
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 10 June 2024
Sec. Inflammation
Volume 15 - 2024 | https://doi.org/10.3389/fimmu.2024.1372079
This article is part of the Research Topic Diagnostic, prognostic and treatment efficacy power of biomarkers of aging for frailty, age-related diseases and multimorbidity View all 15 articles
 Ana M. Valdes1,2*
Ana M. Valdes1,2* Adeel Ikram1,2
Adeel Ikram1,2 Lauren A. Taylor1,2
Lauren A. Taylor1,2 Amy Zheng1,2
Amy Zheng1,2 Afroditi Kouraki1,2
Afroditi Kouraki1,2 Anthony Kelly1,2
Anthony Kelly1,2 Waheed Ashraf1,2
Waheed Ashraf1,2 Amrita Vijay1,2
Amrita Vijay1,2 Suzanne Miller1,2
Suzanne Miller1,2 Jessica Nightingale1,2
Jessica Nightingale1,2 Nicholas M. Selby3
Nicholas M. Selby3 Benjamin J. Ollivere1,2
Benjamin J. Ollivere1,2Background: Hip fractures in frail patients result in excess mortality not accounted for by age or comorbidities. The mechanisms behind the high risk of mortality remain undetermined but are hypothesized to be related to the inflammatory status of frail patients.
Methods: In a prospective observational exploratory cohort study of hospitalized frail hip fracture patients, 92 inflammatory markers were tested in pre-operative serum samples and markers were tested against 6-month survival post-hip fracture surgery and incidence of acute kidney injury (AKI). After correcting for multiple testing, adjustments for comorbidities and demographics were performed on the statistically significant markers.
Results: Of the 92 markers tested, circulating levels of fibroblast growth factor 23 (FGF-23) and interleukin-15 receptor alpha (IL15RA), both involved in renal disease, were significantly correlated with 6-month mortality (27.5% overall) after correcting for multiple testing. The incidence of postoperative AKI (25.4%) was strongly associated with 6-month mortality, odds ratio = 10.57; 95% CI [2.76–40.51], and with both markers plus estimated glomerular filtration rate (eGFR)– cystatin C (CYSC) but not eGFR-CRE. The effect of these markers on mortality was significantly mediated by their effect on postoperative AKI.
Conclusion: High postoperative mortality in frail hip fracture patients is highly correlated with preoperative biomarkers of renal function in this pilot study. The effect of preoperative circulating levels of FGF-23, IL15RA, and eGFR-CYSC on 6-month mortality is in part mediated by their effect on postoperative AKI. Creatinine-derived preoperative renal function measures were very poorly correlated with postoperative outcomes in this group.
Over 1.5 million patients worldwide suffer a hip fracture each year (1). According to the World Health Organization (WHO) hip fractures permanently disable 50% of sufferers and have a 1-year post-fracture mortality around 24% (2–4). The mortality rate after a hip surgery is significantly higher compared to the age and gender-matched population (5). Post-surgical mortality globally has remained mostly static over the past decade despite comprehensive best-practice surgery and rehabilitation packages (6–9). The overall incidence rates and costs of hip fractures are expected to rise (10), with estimated annual incidence rising to 6 million a year by 2050 worldwide (11) and costs estimated at 1.4% of total social and healthcare budgets.
Patients with a hip fracture are at substantial risk of major complications including cardiovascular, infectious, neurocognitive, and mortality (12). Several systematic reviews have found consistently that most of the associated factors for functional recovery and mortality of elderly hip fracture are biological, sociodemographic, or inherent to patients’ baseline characteristics (13–15).
A large proportion of frail individuals who suffer hip fractures have mild to severe impaired kidney function at the time of admission (16). Evidence-based approaches show that around 75% of hip fractures are frail, and preoperative frailty is associated with poorer outcomes and higher risk of postoperative mortality (17). Frailty is defined as a status of extreme vulnerability to stressors and is highly correlated with higher systemic inflammation (18). Importantly, the main causes of 12-month mortality for elderly hip fracture patients are cardiovascular disease and Alzheimer’s disease (19), both of which have inflammation as a central mechanism (20, 21).
Understanding the specific mechanisms involved in the increased risk of mortality associated with delayed surgery and the responsiveness of such mechanisms to surgery and rehabilitation are crucial to the development of interventions that improve outcomes from hip fracture surgery and reduce mortality and complications of fragility fractures. We have hypothesized that higher rates of mortality among individuals with pre-operative frailty might be driven by specific inflammatory pathways.
To explore this possibility, we recruited a pilot cohort of frail hip fracture patients from the busiest major trauma center in the United Kingdom. We compared their mortality, frailty levels, and basic characteristics to those from the same trauma center. Having obtained serum samples at the time of going into the operating theater, we carried out proteomic profiling and identified the molecular markers correlated with 6-month mortality, and assessed whether these were associated with incidence of postoperative acute kidney injury (AKI).
A summary of the study design is presented in Figure 1. In this prospective, observational cohort study, serum preoperative samples were collected from a sub-cohort of frail hip fracture patients, with outcomes assessed at 6 months. Serum markers were tested as predictors of postoperative mortality and incident AKI in the sub-cohort. Levels of the significantly associated serum markers were also compared to positive and negative controls from additional comparator cohorts.
Fifty-nine frail hip fracture patients from the Functioning of Elder Muscle; Understanding Recovery (FEMUR) cohort were consented to give blood samples at induction of anesthesia (clinicaltrials.gov NCT04764617) (see Table 1 for descriptive characteristics). This cohort recruited frail individuals admitted to hospital with a hip fracture. All participants were over 65 years of age, with a clinical frailty score of 4 or above and had suffered a hip fracture following a fall. All patients were recruited from Nottingham University Hospitals National Health Service (NHS) Trust. The CONSORT diagram for recruitment is shown in Supplementary Figure S1. The study protocol has been uploaded to be made publicly available at the University of Nottingham’s research data repository: https://www.nottingham.ac.uk/dts/researcher/managing-data/research-data-repository.aspx.
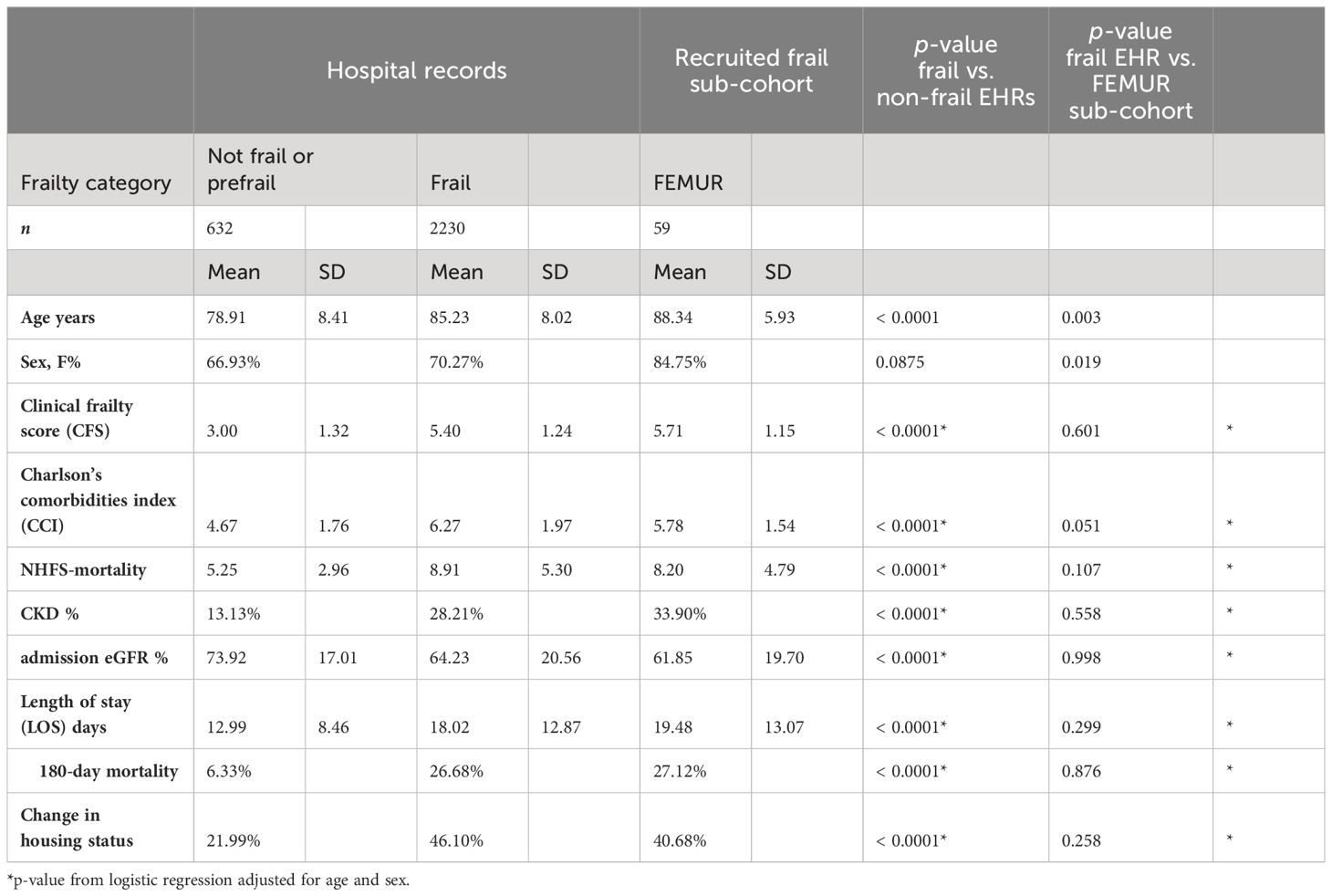
Table 1 Comparison between electronic health records (EHRs) for hip fracture surgeries at Nottingham University Hospitals in the same period and the enrolled sub-cohort.
