- 1Division of Medical Oncology, Institute of Oncology Ljubljana, Ljubljana, Slovenia
- 2Medical Centre for Molecular Biology, Institute of Biochemistry and Molecular Genetics, Faculty of Medicine, University of Ljubljana, Ljubljana, Slovenia
- 3Department of Experimental Oncology, Institute of Oncology Ljubljana, Ljubljana, Slovenia
- 4Faculty of Medicine, University of Ljubljana, Ljubljana, Slovenia
Triple-negative breast cancer (TNBC) accounts for about 10-20% of all breast cancer cases and is associated with an unfavorable prognosis. Until recently, treatment options for TNBC were limited to chemotherapy. A new successful systemic treatment is immunotherapy with immune checkpoint inhibitors, but new tumor-specific biomarkers are needed to improve patient outcomes. Cannabinoids show antitumor activity in most preclinical studies in TNBC models and do not appear to have adverse effects on chemotherapy. Clinical data are needed to evaluate efficacy and safety in humans. Importantly, the endocannabinoid system is linked to the immune system and immunosuppression. Therefore, cannabinoid receptors could be a potential biomarker for immune checkpoint inhibitor therapy or a novel mechanism to reverse resistance to immunotherapy. In this article, we provide an overview of the currently available information on how cannabinoids may influence standard therapy in TNBC.
1 Triple-negative breast cancer
Triple-negative breast cancer (TNBC) accounts for about 10-20% of all breast cancer cases and is associated with the worst prognosis. TNBC is a heterogeneous group of tumors defined by the absence of the estrogen receptor (ER), the progesterone receptor (PR), and the absence of the human epidermal growth factor receptor 2 (HER2). It mainly occurs in younger patients and patients with BRCA germline mutations (1, 2). TNBC has been divided into six different subgroups: basal-like 1 (BL1), basal-like 2 (BL2), mesenchymal (M), mesenchymal stem-like (MSL), immunomodulatory (IM) and luminal androgen receptor (LAR) (3). The TNBC microenvironment (TME) includes tumor-infiltrating lymphocytes (TILs) with CD3+ T and CD20+ B lymphocytes, CD38+/CD138+ plasma cells, tumor-associated macrophages (TAMs), cancer-associated fibroblasts (CAFs), tumor-associated neutrophils (TANs), natural killer (NK) cells and cancer-associated adipocytes (CAAs). The TNBC TME is unique in that it has a high expression of vascular endothelial growth factors and a high infiltration rate of TILs and TAM and plays an important role in the pathogenesis of the disease. Type M2 macrophages are upregulated compared to other subtypes of breast cancer. CAAs also play a greater role. A variety of cytokines and chemokines influence tumor growth, metastasis and drug resistance, mediate immunosuppression and antitumor activity, and play an important role in TNBC TME (4). Dendritic cells (DCs) are antigen-presenting cells (APCs) that play a crucial role in acquired and innate immune responses and are involved in the development of T cell-mediated antitumor immune responses (5). Neoangiogenesis is necessary for the nutrition and oxygen supply of the tumor and thus for the progression of the tumor. The endothelium is involved in the invasion of tumor cells into the vascular lumen and the resulting metastasis. In addition, endothelial cells are one of the main sources of CAFs (6). Until recently, treatment options for TNBC were limited to chemotherapy, e.g. anthracyclines and taxanes. The prognosis of metastatic TNBC is poor, with a median survival of 11 to 17 months. A new successful systemic treatment is immunotherapy with inhibitors of programmed cell death ligand 1 (PD-L1) and programmed cell death protein 1 (PD-1), also known as immune checkpoint inhibitors (ICI). Atezolizumab (PD-L1 inhibitor) and pembrolizumab (PD-1 inhibitor) are in clinical use (7). Atezolizumab is used for the treatment of metastatic or unresectable locally advanced breast cancer and is restricted to patients whose tumors express PD-L1 on immune cells (> 1% PD-L1 positive tumor cells) (8). Pembrolizumab is used in early-stage TNBC (9) and in patients with advanced cancer with a combined positive score (CPS score), defined as the number of PD-L1–staining cells (tumor cells, lymphocytes and macrophages) divided by the total number of viable tumor cells multiplied by 100) > 10 (10). In TNBC PD-1/PD-L1 are used as biomarkers for response to immunotherapy. However, new tumor-specific biomarkers are needed to improve patient outcomes (11). In patients with BRCA mutation, poly (ADP-ribose) polymerase (PARP) inhibitors are used in adjuvant and metastatic treatment. This is an example of synthetic lethality and targeted treatment (12, 13). Newer treatment options are the antibody-drug conjugates sacituzumab govitecan and trastuzumab deruxtecan. Sacituzumab govitecan is an antibody directed against Trop2 to which SN38 (a derivative of irinotecan) is bound (14). Trastuzumab deruxtecan is an antibody-drug conjugate consisting of the humanized monoclonal antibody trastuzumab covalently linked to the topoisomerase I inhibitor deruxtecan (a derivative of exatecan). It is used in a subset of TNBC that is considered HER2-low, a category that blurs the distinction between HER2-positive and TNBC (15).
2 Cannabinoids and the endocannabinoid system
Cannabinoid receptors (CBRs) are membrane-bound G protein-coupled receptors (GPCRs) and were identified over 30 years ago. Cannabinoid receptor 1 (CB1R) is one of the most abundant receptors in the brain. To a lesser extent, CB1R is also expressed in the periphery, including the immune system. Cannabinoid receptor 2 (CB2R) is also expressed in the central nervous system, but to a much lesser extent than CB1R. In addition, CB2R is highly expressed in the periphery, particularly in organs that are part of the immune system and in other peripheral tissues. Natural polymorphisms and alternative splice variants may be important for its function (16). In addition, there is a “non-canonical” extended signaling network of the endocannabinoid system. It consists of fatty acid derivatives, ionotropic cannabinoid receptors (transient receptor potential (TRP) channels) and other GPCRs (GPR18, GPR19, GPR55, peroxisome proliferator-activated receptor alpha (PPARα), eCB), enzymes involved in the biosynthesis and degradation of endocannabinoids (fatty-acid amide hydrolase 1 (FAAH) and monoacylglycerol lipase (MAGL)), and protein transporters fatty-acid-binding proteins (FABPs)) (17–22). Cannabinoid receptors recognize different agonists and antagonists and can be activated by endogenous or exogenous cannabinoids. Endogenous cannabinoids or endocannabinoids are metabolites of arachidonic acid (AA). The best studied are N-arachidonoylethanolamine (anandamide, the Sanskrit word for “bliss”) and 2-arachidonoylglycerol (2-AG), which together with the CBRs form the endocannabinoid system (23). Anandamide binds to CBRs with higher affinity than 2-AG, but acts only as a partial agonist (24). 2-AG, on the other hand, behaves as a full agonist for CBRs (25, 26). 2-AG binds selectively to CBRs, whereas anandamide is less selective (27). It has been suggested that 2-AG and not anandamide is the actual natural ligand for CBRs (28–30). Tetrahydrocannabinol (THC) is the main psychoactive constituent of Cannabis sativa and thus an exogenous phytocannabinoid and a non-selective agonist of CB1R and CB2R (31). Other important phytocannabinoids are cannabidiol (CBD), cannabichromene (CBC), cannabigerol (CBG), cannabidivarin (CBDV) and tetrahydrocannabivarin (THCV) (32). Compared to THC, CBD has a lower CB1R and CB2R affinity and acts as an inverse agonist on CB2R. It is a non-psychoactive substance and therefore a potential therapeutic agent (33). Synthetic cannabinoids (i.e. JWH-015, WIN55,212-2, compound-10, etc.) are a heterogeneous group of substances that can be selective agonists or antagonists of CB1R or CB2R (34). CB1R and CB2R exhibit allosteric binding and biased signaling that influences the biological response mediated by agonists (31, 35).
3 Cannabinoids and the immune system
Various components of the endocannabinoid system act as important regulators of the immune system and the immune response (36–38). Exogenous cannabinoids are generally immunosuppressive and potent immunological mediators (39–43). Immune cells express both CB1R and CB2R (44, 45), although the expression of CB1R is significantly lower compared to CB2R (41, 46). CB2R is mainly found in cells of the immune system and plays an important role as a modulator of immune function (47–49). The activation of CB2R in many immune cells is central to the suppressive effects of cannabinoids. However, the pro-inflammatory effects appear to be linked to the expression of CB1R. Cannabinoids inhibit adenylyl cyclase and activate beta-gamma-mediated signaling pathways and modulate intracellular free calcium levels. These changes may negatively affect the release of inflammatory mediators and the induction of pro-inflammatory transcriptional programs. Exposure to cannabinoids inhibits the release of prostaglandins, histamine, and matrix-active proteases from mast cells (50). Phagocytic function is suppressed by cannabinoids. Cannabinoids also suppress inflammation at a secondary level by downregulating the production of cytokines (e.g. tumor necrosis factor alpha (TNFα), interferon gamma (IFNγ), interleukin-1 (IL-1), and IL-4 (41, 51–54). There are relatively few descriptions of immunological side effects of cannabinoids in humans. However, the human immune system is altered by chronic exposure to cannabinoids (55). Cannabinoid exposure does not accelerate the loss of immunocompetence in HIV-1 infected patients (56, 57). Cannabis use is associated with long-term changes in immunological homeostasis (58, 59). Inhibition of cell-mediated immunity has been found in marijuana smokers (60) and cannabis smoking causes inflammatory changes in the airways (61). Studies have shown that exposure to cannabinoids leads to a suppression of responsiveness to infectious diseases, and a link between cannabis use and increased susceptibility to various infections has been suggested (62–64). These studies suggest a significant potential for immunological side effects of cannabinoid compounds in humans. Interestingly, cannabinoids can affect the gut microbiota (65–67), which also influences immunotherapy for cancer (68–71).
