- 1Dalian Medical University, Dalian, China
- 2Department of Hepatobiliary and Pancreatic Surgery, Northern Jiangsu People’s Hospital Affiliated Yangzhou University, Yangzhou, China
Background: Immune cells play a crucial role in the development and progression of pancreatic cancer, yet the causal relationship remains uncertain due to complex immune microenvironments and conflicting research findings. Mendelian randomization (MR), this study aims to delineate the causal relationships between immune cells and pancreatic cancer while identifying intermediary factors.
Methods: The genome-wide association study (GWAS) data on immune cells, pancreatic cancer, and plasma metabolites are derived from public databases. In this investigation, inverse variance weighting (IVW) as the primary analytical approach to investigate the causal relationship between exposure and outcome. Furthermore, this study incorporates MR-Egger, simple mode, weighted median, and weighted mode as supplementary analytical approaches. To ensure the reliability of our findings, we further assessed horizontal pleiotropy and heterogeneity and evaluated the stability of MR results using the Leave-one-out method. In conclusion, this study employed mediation analysis to elucidate the potential mediating effects of plasma metabolites.
Results: Our investigation revealed a causal relationship between immune cells and pancreatic cancer, highlighting the pivotal roles of CD11c+ monocytes (odds ratio, ORIVW=1.105; 95% confidence interval, 95%CI: 1.002–1.218; P=0.045), HLA DR+ CD4+ antigen-presenting cells (ORIVW=0.920; 95%CI: 0.873–0.968; P=0.001), and HLA DR+ CD8br T cells (ORIVW=1.058; 95%CI: 1.002–1.117; P=0.041) in pancreatic cancer progression. Further mediation analysis indicated that oxalate (proportion of mediation effect in total effect: -11.6%, 95% CI: -89.7%, 66.6%) and the mannose to trans-4-hydroxyproline ratio (-19.4, 95% CI: -136%, 96.8%) partially mediate the relationship between HLA DR+ CD8br T cells and pancreatic cancer in nature. In addition, our analysis indicates that adrenate (-8.39%, 95% CI: -18.3%, 1.54%) plays a partial mediating role in the association between CD11c+ monocyte and pancreatic cancer, while cortisone (-26.6%, 95% CI: 138%, -84.8%) acts as a partial mediator between HLA DR+ CD4+ AC and pancreatic cancer.
Conclusion: This MR investigation provides evidence supporting the causal relationship between immune cell and pancreatic cancer, with plasma metabolites serving as mediators. Identifying immune cell phenotypes with potential causal effects on pancreatic cancer sheds light on its underlying mechanisms and suggests novel therapeutic targets.
Introduction
As a frequently encountered malignant neoplasm, pancreatic cancer is characterized by its high malignancy and dismal prognosis (1). Over the last three decades, there has been a consistent rise in the incidence of pancreatic cancer. Projections indicate that by 2040, the overall incidence of pancreatic cancer is expected to surge by 30%. Pancreatic cancer typically presents as pancreatic adenocarcinoma, characterized by a grim prognosis, with an overall 5-year relative survival rate of approximately 10% (2, 3). In recent years, the ongoing development of immunotherapy has somewhat prolonged the overall survival of patients with advanced pancreatic cancer. Nevertheless, the prognosis remains challenging due to the intricacies of the immune microenvironment (4). Typically, risk factors associated with pancreatic cancer encompass unhealthy lifestyle behaviors, chronic pancreatitis, and a familial history of the disease (5, 6). As mounting evidence suggests, immune dysregulation and remodeling of the immune microenvironment emerge as risk factors contributing to the onset and progression of pancreatic cancer (7–9). Therefore, precise identification of immune-related risk factors and thorough exploration of their association with pancreatic cancer is paramount.
The immune microenvironment encompasses the complex milieu surrounding tumors, comprising cells, cytokines, extracellular matrix, blood vessels, and other factors. It exerts crucial regulatory influence over tumor growth and progression (10). As a complex regulatory network predominantly comprised of immune cells, the immune microenvironment demonstrates a dichotomous role in both anti-tumor and pro-tumor activities (11–13). While the immune system can effectively eradicate tumor cells via tumor immunosurveillance, tumors eventually circumvent immune surveillance by orchestrating an immunosuppressive microenvironment (14). This underscores the crucial need to precisely identify potent anti-tumor immune cells within the immune microenvironment and restore their cytotoxic effects on tumors. Such efforts could significantly bolster the response rates to immunotherapy and foster the development of novel immunotherapeutic strategies. Currently, there have been no randomized controlled trials (RCTs) investigating the relationship between immune cells and pancreatic cancer. Consequently, the potential causal relationships between specific immune cells and pancreatic cancer remain unknown.
Besides the intricate immune microenvironment, the distinctive metabolic traits of pancreatic cancer play a crucial role in fostering tumor cell survival, proliferation, and metastasis (15, 16). Previous studies have indicated that metabolic reprogramming in pancreatic cancer could induce changes in immune cell phenotypes, thereby fostering the establishment of suppressive immune microenvironments (17). Moreover, with the progress of metabolomics, an expanding array of plasma metabolites are being confirmed to play crucial roles in the early diagnosis of pancreatic cancer (18, 19). Previous studies have indicated that alterations in tumor cell metabolism are partially driven by the recruitment of immune cells (20). For instance, research by Pham et al. demonstrated that macrophages in the tumor microenvironment are reprogrammed into a pro-tumor (M2) phenotype through the uptake of lactate, a byproduct of tumor cell glycolysis. This uptake further activates HIF-1α, leading to increased expression of arginase 1 (Arg1) (21). Additionally, Geiger et al. found that high levels of Arg1 in the tumor microenvironment are associated with lower anti-tumor activity (22). Subsequently, a growing body of research has revealed that aberrant metabolites or intermediates of cancer metabolism play a crucial role in regulating the proliferation, differentiation, activation, and function of immune cells (23–25). For instance, in a study by Shane et al., elevated levels of tetrahydrobiopterin (BH4) were found to enhance the responses of CD4 and CD8 T cells, thereby amplifying their antitumor activity in vivo. Furthermore, BH4 administration in mice markedly reduced tumor growth and increased the population of intra-tumoral effector T cells (23). In a comprehensive metabolome-wide association study (MWAS), Zhong et al. systematically investigated the associations between genetically predicted blood metabolite levels and PDAC risk (26). Utilizing a two-sample MR approach, they identified 44 unique plasma metabolites that exhibit significant associations with PDAC risk (27). These findings indicate a potential causal relationship between plasma metabolites and the development, progression, and immune cell infiltration of tumors. Plasma metabolites may serve as intermediaries, mediating the causal association between immune cells and pancreatic cancer.
Despite the scarcity of RCTs evaluating the influence of plasma metabolites on immune cells and pancreatic cancer, it remains imperative to investigate the potential effects of plasma metabolites on both immune cells and pancreatic cancer.
MR provides a robust approach to investigate the causal relationship between exposure and outcomes by leveraging genetic variation, thereby mitigating confounding effects and reverse causation (28–30). Hence, it is apparent that the MR approach shares a conceptual resemblance with RCTs, yet it boasts broader applicability and superior cost-effectiveness in practical implementation. To probe the potential causal link between immune cells and pancreatic cancer, as well as the intermediary role of plasma metabolites, we devised a “two-step” MR analysis. This approach delves into the causal nexus between immune cells and pancreatic cancer, along with the mediating influence of plasma metabolites. Our research offers a fresh perspective on the mechanisms driving the onset and progression of pancreatic cancer, potentially paving the way for innovative metabolic intervention strategies.