Four comparator cohorts were assembled, including an established local registry that has been described elsewhere (22), which includes data collected from the electronic health records (EHRs) for all individuals who underwent a hip fracture surgery during the same period at Nottingham University Hospitals Trust. Patients were sequentially admitted neck of femur fracture patients between 01/01/2019 and 31/12/2022.
EHRs from Nottingham University Hospital’s Trust for individuals who underwent hip fracture surgery during the same period were collected. Patients in this cohort were sequentially admitted neck of femur fracture patients admitted between 01/01/2019 and 31/12/2022. This data was collected as part of an established local registry that has been described elsewhere.28
The PANdemic Tracking of HEalthcare woRkers (PANTHER) cohort involved asymptomatic healthcare workers who were assessed for SARS-CoV-2 infection during the first U.K. wave of the COVID-19 pandemic using symptom questionnaires and antibody testing. These participants donated blood to the Nottingham Tissue Bank for antibody testing and completed up to 16 weeks of symptom questionnaires. A randomly selected subset of this cohort has been included in this study as the negative control group. Sixty-two participants from the PANTHER cohort were randomly selected to be included in this study as the healthy control group, ensuring ethnic diversity of the sample. This study was initially under a Human Tissue Authority license in Nottingham (license number: 11035) and subsequently received ethical approval from North West, Greater Manchester South Research Ethics Committee, reference 20/NW/039 (registration number: NCT04318314). This group was included as a healthy control cohort.
The Orif Procedure mEchanisms of Rib Fixation (OPERA) cohort is an embedded mechanistic sub-study within the ORiF’s randomized controlled trial (RCT) aimed at investigating the mortality, quality of life, and cost effectiveness of operative rib fixation plus supportive management compared to supportive management only for patients admitted with three or more rib fractures. The OPERA sub-study that collects preoperative sera for these impact fracture patients has been included in this study as the positive control group. This group was included as a positive control of severe orthopedic trauma in the absence of frailty.
The prefrail sub-cohort included a subset of participants over 65 years of age who were recruited into an OA study and donated serum samples. Participants who were over 65 years and considered to be prefrail using the FRAIL scale combining the three items addressing Fatigue, Resistance (climbing steps), Ambulation (walking), together with weight loss and morbidity counts. Participants fulfilling one or two criteria may be classified as “pre-frail” and three or more as frail. This study received ethical approval from East Midlands Research Ethics Committee, reference 20/EM/0065 (registration number: NCT0442452). This group was used as a positive control of frailty in the absence of orthopedic trauma.
The key exposure was hip fracture. The primary outcome was 6-month postoperative mortality. The following comorbidities, confounder, and secondary outcomes were included: Charlson comorbidities index (CCI) (23) provides a measure of comorbid condition severity and has been widely validated, (24) was derived from patients’ hospital medical records. Specifically, the CCI includes myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, dementia, chronic pulmonary disease, connective tissue disease, peptic ulcer disease, mild liver disease, diabetes mellitus (with or without complications), hemiplegia or paraplegia, renal disease, any malignancy (except malignant neoplasm of the skin), moderate or severe liver disease, metastatic solid tumors, and AIDS/HIV. Each condition is assigned a based on its severity and impact on mortality risk. The total score is calculated by summing up the scores for each comorbidity present in a patient, with higher scores indicating a higher risk of mortality. The presence of renal disease coded as chronic kidney disease (CKD) along with all the other comorbidities that are part of the CCI were also recorded at admission. The Rockwood Clinical Frailty Scale (CFS) is a judgment-based frailty tool that evaluates specific domains including comorbidity, function, and cognition to generate a frailty score ranging from 1 (very fit) to 9 (terminally ill) (25). The Nottingham Hip Fracture Score (NHFS) is a validated predictor of 30-day mortality in neck of femur fracture patients using demographic and clinical parameters recorded immediately preoperatively (26).
CFS, CCI, NHFS, age, sex, date and time of admission, surgery, and date of death (where applicable) were recorded for all FEMUR participants and were extracted from EHRs from the same hospital.
A measure of estimated glomerular filtration rate (eGFR) at admission, derived from serum creatinine, was available for all FEMUR study participants enrolled. eGFR immediately preoperatively (36.6h on average) from hospital records was available only for a subset; therefore, we obtained two additional measures of preoperative renal function eGFR from creatinine [CRE using CKD-EPI 2009 (27)] and cystatin C [CYSC, using CKD-EPI2012Scr-cys (28)]. Serum CYSC is a low-molecular-weight protein found in all tissues in the body, is filtered at the glomerulus and not secreted into the renal tubules or reabsorbed into the bloodstream, (29) and hence its use is recommended instead of creatinine to estimate GFR in adults with or at risk for CKD (30).
Incident AKI was assessed from creatinine recorded values for all FEMUR patients over the 2 months immediately post-operatively. Incident AKI was defined using the Kidney Disease: Improving Global Outcomes (KDIGO) criteria as either an increase in creatinine from baseline of ≥50% or an absolute rise of ≥27 micromol/l if within 48h (31).
Serum was extracted from blood collected from all FEMUR patients at the time of going into the operating room. Venous blood was collected using a butterfly needle into 5 ml serum separation tubes containing clot activator and polymer gel. Tubes were left at room temperature for 20–30 min before being centrifuged for 5 min at 3000 RPM. The resulting serum was aliquoted into cryo-vials and stored immediately at −80. Inflammatory protein measurements were performed using a proximity extension assay method (Olink Bioscience, Uppsala, Sweden) (32) with the Olink inflammatory panel (33). ELISAs to measure serum levels of creatinine and cystatin C were performed by Affinity Biomarkers, London, using the Clinical Chemistry Analyzer. Preoperative eGFR was assessed from both creatinine and cystatin C levels measured from the same serum aliquot used to measure inflammatory markers (see 34).
We computed the Spearman’s correlation between 6-month mortality and 92 proteins from the Olink inflammatory panel. Results were adjusted for multiple testing using a False Discovery Rate correction (35). We compared results to an age-matched cohort of 62 healthy individuals without injury (negative controls) and a cohort of 32 younger patients with traumatic fracture from the OPERA cohort (positive controls) and 19 prefrail and frail individuals without trauma using unpaired t-tests. Effect size of associations with mortality for clinical parameters and significant biomarkers was further evaluated using logistic regression analyses.
Mediation analysis was performed to assess the mediating effect of incident AKI. For all models, we report the percentage causal mediation effect (ACME), the percentage direct effect (ADE). ACME represents the average size of the effect of the presurgical biomarkers on postoperative mortality that is mediated by incident AKI, while ADE represents the direct effect of biomarkers on mortality.
The statistical power to compute a significant correlation between inflammatory circulating markers and postoperative outcomes was computed using StatsDirect version 3.3.5.
