
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Immunol. , 26 March 2024
Sec. Nutritional Immunology
Volume 15 - 2024 | https://doi.org/10.3389/fimmu.2024.1370707
Background: Hypothyroidism, a prevalent endocrine disorder, carries significant implications for maternal and infant health, especially in the context of maternal hypothyroidism. Despite a gradual surge in recent research, achieving a comprehensive understanding of the current state, focal points, and developmental trends in this field remains challenging. Clarifying these aspects and advancing research could notably enhance maternal-infant health outcomes. Therefore, this study employs bibliometric methods to systematically scrutinize maternal hypothyroidism research, serving as a reference for further investigations.
Objective: Through bibliometric analysis, this study seeks to unveil key research focus areas, developmental trends, and primary contributors in Maternal Hypothyroidism. The findings offer insights and recommendations to inform future research endeavors in this domain.
Methods: Literature metrics analysis was performed on data retrieved and extracted from the Web of Science Core Collection database. The analysis examined the evolution and thematic trends of literature related to Maternal Hypothyroidism. Data were collected on October 28, 2023, and bibliometric analysis was performed using VOSviewer, CiteSpace, and the Bibliometrix software package, considering specific characteristics such as publication year, country/region, institution, authorship, journals, references, and keywords.
Results: Retrieved from 1,078 journals, 4,184 articles were authored by 18,037 contributors in 4,580 institutions across 113 countries/regions on six continents. Maternal Hypothyroidism research publications surged from 44 to 310 annually, a 604.54% growth from 1991 to 2022. The USA (940 articles, 45,233 citations), China Medical University (82 articles, 2,176 citations), and Teng, Weiping (52 articles, 1,347 citations) emerged as the most productive country, institution, and author, respectively. “Thyroid” topped with 233 publications, followed by “Journal of Clinical Endocrinology & Metabolism” (202) with the most citations (18,513). “Pregnancy” was the most cited keyword, with recent high-frequency keywords such as “outcome,” “gestational diabetes,” “iodine intake,” “preterm birth,” “guideline,” and “diagnosis” signaling emerging themes in Maternal Hypothyroidism.
Conclusions: This study unveils developmental trends, global collaboration patterns, foundational knowledge, and emerging frontiers in Maternal Hypothyroidism. Over 30 years, research has predominantly focused on aspects like diagnosis, treatment guidelines, thyroid function during pregnancy, and postpartum outcomes, with a central emphasis on the correlation between maternal and fetal health.
Hypothyroidism is an endocrine system disorder primarily caused by insufficient secretion of thyroid hormones. Dominantly, primary hypothyroidism is characterized by elevated thyroid-stimulating hormone (TSH) levels, accompanied by Free Thyroxine 4 (FT4) concentrations below the reference range. Subclinical hypothyroidism, considered an early sign of thyroid dysfunction, is characterized by elevated TSH levels while FT4 concentrations remain within the reference range (1). Multiple factors, including iodine deficiency (2), Hashimoto’s thyroiditis (3), and rare causes such as congenital, drug-related, iatrogenic, and infiltrative diseases, may contribute to the development of hypothyroidism (1).
During pregnancy, hormonal fluctuations may impact thyroid function in pregnant women. Factors such as the rise in human chorionic gonadotropin, increased synthesis and secretion of thyroxine-binding globulin by the liver (4), and enhanced renal clearance of iodine (5) can influence maternal thyroid function. Maternal hypothyroidism often presents as subclinical hypothyroidism, with most patients lacking significant clinical symptoms. However, a minority may exhibit clinical hypothyroidism or isolated low thyroxine levels (6). The prevalence of hypothyroidism during pregnancy varies from 0.5% to 3.47% (7, 8), highlighting its relatively high incidence among pregnant women and establishing it as one of the common chronic conditions during pregnancy (9).
Maternal hypothyroidism increases the risk of complications during pregnancy, including gestational hypertension (10), preeclampsia (11), gestational diabetes (12), and preterm birth (13). Thyroid hormones play a crucial role in fetal neurological and intellectual development. Maternal hypothyroidism during pregnancy may elevate the risk of intellectual developmental defects (14), preterm birth, low birth weight (15), and fetal hypothyroidism (16). Therefore, maintaining optimal thyroid function is crucial for pregnant women.
Bibliometric analysis, a systematic method aimed at evaluating, summarizing, and interpreting a large body of literature, provides insights into research trends, hotspots, and developmental directions in a specific field or topic (17). The analysis combines principles of statistics and informatics to quantify and assess literature resources, revealing characteristics of a particular research area. Maternal Hypothyroidism has been a subject of clinical and scientific interest. Despite existing reviews focusing on different aspects of Maternal Hypothyroidism (18, 19), bibliometric analysis offers a more comprehensive and intuitive understanding of its development and trends.
This paper aims to conduct a bibliometric analysis using tools such as CiteSpace and VOSviewer on publications related to Maternal Hypothyroidism in the Web of Science Core Collection (WoSCC). The analysis encompasses aspects such as the annual distribution of publications, countries, institutions, authors, source journals, keyword co-occurrence, and reference co-occurrence. Through this analysis, the goal is to gain in-depth insights into the current state, focal areas, and future trends of research on Maternal Hypothyroidism. This study will assist new researchers and experts in delineating the research scope, identifying novel topics, or planning future research directions, thereby providing guidance for both clinical practice and scientific inquiry.
To enhance the representativeness and accessibility of the data, a literature search was conducted within the WoSCC on October 28, 2023, and the data collection and retrieval strategy is illustrated in Figure 1. The search terms, identified through TS (“topic”, covering title, abstract, author keywords, and keywords plus), were set as TS=(“Maternal hypothyroid*” or “Gestational hypothyroidism” or “Hypothyroidism in pregnant women” or “Hypothyroidism and pregnancy” or “Maternal thyroid hormone deficiency” or “Hypothyroidism during gestation” or “Thyroid deficiency and pregnancy” or “Thyroid hormone insufficiency in pregnancy” or “Thyroid insufficiency in pregnant women” or “Thyroid insufficiency during pregnancy” or “Thyroid function deficiency during gestation” or “Thyroid insufficiency during gestation”). The publication type was limited to articles, with no restrictions imposed on time and language. A total of 4184 records were obtained, collecting information on publications, authors, countries, institutions, journals, keywords, and citations, and were exported in the form of complete citation records.
Literature metrics data analysis was performed using VOSviewer (v1.6.19), CiteSpace (v.6.1.R6 Advanced), and the bibliometrics software package (version 4.1.3) based on the R language (4.3.1). Preliminary descriptive statistics of the number of publications and citations by year, country and author were performed using the “Analyze” function in WoSCC. The bibliometrics package was used to visualize publication distribution and collaboration between countries/regions. VOSviewer was used for data extraction and visualization of countries, institutions, authors and keywords. CiteSpace was used to construct dual map overlays of journals related to Maternal Hypothyroidism and visualizations of keyword timelines and cluster maps, detecting the top 25 bursty keywords.
The number of publications over time is indicative of research trends and progress in a given field. According to records from the WoSCC database, literature on Maternal Hypothyroidism stretches back to 1991, with 4184 articles identified in total. Figure 2A charts the yearly publication output concerning “Maternal Hypothyroidism,” with an inaugural count of 44 in 1991. This suggests the early initiation and development of research in this domain. The literature in this field has undergone consistent growth, with an annual growth rate of 5.11%. The overall global annual publication output increased steeply from a meager 44 articles in 1991 to an astonishing 310 articles in 2022, a remarkable 604.54% growth. From 1991 to 2006, the annual publication count remained below 100 articles. However, between 2009 and 2017, the number of publications progressively increased from 115 to 178. In 2018, the publication count surmounted the 200 mark, ultimately exceeding 300 by 2022. The total number of citations for publications collected from 1991 to 2023 was 114,378, with an average of 27.34 citations per article. The consistent increase in citations every year signifies continual research attention towards “Maternal Hypothyroidism” during the past 30 years.
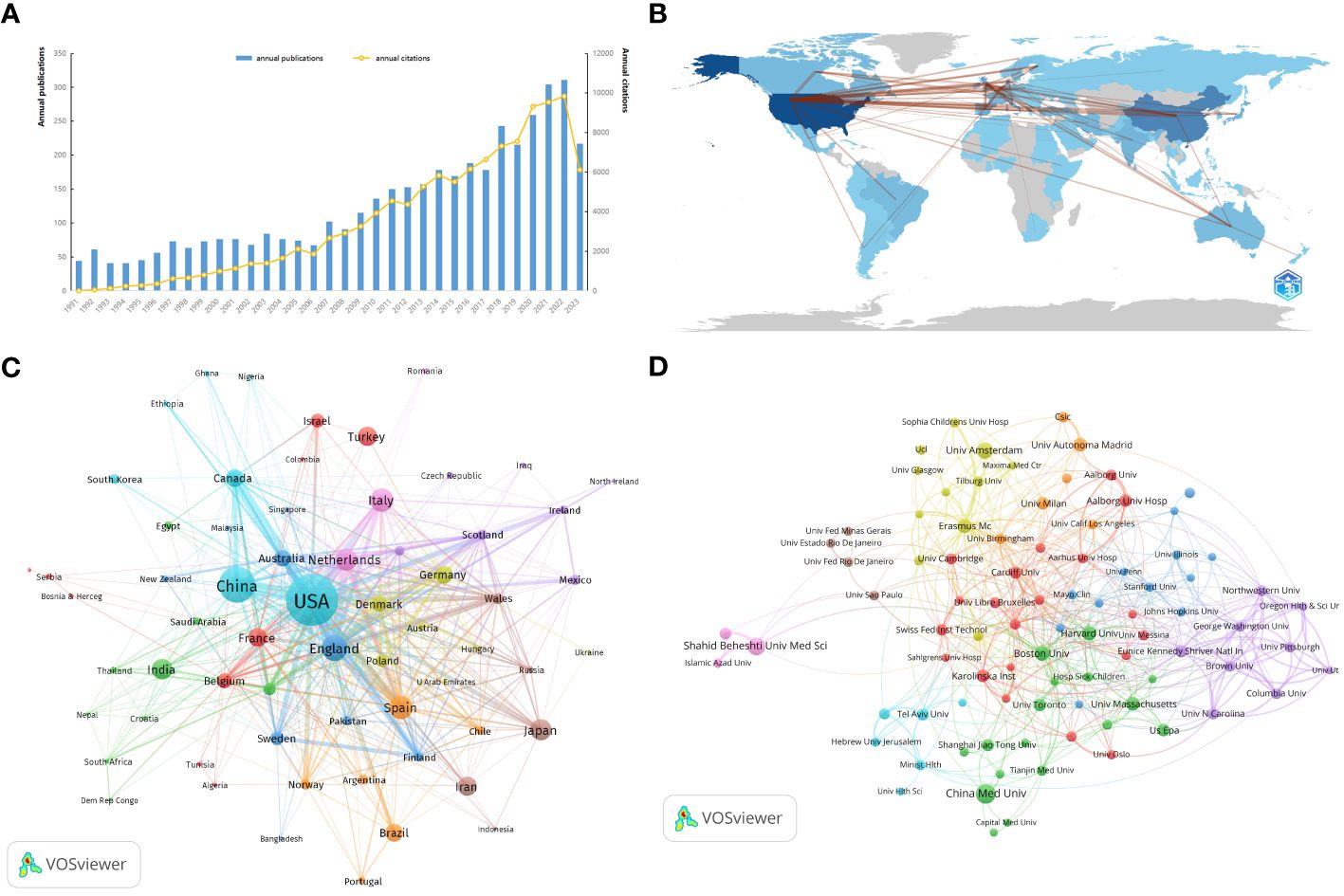
Figure 2 The distribution of Maternal Hypothyroidism publications over time and collaboration networks among countries/regions/institutions related to the subject. (A) Distribution of Maternal Hypothyroidism publications over time. (B) Distribution and collaboration of publications among countries/regions. (C) Collaboration clusters among countries/regions. (D) Visualization map of institutional collaboration.
