
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol., 26 July 2024
Sec. Molecular Innate Immunity
Volume 15 - 2024 | https://doi.org/10.3389/fimmu.2024.1418539
 Yunfang Li1
Yunfang Li1 Jianming Wu1,2*†
Jianming Wu1,2*†CD177 plays an important role in the proliferation and differentiation of myeloid lineage cells including neutrophils, myelocytes, promyelocytes, megakaryocytes, and early erythroblasts in bone marrow. CD177 deficiency is a common phenotype in humans. Our previous studies revealed genetic mechanisms of human CD177 deficiency and expression variations. Up to now, immune functions of CD177 remain undefined. In the current study, we revealed human IgG as a ligand for CD177 by using flow cytometry, bead-rosette formation, and surface plasmon resonance (SPR) assays. In addition, we show that CD177 variants affect the binding capacity of CD177 for human IgG. Furthermore, we show that the CD177 genetic variants significantly affect antibody-dependent cell-mediated cytotoxicity (ADCC) function. The demonstration of CD177 as a functional IgG Fc-receptor may provide new insights into CD177 immune function and genetic mechanism underlying CD177 as biomarkers for human diseases.
IgG Fc receptors (or FcγRs) are surface receptors of immune cells to recognize IgG immune complexes and antibody-opsonized targets. FcγRs are essential for antibody-dependent cell-mediated phagocytosis (ADCP) and antibody-dependent cellular cytotoxicity (ADCC) to eradicate pathogens and infected/mutated target cells. The interaction between FcγRs on immune cells and target-bound antibodies initiates signal cascades for ADCC and ADCP (1). Humans possess a variety of FcγRs to regulate immune responses (2). Genetic polymorphisms of human FcγR genes significantly affect immune responses and disease susceptibility (3–6).
CD177 (NB1 and PRV-1) is a GPI-linked cell surface protein (7, 8) on bone marrow cells (9, 10). CD177 plays an important role in the proliferation and differentiation of myeloid lineage cells including neutrophils, myelocytes, promyelocytes, megakaryocytes, and early erythroblasts (9, 11–14). In peripheral blood, CD177 is exclusively expressed on neutrophils that serve as the first line of defense against infections and the primary mediator for inflammation. CD177 is a biomarker for a number of human diseases. CD177 expression is the most dysregulated in sepsis patients (15), indicating a role in infection. CD177+ neutrophil play a protective role in the development of inflammatory bowel disease (16), indicating a role of CD177 in inflammation. Low percentages of CD177+ neutrophils are significantly associated with myelodysplastic syndrome and chronic myelogenous leukemia (10, 17). As an important biomarker for polycythemia vera and essential thrombocythemia (14, 18, 19), CD177 plays a pivotal role in myeloid proliferation and differentiation (11–13). CD177 overexpression was significantly correlated with a favorable prognosis for gastric cancer (20) and CD177+ neutrophils suppress epithelial cell tumorigenesis in colitis-associated cancer and predict good prognosis in colorectal cancer (21), pointing to a protective role of CD177 in cancer. Up to now, the mechanism with which CD177 influences tumor outcome is unknown (20).
In the previous studies, we delineated genetic mechanisms of CD177 deficiency and expression variations (22, 23). CD177 mediates neutrophil activation triggered by autoantibodies (24), but the physiological ligands and immune functions of CD177 remain incompletely understood. In the current study, we revealed that CD177 is a functional FcγR and that CD177 genetic variants affect CD177 immune function. Our study may provide novel insights into CD177-mediated immune functions and the mechanism underlying the roles of CD177 as a disease biomarker for human diseases.
Human serum IgG (hIgG) and IgA (hIgA) were purchased from Sigma Chemical Co. (St. Louis, MO). FITC (or APC)-conjugated anti-CD177 mAb (clone MEM-166, mouse IgG1) and respective isotype controls were from BioLegend (San Diego, CA). FITC-conjugated goat anti-human Ig(H+L) F(ab’)2 were from Jackson ImmunoResearch (West Grove, PA). Human IgGs (IgG1κ, IgG2κ, IgG3κ, and IgG4κ, purity >95%) and IgG Fc fragment were obtained from Athens Research and Technology (Athens, GA). Human mAb trastuzumab/Herceptin manufactured by Genentech (South San Francisco, CA) was obtained through the University of Minnesota Boynton Pharmacy as previously described (25). This study also used puromycin from InvivoGen (San Diego, CA), geneticin (G418) from Life Technologies (Grand Island, NY), and QuikChange Site-directed mutagenesis kit Stratagene (La Jolla, CA).
The human CD177 expression constructs were generated by cloning Hind III/Xba I-flanked full-length CD177 cDNA into the eukaryotic expression vector pcDNA3 (Gibco BRL) as previously described (22). For the generation of CD177 retroviral expression constructs, CD177 cDNA inserts from CD177-pcDNA3 expression constructs were amplified by PCR using the forward primer (5’- CTC TAG ACT GCC GGA TCC ACC AGC CAC AGA CGG GTC ATG AG-3’) and the reverse primer (5’- CGA GGC CTG CAG GAA TTC GAG GTC AGA GGG AGG TTG AGT GTG-3’) (the underlined nucleotides indicate Bam HI and EcoR I restriction sites, respectively). The full-length CD177 cDNA inserts were sub-cloned into pBMN-I-EGFP retroviral vector digested with Bam HI and EcoR I restriction enzymes using InFusion HD Cloning Kit (Takara Bio USA, Mountain View, Ca) according to the product manual. For the production of soluble CD177, CD177 cDNA coding for amino acids 1-408 was amplified by PCR from the CD177-pcDNA3 expression construct using the forward primer 5’- GAG TCT AGA ACC AGC CAC AGA CGG GTC ATG AG -3’ (the underlined nucleotides indicate an Xba I restriction sites) and the reverse primer 5’- CCG GGA TCC TTA ATG ATG ATG ATG ATG ATG ACC TCC CTC ATG CTG AGA GGC AG-3’ (the underlined nucleotides indicate a Bam HI restriction sites). The amplified CD177 cDNA fragment with the addition of 6×histidine-tag at the carboxyl terminus was digested by Xba I and Bam HI before being cloned into a pLenti-3F vector as previously described (26). Nucleotide sequences of all CD177 expression constructs were confirmed by direct Sanger sequencing on an ABI 3730xl DNA Analyzer with BigDye v3.1 Sequencing kit (Applied Biosystems, Inc., Foster City, CA).
Human embryonic kidney cell line 293 cells (ATCC#CRL-1573, Manassas, VA) were cultured with the DMEM medium containing 10% fetal calf serum, L-glutamine (2 mM), and 1× Pen/Strep (Life Technologies) in 5% CO2 incubator at 37 °C. Transfection reactions were carried out in 100-mm cell culture dishes with the plasmid DNA (20 μg) purified with OMEGA Plasmid Maxi Kit (Omega Bio-Tek, Norcross, GA) and 40 μl of Lipofectamine 2000 reagent (Life Technologies). Transfected cells were cultured in DMEM medium for two days before the supplement of G418 (final concentration: 1 mg/ml) for the selection of stable cell lines. The polyclonal cells surviving the G418 selection were sorted with StemCelll EasySep Cell Sorter and the expression of CD177 on the transfected 293 cell lines was determined with FITC-conjugated anti-CD177 mAb as described previously (22).
For retrovirus production, PhoenixA cells were cultured in DMEM supplemented with 10% FBS to ~ 80% confluence. Transfections of PhoenixA cells were carried out in 100-mm cell culture dishes with pBMN-I-EGFP construct plasmid DNA (20 μg) and 40 μl of Lipofectamine 2000 reagent (Life Technologies). Transfected cells were cultured in DMEM medium for two days before culture supernatants containing retroviral particles were harvested. Cell culture supernatants containing pseudo retrovirus particles were filtered through 0.4 µm syringe filter and used for transduction. IgG Fc receptor-deficient NK-92 (ATCC# CRL-2407, Manassas, VA) cells were maintained in Alpha Minimum Essential medium supplemented with 12.5% heat-inactivated fetal bovine serum (FBS), 12.5% heat-inactivated horse serum, 1× Antibiotic-Antimycotic, and 100 U/ml IL-2 (R&D Systems, Minneapolis, MN). NK-92 cells were passaged in 2–3 day interval to maintain the cell density at 2.5 × 105 – 1 × 106 cells/ml. NK-92 cell transduction was carried out as previously described (27). GFP+ NK-92 cells were sorted with FACSAria II cell sorter (BD Biosciences).
