. According with results from the overall study population, median PFS was in line with data of clinical trials and other real-world experiences whereas median OS was significantly decreased (6, 11, 33, 40–42). Conversely, according with our data, in the study of Verschueren et al. a median OS of about 10 months was reported (43). In the latter subgroup, at a median follow-up of 69.80 months, the median PFS and OS were 4.43 months and 8.17 months, respectively. These results are globally lower than those reported in the literature (4, 5, 21, 25, 28, 31, 33, 34, 37, 38). However, a large portion of patients we have analyzed (about 70%) received ICI as monotherapy in second or subsequent-line of treatment explaining this difference. Overall, further studies are needed to predict more accurately the survival benefit of ICI-based immunotherapy in the real-world populations of advanced non oncogene NSCLC patients.
Toxicity analysis was also performed in our study. Grade 1-2 and grade 3-4 irAEs were reported in 62.02% and 9.38% of treated patients, respectively being in line with data of the literature (3–11). Furthermore, in order to validate the quality of our study population, we demonstrated that patients with complete or partial response, achieved better survival outcomes and received an increased number of ICIs than those with stable or progressive disease. Noteworthy, we reported that age of the patients may influenced the choice of the type of treatment by the clinical oncologists. Specifically, older patients were treated more frequently with ICI as monotherapy than the combination of chemotherapy and ICI (P=0.0480). This result may be explained by the worse toxicity-profile of the combination than monotherapy (6, 11, 21, 44). However, the sample size of our study population was not enough to demonstrate this association (effect size: 0.0473; power: 0.0832). Larger studies are needed to validate this hypothesis. On the same line, our older patients were treated more frequently with anti-PD-L1 therapy as compared to anti-PD-1 therapy. This difference reflected the general idea of the better toxicity-profile of anti-PD-L1 therapy than anti-PD-1 therapy. This issue was investigated by many studies with contrasting results (45–48). Some of them have shown an higher rate of irAEs for anti-PD-1 therapy while no difference was found in others (45–48). In our work, no difference in terms of toxicity was found between patients treated with anti-PD-1 therapy than those treated with anti-PD-L1 therapy.
Although ICI-based immunotherapy represents the cornerstone of the treatment of patients with advanced non-oncogene NSCLC, almost half of treated patients did not obtain any clinical benefit from this novel immunotherapeutic approach (3–11). Our study confirmed this crucial issue. Indeed, about 40% of treated patients achieved a radiologic progression of disease as best response. Then, predictive biomarkers of tumor response are urgently needed to improve the therapeutic algorithms of these patients. We found that specific clinical-pathological characteristics including ECOG PS, smoking status, concomitant administration of antidepressants or opioids, bone metastases, skin metastases, PD-L1 TPS, and the occurrence of irAEs influenced the survival outcomes.
According to our findings, the predictive role of specific clinical-pathological characteristics was already investigated with various results (20, 26). Robust data have already demonstrated that patients with ECOG PS ≥ 2 presented worse survival outcomes than those with ECOG PS < 2 (20, 49, 50). The high tumor load and/or the presence of multiple concomitant comorbidities may explain this difference. In our study no correlation between tumor load or concomitant comorbidities and ECOG PS was demonstrated. However, the presence of comorbidities was not significantly associated with survival outcomes.
The predictive role of smoking status was also investigated with contrasting results. For instance, Wang et al. demonstrated that increased smoking exposure was significantly correlated with improved clinical benefit, regardless PD-L1 status (51). Conversely, Chen et al, in the conclusions of their meta-analysis suggested that smoking status should not be recognized as predictive of ICI-based immunotherapy in patients with advanced NSCLC (52). Beyond clinical results, smoking is well-known to be associated with increased TMB (53) which is in turn correlated with higher likelihood to achieve a clinical benefit from ICIs (54). As a result, it seems reasonable to think that smoking status may influence tumor response. In our study, we did not investigate the role of TMB and its potential correlation with smoking status and/or tumor response. However, the smoking status significantly correlated with PFS and OS. Indeed, never smokers displayed increased PFS and OS than current and previous smokers. In addition, patients who discontinued smoking, achieved decreased PFS than current smokers (this effect is not influenced by age (p=0.4460) and comorbidities of the patients (Supplementary Table S2). These results were also confirmed by multivariate analyses. However, further studies are needed to clarify the predictive role of smoking status in advanced non oncogene addicted NSCLC patients treated with ICIs.