Maternal Hypothyroidism is a globally recognized research focus. Between 1991 and 2023, 113 countries/regions across six continents contributed to the study of Maternal Hypothyroidism. The Countries’ Collaboration World Map in Figure 2B illustrates close collaboration between North America, East Asia, and Western Europe. Table 1 highlights the top 10 most productive countries, showcasing the USA (940 publications, 45233 citations) as the most prolific nation, constituting 22.47% of the total publications. China (471 publications, 6614 citations) follows at 11.26%, and the England (263 publications, 12571 citations) closely trails, representing 6.29% of the total publications. These three countries collectively contribute over 40% of the research output, underscoring a high level of interest in Maternal Hypothyroidism research.
VOSviewer analyzed collaborative authors among countries/regions, utilizing the parameters: Method (Association Strength) and a minimum number of country documents: 5. From the results involving 113 countries, 67 met the threshold. Netherlands, the USA, and England exhibited the highest Total link strength, Average citations, and H-Index (Table 1), indicating their relatively mature research outcomes in Maternal Hypothyroidism. These nations showcase more significant connections, collaborations, and influence compared to others.
Based on co-authorship analysis, VOSviewer categorized countries into different clusters. The visual representation in Figure 2C depicts the collaborative author network for Maternal Hypothyroidism, divided into nine clusters. The light blue cluster comprises 7 countries, centered around the USA, China, and Canada. The blue cluster includes countries such as England, Australia, Sweden, and Finland. The orange cluster consists of Spain, Brazil, Norway, and Argentina among others. The pink cluster includes Italy, the Netherlands, and Romania. Japan frequently collaborates with Iran, Wales, and Russia (brownish). India closely collaborates with Switzerland, Saudi Arabia, Thailand, and other countries (green). The red cluster comprises Turkey, France, Belgium, Israel, Serbia, and other nations. Germany closely collaborates with Denmark, Poland, Austria, and other countries (yellowish-green).
Utilizing VOSviewer, cooperative author analysis was conducted on these institutions, with parameters set as Method (Association Strength) and a minimum number of documents: 15. The results, drawn from 4580 institutions, identified 90 institutions meeting the threshold. Table 2 showcases the top 10 most productive institutions, where China Medical University (n=82, 1.96%) emerges as the institution with the highest publication output, followed by Shahid Beheshti University of Medical Sciences (n=68, 1.63%), Universiteit van Amsterdam (n=59, 1.41%), Boston University (n=49, 1.17%), and Harvard University (n=46, 1.10%). Among the top 10 institutions, three are located in the USA. Universidad Autonoma de Madrid (95.32) boasts the highest Average citations, followed by Harvard University (87.83) and University of Massachusetts (74.08). Erasmus Medical Center leads in Total link strength (79) among the top 10 institutions, followed by Boston University (53) and Aalborg Universitets Hospital (47), indicating these institutions maintain closer connections with others in Maternal Hypothyroidism research.
Figure 2D categorizes leading publishing institutions into nine clusters. The red cluster comprises primarily European institutions, including Karolinska Inst, Aalborg Univ Hosp, and Cardiff Univ. The green cluster is dominated by institutions from China, the USA, and Canada, such as China Med Univ, Boston Univ, Harvard Univ, and Univ Massachusetts. The blue cluster consists mainly of US institutions, including Mayo Clin, Univ Illinois, and Stanford Univ. Yellow cluster is primarily composed of Netherlands institutions, such as Univ Amsterdam, Erasmus Mc, and Tilburg Univ. The purple cluster is made up of US institutions, including Eunice Kennedy Shriver Natl Inst Child Hlth & Hum, Univ N Carolina, and Northwestern Univ. The light blue cluster is predominantly composed of Israeli institutions, including Tel Aviv Univ, Ben Gurion Univ Negev, and Hebrew Univ Jerusalem. The orange cluster is mainly European institutions, including Univ Autonoma Madrid, Univ Milan, Csic, and Univ Birmingham. The brown cluster is dominated by Brazilian institutions, such as Univ Sao Paulo, Univ Fed Rio De Janeiro, and Univ Estado Rio De Janeiro. The relatively distant pink cluster is comprised of Iranian institutions, such as Shahid Beheshti Univ Med Sci, Univ Tehran Med Sci, and Islamic Azad Univ.
The most prolific author is Teng, Weiping (China Medical University) with 52 publications (1.24%), followed by Shan, Zhongyan (China Medical University) with 51 publications (1.22%) and Azizi, Fereidoun (Shahid Beheshti University Medical Sciences) with 50 publications (1.2%) (Table 3). Among the top 10 most productive authors, the highest Average citations are attributed to Lazarus, John H., Laurberg, Peter, and Peeters, Robin P. The authors with the highest H-Index are Peeters, Robin P., and Visser, Theo J. Teng, Weiping, Shan, Zhongyan, and Peeters, Robin P. exhibit the highest Total link strength, indicating close collaboration with other authors in Maternal Hypothyroidism research.
Further collaborative author analysis using VOSviewer, with parameters set as Method (Association Strength) and the minimum number of documents: 9, identified 113 authors from 18037 results who met the threshold. Based on co-authorship frequency and density, authors were categorized into clusters, with overlay visualization using publication timelines for color annotation. Figure 3A depicts each node as an author, with circle size reflecting the number of articles published, and connecting lines representing co-occurrence relationships. Different clusters represent collaborations between authors. Notably, Teng, Weiping collaborates closely with Shan, Zhongyan; Fan, Chenling; Li, Chenyan; Zhang, Xiaomei. Peeters, Robin P. collaborates closely with Tiemeier, Henning; De Rijke, Yolanda B.; Korevaar, Tim I.M.; Jaddoe, Vincent W.V. Pearce, ElizabethN. collaborates closely with Braverman, Lewis E.; Laurberg, Peter; Lazarus, John H. The yellow nodes in Figure 3A represent authors who have remained consistently active and continually contributed to papers in recent times, such as Andersen, Stig; Nazarpour, Sima; Kleynen, Pierre; Rozenberg, Serge; Veltri, Flora; Tehrani, Fahimeh, Ramezani; Zhang, Wanqi.
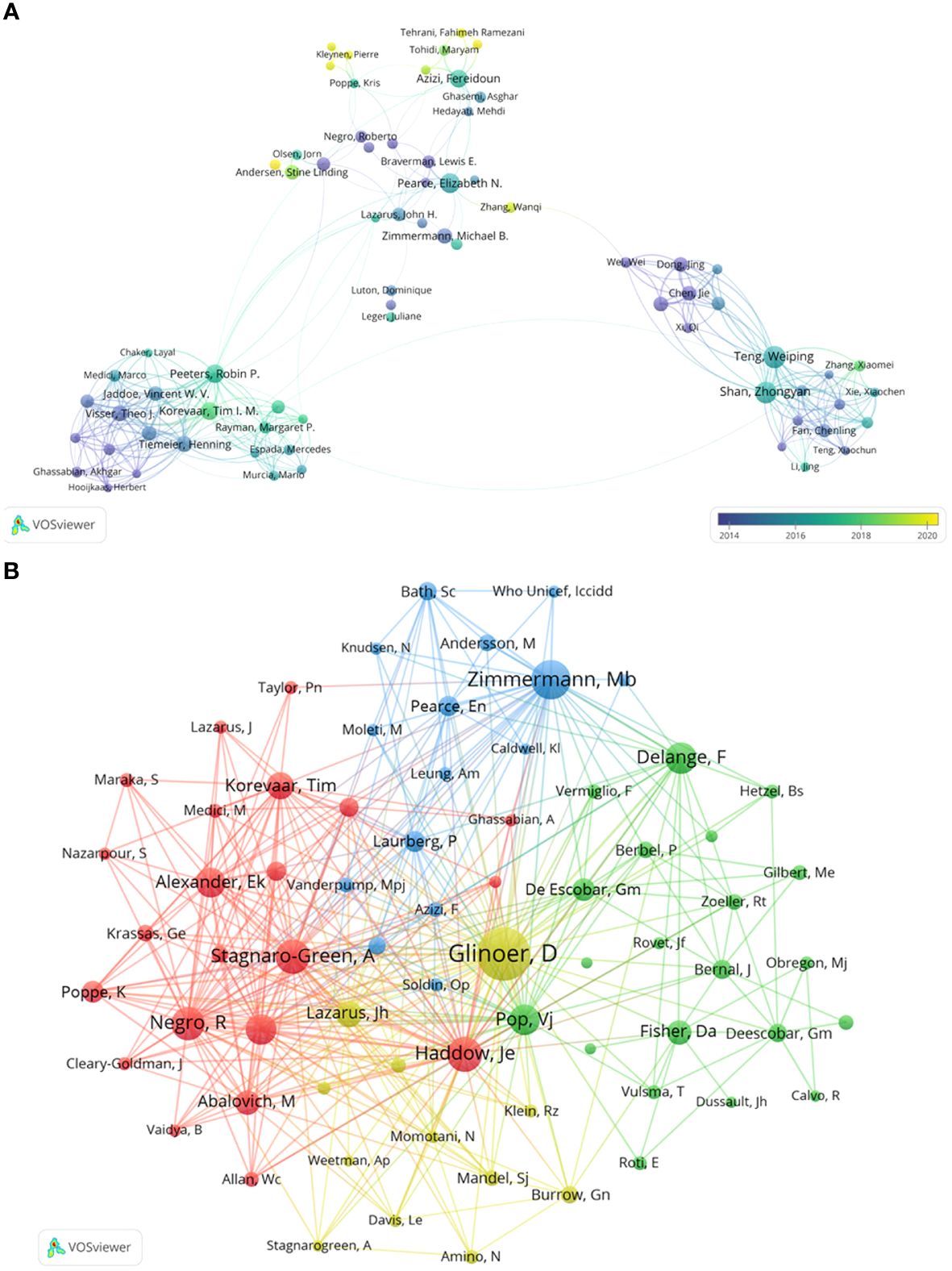
Figure 3 Collaboration networks among authors in Maternal Hypothyroidism. (A) Visualization map of author collaboration. (B) Co-citation analysis of cited authors.
We conducted a co-citation analysis of cited authors to gain deeper insights into the intrinsic connections and knowledge structure among the literature. Figure 3B depicts the citation patterns of prominent researchers on the topic of Maternal Hypothyroidism. The top 5 most cited authors are Glinoer, Daniel; Zimmermann, Mb; Haddow, Je; Stagnaro-Green, A; and Negro, R. These authors hold significant positions within the co-citation network and collaborate closely. Their citation counts far exceed those of other authors, demonstrating their widespread influence and core status within the research field.
