- 1Chaum Life Center, CHA University, Seoul, Republic of Korea
- 2Department of Family Medicine, CHA Bundang Medical Center, CHA University, Seongnam, Republic of Korea
Introduction: Blood pressure is closely linked with immune function. This study examined the association between natural killer (NK) cell activity (NKA) and blood pressure and the development of hypertension according to NKA levels.
Methods: This study enrolled 1543 adults who underwent NKA measurement and serial health check-ups at a medical center in Korea. NKA was estimated as the concentration of IFN-γ in the incubated whole blood containing a patented stimulatory cytokine. The participants were categorized into quartiles according to their NKA levels. Participants without hypertension were followed up, and the development of hypertension was compared according to the quartiles.
Results: The prevalence of hypertension was not different among the NKA quartiles, whereas blood pressures significantly decreased, followed by an increment of quartiles (systolic blood pressure of 119.0 in Q1 and 117.0 in Q4, P-trend = 0.018). Over a mean follow-up period of 2.13 years, hypertension developed in 156 of 1170 individuals without baseline hypertension. The hazard ratio of Q4 compared with Q1 was 0.625 (95% CI: 0.397–0.983; p = 0.042).
Conclusion: In conclusion, our findings indicate a correlation between lower NKA and higher blood pressure and the development of incident hypertension. This may suggest a potential protective role of NK cells against endothelial dysfunction. Further research is necessary to elucidate the specific relationship between immune functions and endothelial function.
1 Introduction
Hypertension is recognized as a risk factor for renal and cardiac diseases and stroke. Blood pressure is closely associated with endothelial inflammation and dysfunction, implying a connection between hypertension and immune function (1, 2). Evidence from animal models indicates that leukocyte subsets alterations may substantially contribute to the development of hypertension (3). Human studies have documented associations between the incidence and prevalence of hypertension and various cytokines and chemokines generated by leukocytes (4, 5).
Natural killer (NK) cells are a type of cytotoxic lymphocyte crucial to the innate immune system. They rapidly express many adhesion molecules common to the hematopoietic lineage, binding to the endothelium, extravasating and responding to chemotactic stimuli, similar to T cells (6). NK cells participate in vascular injury in hypertension (7). NK cell-derived interferon gamma (IFN-γ) plays a major role in activating monocytes/macrophages and driving them toward inflammatory phenotypes, which results in vascular dysfunction (8, 9). Findings from clinical studies are controversial pertaining to the association between circulating NK cells and endothelial function. In the MESA study, an increase in the number of NK cells was related to blood pressure (10). Conversely, patients with coronary heart disease showed a lower NK cytotoxic activity (11).
Established methods that measure NK cell activity (NKA), such as the 51Cr release assay and CD107a degranulation assay, have been widely employed to determine NK cell function. However, these methods are complicated and time-consuming as they require the isolation of peripheral blood mononuclear cells (PBMCs) or NK cells (12). To address these challenges, a relatively simple assay that employs whole blood instead of PBMCs or isolated NK cells was recently developed for commercial use to assess NKA. This innovative assay utilizes serum from ex vivo-stimulated whole blood to detect secreted IFN-γ from NK cells, serving as an NKA indicator (13). Clinical studies have indicated that NKA is a valuable marker for different cancers and is associated with immunological conditions such as ageing and vitamin D insufficiency (14–17).
The beneficial or harmful nature of NK cells in metabolic diseases remains a subject of debate (18). The depletion of NK cells reduces angiotensin II-induced vascular dysfunction, which is in contrast to their role in the inflammatory process (7). Currently, the role of NK cells in hypertension development has not been sufficiently evaluated. Specifically, the measurement of NKA has not been evaluated in this context. In this study, we examined the cross-sectional association between NKA, measured by IFN-γ released from activated NK cells, and blood pressure. Additionally, we examined differences in the incidence of hypertension according to baseline NKA levels over a mean period of 2.13 years.
