
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Immunol. , 24 June 2024
Sec. Molecular Innate Immunity
Volume 15 - 2024 | https://doi.org/10.3389/fimmu.2024.1419540
This article is part of the Research Topic Community Series in the Role of Complement in Health and Disease: Volume II View all 16 articles
 Andrea Balduit1
Andrea Balduit1 Chiara Agostinis1*
Chiara Agostinis1* Alessandro Mangogna1
Alessandro Mangogna1 Gabriella Zito1
Gabriella Zito1 Tamara Stampalija1,2
Tamara Stampalija1,2 Giuseppe Ricci1,2
Giuseppe Ricci1,2 Roberta Bulla3
Roberta Bulla3The complement system (C) is a crucial component of the innate immune system. An increasing body of research has progressively shed light on the pivotal role of C in immunological tolerance at the feto-maternal interface. Excessive C activation or impaired C regulation may determine the onset of pregnancy-related pathological conditions, including pre-eclampsia (PE). Thus, several studies have investigated the presence of C components or split products in blood matrixes (i.e., plasma, serum), urine, and amniotic fluid in PE. In the current study, we systematically reviewed the currently available scientific literature reporting measurements of C components as circulating biomarkers in PE, based on a literature search using Pubmed, Scopus, and Embase databases. A total of 41 out of 456 studies were selected after full-text analysis. Fourteen studies (34.1%) were identified as measuring the blood concentrations of the classical pathway, 5 (12.1%) for the lectin pathway, 28 (68.3%) for the alternative pathway, 17 (41.5%) for the terminal pathway components, and 16 (39%) for C regulators. Retrieved results consistently reported C4, C3, and factor H reduction, and increased circulating levels of C4d, Bb, factor D, C3a, C5a, and C5b-9 in PE compared to normal pregnancies, depicting an overall scenario of excessive C activation and aberrant C regulation. With evidence of C activation and dysregulation, C-targeted therapy is an intriguing perspective in PE management. Moreover, we also discussed emerging pitfalls in C analysis, mainly due to a lack of experimental uniformity and biased cohort selection among different studies and laboratories, aiming to raise a more comprehensive awareness for future standardization.
Systematic review registration: https://www.crd.york.ac.uk/prospero/, identifier CRD42024503070.
Pre-eclampsia (PE) is a pregnancy-related disorder diagnosed by onset of hypertension (systolic blood pressure ≥ 140 mmHg and/or diastolic blood pressure ≥ 90 mmHg) after 20 weeks of gestation, associated with proteinuria (≥ 300 mg in 24 hours) and/or presence of kidney or liver dysfunction, neurological complications, hemolysis, or thrombocytopenia, and/or fetal underdevelopment. It is accounted for significant maternal and neonatal morbidity and mortality (1, 2). Based on gestational age at clinical presentation, PE is usually classified into preterm (< 37 weeks of gestation), term (≥ 37 weeks of gestation), and post-partum (from 48 hours to 6 days after delivery) (3, 4). According to onset, PE has been defined as early-onset PE (EOPE; < 34 weeks) and late-onset PE (LOPE; > 34 weeks) (5). It is now widely accepted that these two entities may differ in etiology and should be accounted for as different phenotypes of the disease (6, 7). Conversely, PE classification into mild and severe (SPE) forms has now lost significance. It can also progress to more severe complications, such as HELLP (Hemolysis, Elevated Liver enzymes, and Low Platelet count) syndrome (8) and eclampsia (9).
PE affects 5–7% of pregnant women worldwide and can occur in any pregnancy, although several associated risk factors exist. These include PE in previous pregnancies, maternal age (< 20 and ≥ 35 years), metabolic disorders, pregnancies achieved through assisted reproductive technology, and pre-existing conditions, such as chronic kidney disease, thyroid dysfunction, and systemic lupus erythematosus (SLE) (10). A family history of PE is associated with an increased risk, suggesting a genetic background (11). However, no single high-risk gene is associated with PE, reflecting the syndromic nature of the disease.
PE management involves outpatient monitoring of blood pressure and proteinuria, or potential hospitalization in more severe cases. Delivery is often the most effective intervention. Up to now, the soluble fms-like tyrosine kinase 1 (sFlt-1)/placental growth factor (PlGF) ratio is the only predictive biomarker for short-term risk of PE and adverse pregnancy outcomes (12). Additional predictive screenings via algorithms have been extensively evaluated and validated by various institutions (13). Thus, there is a critical need for serum biomarkers for early diagnosis and screening as reliable predictors of PE in the short term.
The etiology of PE is complex and multifactorial. A consistent body of evidence supports the involvement of an abnormal placentation process mainly due to immunological dysfunctions occurring at the fetal-maternal interface (13). Dysregulation of the complement system (C) is recognized as one of the main contributors to PE pathogenesis (14, 15). The C is a crucial mediator of the innate immune response, being also involved in cell homeostasis, tissue development and repair, and crosstalk with other endogenous systems (i.e., renin-angiotensin, coagulation, and kinin-kallikrein systems). C consists of more than 50 fluid-phase and membrane-bound proteins. Its activation can be initiated by three distinct pathways (i.e., classical, CP; lectin, LP; and alternative, AP) (Figure 1), converging on the common activation of the major component C3 and in the production of proinflammatory mediators, opsonization, membrane attack complex (MAC) formation, and target cell lysis (16, 17).
