
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol., 21 May 2024
Sec. Nutritional Immunology
Volume 15 - 2024 | https://doi.org/10.3389/fimmu.2024.1383122
This article is part of the Research TopicInfluence of Dietary Factors, Nutrients, and the Gut-Lung Axis on Respiratory HealthView all 11 articles
Background: Presently, numerous studies have indicated that protein consumption and levels of blood albumin serve as important biomarkers for a range of respiratory illnesses. However, there have been few investigations into the correlation between protein consumption, serum albumin, and asthma.
Methods: Our analysis incorporated 2509 asthmatics from the 2011–2018 NHANES dataset. The investigation employed three linear regression models and XGBoost model to investigate the potential link between protein intake, serum albumin levels, and blood eosinophil counts (BEOC) in patients with asthma. The trend test, generalized additive model (GAM), and threshold effect model were utilized to validate this correlation. As well, we undertook stratified analyses to look at the correlation of serum albumin with BEOC among distinct populations.
Results: In the univariable regression model, which did not account for any covariates, we observed a positive correlation between protein intake and BEOC. However, univariable and multivariable regression analyses all suggested a negative connection of serum albumin with BEOC in asthma populations. In Model C, which took into account all possible factors, BEOC dropped by 2.82 cells/uL for every unit increase in serum albumin (g/L). Additionally, the GAM and threshold effect model validated that serum albumin and BEOC showed an inverted U-shaped correlation.
Conclusion: Our investigation discovered there was no independent link between asthmatics’ protein intake and BEOC. However, we observed an inverted U-shaped relationship between serum albumin levels and BEOC, suggesting a possible relationship between the overall nutritional status of asthmatics and immune system changes. Our findings provide new directions for future research in the field of asthma management and therapy.
Asthma, which manifests as a clinical syndrome of inflammation, bronchial hyperresponsiveness, and reversible airflow obstruction, is a prevalent chronic airway disease (1). The prevalence of asthma has witnessed a significant rise in several nations over the last few decades. At present, the prevalence estimates for asthma among children and adults in the United States stand at 10.9% and 18.5%, respectively (2). Asthma was responsible for around 1.6 million visits to emergency departments and 183,000 hospitalizations in the United States in 2017, according to the survey (3). This caused a significant economic burden and a considerable number of missed school days.
Airway eosinophilia is a frequent clinical manifestation of asthma, a chronic inflammatory disease of the airways (4). Eosinophils play a role in multiple pathological processes, including airflow obstruction in asthma and chronic airway remodeling (5, 6). These processes include smooth muscle hypertrophy, neural plasticity, epithelial injury, and impaired tissue repair. The blood eosinophil count is a widely recognized and readily available biomarker that is of paramount importance in the management of asthma (7, 8). The correlation between elevated blood eosinophil levels and compromised disease control, as well as an increased susceptibility to severe asthma exacerbations, has been established by a multitude of studies (9–13). Additionally, blood eosinophils can be utilized to predict the response of asthma therapy and guide treatment decisions (14–17). In conclusion, blood eosinophils are an essential biomarker for asthma management and play a critical role in the onset, progression, and treatment of asthma.
Albumin is the predominant protein found in serum, making up over 60% of all blood proteins (18). It is solely produced in the liver and then released into the bloodstream. Serum albumin, with a half-life of 19 days, is crucial for regulating several physiological processes. Keeping the acid-base balance, transporting important molecules (like long-chain fatty acids, hormones, bilirubin, metal ions, etc.) through the bloodstream and to organs, stopping platelet function, Keeping the acid-base balance, transporting important molecules (like long-chain fatty acids, hormones, bilirubin, metal ions, etc.) through the bloodstream and to organs, stopping platelet function, keeping vascular permeability, and managing colloid osmotic pressure are some of these jobs (19). Furthermore, it demonstrates antioxidant properties and the ability to trap free radicals (20). Furthermore, serum albumin serves as an established clinical indicator for malnutrition (21). Asthmatic persons frequently experience malnutrition, which adversely affects their quality of life, exacerbation risk, duration of hospitalization, and overall healthcare costs (22, 23).
Recent research has shown that there is a correlation between low levels of albumin in the blood (hypoalbuminemia) and an extended duration of hospitalization in patients with acute exacerbations of chronic obstructive pulmonary disease (COPD) (24, 25). In individuals suffering from acute pulmonary embolism, low levels of serum albumin continue to serve as indicators of long-term mortality (20). However, there have been few investigations conducted to explore the connection between protein status and asthma thus far. Consequently, we utilized data from the National Health and Nutrition Examination Survey (NHANES) 2011–2018 cycles to examine the connection between protein intake, serum albumin and BEOC in patients with asthma.
The data for this investigation were collected from the NHANES public database, which was a part of the Centers for Disease Control and Prevention (CDC) in the United States. The NHANES database was responsible for collecting vital and health statistics for the country. The NHANES survey was conducted using a sophisticated multistage stratified sample design to ensure that a diverse and accurate representation of the US population was obtained. The program included interviews, medical exams, and laboratory tests. The collected data was used to analyze the correlation between nutritional status and promoting health and preventing diseases. All participants underwent the necessary procedures for obtaining informed consent and comprehensive health tests. The NHANES study protocol received approval from the Research Ethics Review Board of the National Center for Health Statistics.
