
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Immunol., 31 May 2024
Sec. Cytokines and Soluble Mediators in Immunity
Volume 15 - 2024 | https://doi.org/10.3389/fimmu.2024.1413466
This article is part of the Research TopicUnraveling the Molecular Mechanisms of Cytokine Signaling in Regulating Inflammatory DiseasesView all 16 articles
Leukocyte cell-derived chemotaxin-2 (LECT2) is an important cytokine synthesized by liver. Significant research interest is stimulated by its crucial involvement in inflammatory response, immune regulation, disease occurrence and development. However, bibliometric study on LECT2 is lacking. In order to comprehend the function and operation of LECT2 in human illnesses, we examined pertinent studies on LECT2 investigation in the Web of Science database, followed by utilizing CiteSpace, VOSview, and Scimago Graphica for assessing the yearly quantity of papers, countries/regions involved, establishments, authors, publications, citations, and key terms. Then we summarized the current research hotspots in this field. Our study found that the literature related to LECT2 has a fluctuating upward trend. “Angiogenesis”, “ALECT2”, “diagnosis”, and “biliary atresia” are the current investigative frontiers. Our findings indicated that liver diseases (e.g. liver fibrosis and hepatic cell carcinoma), systemic inflammatory disease, and amyloidosis are the current research focus of LECT2. The current LECT2 research outcomes are not exceptional. We hope to promote the scientific research of LECT2 and exploit its potential for clinical diagnosis and treatment of related diseases through a comprehensive bibliometric review.
Leukocyte-derived chemokines-2 (LECT2) is a 16 kDa chemokine that mediates neutrophil migration, and it belongs to the interleukin-8 family (1). The LECT2 gene is situated on chromosome 5q31.1-q32 (2). It is highly similar to the sequence of the chondromodulin repeat region of the chicken myb-induced myeloid 1 protein (3). This gene has the Val58Ile polymorphism regarding rheumatoid arthritis. Therefore, it is also called chondromodulin-II (ChM-II or CHM2) (4, 5). Human LECT2 protein contains three internal disulfide connections (Cys25-Cys60; Cys36-Cys41; Cys99-Cys142). Zinc binds to its disulfide bonds to inhibit the self-oligomerization of LECT2 in vitro, thereby stabilizing the LECT2 structure (6, 7).
LECT2 primarily produces by hepatocytes, released into the bloodstream (8). It is widely distributed in the liver, kidneys, intestines, skin, and brain (9–15). It is also expressed in many tissues, including muscle cells, endothelial cells, adipocytes, lymph nodes, spleen, bone marrow, and other immune tissues (16–19).
A series of research indicated that the LECT2 protein could play a role in the development and advancement of various conditions. Among immune system diseases like osteoarthritis (20) and rheumatoid arthritis (21), septicemia (22), atherosclerosis, osteoporosis (23), diabetes (18), atopic dermatitis (24), epithelial ovarian cancer (25), and obesity (26), LECT2 can activate and recruit immune cells to regulate inflammatory responses and immune responses (9). LECT2 regulates the bone microenvironment and promotes chondrocyte proliferation (20). New research has uncovered that in the pathogenesis of hepatocellular carcinoma (HCC), LECT2 can improve the tumor microenvironment (27, 28), and prevent vascular invasion and metastasis in HCC (29). Leukocyte chemotactic factor 2 amyloidosis (ALECT2) mainly expressed in the liver or kidney, and the overexpression of LECT2 is one of the important causes of ALECT2 amyloid deposition (30–32). Some researchers have found that LECT2 could promote the development of nerve cells in the brain (33, 34). Regarding obesity and insulin resistance, overexpression of LECT2 reduces insulin receptor substrate (IRS-1) levels (26, 35). The above studies have revealed the potential of LECT2 as a therapeutic target for related diseases. However, demand for more advanced research is critical because more complex mechanisms need to be further elucidated.
Currently, there is no bibliometric research on LECT2. Therefore, we utilize VOSviewer and CiteSpace bibliometric software for visual examination of the LECT2 literature from the Web of Science Core Collection (WoSCC). This method intertwines mathematical statistics, providing a systematic qualitative and quantitative evaluation. One of its benefits is the ability to assess the impact and contributions of various authors, countries, institutions, etc. general information. Additionally, it can illustrate the evolution of the field, identify research trends, and explore cutting-edge topics using the Scientific Knowledge Graph. This paper shows the macro development of LECT2 research, discusses the current research hotspots and frontiers, and provides a reference for follow-up related research.
In order to guarantee the reliability and availability of the information, we conducted a search in the Web of Science Core Collection (WoSCC) database for articles on LECT2. The search term is “ TS= (“LECT2” OR “Leukocyte cell-derived chemotaxin-2” OR “Chondromodulin-II” OR “ChM-II” OR “CHM2”)”. According to the purpose and content of this study, the disciplines of the Web of Science classified as fisheries, veterinary science, marine freshwater biology, zoology, oceanography, remote sensing, agriculture, dairy, and animal science were excluded. The publication period is limited from January 1, 2010 to December 31, 2023, reasonably. For one thing, the earliest publication relevant to the topic of this review was published in 2010. For another thing, the number of publications published in 2024 is too small to have a profound impact. Since the total number of papers on LECT2 is not large, there is no restriction on the type of publications. To avoid bias caused by the daily update of the database, we collected the search data on January 27, 2024, and obtained a total of 181 articles, including article (n=125), meeting abstract (n=33), review article (n=12), editorial material (n=6), letter (n=3), correction (n=1), retraction (n=1). The record format is all records and references. The data is in plain text, and then imported into CiteSpace. These 181 articles obtain 3928 different references, with 2857 citations, an average of 15.78 times per item, and 30 h-indexes.
This study used Microsoft Office Excel 2021, VOSviewer (v1.6.20), CiteSpace (v6.2.R7), Origin, Pajek, and Scimago Graphica to analyze 181 collected documents. Figure 1 is a flowchart of the data acquisition and visual analysis of this study. VOSviewer, a Java-based software created in 2009 by Van Eck and Waltman from the Center for Science and Technology Research (CWTS) at Leiden University in the Netherlands, is available for free. It has great features in displaying bibliometric maps, commonly used to build collaborative, co-quoting, and co-occurrence networks (36). The combination of VOSview with Scimago Graphica and Pajek makes images and data more diverse. CiteSpace, a program created by Professor Chaomei Chen from Drexel University in the US, is used for analyzing and visualizing bibliometric data. The advantage of this approach is that it shows the turning point of the scientific revolution through visually prominent nodes and connections to discover research hotspots and frontiers (37). According to the features and advantages of the above tools, we make use of them to conduct corresponding Scientific Knowledge Graphs.
The annual number of publications can reflect the research process and trend in this field to a certain extent. In Figure 2, the increase in publications and citations is depicted. Between 2010 and 2021, there were notable fluctuations in the yearly numbers, with a general upward trend. The highest annual number of publications is 21 in 2020. The most significant increase is from 2013 to 2014, reaching 13 publications, suggesting a major advancement in LECT2 research during that period. The large number of publications in the past two years indicates that this field has attracted people’s attention and has a good development trend. It is now garnering increasing interest from scholars.
There are 32 countries/regions and 237 institutions in total. The rankings, quantities, proportions, citation frequencies, and centralities of the top ten countries in terms of publications are displayed in Table 1. The top three countries/regions for publication are the United States (n = 66), mainland China (38), and Japan (39). The top three countries/regions in centrality are the United States (1.08), Germany (0.25), and mainland China (0.19), indicating that their research results have a greater impact on LECT2 research.
We employed VOSviewer and Scimago Graphica to generate a national geographic visualization atlas (Figure 3A). CiteSpace was used to generated a collaborative network analysis map amongst 32 countries, visualizing publications and partnerships. Labels denote nations with more than two publications (Figure 3B). Node sizes represent the number of publications; link width denotes partnership strength. As evident from Figures 3A, B, the USA leads in transnational cooperation, partnering with 21 nations including Italy, India, Poland, China, and so on. In Europe, Iceland, Netherlands, Switzerland, Portugal have carried out extensive and close cooperation on LECT2-related research.
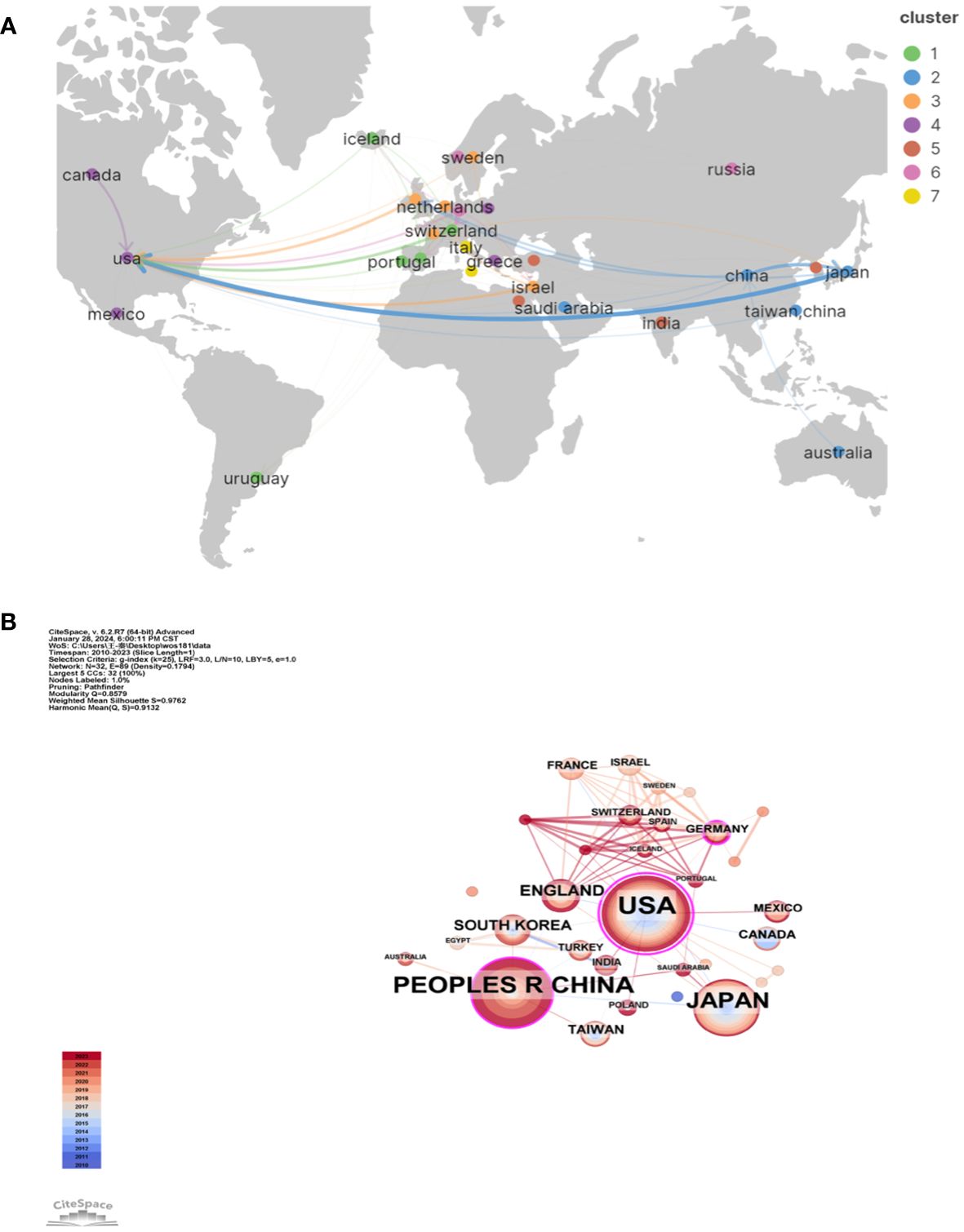
Figure 3 The geographical distribution (A) and visualization of countries (B) on research of leukocyte cell-derived chemotaxin-2.
