- 1Department of Pharmacy, Shanghai Tenth People’s Hospital, Tongji University School of Medicine, Shanghai, China
- 2Department of Obstetrics and Gynecology, Shanghai Tenth People’s Hospital, School of Medicine, Tongji University School of Medicine, Shanghai, China
Drug-induced immune thrombocytopenia is an adverse reaction marked by accelerated destruction of blood platelets. In cancer therapy, thrombocytopenia has many other causes including bone marrow suppression induced by chemotherapeutic agents, infection, and progression of cancer; drug-induced thrombocytopenia can easily be misdiagnosed or overlooked. Here, we present a case of an ovarian cancer patient with a history of mixed connective tissue disease who underwent surgery followed by treatment with paclitaxel, cisplatin, and bevacizumab. The patient developed acute isolated thrombocytopenia after the sixth cycle. Serum antiplatelet antibody testing revealed antibodies against glycoprotein IIb. After we analyzed the whole therapeutic process of this patient, drug-induced immune thrombocytopenia was assumed, and bevacizumab was conjectured as the most probable drug. Thrombocytopenia was ultimately successfully managed using recombinant human thrombopoietin, prednisone, and recombinant human interleukin-11. We provide a summary of existing literature on immune thrombocytopenia induced by bevacizumab and discuss related mechanisms and triggers for drug-induced immune thrombocytopenia. The present case underscores the potential of bevacizumab to induce immune-mediated thrombocytopenia, emphasizing the need for heightened vigilance towards autoimmune diseases or an autoimmune-activated state as plausible triggers for rare drug-induced immune thrombocytopenia in cancer therapy.
Introduction
Thrombocytopenia is a frequent hematological adverse reaction of cancer therapy, which is defined as a platelet count of <100×103/μl (1). Myelosuppression leading to hematopoietic abnormalities induced by chemotherapy is the major cause; immune thrombocytopenia may also occur (2). Many reports indicated that patients with autoimmune diseases were more likely to develop immune thrombocytopenia induced by drugs (3, 4). However, since thrombocytopenia is a condition with many other inducements, the diagnosis of drug-induced immune thrombocytopenia (DITP) can easily be overlooked (5). Here, we report a case of DITP with bevacizumab as the most likely culprit drug in an ovarian cancer patient with a history of mixed connective tissue disease (MCTD). We also review existing literatures on immune thrombocytopenia induced by bevacizumab and discuss related mechanisms. Autoimmune predisposition as a trigger for DITP is also presented.
Case report
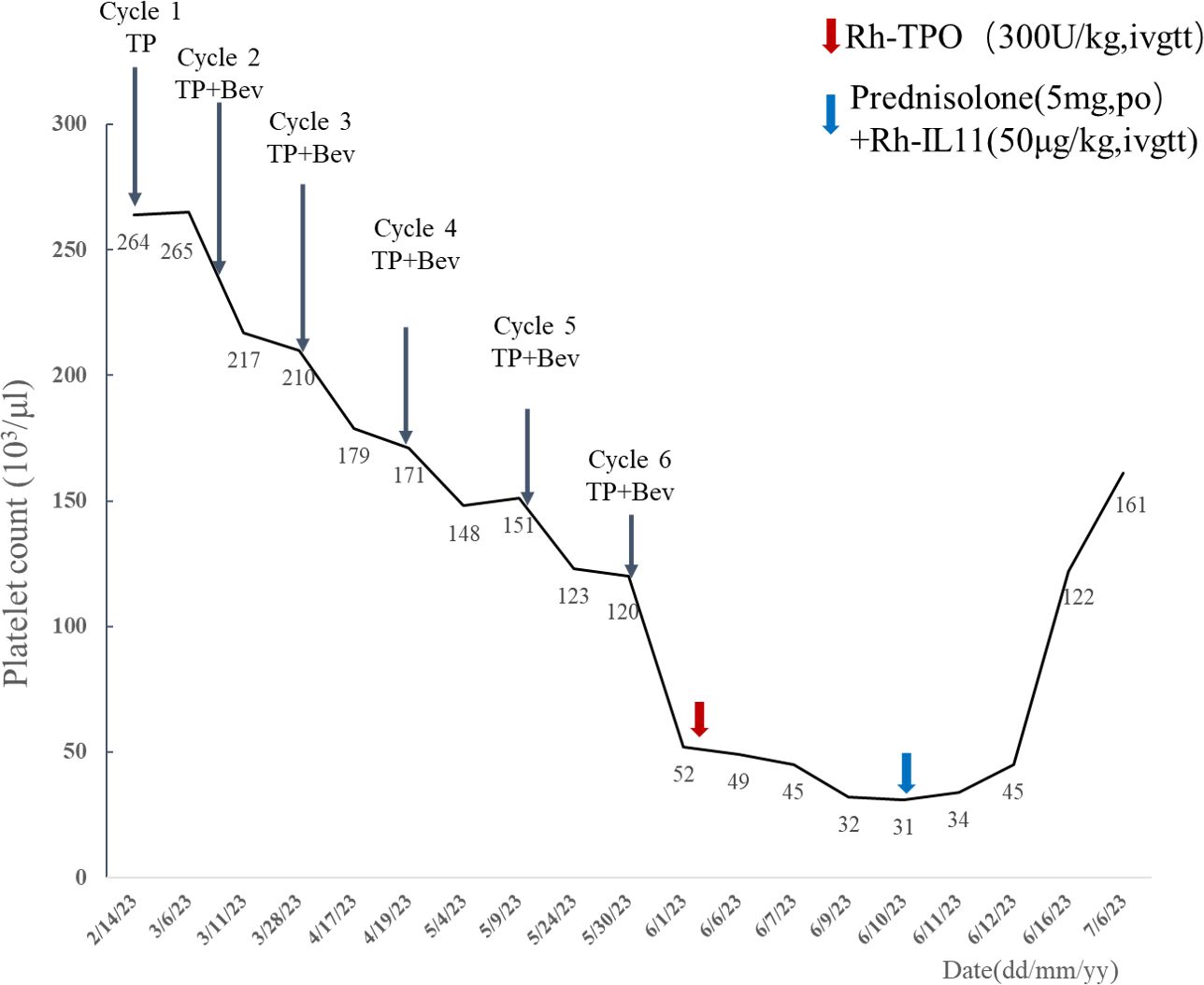