Between 2011 and 2018, the NHANES amassed a total of 39156 individuals of data. The following individuals were excluded from our investigation: (1) under 18 years old (n=15331); (2) missing BEOC (n=2147); (3) missing serum albumin or protein intake data (n=2191); (4) non-asthmatics (n=16486); (5) missing over one of following covariates (n=492): education, marriage, poverty to income ratios (PIR), body mass index (BMI), smoking state, alcohol intake, hypertension, diabetes, liver condition, COPD, cancer history, aspartate aminotransferase (AST), alanine aminotransferase (ALT), serum creatinine, serum globulin, serum total protein, and urine albumin, steroid use. At last, a large, nationally representative group (n=2509) of asthmatic adults in the United States was collected for our investigation. The screening procedure’s flowchart appears in Figure 1.
The bichromatic digital endpoint technique (DcX800) is applied in order to determine the concentration of albumin in the serum. Throughout the procedure, bromcresol purple (BCP) reagent and albumin combine to form a complex. At 600 nanometers, the system logs any fluctuations in absorbance. The change in absorbance is directly proportional to the quantity of albumin present in the sample. The dietary protein intake of the participants was assessed using a 24-hour dietary recall approach. A skilled technician requested information regarding the specific kinds and amounts of food and medication consumed in a single day. This information was then recorded in the NHANES computer-assisted dietary survey system. A Beckman-Coulter MAXM analyzer was utilized to perform a complete blood count and 5-part differential on whole blood samples. For use in post hoc analyses, cell counts of lymphocytes, monocytes, segmented neutrophils, eosinophils, and basophils (1000 cells/uL) were obtained using the 5-part differential measure.
We incorporated numerous covariates in our investigation to lessen the potential impact of confounding variables. These factors were looked at: sex, age, race, education, PIR (low, middle, and high), marriage, BMI, history of smoking, alcohol use, high blood pressure, diabetes, liver disease, COPD, cancer, recent use of steroid drugs, AST, ALT, serum creatinine, serum globulin, serum total protein, and urine albumin. When people came in for their visit, standard surveys were used to confirm whether they had asthma. The intended evaluation question was: “Have you ever received a diagnosis of asthma from a doctor?” People who said “yes” were identified as having asthma.
The statistical analyses were accomplished via the R program (version 4.2.0). A p-value below 0.05 implied statistical significance. To address the complex sampling design of the NHANES, sample weights were incorporated. The BEOC was initially converted into quartiles. The p-value for categorical variables was calculated via the chi-square test, while for continuous variables, the Kruskal-Wallis rank sum test was deployed. Three distinctive linear regression models (Model A, Model B, and Model C) were utilized to evaluate the connection between protein intake, serum albumin, and BEOC while accounting for different covariates. And we employed trend analysis, the generalized additive model (GAM), and the threshold effect model to test the linearity or nonlinearity of the connection of serum albumin with BEOC. If a non-linear correlation was detected, a two-piecewise linear regression model was utilized to ascertain the threshold impact of serum albumin levels on BEOC. When the relationship between serum albumin and BEOC was obvious in a smoothed curve, the recursive technique automatically predicted the inflection point at which the greatest model likelihood would be adopted. As well, stratified analyses were conducted to investigate the connection of serum albumin with BEOC in various groups. Lastly, our investigation applied the XGBoost algorithm model to evaluate the relative significance of different indicators in connection to the impact of BEOC.
We utilized BEOC quartiles to subdivide the weighted characteristics of the 2509 adults with asthma who participated in our investigation (1031 men and 1478 women) (Table 1). The research sample comprised non-Hispanic white individuals, with an average age of 47.1 years, representing the designated participants’ demographic. The distributions of sex, age, race, BMI, hypertension, ALT, serum creatinine, serum total protein, and serum albumin varied significantly between the quartiles of BEOC. However, the distributions of education, marriage, PIR, smoking, alcohol consumption, protein consumption, diabetes, liver condition, COPD, cancer, steroid drug use, AST, serum globulin, and urine albumin did not differ significantly between the quartiles of BEOC. Participants whose BEOC was in the lowest quartile had elevated levels of serum total protein and serum albumin, in addition to lower values of age, BMI, ALT, and serum creatinine (p < 0.05), in comparison to the other groups.
