- 1Department of Medical Oncology, Fujian Medical University Union Hospital, Fuzhou, Fujian, China
- 2Department of Medical Oncology, People’s Hospital Affiliated to Shandong First Medical University, Jinan, Shandong, China
- 3Department of Medical Oncology, Shengli Clinical Medical College, Fujian Medical University, Fujian Provincial Hospital, Fuzhou, Fujian, China
- 4Department of Medical Oncology, Fujian Medical University Cancer Hospital, Fuzhou, Fujian, China
Backgroud: The study aimed to analyze the efficacy and safety of PD-1 inhibitors plus chemotherapy with or without endostatin for stage IV lung squamous cell carcinoma (LUSC).
Methods: A total of 219 patients with stage IV LUSC were included. 120 received PD-1 inhibitors plus chemotherapy with or without endostatin (IC ± A), of which 39 received endostatin (IC+A) and 81 did not receive endostatin (IC-A). 99 received chemotherapy with or without endostatin (C ± A). Endpoints included overall survival (OS), progression-free survival (PFS), adverse events (AEs), and immune-related adverse events (irAEs).
Results: The median PFS in the IC ± A group versus the C ± A group was 8 and 4 months (P < 0.001), and the median OS was 17 and 9 months (P < 0.001). There was no significant difference in any grade AEs between the IC ± A and C ± A groups (P > 0.05). The median PFS in the IC+A group versus the IC-A group was 11 and 7 months (P = 0.024), and the median OS was 34 and 15 months (P = 0.01). There was no significant difference between the IC+A group and the IC-A group for all grade AEs and irAEs (P > 0.05). The subgroup analysis showed that patients with LIPI = 0 had significant OS and PFS benefits in IC+A group, while for patients with LIPI = 1–2, there was no significant difference in OS and PFS benefits between the IC+A group and IC-A group.
Conclusions: PD-1 inhibitors plus chemotherapy with endostatin might be first-line treatment for patients with stage IV LUSC.
1 Introduction
Stage IV lung squamous cell carcinoma (LUSC) typically carries a poor prognosis (1, 2). However, survival rates among LUSC patients have improved with the application of immune checkpoint inhibitors (ICIs), particularly PD-1 inhibitors. Consequent to data unearthed from several clinical studies, the combination of PD-1 inhibitors with chemotherapy has emerged as the standard first-line treatment for stage IV driver gene-negative LUSC (3–6). While this combination enhances survival rates in patients with advanced LUSC, the emergence of drug resistance remains a critical concern, limiting the potential benefits (7, 8).
Endostatin (recombinant human vascular endostatin), targeting the endothelial cells of tumor vasculature, inhibits neovascularization, thereby impeding nutrient supply to tumor cells and curbing their proliferation and metastasis (9). As an anti-angiogenic agent, endostatin influences the tumor immune microenvironment similarly to PD-1 inhibitors, providing a rationale for their concurrent use (9, 10). A retrospective clinical study has demonstrated that the combination of ICIs with endostatin offers greater efficacy and safety than the combination of ICIs with chemotherapy in treating advanced non-small cell lung cancer (NSCLC) (11). Furthermore, Phase II clinical trials in the Lung-MAP S1800A study have shown that combining pembrolizumab with ramucirumab leads to improved efficacy and survival outcomes for patients with advanced LUSC (12).
Our research focuses on determining the potential of endostatin to enhance the efficacy of PD-1 inhibitors in conjunction with chemotherapy for treating stage IV LUSC. Given the insufficient clinical evidence to support the combined usage of PD-1 inhibitors, chemotherapy, and endostatin, our study is designed to evaluate the efficacy and safety of this treatment regimen. Specifically, we aim to elucidate endostatin’s impact on the outcome and adverse effects when simultaneously administered with PD-1 inhibitors and chemotherapy in stage IV LUSC patients.
2 Methods
2.1 Patients
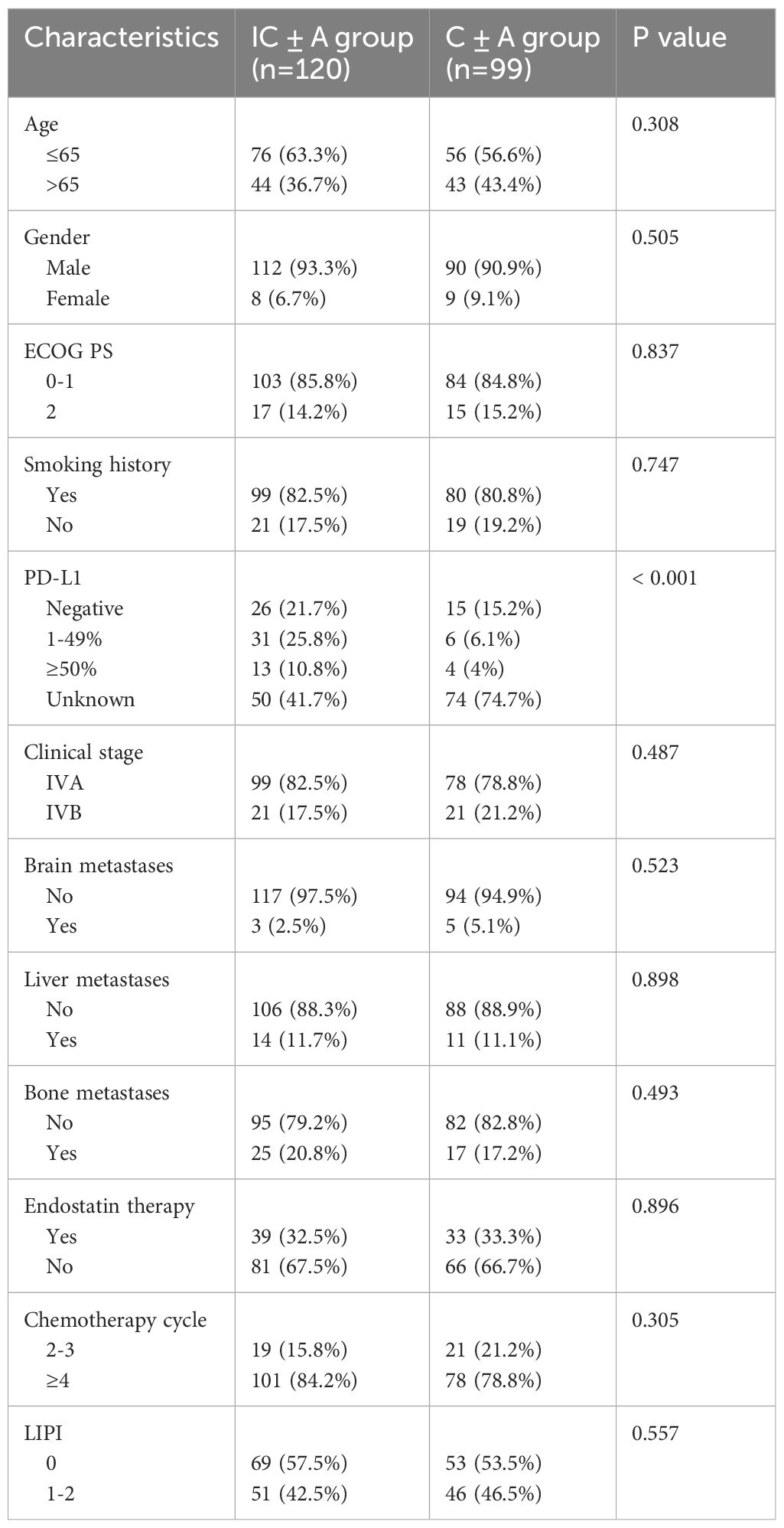
This retrospective study included patients diagnosed with stage IV LUSC at our hospital from 2018 to 2023 who were first-line received PD-1 inhibitor combined with chemotherapy or chemotherapy alone with or without endostatin therapy. Inclusion criteria: (1) pathological diagnosis was LUSC; (2) clinical stage was stage IV; (3) Eastern Cooperative Oncology Group Performance Status (ECOG PS) was 0–2. Exclusion criteria: (1) patients’ age less 18 years or over 85 years; (2) patients with other primary malignancies; (3) lack of clinical hematological and imaging data. All patients were clinically staged using the 8th edition of the American Joint Committee on Cancer (AJCC) TNM staging system. According to the inclusion and exclusion criteria, 219 patients with stage IV LUSC were finally enrolled.
2.2 Data collections
Clinical data included baseline data before receiving anti-tumor therapy: gender, age, ECOG PS, smoking history, histological type, clinical stage, distant metastatic(brain, liver, bone), PD-L1 expression level, and Lung Immune Prognostic Index (LIPI). LIPI is based on the derived neutrophil to lymphocyte ratio (dNLR) and lactate dehydrogenase (LDH), dNLR = baseline neutrophil count/(white blood cell-neutrophil count), and is calculated as 1 point for dNLR greater than 3 or LDH greater than normal. Patients are divided into two groups with good (0 points) and poor (1–2 points) prognosis (13, 14). Other relevant clinical data: anti-tumor drugs, chemotherapy cycle, survival events, treatment efficacy, AEs and irAEs.
