- The First Affiliated Hospital of Guangxi University of Science and Technology, Guangxi University of Science and Technology, Liuzhou, Guangxi, China
Introduction: This systematic review and network meta-analysis(NMA) was designed to compare the long-term outcomes of pembrolizumab monotherapy and pembrolizumab plus chemotherapy as first-line therapy for metastatic non-small-cell lung cancer(NSCLC).
Materials and Methods: Four databases(Medline, Embase, Web of Science and CENTRAL were searched published from establishment of database to August 17, 2023, for articles studying pembrolizumab monotherapy or pembrolizumab plus chemotherapy for non-small cell lung cancer (NSCLC). Network meta-analyses of progression-free survival(PFS), overall survival(OS), objective response rate(ORR), treatment-related adverse events(trAEs) and immune-related adverse events(irAEs) were performed.
Results: A total of five studies were considered for NMA. This NMA includes a cohort of 2878 patients diagnosed with advanced NSCLC. Among them, 791 patients received pembrolizumab monotherapy, 1337 patients received chemotherapy, and 748 patients received pembrolizumab plus chemotherapy. The IPDformKM software was utilized to reconstruct Kaplan-Meier curves for OS and PFS, offering a lucid and intuitive depiction of oncological outcomes. For patients who have high levels of programmed death-ligand 1(PD-L1) expression (≥50%), pembrolizumab plus chemotherapy was more effective than using pembrolizumab alone as first-line therapy in terms of PFS (median survival time: 10.41 months versus 7.41 months, HR: 0.81, 95%CI 0.67 to 0.97, P=0.02) and ORR (RR:1.74, 95% CI: 1.25-2.43). Nevertheless, there was no statistically significant difference observed between the two groups in terms of OS (median survival time: 22.54 months versus 22.62 months, HR: 0.89, 95%CI 0.73 to 1.08, P=0.24). Furthermore, pembrolizumab plus chemotherapy provided a more advantageous long-term survival advantage in terms of OS (median survival time: 20.88 months versus 13.60 months, HR: 0.77, 95%CI: 0.62 to 0.95, P=0.015) compared to pembrolizumab monotherapy in patients with low PD-L1 expression levels (1% to 49%). With regards to safety, there was no statistically significant disparity between the two groups in relation to any irAEs (RD=0.02, 95% CI: -0.12 to 0.16) or Grade≥ 3 irAEs (RD=0.01, 95% CI: -0.10 to 0.12). Nevertheless, pembrolizumab plus chemotherapy exhibited a greater likelihood of encountering any trAEs (RD=0.23, 95% CI: 0.17 to 0.30) and Grade≥ 3 trAEs (RD=0.28, 95% CI: 0.21 to 0.35) in comparison to pembrolizumab monotherapy.
Conclusions: The present network meta-analysis reported comparative long-term outcomes of pembrolizumab plus chemotherapy versus pembrolizumab monotherapy as first-line therapy for metastatic non-small-cell lung cancer. Pembrolizumab plus chemotherapy led to improved PFS and ORR in patients with advanced NSCLC who had a PD-L1 expression level of 50% or above. However, there was no noticeable benefit in terms of OS when pembrolizumab was paired with chemotherapy compared to utilizing pembrolizumab alone. In addition, pembrolizumab plus chemotherapy offered a greater long-term survival benefit in terms of OS when compared to utilizing pembrolizumab alone in patients with PD-L1 expression levels ranging from 1% to 49%. Furthermore, the increased effectiveness of pembrolizumab plus chemotherapy was accompanied by an increase in adverse side effects.
Systematic review registration: https://www.crd.york.ac.uk/prospero/, identifier CRD42024501740.
1 Introduction
Lung cancer is the predominant form of cancer globally and the primary cause of mortality connected to cancer, resulting in about 1.7 million fatalities annually (1). About 80%-85% of lung cancers are pathologically classified as non-small cell lung cancer (2). Currently, surgery is the main approach used to treat early-stage non-small cell lung cancer. Nevertheless, a significant proportion of individuals experience the formation of either local or distant metastases (3). The therapeutic advancement of immune checkpoint blockade has significantly transformed the approach to treating and predicting outcomes for individuals diagnosed with NSCLC (4). Tumor cells possess many methods to resist immune system attacks, including the expression of immunosuppressive molecules on their cell surface, secretion of immunosuppressive substances, and recruitment of other immune cell populations with suppressive properties (5). Specific inhibitors against checkpoint receptors can block this immunosuppression, thereby increasing the specific immune response of T lymphocytes and eliciting an antitumor response (6, 7). Pembrolizumab is a humanized monoclonal antibody targeting programmed death 1 (PD-1), which has been shown to have antitumor activity in advanced non-small cell lung cancer (NSCLC). Single-agent pembrolizumab as first-line therapy is approved for tumors with high expression of PD-L1 (≥50%) while immunotherapy and chemotherapy are approved for any PD-L1 (8).
Pembrolizumab has demonstrated encouraging outcomes in recent clinical studies, particularly in cases where PDL1 staining is equal to or greater than 50% of tumor cells (9). Clinical trials and meta-analyses have shown that this treatment regimen can, in some cases, significantly improve patients’ overall survival and progression-free survival, while having a low toxicity profile (10). Additionally, the utilization of pembrolizumab in conjunction with chemotherapy has garnered considerable interest. The benefit of this combo treatment is its ability to achieve a wider range of effectiveness in patients with limited PD-L1 expression. Combining multiple therapies may offer longer-lasting disease management and improved survival advantages compared to using a single medication. Nevertheless, it is crucial to consider the potential toxicities and medication resistance associated with it (11). The KEYNOTE-042 study was an open label phase II-III randomized trial comparing pembrolizumab monotherapy with chemotherapy in the treatment of advanced NSCLC. The findings indicated that pembrolizumab outperformed chemotherapy in terms of overall survival and progression-free survival in the overall population. Moreover, there were more substantial enhancements in OS and PFS specifically for the subset of tumors with PDL1≥50% (12). The KEYNOTE-189 study was a randomized phase III trial that evaluated the efficacy of pembrolizumab in combination with chemotherapy compared to chemotherapy alone for the treatment of advanced NSCLC. The findings demonstrated that the combination of pembrolizumab and chemotherapy outperformed chemotherapy alone in terms of OS and PFS in both the overall population and the subgroup of tumors with a PDL1≥50% expression (13).
However, there is currently a lack of clinical trials of chemotherapy combined with pembrolizumab versus pembrolizumab monotherapy to determine whether chemotherapy combined with pembrolizumab has a higher benefit than pembrolizumab monotherapy in metastatic non-small cell carcinoma. Network meta-analysis enables the comparison of treatment arms in randomized controlled trials (RCTs) by utilizing relative and absolute measures of treatment efficacy and common treatment arms (14–16). Prior network meta-analyses (17–19) had shown the effectiveness and safety of combining pembrolizumab with chemotherapy compared to using pembrolizumab alone. However, since the original articles only presented short-term outcomes, they failed to provide information on the long-term outcomes of RCTs. Over the course of the last three years, multiple randomized controlled trials (RCTs) have updated their long-term outcomes (13, 20–23). Hence, it is both possible and essential to perform a meta-analysis that compares the long-term outcomes of pembrolizumab plus chemotherapy versus pembrolizumab monotherapy as the initial treatment for metastatic non-small-cell lung cancer.
The aim of our study was to indirectly compare the long-term outcomes of pembrolizumab in combination with chemotherapy versus pembrolizumab monotherapy as first-line therapy. We additionally assessed the disparities in survival rates among patients with tumors exhibiting PD-L1 expression ranging from 1% to 49%.
2 Material and methods
2.1 Search strategy
The present meta-analysis was performed in accordance with the 2020 standards of the Preferred Reporting Project for Systematic Review and Meta-Analysis (PRISMA).This study has been registered at PROSPERO with a registration number of CRD42024501740. Four databases including of PubMed, Embase, Web of science, and the Cochrane Library were systematically searched for literatures published up to August 17, 2023, and a combination of MeSH and free-text words were searched according to the PICOS principle, using the following searching strategy: (“pembrolizumab” AND “Chemotherapy” AND “Non Small Cell Lung” AND “randomized controlled trial”). Supplementary Material 1 presented the searching record in detail.
2.2 Inclusion and exclusion criteria
Inclusion criteria were as follows (1): Comparing pembrolizumab versus chemotherapy, or pembrolizumab plus chemotherapy versus chemotherapy, or pembrolizumab versus pembrolizumab plus chemotherapy; (2) Untreated metastatic non-small-cell lung cancer; (3) Median follow-up time was at least 48 months, and at least one of the following outcomes were reported: PFS, OS, Grade≥ 3 irAEs rate, Grade≥ 3 TRAEs rate; (4) Randomized controlled trials.
