- 1Department of Pharmacy, Zhongda Hospital, School of Medicine, Southeast University, Nanjing, China
- 2Department of Endocrinology, Children’s Hospital of Nanjing Medical University, Nanjing, China
Mast cells (MCs) are bone-marrow-derived haematopoietic cells that are widely distributed in human tissues. When activated, they will release tryptase, histamine and other mediators that play major roles in a diverse array of diseases/disorders, including allergies, inflammation, cardiovascular diseases, autoimmune diseases, cancers and even death. The multiple pathological effects of MCs have made their stabilizers a research hotspot for the treatment of related diseases. To date, the clinically available MC stabilizers are limited. Considering the rapidly increasing incidence rate and widespread prevalence of MC-related diseases, a comprehensive reference is needed for the clinicians or researchers to identify and choose efficacious MC stabilizers. This review analyzes the mechanism of MC activation, and summarizes the progress made so far in the development of MC stabilizers. MC stabilizers are classified by the action mechanism here, including acting on cell surface receptors, disturbing signal transduction pathways and interfering exocytosis systems. Particular emphasis is placed on the clinical applications and the future development direction of MC stabilizers.
1 Introduction
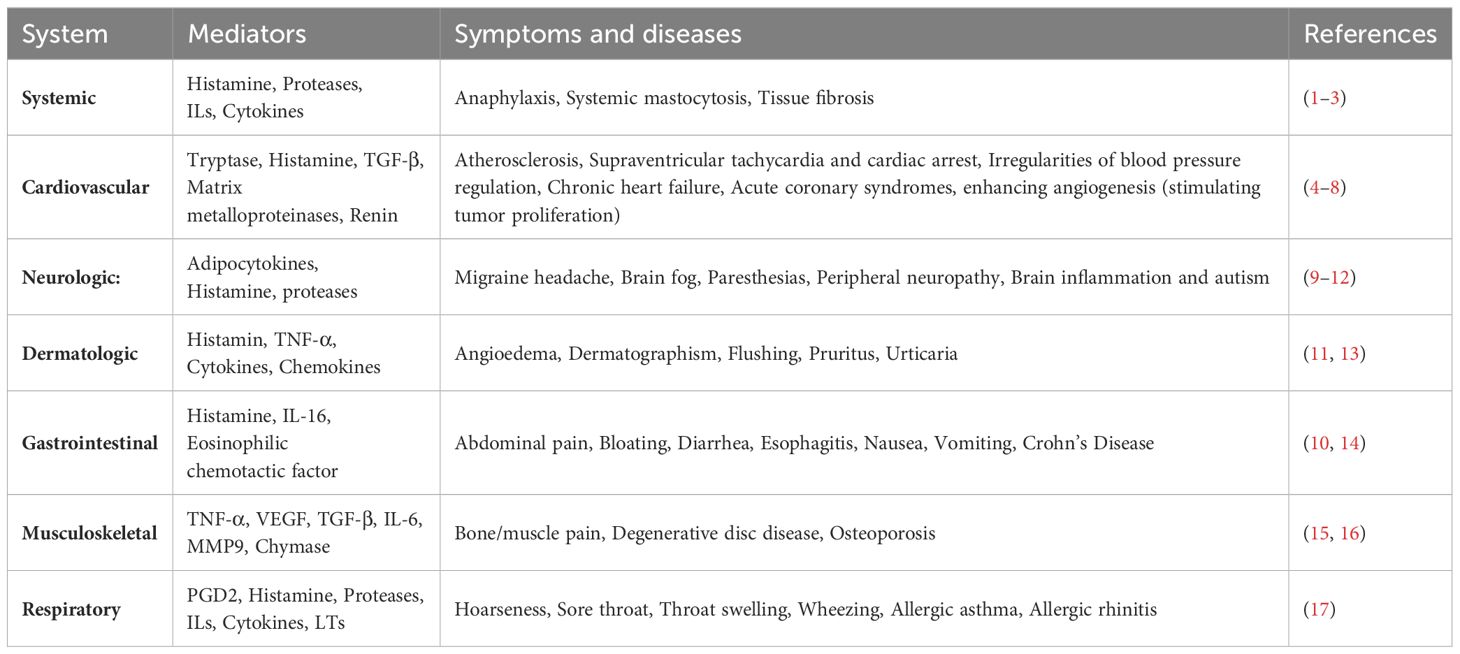
Mast cells (MCs) are bone-marrow-derived haematopoietic cells involved in a multitude of diseases/disorders, including allergies, inflammation, migraine headache, cardiovascular diseases, autoimmune disease, cancer and even death (Table 1) (9, 18). They are widely distributed in tissues, especially at sites exposed to the external environment, such as the skin, digestive tract and respiratory tract (19). As innate immune cells, MCs are involved in the early and rapid sensing of external invaders such as bacteria, viruses, fungi, parasites and other allergic proteins (20).
Upon activation, MCs release biologically active compounds, and exert physiological and pathological functions. The mediators of MCs can be classified into three types: i) preformed mediators stored in secretory granules; ii) neoformed or lipid mediators derived from membrane lipids; and iii) neosynthesized mediators produced following transcriptional activation. Histamine, chymase and tryptase are well-known MC preformed mediators. Histamine can induce vasodilation, bronchoconstriction, smooth muscle contraction and augment mucus secretion (1, 2), all of which are commonly associated with allergic and inflammatory reactions. In addition, histamine can also stimulate tumor proliferation by enhancing angiogenesis (4, 5). Chymases and tryptases are serine proteinases and exclusively expressed by MCs, which can produce the coronary constrictor angiotensin and induce proteolytic changes in high density lipoprotein particles (6, 7). They are related to a number of pathological states including inflammation, arthritis, innate immune defence, glomerulonephritis, abdominal aortic aneurism formation and tumor angiogenesis (21, 22). Based on serine proteinase composition, MCs can be divided into two subsets: a subset that contains the tryptase and chymase (MCTC), and a subset that contains only tryptase (MCT) (23). Histamine and tryptases released by activated meningeal (dural) MCs play important roles in the migraine headache pathophysiology mainly through the complex bidirectional relationship with calcitonin gene-related peptide (CGRP) (9). The activation of mast cell signaling pathways leads to the rapid production and release of neoformed mediators, representing by prostaglandins (PGs) and leukotrienes (LTs) (10). PGs contribute to mucus production, leukocyte recruitment, increased vascular permeability and nerve cell activation (10). LTs exert local effects on the vascular endothelium by promoting the recruitment of eosinophils and neutrophils, which is beneficial to host defend against bacterial infections (10). Neosynthesized mediators produced following transcriptional activation and regulated by the type of stimuli and receptor including cytokines, CXC-chemokine ligands (CXCL) and CC-chemokine ligands (CCL) (11). Transforming growth factor-β (TGF-β) released by MCs has been shown to promote Treg function contributing to controlling autoimmune and allergic inflammation (24). More detrimentally, it is extensively implicated in the pathogenesis of fibrosis (3). Other cytokines, associated with type 1and 2 T-helper cell responses such as interferon-gamma (IFN-γ), IL-2, IL-4, IL-3 and TNF-α, are primarily involved in inflammatory responses that include alopecia areata, obesity, diabetes and laminitis (25). The chemokines CCL5 and CXCL8 can recruit immune cells to sites of infection (26).
The diverse cellular functions and ubiquitous distribution make MCs a hotspot for the treatment of numerous diseases, especially allergic diseases (24). To date, the clinically available MC stabilizers are limited. Due to the rapidly increasing incidence rate and widespread prevalence of MC-related diseases, a comprehensive reference is needed for the clinicians or researchers to identify new effective MC stabilizers. The present review classifies MC stabilizers based on their mechanism. Particular emphasis is placed on the clinical applications and the future development direction of MC stabilizers.
2 Process of MC activation
MC activation is regulated by surface receptors (27), including FcϵRI, KIT, Mas-related G protein coupled receptor X2 (MRGPRX2) and natural killer (NK) receptor. The FcϵRI-dependent pathway is the most recognized MC activation pathway with the crosslink of FcϵRI caused by the recognition of allergens to bound IgE. The crosslink leads to spleen tyrosine kinase (Syk)-dependent phosphorylation and activation of the SRC-family kinases FYN and LYN, causing the phosphorylation of adaptor proteins, including linker For Activation of T-Cells (LAT) and Grb2-related adaptor protein (GAB2) (28). The downstream pathways consist of the PLCγ and the phosphatidylinositol 3-kinase (PI3K) signalling pathways (28). Signal molecule recruitment is followed by activation of second messenger molecules, including inositol triphosphate (IP3), diacylglycerol (DAG) and PtdIns, which activate protein kinase C (PKC) and increase intracellular Ca2+. Along with Ca2+ mobilization, the degranulation machinery is triggered. Granules containing mediators move to the plasma membrane in a microtubule-dependent manner along with the production of lipid mediators and cytokines (Figure 1).
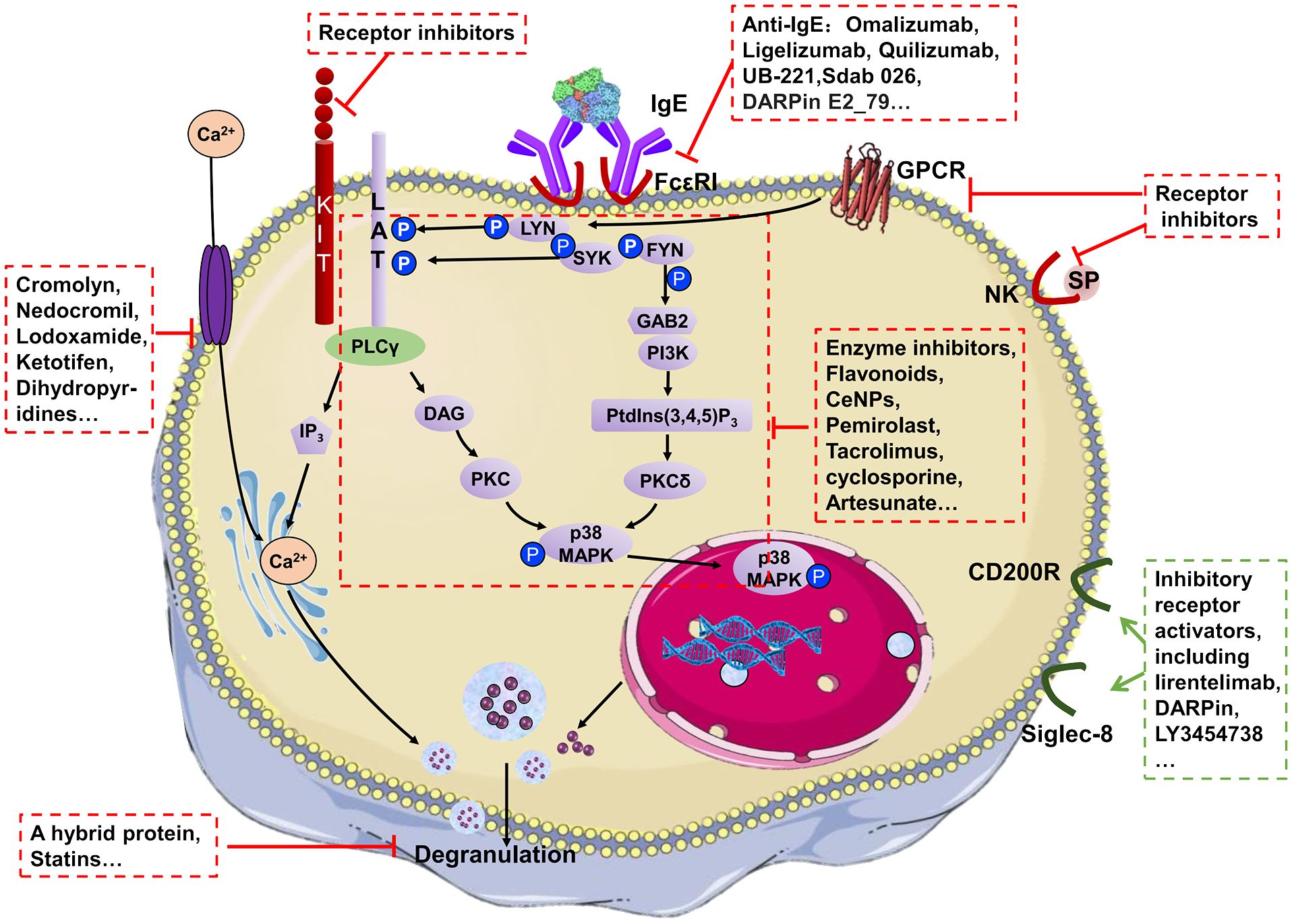
Figure 1 A highly simplified mechanism for MC degranulation and the action mechanism of MC stabilizers.