4 Cells of the TNBC microenvironment and the endocannabinoid system
It appears that most of the cell types present in the TNBC TME express CBRs and may be affected in some way by the exogenous cannabinoids and/or the endocannabinoid system (4, 72). CB2R is expressed in CD8+ T lymphocytes and CD4+ T lymphocytes and CB1R mRNA transcripts are modestly present in human T- lymphocytes (40, 41, 73). The expression of the CB2R receptor is low in circulating T lymphocytes. However, several studies have reported that CB2R receptor expression is increased in activated T lymphocytes and that its activation decreases their proliferation (74–76). This is associated with reduced production of pro-inflammatory cytokines and increased apoptosis (74–78). In addition, CBR agonists can upregulate immunosuppressive cytokines (79, 80) and cause inhibition of chemotaxis (81–83). The antitumor function of T lymphocytes is enhanced in Cnr2 conditional knockout mouse (84). The activation of CB2R appears to have different effects depending on the subtype of T lymphocytes, with the functions of Th1 and Th17 tending to be reduced and those of Th2 promoted in humans (72, 75, 85, 86). In addition, the CB2R receptor is highly expressed in cytokine-induced killer (CIK) cells, a subset of cytotoxic T lymphocytes with a CD3+ CD56+ immunophenotype (4, 87–90). It is important to note that the activation of T lymphocytes is the primary mechanism of action of ICI (91, 92). B-cells express CB1R and CB2R (93, 94). Treatment with a CB2R agonist increased the proliferation of B lymphocytes, a phenomenon that was blocked by a CB2R antagonist (95). In mice, activation of the CB2R receptor was associated with differentiation, migration, proliferation and antibody class switching of B lymphocytes (96–98). These results suggest that CB2R is part of the immune programming of B lymphocytes and plays an important role in the development of B lymphocytes (72, 99). CBRs are overexpressed in chronic lymphocytic leukemia (CLL) compared to healthy B lymphocytes and CBR1 could be a new prognostic marker (100). CB2R is expressed in monocytes and CB1R mRNA transcripts are modestly present (40, 41, 73). CB2R agonists can modulate human monocyte migration (101, 102). Macrophages express both CB1R and CB2R (103, 104). Several studies have shown that cannabinoids negatively regulate phagocytosis, cell-spreading, and antigen presentation by macrophages (105–107). CB2R has been shown to switch the polarization of M1 macrophages to M2 macrophages (108–110). CB2R is expressed in human fibroblasts (111) and transcripts for CB1R and CB2R have been found in both odontoblasts and gingival fibroblasts (112). Fibroblasts also express enzymes that metabolize endocannabinoids. Fibroblasts can be influenced by autocrine signaling of endocannabinoids via CB1R and CB2R and paracrine signaling by neighboring leukocytes (113). CB2R is expressed in polymorphonuclear neutrophils (40, 41, 73). Cannabinoids can influence neutrophil function via CBRs or other mechanisms. The inhibitory effect of cannabinoids on neutrophil functional responses is mostly related to a mechanism other than CBRs, consistent with the absence or very low expression of CB2R (114–119). Neutrophil migration is related to the expression of CB2R and GPR55 (120). Chemotaxis of human neutrophils is inhibited by CBR agonists (114, 121). CB1R and CB2R are expressed in NK cells. The predominance of CB2R in NK cells is illustrated by a striking ratio of 100:1 between CB2R and CB1R (40, 41, 44, 73, 122–125). NK cells release large amounts of endocannabinoids (125) and endocannabinoids influence the chemotaxis of NK cells (126). NK cell activity is inhibited by CBR agonists (127), and cannabis use has been associated with a decrease in NK cell counts (58, 59). Mature adipocytes from visceral and subcutaneous adipose tissue express CBRs on their plasma membranes and CB1R is located at various subcellular levels, including the plasma membrane and mitochondria of the adipocyte (128, 129). However, there is some uncertainty regarding CB2R expression in differentiated adipocytes (130–134). A complete endocannabinoid system has been found in both murine and human adipocytes (135–137). Bone marrow-derived DCs from mice express CB1R and CB2R (138) and the endocannabinoid system is present in human DCs (139). Endocannabinoids act as chemoattractants for DCs and activation of CBRs induces apoptosis (138, 140). Both CB1R and CB2R are expressed in endothelial cells (141, 142). It is possible that an atypical cannabinoid receptor, the endothelial cannabinoid receptor (eCB receptor), is responsible for the vasodilatory effect of cannabinoids (143). A study found that CBRs are expressed in glioblastoma endothelial cells. CB1R expression was detected in about 38% and CB2R expression in 54% of cells. Compared to CB1R, the expression of CB2R was increased in the endothelial cells of glioblastoma (142).
5 Cannabinoid receptor expression in TNBC
The presence of CBRs in human breast tumors was investigated by quantitative real-time PCR and confocal microscopy. Lower levels of CB1R mRNA were detected in low, intermediate and high histologic grade tumors compared to normal, non-cancerous breast tissue. In all tumors examined, CB2R expression was higher than CB1R expression. Hormone receptor-negative (HR-) tumors expressed more CB2R mRNA than ER +/PR + tumors (144). Perez-Gomez et al. performed a histopathologic analysis of tissue samples for the expression of CBRs. A very large proportion of human breast adenocarcinomas (~75%) expressed CB2R and expression was strongly associated with HER2+ tumors, while no association was found between CB2R expression and HR+ or TNBC. There was an association between increased expression of CB2R and poorer prognosis and higher likelihood of local recurrence in HER2+ breast cancer (145). The orphan receptor GRP55 could play an important role in TNBC. In the study by Andradas et al. it was found that the TNBC cell line MDA-MB-231 reduced its invasive behavior when GPR55 expression was knocked down. Similar effects were observed in an animal model of lung metastasis. In breast cancer patients, there is a strong association between GPR55 protein levels and TNBC tumors. Using tissue microarrays and publicly available data from The Cancer Genome Atlas (TCGA), it has been shown that higher GPR55 expression is associated with poorer patient prognosis (lower disease-free survival and metastasis-free survival) (146).
6 Effect of different cannabinoids on TNBC
The antitumor effect of THC on breast cancer cell lines was documented. Among the tumor cells, those with a more aggressive phenotype, including the MDA-MB-231 cell line, were more sensitive to THC. One mechanism of this effect is that THC arrests the cells at the G2-M cell cycle checkpoint via downregulation of the cyclin-dependent kinase 1 (Cdc2), as suggested by the reduced sensitivity of Cdc2-overexpressing cells to THC (144). In addition, CBD has an antitumor and anti-metastatic effect in TNBC cell lines. The antitumor effect of CBD is mediated by the activation of apoptosis. Apoptosis in the MDA-MB-231 cell line can be induced by direct or indirect activation of the CB2R receptor and transient receptor potential vanilloid type-1 (TRPV1) as well as by an increase in intracellular Ca2+ and reactive oxygen species (ROS) independent of the cannabinoid/vanilloid receptors (147). CBD induces an interaction between the PPARγ, the mammalian target of rapamycin (mTOR) and cyclin D1, which promotes apoptosis (148) and can downregulate the expression of an inhibitor of basic helix-loop-helix transcription factors (Id-1) in metastatic TNBC cells, which leads to a reduction in tumor aggressiveness (149). The downregulation of Id-1 expression is the result of differential modulation of extracellular signal-regulated kinase (ERK) and ROS. In addition, the pro-differentiation factor Id-2 is upregulated by CBD. The anti-metastatic activity was also confirmed in animal models, in which CBD significantly reduced the primary tumor mass as well as the size and number of metastases in the lung (150). The study by Elbaz et al. showed that CBD inhibits the growth and metastasis of breast cancer cells by inhibiting epidermal growth factor (EGF)/EGFR signaling and modulating the tumor microenvironment, and that it can significantly inhibit EGF-induced proliferation and chemotaxis of breast cancer cells. Inhibition of EGF-induced activation of the EGFR, ERK, protein kinase B (AKT) and nuclear factor kappa B (NF-κB) signaling pathways and the secretion of the matrix metallopeptidases (MMP2 and MMP9) has been described. In in vivo models, the analysis of the molecular mechanism showed that CBD significantly inhibits the recruitment of TAMs in the primary tumor stroma and in secondary lung metastases (151). The selective CB2R agonist JWH-015 reduced the primary tumor burden and metastasis of the luciferase-labeled murine TNBC 4T1 cell line in immunocompetent mice in vivo and reduced the viability of murine 4T1 cells in vitro by inducing apoptosis. The reduction in cell viability mediated by JWH-015 was not dependent on Gαi signaling in vitro, nor was it altered by classical pharmacological blockade of other CBRs (CB1R, GPR55, TRPV1). The effect of JWH-015 was calcium-dependent and led to changes in mitogen-activated protein kinase (MAPK)/ERK signaling (152). Rimonabant (SR141716), a CB1R antagonist, inhibits the proliferation of the TNBC cell line MDA-MB-231 more effectively than ER+ cell lines. It also shows an antiproliferative effect in vivo by reducing the volume of xenograft tumors induced by injection of MDA-MB-231 in mice. Rimonabant inhibits the growth of human TNBC cells via a CB1R-lipid raft/caveolae-mediated mechanism (153). A strong synergy between rimonabant and erastin in inhibiting the growth of TNBC cells has been described both in vitro and in vivo. This occurred by increasing the levels of lipid peroxides, malondialdehyde (MDA), 4-hydroxynonenal (4-HNE) and ROS production in the cytosol, enhancing intracellular glutathione (GSH) depletion and inducing G1 cell cycle arrest (154). Cannabidiolic acid activates the expression of PPARβ/δ target genes in the MDA-MB-231 cell line (155). The molecular interaction between the cannabinoid agonists of vetiver oil and the CBR2 receptor was found to be the cause of the cytotoxicity of vetiver oil on the 4T1 cell line (156). Styrene-maleic acid nanomicelles encapsulating WIN55,212-2 were synthesized to reduce the side effects and increase the efficacy of the drug. The synthetic cannabinoid analog WIN55,212-2 is a synthetic CBR agonist with a cytotoxic effect on the MDA-MB-231 cell line, but causes psychoactive side effects (157). The synthesis and evaluation of a selective, non-psychotropic CB2R agonist, designated compound 10, with in vivo activity against MDA-MB-231 cells was reported. This novel cannabinoid o-quinone (compound 10) has been described to induce cell apoptosis through CB2R activation and oxidative stress. Importantly, compound 10 showed no toxic effects on non-cancerous human mammary epithelial cells or in vivo (158). Photodynamic therapy for TNBC was combined with CB2R agonists for TNBC. Synergistic effects were observed in both in vitro (MDA-MB-231 cell line) and in vivo experiments. The survival time of tumor-bearing animals was significantly prolonged (159).
7 Cannabinoids and triple-negative breast cancer treatment
7.1 Cannabinoids and chemotherapy in TNBC
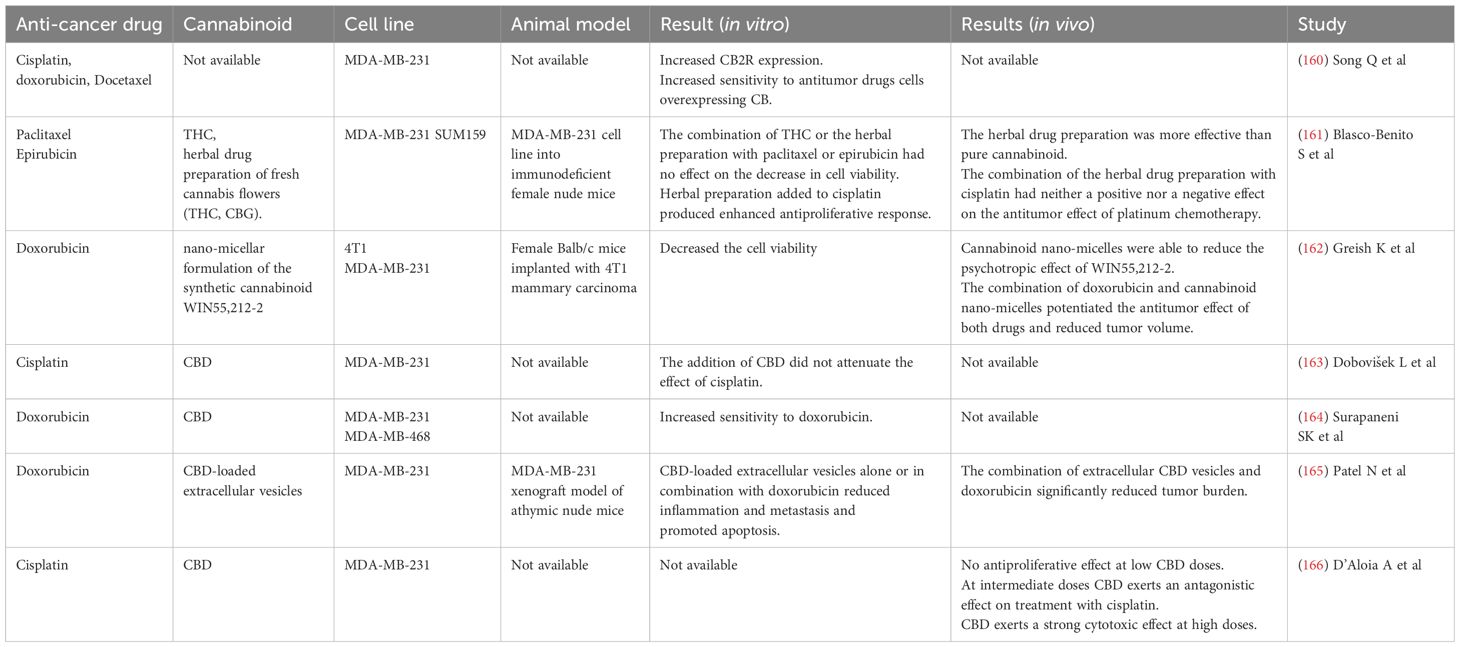
A brief overview of the research on cannabinoids and chemotherapy in TNBC is summarised in Table 1. CB2R expression is increased in the MDA‐MB‐231 cell line when treated with chemotherapy. Overexpression of CB2R had an inhibitory effect on TNBC cells and significantly improved their sensitivity to chemotherapy with cisplatin, doxorubicin and docetaxel. Therefore, CB2R may be involved in the chemosensitivity of the MDA‐MB‐231 cell line to chemotherapy (160). The antitumor efficacy of pure THC was compared with that of an herbal drug preparation of fresh cannabis flowers containing a variety of cannabinoids and terpenes. The herbal drug preparation contained THC and CBG, but no CBD, and was more effective than pure THC in producing antitumor responses in cell cultures and animal models of various breast cancer subtypes, including the TNBC subtype (MDA-MB-231 and SUM159 cell lines). In the study by Blasco-Benito et al. different combinations of chemotherapy with THC and herbal preparations were tested. The combination of THC or the herbal preparation with paclitaxel or epirubicin had no effect on the decrease in cell viability. When the herbal preparation was added to cisplatin treatment, an enhanced antiproliferative response was observed. Furthermore, this was tested in vivo by injecting the MDA-MB-231 cell line into immunodeficient mice. The herbal drug preparation was significantly more potent than pure cannabinoid (the same dose of THC was administered). The combination of the herbal drug preparation with cisplatin had neither a positive nor a negative effect on the antitumor effect of platinum chemotherapy (161).