Exclusion criteria: (1) Other types of articles, such as case reports, letters, reviews, meta-analyses, editorials, animal studies and protocols; (2) Not RCTs; (3) Unable to extract data; (4) Reduplicate cohort of patients.
2.3 Selection of studies
The selection of research, including duplicate removal, was managed using EndNote (Version 20; Clarivate Analytics). Two reviewers independently conducted the initial search, eliminated duplicate records, evaluated the titles and abstracts for relevance, and categorized each study as either included or omitted. We reached a resolution by achieving consensus. In the absence of a consensus, a third review author assumed the role of an arbitrator.
2.4 Data extraction
Two reviewers independently extracted the data. Data retrieved included patient groups and numbers, age, sex, smoking status, Eastern Tumor Cooperative Group(ECOG), brain metastases, histological type, PD-L1 TPS, the name of the study, first author, year of publication, ORR, OS, PFS, trAEs, Grade≥ 3 trAEs, irAEs, Grade≥ 3 irAEs, Kaplan-Meier curves for OS, Kaplan-Meier curves for PFS. Discrepancy was resolved by consulting with a third investigator.
2.5 Risk of bias assessment
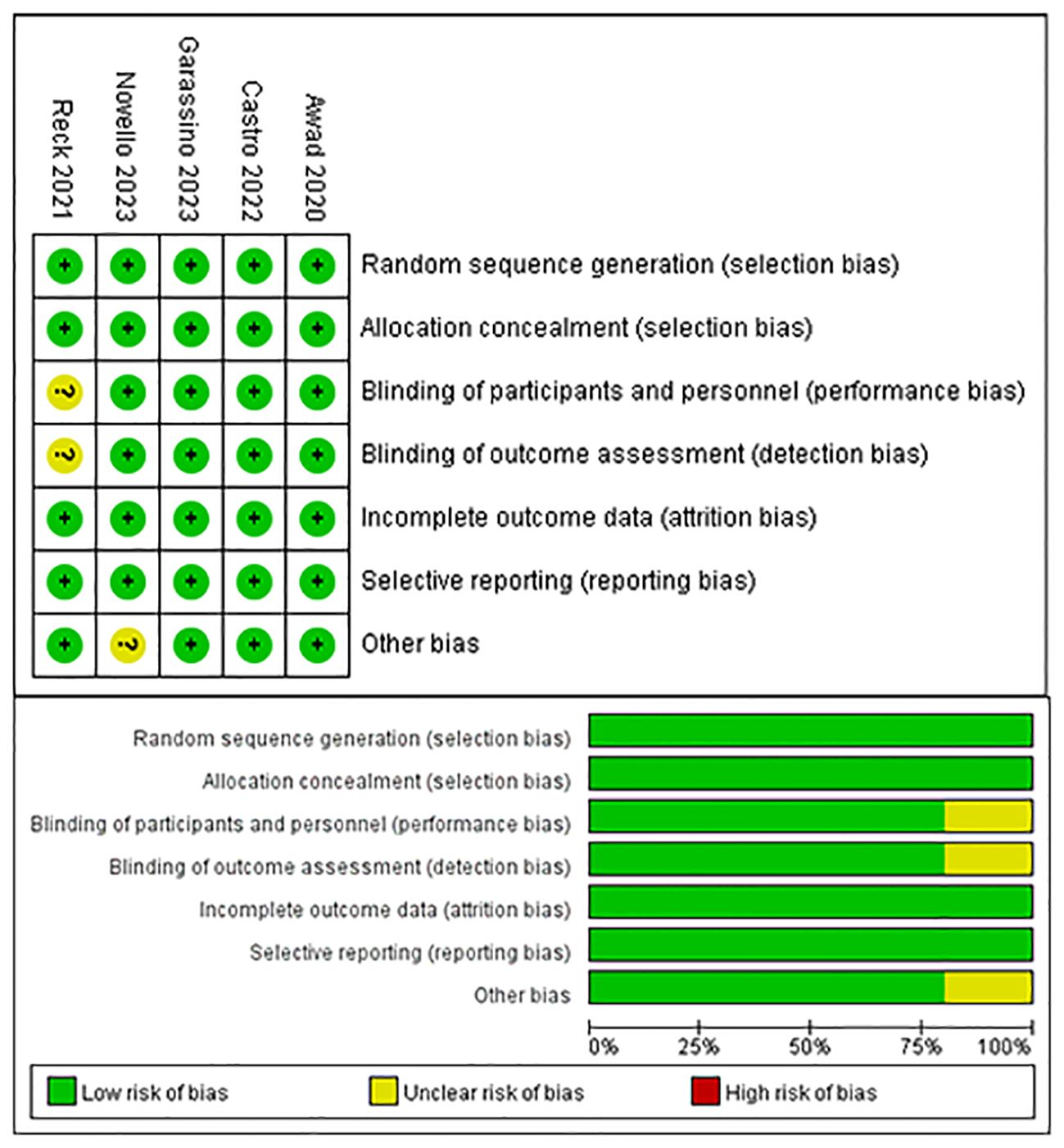
The risk of bias in the trials included was assessed by two independent reviewers using the Cochrane Risk of Bias tool, according to the following domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting and others bias. If there were discrepancies, the controversial results were resolved by group discussion. The quality evaluation of the literature is shown in Figure 1.
2.6 Statistical analysis
The selection duplicate removal of studies included was conducted using EndNote (Version 20; Clarivate Analytics). Review manager 5.3(Cochrane Collaboration, Oxford, UK), Stata 12.0, Statistical software R (version 4.3.1, https://www.r-project.org/), the R package “netmeta” and “IPDformKM” package were used for data analysis (24). We quantified Kaplan-Meier curves for RFS and OS using GetData Graph Digitizer software and reconstructed individual data through the IPDformKM package. Individual patient-level data were reconstructed using the method established by Guyot et al. (25). Upon reconstructing the individual patient data, the patients were categorized into groups. Patients who received chemotherapy were assigned to the chemotherapy cohort, while patients who received pembrolizumab monotherapy were assigned to the pembrolizumab cohort. Another cohort was formed consisting of patients who had pembrolizumab in combination with chemotherapy. Subsequently, we recreated the survival curves for the three groups in order to gain insight into long-term survival following treatment with three distinct interventions. All the results were analyzed by random effects model. P value < 0.05 was considered statistically significant.
3 Results
3.1 Search results
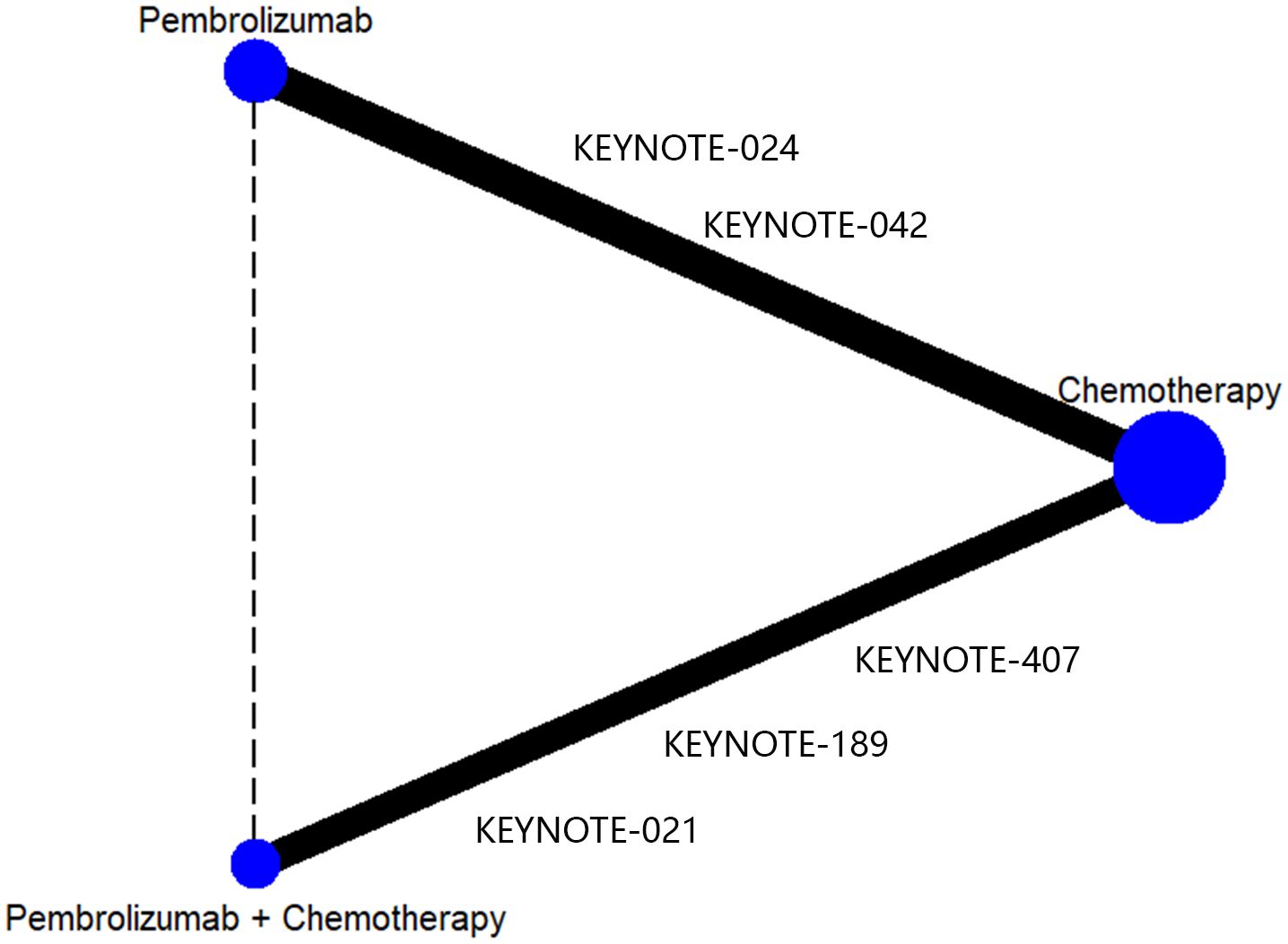
After doing the initial search, a total of 3345 publications were identified. However, after removing duplicate research, only 2618 cases remained. Out of these papers, a total of 2565 were eliminated from consideration after evaluating the titles and abstracts. Ultimately, a total of 53 articles were accessible for a comprehensive examination of their complete content. Following the application of the inclusion criteria, 5 trials were chosen for inclusion. Two of these evaluated pembrolizumab monotherapy versus platinum chemotherapy (21, 22) and three evaluated pembrolizumab combined with platinum chemotherapy versus platinum chemotherapy (13, 20, 23). The detail process of inclusion and exclusion of literature is shown in Figure 1. Data from the included RCT trials were used to construct a network of RCTs that indirectly compared pembrolizumab plus chemotherapy versus pembrolizumab alone, with chemotherapy as the common control group (Figure 2).