In February 2023, a 68-year-old woman was diagnosed with stage IV high-grade serous ovarian carcinoma. The patient had a past medical history of MCTD presented with Raynaud’s phenomenon with fingers cold and white in winter, which was well controlled with oral prednisone (5 mg per 24 h), hydroxychloroquine (0.1 g, per 12 h), and aspirin (100 mg per 24 h) from November to February of the following year. Considering peritoneal metastasis, the patient received intravenous paclitaxel (135mg/m2) plus intraperitoneal cisplatin (75 mg/m2) (TP regimen) for day 1 every 3 weeks for six cycles after cytoreductive surgery. Bevacizumab (15 mg/kg) was administered intravenously at day 2 starting from cycle 2. With no other hematological abnormality found, the patient exhibited a gradual drift downward in platelet count within normal limits (Figure 1). Furthermore, the patient developed acute thrombocytopenia on the first day after the sixth cycle of bevacizumab infusion. Complete blood count showed an isolated decrease in platelet count from 120 × 103/μl (as measured before chemotherapy infusion) to 52 × 103/μl (Figure 1). Other hematological parameters, such as leukocyte (4.27 × 103/μl) and neutrophil count (3.36 × 103/μl) were in the normal range, and no liver function or coagulation disorders were displayed. She subsequently received recombinant human thrombopoietin (Rh-TPO), 300 U/kg intravenously. Despite this treatment, platelet count continued to drop to 31 × 103/μl on day 10 after the administration of bevacizumab (Figure 1). The patient showed no signs of progression or metastases of malignancies with the unremarkable hypogastrium MRI and no elevation in biomarkers of cancers including HE4, CA125, and ROMA algorithm (Table 1). A bone marrow biopsy was disregarded by the patient due to its invasive nature and the absence of hemorrhagic complications. The patient had no fever or Raynaud’s syndrome signs. Autoimmunology parameters were conducted, and tests were negative except for ANA and anti-nRNP, which were similar to the tests before cancer therapy (Table 1). Anti-platelet antibody testing of the peripheral blood samples showed the presence of glycoprotein IIb (GPIIb) antibodies with flow fluorescence microsphere method. After excluding malignancy, myelosuppression, infection, pseudo-thrombocytopenia (PTCP), autoimmune diseases, or other drug-induced thrombocytopenia, DITP was finally diagnosed. According to Naranjo’s algorithm, bevacizumab was deemed the most likely drug with a score of 6 (probable) (see Supplementary Table S1). Therefore, treatment with oral prednisone (5 mg per 24 h) and intravenous recombinant human interleukin-11 (RhIL-11), 25 μg/kg, was started. Platelet count gradually increased up to 122 × 103/μl 6 days later (Figure 1). After 42 days of the infusion, re-evaluation of autoimmunity parameters and platelet count was conducted, and changes in tests were unremarkable except for GPIIb, which was negative. The patient remained stable and was rechecked monthly, with no decrease in platelet count observed.