The potential predictive role of concomitant medications was also investigated. Sieber et al. have shown no significant correlation between concomitant medications such as metformin, antihypertensive and low dose of aspirin and survival outcomes (55). In contrast, concomitant assumption of antibiotics, proton pump inhibitors (PPIs), anticoagulants and opioids was associated with worse survival outcomes (56). In our study, at the univariate analyses, concomitant administration of antidepressants was correlated with increased PFS and OS whereas opioids with decreased PFS and OS. Other studies have already reported the negative predictive role of concomitant assumption of opioids (56–58). However, whether this effect is directly mediated by the opioids or reflects the presence of other negative clinical factors such as the presence of bone metastases or poor ECOG PS should be clarified. Some evidence suggested the negative predictive role of concomitant administration of opioids is caused by the modifications of the gut microbiome induced by their chronic assumption (59, 60). In our population, the concomitant assumption of opioids was significantly associated with the presence of bone metastases (P=0.023) and/or poor ECOG PS (P=0.000). In addition, at the multivariate analyses, no statistically significant association between concomitant administration of opioids and survival outcomes were found. As a result, our findings suggested that the predictive significance of opioids should be further investigated.
On the other hand, few studies have investigated the potential impact of the concomitant administration of antidepressants in advanced non oncogene NSCLC patients treated with ICIs. Preclinical studies have suggested that monoamine oxidase A (MAO-A) inhibitors and SSRIs may influence the tumor microenvironment exerting a synergistic cytotoxic effect with anti-PD-1 therapy on cancer cells (61–63). However, in our study, while the multivariate analysis confirmed the statistically significant correlation between the concomitant administration of antidepressants and OS, no correlation with PFS was found. This discrepancy may be the results of the different subsequent therapies and their potential synergistic effects with the concomitant administration of antidepressants. Further studies should clarify the predictive role of antidepressants in these patients as well as the underlying biological mechanisms. The latter should open the door to develop novel synergistic therapeutic strategies.
Beyond concomitant medications, specific sites of metastasis may also influence the efficacy of ICI-based immunotherapy in this patient population (64–66). In multivariate analyses, the presence of bone metastases was significantly correlated with worse PFS and OS. In line with other studies (67–69), our results confirmed the independent negative predictive role of the bone metastases in patients with advanced non oncogene NSCLC. The “cold” tumor microenvironment of NSCLC patients with bone metastases may explain their negative predictive role (67). A deeper knowledge of the characteristics of tumor microenvironment is crucial to build potential strategies to improve the survival of these patients.
Conversely, in the multivariate analysis, the presence of skin metastases was significantly correlated with better OS. The presence of skin metastases was also numerically correlated with better PFS without reaching the statistically significance at multivariate analysis (P=0.063). To the best of our knowledge, no other study has demonstrated this type of association. The latter was independent by the presence/absence of other sites of metastases (Supplementary Table S3). In addition, all six patients with skin metastases presented at least another site of metastasis. However, in our study population, the presence of skin metastases was reported in only six patients. As a result, larger studies are needed to confirm this type of association.
As mentioned above, among several predictive biomarkers, PD-L1 TPS is the most widely investigated (13, 17–19). To date, many issues about the utilize of PD-L1 TPS as predictive biomarker remain unsolved and the evidence available has provided very heterogenous results (13, 17–19). In our study, patients with PD-L1 TPS < 50% had a shorter PFS than those with PD-L1 TPS ≥ 50% (P=0.0430). This result was confirmed by multivariate analysis, although no significant correlation between PD-L1 TPS and OS was found. This discrepancy has clinical relevance confirming the scarce predictive value of PD-L1 TPS.
In line with other studies (70–74), the occurrence of all grade irAEs was significantly correlated with increased PFS and OS. We have to note that the presence of immortal-time bias in our study population might limit the validity of this result (75). On the other hand, the statistical significance at the multivariate analysis as well as the correlations between the occurrence of irAEs and both ORR and DCR, corroborated this finding. In contrast, other studies have reported no significant association between the occurrence of irAEs and survival outcomes (16, 76). As a result, this correlation, and the underlying biological mechanisms should be further investigated. In addition, it remains also unknown how clinical oncologists may utilize this correlation to improve the therapeutic algorithm of these patients.