3.2 Patient characteristics
This study includes a sample of 2878 patients diagnosed with metastatic NSCLC. Among them, 791 patients were randomly assigned to receive pembrolizumab monotherapy, 1337 patients were assigned to receive chemotherapy alone, and 748 patients were assigned to receive combination therapy consisting of pembrolizumab and chemotherapy. Though the specific chemotherapy regimens were different among five RCTs, all chemotherapy cohorts were administered platinum-based combination treatment (Table 1). The baseline features of the patients, such as age, ECOG performance status, smoking status, masculinity, brain metastases status, and previous treatment, were comparable. All chemotherapy cohorts were administered platinum-based combination treatment. Table 1 displays the characteristics of the studies that were included.
3.3 Risk of bias
Figure 3 provides a summary of the risk of bias assessment results. Among the 5 studies, an adequate randomized sequence was generated in five studies, appropriate allocation concealment was reported in five studies, the blinding of participants was clear in four studies, the blinding of outcome assessors was reported in four studies, outcome data were complete in five studies, five studies had no selective reporting, and four studies had no other bias.
3.4 Progression-free survival - PD-L1 TPS ≥ 50%
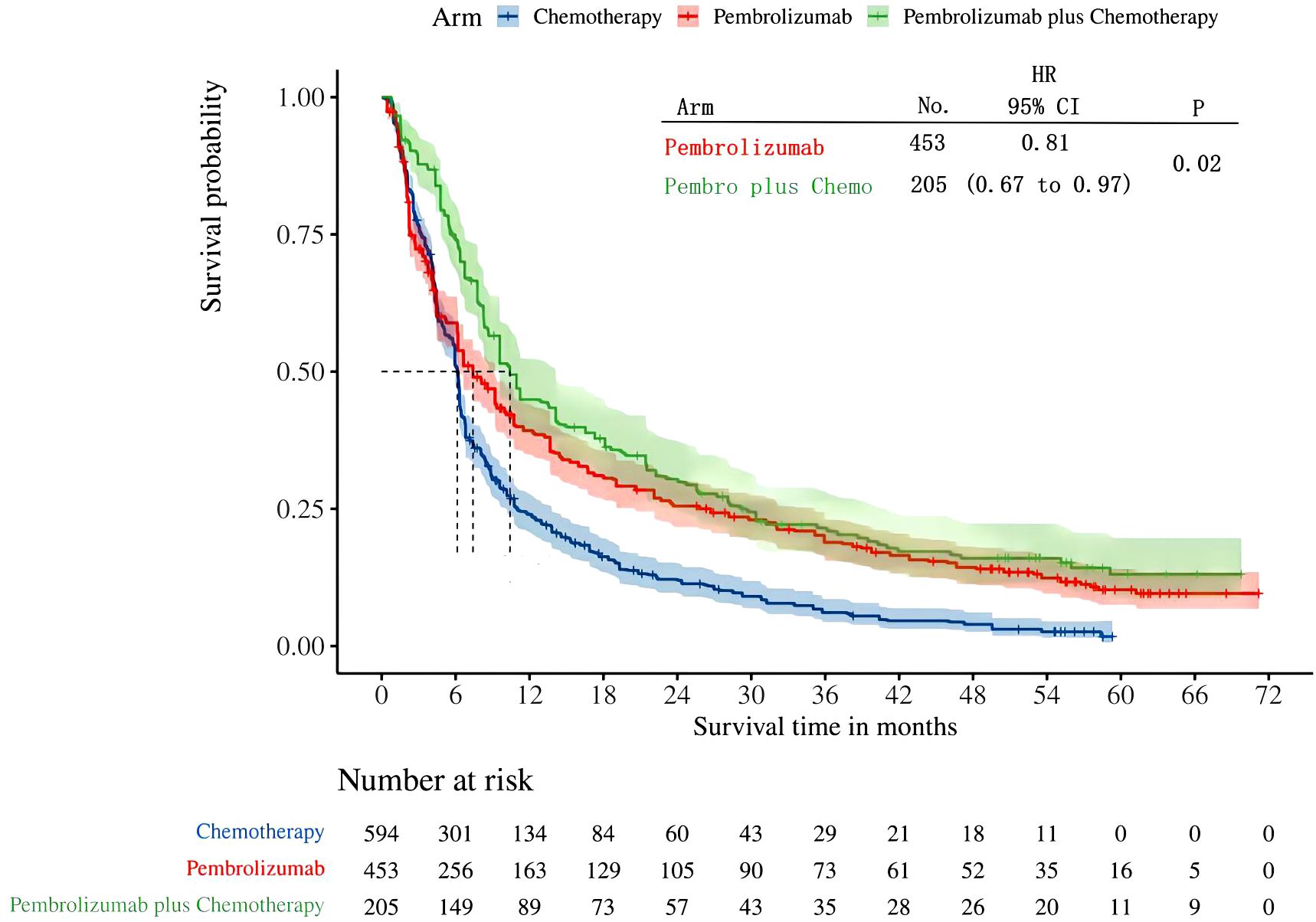
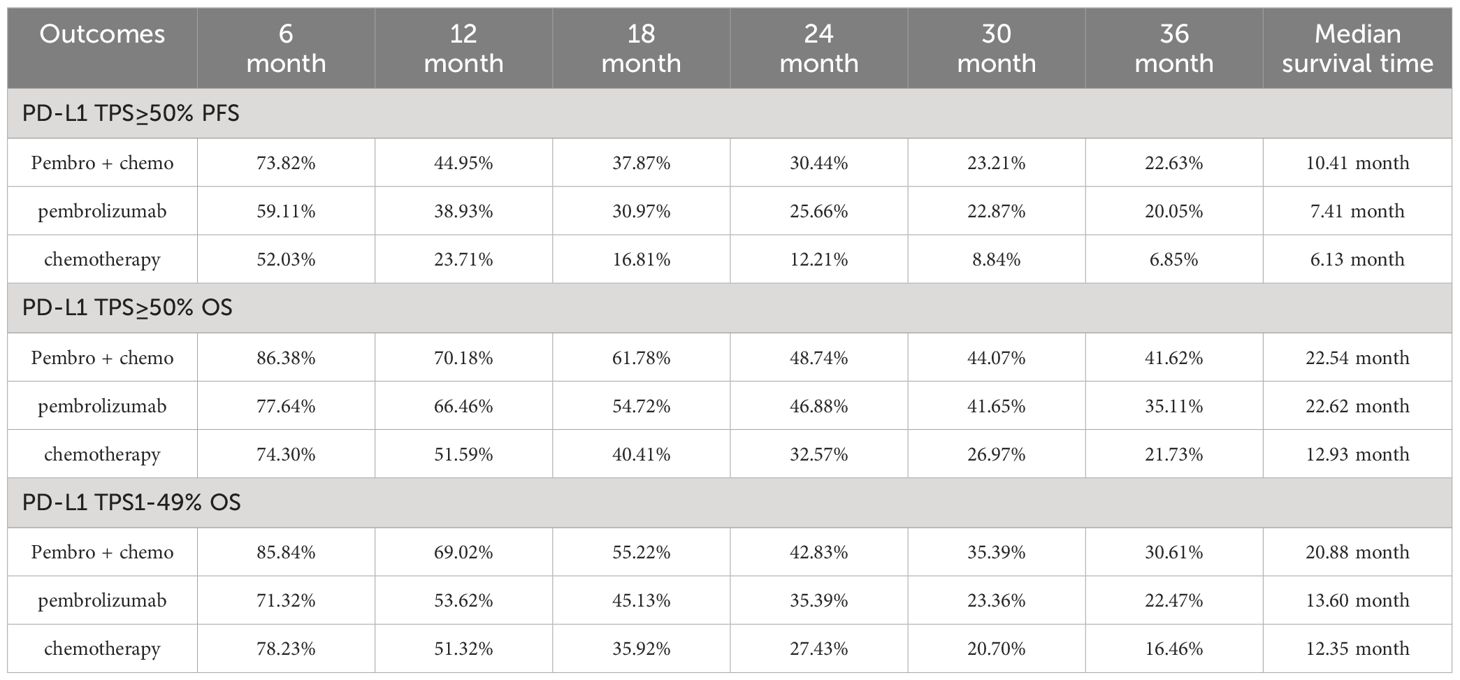
Four studies provided data on PFS in patients with high levels of PD-L1 expression (≥50%). Among these studies, KEYNOTE-024 and KEYNOTE-042 reported PFS outcomes with pembrolizumab monotherapy, while KEYNOTE-407 and KEYNOTE-189 revealed PFS outcomes with pembrolizumab in conjunction with chemotherapy. Following the reconstruction of the cohort, we conducted a new analysis of PFS specifically in patients with a tumor PD-L1 expression level of 50% or higher. The Kaplan-Meier curve demonstrates that the combination of pembrolizumab with chemotherapy provides a superior long-term survival advantage compared to pembrolizumab alone in terms of PFS(HR: 0.81, 95%CI: 0.67 to 0.97, P=0.02) (Figure 4). The PFS median survival time for pembrolizumab combined with chemotherapy was 10.41 months, while it was 7.41 months for pembrolizumab monotherapy and 6.13 months for chemotherapy alone. We provided periodic updates on PFS of each group at 6-month intervals from 0 to 36 months, which are displayed in Table 2.