In terms of the quantity of published papers, Endocrinology Metabolism (36.59%), Obstetrics Gynecology (14.91%), and Medicine General Internal (10.37%) are the top three disciplines (Table 4). Notably, over one-third of papers are published in Endocrinology Metabolism (36.59%). Other disciplines include Pediatrics (9.80%), Nutrition Dietetics (6.02%), Neurosciences (4.42%), among others.
The identified literature on Maternal Hypothyroidism was published in 1078 journals. Using Bradford’s Law, 24 journals were identified as core journals (Figure 4A). Table 5 lists the top 10 journals publishing the most papers on Maternal Hypothyroidism, accounting for 22.90% (958/4184) of the total published papers. “Thyroid” tops the list with 233 papers, followed by “Journal of Clinical Endocrinology & Metabolism” with 202 papers and “Clinical Endocrinology” with 116. “Journal of Clinical Endocrinology & Metabolism” leads in citation count (n=18513), average citation count (91.65), and H-Index, making it the most influential journal. This can also be understood through the co-citation bibliometric map generated by VOSviewer, as shown in Figure 4B. The strength of connections between journals is typically represented by the thickness or darkness of the links. The thicker or darker the link, the closer the connection between two journals, indicating a higher number of co-citations between them. This suggests that these journals may exhibit higher similarity or complementarity in terms of research topics, methodologies, and knowledge dissemination. The co-citation analysis of cited sources indicates that the top three journals with the highest citations are the Journal of Clinical Endocrinology & Metabolism, Thyroid, and Clinical Endocrinology.
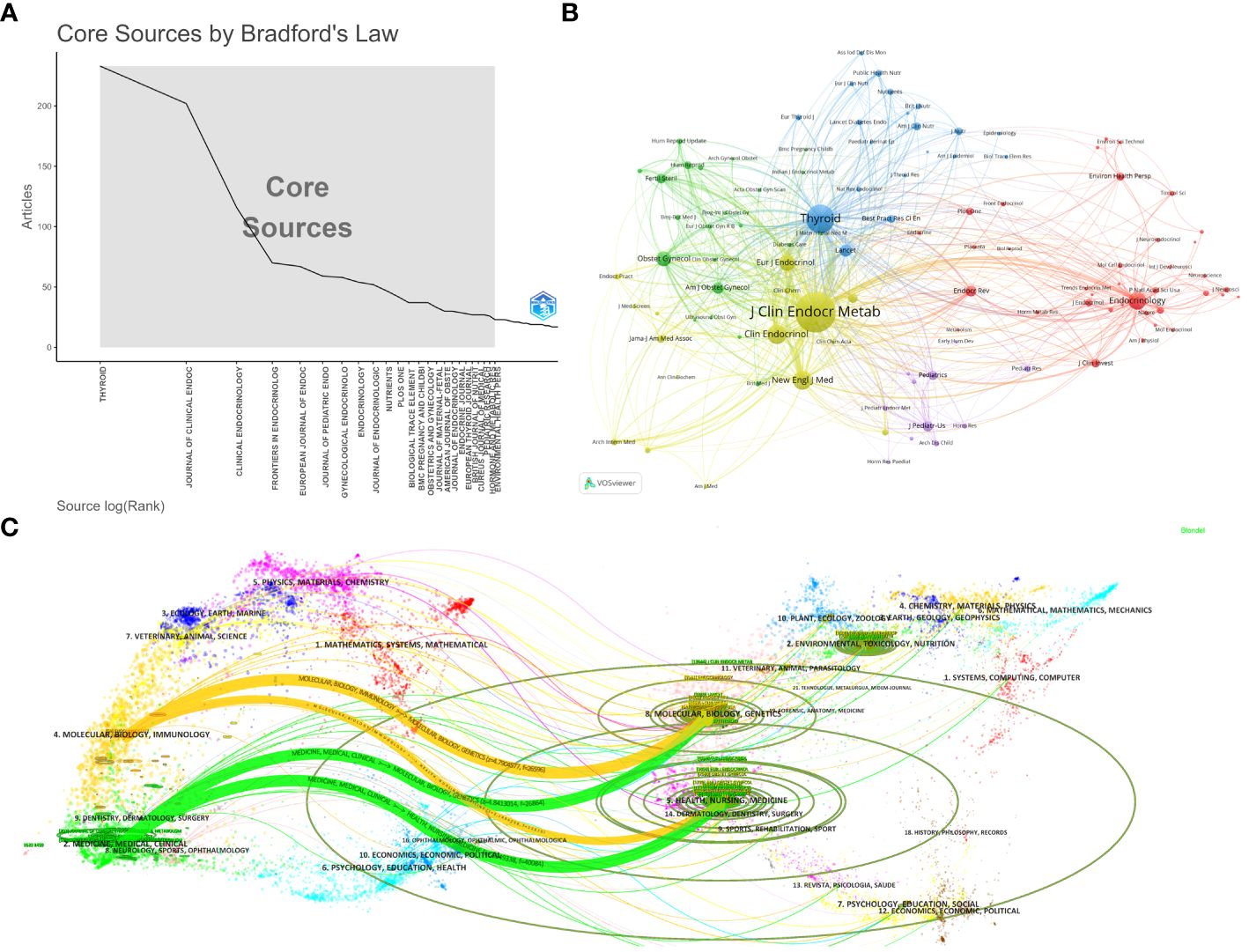
Figure 4 Analysis of journal sources. (A) The distribution of journal sources according to Bradford’s Law. (B) Co-citation analysis of cited sources regarding Maternal Hypothyroidism. (C) Dual-Map overlay of journals publishing research on Maternal Hypothyroidism.
Figure 4C, a dual-map overlay of journals, reveals the distribution of topics. Citing journals are on the left, cited journals on the right. Labels indicate the disciplines covered by the journals, and colored paths represent citation relationships. Four major paths stand out. The orange and green citation paths suggest that research from Molecular, Biology, Genetics journals and Health, Nursing, Medicine journals is often cited by Molecular/Biology/Immunology journals or Medicine/Medical/Clinical journals.
Of the 4184 articles, they have been cited a total of 114378 times, with a median citation count of 10 times. Table 6 and Figure 5A lists the top 10 most cited articles in the field of maternal hypothyroidism, ranging from 536 to 2694 citations. The article “Serum TSH, T (4), and thyroid antibodies in the USA population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III),” published in 2002 in the “Journal of Clinical Endocrinology & Metabolism,” stands out with the highest citation count (2694 citations). The article “2017 Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease During Pregnancy and the Postpartum,” published in 2017 in “Thyroid,” has the highest average yearly citation count (178.14).
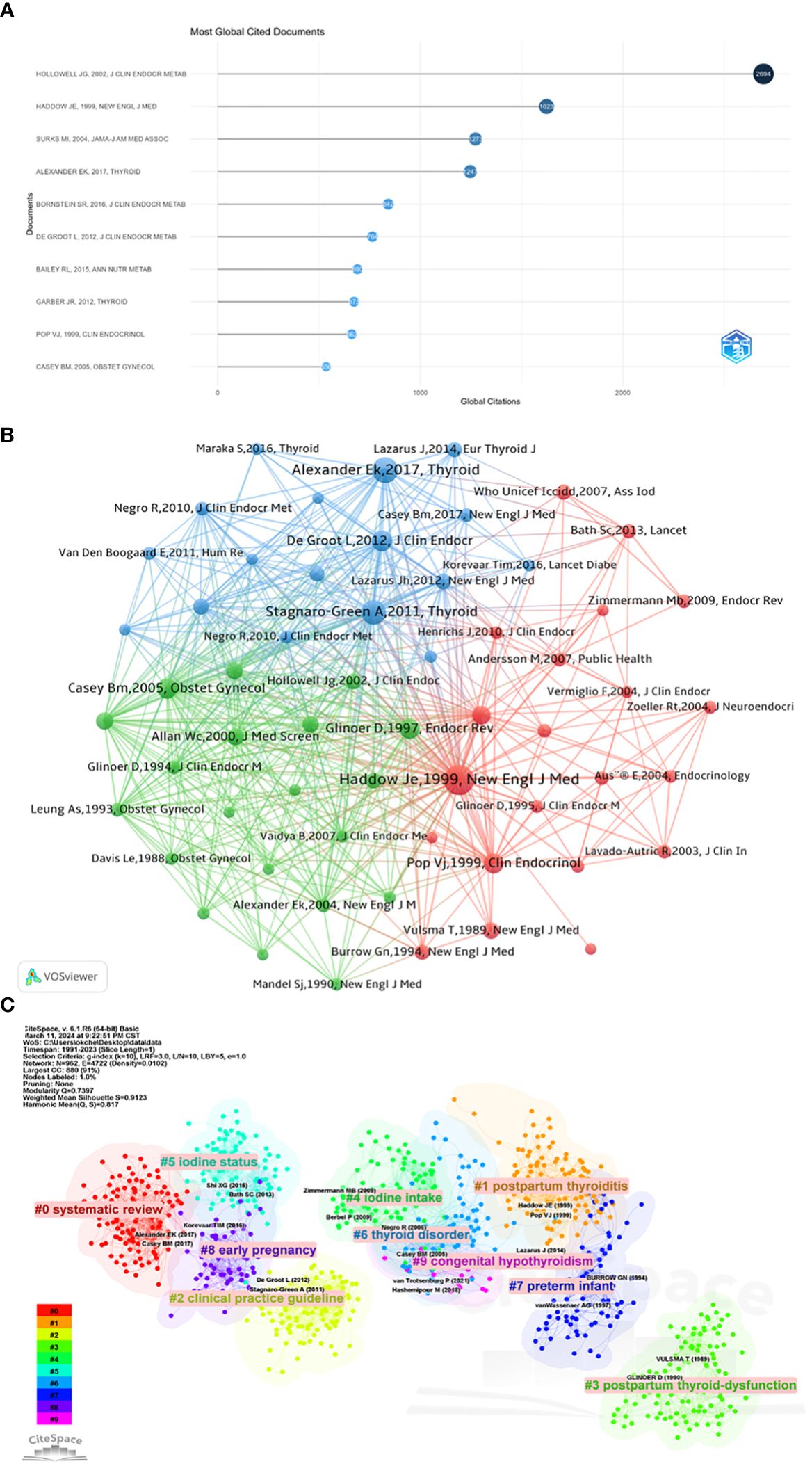
Figure 5 Top 10 highly cited references and co-cited references. (A) Top 10 most cited articles in Maternal Hypothyroidism research. (B) Co-citation network analysis of most cited references. (C) Cluster analysis of co-cited references.
The top 10 co-cited references in the Maternal Hypothyroidism field are listed in Table 7. The most co-cited article is “Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child” (699 citations), followed by “2017 Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease During Pregnancy and the Postpartum” (507 citations). The third most co-cited article is “Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease During Pregnancy and Postpartum” (463 citations).