Analyses were performed using Python 3.11.1 (libraries pyplot, numpy, and scipy), GraphPad Prism 10 and R 4.3.2 (library mediation).
The descriptive characteristics of 59 frail fracture patients from the pilot study (FEMUR) who had serum samples taken immediately pre-operatively were compared to frail and non-frail hip fracture patients in the same hospital (Table 1). The FEMUR sub-cohort of frail patients with preoperative serum is broadly comparable to the overall patient population of frail hip fracture patients operated at this major trauma center between 2019 and 2022 (n = 2230). After adjustment for age and sex, there were no statistically significant differences between the pilot cohort and the overall frail hip fracture population in any of the clinical parameters including preoperative comorbidities and post-operative length of stay and mortality. On the other hand, as expected, we observed highly significant differences in all preoperative clinical parameters and post-operative outcomes between frail and non-frail hip fracture patients operated in the Queen’s Medical Centre in Nottingham. We therefore concluded that the frail fracture pilot sub-cohort was suitably representative of the mortality and comorbidities in the frail hip fracture population.
In this sub-cohort, we then assessed the correlation between 92 inflammatory proteins and 6-month mortality. Thirteen proteins were nominally associated, namely, fibroblast growth factor 23 (FGF-23) and interleukin-15 receptor alpha (IL15RA), CD40, MMP-1, CCL25, IL-17C, VEGFA, IL-10RB, IL-12B, TNFRSF9, MMP-10, SLAMF1, and FGF-21. Of these, FGF-23 and IL15RA passed a multiple testing correction (Figure 2A). The minimum effect size needed to achieve 80% power with a p < 0.00054 (Bonferroni alpha = 0.05 for 92 independent tests) with the current sample size corresponds to correlation coefficient of 0.519 or higher. The strongest correlation observed in our study is 0.473 95% CI [0.25, 0.65] for FGF-23, which has a statistical power of 67.5%, that is, the present study was reasonably powered to detect the effect sizes actually observed.
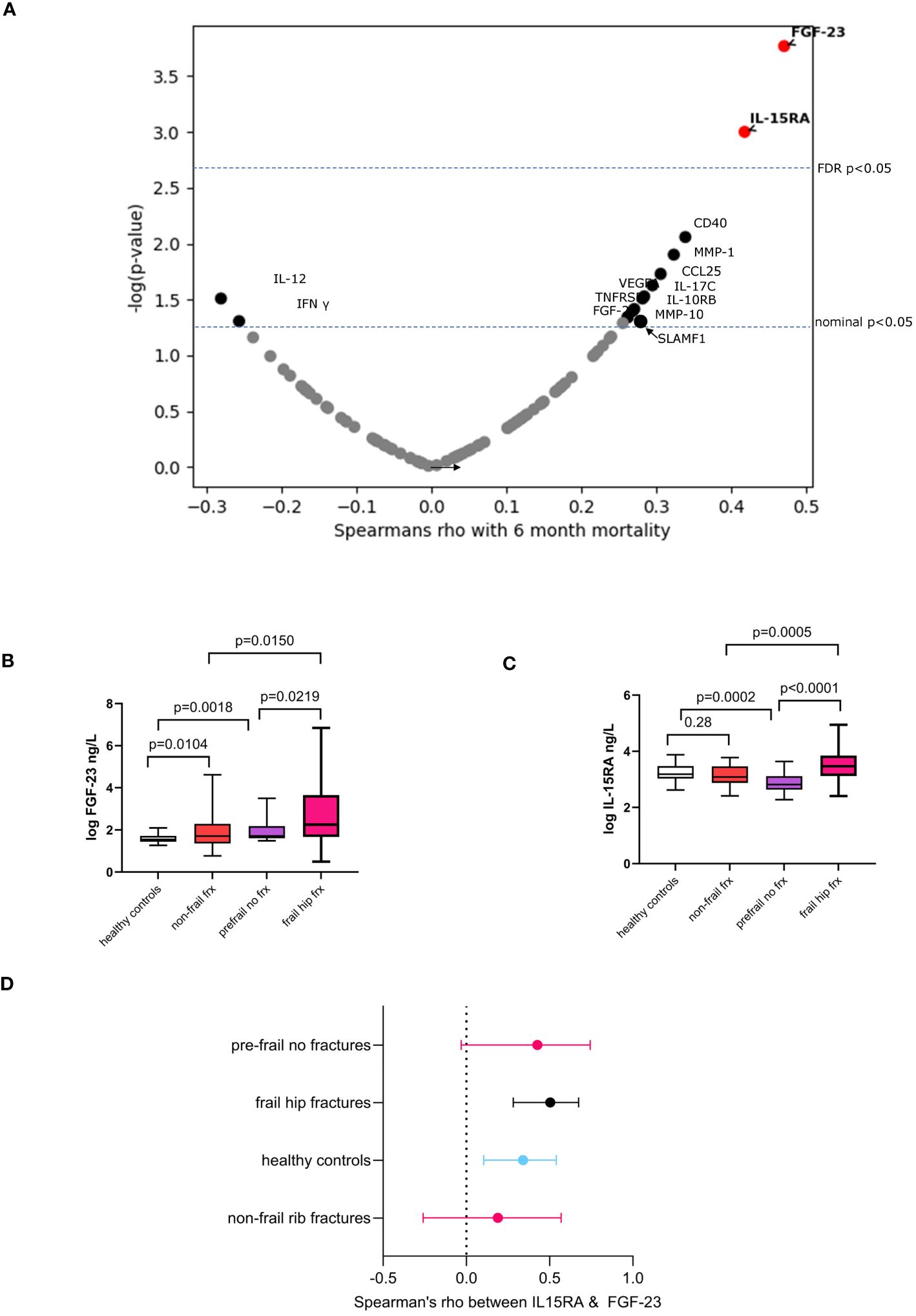
Figure 2 Associations for 6-month mortality and molecular inflammatory markers. (A) Volcano plot showing Spearman’s correlation coefficient and p-values. Boxplots showing the comparison of serum levels of (B) FGF-23 and (C) IL-15RA in frail hip fractures with regards to healthy controls, non-frail (impact) fracture cases and pre-frail individuals with no fractures. Box plots: mean values of molecular marker levels indicated by plus signs and medians indicated by bars in the boxes. The boxes show the interquartile range (IQR) and the whiskers extend from the boxes to indicate the 95% confidence intervals. (D) Forest plot showing the correlations between FGF-23 and IL-15RA in frail hip fracture patients and in the various comparator groups. Dots represent Spearman’s rho, whiskers are 95% confidence intervals.
We compared levels of these markers in frail fracture patients to n = 62 healthy controls (aged 42 ± 11.57 years), n = 30 non-frail multiple rib fracture patients (aged 60 ± 14.2 years, 9% female), and n = 19 frail/prefrail individuals with no fracture (age 70.05 ± 7.16 years) (descriptive statistics in Supplementary Table S1).
Levels of FGF-23 were increased in non-frail trauma compared to healthy controls and in pre-frail individuals without fractures compared to healthy controls (Figure 2B). In contrast, the pattern seen with IL-15RA shows a significant decrease in pre-frail versus healthy controls and no difference in non-frail trauma compared to controls (Figure 2C). Both markers have been implicated in kidney disease (36, 37). though with different mechanisms being involved for the role of these molecules in renal failure (38–40).
To better understand the relationship between FGF-23 and IL-15RA, we also assessed the correlations between the two markers in the various groups (Figure 2D) and find that they are in general very weakly correlated in healthy participants young and old and in individuals with severe orthopedic trauma but who are not frail. The relationship between both appears to be stronger in the frail fracture group that we studied here achieving a Spearman’s rho of 0.50 compared to the other patient and control groups analyzed.
The presence of comorbidities was overall strongly associated with 6-month mortality (Table 2) with the odds ratio for each unit increase of the Charlson’s comorbidities index is 2.73, 95% CI [1.52–4.94] (Table 2).