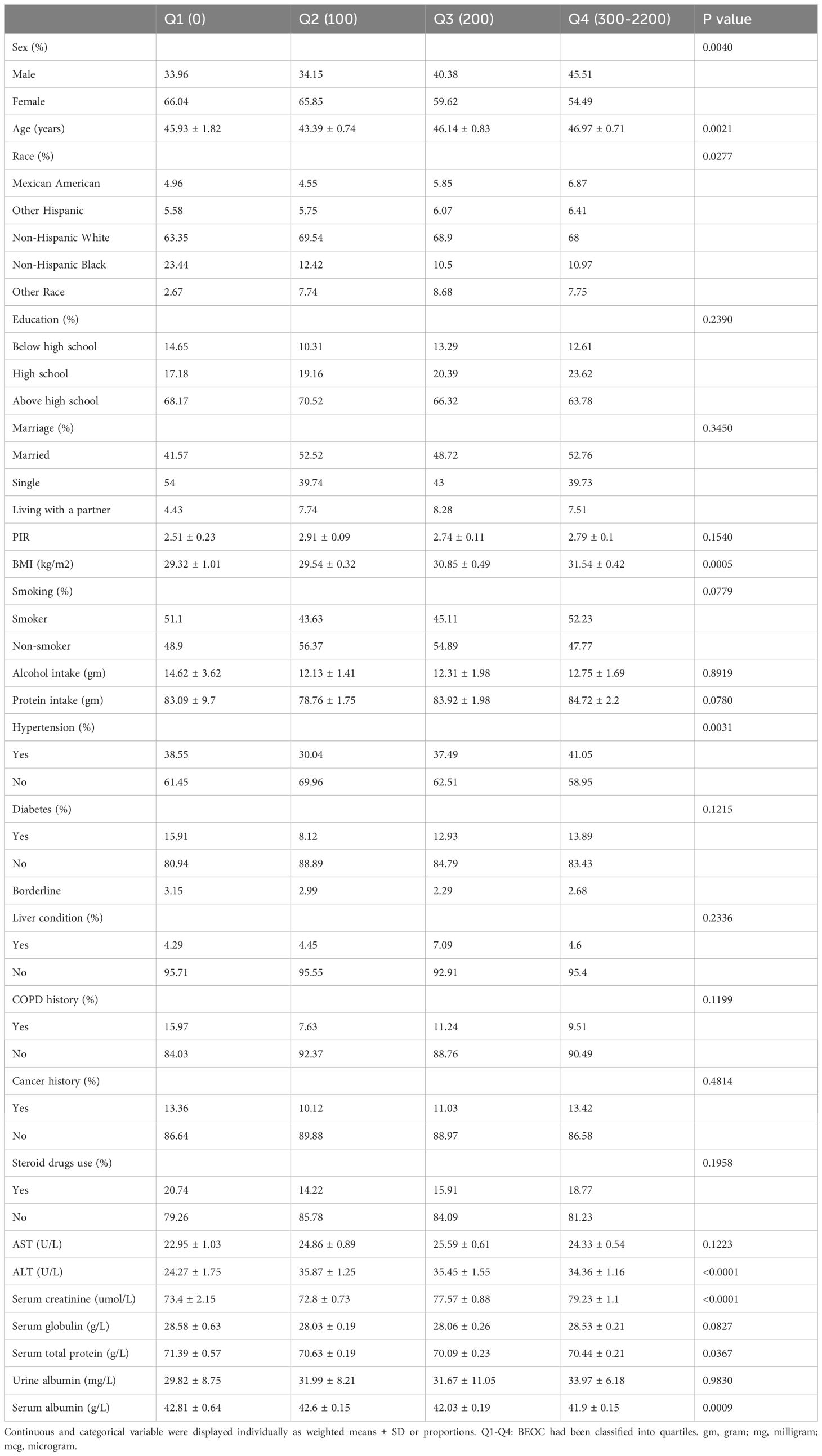
Table 1 The weighted characteristics of the study population were analyzed based on quartiles of BEOC.
We applied both univariable and multivariable linear regression models to investigate the connection among asthma patients’ protein intake, serum albumin, and BEOC. Only Model A, which did not account for any covariates (Supplementary Table 1), revealed a positive correlation of protein intake with BEOC. Nevertheless, we all identified with statistical significance in Models A, B, and C the inverse correlation of serum albumin with BEOC (Table 2). In Model C, which controlled for all covariates, BEOC decreased by 2.82 cells/uL for each extra unit of serum albumin (g/L). Furthermore, the outcomes of the trend test revealed statistical significance in Models A and B (p for trend < 0.05). However, no statistical significance was observed in the trend test of Model C (p for trend> 0.05), which suggesting a potential non-linear correlation of serum albumin with BEOC.
The GAM and threshold effect models were highly effective in identifying whether the correlation demonstrated linearity or nonlinearity. We employed GAM to generate a smooth and continuous curve based on Model C. This enabled us to ascertain if there was a nonlinear correlation between serum albumin and BEOC (Figure 2). After accounting for all covariates except serum albumin, we observed an inverted U-shaped correlation of serum albumin with BEOC. As well, we performed a threshold effect analysis to compare the single-line regression model with the two-segment regression model. The log-likelihood ratio was less than 0.05, indicating that model I (the one-line model) was statistically distinct from model II (the two-piecewise linear regression model). Given the statistical significance of the inflection point (K = 36), the two-piecewise linear regression model was deemed more appropriate, as indicated in Table 3. The highest point of BEOC was seen when the serum albumin reached 36 g/L.