Materials and methods
Research design
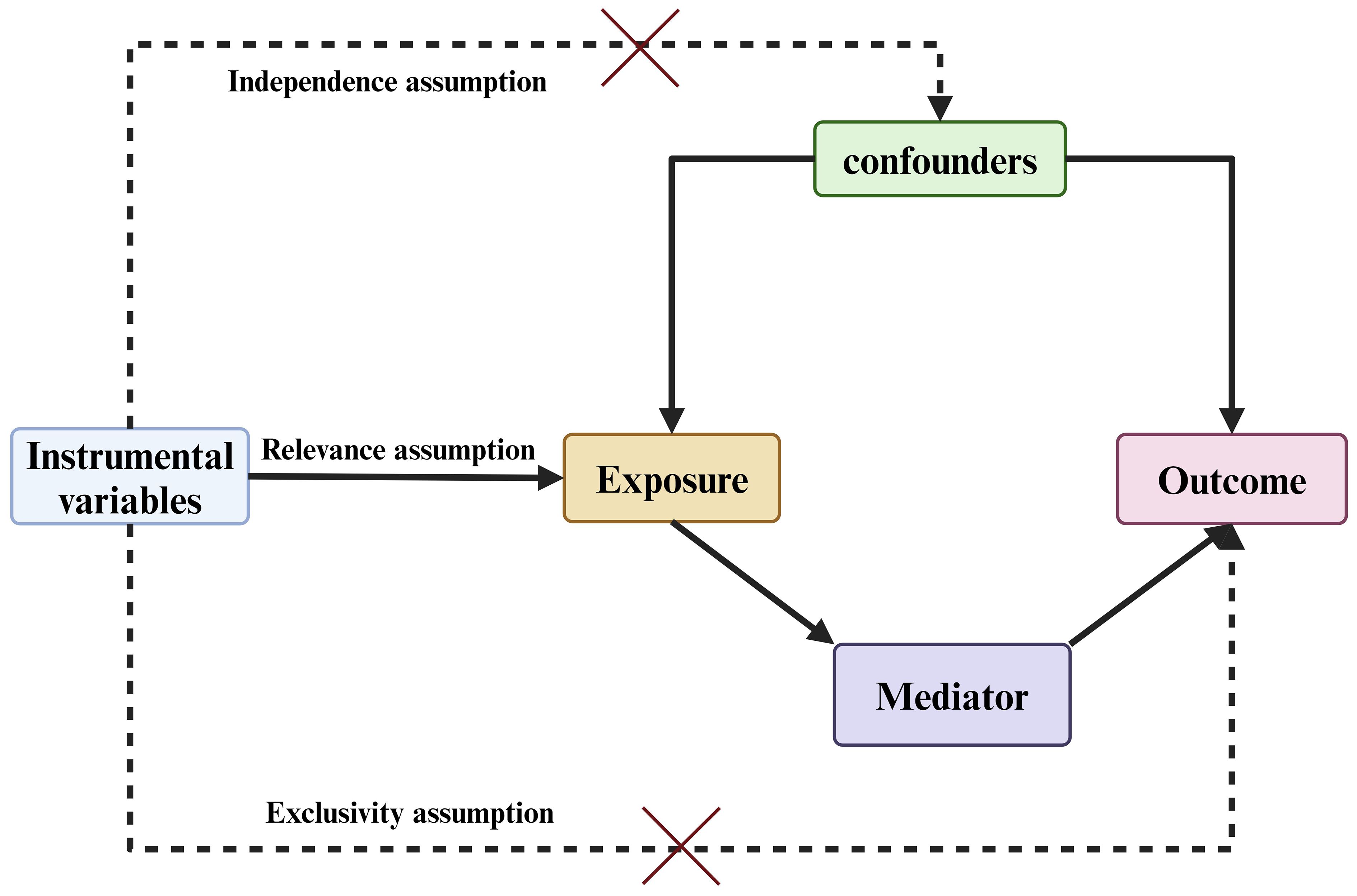
Based on bidirectional MR analysis using two independent samples and mediation analysis, this study investigated the causal relationship between 731 immune cell phenotypes and pancreatic cancer, and explored the potential modulation of this relationship by circulating metabolites. (Figure 1) At the outset, pancreatic cancer was designated as the primary outcome, while 731 immune cell phenotypes were identified as potential exposures, enabling an investigation into the comprehensive causal relationship between immune cell phenotypes and pancreatic cancer. Following this, we examined the respective proportions of causative links between 1400 plasma metabolites, acting as potential mediators, and the connections between pancreatic cancer and immune cell phenotypes. The instrumental variables (IV) employed in this investigation must satisfy the following criteria: 1. They must exhibit a strong correlation with the exposure factors. 2. The selected instrumental variables remain unaffected by confounding factors, and 3. They demonstrate a distinct relationship with the outcome event (29). (Figure 2) Furthermore, the GWAS data employed in this investigation were sourced from publicly available datasets and received approval from the respective institutional review boards in their studies.
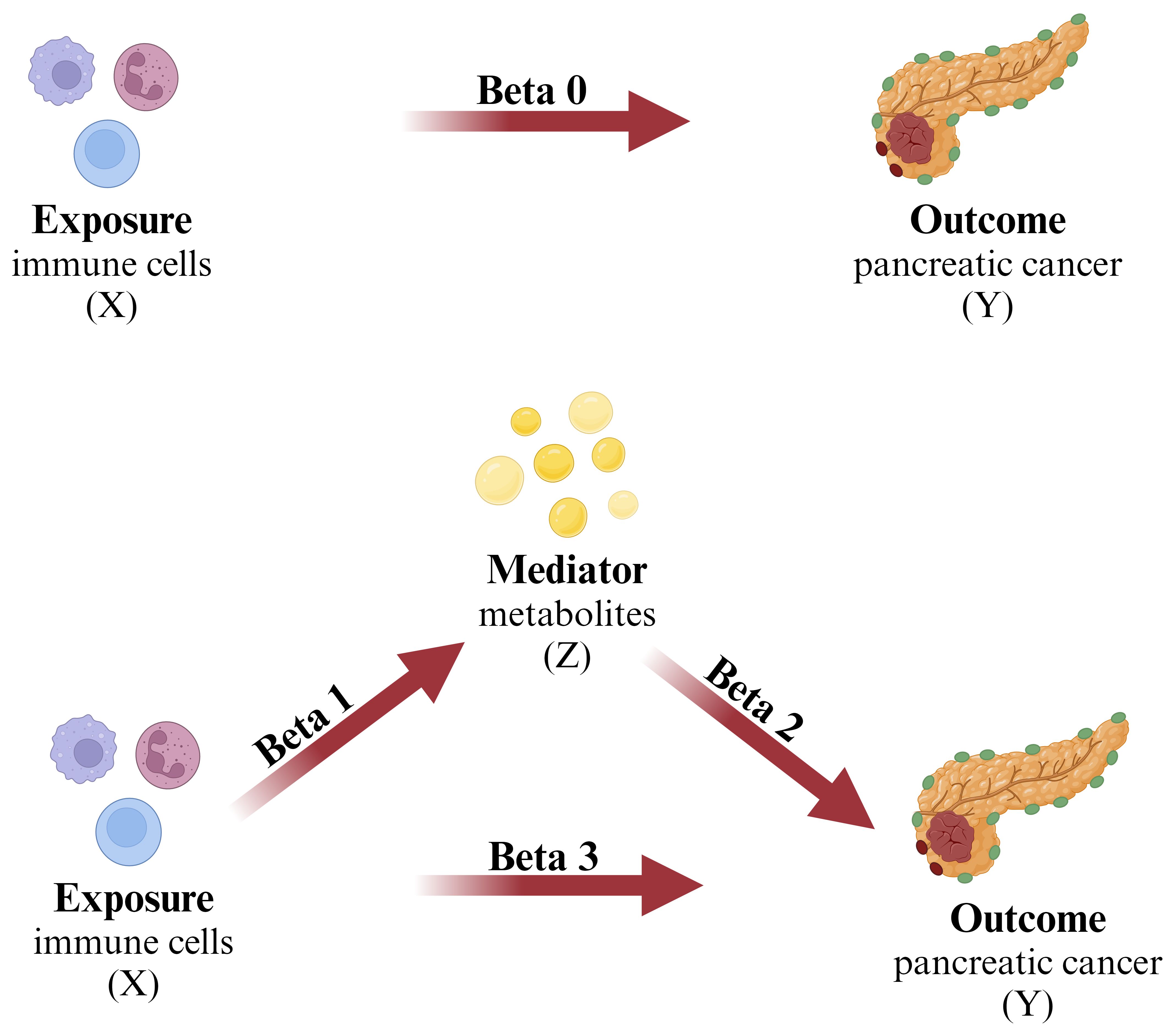
Figure 1 The mediation analysis X (exposure): immune cell; Y (outcome): pancreatic cancer; Z (mediator): plasma metabolites.
Source of data
The origin of the pancreatic cancer GWAS dataset
The statistical summary data for pancreatic cancer GWAS originates from the FinnGen consortium (https://www.finngen.fi/en), a nationwide longitudinal cohort study that encompasses genetic and electronic health record data. We incorporated GWAS data from 1626 pancreatic cancer patients, in accordance with the ICD-10 diagnostic criteria, sourced from the R10 version of FinnGen.
The GWAS data for immune cell phenotypes
The GWAS data on immune cell phenotypes were obtained from a cohort study involving 3,757 individuals of European ancestry (accession numbers from ebi-a-GCST0001391 to ebi-a-GCST0002121) (31). The research investigated the correlation between 731 immune cell phenotypes, covering B cells, dendritic cells, various stages of T cells, monocytes, NK cells, and regulatory T cells. These phenotypes included 118 absolute cell counts (AC), 389 median fluorescence intensities (MFI) reflecting surface antigen levels, 32 morphological parameters (MP), and 192 relative cell counts (RC), and their associations with 22 million single nucleotide polymorphisms (SNPs).
The GWAS data for plasma metabolites
The plasma metabolite GWAS dataset was obtained from the Canadian Longitudinal Study on Aging (CLSA), a cohort study involving 8299 participants (32). In this study, a total of 1458 metabolites were quantified with the Metabolon HD4 platform and were then batch-normalized. Only those with missing measurements in fewer than 50% of samples () were retained (32). Furthermore, the study utilized the Human Metabolome Database (HMDB) to identify 309 pairs of metabolites that share enzymes or transporters () (32, 33). The ratio for each metabolite pair was then calculated by dividing the batch-normalized measurement value of one metabolite by the measurement value of the other metabolite within the same individual. Finally, a total of 1400 unique metabolites were evaluated in this study.