Greish et al. investigated the effects of a nano-micellar formulation of the synthetic cannabinoid WIN55,212-2, a CBR agonist, on TNBC. Cannabinoid nano-micelles decreased the cell viability of TNBC cell lines (4T1 and MDA-MB-231). In addition, the antitumor effect of cannabinoid nano-micelles was investigated in mice with 4T1 tumors. Cannabinoid nano-micelles were more effective compared to free WIN55,212-2 and were able to reduce the psychotropic effect of WIN55,212-2. In addition, administration of cannabinoid nano-micelles sensitized the 4T1 cell line to the effect of doxorubicin. This effect was also observed in vivo. The combination of doxorubicin and cannabinoid nano-micelles potentiated the antitumor effect of both drugs and reduced tumor volume (162). The addition of CBD did not attenuate the effect of cisplatin in the MDA-MB-231 cell line (163). In combination with CBD, increased sensitivity to doxorubicin was observed in MDA-MB-468 cells. This was partly due to activation of apoptosis and inhibition of autophagy. The combination of CBD and doxorubicin decreased lysyl oxidase (LOX) and integrin-α5 and increased caspase-9 protein in the MDA-MB-468 cell line (164). CBD-loaded extracellular vesicles isolated from human umbilical cord mesenchymal stem cells and encapsulated by sonication with CBD sensitize TNBC cells to doxorubicin in both in vitro and in vivo models. CBD-loaded extracellular vesicles alone or in combination with doxorubicin reduced inflammation and metastasis and promoted apoptosis in the MDA-MB-231 cell line through cell cycle arrest in G1 phase and downregulation of IL-17, NF-κB, Twist, phosphorylated transducer and activator of transcription-3 (pSTAT3), STAT3 proteins in vitro. This was confirmed by in vivo studies, which showed that the combination of extracellular CBD vesicles and doxorubicin significantly reduced tumor burden. The combination modulated the tumor microenvironment by decreasing the expression of transforming growth factor-beta (TGFβ), IL-6, NF-κB, Integrin α−5 (ITGA5), Smad-2, GPC 1&6 and Twist and mediated apoptosis by increasing the expression of Bcl-2-associated X protein (BAX) and caspase 9 and decreasing the expression of Bcl2. The study concluded that extracellular CBD vesicles increase the sensitivity of the MDA-MB-231 cell line to doxorubicin, thereby reducing the required effective dose of doxorubicin and thus reducing or eliminating toxicity, and that extracellular vesicles can be used as potential delivery systems for cannabinoids due to their easy internalization by tumors (165). One study found that a threshold mechanism rather than a dose-dependent curve better describes the CBD effects on the MDA-MB-231 cell line. The threshold is reached between 3 and 5 μM. At low doses, CBD exerts no antiproliferative effect. At intermediate doses, near the threshold concentration, CBD induces survival mechanisms, including cell cycle arrest and autophagy, and exerts an antagonistic effect on treatment with cisplatin. In contrast, at high doses (> 5 µM), CBD exerts a strong cytotoxic effect by activating bubbling death (166). Importantly, the major cannabinoids (THC, CBD, and cannabinol) and their metabolites found in the plasma of cannabis users can inhibit several P450 enzymes, including CYP2B6, CYP2C9, and CYP2D6, and cause pharmacokinetic interactions between these cannabinoids and xenobiotics that are extensively metabolized by these enzymes (167). There is evidence that cannabinoids alleviate peripheral neuropathic pain caused by chemotherapy (168–170) and prevent doxorubicin-induced cardiomyopathy (171–173). Both are side effects of taxane and anthracycline chemotherapy, which is frequently used in TNBC (174, 175).
7.2 Cannabinoids and Immunotherapy
Exposure to THC can promote growth and metastasis of the 4T1 cell line. Specifically, THC exposure leads to an accelerated onset of detectable tumors, larger tumor sizes, and a higher number of lung metastases. The suppression of the immune response against the 4T1 tumor by THC may be responsible for the increased tumor growth and metastasis. The study by McKallip et al. showed that MDA-MB-231 expresses low levels of CB1R and undetectable levels of CB2R. Therefore, it was hypothesized that the degree of tumor sensitivity to THC is directly related to the degree of CB1R and CB2R expression and that THC may only kill tumors that express CBRs. However, THC exposure may lead to increased growth and metastasis of tumors with little or no expression of CBRs due to suppression of the antitumor immune response (176). Two studies investigated the effects of cannabis use during immunotherapy with ICI. A clinical retrospective analysis including 140 patients with advanced melanoma, non-small cell lung cancer and clear cell renal carcinoma showed a worse response rate (RR) in cannabis users (37.5% RR with nivolumab alone versus 15.9% in the nivolumab-cannabis group). Cannabis use was not a significant factor for progression-free survival (PFS) or overall survival (OS) (177). A prospective observational study by Bar-Sela et al. included 102 patients with advanced cancers (melanoma, non-small cell lung cancer and clear cell renal carcinoma) treated with ICI (anti-PD1 and anti-PDL1). Cannabis use correlated with a significant reduction in time to tumor progression and OS. Cannabis users were associated with a lower number of immune-related adverse events (iAEs). Blood samples from patients taken before the start of treatment were analyzed for endocannabinoids and endocannabinoid-like substances. A single endocannabinoid-like lipid, 2-oleoyl-glycerol (2-OG), was associated with significantly different levels between groups. The concentrations of AA, 2-AG, N-docsatetraenoyl ethanolamide (DtEA), linolenic acid (LnA), N-linoleoyl ethanolamide (LEA), N-oleoyl amide (O-Am), N-oleoyl ethanolamide (OEA), N-palmitoyl ethanolamide (PEA) and N-stearoyl ethanolamide (SEA) were significantly affected by the onset of immunotherapy. The levels of O-Am and OEA increased, while the levels of the other substances decreased after immunotherapy. Four lipids (measured before immunotherapy) correlated with OS: increased levels of N-arachidonoylamide (A-Am) were associated with better OS expectancy, and an inverse relationship was observed between OS and SEA, 2-AG and 2-linoleoylglycerol (2-LG) (178). Another possible interaction between cannabinoids and ICI therapy is the gut microbiota, as already mentioned in this article (65–69).
7.3 Cannabinoids and other therapies in triple-negative breast cancer
There are no data on cannabinoids interfering with PARPi therapy and antibody-drug conjugates (sacituzumab govitecan and trastuzumab deruxtecan). However, it should be remembered that liver metabolism can be affected by cannabinoids, as mentioned above. PARPi olaparib is subject to extensive hepatic metabolism (mainly by the isoenzymes cytochrome P450 3A4/5 (179). Exatecan is primarily metabolized in the liver by hepatic P450 metabolism (CYP3A4 and CYP1A2) (180) and SN38 is metabolized in the liver by UDP-glucuronosyltransferase (UGT) (UGT1A1 and UGHT1A9) and UGT1A9 and other non-hepatic UGT enzymes (181). The main cannabinoids and their metabolites found in the plasma of cannabis users inhibit several P450 enzymes. THC competitively inhibits CYP1A2 and CBD competitively inhibits CYP3A4. THC-COOH is a substrate for UGT (167).
8 Discussion
Cannabinoids are already being used in palliative care for cancer patients (182). However, the panel of the Multinational Association of Supportive Care in Cancer (MASCC) advises against the use of cannabinoids as an adjuvant analgesic for cancer pain (183). In addition to breast cancer, cannabinoids influence tumor progression in various types of cancer, e.g. glioma/glioblastoma, skin, liver, colon, prostate (184–186). TNBC TME could be influenced by cannabinoids, however the role of CBRs on different cells infiltrating the TME is largely unknown (187). Preclinical and clinical data show that cannabinoids can help with chemotherapy-induced neuropathy (167–170). In addition, there is a worldwide trend toward legalization (e.g., in the United States and Canada) of recreational and medical use of cannabis and cannabis products, with new health concerns arising related to the increasing use of cannabis (188). Exposure to cannabis and the cannabinoids (THC, CBD and CBG) may be a risk factor for the development of breast cancer in the female population (189, 190). Therefore, many breast cancer patients are exposed to cannabinoids in one way or another during and/or after their oncologic treatment, but the safety of this exposure is uncertain and requires further research. Many patients take cannabinoids in the belief that this will help cure their disease, although there is currently no clinical data to support this claim in breast cancer patients, including TNBC. A survey of breast cancer patients found that 42% of survey participants used cannabis to treat symptoms and about half of these participants believed that cannabis could treat the cancer itself. Most participants used cannabis during active cancer treatment (191). There are no clinical data on whether cannabinoids interfere with standard TNBC treatment, although initial clinical trials show that patients with metastatic non-breast cancer who use cannabis while receiving ICI immunotherapy have poorer outcomes. The ability of cannabinoids to shorten the survival of patients treated with immunotherapy suggests the efficacy of cannabinoids and the potentially important role of the endocannabinoid system in generating the immune response triggered by ICI (177, 178). At the preclinical level, there is increasing evidence that the endocannabinoid system may play a role in tumor growth, treatment resistance, and metastasis. In most preclinical studies, various cannabinoids inhibited breast cancer cell lines and improved cancer outcomes in TNBC animal models (148–150, 192–194) usually without interfering with standard treatment (e.g., cisplatin, epirubicin, doxorubicin, paclitaxel) (162–165). However, tumor-promoting effects of cannabinoids have also been reported. An antagonistic effect of CBD on TNBC cell lines treated with cisplatin at intermediate doses was observed (166). THC exposure has been shown to stimulate the growth of the ER+ MCF-7 cell line (195) and can lead to increased growth and metastasis of tumors with little or no expression of cannabinoid receptors due to suppression of the antitumor immune response (176). Importantly, cannabinoids have immunosuppressive properties (39–43). Immunosuppression in the periphery occurs mainly through the modulation of CB2R, while CB1R are less involved in this process (44–46). Due to the current limitations of immunotherapy (non-response, disease progression and hyperprogression), new strategies are needed to sensitize cancer cells to immunotherapy (196) and modulators of CBRs may provide a new mechanism to achieve this goal (Figure 1). CBRs could be a predictive biomarker for ICI therapy or a novel mechanism to reverse resistance to ICI therapy. Importantly, phytocannabinoids are not selective for CBRs and therefore give us little insight into the role of CBRs in the pathophysiology of cancer. Selective CB2R agonists and antagonists are needed to develop potential anti-cancer drugs that target the endocannabinoid system (197).