Using publication volumes as an indicator, each institute’s research output on LECT2 research is counted. The top 10 institutions on research of LECT2 are displayed in Table 2. Kanazawa University had the most publications with 19 articles, while the Mayo Clinic and the National Institute of Infectious Diseases followed with 15 and 10 articles respectively. The institutional collaboration map was created filtering institutions publishing less than ten times. As shown in Figure 4, institutional cooperation is relatively close. Mayo Clinic collaborated with 25 organizations such as Mayo Clinic Phoenix and Memorial Sloan Kettering Cancer Center. The National Institute of Health and Medical Research also maintains numerous collaborations with various institutions.
Table 3 lists the top 10 authors with the most posts. Of these authors, Satoshi Yamagoe has been cited the most. Toshinari Takamura has the highest number of articles with 14, followed by Hirofumi Misu with 13, and Shuichi Kaneko with 11. All of them are from Kanazawa University, and their research on LECT2 is biased toward the association of LECT2 with obesity and insulin resistance. The concept of co-citation is a research method to measure the degree of relationship between documents. Table 3 also displays the top ten authors who have the highest number of co-citations in academic works. The top three co-citations are Yamagoe S (159 articles), Larsen CP (78 articles), and Okumura A (74 articles).
A total of 1005 authors were included in the literature data. The author cooperation network generated by CiteSpace was used to show the collaboration of 310 authors. The lines linking the nodes represent the cooperative relationships between authors, and the sizes of the nodes indicate the quantity of articles published on LECT2 by each author. Based on keywords, the authors’ collaborative research was divided into 7 clusters, labeled #0- #6, and corresponding to the keywords shown in the color bars in Figure 5. Nodes of the same cluster are filled with the same color. In terms of Sigma, a metric that measures importance, it can be concluded that the top ranked author Aithal, Guruprasad P made a very important contribution to the research in this field. According to the lines in the diagram to analyze the collaboration between the authors, Takamura Toshinari and Shuichi Kaneko were the most active collaborators (Figure 5). Satoshi Yamagoe, Toshinari Takamura, and Shuichi Kaneko collaborated to uncover the therapeutic potential of LECT2 in treating infectious diseases and cancer (40). The cooperation between the authors promotes the breadth and depth of LECT2 research directions.
A total of 118 journals published articles on LECT2. HEPATOLOGY had the highest number of publications with 17, while PLOS ONE came in second with 6. KIDNEY INTERNATIONAL ranks first in impact factor among the top 10 journals according to Table 4.
We employed a chord diagram to illustrate journal citation connections (Figure 6A). Each colorful track signifies a journal, positioned radially. The string thickness visually quantifies the correlation strength. KIDNEY INTERNATIONAL boasts an extensive correlation with AMERICAN JOURNAL OF KIDNEY DISEASES, and a a relatively strong one with CLINICAL NEPHROLOGY. It can be concluded that the citation connections between journals are highly active.
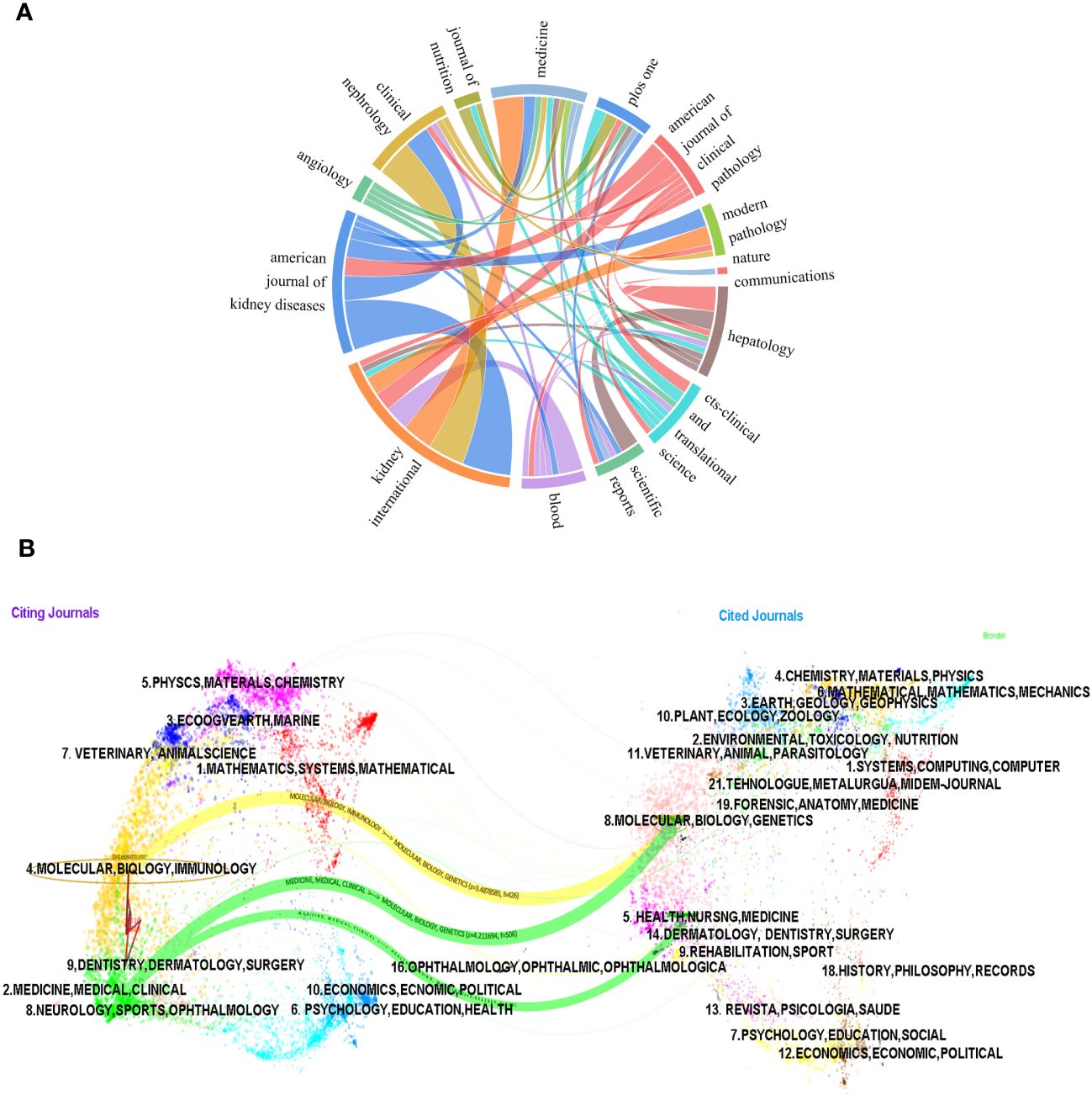
Figure 6 The chord diagram (A) and the dualmap overlay of journals (B) on research of leukocyte cell-derived chemotaxin-2 to illustrate journal citation connections.
The dual-map overlay of journals illustrates the citation connections among the leading edge of knowledge and the knowledge base in the macrostructure. There are four macro structural development models: independence, divergence, convergence, and intersection. Understanding them can help researchers screen important references and grasp the historical development path of the topic. In Figure 6B, on the left is the distribution of journals where the original documents are located; on the right is the distribution of journals where the corresponding cited literature is located. The width of the link reflects how often it is referenced. The graph displays the top three significant pathways. The results of the double graph superposition show that hot journals (such as HEPATOLOGY) are mainly concentrated in the fields of molecular biology and immunology (see the center of the circle on the left). Hot cited journals are mainly concentrated in the fields of molecular biology, genetics (such as HEPATOLOGY, BLOOD) and health, nursing, medicine (such as KIDNEY INT) (see the center of the circle on the right).
The results also confirmed that the research hotspots of LECT2 have evolved from molecular biology and gene fields to immunology and medicine, medical, clinical respectively, while the research in the fields of health, nursing, and medicine has evolved to medicine, medical, and clinical.
Citation coupling is a method to study the internal connection of scientific literature. If both papers cite the same article, then both citations will be considered a citation pair. The degree of coupling reflects the degree of similarity to the literature research topic. In finding relevant literature and determining the direction of research, scholars are given better advice. VOSviewer was used to perform citation coupling analysis on the top 20 most cited articles (with a minimum of 33 citations). Nineteen of them form a total of 91 coupling relationships (Figure 7A). Each node represents a document, marked with the name of the first author and the year of publication. The four nodes marked as Nasr (2015), Dogan (2017), Said (2014), and Murphy (2010) are very tightly connected, indicating that the four articles are highly correlated with each other.