2 Participants and methods
2.1 Study population
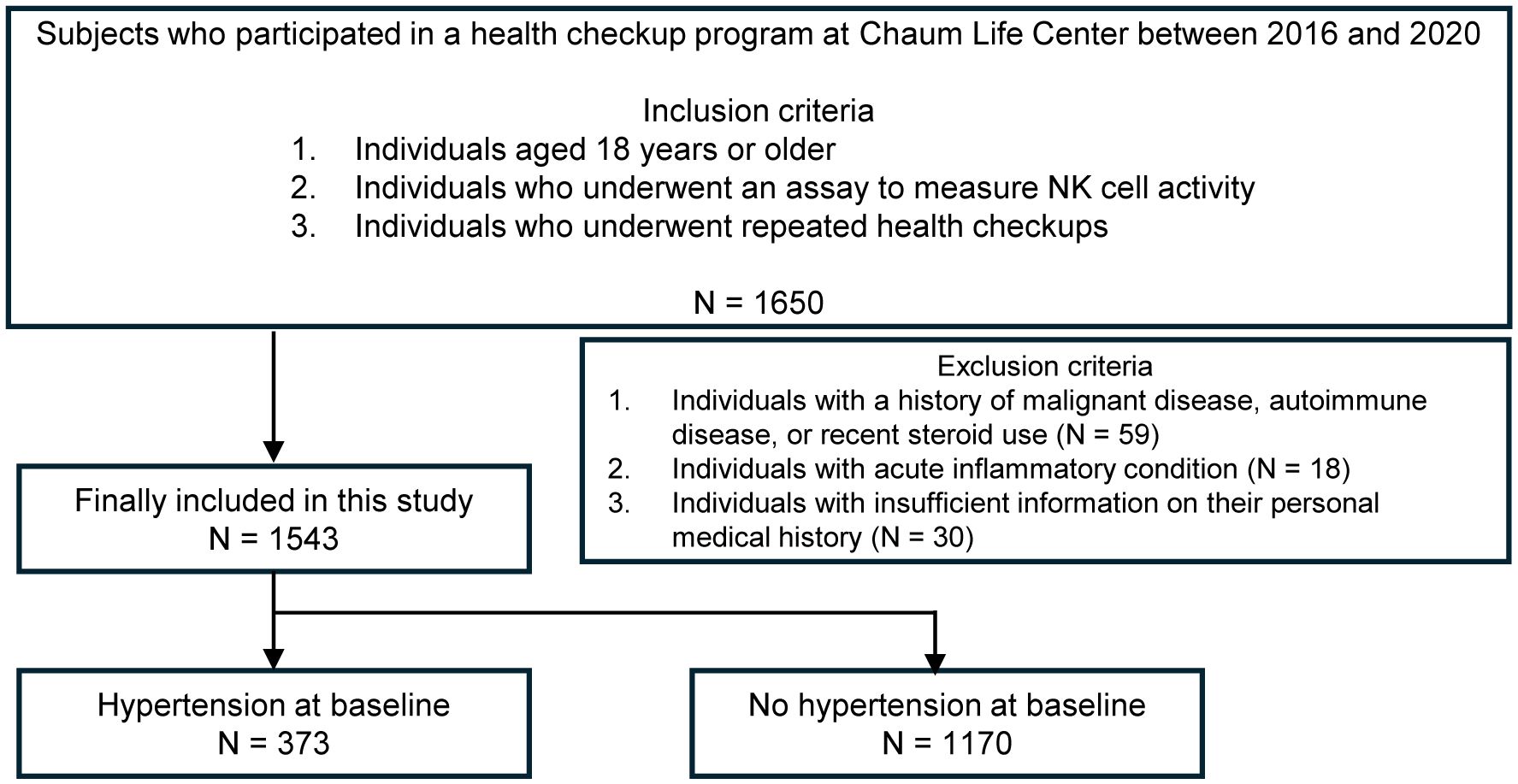
Health check-up data from the Chaum Life Center were analyzed in this study. We screened individuals aged 18 years or older who had participated in health check-up programs between 2016 and 2020. We included those who underwent an assay to measure NKA and attended repeated health check-ups (N = 1650). Among the eligible participants, we excluded individuals with a history of malignant diseases, autoimmune diseases or recent steroid use (N = 59) including those with acute inflammatory conditions (N = 18) and insufficient information on their personal medical history (N = 30), which were surveyed from all visits. Finally, a total of 1543 participants (768 men and 775 women) were included in the analysis (Figure 1).
The study protocol was approved by the Institutional Review Board of the CHA Bundang Medical Center (CHAMC 2020-10-006). Informed consent was waivered because of the retrospective nature of the study. This study was conducted in accordance with the principles of the Declaration of Helsinki.