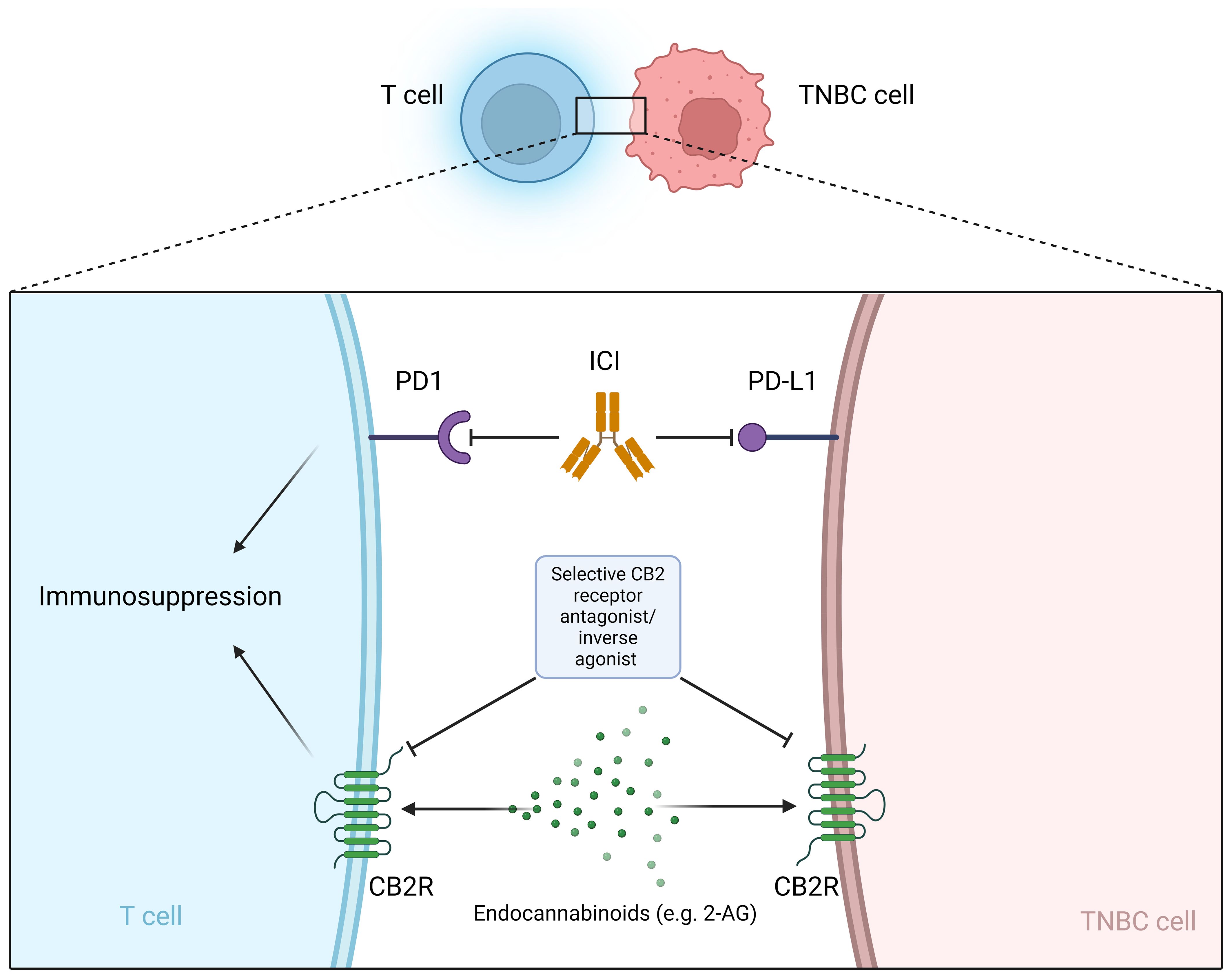
Figure 1 Selective CB2R antagonist/inverse agonist as a potential sensitizer for immunotherapy with ICI. ICI, Immune checkpoint inhibitors; PD-L1, programmed cell death ligand 1; PD-1, programmed cell death protein 1; CB2R, cannabinoid receptor 2; 2-AG, 2-arachidonoylglycerol.
Author contributions
LD: Writing – original draft, Visualization, Validation, Supervision, Investigation, Conceptualization. SB: Writing – review & editing, Validation. ND: Writing – review & editing, Validation. SKB: Writing – review & editing, Visualization, Validation, Supervision, Conceptualization.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The research was supported by the Slovenian Research and Innovation Agency (ARIS) (grant numbers J3-3062 and P3-0003).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Howard FM, Olopade OI. Epidemiology of triple-negative breast cancer: A review. Cancer J. (2021) 27:8–16. doi: 10.1097/PPO.0000000000000500
2. Kumar P, Aggarwal R. An overview of triple-negative breast cancer. Arch Gynecol Obstet. (2016) 293:247–69. doi: 10.1007/s00404-015-3859-y
3. Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. (2011) 121:2750–67. doi: 10.1172/JCI45014
4. Fan Y, He S. The characteristics of tumor microenvironment in triple negative breast cancer. Cancer Manag Res. (2022) 14:1–17. doi: 10.2147/CMAR.S316700
5. Ghorbaninezhad F, Asadzadeh Z, Masoumi J, Mokhtarzadeh A, Kazemi T, Aghebati-Maleki L, et al. Dendritic cell-based cancer immunotherapy in the era of immune checkpoint inhibitors: From bench to bedside. Life Sci. (2022) 297:120466. doi: 10.1016/j.lfs.2022.120466
6. Sobierajska K, Ciszewski WM, Sacewicz-Hofman I, Niewiarowska J. Endothelial cells in the tumor microenvironment. Adv Exp Med Biol. (2020) 1234:71–86. doi: 10.1007/978-3-030-37184-5_6
7. Won KA, Spruck C. Triple-negative breast cancer therapy: Current and future perspectives (Review). Int J Oncol. (2020) 57:1245–61. doi: 10.3892/ijo.2020.5135
8. Emens LA, Molinero L, Loi S, Rugo HS, Schneeweiss A, Diéras V, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer: biomarker evaluation of the IMpassion130 study. J Natl Cancer Inst. (2021) 113:1005–16. doi: 10.1093/jnci/djab004
9. Schmid P, Cortes J, Pusztai L, McArthur H, Kümmel S, Bergh J, et al. Pembrolizumab for early triple-negative breast cancer. N Engl J Med. (2020) 382:810–21. doi: 10.1056/NEJMoa1910549
10. Cortes J, Cescon DW, Rugo HS, Nowecki Z, Im SA, Yusof MM, et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomzsed, placebo-controlled, double-blind, phase 3 clinical trial. Lancet. (2020) 396:1817–28. doi: 10.1016/S0140-6736(20)32531-9
11. Sajjadi E, Venetis K, Scatena C, Fusco N. Biomarkers for precision immunotherapy in the metastatic setting: hope or reality? Ecancermedicalscience. (2020) 14:1150. doi: 10.3332/ecancer.2020.1150
12. Tutt ANJ, Garber JE, Kaufman B, Viale G, Fumagalli D, Rastogi P, et al. Adjuvant olaparib for patients with BRCA1- or BRCA2-mutated breast cancer. N Engl J Med. (2021) 384:2394–405. doi: 10.1056/NEJMoa2105215
13. Robson M, Im SA, Senkus E, Xu B, Domchek SM, Masuda N, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med. (2017) 377:523–33. doi: 10.1056/NEJMoa1706450
14. Bardia A, Hurvitz SA, Tolaney SM, Loirat D, Punie K, Oliveira M, et al. Sacituzumab govitecan in metastatic triple-negative breast cancer. N Engl J Med. (2021) 384:1529–41. doi: 10.1056/NEJMoa2028485
15. Modi S, Jacot W, Yamashita T, Sohn J, Vidal M, Tokunaga E, et al. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N Engl J Med. (2022) 387:9–20. doi: 10.1056/NEJMoa2203690
16. Howlett AC, Abood ME. CB1R and CB2R receptor pharmacology. Adv Pharmacol. (2017) 80:169–206. doi: 10.1016/bs.apha.2017.03.007
17. Pisanti S, Picardi P, D’Alessandro A, Laezza C, Bifulco M. The endocannabinoid signaling system in cancer. Trends Pharmacol Sci. (2013) 34:273–82. doi: 10.1016/j.tips.2013.03.003
18. Contino M, McCormick PJ. Editorial: the canonical and non-canonical endocannabinoid system as a target in cancer and acute and chronic pain. Front Pharmacol. (2020) 11:312. doi: 10.3389/fphar.2020.00312
19. Ryberg E, Larsson N, Sjögren S, Hjorth S, Hermansson NO, Leonova J, et al. The orphan receptor GPR55 is a novel cannabinoid receptor. Br J Pharmacol. (2007) 152:1092–101. doi: 10.1038/sj.bjp.0707460
20. McHugh D, Hu SS, Rimmerman N, Juknat A, Vogel Z, Walker JM, et al. N-arachidonoyl glycine, an abundant endogenous lipid, potently drives directed cellular migration through GPR18, the putative abnormal cannabidiol receptor. BMC Neurosci. (2010) 11:44. doi: 10.1186/1471-2202-11-44
21. Brown AJ. Novel cannabinoid receptors. Br J Pharmacol. (2007) 152:567–75. doi: 10.1038/sj.bjp.0707481
22. Bondarenko AI. Endothelial atypical cannabinoid receptor: do we have enough evidence? Br J Pharmacol. (2014) 171:5573–88. doi: 10.1111/bph.12866
23. Di Marzo V, Piscitelli F. The endocannabinoid system and its modulation by phytocannabinoids. Neurotherapeutics. (2015) 12:692–8. doi: 10.1007/s13311-015-0374-6
24. Mackie K, Devane WA, Hille B. Anandamide, an endogenous cannabinoid, inhibits calcium currents as a partial agonist in N18 neuroblastoma cells. Mol Pharmacol. (1993) 44(3):498–503.
25. Sugiura T, Kodaka T, Nakane S, Miyashita T, Kondo S, Suhara Y, et al. Evidence that the cannabinoid CB1R receptor is a 2-arachidonoylglycerol receptor. Structure-activity relationship of 2-arachidonoylglycerol, ether-linked analogues, and related compounds. J Biol Chem. (1999) 274:2794–801. doi: 10.1074/jbc.274.5.2794
26. Gonsiorek W, Lunn C, Fan X, Narula S, Lundell D, Hipkin RW. Endocannabinoid 2-arachidonyl glycerol is a full agonist through human type 2 cannabinoid receptor: antagonism by anandamide. Mol Pharmacol. (2000) 57:1045–50.