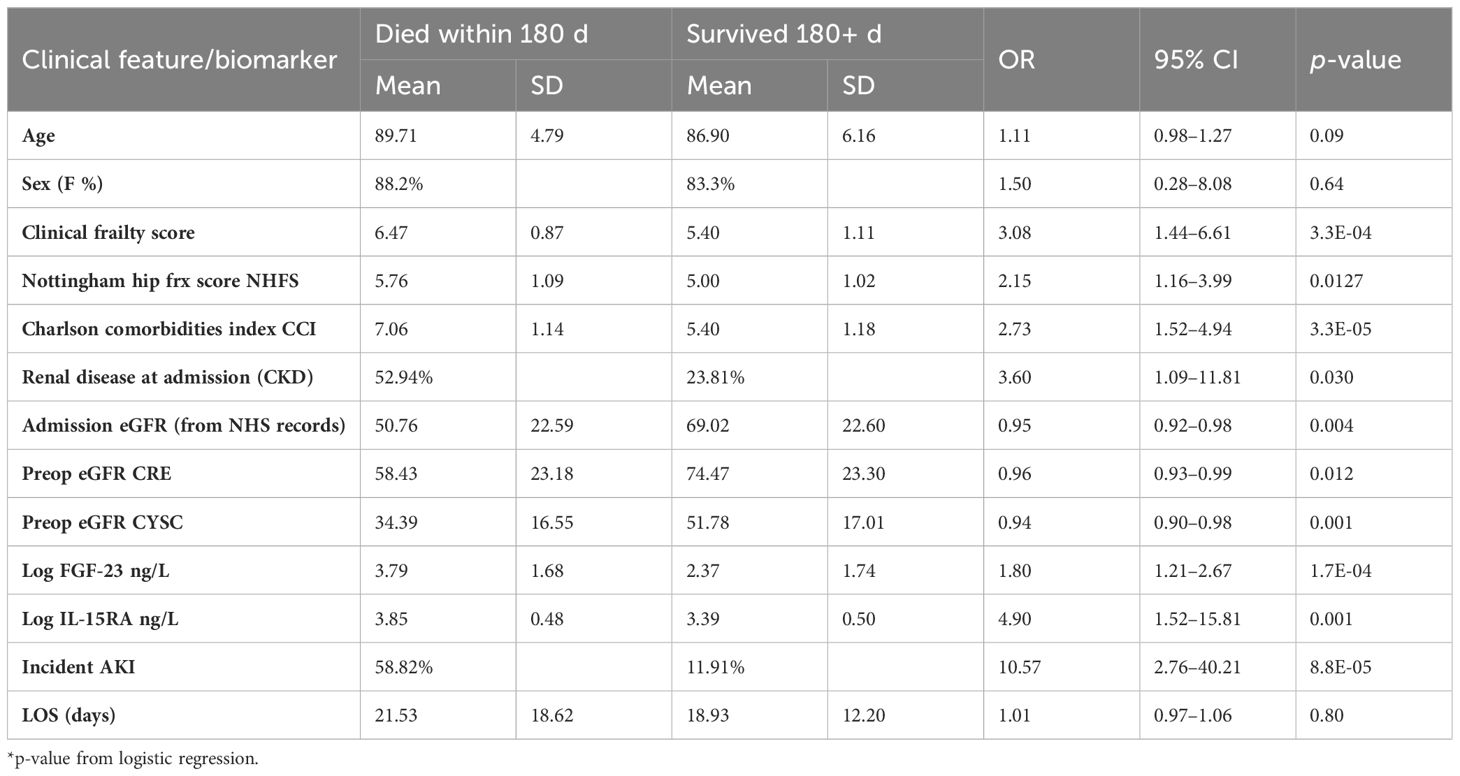
Table 2 Association between clinical traits/biomarkers pre and post operative with 6-month mortality post hip fracture surgery in the FEMUR cohort.
In addition, in this cohort, preoperative eGFR-CRE is only modestly associated with 6-month mortality, but eGFR-CYSC is more strongly associated (Table 2). Fifteen individuals (25.4%) developed AKI within the first 60 days after surgery, seven (11.8%) within 1-week postsurgery. Incidence of AKI was found to be strongly correlated with 6-month mortality (Table 2) corresponding to an odds ratio of 10.57, 95% CI [2.76–40.51].
We investigated the links between FGF-23 and IL15-RA, other markers of renal function, incident AKI, and 6-month post-surgical mortality. Overall, we find that of the 92 inflammatory markers measured preoperatively these are the two most strongly associated with post-operative incidence of AKI (Supplementary Figure S2).
A comparison of preoperative eGFR-CRE to admission values in hospital records showed that eGFR-CRE was significantly higher immediately preoperatively (Figure 3A). On the other hand, preoperative eGFR-CYSC was significantly lower compared to preoperative eGFR-CRE in both the survivor and deceased groups (Figure 3B). We then assessed the association between preoperative eGFR measures, FGF-23 and IL-15RA, and incidence of AKI. Preoperative eGFR-CRE was not significantly associated with postoperative incidence of AKI (Figure 3D) whereas preoperative FGF-23, IL-15RA, and eGFR-CYSC were all significantly correlated with incident AKI (Figures 3E–G).
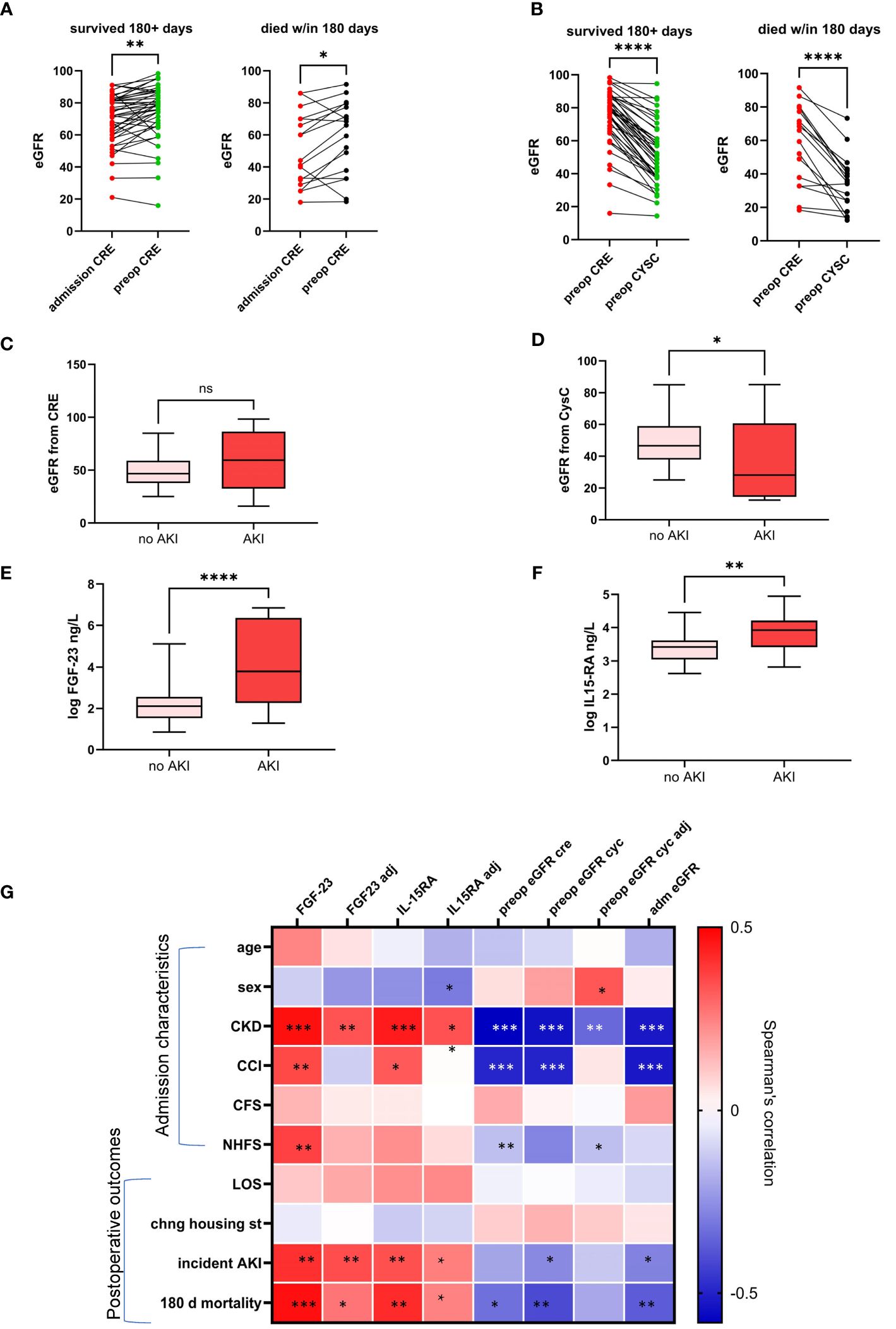
Figure 3 Change in eGFR from admission to immediately preoperatively measured from serum creatinine (A) and difference in eGFR preoperative estimates from serum creatinine and serum cystatin C levels (B). Box plots showing the association between incident postoperative acute kidney injury (AKI) and preoperative eGFR from creatinine (C) and cystatin C (D) circulating IL15RA (E) and FGF-23 (F). Heatmap showing correlations of admission characteristics and postoperative clinical outcomes with circulating biomarkers measured preoperatively with and without adjustment for age and comorbidities (G). * p<0.05; ** p<0.01; *** p<0.001; **** p<0.0005; NS, not statistically significant.