It is noteworthy that five articles among the top 10 co-cited references also appear in the top 10 highly cited articles. These articles, including “Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child,” “2017 Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease During Pregnancy and the Postpartum,” “Management of Thyroid Dysfunction during Pregnancy and Postpartum: An Endocrine Society Clinical Practice Guideline,” “Subclinical hypothyroidism and pregnancy outcomes,” and “Low maternal free thyroxine concentrations during early pregnancy are associated with impaired psychomotor development in infancy,” underscore their paramount importance and influence in the field. Widely acknowledged and frequently cited, these articles collectively serve as cornerstone contributions to Maternal Hypothyroidism research.
Figure 5B displays the co-citation network analysis of the most frequently cited references. The size of nodes represents the total number of citations of references, and the thickness of lines connecting two nodes represents the strength of co-citation relationships. The color of nodes represents the clusters to which the articles belong. Each reference in Figure 5B is represented by its authors, publication year, and journal. VOSviewer parameters were set as follows: Method (association strength), Minimum number of citations of a cited reference: 100. We retrieved 77162 cited references, of which 58 meet the threshold. Figure 5B shows three clusters: red, blue, and green. The first cluster (red) primarily focuses on the prevention and control of iodine deficiency in pregnant and lactating women, and how this deficiency affects maternal thyroid function and neurodevelopment of children. For example, the study by Haddow, et al. (20) focuses on “Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child.” The second cluster (blue) mainly focuses on the diagnosis, management, and impact on maternal and infant health of maternal hypothyroidism during pregnancy. Alexander, E.K., et al., participated in the 2017 American Thyroid Association guidelines for the diagnosis and treatment of thyroid diseases during pregnancy and postpartum, providing valuable references for healthcare professionals to accurately diagnose and manage thyroid diseases during pregnancy and postpartum. Cluster 3 (green) primarily focuses on the impact and management of clinical hypothyroidism and subclinical hypothyroidism during pregnancy. It covers a range of topics from the regulation of thyroid function during pregnancy to the effects of hypothyroidism on pregnant women and fetuses, to screening and management strategies for these conditions.
We further employed CiteSpace for visualizing co-cited references, as illustrated in Figure 5C. The time span was set from 1991 to 2023, with a time slice of 1 year, focusing on reference nodes. We applied the selection criterion (g-index k=10). The resulting network comprises 962 nodes and 4722 links. The top 10 largest clusters include: systematic review, clinical practice guideline, postpartum thyroid dysfunction, iodine intake, iodine status, thyroid disorder, preterm infant, thyroid disorder, congenital hypothyroidism.
Utilizing VOSviewer with parameters set to Method (Association Strength) and a minimum keyword occurrence of 50, a total of 9952 keywords were identified, with 111 meeting the specified threshold. The keyword co-occurrence network map (Figure 6A) illustrates the varying frequencies of simultaneous keyword appearances. Among the top 50 keywords, four distinct clusters were identified based on co-occurrence rates. In Figure 6A, the red cluster, led by “Hypothyroidism,” primarily encompasses keywords such as “Thyroid Hormone,” “Congenital Hypothyroidism,” “Thyroxine,” “Hormone,” “Hypothyroxinemia,” “Early-Pregnancy,” “Expression,” “Thyrotropin,” and “Infant.” The green cluster predominantly includes keywords like “Women,” “Disease,” “Subclinical Hypothyroidism,” “Management,” “Dysfunction,” “Association,” “Diagnosis,” “Risk,” “Prevalence,” and “Guidelines.” The blue cluster comprises keywords such as “Pregnancy,” “Deficiency,” “Thyroid Function,” “Children,” “Iodine Deficiency,” “Iodine,” “Thyroid,” “Pregnant Women,” “Population,” and “Nutrition.” Lastly, the yellow cluster includes keywords like “Hyperthyroidism,” “Fetal,” “Graves’ Disease,” “Therapy,” “Thyrotoxicosis,” “Follow-Up,” “Propylthiouracil,” “Methimazole,” and “Thyroid Disease.”
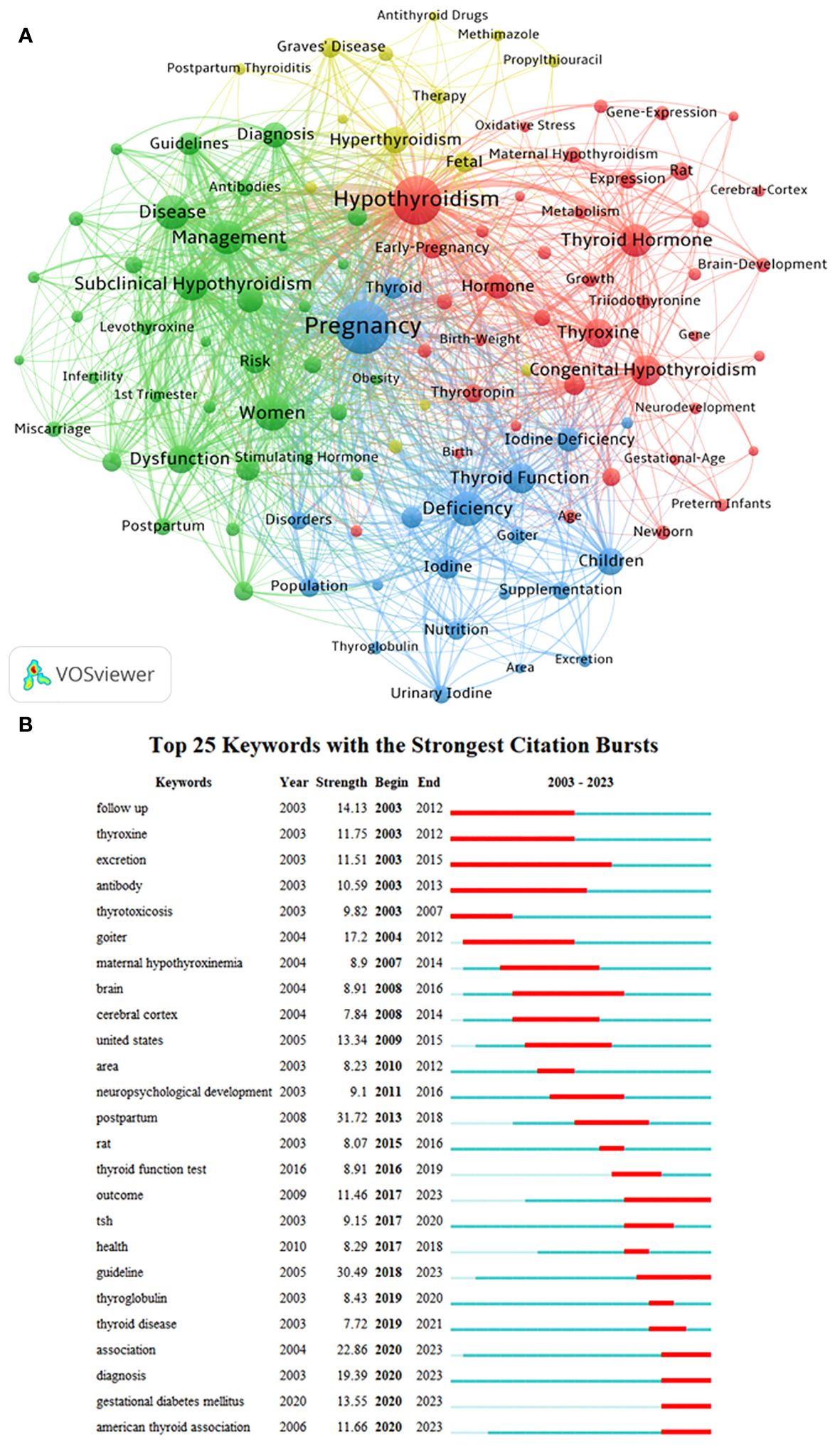
Figure 6 Analysis of keywords associated with Maternal Hypothyroidism. (A) Clustering of key terms. (B) Burst detection of keywords.
The burst detection of keywords over the past 20 years reveals the most impactful terms in different periods (Figure 6B). “Postpartum” emerged as a prominent research focus from 2013 to 2018, displaying the highest burst strength (31.72). Following closely are bursts related to “Guideline” (30.49), “Association” (22.86), “Diagnosis” (19.39), “Goiter” (17.2), “Follow-Up” (14.13), “Gestational Diabetes Mellitus” (13.55), “United States” (13.34), “Thyroxine” (11.75), and “American Thyroid Association” (11.66). Examining the start times of these bursts indicates that terms like “Follow-Up,” “Thyroxine,” “Excretion,” “Antibody,” “Thyrotoxicosis,” and “Goiter” drew attention over a decade ago. On the other hand, “Outcome,” “Guideline,” “Gestational Diabetes Mellitus,” “American Thyroid Association,” “Association,” and “Diagnosis” represent recent frontiers in Maternal Hypothyroidism research.
We further conducted Trend Topics analysis using the R language bibliometrix package to perform time series analysis of keywords in the Maternal Hypothyroidism literature dataset, aiming to identify evolving research trends and topics over time. In Figure 7A, the plotted data illustrates the evolution of various topics over time. Each line represents the lifecycle of a topic from emergence, peak, to decline. It is noteworthy that some topics have short spans (thin lines), indicating rapid fluctuations in interest, while others exhibit longer relevancy (long lines). Top Topics from 1992-2002: Onset, 3,5,3’-triiodo-L-thyronine, Neonatal rat, Fetal brain-development, Embryonic-tissues. From 2002-2012, Top Topics: Thyroxine, Graves-disease, Therapy, Thyrotoxicosis, Metabolism. From 2012-2022, Top Topics: Pregnancy, Hypothyroidism, Women, Disease, Deficiency. From 2022-2032, Top Topics: Association, Perinatal outcomes, Diagnosis, Guidelines, Evolution.