27. Pertwee RG. Ligands that target cannabinoid receptors in the brain: from THC to anandamide and beyond. Addict Biol. (2008) 13:147–59. doi: 10.1111/j.1369-1600.2008.00108.x
28. Justinová Z, Yasar S, Redhi GH, Goldberg SR. The endogenous cannabinoid 2-arachidonoylglycerol is intravenously self-administered by squirrel monkeys. J Neurosci. (2011) 31:7043–8. doi: 10.1523/JNEUROSCI.6058-10.2011
29. Sugiura T, Kishimoto S, Oka S, Gokoh M. Biochemistry, pharmacology and physiology of 2-arachidonoylglycerol, an endogenous cannabinoid receptor ligand. Prog Lipid Res. (2006) 45:405–46. doi: 10.1016/j.plipres.2006.03.003
30. Hillard CJ. Circulating endocannabinoids: from whence do they come and where are they going? Neuropsychopharmacology. (2018) 43:155–72. doi: 10.1038/npp.2017.130
31. Console-Bram L, Marcu J, Abood ME. Cannabinoid receptors: nomenclature and pharmacological principles. Prog Neuropsychopharmacol Biol Psychiatry. (2012) 38:4–15. doi: 10.1016/j.pnpbp.2012.02.009
32. Pisanti S, Malfitano AM, Ciaglia E, Lamberti A, Ranieri R, Cuomo G, et al. Cannabidiol: State of the art and new challenges for therapeutic applications. Pharmacol Ther. (2017) 175:133–50. doi: 10.1016/j.pharmthera.2017.02.041
33. Thomas A, Baillie GL, Phillips AM, Razdan RK, Ross RA, Pertwee RG. Cannabidiol displays unexpectedly high potency as an antagonist of CB1R and CB2R receptor agonists in vitro. Br J Pharmacol. (2007) 150:613–23. doi: 10.1038/sj.bjp.0707133
34. Castaneto MS, Gorelick DA, Desrosiers NA, Hartman RL, Pirard S, Huestis MA. Synthetic cannabinoids: epidemiology, pharmacodynamics, and clinical implications. Drug Alcohol Depend. (2014) 144:12–41. doi: 10.1016/j.drugalcdep.2014.08.005
35. Ye L, Cao Z, Wang W, Zhou N. New insights in cannabinoid receptor structure and signaling. Curr Mol Pharmacol. (2019) 12:239–48. doi: 10.2174/1874467212666190215112036
36. Almogi-Hazan O, Or R. Cannabis, the endocannabinoid system and immunity-the journey from the bedside to the bench and back. Int J Mol Sci. (2020) 21:4448. doi: 10.3390/ijms21124448
37. Köse S, Aerts-Kaya F, Köprü ÇZ, Nemutlu E, Kuşkonmaz B, Karaosmanoğlu B, et al. Human bone marrow mesenchymal stem cells secrete endocannabinoids that stimulate in vitro hematopoietic stem cell migration effectively comparable to beta-adrenergic stimulation. Exp Hematol. (2018) 57:30–41.e1. doi: 10.1016/j.exphem.2017.09.009
38. Tanasescu R, Constantinescu CS. Cannabinoids and the immune system: an overview. Immunobiology. (2010) 215:588–97. doi: 10.1016/j.imbio.2009.12.005
39. Kumar RN, Chambers WA, Pertwee RG. Pharmacological actions and therapeutic uses of cannabis and cannabinoids. Anesthesia. (2001) 56:1059–68. doi: 10.1046/j.1365-2044.2001.02269.x
40. Klein TW, Newton C, Larsen K, Lu L, Perkins I, Nong L, et al. The cannabinoid system and immune modulation. J Leukoc Biol. (2003) 74:486–96. doi: 10.1189/jlb.0303101
41. Berdyshev EV. Cannabinoid receptors and the regulation of immune response. Chem Phys Lipids. (2000) 108:169–90. doi: 10.1016/S0009-3084(00)00195-X
42. Cabral GA, Staab A. Effects on the immune system. Handb Exp Pharmacol. (2005) 168):385–423. doi: 10.1007/3-540-26573-2_13
43. Greineisen WE, Turner H. Immunoactive effects of cannabinoids: considerations for the therapeutic use of cannabinoid receptor agonists and antagonists. Int Immunopharmacol. (2010) 10:547–55. doi: 10.1016/j.intimp.2010.02.012
44. Galiègue S, Mary S, Marchand J, Dussossoy D, Carrière D, Carayon P, et al. Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur J Biochem. (1995) 232:54–61. doi: 10.1111/j.1432-1033.1995.tb20780.x
45. Pettit DA, Anders DL, Harrison MP, Cabral GA. Cannabinoid receptor expression in immune cells. Adv Exp Med Biol. (1996) 402:119–29. doi: 10.1007/978-1-4613-0407-4_17
46. Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. (1993) 365:61–5. doi: 10.1038/365061a0
47. Grabon W, Rheims S, Smith J, Bodennec J, Belmeguenai A, Bezin L. CB2 receptor in the CNS: From immune and neuronal modulation to behavior. Neurosci Biobehav Rev. (2023) 150:105226. doi: 10.1016/j.neubiorev.2023.105226
48. Howlett AC. Pharmacology of cannabinoid receptors. Annu Rev Pharmacol Toxicol. (1995) 35:607–34. doi: 10.1146/annurev.pa.35.040195.003135
49. Pertwee RG, Ross RA. Cannabinoid receptors and their ligands. Prostaglandins Leukot Essent Fatty Acids. (2002) 66:101–21. doi: 10.1054/plef.2001.0341
50. Small-Howard AL, Shimoda LM, Adra CN, Turner H. Anti-inflammatory potential of CB1R-mediated cAMP elevation in mast cells. Biochem J. (2005) 388:465–73. doi: 10.1042/BJ20041682
51. Hollister LE. Marijuana and immunity. J Psychoactive Drugs. (1992) 24:159–64. doi: 10.1080/02791072.1992.10471635
52. Zurier RB. Prospects for cannabinoids as anti-inflammatory agents. J Cell Biochem. (2003) 88:462–6. doi: 10.1002/jcb.10291
53. Cabral GA, Dove Pettit DA. Drugs and immunity: cannabinoids and their role in decreased resistance to infectious disease. J Neuroimmunol. (1998) 83:116–23. doi: 10.1016/S0165-5728(97)00227-0
54. De Filippis D, D’Amico A, Iuvone T. Cannabinomimetic control of mast cell mediator release: new perspective in chronic inflammation. J Neuroendocrinol. (2008) 20:20–5. doi: 10.1111/j.1365-2826.2008.01674.x
55. Kaslow RA, Blackwelder WC, Ostrow DG, Yerg D, Palenicek J, Coulson AH, et al. No evidence for a role of alcohol or other psychoactive drugs in accelerating immunodeficiency in HIV-1-positive individuals. A report from the Multicenter AIDS Cohort Study. JAMA. (1989) 261:3424–9. doi: 10.1001/jama.1989.03420230078030
56. Abrams DI, Hilton JF, Leiser RJ, Shade SB, Elbeik TA, Aweeka FT, et al. Short-term effects of cannabinoids in patients with HIV-1 infection: a randomized, placebo-controlled clinical trial. Ann Intern Med. (2003) 139:258–66. doi: 10.7326/0003-4819-139-4-200308190-00008
57. Bredt BM, Higuera-Alhino D, Shade SB, Hebert SJ, McCune JM, Abrams DI. Short-term effects of cannabinoids on immune phenotype and function in HIV-1-infected patients. J Clin Pharmacol. (2002) 42:82S–9S. doi: 10.1002/j.1552-4604.2002.tb06007.x
58. Pacifici R, Zuccaro P, Farré M, Poudevida S, Abanades S, Pichini S, et al. Combined immunomodulating properties of 3,4-methylenedioxymethamphetamine (MDMA) and cannabis in humans. Addiction. (2007) 102:931–6. doi: 10.1111/j.1360-0443.2007.01805.x
59. Pacifici R, Zuccaro P, Pichini S, Roset PN, Poudevida S, Farré M, et al. Modulation of the immune system in cannabis users. JAMA. (2003) 289:1929–31. doi: 10.1001/jama.289.15.1929-b
60. Nahas GG, Suciu-Foca N, Armand JP, Morishima A. Inhibition of cellular mediated immunity in marihuana smokers. Science. (1974) 183:419–20. doi: 10.1126/science.183.4123.419
61. Tashkin DP. Smoked marijuana as a cause of lung injury. Monaldi Arch Chest Dis. (2005) 63:93–100. doi: 10.4081/monaldi.2005.645
62. Newton CA, Klein TW, Friedman H. Secondary immunity to Legionella pneumophila and Th1 activity are suppressed by delta-9-tetrahydrocannabinol injection. Infect Immun. (1994) 62:4015–20. doi: 10.1128/iai.62.9.4015-4020.1994
63. Maykut MO. Health consequences of acute and chronic marihuana use. Prog Neuropsychopharmacol Biol Psychiatry. (1985) 9:209–38. doi: 10.1016/0278-5846(85)90085-5
64. Klein TW, Newton C, Friedman H. Cannabinoid receptors and immunity. Immunol Today. (1998) 19:373–81. doi: 10.1016/S0167-5699(98)01300-0
65. Karoly HC, Mueller RL, Bidwell LC, Hutchison KE. Cannabinoids and the microbiota-gut-brain axis: emerging effects of cannabidiol and potential applications to alcohol use disorders. Alcohol Clin Exp Res. (2020) 44:340–53. doi: 10.1111/acer.14256
66. McDew-White M, Lee E, Premadasa LS, Alvarez X, Okeoma CM, Mohan M. Cannabinoids modulate the microbiota-gut-brain axis in HIV/SIV infection by reducing neuroinflammation and dysbiosis while concurrently elevating endocannabinoid and indole-3-propionate levels. J Neuroinflamm. (2023) 20:62. doi: 10.1186/s12974-023-02729-6
67. Ibrahim I, Syamala S, Ayariga JA, Xu J, Robertson BK, Meenakshisundaram S, et al. Gut-brain, gut-bone axes, and the impact of cannabinoids. Metabolites. (2022) 12(12):1247. doi: 10.3390/metabo12121247
68. Lu Y, Yuan X, Wang M, He Z, Li H, Wang J, et al. Gut microbiota influence immunotherapy responses: mechanisms and therapeutic strategies. J Hematol Oncol. (2022) 15:47. doi: 10.1186/s13045-022-01273-9
69. Zhou CB, Zhou YL, Fang JY. Gut microbiota in cancer immune response and immunotherapy. Trends Cancer. (2021) 7:647–60. doi: 10.1016/j.trecan.2021.01.010
70. Bibbò S, Porcari S, Del Vecchio LE, Severino A, Mullish BH, Ianiro G, et al. Gut microbiota and immunotherapy of renal cell carcinoma. Hum Vaccin Immunother. (2023) 19:2268982. doi: 10.1080/21645515.2023.2268982
71. Wang M, Yang G, Tian Y, Zhang Q, Liu Z, Xin Y. The role of the gut microbiota in gastric cancer: the immunoregulation and immunotherapy. Front Immunol. (2023) 14:1183331. doi: 10.3389/fimmu.2023.1183331
72. Simard M, Rakotoarivelo V, Di Marzo V, Flamand N. Expression and functions of the CB2R receptor in human leukocytes. Front Pharmacol. (2022) 13:826400. doi: 10.3389/fphar.2022.826400
73. Rieder SA, Chauhan A, Singh U, Nagarkatti M, Nagarkatti P. Cannabinoid-induced apoptosis in immune cells as a pathway to immunosuppression. Immunobiology. (2010) 215:598–605. doi: 10.1016/j.imbio.2009.04.001
74. Börner C, Smida M, Höllt V, Schraven B, Kraus J. Cannabinoid receptor type 1- and 2-mediated increase in cyclic AMP inhibits T cell receptor-triggered signaling. J Biol Chem. (2009) 284:35450–60. doi: 10.1074/jbc.M109.006338
75. Cencioni MT, Chiurchiù V, Catanzaro G, Borsellino G, Bernardi G, Battistini L, et al. Anandamide suppresses proliferation and cytokine release from primary human T-lymphocytes mainly via CB2R receptors. PloS One. (2010) 5:e8688. doi: 10.1371/journal.pone.0008688
76. Capozzi A, Mattei V, Martellucci S, Manganelli V, Saccomanni G, Garofalo T, et al. Anti-proliferative properties and proapoptotic function of new CB2R selective cannabinoid receptor agonist in jurkat leukemia cells. Int J Mol Sci. (2018) 19:1958. doi: 10.3390/ijms19071958
77. Herrera B, Carracedo A, Diez-Zaera M, Gómez del Pulgar T, Guzmán M, Velasco G. The CB2R cannabinoid receptor signals apoptosis via ceramide-dependent activation of the mitochondrial intrinsic pathway. Exp Cell Res. (2006) 312:2121–31. doi: 10.1016/j.yexcr.2006.03.009
78. Huang ZB, Zheng YX, Li N, Cai SL, Huang Y, Wang J, et al. Protective effects of specific cannabinoid receptor 2 agonist GW405833 on concanavalin A-induced acute liver injury in mice. Acta Pharmacol Sin. (2019) 40:1404–11. doi: 10.1038/s41401-019-0213-0
79. Zhu LX, Sharma S, Stolina M, Gardner B, Roth MD, Tashkin DP, et al. Delta-9-tetrahydrocannabinol inhibits antitumor immunity by a CB2 receptor-mediated, cytokine-dependent pathway. J Immunol. (2000) 165:373–80. doi: 10.4049/jimmunol.165.1.373