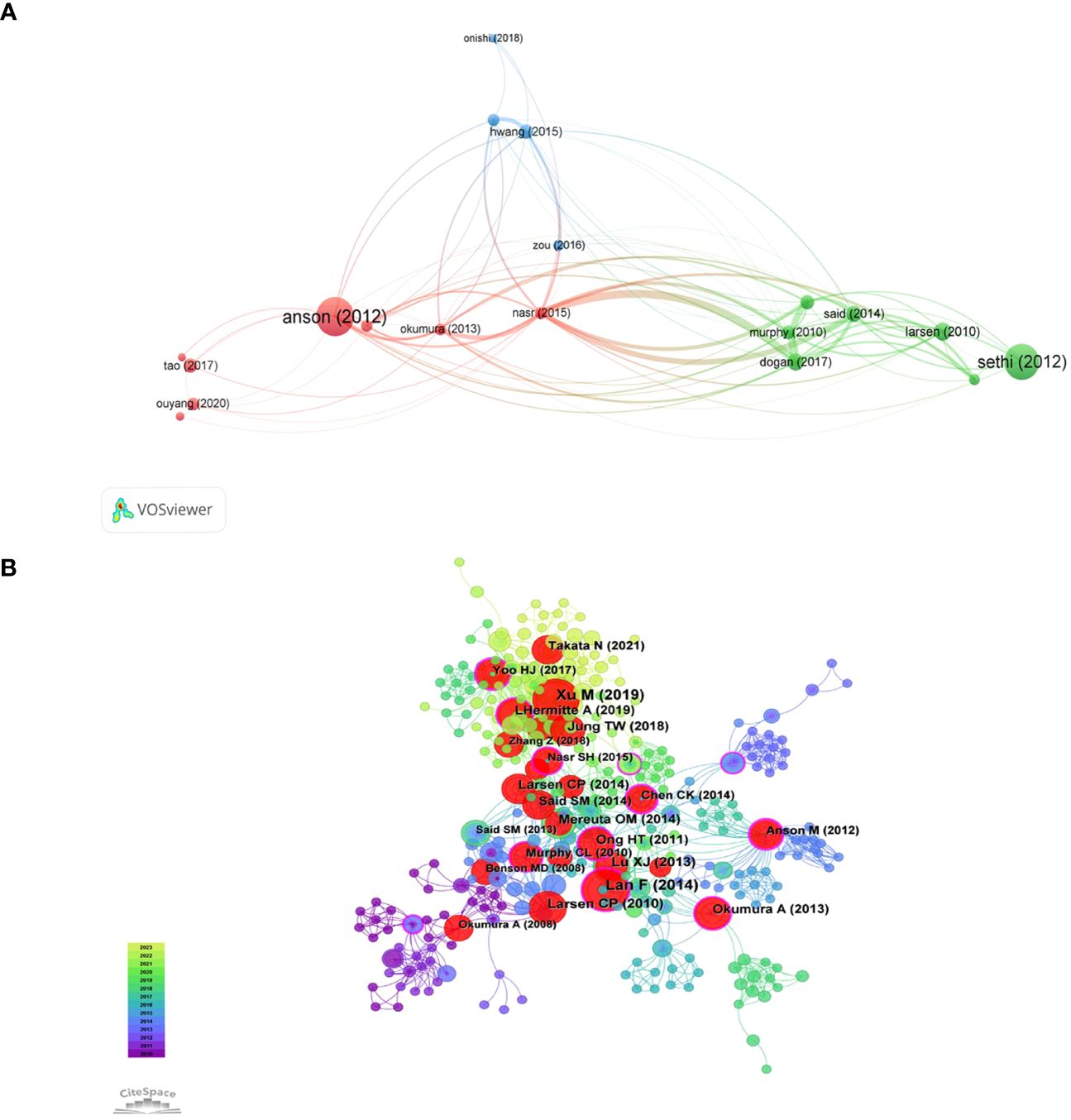
Figure 7 The visualization of f citation coupling (A) and and highly co-cited references (B) on research of leukocyte cell-derived chemotaxin-2.
When multiple papers are referenced by another paper simultaneously, it forms a co-citation connection. Co-citation analysis is an evolving framework that can assist researchers in comprehending the latest advancements in the field of study. The Supplementary Materials show the top ten co-cites in the literature. The article titled “LECT2, a Ligand for Tie1, Plays a Crucial Role in Liver Fibrogenesis” is the most frequently cited. We use CiteSpace to generate a co-citation network of 413 nodes and 1523 connections, with a density of 0.0179 (Figure 7B). Overall, the co-occurrence relationship between pieces of literature is strong, and multiple nodes act as bridges between subgroups. The 12 purple outer ring nodes are the literatures with high intermediary centrality (Centrality greater than 0.1), which explains the significance. Further analysis of the main content of the literature, there are 5 articles related to liver diseases, 4 articles related to inflammatory immune mechanisms, and 3 articles related to obesity and amyloid lesions.
Through the above two analysis methods, representative documents can be screened by combining the frequencies of citations, the frequencies of co-citations, and the centrality of literature. In the article titled “LECT2 Controls Inflammatory Monocytes to Constrain Growth and Progression of Hepatocellular Carcinoma”, the authors analyze how LECT2 influences the progression of HCC and suggest that LECT2 could be a beneficial immunotherapy choice for HCC. Another study revealed that LECT2 acts as a hepatokine connecting obesity with insulin resistance in skeletal muscle. It was discovered that the LECT2 protein hindered insulin signaling by phosphorylating Jun NH2-terminal kinase in C2C12 myocytes.
Keyword cooccurrence analysis reveals research hotspots by analyzing high-frequency keywords and their correlations. Table 5 lists the keywords, centrality, and first appearance for the top 20 cooccurrences. Intermediary centrality analysis reveals the mutation or transformation of research hotspots. CiteSpace was used, resulting in 294 keywords. Larger nodes correspond to more frequent cooccurrences. Purple outer ring node is an important keyword: expression, amino acid sequence, activation, beta-catenin, protein, obesity (Figure 8).
In the timeline (Supplementary Figure 1), each line is a cluster, numbered #0 #1 #2 #3, etc. and a total of 8 clusters are formed. The cluster reflects the temporal characteristics of the research field. The number of keywords in cluster #0 #1 #2 #3 #4 are the largest. The first group of human hepatocellular carcinoma (cluster #0) contains the keywords beta-catenin, gene expression, and diagnosis. etc. This indicates that hepatocellular carcinoma may be related to LECT2. The second group is systemic inflammation (cluster #1). Its keywords are expression, hepatocellular carcinoma, chondromodulin II. This group focused on the association of leukocyte-derived chemotaxin 2 expressions with systemic inflammation or immune regulation. The third group is chemotactic factor (cluster #2). The 4th group (cluster #3) is JNK-dependent inhibition. The three most cited keywords are obesity, fatty liver disease, risk. This group of studies revealed the chemotactic effect of LECT2 on leukocytes. The 5th group (cluster #4) is selenoprotein p. Its research is linked to insulin resistance and green tea extracts. The keywords in the first five clusters indicate that LECT2 research has achieved more significant results in human hepatocellular carcinoma, systemic inflammation, chemotactic factor, selenoprotein p and JNK-dependent inhibition.
CiteSpace software can extract and detect burst terms to understand the research frontier, changes in research focus, and the latest research hotspot trends, and help predict future trends in the field. As shown in Supplementary Figure 2, 25 emergent words are obtained, showing the year of the initial appearance of the keyword, while Begin and End signify the beginning and conclusion of its relevance as a cutting-edge concept, with Strength denoting its emerging power. The red line indicates the particular period in history when the term gained popularity in academic research. Nodes that have not yet appeared are shown in a light blue color, while nodes that have started to appear are shown in a dark blue color. It is worth noting that insulin resistance (5.5) has the greatest emergent strength, followed by cell-derived chemotaxin 2 (2.7), selenoprotein p (2.67), and risk (2.56). From the time of appearance, diseases, and newly recognized proteins first appeared. Current research on LECT2 is focused on cell-derived chemotaxin 2, angiogenesis, leukocyte chemotactic factor 2 amyloidosis (ALECT2), diagnosis, and biliary atresia (BA), marking an emerging period of study.
Our bibliometric research assess the impact and contributions of various countries, institutions, authors, journals. The number of published articles exploded from 2013 to 2014, indicating a surge in interest among scholars. The countries with the most published papers are the United States, China, and Japan, demonstrating their maturity in this area. 118 journals have published research literature related to LECT, with Hepatology being the most prolific and PLOS ONE closely following. These journal data can be sued as a navigation for scholars aiming to publish in specific journals. Toshinari Takamura, Hirofumi Misu, and Shuichi Kaneko are the top three authors. The most cited author is Satoshi Yamagoe (n = 159) of the National Institute of Infectious Diseases (NIID). He and his research team firstly reported LECT2 protein in 1998, which is mainly produced by hepatocytes (2). Satoshi Yamagoe, Toshinari Takamura, and Shuichi Kaneko discovered that LECT2 interacts with MET (Mesenchymal to epithelial transition factor) to promote RIG-L-mediated innate immune responses. The findings hold promise for therapeutic applications in several infectious diseases and cancers (41).
According to the result of co-citation network analysis in the literature, we can roughly determine the research basis of LECT2. In these 10 co-cited papers, the first, eighth, and tenth were on liver fibrosis and liver cancer; the second, sixth, and seventh were on obesity and insulin resistance; and the third, fifth, and ninth were on amyloidosis. Meng Xu et al. published the most co-cited study in Cell in 2019 (42). They found that LECT2 promoted the transformation of Tie1/Tie2 heterodimer to Tie2/Tie2. Tie1 and Tie2, two types of receptor tyrosine kinase (RTKs) on the surface of hepatic sinusoidal endothelial cells, are members of the TIE (Tyrosine kinase with immunoglobulin-like and EGF-like domains 1) receptor family that regulates angiogenesis. Overexpression of LECT2 leads to the deterioration of liver fibrosis by inhibiting portal vein angiogenesis and promoting sinus capillary formation (42). Additionally, Antoine L’Hermitte et al. and H T Ong all discovered the ability of LECT2 to inhibit the migration and growth of liver cancer cells (29, 39). Fei Lan, Okumura A and Tae Woo Jung proposed that LECT2 is a metabolism-related hepatokine, and predicted it as a therapeutic target for insulin resistance, respectively. It exerts a positive influence on Jun NH2-terminal kinase (JNK) stavation-sensing kinase adenosine monophosphate-activated protein kinase negatively regulates its expression (26). Tae Woo Jung found that LECT2 enhanced inflammation by acting on downstream targets such as P38 and CD209a. It also promotes lipid synthesis by regulating sterol regulatory element-binding protein 1c (SREBP1c) (35). Christopher P Larsen published 2 papers in these 10 total cited papers. He first discovered an amyloid deposit composed of LECT2 (ALECT2). It is considered to be the third form of renal amyloidosis (31, 43). Based on that basis, Oana M Mereuta typed the liver amyloid deposits (44). Their findings lay the foundation for further research into the effects of LECT2 on amyloidosis. Researchers from the Laboratory of Biochemistry and Molecular Biology, Ningbo University found that LECT2 specifically binds to the CD209a receptor and activates macrophages (22).