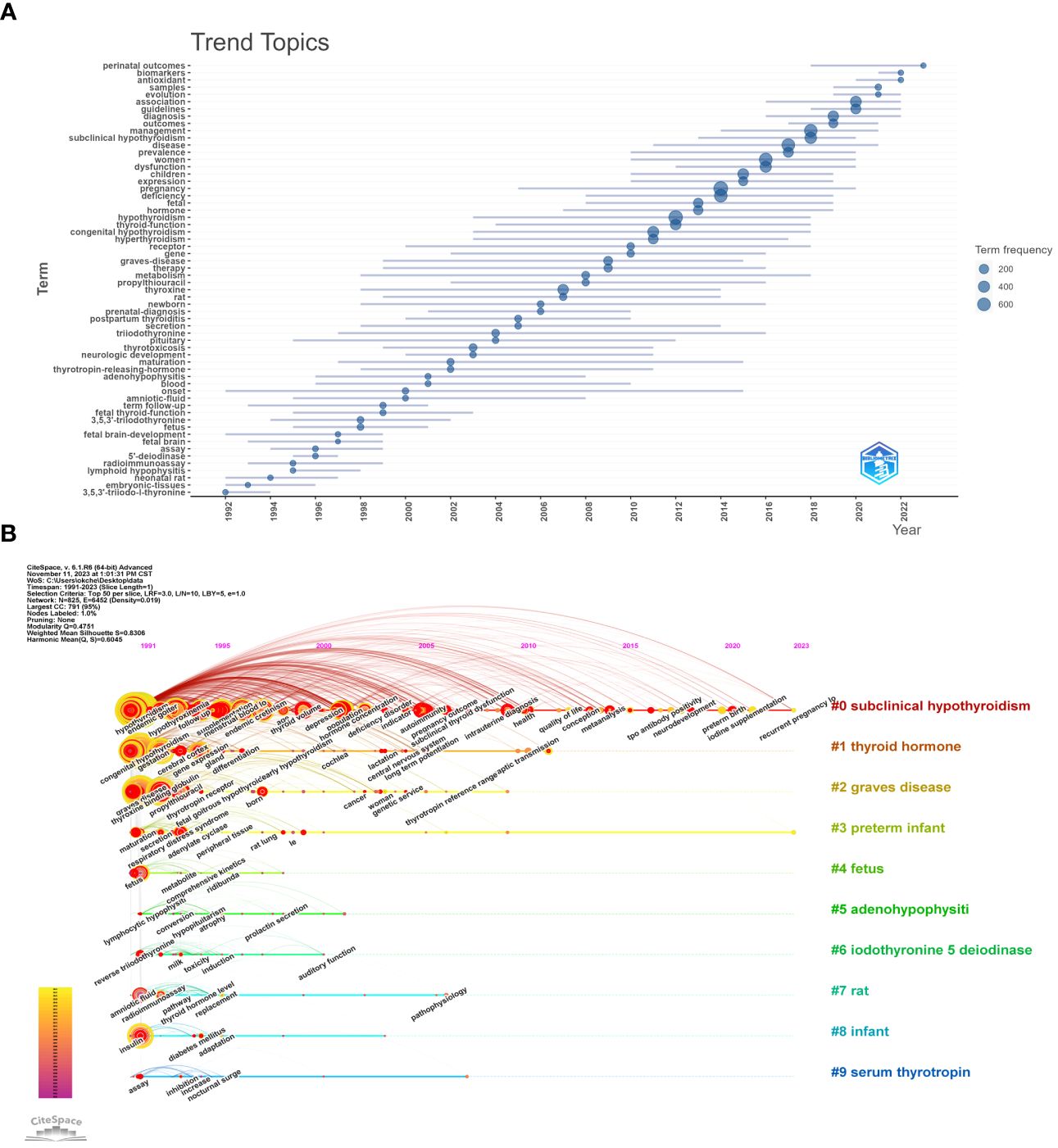
Figure 7 Trend analysis and Timeline viewer of Maternal Hypothyroidism topics. (A) Trend topics analysis. (B) Timeline viewer related to Maternal Hypothyroidism.
The timeline viewer in CiteSpace is also utilized to depict the evolving trends of research fields over time. Configured with parameters set to a time slice (1991–2023), one year per slice, node type (keywords), selection criteria (top N=50), and no pruning, it generated a network with 825 nodes, 6452 connections, and a density of 0.019 (Figure 7B). The top 10 clusters include “Subclinical Hypothyroidism,” “Thyroid Hormone,” “Graves Disease,” “Preterm Infant,” “Fetus,” “Adenohypophysiti,” “Iodothyronine 5 Deiodinase,” “Rat,” “Infant,” and “Serum Thyrotropin.” The keywords prevalent in 1991 and 2000 revolved around “Pregnancy,” “Hypothyroidism,” “Disease,” “Women,” “Deficiency,” “Subclinical Hypothyroidism,” “Management,” “Thyroid Hormone,” “Congenital Hypothyroidism,” and “Thyroid Function.” In the years 2020-2022, emerging keywords include “Gestational Diabetes Mellitus,” “Urinary Iodine Concentration,” “Preterm Birth,” “Sample,” “Iodine Supplementation,” “Obesity,” “Physiology,” “Recurrent Pregnancy Loss,” “Trimester,” “Small for Gestational Age,” and “Perinatal Outcome.”
In this study, we employed CiteSpace, VOSviewer, and bibliometrix analysis software to examine the literature related to Maternal Hypothyroidism, providing a comprehensive review of research achievements and advancements. As shown in the schematic diagram in Figure 8, we conducted a quantitative analysis of basic information such as annual publication count, country, institution, author, discipline, and journal. The analysis covered 4,184 articles published since 1991, accumulating a total citation count of 114,378, indicating a rising trend in the field. Researchers from 113 countries/regions across six continents participated in Maternal Hypothyroidism studies, underscoring the global significance and relevance of this topic to public health worldwide.
The leading nations in Maternal Hypothyroidism research were identified as the USA, China, England, and the Netherlands. Among the top 10 institutions, three were from the USA and two from the Netherlands, with China Medical University having the highest number of published papers and Harvard University receiving the highest citation count. Collaborations between countries and institutions were notably close, fostering a conducive environment for academic collaboration and facilitating further research in the field of Maternal Hypothyroidism.
Among the top 10 authors, Professor Teng, Weiping, with 52 published papers (1.243%), emerged as the most prolific, followed by Shan, Zhongyan (51 papers, 1.219%) and Pearce, Elizabeth N. (50 papers, 1.195%). This indicates a sustained and in-depth commitment to Maternal Hypothyroidism research by these three authors. Professors Peeters, Robin P. and Visser, Theo J. from Erasmus University Medical Center had the highest H-indices, signifying their significant contributions to the field. The collaboration between Professor Teng, Weiping, and Professor Shan, Zhongyan, focused on exploring the associations between maternal thyroid health during pregnancy in China and its correlation with offspring health. They investigated the potential impact and underlying mechanisms of maternal hypothyroidism, nutritional iodine (21), iron (22, 23), environmental factors (polybrominated diphenyl ethers) (24), and autoimmunity (23) on thyroid function during pregnancy, miscarriage (25), offspring neurodevelopment (26–28), cognition (29), and related disease mechanisms. They provided valuable insights for maternal health during pregnancy in China (30, 31).
Professor Pearce, Elizabeth N. concentrated on the diagnosis, management, and iodine nutrition of thyroid diseases during pregnancy and postpartum. She actively participated in formulating the 2017 American Thyroid Association guidelines for the diagnosis and management of thyroid diseases during pregnancy and postpartum, contributing to the standardization and regulation of this field (32). Her extensive research covered global and regional iodine nutrition status (33), including pregnant women (34) and iodine nutrition during breastfeeding (35). She investigated the impact of environmental pollutants such as perchlorate and thiocyanate on thyroid function in pregnant women (36). Furthermore, she explored the relationships between maternal thyroid function and complications during pregnancy, such as preterm birth (13), pregnancy-induced hypertension, and preeclampsia (37). Professor Pearce, Elizabeth N. proposed a series of clinical practice and research recommendations for thyroid diseases during pregnancy and lactation, providing crucial references for healthcare professionals and researchers.
Professors Visser, Theo J., and Peeters, Robin P., collaborated extensively, focusing on the association between maternal thyroid function and pregnancy complications [preeclampsia (11, 38), pregnancy-induced hypertension (10), and preterm birth (13, 39)], placenta (40), and offspring health [birth weight (41), attention deficit/hyperactivity disorder (42), cognition (43), intelligence and brain morphology (44), neurodevelopment, and behavioral issues (45, 46)]. Their research delved into various factors influencing thyroid function during pregnancy, such as thyroid autoimmunity (47), iodine levels (48), and their effects on the mother and fetus. They conducted studies on the reference range of childhood thyroid function and reviewed the determinants of thyroid function through literature and prospective cohort studies (49). Their research guided the monitoring of thyroid function and iodine intake in pregnant women, aiming to improve pregnancy outcomes and promote healthy offspring development. They made significant contributions to the diagnosis, management, prevention, and treatment of thyroid diseases during pregnancy and postpartum.
Further, we conducted co-citation analysis using the cited authors as units of analysis. The top five authors with the most citations are Glinoer, Daniel; Zimmermann, Mb; Haddow, Je; Stagnaro-Green, A; and Negro, R. Professor Glinoer’s research primarily focuses on the intersection of thyroid function, pregnancy, and reproductive health, including thyroid regulation and dysfunction during pregnancy and their management (50–52), autoimmune thyroid diseases and reproductive health (53), iodine nutrition and thyroid health (54), thyroid hormones and pregnancy outcomes, interventions, and recommendations for thyroid disease management during pregnancy (55, 56). Their work aims to improve clinical practice (57), particularly in the context of thyroid-related issues. Professor Zimmermann’s research primarily focuses on nutritional deficiencies, particularly iodine (58, 59) and iron (60), and their impact on public health (61), focusing on nutritional status assessments in pregnant women, infants, and children in different geographical settings (62, 63). His research deepens the understanding of the impact of iodine and iron deficiencies on public health, informs strategies to alleviate these deficiencies, and influences policies and guidelines to improve global nutrition status and health outcomes. Professor Haddow’s research primarily focuses on prenatal screening and maternal health, particularly addressing hypothyroidism, Down syndrome, trisomy syndromes, cystic fibrosis, and the impact of environmental factors such as tobacco smoke on child development. Works related to Maternal Hypothyroidism include the effects of maternal hypothyroidism on child neurodevelopment (20, 64, 65), reference ranges and individual variations in thyroid-stimulating hormone levels in early to mid-pregnancy (66), and the importance of adequate maternal iodine intake and its impact on fetal outcomes (67, 68). Professor Stagnaro-Green, A’s research primarily focuses on thyroid diseases during pregnancy and their impact on maternal and infant health. His contributions can be categorized into several key areas: diagnosis and management of thyroid diseases during pregnancy and postpartum (69, 70), evaluating the effectiveness and cost-effectiveness of universal screening and targeted case finding for thyroid disorders in pregnant women (71), aimed at optimizing outcomes for mothers and offspring, prevalence of thyroid diseases during pregnancy (7), and impact on pregnancy complications (72–74). Professor Negro, R’s research primarily focuses on thyroid diseases during pregnancy and their management, emphasizing understanding the impact of thyroid function and autoimmune thyroid diseases on pregnancy outcomes. He has made significant contributions to establishing and updating guidelines for the diagnosis and management of thyroid diseases during pregnancy and postpartum (69, 75, 76). This includes developing clinical practice guidelines to aid in identifying, treating, and monitoring thyroid dysfunction in pregnant and postpartum women. These authors hold significant positions in the co-citation network and collaborate closely.
In terms of disciplinary distribution, as outlined in Table 5, more than one-third of the articles on Maternal Hypothyroidism have been published in the field of Endocrinology Metabolism (1,531 articles, 36.592%). The journal “Thyroid” has emerged as the primary outlet for articles related to Maternal Hypothyroidism, with 233 publications (5.57%), followed by the “Journal of Clinical Endocrinology & Metabolism” (202 publications, 4.83%), and “Clinical Endocrinology” (116 publications, 2.77%). Among the top 10 journals in the field of Maternal Hypothyroidism, six are positioned within the JCR Q1 zone. Analyzing the distribution of literature sources is instrumental in identifying core journals for the publication of Maternal Hypothyroidism-related papers, aiding future scholars in selecting appropriate outlets for their research. Figure 4C, presenting the dual-map overlay of journals in Molecular Biology Genetics and Health Nursing Medicine research, indicates that studies in Maternal Hypothyroidism are not only frequently cited in Molecular/Biology/Immunology journals but also extensively referenced in Medicine/Medical/Clinical journals. This implies that research on maternal hypothyroidism involves in-depth exploration at the molecular and biological levels and is directly relevant to medical, healthcare, and clinical practices. This comprehensiveness likely renders these studies appealing to scholars and professionals in different fields, resulting in frequent citations across various journals. The majority of the top 10 cited literature predominantly revolves around clinical practice guidelines, primarily because these guidelines provide specific treatment protocols and decision support for healthcare practitioners. Grounded in extensive research and expert consensus, these guidelines are widely acknowledged, authoritative, and consequently, extensively cited.