2.2 Medical history of the participants
Medical histories and lifestyle habits of the participants were collected. The participants were classified into three categories according to their smoking habits: non-smokers, ex-smokers and current smokers. Significant alcohol consumption was defined as consuming >21 standard drinks/week in men and >14 standard drinks/week in women over a period of 2 years.
2.3 IFN-γ measurement for NKA
Using a 1-mL sample of whole blood, NKA was evaluated through measurement of IFN-γ, released by activated NK cells. This was performed by transferring whole blood directly into a tube containing a patented stimulatory cytokine (Promoca®, NKMAX, Seongnam, Korea), which causes secretion of IFN-γ into the plasma during the incubation period. The IFN-γ secretion predominantly occurs via the NK cells rather than through other innate or adaptive immune cells (13, 19–22). Within 30 min of collection, the tube was gently and repeatedly mixed and incubated for 20–24 h in a 37.0°C chamber. After incubation, the supernatant was obtained, centrifuged at 3000 × g for 3 min and then loaded onto enzyme-linked immunosorbent assay (ELISA) plates. The IFN-γ level was measured (pg/mL) using the designed ELISA.
2.4 Measurements
Height and weight were measured in a standing position without shoes and were recorded to the first decimal point in centimeters and kilograms, respectively. Body mass index (BMI) was defined as body weight in kilograms divided by height squared in meters. Blood pressure (BP) was measured using an automatic sphygmomanometer after resting for 10 min in a sitting position. The mean arterial pressure (MAP) was estimated using the following formula: (systolic BP + 2 * diastolic BP)/3.
2.5 Definition of hypertension
Hypertension was defined as meeting any of the following criteria: 1) Systolic BP ≥ 140 mmHg; 2) diastolic BP ≥ 90 mmHg; and 3) current use of antihypertensive medication (23).
2.6 Statistical analysis
Variables were summarized using mean and standard deviation or number and percentage as appropriate. Subsequently, the participants were stratified based on the presence of hypertension, and the variables were compared between the groups. The differences between the groups were compared using an independent sample t-test and a chi-square test.
To visualize the distribution of NKA, a histogram was drawn. The participants were further stratified into quartiles based on their IFN-γ levels and BP levels and the prevalence of hypertension were evaluated across these quartiles and compared using Pearson’s correlation test and Cochran–Armitage test for trend. To explore the association between risk factors and hypertension, logistic regression models were constructed.