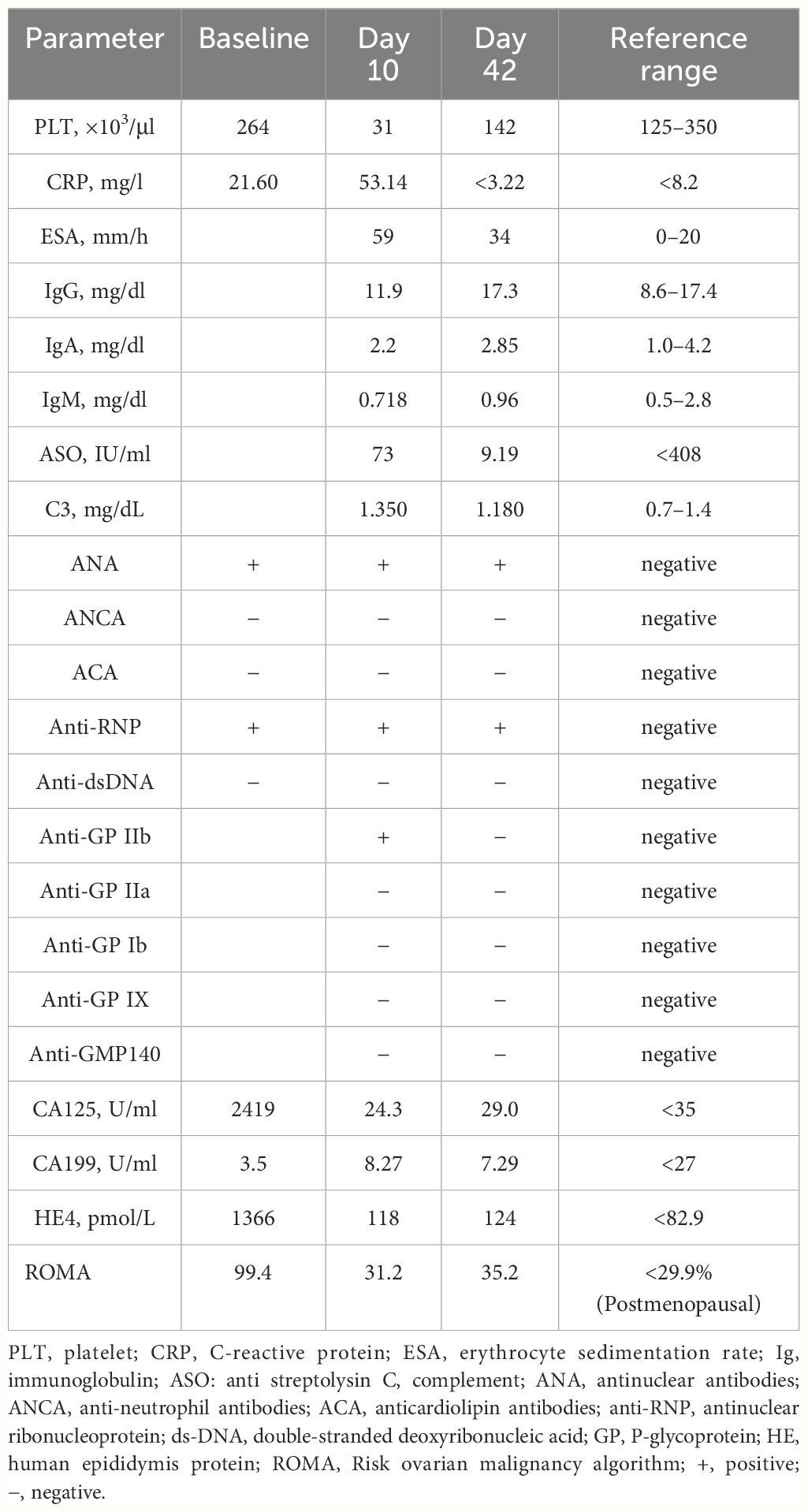
Table 1 Autoimmunity findings, platelet count, and biological markers of cancers prior to cancer therapy (baselines) and on day 10 and 42 after administration of bevacizumab (cycle 6).