3.5 Overall survival - PD-L1 TPS ≥ 50%
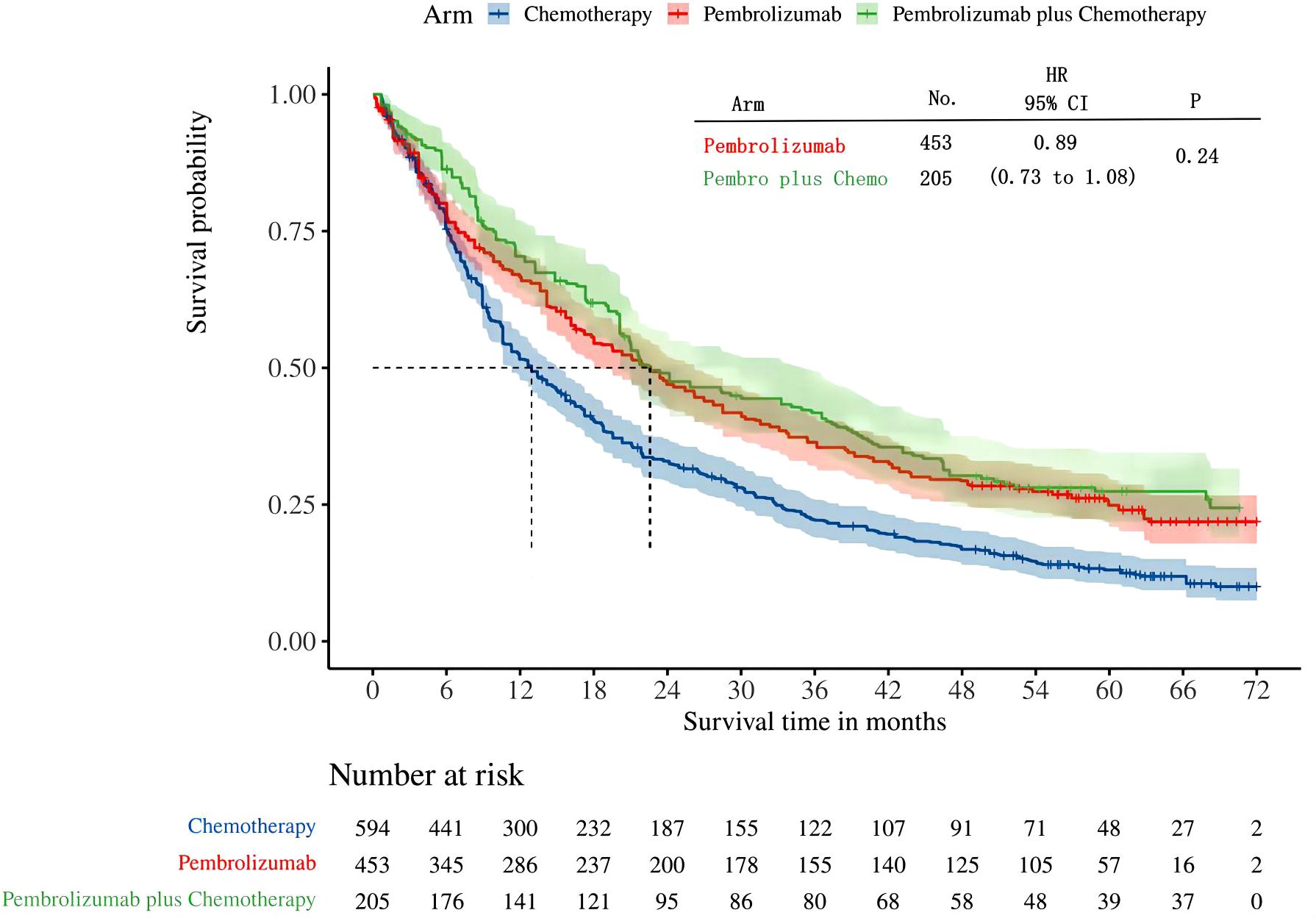
A total of four studies reported overall survival in patients with high levels of PD-L1 expression (≥50%). Among these studies, KEYNOTE-024 and KEYNOTE-042 reported OS with pembrolizumab monotherapy, and KEYNOTE-407 and KEYNOTE-189 reported OS with pembrolizumab in combination with chemotherapy. Following the reconstruction of the cohort, we conducted a new analysis of OS specifically in patients with a tumor PD-L1 expression level of 50% or higher. The Kaplan-Meier curve demonstrates that there was no statistical significance in terms of OS between two groups(HR: 0.89, 95%CI: 0.73 to 1.08, P=0.24) (Figure 5). The median survival time for OS was 22.54 months for the combination of pembrolizumab and chemotherapy, 22.62 months for pembrolizumab monotherapy, and 12.93 months for chemotherapy alone. We provided periodic updates on OS of each group at 6-month intervals from 0 to 36 months, which are displayed in Table 2.
3.6 Objective response rate–PD-L1 TPS > 50%
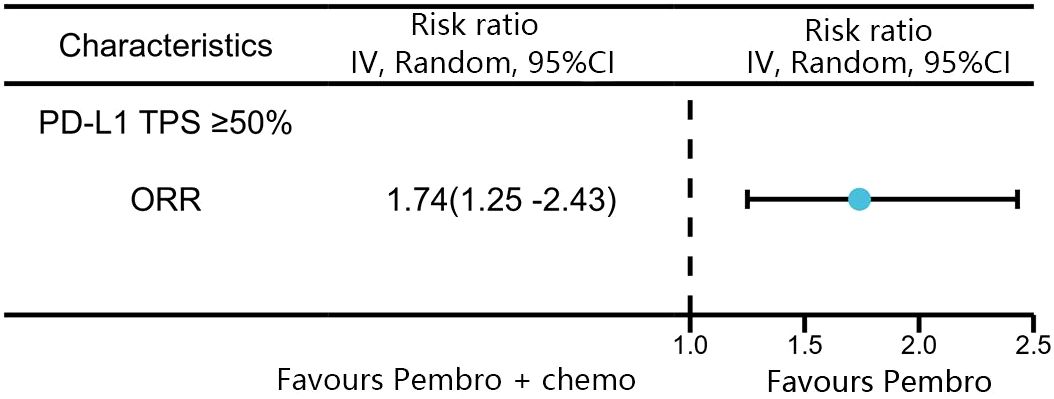
A total of four studies reported ORR in patients with high levels of PD-L1 expression (≥50%). Among these studies, KEYNOTE-024 and KEYNOTE-042 reported ORR with pembrolizumab monotherapy, and KEYNOTE-407 and KEYNOTE-189 reported ORR with pembrolizumab in combination with chemotherapy. The network meta-analysis revealed that the combination of pembrolizumab with chemotherapy had a superior response rate compared to pembrolizumab alone in patients with high levels of PD-L1 expression (≥50%)(RR:1.74, 95% CI: 1.25-2.43) (Figure 6).