Our methodological review focuses on the top 25 terms with the strongest citation bursts to identify the frontiers of LECT2, including Angiogenesis, ALECT2, cell-derived chemotaxin 2, diagnosis, and BA (Biliary Atresia). Angiogenesis affects the pathological stages of liver fibrosis and hepatocarcinogenesis, associated with RA, inflammation (45). Therefore, we can predict the pathway in which LECT2 affects these diseases. ALECT2 is an amyloidosis that occurs frequently in the kidney and liver. It is commonly seen in patients with nephrotic syndrome and azotemia triggered by overexpression of LECT2 (46). Cell-derived chemotaxin 2 is the third research hotspot, indicating that LECT2 mainly plays the biological function of chemotactic centrocytes in immune response. The diagnosis of diseases is also a hot research direction of LECT2, especially for tumors like HCC and breast cancer (13, 40, 47, 48). BA is the fifth research hotspot to study the mechanism of LECT2-induced liver fibrosis in patients with Biliary Atresia (49). We drew a mechanism diagram by Figdraw to illustrate how LECT2 affects the development of related diseases, including HCC, liver fibrogenesis, bacterial sepsis, inflammatory response (Figure 9). According to disease classification and the specific mechanism, the current research hotspots of LECT2 will be elucidated in each of the following sections.
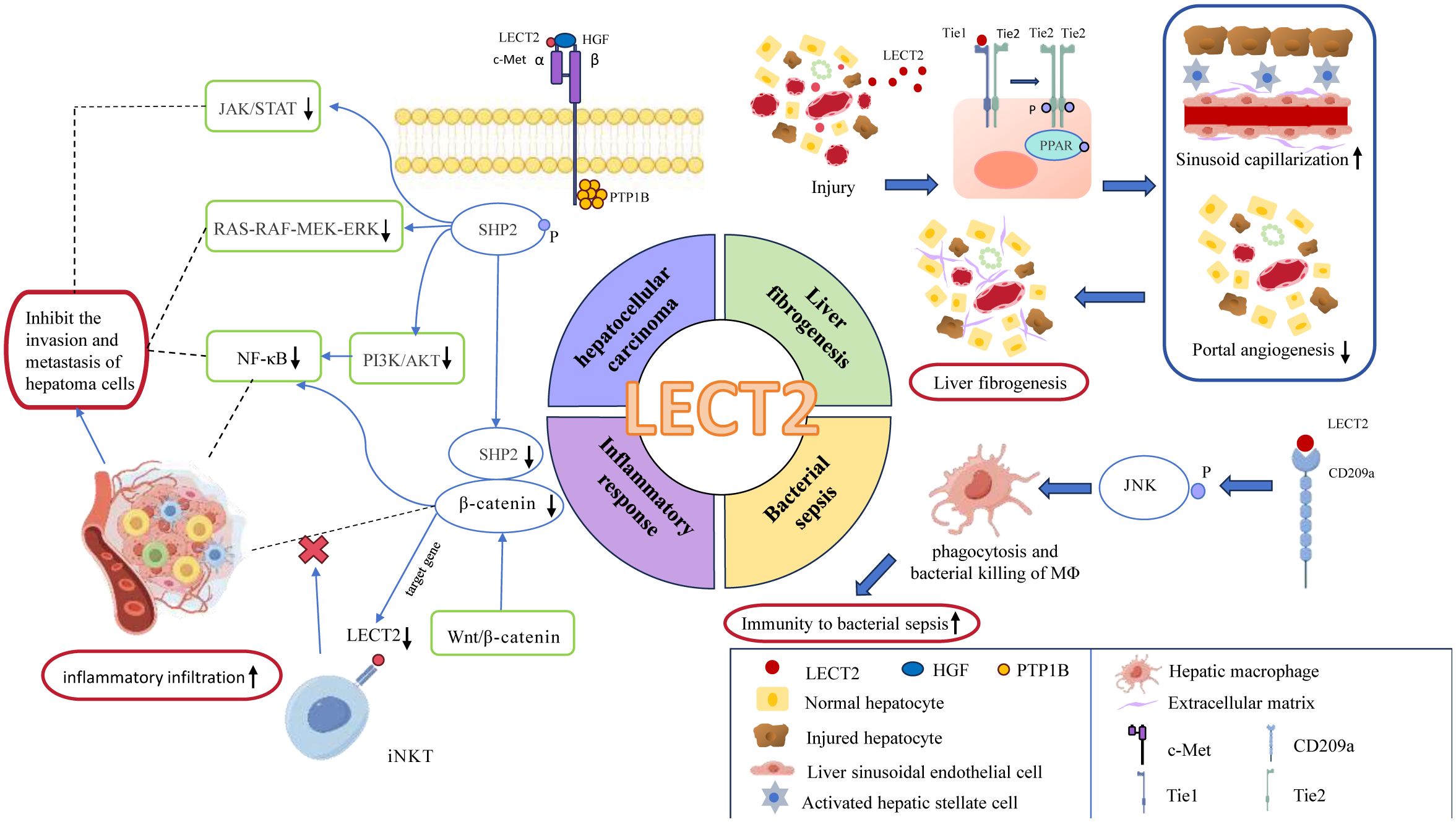
Figure 9 the mechanism diagram of LECT2’s involvement in the regulation of related diseases, including hepatocellular carcinoma, liver fibrogenesis, bacterial sepsis, inflammatory response. In this figure, blue ovals represent enzymes or proteins; red rounded rectangles represent effects; green rounded rectangles represent the signaling pathways; Tie1, Tie2: as tyrosine kinase receptors, they are subtypes of Tie; PPAR, peroxisome proliferators-activated receptors; JNK, c-Jun N-terminal kinase; c-Met, cellular-mesenchymal epithelial transition factor; HGF, hepatocyte growth factor; PTP1B, Protein Tyrosine Phosphatase-1B; SHP2, Src homology 2 domain-containing protein tyrosine phosphatase.
Systemic inflammation is one of the research hotspots of LECT2. LECT2 has neutrophil chemotaxis and is involved in inflammatory and immune regulation processes. In general, LECT2 has immune cell activation, inflammatory mediator regulation, and anti-inflammatory effects (50, 51). Systemic inflammatory diseases related to LECT2 mainly include bacterial sepsis (22), atherosclerosis (52), osteoporosis (23), arthritis (38), and atopic dermatitis (24).
LECT2 can chemotaxis immune cells and pathogenic phagocytose microorganisms, but the exact mechanism by which LECT2 exerts its anti-inflammatory effects is unclear. A large number of research results can provide some clues. LECT2 can directly interact with CD209a receptors to boost macrophages’ capacity to phagocytose and eliminate bacteria through JNK phosphorylation (53). Then hepatic macrophages exert phagocytosis and bactericidal effects, thereby improving the body’s immunity to bacterial sepsis (22). This finding provides a basis for LECT2 as a new targeted therapy for sepsis. Furthermore, LECT2 can also reduce tumor necrosis factor (TNF) expression via CD209a receptor and maintain HSC (hematopoietic stem cells) homeostasis (54). These findings may help in the treatment of various blood diseases.
AS is a chronic immune inflammatory disease. The development of this condition involves various factors like dysfunctional endothelial cells, heightened levels of vascular adhesion molecules, elevated production of inflammatory cytokines, and accumulation of cholesterol in the walls of blood vessels. LECT2 may participate in these pathological processes, thus affecting the development of atherosclerotic lesions (52). Fatma Cavide Sonmez et al. proposed that LECT2 could be used as a new marker of AS (55). They found that LECT2 in atherosclerotic areas tended to be overexpressed through aortic wall perforation biopsy and immunohistochemical staining, which was confirmed in later studies (17, 56). Additional research has indicated that increased levels of LECT2 can impede the progression of AS. A genetically modified animal model containing the LECT2 gene is utilized to evaluate metabolic factors associated with the development of AS, including reduced levels of total cholesterol and low-density lipoprotein in the blood; lower levels of inflammatory cytokines and mRNA for monocyte chemoattractant protein-1 (MCP-1), matrix metallopeptidase 1 (MMP-1), tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and interleukin-8 (IL-8); higher quantities of smooth muscle cells and fewer CD68 macrophages, while CD31 endothelial cells remain unchanged (17). In contrast, another study found that the levels of intercellular adhesion molecule-1 and TNF-α, MCP-1, and IL-1β are elevated in LECT2-treated human umbilical vein endothelial cells and Tohoku Hospital Pediatrics-1 (THP-1 cells). These results indicated that LECT2 promotes the inflammatory response in AS. Therefore, the role of LECT2 in AS is controversial and needs to be confirmed by further researches (53).
As a chronic systemic disease, the key pathophysiological process of RA is the progressive destruction of articular cartilage and bone. This process also includes the imbalance of osteoclasts, mesenchymal stem cells, osteoblasts, and endothelial cells within the bone environment, as well as irregular chemotaxis and infiltration of inflammatory cells (20).
The occurrence of RA and osteoarthritis is connected to the G/A polymorphism at nucleotide 172 in exon 3 of the ChM-II gene (21). Research has analyzed the genetic code and X-ray injuries of individuals with RA and discovered that having the 172A gene variant notably raises the risk of developing RA and the severity of joint deterioration (38). In line with this finding, the arthritis severity was notably higher in LECT2-deficient mice compared to the wild-type control group in the collagen antibody-induced arthritis (CAIA model) induced by anti-type II collagen antibody. The distinct signs included damage to cartilage and bone, increased inflammation, and elevated levels of proinflammatory cytokines like IL-1β and IL-6 (57). In subsequent studies, the exogenous expression of LECT2 reduced the RA phenotype of LECT2 (-/-) mice (20).
Collectively, the aforementioned researches suggested that LECT2 acts as a suppressor in regulating RA. The precise molecular mechanism by which LECT2 regulates RA remains unknown. Further laboratory experiments are needed to investigate how LECT2 influences arthritis-related cytokines.
The development of osteoporosis could also be linked to LECT2. Recent research indicated that there was a notable increase in serum LECT2 levels in individuals with osteoporosis, which impacted the severity of osteopenia (23). Certain academics forecast that serum LECT2 could serve as a promising indicator for evaluating the likelihood of bone deterioration (58).
Research on RA, osteoarthritis, and osteoporosis suggests that LECT2 plays a role in controlling bone immune responses, making it crucial for bone health.