Discussion
Possible causes of thrombocytopenia in the present patient included cancer-therapy-related thrombocytopenia, exacerbation of MCTD, primary immune thrombocytopenia (ITP), and PTCP. Thrombocytopenia maybe caused by cancer directly with tumor involvement of bone marrow and spleen, which occurred most often in patients with known metastatic cancer and exacerbation of the disease (6, 7). It was ruled out that the thrombocytopenia was directly caused by the ovarian cancer or cancer-associated factors with unremarkable biomarkers and MRI results. Severe thrombocytopenia was a rare risk of paclitaxel plus cisplatin and bevacizumab in ovarian cancer, occurring in up to 2%–4% of patients as reported in clinical trials (8, 9). Thrombocytopenia due to myelosuppression is typically accompanied by leukopenia (10). In this case, the platelet count decreased progressively and showed acute isolated thrombocytopenia after several cycles, which was similar to the gradual thrombocytopenia induced by long-term exposure to trastuzumab (11). Although thrombocytopenia occurred in MCTD, the frequency was rare, as most were case reports, and thrombocytopenia was usually correlated with positive ACA (12). Thrombocytopenia secondary to MCTD was not present in the patient, as there were no clinical features of Raynaud’s syndrome and autoimmunity parameters showing unremarkable changes. ITP was also excluded as well since thrombocytopenia promptly recovered within 16 days after the discontinuation of bevacizumab, and this clinical manifestation is not consistent with ITP. PTCP was ruled out, as no platelet aggregation was observed in a peripheral blood smear (13). Then, clinical and laboratory criteria for establishing a causative relationship between a drug and thrombocytopenia were used, and possible score was found (5, 14). In the present case, drug exposure precedes thrombocytopenia, and recovery from thrombocytopenia was complete and sustained after drugs withdrawal. Other etiological factors of thrombocytopenia were excluded. Serum antiplatelet antibody testing revealed antibodies against GPIIb.
In the context of advanced cancer therapy, distinguishing the specific drug causing thrombocytopenia in patients receiving many medication treatments simultaneously is a challenging issue (15). Several elements were provided to analyze that acute thrombocytopenia in the current patient was probably triggered by bevacizumab. 1) Neither cases of cisplatin and paclitaxel have been reported nor have they been documented in the adverse reaction systems. Bevacizumab associated with thrombocytopenia has been reported in several cases, which was considered to be related to immunity, and the French PharmacoVigilance Database showed relevant records. 2) The administration’s relationship in time to the onset of clinical manifestations was clear. 3) Recovery from thrombocytopenia did not occur rapidly after withdrawal of bevacizumab. The presence of antiplatelet antibodies against GPIIb, which may be a chronic effect of immune thrombocytopenia caused by bevacizumab (a drug with a half-life of 10 days) could be the reason for this. It was possible that the absence of GPIIb in a later period and the subsequent recovery after corticosteroid treatment could be attributed to this. 4) The probable score of the relationship between the drug and development of thrombocytopenia (Naranjo scale = 6) was found with Naranjo’s algorithm (11).