Lastly, we explored potential correlations between clinical-pathological characteristics and irAEs. Noteworthy, in patients who assumed antiplatelet drugs or statin we reported a higher rate of grade 3-4 irAEs. Moreover, in those assuming low dose of aspirin a higher rate of all grade irAEs was reported. This correlation has clinical relevance since a high percentage of patients with advanced NSCLC assumed these drugs. Various studies have already investigated whether concomitant medications influenced the occurrence of irAEs with contrasting results. For instance, Yang et al, demonstrated the correlation between the use of aspirin and the occurrence of irAEs in a pan-cancer study population (77). In contrast, Kostine et al, showed that use of aspirin, antibiotics, glucocorticoids, PPIs, opioid, non-steroidal anti-inflammatory drugs (NSAIDs), and psychotropic drugs was associated with decreased occurrence of irAEs (78). Further studies are needed to clarify this difference as well as the potential implications in the clinical management of this patient population.
In our study, patients treated with ICIs as first-line reported a lower rate of all grade and grade 1-2 irAEs as compared to those treated with ICIs as second or subsequent lines. This correlation suggests that previous treatments may influence the predisposition to irAEs. To the best of our knowledge, no study demonstrated the biological mechanisms underlying this type of association. However, we have to highlight that all patients with advanced non oncogene NSCLC received ICI in the first-line setting as monotherapy or in combination with chemotherapy, so far. As a result, although of biological interest, this finding has no high clinical relevance.
In this study we reported our real-world experience of ICIs for the treatment of patients with advanced non oncogene NSCLC. Decreased clinical benefit in terms of overall survival as compared to that of patients included in the clinical trials was reported in our study population. However, in the specific subgroup of patients treated with ICI as monotherapy in second or subsequent-line setting, our results are in line with clinical trials as well as other real-world experiences. Noteworthy, some correlations between clinical-pathological characteristics and survival outcomes emerged. However, we have to highlight that these correlations emerged from a retrospective analysis and should be read for generating hypotheses. The potential integration of clinical-pathological characteristics in more accurate predictive algorithms as well as the underlying biological mechanisms should be further validated in ad hoc prospective studies.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by ASL Napoli 3 sud Servizio Coordinamento Etico Campania Sud. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
LL: Writing – original draft, Data curation, Formal analysis, Investigation, Methodology. GG: Writing – review & editing, Data curation. GP: Writing – review & editing. VP: Writing – review & editing. DM: Writing – review & editing. FP: Writing – review & editing. MC: Writing – review & editing. AO: Writing – review & editing. VC: Writing – review & editing. AS: Writing – review & editing. RB: Writing – review & editing, Visualization. SP: Writing – review & editing, Supervision, Validation. FS: Conceptualization. Writing – original draft, Writing – review & editing, Project administration, Methodology.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors wish to gratefully acknowledge the patients for allowing us to publish this work.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1419544/full#supplementary-material
1. Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. (2024) 74:12–49. doi: 10.3322/caac.21820
2. Punekar SR, Shum E, Grello CM, Lau SC, Velcheti V. Immunotherapy in non-small cell lung cancer: Past, present, and future directions. Front Oncol. (2022) 12:877594. doi: 10.3389/fonc.2022.877594