Numerous studies have demonstrated the involvement of LECT2 in various liver conditions, including hepatitis, acute liver failure (50), liver fibrosis (59), nonalcoholic fatty liver disease (60), cirrhosis, and HCC (29).
Previous research has shown that removing LECT2 during concanavalin A (ConA) -induced hepatitis can disturb the balance of Natural killer T cell (NKT cells) in the liver. In LECT2 (-/-) mice, NKT cells are significantly increased. Elevated levels of IL-4, Interferon-gamma (IFN-γ), and cytotoxicity towards syngeneic thymocytes are observed as well (61). Nevertheless, the quantity of traditional T cells, natural killer cells, and various other cell varieties stays constant according to a study (50). Additional research has uncovered how LECT2 controls the progression of hepatitis. LECT2 has the ability to trigger the signaling pathways of TGF-β-activated kinase 1 (TAK1), Mitogen-activated protein (MAP) kinase kinase 4 (MKK4), and JNK, converting remaining liver macrophages into an m1-like state and advancing liver inflammation (62).
Yuan Xie, Ke-Bo Zhong et al. found that the function of LECT2 in the process of acute liver damage and recovery (50). They simulated liver damage caused by toxins and autoimmune reactions using carbon tetrachloride and concanavalin A (ConA) respectively. The degree of liver injury was evaluated by serum levels of Alanine Transaminase (ALT), Aspartate Aminotransferase (AST), and total bilirubin (Tbil). The results showed that LECT2 mRNA and serum LECT2 increased during the first to second days of exacerbation of liver injury. Compared with WT mice, LECT2-ko mice had less liver injury and less macrophage infiltration. These results confirmed the findings of Rachel J Church et al. (63).
Several research studies have indicated that LECT2 played a part in controlling acute liver damage and healing by managing the entry of various immune cells (64), and it could also serve as a potential biomarker for diagnosing liver injuries in clinical settings (10, 63, 65).
LECT2 has the effect of promoting liver fibrosis. The use of ICG-001 and LF3 to block β-catenin/TCF4 transcriptional activity resulted in decreased levels of LECT2, pSer 675 β-catenin, and nuclear β-catenin. The results showed that LECT2 was regulated by β-catenin/TCF4 signaling, thereby regulating angiogenesis and participating in liver fibrosis (59). LECT2 can bind to Tie1 and activate peroxisome proliferators-activated receptors (PPAR) signaling to transform Tie1/Tie2 heterodimers into Tie2-Tie2 homodimers, thereby inhibiting endothelial cell invasion and metastasis. Knockdown of the LECT2 gene significantly promotes portal vein angiogenesis and sinusoidal capillary reduction, which alleviates the symptoms of liver fibrosis (42). Moreover, LECT2 is also considered a pivotal gene in BA liver fibrosis to boost fibrosis by triggering fibrous genes such as α-smooth muscle actin and collagen type I alpha 1 Chain (66). The above studies indicated that LECT2 could potentially be used as a biomarker to predict liver fibrogenesis. The use of AAV9-LECT2-shRNA combined with bevacizumab has shown promising outcomes in treating liver fibrosis during the development of new drugs (67).
NAFLD is not a typical systemic inflammatory disease (68). However, inflammation plays a pivotal role in its progression, from hepatic steatosis to nonalcoholic steatohepatitis and cirrhosis (69). LECT2 levels significantly increased in NAFLD (26, 35, 70). Studies have found two ways in which LECT2 promotes liver fat accumulation, including the STAT-1 pathway and transforming residual hepatic macrophages into an m1-like phenotype (71). Hwan-Jin Hwang and Tae Woo Jung have discovered that in the development of hepatic steatosis, adenosine 5’-monophosphate (AMP)-activated protein kinase (AMPK) and JNK mechanisms could play a role in regulating LECT2 expression and inducing liver inflammation (72). Meanwhile, LECT2 can stimulate LPS-triggered MKK4 and TGF-beta activated kinase 1 (MAP3K7) binding protein 2 (TAB2), activating JNK pathway (16). Therefore, LECT2 has the function of promotion on NAFLD progression.
HCC is the first focus of LECT2 research at present. LECT2 affects the evolution of liver cancer by altering the tumor phenotype and tumor microenvironment (73). It effectively suppresses endothelial cell proliferation, migration, and angiogenesis (15, 74).
L’Hermitte A et al. found that the absence of LECT2 leads to an increase in immune infiltrations and promotes the epithelial-mesenchymal transformation of Ctnnb-1 mutant tumor hepatocytes (29). Other research findings indicate that LECT2 expression appears to contradict angiogenesis in HCC patients (39, 75). The growth and spread of HCC depend on angiogenesis (76). So individuals with elevated LECT2 levels tend to experience decreased vascular invasion and prolonged survival in cases of HCC (15). Prior research has revealed how LECT2 hinders the process of angiogenesis. LECT2 directly binds to vascular endothelial growth factor receptor 2 (VEGFR2), resulting in the inhibition of VEGF165-induced tyrosine phosphorylation of VEGFR2 and subsequent extracellular signal-regulated kinase and serine/threonine kinase AKT (also known as protein kinase B or PKB) phosphorylation (77). This provides insights into LECT2’s anti-tumor activity.
LECT2 can also directly bind to the MET receptor on liver tumor cells at aa 159–175 of the α chain, preventing the interaction between the tyrosine kinase receptor MET and its ligand hepatocyte growth factor (HGF). Suppressing the downstream pathway of the HGF/MET axis, including phosphoinositide 3-kinase (PI3K)/AKT, RAS-RAF-MEK-ERK, and JAK/STAT, hinders the invasion and metastasis of hepatoma cells (74). Interaction between LECT2 and MET leads to the involvement of protein tyrosine phosphatase-1B (PTP1B) and the separation of adaptor proteins (such as Gab1, Grb2, Src, and PI3K), which inhibits MET dephosphorylation and enhances Src homology-2 domain-containing protein tyrosine phosphatase-2 (SHP2) phosphorylation (78). This shields RIG-I from degradation by SHP2/c-Cbl, enhancing IFN generation, and suppressing the replication of lymphocytic choriomeningitis virus (LCMV) (41), which implies that LECT2’s impact on metabolism and tumors arises through affecting MET and PTP1B (79).
Furthermore, MET can regulate LECT2 expression by activating β-catenin in turn (80). In mouse liver, LECT2 is directly targeted by Wnt/β-catenin, triggering LECT2 expression exclusively during β-catenin-dependent hepatocyte proliferation (28). However, LECT2 expression is not up-regulated in some HCC specimens containing β-catenin-activating mutations, indicating that Wnt/β-catenin is not the only pathway that regulates LECT2 expression in liver cancer. LECT2 can also interconnect with iNKT cells, which blocks β-catenin-induced inflammation and influencing liver tumor invasion in turn (51). Other studies have shown that LECT2’s tumor inhibition can also be achieved by inhibiting HCC glycolysis (81).
From the above studies, we can conclude that LECT2 may control HCC by three mechanisms: endothelial VEGF receptor signaling, MET signaling, and Wnt/β-catenin pathway. LECT2 may be a promising candidate for cancer therapy.
ALECT2, a form of renal amyloidosis, is ranked as the third most prevalent type and was initially described by Benson et al.in 2008 (30). LECT2, a subunit protein in amyloid fibrils, has been identified as a protein capable of inducing systemic amyloidosis in cases of nephrotic syndrome and azotemia (31). This form of amyloidosis is common, accounting for 3 to 10 percent of amyloidosis reported abroad (82). Amyloidogenic LECT2 (ALECT2) forms clumps in various organs such as the lungs, spleen, adrenal glands, small intestine, gallbladder, ovaries, and uterus, but not in the heart or brain (83–86). Frequently diagnosed through clinical observation, chronic kidney failure is commonly accompanied by a mild presence of particles in the urine, with or without protein in the urine. Amyloid deposits in glomeruli, renal vessels, and stroma are common features in individuals suffering from nephrotic syndrome and renal failure (32, 87, 88).
LECT2-derived amyloidosis is ethnicity-related with a high prevalence in Hispanics (44, 89, 90). Excessive production of LECT2 leading to ALECT2 deposition is a prevalent factor in hepatic amyloidosis cases in the United States (44). Improved understanding and identification methods of this form of amyloidosis have led to a small amount of detection of ALECT2-induced amyloidosis in patients from Canadian Aboriginal northern British Columbia, Pakistan, Syria, and China who have chronic kidney disease (91–94). The implementation of laser microdissection and mass spectrometry (LMD/MS) has offered significant assistance in identifying and categorizing amyloidosis (87, 95). Analysis of LECT2 gene sequence in peripheral blood samples can serve as a predictive tool for LECT2 amyloidosis. In cases where all AL and AA markers yield negative results, further diagnostic measures should be taken to determine appropriate therapeutic interventions (96). The principle of non-maleficence should be prioritized, with cautious selection of potentially toxic treatments unless dealing with immunoglobulin light chain amyloidosis (46, 97–99). However, it is worth noting that ALECT2 remains inadequately investigated to date, leaving its pathogenesis still unclear.
There are no drugs on the market based on LECT2 as a therapeutic target. In the published study, there is only one drug development project for the LECT2 target by Alnylam Pharmaceuticals, Inc. The drug inhibits the expression of the LECT2 gene through RNA interference to treat amyloidosis. An existing patent provides a novel method for the treatment or prevention of tumors characterized by excessive activity of the Met receptor. Phosphorylation of MET in HCC cells is reduced by administration of agents that can increase the level or biological activity of the active fragment of the LECT2 protein in HCC cells. This method not only inhibits the proliferation, migration and invasiveness of HCC cells, but even includes human lung cancer cells, breast cancer cells, gastric cancer cells, ovarian cancer cells, hypopharyngeal cancer cells, colon cancer cells and glioma cells (100, 101). Gene therapy approaches targeting the LECT2 gene are also being explored. The DNA encoding the human LECT2 protein is transferred to cells for gene therapy by retroviral vectors or non-viral techniques such as receptor-mediated targeted DNA transfer, the use of ligand-DNA conjugates or adenovirus-ligand-DNA conjugates, lipid membrane fusion, or direct microinjection (102). LECT2 plays an important role in inflammation, immune response and tumor development, so it has the potential to be a target for future therapies. But more research is needed to verify its effectiveness and safety.
There are multiple advantages to this research. The study utilized the Web of Science Core Collection Database, a comprehensive academic literature index database that includes reputable journals from multiple disciplines and is updated daily. As a literature search tool, it can ensure the credibility and high quality of bibliometric analysis data. Second, we used three bibliometric tools (Microsoft Office Excel 2021, VOSviewer, and CiteSpace) for visual analysis of bibliometrics. This method can reduce the distortion and bias caused by subjective information filtering. Finally, compared with the traditional review, the visual analysis and review of LECT2-related research in this study can more intuitively and comprehensively show the hot spots and frontiers in this field.