The CD177 expression on cells were determined with flow cytometry analysis as previously described (22). Cells stained with anti-CD177 mAb or isotype control were analyzed on a FACSCelesta flow cytometer (BD Biosciences). The FlowJo software (Tree Star Inc.) was used to evaluate flow cytometry data.
The binding of human IgG (hIgG) to 293 cells expressing CD177 was measured by quantitative flow cytometry as previously described (28, 29). 293 cells were washed twice with cold PBS containing 1% BSA (wash buffer) and re-suspended at the density of 2×106/ml in wash buffer. An aliquot of 100 µl (2×105) of 293 cells was transferred to a flow tube and centrifuged at 400 ×g for 6 min to pellet cells. After removal of supernatant, 100 µl of hIgG or hIgA diluted at various concentrations in wash buffer was added to each tube. The cells were vortexed and incubated on ice for 30 minutes. Cells were then washed three times with wash buffer and were stained with 100 µl of FITC-conjugated goat anti-human IgG (H+L) antibody (1 μg/ml final concentration) on ice for 30 min. The cells were washed and fixed with 1% formaldehyde before being analyzed on a Celesta flow cytometer (BD Biosciences). For phosphatidylinositol-specific phospholipase C (PI-PLC) treatment, 293 cells (106 cells) expressing CD177 were treated by 0.2 unit PI-PLC (cat# P-6466, ThermoFisher Scientific, Waltham, Massachusetts) in 0.5 ml PBS for 1 h at 37°C. PI-PLC treated cells were washed twice with wash buffer for the IgG binding assay at the final IgG concentration of 100 µg/ml. For mAb blockade analysis, 293 cells expressing CD177 were incubated with anti-CD177 mAb MEM-166 (10 ug/ml final concentration) for 30 min at room temperature. MEM-166 mAb treated cells were washed twice with wash buffer before being used for the IgG binding assay at the final IgG concentration of 200 µg/ml.
Pseudo-lentiviral particles containing sCD177 construct were generated using 293T cells and packaging vectors pMD2.G and pCMV-dR8.74psPAX2 (Addgene). Pseudo-lentiviral particles were transduced into the FreeStyle 293-F cells (ThermoFisher Scientific). The stable 293-F cell line was established under 1.0 µg/ml puromycin selection. The 293-F cells stably expressing sCD177 were cultured in the FreeStyle™ 293 Expression Medium (ThermoFisher Scientific) and cell culture supernatant was harvested when cell density reached 2.5×106/ml. sCD177 was purified from cell culture supernatants using a two-step purification procedure including Ni‐affinity chromatography and Fast protein liquid chromatography. For the first step, His-tagged proteins in cell culture supernatant was purified on a HisTrap HP His tag protein purification column (Cytiva, Marlborough, MA) according to the manufacture’s protocol. sCD177 from the first step purification was injected into a Superdex 200 Increase 10/300 GL column (Millipore-Sigma) on an AKTA pure protein purification system (Cytiva) for the second step purification. The purity of proteins was >95% as determined by SDS-PAGE (Supplementary Figure 1).
A Biacore S200 instrument (GE Healthcare) was used to determine interaction affinity between sCD177 with human IgG subclasses (IgG1, IgG2, IgG3, and IgG4). In SPR analyses, pure CD177 protein was dialyzed against 1×HBS-P+ buffer (cat# BR100671, Cytiva, Uppsala, Sweden) as previously described (30). An N-hydroxysuccinimide ester was formed on a CM5 sensor chip (GE Healthcare) surface according to a procedure recommended by the manufacturer. Pure human IgG proteins diluted in 100 mM Sodium Acetate (pH 4.5) were immobilized at acidic pH, resulting in the following densities: IgG1: 254 RU, IgG2: 259 RU, IgG3: 185 RU, and IgG4: 239 RU on a CM5 chip. In multicycle SPR kinetic analysis, sCD177 at concentrations of 125, 250, 500, 1,000, 2,000, 4,000, 8,000, and 16,000 nM in 1×HBS-P+ buffer were injected into flow cells at a flow rate of 30 µl/min, with a contact and dissociation time of 250 and 600 seconds, respectively. After each assay cycle, the sensor chip surface was regenerated by 10 mM NaOH. Binding response was recorded as resonance units continuously, with background binding automatically subtracted. The kinetic constants (kon, koff, t1/2) were determined. Association (kon) and dissociation (koff) rate constants were calculated by a global fitting analysis assuming a Langmuir binding model and a stoichiometry of (1:1). The kinetic dissociation constant (KD) was determined from the ratio of the rate constants (KD = koff/kon). KD was calculated by studying the concentration-dependence of the steady-state signal reached at the end of the injection using BIA evaluation software (GE Healthcare).
Human IgG (hIgG) was coupled to M-450 Epoxy Dynabeads (cat# 14011, Thermo Fisher Scientific) according to the product manual. Briefly, 1 ml (4×108) Dynabeads were transferred to a microfuge tube and washed twice with 1 ml PBS (pH 7.4) on a magnet. The washed beads were mixed with 200 µg of hIgG in 1 ml of PBS and incubated at room temperature for 15 min. Following the incubation, 20 µl of 5% BSA (bovine serum albumin) was added to the mixture to the final concentration of 0.1% BSA. The beads-hIgG-BSA mixture was continuously incubated for 24 hours at room temperature with rotations on a vertical rotator. After the completion of coupling process, beads were placed on a magnet for the removal of supernatant containing unbound hIgG and BSA. hIgG-coupled beads were washed three times on a magnet with 1 ml of pH 7.4 PBS containing 0.1% BSA and 2 mM EDTA. The hIgG-coupled beads was re-suspended in 1 ml of pH 7.4 PBS containing 0.1% BSA and 2 mM EDTA to obtain 4×108 beads/ml. The rosette formation assay was performed as described (31). IgG-coated beads were mixed with 293 cells at a ratio of 3.5 (3.5 beads per cell). All cells with the attachment of two or more beads were counted in the beads-rosette assay to determine the binding capacity of CD177 alleles.
DELFIA EuTDA Cytotoxicity Reagents kit (PerkinElmer, Waltham, MA) was used for ADCC assay as previously described (25, 32). Briefly, SKOV-3 target cells were labeled with Bis(acetoxymethyl)-2-2:6,2 terpyridine 6,6 dicarboxylate (BATDA) for 30 min in their culture medium before being extensively washed in 10 ml DMEM culture medium for three times. The BATDA-labeled cells were transferred into a 96-well non-tissue culture-treated U-bottom plates at a density of 8 × 103 cells/well. Trastuzumab was added directly to the SKOV-3 cells at the final concentration of 5 μg/ml and NK-92 cells were added at the E:T ratio of 20:1. Effector and target cells in each well were mixed by pipetting up-and-down five times. The plates were then centrifuged at 400 × g for 1 min and subsequently incubated for 2 h in a humidified 5% CO2 atmosphere at 37°C. At the end of the incubation, the plates were centrifuged at 500 × g for 5 min and 20 µl of supernatant from each well was transferred to a 96 well DELFIA Yellow Plate (PerkinElmer) and combined with 200 µl of europium. Fluorescence was measured by time-resolved fluorometry using a BMG Labtech CLARIOstar plate reader (Cary, NC). BATDA-labeled target cells alone with or without therapeutic antibodies were cultured in parallel to assess spontaneous lysis and in the presence of 1% Triton-X to measure maximum lysis. ADCC for each sample is represented as % specific release and was calculated using the following formula: Percent Specific Release = (Experimental release – Spontaneous release)/(Maximal release – Spontaneous release)*100.
GraphPad Prism v10 (La Jolla, CA, USA) was used for data analyses. One-way ANOVA followed by Tukey honest significance post hoc test was used to analyze binding capacity and ADCC differences among CD177 alleles.