80. Gardner B, Zu LX, Sharma S, Liu Q, Makriyannis A, Tashkin DP, et al. Autocrine and paracrine regulation of lymphocyte CB2 receptor expression by TGF-beta. Biochem Biophys Res Commun. (2002) 290:91–6. doi: 10.1006/bbrc.2001.6179
81. Ghosh S, Preet A, Groopman JE, Ganju RK. Cannabinoid receptor CB2 modulates the CXCL12/CXCR4-mediated chemotaxis of T lymphocytes. Mol Immunol. (2006) 43:2169–79. doi: 10.1016/j.molimm.2006.01.005
82. Coopman K, Smith LD, Wright KL, Ward SG. Temporal variation in CB2R levels following T lymphocyte activation: evidence that cannabinoids modulate CXCL12-induced chemotaxis. Int Immunopharmacol. (2007) 7:360–71. doi: 10.1016/j.intimp.2006.11.008
83. Eisenstein TK, Meissler JJ. Effects of cannabinoids on T-cell function and resistance to infection. J Neuroimmune Pharmacol. (2015) 10:204–16. doi: 10.1007/s11481-015-9603-3
84. Xiong X, Chen S, Shen J, You H, Yang H, Yan C, et al. Cannabis suppresses antitumor immunity by inhibiting JAK/STAT signaling in T cells through CNR2. Signal Transduct Target Ther. (2022) 7:99. doi: 10.1038/s41392-022-00918-y
85. Yuan M, Kiertscher SM, Cheng Q, Zoumalan R, Tashkin DP, Roth MD. Delta 9-Tetrahydrocannabinol regulates Th1/Th2 cytokine balance in activated human T cells. J Neuroimmunol. (2002) 133:124–31. doi: 10.1016/S0165-5728(02)00370-3
86. Tiberi M, Evron T, Saracini S, Boffa L, Mercuri NB, Chintalacharuvu SR, et al. Potent T cell-mediated anti-inflammatory role of the selective CB2R agonist lenabasum in multiple sclerosis. Neuropathol Appl Neurobiol. (2022) 48:e12768. doi: 10.1111/nan.12768
87. Pan MR, Wu CC, Kan JY, Li QL, Chang SJ, Wu CC, et al. Impact of FAK expression on the cytotoxic effects of CIK therapy in triple-negative breast cancer. Cancers (Basel). (2019) 12:94. doi: 10.3390/cancers12010094
88. Gao X, Mi Y, Guo N, Xu H, Xu L, Gou X, et al. Cytokine-induced killer cells as pharmacological tools for cancer immunotherapy. Front Immunol. (2017) 8:774. doi: 10.3389/fimmu.2017.00774
89. Sommaggio R, Cappuzzello E, Dalla Pietà A, Tosi A, Palmerini P, Carpanese D, et al. Adoptive cell therapy of triple negative breast cancer with redirected cytokine-induced killer cells. Oncoimmunology. (2020) 9:1777046. doi: 10.1080/2162402X.2020.1777046
90. Garofano F, Schmidt-Wolf IGH. High expression of cannabinoid receptor 2 on cytokine-induced killer cells and multiple myeloma cells. Int J Mol Sci. (2020) 21:3800. doi: 10.3390/ijms21113800
91. Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. (2007) 27:111–22. doi: 10.1016/j.immuni.2007.05.016
92. Karwacz K, Bricogne C, MacDonald D, Arce F, Bennett CL, Collins M, et al. PD-L1 co-stimulation contributes to ligand-induced T cell receptor down-modulation on CD8+ T cells. EMBO Mol Med. (2011) 3:581–92. doi: 10.1002/emmm.201100165
93. Wolfson ML, Muzzio DO, Ehrhardt J, Franchi AM, Zygmunt M, Jensen F. Expression analysis of cannabinoid receptors 1 and 2 in B cells during pregnancy and their role on cytokine production. J Reprod Immunol. (2016) 116:23–7. doi: 10.1016/j.jri.2016.05.001
94. Castaneda JT, Harui A, Kiertscher SM, Roth JD, Roth MD. Differential expression of intracellular and ex-tracellular cb(2) cannabinoid receptor protein by human peripheral blood leukocytes. J Neuroimmun Pharmacol. (2013) 8:323–32. doi: 10.1007/s11481-012-9430-8
95. Carayon P, Marchand J, Dussossoy D, Derocq JM, Jbilo O, Bord A, et al. Modulation and functional involvement of CB2R peripheral cannabinoid receptors during B-cell differentiation. Blood. (1998) 92:3605–15. doi: 10.1182/blood.V92.10.3605
96. Jordà MA, Verbakel SE, Valk PJ, Vankan-Berkhoudt YV, Maccarrone M, Finazzi-Agrò A, et al. Hematopoietic cells expressing the peripheral cannabinoid receptor migrate in response to the endocannabinoid 2-arachidonoylglycerol. Blood. (2002) 99:2786–93. doi: 10.1182/blood.V99.8.2786
97. Tanikawa T, Kurohane K, Imai Y. Induction of preferential chemotaxis of unstimulated B-lymphocytes by 2-arachidonoylglycerol in immunized mice. Microbiol Immunol. (2007) 51:1013–9. doi: 10.1111/j.1348-0421.2007.tb03985.x
98. Agudelo M, Newton C, Widen R, Sherwood T, Nong L, Friedman H, et al. Cannabinoid receptor 2 (CB2R) mediates immunoglobulin class switching from IgM to IgE in cultures of murine-purified B lymphocytes. J Neuroimmune Pharmacol. (2008) 3:35–42. doi: 10.1007/s11481-007-9088-9
99. Pereira JP, An J, Xu Y, Huang Y, Cyster JG. Cannabinoid receptor 2 mediates the retention of immature B cells in bone marrow sinusoids. Nat Immunol. (2009) 10:403–11. doi: 10.1038/ni.1710
100. Freund P, Porpaczy EA, Le T, Gruber M, Pausz C, Staber P, et al. Cannabinoid receptors are overexpressed in CLL but of limited potential for therapeutic exploitation. PloS One. (2016) 11:e0156693. doi: 10.1371/journal.pone.0156693
101. Montecucco F, Burger F, Mach F, Steffens S. CB2 cannabinoid receptor agonist JWH-015 modulates human monocyte migration through defined intracellular signaling pathways. Am J Physiol Circ Physiol. (2008) 294:H1145–55. doi: 10.1152/ajpheart.01328.2007
102. Kishimoto S, Gokoh M, Oka S, Muramatsu M, Kajiwara T, Waku K, et al. 2-Arachidonoylglycerol Induces the Migration of HL-60 Cells Differentiated into Macrophage-like Cells and Human Peripheral Blood Monocytes through the Cannabinoid CB2 Receptor-dependent Mechanism. J Biol Chem. (2003) 278:24469–75. doi: 10.1074/jbc.M301359200
103. Sacerdote P, Massi P, Panerai AE, Parolaro D. In vivo and in vitro treatment with the synthetic cannabinoid CP55,940 decreases the in vitro migration of macrophages in the rat: Involvement of both CB1 and CB2 receptors. J Neuroimmunol. (2000) 109:155–63. doi: 10.1016/S0165-5728(00)00307-6
104. Di Marzo V, Bisogno T, De Petrocellis L, Melck D, Orlando P, Wagner JA, et al. Biosynthesis and inactivation of the endocannabinoid 2-arachidonoylglycerol in circulating and tumoral macrophages. JBIC J Biol Inorg Chem. (1999) 264:258–67. doi: 10.1046/j.1432-1327.1999.00631.x
105. Cabral GA, Mishkin EM. Delta-9-tetrahydrocannabinol inhibits macrophage protein expression in response to bacterial immunomodulators. J Toxicol Environ Health. (1989) 26:175–82. doi: 10.1080/15287398909531243
106. Lopez-Cepero M, Friedman M, Klein T, Friedman H. Tetrahydrocannabinol-induced suppression of macrophage spreading and phagocytic activity in vitro. J Leukoc Biol. (1986) 39:679–86. doi: 10.1002/jlb.39.6.679
107. McKallip RJ, Lombard C, Fisher M, Martin BR, Ryu S, Grant S, et al. Targeting CB2R cannabinoid receptors as a novel therapy to treat Malignant lymphoblastic disease. Blood. (2002) 100:627–34. doi: 10.1182/blood-2002-01-0098
108. Duerr GD, Heinemann JC, Suchan G, Kolobara E, Wenzel D, Geisen C, et al. The endocannabinoid-CB2R receptor axis protects the ischemic heart at the early stage of cardiomyopathy. Basic Res Cardiol. (2014) 109:425. doi: 10.1007/s00395-014-0425-x
109. Denaës T, Lodder J, Chobert MN, Ruiz I, Pawlotsky JM, Lotersztajn S, et al. The cannabinoid receptor 2 protects against alcoholic liver disease via a macrophage autophagy-dependent pathway. Sci Rep. (2016) 6:28806. doi: 10.1038/srep28806
110. Du Y, Ren P, Wang Q, Jiang SK, Zhang M, Li JY, et al. Cannabinoid 2 receptor attenuates inflammation during skin wound healing by inhibiting M1 macrophages rather than activating M2 macrophages. J Inflamm (Lond). (2018) 15:25. doi: 10.1186/s12950-018-0201-z
111. Correia-Sá I, Carvalho C A, MaChado V, Carvalho S, Serrão P, Marques M, et al. Targeting cannabinoid receptor 2 (CB2R) limits collagen production-An in vitro study in a primary culture of human fibroblasts. Fundam Clin Pharmacol. (2022) 36:89–99. doi: 10.1111/fcp.12716
112. Navarro-Saiz LM, Bernal-Cepeda LJ, Castellanos JE. Immune challenges upregulate the expression of cannabinoid receptors in cultured human odontoblasts and gingival fibroblasts. Acta Odontol Latinoam. (2022) 35:80–9. doi: 10.54589/aol.35/2/80
113. McPartland JM. Expression of the endocannabinoid system in fibroblast s and myofascial tissues. J Bodyw Mov Ther. (2008) 12:169–82. doi: 10.1016/j.jbmt.2008.01.004
114. Deusch E, Kraft B, Nahlik G, Weigl L, Hohenegger M, Kress HG. No evidence for direct modulatory effects of delta 9-tetrahydrocannabinol on human polymorphonuclear leukocytes. J Neuroimmunol. (2003) 141:99–103. doi: 10.1016/S0165-5728(03)00259-5
115. Kraft B, Wintersberger W, Kress HG. Cannabinoid receptor-independent suppression of the superoxide generation of human neutrophils (PMN) by CP55 940, but not by anandamide. Life Sci. (2004) 75:969–77. doi: 10.1016/j.lfs.2004.02.007
116. Oka S, Ikeda S, Kishimoto S, Gokoh M, Yanagimoto S, Waku K, et al. 2-arachidonoylglycerol, an endogenous cannabinoid receptor ligand, induces the migration of EoL-1 human eosinophilic leukemia cells and human peripheral blood eosinophils. J Leukoc Biol. (2004) 76:1002–9. doi: 10.1189/jlb.0404252
117. McHugh D, Tanner C, Mechoulam R, Pertwee RG, Ross RA. Inhibition of human neutrophil chemotaxis by endogenous cannabinoids and phytocannabinoids: evidence for a site distinct from CB1R and CB2R. Mol Pharmacol. (2008) 73:441–50. doi: 10.1124/mol.107.041863
118. Chouinard F, Turcotte C, Guan X, Larose MC, Poirier S, Bouchard L, et al. 2-Arachidonoyl-glycerol- and arachidonic acid-stimulated neutrophils release antimicrobial effectors against E. coli, S. aureus, HSV-1, and RSV. J Leukoc Biol. (2013) 93:267–76. doi: 10.1189/jlb.0412200
119. Montecucco F, Di Marzo V, da Silva RF, Vuilleumier N, Capettini L, Lenglet S, et al. The activation of the cannabinoid receptor type 2 reduces neutrophilic protease-mediated vulnerability in atherosclerotic plaques. Eur Heart J. (2012) 33:846–56. doi: 10.1093/eurheartj/ehr449
120. Balenga NA, Aflaki E, Kargl J, Platzer W, Schröder R, Blättermann S, et al. GPR55 regulates cannabinoid 2 receptor-mediated responses in human neutrophils. Cell Res. (2011) 21:1452–69. doi: 10.1038/cr.2011.60
121. Coffelt SB, Wellenstein MD, De Visser KE. Neutrophils in cancer: Neutral no more. Nat Rev Cancer. (2016) 16:431–46. doi: 10.1038/nrc.2016.52
122. Thul PJ, Åkesson L, Wiking M, Mahdessian D, Geladaki A, Ait Blal H, et al. A subcellular map of the human proteome. Science. (2017) 356:eaal3321. doi: 10.1126/science.aal3321
123. Graham ES, Angel CE, Schwarcz LE, Dunbar PR, Glass M. Detailed characterisation of CB2R receptor protein expression in peripheral blood immune cells from healthy human volunteers using flow cytometry. Int Immunopathol Pharmacol. (2009) 23:25–34. doi: 10.1177/039463201002300103
124. Olivas-Aguirre M, Gutiérrez-Iñiguez C, Pottosin I, Dobrovinskaya O. Molecular targets for cannabinoids in natural killer cells: do they modulate the antitumor activity? Receptors. (2024) 3:122–44. doi: 10.3390/receptors3020007
125. Chiurchiù V, Battistini L, Maccarrone M. Endocannabinoid signaling in innate and adaptive immunity. Immunology. (2015) 144:352–64.