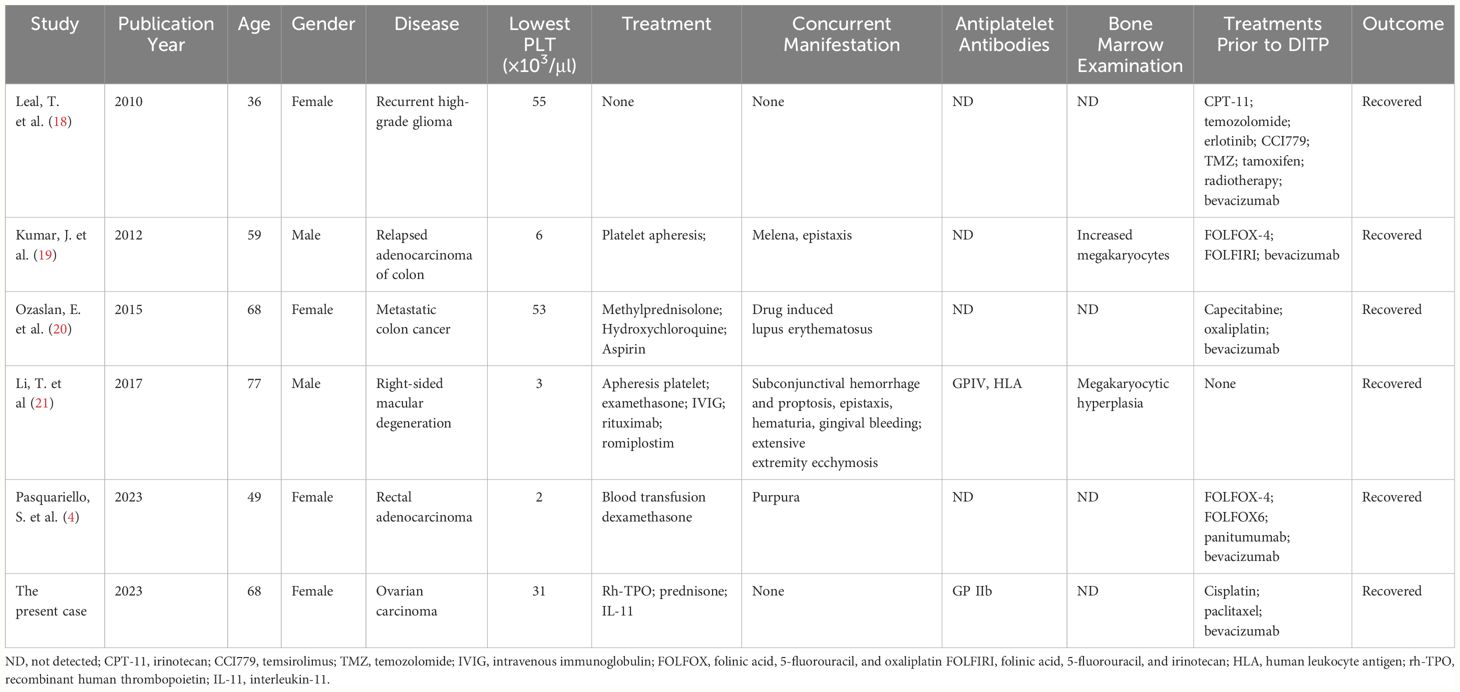
Immune-mediated thrombocytopenia is a rare complication of bevacizumab that had only few reports in the literature about glioma, colon cancer, and macular degeneration, which was an off-label use. Glioma cells were reported to have the ability to evade the immune system and foster an immunosuppressive milieu by various mechanisms, notably by diminishing the recruitment of immune cells, secreting immunosuppressive cytokines, and activating the STAT3 pathway. The STAT3 pathway facilitated the synthesis of vascular endothelial growth factor (VEGF), which was the target of bevacizumab (16). VEGF was often overexpressed in epithelial ovarian cancer and therefore an attractive target for therapy (17). The reported cases of malignancies all received many prior therapies with resulting decreased bone marrow reserve (see Table 2). Our patient received five cycles of bevacizumab and paclitaxel-cisplatin chemotherapy before. The clinical features of bevacizumab-induced thrombocytopenia varied. Both were diagnosed with colon cancer; the case of bevacizumab-induced thrombocytopenia reported by Leal et al. had no bleeding with mild thrombocytopenia (18). In the other case reported by Kumar et al., the patient developed severe thrombocytopenia with melena and epistaxis, attributable to bevacizumab (19). Our patient had no bleeding, and platelet count was slightly decreased. Although it was not widely adapted in the management of thrombocytopenia in cancer patients, a bone marrow biopsy was a favorable method to confirm the immune thrombocytopenia especially in the cases where the diagnosis remained uncertain (21, 22). Immune-related thrombocytopenia induced by bevacizumab was usually reversible with the treatment with corticosteroids and thrombopoietic growth factors, such as Rh-TPO or Rh-IL11. Under certain circumstances, mild thrombocytopenia may not require specific treatment.
Bevacizumab is a highly effective humanized monoclonal antibody against VEGF that disrupts the blood supply to the tumor, making it an essential treatment option for cancer patients (23). Albeit the exact mechanisms that lead to these side effects are not well understood, increasing evidence represented the immunological characteristics of bevacizumab (23, 24). It has been concluded that bevacizumab was concentrated in platelets and formed immune complexes with VEGF in vitro and in vivo (25, 26). Meyer et al. and Nomura et al. (24, 27) described that the bevacizumab associated immune complexes activated platelets via the IgG receptor FcγRIIa and directly caused thrombocyte aggregation, which was similar to the mechanism of heparin-induced thrombocytopenia (HIT), which was typically an immune-mediated thrombocytopenia (28).