For individuals without baseline hypertension, a longitudinal follow-up was conducted to observe the incidence of hypertension. Kaplan–Meier survival curves for each quartile were constructed and the Mantel–Haenszel test was used to compare hypertension development.
To elucidate the relationship between quartiles and the incidence of hypertension while adjusting for potential confounding factors, Cox regression models were developed.
All statistical analyses were conducted using R statistical packages version 4.3.3 (R Core Team, Vienna, Austria) and R studio 2023.06.0 + 421 with the gglot2 library. Statistical significance was determined at a conventional threshold of P < 0.05.
3 Results
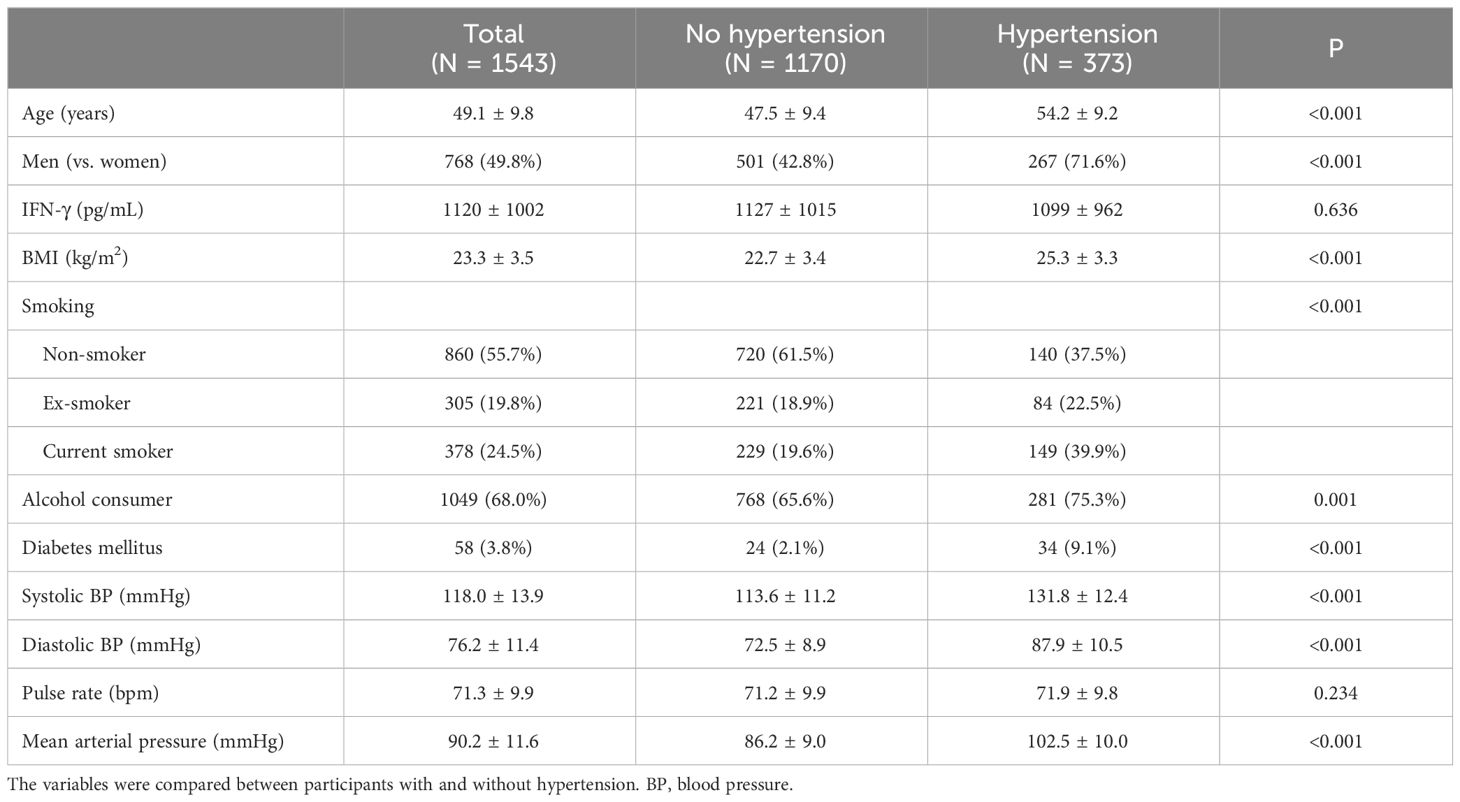
Hypertensive participants were older, with a mean age of 54.2 years compared with 47.5 years in nonhypertensive participants (Table 1). The proportion of men was higher among hypertensive individuals. Furthermore, hypertensive individuals exhibited a higher BMI, with a mean of 25.3 compared to 22.7 in nonhypertensive individuals. While systolic BP, diastolic BP and MAP were higher in the hypertensive group, no difference was observed in the pulse rate between the two groups. Moreover, no significant difference was observed in the IFN-γ levels between the two groups.
Based on the distribution of IFN-γ levels, the participants were divided into quartiles (Figure 2). According to this distribution, significant trends were observed in systolic BP, diastolic BP and MAP, all of which displayed meaningful differences across quartiles (Figure 3; p-values for trend were all statistically significant). In the multivariate logistic regression model (Supplementary Table), age, DM, BMI, and smoking emerged as significant predictors.

Figure 2 Distribution of IFN-γ and density plot of the study participants. Quartiles are drawn in the plot (Q1–Q4).

Figure 3 Blood pressure levels and the prevalence of hypertension are compared across the quartiles using Pearson’s correlation test and Cochran–Armitage test for trend. SBP, systolic blood pressure; MAP, mean arterial pressure; DBP, diastolic blood pressure; HT, hypertension; NKA, natural killer cell activity expressed in IFN-γ levels.
A total of 1170 individuals without baseline hypertension were included in the longitudinal analyses. Over a mean follow-up period of 2.13 years, hypertension developed in 156 individuals (13.3%). The incidence rate was calculated as 62.4 per 1000 person-years.