To establish CD177 as a bona fide IgG Fc receptor, we carried out flow cytometry-based IgG binding assay using 293 cells expressing CD177 and vector control. As shown in Figure 1, cells transfected with vector control do not bind human IgG (Figure 1B). In addition, human IgA does not bind to cells expressing CD177 (Figure 1C). However, cells expressing CD177 bound human IgG in a dose-dependent fashion (Figure 1D), indicating that CD177 is an IgG receptor. CD177 is a GPI-linked cell surface glycoprotein and PI-PLC could cleave CD177 from the cell surface (8). As shown in Figure 2A, PI-PLC treatment dramatically reduced CD177 intensity on cell surface. Importantly, cells treated with PI-PLC almost lost the binding ability to human IgG (Figure 2B) compared to the same cell without PI-PLC treatment. In addition, treatment of cells with anti-CD177 monoclonal antibody (mAb MEM-166) significantly inhibited the binding of human IgG to the cells expressing CD177 (Figure 2C). Taken together, our data confirmed the interaction between CD177 and human IgG.
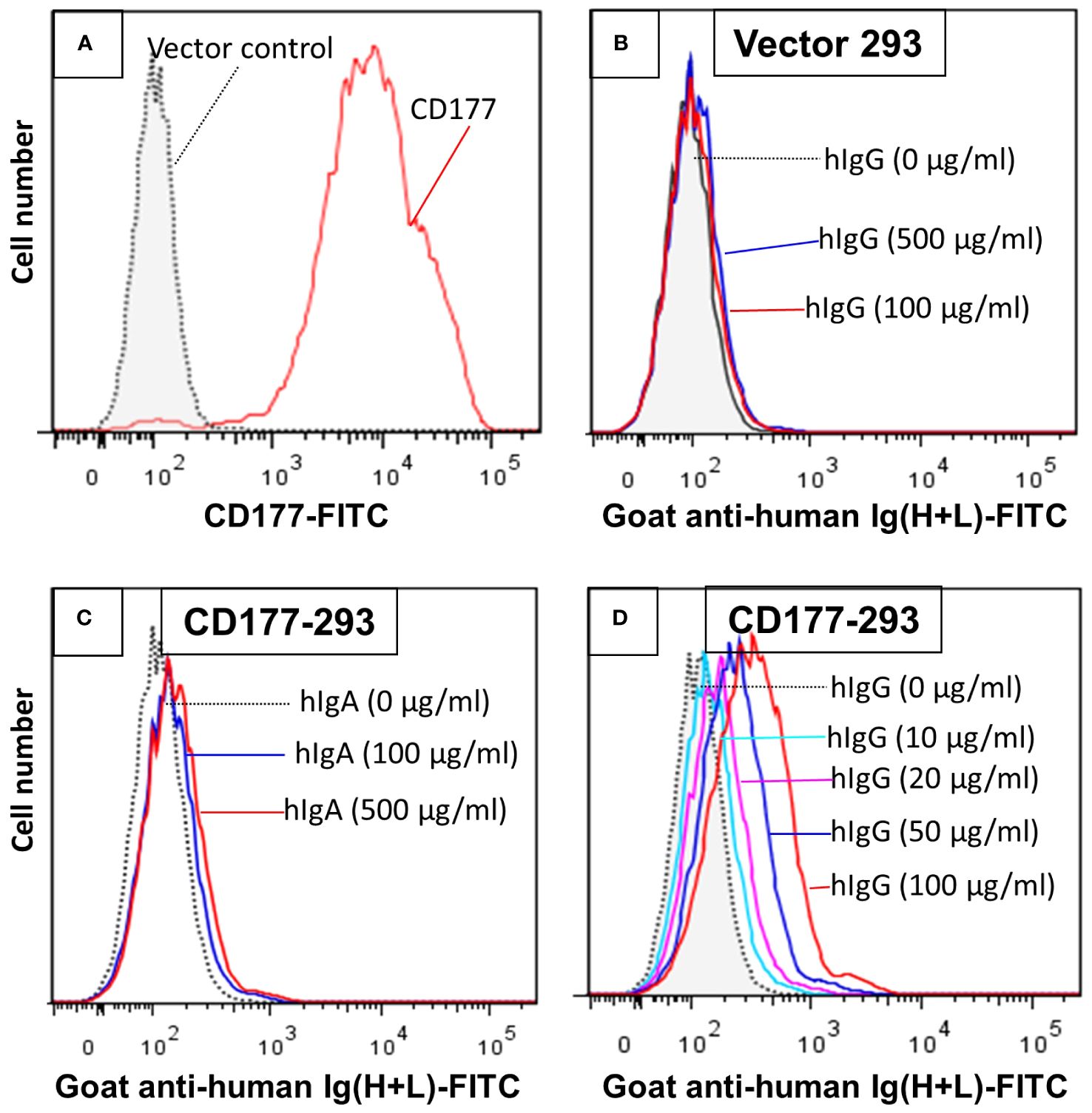
Figure 1 293 cells expressing CD177 bind human IgG (hIgG) in a dose-dependent manner. (A) Expression of CD177 on 293 cells. 293 cells transfected with the vector do not express CD177. (B) Vector control 293 cells were unable to bind hIgG. IgG binding assay was carried out as described in “Materials and Methods”. No binding could be observed with hIgG at concentrations of 100 or 500 µg/ml. (C) 293 cells expressing CD177 were unable to bind human IgA (hIgA). (D) 293 cells expressing CD177 bound hIgG in dose-dependent manner. Increased hIgG concentrations led to more IgG bound on 293 cells expressing CD177.
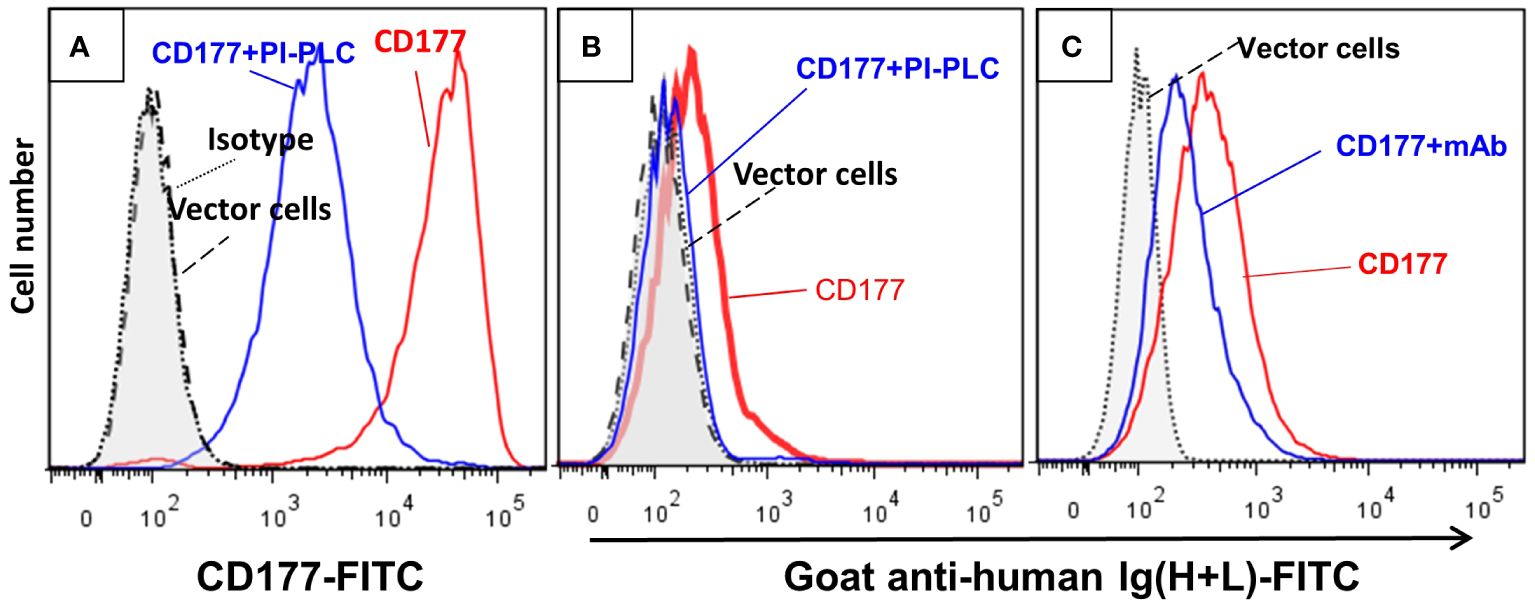
Figure 2 Cleavage of CD177 from cell surface and treatment of cells with anti-CD177 monoclonal antibody inhibit the binding of CD177 to hIgG. (A) Treatment of the 293 cells expressing CD177 with PI-PLC (CD177+PI-PLC) removed most of CD177 from cell surface compared to the same cells without PI-PLC treatment (CD177). (B) IgG binding assay was carried out as described in “Materials and Methods”. The removal of CD177 from the cell surface (CD177+PI-PLC) abolished hIgG binding to cells compared to the same cells without PI-PLC treatment (CD177). (C) Cells treated with anti-CD177 mAb (CD177+mAb) significantly inhibited hIgG binding compared to the cells without mAb treatment (CD177).