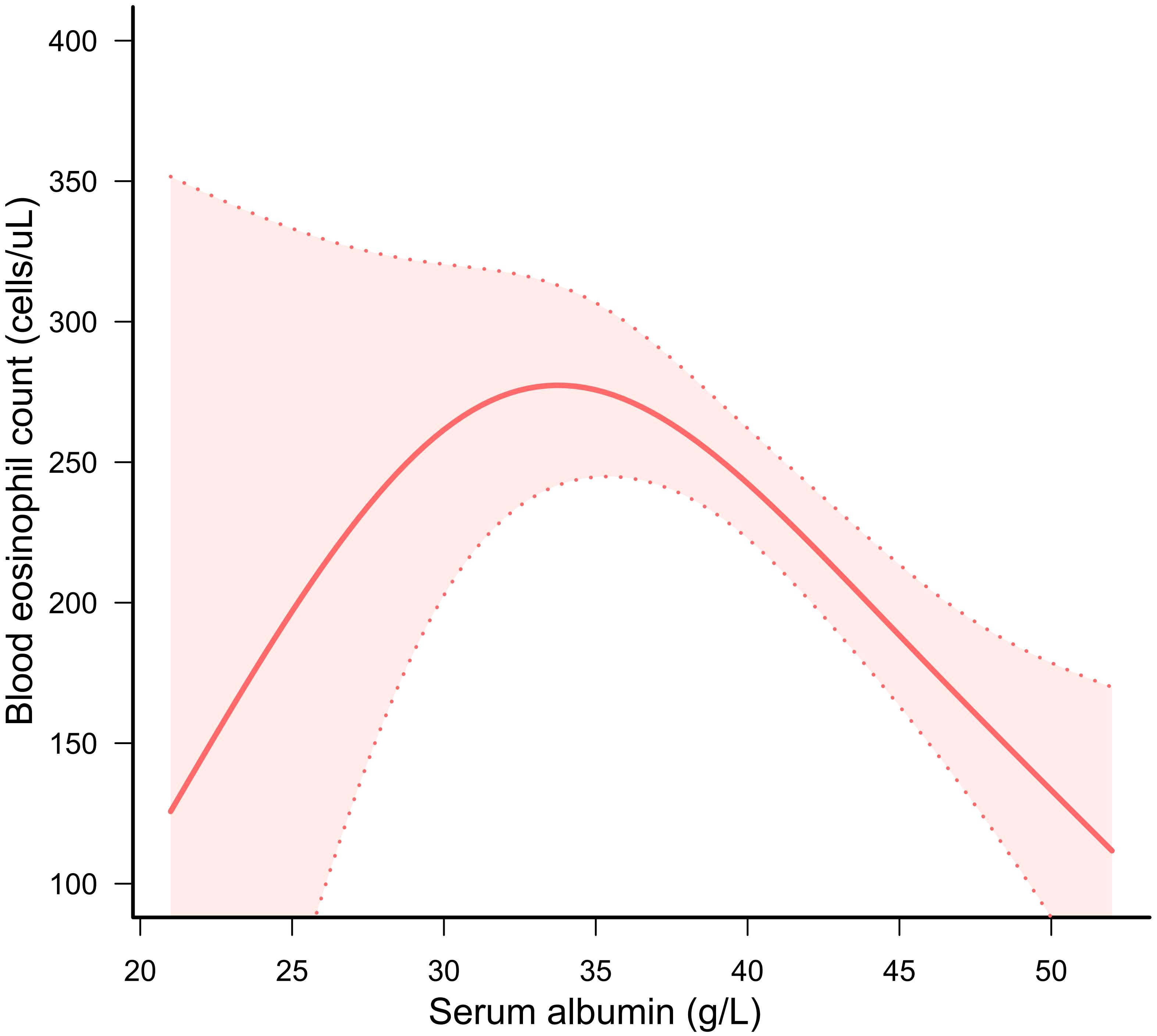
Figure 2 A solid red line displayed the correlation of serum albumin with BEOC. A red area indicated suitable 95% confidence ranges.
Stratified analyses were carried out to evaluate the connection between serum albumin and BEOC in various subgroups. Supplementary Table 2 displayed the results, stratified by sex, age, race, education, marriage, PIR, BMI, smoking, hypertension, diabetes, COPD, cancer, liver condition, and steroid use. We found that serum albumin was negatively linked to BEOC in women younger than 40, non-Hispanic Whites with low PIR, non-smokers, and people who did not have COPD, cancer, diabetes, liver disease, or use steroid. All stratified analyses showed no interaction (all p-values for interaction > 0.05).
When assessing the significance of the selected variable with regard to its impact on the BEOC, we adopted the XGBoost model. The selected variables included age, PIR, BMI, alcohol consumption, protein intake, AST, ALT, serum creatinine, serum albumin, serum globulin, serum total protein, and urine albumin. Ten variables, ranked in descending order of relative importance, primarily influenced the BEOC, according to the XGBoost model: urine albumin, protein consumption, BMI, serum creatinine, PIR, ALT, AST, age, serum globulin, and serum albumin (Figure 3).