3.7 Overall survival - PD-L1 TPS 1%-49%
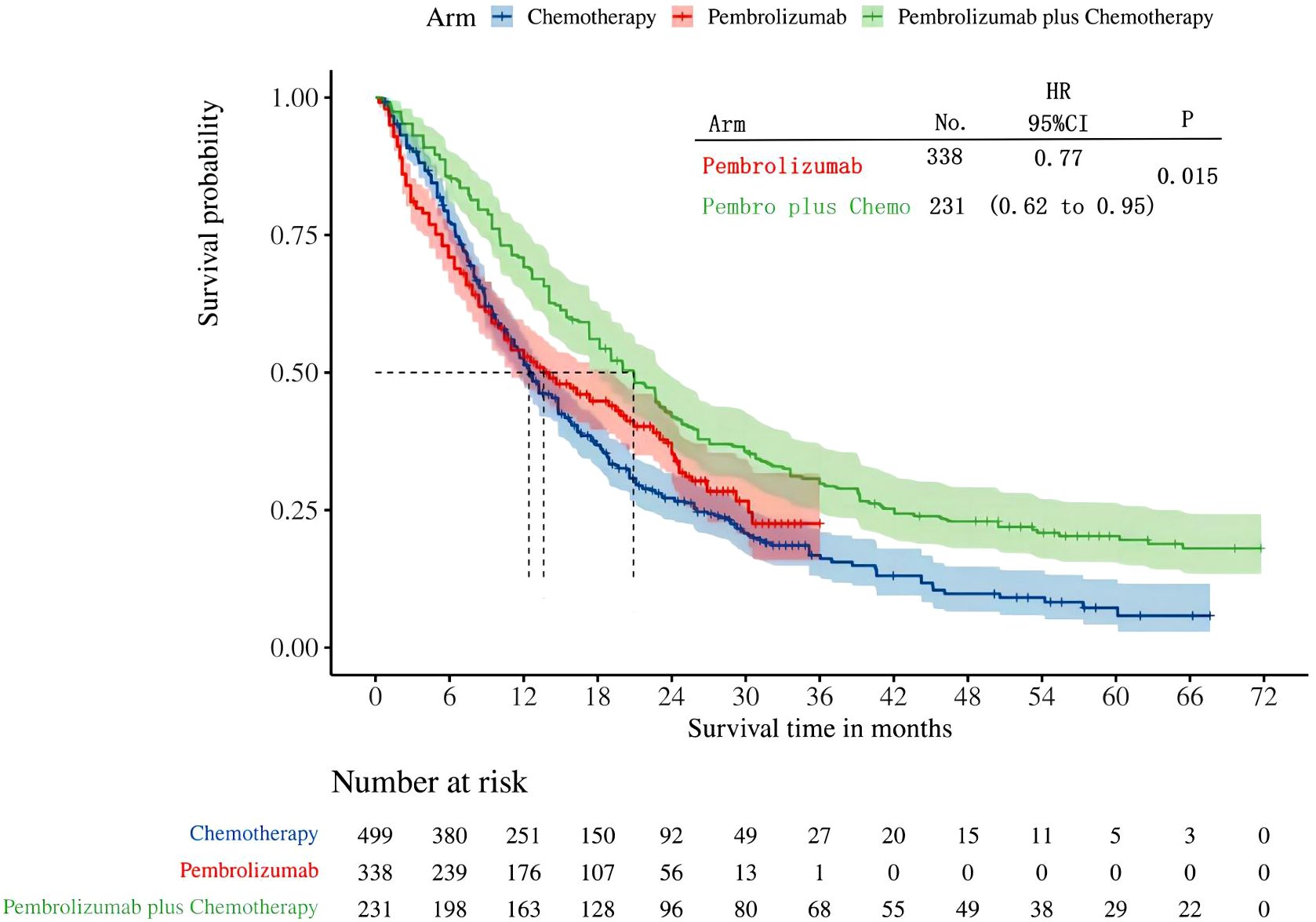
Three studies in all reported OS in individuals whose tumors expressed PD-L1 in the range of 1-49%. Among these studies, the KEYNOTE-042 trial presented OS data for pembrolizumab monotherapy, while the KEYNOTE-407 and KEYNOTE-189 trials presented OS for pembrolizumab plus chemotherapy. After reconstructing the cohort, we performed an updated evaluation of OS especially in patients with a tumor PD-L1 expression level of 1-49%. The Kaplan-Meier curves demonstrate that the combination of pembrolizumab with chemotherapy provides a superior long-term survival advantage compared to pembrolizumab alone in patients with tumor PD-L1 expression ranging from 1% to 49% (HR: 0.77, 95%CI: 0.62 to 0.95, P=0.015) (Figure 7). The median survival time for OS was 20.88 months for pembrolizumab plus chemotherapy, 13.60 months for pembrolizumab monotherapy, and 12.35 months for chemotherapy. We provided periodic updates on OS of each group at 6-month intervals from 0 to 36 months, which are displayed in Table 2. Insufficient relevant data prevented us from conducting a comparison of PFS between pembrolizumab and pembrolizumab plus chemotherapy.
3.8 Treatment-related adverse events
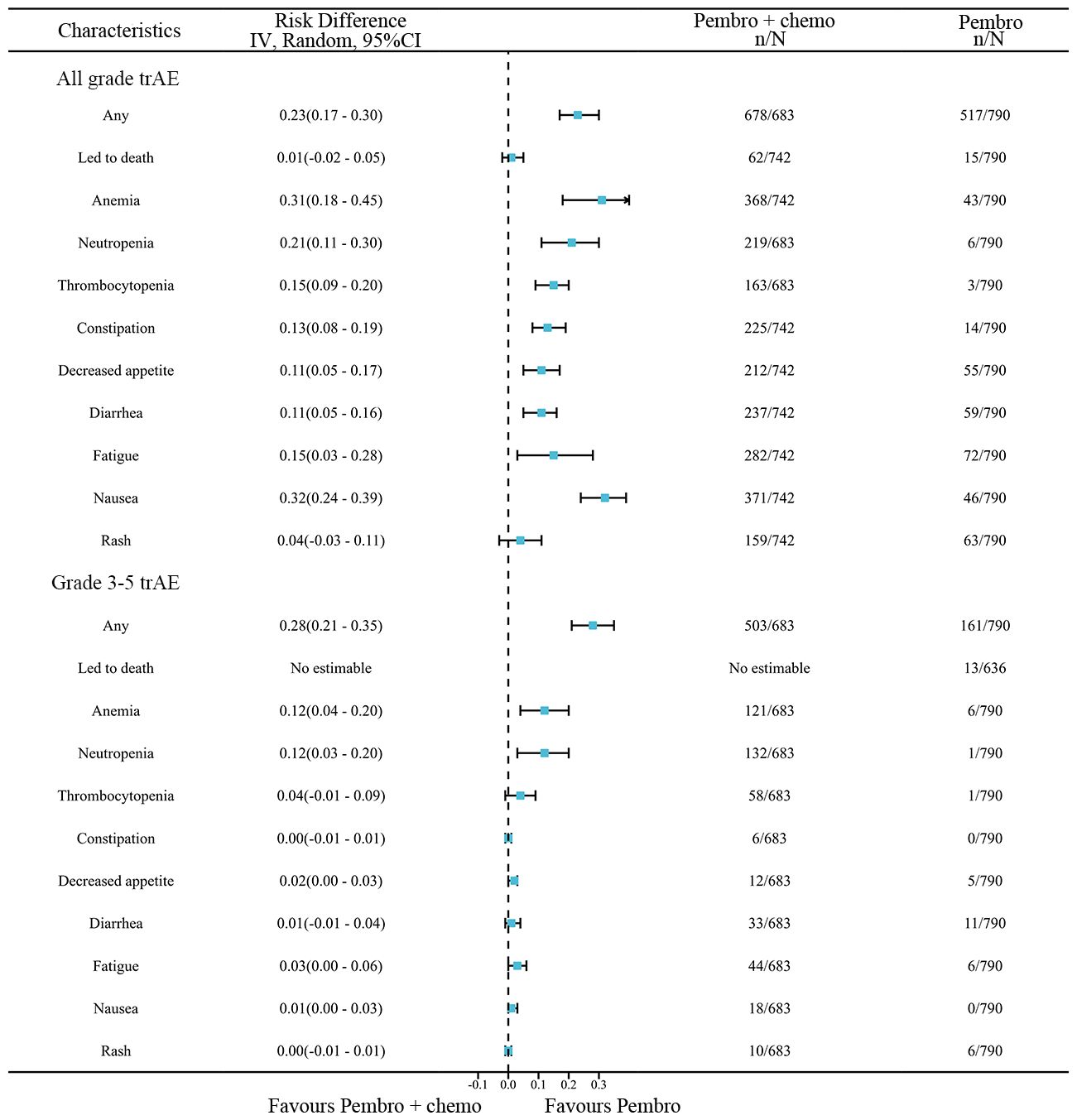
A network meta-analysis was conducted to compare any trAEs and Grade≥ 3 trAEs between pembrolizumab monotherapy and chemotherapy plus pembrolizumab (Figure 8). The meta-analysis results indicate that chemotherapy plus pembrolizumab was linked to a greater likelihood of any trAEs compared to pembrolizumab monotherapy(RD=0.23, 95% CI: 0.17 to 0.30). Chemotherapy combined with pembrolizumab had a greater occurrence of the following trAEs compared to pembrolizumab alone: mortality, anemia, neutropenia, thrombocytopenia, constipation, reduced appetite, diarrhea, fatigue, nausea, and rash. The meta-analysis findings suggest that the combination of chemotherapy and pembrolizumab is associated with a higher probability of Grade≥ 3 trAEs compared to pembrolizumab monotherapy(RD=0.28, 95% CI: 0.21 to 0.35). The combination of chemotherapy and pembrolizumab resulted in a higher incidence of the following Grade≥ 3 trAEs compared to pembrolizumab monotherapy: anemia and neutropenia.