Mendelian randomization analysis
IVs screening
The assumptions regarding IVs entail a substantial correlation with the exposure factor, while maintaining independence from the outcome variable. Moreover, only the exposure factor is presumed to have a direct impact on the outcome. Therefore, in order to screen for IVs associated with the exposure factor, we identified SNPs linked to the exposure factor, with a significance level set at . Additionally, we further removed linkage disequilibrium (LD) effects for each SNP based on and an interval of 10,000 kb (34, 35). Furthermore, to ensure the strong correlation between IVs and exposure factors, this study employed F-statistics to assess the strength of IVs. A causal inference was considered to have significant bias when the F-value was less than 10 (36). Finally, to control for the impact of confounding factors on instrumental variables, this study employed the Phenoscanner and R software packages, setting a to eliminate palindromic SNPs and incompatible SNPs. This process excluded closely related to the occurrence of pancreatic cancer [BMI, smoking, drinking, diabetes, and pancreatitis, etc (37).] ensuring that the resulting instrumental variables were suitable for subsequent analysis.
MR analysis
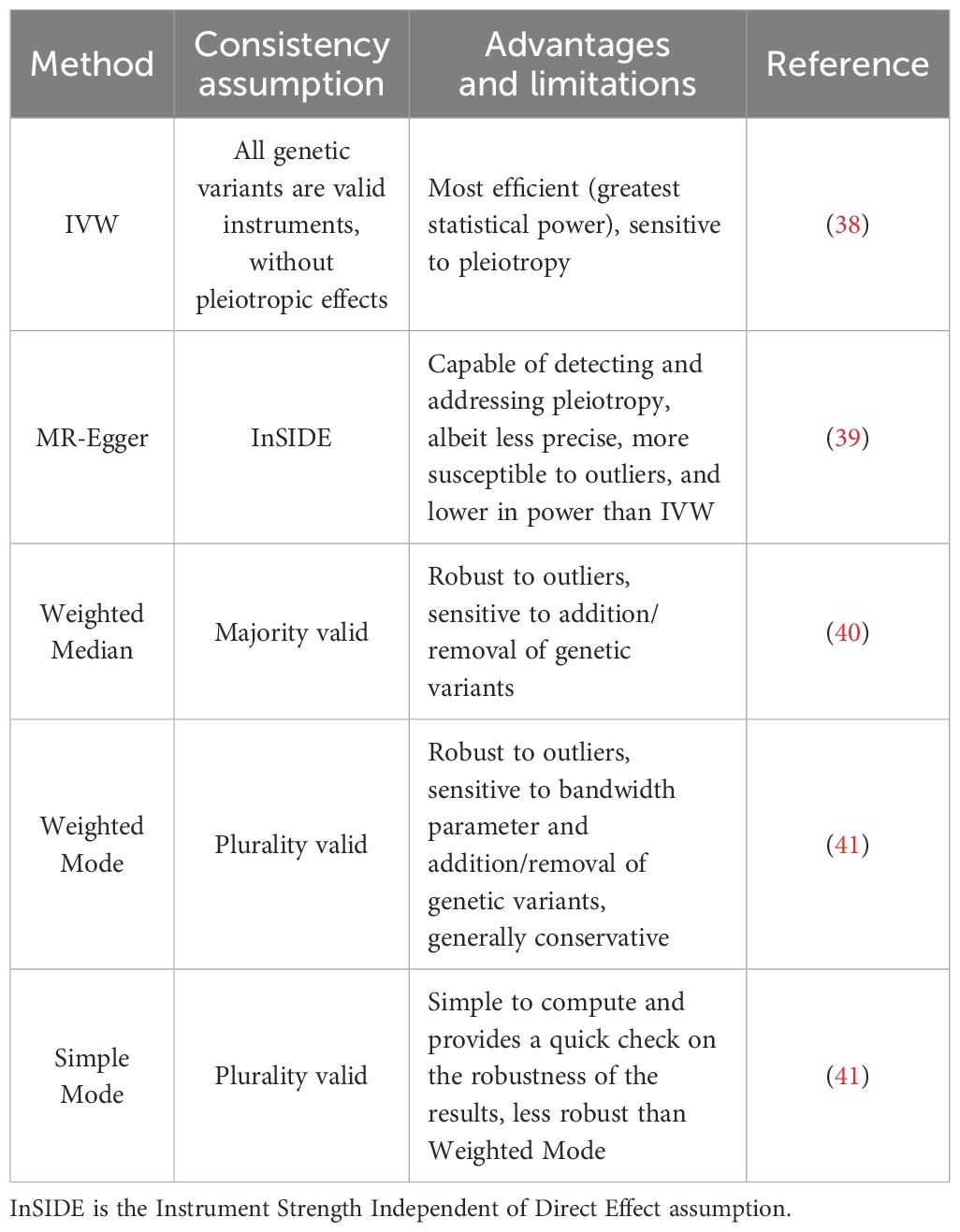
To investigate the causal effects of exposure factors on outcome events and ensure robust results, we conducted a two-sample MR analysis using five different methods: IVW, Weighted Median, Simple Mode, Weighted Mode, and MR-Egger. The respective advantages and limitations of these methods are summarized in Table 1.
The assessment of heterogeneity and horizontal pleiotropy
To maintain the validity of the independence and exclusivity assumptions, it is essential to ensure that IVs do not affect the outcome through factors unrelated to the exposure variable. Employing the MR-Egger intercept test to assess horizontal pleiotropy and ensure the robustness of the research findings. When the P-value of the MR-Egger intercept is less than 0.05, horizontal pleiotropy is deemed to be present; conversely, if the P-value is greater than 0.05, horizontal pleiotropy is considered to be absent (42). Employing Cochran’s Q statistic along with its corresponding P-value provides a quantitative evaluation of the heterogeneity among the selected IVs (43). In a final step, we conducted a sensitivity analysis using the “leave-one-out” approach to assess the impact of each SNP on the outcomes of the MR analysis (44). Statistical analysis and visualization of the results were conducted using the R software packages “TwoSample MR”, “Mendelian-Randomization”, and “ggplot2”.
Analysis of the overall causal effect
To obtain the overall causal effect between immune cell phenotypes and pancreatic cancer, we executed a bidirectional two-sample MR analysis encompassing 731 immune cell phenotypes and their association with pancreatic cancer. All analyses were performed using R version 4.3.1 software (http://www.Rproject.org). In the absence of considering heterogeneity and horizontal pleiotropy, we employed the “Mendelian Randomization” R package to preliminarily evaluate the causal relationship between 731 immune cell phenotypes and pancreatic cancer using IVW (45). Moreover, this study employed MR-Egger regression analysis and weighted median-based analysis to further assess the reliability of the study findings (40, 46).
Mediation analysis
Using a two-step MR approach, this study conducted mediation analysis to explore whether plasma metabolites mediate the causal pathway from immune cell phenotypes to pancreatic cancer outcomes. The overall effect of immune cell phenotypes on pancreatic cancer can be decomposed into the direct effect of immune cell phenotypes on pancreatic cancer and the indirect effect mediated by intermediates (47). In the first step of analysis, we computed the causal impact (Beta1) of immune cell phenotypes on the mediators. Following this, in the second step of analysis, we evaluated the causal impact (Beta2) of the mediators on pancreatic cancer. The proportion of mediated effect in the total effect was then obtained by calculating . The direct impact of the exposure on the outcome is represented as .
Results
Exploring the overall causal impact of immune cell phenotypes on pancreatic cancer
MR analysis
In order to investigate the overall causal impact of immune cell phenotypes on pancreatic cancer, we conducted two-sample MR analyses using five methods: IVW, weighted median, simple mode, weighted mode, and MR Egger. Subsequently, we employed a threshold of derived from the IVW method to detect 27 immune cell phenotypes causally associated with pancreatic cancer. Among these, 16 immune cell phenotypes, including CD11c+ monocyte % monocyte, are regarded as risk factors for pancreatic cancer (Supplementary Figure 1), while 11 immune cell phenotypes, such as CD4 Treg AC are regarded as protective factors for pancreatic cancer (Supplementary Figure 2). The summarized results of the MR analysis are provided in Supplementary Tables 1, 2.
Analysis for horizontal pleiotropy and heterogeneity
To mitigate potential heterogeneity stemming from varying analysis platforms, experiments, populations, and other factors that may affect the results of MR analysis.
We evaluated heterogeneity using both the IVW and MR-Egger test methods, with indicating the presence of heterogeneity in the study (Supplementary Table 4). The results revealed that none of the intercepts from MR-Egger and IVW for the 27 immune cell phenotypes mentioned above were not statistically significant, indicating that the findings remained unaffected by any potential bias stemming from heterogeneity (Supplementary Figure 3). Furthermore, in order to mitigate potential confounding effects of IVs on outcomes via pathways beyond exposure, thereby safeguarding the assumptions of independence and exclusivity, we additionally employed the MR-Egger intercept method to assess the presence of horizontal pleiotropy in the data. The study findings indicate that the MR-Egger intercept for CX3CR1 on CD14- CD16- () exhibited horizontal pleiotropy and was consequently excluded from subsequent analyses (Supplementary Table 3). The MR-Egger intercepts for the remaining 26 immune cell phenotypes () were not statistically significant, suggesting an absence of genetic pleiotropy bias. Through leave-one-out sensitivity analysis, the study results were further validated, revealing no indication of SNPs impacting the overall causal relationship (Supplementary Figure 4).