It’s worth noting that some limitations do exist in this study. Firstly, publications in non-SCI journals or other databases may not be included in the statistics. In addition, our study solely reflected the citation frequency or publication count to gauge the importance of a paper, which might not fully account for other factors.
A thorough bibliometric analysis was carried out in this research, examining the current literature on LECT2, including an assessment of publication dates, countries, organizations, authors, and publications. An in-depth examination of trends, research hotspots, and frontiers was also performed. The analysis results show that the current research has made a breakthrough thanks to the cooperation between countries and institutions, and more scholars have participated in LECT2 research. Research has shown that LECT2 holds significant potential for the treatment of immune diseases, liver fibrosis, liver cancer, amyloidosis, and other illnesses. However, the findings are preliminary and need more exploration for a clearer understanding.
Publicly available datasets were analyzed in this study. This data can be found here: Web of science.
WL: Writing – original draft, Visualization. QW: Writing – original draft, Visualization. JY: Writing – original draft. YY: Writing – original draft. LX: Writing – original draft. CJ: Writing – review & editing. XL: Writing – review & editing, Funding acquisition. LZ: Writing – review & editing, Supervision, Project administration, Funding acquisition.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Sichuan Geriatrics Clinical Medical Research Center (23LHNBZZD05), the National College Student Innovation and Entrepreneurship Training Program Project (202213705036), and Sichuan Provincial College Student Innovation and Entrepreneurship Training Program Project (S202313705089, S202313705066).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1413466/full#supplementary-material
Supplementary File 1 | Contains a table (Word) of the top 10 references that are frequently cited together in studies on LECT2 research and two images (TIF). One of the images shows the top 25 keywords with the strongest citation bursts. Another image is timeline viewer related to LECT2.
1. Yamagoe S, Yamakawa Y, Matsuo Y, Minowada J, Mizuno S, Suzuki K. Purification and primary amino acid sequence of a novel neutrophil chemotactic factor lect2. Immunol Lett. (1996) 52:9–13. doi: 10.1016/0165–2478(96)02572–2
2. Yamagoe S, Kameoka Y, Hashimoto K, Mizuno S, Suzuki K. Molecular cloning, structural characterization, and chromosomal mapping of the human lect2 gene. Genomics. (1998) 48:324–9. doi: 10.1006/geno.1997.5198
3. Hiraki Y, Inoue H, Kondo J, Kamizono A, Yoshitake Y, Shukunami C, et al. A novel growth-promoting factor derived from fetal bovine cartilage, chondromodulin ii. Purification and amino acid sequence. J Biol Chem. (1996) 271:22657–62. doi: 10.1074/jbc.271.37.22657
4. Mori Y, Hiraki Y, Shukunami C, Kakudo S, Shiokawa M, Kagoshima M, et al. Stimulation of osteoblast proliferation by the cartilage-derived growth promoting factors chondromodulin-I and -ii. FEBS Lett. (1997) 406:310–4. doi: 10.1016/s0014–5793(97)00291–3
5. Yamagoe S, Watanabe T, Mizuno S, Suzuki K. The mouse lect2 gene: cloning of cdna and genomic DNA, structural characterization and chromosomal localization. Gene. (1998) 216:171–8. doi: 10.1016/s0378–1119(98)00294–7
6. Okumura A, Suzuki T, Dohmae N, Okabe T, Hashimoto Y, Nakazato K, et al. Identification and assignment of three disulfide bonds in mammalian leukocyte cell-derived chemotaxin 2 by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Biosci Trends. (2009) 3:139–43.
7. Okumura A, Suzuki T, Miyatake H, Okabe T, Hashimoto Y, Miyakawa T, et al. Leukocyte cell-derived chemotaxin 2 is a zinc-binding protein. FEBS Lett. (2013) 587:404–9. doi: 10.1016/j.febslet.2013.01.025
8. Xie Y, Fan KW, Guan SX, Hu Y, Gao Y, Zhou WJ. Lect2: A pleiotropic and promising hepatokine, from bench to bedside. J Cell Mol Med. (2022) 26:3598–607. doi: 10.1111/jcmm.17407
9. Zhu MH, Liu YJ, Li CY, Tao F, Yang GJ, Chen J. The emerging roles of leukocyte cell-derived chemotaxin-2 in immune diseases: from mechanisms to therapeutic potential. Front Immunol. (2023) 14:1158083. doi: 10.3389/fimmu.2023.1158083
10. Sato Y, Watanabe H, Kameyama H, Kobayashi T, Yamamoto S, Takeishi T, et al. Serum lect2 level as a prognostic indicator in acute liver failure. Transplant Proc. (2004) 36:2359–61. doi: 10.1016/j.transproceed.2004.07.007
11. Ando K, Kato H, Kotani T, Ozaki M, Arimura Y, Yagi J. Plasma leukocyte cell-derived chemotaxin 2 is associated with the severity of systemic inflammation in patients with sepsis. Microbiol Immunol. (2012) 56:708–18. doi: 10.1111/j.1348-0421.2012.00488.x
12. Ikeda D, Ageta H, Tsuchida K, Yamada H. Itraq-based proteomics reveals novel biomarkers of osteoarthritis. J Orthop Surg Res. (2013) 18:565–72. doi: 10.3109/1354750x.2013.810667
13. Andres SA, Bickett KE, Alatoum MA, Kalbfleisch TS, Brock GN, Wittliff JL. Interaction between smoking history and gene expression levels impacts survival of breast cancer patients. Breast Cancer Res Treat. (2015) 152:545–56. doi: 10.1007/s10549-015-3507-z
14. MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. (2009) 17:9–26. doi: 10.1016/j.devcel.2009.06.016
15. Chen CK, Yang CY, Hua KT, Ho MC, Johansson G, Jeng YM, et al. Leukocyte cell-derived chemotaxin 2 antagonizes met receptor activation to suppress hepatocellular carcinoma vascular invasion by protein tyrosine phosphatase 1b recruitment. Hepatology. (2014) 59:974–85. doi: 10.1002/hep.26738
16. Takata N, Ishii KA, Takayama H, Nagashimada M, Kamoshita K, Tanaka T, et al. Lect2 as a hepatokine links liver steatosis to inflammation via activating tissue macrophages in nash. Sci Rep. (2021) 11:555. doi: 10.1038/s41598–020-80689–0
17. He WM, Dai T, Chen J, Wang JA. Leukocyte cell-derived chemotaxin 2 inhibits development of atherosclerosis in mice. Zool Res. (2019) 40:317–23. doi: 10.24272/j.issn.2095–8137.2019.030
18. Kim J, Lee SK, Kim D, Lee E, Park CY, Choe H, et al. Adipose tissue lect2 expression is associated with obesity and insulin resistance in korean women. Obes (Silver Spring). (2022) 30:1430–41. doi: 10.1002/oby.23445
19. Berthou F, Sobolewski C, Abegg D, Fournier M, Maeder C, Dolicka D, et al. Hepatic pten signaling regulates systemic metabolic homeostasis through hepatokines-mediated liver-to-peripheral organs crosstalk. Int J Mol Sci. (2022) 23(7): 3959. doi: 10.3390/ijms23073959
20. Zhu S, Bennett S, Li Y, Liu M, Xu J. The molecular structure and role of lect2 or chm-ii in arthritis, cancer, and other diseases. J Cell Physiol. (2022) 237:480–8. doi: 10.1002/jcp.30593
21. Graessler J, Verlohren M, Graessler A, Zeissig A, Kuhlisch E, Kopprasch S, et al. Association of chondromodulin-ii val58ile polymorphism with radiographic joint destruction in rheumatoid arthritis. J Rheumatol. (2005) 32:1654–61. doi: 0315162X-32-1654
22. Lu XJ, Chen J, Yu CH, Shi YH, He YQ, Zhang RC, et al. Lect2 protects mice against bacterial sepsis by activating macrophages via the cd209a receptor. J Exp Med. (2013) 210:5–13. doi: 10.1084/jem.20121466
23. Wang Q, Xu F, Chen J, Xie YQ, Xu SL, He WM. Serum leukocyte cell-derived chemotaxin 2 (Lect2) level is associated with osteoporosis. Lab Med. (2023) 54:106–11. doi: 10.1093/labmed/lmac080
24. Zhao K, Xu F, Jiang X, Chen J, Zhu X, Zhou Q, et al. Serum leukocyte cell-derived chemotaxin 2 level is associated with atopic dermatitis patients. Ann Palliat Med. (2021) 10:11006–12. doi: 10.21037/apm-21–2690
25. Wu C-J, Pan K-F, Chen J-Q, Tao YC, Liu Y-C, Chen B-R, et al. Loss of lect2 promotes ovarian cancer progression by inducing cancer invasiveness and facilitating an immunosuppressive environment. Oncogene. (2024) 43(7):511–23. doi: 10.1038/s41388-023-02918-w
26. Lan F, Misu H, Chikamoto K, Takayama H, Kikuchi A, Mohri K, et al. Lect2 functions as a hepatokine that links obesity to skeletal muscle insulin resistance. Diabetes. (2014) 63:1649–64. doi: 10.2337/db13–0728
27. Gajos-Michniewicz A, Czyz M. Wnt/beta-catenin signaling in hepatocellular carcinoma: the aberrant activation, pathogenic roles, and therapeutic opportunities. Genes Dis. (2024) 11:727–46. doi: 10.1016/j.gendis.2023.02.050