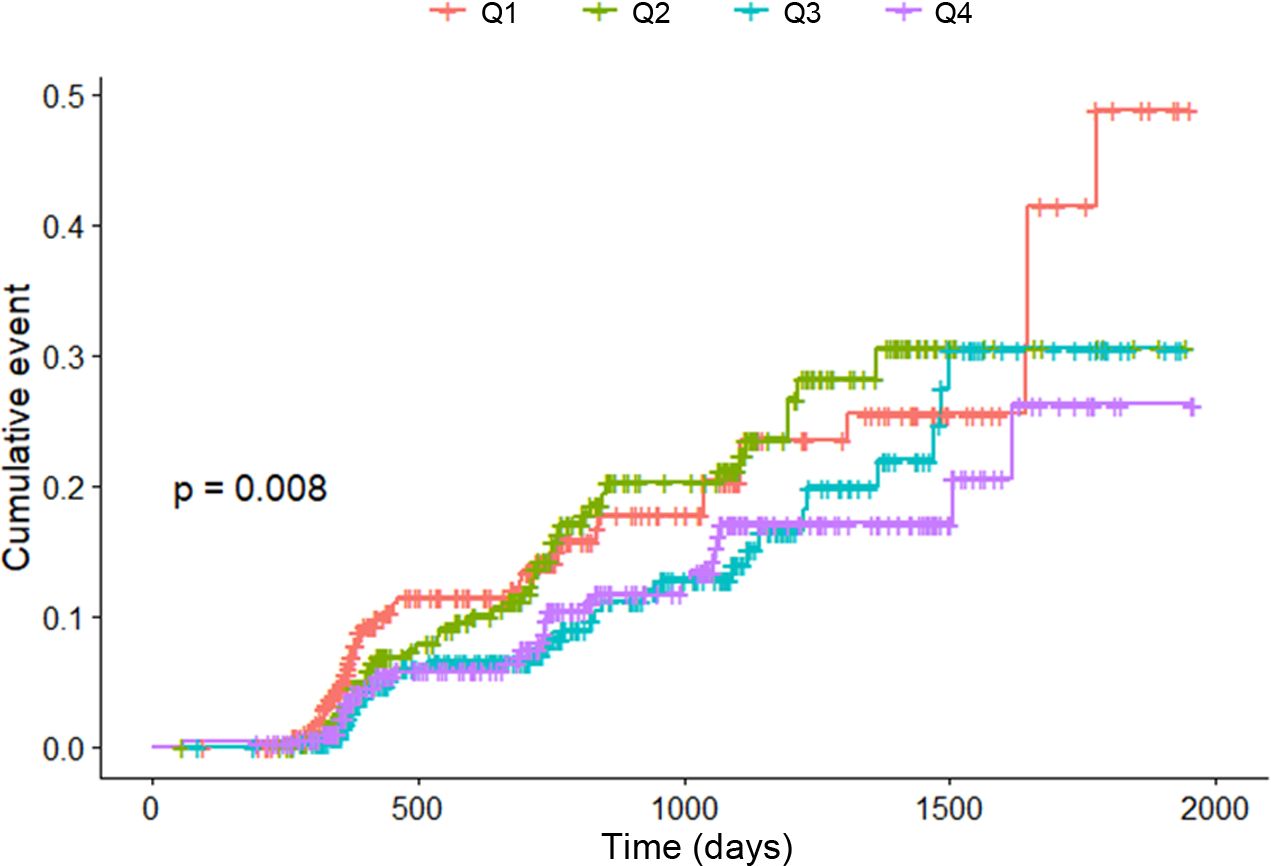
Kaplan–Meier curves were employed in the analysis (Figure 4). The occurrence of hypertension was most frequent in the first quartile (Q1) and least frequent in the fourth quartile (Q4). The p-value for trend, calculated using the Mantel–Haenszel test, was determined to be 0.008.
Table 2 shows the Cox proportional hazards model. In model 2, the HR for the third quartile (Q3) was 0.590 (95% CI: 0.376–0.926; p = 0.022) compared with Q1. Similarly, for the fourth quartile (Q4), HR was 0.625 (95% CI: 0.397–0.983; p = 0.042) compared with that for Q1. With each increase in the quartile, the HR was calculated as 0.836 (p = 0.011). In addition to NKA, age, sex, and BMI were also found to be significant factors (Supplementary Table).
4 Discussion
This study showed that a higher NKA was associated with a lower BP in a cross-sectional manner. Moreover, longitudinally, an increase in baseline NKA was linked to a reduced risk of developing hypertension. Specifically, individuals with an IFN-γ level below 300 pg/mL exhibited a 1.6-fold higher risk of incident hypertension than those with an IFN-γ level above 1700 pg/mL.