2.3 Treatment regimen
IC ± A group received PD-1 inhibitor plus chemotherapy with or without endostatin, IC + A group received PD-1 inhibitor plus chemotherapy plus endostatin, IC-A group received PD-1 inhibitor plus chemotherapy without endostatin, C ± A group received chemotherapy with or without endostatin. PD-1 inhibitors: pembrolizumab(200 mg iv q3w d1) or sintilimab (200 mg iv q3w d1) or camrelizumab (200 mg iv q3w d1) or tislelizumab (200 mg iv q3w d1). Chemotherapy: paclitaxel (175 mg/m2 iv q3w d1) plus carboplatin (400 mg/m2 iv q3w d1) or cisplatin (100 mg/m2 iv q3w d1). Endostatin:(15 mg qd iv q3w d0–6) was given intravenously at a dose of 15 mg for 3 hours once daily for 7 days. All patients received two cycles of treatment at least.
2.4 Outcomes
Overall survival (OS) is defined as the time between the first treatment and death from any cause or the last follow-up. Progression-free survival (PFS) is the time from the first treatment to disease progression, death from any cause, or the last follow-up. Objective response rate (ORR) is defined as the proportion of patients who achieve complete response (CR) or partial response (PR). Disease control rate (DCR) is defined as the proportion of patients who achieve CR, PR, and stable disease (SD). RECIST1.1 solid tumor evaluation criteria were used for short-term efficacy evaluation. All patients were followed up until September 2023.
2.5 Adverse events
Adverse events (AEs) and immune-related adverse events (irAEs) occurred during treatment were collected through the medical record system. AEs included anemia, neutropenia, thrombocytopenia, alanine aminotransferase(ALT) elevation, creatinine elevation, nausea, vomiting, decreased appetite, bronchial or pulmonary infection, rash, diarrhea, pain, and insomnia. irAEs included hyperthyroidism, hypothyroidism, adrenocortical insufficiency, pneumonia, severe skin reaction, hepatitis, nephritis, colitis, myocarditis, hypophysitis, pancreatitis, arthritis, peripheral neuropathy, and cardiac arrhythmias. All adverse events were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE version 5.0).
2.6 Statistical analysis
We used Pearson’s chi-square test or Fisher’s exact test to compare categorical variables. The Kaplan-Meier method was used to plot survival curves, and the log-rank test was used for differences between survival curves. Variables with a P value ≤ 0.05 in univariate Cox analysis were included in the multivariate Cox analysis. Multivariate Cox analysis was used to determine independent prognostic factors affecting OS and PFS. Therefore, a statistical result P value < 0.05 was considered statistically significant. All analyses were performed using SPSS 25.0 (IBM, Armonk, NY, USA) for all of the above statistical analyses.
3 Result
3.1 Patient characteristics
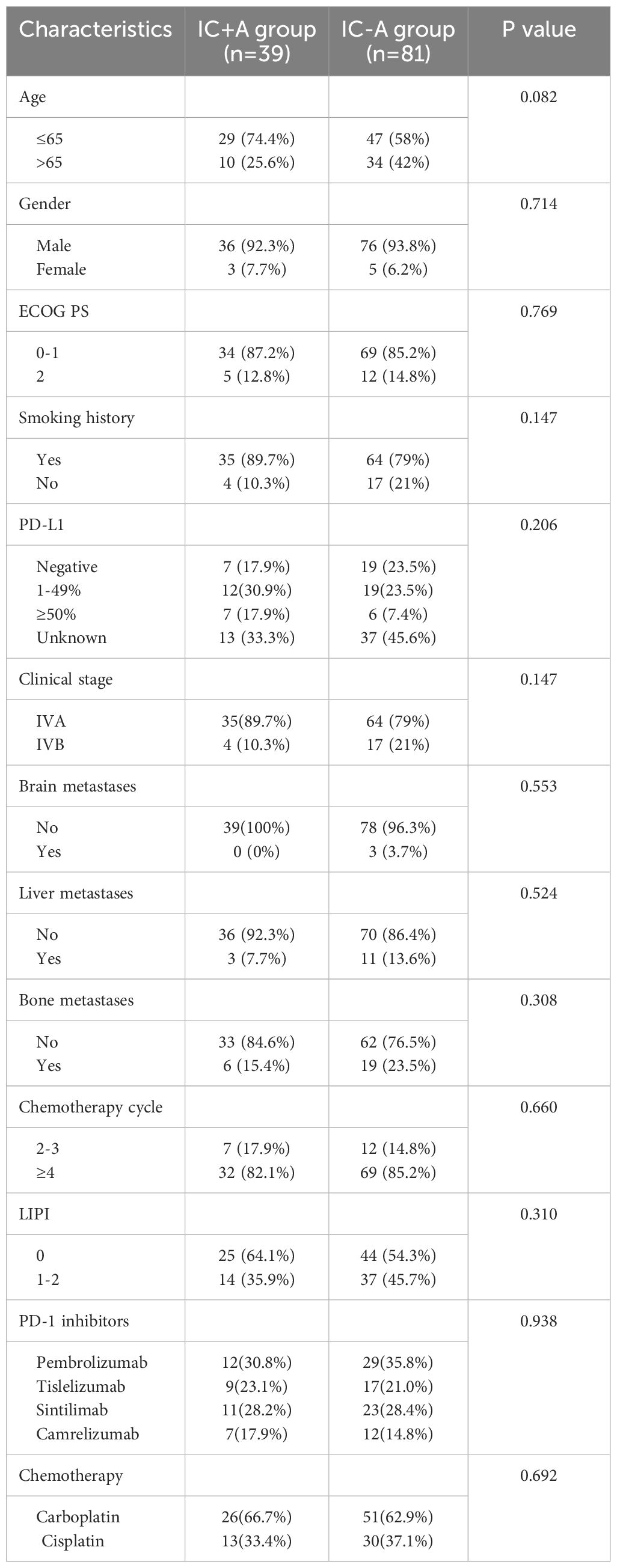
A total of 219 patients with stage IV LUSC were enrolled in our study. The baseline characteristics are shown in Tables 1, 2. There were 120 patients in the IC ± A group and 99 patients in the C ± A group. There were 39 patients in the IC+A group and 81 patients in the IC-A group. The IC ± A group and the C ± A group, the IC+A group and the IC-A group were mostly male, ECOG PS 0–1, smoking history, stage IVA, no brain metastasis, no liver metastasis, no bone metastasis, and the chemotherapy cycle ≥ 4. Except for the PD-L1 expression status, there were no statistical differences in other baseline characteristics between the IC ± A group and the C ± A group. There was no statistical difference in baseline characteristics between the IC+A group and the IC-A group.
3.2 Outcome and efficacy analysis
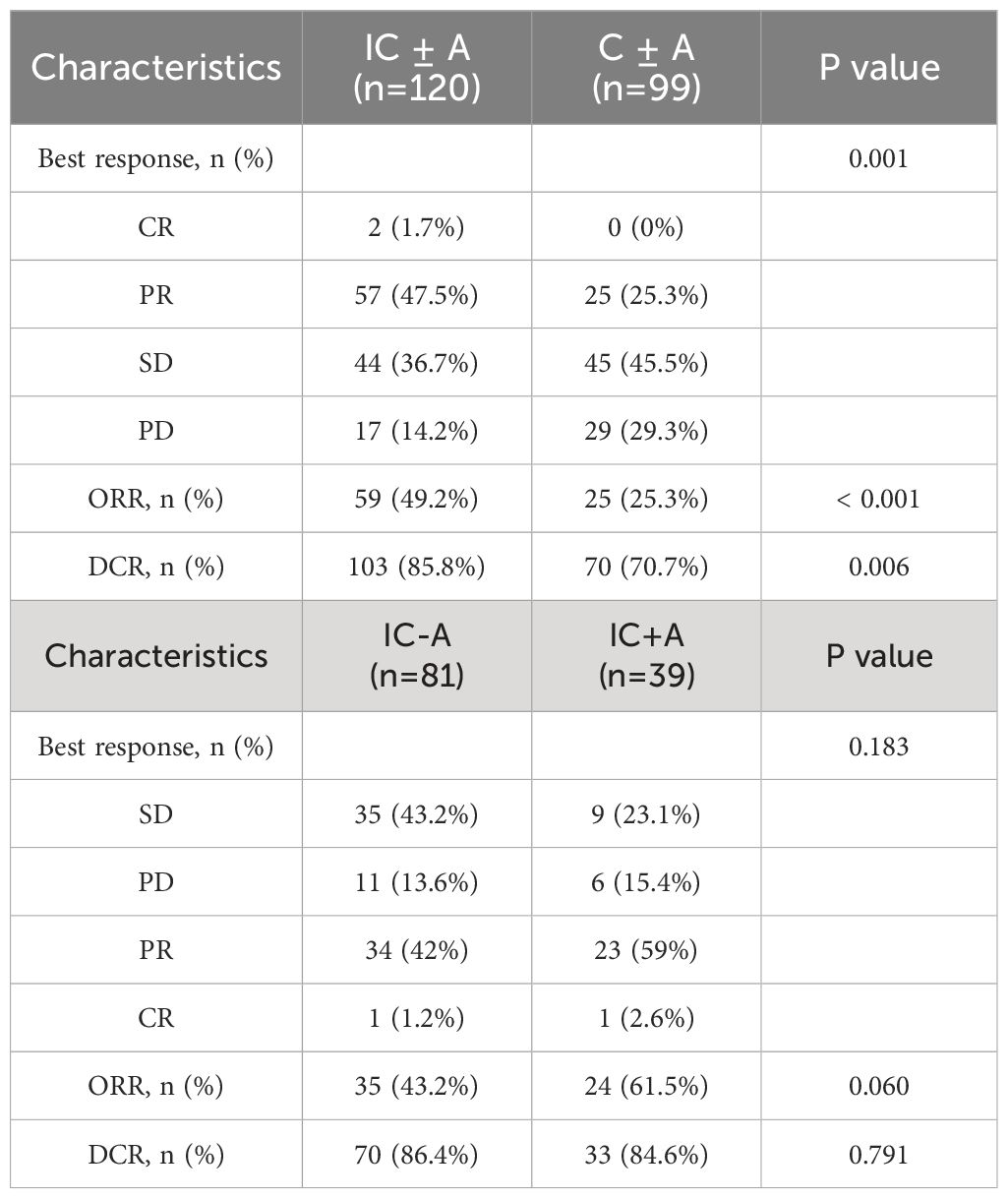
Until September 2023, 105 PFS events (87.5%) and 76 OS events (63.4%) occurred in the IC ± A group and 99 PFS events (100%) and 96 OS events (97%) occurred in the C ± A group. The median OS was 17 (95% CI: 15–19) and 9 (95% CI: 6.6–11.4) months (P < 0.001, Figure 1A), and the median PFS of the IC ± A group and the C ± A group was 8 (95% CI: 6.9–9.1) and 4 (95% CI: 3.2–4.8) months respectively (P < 0.001, Figure 1B). The IC ± A group had longer median PFS and median OS than the C ± A group. In addition, 2 patients (1.6%) achieved CR, 57 patients (47.5%) achieved PR, 44 patients (36.7%) achieved SD, and 17 patients (14.2%) achieved PD in the IC ± A group, with an ORR of 49.2% and a DCR of 85.8%. In the C ± A group, 33 patients (30.3%) achieved PR, 45 patients (45.5%) achieved SD, and 29 patients (29.3%) achieved PD, with an ORR of 25.3% and a DCR of 70.7% (Table 3). The ORR (P < 0.001) and DCR (P = 0.006) were better in the IC ± A group than in the C ± A group.