28. Ovejero C, Cavard C, Périanin A, Hakvoort T, Vermeulen J, Godard C, et al. Identification of the leukocyte cell-derived chemotaxin 2 as a direct target gene of beta-catenin in the liver. Hepatology. (2004) 40:167–76. doi: 10.1002/hep.20286
29. L'Hermitte A, Pham S, Cadoux M, Couchy G, Caruso S, Anson M, et al. Lect2 controls inflammatory monocytes to constrain the growth and progression of hepatocellular carcinoma. Hepatology. (2019) 69:160–78. doi: 10.1002/hep.30140
30. Benson MD, James S, Scott K, Liepnieks JJ, Kluve-Beckerman B. Leukocyte chemotactic factor 2: A novel renal amyloid protein. Kidney Int. (2008) 74:218–22. doi: 10.1038/ki.2008.152
31. Larsen CP, Walker PD, Weiss DT, Solomon A. Prevalence and morphology of leukocyte chemotactic factor 2-associated amyloid in renal biopsies. Kidney Int. (2010) 77:816–9. doi: 10.1038/ki.2010.9
32. Holanda DG, Acharya VK, Dogan A, Racusen LC, Atta MG. Atypical presentation of atypical amyloid. Nephrol Dial Transplant. (2011) 26:373–6. doi: 10.1093/ndt/gfq638
33. Koshimizu Y, Ohtomi M. Regulation of neurite extension by expression of lect2 and neurotrophins based on findings in lect2-knockout mice. Brain Res. (2010) 1311:1–11. doi: 10.1016/j.brainres.2009.11.010
34. Koshimizu Y, Ohtomi M. Regulation of katanin-P60 levels by lect2 adjusts microtubular morphology. Neuroreport. (2010) 21:646–50. doi: 10.1097/WNR.0b013e32833a7d6f
35. Jung TW, Chung YH, Kim HC, Abd El-Aty AM, Jeong JH. Lect2 promotes inflammation and insulin resistance in adipocytes via P38 pathways. J Mol Endocrinol. (2018) 61:37–45. doi: 10.1530/jme-17–0267
36. van Eck NJ, Waltman L. Software survey: vosviewer, a computer program for bibliometric mapping. Scientometrics. (2010) 84:523–38. doi: 10.1007/s11192–009-0146–3
37. Chen C. Searching for intellectual turning points: progressive knowledge domain visualization. Proc Natl Acad Sci U.S.A. (2004) 101 Suppl 1:5303–10. doi: 10.1073/pnas.0307513100
38. Kameoka Y, Yamagoe S, Hatano Y, Kasama T, Suzuki K. Val58ile polymorphism of the neutrophil chemoattractant lect2 and rheumatoid arthritis in the Japanese population. Arthritis Rheum. (2000) 43:1419–20. doi: 10.1002/1529–0131(200006)43:6<1419::Aid-anr28>3.0.Co;2-i
39. Ong HT, Tan PK, Wang SM, Hian Low DT, Ooi LL, Hui KM. The tumor suppressor function of lect2 in human hepatocellular carcinoma makes it a potential therapeutic target. Cancer Gene Ther. (2011) 18:399–406. doi: 10.1038/cgt.2011.5
40. Deacon CF. Dipeptidyl peptidase-4 inhibitors in the treatment of type 2 diabetes: A comparative review. Diabetes Obes Metab. (2011) 13:7–18. doi: 10.1111/dom.2011.13.issue-1
41. Shirasaki T, Yamagoe S, Shimakami T, Murai K, Imamura R, Ishii KA, et al. Leukocyte cell-derived chemotaxin 2 is an antiviral regulator acting through the proto-oncogene met. Nat Commun. (2022) 13(1):15. doi: 10.1038/s41467–022-30879–3
42. Xu M, Xu HH, Lin Y, Sun X, Wang LJ, Fang ZP, et al. Lect2, a ligand for tie1, plays a crucial role in liver fibrogenesis. Cell. (2019) 178:1478–92.e20. doi: 10.1016/j.cell.2019.07.021
43. Larsen CP, Kossmann RJ, Beggs ML, Solomon A, Walker PD. Clinical, morphologic, and genetic features of renal leukocyte chemotactic factor 2 amyloidosis. Kidney Int. (2014) 86:378–82. doi: 10.1038/ki.2014.11
44. Mereuta OM, Theis JD, Vrana JA, Law ME, Grogg KL, Dasari S, et al. Leukocyte cell-derived chemotaxin 2 (Lect2)-associated amyloidosis is a frequent cause of hepatic amyloidosis in the United States. Blood. (2014) 123:1479–82. doi: 10.1182/blood-2013–07-517938
45. Thomas H. Liver: delineating the role of angiogenesis in liver fibrosis. Nat Rev Gastroenterol Hepatol. (2018) 15:6. doi: 10.1038/nrgastro.2017.168
46. Mann BK, Bhandohal JS, Cobos E, Chitturi C, Eppanapally S. Lect-2 amyloidosis: what do we know? J Investig Med. (2022) 70:348–53. doi: 10.1136/jim-2021–002149
47. Slowik V, Apte U. Leukocyte cell-derived chemotaxin-2: it's role in pathophysiology and future in clinical medicine. Clin Transl Sci. (2017) 10:249–59. doi: 10.1111/cts.12469
48. Okabe H, Delgado E, Lee JM, Yang J, Kinoshita H, Hayashi H, et al. Role of leukocyte cell-derived chemotaxin 2 as a biomarker in hepatocellular carcinoma. PloS One. (2014) 9:11. doi: 10.1371/journal.pone.0098817
49. Zhao JF, Xu XD, Gou QY, Zheng QP, Ge L, Chen LZ, et al. Tgf-B1-mediated leukocyte cell-derived chemotaxin 2 is associated with liver fibrosis in biliary atresia. Front Pediatr. (2022) 10:901888. doi: 10.3389/fped.2022.901888
50. Xie Y, Zhong KB, Hu Y, Xi YL, Guan SX, Xu M, et al. Liver infiltration of multiple immune cells during the process of acute liver injury and repair. World J Gastroenterol. (2022) 28:6537–50. doi: 10.3748/wjg.v28.i46.6537
51. Anson M, Crain-Denoyelle AM, Baud V, Chereau F, Gougelet A, Terris B, et al. Oncogenic B-catenin triggers an inflammatory response that determines the aggressiveness of hepatocellular carcinoma in mice. J Clin Invest. (2012) 122:586–99. doi: 10.1172/jci43937
52. Wei M, Liu J, Pan H, Zhou Z, Guo K. Plasma leukocyte cell-derived chemotaxin 2 (Lect2) as a risk factor of coronary artery disease: A cross-sectional study. Angiology. (2022) 73:265–74. doi: 10.1177/00033197211028023
53. Hwang HJ, Jung TW, Hong HC, Seo JA, Kim SG, Kim NH, et al. Lect2 induces atherosclerotic inflammatory reaction via cd209 receptor-mediated jnk phosphorylation in human endothelial cells. Metabolism. (2015) 64:1175–82. doi: 10.1016/j.metabol.2015.06.001
54. Lu XJ, Chen Q, Rong YJ, Yang GJ, Li CH, Xu NY, et al. Lect2 drives haematopoietic stem cell expansion and mobilization via regulating the macrophages and osteolineage cells. Nat Commun. (2016) 7:13. doi: 10.1038/ncomms12719
55. Sonmez FC, Yildiz P, Akhtar MS, Aydin C, Sonmez O, Ay N, et al. New markers in atherosclerosis: thrombospondin-2 (Thbs-2) and leukocyte cell-derived chemotaxin-2 (Lect-2); an immunohistochemical study. Med Sci Monit. (2016) 22:5234–9. doi: 10.12659/msm.898889
56. Yoo HJ, Hwang SY, Choi JH, Lee HJ, Chung HS, Seo JA, et al. Association of leukocyte cell-derived chemotaxin 2 (Lect2) with nafld, metabolic syndrome, and atherosclerosis. PloS One. (2017) 12:e0174717. doi: 10.1371/journal.pone.0174717
57. Okumura A, Saito T, Otani I, Kojima K, Yamada Y, Ishida-Okawara A, et al. Suppressive role of leukocyte cell-derived chemotaxin 2 in mouse anti-type ii collagen antibody-induced arthritis. Arthritis Rheum. (2008) 58:413–21. doi: 10.1002/art.23215
58. Yeung AWK, Mozos I. The innovative and sustainable use of dental panoramic radiographs for the detection of osteoporosis. Int J Environ Res Public Health. (2020) 17(7):2449. doi: 10.3390/ijerph17072449
59. Gao Y, Fan SC, Zhao PF, Li HL, Cai CH, Li X, et al. 13-catenin/tcf4 inhibitors icg-001 and lf3 alleviate bdl-induced liver fibrosis by suppressing lect2 signaling. Chem Biol Interact. (2023) 371:10. doi: 10.1016/j.cbi.2023.110350
60. Park CY, Lee SK, Kim J, Kim D, Choe H, Jeong JH, et al. Endoplasmic reticulum stress increases lect2 expression via atf4. Biochem Biophys Res Commun. (2021) 585:169–76. doi: 10.1016/j.bbrc.2021.11.038
61. Segawa Y, Itokazu Y, Inoue N, Saito T, Suzuki K. Possible Changes in Expression of Chemotaxin Lect2 Mrna in Mouse Liver after Concanavalin a-Induced Hepatic Injury. Biol Pharm Bull. (2001) 24:425–8. doi: 10.1248/bpb.24.425
62. Maki K, Katsumi T, Hanatani T, Uchiyama F, Suzuki F, Hoshikawa K, et al. Elucidation of pericholangitis and periductal fibrosis in cholestatic liver diseases via extracellular vesicles released by polarized biliary epithelial cells. Am J Physiol Cell Physiol. (2024) 326:C1094–C1105. doi: 10.1152/ajpcell.00655.2023
63. Church RJ, Kullak-Ublick GA, Aubrecht J, Bonkovsky HL, Chalasani N, Fontana RJ, et al. Candidate biomarkers for the diagnosis and prognosis of drug-induced liver injury: an international collaborative effort. Hepatology. (2019) 69:760–73. doi: 10.1002/hep.29802
64. Dang MH, Kato H, Ueshiba H, Omori-Miyake M, Yamagoe S, Ando K, et al. Possible role of lect2 as an intrinsic regulatory factor in sea-induced toxicity in D-galactosamine-sensitized mice. Clin Immunol. (2010) 137:311–21. doi: 10.1016/j.clim.2010.08.002
65. Ravindra KC, Vaidya VS, Wang ZY, Federspiel JD, Virgen-Slane R, Everley RA, et al. Tandem mass tag-based quantitative proteomic profiling identifies candidate serum biomarkers of drug-induced liver injury in humans. Nat Commun. (2023) 14(1):1215. doi: 10.1038/s41467–023-36858–6
66. Kong M, Xiang B. Identifying biomarkers to predict the prognosis of biliary atresia by weighted gene co-expression network analysis. Front Genet. (2021) 12:760182. doi: 10.3389/fgene.2021.760182