126. Kishimoto S, Muramatsu M, Gokoh M, Oka S, Waku K, Sugiura T. Endogenous cannabinoid receptor ligand induces the migration of human natural killer cells. J Biochem. (2005) 137:217–23. doi: 10.1093/jb/mvi021
127. Wang M, Richards AL, Friedman H, Djeu JY. Selective inhibition of natural killer but not natural cytotoxic activity in a cloned cell line by delta-9-tetrahydrocannabinol. J Leukoc Biol. (1991) 50:192–7. doi: 10.1002/jlb.50.2.192
128. Pagano Zottola AC, Severi I, Cannich A, Ciofi P, Cota D, Marsicano G, et al. Expression of functional cannabinoid type-1 (CB1R) receptor in mitochondria of white adipocytes. Cells. (2022) 11:2582. doi: 10.3390/cells11162582
129. Roche R, Hoareau L, Bes-Houtmann S, Gonthier MP, Laborde C, Baron JF, et al. Presence of the cannabinoid receptors, CB1R and CB2R, in human omental and subcutaneous adipocytes. Histochem Cell Biol. (2006) 126:177–87. doi: 10.1007/s00418-005-0127-4
130. Bouaboula M, Hilairet S, Marchand J, Fajas L, Le Fur G, Casellas P. Anandamide induced PPARgamma transcriptional activation and 3T3-L1 preadipocyte differentiation. Eur J Pharmacol. (2005) 517:174–81. doi: 10.1016/j.ejphar.2005.05.032
131. Karaliota S, Siafaka-Kapadai A, Gontinou C, Psarra K, Mavri-Vavayanni M. Anandamide increases the differentiation of rat adipocytes and causes PPARgamma and CB1R receptor upregulation. Obes (Silver Spring). (2009) 17:1830–8. doi: 10.1038/oby.2009.177
132. Lindborg KA, Teachey MK, Jacob S, Henriksen EJ. Effects of in vitro antagonism of endocannabinoid-1 receptors on the glucose transport system in normal and insulin-resistant rat skeletal muscle. Diabetes Obes Metab. (2010) 12:722–30. doi: 10.1111/j.1463-1326.2010.01227.x
133. Sam AH, Salem V, Ghatei MA. Rimonabant: from RIO to ban. J Obes. (2011) 2011:432607. doi: 10.1155/2011/432607
134. Moreno E, Cavic M, Canela EI. Functional fine-tuning of metabolic pathways by the endocannabinoid system-implications for health and disease. Int J Mol Sci. (2021) 22:3661. doi: 10.3390/ijms22073661
135. Osei-Hyiaman D, DePetrillo M, Pacher P, Liu J, Radaeva S, Bátkai S, et al. Endocannabinoid activation at hepatic CB1R receptors stimulates fatty acid synthesis and contributes to diet-induced obesity. J Clin Invest. (2005) 115:1298–305. doi: 10.1172/JCI23057
136. Silvestri C, Di Marzo V. The endocannabinoid system in energy homeostasis and the etiopathology of metabolic disorders. Cell Metab. (2013) 17:475–90. doi: 10.1016/j.cmet.2013.03.001
137. Côté M, Matias I, Lemieux I, Petrosino S, Alméras N, Després JP, et al. Circulating endocannabinoid levels, abdominal adiposity and related cardiometabolic risk factors in obese men. Int J Obes (Lond). (2007) 31:692–9. doi: 10.1038/sj.ijo.0803539
138. Do Y, McKallip RJ, Nagarkatti M, Nagarkatti PS. Activation through cannabinoid receptors 1 and 2 on den-dritic cells triggers Nf-kappab-dependent apoptosis: novel role for endogenous and exogenous cannabinoids in immunoregulation. J Immunol. (2004) 173:2373–82. doi: 10.4049/jimmunol.173.4.2373
139. Matias I, Pochard P, Orlando P, Salzet M, Pestel J, Di Marzo V. Presence and regulation of the endocannabinoid system in human dendritic cells. JBIC J Biol Inorg Chem. (2002) 269:3771–8. doi: 10.1046/j.1432-1033.2002.03078.x
140. Maestroni GJM. The endogenous cannabinoid 2-arachidonoyl glycerol as in vivo chemoattractant for dendritic cells and adjuvant for Th1 response to a soluble protein. FASEB J. (2004) 18:1914–6. doi: 10.1096/fj.04-2190fje
141. Liu J, Gao B, Mirshahi F, Sanyal AJ, Khanolkar AD, Makriyannis A, et al. Functional CB1R cannabinoid receptors in human vascular endothelial cells. Biochem J. (2000) 346 Pt 3:835–40.
142. Schley M, Ständer S, Kerner J, Vajkoczy P, Schüpfer G, Dusch M, et al. Predominant CB2R receptor expression in endothelial cells of glioblastoma in humans. Brain Res Bull. (2009) 79:333–7. doi: 10.1016/j.brainresbull.2009.01.011
143. Bondarenko AI, Panasiuk O, Drachuk K, Montecucco F, Brandt KJ, Mach F. The quest for endothelial atypical cannabinoid receptor: BKCa channels act as cellular sensors for cannabinoids in in vitro and in situ endothelial cells. Vascul Pharmacol. (2018) 102:44–55. doi: 10.1016/j.vph.2018.01.004
144. Caffarel MM, Sarrió D, Palacios J, Guzmán M, Sánchez C. Delta9-tetrahydrocannabinol inhibits cell cycle progression in human breast cancer cells through Cdc2 regulation. Cancer Res. (2006) 66:6615–21. doi: 10.1158/0008-5472.CAN-05-4566
145. Pérez-Gómez E, Andradas C, Blasco-Benito S, Caffarel MM, García-Taboada E, Villa-Morales M, et al. Role of cannabinoid receptor CB2R in HER2 pro-oncogenic signaling in breast cancer. J Natl Cancer Inst. (2015) 107:djv077. doi: 10.1093/jnci/djv077
146. Andradas C, Blasco-Benito S, Castillo-Lluva S, Dillenburg-Pilla P, Diez-Alarcia R, Juanes-García A, et al. Activation of the orphan receptor GPR55 by lysophosphatidylinositol promotes metastasis in triple-negative breast cancer. Oncotarget. (2016) 7:47565–75. doi: 10.18632/oncotarget.v7i30
147. Ligresti A, Moriello AS, Starowicz K, Matias I, Pisanti S, De Petrocellis L, et al. Antitumor activity of plant cannabinoids with emphasis on the effect of cannabidiol on human breast carcinoma. J Pharmacol Exp Ther. (2006) 318:1375–87. doi: 10.1124/jpet.106.105247
148. Sultan AS, Marie MA, Sheweita SA. Novel mechanism of cannabidiol-induced apoptosis in breast cancer cell lines. Breast. (2018) 41:34–41. doi: 10.1016/j.breast.2018.06.009
149. McAllister SD, Christian RT, Horowitz MP, Garcia A, Desprez PY. Cannabidiol as a novel inhibitor of Id-1 gene expression in aggressive breast cancer cells. Mol Cancer Ther. (2007) 6:2921–7. doi: 10.1158/1535-7163.MCT-07-0371
150. McAllister SD, Murase R, Christian RT, Lau D, Zielinski AJ, Allison J, et al. Pathways mediating the effects of cannabidiol on the reduction of breast cancer cell proliferation, invasion, and metastasis. Breast Cancer Res Treat. (2011) 129:37–47. doi: 10.1007/s10549-010-1177-4
151. Elbaz M, Nasser MW, Ravi J, Wani NA, Ahirwar DK, Zhao H, et al. Modulation of the tumor microenvironment and inhibition of EGF/EGFR pathway: novel anti-tumor mechanisms of Cannabidiol in breast cancer. Mol Oncol. (2015) 9:906–19. doi: 10.1016/j.molonc.2014.12.010
152. Hanlon KE, Lozano-Ondoua AN, Umaretiya PJ, Symons-Liguori AM, Chandramouli A, Moy JK, et al. Modulation of breast cancer cell viability by a cannabinoid receptor 2 agonist, JWH-015, is calcium dependent. Breast Cancer. (2016) 8:59–71. doi: 10.2147/BCTT.S100393
153. Sarnataro D, Pisanti S, Santoro A, Gazzerro P, Malfitano AM, Laezza C, et al. The cannabinoid CB1R receptor antagonist rimonabant (SR141716) inhibits human breast cancer cell proliferation through a lipid raft-mediated mechanism. Mol Pharmacol. (2006) 70:1298–306. doi: 10.1124/mol.106.025601
154. Li P, Lin Q, Sun S, Yang N, Xia Y, Cao S, et al. Inhibition of cannabinoid receptor type 1 sensitizes triple-negative breast cancer cells to ferroptosis via regulating fatty acid metabolism. Cell Death Dis. (2022) 13:808. doi: 10.1038/s41419-022-05242-5
155. Hirao-Suzuki M, Takayuki K, Takiguchi M, Peters JM, Takeda S. Cannabidiolic acid activates the expression of the PPARβ/δ target genes in MDA-MB-231 cells. Arch Biochem Biophys. (2022) 731:109428. doi: 10.1016/j.abb.2022.109428
156. Hanifa M, Wulandari R, Zulfin UM, Nugroho EP, Haryanti S, Meiyanto E. Different cytotoxic effects of vetiver oil on three types of cancer cells, mainly targeting CNR2 on TNBC. Asian Pac J Cancer Prev. (2022) 23:241–51. doi: 10.31557/APJCP.2022.23.1.241
157. Xian S, Parayath NN, Nehoff H, Giles NM, Greish K. The use of styrene maleic acid nanomicelles encapsulating the synthetic cannabinoid analog WIN55,212-2 for the treatment of cancer. Anticancer Res. (2015) 35:4707–12.