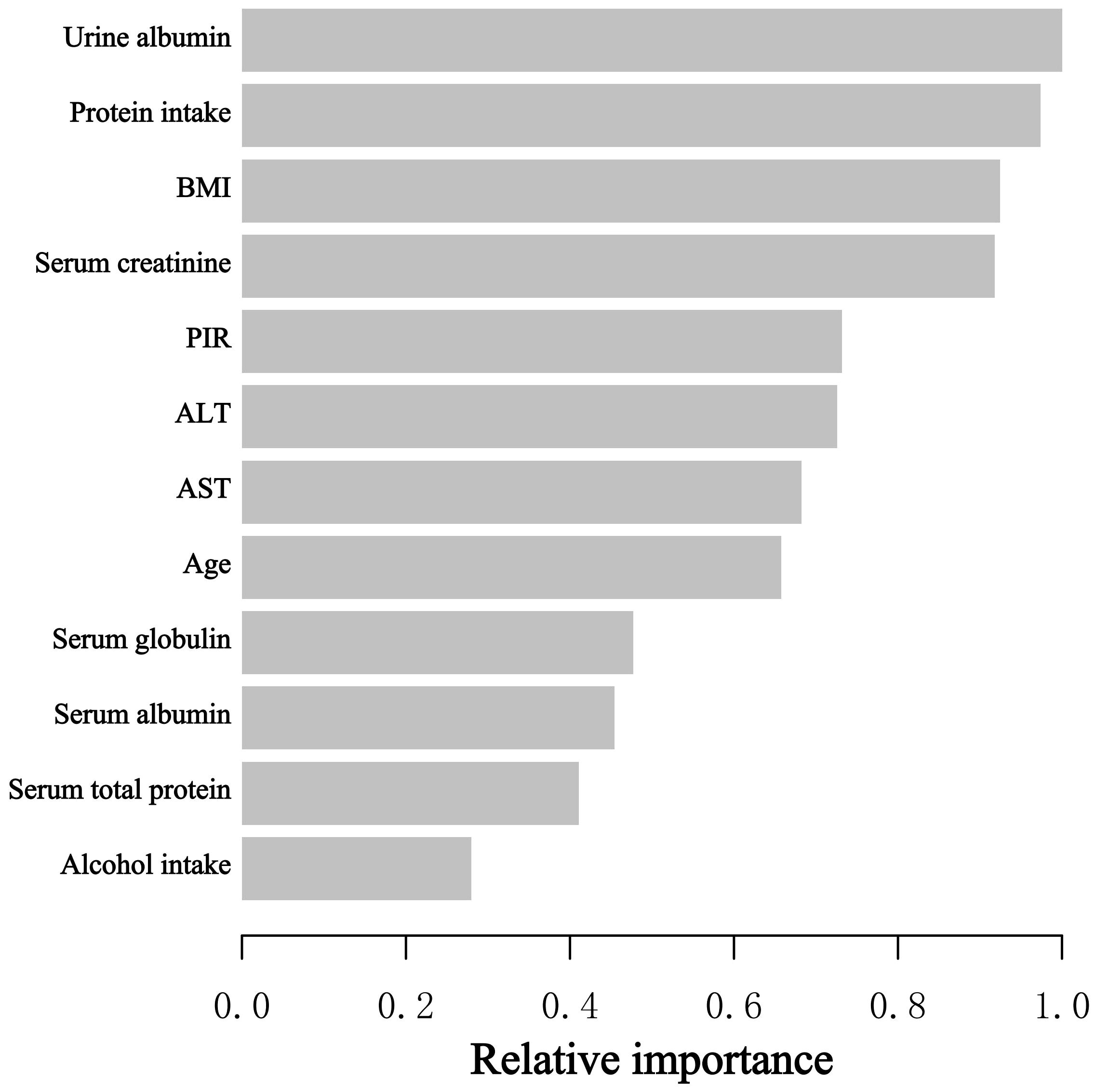
Figure 3 The XGBoost model provided the relative importance of each indicator on BEOC, along with the corresponding indicator importance score for every indicator.
The investigation is the first and most extensive cross-sectional investigation to quantify the connection between protein intake, serum albumin, and BEOC in asthma patients, to the best of our knowledge. In our study, we found a positive correlation of protein intake with BEOC, but only in the univariable regression model. However, in univariate and multivariate regression models, there was a negative correlation of serum albumin with BEOC in asthma people. In addition, we utilized the GAM and threshold effect model to verify the link between serum albumin and BEOC, which exhibited an inverted U-shaped correlation. The maximal BEOC occurred at a serum albumin concentration of 36 g/L. The XGBoost model proved that urine albumin, protein consumption, BMI, serum creatinine, PIR, ALT, AST, age, serum globulin, and serum albumin are the top 10 influential factors affecting BEOC. Our investigation gave unique insights into the connection of serum albumin with BEOC in patients with asthma.
Serum albumin is the most abundant plasma protein in the body, managing the distribution of vascular fluid and maintaining plasma colloid osmotic pressure. And serum albumin also possesses powerful anti-inflammatory and antioxidant properties due to its multiple binding sites, which provide an ideal substrate for free radical removal (26). In addition, it can still bind diverse inflammatory mediators and regulate the immune response during systemic inflammation (27). It has been postulated that serum albumin is responsible for over 70 percent of the total free radical–trapping activity, making it the predominant antioxidant in the circulatory system. Besides, albumin also has various biologic functions, such as the binding and transport of endogenous and exogenous molecules, endothelial stabilization, anti-thrombotic effects, and so on (26). Consequently, serum albumin is also a valuable biomarker for a variety of illnesses, which include obesity, diabetes, rheumatoid arthritis, and carcinoma, while it can be employed to treat various diseases, such as shock, trauma, hemorrhage, acute respiratory distress syndrome, hemodialysis, acute liver failure, chronic liver disease, hypoalbuminemia, and so on (28).