67. Lin Y, Dong MQ, Liu ZM, Xu M, Huang ZH, Liu HJ, et al. A strategy of vascular-targeted therapy for liver fibrosis. Hepatology. (2022) 76:660–75. doi: 10.1002/hep.32299
68. Wang XJ, Malhi H. Nonalcoholic fatty liver disease. Ann Intern Med. (2018) 169:Itc65–itc80. doi: 10.7326/aitc201811060
69. Paternostro R, Trauner M. Current treatment of non-alcoholic fatty liver disease. J Intern Med. (2022) 292:190–204. doi: 10.1111/joim.13531
70. Okumura A, Unoki-Kubota H, Matsushita Y, Shiga T, Moriyoshi Y, Yamagoe S, et al. Increased serum leukocyte cell-derived chemotaxin 2 (Lect2) levels in obesity and fatty liver. Biosci Trends. (2013) 7:276–83. doi: 10.5582/bst.2013.v7.6.276
71. Wang J, Chen Y, Pan R, Wu C, Chen S, Li L, et al. Leukocyte cell-derived chemotaxin 2 promotes the development of nonalcoholic fatty liver disease through stat-1 pathway in mice. Liver Int. (2021) 41:777–87. doi: 10.1111/liv.14816
72. Hwang HJ, Jung TW, Kim BH, Hong HC, Seo JA, Kim SG, et al. A dipeptidyl peptidase-iv inhibitor improves hepatic steatosis and insulin resistance by ampk-dependent and jnk-dependent inhibition of lect2 expression. Biochem Pharmacol. (2015) 98:157–66. doi: 10.1016/j.bcp.2015.08.098
73. Qin J, Sun W, Zhang H, Wu Z, Shen J, Wang W, et al. Prognostic value of lect2 and relevance to immune infiltration in hepatocellular carcinoma. Front Genet. (2022) 13:951077. doi: 10.3389/fgene.2022.951077
74. Zhang Y, Xia M, Jin K, Wang S, Wei H, Fan C, et al. Function of the C-met receptor tyrosine kinase in carcinogenesis and associated therapeutic opportunities. Mol Cancer. (2018) 17:45. doi: 10.1186/s12943-018-0796-y
75. Chu TH, Ko CY, Tai PH, Chang YC, Huang CC, Wu TY, et al. Leukocyte cell-derived chemotaxin 2 regulates epithelial-mesenchymal transition and cancer stemness in hepatocellular carcinoma. J Biol Chem. (2022) 298(10):18. doi: 10.1016/j.jbc.2022.102442
76. Cheng K, Cai N, Zhu J, Yang X, Liang H, Zhang W. Tumor-associated macrophages in liver cancer: from mechanisms to therapy. Cancer Commun (Lond). (2022) 42:1112–40. doi: 10.1002/cac2.12345
77. Chen CK, Yu WH, Cheng TY, Chen MW, Su CY, Yang YC, et al. Inhibition of vegf165/vegfr2-dependent signaling by lect2 suppresses hepatocellular carcinoma angiogenesis. Sci Rep. (2016) 6:31398. doi: 10.1038/srep31398
78. Liu R, Mathieu C, Berthelet J, Zhang W, Dupret JM, Rodrigues Lima F. Human protein tyrosine phosphatase 1b (Ptp1b): from structure to clinical inhibitor perspectives. Int J Mol Sci. (2022) 23(13):7027. doi: 10.3390/ijms23137027
79. Wiede F, Lu KH, Du X, Zeissig MN, Xu R, Goh PK, et al. Ptp1b is an intracellular checkpoint that limits T-cell and car T-cell antitumor immunity. Cancer Discovery. (2022) 12:752–73. doi: 10.1158/2159–8290.Cd-21–0694
80. Purcell R, Childs M, Maibach R, Miles C, Turner C, Zimmermann A, et al. Hgf/C-met related activation of B-catenin in hepatoblastoma. J Exp Clin Cancer Res. (2011) 30:96. doi: 10.1186/1756–9966-30–96
81. Lu CY, Fang SJ, Weng QY, Lv XL, Meng MM, Zhu JY, et al. Integrated analysis reveals critical glycolytic regulators in hepatocellular carcinoma. Cell Commun Signal. (2020) 18(1):14. doi: 10.1186/s12964–020-00539–4
82. Nasr SH, Dogan A, Larsen CP. Leukocyte cell-derived chemotaxin 2-associated amyloidosis: A recently recognized disease with distinct clinicopathologic characteristics. Clin J Am Soc Nephrol. (2015) 10:2084–93. doi: 10.2215/cjn.12551214
83. Mejia-Vilet JM, Cárdenas-Mastrascusa LR, Palacios-Cebreros EJ, Reyes-Macedo M, Portilla-Jiménez A, Morales-Buenrostro LE, et al. Lect2 amyloidosis in kidney transplantation: A report of 5 cases. Am J Kidney Dis. (2019) 74:563–6. doi: 10.1053/j.ajkd.2018.10.016
84. Khalighi MA, Yue A, Hwang MT, Wallace WD. Leukocyte chemotactic factor 2 (Lect2) amyloidosis presenting as pulmonary-renal syndrome: A case report and review of the literature. Clin Kidney J. (2013) 6:618–21. doi: 10.1093/ckj/sft126
85. Larsen CP, Beggs ML, Wilson JD, Lathrop SL. Prevalence and organ distribution of leukocyte chemotactic factor 2 amyloidosis (Alect2) among decedents in new Mexico. Amyloid. (2016) 23:119–23. doi: 10.3109/13506129.2016.1145110
86. Tariq H, Sharkey FE. Leukocyte cell-derived chemotaxin-2 amyloidosis (Alect2) in a patient with lung adenocarcinoma: an autopsy report and literature review. Int J Surg Pathol. (2018) 26:271–5. doi: 10.1177/1066896917735893
87. Said SM, Sethi S, Valeri AM, Leung N, Cornell LD, Fidler ME, et al. Renal amyloidosis: origin and clinicopathologic correlations of 474 recent cases. Clin J Am Soc Nephrol. (2013) 8:1515–23. doi: 10.2215/cjn.10491012
88. Said SM, Sethi S, Valeri AM, Chang A, Nast CC, Krahl L, et al. Characterization and outcomes of renal leukocyte chemotactic factor 2-associated amyloidosis. Clin J Am Soc Nephrol. (2014) 86:370–7. doi: 10.1038/ki.2013.558
89. de la Cruz Jasso MA, Mejía-Vilet JM, Del Toro-Cisneros N, Aguilar-León DE, Morales-Buenrostro LE, Herrera G, et al. Leukocyte chemotactic factor 2 amyloidosis (Alect2) distribution in a Mexican population. Am J Clin Pathol. (2023) 159:89–97. doi: 10.1093/ajcp/aqac138
90. Murphy CL, Wang S, Kestler D, Larsen C, Benson D, Weiss DT, et al. Leukocyte chemotactic factor 2 (Lect2)-associated renal amyloidosis: A case series. Am J Kidney Dis. (2010) 56:1100–7. doi: 10.1053/j.ajkd.2010.08.013
91. Li DY, Liu D, Wang SX, Yu XJ, Cui Z, Zhou FD, et al. Renal leukocyte chemotactic factor 2 (Alect2)-associated amyloidosis in chinese patients. Amyloid. (2020) 27:134–41. doi: 10.1080/13506129.2020.1722097
92. Hutton HL, DeMarco ML, Magil AB, Taylor P. Renal leukocyte chemotactic factor 2 (Lect2) amyloidosis in first nations people in northern British Columbia, Canada: A report of 4 cases. Am J Kidney Dis. (2014) 64:790–2. doi: 10.1053/j.ajkd.2014.06.017
93. Law S, Gillmore J, Gilbertson JA, Bass P, Salama AD. Karyomegalic interstitial nephritis with a novel fan1 gene mutation and concurrent alect2 amyloidosis. BMC Nephrol. (2020) 21(1):74. doi: 10.1186/s12882–020-01733–9
94. Gibier JB, Gilbertson JA, Perbet R, Leriche W, Truant S, Leteurtre E, et al. Incidental finding of leukocyte cell-derived chemotaxin 2-associated amyloidosis in a liver. Ann Pathol. (2020) 40:472–7. doi: 10.1016/j.annpat.2020.04.007
95. Dogan A. Amyloidosis: Insights from Proteomics. Annu Rev Pathol. (2017) 12:277–304. doi: 10.1146/annurev-pathol-052016-100200
96. Paueksakon P, Fogo AB, Sethi S. Leukocyte chemotactic factor 2 amyloidosis cannot be reliably diagnosed by immunohistochemical staining. Hum Pathol. (2014) 45:1445–50. doi: 10.1016/j.humpath.2014.02.020
97. Nasr SH, Said SM, Valeri AM, Sethi S, Fidler ME, Cornell LD, et al. The diagnosis and characteristics of renal heavy-chain and heavy/light-chain amyloidosis and their comparison with renal light-chain amyloidosis. Kidney Int. (2013) 83:463–70. doi: 10.1038/ki.2012.414
98. Chandan VS, Shah SS, Lam-Himlin DM, De Petris G, Mereuta OM, Dogan A, et al. Globular hepatic amyloid is highly sensitive and specific for lect2 amyloidosis. Am J Surg Pathol. (2015) 39:558–64. doi: 10.1097/pas.0000000000000373
99. Kowalski A, Cabrera J, Nasr S, Lerma E. Renal lect2 amyloidosis: A newly described disorder gaining greater recognition. Clin Nephrol. (2015) 84:236–40. doi: 10.5414/cn108549
100. Kuo ML WY. Methods and compositions related to reduced met phosphorylation by leukocyte cell-derived chemotaxin 2 in tumor cells. (2010). WO 2011076095A1.
101. Yin ZJ, Yan XM, Wang QM, Deng ZL, Tang KL, Cao ZW, et al. Detecting prognosis risk biomarkers for colon cancer through multi-omics-based prognostic analysis and target regulation simulation modeling. Front Genet. (2020) 11:16. doi: 10.3389/fgene.2020.00524
Keywords: LECT2, bibliometrics, CiteSpace, VOSviewer, visualization
Citation: Liu W, Wang Q, Yeerlan J, Yan Y, Xu L, Jia C, Liu X and Zhang L (2024) Global research trends and hotspots for leukocyte cell-derived chemotaxin-2 from the past to 2023: a combined bibliometric review. Front. Immunol. 15:1413466. doi: 10.3389/fimmu.2024.1413466
Received: 07 April 2024; Accepted: 15 May 2024;
Published: 31 May 2024.
Edited by:
Wai Po Chong, Hong Kong Baptist University, ChinaCopyright © 2024 Liu, Wang, Yeerlan, Yan, Xu, Jia, Liu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xinlian Liu, bHhsZGx5QGNtYy5lZHUuY24=; Lushun Zhang, emhhbmdsczIwMTJAY21jLmVkdS5jbg==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.