3. Borghaei H, Gettinger S, Vokes EE, Chow LQM, Burgio MA, de Castro Carpeno J, et al. Five-year outcomes from the randomized, phase III trials checkMate 017 and 057: nivolumab versus docetaxel in previously treated non-small-cell lung cancer. J Clin Oncol. (2021) 39:723–33. doi: 10.1200/JCO.20.01605
4. Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. (2015) 373:1627–39. doi: 10.1056/NEJMoa1507643
5. Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WEE, Poddubskaya E, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. (2015) 373:123–35. doi: 10.1056/NEJMoa1504627
6. Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. (2018) 378:2078–92. doi: 10.1056/NEJMoa1801005
7. Garassino MC, Gadgeel S, Speranza G, Felip E, Esteban E, Dómine M, et al. Pembrolizumab plus pemetrexed and platinum in nonsquamous non-small-cell lung cancer: 5-year outcomes from the phase 3 KEYNOTE-189 study. J Clin Oncol. (2023) 41:1992–8. doi: 10.1200/JCO.22.01989
8. Novello S, Kowalski DM, Luft A, Gümüş M, Vicente D, Mazières J, et al. Pembrolizumab plus chemotherapy in squamous non-small-cell lung cancer: 5-year update of the phase III KEYNOTE-407 study. J Clin Oncol. (2023) 41:1999–2006. doi: 10.1200/JCO.22.01990
9. Paz-Ares L, Ciuleanu T-E, Cobo M, Schenker M, Zurawski B, Menezes J, et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): an international, randomised, open-label, phase 3 trial. Lancet Oncol. (2021) 22:198–211. doi: 10.1016/S1470-2045(20)30641-0
10. Paz-Ares L, Ciuleanu T-E, Cobo M, Schenker M, Zurawski B, Menezes J, et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): an international, randomised, open-label, phase 3 trial. Lancet Oncol. (2021) 22:198–211. doi: 10.1016/S1470-2045(20)30641-0
11. Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gümüş M, Mazières J, et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med. (2018) 379:2040–51. doi: 10.1056/NEJMoa1810865
12. Gibney GT, Weiner LM, Atkins MB. Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol. (2016) 17:e542–51. doi: 10.1016/S1470-2045(16)30406-5
13. Sholl LM. Biomarkers of response to checkpoint inhibitors beyond PD-L1 in lung cancer. Mod Pathol. (2022) 35:66–74. doi: 10.1038/s41379-021-00932-5
14. Sabbatino F, Liguori L, Polcaro G, Salvato I, Caramori G, Salzano FA, et al. Role of human leukocyte antigen system as A predictive biomarker for checkpoint-based immunotherapy in cancer patients. Int J Mol Sci. (2020) 21:7295. doi: 10.3390/ijms21197295
15. Bodor JN, Boumber Y, Borghaei H. Biomarkers for immune checkpoint inhibition in non-small cell lung cancer (NSCLC). Cancer. (2020) 126:260–70. doi: 10.1002/cncr.32468
16. Polcaro G, Liguori L, Manzo V, Chianese A, Donadio G, Caputo A, et al. rs822336 binding to C/EBPβ and NFIC modulates induction of PD-L1 expression and predicts anti-PD-1/PD-L1 therapy in advanced NSCLC. Mol Cancer. (2024) 23:63. doi: 10.1186/s12943-024-01976-2
17. Patel SP, Kurzrock R. PD-L1 expression as a predictive biomarker in cancer immunotherapy. Mol Cancer Ther. (2015) 14:847–56. doi: 10.1158/1535-7163.MCT-14-0983
18. Uruga H, Mino-Kenudson M. Predictive biomarkers for response to immune checkpoint inhibitors in lung cancer: PD-L1 and beyond. Virchows Arch. (2021) 478:31–44. doi: 10.1007/s00428-021-03030-8
19. O’Brien M, Paz-Ares L, Marreaud S, Dafni U, Oselin K, Havel L, et al. Pembrolizumab versus placebo as adjuvant therapy for completely resected stage IB-IIIA non-small-cell lung cancer (PEARLS/KEYNOTE-091): an interim analysis of a randomised, triple-blind, phase 3 trial. Lancet Oncol. (2022) 23:1274–86. doi: 10.1016/S1470-2045(22)00518-6
20. Brueckl WM, Ficker JH, Zeitler G. Clinically relevant prognostic and predictive markers for immune-checkpoint-inhibitor (ICI) therapy in non-small cell lung cancer (NSCLC). BMC Cancer. (2020) 20:1185. doi: 10.1186/s12885-020-07690-8
21. Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. (2016) 375:1823–33. doi: 10.1056/NEJMoa1606774
22. Common Terminology Criteria for Adverse Events (CTCAE) | Protocol Development | CTEP. Available online at: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm (Accessed February 18, 2024).
23. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. (2009) 45:228–47. doi: 10.1016/j.ejca.2008.10.026
24. Manrique MCA, Martínez JM, González JG, Afonso FJA, Quintela ML, Núñez NF, et al. Real world data of nivolumab for previously treated non-small cell lung cancer patients: a Galician lung cancer group clinical experience. Trans Lung Cancer Res. (2018) 7:404–15. doi: 10.21037/tlcr.2018.04.03
25. Miao K, Zhang X, Wang H, Si X, Ni J, Zhong W, et al. Real-world data of different immune checkpoint inhibitors for non-small cell lung cancer in China. Front Oncol. (2022) 12:859938. doi: 10.3389/fonc.2022.859938
26. Youn B, Trikalinos NA, Mor V, Wilson IB, Dahabreh IJ. Real-world use and survival outcomes of immune checkpoint inhibitors in older adults with non–small cell lung cancer. Cancer. (2020) 126:978–85. doi: 10.1002/cncr.32624
27. Khozin S, Miksad RA, Adami J, Boyd M, Brown NR, Gossai A, et al. Real-world progression, treatment, and survival outcomes during rapid adoption of immunotherapy for advanced non–small cell lung cancer. Cancer. (2019) 125:4019–32. doi: 10.1002/cncr.32383
28. Velcheti V, Hu X. Long-term real-world outcomes of first-line pembrolizumab monotherapy for metastatic non-small cell lung cancer with ≥50% Expression of programmed cell death-ligand 1. Front Oncol. (2022) 12:834761. doi: 10.3389/fonc.2022.834761
29. Zhang P, Ma M, Nie J, Dai L, Hu W, Zhang J, et al. Real-world data on the first-line immune checkpoint inhibitors or in combination with chemotherapy in older patients (aged ≥ 75 years) with advanced non-small cell lung cancer. Heliyon. (2024) 10:e26026. doi: 10.1016/j.heliyon.2024.e26026
30. Muchnik E, Loh KP, Strawderman M, Magnuson A, Mohile SG, Estrah V, et al. Immune checkpoint inhibitors in real-world treatment of older adults with non-small cell lung cancer. J Am Geriatr Soc. (2019) 67:905–12. doi: 10.1111/jgs.15750
31. Mouritzen MT, Junker KF, Carus A, Ladekarl M, Meldgaard P, Nielsen AWM, et al. Clinical features affecting efficacy of immune checkpoint inhibitors in pretreated patients with advanced NSCLC: a Danish nationwide real-world study. Acta Oncol. (2022) 61:409–16. doi: 10.1080/0284186X.2021.2023213
32. Facchinetti F, Mazzaschi G, Barbieri F, Passiglia F, Mazzoni F, Berardi R, et al. First-line pembrolizumab in advanced non-small cell lung cancer patients with poor performance status. Eur J Cancer. (2020) 130:155–67. doi: 10.1016/j.ejca.2020.02.023
33. Gadgeel S, Rodríguez-Abreu D, Speranza G, Esteban E, Felip E, Dómine M, et al. Updated analysis from KEYNOTE-189: pembrolizumab or placebo plus pemetrexed and platinum for previously untreated metastatic nonsquamous non–small-cell lung cancer. JCO. (2020) 38:1505–17. doi: 10.1200/JCO.19.03136
34. Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Five-year outcomes with pembrolizumab versus chemotherapy for metastatic non-small-cell lung cancer with PD-L1 tumor proportion score ≥ 50. J Clin Oncol. (2021) 39:2339–49. doi: 10.1200/JCO.21.00174
35. Chen H, Yumoto K, Kashizaki F, Koizumi H, Ikeda I, Horita N, et al. KEYNOTE-407: an effective and safe first-line treatment option for metastatic squamous non-small cell lung cancer. Transl Lung Cancer Res. (2023) 12:1830–3. doi: 10.21037/tlcr-23-271
36. Reck M, Ciuleanu T-E, Cobo M, Schenker M, Zurawski B, Menezes J, et al. First-line nivolumab plus ipilimumab with two cycles of chemotherapy versus chemotherapy alone (four cycles) in advanced non-small-cell lung cancer: CheckMate 9LA 2-year update. ESMO Open. (2021) 6:100273. doi: 10.1016/j.esmoop.2021.100273
37. Herbst RS, Baas P, Kim D-W, Felip E, Pérez-Gracia JL, Han J-Y, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. (2016) 387:1540–50. doi: 10.1016/S0140-6736(15)01281-7
38. Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. (2017) 389:255–65. doi: 10.1016/S0140-6736(16)32517-X
39. Pasello G, Pavan A, Attili I, Bortolami A, Bonanno L, Menis J, et al. Real world data in the era of Immune Checkpoint Inhibitors (ICIs): Increasing evidence and future applications in lung cancer. Cancer Treat Rev. (2020) 87:102031. doi: 10.1016/j.ctrv.2020.102031
40. Paz-Ares L, Vicente D, Tafreshi A, Robinson A, Parra HS, Mazières J, et al. A randomized, placebo-controlled trial of pembrolizumab plus chemotherapy in patients with metastatic squamous NSCLC: protocol-specified final analysis of KEYNOTE-407. J Thorac Oncol. (2020) 15:1657–69. doi: 10.1016/j.jtho.2020.06.015
41. Pelicon V, Cufer T, Knez L. Real-world outcomes of immunotherapy with or without chemotherapy in first-line treatment of advanced non-small cell lung cancer. Front Oncol. (2023) 13:1182748. doi: 10.3389/fonc.2023.1182748
42. Izano MA, Sweetnam C, Zhang C, Weese JL, Reding D, Treisman J, et al. Brief report on use of pembrolizumab with or without chemotherapy for advanced lung cancer: A real-world analysis. Clin Lung Cancer. (2023) 24:362–5. doi: 10.1016/j.cllc.2023.01.011
43. Verschueren MV, Peters BJM, Bloem LT, Kruik VR, Uitvlugt EB, Bijsmans AR, et al. Pembrolizumab plus chemotherapy per PD-L1 stratum in patients with metastatic non–small cell lung cancer: real-world effectiveness versus trial efficacy. Clin Lung Cancer. (2024) 25:119–127.e1. doi: 10.1016/j.cllc.2023.12.011
44. Li L, Xu F, Chen Y, Ren X, Liu Y, Chen Y, et al. Indirect comparison between immunotherapy alone and immunotherapy plus chemotherapy as first-line treatment for advanced non-small cell lung cancer: a systematic review. BMJ Open. (2020) 10. doi: 10.1136/bmjopen-2019-034010
45. Pillai RN, Behera M, Owonikoko TK, Kamphorst AO, Pakkala S, Belani CP, et al. Comparison of the toxicity profile of PD-1 versus PD-L1 inhibitors in non-small cell lung cancer: A systematic analysis of the literature. Cancer. (2018) 124:271–7. doi: 10.1002/cncr.31043
46. Li B, Jiang C, Pang L, Zou B, Ding M, Sun X, et al. Toxicity profile of combining PD-1/PD-L1 inhibitors and thoracic radiotherapy in non-small cell lung cancer: A systematic review. Front Immunol. (2021) 12:627197. doi: 10.3389/fimmu.2021.627197
47. Banna GL, Cantale O, Bersanelli M, Re MD, Friedlaender A, Cortellini A, et al. Are anti-PD1 and anti-PD-L1 alike? The non-small-cell lung cancer paradigm. Oncol Rev. (2020) 14:135–43. doi: 10.4081/oncol.2020.490
48. Brito ABC, Camandaroba MPG, de Lima VCC. Anti-PD1 versus anti-PD-L1 immunotherapy in first-line therapy for advanced non-small cell lung cancer: A systematic review and meta-analysis. Thorac Cancer. (2021) 12:1058–66. doi: 10.1111/1759-7714.13867
49. Kawachi H, Tamiya M, Tamiya A, Ishii S, Hirano K, Matsumoto H, et al. Association between metastatic sites and first-line pembrolizumab treatment outcome for advanced non-small cell lung cancer with high PD-L1 expression: a retrospective multicenter cohort study. Invest New Drugs. (2020) 38:211–8. doi: 10.1007/s10637-019-00882-5
50. Dall’Olio FG, Maggio I, Massucci M, Mollica V, Fragomeno B, Ardizzoni A. ECOG performance status ≥2 as a prognostic factor in patients with advanced non small cell lung cancer treated with immune checkpoint inhibitors-A systematic review and meta-analysis of real world data. Lung Cancer. (2020) 145:95–104. doi: 10.1016/j.lungcan.2020.04.027
51. Wang X, Ricciuti B, Alessi JV, Nguyen T, Awad MM, Lin X, et al. Smoking history as a potential predictor of immune checkpoint inhibitor efficacy in metastatic non-small cell lung cancer. J Natl Cancer Inst. (2021) 113:1761–9. doi: 10.1093/jnci/djab116