FcϵRI -independent pathways mediated by KIT, NK or MRGPRX2 have also been found to serve pivotal roles in the pathophysiology of various allergic and inflammatory conditions (Figure 1) (29). KIT, NK and MRGPRX2 can also induce autophosphorylation at multiple tyrosine residues in the cytoplasmic tail resulting in the recruitment of signal molecules and leading to MC degranulation (Figure 1). These receptors can both be the ‘prime’ method for MC activation and complementary of FcϵRI-dependent pathways. Among them, MRGPRX2 is a prominent receptor responsible for FcϵRI-independent allergic reactions, including itch, rosacea, urticaria and adverse drug reactions (30). It can be activated by a wide range of stimuli including cysteine proteases, neuropeptides, small cationic molecules and peptides with amphipathic properties, and drugs, playing important roles in host defence, immunomodulation, inflammatory diseases and pseudo-allergic drug reactions (31, 32). The pathway of MRGPRX2-mediated activation is similar to that of the FcϵRI-dependent pathway, including Ca2+ mobilizing and activation of downstream signals such as Erk1/2, JNK, p38 and PI3K/AKT (33).
3 MC-stabilizing agents
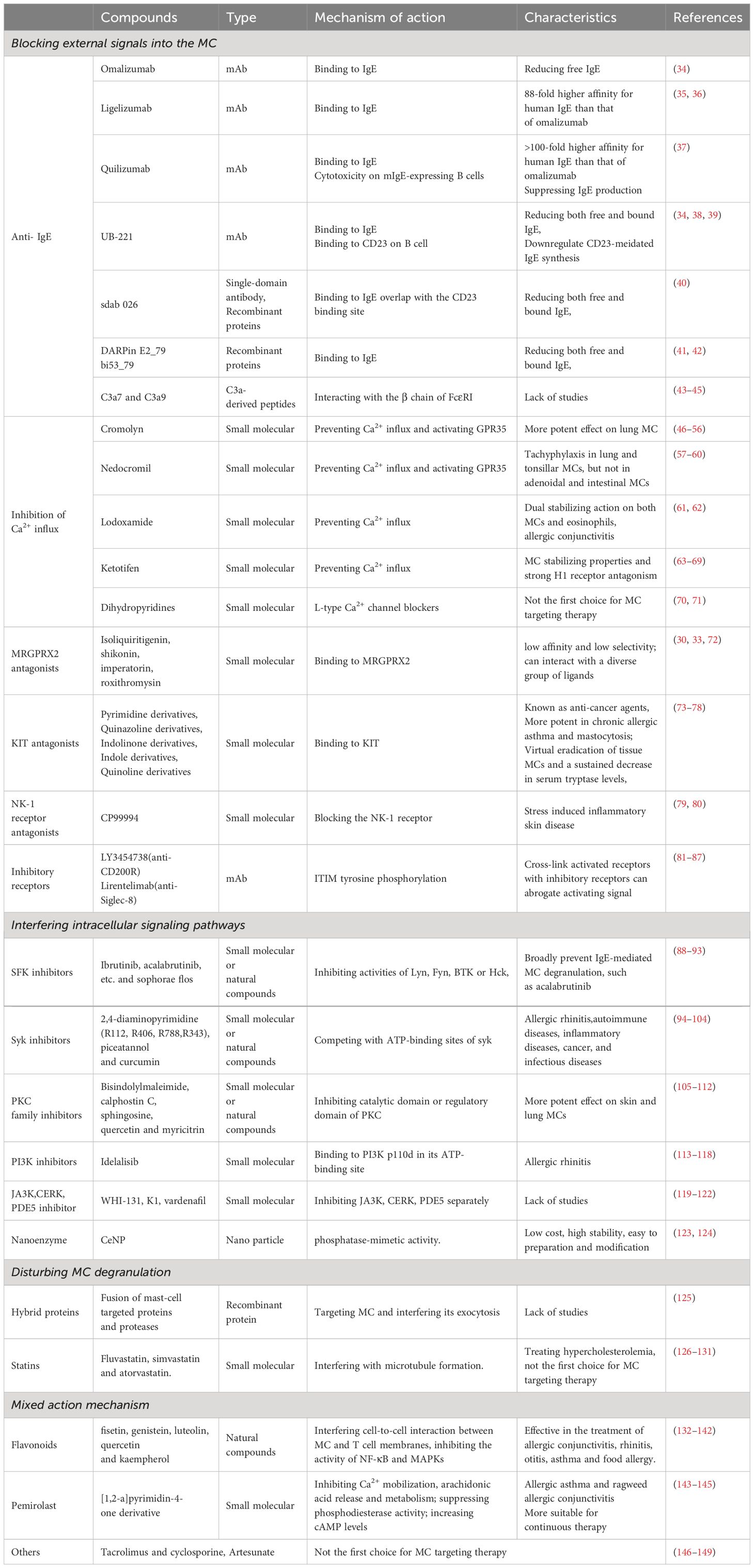
Regarding various MC activation pathways, a diverse array of MC stabilizers has been reported. The mechanism of action for MC stabilizers can be classified into three categories: i) Blocking external stimulus signals into cells; ii) inhibiting intracellular signalling pathways; and iii) disturbing degranulation (Figure 1, Table 2).
3.1 Blocking external signals into the MCs
3.1.1 Anti- IgE
IgE plays a central role in MC activation. The ability to reduce circulating IgE with a humanized monoclonal antibody (mAb) such as omalizumab, ligelizumab, quilizumab and UB-221 represents a new approach for stabilizing MCs (150). Omalizumab is a first generation anti-IgE mAb that was originally designed to reduce patients’ sensitivity to inhaled or ingested allergens (34). It selectively binds to the Cϵ3 domain of soluble IgE, thus immobilizing and preventing IgE-FcϵRI binding (151). Combining omalizumab (300 mg/month subcutaneously) with anti-inflammatory agents and/or pimecrolimus, a calmodulin inhibitor, can achieve better therapeutic efficacy in MC-associated diseases (152), including allergic asthma, allergic rhinitis and urticaria. Apart from allergic diseases, omalizumab also plays an important role in infectious diseases, such as aspergillosis. Omalizumab treatment reduced exacerbations and oral corticosteroid use, improved lung function and asthma control in patients with allergic bronchopulmonary aspergillosis (ABPA) and was well-tolerated (153). It is also demonstrated to enhance plasmacytoid dendritic cell antiviral responses (154).
Ligelizumab is a second-generation anti-IgE mAb with an 88-fold higher affinity for human IgE than omalizumab (35, 36). In a phase 2b clinical trial (NCT02477332), ligelizumab therapy of 72 mg or 240 mg had a higher percentage of complete control of symptoms in patients with chronic spontaneous urticaria compared to omalizumab at a dose of 300 mg, or placebo, administered subcutaneously every 4 weeks (155). Different from omalizumab, ligelizumab cannot dissociate the combined IgE from FcϵRI. Quilizumab is also classified as a second-generation anti-IgE mAb. It selectively binds to the Cϵ3 and Cϵ4 domains of human IgE with a higher affinity (>100-fold) than omalizumab (37). Quilizumab can suppress IgE production by its antibody-dependent cell mediated cytotoxicity on IgE-expressing B cells. However, the results of clinical trials (NCT01987947, NCT01582503) showed that its effect on IgE production and B cells could not bring a clinically meaningful benefit for adults with refractory chronic spontaneous urticaria (CSU) and allergic asthma when used at a dose of 300 mg monthly (156, 157). UB-221 is a third-generation anti-IgE mAb (38). Compared with the previous two generations, UB-221 binds to IgE with a higher affinity and reduces faster the IgE levels in circulation (34). UB-221 binds to CD23 on B cells and downregulates CD23-mediated IgE synthesis (39). Phase 1 studies are investigating the characteristics and effect of intravenous UB-221 in patients with CSU (150). Treatment with IgE mAb provides a new era for the management of severe allergic conditions (158).
Apart from traditional mAbs, recombinant proteins have been reported to possess anti-IgE activity, including recombinant humanized single-domain antibody (sdab) and designed ankyrin repeat proteins (DARPins) (159). The sdab 026 was reported to reduce both free and bound IgE, which is more efficient than omalizumab (40). Sdab 026 targets IgE by binding to an epitope within the Fc domains that markedly overlap with the CD23-binding site instead of the FcϵRI-binding site (40). The DARPin E2_79 is a fusion of two anti-IgE DARPins that not only prevents binding of free IgE to FcϵRI, but also removes receptor-bound IgE from the cells in a concentration- and time-dependent manner (41). Moreover, the fusion of DARPin E2_79 with the non-inhibitory anchor DARPin E3_53 results in a bi-paratopic anti-IgE binder, bi53_79, with markedly enhanced disruptive efficacy (42). Compared with mAbs, these recombinant proteins exhibit increased stability and high production yield in simple expression systems. Advanced strategies for multiple targeting and half-life extension can be easily applied to them. Delivery in functional form via mucosal and airway tissues may be possible using these small-size IgE inhibitors promoting anti-IgE application.
A patent reported that the C3a-derived peptides C3a7 and C3a9 can interact with the β-chain of FcϵRI on MCs and decrease the probability of IgE FcϵRI binding, resulting in suppression of signal transduction (43–45). Although C3a7 and C3a9 can inhibit MCs’ function, in-depth studies are lacking and their specific functions are largely unknown.
3.1.2 Prevention of Ca2+ influx into MCs
The increase in intracellular Ca2+ is a key step for MC activation. Intracellular Ca2+ is necessary for microtubule assembly, microfilament contraction, vesicle fusion to the cell membrane and subsequent degranulation (160). Prevention of Ca2+ influx into MCs is the action of mode of most clinically approved MC stabilizers.
3.1.2.1 Cromolyn
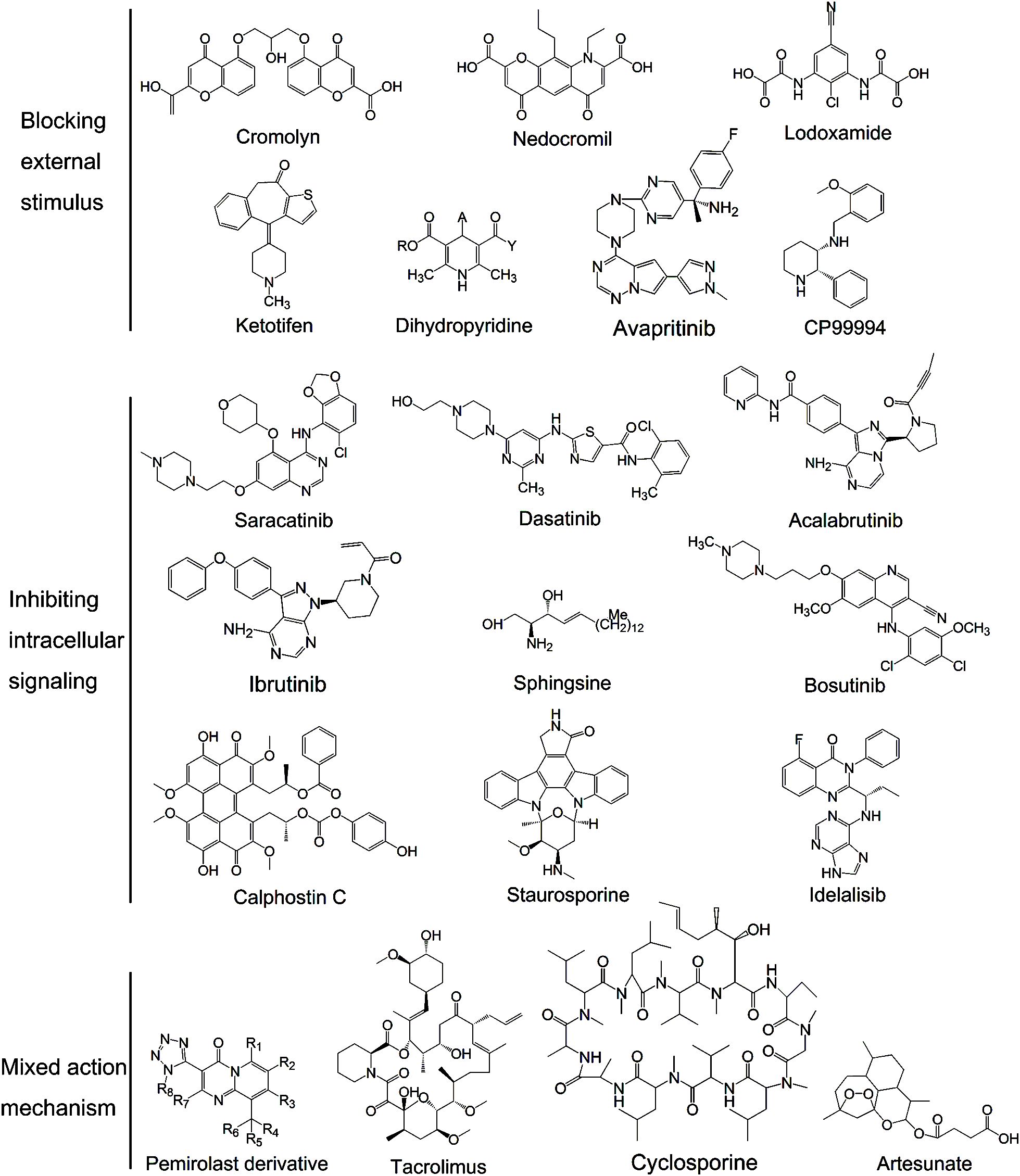
In 1965, cromolyn, also known as 5,5 - [(2 - hydroxytrimethylene) bis - (oxy)]4 - oxo - 4H-1-benzopyran-2-carbox-ylic acid (Figure 2), was first synthesized during experiments for the drug khellin used for cardiovascular disease treatment. It is the most commonly used MC stabilizer that binds specifically to the Ca2+-binding protein on MC membranes, forming a ternary complex with Ca2+. The ternary complex creates a blockage that stabilizes MC membranes and prevents degranulation (46). Recently, it was also shown to have agonist activity at GPR35 (47) which is predominantly expressed in the gastrointestinal tract and is closely related to inflammatory bowel diseases (48). GPR35 is coupled with numerous effectors after agonist stimulation, causing a series of downstream events, including ion channel inhibition and transient Ca2+ reduction (49). However, this discovery differs from previous findings showing that GPRs are activated receptors and GPR35 is upregulated upon stimulation with allergens (50). Therefore, further investigation concerning this potential mechanism is necessary.