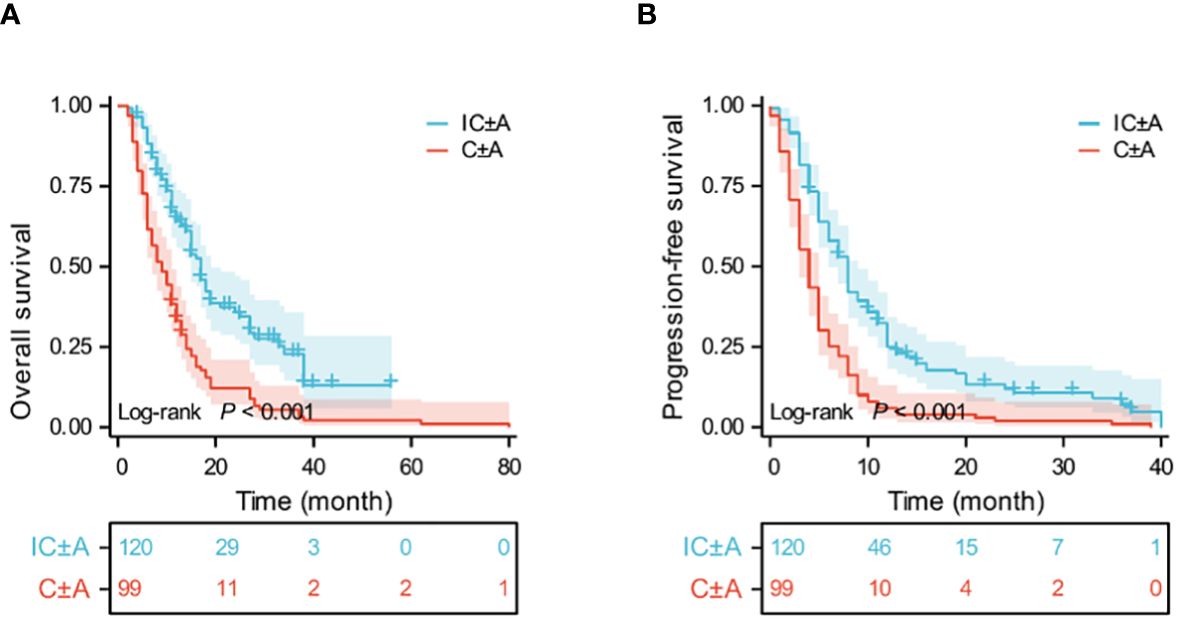
Figure 1 Kaplan-Meier estimates of OS (A) and PFS (B) in the IC ± A and C ± A population; IC ± A, PD-1 inhibitor plus chemotherapy with or without endostatin; C ± A, chemotherapy with or without endostatin; OS, overall survival; PFS, progression-free survival; Time, month.
Thirty-nine patients in the IC+A group had PFS events in 33 (84.6%) and OS events in 20 (51.2%). Eighty-one patients in the IC-A group had PFS events in 72 (88.8%) and OS events in 56 (69.1%). The median OS was 34 (95% CI: 9.6–58.4) and 15 (95% CI: 13.1–16.9) months (P = 0.01, Figure 2A), and the median PFS in the IC+A group and the IC-A group was 11 (95% CI: 7.8–14.2) and 7 (95% CI: 5.5–8.5) months respectively (P = 0.024, Figure 2B), and The median PFS and median OS of the IC+A group were longer than those of the IC-A. In the IC+A group, 1 patient (2.6%) achieved CR, 23 patients (59%) achieved PR, 9 patients (23.1%) achieved SD, and 6 patients (15.4%) achieved PD, with an ORR of 61.5% and a DCR of 84.6%. In the IC-A group, 1 patient (1.2%) achieved CR, 34 patients (42%) achieved PR, 35 patients (43.2%) achieved SD, and 11 patients (13.6%) achieved PD, with an ORR of 43.2% and a DCR of 86.4% (Table 3). There was no statistical difference in ORR and DCR between the IC+A group and the IC-A group.
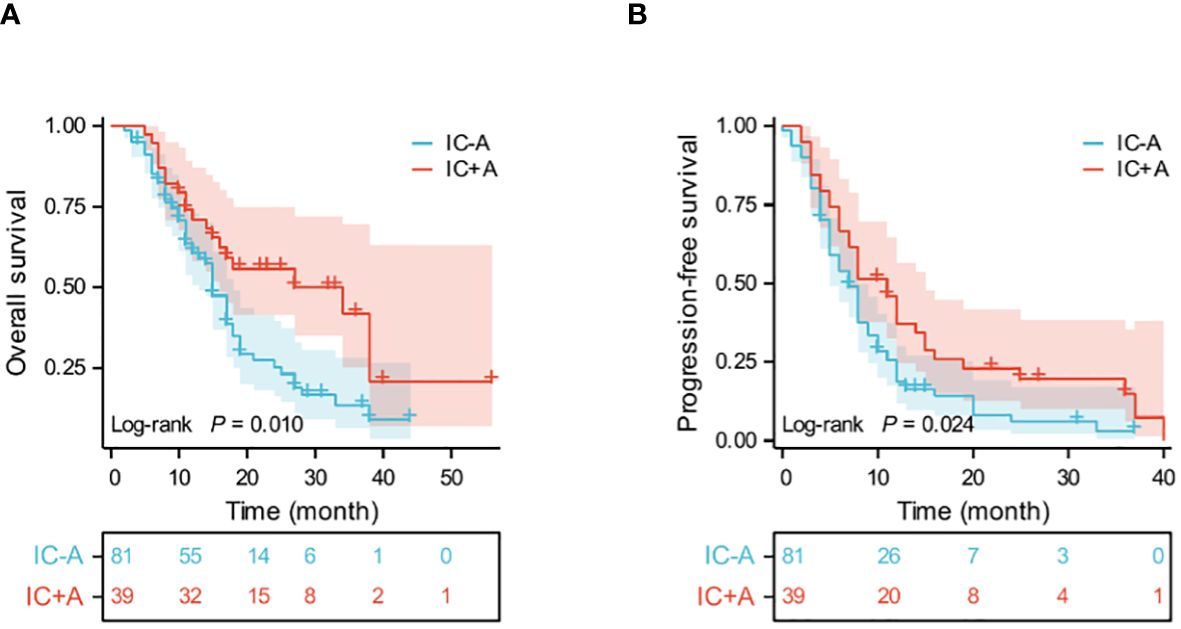
Figure 2 Kaplan-Meier estimates of OS (A) and PFS (B) in the IC+A and IC-A population; IC+A,PD-1 inhibitor plus chemotherapy with endostatin; IC-A,PD-1 inhibitor plus chemotherapy without endostatin; OS, overall survival; PFS, progression-free survival; Time, month.