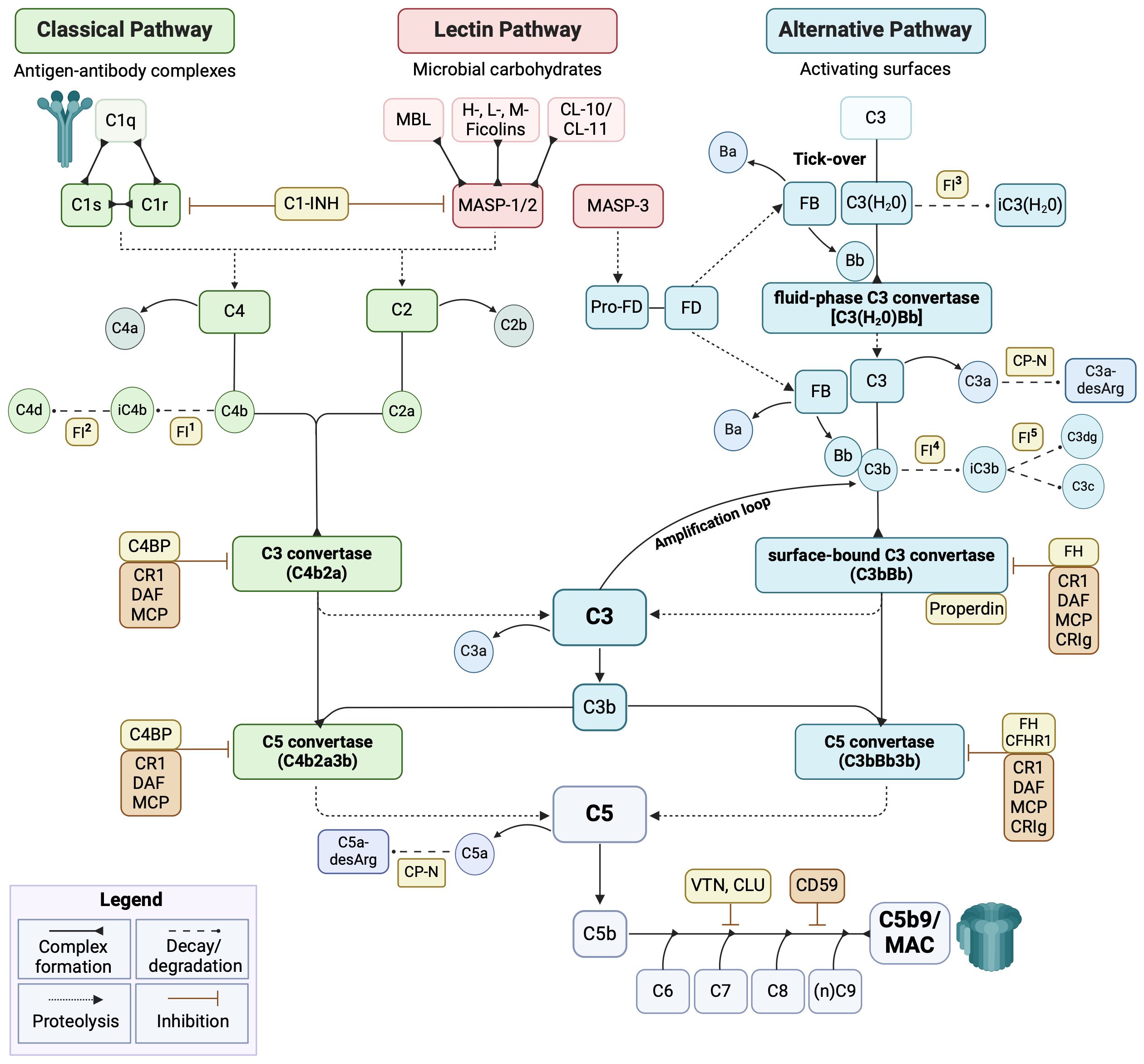
Figure 1 Schematic overview of the complement system cascade. The complement system (C) operates via three pathways: classical (green), lectin (pink), and alternative (cyan). The classical pathway is mainly triggered by the binding of C1q to antigen-antibody complexes. The lectin pathway is activated by mannan-binding lectin (MBL), ficolins, or collectins (CLs), which recognize microbial carbohydrates and form a complex with MASP-1 and MASP-2. The alternative pathway is constitutively active at basal levels, with C3 undergoing spontaneous hydrolysis (tick-over). All three pathways converge on the common component C3 and proceed with the terminal pathway (grey). The C activation is tightly controlled by several fluid-phase (yellow) and membrane-bound (orange) regulators. FI activity is supported by several cofactors: 1-2) C4BP, CD46, CR1; 3) FH/FH-L1; 4) FH, FH-L1, CD46, CR1; and 5) CD46, CR1. C1-INH, C1-inhibitor; C4BP, C4b-binding protein; CFHR1, complement factor H-related protein 1; CLU, clusterin; CP-N, carboxypeptidase-N; CR1, complement receptor 1; CRIg, complement receptor immunoglobulin; DAF, decay-accelerating factor; FB, factor B; FD, factor D; FH, factor H; FI, factor I; MAC, membrane attack complex; MCP, membrane cofactor protein; VTN, vitronectin. Image created with BioRender.com.
An accumulating body of evidence has extensively pointed out the pivotal role of C in immunological tolerance at the feto-maternal interface (18). A shift towards excessive C activation or impaired C regulation may determine the onset of pregnancy-related pathological conditions, including PE (15). An aberrant or excessive C activation in the placenta can be responsible for PE pathogenesis, leading to inflammation and endothelial dysfunction (14, 18, 19). This may be mainly due to increased circulating anaphylatoxins, enhanced deposition of C factors in the placenta, or consumption of circulating C factors, leading to placental dysfunction. C activation products can damage endothelial cells, impairing their functions and contributing to the high blood pressure and other symptoms frequently observed in PE (20). This inflammatory condition may also impact the function of other organs, such as liver and kidney, leading to complications. Interestingly, recent research has explored the possibility of targeting the C as a novel therapeutic approach for PE (21, 22).
C proteins and their activation products have been frequently found in the placenta of women with PE (23), but the relationship between circulating C components and placental C deposition remains elusive. In addition to excessive C activation leading to a simultaneous reduction in C component levels and an increase in activation products, different levels of C components between healthy pregnancies and PE may also be caused by the different hepatic synthesis due to systemic inflammation. Furthermore, one should consider the presence of a massive quantity of circulating microvesicles in the bloodstream of PE patients; they can act as decoys for C components and contribute to an aberrant concentration of C proteins and inhibitors. Thus, research interest has been focused on the evaluation of C components as PE biomarkers before the disease onset (predictive biomarkers) or during the active disease (diagnostic biomarkers). Several studies have investigated the presence of C components or split products in blood matrixes (i.e., plasma, serum), urine, and amniotic fluid in PE.
This systematic review aims to evaluate the current state of the art of C components as potential circulating biomarkers in PE women, provides a general overview of C status in PE, and raises awareness about measuring C components in a reliable and standardized manner. This may help to explore novel predictive approaches and therapeutic opportunities in PE management.
A systematic literature search was carried out to identify original articles reporting the values of circulating C components in PE pregnancies compared to healthy pregnancies. The search strategy was developed following the recommendations of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (24). PubMed, Scopus, and Embase databases were interrogated for relevant articles published until the 13th March 2024. The search terms included: “preeclampsia”, “complement”, “serum”, “plasma”, “blood”, and “biomarker”. The specific search strategy is reported in the Supplementary Material. The review was registered in the PROSPERO database (CRD42024503070).
Removal of duplicates and screening of records were performed using Rayyan systematic review tool (25). Two independent investigators (AB and AM) screened the articles by title and abstract, followed by full-text checking for their eligibility. Relevant articles were included if they met all the following eligibility criteria: 1) English language; 2) original peer-reviewed article; and 3) study measuring the levels of at least one C component in the blood of PE vs healthy women. Articles were not included if they were as follows: 1) articles published in languages other than English; 2) case reports/case series; 3) reviews, systematic reviews, meta-analyses, book chapters, conference or comment papers, prospective or retrospective studies; 4) studies reporting biomarkers in urine, cord or fetal blood; 5) studies which did not report numerical values for the measurements of circulating C components; and 6) animal studies. Disparities were resolved by a third investigator (CA).
All considered articles of interest among those identified were reported in this review.
The reported variables include: the first author’s name, year of publication, and reference of each study; the values of the biomarkers in PE and control groups, with sample size and units of measurement; p-values and the statistical test used; the time of sample collection and type of blood sample (i.e., serum, plasma); the techniques used for biomarker measurement; notes about EOPE, LOPE, and/or SPE. The recorded statistical measures were reported as mean with standard deviation (mean ± SD) or median with interquartile range [median (IQR)].
Most articles reported a single value for a biomarker, which was included in the study. In a few research articles, multiple values obtained in different cohorts were reported for a single biomarker; in such cases, all cohorts were separately reported.