Identifying immune cell phenotypes associated with pancreatic cancer
Based on the study findings, in order to identify immune cell phenotypes associated with pancreatic cancer for further analysis, we initially screened for immune cell phenotypes showing consistent directional effects () across five different MR analysis methods. This screening process resulted in the identification of 22 immune cell phenotypes (Supplementary Figure 5). Subsequently, we conducted reverse MR analysis on the 22 immune cell phenotypes in relation to pancreatic cancer, excluding those with reverse causal effects (Supplementary Table 5). Ultimately, we identified 20 immune cell phenotypes associated with pancreatic cancer for further mediation analysis.
Mediating effects of plasma metabolites on immune cell−pancreatic cancer risk
To investigate the mediating effect of plasma metabolites on the association between immune cell and pancreatic cancer, with the aim of elucidating the underlying mechanisms by which immune cells influence pancreatic cancer. Based on two-step MR analysis, we first performed two-sample MR analysis on 1400 plasma metabolites with pancreatic cancer and then, plasma metabolites with consistent OR values were selected as pancreatic cancer-associated plasma metabolites for further analysis (Supplementary Table 6).
In our investigation, we first examined the mediating effect (Beta1) of immune cell phenotype on plasma metabolites (Supplementary Table 7). Subsequently, employing the same methodology, we analyzed the mediating effect (Beta2) of plasma metabolites on pancreatic cancer (Supplementary Table 8). Ultimately, our analysis identified four pairs of plasma metabolites that serve as mediators in the relationship between immune cell phenotype and pancreatic cancer (Figure 3).
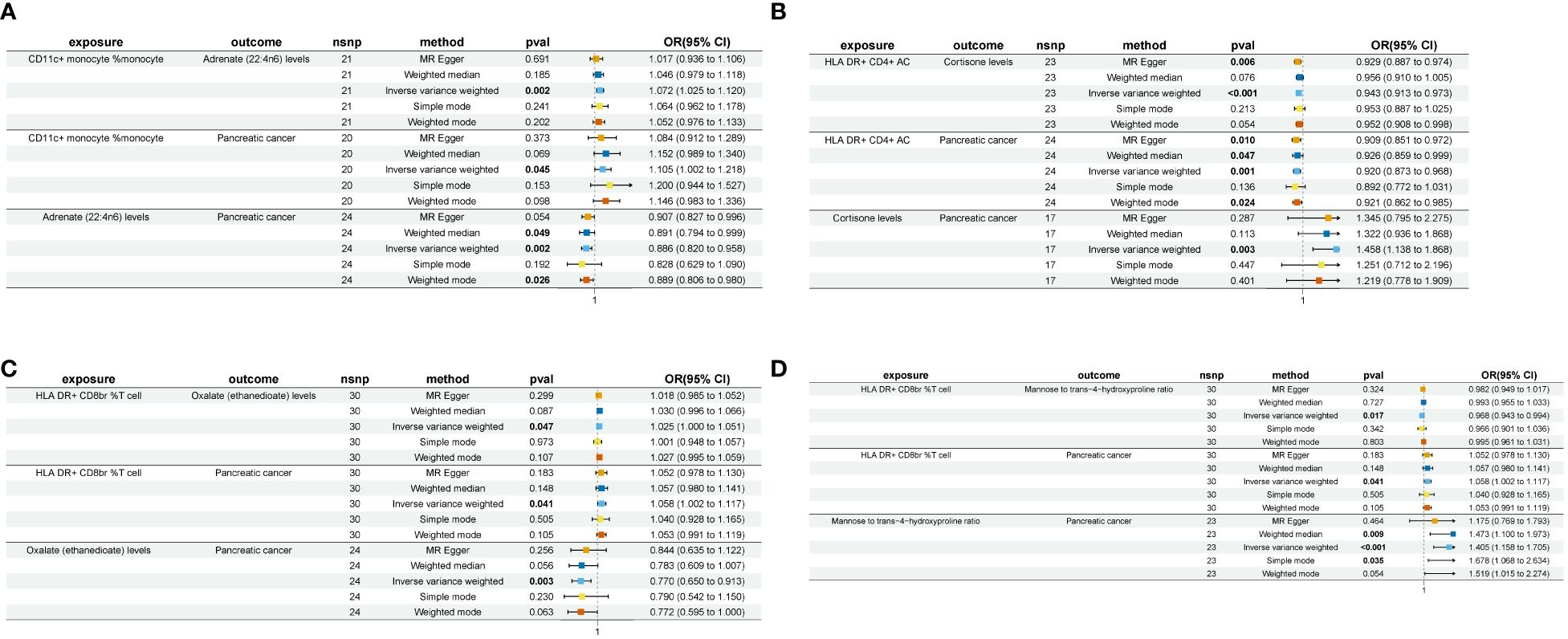
Figure 3 A forest plot of the four sets of plasma metabolites exhibiting “immune cell phenotype - pancreatic cancer” mediating effects nsnp, number of single nucleotide polymorphism; pval, p-value; OR, odds ratio. (A) CD11c+ monocyte % monocyte-Adrenate (22:4n6)-Pancreatic cancer. (B) HLA DR+ CD4+ AC-Cortisone-Pancreatic cancer. (C) HLA DR+ CD8br %T cell-Oxalate (ethanedioate)-Pancreatic cancer. (D) HLA DR+ CD8br %T cell-Mannose to trans-4-hydroxyproline ratio-Pancreatic cancer.
The levels of adrenate (22:4n6) were found to negatively modulate pancreatic cancer concerning CD11c+ monocyte %monocyte (Mediated effect, ME=-0.00837; Mediated proportion, MP=-8.39%) (Table 2). Oxalate (ethanedioate) levels exhibited a negative regulatory effect on pancreatic cancer with respect to HLA DR+ CD8br %T cell (ME=-0.00655; MP=-11.6%) (Table 2). Cortisone levels negatively regulated pancreatic cancer in conjunction with HLA DR+ CD4+ AC (ME=-0.0223; MP=-26.6%) (Table 2). Lastly, the mannose to trans-4-hydroxyproline ratio negatively influenced pancreatic cancer in association with HLA DR+ CD8br %T cell (ME=-0.011; MP=-19.4%) (Table 2).
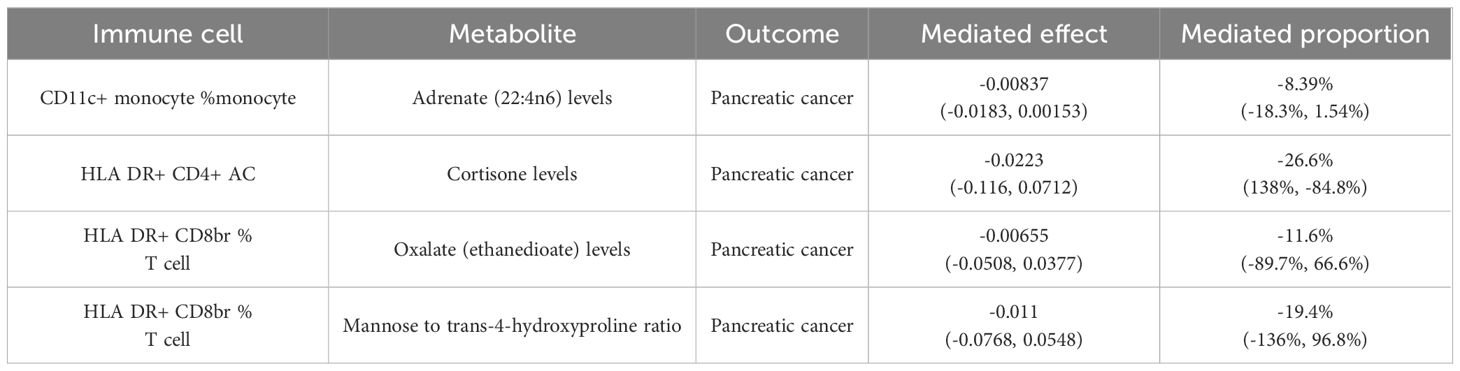
Table 2 Four pairs of plasma metabolites with “immune cell phenotype - pancreatic cancer” mediating effects.
Discussion
Pancreatic cancer, characterized by its high malignancy, aggressive biological behavior, and poor prognosis, presents a persistent threat to global health (2, 48). Immune cells play a crucial role in the initiation and progression of pancreatic cancer (49). Despite the growing interest in immune cells and immunotherapy in pancreatic cancer research, the intricate dynamics between immune cells and pancreatic cancer remain largely unexplored. Metabolic reprogramming, as a pivotal biological phenomenon in tumor tissue, exerts a profound influence on tumor cell growth, proliferation, and survival (50).