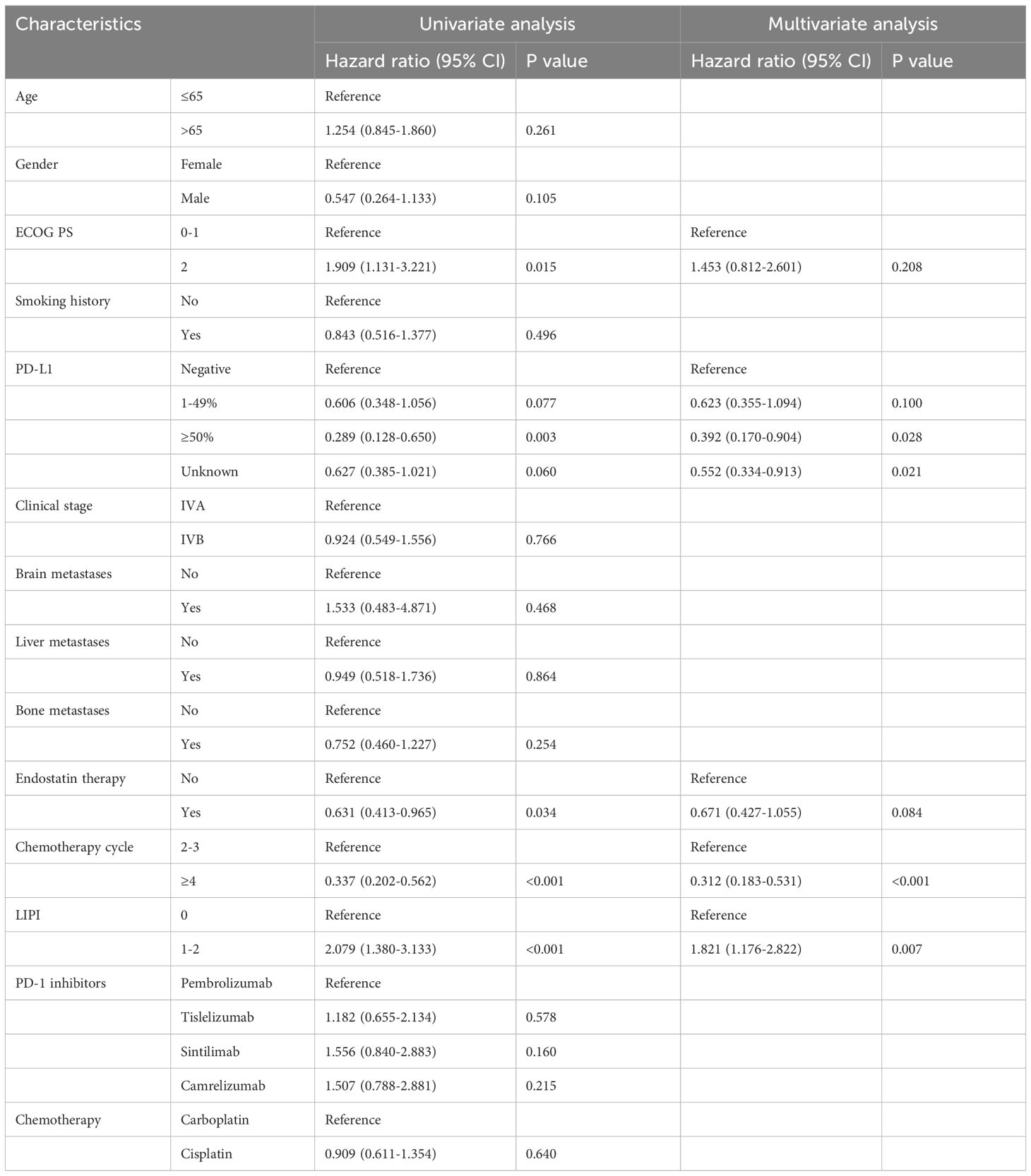
3.3 Predictors affecting efficacy in the IC ± A group
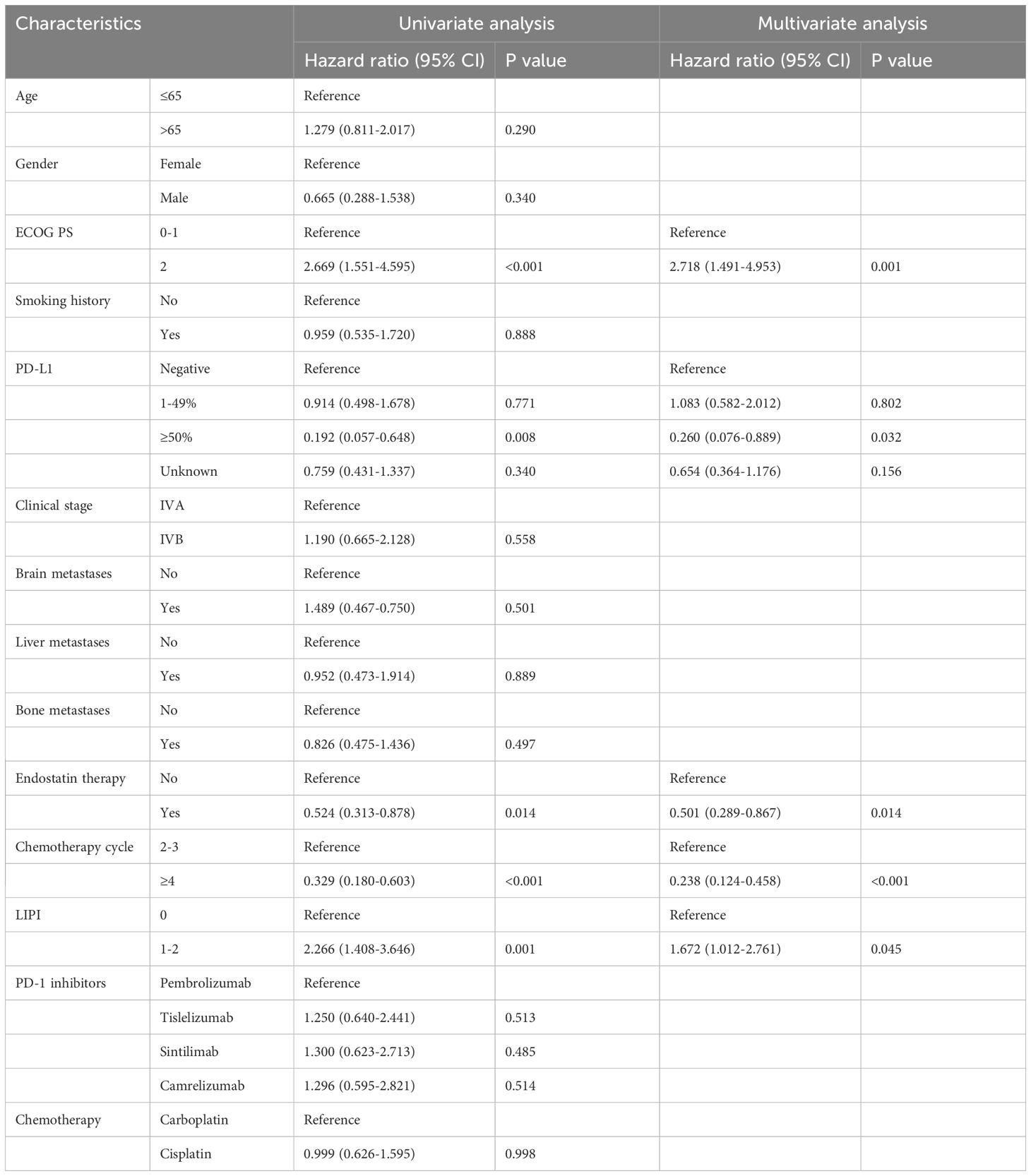
Multivariate analysis showed that ECOG PS (HR:2.718, 95%CI:1.491–4.953, P=0.001), PD-L1 ≥50% (HR:0.260, 95%CI:0.076–0.889, P=0.032), plus endostatin (HR:0.501, 95%CI:0.289–0.867, P=0.014), chemotherapy cycle ≥4 (HR:0.238, 95%CI: 0.124–0.458, P<0.001), LIPI 0 score (HR:1.672, 95%CI:1.012–2.761, P=0.045) were independent prognostic factors for OS (Table 4). In addition, PD-L1 ≥50% (HR:0.392, 95%CI:0.170–0.904, P=0.028), chemotherapy cycle ≥4 (HR:0.312, 95%CI:0.183–0.531, P<0.001), LIPI 0 score (HR:1.821, 95%CI:1.176–2.822, P=0.007) were independent prognostic factors for PFS (Table 5).
3.4 Subgroup analysis
Subgroup analysis of IC ± A and C ± A groups showed that IC ± A had an OS benefit in all subgroups except women (P = 0.68), ECOG PS score 2 (P = 0.322), no history of smoking (P = 0.212), PD-L1 expression 1–49% (P = 0.338), brain metastases (P = 0.51), and LIPI score 1–2 (P = 0.08) (Figure 3A). Subgroup analysis in the IC ± A and C ± A groups showed that IC ± A had a PFS benefit, except for women (P = 0.966), no history of smoking (P = 0.194), PD-L1 expression 1–49% (P = 0.082), brain metastases (P = 0.856), and LIPI scores 1–2 (P = 0.347) (Figure 3B).