The analysis of high-frequency keywords and emergent terms reflects the hotspots and frontiers of a specific research field. Through keyword co-occurrence analysis, we identified the primary directions and hotspots of Maternal Hypothyroidism, revealing the development and evolution of its thematic structure. VOSviewer’s keyword clustering analysis ultimately yielded four clusters represented by distinct colors:
The red cluster’s keywords predominantly focus on the association between maternal hypothyroidism in pregnant women and offspring birth weight, intelligence quotient, attention-deficit/hyperactivity disorder (ADHD), cognition, intelligence, brain morphology, neural development, and behavioral issues. Mechanisms, physiological processes, and environmental exposures are also implicated. Insufficient maternal thyroid hormones may lead to intrauterine growth retardation, potentially resulting in low birth weight (77). Thyroid hormones play a crucial role in fetal brain development, and maternal hypothyroidism may impact normal development, increasing the risk of intellectual disabilities in offspring (78), diminished learning abilities (79), and other neurological issues. Some studies suggest a link between maternal hypothyroidism and an increased risk of autism (80), ADHD (81), and other neurodevelopmental problems in offspring. Hypothyroidism during pregnancy may elevate the risk of metabolic disorders in children (82). These advancements underscore the potential impact of Maternal Hypothyroidism on various aspects of offspring health. Ongoing research aims to comprehensively understand this relationship, providing more accurate guidance for clinical practice and prenatal management.
The green cluster’s co-occurring keywords center around the diagnosis, management, guidelines, treatment, risk assessment, autoimmune aspects, and various types of pregnancy complications related to maternal hypothyroidism. These complications include preterm birth, gestational hypertension, preeclampsia/eclampsia, gestational diabetes, miscarriage, and placental abruption. Preterm birth is a common complication of maternal hypothyroidism (83). Studies indicate a close association between subclinical hypothyroidism, isolated hypothyroidism, positive thyroid peroxidase antibodies (TPOAb), and the risk of preterm birth. Isolated hypothyroidism and positive TPOAb are more significantly linked to severe preterm birth (13). Furthermore, hypothyroidism may lead to metabolic abnormalities and cardiovascular dysfunction, increasing the risk of preeclampsia/eclampsia. Dominant hypothyroidism is significantly correlated with severe preeclampsia risk (84). Subclinical hypothyroidism is associated with a higher risk of preeclampsia (37), whereas isolated hypothyroidism or positive TPOAb shows no clear association with gestational hypertension or preeclampsia (11). Some studies suggest that the risk of preeclampsia-eclampsia in women with isolated hypothyroidism increases with the severity of hypothyroidism (85). Hypothyroidism also increases the risk of gestational diabetes (86). Increased thyroid-stimulating hormone levels (87) and subclinical hypothyroidism are associated with an increased risk of gestational diabetes (88). The early levels of free T4 are associated with gestational diabetes, and as the levels increase, the incidence of gestational diabetes decreases (12). Miscarriage risk is also associated with thyroid dysfunction. Women with subclinical hypothyroidism and thyroid autoimmunity have an increased risk of miscarriage during weeks 4-8 of pregnancy, especially those with both conditions, experiencing higher risks and earlier gestational ages (25). Placental abruption, a severe pregnancy complication, is associated with subclinical hypothyroidism and isolated maternal hypothyroidism (89). In 2005, Casey et al. found that at 20 weeks of pregnancy, subclinical hypothyroidism patients had a threefold increase in the risk of placental abruption and nearly a twofold increase in the risk of preterm birth (90). Studies emphasize autoimmune thyroid disease (AITD), particularly Hashimoto’s disease, which is often accompanied by the production of thyroid antibodies. Pregnant women with AITD have an increased postpartum demand for levothyroxine compared to the pre-pregnancy period (91). Researchers strive to optimize treatment strategies during pregnancy in cases of AITD, addressing how to maintain optimal thyroid function and reduce adverse effects on both patients and infants.
Research on Maternal Hypothyroidism focuses on early screening and diagnosis of thyroid function in pregnant women to ensure the timely detection and management of potential thyroid dysfunction (92). Through large-scale studies, researchers have conducted a more detailed analysis of the dynamic changes in thyroid hormone levels during pregnancy to better understand the fluctuations in thyroid hormones (93). Researchers have compared and analyzed reference values for thyroid hormones during pregnancy in different regions, populations, and gestational weeks to establish more adaptive reference ranges for various populations (94–97). Attention has been given to the relationship between thyroid hormone levels during pregnancy and pregnancy outcomes (such as preterm birth and low birth weight) (98), aiming to establish more suitable reference ranges (32). This ensures a more accurate reflection of situations that pose risks to maternal and infant health. Additionally, researchers have established thyroid hormone reference ranges applicable to infants in different seasons, regions, races, and circumstances, including preterm birth, through large-scale cross-sectional and longitudinal studies (49, 99–101). These efforts aim to ensure a more accurate reflection of the normal physiological state of this age group. Studies emphasize the correlation between thyroid hormone levels in infants and aspects such as intelligence and growth (102), contributing to a deeper understanding of the impact of thyroid hormones on child development. In summary, these studies contribute to a more accurate determination of normal thyroid hormone ranges during pregnancy and infancy in different contexts. They underscore the importance of establishing accurate thyroid hormone reference values for clinical management, especially in high-risk pregnant women, to detect and address potential thyroid issues early and provide more precise assessment and management for the health of both pregnant women and fetuses.
For a long time, the safety of thyroid hormone replacement therapy has been a focal point. Pregnant women with hypothyroidism need to increase levothyroxine (T4) dosage (an average increase of 50%), with this increased dosage mainly occurring in the first half of pregnancy and stabilizing by the 16th week of pregnancy (103). However, both high and low maternal FT4 concentrations are associated with lower child IQ, suggesting that overcorrection of maternal hypothyroidism may itself have adverse effects (44). In recent years, progress has been made regarding the safety of thyroid hormone replacement therapy, including studies on different dosages (104, 105). Research also emphasizes adjusting thyroid hormone dosage and administration methods based on the specific circumstances of patients (106). Additionally, some studies are dedicated to developing new types of thyroid hormone medications to improve the drawbacks of traditional thyroid hormone replacement therapy, such as enhancing formulations (107). Several large clinical trials have confirmed the effectiveness of thyroid hormone replacement therapy in the treatment of hypothyroidism, making it the standard treatment method (108, 109). These studies demonstrate that thyroid hormone replacement therapy significantly improves patients’ quality of life and alleviates symptoms associated with hypothyroidism. Recent research has also explored the combined use of levothyroxine (T4) and triiodothyronine (T3), indicating that monotherapy with levothyroxine (T4) is the standard treatment, and there is not enough evidence to widely recommend combination therapy (110).
Research has also delved into the management and treatment strategies for hypothyroidism during pregnancy to ensure the maintenance of appropriate thyroid hormone levels and reduce the risk of adverse effects on both mother and infant (111). Updates and revisions to clinical practice guidelines provide more accurate and up-to-date diagnostic and treatment recommendations, enabling healthcare professionals to better manage hypothyroidism during pregnancy (32).
The co-occurrence clustering in the blue region highlights various themes in the research on Maternal Hypothyroidism, including thyroid function during pregnancy, iodine deficiency, potential impacts on offspring health, and regional disparities. Iodine is a crucial element for thyroid hormone synthesis (112), and some studies emphasize the importance for pregnant women to maintain sufficient iodine intake to prevent thyroid issues caused by iodine deficiency (32). Severe iodine deficiency in pregnant women is associated with adverse pregnancy outcomes (113, 114). Additionally, iodine plays a critical role in the development of the fetal brain and nervous system (21). Iodine deficiency in pregnant women and fetuses can have detrimental effects on cognitive function in the offspring (115, 116). Both insufficient (112) and excessive (117) iodine intake may lead to maternal hypothyroidism. Researchers have explored the relationship between iodine intake and maternal thyroid hormone levels, with adequate iodine intake considered helpful in maintaining normal thyroid function (118, 119) Multiple studies focus on monitoring postpartum mothers’ iodine nutrition status (120–122), aiding in the formulation of iodine supplementation strategies and providing guidance on iodine intake recommendations. Overall, researchers have conducted in-depth investigations into the relationship between iodine and Maternal Hypothyroidism to promote effective management of the health of pregnant and postpartum women. These studies contribute to guiding public health policies and providing more comprehensive advice for antenatal care.
The co-occurrence clustering in the yellow region points to various themes in the research on maternal thyroid dysfunction, including hyperthyroidism-related disorders. These themes encompass “treatment methods, fetal effects”, “epidemiological data”, and “therapeutic drugs”, focusing on understanding the impact of maternal thyroid dysfunction on maternal and infant health and providing relevant guidance and recommendations for clinical practice. Keywords such as “Hyperthyroidism”, “Thyrotoxicosis”, “Graves’ Disease”, “Postpartum Thyroiditis”, “Antithyroid Drugs” involve maternal thyroid dysfunction and corresponding treatment methods like antithyroid drugs (such as methimazole, propylthiouracil). “Fetal”, “Mothers”, “Human Chorionic-Gonadotropin” indicate the potential effects of maternal thyroid dysfunction on the fetus and the related physiological mechanisms. “Epidemiology”, “Follow-Up” suggest research on the epidemiology of maternal thyroid dysfunction and patient follow-up.
Subsequently, we identified the research focal points and frontiers of Maternal Hypothyroidism through an analysis of the Timeline viewer related to Maternal Hypothyroidism and the top 25 keywords with the most substantial citation bursts. The primary research focal points are as follows (1):Pregnancy and postpartum outcomes: Research places emphasis on postpartum and pregnancy outcomes, considering the impact of thyroid disorders on maternal and infant health (2).Diagnosis and guidelines: The roles of diagnosis and guidelines are pivotal in managing thyroid disorders during pregnancy (3).Thyroid hormones and function: The influence of thyroxine concentration and thyroid dysfunction (thyrotoxicosis, hypothyroidism) on maternal health (4).United States research and collaborations: United States research and international cooperation (meta-analysis, American Thyroid Association) hold significant positions in the studies (5).Rodent models and physiology: Keywords such as adult rat and physiology are commonly observed, underscoring the importance of animal models in research (6).Thyroid disorders and autoimmune diseases: Crucial aspects of the research encompass thyroid disorders, including autoimmune thyroiditis and Graves’ disease. The forefront trends include (1):Neurological system and cognitive development: Research delves deeper into the relationship between pregnancy, thyroid dysfunction, and the brain, as well as neuropsychological development (2).Biomarkers and health outcomes: Biomarkers such as antibodies and thyroglobulin play a significant role in disease prognosis and health outcomes (3).Models and brain regions: Key areas such as the cerebral cortex, dentate gyrus, and adult rodent models hold substantial importance in research (4).Gestational diabetes and health: There is a close association between gestational diabetes mellitus and health in Maternal Hypothyroidism research.