The results of the literature search and selection process are illustrated in Figure 2. A total of 812 records were retrieved after the first search in the above-mentioned databases. Duplicates (n = 356) were removed via Rayyan, and the remaining 456 records were screened. During the screening phase, involving the examination of the title and abstract, 330 records were excluded for the following reasons: wrong topic (n = 121), wrong publication type (n = 140), and wrong study design (n = 69). Full-text screening resulted in 126 articles, which were assessed for eligibility. During the eligibility phase, 85 full-text articles were excluded since they did not meet the inclusion criteria: wrong publication type (n = 46), no values were reported (n = 19), wrong topic (n = 7), wrong study design (n = 4), languages other than English (n = 4), and presence of comorbidities (n = 5). Forty-one studies were included in this systematic review. The included papers and relative values are reported in Tables 1–5.
The C markers can be divided into categories based on their involvement in the C cascade.
Fourteen studies (34.1%) were identified as measuring the blood concentrations of CP components, including C1q, C4, C4a, and C4d, as summarized in Table 1. Five studies measured C1q levels (12.1%), nine C4 (21.9%), one C4a (2.4%), and four C4d (9.7%). Regarding C1q, two studies reported no statistically significant differences in C1q levels between PE and control pregnancies (26, 28). One study showed significantly higher C1q levels in SPE (27). Two studies analyzing two different PE cohorts (EOPE and LOPE) reported significantly lower or higher C1q levels in PE, respectively (29, 30). Conversely, the majority of the studies were coherent with reporting a significantly lower level of C4 in PE women (29, 31, 32, 34, 35, 37), also when analyzing EOPE and LOPE separately (29). Only one article measured C4a levels, not detecting any significant difference in PE women, except for those experiencing the delivery of a small-for-gestational-age newborn (38). Four studies related to C4d fulfilled the inclusion criteria and suggested an increase in C4d levels in PE women (27, 30, 33, 39), particularly in LOPE (30).
Our systematic review of currently available research highlighted very few studies (5/41; 12.1%) analyzing blood concentrations of LP components’ serum levels, as shown in Table 2. LP components were mainly measured in one study by Larsen and colleagues. They reported significantly higher serum concentrations of CL-L1, Map44, MASP-1, and MASP-3 in PE women than in healthy pregnancies, while significantly lower H- and M-ficolin levels. At the same time, no significant differences were observed in Map19, MASP-2, and MBL (41). MBL levels were further investigated in four additional studies (27, 28, 30, 42), but only one demonstrated an association between increased MBL levels and PE pregnancies (42).
The vast majority of studies focused their interest on the assessment of AP components in serum or plasma samples (28/41; 68.3%), including Ba, Bb, C3, C3a, C3c, C3d, Factor B (FB), Factor D (FD), and iC3b, as reported in Table 3. Fragment Ba levels were measured by Blakey and colleagues in two different cohorts of PE patients matched with controls, reporting significantly higher levels of Ba in PE women only in one cohort (31). Seven studies (17.1%) related to Bb concentrations were evaluated, and they consistently demonstrated higher levels of Bb in PE, both in EOPE and LOPE (27, 29, 30, 33, 43–45). The central component C3 was analyzed in eight articles (19.5%), with a significant decrease in PE patients (29, 31, 33), even though not all studies reached statistical significance (32, 34–36, 46). Twelve articles (29.3%) measured C3a levels in the blood of PE women compared to healthy pregnancies, detecting an overall significant increase of C3a in PE (27, 30, 33, 39, 49, 50). Conversely, neither C3c (28, 53) nor C3d (36) plasma assessment showed significant differences between PE and normal pregnancies. FB was assessed by two studies, which determined a significant increase in serum levels (26) and not significant in plasma (28) of pregnant women with PE. Four studies consistently determined a greater concentration of adipsin/FD in serum (54, 55, 57) or plasma (56) of women with PE, particularly increased in SPE (54). Only one study by Blakey et al. reported a significant reduction of iC3b levels in PE (31).
Terminal pathway components were investigated in 17/41 studies (41.5%) retrieved from the literature, mainly focusing on C5a and C5b-9 (Table 4). Circulating levels of the anaphylatoxin C5a were evaluated by twelve studies (29.3%). Most studies accordingly reported higher levels of C5a in the PE group (27, 30, 38, 48–51). Only one study by He and colleagues reported a significant reduction (28), while circulating C5a levels did not differ significantly between PE and control groups in the remaining studies (47, 52, 60, 61). Circulating levels of C5b-9 were measured by thirteen research articles (31.7%). Almost all of them were consistent with significantly higher concentrations of C5b-9 in blood samples of women with PE (27, 30, 33, 39, 49, 51, 54, 60–62). Interestingly, Burwick et al. analyzed C5b-9 in four different PE cohorts (PE, SPE, EOPE, and EOSPE), highlighting significantly higher plasmatic levels in PE compared to matched healthy groups, except for EOPE (62).
Several studies (16/41; 39.0%) assessed the circulating levels of different C regulators, mainly focusing on C1-inhibitor and Factor H (FH), as summarized in Table 5. Four studies investigated the association between circulating C1-inhibitor levels and PE (33, 34, 63), but only one reported a significant reduction of C1-inhibitor in women with PE (37). Factor H levels were evaluated in seven studies, which determined an overall reduction of this C regulator in patients with PE (26, 28, 29, 46, 47, 66) in both EOPE and LOPE (29). A statistically significant reduction in PE cohorts was observed also for Factor I (FI) (67), properdin (31), and vitronectin (68). Velasquez and colleagues reported higher plasma levels of CD59 in PE (64). Conversely, C4b-binding protein (C4BP) (33) and complement receptor 1 (CR1)/CD35 (65) did not show any statistical differences in PE and control groups.
Even though C dysregulation may have a role in affecting placental formation before the disease onset, the contribution of C in PE pathophysiology mainly consists in a secondary mechanism of amplification of tissue injury and inflammation, following endothelial damage and local placental ischemia and hypoxia, and results in a cascade of reactions that contribute to the rapid development and symptom exacerbation of PE at the systemic level (69, 70). When discussing C testing, one should always bear in mind the complexity of C and the interdependence among its individual components. For ease of comprehension, our systematic review dissected the involvement of C components as biomarkers of PE, initially focusing on the contribution of the different C pathways as separate entities, and then moving to a more extensive overview.
As regards the CP and LP, studies were coherent in reporting lower levels of C4 and higher levels of C4d in PE compared to healthy pregnancies. This supports the hypothesis that the relatively low C4 values in PE may indicate a low grade of CP and LP activation with consumption of C4, as suggested by the increase in C4d levels. Agostinis and co-authors also demonstrated that lower levels of C4 were associated with PE (71). Consistently, excessive deposition of C4d at the placental site was frequently reported in PE (23, 31, 72) as a marker of CP and LP activation, even though C4d deposits seem more likely related to CP activation due to the co-localization with C1q (73). The vast majority of the resulting proteolytic cleavage product C4d remains at the activation site, while part of it may eventually enter the circulation as free C4d.