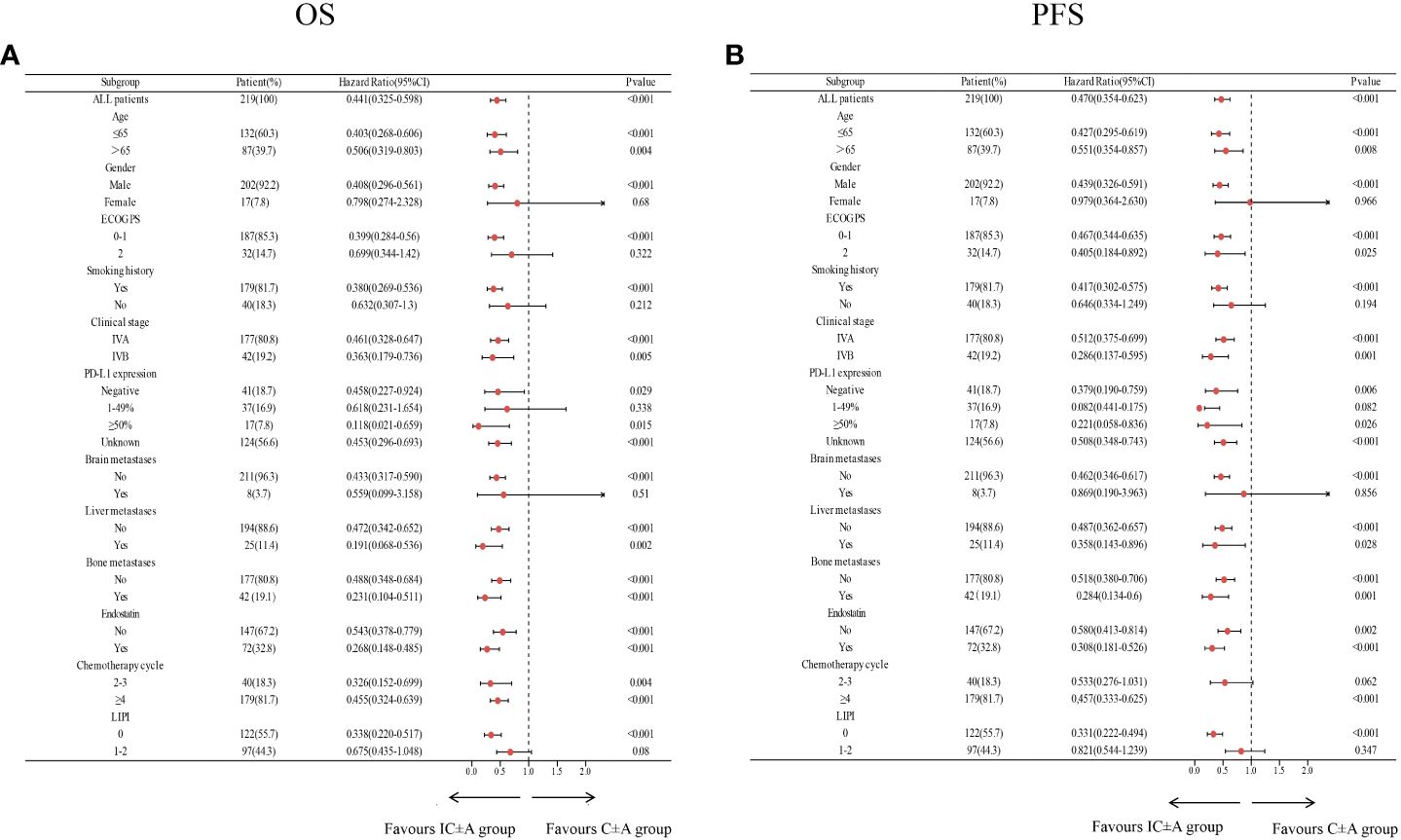
Figure 3 Subgroup analysis of the IC ± A group versus the C ± A group for OS (A) and PFS (B) based on baseline characteristics; IC ± A,PD-1 inhibitor plus chemotherapy with or without endostatin; C ± A, chemotherapy with or without endostatin; OS, overall survival; PFS, progression-free survival, LIPI, lung immune prognostic index.
The results of the subgroup analysis of IC+A and IC-A are shown in Figure 4. Patients with age ≤ 65 (P = 0.017), male (P = 0.018), ECOG PS = 0–1 (P = 0.021), smoking history (P = 0.011), stage IVA (P = 0.004), no brain metastasis (P = 0.017), no liver metastasis (P = 0.012), no bone metastasis (P = 0.01), chemotherapy cycles 2–3 (P = 0.042), chemotherapy cycles ≥ 4 (P = 0.03), and LIPI score of 0 (P = 0.021) had better OS when receiving IC+A treatment (Figure 4A). Patients who were male (P = 0.043), history of smoking (P = 0.038), IVA (P = 0.03), no brain metastasis (P = 0.04), no liver metastasis (P = 0.02), no bone metastasis (P = 0.02), chemotherapy cycles ≥4 (P = 0.03), and LIPI score of 0 (P = 0.029) had a better PFS when treated with IC+A (Figure 4B).
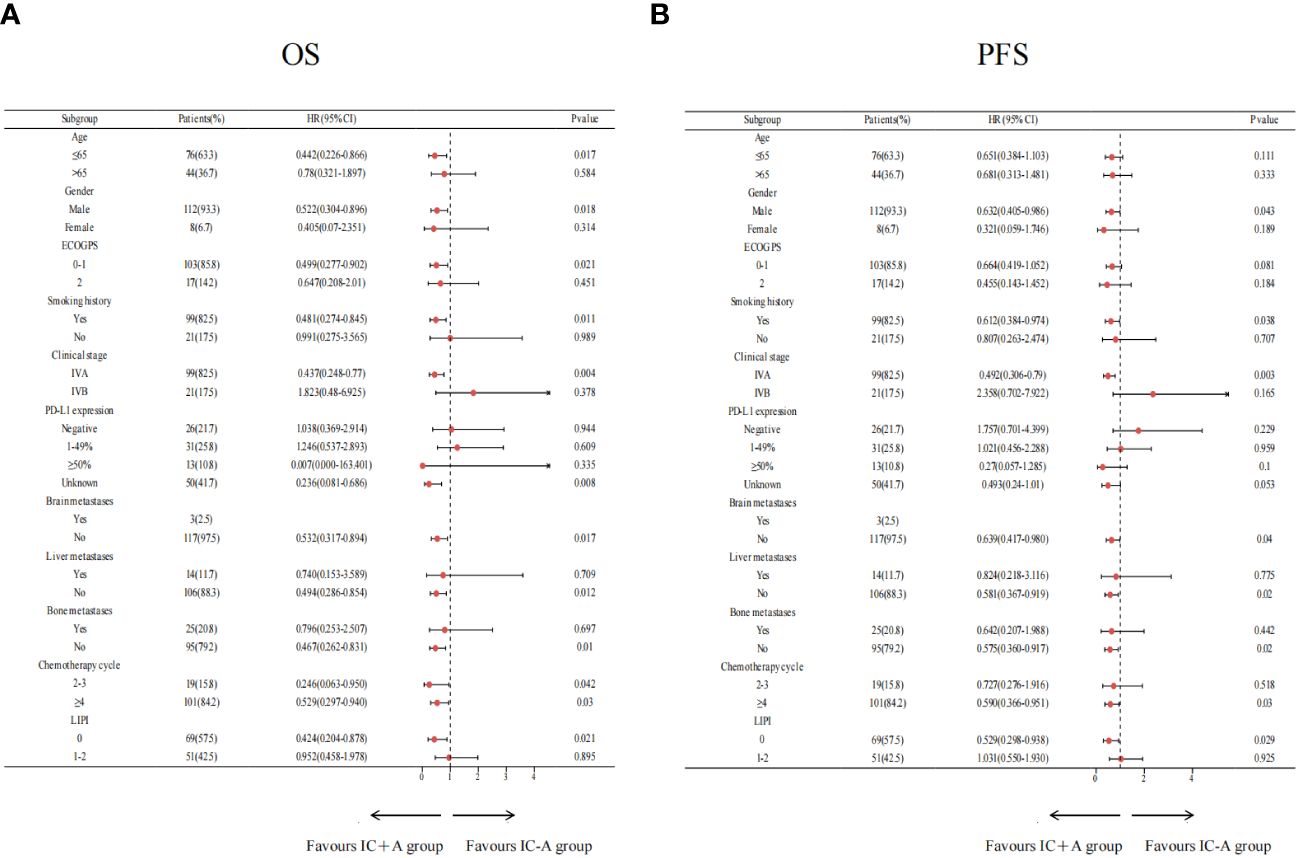
Figure 4 Subgroup analysis of the IC+A group versus the IC-A group for OS (A) and PFS (B) based on baseline characteristics; IC+A,PD-1 inhibitor plus chemotherapy with endostatin; IC-A,PD-1 inhibitor plus chemotherapy without endostatin; OS, overall survival; PFS, progression-free survival, LIPI, lung immune prognostic index.