Cromolyn has a potent MC stabilizing effect, especially on lung MCs. Thus, it is widely used for the treatment of allergic diseases such as rhinitis and asthma. It can improve acute and chronic injury in lung transplant animal models, but the precise mechanism of action remains unknown (51). Due to its large molecular weight, highly hydrophilic and ionisable character, cromolyn is poorly absorbed by the gastrointestinal tract. Thus, inhalation is the preferred delivery method. Cromolyn must be taken 4-8 times daily due to its short half-life (52) which results in a poor compliance. Recent studies (53) found that cromolyn was able to form niosomes, giving this drug a higher percutaneous permeation profile and the possibility for passive transdermal delivery. Besides, a series of metal complexes derived from cromolyn were recently designed, including cromolyn-Zn, -Mg and -Ca. These new metal complexes can prolong the action time and reduce adverse reactions (54). Compared with other MC stabilizers, cromolyn is preferred for patients with cardiovascular diseases (55), obesity and diabetes (56).
3.1.2.2 Nedocromil
Nedocromil sodium, also known as [9-ethyl-6, 9-dihydro-4, 6-dioxo-10-propyl-4H- pyrano (3,2-g) quinoline-2,8- dicarboxylic acid; Figure 2], is classified as a benzopyrone and is a second-generation cromolyn drug. Its mechanism of action is similar to cromolyn in preventing Ca2+ influx and activating GPR35. Apart from Ca2+, nedocromil is capable of inhibiting chloride ion flux in MCs (57). Electrolytes with low ion numbers, such as K+ and Cl-, are likely to affect cell membrane potential and inhibit Ca2+ influx indirectly.
Nedocromil shows tachyphylaxis in lung and tonsillar MCs, but not in adenoidal and intestinal MCs (58). It is more powerful than cromolyn sodium in inhibiting inflammatory mediator release in bronchial mucosa with a better safety profile (59). Thus, nedocromil can be used as an effective therapeutic for tonsillitis, asthma and asthmatic bronchitis. Moreover, a patent stated that nedocromil sodium was the first choice MC stabilizer for animal laminitis (60). However, detailed experiments were not provided, and the application requires more evidence.
3.1.2.3 Lodoxamide
Lodoxamide or 2-[2-Chloro-5-cyano-3-(oxaloamino)anilino]-2- oxoacetic acid (Figure 2), is another clinically effective MC stabilizer. Its derivatives, lodoxamide ethyl and lodoxamide tromethamine, have high anti-allergic activity. They can inhibit Ca2+ influx into MCs in response to antigen and activating GPR35 (48, 161). Lodoxamide can be used to control various allergic responses due to its dual stabilizing action on both MCs and eosinophils. Especially, it is widely used for allergic conjunctivitis with a better effect than cromolyn and nedocromil (61). In addition, animal studies (62) showed that injection of lodoxamide inhibited CP48/80-induced hypotension, which indicated the therapeutic effect of lodoxamide on cardiovascular disease.
3.1.2.4 Ketotifen
Ketotifen or [4-(1-methyl-4-piperidylidene)-4h-benzo[4,5]cyclohepta [1,2-b]thiophen-10(9H)-one fumarate; Figure 2], is a benzocycloheptathiophene derivative with MC stabilizing properties and strong Histamine type 1 (H1) receptor antagonism (63). It blocks Ca2+ channels essential for MC degranulation resembling the function of cromolyn, which is its main mechanism of action (64). Besides, ketotifen was also reported to suppress the process of exocytosis in a dose-dependent manner by counteracting the plasma membrane deformation in degranulating MCs (65). Ketotifen is widely used in IgE-mediated allergic reactions, including dermatitis, urticaria, asthma, and food or drug allergy (66). Its ability to inhibit passively induced skin allergy and allergic airway obstruction is 6 and 50 times stronger than that of cromolyn, respectively. Apart from the anti-allergy effects, it also plays major roles in prevention of UV-induced wrinkle formation, reduction of joint capsule fibrosis and improvement of sperm quality, chromatin integrity and pregnancy rate after varicocelectomy (67–69). Due to its central nervous system inhibitory effects and anticholinergic effects, ketotifen should not be taken prior to driving, operating heavy machinery or performing athletic endeavours.
3.1.2.5 Dihydropyridines
Dihydropyridines (Figure 2) are L-type Ca2+ channel (LTCC) blockers usually used in the treatment of hypertension. MCs express dihydropyridine-sensitive LTCCs, which is likely the mechanism by which dihydropyridines stabilize MCs (70). In animal experiments, dihydropyridines showed therapeutic effects in the treatment of bronchoconstriction and ocular allergies (71). However, these drugs are not the first choice in the treatment of MC-related diseases and further clinical studies are needed.
3.1.3 Promoting the potassium efflux
Apart from Ca2+ channels, the K+ and Cl − channels may play a key role in activation processes of MCs since these channels modulate cell membrane potential and inhibit Ca2+ influx indirectly. A recent study showed that a selective TWIK-related spinal cord potassium (TRESK) channel activator, cloxyquin, dose-dependently prevented excitotoxicity-induced degranulation of brain MCs and decreased the number of MCs (162). However, it is unknown whether TRESK channels are expressed in MCs, and the MC stabilizing effect of cloxyquin needs to be further studied.
3.1.4 Targeting receptors on the MC surface
3.1.4.1 MRGPRX2 antagonists
MRGPRX2 is a member of the Mas-related gene receptor family and it is emerging as a prominent receptor involved in non-IgE-mediated allergic reactions, including urticaria, rosacea, itch, atopic dermatitis and adverse drug reactions (30). Due to its low selectivity and affinity, MRGPRX2 can interact with a diverse group of ligands such as neuropeptides, antimicrobial peptides and FDA-approved drugs (72), playing a critical role in promoting MC-mediated host defence. Naturally occurring compounds such as isoliquiritigenin, shikonin, imperatorin and roxithromysin were shown to bind to MRGPRX2 in molecular docking studies and surface plasmon resonance (33). They could inhibit C48/80 or substance P (SP)-induced passive cutaneous anaphylaxis in mice. Although a series of natural and small-molecule MRGPRX2 antagonists were discovered, none of them is in clinical use. Combining new protein analysis techniques such as cryoEM and X-ray crystallography with three-dimensional (3D) structure analysis of MRGPRX2 antagonist complexes will enable the rational design of MRGPRX2 antagonists with higher affinity.
3.1.4.2 KIT antagonists
KIT inhibitors can inhibit the binding of stem cell factor (SCF) to the KIT receptor thereby inhibit KIT-dependent MC activation. These agents can be classified on the basis of their parent scaffolds into six categories, including pyrimidine derivatives, particularly N-phenyl-2-pyrimidine-amine, quinazoline derivatives, indolinone derivatives, particularly pyrrol-substituted indolinones, indole derivatives, quinoline derivatives and others (73–77). KIT inhibitors are usually known as anti-cancer agents. They also have therapeutic value for the treatment of MC-related diseases, especially chronic allergic asthma and mastocytosis. The long-term treatment with KIT antagonists such as avapritinib can cause a sustained decrease in serum tryptase levels and the virtual eradication of tissue MCs (78). However, the use of KIT inhibitors must be balanced against their potential side effects. Although KIT inhibitors have the advantages of immediate, complete, sustained and non-toxic remission in anti-allergy, this new drug indication warrants further studies in patients with allergic diseases (74–76).
3.1.4.3 NK-1 receptor antagonists
NK-1 receptor is generally localized to skin MCs and considered to play a vital role in stress-induced inflammation when it combines with the substance P (79). CP99994, (2S,3S)-3-(2-methoxybenzylamino)-2-phenylpiperidine (Figure 2) (80), is a representative drug that blocks the binding of substance P to the NK-1 receptor and inhibits NK-1-dependent MC degranulation. As an NK antagonist, CP99994 holds therapeutic potential in the treatment of stress-induced inflammatory skin diseases. No evidence showed that CP99994 also has an inhibitory effect on other activation pathways (79).
3.1.4.4 Silencing MC through inhibitory receptors
Inhibitory surface receptors such as Siglec-6, Siglec-8, FcγRIIB, CD200R and CD300a are able to inhibit MC activity (81–83). Inhibition is mainly accomplished through immunoreceptor tyrosine-based inhibitory motifs (ITIMs) in their cytoplasmic tail that can reverse one or more tyrosine phosphorylation steps critical to progressive signal transduction. The anti-Siglec-8 mAb (lirentelimab) can selectively inhibit MCs and deplete eosinophils. In omalizumab-naive and omalizumab-refractory patients with CSU, lirentelimab decreased the degree of disease activity by 77% and 45% respectively at week 22 according to urticaria activity score (NCT03436797) (84). As the Siglec-8 receptor is also expressed on eosinophils, lirentelimab can reduce the numbers of both blood eosinophil and gastrointestinal MCs in patients with eosinophilic gastrointestinal diseases. Apart from lirentelimab, the CD200R agonist LY3454738 was also developed for the treatment of atopic dermatitis and CSU (85). Bispecific antibodies, that cross-link activated receptors, FcϵRI or KIT, with inhibitory receptors (CD300a or FcγRIIb), could abrogate FcϵRI- or KIT-induced signalling (86, 87). For example, the DARPin-Fc fusion protein can aggregate FcϵRI-bound IgE with FcγRIIb and block IgE-FcϵRI binding faster, indicating that it is more efficient than omalizumab, causing the dissociation of preformed ligand-receptor complexes (85).
3.2 Interfering intracellular signalling pathways
MC activation is accomplished by various signalling enzymes. Inhibiting the activity of these vital enzymes can disturb the signalling pathways and stabilize MCs to some extent.
3.2.1 Src family kinase inhibitors
The SFKs Lyn, Fyn, Bruton’s tyrosine kinase (BTK) and Hck participate at the start of activation-induced MC signalling (88). Suppressing the activity of SFKs can reduce the symptoms of allergic diseases especially asthma and rhinitis (89). A total of >20 different drugs were proved to inhibit SFKs, including synthetic drugs such as saracatinib, dasatinib, ibrutinib and acalabrutinib, and natural drugs such as sophorae flos (Figure 2) (90). BTK inhibitors can broadly inhibit FcϵRI-dependent MC activation and cytokine production, thus preventing allergen-induced contraction of isolated human bronchi (91). Acalabrutinib, a BTKi, can completely prevent moderate IgE-mediated anaphylaxis in mice and protect against death during severe anaphylaxis (92). Apart from MC activation, SFKs also play crucial roles in signal transduction and regulation of other cell biological processes, such as proliferation, differentiation and apoptosis; research is focused on the role and mechanism of action of SFKs in tumorigenesis instead of allergic diseases (93).