158. Morales P, Blasco-Benito S, Andradas C, Gómez-Cañas M, Flores JM, Goya P, et al. Selective, nontoxic CB(2) cannabinoid o-quinone with in vivo activity against triple-negative breast cancer. J Med Chem. (2015) 58:2256–64. doi: 10.1021/acs.jmedchem.5b00078
159. Zhang J, Zhang S, Liu Y, Su M, Ling X, Liu F, et al. Combined CB2R receptor agonist and photodynamic therapy synergistically inhibit tumor growth in triple negative breast cancer. Photodiagnosis Photodyn Ther. (2018) 24:185–91. doi: 10.1016/j.pdpdt.2018.09.006
160. Song Q, Zhang W, Shi D, Zhang Z, Zhao Q, Wang M, et al. Overexpressi on of cannabinoid receptor 2 is associated with human breast cancer proliferation, apoptosis, chemosensitivity and prognosis via the PI3K/Akt/mTOR signaling pathway. Cancer Med. (2023) 12:13538–50. doi: 10.1002/cam4.6037
161. Blasco-Benito S, Seijo-Vila M, Caro-Villalobos M, Tundidor I, Andradas C, García-Taboada E, et al. Appraising the “entourage effect”: Antitumor action of a pure cannabinoid versus a botanical drug preparation in preclinical models of breast cancer. Biochem Pharmacol. (2018) 157:285–93. doi: 10.1016/j.bcp.2018.06.025
162. Greish K, Mathur A, Al Zahrani R, Elkaissi S, Al Jishi M, Nazzal O, et al. Synthetic cannabinoids nano-micelles for the management of triple negative breast cancer. J Control Release. (2018) 291:184–95. doi: 10.1016/j.jconrel.2018.10.030
163. Dobovišek L, Novak M, Krstanović F, Borštnar S, Turnšek TL, Debeljak N. Effect of combining CBD with standard breast cancer therapeutics. Adv Cancer Biology-Metastasis. (2022) 4:100038. doi: 10.1016/j.adcanc.2022.100038
164. Surapaneni SK, Patel N, Sun L, Kommineni N, Kalvala AK, Gebeyehu A, et al. Anticancer and chemosensitization effects of cannabidiol in 2D and 3D cultures of TNBC: involvement of GADD45α, integrin-α5, -β5, -β1, and autophagy. Drug Deliv Transl Res. (2022) 12:2762–77. doi: 10.1007/s13346-022-01137-2
165. Patel N, Kommineni N, Surapaneni SK, Kalvala A, Yaun X, Gebeyehu A, et al. Cannabidiol loaded extracellular vesicles sensitize triple-negative breast cancer to doxorubicin in both in-vitro and in vivo models. Int J Pharm. (2021) 607:120943. doi: 10.1016/j.ijpharm.2021.120943
166. D’Aloia A, Ceriani M, Tisi R, Stucchi S, Sacco E, Costa B. Cannabidiol antiproliferative effect in triple-negative breast cancer MDA-MB-231 cells is modulated by its physical state and by IGF-1. Int J Mol Sci. (2022) 23:7145. doi: 10.3390/ijms23137145
167. Nasrin S, Watson CJW, Perez-Paramo YX, Lazarus P. Cannabinoid metabolites as inhibitors of major hepatic CYP450 enzymes, with implications for cannabis-drug interactions. Drug Metab Dispos. (2021) 49:1070–80. doi: 10.1124/dmd.121.000442
168. O’Hearn S, Diaz P, Wan BA, DeAngelis C, Lao N, Malek L, et al. Modulating the endocannabinoid pathway as treatment for peripheral neuropathic pain: a selected review of preclinical studies. Ann Palliat Med. (2017) 6:S209–S14. doi: 10.21037/apm
169. Blanton HL, Brelsfoard J, DeTurk N, Pruitt K, Narasimhan M, Morgan DJ, et al. Cannabinoids: current and future options to treat chronic and chemotherapy-induced neuropathic pain. Drugs. (2019) 79:969–95. doi: 10.1007/s40265-019-01132-x
170. Zaiss M, Uhlig J, Zahn MO, Decker T, Lehmann HC, Harde J, et al. Improving chemotherapy-induced peripheral neuropathy in patients with breast or colon cancer after end of (Neo)adjuvant therapy: results from the observational study STEFANO. Oncol Res Treat. (2021) 44:613–21. doi: 10.1159/000519000
171. Hao E, Mukhopadhyay P, Cao Z, Erdelyi K, Holovac E, Liaudet L, et al. Cannabidiol protects against doxorubicin-induced cardiomyopathy by modulating mitochondrial function and biogenesis. Mol Med. (2015) 21:38–45. doi: 10.2119/molmed.2014.00261
172. Fouad AA, Albuali WH, Al-Mulhim AS, Jresat I. Cardioprotective effect of cannabidiol in rats exposed to doxorubicin toxicity. Environ Toxicol Pharmacol. (2013) 36:347–57. doi: 10.1016/j.etap.2013.04.018
173. Hydock DS, Lien CY, Hayward R. Anandamide preserves cardiac function and geometry in an acute doxorubicin cardiotoxicity rat model. J Cardiovasc Pharmacol Ther. (2009) 14:59–67. doi: 10.1177/1074248408329449
174. Zhang S. Chemotherapy-induced peripheral neuropathy and rehabilitation: A review. Semin Oncol. (2021) 48:193–207. doi: 10.1053/j.seminoncol.2021.09.004
175. Padegimas A, Clasen S, Ky B. Cardioprotective strategies to prevent breast cancer therapy-induced cardiotoxicity. Trends Cardiovasc Med. (2020) 30:22–8. doi: 10.1016/j.tcm.2019.01.006
176. McKallip RJ, Nagarkatti M, Nagarkatti PS. Delta-9-tetrahydrocannabinol enhances breast cancer growth and metastasis by suppression of the antitumor immune response. J Immunol. (2005) 174:3281–9. doi: 10.4049/jimmunol.174.6.3281
177. Taha T, Meiri D, Talhamy S, Wollner M, Peer A, Bar-Sela G. Cannabis impacts tumor response rate to nivolumab in patients with advanced Malignancies. Oncologist. (2019) 24:549–54. doi: 10.1634/theoncologist.2018-0383
178. Bar-Sela G, Cohen I, Campisi-Pinto S, Lewitus GM, Oz-Ari L, Jehassi A, et al. Cannabis consumption used by cancer patients during immunotherapy correlates with poor clinical outcome. Cancers (Basel). (2020) 12:2447. doi: 10.3390/cancers12092447
179. Rolfo C, Isambert N, Italiano A, Molife LR, Schellens JHM, Blay JY, et al. Pharmacokinetics and safety of olaparib in patients with advanced solid tumors and mild or moderate hepatic impairment. Br J Clin Pharmacol. (2020) 86:1807–18. doi: 10.1111/bcp.14283
180. De Jager R, Cheverton P, Tamanoi K, Coyle J, Ducharme M, Sakamoto N, et al. DX-8951f: summary of phase I clinical trials. Ann N Y Acad Sci. (2000) 922:260–73. doi: 10.1111/j.1749-6632.2000.tb07044.x
181. Kciuk M, Marciniak B, Kontek R. Irinotecan—Still an important player in cancer chemotherapy: A comprehensive overview. Int J Mol Sci. (2020) 21:4919. doi: 10.3390/ijms21144919
182. Doppen M, Kung S, Maijers I, John M, Dunphy H, Townsley H, et al. Cannabis in palliative care: A systematic review of current evidence. J Pain Symptom Manage. (2022) 64:e260–84. doi: 10.1016/j.jpainsymman.2022.06.002
183. To J, Davis M, Sbrana A, Alderman B, Hui D, Mukhopadhyay S, et al. MASCC guideline: cannabis for cancer-related pain and risk of harms and adverse events. Support Care Cancer. (2023) 31:202. doi: 10.1007/s00520-023-07662-1
184. Lah TT, Novak M, Pena Almidon MA, Marinelli O, Žvar Baškovič B, Majc B, et al. Cannabigerol is a potential therapeutic agent in a novel combined therapy for glioblastoma. Cells. (2021) 10:340. doi: 10.3390/cells10020340
185. Wang F, Multhoff G. Repurposing cannabidiol as a potential drug candidate for anti-tumor therapies. Biomolecules. (2021) 11:582. doi: 10.3390/biom11040582
186. Singh K, Jamshidi N, Zomer R, Piva TJ, Mantri N. Cannabinoids and prostate cancer: A systematic review of animal studies. Int J Mol Sci. (2020) 21:6265. doi: 10.3390/ijms21176265
187. Braile M, Marcella S, Marone G, Galdiero MR, Varricchi G, Loffredo S. The interplay between the immune and the endocannabinoid systems in cancer. Cells. (2021) 10:1282. doi: 10.3390/cells10061282
188. Monte AA, Zane RD, Heard KJ. The implications of marijuana legalization in Colorado. JAMA. (2015) 313:241–2. doi: 10.1001/jama.2014.17057
189. Reece AS, Hulse GK. Geospatiotemporal and causal inference study of cannabis and other drugs as risk factors for female breast cancer USA 2003-2017. Environ Epigenet. (2022) 8:dvac006. doi: 10.1093/eep/dvac006
190. Huang P, Zhang PF, Li Q. Causal relationship between cannabis use and cancer: a genetically informed perspective. J Cancer Res Clin Oncol. (2023) 149:8631–8. doi: 10.1007/s00432-023-04807-x
191. Weiss MC, Hibbs JE, Buckley ME, Danese SR, Leitenberger A, Bollmann-Jenkins M, et al. A Coala-T-Cannabis Survey Study of breast cancer patients’ use of cannabis before, during, and after treatment. Cancer. (2022) 128:160–8. doi: 10.1002/cncr.33906
192. Melck D, Rueda D, Galve-Roperh I, De Petrocellis L, Guzmán M, Di Marzo V. Involvement of the cAMP/protein kinase A pathway and of mitogen-activated protein kinase in the anti-proliferative effects of anandamide in human breast cancer cells. FEBS Lett. (1999) 463:235–40. doi: 10.1016/S0014-5793(99)01639-7
193. Melck D, De Petrocellis L, Orlando P, Bisogno T, Laezza C, Bifulco M, et al. Suppression of nerve growth factor Trk receptors and prolactin receptors by endocannabinoids leads to inhibition of human breast and prostate cancer cell proliferation. Endocrinology. (2000) 141:118–26. doi: 10.1210/endo.141.1.7239
194. De Petrocellis L, Melck D, Palmisano A, Bisogno T, Laezza C, Bifulco M, et al. The endogenous cannabinoid anandamide inhibits human breast cancer cell proliferation. Proc Natl Acad Sci USA. (1998) 95:8375–80. doi: 10.1073/pnas.95.14.8375
195. Takeda S, Yamamoto I, Watanabe K. Modulation of Delta9-tetrahydrocannabinol-induced MCF-7 breast cancer cell growth by cyclooxygenase and aromatase. Toxicology. (2009) 259:25–32. doi: 10.1016/j.tox.2009.01.024
196. George A, Sahin I, Carneiro BA, Dizon DS, Safran HP, El-Deiry WS. Strategies to sensitize cancer cells to immunotherapy. Hum Vaccin Immunother. (2021) 17:2595–601. doi: 10.1080/21645515.2021.1891817
Keywords: triple-negative breast cancer, cannabinoids, endocannabinoid system, chemotherapy, immunotherapy, immune system, tumor microenvironment, immune checkpoint inhibitors
Citation: Dobovišek L, Borštnar S, Debeljak N and Kranjc Brezar S (2024) Cannabinoids and triple-negative breast cancer treatment. Front. Immunol. 15:1386548. doi: 10.3389/fimmu.2024.1386548
Received: 15 February 2024; Accepted: 15 July 2024;
Published: 08 August 2024.
Edited by:
Xiangyi Kong, Chinese Academy of Medical Sciences and Peking Union Medical College, ChinaReviewed by:
Marialessandra Contino, University of Bari Aldo Moro, ItalyNabanita Chatterjee, The Ohio State University, United States
Copyright © 2024 Dobovišek, Borštnar, Debeljak and Kranjc Brezar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Simona Kranjc Brezar, c2tyYW5qY0BvbmtvLWkuc2k=
 Luka Dobovišek
Luka Dobovišek Simona Borštnar1
Simona Borštnar1 Nataša Debeljak
Nataša Debeljak Simona Kranjc Brezar
Simona Kranjc Brezar