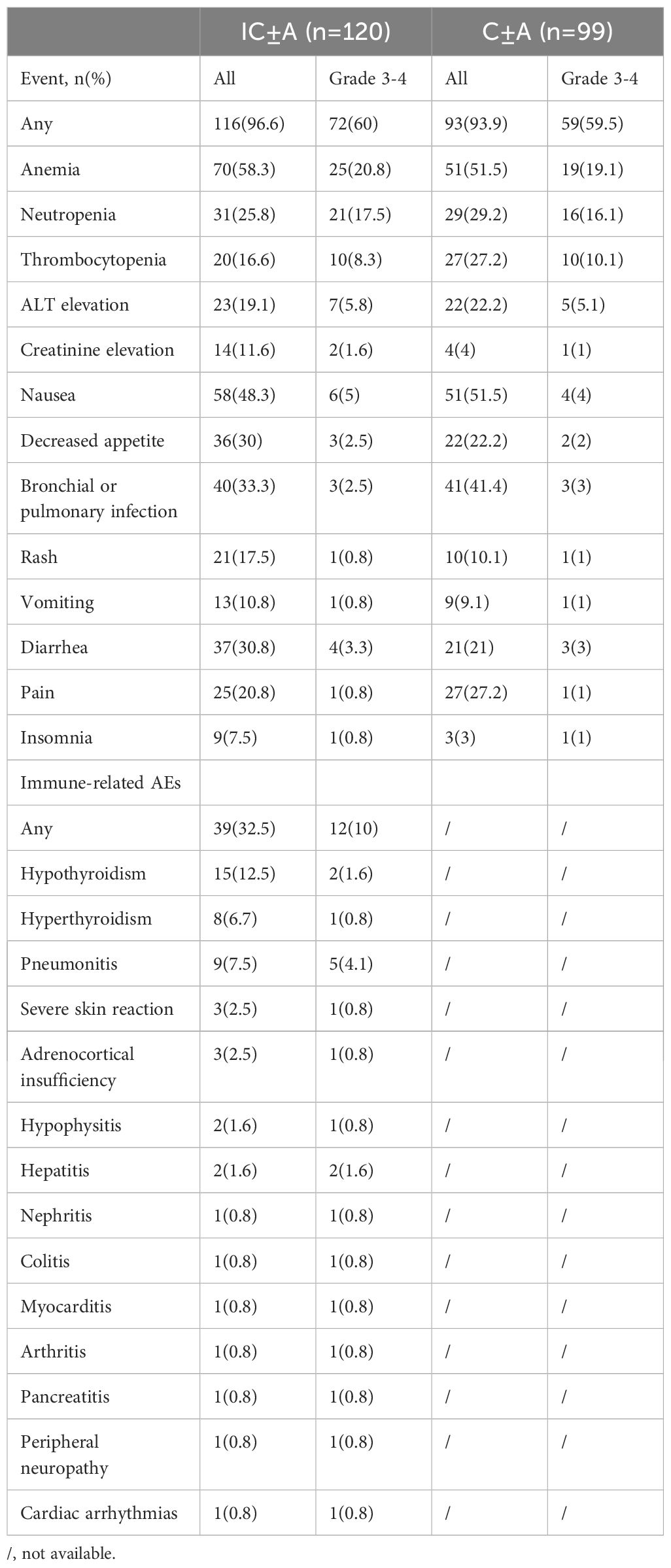
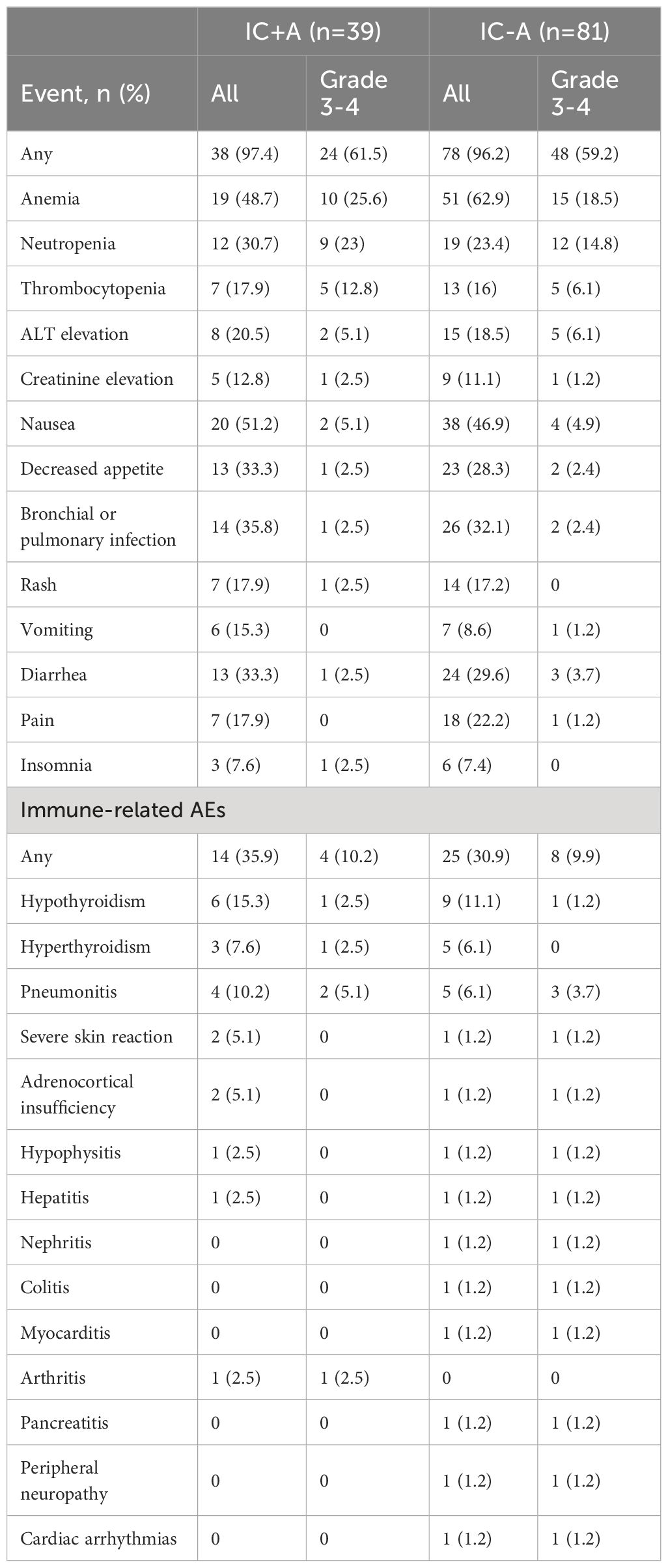
3.5 Safety and adverse events
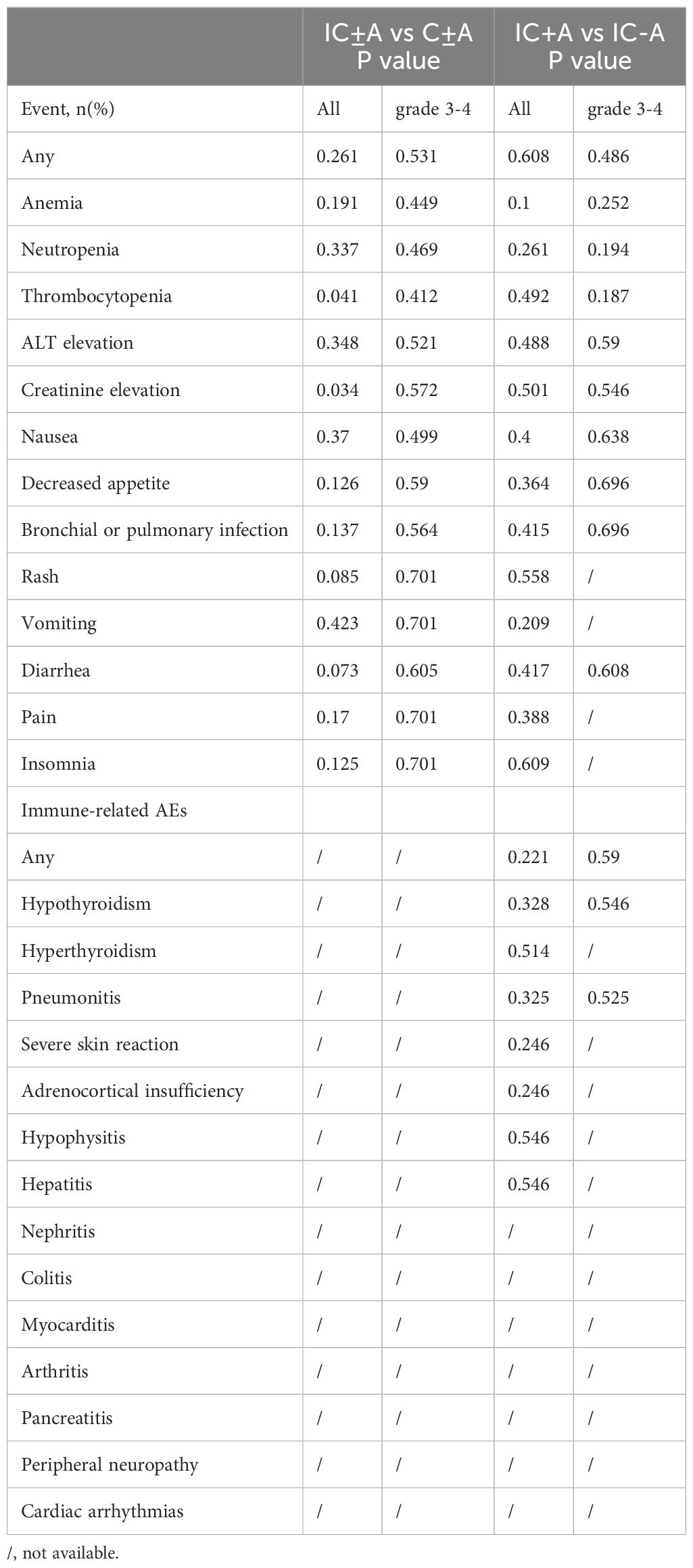
The adverse events in each group are shown in Tables 6, 7, and the chi-square test for adverse events in each group is shown in Table 8. There were 116 (96.6%) any grade AEs and 72 (60%) grade 3–4 AEs in the IC ± A group, while there were 93 (93.9%) any grade AEs and 59 (59.5%) grade 3–4 AEs in the IC-A group. There was no statistical difference in any grade AEs and grade 3–4 AEs between IC ± A and C ± A groups (P > 0.05). In addition, there were 38 (97.4%) and 78 (96.2%) any grade AEs, 24 (61.5%) and 48 (59.2%) grade 3–4 AEs, 14 (35.9%) and 25 (30.9%) any grade irAEs, and 4 (10.2%) and 8 (9.9%) grade 3–4 irAEs in the IC+A group and the IC-A group, respectively. No statistical differences were observed in all AEs, grade 3–4 AEs, any grade irAEs and grade 3–4 irAEs between IC + A and IC-A groups.