3.9 Immune-related adverse events
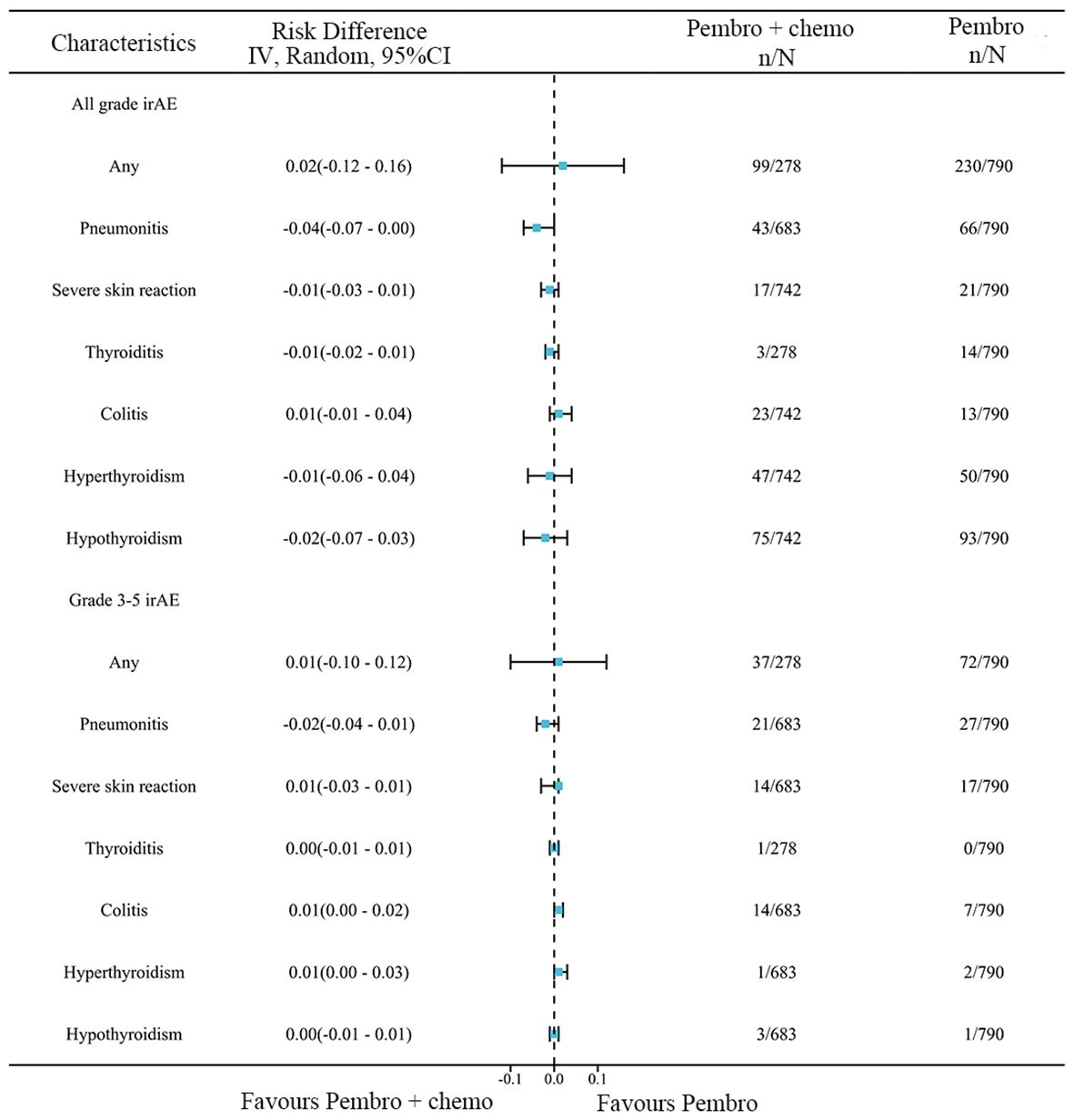
A network meta-analysis was performed to assess any irAEs and Grade≥ 3 irAEs between pembrolizumab monotherapy and chemotherapy plus pembrolizumab (Figure 9). The meta-analysis findings suggest that there was no statistically significant difference between the two groups in terms of any irAEs(RD=0.02, 95% CI: -0.12 to 0.16) or Grade≥ 3 irAEs(RD=0.01, 95% CI: -0.10 to 0.12).
4 Discussion
Activated T cells, B cells, natural killer cells, monocytes, and dendritic cells express programmed cell death receptor-1 (PD-1), a type I transmembrane glycoprotein belonging to the Ig superfamily (26). As an important immune checkpoint proteins, PD-1 interacts with two ligands, PD-L1 (B7-H1) and PD-L2(B7-H2), respectively. Immune and epithelial cells inductively express PD-L1, while antigen-presenting cells express PD-L2. In a physiological sense, PD-1 prevents immune system dysregulation by interacting with antigen-presenting cell surface PD-L1 and PD-L2. By overexpressing PD-L1, tumor cells encourage PD-1 binding to surface-expressed PD-L1 molecules, which in turn impairs immune surveillance of T cells, making it more difficult for tumor cells to be recognized and killed, and encouraging tumor immune escape (27). By disrupting interactions of PD-1/PD-L1, tumor immune tolerance can be broken, tumor specific T cells can regain their killing ability, and tumor clearance can be achieved by PD-1/PD-L1 monoclonal antibodies (28). Pembrolizumab, a PD-L1 inhibitor, has received approval for the treatment of NSCLC due to its notable clinical efficacy (29). The KEYNOTE-189 study was a randomized phase III trial that assessed the effectiveness of pembrolizumab in conjunction with chemotherapy in comparison to chemotherapy alone for treating advanced NSCLC. The results showed that the combination of pembrolizumab plus chemotherapy was more effective than chemotherapy alone in terms of OS and PFS in both the entire study population and the subset of tumors with a PDL1≥50% expression (13).
The results of our study offer robust evidence-based recommendations about the long-term prognosis for choosing between pembrolizumab monotherapy or pembrolizumab in combination with chemotherapy in clinical practice. Notably, the IPDformKM software was used to reconstruct Kaplan-Meier curves for OS and PFS, providing a clear and intuitive representation of oncological outcomes. Our results indicate that in patients with advanced NSCLC who have high levels of PD-L1 expression, the combination of pembrolizumab and chemotherapy is more efficacious than pembrolizumab alone as the first-line therapy in terms of PFS (median survival time: 10.41 months versus 7.41 months) and ORR (RR:1.74). However, there was no statistically significant distinction between the two groups in terms of OS. The notable enhancement in terms of ORR and PFS might be attributed to the stimulation of neoantigen release induced by chemotherapy, as well as the synergistic impact of immunotherapy and chemotherapy. However, the chemotherapy regimens are generally maintained for only three to four months(every 3 weeks, 4 to 6 cycles), while the pembrolizumab regimens are generally maintained for two years (every 3 weeks, 35 cycles). Over time, the residual effects of chemotherapy will progressively diminish, leaving only the lingering effects of pembrolizumab. In addition, pembrolizumab in combination with chemotherapy offers a more favorable long-term survival benefit in relation to OS (median survival time: 20.88 months versus 13.60 months) when compared to pembrolizumab monotherapy in patients with PD-L1 expression levels ranging from 1% to 49%. Since the data was not available, we could not provide the progression-free survival data among the PD-L1 TPS 1-49%. Regarding safety, there was no statistically significant difference between the two groups in terms of any irAEs or Grade≥ 3 irAEs. However, the combination of chemotherapy and pembrolizumab was associated with a higher probability of experiencing any trAEs and Grade≥ 3 trAEs compared to using pembrolizumab alone, suggesting that the enhanced effectiveness of pembrolizumab plus chemotherapy came with the drawback of increased adverse reactions.