The CP or LP may be triggered by repeated ischemia–reperfusion injury and oxidative stress caused by defective placentation in PE (72, 73), while AP exacerbates the activation cycle via an amplification loop. In human PE, C has been shown to be locally activated via the LP triggered by H-ficolin, while MBL did not appear to be particularly involved (23). Larsen and colleagues reported that LP protein concentrations in the serum generally increased throughout the trimesters of normal pregnancy and reduced after delivery in healthy pregnant women and in women with PE (41). A study by Celik and Ozan also reported higher MBL levels in SPE patients compared to women with uncomplicated pregnancies (74), in accordance with further studies (42, 75). Conversely, herein reported results about C1q levels were inconclusive. Dijkstra and colleagues observed no significant differences in serum C1q between PE and control pregnancies in three different cohorts (76),while Agostinis et al. reported a decrease in PE patients, both in serum and plasma samples, maybe due to C1q binding to circulating immunocomplexes or to syncytiotrophoblast extracellular vesicles derived from PE placentas (71, 77, 78). C1q reduction is also supported by the findings of Jia et al., both in EOPE and LOPE (29). In the occurrence of PE related to antibody-mediated autoimmune diseases (e.g., antiphospholipid syndrome), C1q reduction could be associated with CP activation induced by the deposition of pathogenic immunoglobulins in the placenta (79).
The AP is also consistently activated in PE, as demonstrated by the reduction in circulating C3 levels and the increased levels of Ba, Bb, and C3a fragments, being markers of AP activation. Similar results are also consistent with the higher levels of circulating FD in PE (54–57), which is responsible for excessive C activation. Interestingly, the increase in Bb levels is consistent with increased FD levels in PE, considering that FD serves as a serine protease acting on FB to yield the active fragment Bb (57). It is also noteworthy to consider that FD levels may increase in parallel to decreased kidney function and be responsible for enhanced AP activation, particularly in the circulation (80). Elevated circulating levels of C3a in the first trimester of pregnancy were demonstrated as an independent predictive factor for PE (45). Moreover, C3a levels were particularly increased in LOPE (30). Accordingly, local C3 deposition in the placentae of PE patients is significantly higher compared to the control placentae (81).
Evidence about terminal pathway components in PE is rather coherent among studies, reporting increased C5a and C5b-9 levels in the maternal circulation, both in EOPE and LOPE (30, 49). Interestingly, a significant correlation was found between C5b-9 levels and anti-angiogenetic factors (i.e., soluble endoglin and sFlt-1). We should keep in mind that the increase in C5a and C5b-9 may also be directly influenced by additional factors, such as coagulation and fibrinolysis. Thus, urinary excretion of C5b-9 has been proposed as a more valuable marker of SPE than plasma evaluation (51).
Taken together, existing data show that C regulation is also impaired in PE. During normal pregnancy, FH levels usually increase throughout trimesters of healthy pregnancies (28), suggesting that a balanced regulatory response is crucial to avoid excessive systemic AP activation, despite allowing local C3-dependent mechanisms required to support a normal pregnancy. Interestingly, three studies consistently reported that circulating levels of FH were significantly reduced in PE (29, 47, 66). Yasmin et al. identified lower FH levels in sera collected in the first trimester of pregnancies with PE, proposing FH as a potential predictive biomarker of PE (66). The relative abundance of FH in the third trimester of pregnancy, and particularly the increased FH/C3 ratio, could prevent AP activation, while a reduction in FH may participate in the C dysregulation observed in PE. This scenario is also consistent with an overall reduction of other C regulators, i.e., properdin (31) and FI (67). Reduced concentrations of properdin in maternal plasma allowed to distinguish cases of PE from healthy pregnancies with excellent diagnostic accuracy (31). Excessive AP activity may determine reduced circulating levels of properdin via consumption from tissue deposition.
The overall emerging scenario is an abnormal C activation and regulation in PE. Decreased circulating levels of C1q are frequently observed in combination with lower levels of C4, which may be due to increased consumption of C4 via aberrant activation of the CP or LP. Reduced concentrations of early AP components (i.e., C3, FB, or FH) are also consistent with an extensive depletion of the cascade components due to excessive activation or poor inhibition, while C activation products (e.g., Bb, C3a, C5a, or C5b-9) are increased as a result of the extensive cleavage of C components. A pivotal role in the dysregulation of C activation seems to be carried out by the reduction in FH levels, which could be caused by deficiency or consumption of FH. Interestingly, a recent study by Lokki et al. identified FH variants that predispose to PE onset, confirming the multifactorial nature of the pathophysiological mechanisms underlying PE (82).
Jia and colleagues demonstrated that the best predictive indicator of PE was the combination of five C factors (C1q, Bb, FH, C3, and C4), both in EOPE and LOPE, displaying a potential as diagnostic markers for severe PE (29).
An accurate analysis of a wide range of C components in PE is of utmost importance for prediction and diagnosis, although the reliability of these measurements is often challenging. The main concern of C assessment for diagnosing disease and monitoring therapy is that measured concentrations of C components may widely vary among different laboratories due to a lack of protocol uniformity for pre-analytical sample handling (e.g., collection, processing, and storage), use of different calibrators and techniques (e.g., nephelometry, turbidimetry, and ELISA), or antibodies targeting different epitopes. These pitfalls unveil an urgent need for standardization, in concert with adequate pre-analytical sample handling and storage. However, up to now, performing ELISA with well-defined antibodies has proven to be the most reproducible method for reflecting the actual state of C activation via quantification of C-derived split products next to the native proteins, while nephelometry and turbidimetry do not allow to distinguish between the native non-activated C proteins and their activated split products (83).
Furthermore, laboratories do not always analyze the proper sample type for C analysis. The use of plasma or serum can lead to different results when certain C factors are considered. In particular, plasma is preferentially chosen for assessing C byproducts to avoid in vitro C activation that occurs during serum preparation due to coagulation and fibrinolysis activation. EDTA-plasma (at least a final concentration of 10 mM), any activation of C is minimized (83). For other factors, the binding of C components to fibrinogen and fibrin in plasma should be taken into account (e.g., C1q) (84). Inconsistences may also be due to sample storage, thawing conditions, and repeated freezing-thawing cycles, which could be crucial variables for specific C components, particularly activation products (85).
Non-uniformity in the timing of sample collection is another issue that needs to be discussed when examining PE, and a serious limitation when comparing the studies collected in this systematic review. Although it may be assumed that the closer the disease onset, the more the potential biomarkers are expected to vary, a different matter is the potential predictive value of the C-components in PE. It is noteworthy that the vast majority of studies gathered in the current review measured C-component expression at the time of PE diagnosis (i.e., in the third trimester of pregnancy). Only a few studies also measured C-components during the first trimester, with limited but encouraging results. He et al. observed significant fluctuations in circulating levels of FB, FH, C1q, C3c, and C4 in PE pregnancies from the first trimester onwards (28). Altered FD and C5a levels in women with PE in the first trimester were also reported (56), as well as FH (66). These preliminary findings emphasize the urgent need to investigate variations in the C components during pregnancy to identify potential predictive markers at early stages. As PE can have multiple causes, it is unlikely that a single timing strategy for biomarker testing can be used to predict all cases of PE (86). Despite requiring a massive effort from clinicians and researchers in terms of number of enrolled women, it is crucial that we embark on prospective longitudinal studies involving blood sample collection at various stages of pregnancy. These studies may allow us to explore the actual time threshold of biomarkers with predictive significance and ensure proper matching for gestational age between healthy and pregnancies with PE.