The effects of autoimmune disorders and their corresponding therapeutic medications on individuals are complex and fascinating. Some of the immunosuppressive agents used to treat autoimmune diseases may directly or indirectly be associated with the subsequent development of malignancies (29). The autoimmune status of patients should also be considered in the setting of drug-mediated immune-like complexes as a triggering factor for immune thrombocytopenia. Patients diagnosed with HIT have a high incidence of comorbid autoimmune diseases, namely, systemic lupus erythematosus, rheumatoid arthritis, and Hashimoto’s thyroiditis, with age being a significant variable (30, 31, 32). In the case reported by Pasquariello et al., the patient with colorectal cancer developed sever thrombocytopenia when treated with panitumumab or bevacizumab, whose immunology tests of anti-nucleus antibody was positive (4). According to cases reported in the French PharmacoVigilance database, 25% of patients with ITP induced by serotonin reuptake inhibitors or bevacizumab had autoimmune antecedents (33). In another case, the patient with colorectal cancer developed methylprednisolone-induced thrombocytopenia after the oxaliplatin-induced thrombocytopenia, attributable to the oxaliplatin immune-induced syndrome owing to anti-GPIIbIIIa and anti-CD36 antibodies (34). Drugs including antineoplastic drug-related immune thrombocytopenia may be more likely to occur in patients with autoimmune diseases or an autoimmune activated state. More research will be needed to support this idea.
Here, we report the first case of an ovarian cancer patient with mixed connective tissue disease who developed an immune-related thrombocytopenia during chemotherapy. However, due to the rarity of this clinical occurrence and the scarcity of existing literature, it is challenging to comprehensively document the event and fully confirm the correct diagnostic and therapeutic approach. Our case highlights an attention on bevacizumab-induced immune thrombocytopenia. In addition, the current case report provides clinical data relevant to the largely unexplored question of DITP in patients with a pre-existing autoimmune state.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
YZ: Writing – original draft, Writing – review & editing, Data curation, Formal analysis, Project administration. FY: Writing – review & editing, Investigation, Project administration. JW: Data curation, Writing – review & editing. HF: Data curation, Funding acquisition, Writing – review & editing. FS: Formal analysis, Supervision, Writing – review & editing. JL: Formal analysis, Writing – review & editing. DL: Formal analysis, Project administration, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (82003759), Chenguang Program of Shanghai Education Development Foundation and Shanghai Municipal Education Commission (21CGA21), the Young Elite Scientist Sponsorship Program by CAST (2023QNRC001, No.YESS20230047), and Antitumor Drug Clinical Application Management Project of Shanghai Hospital Association Clinical Pharmaceutical Management Committee (YS 2021002).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1382964/full#supplementary-material
References
1. Mones JV, Soff G. Management of thrombocytopenia in cancer patients. In: Soff G, editor. Thrombosis and Hemostasis in Cancer. Cancer Treatment and Research. Springer International Publishing, Cham (2019). p. 139–50. doi: 10.1007/978–3-030–20315-3_9
2. Kuter DJ. Treatment of chemotherapy-induced thrombocytopenia in patients with non-hematologic Malignancies. Haematologica. (2022) 107:1243–63. doi: 10.3324/haematol.2021.279512
3. Hayden A, Vyas-Lahar A, Rella V, Rudinskaya A. Severe refractory thrombocytopenia in a woman positive for coronavirus disease 2019 with lupus and antiphospholipid syndrome. Lupus. (2020) 29:1472–4. doi: 10.1177/0961203320940389