Additionally, serum albumin is also a valuable biomarker for a variety of respiratory disorders, including lung cancer, chronic obstructive pulmonary disease, acute pulmonary embolism, and bronchiectasis (20, 29–31). Meanwhile, some studies have reported the role of albumin in asthma, too. A case-control study in Nigeria involving 37 asthma cases and 30 controls has discovered that children with asthma have significantly lower serum albumin concentrations in comparison to controls and that there is a negative association between serum IgE and serum albumin, but that there is no significant association between serum albumin and blood eosinophil counts or eosinophil percentage (32). According to a Turkish case-control study involving 40 asthma cases and 40 healthy subjects, there is a significant decrease in serum albumin concentration among bronchial asthma patients compared to controls (33). Likewise, a Japanese investigation has observed that the serum albumin levels of asthmatic children are substantially lower than those of children without asthma, whereas the levels of children with wheezing symptoms or a history of allergic diseases are not significantly different (34). And another cross-sectional study in Australia has found that serum albumin is lower in people with more severe asthma and is positively related to lung function (35). Similarly, Khatri SB et al. have discovered that plasma albumin levels are substantially lower in asthmatics compared to nonasthmatics and are positively correlated with lung function (% forced expiratory volume in 1 second) in asthmatics (36). And a prospective, multicenter study in Spain has demonstrated that the onset of asthma exacerbations is negatively correlated with serum albumin levels (37). However, AbdulWahab et al. have reported that there is no significant difference in serum albumin levels between asthmatic children and control groups (38). In the same way, another study in Turkey found that serum albumin levels are not significantly different between asthmatic children and control groups (39). As well, a few investigations have looked into the connection between protein consumption and asthma. Huang SL et al. reported that protein-rich and fat-rich foods of animal origin were associated with a higher prevalence of asthma in teenagers (40). However, Schwartz J. et al. found no connection between dietary protein consumption and wheezing or asthma in children in the USA (41). Another South Korean study proved that protein consumption was shown to be slightly but significantly connected with allergic rhinitis but not with asthma (42). And Han YY et al. reported no significant connection between protein food consumption and the prevalence of asthma in Puerto Rican kids (43). Due to the inclusion of various confounders in previous studies, inconsistent conclusions have been drawn. Our investigation found no independent connection between asthmatics’ protein consumption and BEOC. But serum albumin and BEOC had an inverted U-shaped association.
In contrast to prior research, ours has some advantages. First, our inquiry provides a relatively large, nationally representative sample of adult asthmatics. Secondly, because confounders may influence the results, we use stratified analysis to determine the relationship of serum albumin with BEOC in various populations. Then, we employ the XGBoost model to determine the relative significance of selected indicators on BEOC. Lastly, the inflection point in the nonlinear relationship of serum albumin with BEOC is determined by using GAM and a two-piecewise linear regression model. But we have to acknowledge the inquiry’s shortcomings. Cross-sectional studies cannot prove a causal link between serum albumin and BEOC. Additionally, there are certain potential confounding factors that we may disregard. The medications that influenced blood eosinophils in our investigation were predominantly cortisol medications and other anti-allergy medications, excluding biologics. Asthma diagnosis is dependent on questionnaires. Future research endeavors should explore the potential influence of serum albumin in regulating and managing asthma while also elucidating potential mechanisms of action.
Our investigation discovered there was no independent link between asthmatics’ protein intake and BEOC. However, we observed an inverted U-shaped relationship of serum albumin with BEOC, suggesting a possible correlation between the overall nutritional status of asthmatics and immune system changes. Our findings provide new directions for future research in the field of asthma management and therapy.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: The official website of NHANES provides access to all available data (http://www.cdc.gov/nchs/nhanes/index.htm).
The studies involving humans were approved by National Center for Health Statistics’ Research Ethics Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
JW: Conceptualization, Data curation, Formal analysis, Methodology, Writing – original draft. JX: Data curation, Formal analysis, Writing – original draft. QH: Data curation, Formal analysis, Writing – review & editing. MG: Data curation, Formal analysis, Writing – review & editing. SG: Conceptualization, Funding acquisition, Project administration, Supervision, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1383122/full#supplementary-material