Diverging results among studies may also be biased by the cohort selection due to different ethnicity, sample size, symptom severity (mild/severe), onset (preterm/term), and presence of comorbidities. For instance, women with African ancestry are at greater risk of PE, likely due to the involvement of specific factors, among which Bb fragment has been accounted (43), while factor B activation has not been observed in specific Caucasian cohorts (33). This evidence suggests that some C factors may serve as predictors of PE only in specific sub-groups of patients, offering a promising avenue for further investigation and potentially explaining the variations among the cohorts analyzed in this review.
Several lines of evidence support the concept that PE should not be considered a single disorder, and the guidelines no longer support the classification into “mild” and “severe” PE. Therefore, the definition of PE severity may not be consistent across studies, based on different updates of the American College of Obstetricians and Gynecologists (ACOG) hypertension in pregnancy guidelines (40, 58, 59), and may lead to bias in the cohort selection. It is also worth considering that EOPE and LOPE may be explored as separate entities reflecting underlying differences in etiology. The complexity of defining and classifying PE was further highlighted by Than et al., who described four molecular clusters of PE (i.e., canonical/placental, metabolic, immunological, and maternal PE) by proteomic analysis that exhibit distinct clinical phenotypes (87), in line with maternal hemodynamic characteristics of different hypertensive disorders of pregnancy phenotypes (88).
The presence of comorbidities is a crucial factor that must be taken into consideration. Interestingly, several studies that were specifically excluded from the current systematic search report the usefulness of C-component assessment in specific subgroups of patients. For example, predictive value was found for FB and FH serum levels in PE associated with gestational diabetes mellitus (89).
To our knowledge, this is the first systematic literature review focusing on currently available research about the assessment of C components as circulating biomarkers of PE. The main strength of the current systematic search includes the comprehensive coverage of available measurements of circulating C components in PE, which were summarized as ready-to-use information.
Nevertheless, we can recognize some methodological limitations. First, not all scientific information may be included in bibliographic databases being reported as unpublished conference papers or congress proceedings. In addition, we included only research papers in which numerical values for the measurement of the C-component were reported, thus possibly neglecting other seminal studies in which numerical values were not explicitly reported. Lastly, only publications in English were included, which may lead to language and country-specific biases, potentially limiting the generalizability of our findings.
Since C activation can occur in several scenarios, the specificity of a single C-component test is low, so a panel of tests should be preferred (90). C multiplex ELISA assays, such as panels for simultaneous testing of C activation fragments and intact components or regulators (91), may be particularly advantageous from a technical and reproducibility standpoint. Thus, multiplex assays can help to address the need for a thorough analysis of the status of C activation and regulation and ensure a comprehensive overview of the C cascade. Here, we propose that multiplex C testing should be performed throughout pregnancy to raise awareness of the importance of planning prospective longitudinal studies to identify early predictive markers for PE.
With the mounting evidence of C activation, the potential of C-targeted therapy for the treatment of PE is a promising avenue. The emergence of new drugs specifically targeting C underscores the growing necessity for soluble biomarkers as companion tests to select and monitor patients who would benefit from these innovative treatments, instilling hope for improved outcomes in the management of PE.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
AB: Conceptualization, Data curation, Funding acquisition, Methodology, Writing – original draft, Writing – review & editing. CA: Conceptualization, Data curation, Funding acquisition, Writing – original draft. AM: Data curation, Methodology, Writing – original draft. GZ: Writing – review & editing. TS: Writing – review & editing. GR: Funding acquisition, Supervision, Writing – review & editing. RB: Conceptualization, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Italian Ministry of Health, through the contribution given to the Institute for Maternal and Child Health IRCCS Burlo Garofolo-Trieste, Italy (RC 01/23 to CA, RC 03/23 to AB, and RC 23/24 to GR).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1419540/full#supplementary-material
1. Chappell LC, Cluver CA, Kingdom J, Tong S. Pre-eclampsia. Lancet. (2021) 398:341–54. doi: 10.1016/S0140–6736(20)32335–7
2. Magee LA, Brown MA, Hall DR, Gupte S, Hennessy A, Karumanchi SA, et al. The 2021 International Society for the Study of Hypertension in Pregnancy classification, diagnosis & management recommendations for international practice. Pregnancy Hypertens. (2022) 27:148–69. doi: 10.1016/j.preghy.2021.09.008
3. Hauspurg A, Jeyabalan A. Postpartum preeclampsia or eclampsia: defining its place and management among the hypertensive disorders of pregnancy. Am J Obstet Gynecol. (2022) 226:S1211–21. doi: 10.1016/j.ajog.2020.10.027
4. von Dadelszen P, Syngelaki A, Akolekar R, Magee LA, Nicolaides KH. Preterm and term pre-eclampsia: Relative burdens of maternal and perinatal complications. BJOG. (2023) 130:524–30. doi: 10.1111/1471–0528.17370
5. Staff AC, Redman CWG. The Differences Between Early- and Late-Onset Pre-eclampsia. In: Saito S, editor. Preeclampsia: Basic, Genomic, and Clinical. Springer Singapore, Singapore (2018). doi: 10.1007/978–981-10–5891-2_10
6. Raymond D, Peterson E. A critical review of early-onset and late-onset preeclampsia. Obstet Gynecol Surv. (2011) 66:497–506. doi: 10.1097/OGX.0b013e3182331028
7. Roberts JM, Rich-Edwards JW, McElrath TF, Garmire L, Myatt L, Collaboration GP. Subtypes of preeclampsia: recognition and determining clinical usefulness. Hypertension. (2021) 77:1430–41. doi: 10.1161/HYPERTENSIONAHA.120.14781