Conventional risk factors, including age, sex, obesity, metabolic disorders, dietary habits, physical activity, smoking, and alcohol consumption, are widely recognized as influential factors in blood pressure regulation and the development of hypertension (23, 24). In our study, several risk factors among these were found to be statistically significant. Unfortunately, data on dietary patterns and physical activity were not collected in our study. Literature indicates that diet and exercise may modulate immune function (25, 26). Previous research has examined the association between exercise and NKA, revealing disparities in NKA between women who engage in regular exercise and those who do not (17). Consequently, exercise may potentially influence the interplay between immune response and hypertension. Therefore, it can be suggested that the possibility of an exercise-immune interaction may have influenced our study findings.
In previous studies, the association between NK cells and BP has been controversial. NK cells have also been recognized as participants in the inflammatory process (27). In hypertension, NK cells are implicated in vascular injury and cytokines derived from NK cells impact vascular dysfunction (7). Notably, an increase in the number of peripheral NK cells has been linked to elevated BP (10). Conversely, NK cell dysfunction can render an individual more susceptible to atherogenic pathogens and potentially contribute to coronary artery disease (11). Consequently, a reduced NK cytotoxic activity is associated with coronary heart disease and atherosclerosis (28). These findings corroborate with the outcomes of our investigation.
Endothelial dysfunction may play a role in initiating and advancing vascular inflammation, vascular remodeling and atherosclerosis in hypertension, and it is independently linked to elevated cardiovascular risk (1). Various cytokines originating from NK cells could impact vascular inflammation and endothelial dysfunction (7). Conversely, NK cells might indirectly contribute to the preservation of endothelial function by regulating inflammation and angiogenesis (29–31). Our study provides support for the notion that NKA could play a role in attenuating the progression of hypertension.
Our study has several limitations. First, as it was conducted in a single center in Korea, the generalizability of our findings may be limited. However, it is important to note that our analysis involved a substantial number of participants and a comprehensive follow-up period. Subsequent investigations could help confirm the broader relevance of the correlation between NKA and hypertension. Second, our study did not use methods of quantifying NK cells. As recognized, the conventional method for assessing NKA is not suitable for accommodating a large sample size. Our employed method enabled the analysis of NKA in a substantial number of participants. The method used to measure IFN-γ levels as a surrogate marker for NKA also has limitation. IFN-γ concentrations may show slight variations depending on the specific duration of incubation within the range of 20–24 hours. However, minor deviations in incubation time are generally acceptable within clinical settings. Thirdly, several conventional risk factors such as diet and exercise were not included as covariates. Diet and physical factors likely influence the endothelium, potentially through mechanisms involving oxidative stress and vascular remodeling (32). Future studies should explore the interaction between diet/exercise and immune function.
Additionally, exploring how diet and exercise interact with the immune system to impact endothelial function warrants investigation.
In conclusion, our findings show a correlation between a lower NKA and higher BP, and the development of incident hypertension. Although our study found encouraging findings suggesting a potential protective role of NK cells against hypertension and endothelial dysfunction, further research is necessary to elucidate the specific relationship, particularly how NKA, and other immune functions, influence endothelial function. Further longitudinal studies could evaluate cardiovascular events according to NKA levels.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Institutional Review Board of the CHA Bundang Medical Centre (CHAMC 2020-10-006). The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because We included the participants among the examinees with previous visit history with retrospective analysis design.