1. Wang Y, Guo D, Chen X, Wang S, Hu J, Liu X. Trends in asthma among adults in the United States, National Health and Nutrition Examination Survey 2005 to 2018. Ann Allergy Asthma Immunol: Off Publ Am Coll Allergy Asthma Immunol. (2022) 129:71–8.e2. doi: 10.1016/j.anai.2022.02.019
2. Forno E, Brandenburg DD, Castro-Rodriguez JA, Celis-Preciado CA, Holguin F, Licskai C, et al. Asthma in the americas: an update: A joint perspective from the Brazilian thoracic society, canadian thoracic society, latin american thoracic society, and american thoracic society. Ann Am Thorac Soc. (2022) 19:525–35. doi: 10.1513/AnnalsATS.202109-1068CME
3. Pate CA, Zahran HS, Qin X, Johnson C, Hummelman E, Malilay J. Asthma surveillance - United States, 2006-2018. Morbidity Mortal Weekly Rep Surveillance summaries. (2021) 70:1–32. doi: 10.15585/mmwr.ss7005a1
4. Wechsler ME, Munitz A, Ackerman SJ, Drake MG, Jackson DJ, Wardlaw AJ, et al. Eosinophils in health and disease: A state-of-the-art review. Mayo Clin Proc. (2021) 96:2694–707. doi: 10.1016/j.mayocp.2021.04.025
5. Drake MG, Lebold KM, Roth-Carter QR, Pincus AB, Blum ED, Proskocil BJ, et al. Eosinophil and airway nerve interactions in asthma. J leukocyte Biol. (2018) 104:61–7. doi: 10.1002/JLB.3MR1117-426R
6. Sastre B, Rodrigo-Muñoz JM, Garcia-Sanchez DA, Cañas JA, Del Pozo V. Eosinophils: old players in a new game. J investigational allergology Clin Immunol. (2018) 28:289–304. doi: 10.18176/jiaci
7. Wen J, Giri M, Xu L, Guo S. Association between exposure to selected heavy metals and blood eosinophil counts in asthmatic adults: results from NHANES 2011-2018. J Clin Med. (2023) 12. doi: 10.3390/jcm12041543
8. Pavord ID. Blood eosinophil-directed management of airway disease. The past, present, and future. Am J Respir Crit Care Med. (2020) 202:637–9. doi: 10.1164/rccm.202004-1013ED
9. Yancey SW, Keene ON, Albers FC, Ortega H, Bates S, Bleecker ER, et al. Biomarkers for severe eosinophilic asthma. J Allergy Clin Immunol. (2017) 140:1509–18. doi: 10.1016/j.jaci.2017.10.005
10. Zeiger RS, Schatz M, Dalal AA, Chen W, Sadikova E, Suruki RY, et al. Blood eosinophil count and outcomes in severe uncontrolled asthma: A prospective study. J Allergy Clin Immunol In Pract. (2017) 5:144–53.e8. doi: 10.1016/j.jaip.2016.07.015
11. Solomon Y, Malkamu B, Berhan A, Eyayu T, Almaw A, Legese B, et al. Peripheral blood eosinophilia in adult asthmatic patients and its association with the severity of asthma. BMC pulmonary Med. (2023) 23:96. doi: 10.1186/s12890-023-02383-x
12. Wen J, Wang C, Giri M, Guo S. Association between serum folate levels and blood eosinophil counts in American adults with asthma: Results from NHANES 2011-2018. Front Immunol. (2023) 14:1134621. doi: 10.3389/fimmu.2023.1134621
13. Lima-Matos A, Ponte EV, de Jesus JPV, Almeida PCA, Lima VB, Kwon N, et al. Eosinophilic asthma, according to a blood eosinophil criterion, is associated with disease severity and lack of control among underprivileged urban Brazilians. Respir Med. (2018) 145:95–100. doi: 10.1016/j.rmed.2018.10.025
14. Kostikas K, Brindicci C, Patalano F. Blood eosinophils as biomarkers to drive treatment choices in asthma and COPD. Curr Drug Targets. (2018) 19:1882–96. doi: 10.2174/1389450119666180212120012
15. Busse W, Chupp G, Nagase H, Albers FC, Doyle S, Shen Q, et al. Anti-IL-5 treatments in patients with severe asthma by blood eosinophil thresholds: Indirect treatment comparison. J Allergy Clin Immunol. (2019) 143:190–200.e20. doi: 10.1016/j.jaci.2018.08.031
16. Coverstone AM, Seibold MA, Peters MC. Diagnosis and management of T2-high asthma. J Allergy Clin Immunol In Pract. (2020) 8:442–50. doi: 10.1016/j.jaip.2019.11.020
17. Shrimanker R, Keene O, Hynes G, Wenzel S, Yancey S, Pavord ID. Prognostic and predictive value of blood eosinophil count, fractional exhaled nitric oxide, and their combination in severe asthma: A post hoc analysis. Am J Respir Crit Care Med. (2019) 200:1308–12. doi: 10.1164/rccm.201903-0599LE
18. Sheinenzon A, Shehadeh M, Michelis R, Shaoul E, Ronen O. Serum albumin levels and inflammation. Int J Biol Macromol. (2021) 184:857–62. doi: 10.1016/j.ijbiomac.2021.06.140
19. Tabata F, Wada Y, Kawakami S, Miyaji K. Serum albumin redox states: more than oxidative stress biomarker. Antioxidants. (2021) 10. doi: 10.3390/antiox10040503
20. Tanık VO, Çınar T, Karabağ Y, Şimşek B, Burak C, Çağdaş M, et al. The prognostic value of the serum albumin level for long-term prognosis in patients with acute pulmonary embolism. Clin Respir J. (2020) 14(6):578–85. doi: 10.1111/crj.13176
21. Alcorta MD, Alvarez PC, Cabetas RN, Martín MA, Valero M, Candela CG. The importance of serum albumin determination method to classify patients based on nutritional status. Clin Nutr ESPEN. (2018) 25:110–3. doi: 10.1016/j.clnesp.2018.03.124
22. Kang M, Sohn SJ, Shin MH. Association between body mass index and prevalence of asthma in korean adults. Chonnam Med J. (2020) 56:62–7. doi: 10.4068/cmj.2020.56.1.62
23. Nantanda R, Ostergaard MS, Ndeezi G, Tumwine JK. Clinical outcomes of children with acute asthma and pneumonia in Mulago hospital, Uganda: a prospective study. BMC Pediatr. (2014) 14:285. doi: 10.1186/s12887-014-0285-4