Incidence rates for all adverse events (AEs) were comparable between the IC+A and IC-A cohorts. Common grade 3–4 AEs for patients undergoing IC+A versus IC-A treatment were as follows: anemia (25.6% vs 18.5%, P = 0.252), neutropenia (23.0% vs 14.8%, P = 0.194), thrombocytopenia (12.8% vs 6.1%, P = 0.187), and ALT elevation (5.1% vs 6.1%, P = 0.59). Regarding immune-related adverse events (irAEs), the prevalences in patients treated with IC+A versus IC-A were hypothyroidism (15.3% vs 11.1%, P = 0.328), hyperthyroidism (7.6% vs 6.1%, P = 0.514), and pneumonia (10.2% vs 6.1%, P = 0.325).
4 Discussion
In patients with early-stage LUSC, the combination of PD-1 inhibitors and chemotherapy markedly decreases recurrence rates and improves prognosis. Similarly, for patients with locally advanced unresectable LUSC, this combined therapeutic approach reduces the risk of metastasis and enhances prognosis. However, the majority of patients present with advanced-stage disease at diagnosis, and the median OS for those receiving first-line PD-1 inhibitors and chemotherapy is only 17.2 months (1–3). Endostatin, an antiangiogenic agent, has been demonstrated in preclinical studies to synergistically enhance the impact of PD-1 inhibitors on lung tumor suppression (15). However, clinical evidence is sparse regarding the additional survival benefits conferred by endostatin in patients with stage IV LUSC, who are also receiving PD-1 inhibitors and chemotherapy. Thus, our research represents the initial effort to substantiate the applicability of combining a PD-1 inhibitor with chemotherapy and endostatin for first-line treatment in this patient population. Our findings indicate that the median OS and PFS for this regimen were 34 months and 11 months, respectively. Overall, AEs and irAEs were within acceptable safety margins and manageable. These results imply that incorporating endostatin with PD-1 inhibitors and chemotherapy may offer a novel frirst-line therapeutic option for stage IV LUSC.
In advanced LUSC, the combination of PD-1 inhibitors with chemotherapy has become a standard approach in clinical settings. Data from several clinical trials have established that this combination therapy provides superior ORR, DCR, PFS, and OS compared with chemotherapy alone (3–6). Our study aligns with these findings, demonstrating that, regardless of PD-L1 expression levels, the incorporation of PD-1 inhibitors with chemotherapy confers a greater survival advantage in the first-line management of stage IV LUSC. Despite these improvements, the survival benefit of anti-PD-1 therapy in combination with chemotherapy remains modest for patients with stage IV LUSC.
In 1971, Judah Folkman pioneered the concept of tumor treatment by inhibiting angiogenesis, proposing the theory that tumor proliferation depends on the formation of new blood vessels to supply essential nutrients. He posited that interrupting the tumor’s blood supply could effectively starve the tumor (16). As an angiogenesis inhibitor, endostatin has undergone extensive clinical trials, demonstrating its capacity to target neovascular endothelial cells and suppress tumor growth (17). Notably, one case study reported that the addition of endostatin to PD-1 inhibitors and chemotherapy yielded significant results in treating stage IV LUSC (18). Furthermore, the combination has been attributed with promising efficacy and acceptable safety in the primary treatment of advanced NSCLC (10, 19). Consequently, our retrospective analysis scrutinized the efficacy and safety of PD-1 inhibition with chemotherapy, both with and without the addition of endostatin, in stage IV LUSC treatment. The addition of endostatin was found to markedly enhance OS and PFS in patients. Multivariate Cox regression analysis reinforced the view that endostatin’s synergistic use constitutes an independent prognostic indicator for stage IV LUSC patients undergoing PD-1 inhibitor and chemotherapy treatment. These findings endorse the combination of PD-1 inhibitors, chemotherapy, and endostatin as an emergent first-line treatment modality for stage IV LUSC, meriting adoption in clinical practice.
It is unclear whether the combination of PD-1 inhibitors with chemotherapy and endostatin is effective for all stage IV LUSC patients. Our study conducted a subgroup stratification analysis and found that, in most subgroups—including male patients, smokers, those with an ECOG PS 0–1, stage IVA, and patients without liver, brain, or bone metastases—the OS and PFS were more favorable with the combined treatment of PD-1 inhibitors, chemotherapy, and endostatin than without endostatin. Interestingly, patients with a LIPI score of 0 showed a benefit from the combined treatment, whereas those with LIPI scores of 1–2 did not experience significant advantages from the addition of endostatin. LIPI is assessed on the basis of two hematologic markers, LDH and dNLR, which reflect the systemic immune response to cancer-related inflammation (13, 14, 20). High levels of LDH are associated with cancer cell invasion and metastasis, and patients who have high levels of LDH before immunotherapy have relatively short PFS and OS (21–24). The dNLR reflects the body’s neutrophil levels, which are associated with immunosuppression and promote cancer cell metastasis (25–27). An exploratory pooled analysis of data from 4914 metastatic non-small cell lung cancer patients from 11 randomized multinational clinical trials showed that LIPI is important for predicting the prognosis of patients with metastatic non-small cell lung cancer, and represents a different prognosis by its stratification, which is particularly significant in patients receiving ICIs therapy (14). Consequently, the LIPI score is a vital prognostic marker for immunotherapy and a significant guide for optimizing anti-PD-1 therapy with chemotherapy and endostatin in clinical practice.
Our study is subject to several limitations. Primarily, it is single-centered and retrospective in nature, characterized by a limited sample size, and incomplete detection of PD-L1 expression levels across the study population is a notable deficiency. Moreover, the incidence of survival events was not ubiquitously observed within our cohort, necessitating extended follow-up to amass comprehensive data on survival and adverse events, which would enable a more precise evaluation of the combined efficacy and toxicity of PD-1 inhibitors and chemotherapy with endostatin. Consequently, to elucidate the therapeutic potential of this combination, prospective clinical trials with more extensive participant numbers are indispensable.
5 Conclusions
Endostatin, administered concomitantly with chemotherapy and PD-1 inhibitors, yield substantial benefits in OS and PFS and are associated with manageable adverse events. This combination therapy is anticipated to become the preferred initial treatment option for stage IV LUSC, particularly in patients presenting with a LIPI score of 0.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Fujian Medical University Union Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because The requirement for informed consent of the patients was waived due to the retrospective nature of this study.
Author contributions
CL: Conceptualization, Data curation, Methodology, Software, Writing – original draft, Writing – review & editing. YW: Conceptualization, Data curation, Methodology, Software, Writing – original draft, Writing – review & editing. WG: Data curation, Formal analysis, Investigation, Visualization, Writing – review & editing. BD: Data curation, Formal analysis, Investigation, Visualization, Writing – review & editing. NY: Resources, Software, Validation, Writing – review & editing. YZ: Resources, Software, Validation, Writing – review & editing. JZ: Resources, Software, Validation, Writing – review & editing. YH: Data curation, Formal analysis, Investigation, Visualization, Writing – review & editing. JL: Conceptualization, Funding acquisition, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The study was supported by the Joint Funds for the Innovation of Science and Technology of Fujian Province (2018Y9063).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
LUSC, lung squamous cell carcinoma; ICIs, immune checkpoint inhibitors; LIPI, lung immune prognostic index; dNLR, derived neutrophil to lymphocyte ratio; LDH, lactate dehydrogenase; ALT, alanine aminotransferase; PFS, progression-free survival; OS, overall survival; ECOG PS, Eastern Cooperative Oncology Group performance status; AEs, adverse events; irAEs, immune-related adverse events; ORR, objective response rate; DCR, disease control rate; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease.