8. Wallace K, Harris S, Addison A, Bean C. HELLP syndrome: pathophysiology and current therapies. Curr Pharm Biotechnol. (2018) 19:816–26. doi: 10.2174/1389201019666180712115215
9. Fishel Bartal M, Sibai BM. Eclampsia in the 21st century. Am J Obstet Gynecol. (2022) 226:S1237–53. doi: 10.1016/j.ajog.2020.09.037
10. English FA, Kenny LC, McCarthy FP. Risk factors and effective management of preeclampsia. Integr Blood Press Control. (2015) 8:7–12. doi: 10.2147/IBPC.S50641
11. Williams PJ, Broughton Pipkin F. The genetics of pre-eclampsia and other hypertensive disorders of pregnancy. Best Pract Res Clin Obstet Gynaecol. (2011) 25:405–17. doi: 10.1016/j.bpobgyn.2011.02.007
12. Zeisler H, Llurba E, Chantraine F, Vatish M, Staff AC, Sennström M, et al. Predictive value of the sFlt-1:PlGF ratio in women with suspected preeclampsia. N Engl J Med. (2016) 374:13–22. doi: 10.1056/NEJMoa1414838
13. Dimitriadis E, Rolnik DL, Zhou W, Estrada-Gutierrez G, Koga K, Francisco RPV, et al. Pre-eclampsia. Nat Rev Dis Primers. (2023) 9:8. doi: 10.1038/s41572–023-00417–6
14. Teirilä L, Heikkinen-Eloranta J, Kotimaa J, Meri S, Lokki AI. Regulation of the complement system and immunological tolerance in pregnancy. Semin Immunol. (2019) 45:101337. doi: 10.1016/j.smim.2019.101337
15. Burwick RM, Feinberg BB. Complement activation and regulation in preeclampsia and hemolysis, elevated liver enzymes, and low platelet count syndrome. Am J Obstet Gynecol. (2022) 226:S1059–70. doi: 10.1016/j.ajog.2020.09.038
16. Walport MJ. Complement. First of two parts. N Engl J Med. (2001) 344:1058–66. doi: 10.1056/NEJM200104053441406
17. Agostinis C, Mangogna A, Balduit A, Aghamajidi A, Ricci G, Kishore U, et al. COVID-19, pre-eclampsia, and complement system. Front Immunol. (2021) 12:775168. doi: 10.3389/fimmu.2021.775168
18. Girardi G, Lingo JJ, Fleming SD, Regal JF. Essential role of complement in pregnancy: from implantation to parturition and beyond. Front Immunol. (2020) 11:1681. doi: 10.3389/fimmu.2020.01681
19. Matsuyama T, Tomimatsu T, Mimura K, Yagi K, Kawanishi Y, Kakigano A, et al. Complement activation by an angiogenic imbalance leads to systemic vascular endothelial dysfunction: A new proposal for the pathophysiology of preeclampsia. J Reprod Immunol. (2021) 145:103322. doi: 10.1016/j.jri.2021.103322
20. Fischetti F, Tedesco F. Cross-talk between the complement system and endothelial cells in physiologic conditions and in vascular diseases. Autoimmunity. (2006) 39:417–28. doi: 10.1080/08916930600739712
21. Regal JF, Burwick RM, Fleming SD. The complement system and preeclampsia. Curr Hypertens Rep. (2017) 19:87. doi: 10.1007/s11906–017-0784–4
22. Pierik E, Prins JR, van Goor H, Dekker GA, Daha MR, Seelen MAJ, et al. Dysregulation of complement activation and placental dysfunction: A potential target to treat preeclampsia? Front Immunol. (2019) 10:3098. doi: 10.3389/fimmu.2019.03098
23. Belmonte B, Mangogna A, Gulino A, Cancila V, Morello G, Agostinis C, et al. Distinct roles of classical and lectin pathways of complement in preeclamptic placentae. Front Immunol. (2022) 13:882298. doi: 10.3389/fimmu.2022.882298
24. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
25. Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. (2016) 5:210. doi: 10.1186/s13643–016-0384–4
26. Wang J, Hu H, Liu X, Zhao S, Zheng Y, Jia Z, et al. Predictive values of various serum biomarkers in women with suspected preeclampsia: A prospective study. J Clin Lab Anal. (2021) 35:e23740. doi: 10.1002/jcla.23740
27. Chen S, Li Z, He Y, Chen Q. Dysregulation of complement system in HELLP syndrome. Hypertens Pregnancy. (2021) 40:303–11. doi: 10.1080/10641955.2021.1983593
28. He YD, Xu BN, Wang ML, Wang YQ, Yu F, Chen Q, et al. Dysregulation of complement system during pregnancy in patients with preeclampsia: A prospective study. Mol Immunol. (2020) 122:69–79. doi: 10.1016/j.molimm.2020.03.021
29. Jia K, Ma L, Wu S, Yang W. Serum levels of complement factors C1q, bb, and H in normal pregnancy and severe pre-eclampsia. Med Sci Monit. (2019) 25:7087–93. doi: 10.12659/MSM.915777
30. He Y, Xu B, Song D, Yu F, Chen Q, Zhao M. Expression of the complement system's activation factors in plasma of patients with early/late-onset severe pre-eclampsia. Am J Reprod Immunol. (2016) 76:205–11. doi: 10.1111/aji.12541
31. Blakey H, Sun R, Xie L, Russell R, Sarween N, Hodson J, et al. Lipkin: Pre-eclampsia is associated with complement pathway activation in the maternal and fetal circulation, and placental tissue. Pregnancy Hypertens. (2023) 32:43–9. doi: 10.1016/j.preghy.2023.04.001
32. Sarween N, Drayson MT, Hodson J, Knox EM, Plant T, Day CJ, et al. Humoral immunity in late-onset Pre-eclampsia and linkage with angiogenic and inflammatory markers. Am J Reprod Immunol. (2018) 80:e13041. doi: 10.1111/aji.13041
33. Derzsy Z, Prohászka Z, Rigó J, Füst G, Molvarec A. Activation of the complement system in normal pregnancy and preeclampsia. Mol Immunol. (2010) 47:1500–6. doi: 10.1016/j.molimm.2010.01.021
34. Mellembakken JR, Høgåsen K, Mollnes TE, Hack CE, Abyholm T, Videm V. Increased systemic activation of neutrophils but not complement in preeclampsia. Obstet Gynecol. (2001) 97:371–4. doi: 10.1016/s0029–7844(00)01179–0
35. Buyon JP, Cronstein BN, Morris M, Tanner M, Weissmann G. Serum complement values (C3 and C4) to differentiate between systemic lupus activity and pre-eclampsia. Am J Med. (1986) 81:194–200. doi: 10.1016/0002–9343(86)90251–2
36. Massobrio M, Benedetto C, Bertini E, Tetta C, Camussi G. Immune complexes in preeclampsia and normal pregnancy. Am J Obstet Gynecol. (1985) 152:578–83. doi: 10.1016/0002–9378(85)90631–3
37. Hsieh C, Cauchi MN. Platelet and complement changes in pre-eclampsia. J Obstetrics Gynaecology. (1983) 3:165–9. doi: 10.3109/01443618309081135
38. Soto E, Romero R, Richani K, Espinoza J, Chaiworapongsa T, Nien JK, et al. Preeclampsia and pregnancies with small-for-gestational age neonates have different profiles of complement split products. J Matern Fetal Neonatal Med. (2010) 23:646–57. doi: 10.3109/14767050903301009
39. Halmos A, Rigó J, Szijártó J, Füst G, Prohászka Z, Molvarec A. Circulating ficolin-2 and ficolin-3 in normal pregnancy and pre-eclampsia. Clin Exp Immunol. (2012) 169:49–56. doi: 10.1111/j.1365-2249.2012.04590.x