52. Chen D-L, Li Q-Y, Tan Q-Y. Smoking history and the efficacy of immune checkpoint inhibitors in patients with advanced non-small cell lung cancer: a systematic review and meta-analysis. J Thorac Dis. (2021) 13:220. doi: 10.21037/jtd-20-1953
53. Alexandrov LB, Ju YS, Haase K, Van Loo P, Martincorena I, Nik-Zainal S, et al. Mutational signatures associated with tobacco smoking in human cancer. Science. (2016) 354:618–22. doi: 10.1126/science.aag0299
54. Ricciuti B, Wang X, Alessi JV, Rizvi H, Mahadevan NR, Li YY, et al. Association of high tumor mutation burden in non-small cell lung cancers with increased immune infiltration and improved clinical outcomes of PD-L1 blockade across PD-L1 expression levels. JAMA Oncol. (2022) 8:1160–8. doi: 10.1001/jamaoncol.2022.1981
55. Sieber B, Strauss J, Li Z, Gatti-Mays ME. Concomitant medication effects on immune checkpoint inhibitor efficacy and toxicity. Front Oncol. (2022) 12:836934. doi: 10.3389/fonc.2022.836934
56. Cortellini A, Tucci M, Adamo V, Stucci LS, Russo A, Tanda ET, et al. Integrated analysis of concomitant medications and oncological outcomes from PD-1/PD-L1 checkpoint inhibitors in clinical practice. J Immunother Cancer. (2020) 8:e001361. doi: 10.1136/jitc-2020-001361
57. Guo H, Li Y, Lin J, Li D, Yang J, Wang J, et al. A novel investigation into the negative impact of opioid use on the efficacy of immune checkpoint inhibitors in advanced non-small cell lung cancer patients. Int Immunopharmacol. (2024) 129:111611. doi: 10.1016/j.intimp.2024.111611
58. Yu X, Zhao L, Song B. Impact of opioid analgesics on the efficacy of immune checkpoint inhibitors in a lung cancer population. BMC Pulm Med. (2022) 22:431. doi: 10.1186/s12890-022-02210-9
59. Ren M, Lotfipour S. The role of the gut microbiome in opioid use. Behav Pharmacol. (2020) 31:113–21. doi: 10.1097/FBP.0000000000000538
60. Banerjee S, Sindberg G, Wang F, Meng J, Sharma U, Zhang L, et al. Opioid-induced gut microbial disruption and bile dysregulation leads to gut barrier compromise and sustained systemic inflammation. Mucosal Immunol. (2016) 9:1418–28. doi: 10.1038/mi.2016.9
61. Schneider MA, Heeb L, Beffinger MM, Pantelyushin S, Linecker M, Roth L, et al. Attenuation of peripheral serotonin inhibits tumor growth and enhances immune checkpoint blockade therapy in murine tumor models. Sci Transl Med. (2021) 13:eabc8188. doi: 10.1126/scitranslmed.abc8188
62. Wang X, Li B, Kim YJ, Wang Y-C, Li Z, Yu J, et al. Targeting monoamine oxidase A for T cell-based cancer immunotherapy. Sci Immunol. (2021) 6:eabh2383. doi: 10.1126/sciimmunol.abh2383
63. Yang Z, Li Z, Guo Z, Ren Y, Zhou T, Xiao Z, et al. Antitumor effect of fluoxetine on chronic stress-promoted lung cancer growth via suppressing kynurenine pathway and enhancing cellular immunity. Front Pharmacol. (2021) 12:685898. doi: 10.3389/fphar.2021.685898
64. Qiao M, Zhou F, Hou L, Li X, Zhao C, Jiang T, et al. Efficacy of immune-checkpoint inhibitors in advanced non-small cell lung cancer patients with different metastases. Ann Transl Med. (2021) 9:34. doi: 10.21037/atm-20-1471
65. Hendriks LEL, Henon C, Auclin E, Mezquita L, Ferrara R, Audigier-Valette C, et al. Outcome of patients with non-small cell lung cancer and brain metastases treated with checkpoint inhibitors. J Thorac Oncol. (2019) 14:1244–54. doi: 10.1016/j.jtho.2019.02.009
66. Shiroyama T, Suzuki H, Tamiya M, Tamiya A, Tanaka A, Okamoto N, et al. Clinical characteristics of liver metastasis in nivolumab-treated patients with non-small cell lung cancer. Anticancer Res. (2018) 38:4723–9. doi: 10.21873/anticanres.12779
67. Del Conte A, De Carlo E, Bertoli E, Stanzione B, Revelant A, Bertola M, et al. Bone metastasis and immune checkpoint inhibitors in non-small cell lung cancer (NSCLC): microenvironment and possible clinical implications. Int J Mol Sci. (2022) 23:6832. doi: 10.3390/ijms23126832