GPI moiety attaches to the residue 408 of CD177 and the extracellular domain of CD177 on the cell membrane will only contain residue 1 – 408 (7, 9). To measure the affinity of CD177 for human IgG subclasses, we produced soluble CD177 (sCD177) containing CD177 extracellular domain (residue 1 – 408) (Supplementary Figure S1) for surface plasmon resonance (SPR) analysis. In multicycle SPR kinetic analysis, different concentrations (125, 250, 500, 1,000, 2,000, 4,000, 8,000, and 16,000 nM) of sCD177 were injected into flow cells at a flow rate of 30 µl/min, with a contact and dissociation time of 250 and 600 seconds, respectively (Figure 3). As shown in Table 1, all human IgG subclasses bound to CD177 ectodomains with a narrow range of affinity (kinetic KD between 1.97×10-6 and 2.798×10-6 M and steady state KD between 2.97×10-5 and 4.12×10-5 M). Interaction between human IgG Fc fragment and CD177 (KD = 1.022 × 10-6 M) was also confirmed by SPR (Supplementary Figure S2). Results from the SPR assay demonstrate that CD177 is a low affinity IgG Fc receptor capable of binding to all human IgG subclasses.
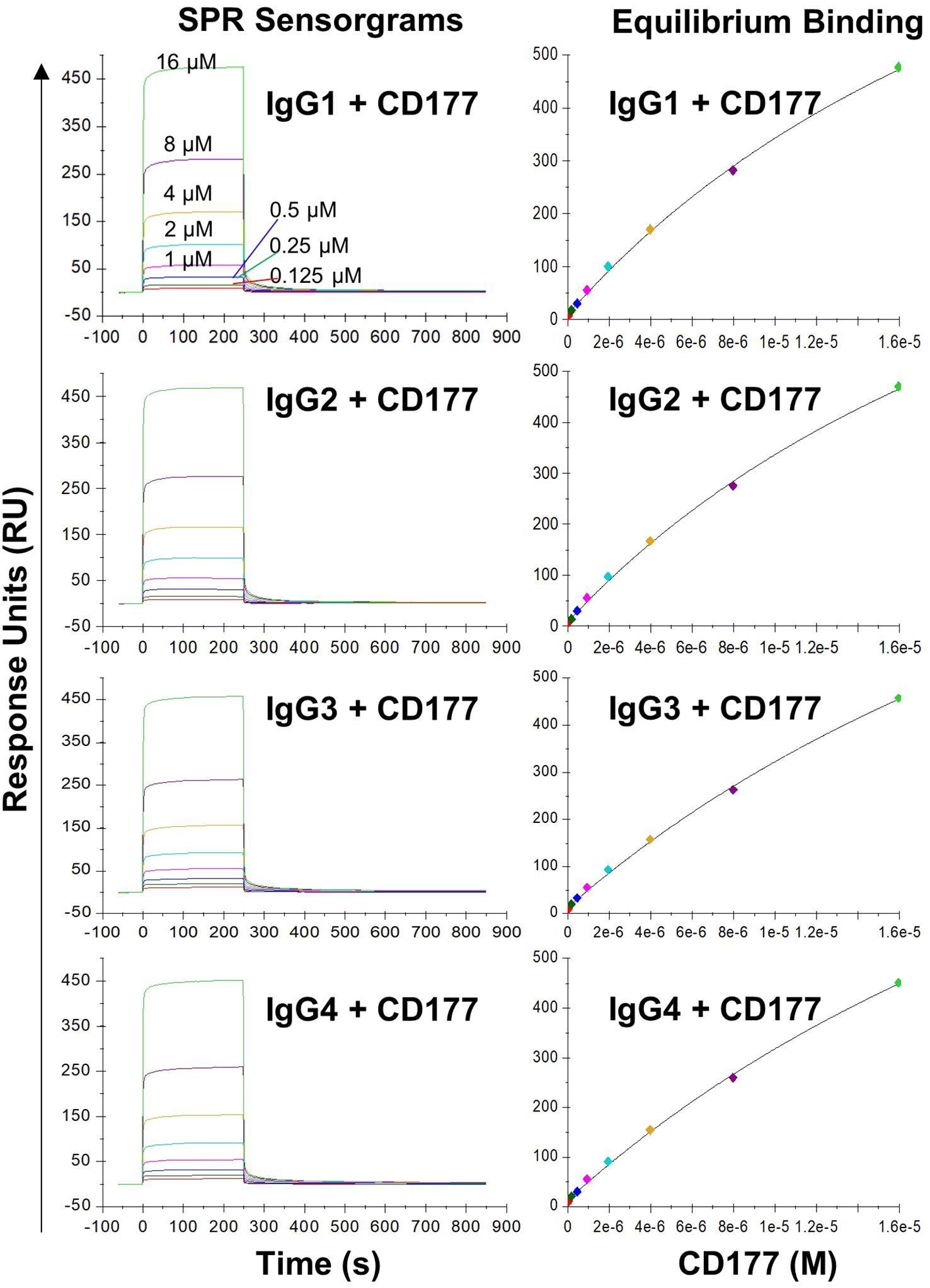
Figure 3 Binding of IgG subclasses to human CD177 by SPR. The left column shows representative SPR sensorgrams and the right column shows steady state binding curves. The experiments were repeated three times and equivalent results were obtained.
We previously demonstrated that the common nonsense CD177 SNP 263K>* (rs201821720) is the primary genetic determinant for CD177 null and expression variations (22, 23). We also found that the CD177 SNP 431G>R (rs78718189) leads to low CD177 expression on neutrophils (23). Haplotype analyses revealed that the nonsense SNP 263* allele is in complete linkage disequilibrium with SNP 431G allele while 263K is in complete linkage disequilibrium with 431R. These two CD177 SNPs form three haplotypes/alleles (263K431G, 263*431G, and 263K431R) (Figure 4). All homozygous 263*431G genotype donors were CD177 deficient (Supplementary Figure 3). In addition, homozygous 263K431R genotype donors (Supplementary Figure 3B) had significantly lower CD177 density on neutrophils (Supplementary Figure 3D) than homozygous 263K431G donors (Supplementary Figure 3C). We hypothesize that CD177-263K431R allele leads to low CD177 expression, which may affect the interaction between human IgG and CD177. To determine effect of CD177 alleles on the binding capacity to human IgG, we used 293 cells stably expressing three CD177 alleles and vector control (Figure 4A) for IgG binding assay. The nonsense CD177-263*431G allele is unable to express CD177 on cell surface. On the hand, CD177-263K431R allele expressed a much lower level of CD177 on cell surface than the wild type CD177-263K431G allele (Figure 4A), consistent with CD177 phenotypes on neutrophils (Supplementary Figure 3D). Our data confirm that CD177 alleles significantly affect cell surface CD177 expressions and that CD177 263K431R is defective allele for CD177 expression as previously reported (23).