Emerging evidence suggests that intricate metabolic alterations within the tumor microenvironment activate immune cells via diverse intrinsic or extrinsic pathways. These complex interactions are pivotal in driving multiple phenotypes such as tumor proliferation, migration, and drug resistance (51–53). Therefore, the reciprocal interplay between immune cells and metabolism emerges as an intriguing topic warranting in-depth exploration. Nevertheless, due to the relatively small number of pancreatic cancer samples available, acquiring high-quality tissue specimens remains a formidable challenge. Furthermore, the complexity of metabolic products in vivo imposes limitations on the depth and breadth of research endeavors. Moreover, the absence of standardized criteria for investigating immune cell phenotypes has contributed to challenges in comparing and verifying findings. Therefore, in order to clarify whether immune cells impact the development of pancreatic cancer by mediating endogenous metabolites, we employed a two-step MR analysis to investigate whether plasma metabolites act as mediating factors affecting the causal relationship between immune cells and pancreatic cancer. The outcomes of our investigation reveal a causal relationship between immune cells and pancreatic cancer. Furthermore, mediation analysis provides additional evidence supporting the mediating effect of plasma metabolites in the causal relationship between immune cells and pancreatic cancer. This discovery may provide fresh insights into the mechanisms driving the onset and progression of pancreatic cancer, thereby offering novel intervention targets for therapeutic strategies.
Adrenate (22:4n6) is found to negatively mediate the causal relationship between CD11c+ monocyte and pancreatic cancer (ME=-0.00837, MP=-8.39%) (Table 2). Recent investigations have revealed the significant involvement of CD11c+ monocytes in the early metastatic process of pancreatic cancer (54), aligning with our own research findings (ORIVW=1.105, 95%CI=1.002–1.218) (Figure 3A). Adrenate (22:4n6), a 22-carbon, 4-double-bond ω-6 fatty acid also known as adrenic acid, plays a crucial biological role in the human body. It contributes to various essential functions, including the structural and functional integrity of cellular membranes, cell signaling processes, and the regulation of inflammation (55). Research findings indicate that its peroxidation plays a critical role in regulating the sensitivity of various drug-resistant tumor cells to ferroptosis (56, 57). The observations indicate that adrenate (22:4n6) exhibits anti-tumor effects, consistent with the outcomes of our study (ORIVW=0.886, 95%CI=0.820–0.958) (Figure 3A). In a study of murine peritonitis, adrenate (22:4n6) was found to suppress leukotriene B4 (LBT4) synthesis in neutrophils and promote their apoptosis, thereby facilitating phagocytosis by monocyte-derived macrophages and ultimately exerting an anti-inflammatory effect (58). The findings above suggest that adrenate (22:4n6) may act as a mediator, mitigating local inflammatory responses within the tumor microenvironment by inhibiting leukotriene synthesis in immune cells, thereby modulating immune cell effects on pancreatic cancer.
HLA-DR, a key component of the major histocompatibility complex (MHC) class II molecules, is commonly found on specialized antigen-presenting cells. It binds external peptides and delivers them to antigen-specific CD4+ helper (Th) T lymphocytes (59). Research findings suggest that HLA-DR+ CD4+ T lymphocytes play a critical role as activated T cells in anti-tumor immune responses (60). They regulate and enhance the activities of other immune cells while also directly targeting tumor cells for cytotoxic effects (61). Our study supports this viewpoint, suggesting an inverse correlation between HLA-DR+ CD4+ antigen-presenting cells and pancreatic cancer (ORIVW=0.920, 95%CI=0.873–0.968) (Figure 3B). Further mediation analysis revealed that cortisone exerts a negative mediating effect (ME=-0.0223, MP=-26.6%) (Table 2). Cortisone, a natural glucocorticoid present in the human body, plays a critical role in maintaining metabolic balance, regulating cell growth, and modulating inflammatory responses (62). Research has uncovered that cortisone binding to glucocorticoid receptors (GR) in pancreatic cancer elicits tumor proliferation, metastasis, and gemcitabine resistance via diverse signaling cascades (63). Cortisol, the most representative hormone among glucocorticoids, is commonly used to treat inflammatory diseases. Traditionally, exogenous glucocorticoids (GCs) have been widely recognized for their immunosuppressive effects (62). However, recent studies on endogenous GCs have revealed that they can both promote and inhibit T cell immunity (64). For instance, current research indicates that endogenous GCs induce differential T cell differentiation to maintain a balance, thereby preventing excessive immune responses (65). The results reported in the previous study are consistent with our own findings (ORIVW=1.458, 95%CI=1.138–1.868) (Figure 3B), indicating that cortisone acts as a mediator, attenuating the impact of HLA DR+ CD4+ T lymphocytes on pancreatic cancer.
HLA DR+ CD8br T cells are commonly acknowledged as activated cytotoxic T lymphocytes (CTLs), with the conventional viewpoint asserting their significance as a key element in cellular immunity, playing a crucial role in the antitumor process (66). Nevertheless, recent investigations have revealed that excessive activation of CTLs could foster the development of an immunosuppressive microenvironment. In Yang et al.’s investigation, it was observed that in lung adenocarcinoma, hyperactivated CTLs induce tumor cells to produce prostaglandin E2 (PGE2) and recruit myeloid-derived suppressor cells (MDSCs) via the Fas/FasL signaling axis, thereby fostering tumor immune evasion (67). The above viewpoint is consistent with the findings of this study (ORIVW=1.058, 95%CI=1.002–1.117) (Figures 3C, 3D). Further mediation analysis revealed the participation of two plasma metabolites in mediating the causal effect of HLA DR+ CD8br T cells on pancreatic cancer. Intracellular accumulation of oxalate, a passive metabolite synthesized by various cells (68), has been found to activate nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, thereby inducing reactive oxygen species (ROS)-mediated oxidative stress (OS) and subsequent tissue damage (69, 70). In light of OS emerging as a pivotal biological phenomenon in tumor progression, the dual therapeutic strategy aimed at modulating the redox status (i.e., pro-oxidant therapy and/or antioxidant therapy) is gaining increasing attention (71, 72). OS acts as a double-edged sword in tumor biology. On one hand, it promotes malignant behaviors in tumors, while on the other hand, it triggers cytotoxic OS within tumor cells, which can be exploited for therapeutic purposes (73, 74). For instance, commonly used chemotherapy drugs like doxorubicin, cisplatin, and 5-fluorouracil can either directly or indirectly enhance the accumulation of ROS, leading to tumor cell death (75, 76). A cohort study on dialysis patients revealed a significant elevation in IL-16 levels, which correlated with plasma oxalate concentrations (77). Similarly, research on colorectal cancer indicated that high IL-16 levels inhibited the cytotoxicity of CD8+ T cells in the immune microenvironment (78). These findings suggest that plasma oxalate may impair CD8+ T cell cytotoxicity through the upregulation of cytokines like IL-16.
In summary, oxalate might trigger apoptosis in tumor cells through OS, consequently attenuating the positive causal relationship between HLA DR+ CD8br T cells and pancreatic cancer.
Intracellular uptake of mannose, a natural C-2 epimer of glucose, occurs via facilitated diffusion through glucose transporters (GLUTs) situated on the cell membrane (79). Subsequently, within the cell, mannose undergoes enzymatic conversion catalyzed by phosphomannose isomerase (MPI) to yield fructose-6-phosphate, thereby entering the glycolytic pathway (80). However, the limited endogenous levels of mannose result in minimal energy-providing capacity. In recent years, research has shown that the combination of mannose with conventional chemotherapy impacts the levels of Bcl-2 family anti-apoptotic proteins, resulting in increased sensitivity of cells to chemotherapy drugs (81). In addition to its direct anti-tumor effects, mannose also possesses immunomodulatory properties. In a study conducted by Zhang et al., it was found that increasing the dosage of mannose in the drinking water of mice suppressed immune cell infiltration in autoimmune diabetes and airway inflammation, while enhancing the infiltration of regulatory T cells (Tregs) (82). Further in vitro experiments confirmed that mannose stimulates the differentiation of Tregs in both humans and mice by promoting the activation of TGF-β (82, 83). In a separate investigation focusing on mannose, it was revealed that supra-physiological concentrations of mannose can inhibit macrophage activation (84). From this perspective, mannose not only exerts direct anti-tumor effects but also demonstrates negative immunomodulatory effects.
Hydroxyproline, an essential structural amino acid in the body, plays a crucial role in collagen synthesis. Studies have indicated that an increase in hydroxyproline concentration in the blood promotes the distant metastasis of pancreatic cancer (85). Moreover, studies have demonstrated that hydroxyproline enhances IFN-γ levels, thereby promoting the expression of PD-L1 and inhibiting tumor autophagy (86).
The specific role of trans-4-hydroxyproline, a variant of hydroxyproline, in cancer pathogenesis remains largely elusive. A large-scale prospective study on plasma metabolites in prostate cancer identified a significant association between elevated plasma levels of trans-4-hydroxyproline and heightened risk of prostate cancer (87). In summary, further investigations are needed to fully elucidate the specific roles of mannose and trans-4-hydroxyproline in tumorigenesis. Our study highlights the mannose to trans-4-hydroxyproline ratio as a risk factor, exerting a negative regulatory effect on HLA DR+ CD8br % T cell responses in pancreatic cancer (ME=-0.011, MP=-19.4%) (Table 2).