68. Zhu Y-J, Chang X-S, Zhou R, Chen Y-D, Ma H-C, Xiao Z-Z, et al. Bone metastasis attenuates efficacy of immune checkpoint inhibitors and displays “cold” immune characteristics in Non-small cell lung cancer. Lung Cancer. (2022) 166:189–96. doi: 10.1016/j.lungcan.2022.03.006
69. Landi L, D’Incà F, Gelibter A, Chiari R, Grossi F, Delmonte A, et al. Bone metastases and immunotherapy in patients with advanced non-small-cell lung cancer. J Immunother Cancer. (2019) 7:316. doi: 10.1186/s40425-019-0793-8
70. Haratani K, Hayashi H, Chiba Y, Kudo K, Yonesaka K, Kato R, et al. Association of immune-related adverse events with nivolumab efficacy in non-small-cell lung cancer. JAMA Oncol. (2018) 4:374–8. doi: 10.1001/jamaoncol.2017.2925
71. Cortellini A, Chiari R, Ricciuti B, Metro G, Perrone F, Tiseo M, et al. Correlations between the immune-related adverse events spectrum and efficacy of anti-PD1 immunotherapy in NSCLC patients. Clin Lung Cancer. (2019) 20:237–247.e1. doi: 10.1016/j.cllc.2019.02.006
72. Ricciuti B, Genova C, De Giglio A, Bassanelli M, Dal Bello MG, Metro G, et al. Impact of immune-related adverse events on survival in patients with advanced non-small cell lung cancer treated with nivolumab: long-term outcomes from a multi-institutional analysis. J Cancer Res Clin Oncol. (2019) 145:479–85. doi: 10.1007/s00432-018-2805-3
73. Grangeon M, Tomasini P, Chaleat S, Jeanson A, Souquet-Bressand M, Khobta N, et al. Association between immune-related adverse events and efficacy of immune checkpoint inhibitors in non-small-cell lung cancer. Clin Lung Cancer. (2019) 20:201–7. doi: 10.1016/j.cllc.2018.10.002
74. Teraoka S, Fujimoto D, Morimoto T, Kawachi H, Ito M, Sato Y, et al. Early immune-related adverse events and association with outcome in advanced non-small cell lung cancer patients treated with nivolumab: A prospective cohort study. J Thorac Oncol. (2017) 12:1798–805. doi: 10.1016/j.jtho.2017.08.022
75. Kfoury M, Najean M, Lappara A, Voisin A-L, Champiat S, Michot J-M, et al. Analysis of the association between prospectively collected immune-related adverse events and survival in patients with solid tumor treated with immune-checkpoint blockers, taking into account immortal-time bias. Cancer Treat Rev. (2022) 110:102452. doi: 10.1016/j.ctrv.2022.102452
76. Owen DH, Wei L, Bertino EM, Edd T, Villalona-Calero MA, He K, et al. Incidence, risk factors, and effect on survival of immune-related adverse events in patients with non-small-cell lung cancer. Clin Lung Cancer. (2018) 19:e893–900. doi: 10.1016/j.cllc.2018.08.008
77. Yang H, Liu Z, Li R, Huang R, Peng X. The association between aspirin use and immune-related adverse events in specific cancer patients receiving ICIs therapy: analysis of the FAERS database. Front Pharmacol. (2023) 14:1259628. doi: 10.3389/fphar.2023.1259628
Keywords: biomarker, ICI, immunotherapy, NSCLC, PD-1, PD-L1, predictive, real-world
Citation: Liguori L, Giorgio G, Polcaro G, Pagliara V, Malandrino D, Perri F, Cascella M, Ottaiano A, Conti V, Servetto A, Bianco R, Pepe S and Sabbatino F (2024) Checkpoint based immunotherapy in non-small cell lung cancer: a real-world retrospective study. Front. Immunol. 15:1419544. doi: 10.3389/fimmu.2024.1419544
Received: 18 April 2024; Accepted: 06 November 2024;
Published: 27 November 2024.
Edited by:
Sumit Kumar Hira, University of Burdwan, IndiaReviewed by:
Silvia Guglietta, Medical University of South Carolina, United StatesCopyright © 2024 Liguori, Giorgio, Polcaro, Pagliara, Malandrino, Perri, Cascella, Ottaiano, Conti, Servetto, Bianco, Pepe and Sabbatino. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Francesco Sabbatino, ZnNhYmJhdGlub0B1bmlzYS5pdA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.