The correlations between markers of renal function with and without adjustment for age and comorbidities on clinical measures at admission and surgical outcomes is summarized in a heatmap in Figure 3G. FGF-23 and IL-15RA remain associated with AKI after adjustment for age and comorbidities.
We also find that both markers are significantly associated with 6-month mortality after adjustment for age, sex and CCI (i.e., comorbidities). FGF-23 had an OR = 1.77, 95% CI [1.00–3.14] in units of standard deviations, and IL15-RA OR = 1.81, 95% CI [1.00–3.42] also in units of standard deviations).
We performed formal mediation analyses to assess whether the effect of these markers on mortality was mediated by their effect on AKI. We find that the proportion of the effect of FGF-23 (Figure 4A) and IL-15RA (Figure 4B) on postoperative mortality mediated by incident postoperative AKI is statistically significant, with the direct effects not quite achieving statistical significance (p < 0.053 and p < 0.070). Neither the direct nor indirect effects alone for eGFR-CYSC achieved statistical significance (Figure 4C).
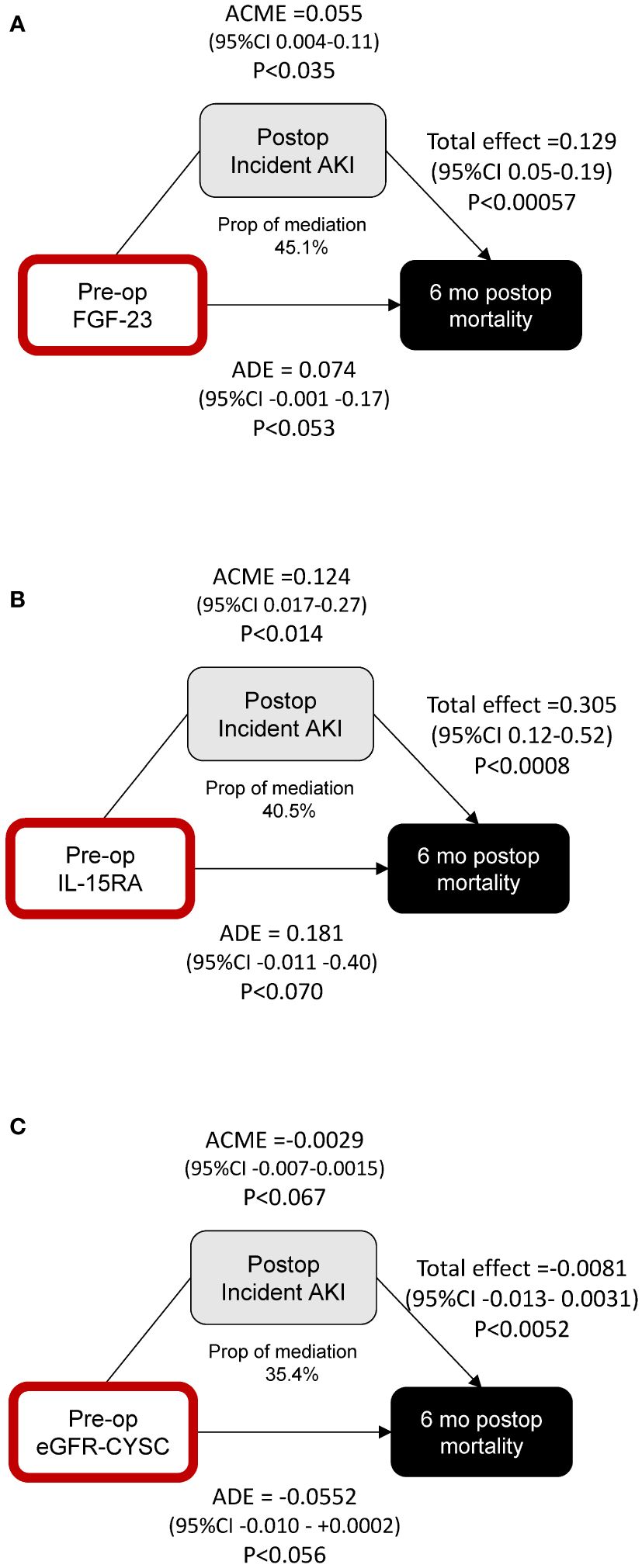
Figure 4 Mediation analysis of the effects of preoperative FGF-23 (A), IL-15RA (B), and eGFR-CYSC (C) on postoperative mortality, and the mediating effect of postoperative incident AKI. ROC curve analyses using clinical indices and biomarkers.
In this exploratory pilot study, having tested 92 circulating inflammatory proteins, preoperative circulating levels of FGF-23 and IL-15RA were found to be significantly predictive of 6-month mortality post-surgery and of incident postoperative AKI. Having tested a large array of inflammatory markers, including various pro and anti-inflammatory cytokines and growth factors, it is notable that only proteins with links to renal function were predictive of postoperative mortality. This suggests that reducing the risk of AKI and its consequences should be a top priority in fragility fracture perioperative management which requires accurate preoperative renal function measurements. We also report that creatinine-based preoperative measures of renal function are only weakly associated with both outcomes whereas cystatin C-derived eGFR appears more informative and predictive.
Fibroblast growth factor–23 (FGF-23) is secreted by bone cells and is a key regulator of serum phosphate and active vitamin D3 levels (36). In late-stage CKD, FGF-23 cannot reduce serum phosphate levels, and high FGF-23 concentrations result in left ventricular hypertrophy (LVH), faster CKD progression, and mortality (41). FGF-23 is an independent predictor for LVH adjusting for eGFR (42, 43) and appears to be the molecular link between the bone’s physiological response to trauma (44, 45) and AKI (45) or an exacerbation of renal insufficiency in frail individuals (44). FGF-23 has also been identified as a predictor of AKI and death following cardiac surgery (46–48).
Two recent meta-analyses have found that FGF-23 can predict incident AKI in various patient populations in adults and children, combining data from critically ill patients, acute heart failure patients, patients with sepsis, and patients with acute respiratory distress syndrome (47, 48). FGF-23 has also been identified as a predictor of AKI and death following cardiac surgery (46–48). However, none of the studies had investigated the role of FGF-23 as a biomarker of AKI in hip fracture patients, even though post-operative incidence of AKI is known to risk factor of hip fracture mortality (49–53).
In addition to FGF-23, we also report for the first time that IL-15RA might also be a biomarker of post-operative incident AKI. IL-15RA mediates pleiotropic proinflammatory signals involved in several inflammatory and cardiovascular disorders (54). IL-15RA was identified (37) as one of 17 proteins involved in 10-year risk of end-stage renal disease in two large U.S. cohorts of individuals with type 2 diabetes. It is also one of 21 proteomic biomarkers associated both with kidney function decline and incident CKD over a 13-year period in a large longitudinal German study (55). IL-15, the natural ligand of IL-15RA is one of the key cytokines regulating the biology of natural killer (NK) cells (56–58).
There is now a substantial body of evidence showing that NK cells contribute to kidney injury and kidney failure by contributing to acute tubular necrosis in humans (57, 59, 60). Thus, it is likely that the effect of IL-15RA might be mediated by the role of its ligand on NK cells.
The data presented here show, for the first time to the best of our knowledge, that circulating levels of IL-15RA are linked to incidence of AKI. We also report the novel finding that this molecule is a biomarker of postoperative mortality in hip fracture patients.