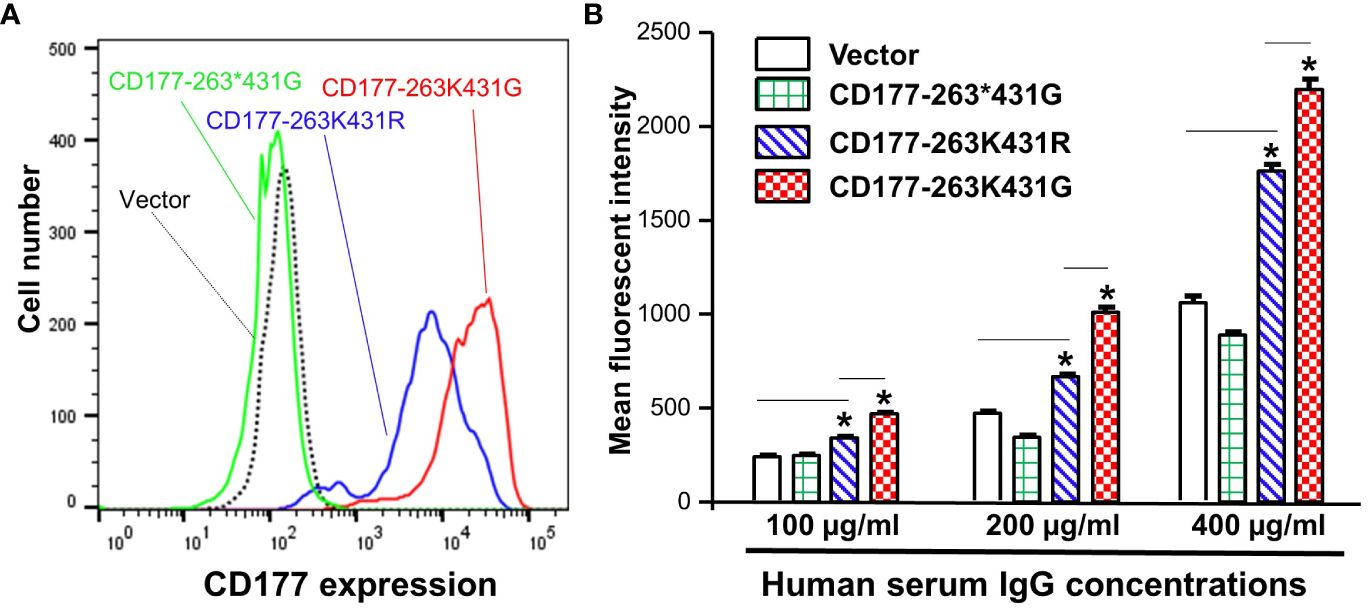
Figure 4 CD177 alleles affect CD177 expressions and binding to human IgG. (A) 293 cell lines stably expressing three CD177 alleles (263K431G, 263*431G, and 263K431R) and vector control. CD177-263*431G cells do not express CD177 as compared to vector control cells. CD177-263K431R cells expressed much lower CD177 compared to CD177-263K431G cells. (B) Binding capacity of cells with three CD177 alleles to hIgG. Flow cytometry-based IgG binding assay were carried out as described in “Materials and Methods”. The mean fluorescent intensities (MFI) represented the binding capacity of CD177 alleles for human IgG. CD177-263*431G cells failed to express CD177 on 293 cells were unable to bind hIgG, similar to vector control cells. CD177-263K431R cells had significantly lower binding capacity compared to CD177-263K431G cells. Results Data were means ± SD of three independent experiments (* P < 0.05).
Subsequently, we used flow cytometry-based binding assays to determine IgG binding capacity of 293 cell lines stably expressing CD177 alleles (263K431G, 263*431G, and 263K431R). As shown in Figure 4B, the common 263K431G allele had the highest IgG binding capacity among three CD177 alleles. Cells expressing nonsense CD177-263*431G allele were unable to bind IgG as mean fluorescent intensities of those cells were statistically not different from those of vector control cells under various IgG concentrations. On the other hand, cells expressing 263K431R allele had significantly lower binding capacity than those expressing the wild-type 263K431G allele for IgG at the concentrations of 100, 200, and 400 µg/ml. Our data demonstrate that CD177 alleles significantly affect cell surface CD177 expressions and the interaction with human IgG.
We carried out beads-rosette formation assay to determine the ability of CD177 to bind IgG-coated beads (particle targets) and effects of CD177 alleles on IgG binding. As shown in Figure 5D, cells expressing the wild-type CD177-263K431G allele are capable of forming typical beads-rosettes with multiple IgG-bound beads on a single cell (≥ 4 beads per cell). On the other hand, large beads-rosettes (a single cell bound more than four IgG-coated beads) were absent with the cells expressing nonsense 263*431G allele (Figure 5B), similar to the vector control cells (Figure 5A). In addition, cells expressing CD177-263K431R allele also could not effectively form large rosettes with IgG-coated beads (Figure 5C). To quantitate the binding capacity of three CD177 alleles for IgG-coated beads, bead number on cells with the attachment of two or more beads was counted from one hundred random cells. As shown in Figure 5E, cells expressing CD177-263K431G bound significantly more IgG-coated beads (123 ± 12) than those expressing CD177-263*431G (15 ± 4) and CD177-263K431R (13 ± 5) cells alleles (P < 0.0001). A low number of beads also bound to vector control cells (10 ± 6) due to the background or non-specific interaction between beads and cells. Interestingly, no significant differences of bound beads per 100 cells were observed among vector control, nonsense CD177-263*431G, and CD177-263K431R cells (P > 0.05). Our data clearly demonstrate that CD177 is an IgG receptor and that CD177 alleles significantly affect the binding capacity of CD177 to IgG-bound particles.
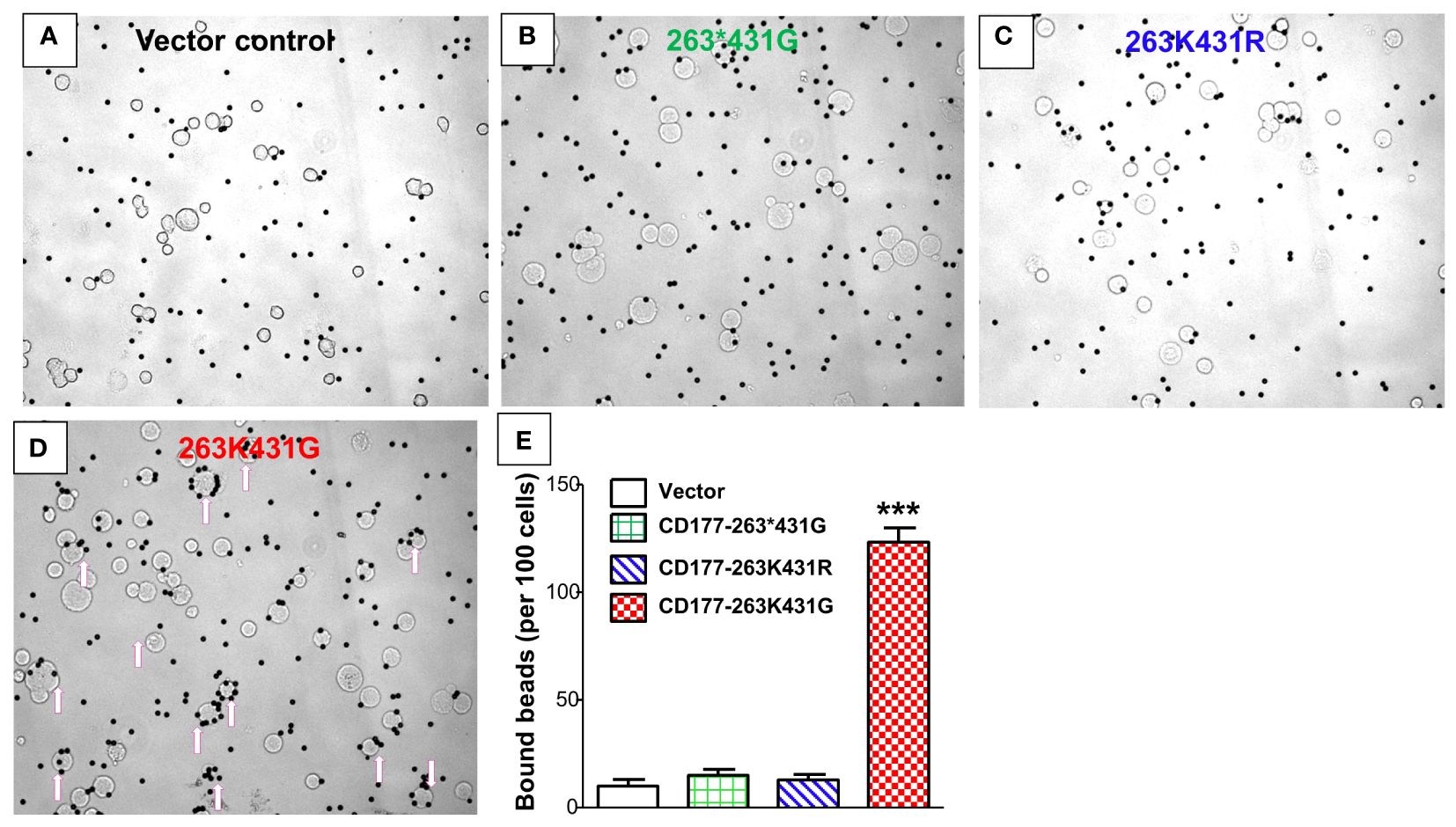
Figure 5 CD177 alleles affect binding capacity for IgG-coated particles. Beads-rosette formation assay was carried out to determine the ability of CD177 to bind IgG-coated particles. Large beads-rosettes (a single cell bound more than four IgG-coated beads) were absent for vector control cells (A), nonsense 263*431G allele cells (B), and 263K431R allele cells (C) while cells with 263K431G allele are capable of forming large beads-rosettes (arrows pointed cells) (D). (E) Cells expressing 263K431G allele bound significantly more IgG-coated beads (123 ± 12) than those expressing CD177-263*431G (15 ± 4) and CD177-263K431R (13 ± 5) cells alleles (***P < 0.0001). Results were mean ± SD of three experiments.