3.2.2 Syk inhibitors
Syk is a 72 kDa non-receptor tyrosine kinase containing two SRC homology 2 domains and a kinase domain. Its expression is highest in haematopoietic cells (94). Syk acts as a central initiator in the MC activation signal pathway. Inhibitors of Syk can be classified on the basis of their parent scaffolds, represented by pyrimidines, prazolyl, 1,6-naphthyridones, pyrido[3,4-b]pyrazine and their derivatives (95–98). These compounds inhibit Syk kinase by competing with ATP-binding sites (99, 100). The most widely studied Syk inhibitors in clinical trials are R112, R406, R788 and R343 (94, 101) which belong to the 2,4-diaminopyrimidine family. Except for small molecule compounds, natural substances such as piceatannol and curcumin also possess inhibitory activity of Syk (102, 103). A phase II study showed that intranasal dosing of Syk inhibitors showed a rapid onset of action without serious side effects in the treatment of allergic rhinitis (94). However, as in the case of SFK inhibitors, Syk is also a vital signal transducer of activated immunoreceptors in multiple downstream events, which differ depending on the cell type, including proliferation, differentiation and phagocytosis. Therefore, except for allergic diseases, Syk inhibitors were also reported to be an attractive target for therapeutic interventions for autoimmune and inflammatory diseases, cancer and infectious diseases, including rheumatoid arthritis, leukemias and plasmodium falciparum malaria (104).
3.2.3 PKC family inhibitors
PKC is a family of protein kinase enzymes that phosphorylate hydroxyl groups on threonine and serine amino acid residues to control the protein function in MC activation pathways (105). The PKC structure consists of a catalytic C-terminal domain and a regulatory N-terminal held together by a hinge region (106). Based on their different sites of action, PKC inhibitors can be classified into catalytic and regulatory domain inhibitors. Clinical trials of PKC candidates are mainly focused on those that inhibit the catalytic domain. The catalytic domain is a highly conserved region throughout the PKC family, making it challenging to selectively target a particular isoform (107). The optimal structure of the catalytic domain inhibitor is bisindolylmaleimide, a staurosporine analog, while the two optimal inhibitors of the regulatory domain are calphostin C and sphingosine (Figure 2) (106, 107). Apart from synthetic small-molecule inhibitors, a couple of natural compounds also have inhibitory activity, including quercetin and myricitrin (108, 109). PKC inhibitors can be used in systemic mastocytosis and asthma (110). Skin and lung MCs were shown to be more sensitive to PKC downregulation than other MCs (111), indicating a good therapeutic effect of PKC inhibitors for patients with skin and airway allergic diseases. Except for the MC activation pathway, PKCs are also involved in multiple signal transduction systems that control cell proliferation, differentiation, apoptosis, survival, migration and invasion. Studies recommend that PKC inhibitors can be applied to other diseases including cancer, neurological and cardiovascular diseases, and infections (112).
3.2.4 PI3K inhibitors
PI3Ks are a family of intracellular heterodimeric lipid kinases responding to environmental factors such as nutrition and growth factors. They regulate a variety of biological functions, including cell growth, differentiation, proliferation, metabolism, genomic stability, motility, angiogenesis and protein synthesis (113). Idelalisib (Figure 2) is a potent and representative PI3K inhibitor that was approved for the treatment of non-Hodgkin lymphoma and chronic lymphocytic leukemia (114–116). It binds noncovalently and reversibly to PI3K p110d in its ATP-binding site (117). To assess its therapeutic effect in allergic disease, 41 patients with allergic rhinitis received idelalisib (100 mg twice daily) or a placebo for 7 days, and then received an allergen challenge on day 7 (NCT00836914) (118). After a 2-week washout period, subjects received the alternate treatment, and the allergen challenge was repeated. The study demonstrated that idelalisib reduced the allergic response after the allergen challenge with notable therapeutic effects regarding nasal symptoms, airflow and secretion weight compared with the placebo. No marked side effects were observed in patients at a dose of 100 mg idelalisib twice daily over the period of 7 days (118).
3.2.5 Other enzyme inhibitors
Apart from the aforementioned enzymes, a number of other enzymes involved in MC signalling pathways can also be the target of MC stabilizers: i) Janus kinase 3 (JAK3) is a member of the JAK family of tyrosine kinases and is involved in cytokine receptor-mediated intracellular signal transduction. WHI-131 is the inhibitor of JAK3 which inhibits both Ca2+ ionophore and IgE-mediated MC degranulation (119); ii) Ceramide Kinase (CERK) acts as a Ca2+-sensor for MC activation. K1 is the inhibitor of CERK which can notably suppress both Ca2+ ionophore and IgE-mediated MC activation (120). K1 does not inhibit IgE-/antigen-induced tyrosine phosphorylation or subsequent Ca2+ increase, indicating a distinctive pathway (121); iii) PDEs catalyse the hydrolysis of 3’,5’-cyclic adenosine monophosphate (cAMP) and 3’,5’-cyclic guanosine monophosphate (cGMP) in cells. Both cAMP and cGMP are important secondary messengers involved in MC activation. As an inhibitor of PDE5, vardenafil was found to ameliorate MC-mediated allergic reactions and reduce histamine release (122), providing evidence for the potential MC-stabilizing properties of PDE inhibitors.
3.2.6 Nanoenzyme
Recently, ceria nanoparticles (CeNPs) were reported to be an effective phosphatase-mimetic MC nano-stabilizer protecting against allergic diseases (123). The regenerable catalytic hotspots of surface oxygen vacancies endow CeNPs with sustainable and excellent phosphatase-mimetic activity. The CeNPs can block the phospho-signalling cascades of MC activation, and thus inhibit the degranulation of allergic mediators and the resulting pathological responses (123). Compared with natural enzymes, the nanoenzyme shows advantages such as low-cost, easy preparation and high stability under harsh conditions (124). Its structure can further be modified to improve its targeting, providing a new research foundation for the development of MC stabilizers.
3.3 MC stabilizers disturbing MC degranulation
3.3.1 Hybrid proteins
MC exocytosis is accomplished by a number of proteins, such as SNAP 25, synapbrevin, syntaxine and others (125). If one of these proteins is inactivated, for example via protease cleavage, the degranulation machine will be broken. A hybrid protein that consists of at least one MC-targeted protein and one protease was reported to disturb MC exocytosis. The protein must attach to the MC or be taken up by the MC, including IgE or anti-IgE. The protease cleaves one or several proteins involved in MC secretion, which includes botulinum or tetanus toxin light chains and Neisseria gonorrhoea IgA protease. This hybrid protein can be synthesized in vitro or in the appropriate host cells by the gene recombination technology (fusion of protease and light chain genes). To date, these hybrid proteins have not entered clinical application. From animal studies, the recommended uses for allergic diseases include asthma, allergic dermatitis and allergic desensitization. It can also be taken as a preventive treatment when taking drugs with fatal allergic side effects (125).
3.3.2 Statins
Statins are a class of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors commonly used to treat hypercholesterolemia, including fluvastatin, simvastatin and atorvastatin. They bind to low-density lipoprotein (LDL) receptors expressed on MCs and suppress geranylgeranyl transferase by depletion of intracellular mevalonic acid, which deactivates small GTP-binding proteins (126). GTP-binding proteins are involved in microtubule formation. Owing to the importance of microtubules in granule translocation, granule secretion will be inhibited.
A number of studies have identified the therapeutic values of statins for the treatment of asthma. Fluvastatin was reported to suppress peripheral blood mononuclear cell proliferation and inflammatory responses in patients with allergic asthma (127). Simvastatin was shown to inhibit airway hyper-responsiveness in a murine model (128). The oral atorvastatin 40 mg daily, in conjunction with inhaled corticosteroids, was shown to improve lung function and reduce sputum macrophage counts in patients with mild-to-moderate atopic asthma (NCT00126048) (129). Furthermore, statins were demonstrated to have potential therapeutic value in the treatment of MC-related skin diseases, including alopecia areata, atopic dermatitis, psoriasis and mastocytosis (130). Fluvastatin was the most potent MC activation inhibitor among statins, suppressing IgE-induced cytokine secretion (131). Although statins may be useful for the treatment of MC-related diseases, they are mainly used to treat hypercholesterolemia.
3.4 MC stabilizers with mixed action mechanism
3.4.1 Flavonoids
Flavonoids are naturally occurring compounds with potent anti-inflammatory, antioxidant and MC-blocking activities, including fisetin, genistein, quercetin, luteolin and kaempherol. The backbone of flavonoids is similar to a part of the structure of cromolyn, and numerous flavonoids were identified possessing MC stabilization activity. Fisetin was proved to interfere cell-to-cell interaction between MC and T cell membranes, and inhibits the activity of NF-κB and MAPKs (132). Genistein inhibits proinflammatory cytokine production of human MCs through the suppression of the ERK pathway (133). Quercetin can decrease the activity of the PKC family. Compared with cromolyn, it is more effective in blocking MC cytokine release and treating contact dermatitis and photosensitivity (134). Luteolin (lut) and its novel structural analog 3’,4’,5,7-tetramethoxyluteolin (methlut) were also proposed as more effective MC stabilizers than cromolyn regardless of the trigger and the mediator measured (135). Methlut was more effective in inhibiting β-hexosaminidase (β-hex), TNF and histamine secretion. The mechanism of action for methlut may be due to its ability to inhibit intracellular Ca2+ increase, as well as NF-κB induction at both the transcriptional and translational levels without affecting cell viability.
Flavonoids were reported to be effective in the treatment of allergic conjunctivitis, rhinitis, otitis, asthma and food allergy. A patent demonstrated that flavonoids can also be used together with proteoglycans including chondroitin, keratan and dermatan sulfates to treat MC activation-induced diseases (136).However, the hypothesis has not been verified in vivo. Apart from flavonoids, a number of other naturally occurring compounds were reported to stabilize MCs including thymoquinone, capsaicin, coumarins, phenols, terpenoids and amino acids (137–142). They were proved to inhibit MC degranulation and decrease the number of MCs, such as thymoquinone and capsaicin (141, 142). Most of them are complex structures, and the precise mechanism by which they act remains largely unknown. It is hypothesised that, as in the case of flavonoids, a number of segments of the allergic signal cascade are targeted.
3.4.2 Pemirolast
Pemirolast or (9-Methyl-3-(1H-tetrazol-5-yl)-pyrido[1,2-a]pyrimidin-4-one; Figure 2), is a [1,2-a]pyrimidin-4-one derivative with MC stabilizing properties. Novel compounds derived from pemirolast have been found according to the deuterium kinetic isotope effect. The structural formula is shown in Figure 2. R1-R8 can be hydrogen- or deuterium-independent with at least one deuterium. Deuterium-enriched compound would not cause any additional toxicity since D2O or DHO is formed during drug metabolism. Other elements may also be selected from less prevalent isotopes including 13C or 14C for carbon, 33S, 34S, or 36S for sulphur, 15N for nitrogen, and 17O or 18O for oxygen. Pemirolast and its derivatives can be potent inhibitors of Ca2+ uptake and release from intracellular stores. They can also suppress phosphodiesterase activity, increase intracellular cAMP levels, and inhibit arachidonic acid release and metabolism (143).
Pemirolast can inhibit both antigen and CP48/80-induced MC degranulation (144). It can be used in allergic diseases, especially allergic asthma and ragweed allergic conjunctivitis. In the treatment of ragweed allergic conjunctivitis, pemirolast potassium 0.1% is as efficacious and safe as nedocromil sodium 2% but is superior in comfort during topical application (145). Thus, pemirolast may be more suitable for continuous therapy (145). To obtain a better curative effect, pemirolast can be combined with other agents used in the treatment of MC degranulation-mediated diseases.
3.4.3 Tacrolimus and cyclosporine
Tacrolimus and cyclosporine (Figure 2) are immunomodulatory agents usually used to treat autoimmune diseases. Both cyclosporine (5mM) and tacrolimus (5mM) were shown to inhibit cytokine release from MCs. Cyclosporine effectively inhibits Ca2+-dependent protein phosphatase activity in MCs, while tacrolimus is considerably less effective (146). However, tacrolimus is ~100 times more potent than cyclosporine as an inhibitor of IgE-dependent MC degranulation, meaning that tacrolimus can stabilize MCs in a variety of ways (146). Although tacrolimus can stabilize MCs, it is not typically used in the treatment of MC related diseases (146).
3.4.4 Artesunate
Artesunate (Figure 2) is usually used in the treatment of malaria. Besides, artesunate was found to inhibit Syk and PLCγ1 phosphorylation, IP3 formation, intracellular Ca2+ increase in MCs (147) as well as downregulate T helper 17 cell responses (148). It can block IgE-mediated MC degranulation in a dose-dependent manner (147). In animal models, artesunate has a protective effect in allergic asthma and showed anti-inflammatory effects similar with dexamethasone (149).
3.4.5 Endogenous stabilizers of MCs
Endogenous stabilizers of MCs play a vital role in controlling activation of MCs and consequently in the immune homeostasis of the body. For instance, as an endogenous cannabinoid, anandamide inhibited the degranulation of dural MCs through CB2 receptors on the surface of MCs (163). Heparin, chondroitin sulphate and spermine from MCs can inhibit the activation of MCs (164). Additionally, there are different endogenous molecules which can inhibit MC activation such as progesterone, testosterone, corticosterone and 2-arachidonoyl glycerol (2-AG) (164).