Furthermore, we report very different patterns of these two markers in the comparator groups with, for example, IL-15RA being lower in pre-frail elderly individuals than in healthy controls, unlike the situation seen with FGF-23. 1L-15 has been shown to be reduced in ageing animal models and given that IL-1RA regulates the localization in immune cells of IL-15 (40, 61). This might explain why IL-15RA is lower in a pre-frail or frail individuals than in healthy controls. On the other hand FGF-23 increases with age and is in fact associated with incidence and prevalence of frailty in our data (62, 63).
In our data, eGFR-CYS is much more strongly associated with postoperative mortality than eGFR-CRE. The shortcomings of creatinine-based eGFR in frail individuals have been extensively described (29, 30, 64). As frail individuals lose muscle mass, a process accelerated by trauma, the amount of creatinine released from muscle into serum declines, resulting in over-estimation of eGFR and reduced accuracy for measurement of kidney function (34). The use of eGFR-CYSC is not affected by alterations in muscle mass and may offer advantages in such scenarios compared to eGFR-CRE (29, 30, 34, 64).
As already mentioned, AKI is a common complication in patients with hip fracture (51, 65, 66). Identifying and monitoring patients at increased risk may underpin strategies to improve outcomes. The value of targeting AKI in hip fracture patients has been recently demonstrated in an interventional study of withholding certain medications preoperatively in emergency and elective T&O patients (67). The investigators found a significant reduction in postoperative AKI (67).
Preoperative measures of eGFR based on creatinine levels appear poorly associated with mortality and incidence of AKI in the frail hip fracture patients in our cohort. This is consistent with various studies showing that eGFR-CYS is more reliable than eGFR-CRE in several patient populations with a variety of acute and chronic illnesses (68, 69). Our results, showing fairly poor performance of preoperative creatinine as a predictor of AKI, are therefore in agreement with what is widely accepted in the literature given the high levels of inflammation and of muscle loss seen in frail hip fracture patients.
Importantly, the strongest marker of incident AKI in our study was FGF-23, followed by IL-15RA. We show here that a substantial proportion of the effect of these biomarkers is mediated by the postoperative incidence of AKI. If these biomarkers were validated in larger study samples, this would be a strong incentive to incorporate such measures within clinical practice to improve patient care. We also find that, along with FGF-23 and IL-15RA, eGFR of cystatin C is also much more informative of AKI incidence than creatinine derived eGFR. Although cystatin C has already been assigned more significant role in estimating glomerular filtration rate (GFR) in the recent KDIGO guidelines (70), this marker is up to 10 times as expensive as creatinine, which might explain why its use has not been more widely adopted in various clinical settings (71). Future studies should focus on defining the health economic benefits of obtaining more reliable predictors of post-operative AKI incidence in this patient population and on exploring how to improve clinical management to reduce the incidence of AKI and therefore also post-operative mortality.
The current study has several strengths. The pilot sub-cohort analyzed for biomarkers is comparable in clinical and demographic characteristics to a much larger cohort of frail hip fracture patients from the largest trauma centre in the United Kingdom, suggesting that the data from our study should be generalizable to the wider hip fracture populations. We were able to compare the biomarkers tested to a series of relevant positive and negative controls, namely healthy controls, non-frail severe fractures, and non-fractured prefrail and frail individuals. Through the analysis of EHRs, we were also able to compare the predictive value of biomarkers for mortality to that of combined clinical and demographic indices fitted in the large EHR database. Overall, the conclusions of the study, namely, that biomarkers of renal failure and AKI are the most highly associated with post-operative mortality after hip fracture surgery, are consistent with a large body of literature showing the role of these biomarkers in renal disease and of kidney injury in post-surgical mortality.
The key limitation of the present study is the pilot nature of the data, that is, the sample size of fracture cases with preoperative serum. The small sample size also means that there may be other preoperative inflammatory markers in the 92-protein panel that are associated with post-operative mortality but these are not detected as statistically significant after adjusting for multiple tests due to the statistical power available in this study. On the other hand, some of the limitations of a small sample size, specifically lack of representativeness, have been compensated for by ensuring that key clinical parameters are comparable to those in the larger frail hip fracture patient population from the largest Major Trauma Centre in England. Furthermore, we note that the study was sufficiently powered to detect the size effects that were actually measured and that there is strong face validity for the two markers identified given their links to kidney injury.
In conclusion, an exploratory study investigating inflammatory mediators of high-postoperative mortality in hip fracture patients identified renal-related factors as strong predictors of mortality. Our data suggest that either FGF-23 or IL-15RA or both should be assessed as potential biomarkers to make accurate clinical decisions regarding risk of postoperative AKI and to evaluate the risk of mortality and complications in frail hip fracture patients.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
The studies involving humans were approved by Camden and Kings Cross Research Ethics Committee. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
AVa: Writing – original draft, Writing – review & editing, Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Supervision. AI: Conceptualization, Funding acquisition, Investigation, Writing – original draft, Writing – review & editing, Data curation. LT: Writing – original draft, Writing – review & editing. AZ: Data curation, Writing – original draft, Writing – review & editing. AKo: Writing – original draft, Writing – review & editing. AKe: Writing – original draft, Writing – review & editing. WA: Writing – original draft, Writing – review & editing. AVi: Writing – original draft, Writing – review & editing. SM: Writing – original draft, Writing – review & editing. JN: Writing – original draft, Writing – review & editing. NS: Writing – original draft, Writing – review & editing. BO: Funding acquisition, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Institute for Health and Care Research (NIHR) Nottingham Biomedical Research Centre and by NIHR grants NIHR132240 (OPERA) and 16/61/10 (ORiF) to BO. Support was also provided by UKRI/MRC grants MR/W026813/1 and MR/Y010175/1 to AVa.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2024.1372079/full#supplementary-material
1. Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int. (2006) 17:1726–33. doi: 10.1007/s00198–006-0172–4
2. Hu F, Jiang C, Shen J, Tang P, Wang Y. Preoperative predictors for mortality following hip fracture surgery: a systematic review and meta-analysis. Injury. (2012) 43:676–85. doi: 10.1016/j.injury.2011.05.017
3. HIP ATTACK Investigators. Accelerated surgery versus standard care in hip fracture (HIP ATTACK): an international, randomised, controlled trial. Lancet. (2020) 395:698–708. doi: 10.1016/S0140–6736(20)30058–1
4. Tran Z, Hsiue PP, Pan C, Verma A, Rahimtoola R, Stavrakis A, et al. Impact of delayed intervention on clinical outcomes following traumatic hip fracture in the elderly: A national analysis. J Orthop. (2021) 27:74–8. doi: 10.1016/j.jor.2021.09.006
5. Miralles-Muñoz FA, Perez-Aznar A, Gonzalez-Parreño S, Sebastia-Forcada E, Mahiques-Segura G, Lizaur-Utrilla A, et al. Change in 1-year mortality after hip fracture surgery over the last decade in a European population. Arch Orthop Trauma Surg. (2023) 143:4173–9. doi: 10.1007/s00402–022-04719–4
6. Ricci G, Longaray MP, Gonçalves RZ, Neto A, da SU, Manente M, et al. EVALUATION OF THE MORTALITY RATE ONE YEAR AFTER HIP FRACTURE AND FACTORS RELATING TO DIMINISHED SURVIVAL AMONG ELDERLY PEOPLE. Rev Bras Ortop. (2012) 47:304–9. doi: 10.1016/S2255–4971(15)30103–8