4. Pasquariello S, Clavarezza M, Piredda S, Foppiani L, Pesce G, Antonucci G, et al. Drug-induced thrombocytopenia in a patient with colorectal cancer: A case report. Oncol Lett. (2023) 26:398. doi: 10.3892/ol.2023.13984
5. Aster RH. Drug-induced immune thrombocytopenia. N Engl J Med. (2007) 357:580–7. doi: 10.1056/NEJMra066469
6. Liebman HA. Thrombocytopenia in cancer patients. Thromb Res. (2014) 133:S63–9. doi: 10.1016/S0049–3848(14)50011–4
7. Font C, De Herreros MG, Tsoukalas N, Brito-Dellan N, Espósito F, Escalante C, et al. Thrombotic microangiopathy (TMA) in adult patients with solid tumors: a challenging complication in the era of emerging anticancer therapies. Support Care Cancer. (2022) 30:8599–609. doi: 10.1007/s00520–022-06935–5
8. Konner JA, Grabon DM, Gerst SR, Iasonos A, Thaler H, Pezzulli SD, et al. Phase II study of intraperitoneal paclitaxel plus cisplatin and intravenous paclitaxel plus bevacizumab as adjuvant treatment of optimal stage II/III epithelial ovarian cancer. J Clin Oncol. (2011) 29:4662–8. doi: 10.1200/JCO.2011.36.1352
9. Armstrong DK, Huang HQ, Copeland LJ. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. (2006) 354:34–43. doi: 10.1056/NEJMoa052985
10. Pan E, Hsieh E, Piatek C. Case report: oxaliplatin-induced immune-mediated thrombocytopenia. Case Rep Oncol. (2018) 11:880–2. doi: 10.1159/000495032
11. Miarons M, Velasco M, Campins L, Fernández S, Gurrera T, Lopez-Viaplana L. Gradual thrombocytopenia induced by long-term trastuzumab exposure. J Clin Pharm Ther. (2016) 41:563–5. doi: 10.1111/jcpt.12416
12. Pope JE. Other manifestations of mixed connective tissue disease. Rheum Dis Clin N Am. (2005) 31:519–33. doi: 10.1016/j.rdc.2005.04.011
13. Schuff-Werner P, Mansour J, Gropp A. Pseudo-thrombocytopenia (PTCP). A challenge in the daily laboratory routine? J Lab Med. (2020) 44:295–304. doi: 10.1515/labmed-2020–0099
14. Arnold D, Kukaswadia S, Nazi I, Esmail A, Dewar L, Smith J, et al. A systematic evaluation of laboratory testing for drug-induced immune thrombocytopenia. J Thromb Haemost JTH. (2013) 11:169–76. doi: 10.1111/jth.12052
15. Dior M, Coriat R, Mir O, Brezault C, Perkins G, Dhooge M, et al. A rare hematological adverse event induced by bevacizumab: severe thrombocytopenia. Am J Med. (2012) 125:828–30. doi: 10.1016/j.amjmed.2012.04.026
16. Linares CA, Varghese A, Ghose A, Shinde SD, Adeleke S, Sanchez E, et al. Hallmarks of the tumour microenvironment of gliomas and its interaction with emerging immunotherapy modalities. Int J Mol Sci. (2023) 24:13215. doi: 10.3390/ijms241713215
17. Kasherman L, Liu S, Karakasis K, Lheureux S. Angiogenesis: A pivotal therapeutic target in the drug development of gynecologic cancers. Cancers. (2022) 14:1122. doi: 10.3390/cancers14051122
18. Leal T, Robins HI. Bevacizumab induced reversible thrombocytopenia in a patient with recurrent high-grade glioma: a case report. Cancer Chemother Pharmacol. (2010) 65:399–401. doi: 10.1007/s00280-009-1118-2
19. Kumar J, Bhargava M, Aggarwal S. Bevacizumab-Induced Reversible Thrombocytopenia in a Patient with Adenocarcinoma of Colon: Rare Adverse Effect of Bevacizumab. Case Rep Oncol Med. (2012) 2012:1–3. doi: 10.1155/2012/695430
20. Ozaslan E, Eroglu E, Gok K, Senel S, Baldane S, Akyol L, et al. Drug induced lupus erythematosus due to capecitabine and bevacizumab treatment presenting with prolonged thrombocytopenia. Rom J Intern Med. (2015) 53:282–5. doi: 10.1515/rjim-2015–0037
21. Li T, Witteman DT, Weber ED, Alexander WL, Schaber JD. Severe immune-mediated thrombocytopenia after intravitreal bevacizumab injection. Retin Cases Brief Rep. (2020) 14:251–254. doi: 10.1097/ICB.0000000000000687
22. Liao T, Li M, Yuan T, Hong Q, Zeng Y, Yu D, et al. Case Report: Severe thrombocytopenia induced by adalimumab in rheumatoid arthritis: A case report and literature review. Front Pharmacol. (2022) 13:1041884. doi: 10.3389/fphar.2022.1041884