4 Discussion
The widespread tissue distribution and versatility of MCs make them a hotspot in the studies of related diseases/disorders. They exert their function mainly through mediators released during activation. Inhibiting one of the activated receptors on the MC surface will prevent MCs from being activated by certain substances. Apart from inhibiting activated receptors, activating inhibitory receptors on MCs can also stabilize them. Co-aggregating inhibitory receptors with activated receptors can reverse the activation mediated by the latter, providing a new direction for research and development of MC stabilizers.
The influx of extracellular Ca2+ is a vital event of MC degranulation. Inhibiting Ca2+ influx is the mechanism of action of most clinical MC stabilizers, represented by cromolyn sodium. Besides, interfering Cl- and K+channels can also influence the Ca2+ influx indirectly through their effects on cell membrane potential, which may offer a novel method for the treatment of MC-related diseases/disorders. Together with the influx of extracellular Ca2+, a series of phosphorylation cascades contribute to the activation of MC. Inhibiting the enzymes involved in MC activation signal pathway can prevent MC degranulation. However, these enzymes are not usually specific to MC activation pathway. They always participate in other cell biological processes, such as proliferation, differentiation and apoptosis. This may explain why enzyme inhibitors without MC-targeting are often not used as MC stabilizers in clinical practice, and clinical drugs for other indications including stains, tacrolimus, cyclosporine and others may have a MC stabilization activity. Low targeting is an urgent problem for MC stabilizers. The mAbs and humanized sdabs are the epoch-making progress of the development of MC stabilizers with high targeting and minimal side effects. There are three generations of anti-IgE mAbs including omalizumab, ligelizumab, quilizumab and UB-221. The improvement of each generation is mainly focused on the affinity for IgE and the reduction of IgE production. Compared with mAbs, sdabs exhibit high production yield in simple expression systems and extraordinary stability. State-of-the-art strategies for multiple targeting and half-life extension can be easily applied to them.
Progress in new material therapy technology has promoted the development of MC stabilizers. Normally, nanomaterials used for medical applications are synthesized using specific polymers, lipids, nano enzymes or proteins. Cell surface receptors, such as FcϵRI or MRGPRX2, can be applied to target nanoparticles (NPs) to specific subsets of MCs. By recognizing the receptors on MCs in their tissue environments, NPs could be tailored to alleviate symptoms such as specific allergies in individual patients, thereby opening a new frontier in precision therapeutics. Furthermore, with the increasing interest in genetic modification, nucleic acid-containing NPs could be engineered to modify the gene necessary for regulating MC activation. MC activation does not always lead to pathology. A recent study showed that MC degranulation suppresses epileptic seizures through the serotonin in their granules (165). Thus, the approach based on genetic modification may interfere with MC-mediated inflammatory responses while preserving protective innate immune or tissue homeostatic functions (166). At present, the development of nano MC stabilizers is still at an early stage and has a broad scope in field of allergy research in the future.
MC leukemia (MCL) is a rare form of systemic mastocytosis with poor prognosis (167). Patients with MCL may benefit from MC-targeted therapies, including controlling MC-related symptoms and cytoreductive therapy. Interferon-α and steroids are usually used to control clinical symptoms. However, their effect is transient and limited (167). With the discovery of KIT mutations in MCL, KIT antagonist have become a hot spot in MCL therapy (168). KIT antagonist represented by midostaurin has been approved for patients with MCL (169) due to its strong inhibitory activity on neoplastic human MC carrying the KIT D816V mutation in preclinical and clinical settings (167). Avapritinib, dasatinib, masitinib and imatinib also hold therapeutic effects in MCL with different KIT mutated forms (167). Data detailing mutations are useful in supporting individualized treatment. Despite achieving initial success, the efficacy of these KIT-targeting agents on MCL prognosis requires further evaluation. Cytostatic drugs (represented by 2-CdA) have been shown to induce apoptosis of the human MC (169). However, these drugs may have unpredictable toxicity and adverse effects. Whether patients with MCL may benefit from combination polychemotherapy remains unknown. Chemotherapy combined with targeted therapy might be an interesting direction for MCL treatment.
Future studies of MC stabilizers should focus on the following: i) Using targeted agents or new material technology to develop more effective and targeted MC stabilizers; ii) preventing the detrimental response caused by MC activation while preserving its vital roles in host defence; iii) exploring and developing novel humanized MC culture technology and construction of humanized animal models to investigate the application of MC stabilizers; iv) increasing the number of clinical trials; and v) isolating compounds from biological agents as a promising way to develop new MC stabilizers.
Author contributions
MC: Writing – original draft, Writing – review & editing. YG: Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was partially supported by the National Natural Science Foundation of China (82302013), Natural Science Foundation of Jiangsu province (BK20230834), Changzhou Fourth Pharmaceutical Hospital Pharmaceutical Research Foundation of Nanjing Pharmaceutical Association (2022YX001). Research Personnel Cultivation Programme of Zhongda Hospital Southeast University (CZXM-GSP-RC107).
Acknowledgments
We appreciate the professional guidance provided by Ji-Fu Wei (worked at Jiangsu Cancer Hospital) and Hua Shao (worked at Zhongda Hospital) during the preparation and revision process of this paper.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Rozenberg I, Sluka SH, Rohrer L, Hofmann J, Becher B, Akhmedov A, et al. Histamine H1 receptor promotes atherosclerotic lesion formation by increasing vascular permeability for low-density lipoproteins. Arterioscler Thromb Vasc Biol. (2010) 30:923–30. doi: 10.1161/ATVBAHA.109.201079
2. Pettipher R. The roles of the prostaglandin D(2) receptors DP(1) and CRTH2 in promoting allergic responses. Br J Pharmacol. (2008) 153 Suppl 1:S191–9. doi: 10.1038/sj.bjp.0707488
3. Frangogiannis N. Transforming growth factor-beta in tissue fibrosis. J Exp Med. (2020) 217:e20190103. doi: 10.1084/jem.20190103
4. Zhang E, Zhang Y, Fan Z, Cheng L, Han S, Che H. Apigenin inhibits histamine-induced cervical cancer tumor growth by regulating estrogen receptor expression. Molecules. (2020) 25:1960. doi: 10.3390/molecules25081960
5. Ribatti D. Mast cells and macrophages exert beneficial and detrimental effects on tumor progression and angiogenesis. Immunol Lett. (2013) 152:83–8. doi: 10.1016/j.imlet.2013.05.003
6. Lee M, Kovanen PT, Tedeschi G, Oungre E, Franceschini G, Calabresi L. Apolipoprotein composition and particle size affect HDL degradation by chymase: effect on cellular cholesterol efflux. J Lipid Res. (2003) 44:539–46. doi: 10.1194/jlr.M200420-JLR200
7. Banafea GH, Bakhashab S, Alshaibi HF, Natesan Pushparaj P, Rasool M. The role of human mast cells in allergy and asthma. Bioengineered. (2022) 13:7049–64. doi: 10.1080/21655979.2022.2044278
8. Hermans M, Lennep JRV, van Daele P, Bot I. Mast cells in cardiovascular disease: from bench to bedside. Int J Mol Sci. (2019) 20:3395. doi: 10.3390/ijms20143395
9. Koyuncu Irmak D, Kilinc E, Tore F. Shared fate of meningeal mast cells and sensory neurons in migraine. Front Cell Neurosci. (2019) 13:136. doi: 10.3389/fncel.2019.00136
10. da Silva EZ, Jamur MC, Oliver C. Mast cell function: a new vision of an old cell. J Histochem Cytochem. (2014) 62:698–738. doi: 10.1369/0022155414545334
11. Theoharides TC, Tsilioni I, Ren H. Recent advances in our understanding of mast cell activation - or should it be mast cell mediator disorders? Expert Rev Clin Immunol. (2019) 15:639–56. doi: 10.1080/1744666X.2019.1596800
12. Toyoshima S, Okayama Y. Neuro-allergology: Mast cell-nerve cross-talk. Allergology Int. (2022) 71:288–93. doi: 10.1016/j.alit.2022.04.002
13. Radonjic-Hoesli S, Hofmeier KS, Micaletto S, Schmid-Grendelmeier P, Bircher A, Simon D. Urticaria and angioedema: an update on classification and pathogenesis. Clin Rev Allergy Immunol. (2018) 54:88–101. doi: 10.1007/s12016-017-8628-1
14. Frieri M. Mast cell activation syndrome. Clin Rev Allergy Immunol. (2018) 54:353–65. doi: 10.1007/s12016-015-8487-6
15. Wiet MG, Piscioneri A, Khan SN, Ballinger MN, Hoyland JA, Purmessur D. Mast Cell-Intervertebral disc cell interactions regulate inflammation, catabolism and angiogenesis in Discogenic Back Pain. Sci Rep. (2017) 7:12492. doi: 10.1038/s41598-017-12666-z
16. Lind T, Melo FR, Gustafson AM, Sundqvist A, Zhao XO, Moustakas A, et al. Mast cell chymase has a negative impact on human osteoblasts. Matrix Biol. (2022) 112:1–19. doi: 10.1016/j.matbio.2022.07.005
17. Elieh Ali Komi D, Bjermer L. Mast cell-mediated orchestration of the immune responses in human allergic asthma: current insights. Clin Rev Allergy Immunol. (2019) 56:234–47. doi: 10.1007/s12016-018-8720-1
18. Pejler G. Novel Insight into the in vivo Function of Mast Cell Chymase: Lessons from Knockouts and Inhibitors. J Innate Immun. (2020) 12:357–72. doi: 10.1159/000506985
19. Yang YS, Cao MD, Wang A, Liu QM, Zhu DX, Zou Y, et al. Nano-silica particles synergistically IgE-mediated mast cell activation exacerbating allergic inflammation in mice. Front Immunol. (2022) 13:911300. doi: 10.3389/fimmu.2022.911300
20. Sobiepanek A, Kuryk L, Garofalo M, Kumar S, Baran J, Musolf P, et al. The multifaceted roles of mast cells in immune homeostasis, infections and cancers. Int J Mol Sci. (2022) 23:2249. doi: 10.3390/ijms23042249
21. de Souza Junior DA, Santana AC, da Silva EZ, Oliver C, Jamur MC. The role of mast cell specific chymases and tryptases in tumor angiogenesis. BioMed Res Int. (2015) 2015:142359. doi: 10.1155/2015/142359
22. Caughey GH. Mast cell tryptases and chymases in inflammation and host defense. Immunol Rev. (2007) 217:141–54. doi: 10.1111/j.1600-065X.2007.00509.x
23. Schechter NM, Irani AM, Sprows JL, Abernethy J, Wintroub B, Schwartz LB. Identification of a cathepsin G-like proteinase in the MCTC type of human mast cell. J Immunol. (1990) 145:2652–61. doi: 10.4049/jimmunol.145.8.2652
24. Paivandy A, Pejler G. Novel strategies to target mast cells in disease. J Innate Immun. (2021) 13:131–47. doi: 10.1159/000513582
25. Mukai K, Tsai M, Saito H, Galli SJ. Mast cells as sources of cytokines, chemokines, and growth factors. Immunol Rev. (2018) 282:121–50. doi: 10.1111/imr.12634