Further Trend Topics analysis revealed significant evolution in the hot topics within the field of Maternal hypothyroidism from 1992 to 2032. Early years (1992–2002) primarily focused on basic biology such as 3,5,3’-Triiodo-L-thyronine, neonatal rats, and fetal brain development. Subsequently (2002–2012), research shifted towards specific health issues like thyroid hormones, Graves’ disease, and their treatments. In recent years (2012–2022), women’s health issues such as pregnancy and hypothyroidism have become research hotspots. Looking ahead, there is expected emphasis on diagnostics, guideline development, and the association between diseases and antioxidants. This evolution highlights the dynamism of scientific research and its response to new technologies and societal needs. As scientific exploration deepens, understanding of health and diseases is progressing towards a more comprehensive and preventative direction.
This study marks the pioneering application of bibliometric analysis to the Maternal Hypothyroidism field, providing a fresh perspective and aiding a profound understanding of its developmental trends. In contrast to traditional literature reviews, our study employed various bibliometric analysis tools, including CiteSpace, VOSviewer, and the R software package bibliometrix. The integrated use of these tools enables a comprehensive and systematic extraction and analysis of data, thereby revealing the dynamic research landscape of the Maternal Hypothyroidism field. However, limitations exist, primarily reflected in the data source being restricted to the WoSCC database, potentially leading to the oversight of relevant information in other databases. Furthermore, while bibliometric analysis can unveil the impact of literature, they often cannot independently assess the quality of each study due to factors like citation counts being influenced by time. Despite these limitations, the bibliometric analysis employed in this study still offer valuable insights into the research trends of the Maternal Hypothyroidism field and provide new avenues for future research directions. To further enhance the analysis of this research field, future studies may consider integrating multiple databases and analysis methods to gain more comprehensive and in-depth insights.
This study conducted an in-depth bibliometric analysis of Maternal Hypothyroidism to explore thematic development and future research focal points. We provided foundational information for researchers interested in the field and identified potential collaborators. Overall, Maternal Hypothyroidism research has historically focused on thyroid function diagnosis during pregnancy, treatment guidelines, iodine nutrition, the correlation between maternal and offspring health, and postpartum aspects. Recently, the research has shifted towards gestational diabetes, iodine intake, preterm birth, obesity, and the impact of iodine supplementation on postpartum outcomes, indicating these areas as crucial directions for future research. Simultaneously, sustained attention to subclinical hypothyroidism, thyroid hormones, Graves’ disease, and related disorders will continue to be research focal points. In summary, the bibliometric analysis of Maternal Hypothyroidism underscores the sustained importance of maternal thyroid health during pregnancy and postpartum. The shift towards emerging topics like gestational diabetes, iodine intake, and preterm birth opens new directions for future research, emphasizing the multifaceted impact of Maternal Hypothyroidism on maternal and infant health.
AC: Investigation, Data curation, Writing – original draft. ZL: Writing – original draft, Data curation. JZ: Writing – review & editing. XC: Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Top Talent Support Program for young and middle-aged people of Wuxi Health Committee (No. BJ2020079; BJ2023076) and the Scientific Research Program of Wuxi Health Commission (M202107).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
ADHD, attention-deficit/hyperactivity disorder; AITD, autoimmune thyroid disease; FT4, Free Thyroxine 4; TSH, thyroid-stimulating hormone; TPOAb, thyroid peroxidase antibodies; WoSCC, Web of Science Core Collection.
1. Chaker L, Razvi S, Bensenor IM, Azizi F, Pearce EN, Peeters RP. Hypothyroidism. Nat Rev Dis Primers. (2022) 8:30. doi: 10.1038/s41572-022-00357-7
2. Mallawa KO, Zhou Q, Li X. Understanding the pathogenesis of gestational hypothyroidism. Front Endocrinol (Lausanne). (2021) 12:653407. doi: 10.3389/fendo.2021.653407
3. Alemu A, Terefe B, Abebe M, Biadgo B. Thyroid hormone dysfunction during pregnancy: A review. Int J Reprod BioMed. (2016) 14:677–86. doi: 10.29252/ijrm.14.11.677
4. Visser WE, Peeters RP. Interpretation of thyroid function tests during pregnancy. Best Pract Res Clin Endocrinol Metab. (2020) 34:101431. doi: 10.1016/j.beem.2020.101431
5. Rodriguez-Diaz E, Pearce EN. Iodine status and supplementation before, during, and after pregnancy. Best Pract Res Clin Endocrinol Metab. (2020) 34:101430. doi: 10.1016/j.beem.2020.101430
6. Lopez-Munoz E, Mateos-Sanchez L, Mejia-Terrazas GE, Bedwell-Cordero SE. Hypothyroidism and isolated hypothyroxinemia in pregnancy, from physiology to the clinic. Taiwan J Obstet Gynecol. (2019) 58:757–63. doi: 10.1016/j.tjog.2019.09.005
7. Dong AC, Stagnaro-Green A. Differences in diagnostic criteria mask the true prevalence of thyroid disease in pregnancy: A systematic review and meta-analysis. Thyroid. (2019) 29:278–89. doi: 10.1089/thy.2018.0475
8. AlGhalayini K. Prevalence of hypothyroidism in a cohort of Saudi women with heart failure and effect on systolic and diastolic function. J Pak Med Assoc. (2015) 65:1300–04.
9. Jolving LR, Nielsen J, Kesmodel US, Nielsen RG, Beck-Nielsen SS, Norgard BM. Prevalence of maternal chronic diseases during pregnancy - a nationwide population based study from 1989 to 2013. Acta Obstet Gynecol Scand. (2016) 95:1295–304. doi: 10.1111/aogs.13007
10. Medici M, Korevaar TI, Schalekamp-Timmermans S, Gaillard R, de Rijke YB, Visser WE, et al. Maternal early-pregnancy thyroid function is associated with subsequent hypertensive disorders of pregnancy: the generation R study. J Clin Endocrinol Metab. (2014) 99:E2591–98. doi: 10.1210/jc.2014-1505
11. Toloza F, Derakhshan A, Mannisto T, Bliddal S, Popova PV, Carty DM, et al. Association between maternal thyroid function and risk of gestational hypertension and pre-eclampsia: a systematic review and individual-participant data meta-analysis. Lancet Diabetes Endocrinol. (2022) 10:243–52. doi: 10.1016/S2213-8587(22)00007-9
12. Yang S, Shi FT, Leung PC, Huang HF, Fan J. Low thyroid hormone in early pregnancy is associated with an increased risk of gestational diabetes mellitus. J Clin Endocrinol Metab. (2016) 101:4237–43. doi: 10.1210/jc.2016-1506
13. Korevaar T, Derakhshan A, Taylor PN, Meima M, Chen L, Bliddal S, et al. Association of thyroid function test abnormalities and thyroid autoimmunity with preterm birth: A systematic review and meta-analysis. Jama. (2019) 322:632–41. doi: 10.1001/jama.2019.10931
14. Williams FL, Watson J, Ogston SA, Visser TJ, Hume R, Willatts P. Maternal and umbilical cord levels of T4, FT4, TSH, TPOAb, and TgAb in term infants and neurodevelopmental outcome at 5.5 years. J Clin Endocrinol Metab. (2013) 98:829–38. doi: 10.1210/jc.2012-3572
15. Zhang C, Yang X, Zhang Y, Guo F, Yang S, Peeters RP, et al. Association between maternal thyroid hormones and birth weight at early and late pregnancy. J Clin Endocrinol Metab. (2019) 104:5853–63. doi: 10.1210/jc.2019-00390
17. Adunlin G, Diaby V, Xiao H. Application of multicriteria decision analysis in health care: a systematic review and bibliometric analysis. Health Expect. (2015) 18:1894–905. doi: 10.1111/hex.12287
18. Taylor PN, Lazarus JH. Hypothyroidism in pregnancy. Endocrinol Metab Clin North Am. (2019) 48:547–56. doi: 10.1016/j.ecl.2019.05.010
19. Maraka S, Ospina NM, O'Keeffe DT, Espinosa DYA, Gionfriddo MR, Erwin PJ, et al. Subclinical hypothyroidism in pregnancy: A systematic review and meta-analysis. Thyroid. (2016) 26:580–90. doi: 10.1089/thy.2015.0418
20. Haddow JE, Palomaki GE, Allan WC, Williams JR, Knight GJ, Gagnon J, et al. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N Engl J Med. (1999) 341:549–55. doi: 10.1056/NEJM199908193410801
21. Berghout A, Wiersinga W. Thyroid size and thyroid function during pregnancy: an analysis. Eur J Endocrinol. (1998) 138:536–42. doi: 10.1530/eje.0.1380536
22. Yu X, Shan Z, Li C, Mao J, Wang W, Xie X, et al. Iron deficiency, an independent risk factor for isolated hypothyroxinemia in pregnant and nonpregnant women of childbearing age in China. J Clin Endocrinol Metab. (2015) 100:1594–601. doi: 10.1210/jc.2014-3887
23. Zhang HY, Teng XC, Shan ZY, Wang ZJ, Li CY, Yu XH, et al. Association between iron deficiency and prevalence of thyroid autoimmunity in pregnant and non-pregnant women of childbearing age: a cross-sectional study. Chin Med J (Engl). (2019) 132:2143–49. doi: 10.1097/CM9.0000000000000409
24. Zhao X, Wang H, Li J, Shan Z, Teng W, Teng X. The correlation between polybrominated diphenyl ethers (PBDEs) and thyroid hormones in the general population: A meta-analysis. PloS One. (2015) 10:e126989. doi: 10.1371/journal.pone.0126989
25. Liu H, Shan Z, Li C, Mao J, Xie X, Wang W, et al. Maternal subclinical hypothyroidism, thyroid autoimmunity, and the risk of miscarriage: a prospective cohort study. Thyroid. (2014) 24:1642–49. doi: 10.1089/thy.2014.0029
26. Liu D, Teng W, Shan Z, Yu X, Gao Y, Wang S, et al. The effect of maternal subclinical hypothyroidism during pregnancy on brain development in rat offspring. Thyroid. (2010) 20:909–15. doi: 10.1089/thy.2009.0036