Author contributions
YL: Data curation, Funding acquisition, Writing – original draft, Writing – review & editing. ES: Resources, Validation, Writing – original draft, Writing – review & editing. HO: Methodology, Software, Writing – original draft, Writing – review & editing. JH: Supervision, Validation, Writing – original draft, Writing – review & editing. YK: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Korean Fund for Regenerative Medicine (KFRM) grant funded by the Korea government (the Ministry of Science and ICT, the Ministry of Health & Welfare, grant number 23C0102L1) and NKMAX Co., Ltd. (grant number 2021-09-047). The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1376421/full#supplementary-material
References
1. Gallo G, Volpe M, Savoia C. Endothelial dysfunction in hypertension: current concepts and clinical implications. Front Med (Lausanne). (2021) 8:798958. doi: 10.3389/fmed.2021.798958
2. Zhang Z, Zhao L, Zhou X, Meng X, Zhou X. Role of inflammation, immunity, and oxidative stress in hypertension: New insights and potential therapeutic targets. Front Immunol. (2022) 13:1098725. doi: 10.3389/fimmu.2022.1098725
3. Kresovich JK, Xu Z, O'Brien KM, Parks CG, Weinberg CR, Sandler DP, et al. Peripheral immune cell composition is altered in women before and after a hypertension diagnosis. Hypertension. (2023) 80:43–53. doi: 10.1161/HYPERTENSIONAHA.122.20001
4. Ishida S, Kondo S, Funakoshi S, Satoh A, Maeda T, Kawazoe M, et al. White blood cell count and incidence of hypertension in the general Japanese population: ISSA-CKD study. PloS One. (2021) 16:e0246304. doi: 10.1371/journal.pone.0246304
5. Mirhafez SR, Mohebati M, Feiz Disfani M, Saberi Karimian M, Ebrahimi M, Avan A, et al. An imbalance in serum concentrations of inflammatory and anti-inflammatory cytokines in hypertension. J Am Soc Hypertens. (2014) 8:614–23. doi: 10.1016/j.jash.2014.05.007
6. Timonen T. Natural killer cells: endothelial interactions, migration, and target cell recognition. J Leukoc Biol. (1997) 62:693–701. doi: 10.1002/jlb.62.6.693
7. Kossmann S, Schwenk M, Hausding M, Karbach SH, Schmidgen MI, Brandt M, et al. Angiotensin II-induced vascular dysfunction depends on interferon-gamma-driven immune cell recruitment and mutual activation of monocytes and NK-cells. Arterioscler Thromb Vasc Biol. (2013) 33:1313–9. doi: 10.1161/ATVBAHA.113.301437
8. Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol. (2004) 75:163–89. doi: 10.1189/jlb.0603252
9. Shereck E, Satwani P, Morris E, Cairo MS. Human natural killer cells in health and disease. Pediatr Blood Cancer. (2007) 49:615–23. doi: 10.1002/pbc.21158
10. Delaney JAC, Olson NC, Sitlani CM, Fohner AE, Huber SA, Landay AL, et al. Natural killer cells, gamma delta T cells and classical monocytes are associated with systolic blood pressure in the multi-ethnic study of atherosclerosis (MESA). BMC Cardiovasc Disord. (2021) 21:45. doi: 10.1186/s12872-021-01857-2
11. Hak L, Mysliwska J, Wieckiewicz J, Szyndler K, Trzonkowski P, Siebert J, et al. NK cell compartment in patients with coronary heart disease. Immun Ageing. (2007) 4:3. doi: 10.1186/1742-4933-4-3
12. Valiathan R, Lewis JE, Melillo AB, Leonard S, Ali KH, Asthana D. Evaluation of a flow cytometry-based assay for natural killer cell activity in clinical settings. Scand J Immunol. (2012) 75:455–62. doi: 10.1111/j.1365-3083.2011.02667.x
13. Lee SB, Cha J, Kim IK, Yoon JC, Lee HJ, Park SW, et al. A high-throughput assay of NK cell activity in whole blood and its clinical application. Biochem Biophys Res Commun. (2014) 445:584–90. doi: 10.1016/j.bbrc.2014.02.040
14. Jobin G, Rodriguez-Suarez R, Betito K. Association between natural killer cell activity and colorectal cancer in high-risk subjects undergoing colonoscopy. Gastroenterology. (2017) 153:980–7. doi: 10.1053/j.gastro.2017.06.009
15. Barkin J, Rodriguez-Suarez R, Betito K. Association between natural killer cell activity and prostate cancer: a pilot study. Can J Urol. (2017) 24:8708–13.