26. Marshall JS. Mast-cell responses to pathogens. Nat Rev Immunol. (2004) 4:787–99. doi: 10.1038/nri1460
27. Xu H, Bin NR, Sugita S. Diverse exocytic pathways for mast cell mediators. Biochem Soc Trans. (2018) 46:235–47. doi: 10.1042/BST20170450
28. Gilfillan AM, Tkaczyk C. Integrated signalling pathways for mast-cell activation. Nat Rev Immunol. (2006) 6:218–30. doi: 10.1038/nri1782
29. Kunimura K, Akiyoshi S, Uruno T, Matsubara K, Sakata D, Morino K, et al. DOCK2 regulates MRGPRX2/B2-mediated mast cell degranulation and drug-induced anaphylaxis. J Allergy Clin Immunol. (2023) 151:1585–94. doi: 10.1016/j.jaci.2023.01.029
30. Baldo BA. MRGPRX2, drug pseudoallergies, inflammatory diseases, mechanisms and distinguishing MRGPRX2- and IgE/FcepsilonRI-mediated events. Br J Clin Pharmacol. (2023) 89:3232–46. doi: 10.1111/bcp.15845
31. Thapaliya M, Chompunud Na Ayudhya C, Amponnawarat A, Roy S, Ali H. Mast cell-specific MRGPRX2: a key modulator of neuro-immune interaction in allergic diseases. Curr Allergy Asthma Rep. (2021) 21:3. doi: 10.1007/s11882-020-00979-5
32. Foer D, Wien M, Karlson EW, Song W, Boyce JA, Brennan PJ. Patient characteristics associated with reactions to mrgprx2-activating drugs in an electronic health record-linked biobank. J Allergy Clin Immunol In practice. (2022) 11:492–9. doi: 10.1016/j.jaip.2022.11.001
33. Mi YN, Ping NN, Cao YX. Ligands and signaling of mas-related G protein-coupled receptor-X2 in mast cell activation. Rev Physiol Biochem Pharmacol. (2021) 179:139–88. doi: 10.1007/112_2020_53
34. Ling XJ, Wei JF, Zhu Y. Aiming to IgE: Drug development in allergic diseases. Int Immunopharmacol. (2023) 121:110495. doi: 10.1016/j.intimp.2023.110495
35. Wood RA, Chinthrajah RS, Eggel A, Bottoli I, Gautier A, Woisetschlaeger M, et al. The rationale for development of ligelizumab in food allergy. World Allergy Organ J. (2022) 15:100690. doi: 10.1016/j.waojou.2022.100690
36. Maurer M, Giménez-Arnau A, Bernstein JA, Chu CY, Danilycheva I, Hide M, et al. Sustained safety and efficacy of ligelizumab in patients with chronic spontaneous urticaria: A one-year extension study. Allergy. (2022) 77:2175–84. doi: 10.1111/all.15175
37. Cohen ES, Dobson CL, Käck H, Wang B, Sims DA, Lloyd CO, et al. A novel IgE-neutralizing antibody for the treatment of severe uncontrolled asthma. mAbs. (2014) 6:756–64. doi: 10.4161/mabs.28394
38. Nyborg AC, Zacco A, Ettinger R, Jack Borrok M, Zhu J, Martin T, et al. Development of an antibody that neutralizes soluble IgE and eliminates IgE expressing B cells. Cell Mol Immunol. (2016) 13:391–400. doi: 10.1038/cmi.2015.19
39. Kuo BS, Li CH, Chen JB, Shiung YY, Chu CY, Lee CH, et al. IgE-neutralizing UB-221 mAb, distinct from omalizumab and ligelizumab, exhibits CD23-mediated IgE downregulation and relieves urticaria symptoms. J Clin Invest. (2022) 132:e157765. doi: 10.1172/JCI157765
40. Jabs F, Plum M, Laursen NS, Jensen RK, Molgaard B, Miehe M, et al. Trapping IgE in a closed conformation by mimicking CD23 binding prevents and disrupts FcepsilonRI interaction. Nat Commun. (2018) 9:7. doi: 10.1038/s41467-017-02312-7
41. Eggel A, Baravalle G, Hobi G, Kim B, Buschor P, Forrer P, et al. Accelerated dissociation of IgE-FcepsilonRI complexes by disruptive inhibitors actively desensitizes allergic effector cells. J Allergy Clin Immunol. (2014) 133:1709–19 e8. doi: 10.1016/j.jaci.2014.02.005
42. Pennington LF, Gasser P, Brigger D, Guntern P, Eggel A, Jardetzky TS. Structure-guided design of ultrapotent disruptive IgE inhibitors to rapidly terminate acute allergic reactions. J Allergy Clin Immunol. (2021) 148:1049–60. doi: 10.1016/j.jaci.2021.03.050
43. Erdei A, Toth GK, Andrasfalvy M, Matko J, Bene L, Bajtay Z, et al. Inhibition of IgE-mediated triggering of mast cells by complement-derived peptides interacting with the Fc epsilon RI. Immunol Lett. (1999) 68:79–82. doi: 10.1016/S0165-2478(99)00033-4
44. Peterfy H, Toth G, Pecht I, Erdei A. C3a-derived peptide binds to the type I FcepsilonR and inhibits proximal-coupling signal processes and cytokine secretion by mast cells. Int Immunol. (2008) 20:1239–45. doi: 10.1093/intimm/dxn083
45. Andrasfalvy M, Peterfy H, Toth G, Matko J, Abramson J, Kerekes K, et al. The beta subunit of the type I Fcepsilon receptor is a target for peptides inhibiting IgE-mediated secretory response of mast cells. J Immunol. (2005) 175:2801–6. doi: 10.4049/jimmunol.175.5.2801
46. Geller-Bernstein C, Mazurek N, Berger G, Hemerich S, Loyter A, Berebi A, et al. The relationship between Ca++ ions and a protein specific to cromolyn in the degranulation of mast cells and basophils in the rat. Allerg Immunol (Paris). (1987) 19:56–8.
47. MacKenzie AE, Caltabiano G, Kent TC, Jenkins L, McCallum JE, Hudson BD, et al. The antiallergic mast cell stabilizers lodoxamide and bufrolin as the first high and equipotent agonists of human and rat GPR35. Mol Pharmacol. (2014) 85:91–104. doi: 10.1124/mol.113.089482
48. Duan J, Liu Q, Yuan Q, Ji Y, Zhu S, Tan Y, et al. Insights into divalent cation regulation and G(13)-coupling of orphan receptor GPR35. Cell Discovery. (2022) 8:135. doi: 10.1038/s41421-022-00499-8
49. Mackenzie AE, Milligan G. The emerging pharmacology and function of GPR35 in the nervous system. Neuropharmacology. (2015) 113:661–71. doi: 10.1016/j.neuropharm.2015.07.035
50. Yang Y, Lu JY, Wu X, Summer S, Whoriskey J, Saris C, et al. G-protein-coupled receptor 35 is a target of the asthma drugs cromolyn disodium and nedocromil sodium. Pharmacology. (2010) 86:1–5. doi: 10.1159/000314164
51. Chang JC, Leung J, Tang T, Holzknecht ZE, Hartwig MG, Duane Davis R, et al. Cromolyn ameliorates acute and chronic injury in a rat lung transplant model. J Heart Lung Transplant. (2014) 33:749–57. doi: 10.1016/j.healun.2014.03.004
52. Sinniah A, Yazid S, Flower RJ. The anti-allergic cromones: past, present, and future. Front Pharmacol. (2017) 8:827. doi: 10.3389/fphar.2017.00827
53. Tavano L, Nicoletta FP, Picci N, Muzzalupo R. Cromolyn as surface active drug (surfadrug): Effect of the self-association on diffusion and percutaneous permeation. Colloids Surf B Biointerfaces. (2016) 139:132–7. doi: 10.1016/j.colsurfb.2015.12.010
54. Rodriguez I, Flores Bello J, Marie Serrano Valcarcel J, Lopez-Mejias V. Design of potential pharmaceutical-based metal complexes derived from cromolyn a mast cell stabilizer. ACS omega. (2020) 5:29714–21. doi: 10.1021/acsomega.0c03320
55. Shi GP. Treatment and prevention of cardiovascular disease using mast cell stabilizers. (2008), US2008027111A1, Alexandria, Virginia, USA: U.S. Patent and Trademark Office.
56. The Brigham and Women’s Hospital I. Mast cell stabilizers in the treatment of obesity. (2014), US08785383B2, Alexandria, Virginia, USA: U.S. Patent and Trademark Office.
57. Alton EW, Norris AA. Chloride transport and the actions of nedocromil sodium and cromolyn sodium in asthma. J Allergy Clin Immunol. (1996) 98:S102–5;discussion S5-6. doi: 10.1016/S0091-6749(96)70024-6
58. Okayama Y, Benyon RC, Rees PH, Lowman MA, Hillier K, Church MK. Inhibition profiles of sodium cromoglycate and nedocromil sodium on mediator release from mast cells of human skin, lung, tonsil, adenoid and intestine. Clin Exp Allergy. (1992) 22:401–9. doi: 10.1111/j.1365-2222.1992.tb03102.x
59. Francesca LS, Saar M, Micha BZ, Laila K. Treatment Of Mast Cell Related Pathologies. (2014), WO2014188423/A1, Geneva, Switzerland, World Intellectual Property Organization.
60. Owen CF. Mast cell stabilizers used to inhibit laminitis. (2006). US 20060173071A1, Alexandria, Virginia, USA: U.S. Patent and Trademark Office.
61. Yanni JM, Weimer LK, Glaser RL, Lang LS, Robertson SM, Spellman JM. Effect of lodoxamide on in vitro and in vivo conjunctival immediate hypersensitivity responses in rats. Int Arch Allergy Immunol. (1993) 101:102–6. doi: 10.1159/000236505
62. Reilly FD, Dimlich RV. Hepatic microvascular regulatory mechanisms. IV. Effect of lodoxamide tromethamine and arterenol-HCl on vascular responses evoked by compound 48/80. Microcirc Endothelium Lymphatics. (1984) 1:87–106.
63. Castillo M, Scott NW, Mustafa MZ, Mustafa MS, Azuara-Blanco A. Topical antihistamines and mast cell stabilisers for treating seasonal and perennial allergic conjunctivitis. Cochrane Database systematic Rev. (2015) 6:Cd009566. doi: 10.1002/14651858.CD009566.pub2
64. Ramirez-Ponce MP, Flores JA, Barrella L, Ales E. Ketotifen is a microglial stabilizer by inhibiting secretory vesicle acidification. Life Sci. (2023) 319:121537. doi: 10.1016/j.lfs.2023.121537
65. Baba A, Tachi M, Ejima Y, Endo Y, Toyama H, Matsubara M, et al. Anti-allergic drugs tranilast and ketotifen dose-dependently exert mast cell-stabilizing properties. Cell Physiol Biochem. (2016) 38:15–27. doi: 10.1159/000438605
66. Grant SM, Goa KL, Fitton A, Sorkin EM. Ketotifen. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use in asthma and allergic disorders. Drugs. (1990) 40:412–48. doi: 10.2165/00003495-199040030-00006
67. Kim MS, Lee DH, Lee CW, Kim YK, Lee MJ, Shin CY, et al. Mast cell stabilizer, ketotifen, prevents UV-induced wrinkle formation. J Invest Dermatol. (2013) 133:1104–7. doi: 10.1038/jid.2012.424
68. Monument MJ, Hart DA, Befus AD, Salo PT, Zhang M, Hildebrand KA. The mast cell stabilizer ketotifen reduces joint capsule fibrosis in a rabbit model of post-traumatic joint contractures. Inflammation Res. (2012) 61:285–92. doi: 10.1007/s00011-011-0409-3
69. Azadi L, Abbasi H, Deemeh MR, Tavalaee M, Arbabian M, Pilevarian AA, et al. Zaditen (Ketotifen), as mast cell blocker, improves sperm quality, chromatin integrity and pregnancy rate after varicocelectomy. Int J Androl. (2011) 34:446–52. doi: 10.1111/j.1365-2605.2010.01112.x
70. Suzuki Y, Yoshimaru T, Inoue T, Ra C. Ca v 1.2 L-type Ca2+ channel protects mast cells against activation-induced cell death by preventing mitochondrial integrity disruption. Mol Immunol. (2009) 46:2370–80. doi: 10.1016/j.molimm.2009.03.017
71. Fanta CH. Calcium-channel blockers in prophylaxis and treatment of asthma. Am J Cardiol. (1985) 55:202b–9b. doi: 10.1016/0002-9149(85)90632-0
72. Ogasawara H, Noguchi M. Therapeutic potential of MRGPRX2 inhibitors on mast cells. Cells. (2021) 10:2906. doi: 10.3390/cells10112906
73. Chen N HE, Kunz R, Rumfelt S, Tasker A. Benzisoxazole and isoxazolo-pyridine compounds and method of use. (2011), US07872128B2, Alexandria, Virginia, USA: U.S. Patent and Trademark Office.
74. Moussy A, Kinet JP. Use of potent, selective and non toxic c-kit inhibitors for treating mastocytosis. (2005), US20050054617A1, Alexandria, Virginia, USA: U.S. Patent and Trademark Office.
75. Moussy A, Kinet JP. Use of c-kit inhibitors for treating acne. (2005), WO/2005/115385. Geneva, Switzerland: World Intellectual Property Organization.
76. Moussy A, Kinet JP. Use of C-kit inhibitors for treating inflammatory muscle disorders including myositis and muscular dystrophy. (2008), US20080146585A1, Alexandria, Virginia, USA: U.S. Patent and Trademark Office.
77. Chen N, Hu E. Di-amino-substituted heterocyclic compounds and methods of use. (2008), CA2658190A1, Gatineau, Quebec, Canada: Canadian Intellectual Property Office.