To determine effect of CD177 alleles on immune function, we generated three CD177-pBMN-IRES-EGFP retroviral constructs (263K431G, 263*431G, and 263K431R) and established NK-92 stable cell lines expressing CD177 alleles. CD177-263*431G allele failed to express CD177 on NK-92 cells and NK-92 cells with CD177-263K431R allele express a much lower density of CD177 compared to those with wild-type CD177-263K431G allele (Figure 6A), consistent with the phenotypes of those two alleles in peripheral blood neutrophils (Supplementary Figure 3). Both CD177-263K431R and nonsense 263*431G allele failed to mediate ADCC in NK-92 cells and the percentages of specific release (or ADCC activity) are near zero and similar to those without Trastuzumab. On the other hand, cells expressing the common CD177-263K431G allele had significantly higher ADCC activity (% specific release = 8.93 ± 4.30%) than those expressing either 263K431R (% specific release = 0.11 ± 0.29 or 263*431G alleles (% specific release = 0.04 ± 0.05) (Figure 6B) (P = 0.0004). CD177-263K431G allele cells has a higher background natural cytotoxicity (% specific release = 1.45 ± 1.42) as compared to 263K431R (% specific release = 0.03 ± 2.31) or 263*431G alleles (% specific release = 0.03 ± 0.06) in the absence of Trastuzumab, but the differences were statistically insignificant (P = 0.2224). Our data suggest that CD177 is a functional IgG Fc receptor capable of mediating ADCC and CD177 alleles affect IgG-mediated function.
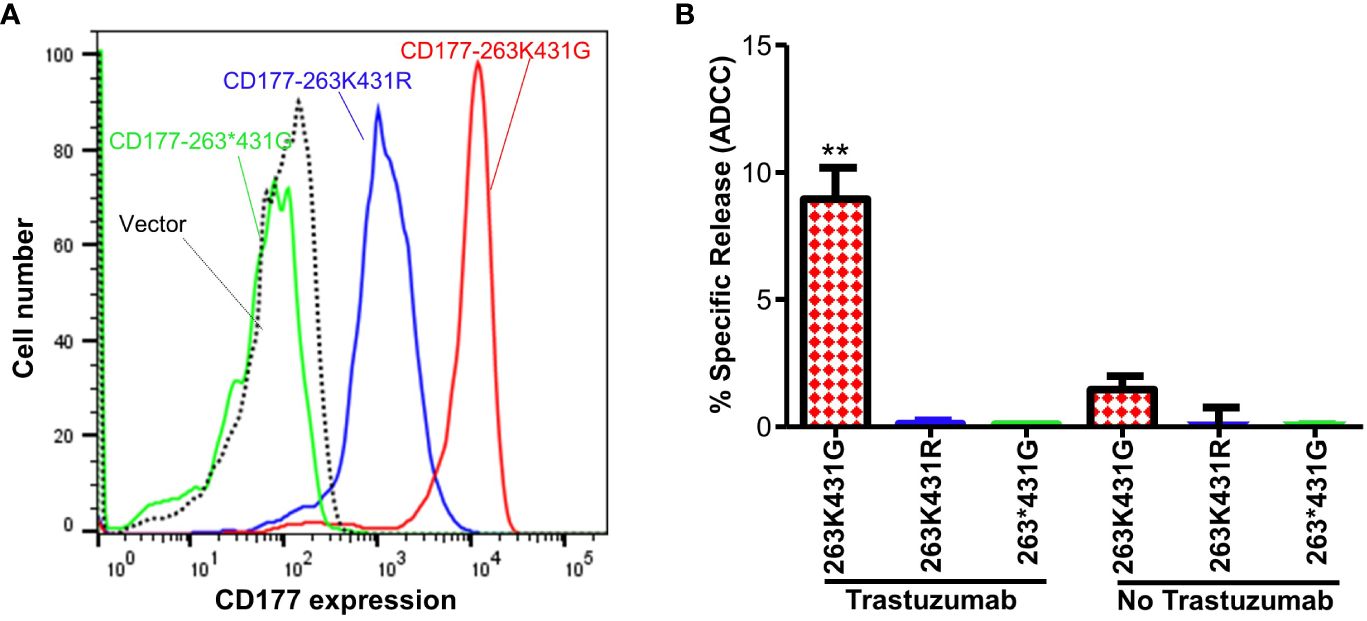
Figure 6 CD177 alleles affect surface CD177 expression and ADCC function of NK-92 cells. (A) Expressions of CD177 on stably NK-92 cell lines carrying CD177 alleles. NK-92 cells carrying 263*431G allele failed to express CD177 on NK92 cells. NK-92 cells with 263K431R allele express a much lower density of CD177 compared to those with 263K431G allele. (B) ADCC activity of NK-92 cells expressing CD177 alleles. NK-92 cells expressing 263K431G allele had the highest ADCC activity among three CD177 alleles (**P < 0.001). Both 263K431R and the null allele CD177-263*431G failed to mediate ADCC, similar to the vector control cells. Results from four experiments are shown.
Inherited CD177 deficiency is common in human populations but its potential impact on immune functions remains unknown. In the current study, we revealed CD177 as a novel IgG Fc receptor that mediates ADCC function. Notably, we observed that CD177 genetic alleles significantly affect its interaction with human IgG and ADCC functions. The definition of CD177 as a novel functional IgG Fc-receptor may fill a knowledge gap about the full repertoire of human FcγRs. Our study provided novel insights into CD177-mediated immune functions and into the potential genetic mechanisms underlying variability of responses to vaccination and antibody therapy.
Our experimental data show that CD177 is a low affinity IgG receptor. However, CD177 differs from classical low affinity IgG Fc receptors in three features. First, the binding affinity of CD177 for human IgGs (steady state KD between 2.97×10-5 and 4.12×10-5 M) is much lower than those of the classical low-affinity IgG Fc receptors (the steady state KD between 10-6 to 10-7) (30). Second, CD177 binds all IgG subclasses with similar affinity while classical IgG Fc receptors bind IgG subclasses with significantly different affinities (30). Third, the reduction of CD177 density on cell surface had a dramatic effect on the interaction between CD177 and IgGs, which could be explained by very low affinity of CD177 for IgGs compared to classical low affinity FcγRs.
The common nonsense CD177 SNP 263K>* is the primary genetic determinant for CD177 null and expression variations (22) while the CD177 SNP 431G>R also leads to low CD177 expression on neutrophils (23). Traum et al. recently demonstrated that that CD177 SNP 431G>R (c.1291G>A) affects the membrane expression of CD177, leading to low level of CD177 protein on the cellular surface for the CD177 431R allele (33). The nonsense SNP 263* allele is in complete linkage disequilibrium with SNP 431G allele and 263K is in complete linkage disequilibrium with 431R, leading to three haplotypes or alleles (263K431G, 263*431G, and 263K431R in human population. All homozygous 263*431G genotype donors were CD177 deficient (23). In addition, homozygous 263K431R genotype donors had significantly lower CD177 density on neutrophils than homozygous 263K431G donors (23). In the current study, CD177-263K431R allele consistently leads to lower CD177 expression than the common/wild-type CD177-263K431G allele in stable transfected 293 and NK-92 cell lines, confirming that CD177-263K431R is also a defective CD177 allele for expression (33). Importantly, cells expressing 263K431R allele had significantly lower binding capacity for human IgG and IgG-coated beads compared to those expressing the 263K431G allele due to low expression of 263K431R allele on cell surface. Notably, CD177-263K431R allele failed to mediate ADCC, indicating that CD177-263K431R allele not only affects CD177 surface expression and binding capacity for IgG but also influence the CD177 immune function. We conclude that both CD177 SNP 263K>* and SNP 431G>R affect CD177 functions.