24. Chen CW, Chen YY, Lu CL, Chen SC, Chen YJ, Lin MS, et al. Severe hypoalbuminemia is a strong independent risk factor for acute respiratory failure in COPD: a nationwide cohort study. Int J Chron Obstrucy Pulmonary Dis. (2015) 10:1147–54. doi: 10.2147/COPD
25. Wang Y, Stavem K, Dahl FA, Humerfelt S, Haugen T. Factors associated with a prolonged length of stay after acute exacerbation of chronic obstructive pulmonary disease (AECOPD). Int J Chron Obstrucy Pulmonary Dis. (2014) 9:99–105. doi: 10.2147/COPD
26. Ward ES, Gelinas D, Dreesen E, Van Santbergen J, Andersen JT, Silvestri NJ, et al. Clinical significance of serum albumin and implications of FcRn inhibitor treatment in igG-mediated autoimmune disorders. Front Immunol. (2022) 13:892534. doi: 10.3389/fimmu.2022.892534
27. Sun L, Yin H, Liu M, Xu G, Zhou X, Ge P, et al. Impaired albumin function: a novel potential indicator for liver function damage? Ann Med. (2019) 51:333–44. doi: 10.1080/07853890.2019.1693056
28. De Simone G, di Masi A, Ascenzi P. Serum albumin: A multifaced enzyme. Int J Mol Sci. (2021) 22. doi: 10.3390/ijms221810086
29. Inoue M, Nakashima R, Enomoto M, Koike Y, Zhao X, Yip K, et al. Plasma redox imbalance caused by albumin oxidation promotes lung-predominant NETosis and pulmonary cancer metastasis. Nat Commun. (2018) 9:5116. doi: 10.1038/s41467-018-07550-x
30. Zinellu E, Fois AG, Sotgiu E, Mellino S, Mangoni AA, Carru C, et al. Serum albumin concentrations in stable chronic obstructive pulmonary disease: A systematic review and meta-analysis. J Clin Med. (2021) 10. doi: 10.3390/jcm10020269
31. Ju S, Jeong JH, Heo M, Heo IR, Kim TH, Kim HC, et al. Serum albumin is a predictor of respiratory hospitalization in patients with bronchiectasis. Chronic Respir Dis. (2021) 18:14799731211017548. doi: 10.1177/14799731211017548
32. Oluwole O, Arinola OG, Adu MD, Adepoju A, Adedokun BO, Olopade OI, et al. Relationships between plasma micronutrients, serum IgE, and skin test reactivity and asthma among school children in rural southwest Nigeria. J Biomark. (2014) 2014:106150. doi: 10.1155/2014/106150
33. Vural H, Uzun K, Uz E, Koçyigit A, Cigli A, Akyol O. Concentrations of copper, zinc and various elements in serum of patients with bronchial asthma. J Trace elements Med biology: Organ Soc Minerals Trace Elements (GMS). (2000) 14:88–91. doi: 10.1016/S0946-672X(00)80036-X
34. Shima M, Adachi M. Association of respiratory symptoms with serum protease inhibitors and albumin levels in Japanese children. Int J Epidemiol. (1996) 25:1213–9. doi: 10.1093/ije/25.6.1213
35. Misso NL, Brooks-Wildhaber J, Ray S, Vally H, Thompson PJ. Plasma concentrations of dietary and nondietary antioxidants are low in severe asthma. Eur Respir J. (2005) 26:257–64. doi: 10.1183/09031936.05.00006705
36. Khatri SB, Peabody J, Burwell L, Harris F, Brown LS. Systemic antioxidants and lung function in asthmatics during high ozone season: a closer look at albumin, glutathione, and associations with lung function. Clin Trans Sci. (2014) 7:314–8. doi: 10.1111/cts.12152
37. Bellido-Casado J, Plaza V, Perpiñá M, Picado C, Bardagí S, Martínez-Brú C, et al. [Inflammatory response of rapid onset asthma exacerbation]. Archivos bronconeumologia. (2010) 46:587–93. doi: 10.1016/S1579-2129(10)70126-9
38. AbdulWahab A, Zeidan A, Avades T, Chandra P, Soliman A. Serum zinc level in asthmatic and non-asthmatic school children. Children. (2018) 5. doi: 10.3390/children5030042
39. Kocyigit A, Armutcu F, Gurel A, Ermis B. Alterations in plasma essential trace elements selenium, manganese, zinc, copper, and iron concentrations and the possible role of these elements on oxidative status in patients with childhood asthma. Biol Trace element Res. (2004) 97:31–41. doi: 10.1385/BTER:97:1
40. Huang SL, Lin KC, Pan WH. Dietary factors associated with physician-diagnosed asthma and allergic rhinitis in teenagers: analyses of the first Nutrition and Health Survey in Taiwan. Clin Exp allergy: J Br Soc Allergy Clin Immunol. (2001) 31:259–64. doi: 10.1046/j.1365-2222.2001.00938.x
41. Schwartz J, Gold D, Dockery DW, Weiss ST, Speizer FE. Predictors of asthma and persistent wheeze in a national sample of children in the United States. Assoc Soc class perinatal events race. Am Rev Respir Dis. (1990) 142:555–62. doi: 10.1164/ajrccm/142.3.555
42. Kim SY, Sim S, Park B, Kim JH, Choi HG. High-fat and low-carbohydrate diets are associated with allergic rhinitis but not asthma or atopic dermatitis in children. PloS One. (2016) 11:e0150202. doi: 10.1371/journal.pone.0150202
Keywords: protein intake, albumin, eosinophil, asthma, National Health and Nutrition Examination Survey (NHANES), XGBoost
Citation: Wen J, Xia J, He Q, Giri M and Guo S (2024) Association between protein intake, serum albumin and blood eosinophil in US asthmatic adults. Front. Immunol. 15:1383122. doi: 10.3389/fimmu.2024.1383122
Received: 06 February 2024; Accepted: 30 April 2024;
Published: 21 May 2024.
Edited by:
Tomoko Suzuki, Saitama Medical University, JapanReviewed by:
Jia Wang, Sichuan University, ChinaCopyright © 2024 Wen, Xia, He, Giri and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shuliang Guo, Z3Vvc2h1bDY2NkAxNjMuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.