40. Hypertension in pregnancy. Report of the american college of obstetricians and gynecologists’ Task force on hypertension in pregnancy. Obstet Gynecol. (2013) 122:1122–31. doi: 10.1097/01.AOG.0000437382.03963.88
41. Larsen JB, Andersen AS, Hvas CL, Thiel S, Lassen MR, Hvas AM, et al. Lectin pathway proteins of the complement system in normotensive pregnancy and pre-eclampsia. Am J Reprod Immunol. (2019) 81:e13092. doi: 10.1111/aji.13092
42. Than NG, Romero R, Erez O, Kusanovic JP, Tarca AL, Edwin SS, et al. A role for mannose-binding lectin, a component of the innate immune system in pre-eclampsia. Am J Reprod Immunol. (2008) 60:333–45. doi: 10.1111/j.1600-0897.2008.00631.x
43. Velickovic I, Dalloul M, Wong KA, Bakare O, Schweis F, Garala M, et al. Complement factor B activation in patients with preeclampsia. J Reprod Immunol. (2015) 109:94–100. doi: 10.1016/j.jri.2014.12.002
44. Hoffman MC, Rumer KK, Kramer A, Lynch AM, Winn VD. Maternal and fetal alternative complement pathway activation in early severe preeclampsia. Am J Reprod Immunol. (2014) 71:55–60. doi: 10.1111/aji.12162
45. Lynch AM, Murphy JR, Byers T, Gibbs RS, Neville MC, Giclas PC, et al. Alternative complement pathway activation fragment Bb in early pregnancy as a predictor of preeclampsia. Am J Obstet Gynecol. (2008) 198:385.e1–9. doi: 10.1016/j.ajog.2007.10.793
46. Ari E, Yilmaz Y, Gul A, Alahdab YO, Kedrah AE, Macunluoglu B, et al. Human serum complement C3 and factor H in the syndrome of hemolysis, elevated liver enzymes, and low platelet count. Am J Reprod Immunol. (2009) 62:238–42. doi: 10.1111/j.1600-0897.2009.00731.x
47. Wiles K, Bramham K, Seed PT, Kurlak LO, Mistry HD, Nelson-Piercy C, et al. Diagnostic indicators of superimposed preeclampsia in women with CKD. Kidney Int Rep. (2019) 4:842–53. doi: 10.1016/j.ekir.2019.03.012
48. Ma Y, Kong LR, Ge Q, Lu YY, Hong MN, Zhang Y, et al. Complement 5a-mediated trophoblasts dysfunction is involved in the development of pre-eclampsia. J Cell Mol Med. (2018) 22:1034–46. doi: 10.1111/jcmm.13466
49. He Y, Xu B, Song D, Yu F, Chen Q, Zhao M. Correlations between complement system's activation factors and anti-angiogenesis factors in plasma of patients with early/late-onset severe preeclampsia. Hypertens Pregnancy. (2016) 35:499–509. doi: 10.1080/10641955.2016.1190845
50. Ye Y, Kong Y, Zhang Y. Complement split products C3a/C5a and receptors: are they regulated by circulating angiotensin II type 1 receptor autoantibody in severe preeclampsia? Gynecol Obstet Invest. (2016) 81:28–33. doi: 10.1159/000440651
51. Burwick RM, Fichorova RN, Dawood HY, Yamamoto HS, Feinberg BB. Urinary excretion of C5b-9 in severe preeclampsia: tipping the balance of complement activation in pregnancy. Hypertension. (2013) 62:1040–5. doi: 10.1161/HYPERTENSIONAHA.113.01420
52. Denny KJ, Coulthard LG, Finnell RH, Callaway LK, Taylor SM, Woodruff TM. Elevated complement factor C5a in maternal and umbilical cord plasma in preeclampsia. J Reprod Immunol. (2013) 97:211–6. doi: 10.1016/j.jri.2012.11.006
53. Griffin JF. Pregnancy-associated plasma protein levels at term in normal pregnancy, preeclampsia and essential hypertension. Aust N Z J Obstet Gynaecol. (1983) 23:11–4. doi: 10.1111/j.1479-828X.1983.tb00150.x
54. Assaf DM, Deaf EA, Elgendy SG, Abbas AM, Shaltout AS. Assessment of the diagnostic value of the terminal complement complex, factor D and factor H in preeclampsia. Egyptian J Med Microbiol. (2024) 33:49–56. doi: 10.21608/ejmm.2024.326879
55. David M, Moodley J, Naicker T. The function of adipsin and C9 protein in the complement system in HIV-associated preeclampsia. Arch Gynecol Obstet. (2021) 304:1467–73. doi: 10.1007/s00404–021-06069–9
56. Liu M, Luo X, Xu Q, Yu H, Gao L, Zhou R, et al. Adipsin of the alternative complement pathway is a potential predictor for preeclampsia in early pregnancy. Front Immunol. (2021) 12:702385. doi: 10.3389/fimmu.2021.702385
57. Poveda NE, Garcés MF, Ruiz-Linares CE, Varón D, Valderrama S, Sanchez E, et al. Serum adipsin levels throughout normal pregnancy and preeclampsia. Sci Rep. (2016) 6:20073. doi: 10.1038/srep20073
58. A. C. o. O. Practice. ACOG practice bulletin. Diagnosis and management of preeclampsia and eclampsia. Number 33, January 2002. American College of Obstetricians and Gynecologists. Int J Gynaecol Obstet. (2002) 77:67–75. doi: 10.1016/S0020-7292(02)80002-9
59. Croke L. Gestational hypertension and preeclampsia: A practice bulletin from ACOG. Am Fam Physician. (2019) 100:649–50.
60. Burwick RM, Togioka BM, Speranza RJ, Gaffney JE, Roberts VHJ, Frias AE, et al. Assessment of blood-brain barrier integrity and neuroinflammation in preeclampsia. Am J Obstet Gynecol. (2019) 221:269.e1–8. doi: 10.1016/j.ajog.2019.06.024
61. Haeger M, Unander M, Bengtsson A. Neopterin, PMN elastase, and complement components as monitoring parameters in women with the syndrome of hemolysis, elevated liver enzymes and low platelet count. Pteridines. (1993) 4:138–43. doi: 10.1515/pteridines.1993.4.3.138
62. Burwick RM, Velásquez JA, Valencia CM, Gutiérrez-Marín J, Edna-Estrada F, Silva JL, et al. Terminal complement activation in preeclampsia. Obstet Gynecol. (2018) 132:1477–85. doi: 10.1097/AOG.0000000000002980
63. Godtfredsen AC, Gram JB, Pham STD, Dolleris BB, Jørgensen JS, Sidelmann JJ, et al. Depressed kallikrein generation in women with preeclampsia: A matched cross-sectional study. Front Med (Lausanne). (2022) 9:896811. doi: 10.3389/fmed.2022.896811
64. Velásquez JA, Burwick RM, Hersh AR, Silva JL, Lenis V, Bernal Y, et al. Plasma CD59 concentrations are increased in preeclampsia with severe features and correlate with laboratory measures of end-organ injury. Pregnancy Hypertens. (2020) 22:204–9. doi: 10.1016/j.preghy.2020.10.004
65. Feinberg BB, Jack RM, Mok SC, Anderson DJ. Low erythrocyte complement receptor type 1 (CR1, CD35) expression in preeclamptic gestations. Am J Reprod Immunol. (2005) 54:352–7. doi: 10.1111/j.1600-0897.2005.00318.x
66. Yasmin H, Agostinis C, Toffoli M, Roy T, Pegoraro S, Balduit A, et al. Protective role of complement factor H against the development of preeclampsia. Front Immunol. (2024) 15:1351898. doi: 10.3389/fimmu.2024.1351898
67. Mei Z, Zhou S, Huang B, Mo Y, Hou H. Proteomic identification of candidate plasma biomarkers for preeclampsia in women with pregnancy-induced hypertension. Int J Clin Exp Pathol. (2017) 10:10383–91.