7. Cenzer IS, Tang V, Boscardin WJ, Smith AK, Ritchie C, Wallhagen MI, et al. One-year mortality after hip fracture: development and validation of a prognostic index. J Am Geriatr Soc. (2016) 64:1863–8. doi: 10.1111/jgs.14237
8. Mitchell R, Harvey L, Brodaty H, Draper B, Close J. One-year mortality after hip fracture in older individuals: the effects of delirium and dementia. Arch Gerontol Geriatr. (2017) 72:135–41. doi: 10.1016/j.archger.2017.06.006
9. Morri M, Ambrosi E, Chiari P, Orlandi Magli A, Gazineo D, D’ Alessandro F, et al. One-year mortality after hip fracture surgery and prognostic factors: a prospective cohort study. Sci Rep. (2019) 9:18718. doi: 10.1038/s41598–019-55196–6
10. Veronese N, Maggi S. Epidemiology and social costs of hip fracture. Injury. (2018) 49:1458–60. doi: 10.1016/j.injury.2018.04.015
11. WHO. Musculoskeletal conditions affect millions. Arch Pathol Lab Med. (2004) 128:480. doi: 10.5858/2004–128-480-MCAM
12. LeBlanc ES, Hillier TA, Pedula KL, Rizzo JH, Cawthon PM, Fink HA, et al. Hip fracture and increased short-term but not long-term mortality in healthy older women. Arch Intern Med. (2011) 171:1831–7. doi: 10.1001/archinternmed.2011.447
13. Xu BY, Yan S, Low LL, Vasanwala FF, Low SG. Predictors of poor functional outcomes and mortality in patients with hip fracture: a systematic review. BMC Musculoskelet Disord. (2019) 20:568. doi: 10.1186/s12891–019-2950–0
14. Araiza-Nava B, Méndez-Sánchez L, Clark P, Peralta-Pedrero ML, Javaid MK, Calo M, et al. Short- and long-term prognostic factors associated with functional recovery in elderly patients with hip fracture: A systematic review. Osteoporos Int. (2022) 33:1429–44. doi: 10.1007/s00198–022-06346–6
15. Li J, Li D, Wang X, Zhang L. The impact of body mass index on mortality rates of hip fracture patients: a systematic review and meta-analysis. Osteoporos Int. (2022) 33(9):1859–69. doi: 10.1007/s00198–022-06415-w
16. Wilhelm-Leen ER, Hall YN, K Tamura M, Chertow GM. Frailty and chronic kidney disease: the Third National Health and Nutrition Evaluation Survey. Am J Med. (2009) 122:664–71.e2. doi: 10.1016/j.amjmed.2009.01.026
17. Ma Y, Wang A, Lou Y, Peng D, Jiang Z, Xia T. Effects of frailty on outcomes following surgery among patients with hip fractures: A systematic review and meta-analysis. Front Med. (2022) 9:829762. doi: 10.3389/fmed.2022.829762
18. Marcos-Pérez D, Sánchez-Flores M, Proietti S, Bonassi S, Costa S, Teixeira JP, et al. Association of inflammatory mediators with frailty status in older adults: results from a systematic review and meta-analysis. Geroscience. (2020) 42:1451–73. doi: 10.1007/s11357–020-00247–4
19. Panula J, Pihlajamäki H, Mattila VM, Jaatinen P, Vahlberg T, Aarnio P, et al. Mortality and cause of death in hip fracture patients aged 65 or older: a population-based study. BMC Musculoskelet Disord. (2011) 12:105. doi: 10.1186/1471–2474-12–105
20. Kinney JW, Bemiller SM, Murtishaw AS, Leisgang AM, Salazar AM, Lamb BT. Inflammation as a central mechanism in Alzheimer’s disease. Alzheimers Dement. (2018) 4:575–90. doi: 10.1016/j.trci.2018.06.014
21. Soysal P, Arik F, Smith L, Jackson SE, Isik AT. Inflammation, frailty and cardiovascular disease. In: Veronese N, editor. Frailty and Cardiovascular Diseases: Research into an Elderly Population. Springer International Publishing, Cham (2020). p. 55–64. doi: 10.1007/978–3-030–33330-0_7
22. Maxwell MJ, Moran CG, Moppett IK. Development and validation of a preoperative scoring system to predict 30 day mortality in patients undergoing hip fracture surgery. Br J Anaesth. (2008) 101:511–7. doi: 10.1093/bja/aen236
23. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. (1987) 40:373–83. doi: 10.1016/0021–9681(87)90171–8
24. Charlson ME, Carrozzino D, Guidi J, Patierno C. Charlson comorbidity index: A critical review of clinimetric properties. Psychother Psychosom. (2022) 91:8–35. doi: 10.1159/000521288
25. Church S, Rogers E, Rockwood K, Theou O. A scoping review of the Clinical Frailty Scale. BMC Geriatr. (2020) 20:393. doi: 10.1186/s12877–020-01801–7
26. Archunan MW, Subhash S, Attwood J, Kumar S, Choudhry N, Fountain J, et al. Nottingham hip fracture score: does it predict mortality in distal femoral fracture patients? Cureus. (2021) 13:e19139. doi: 10.7759/cureus.19139
27. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. (2009) 150:604–12. doi: 10.7326/0003–4819-150–9-200905050–00006
28. Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. (2012) 367:20–9. doi: 10.1056/NEJMoa1114248
29. Shlipak MG, Inker LA, Coresh J. Serum cystatin C for estimation of GFR. JAMA. (2022) 328:883–4. doi: 10.1001/jama.2022.12407
30. Delgado C, Baweja M, Crews DC, Eneanya ND, Gadegbeku CA, Inker LA, et al. A unifying approach for GFR estimation: recommendations of the NKF-ASN task force on reassessing the inclusion of race in diagnosing kidney disease. Am J Kidney Dis. (2022) 79:268–288.e1. doi: 10.1053/j.ajkd.2021.08.003
31. Kdigo AKI. Kidney Disease Improving Global Outcomes. AKI Work Group: Clinical practice guideline for acute kidney injury. Kidney Int Suppl. (n.) 2, 1. doi: 10.1038/kisup.2012.1
32. Lundberg M, Eriksson A, Tran B, Assarsson E, Fredriksson S. Homogeneous antibody-based proximity extension assays provide sensitive and specific detection of low-abundant proteins in human blood. Nucleic Acids Res. (2011) 39:e102. doi: 10.1093/nar/gkr424
33. Olink target 96 inflammation panels. Olink(2021). Available online at: https://olink.com/products-services/target/inflammation/ (Accessed October 26, 2023).
34. Ballew SH, Zhou L, Surapaneni A, Grams ME, Windham BG, Selvin E, et al. A novel creatinine muscle index based on creatinine filtration: associations with frailty and mortality. J Am Soc Nephrol. (2023) 34:495–504. doi: 10.1681/ASN.0000000000000037
35. Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Stat Soc. (1995) 57:289–300. doi: 10.1111/j.2517–6161.1995.tb02031.x
36. Fauconnier C, Roy T, Gillerot G, Roy C, Pouleur A-C, Gruson D. FGF23: Clinical usefulness and analytical evolution. Clin Biochem. (2019) 66:1–12. doi: 10.1016/j.clinbiochem.2019.03.002
37. Niewczas MA, Pavkov ME, Skupien J, Smiles A, Md Dom ZI, Wilson JM, et al. A signature of circulating inflammatory proteins and development of end-stage renal disease in diabetes. Nat Med. (2019) 25:805–13. doi: 10.1038/s41591–019-0415–5
38. Kimura A, Ono S, Hiraki S, Takahata R, Tsujimoto H, Miyazaki H, et al. The postoperative serum interleukin-15 concentration correlates with organ dysfunction and the prognosis of septic patients following emergency gastrointestinal surgery. J Surg Res. (2012) 175:e83–8. doi: 10.1016/j.jss.2011.12.003
39. Christov M, Neyra JA, Gupta S, Leaf DE. Fibroblast growth factor 23 and Klotho in AKI. Semin Nephrol. (2019) 39:57–75. doi: 10.1016/j.semnephrol.2018.10.005
40. Yoshida S, Fujimoto T, Takahashi T, Sugimoto K, Akasaka H, Tanaka M, et al. Interleukin-15 receptor subunit alpha regulates interleukin-15 localization and protein expression in skeletal muscle cells. Exp Physiol. (2022) 107:222–32. doi: 10.1113/EP090205
42. Kirkpantur A, Balci M, Gurbuz OA, Afsar B, Canbakan B, Akdemir R, et al. Serum fibroblast growth factor-23 (FGF-23) levels are independently associated with left ventricular mass and myocardial performance index in maintenance haemodialysis patients. Nephrol Dial Transplant. (2011) 26:1346–54. doi: 10.1093/ndt/gfq539
43. Unver S, Kavlak E, Gümüsel HK, Celikbilek F, Esertas K, Muftuoglu T, et al. Correlation between hypervolemia, left ventricular hypertrophy and fibroblast growth factor 23 in hemodialysis patients. Ren Fail. (2015) 37:951–6. doi: 10.3109/0886022X.2015.1052945
44. Neyra JA, Hu MC, Moe OW. Fibroblast growth factor 23 and αKlotho in acute kidney injury: current status in diagnostic and therapeutic applications. Nephron. (2020) 144:665–72. doi: 10.1159/000509856
45. Cannata-Andía JB, Martín-Carro B, Martín-Vírgala J, Rodríguez-Carrio J, Bande-Fernández JJ, Alonso-Montes C, et al. Chronic kidney disease-mineral and bone disorders: pathogenesis and management. Calcif Tissue Int. (2021) 108:410–22. doi: 10.1007/s00223–020-00777–1
46. Leaf DE, Christov M, Jüppner H, Siew E, Ikizler TA, Bian A, et al. Fibroblast growth factor 23 levels are elevated and associated with severe acute kidney injury and death following cardiac surgery. Kidney Int. (2016) 89:939–48. doi: 10.1016/j.kint.2015.12.035
47. Sun S, Liu Z, Chen C, Wang Z, Jin H, Meng X, et al. Serum fibroblast growth factor 23 for early detection of acute kidney injury in critical illness. Am J Transl Res. (2021) 13:12141–51.