One of the major strengths of our study lies in the application of MR, which employs SNPs as IVs to analyze the relationship between exposure and outcome. To our knowledge, this study represents the first attempt to employ MR analysis using the most recent and comprehensive GWAS data to investigate the causal relationship between immune cells and pancreatic cancer. Compared to RCTs, MR helps mitigate confounding factors and prevents interference from reverse causality. We employ two-steps MR to investigate the linear relationship between exposure and outcome, and employ mediation analysis to explore potential non-linear associations of plasma metabolites. Furthermore, the utilization of data samples derived from the identical population minimizes biases originating from population heterogeneity. Conducting multiple sensitivity analyses enhances the resilience and dependability of the outcomes. Nevertheless, our study still faces several noteworthy limitations. Firstly, the bulk of statistical data in GWAS originates from individuals of European descent, limiting the generalizability of our findings to diverse ethnic populations. Consequently, additional research is needed to extend our results to other racial groups. Secondly, regarding the selection of outcome GWAS data, this study utilized GWAS data from FinnGen. Although the FinnGen dataset is smaller in scale compared to the larger GWAS datasets from the Pancreatic Cancer Cohort Consortium (PanScan) and the Pancreatic Cancer Case-Control Consortium (PanC4), FinnGen, which commenced in the fall of 2017, offers an advantage in terms of data timeliness (88). Additionally, Zhong et al. have already analyzed the association of plasma metabolites with pancreatic cancer using PanScan and PanC4 data (26, 27). Our study aims to explore different GWAS datasets to identify new potential mediators, thereby providing complementary insights and enhancing the robustness of our findings. Thirdly, the causal relationship between immune cells and pancreatic cancer is influenced by numerous factors, and there may still be unidentified confounding variables between exposure and outcome, which could introduce bias into our results.
Conclusion
In summary, this study presents genetic evidence of the causal relationship between immune cells and pancreatic cancer through MR analysis. In our investigation, we found that higher levels of HLA-DR+ CD4+ T lymphocytes were associated with a decreased risk of pancreatic cancer. Notably, cortisone was found to significantly modulate this effect, explaining 26.6% of the association. Furthermore, the investigation revealed a direct association between HLA DR+ CD8 T cells and pancreatic cancer. Notably, the mannose to trans-4-hydroxyproline ratio emerged as a key mediator, explaining 19.4% of the total effect. Additionally, oxalate showed significant mediation, accounting for 11.6% of the total effect. In essence, higher levels of HLA DR+ CD8 T cells corresponded to an increased risk of pancreatic cancer, with the mannose to trans-4-hydroxyproline ratio and oxalate serving as intermediaries. In addition, the findings revealed a positive correlation between CD11c+ monocytes and pancreatic cancer incidence, with adrenate serving as a mediator, accounting for 8.39% of the effect. In conclusion, the findings from the above study may offer fresh insights into the mechanisms underlying the initiation and advancement of pancreatic cancer, thus presenting novel metabolic intervention targets for therapeutic strategies.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: https://www.finngen.fi/en.
Ethics statement
Ethical approval was deemed unnecessary for the human studies as the original studies had undergone ethical review and approval, as documented in their respective publications. Informed consent was obtained from all participants involved in the original genome-wide association studies. The studies were conducted in accordance with local legislation and institutional requirements. Participants provided written informed consent to participate in this study.
Author contributions
KZ: Writing – original draft, Writing – review & editing. JZ: Conceptualization, Data curation, Investigation, Validation, Writing – review & editing. PW: Writing – review & editing. YC: Writing – review & editing. ZW: Writing – review & editing. XG: Writing – review & editing. JW: Writing – review & editing. LC: Writing – review & editing. YL: Writing – review & editing. PX: Conceptualization, Methodology, Writing – review & editing. JY: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors express their sincere gratitude to the researchers who generously provided the original dataset used in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1402113/full#supplementary-material
References
1. Kamisawa T, Wood LD, Itoi T, Takaori K. Pancreatic cancer. Lancet. (2016) 388:73–85. doi: 10.1016/s0140–6736(16)00141–0
2. Cai J, Chen H, Lu M, Zhang Y, Lu B, You L, et al. Advances in the epidemiology of pancreatic cancer: trends, risk factors, screening, and prognosis. Cancer Lett. (2021) 520:1–11. doi: 10.1016/j.canlet.2021.06.027
3. Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA: A Cancer J Clin. (2023) 73:17–48. doi: 10.3322/caac.21763
4. Morrison AH, Byrne KT, Vonderheide RH. Immunotherapy and prevention of pancreatic cancer. Trends Cancer. (2018) 4:418–28. doi: 10.1016/j.trecan.2018.04.001
5. Yang J, Xu R, Wang C, Qiu J, Ren B, You L. Early screening and diagnosis strategies of pancreatic cancer: A comprehensive review. Cancer Commun. (2021) 41:1257–74. doi: 10.1002/cac2.12204
6. Yuan C, Kim J, Wang QL, Lee AA, Babic A, Amundadottir LT, et al. The age-dependent association of risk factors with pancreatic cancer. Ann Oncol. (2022) 33:693–701. doi: 10.1016/j.annonc.2022.03.276
7. Hosein AN, Dangol G, Okumura T, Roszik J, Rajapakshe K, Siemann M, et al. Loss of rnf43 accelerates kras-mediated neoplasia and remodels the tumor immune microenvironment in pancreatic adenocarcinoma. Gastroenterology. (2022) 162:1303–18.e18. doi: 10.1053/j.gastro.2021.12.273
8. Huber M, Brehm CU, Gress TM, Buchholz M, Alashkar Alhamwe B, von Strandmann E, et al. The immune microenvironment in pancreatic cancer. Int J Mol Sci. (2020) 21(19):7307. doi: 10.3390/ijms21197307
9. Yousuf S, Qiu M, Voith von Voithenberg L, Hulkkonen J, Macinkovic I, Schulz AR, et al. Spatially resolved multi-omics single-cell analyses inform mechanisms of immune dysfunction in pancreatic cancer. Gastroenterology. (2023) 165:891–908.e14. doi: 10.1053/j.gastro.2023.05.036
10. Lv B, Wang Y, Ma D, Cheng W, Liu J, Yong T, et al. Immunotherapy: reshape the tumor immune microenvironment. Front Immunol. (2022) 13:844142. doi: 10.3389/fimmu.2022.844142
11. Kemp SB, Pasca di Magliano M, Crawford HC. Myeloid cell mediated immune suppression in pancreatic cancer. Cell Mol Gastroenterol Hepatol. (2021) 12:1531–42. doi: 10.1016/j.jcmgh.2021.07.006
12. Mahadevan KK, McAndrews KM, LeBleu VS, Yang S, Lyu H, Li B, et al. Krasg12d inhibition reprograms the microenvironment of early and advanced pancreatic cancer to promote fas-mediated killing by cd8+ T cells. Cancer Cell. (2023) 41:1606–20.e8. doi: 10.1016/j.ccell.2023.07.002
13. Zhang Y, Lazarus J, Steele NG, Yan W, Lee H-J, Nwosu ZC, et al. Regulatory T-cell depletion alters the tumor microenvironment and accelerates pancreatic carcinogenesis. Cancer Discovery. (2020) 10:422–39. doi: 10.1158/2159–8290.Cd-19–0958
14. El-Kenawi A, Hänggi K, Ruffell B. The immune microenvironment and cancer metastasis. Cold Spring Harbor Perspect Med. (2020) 10(4):a037424. doi: 10.1101/cshperspect.a037424
15. Kao K-C, Vilbois S, Tsai C-H, Ho P-C. Metabolic communication in the tumour–immune microenvironment. Nat Cell Biol. (2022) 24:1574–83. doi: 10.1038/s41556-022-01002-x