68. Kolialexi A, Tsangaris GT, Sifakis S, Gourgiotis D, Katsafadou A, Lykoudi A, et al. Plasma biomarkers for the identification of women at risk for early-onset preeclampsia. Expert Rev Proteomics. (2017) 14:269–76. doi: 10.1080/14789450.2017.1291345
69. Lynch AM, Salmon JE. Dysregulated complement activation as a common pathway of injury in preeclampsia and other pregnancy complications. Placenta. (2010) 31:561–7. doi: 10.1016/j.placenta.2010.03.010
70. David M, Naicker T. The complement system in preeclampsia: a review of its activation and endothelial injury in the triad of COVID-19 infection and HIV-associated preeclampsia. Obstet Gynecol Sci. (2023) 66:253–69. doi: 10.5468/ogs.22175
71. Agostinis C, Stampalija T, Tannetta D, Loganes C, Vecchi Brumatti L, De Seta F, et al. Complement component C1q as potential diagnostic but not predictive marker of preeclampsia. Am J Reprod Immunol. (2016) 76:475–81. doi: 10.1111/aji.12586
72. Kim EN, Yoon BH, Lee JY, Hwang D, Kim KC, Lee J, et al. Placental C4d deposition is a feature of defective placentation: observations in cases of preeclampsia and miscarriage. Virchows Arch. (2015) 466:717–25. doi: 10.1007/s00428-015-1759-y
73. Buurma A, Cohen D, Veraar K, Schonkeren D, Claas FH, Bruijn JA, et al. Preeclampsia is characterized by placental complement dysregulation. Hypertension. (2012) 60:1332–7. doi: 10.1161/HYPERTENSIONAHA.112.194324
74. Celik N, Ozan H. Maternal serum mannose-binding lectin in severe preeclampsia. Clin Exp Obstet Gynecol. (2008) 35:179–82.
75. Agostinis C, Bossi F, Masat E, Radillo O, Tonon M, De Seta F, et al. MBL interferes with endovascular trophoblast invasion in pre-eclampsia. Clin Dev Immunol. (2012) 2012:484321. doi: 10.1155/2012/484321
76. Dijkstra DJ, Lokki AI, Gierman LM, Borggreven NV, van der Keur C, Eikmans M, et al. Circulating levels of anti-C1q and anti-factor H autoantibodies and their targets in normal pregnancy and preeclampsia. Front Immunol. (2022) 13:842451. doi: 10.3389/fimmu.2022.842451
77. Agostinis C, Zito G, Toffoli M, Peterlunger I, Simoni L, Balduit A, et al. A longitudinal study of C1q and anti-C1q autoantibodies in homologous and heterologous pregnancies for predicting pre-eclampsia. Front Immunol. (2022) 13:1037191. doi: 10.3389/fimmu.2022.1037191
78. Agostinis C, Mangogna A, Balduit A, Kishore U, Bulla R. A non-redundant role of complement protein C1q in normal and adverse pregnancy. Explor Immunol. (2022) 2:622–36. doi: 10.37349/ei.2022.00072
79. Tedesco F, Borghi MO, Gerosa M, Chighizola CB, Macor P, Lonati PA, et al. Pathogenic role of complement in antiphospholipid syndrome and therapeutic implications. Front Immunol. (2018) 9:1388. doi: 10.3389/fimmu.2018.01388
80. Pascual M, Steiger G, Estreicher J, Macon K, Volanakis JE, Schifferli JA. Metabolism of complement factor D in renal failure. Kidney Int. (1988) 34:529–36. doi: 10.1038/ki.1988.214
81. Wang W, Irani RA, Zhang Y, Ramin SM, Blackwell SC, Tao L, et al. Autoantibody-mediated complement C3a receptor activation contributes to the pathogenesis of preeclampsia. Hypertension. (2012) 60:712–21. doi: 10.1161/HYPERTENSIONAHA.112.191817
82. Lokki AI, Ren Z, Triebwasser M, Daly E, Perola M, Auro K, et al. and FINNPEC: Identification of complement factor H variants that predispose to pre-eclampsia: A genetic and functional study. BJOG. (2023) 130:1473–82. doi: 10.1111/1471–0528.17529
83. Brandwijk RJMG, Michels MAHM, van Rossum M, de Nooijer AH, Nilsson PH, de Bruin WCC, et al. Pitfalls in complement analysis: A systematic literature review of assessing complement activation. Front Immunol. (2022) 13:1007102. doi: 10.3389/fimmu.2022.1007102
84. Entwistle RA, Furcht LT. C1q component of complement binds to fibrinogen and fibrin. Biochemistry. (1988) 27:507–12. doi: 10.1021/bi00401a073
85. Yang S, McGookey M, Wang Y, Cataland SR, Wu HM. Effect of blood sampling, processing, and storage on the measurement of complement activation biomarkers. Am J Clin Pathol. (2015) 143:558–65. doi: 10.1309/AJCPXPD7ZQXNTIAL
86. Han L, Holland OJ, Da Silva Costa F, Perkins AV. Potential biomarkers for late-onset and term preeclampsia: A scoping review. Front Physiol. (2023) 14:1143543. doi: 10.3389/fphys.2023.1143543
87. Than NG, Posta M, Györffy D, Orosz L, Orosz G, Rossi SW, et al. Early pathways, biomarkers, and four distinct molecular subclasses of preeclampsia: The intersection of clinical, pathological, and high-dimensional biology studies. Placenta. (2022) 125:10–9. doi: 10.1016/j.placenta.2022.03.009
88. Vasapollo B, Zullino S, Novelli GP, Farsetti D, Ottanelli S, Clemenza S, et al. Maternal hemodynamics from preconception to delivery: research and potential diagnostic and therapeutic implications: position statement by italian association of pre-eclampsia and italian society of perinatal medicine. Am J Perinatol. (2024). doi: 10.1055/a-2267–3994
89. Xue Y, Yang N, Ma L, Gu X, Jia K. Predictive value of the complement factors B and H for women with gestational diabetes mellitus who are at risk of preeclampsia. Pregnancy Hypertens. (2022) 30:210–4. doi: 10.1016/j.preghy.2022.10.010
90. Prohászka Z, Frazer-Abel A. Complement multiplex testing: Concept, promises and pitfalls. Mol Immunol. (2021) 140:120–6. doi: 10.1016/j.molimm.2021.10.006
Keywords: pre-eclampsia, complement system, systematic review, biomarker, pregnancy
Citation: Balduit A, Agostinis C, Mangogna A, Zito G, Stampalija T, Ricci G and Bulla R (2024) Systematic review of the complement components as potential biomarkers of pre-eclampsia: pitfalls and opportunities. Front. Immunol. 15:1419540. doi: 10.3389/fimmu.2024.1419540
Received: 18 April 2024; Accepted: 07 June 2024;
Published: 24 June 2024.
Edited by:
Marcin Okrój, Intercollegiate Faculty of Biotechnology of University of Gdańsk and Medical University of Gdańsk, PolandReviewed by:
Marina Noris, Mario Negri Institute for Pharmacological Research (IRCCS), ItalyCopyright © 2024 Balduit, Agostinis, Mangogna, Zito, Stampalija, Ricci and Bulla. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chiara Agostinis, Y2Fnb3N0aW5pc0B1bml0cy5pdA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.