16. Choi SI, Lee SH, Park JY, Kim KA, Lee EJ, Lee SY, et al. Clinical utility of a novel natural killer cell activity assay for diagnosing non-small cell lung cancer: a prospective pilot study. Onco Targets Ther. (2019) 12:1661–9. doi: 10.2147/OTT.S194473
17. Oh S, Chun S, Hwang S, Kim J, Cho Y, Lee J, et al. Vitamin D and exercise are major determinants of natural killer cell activity, which is age- and gender-specific. Front Immunol. (2021) 12:594356. doi: 10.3389/fimmu.2021.594356
18. Li Y, Wang F, Imani S, Tao L, Deng Y, Cai Y. Natural killer cells: friend or foe in metabolic diseases? Front Immunol. (2021) 12:614429. doi: 10.3389/fimmu.2021.614429
19. Du N, Zhou J, Lin X, Zhang Y, Yang X, Wang Y, et al. Differential activation of NK cells by influenza A pseudotype H5N1 and 1918 and 2009 pandemic H1N1 viruses. J Virol. (2010) 84:7822–31. doi: 10.1128/JVI.00069-10
20. Koo KC, Shim DH, Yang CM, Lee SB, Kim SM, Shin TY, et al. Reduction of the CD16(-)CD56bright NK cell subset precedes NK cell dysfunction in prostate cancer. PloS One. (2013) 8:e78049. doi: 10.1371/journal.pone.0078049
21. Nederby L, Jakobsen A, Hokland M, Hansen TF. Quantification of NK cell activity using whole blood: Methodological aspects of a new test. J Immunol Methods. (2018) 458:21–5. doi: 10.1016/j.jim.2018.04.002
22. Jurisic V. Multiomic analysis of cytokines in immuno-oncology. Expert Rev Proteomics. (2020) 17:663–74. doi: 10.1080/14789450.2020.1845654
23. Unger T, Borghi C, Charchar F, Khan NA, Poulter NR, Prabhakaran D, et al. International society of hypertension global hypertension practice guidelines. Hypertension. (2020) 75:1334–57. doi: 10.1161/HYPERTENSIONAHA.120.15026
24. Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J Hypertens. (2018) 36:1953–2041. doi: 10.1097/HJH.0000000000001940
25. Singh DN, Bohra JS, Dubey TP, Shivahre PR, Singh RK, Singh T, et al. Common foods for boosting human immunity: A review. Food Sci Nutr. (2023) 11:6761–74. doi: 10.1002/fsn3.3628
26. Salimans L, Liberman K, Njemini R, Kortekaas Krohn I, Gutermuth J, Bautmans I. The effect of resistance exercise on the immune cell function in humans: A systematic review. Exp Gerontol. (2022) 164:111822. doi: 10.1016/j.exger.2022.111822
27. Zitti B, Bryceson YT. Natural killer cells in inflammation and autoimmunity. Cytokine Growth Factor Rev. (2018) 42:37–46. doi: 10.1016/j.cytogfr.2018.08.001
28. Bruunsgaard H, Pedersen AN, Schroll M, Skinhoj P, Pedersen BK. Decreased natural killer cell activity is associated with atherosclerosis in elderly humans. Exp Gerontol. (2001) 37:127–36. doi: 10.1016/s0531-5565(01)00162-0
29. Tosello-Trampont A, Surette FA, Ewald SE, Hahn YS. Immunoregulatory role of NK cells in tissue inflammation and regeneration. Front Immunol. (2017) 8:301. doi: 10.3389/fimmu.2017.00301
30. Radomska-Lesniewska DM, Bialoszewska A, Kaminski P. Angiogenic properties of NK cells in cancer and other angiogenesis-dependent diseases. Cells. (2021) 10:1621. doi: 10.3390/cells10071621
31. Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. (2008) 9:503–10. doi: 10.1038/ni1582
Keywords: natural killer cells, natural killer cell activity, blood pressure, hypertension, incidence
Citation: Lee Y-K, Suh E, Oh H, Haam J-H and Kim Y-S (2024) Decreased natural killer cell activity as a potential predictor of hypertensive incidence. Front. Immunol. 15:1376421. doi: 10.3389/fimmu.2024.1376421
Received: 25 January 2024; Accepted: 11 April 2024;
Published: 23 April 2024.
Edited by:
Lan Wu, Vanderbilt University Medical Center, United StatesReviewed by:
Mark L. Ormiston, Queen’s University, CanadaJuan Carlos Quintana-Castillo, Universidad Cooperativa de Colombia, Colombia
Copyright © 2024 Lee, Suh, Oh, Haam and Kim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Young-Sang Kim, emVyb3VwQGNoYS5hYy5rcg==
 Yun-Kyong Lee
Yun-Kyong Lee Eunkyung Suh
Eunkyung Suh Hyoju Oh
Hyoju Oh Ji-Hee Haam
Ji-Hee Haam Young-Sang Kim
Young-Sang Kim