16. Zou W, Green DR. Beggars banquet: metabolism in the tumor immune microenvironment and cancer therapy. Cell Metab. (2023) 35:1101–13. doi: 10.1016/j.cmet.2023.06.003
17. Tang R, Xu J, Wang W, Meng Q, Shao C, Zhang Y, et al. Targeting neoadjuvant chemotherapy-induced metabolic reprogramming in pancreatic cancer promotes anti-tumor immunity and chemo-response. Cell Rep Med. (2023) 4(101234):a037424. doi: 10.1016/j.xcrm.2023.101234
18. Muranaka H, Hendifar A, Osipov A, Moshayedi N, Placencio-Hickok V, Tatonetti N, et al. Plasma metabolomics predicts chemotherapy response in advanced pancreatic cancer. Cancers. (2023) 15(11):3020. doi: 10.3390/cancers15113020
19. Winnard PT, Bharti SK, Sharma RK, Krishnamachary B, Mironchik Y, Penet MF, et al. Brain metabolites in cholinergic and glutamatergic pathways are altered by pancreatic cancer cachexia. J Cachexia Sarcopenia Muscle. (2020) 11:1487–500. doi: 10.1002/jcsm.12621
20. Ramapriyan R, Caetano MS, Barsoumian HB, Mafra ACP, Zambalde EP, Menon H, et al. Altered cancer metabolism in mechanisms of immunotherapy resistance. Pharmacol Ther. (2019) 195:162–71. doi: 10.1016/j.pharmthera.2018.11.004
21. Pham TN, Liagre B, Girard-Thernier C, Demougeot C. Research of novel anticancer agents targeting arginase inhibition. Drug Discovery Today. (2018) 23:871–8. doi: 10.1016/j.drudis.2018.01.046
22. Geiger R, Rieckmann JC, Wolf T, Basso C, Feng Y, Fuhrer T, et al. L-arginine modulates T cell metabolism and enhances survival and anti-tumor activity. Cell. (2016) 167:829–42 e13. doi: 10.1016/j.cell.2016.09.031
23. Cronin SJF, Seehus C, Weidinger A, Talbot S, Reissig S, Seifert M, et al. The metabolite bh4 controls T cell proliferation in autoimmunity and cancer. Nature. (2018) 563:564–8. doi: 10.1038/s41586–018-0701–2
24. Moon JY, Zolnik CP, Wang Z, Qiu Y, Usyk M, Wang T, et al. Gut Microbiota and Plasma Metabolites Associated with Diabetes in Women with, or at High Risk for, Hiv Infection. EBioMedicine. (2018) 37:392–400. doi: 10.1016/j.ebiom.2018.10.037
25. Oliveira LM, Teixeira FME, Sato MN. Impact of retinoic acid on immune cells and inflammatory diseases. Mediators Inflammation. (2018) 2018:3067126. doi: 10.1155/2018/3067126
26. Zhong H, Liu S, Zhu J, Wu L. Associations between genetically predicted levels of blood metabolites and pancreatic cancer risk. Int J Cancer. (2023) 153:103–10. doi: 10.1002/ijc.34466
27. Zhong H, Liu S, Zhu J, Xu TH, Yu H, Wu L. Elucidating the role of blood metabolites on pancreatic cancer risk using two-sample mendelian randomization analysis. Int J Cancer. (2024) 154:852–62. doi: 10.1002/ijc.34771
28. O’Donnell CJ, Sabatine MS. Opportunities and challenges in mendelian randomization studies to guide trial design. JAMA Cardiol. (2018) 3(10):967. doi: 10.1001/jamacardio.2018.2863
29. Sanderson E, Glymour MM, Holmes MV, Kang H, Morrison J, Munafò MR, et al. Mendelian randomization. Nat Rev Methods Primers. (2022) 2:6. doi: 10.1038/s43586–021-00092–5
30. Verduijn M, Siegerink B, Jager KJ, Zoccali C, Dekker FW. Mendelian randomization: use of genetics to enable causal inference in observational studies. Nephrol Dialysis Transplant. (2010) 25:1394–8. doi: 10.1093/ndt/gfq098
31. Orrù V, Steri M, Sidore C, Marongiu M, Serra V, Olla S, et al. Complex genetic signatures in immune cells underlie autoimmunity and inform therapy. Nat Genet. (2020) 52:1036–45. doi: 10.1038/s41588–020-0684–4
32. Chen Y, Lu T, Pettersson-Kymmer U, Stewart ID, Butler-Laporte G, Nakanishi T, et al. Genomic atlas of the plasma metabolome prioritizes metabolites implicated in human diseases. Nat Genet. (2023) 55:44–53. doi: 10.1038/s41588–022-01270–1
33. Wishart DS, Feunang YD, Marcu A, Guo AC, Liang K, Vazquez-Fresno R, et al. Hmdb 4.0: the human metabolome database for 2018. Nucleic Acids Res. (2018) 46:D608–D17. doi: 10.1093/nar/gkx1089
34. Pierce BL, Ahsan H, VanderWeele TJ. Power and instrument strength requirements for mendelian randomization studies using multiple genetic variants. Int J Epidemiol. (2011) 40:740–52. doi: 10.1093/ije/dyq151
35. Sved JA, Hill WG. One hundred years of linkage disequilibrium. Genetics. (2018) 209:629–36. doi: 10.1534/genetics.118.300642
36. Hartwig FP, Davies NM, Hemani G, Davey Smith G. Two-sample mendelian randomization: avoiding the downsides of a powerful, widely applicable but potentially fallible technique. Int J Epidemiol. (2016) 45:1717–26. doi: 10.1093/ije/dyx028
37. Klein AP. Pancreatic cancer epidemiology: understanding the role of lifestyle and inherited risk factors. Nat Rev Gastroenterol Hepatol. (2021) 18:493–502. doi: 10.1038/s41575-021-00457-x
38. Burgess S, Butterworth A, Thompson SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol. (2013) 37:658–65. doi: 10.1002/gepi.21758
39. Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through egger regression. Int J Epidemiol. (2015) 44:512–25. doi: 10.1093/ije/dyv080
40. Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. (2016) 40:304–14. doi: 10.1002/gepi.21965
41. Hartwig FP, Davey Smith G, Bowden J. Robust inference in summary data mendelian randomization via the zero modal pleiotropy assumption. Int J Epidemiol. (2017) 46:1985–98. doi: 10.1093/ije/dyx102
42. Burgess S, Thompson SG. Interpreting findings from mendelian randomization using the mr-egger method. Eur J Epidemiol. (2017) 32:377–89. doi: 10.1007/s10654-017-0255-x
43. Bowden J, Tierney JF, Copas AJ, Burdett S. Quantifying, displaying and accounting for heterogeneity in the meta-analysis of rcts using standard and generalised qstatistics. BMC Med Res Method. (2011) 11:41. doi: 10.1186/1471–2288-11–41
44. Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21:1539–58. doi: 10.1002/sim.1186
45. Yavorska OO, Burgess S. Mendelianrandomization: an R package for performing mendelian randomization analyses using summarized data. Int J Epidemiol. (2017) 46:1734–9. doi: 10.1093/ije/dyx034
46. Bowden J, Del Greco MF, Minelli C, Davey Smith G, Sheehan NA, Thompson JR. Assessing the suitability of summary data for two-sample mendelian randomization analyses using mr-egger regression: the role of the I2 statistic. Int J Epidemiol. (2016) 45(6):1961–74. doi: 10.1093/ije/dyw220
47. Sanderson E. Multivariable mendelian randomization and mediation. Cold Spring Harbor Perspect Med. (2021) 11(2):a038984. doi: 10.1101/cshperspect.a038984
48. Duan H, Li L, He S. Advances and prospects in the treatment of pancreatic cancer. Int J Nanomed. (2023) 18:3973–88. doi: 10.2147/ijn.S413496
49. Burdziak C, Alonso-Curbelo D, Walle T, Reyes J, Barriga FM, Haviv D, et al. Epigenetic plasticity cooperates with cell-cell interactions to direct pancreatic tumorigenesis. Science. (2023) 380. doi: 10.1126/science.add5327
50. Qin C, Yang G, Yang J, Ren B, Wang H, Chen G, et al. Metabolism of pancreatic cancer: paving the way to better anticancer strategies. Mol Cancer. (2020) 19(1):50. doi: 10.1186/s12943–020-01169–7
51. Liu C, Li C, Liu Y. The role of metabolic reprogramming in pancreatic cancer chemoresistance. Front Pharmacol. (2023) 13:1108776. doi: 10.3389/fphar.2022.1108776
52. Søreide K, Sund M. Epidemiological-molecular evidence of metabolic reprogramming on proliferation, autophagy and cell signaling in pancreas cancer. Cancer Lett. (2015) 356:281–8. doi: 10.1016/j.canlet.2014.03.028
53. Yang J, Ren B, Yang G, Wang H, Chen G, You L, et al. The enhancement of glycolysis regulates pancreatic cancer metastasis. Cell Mol Life Sci. (2019) 77:305–21. doi: 10.1007/s00018-019-03278-z
54. Kenkel JA, Tseng WW, Davidson MG, Tolentino LL, Choi O, Bhattacharya N, et al. An immunosuppressive dendritic cell subset accumulates at secondary sites and promotes metastasis in pancreatic cancer. Cancer Res. (2017) 77:4158–70. doi: 10.1158/0008–5472.CAN-16–2212