23. Aguiar RBD, Moraes JZD. Exploring the immunological mechanisms underlying the anti-vascular endothelial growth factor activity in tumors. Front Immunol. (2019) 10:1023. doi: 10.3389/fimmu.2019.01023
24. Meyer T, Robles-Carrillo L, Robson T, Langer F, Desai H, Davila M, et al. Bevacizumab immune complexes activate platelets and induce thrombosis in FCGR2A transgenic mice. J Thromb Haemost. (2009) 7:171–81. doi: 10.1111/j.1538-7836.2008.03212.x
25. Kut C, Mac Gabhann F, Popel AS. Where is VEGF in the body? A meta-analysis of VEGF distribution in cancer. Br J Cancer. (2007) 97:978–85. doi: 10.1038/sj.bjc.6603923
26. Verheul HMW, Lolkema MPJ, Qian DZ, Hilkes YHA, Liapi E, Akkerman J-WN, et al. Platelets take up the monoclonal antibody bevacizumab. Clin Cancer Res. (2007) 13:5341–7. doi: 10.1158/1078–0432.CCR-07–0847
27. Nomura Y, Kaneko M, Miyata K, Yatomi Y, Yanagi Y. Bevacizumab and aflibercept activate platelets via fcγRIIa. Investig Opthalmol Vis Sci. (2015) 56:8075. doi: 10.1167/iovs.15–17814
28. Cheng J, Zeng H, Chen H, Fan L, Xu C, Huang H, et al. Current knowledge of thrombocytopenia in sepsis and COVID-19. Front Immunol. (2023) 14:1213510. doi: 10.3389/fimmu.2023.1213510
29. Boussios S, Pentheroudakis G, Somarakis G, Markatseli TE, Drosos AA, Pavlidis N. Cancer Diagnosis in a Cohort of Patients with Sjogren’s Syndrome and Rheumatoid Arthritis: A Single-center Experience and Review of the Literature. Anticancer Res. (2014) 34:6669–6676.
30. Klinkhammer B, Gruchalla M. Is There an Association Between Heparin-Induced Thrombocytopenia (HIT) and Autoimmune Disease? WMJ (2018) 117:13–17.
31. Kaur J, Arsene C, Yadav SK, Ogundipe O, Malik A, Sule AA, et al. Risk factors in hospitalized patients for heparin-induced thrombocytopenia by real world database: A new role for primary hypercoagulable states. J Hematol. (2021) 10:171–7. doi: 10.14740/jh876
32. Rytel A, Nowak M, Kukawska-Rytel M, Morawiec K, Niemczyk S. Different types of vasculitis complicated by heparin-induced thrombocytopenia—Analysis of four cases and literature review. J Clin Med. (2023) 12:6176. doi: 10.3390/jcm12196176
33. Moulis G, Sommet A, Sailler L, Lapeyre-Mestre M, Montastruc J-L. The french association of regional. Drug-induced immune thrombocytopenia: A descriptive survey in the French PharmacoVigilance database. Platelets. (2012) 23:490–4. doi: 10.3109/09537104.2011.633179
34. Laurichesse M, Pedrono M, Guilleray G, Gallienne L, Cherel M, Schmitt F, et al. Oxaliplatin and methylprednisolone-induced thrombocytopenia and monocytopenia, owing to anti-GPIIbIIIa and -CD36 antibodies in a patient with colorectal cancer. Clin Colorectal Cancer. (2020) 19:e277–80. doi: 10.1016/j.clcc.2020.06.007
Keywords: drug-induced immune thrombocytopenia, bevacizumab, autoimmune diseases, ovarian cancer, mixed connective tissue disease, case report
Citation: Zhang Y, Yang F, Wang J, Fu H, Shen F, Liu J and Li D (2024) Bevacizumab-induced immune thrombocytopenia in an ovarian cancer patient with mixed connective tissue disease: case report and literature review. Front. Immunol. 15:1382964. doi: 10.3389/fimmu.2024.1382964
Received: 06 February 2024; Accepted: 17 May 2024;
Published: 05 June 2024.
Edited by:
Chris Wincup, King’s College Hospital NHS Foundation Trust, United KingdomReviewed by:
Stergios Boussios, Canterbury Christ Church University, United KingdomWeir Chiang You, Taichung Veterans General Hospital, Taiwan
Donglei Zhang, Zhongnan Hospital of Wuhan University, China
Copyright © 2024 Zhang, Yang, Wang, Fu, Shen, Liu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dongjie Li, ZGpsaUB0b25namkuZWR1LmNu; Jie Liu, TGl1amllMTk5N0B0b25namkuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
 Yunting Zhang
Yunting Zhang Fanchun Yang
Fanchun Yang Jining Wang1
Jining Wang1 Fuming Shen
Fuming Shen Dongjie Li
Dongjie Li