78. Valent P, Akin C, Hartmann K, Reiter A, Gotlib J, Sotlar K, et al. Drug-induced mast cell eradication: A novel approach to treat mast cell activation disorders? J Allergy Clin Immunol. (2022) 149:1866–74. doi: 10.1016/j.jaci.2022.04.003
79. Erin N, Ersoy Y, Ercan F, Akici A, Oktay S. NK-1 antagonist CP99994 inhibits stress-induced mast cell degranulation in rats. Clin Exp Dermatol. (2004) 29:644–8. doi: 10.1111/j.1365-2230.2004.01613.x
80. Lau AH, Chow SS, Ng YS. Immunologically induced histamine release from rat peritoneal mast cells is enhanced by low levels of substance P. Eur J Pharmacol. (2001) 414:295–303. doi: 10.1016/S0014-2999(01)00805-6
81. Babolewska E, Brzezinska-Blaszczyk E. Mast cell inhibitory receptors. Postepy higieny i medycyny doswiadczalnej (Online). (2012) 66:739–51. doi: 10.5604/17322693.1015039
82. Treudler R, Simon JC. Developments and perspectives in allergology. J Dtsch Dermatol Ges. (2023) 21:399–403. doi: 10.1111/ddg.15034
83. Miralda I, Samanas NB, Seo AJ, Foronda JS, Sachen J, Hui Y, et al. Siglec-9 is an inhibitory receptor on human mast cells in vitro. J Allergy Clin Immunol. (2023) 152:711–24. doi: 10.1016/j.jaci.2023.04.007
84. Altrichter S, Staubach P, Pasha M, Singh B, Chang AT, Bernstein JA, et al. An open-label, proof-of-concept study of lirentelimab for antihistamine-resistant chronic spontaneous and inducible urticaria. J Allergy Clin Immunol. (2022) 149:1683–90.e7. doi: 10.1016/j.jaci.2021.12.772
85. Kolkhir P, Elieh-Ali-Komi D, Metz M, Siebenhaar F, Maurer M. Understanding human mast cells: lesson from therapies for allergic and non-allergic diseases. Nat Rev Immunol. (2022) 22:294–308. doi: 10.1038/s41577-021-00622-y
86. Sabato V, Verweij MM, Bridts CH, Levi-Schaffer F, Gibbs BF, De Clerck LS, et al. CD300a is expressed on human basophils and seems to inhibit IgE/FcepsilonRI-dependent anaphylactic degranulation. Cytometry B Clin Cytom. (2012) 82:132–8. doi: 10.1002/cyto.b.21003
87. Bachelet I, Munitz A, Berent-Maoz B, Mankuta D, Levi-Schaffer F. Suppression of normal and Malignant kit signaling by a bispecific antibody linking kit with CD300a. J Immunol. (2008) 180:6064–9. doi: 10.4049/jimmunol.180.9.6064
88. Gilfillan AM, Rivera J. The tyrosine kinase network regulating mast cell activation. Immunol Rev. (2009) 228:149–69. doi: 10.1111/j.1600-065X.2008.00742.x
89. Robak T, Robak E. Tyrosine kinase inhibitors as potential drugs for B-cell lymphoid Malignancies and autoimmune disorders. Expert Opin Investig Drugs. (2012) 21:921–47. doi: 10.1517/13543784.2012.685650
90. Lee JH, Kim JW, Ko NY, Mun SH, Kim do K, Kim JD, et al. Mast cell-mediated allergic response is suppressed by Sophorae flos: inhibition of SRC-family kinase. Exp Biol Med (Maywood). (2008) 233:1271–9. doi: 10.3181/0803-RM-89
91. Dispenza MC, Krier-Burris RA, Chhiba KD, Undem BJ, Robida PA, Bochner BS. Bruton’s tyrosine kinase inhibition effectively protects against human IgE-mediated anaphylaxis. J Clin Invest. (2020) 130:4759–70. doi: 10.1172/JCI138448
92. Dispenza MC. The use of bruton’s tyrosine kinase inhibitors to treat allergic disorders. Curr Treat Options Allergy. (2021) 8:261–73. doi: 10.1007/s40521-021-00286-y
93. Roskoski R Jr. Src protein-tyrosine kinase structure, mechanism, and small molecule inhibitors. Pharmacol Res. (2015) 94:9–25. doi: 10.1016/j.phrs.2015.01.003
94. Ruzza P, Biondi B, Calderan A. Therapeutic prospect of Syk inhibitors. Expert Opin Ther Pat. (2009) 19:1361–76. doi: 10.1517/13543770903207039
95. Atkinson FL, Patel VK. Pyrimidinecarboxamide derivatives as inhibitors of Syk kinase. (2013), US08470835B2, Alexandria, Virginia, USA: U.S. Patent and Trademark Office.
96. Charles LC, Scott AE, Joachim H, Mark AB, Desjarlais RL, Mark P, et al. 1,6-naphthyridones,yseful as inhibitors of syk kinase. (2003), US2003/0229090A1, Alexandria, Virginia, USA: U.S. Patent and Trademark Office.
97. Michelle RM, Michelle DA, Eric TR, Dilrukshi V, Brandon C, Tony S, et al. Pyrazolyl derivatives as Syk inhibitors. (2016), US09242984B2, Alexandria, Virginia, USA: U.S. Patent and Trademark Office.
98. Shipley TJ, Wilson DM, Watson RJ, Atkinson FL, Atkinson SJ, Baker MD, et al. Pyrido[3,4-b]pyrazine derivatives as syk inhibitors. (2012), WO2012/123312A1, Geneva, Switzerland: World Intellectual Property Organization.
99. Oliver JM, Burg DL, Wilson BS, McLaughlin JL, Geahlen RL. Inhibition of mast cell Fc epsilon R1-mediated signaling and effector function by the Syk-selective inhibitor, piceatannol. J Biol Chem. (1994) 269:29697–703. doi: 10.1016/S0021-9258(18)43936-1
100. Uckun FM, Ma H, Ozer Z, Goodman P, Zhang J, Qazi S. A previously unknown unique challenge for inhibitors of syk atp-binding site: Role of syk as a cell cycle checkpoint regulator. EBioMedicine. (2014) 1:16–28. doi: 10.1016/j.ebiom.2014.10.019
101. Rossi AB, Herlaar E, Braselmann S, Huynh S, Taylor V, Frances R, et al. Identification of the Syk kinase inhibitor R112 by a human mast cell screen. J Allergy Clin Immunol. (2006) 118:749–55. doi: 10.1016/j.jaci.2006.05.023
102. Lu Y, Yang JH, Li X, Hwangbo K, Hwang SL, Taketomi Y, et al. Emodin, a naturally occurring anthraquinone derivative, suppresses IgE-mediated anaphylactic reaction and mast cell activation. Biochem Pharmacol. (2011) 82:1700–8. doi: 10.1016/j.bcp.2011.08.022
103. Ramis I, Otal R, Carreno C, Domenech A, Eichhorn P, Orellana A, et al. A novel inhaled Syk inhibitor blocks mast cell degranulation and early asthmatic response. Pharmacol Res. (2015) 99:116–24. doi: 10.1016/j.phrs.2015.05.011
104. Efremov DG, Laurenti L. The Syk kinase as a therapeutic target in leukemia and lymphoma. Expert Opin investigational Drugs. (2011) 20:623–36. doi: 10.1517/13543784.2011.570329
105. Sobhia ME, Grewal BK, Paul ML, Patel J, Kaur A, Haokip T, et al. Protein kinase C inhibitors: a patent review (2010 - present). Expert Opin Ther Pat. (2013) 23:1451–68. doi: 10.1517/13543776.2013.812073
106. Khalil RA. Protein kinase C inhibitors as modulators of vascular function and their application in vascular disease. Pharm (Basel). (2013) 6:407–39. doi: 10.3390/ph6030407
107. Sobhia ME, Grewal BK, Paul ML, Patel J, Kaur A, Haokip T, et al. Protein kinase C inhibitors: a patent review (2008 - 2009). Expert Opin Ther Pat. (2013) 23:1297–315. doi: 10.1517/13543776.2013.805205
108. Kim M, Lim SJ, Kang SW, Um BH, Nho CW. Aceriphyllum rossii extract and its active compounds, quercetin and kaempferol inhibit IgE-mediated mast cell activation and passive cutaneous anaphylaxis. J Agric Food Chem. (2014) 62:3750–8. doi: 10.1021/jf405486c
109. Pereira M, Siba IP, Chioca LR, Correia D, Vital MA, Pizzolatti MG, et al. Myricitrin, a nitric oxide and protein kinase C inhibitor, exerts antipsychotic-like effects in animal models. Prog Neuropsychopharmacol Biol Psychiatry. (2011) 35:1636–44. doi: 10.1016/j.pnpbp.2011.06.002
110. Wagner J, Van EM, Von MP, Evenou JP, Schuler W, Novartis AG. Indolymaleimide deravatives. (2010), US20100273774A1, Alexandria, Virginia, USA: U.S. Patent and Trademark Office.
111. Massey WA, Cohan VL, MacGlashan DW Jr., Gittlen SW, Kagey-Sobotka A, Lichtenstein LM, et al. Protein kinase C modulates immunoglobulin E-mediated activation of human mast cells from lung and skin. I. Pharmacologic inhibition. J Pharmacol Exp Ther. (1991) 258:824–9.
112. Kawano T, Inokuchi J, Eto M, Murata M, Kang JH. Activators and inhibitors of protein kinase C (PKC): their applications in clinical trials. Pharmaceutics. (2021) 13:1748. doi: 10.3390/pharmaceutics13111748
113. Ellis H, Ma CX. PI3K inhibitors in breast cancer therapy. Curr Oncol Rep. (2019) 21:110. doi: 10.1007/s11912-019-0846-7
114. Yang Q, Modi P, Newcomb T, Queva C, Gandhi V. Idelalisib: first-in-class PI3K delta inhibitor for the treatment of chronic lymphocytic leukemia, small lymphocytic leukemia, and follicular lymphoma. Clin Cancer Res. (2015) 21:1537–42. doi: 10.1158/1078-0432.CCR-14-2034
115. Graf SA, Gopal AK. Idelalisib for the treatment of non-Hodgkin lymphoma. Expert Opin Pharmacother. (2016) 17:265–74. doi: 10.1517/14656566.2016.1135130
116. Lannutti BJ, Meadows SA, Herman SE, Kashishian A, Steiner B, Johnson AJ, et al. CAL-101, a p110delta selective phosphatidylinositol-3-kinase inhibitor for the treatment of B-cell Malignancies, inhibits PI3K signaling and cellular viability. Blood. (2011) 117:591–4. doi: 10.1182/blood-2010-03-275305
117. Somoza JR, Koditek D, Villasenor AG, Novikov N, Wong MH, Liclican A, et al. Structural, biochemical, and biophysical characterization of idelalisib binding to phosphoinositide 3-kinase delta. J Biol Chem. (2015) 290:8439–46. doi: 10.1074/jbc.M114.634683
118. Horak F, Puri KD, Steiner BH, Holes L, Xing G, Zieglmayer P, et al. Randomized phase 1 study of the phosphatidylinositol 3-kinase delta inhibitor idelalisib in patients with allergic rhinitis. J Allergy Clin Immunol. (2016) 137:1733–41. doi: 10.1016/j.jaci.2015.12.1313
119. Linwong W, Hirasawa N, Aoyama S, Hamada H, Saito T, Ohuchi K. Inhibition of the antigen-induced activation of rodent mast cells by putative Janus kinase 3 inhibitors WHI-P131 and WHI-P154 in a Janus kinase 3-independent manner. Br J Pharmacol. (2005) 145:818–28. doi: 10.1038/sj.bjp.0706240
120. Kim JW, Inagaki Y, Mitsutake S, Maezawa N, Katsumura S, Ryu YW, et al. Suppression of mast cell degranulation by a novel ceramide kinase inhibitor, the F-12509A olefin isomer K1. Biochim Biophys Acta. (2005) 1738:82–90. doi: 10.1016/j.bbalip.2005.10.007
121. Mitsutake S, Kumada H, Soga M, Hurue Y, Asanuma F, Nagira M, et al. Ceramide kinase is not essential but might act as an Ca2+-sensor for mast cell activation. Prostaglandins Other Lipid Mediat. (2010) 93:109–12. doi: 10.1016/j.prostaglandins.2010.07.003
122. El-Awady MS, Said E. Vardenafil ameliorates immunologic- and non-immunologic-induced allergic reactions. Can J Physiol Pharmacol. (2014) 92:175–80. doi: 10.1139/cjpp-2013-0316
123. Lin P, Cao M, Xia F, Liao H, Sun H, Wang Q, et al. A phosphatase-mimetic nano-stabilizer of mast cells for long-term prevention of allergic disease. Advanced Sci (Weinheim Baden-Wurttemberg Germany). (2021) 8:2004115. doi: 10.1002/advs.202004115
124. Hu R, Dai C, Dong C, Ding L, Huang H, Chen Y. Living macrophage-delivered tetrapod pdH nanoenzyme for targeted atherosclerosis management by ROS scavenging, hydrogen anti-inflammation, and autophagy activation. Advanced healthcare materials. (2022) 16:15959–76. doi: 10.1002/adhm.20220241810.1021/acsnano.2c03422
125. Bigalke H, Frevert J. A hybrid protein used to inhibit mast cell degranulation. (1999), WO99/58571, Geneva, Switzerland: World Intellectual Property Organization.