55. Crawford MA, Sinclair AJ, Hall B, Ogundipe E, Wang Y, Bitsanis D, et al. The imperative of arachidonic acid in early human development. Prog Lipid Res. (2023) 91:101222. doi: 10.1016/j.plipres.2023.101222
56. Doll S, Proneth B, Tyurina YY, Panzilius E, Kobayashi S, Ingold I, et al. Acsl4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat Chem Biol. (2016) 13:91–8. doi: 10.1038/nchembio.2239
57. Lee J-Y, Nam M, Son HY, Hyun K, Jang SY, Kim JW, et al. Polyunsaturated fatty acid biosynthesis pathway determines ferroptosis sensitivity in gastric cancer. Proc Natl Acad Sci. (2020) 117:32433–42. doi: 10.1073/pnas.2006828117
58. Brouwers H, Jonasdottir HS, Kuipers ME, Kwekkeboom JC, Auger JL, Gonzalez-Torres M, et al. Anti-inflammatory and proresolving effects of the omega-6 polyunsaturated fatty acid adrenic acid. J Immunol. (2020) 205:2840–9. doi: 10.4049/jimmunol.1801653
59. Momburg F, Ziegler A, Harpprecht J, Möller P, Moldenhauer G, Hämmerling GJ. Selective loss of hla-a or hla-B antigen expression in colon carcinoma. J Immunol. (1989) 142:352–8. doi: 10.4049/jimmunol.142.1.352
60. Hung K, Hayashi R, Lafond-Walker A, Lowenstein C, Pardoll D, Levitsky H. The central role of cd4(+) T cells in the antitumor immune response. J Exp Med. (1998) 188:2357–68. doi: 10.1084/jem.188.12.2357
61. Baleeiro RB, Bouwens CJ, Liu P, Di Gioia C, Dunmall LSC, Nagano A, et al. Mhc class ii molecules on pancreatic cancer cells indicate a potential for neo-antigen-based immunotherapy. OncoImmunology. (2022) 11(1):2080329. doi: 10.1080/2162402x.2022.2080329
62. Yang R, Yu Y. Glucocorticoids are double-edged sword in the treatment of covid-19 and cancers. Int J Biol Sci. (2021) 17:1530–7. doi: 10.7150/ijbs.58695
63. Liu L, Aleksandrowicz E, Schönsiegel F, Gröner D, Bauer N, Nwaeburu CC, et al. Dexamethasone mediates pancreatic cancer progression by glucocorticoid receptor, tgfβ and jnk/ap-1. Cell Death Dis. (2017) 8:e3064–e. doi: 10.1038/cddis.2017.455
64. Taves MD, Ashwell JD. Glucocorticoids in T cell development, differentiation and function. Nat Rev Immunol. (2021) 21:233–43. doi: 10.1038/s41577–020-00464–0
65. Kugler DG, Mittelstadt PR, Ashwell JD, Sher A, Jankovic D. Cd4+ T cells are trigger and target of the glucocorticoid response that prevents lethal immunopathology in toxoplasma infection. J Exp Med. (2013) 210:1919–27. doi: 10.1084/jem.20122300
66. Farhood B, Najafi M, Mortezaee K. Cd8+ Cytotoxic T lymphocytes in cancer immunotherapy: A review. J Cell Physiol. (2018) 234:8509–21. doi: 10.1002/jcp.27782
67. Yang F, Wei Y, Cai Z, Yu L, Jiang L, Zhang C, et al. Activated cytotoxic lymphocytes promote tumor progression by increasing the ability of 3ll tumor cells to mediate mdsc chemoattraction via fas signaling. Cell Mol Immunol. (2014) 12:66–76. doi: 10.1038/cmi.2014.21
68. Stewart Robertson D. The function of oxalic acid in the human metabolism. Clin Chem Lab Med. (2011) 49(9):1405–12. doi: 10.1515/cclm.2011.238
69. Joshi S, Peck AB, Khan SR. Nadph oxidase as a therapeutic target for oxalate induced injury in kidneys. Oxid Med Cell Longevity. (2013) 2013:1–18. doi: 10.1155/2013/462361
70. Tsuji H, Wang W, Sunil J, Shimizu N, Yoshimura K, Uemura H, et al. Involvement of renin–angiotensin–aldosterone system in calcium oxalate crystal induced activation of nadph oxidase and renal cell injury. World J Urol. (2015) 34:89–95. doi: 10.1007/s00345–015-1563-y
71. Forman HJ, Zhang H. Targeting oxidative stress in disease: promise and limitations of antioxidant therapy. Nat Rev Drug Discovery. (2021) 20:689–709. doi: 10.1038/s41573–021-00233–1
72. Jiang H, Zuo J, Li B, Chen R, Luo K, Xiang X, et al. Drug-induced oxidative stress in cancer treatments: angel or devil? Redox Biol. (2023) 63:102754. doi: 10.1016/j.redox.2023.102754
73. Hayes JD, Dinkova-Kostova AT, Tew KD. Oxidative stress in cancer. Cancer Cell. (2020) 38:167–97. doi: 10.1016/j.ccell.2020.06.001
74. Le A, Cooper CR, Gouw AM, Dinavahi R, Maitra A, Deck LM, et al. Inhibition of lactate dehydrogenase a induces oxidative stress and inhibits tumor progression. Proc Natl Acad Sci U.S.A. (2010) 107:2037–42. doi: 10.1073/pnas.0914433107
75. Rawat L, Hegde H, Hoti SL, Nayak V. Piperlongumine induces ros mediated cell death and synergizes paclitaxel in human intestinal cancer cells. Biomed Pharmacother. (2020) 128:110243. doi: 10.1016/j.biopha.2020.110243
76. Zaidieh T, Smith JR, Ball KE, An Q. Ros as a novel indicator to predict anticancer drug efficacy. BMC Cancer. (2019) 19(1):1224. doi: 10.1186/s12885-019-6438-y
77. Brosecke F, Pfau A, Ermer T, Dein Terra Mota Ribeiro AB, Rubenbauer L, Rao VS, et al. Interleukin-16 is increased in dialysis patients but is not a cardiovascular risk factor. Sci Rep. (2024) 14:11323. doi: 10.1038/s41598–024-61808–7
78. Yang SJ, Chang ST, Chang KC, Lin BW, Chang KY, Liu YW, et al. Neutralizing il-16 enhances the efficacy of targeting aurora-a therapy in colorectal cancer with high lymphocyte infiltration through restoring anti-tumor immunity. Cell Death Dis. (2024) 15:103. doi: 10.1038/s41419-023-06381-z
79. Jin H, Liu X, Liu HX. Biological function, regulatory mechanism, and clinical application of mannose in cancer. Biochim Biophys Acta Rev Cancer. (2023) 1878:188970. doi: 10.1016/j.bbcan.2023.188970
80. Sharma V, Ichikawa M, Freeze HH. Mannose metabolism: more than meets the eye. Biochem Biophys Res Commun. (2014) 453:220–8. doi: 10.1016/j.bbrc.2014.06.021
81. Gonzalez PS, O’Prey J, Cardaci S, Barthet VJA, Sakamaki J-i, Beaumatin F, et al. Mannose impairs tumour growth and enhances chemotherapy. Nature. (2018) 563:719–23. doi: 10.1038/s41586–018-0729–3
82. Zhang D, Chia C, Jiao X, Jin W, Kasagi S, Wu R, et al. D-mannose induces regulatory T cells and suppresses immunopathology. Nat Med. (2017) 23:1036–45. doi: 10.1038/nm.4375
83. Zhang W, Cheng H, Gui Y, Zhan Q, Li S, Qiao W, et al. Mannose treatment: A promising novel strategy to suppress inflammation. Front Immunol. (2021) 12:756920. doi: 10.3389/fimmu.2021.756920
84. Torretta S, Scagliola A, Ricci L, Mainini F, Di Marco S, Cuccovillo I, et al. D-mannose suppresses macrophage il-1β Production. Nat Commun. (2020) 11(1):6343. doi: 10.1038/s41467–020-20164–6
85. Shoulders MD, Raines RT. Collagen structure and stability. Annu Rev Biochem. (2009) 78:929–58. doi: 10.1146/annurev.biochem.77.032207.120833
86. Spangenberg SH, Palermo A, Gazaniga NR, Martínez-Peña F, Guijas C, Chin EN, et al. Hydroxyproline metabolism enhances ifn-Γ-induced pd-L1 expression and inhibits autophagic flux. Cell Chem Biol. (2023) 30:1115–34.e10. doi: 10.1016/j.chembiol.2023.06.016
87. Schmidt JA, Fensom GK, Rinaldi S, Scalbert A, Appleby PN, Achaintre D, et al. Pre-diagnostic metabolite concentrations and prostate cancer risk in 1077 cases and 1077 matched controls in the European prospective investigation into cancer and nutrition. BMC Med. (2017) 15(1):122. doi: 10.1186/s12916–017-0885–6
Keywords: pancreatic cancer, immune cells, Mendelian randomization, mediation analysis, plasma metabolites
Citation: Zhang K, Zhu J, Wang P, Chen Y, Wang Z, Ge X, Wu J, Chen L, Lu Y, Xu P and Yao J (2024) Plasma metabolites as mediators in immune cell-pancreatic cancer risk: insights from Mendelian randomization. Front. Immunol. 15:1402113. doi: 10.3389/fimmu.2024.1402113
Received: 16 March 2024; Accepted: 31 May 2024;
Published: 12 June 2024.
Edited by:
Annalisa Marcuzzi, University of Ferrara, ItalyReviewed by:
Katrin Richter, University of Giessen, GermanyRishat Ruzi, First Affiliated Hospital of Xinjiang Medical University, China
Copyright © 2024 Zhang, Zhu, Wang, Chen, Wang, Ge, Wu, Chen, Lu, Xu and Yao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peng Xu, cGVuZ3h1MTk4NkAxMjYuY29t; Jie Yao, ZHJ5YW9qaWVAeXp1LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
 Ke Zhang
Ke Zhang Jie Zhu
Jie Zhu Peng Wang2
Peng Wang2