48. Tao W, Guo S-W, Fan Y-J, Zheng F. Prediction of acute kidney injury by fibroblast growth factor 23 (FGF-23) in adult patients, A meta-analysis study. Iran J Kidney Dis. (2023) 1:1–8.
49. Porter CJ, Moppett IK, Juurlink I, Nightingale J, Moran CG, Devonald MAJ. Acute and chronic kidney disease in elderly patients with hip fracture: prevalence, risk factors and outcome with development and validation of a risk prediction model for acute kidney injury. BMC Nephrol. (2017) 18:20. doi: 10.1186/s12882–017-0437–5
50. Braüner Christensen J, Aasbrenn M, Sandoval Castillo L, Ekmann A, Giver Jensen T, Pressel E, et al. Predictors of acute kidney injury after hip fracture in older adults. Geriatr Orthop Surg Rehabil. (2020) 11:2151459320920088. doi: 10.1177/2151459320920088
51. Berar Yanay N, Abu Arisha M, Berkowitz Y. Acute kidney injury in patients undergoing hip fracture surgery. Isr Med Assoc J. (2021) 23:815–8.
52. Chiang M-H, Lee H-J, Kuo Y-J, Chien P-C, Chang W-C, Wu Y, et al. Predictors of in-hospital mortality in older adults undergoing hip fracture surgery: A case-control study. Geriatr Orthop Surg Rehabil. (2021) 12:21514593211044644. doi: 10.1177/21514593211044644
53. Chen M, Du Y, Tang W, Yu W, Li H, Zheng S, et al. Risk factors of mortality and second fracture after elderly hip fracture surgery in Shanghai, China. J Bone Miner Metab. (2022) 40:951–9. doi: 10.1007/s00774–022-01358-y
54. Guo L, Liu M-F, Huang J-N, Li J-M, Jiang J, Wang J-A. Role of interleukin-15 in cardiovascular diseases. J Cell Mol Med. (2020) 24:7094–101. doi: 10.1111/jcmm.15296
55. Lin J-S, Nano J, Petrera A, Hauck SM, Zeller T, Koenig W, et al. Proteomic profiling of longitudinal changes in kidney function among middle-aged and older men and women: the KORA S4/F4/FF4 study. BMC Med. (2023) 21:245. doi: 10.1186/s12916–023-02962-z
56. Becknell B, Caligiuri MA. Interleukin-2, interleukin-15, and their roles in human natural killer cells. Adv Immunol. (2005) 86:209–39. doi: 10.1016/S0065–2776(04)86006–1
57. Turner J-E, Rickassel C, Healy H, Kassianos AJ. Natural killer cells in kidney health and disease. Front Immunol. (2019) 10:587. doi: 10.3389/fimmu.2019.00587
58. Ma S, Caligiuri MA, Yu J. Harnessing IL-15 signaling to potentiate NK cell-mediated cancer immunotherapy. Trends Immunol. (2022) 43:833–47. doi: 10.1016/j.it.2022.08.004
59. Zhang Z-X, Wang S, Huang X, Min W-P, Sun H, Liu W, et al. NK cells induce apoptosis in tubular epithelial cells and contribute to renal ischemia-reperfusion injury. J Immunol. (2008) 181:7489–98. doi: 10.4049/jimmunol.181.11.7489
60. Uchida T, Seki S, Oda T. Infections, reactions of natural killer T cells and natural killer cells, and kidney injury. Int J Mol Sci. (2022) 23, 479. doi: 10.3390/ijms23010479
61. Saito M, Inoue S, Yamashita K, Kakeji Y, Fukumoto T, Kotani J. IL-15 improves aging-induced persistent T cell exhaustion in mouse models of repeated sepsis. Shock. (2020) 53:228–35. doi: 10.1097/SHK.0000000000001352
62. Beben T, Ix JH, Shlipak MG, Sarnak MJ, Fried LF, Hoofnagle AN, et al. Fibroblast growth factor-23 and frailty in elderly community-dwelling individuals: the cardiovascular health study. J Am Geriatr Soc. (2016) 64:270–6. doi: 10.1111/jgs.13951
63. Wang R, Shlipak MG, Ix JH, Brown TT, Jacobson LP, Palella FJ Jr., et al. Association of fibroblast growth factor-23 (FGF-23) with incident frailty in HIV-infected and HIV-uninfected individuals. J Acquir Immune Defic Syndr. (2019) 80:118–25. doi: 10.1097/QAI.0000000000001868
64. Potok OA, Ix JH, Shlipak MG, Katz R, Hawfield AT, Rocco MV, et al. The difference between cystatin C- and creatinine-based estimated GFR and associations with frailty and adverse outcomes: A cohort analysis of the systolic blood pressure intervention trial (SPRINT). Am J Kidney Dis. (2020) 76:765–74. doi: 10.1053/j.ajkd.2020.05.017
65. Hong SE, Kim T-Y, Yoo J-H, Kim J-K, Kim SG, Kim HJ, et al. Acute kidney injury can predict in-hospital and long-term mortality in elderly patients undergoing hip fracture surgery. PloS One. (2017) 12:e0176259. doi: 10.1371/journal.pone.0176259
66. Agar A, Gulabi D, Sahin A, Gunes O, Hancerli CO, Kılıc B, et al. Acute kidney injury after hip fracture surgery in patients over 80 years of age. Arch Orthop Trauma Surg. (2022) 142:2245–52. doi: 10.1007/s00402–021-03969-y
67. Kulkarni S, Richardson T, Green A, Acharya R, Latif A, Johnson D, et al. Reducing incidence of postoperative acute kidney injury in trauma and orthopaedics patients: a quality improvement project. BMJ Open Qual. (2023) 12:e002124. doi: 10.1136/bmjoq-2022–002124
68. Aldenbratt A, Lindberg C, Johannesson E, Hammarsten O, Svensson MK. Estimation of kidney function in patients with primary neuromuscular diseases: is serum cystatin C a better marker of kidney function than creatinine? J Nephrol. (2022) 35:493–503. doi: 10.1007/s40620–021-01122-x
69. Adingwupu OM, Barbosa ER, Palevsky PM, Vassalotti JA, Levey AS, Inker LA. Cystatin C as a GFR estimation marker in acute and chronic illness: A systematic review. Kidney Med. (2023) 5:100727. doi: 10.1016/j.xkme.2023.100727
70. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2024 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. (2024) 105:S117–314. doi: 10.1016/j.kint.2023.09.002
Keywords: hip fracture, frailty, inflammation, FGF23, renal, surgery, acute kidney injury, mortality
Citation: Valdes AM, Ikram A, Taylor LA, Zheng A, Kouraki A, Kelly A, Ashraf W, Vijay A, Miller S, Nightingale J, Selby NM and Ollivere BJ (2024) Preoperative inflammatory biomarkers reveal renal involvement in postsurgical mortality in hip fracture patients: an exploratory study. Front. Immunol. 15:1372079. doi: 10.3389/fimmu.2024.1372079
Received: 17 January 2024; Accepted: 20 May 2024;
Published: 10 June 2024.
Edited by:
Florencia Maria Barbé-Tuana, Pontifical Catholic University of Rio Grande do Sul, BrazilReviewed by:
Pengbin Yin, People’s Liberation Army General Hospital, ChinaCopyright © 2024 Valdes, Ikram, Taylor, Zheng, Kouraki, Kelly, Ashraf, Vijay, Miller, Nightingale, Selby and Ollivere. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ana M. Valdes, YW5hLnZhbGRlc0Bub3R0aW5naGFtLmFjLnVr
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.