The function of CD177 remains poorly understood. CD177 is a GPI-anchored membrane protein tethered to the neutrophil surface by a glycosylphosphatidylinositol anchor lacking a transmembrane domain. CD177 employs Mac-1 (CD11b/CD18) to transduce signals for cell functions (24, 34–36), reminiscent of the low affinity IgG Fc receptor FcγRIIIB (37–40). CD177 is selectively expressed in bone marrow cells and plays an important role in the proliferation and differentiation of myeloid lineage cells including neutrophils, myelocytes, promyelocytes, megakaryocytes, and early erythroblasts. In a CD177-genetic knockout mouse model, CD177-deficiency leads to increased neutrophil cell death in bone marrow, suggesting that CD177 could provide survival signal during neutrophil differentiation (13). CD177 is over-expressed in neutrophils from patients with myeloproliferative disorders including polycythemia vera, essential thrombocythemia, idiopathic myelofibrocythemia, and hypereosinophilic syndrome (14, 18). CD177 was an indicator of increased erythropoietic activity in thalassemia syndromes as CD177 expression was significantly elevated in β-thalassemia patients compared to healthy controls (41). CD177 overexpression may also have a direct role in the pathogenesis of myeloproliferative disorders as CD177 enhances cell proliferation in vitro (11, 12). On the other hand, the low percentage of CD177 positive neutrophil is significantly associated with myelodysplastic syndrome and chronic myelogenous leukemia (10, 17). In vitro studies show that CD177 enhances cell growth/proliferation (11, 12). The current study suggests that IgGs may be natural ligands for CD177. As a low affinity IgG Fc receptor (FcγR), CD177 may only recognize multivalent IgG complexes. Accordingly, CD177 could serve as a sensor for IgG immune complexes (or IgG-opsonized targets) in bone marrow or in circulation. We speculate that that interaction between CD177 of myeloid progenitor cells and IgG immune complexes may provide a stimulatory signal to drive promyeloid cell proliferation and differentiation into mature neutrophils during infections and diseases. The limitation of the current study is that NK cells were used to determine CD177 ADCC function. The exact role of interaction between CD177 and IgG immune complexed in myeloid cell development and function requires further investigation. Future studies need to focus on direct effects of CD177 expression and deficiency on immune system in human subjects.
CD177 is a novel IgG Fc receptor and the CD177 genetic variants significantly affect the binding of IgG. Additionally, CD177 could mediate ADCC function and genetic variants of CD177 significantly affect the immune function. The definition of CD177 as novel functional IgG Fc-receptor fills the critical knowledge gap about the full repertoire of human FcγRs. Our study may provide novel insights into CD177-mediated immune functions and into the genetic mechanisms underlying response variability of antibody therapy. CD177 genetic variants may also serve as valuable biomarkers in antibody therapy and vaccinations.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
YL: Data curation, Investigation, Methodology, Writing – review & editing, Formal analysis, Validation. JW: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing – original draft.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by National Institute of Health grant R21 AI125729 and R21 AI149395. The funders had no role in study design data collection and analysis, decision to publish, or preparation of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1418539/full#supplementary-material
1. Dahal LN, Roghanian A, Beers SA, Cragg MS. FcgammaR requirements leading to successful immunotherapy. Immunol Rev. (2015) 268:104–22. doi: 10.1111/imr.12342
2. Nimmerjahn F, Ravetch JV. Fcgamma receptors as regulators of immune responses. Nat Rev Immunol. (2008) 8:34–47. doi: 10.1038/nri2206
3. Wu J, Edberg JC, Redecha PB, Bansal V, Guyre PM, Coleman K, et al. A novel polymorphism of FcgammaRIIIa (CD16) alters receptor function and predisposes to autoimmune disease. J Clin Invest. (1997) 100:1059–70. doi: 10.1172/JCI119616
4. Wu J, Li Y, Rendahl A, Bhargava M. Novel human FCGR1A variants affect CD64 functions and are risk factors for sarcoidosis. Front Immunol. (2022) 13:841099. doi: 10.3389/fimmu.2022.841099
5. Li X, Wu J, Ptacek T, Redden DT, Brown EE, Alarcon GS, et al. Allelic-dependent expression of an activating Fc receptor on B cells enhances humoral immune responses. Sci Transl Med. (2013) 5:216ra175. doi: 10.1126/scitranslmed.3007097
6. Li X, Wu J, Carter RH, Edberg JC, Su K, Cooper GS, et al. A novel polymorphism in the Fcgamma receptor IIB (CD32B) transmembrane region alters receptor signaling. Arthritis Rheum. (2003) 48:3242–52. doi: 10.1002/art.11313
7. Kissel K, Santoso S, Hofmann C, Stroncek D, Bux J. Molecular basis of the neutrophil glycoprotein NB1 (CD177) involved in the pathogenesis of immune neutropenias and transfusion reactions. Eur J Immunol. (2001) 31:1301–9. doi: 10.1002/1521-4141(200105)31:5<1301::AID-IMMU1301>3.0.CO;2-J
8. Skubitz KM, Stroncek DF, Sun B. Neutrophil-specific antigen NB1 is anchored via a glycosyl-phosphatidylinositol linkage. J Leukoc Biol. (1991) 49:163–71. doi: 10.1002/jlb.49.2.163
9. Temerinac S, Klippel S, Strunck E, Roder S, Lubbert M, Lange W, et al. Cloning of PRV-1, a novel member of the uPAR receptor superfamily, which is overexpressed in polycythemia rubra vera. Blood. (2000) 95:2569–76. doi: 10.1182/blood.V95.8.2569
10. Stroncek DF, Shankar R, Litz C, Clement L. The expression of the NB1 antigen on myeloid precursors and neutrophils from children and umbilical cords. Transfus Med. (1998) 8:119–23. doi: 10.1046/j.1365-3148.1998.00136.x
11. Dillon M, Minear J, Johnson J, Lannutti BJ. Expression of the GPI-anchored receptor Prv-1 enhances thrombopoietin and IL-3-induced proliferation in hematopoietic cell lines. Leuk Res. (2008) 32:811–9. doi: 10.1016/j.leukres.2007.09.018
12. Mnjoyan Z, Li J, Afshar-Kharghan V. Expression of polycythemia rubra vera-1 decreases the dependency of cells on growth factors for proliferation. Haematologica. (2005) 90:405–6.