Chemotherapeutics possess the capacity to enhance the immune system’s capability to identify and react to malignancies, or they can eliminate cells that inhibit the immune system. Furthermore, they have the potential to alter certain elements of the tumor microenvironment (30). It is crucial for us to distinguish these effects as we progress since chemotherapeutics have the ability to postpone the development of drug resistance, which could potentially change the chances of survival. Our meta-analysis consistently confirms that combining chemotherapy with first-line immune checkpoint medicines, such as pembrolizumab, enhances the efficacy of treatment for patients with advanced NSCLC. This phenomenon can be partially elucidated by the synergistic impact of immunotherapies and the induction of neoantigen release prompted by chemotherapy (31). There is variability among immune checkpoint inhibitors. Nivolumab and Pembrolizumab are immunosuppressive medications that inhibit the PD-1 protein, while Durvalumab and Atezolizumab were specifically engineered to target the PD-1, PD-L1 ligand (32). This approach has the potential to be used more extensively in order to reduce the impact on particular subgroups of NSCLC. However, further research is required. Genetic alterations of PD-L1 have been observed, which often result in the over-expression of PD-L1 (33). Xianhuo Wang et al. discovered that the genetic mutations of PD-L1 were specifically positioned in the exons of PD-L1 (34). These changes could impact the function of immunoglobulins and the transmembrane action of PD-L1, thus altering the immune response against tumors. Multiple studies have indicated that the expression of PD-L1, a protein associated with lung and other solid tumors, can be modified following treatment with platinum-based chemotherapy or concomitant chemoradiation (35). Fujimoto et al. conducted a study where they found that the expression of PD-L1 dropped dramatically after concurrent chemoradiation therapy in patients with locally advanced non-small lung cancer. This decrease in PD-L1 expression was linked to a positive prognosis (36). Toshiaki Takahashi et al. observed a considerable decrease in PD-L1 expression after treatment with pembrolizumab (37). This connection could potentially be one of the contributing factors to resistance against ICI and warrants additional exploration in extensive investigations.
An major strength of this study is that it is the first network meta-analysis to compare the long-term outcomes of pembrolizumab plus chemotherapy versus pembrolizumab monotherapy as first-line therapy for metastatic non-small-cell lung cancer. Previous network meta-analyses (17–19) exclusively reported outcomes that were limited to the short-term. Our findings support the existing scientific evidence about the long-term prognosis for pembrolizumab plus chemotherapy as first-line therapy for metastatic non-small-cell lung cancer. Besides, the Kaplan-Meier curves for OS and PFS were recreated to allow for a clear and comprehensible representation of the oncological outcomes.
This network meta-analysis possesses inherent limitations. The IPDformKM software was used to obtain reconstructed individual patient data from published KM curves of different quality. The quality of the analysis may be impacted. it is important to exercise caution when interpreting the results due to the potential for errors in reconstructing individual data. However, earlier research has demonstrated that HR obtained from rebuilt data has exhibited superior accuracy compared to published HR (38). Inconsistent baseline characteristics of patients in different clinical trials, such as doses and schedules of chemotherapeutic regimens, PD-L1 expression, gender, ECOG PS, smoking status, histology, metastases, and neoadjuvant therapy, may lead to heterogenicity in term of efficacy assessment and long-term survival assessment. Chemotherapy alone showed similar treatment effects in the five RCTs in terms of median PFS and OS (Supplementary Material 2), implying the that the types of chemotherapeutic agent would not lead to no obvious impact. By stratifying patients based on PD-L1 TPS, the heterogeneity caused by PD-L1 expression was minimized, leading to improved reliability in pooling the results. Unfortunately, this meta-analysis did not have access to data on individual patients, which means that it was not possible to conduct subgroup analysis based on other inconsistent baseline characteristics. To address these constraints, it is imperative to conduct controlled randomized trials to directly assess the effectiveness of pembrolizumab combination chemotherapy in comparison to pembrolizumab monotherapy.
In conclusion, the present network meta-analysis reported comparative long-term outcomes of pembrolizumab plus chemotherapy versus pembrolizumab monotherapy as first-line therapy for metastatic non-small-cell lung cancer. Pembrolizumab plus chemotherapy resulted in enhanced PFS and ORR among patients with advanced NSCLC who had a PD-L1 expression level of 50% or above. Nevertheless, there was no discernible advantage in terms of OS when pembrolizumab was combined with chemotherapy in comparison to using pembrolizumab alone. Furthermore, pembrolizumab plus chemotherapy provided a more advantageous long-term survival advantage in terms of OS compared to using pembrolizumab alone in patients with PD-L1 expression levels ranging from 1% to 49%. In addition, the heightened efficacy of pembrolizumab in combination with chemotherapy was accompanied by a rise in undesirable side effects.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
SH: Conceptualization, Data curation, Investigation, Software, Writing – original draft. ZH: Conceptualization, Data curation, Investigation, Software, Writing – original draft. XH: Data curation, Formal analysis, Writing – original draft. RL: Data curation, Formal analysis, Writing – original draft. WL: Funding acquisition, Methodology, Writing – review & editing. TQ: Funding acquisition, Methodology, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Scientific Research Foundation of Guangxi University of Science and Technology(20Z13),the Scientific Research Foundation of Guangxi Health Commission (Z-B20220927) and the Scientific Research Foundation of Guangxi Health Commission (Z-B20220930).
Acknowledgments
Everyone who contributed significantly to this study has been listed.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1375136/full#supplementary-material
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin. (2018) 68:394–424. doi: 10.3322/caac.21492