126. Li S, Dudczak R, Koller E, Baghestanian M, Ghannadan M, Minar E, et al. Effect of statins on lipoprotein receptor expression in cell lines from human mast cells and basophils. Eur J Clin Pharmacol. (2003) 59:507–16. doi: 10.1007/s00228-003-0668-1
127. Samson KT, Minoguchi K, Tanaka A, Oda N, Yokoe T, Yamamoto Y, et al. Inhibitory effects of fluvastatin on cytokine and chemokine production by peripheral blood mononuclear cells in patients with allergic asthma. Clin Exp Allergy. (2006) 36:475–82. doi: 10.1111/j.1365-2222.2006.02470.x
128. Ahmad T, Mabalirajan U, Sharma A, Aich J, Makhija L, Ghosh B, et al. Simvastatin improves epithelial dysfunction and airway hyperresponsiveness: from asymmetric dimethyl-arginine to asthma. Am J Respir Cell Mol Biol. (2011) 44:531–9. doi: 10.1165/rcmb.2010-0041OC
129. Hothersall EJ, Chaudhuri R, McSharry C, Donnelly I, Lafferty J, McMahon AD, et al. Effects of atorvastatin added to inhaled corticosteroids on lung function and sputum cell counts in atopic asthma. Thorax. (2008) 63:1070–5. doi: 10.1136/thx.2008.100198
130. Egesi A, Sun G, Khachemoune A, Rashid RM. Statins in skin: research and rediscovery, from psoriasis to sclerosis. J Drugs dermatology: JDD. (2010) 9:921–7.
131. Kolawole EM, McLeod JJ, Ndaw V, Abebayehu D, Barnstein BO, Faber T, et al. Fluvastatin suppresses mast cell and basophil igE responses: genotype-dependent effects. J Immunol. (2016) 196:1461–70. doi: 10.4049/jimmunol.1501932
132. Nagai K, Takahashi Y, Mikami I, Fukusima T, Oike H, Kobori M. The hydroxyflavone, fisetin, suppresses mast cell activation induced by interaction with activated T cell membranes. Br J Pharmacol. (2009) 158:907–19. doi: 10.1111/j.1476-5381.2009.00365.x
133. Kim DH, Jung WS, Kim ME, Lee HW, Youn HY, Seon JK, et al. Genistein inhibits proinflammatory cytokines in human mast cell activation through the inhibition of the ERK pathway. Int J Mol Med. (2014) 34:1669–74. doi: 10.3892/ijmm.2014.1956
134. Weng Z, Zhang B, Asadi S, Sismanopoulos N, Butcher A, Fu X, et al. Quercetin is more effective than cromolyn in blocking human mast cell cytokine release and inhibits contact dermatitis and photosensitivity in humans. PloS One. (2012) 7:e33805. doi: 10.1371/journal.pone.0033805
135. Weng Z, Patel AB, Panagiotidou S, Theoharides TC. The novel flavone tetramethoxyluteolin is a potent inhibitor of human mast cells. J Allergy Clin Immunol. (2015) 135:1044–52.e5. doi: 10.1016/j.jaci.2014.10.032
136. Theoharides TC. Method of treating mast cell activation-induced diseases with a proteoglycan. (2004), US06689748, Alexandria, Virginia, USA: U.S. Patent and Trademark Office.
137. de Souza Santos M, Freire de Morais Del Lama MP, Deliberto LA, da Silva Emery F, Tallarico Pupo M, Zumstein Georgetto Naal RM. In situ screening of 3-arylcoumarin derivatives reveals new inhibitors of mast cell degranulation. Arch Pharm Res. (2013) 36:731–8. doi: 10.1007/s12272-013-0084-8
138. Yuan M, Li J, Lv J, Mo X, Yang C, Chen X, et al. Polydatin (PD) inhibits IgE-mediated passive cutaneous anaphylaxis in mice by stabilizing mast cells through modulating Ca(2)(+) mobilization. Toxicol Appl Pharmacol. (2012) 264:462–9. doi: 10.1016/j.taap.2012.08.024
139. Kim SH, Shin TY. Amomum xanthiodes inhibits mast cell-mediated allergic reactions through the inhibition of histamine release and inflammatory cytokine production. Exp Biol Med (Maywood). (2005) 230:681–7. doi: 10.1177/153537020523000911
140. Finn DF, Walsh JJ. Twenty-first century mast cell stabilizers. Br J Pharmacol. (2013) 170:23–37. doi: 10.1111/bph.12138
141. Kilinc E, Tore F, Dagistan Y, Bugdayci G. Thymoquinone inhibits neurogenic inflammation underlying migraine through modulation of calcitonin gene-related peptide release and stabilization of meningeal mast cells in glyceryltrinitrate-induced migraine model in rats. Inflammation. (2020) 43:264–73. doi: 10.1007/s10753-019-01115-w
142. Baranoglu Kilinc Y, Dilek M, Kilinc E, Torun IE, Saylan A, Erdogan Duzcu S. Capsaicin attenuates excitotoxic-induced neonatal brain injury and brain mast cell-mediated neuroinflammation in newborn rats. Chem Biol Interact. (2023) 376:110450. doi: 10.1016/j.cbi.2023.110450
143. Thomas G. Gant.Pyrido[1,2-a]pyrimidin-4-one inhibitors of mast cell degranulation. (2010), US2010/0160347A1, Alexandria, Virginia, USA: U.S. Patent and Trademark Office.
144. Fujimiya H, Nakashima S, Miyata H, Nozawa Y. Effect of a novel antiallergic drug, pemirolast, on activation of rat peritoneal mast cells: inhibition of exocytotic response and membrane phospholipid turnover. Int Arch Allergy Appl Immunol. (1991) 96:62–7. doi: 10.1159/000235536
145. Shulman DG. Two mast cell stabilizers, pemirolast potassium 0.1% and nedocromil sodium 2%, in the treatment of seasonal allergic conjunctivitis: a comparative study. Adv Ther. (2003) 20:31–40. doi: 10.1007/BF02850117
146. Harrison CA, Bastan R, Peirce MJ, Munday MR, Peachell PT. Role of calcineurin in the regulation of human lung mast cell and basophil function by cyclosporine and FK506. Br J Pharmacol. (2007) 150:509–18. doi: 10.1038/sj.bjp.0707002
147. Cheng C, Ng DS, Chan TK, Guan SP, Ho WE, Koh AH, et al. Anti-allergic action of anti-malarial drug artesunate in experimental mast cell-mediated anaphylactic models. Allergy. (2013) 68:195–203. doi: 10.1111/all.12077
148. Bai XY, Liu P, Chai YW, Wang Y, Ren SH, Li YY, et al. Artesunate attenuates 2, 4-dinitrochlorobenzene-induced atopic dermatitis by down-regulating Th17 cell responses in BALB/c mice. Eur J Pharmacol. (2020) 874:173020. doi: 10.1016/j.ejphar.2020.173020
149. Ho WE, Peh HY, Chan TK, Wong WS. Artemisinins: pharmacological actions beyond anti-malarial. Pharmacol Ther. (2014) 142:126–39. doi: 10.1016/j.pharmthera.2013.12.001
150. Wedi B, Traidl S. Anti-igE for the treatment of chronic urticaria. Immunotargets Ther. (2021) 10:27–45. doi: 10.2147/ITT.S261416
151. El-Qutob D. Off-label uses of omalizumab. Clin Rev Allergy Immunol. (2016) 50:84–96. doi: 10.1007/s12016-015-8490-y
152. Owen CE, Howard F, Walker C. Synergistic combination of omalizumab (Xolair) and immunosuppressive agents. (2008), CN101111265, Beijing, China: National Intellectual Property Administration of the People’s Republic of China.
153. Jin M, Douglass JA, Elborn JS, Agarwal R, Calhoun WJ, Lazarewicz S, et al. Omalizumab in allergic bronchopulmonary aspergillosis: A systematic review and meta-analysis. J Allergy Clin Immunol In practice. (2023) 11:896–905. doi: 10.1016/j.jaip.2022.12.012
154. Gill MA, Liu AH, Calatroni A, Krouse RZ, Shao B, Schiltz A, et al. Enhanced plasmacytoid dendritic cell antiviral responses after omalizumab. J Allergy Clin Immunol. (2018) 141:1735–43.e9. doi: 10.1016/j.jaci.2017.07.035
155. Maurer M, Gimenez-Arnau AM, Sussman G, Metz M, Baker DR, Bauer A, et al. Ligelizumab for chronic spontaneous urticaria. N Engl J Med. (2019) 381:1321–32. doi: 10.1056/NEJMoa1900408
156. Harris JM, Cabanski CR, Scheerens H, Samineni D, Bradley MS, Cochran C, et al. A randomized trial of quilizumab in adults with refractory chronic spontaneous urticaria. J Allergy Clin Immunol. (2016) 138:1730–2. doi: 10.1016/j.jaci.2016.06.023
157. Harris JM, Maciuca R, Bradley MS, Cabanski CR, Scheerens H, Lim J, et al. A randomized trial of the efficacy and safety of quilizumab in adults with inadequately controlled allergic asthma. Respir Res. (2016) 17:29. doi: 10.1186/s12931-016-0347-2
158. Okayama Y, Matsumoto H, Odajima H, Takahagi S, Hide M, Okubo K. Roles of omalizumab in various allergic diseases. Allergology international: Off J Japanese Soc Allergology. (2020) 69:167–77. doi: 10.1016/j.alit.2020.01.004
159. Jabs F, Plum M, Laursen NS, Jensen RK, Mølgaard B, Miehe M, et al. Trapping IgE in a closed conformation by mimicking CD23 binding prevents and disrupts FcϵRI interaction. Nat Commun. (2018) 9:7. doi: 10.1038/s41467-017-02312-7
160. Lambiase A, Micera A, Bonini S. Multiple action agents and the eye: do they really stabilize mast cells? Curr Opin Allergy Clin Immunol. (2009) 9:454–65. doi: 10.1097/ACI.0b013e3283303ebb
161. Johnson HG, Sheridan AQ. The characterization of lodoxamide, a very active inhibitor of mediator release, in animal and human models of asthma. Agents Actions. (1986) 18:301–5. doi: 10.1007/BF01964988
162. Dilek M, Kilinc YB, Kilinc E, Torun IE, Saylan A, Duzcu SE. Activation of TRESK background potassium channels by cloxyquin exerts protective effects against excitotoxic-induced brain injury and neuroinflammation in neonatal rats. J Neuroimmunol. (2022) 368:577894. doi: 10.1016/j.jneuroim.2022.577894
163. Kilinc E, Ankarali S, Torun IE, Dagistan Y. Receptor mechanisms mediating the anti-neuroinflammatory effects of endocannabinoid system modulation in a rat model of migraine. Eur J Neurosci. (2022) 55:1015–31. doi: 10.1111/ejn.14897
164. Kilin E, Baranolu Y, Kilin YB. Mast cell stabilizers as a supportive therapy can contribute to alleviate fatal inflammatory responses and severity of pulmonary complications in COVID-19 infection. Anadolu Kliniği Tıp Bilimleri Dergisi. (2020) 25:111–8. doi: 10.21673/anadoluklin.720116
165. Kilinc E, Torun IE, Cetinkaya A, Tore F. Mast cell activation ameliorates pentylenetetrazole-induced seizures in rats: The potential role for serotonin. Eur J Neurosci. (2022) 55:2912–24. doi: 10.1111/ejn.15145
166. Duguay BA, Lu L, Arizmendi N, Unsworth LD, Kulka M. The possible uses and challenges of nanomaterials in mast cell research. J Immunol. (2020) 204:2021–32. doi: 10.4049/jimmunol.1800658
167. Georgin-Lavialle S, Lhermitte L, Dubreuil P, Chandesris MO, Hermine O, Damaj G. Mast cell leukemia. Blood. (2013) 121:1285–95. doi: 10.1182/blood-2012-07-442400
168. Laforgia M, Calabro C, Scattone A, Laface C, Porcelli M, Gadaleta CD, et al. Pharmacotherapy in mast cell leukemia. Expert Opin Pharmacother. (2020) 21:1059–69. doi: 10.1080/14656566.2020.1744566
Keywords: MC stabilizers, activation, mechanism, targeted, clinical application
Citation: Cao M and Gao Y (2024) Mast cell stabilizers: from pathogenic roles to targeting therapies. Front. Immunol. 15:1418897. doi: 10.3389/fimmu.2024.1418897
Received: 17 April 2024; Accepted: 16 July 2024;
Published: 01 August 2024.
Edited by:
Virginia Calder, University College London, United KingdomReviewed by:
Erkan Kilinc, Abant Izzet Baysal University, TürkiyeAnna Sobiepanek, Warsaw University of Technology, Poland
Monika Staniszewska, Warsaw University of Technology, Poland
Copyright © 2024 Cao and Gao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mengda Cao, MzU4MTY3NjY5QHFxLmNvbQ==; Yao Gao, MTU1NDU3OTQyMkBxcS5jb20=
 Mengda Cao
Mengda Cao Yao Gao
Yao Gao