13. Xie Q, Klesney-Tait J, Keck K, Parlet C, Borcherding N, Kolb R, et al. Characterization of a novel mouse model with genetic deletion of CD177. Protein Cell. (2015) 6:117–26. doi: 10.1007/s13238-014-0109-1
14. Stroncek DF, Caruccio L, Bettinotti M. CD177: A member of the Ly-6 gene superfamily involved with neutrophil proliferation and polycythemia vera. J Transl Med. (2004) 2:8. doi: 10.1186/1479-5876-2-8
15. Demaret J, Venet F, Plassais J, Cazalis MA, Vallin H, Friggeri A, et al. Identification of CD177 as the most dysregulated parameter in a microarray study of purified neutrophils from septic shock patients. Immunol Lett. (2016) 178:122–30. doi: 10.1016/j.imlet.2016.08.011
16. Zhou G, Yu L, Fang L, Yang W, Yu T, Miao Y, et al. CD177(+) neutrophils as functionally activated neutrophils negatively regulate IBD. Gut. (2018) 67:1052–63. doi: 10.1136/gutjnl-2016-313535
17. Meyerson HJ, Osei E, Schweitzer K, Blidaru G, Edinger A, Balog A. CD177 expression on neutrophils: in search of a clonal assay for myeloid neoplasia by flow cytometry. Am J Clin Pathol. (2013) 140:658–69. doi: 10.1309/AJCPDFBEBQZW1OI7
18. Stroncek DF. Neutrophil-specific antigen HNA-2a, NB1 glycoprotein, and CD177. Curr Opin Hematol. (2007) 14:688–93. doi: 10.1097/MOH.0b013e3282efed9e
19. Teofili L, Martini M, Luongo M, Di Mario A, Leone G, De Stefano V, et al. Overexpression of the polycythemia rubra vera-1 gene in essential thrombocythemia. J Clin Oncol. (2002) 20:4249–54. doi: 10.1200/JCO.2002.11.507
20. Toyoda T, Tsukamoto T, Yamamoto M, Ban H, Saito N, Takasu S, et al. Gene expression analysis of a Helicobacter pylori-infected and high-salt diet-treated mouse gastric tumor model: identification of CD177 as a novel prognostic factor in patients with gastric cancer. BMC Gastroenterol. (2013) 13:122. doi: 10.1186/1471-230X-13-122
21. Zhou G, Peng K, Song Y, Yang W, Shu W, Yu T, et al. CD177+ neutrophils suppress epithelial cell tumourigenesis in colitis-associated cancer and predict good prognosis in colorectal cancer. Carcinogenesis. (2018) 39:272–82. doi: 10.1093/carcin/bgx142
22. Li Y, Mair DC, Schuller RM, Li L, Wu J. Genetic mechanism of human neutrophil antigen 2 deficiency and expression variations. PloS Genet. (2015) 11:e1005255. doi: 10.1371/journal.pgen.1005255
23. Wu J, Li Y, Schuller RM, Li L, Litmeyer AS, Bein G, et al. The nonconservative CD177 single-nucleotide polymorphism c.1291G>A is a genetic determinant for human neutrophil antigen-2 atypical/low expression and deficiency. Transfusion. (2019) 59:1836–42. doi: 10.1111/trf.15222
24. Jerke U, Rolle S, Dittmar G, Bayat B, Santoso S, Sporbert A, et al. Complement receptor Mac-1 is an adaptor for NB1 (CD177)-mediated PR3-ANCA neutrophil activation. J Biol Chem. (2011) 286:7070–81. doi: 10.1074/jbc.M110.171256
25. Snyder KM, Hullsiek R, Mishra HK, Mendez DC, Li Y, Rogich A, et al. Expression of a recombinant high affinity igG fc receptor by engineered NK cells as a docking platform for therapeutic mAbs to target cancer cells. Front Immunol. (2018) 9:2873. doi: 10.3389/fimmu.2018.02873
26. Hullsiek R, Li Y, Snyder KM, Wang S, Di D, Borgatti A, et al. Examination of igG fc receptor CD16A and CD64 expression by canine leukocytes and their ADCC activity in engineered NK cells. Front Immunol. (2022) 13:841859. doi: 10.3389/fimmu.2022.841859
27. Jing Y, Ni Z, Wu J, Higgins L, Markowski TW, Kaufman DS, et al. Identification of an ADAM17 cleavage region in human CD16 (FcgammaRIII) and the engineering of a non-cleavable version of the receptor in NK cells. PloS One. (2015) 10:e0121788. doi: 10.1371/journal.pone.0121788
28. Armour KL, van de Winkel JG, Williamson LM, Clark MR. Differential binding to human FcgammaRIIa and FcgammaRIIb receptors by human IgG wildtype and mutant antibodies. Mol Immunol. (2003) 40:585–93. doi: 10.1016/j.molimm.2003.08.004
29. Dong C, Ptacek TS, Redden DT, Zhang K, Brown EE, Edberg JC, et al. Fcgamma receptor IIIa single-nucleotide polymorphisms and haplotypes affect human IgG binding and are associated with lupus nephritis in African Americans. Arthritis Rheumatol. (2014) 66:1291–9. doi: 10.1002/art.38337
30. Bruhns P, Iannascoli B, England P, Mancardi DA, Fernandez N, Jorieux S, et al. Specificity and affinity of human Fcgamma receptors and their polymorphic variants for human IgG subclasses. Blood. (2009) 113:3716–25. doi: 10.1182/blood-2008-09-179754
31. Bracke M, Dubois GR, Bolt K, Bruijnzeel PL, Vaerman JP, Lammers JW, et al. Differential effects of the T helper cell type 2-derived cytokines IL-4 and IL-5 on ligand binding to IgG and IgA receptors expressed by human eosinophils. J Immunol. (1997) 159:1459–65.
32. Hintz HM, Snyder KM, Wu J, Hullsiek R, Dahlvang JD, Hart GT, et al. Simultaneous engagement of tumor and stroma targeting antibodies by engineered NK-92 cells expressing CD64 controls prostate cancer growth. Cancer Immunol Res. (2021) 9:1270–82. doi: 10.1158/2326-6066.CIR-21-0178
33. Traum A, Jehle S, Waxmann Y, Litmeyer AS, Berghofer H, Bein G, et al. The CD177 c.1291A allele leads to a loss of membrane expression and mimics a CD177-null phenotype. Int J Mol Sci. (2024) 25:2877. doi: 10.3390/ijms25052877
34. Bai M, Grieshaber-Bouyer R, Wang J, Schmider AB, Wilson ZS, Zeng L, et al. CD177 modulates human neutrophil migration through activation-mediated integrin and chemoreceptor regulation. Blood. (2017) 130:2092–100. doi: 10.1182/blood-2017-03-768507
35. Bayat B, Werth S, Sachs UJ, Newman DK, Newman PJ, Santoso S. Neutrophil transmigration mediated by the neutrophil-specific antigen CD177 is influenced by the endothelial S536N dimorphism of platelet endothelial cell adhesion molecule-1. J Immunol. (2010) 184:3889–96. doi: 10.4049/jimmunol.0903136
36. Sachs UJ, Andrei-Selmer CL, Maniar A, Weiss T, Paddock C, Orlova VV, et al. The neutrophil-specific antigen CD177 is a counter-receptor for platelet endothelial cell adhesion molecule-1 (CD31). J Biol Chem. (2007) 282:23603–12. doi: 10.1074/jbc.M701120200
37. Krauss JC, PooH, Xue W, Mayo-Bond L, Todd 3RF, Petty HR. Reconstitution of antibody-dependent phagocytosis in fibroblasts expressing Fc gamma receptor IIIB and the complement receptor type 3. J Immunol. (1994) 153:1769–77.
38. Poo H, Krauss JC, Mayo-Bond L, Todd 3RF, Petty HR. Interaction of Fc gamma receptor type IIIB with complement receptor type 3 in fibroblast transfectants: evidence from lateral diffusion and resonance energy transfer studies. J Mol Biol. (1995) 247:597–603. doi: 10.1006/jmbi.1995.0166
39. Zhou MJ, Brown EJ. CR3 (Mac-1, alpha M beta 2, CD11b/CD18) and Fc gamma RIII cooperate in generation of a neutrophil respiratory burst: requirement for Fc gamma RIII and tyrosine phosphorylation. J Cell Biol. (1994) 125:1407–16. doi: 10.1083/jcb.125.6.1407
40. Stockl J, Majdic O, Pickl WF, Rosenkranz A, Prager E, Gschwantler E, et al. Granulocyte activation via a binding site near the C-terminal region of complement receptor type 3 alpha-chain (CD11b) potentially involved in intramembrane complex formation with glycosylphosphatidylinositol-anchored Fc gamma RIIIB (CD16) molecules. J Immunol. (1995) 154:5452–63.
Keywords: CD177, IgG Fc receptor, genetic variants, ADCC - antibody dependent cellular cytotoxicity, immune function
Citation: Li Y and Wu J (2024) CD177 is a novel IgG Fc receptor and CD177 genetic variants affect IgG-mediated function. Front. Immunol. 15:1418539. doi: 10.3389/fimmu.2024.1418539
Received: 16 April 2024; Accepted: 08 July 2024;
Published: 26 July 2024.
Edited by:
Marcin Okrój, Intercollegiate Faculty of Biotechnology of University of Gdańsk and Medical University of Gdańsk, PolandReviewed by:
Daniel Ajona, University of Navarra, SpainCopyright © 2024 Li and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianming Wu, am13dUB1bW4uZWR1
†ORCID: Jianming Wu, orcid.org/0000-0001-9142-7066
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.