2. Basumallik N, Agarwal M. Small Cell Lung Cancer Vol. 2023. . Treasure Island (FL: StatPearls Publishing (2023).
3. Wang MC, Liang X, Liu ZY, Cui J, Liu Y, Jing L, et al. In vitro synergistic antitumor efficacy of sequentially combined chemotherapy/icotinib in non−small cell lung cancer cell lines. Oncol Rep. (2015) 33:239–49. doi: 10.3892/or.2014.3583
4. Patel SA, Weiss J. Advances in the treatment of non-small cell lung cancer: Immunotherapy. Clinics Chest Med. (2020) 41:237–47. doi: 10.1016/j.ccm.2020.02.010
5. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. (2012) 12:252–64. doi: 10.1038/nrc3239
6. Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. (2002) 3:991–8. doi: 10.1038/ni1102-991
7. Pardoll D. Cancer and the immune system: Basic concepts and targets for intervention. Semin Oncol. (2015) 42:523–38. doi: 10.1053/j.seminoncol.2015.05.003
8. Cella E, Zullo L, Marconi S, Rossi G, Coco S, Dellepiane C, et al. Immunotherapy-chemotherapy combinations for non-small cell lung cancer: current trends and future perspectives. Expert Opin Biol Ther. (2022) 22:1259–73. doi: 10.1080/14712598.2022.2116273
9. Aguilar EJ, Ricciuti B, Gainor JF, Kehl KL, Kravets S, Dahlberg S, et al. Outcomes to first-line pembrolizumab in patients with non-small-cell lung cancer and very high PD-L1 expression. Ann Oncol: Off J Eur Soc Med Oncol. (2019) 30:1653–9. doi: 10.1093/annonc/mdz288
10. Ninomiya K, Hotta K. Pembrolizumab for the first-line treatment of non-small cell lung cancer. Expert Opin Biol Ther. (2018) 18:1015–21. doi: 10.1080/14712598.2018.1522300
11. Borghaei H, Langer CJ, Paz-Ares L, Rodríguez-Abreu D, Halmos B, Garassino MC, et al. Pembrolizumab plus chemotherapy versus chemotherapy alone in patients with advanced non-small cell lung cancer without tumor PD-L1 expression: A pooled analysis of 3 randomized controlled trials. Cancer. (2020) 126:4867–77. doi: 10.1002/cncr.33142
12. Herbst RS, Garon EB, Kim DW, Cho BC, Gervais R, Perez-Gracia JL, et al. Five year survival update from KEYNOTE-010: pembrolizumab versus docetaxel for previously treated, programmed death-ligand 1-positive advanced NSCLC. J Thorac Oncol: Off Publ Int Assoc Study Lung Cancer. (2021) 16:1718–32. doi: 10.1016/j.jtho.2021.05.001
13. Garassino MC, Gadgeel S, Speranza G, Felip E, Esteban E, Dómine M, et al. Pembrolizumab plus pemetrexed and platinum in nonsquamous non-small-cell lung cancer: 5-year outcomes from the phase 3 KEYNOTE-189 study. J Clin Oncol: Off J Am Soc Clin Oncol. (2023) 41:1992–8. doi: 10.1200/JCO.22.01989
14. Song F, Altman DG, Glenny AM, Deeks JJ. Validity of indirect comparison for estimating efficacy of competing interventions: empirical evidence from published meta-analyses. BMJ (Clinical Res ed). (2003) 326:472. doi: 10.1136/bmj.326.7387.472
15. Lumley T. Network meta-analysis for indirect treatment comparisons. Stat Med. (2002) 21:2313–24. doi: 10.1002/sim.1201
16. Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med. (2004) 23:3105–24. doi: 10.1002/sim.1875
17. Kim R, Keam B, Hahn S, Ock CY, Kim M, Kim TM, et al. First-line pembrolizumab versus pembrolizumab plus chemotherapy versus chemotherapy alone in non-small-cell lung cancer: A systematic review and network meta-analysis. Clin Lung Cancer. (2019) 20:331–8.e4. doi: 10.1016/j.cllc.2019.05.009
18. Zhou Y, Lin Z, Zhang X, Chen C, Zhao H, Hong S, et al. First-line treatment for patients with advanced non-small cell lung carcinoma and high PD-L1 expression: pembrolizumab or pembrolizumab plus chemotherapy. J Immunother Cancer. (2019) 7:120. doi: 10.1186/s40425-019-0600-6
19. Udayakumar S, Parmar A, Leighl NB, Everest L, Arciero VS, Santos SD, et al. Pembrolizumab alone or with chemotherapy for metastatic non-small-cell lung cancer: a systematic review and network meta-analysis. Crit Rev Oncol/Hematol. (2022) 173:103660. doi: 10.1016/j.critrevonc.2022.103660
20. Awad MM, Gadgeel SM, Borghaei H, Patnaik A, Yang JC, Powell SF, et al. Long-term overall survival from KEYNOTE-021 cohort G: Pemetrexed and carboplatin with or without pembrolizumab as first-line therapy for advanced nonsquamous NSCLC. J Thorac Oncol: Off Publ Int Assoc Study Lung Cancer. (2021) 16:162–8. doi: 10.1016/j.jtho.2020.09.015
21. Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Five-year outcomes with pembrolizumab versus chemotherapy for metastatic non-small-cell lung cancer with PD-L1 tumor proportion score ≥ 50. J Clin Oncol: Off J Am Soc Clin Oncol. (2021) 39:2339–49. doi: 10.1200/JCO.21.00174
22. de Castro G Jr., Kudaba I, Wu YL, Lopes G, Kowalski DM, Turna HZ, et al. Five-year outcomes with pembrolizumab versus chemotherapy as first-line therapy in patients with non-small-cell lung cancer and programmed death ligand-1 tumor proportion score ≥ 1% in the KEYNOTE-042 study. J Clin Oncol: Off J Am Soc Clin Oncol. (2023) 41:1986–91. doi: 10.1200/JCO.21.02885
23. Novello S, Kowalski DM, Luft A, Gümüş M, Vicente D, Mazières J, et al. Pembrolizumab plus chemotherapy in squamous non-small-cell lung cancer: 5-year update of the phase III KEYNOTE-407 study. J Clin Oncology: Off J Am Soc Clin Oncol. (2023) 41:1999–2006. doi: 10.1200/JCO.22.01990
24. Neupane B, Richer D, Bonner AJ, Kibret T, Beyene J. Network meta-analysis using R: a review of currently available automated packages. PLoS One. (2014) 9:e115065. doi: 10.1371/journal.pone.0123364
25. Liu N, Zhou Y, Lee JJ. IPDfromKM: reconstruct individual patient data from published Kaplan-Meier survival curves. BMC Med Res Methodology. (2021) 21:111. doi: 10.1186/s12874-021-01308-8
26. He J, Hu Y, Hu M, Li B. Development of PD-1/PD-L1 pathway in tumor immune microenvironment and treatment for non-small cell lung cancer. Sci Rep. (2015) 5:13110. doi: 10.1038/srep13110
27. Pedoeem A, Azoulay-Alfaguter I, Strazza M, Silverman GJ, Mor A. Programmed death-1 pathway in cancer and autoimmunity. Clin Immunol (Orlando Fla). (2014) 153:145–52. doi: 10.1016/j.clim.2014.04.010
28. Peters S, Kerr KM, Stahel R. PD-1 blockade in advanced NSCLC: A focus on pembrolizumab. Cancer Treat Rev. (2018) 62:39–49. doi: 10.1016/j.ctrv.2017.10.002
29. Pai-Scherf L, Blumenthal GM, Li H, Subramaniam S, Mishra-Kalyani PS, He K, et al. FDA approval summary: pembrolizumab for treatment of metastatic non-small cell lung cancer: first-line therapy and beyond. Oncologist. (2017) 22:1392–9. doi: 10.1634/theoncologist.2017-0078
30. Liu J, Li C, Seery S, Yu J, Meng X. Identifying optimal first-line interventions for advanced non-small cell lung carcinoma according to PD-L1 expression: a systematic review and network meta-analysis. Oncoimmunology. (2020) 9:1746112. doi: 10.1080/2162402X.2020.1746112
31. Zitvogel L, Galluzzi L, Smyth MJ, Kroemer G. Mechanism of action of conventional and targeted anticancer therapies: reinstating immunosurveillance. Immunity. (2013) 39:74–88. doi: 10.1016/j.immuni.2013.06.014
32. Liu L, Bai H, Wang C, Seery S, Wang Z, Duan J, et al. Efficacy and safety of first-line immunotherapy combinations for advanced NSCLC: A systematic review and network meta-analysis. J Thorac oncology: Off Publ Int Assoc Study Lung Cancer. (2021) 16:1099–117. doi: 10.1016/j.jtho.2021.03.016
33. Georgiou K, Chen L, Berglund M, Ren W, de Miranda NF, Lisboa S, et al. Genetic basis of PD-L1 overexpression in diffuse large B-cell lymphomas. Blood. (2016) 127:3026–34. doi: 10.1182/blood-2015-12-686550
34. Zhang T, Liu H, Jiao L, Zhang Z, He J, Li L, et al. Genetic characteristics involving the PD-1/PD-L1/L2 and CD73/A2aR axes and the immunosuppressive microenvironment in DLBCL. J Immunother Cancer. (2022) 10:1–13. doi: 10.1136/jitc-2021-004114
35. Rojkó L, Reiniger L, Téglási V, Fábián K, Pipek O, Vágvölgyi A, et al. Chemotherapy treatment is associated with altered PD-L1 expression in lung cancer patients. J Cancer Res Clin Oncol. (2018) 144:1219–26. doi: 10.1007/s00432-018-2642-4
36. Fujimoto D, Uehara K, Sato Y, Sakanoue I, Ito M, Teraoka S, et al. Alteration of PD-L1 expression and its prognostic impact after concurrent chemoradiation therapy in non-small cell lung cancer patients. Sci Rep. (2017) 7:11373. doi: 10.1038/s41598-017-11949-9
37. Takahashi T, Tateishi A, Bychkov A, Fukuoka J. Remarkable alteration of PD-L1 expression after immune checkpoint therapy in patients with non-small-cell lung cancer: Two autopsy case reports. Int J Mol Sci. (2019) 20:1–10. doi: 10.3390/ijms20102578
Keywords: pembrolizumab, chemotherapy, non-small-cell lung cancer, metastatic, meta-analysis
Citation: Huang S, Huang Z, Huang X, Luo R, Liang W and Qin T (2024) Comparative long-term outcomes of pembrolizumab plus chemotherapy versus pembrolizumab monotherapy as first-line therapy for metastatic non-small-cell lung cancer: a systematic review and network meta-analysis. Front. Immunol. 15:1375136. doi: 10.3389/fimmu.2024.1375136
Received: 23 January 2024; Accepted: 01 July 2024;
Published: 11 July 2024.
Edited by:
Jesse Haramati, University of Guadalajara, MexicoReviewed by:
Tai Hato, Saitama Medical University, JapanXianhuo Wang, Tianjin Medical University Cancer Institute and Hospital, China
Copyright © 2024 Huang, Huang, Huang, Luo, Liang and Qin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weiming Liang, TGlhbmd3bTIyQGljbG91ZC5jb20=; Tian Qin, cWludGlhbjIwMjRrZEAxNjMuY29t
†These authors share first authorship
 Shibo Huang
Shibo Huang Zhilong Huang
Zhilong Huang Xiaolong Huang
Xiaolong Huang Weiming Liang
Weiming Liang Tian Qin
Tian Qin