References
1. Chen P, Liu Y, Wen Y, Zhou C. Non-small cell lung cancer in China. Cancer Commun (Lond). (2022) 42:937–70. doi: 10.1002/cac2.12359
2. Lau SCM, Pan Y, Velcheti V, Wong KK. Squamous cell lung cancer: Current landscape and future therapeutic options. Cancer Cell. (2022) 40:1279–93. doi: 10.1016/j.ccell.2022.09.018
3. Novello S, Kowalski DM, Luft A, Gümüş M, Vicente D, Mazières J, et al. Pembrolizumab plus chemotherapy in squamous non-small-cell lung cancer: 5-year update of the phase III KEYNOTE-407 study. J Clin Oncol. (2023) 41:1999–2006. doi: 10.1200/JCO.22.01990
4. Ren S, Chen J, Xu X, Jiang T, Cheng Y, Chen G, et al. Camrelizumab plus carboplatin and paclitaxel as first-line treatment for advanced squamous NSCLC (CameL-Sq): A phase 3 trial. J Thorac Oncol. (2022) 17:544–57. doi: 10.1016/j.jtho.2021.11.018
5. Shi Y, Wu L, Yu X, Xing P, Wang Y, Zhou J, et al. Sintilimab versus docetaxel as second-line treatment in advanced or metastatic squamous non-small-cell lung cancer: an open-label, randomized controlled phase 3 trial (ORIENT-3). Cancer Commun (Lond). (2022) 42:1314–30. doi: 10.1002/cac2.12385
6. Wang J, Lu S, Yu X, Hu Y, Sun Y, Wang Z, et al. Tislelizumab plus chemotherapy vs chemotherapy alone as first-line treatment for advanced squamous non-small-cell lung cancer: A phase 3 randomized clinical trial. JAMA Oncol. (2021) 7:709–17. doi: 10.1001/jamaoncol.2021.0366
7. Passaro A, Brahmer J, Antonia S, Mok T, Peters S. Managing resistance to immune checkpoint inhibitors in lung cancer: treatment and novel strategies. J Clin Oncol. (2022) 40:598–610. doi: 10.1200/JCO.21.01845
8. Zhou K, Li S, Zhao Y, Cheng K. Mechanisms of drug resistance to immune checkpoint inhibitors in non-small cell lung cancer. Front Immunol. (2023) 14:1127071. doi: 10.3389/fimmu.2023.1127071
9. Poluzzi C, Iozzo RV, Schaefer L. Endostatin and endorepellin: A common route of action for similar angiostatic cancer avengers. Adv Drug Delivery Rev. (2016) 97:156–73. doi: 10.1016/j.addr.2015.10.012
10. Fu S, Huang H, Shang K, Tu G, Zhong P, Li S, et al. Efficacy and safety of immune checkpoint inhibitors combined with recombinant human endostatin and chemotherapy as the first-line treatment of advanced non-small-cell lung cancer. Future Oncol. (2023) 19:147–58. doi: 10.2217/fon-2022–0861
11. Huang H, Zhong P, Zhu X, Fu S, Li S, Peng S, et al. Immunotherapy combined with rh-endostatin improved clinical outcomes over immunotherapy plus chemotherapy for second-line treatment of advanced NSCLC. Front Oncol. (2023) 13:1137224. doi: 10.3389/fonc.2023.1137224
12. Reckamp KL, Redman MW, Dragnev KH, Minichiello K, Villaruz LC, Faller B, et al. Phase II randomized study of ramucirumab and pembrolizumab versus standard of care in advanced non-small-cell lung cancer previously treated with immunotherapy-lung-MAP S1800A. J Clin Oncol. (2022) 40:2295–306. doi: 10.1200/JCO.22.00912
13. Sorich MJ, Rowland A, Karapetis CS, Hopkins AM. Evaluation of the lung immune prognostic index for prediction of survival and response in patients treated with atezolizumab for NSCLC: pooled analysis of clinical trials. J Thorac Oncol. (2019) 14:1440–6. doi: 10.1016/j.jtho.2019.04.006
14. Kazandjian D, Gong Y, Keegan P, Pazdur R, Blumenthal GM. Prognostic value of the lung immune prognostic index for patients treated for metastatic non-small cell lung cancer. JAMA Oncol. (2019) 5:1481–5. doi: 10.1001/jamaoncol.2019.1747
15. Wu J, Zhao X, Sun Q, Jiang Y, Zhang W, Luo J, et al. Synergic effect of PD-1 blockade and endostar on the PI3K/AKT/mTOR-mediated autophagy and angiogenesis in Lewis lung carcinoma mouse model. BioMed Pharmacother. (2020) 125:109746. doi: 10.1016/j.biopha.2019.109746
16. Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. (1971) 285:1182–6. doi: 10.1056/NEJM197111182852108
17. Ou-Yang F, Lan KL, Chen CT, Liu JC, Weng CL, Chou CK, et al. Endostatin-cytosine deaminase fusion protein suppresses tumor growth by targeting neovascular endothelial cells. Cancer Res. (2006) 66:378–84. doi: 10.1158/0008–5472.CAN-05–1578
18. Fang Y, Sun H, Chen Y, Jiang N, Ji L, Shi J. A rapid response of lung squamous cell carcinoma following treatment with sintilimab combined with recombinant humane endostatin injection and nab-paclitaxel in an elderly patient: A case report. Med (Baltimore). (2021) 100:e26801. doi: 10.1097/MD.0000000000026801
19. Pu X, Wang Q, Liu L, Chen B, Li K, Zhou Y, et al. Rh-endostatin plus camrelizumab and chemotherapy in first-line treatment of advanced non-small cell lung cancer: A multicenter retrospective study. Cancer Med. (2023) 12:7724–33. doi: 10.1002/cam4.5526
20. Mezquita L, Auclin E, Ferrara R, Charrier M, Remon J, Planchard D, et al. Association of the lung immune prognostic index with immune checkpoint inhibitor outcomes in patients with advanced non-small cell lung cancer. JAMA Oncol. (2018) 4:351–7. doi: 10.1001/jamaoncol.2017.4771
21. Jurisic V, Radenkovic S, Konjevic G. The actual role of LDH as tumor marker, biochemical and clinical aspects. Adv Exp Med Biol. (2015) 867:115–24. doi: 10.1007/978–94-017–7215-0_8
22. Sharma D, Singh M, Rani R. Role of LDH in tumor glycolysis: Regulation of LDHA by small molecules for cancer therapeutics. Semin Cancer Biol. (2022) 87:184–95. doi: 10.1016/j.semcancer.2022.11.007
23. Zhang Z, Li Y, Yan X, Song Q, Wang G, Hu Y, et al. Pretreatment lactate dehydrogenase may predict outcome of advanced non small-cell lung cancer patients treated with immune checkpoint inhibitors: A meta-analysis. Cancer Med. (2019) 8:1467–73. doi: 10.1002/cam4.2024
24. Adachi Y, Tamiya A, Taniguchi Y, Enomoto T, Azuma K, Kouno S, et al. Predictive factors for progression-free survival in non-small cell lung cancer patients receiving nivolumab based on performance status. Cancer Med. (2020) 9:1383–91. doi: 10.1002/cam4.2807
25. Ma T, Tang Y, Wang T, Yang Y, Zhang Y, Wang R, et al. Chronic pulmonary bacterial infection facilitates breast cancer lung metastasis by recruiting tumor-promoting MHCIIhi neutrophils. Signal Transduct Target Ther. (2023) 8:296. doi: 10.1038/s41392–023-01542–0
26. Xiong S, Dong L, Cheng L. Neutrophils in cancer carcinogenesis and metastasis. J Hematol Oncol. (2021) 14:173. doi: 10.1186/s13045-021-01187-y
Keywords: lung squamous cell carcinoma, PD-1 inhibitor, chemotherapy, endostatin, lung immune prognostic index
Citation: Lv C, Wu Y, Gu W, Du B, Yao N, Zhu Y, Zheng J, Hong Y and Lai J (2024) Efficacy and safety of PD-1 inhibitors plus chemotherapy with or without endostatin for stage IV lung squamous cancer: a retrospective study. Front. Immunol. 15:1413204. doi: 10.3389/fimmu.2024.1413204
Received: 06 April 2024; Accepted: 24 May 2024;
Published: 07 June 2024.
Edited by:
Subhadeep Roy, National Institute of Pharmaceutical Education and Research, IndiaReviewed by:
Chandan Mandal, Chittaranjan National Cancer Institute (CNCI), IndiaMayank Kumar, Columbia University, United States
Copyright © 2024 Lv, Wu, Gu, Du, Yao, Zhu, Zheng, Hong and Lai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinhuo Lai, bGFpamluaHVvX2ZqeGhAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Chengliu Lv
Chengliu Lv Yahua Wu
Yahua Wu Weiwei Gu2
Weiwei Gu2 Jinhuo Lai
Jinhuo Lai