27. Zhang Y, Fan Y, Yu X, Wang X, Bao S, Li J, et al. Maternal subclinical hypothyroidism impairs neurodevelopment in rat offspring by inhibiting the CREB signaling pathway. Mol Neurobiol. (2015) 52:432–41. doi: 10.1007/s12035-014-8855-x
28. Wang Y, Wang Y, Dong J, Wei W, Song B, Min H, et al. Developmental hypothyroxinemia and hypothyroidism reduce proliferation of cerebellar granule neuron precursors in rat offspring by downregulation of the sonic hedgehog signaling pathway. Mol Neurobiol. (2014) 49:1143–52. doi: 10.1007/s12035-013-8587-3
29. Zhang L, Teng W, Liu Y, Li J, Mao J, Fan C, et al. Effect of maternal excessive iodine intake on neurodevelopment and cognitive function in rat offspring. BMC Neurosci. (2012) 13:121. doi: 10.1186/1471-2202-13-121
30. Shi X, Han C, Li C, Mao J, Wang W, Xie X, et al. Optimal and safe upper limits of iodine intake for early pregnancy in iodine-sufficient regions: a cross-sectional study of 7190 pregnant women in China. J Clin Endocrinol Metab. (2015) 100:1630–38. doi: 10.1210/jc.2014-3704
31. Li C, Shan Z, Mao J, Wang W, Xie X, Zhou W, et al. Assessment of thyroid function during first-trimester pregnancy: what is the rational upper limit of serum TSH during the first trimester in Chinese pregnant women? J Clin Endocrinol Metab. (2014) 99:73–9. doi: 10.1210/jc.2013-1674
32. Alexander EK, Pearce EN, Brent GA, Brown RS, Chen H, Dosiou C, et al. 2017 Guidelines of the american thyroid association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid. (2017) 27:315–89. doi: 10.1089/thy.2016.0457
33. Pearce EN, Andersson M, Zimmermann MB. Global iodine nutrition: Where do we stand in 2013? Thyroid. (2013) 23:523–28. doi: 10.1089/thy.2013.0128
34. Pearce EN, Lazarus JH, Moreno-Reyes R, Zimmermann MB. Consequences of iodine deficiency and excess in pregnant women: an overview of current knowns and unknowns. Am J Clin Nutr. (2016) 104 Suppl 3:918S–23S. doi: 10.3945/ajcn.115.110429
35. Becker DV, Braverman LE, Delange F, Dunn JT, Franklyn JA, Hollowell JG, et al. Iodine supplementation for pregnancy and lactation-United States and Canada: recommendations of the American Thyroid Association. Thyroid. (2006) 16:949–51. doi: 10.1089/thy.2006.16.949
36. Pearce EN, Alexiou M, Koukkou E, Braverman LE, He X, Ilias I, et al. Perchlorate and thiocyanate exposure and thyroid function in first-trimester pregnant women from Greece. Clin Endocrinol (Oxf). (2012) 77:471–74. doi: 10.1111/j.1365-2265.2012.04407.x
37. Wilson KL, Casey BM, McIntire DD, Halvorson LM, Cunningham FG. Subclinical thyroid disease and the incidence of hypertension in pregnancy. Obstet Gynecol. (2012) 119:315–20. doi: 10.1097/AOG.0b013e318240de6a
38. Kurlak LO, Mistry HD, Kaptein E, Visser TJ, Broughton PF. Thyroid hormones and their placental deiodination in normal and pre-eclamptic pregnancy. Placenta. (2013) 34:395–400. doi: 10.1016/j.placenta.2013.02.009
39. Korevaar TI, Schalekamp-Timmermans S, de Rijke YB, Visser WE, Visser W, de Muinck KS, et al. Hypothyroxinemia and TPO-antibody positivity are risk factors for premature delivery: the generation R study. J Clin Endocrinol Metab. (2013) 98:4382–90. doi: 10.1210/jc.2013-2855
40. Chan S, KaChilele S, Hobbs E, Bulmer JN, Boelaert K, McCabe CJ, et al. Placental iodothyronine deiodinase expression in normal and growth-restricted human pregnancies. J Clin Endocrinol Metab. (2003) 88:4488–95. doi: 10.1210/jc.2003-030228
41. Derakhshan A, Peeters RP, Taylor PN, Bliddal S, Carty DM, Meems M, et al. Association of maternal thyroid function with birthweight: a systematic review and individual-participant data meta-analysis. Lancet Diabetes Endocrinol. (2020) 8:501–10. doi: 10.1016/S2213-8587(20)30061-9
42. Modesto T, Tiemeier H, Peeters RP, Jaddoe VW, Hofman A, Verhulst FC, et al. Maternal mild thyroid hormone insufficiency in early pregnancy and attention-deficit/hyperactivity disorder symptoms in children. JAMA Pediatr. (2015) 169:838–45. doi: 10.1001/jamapediatrics.2015.0498
43. Henrichs J, Bongers-Schokking JJ, Schenk JJ, Ghassabian A, Schmidt HG, Visser TJ, et al. Maternal thyroid function during early pregnancy and cognitive functioning in early childhood: the generation R study. J Clin Endocrinol Metab. (2010) 95:4227–34. doi: 10.1210/jc.2010-0415
44. Korevaar TI, Muetzel R, Medici M, Chaker L, Jaddoe VW, de Rijke YB, et al. Association of maternal thyroid function during early pregnancy with offspring IQ and brain morphology in childhood: a population-based prospective cohort study. Lancet Diabetes Endocrinol. (2016) 4:35–43. doi: 10.1016/S2213-8587(15)00327-7
45. Ghassabian A, Bongers-Schokking JJ, Henrichs J, Jaddoe VW, Visser TJ, Visser W, et al. Maternal thyroid function during pregnancy and behavioral problems in the offspring: the generation R study. Pediatr Res. (2011) 69:454–59. doi: 10.1203/PDR.0b013e3182125b0c
46. Ghassabian A, Bongers-Schokking JJ, de Rijke YB, van Mil N, Jaddoe VW, de Muinck KS, et al. Maternal thyroid autoimmunity during pregnancy and the risk of attention deficit/hyperactivity problems in children: the Generation R Study. Thyroid. (2012) 22:178–86. doi: 10.1089/thy.2011.0318
47. Korevaar TI, Steegers EA, Pop VJ, Broeren MA, Chaker L, de Rijke YB, et al. Thyroid autoimmunity impairs the thyroidal response to human chorionic gonadotropin: two population-based prospective cohort studies. J Clin Endocrinol Metab. (2017) 102:69–77. doi: 10.1210/jc.2016-2942
48. Kester MH, Martinez DMR, Obregon MJ, Marinkovic D, Howatson A, Visser TJ, et al. Iodothyronine levels in the human developing brain: major regulatory roles of iodothyronine deiodinases in different areas. J Clin Endocrinol Metab. (2004) 89:3117–28. doi: 10.1210/jc.2003-031832
49. Onsesveren I, Barjaktarovic M, Chaker L, de Rijke YB, Jaddoe V, van Santen HM, et al. Childhood thyroid function reference ranges and determinants: A literature overview and a prospective cohort study. Thyroid. (2017) 27:1360–69. doi: 10.1089/thy.2017.0262
50. Glinoer D. The regulation of thyroid function in pregnancy: pathways of endocrine adaptation from physiology to pathology. Endocr Rev. (1997) 18:404–33. doi: 10.1210/edrv.18.3.0300
51. Tanguy F, Hamdi S, Chikh K, Glinoer D, Caron P. Central hypothyroidism during pregnancy in a woman with Graves' disease. Clin Endocrinol (Oxf). (2022) 96:89–91. doi: 10.1111/cen.14600
52. Glinoer D, Spencer CA. Serum TSH determinations in pregnancy: how, when and why? Nat Rev Endocrinol. (2010) 6:526–29. doi: 10.1038/nrendo.2010.91
53. Poppe K, Velkeniers B, Glinoer D. Thyroid disease and female reproduction. Clin Endocrinol (Oxf). (2007) 66:309–21. doi: 10.1111/j.1365-2265.2007.02752.x
54. Moreno-Reyes R, Glinoer D, Van Oyen H, Vandevijvere S. High prevalence of thyroid disorders in pregnant women in a mildly iodine-deficient country: a population-based study. J Clin Endocrinol Metab. (2013) 98:3694–701. doi: 10.1210/jc.2013-2149
55. Borson-Chazot F, Caron P, Glinoer D, Wemeau JL. [Antithyroid drugs: what kind of prescription?]. Presse Med. (2014) 43:105–08. doi: 10.1016/j.lpm.2014.01.003
56. Glinoer D, Cooper DS. The propylthiouracil dilemma. Curr Opin Endocrinol Diabetes Obes. (2012) 19:402–07. doi: 10.1097/MED.0b013e3283565b49
57. Glinoer D. Personal considerations on the 2011 American Thyroid Association and the 2007 Endocrine Society pregnancy and thyroid disease guidelines. Thyroid. (2011) 21:1049–51. doi: 10.1089/thy.2011.2110.ed2
59. Zimmermann MB, Jooste PL, Pandav CS. Iodine-deficiency disorders. Lancet. (2008) 372:1251–62. doi: 10.1016/S0140-6736(08)61005-3
60. Zimmermann MB, Hurrell RF. Nutritional iron deficiency. Lancet. (2007) 370:511–20. doi: 10.1016/S0140-6736(07)61235-5
61. Zimmermann MB, Boelaert K. Iodine deficiency and thyroid disorders. Lancet Diabetes Endocrinol. (2015) 3:286–95. doi: 10.1016/S2213-8587(14)70225-6
62. Andersson M, Karumbunathan V, Zimmermann MB. Global iodine status in 2011 and trends over the past decade. J Nutr. (2012) 142:744–50. doi: 10.3945/jn.111.149393
63. Zimmermann MB, Andersson M. Assessment of iodine nutrition in populations: past, present, and future. Nutr Rev. (2012) 70:553–70. doi: 10.1111/nure.2012.70.issue-10
64. Allan WC, Haddow JE, Palomaki GE, Williams JR, Mitchell ML, Hermos RJ, et al. Maternal thyroid deficiency and pregnancy complications: implications for population screening. J Med Screen. (2000) 7:127–30. doi: 10.1136/jms.7.3.127
65. Craig WY, Allan WC, Kloza EM, Pulkkinen AJ, Waisbren S, Spratt DI, et al. Mid-gestational maternal free thyroxine concentration and offspring neurocognitive development at age two years. J Clin Endocrinol Metab. (2012) 97:E22–28. doi: 10.1210/jc.2011-1772
66. Haddow JE, Knight GJ, Palomaki GE, McClain MR, Pulkkinen AJ. The reference range and within-person variability of thyroid stimulating hormone during the first and second trimesters of pregnancy. J Med Screen. (2004) 11:170–74. doi: 10.1258/0969141042467340
67. LaFranchi SH, Haddow JE, Hollowell JG. Is thyroid inadequacy during gestation a risk factor for adverse pregnancy and developmental outcomes? Thyroid. (2005) 15:60–71. doi: 10.1089/thy.2005.15.60
68. Hollowell JG, Haddow JE. The prevalence of iodine deficiency in women of reproductive age in the United States of America. Public Health Nutr. (2007) 10:1532–39. doi: 10.1017/S1368980007360862
69. Stagnaro-Green A, Abalovich M, Alexander E, Azizi F, Mestman J, Negro R, et al. Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid. (2011) 21:1081–125. doi: 10.1089/thy.2011.0087
70. Abalovich M, Amino N, Barbour LA, Cobin RH, De Groot LJ, Glinoer D, et al. Management of thyroid dysfunction during pregnancy and postpartum: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. (2007) 92:S1–47. doi: 10.1210/jc.2007-0141
71. Negro R, Schwartz A, Gismondi R, Tinelli A, Mangieri T, Stagnaro-Green A. Universal screening versus case finding for detection and treatment of thyroid hormonal dysfunction during pregnancy. J Clin Endocrinol Metab. (2010) 95:1699–707. doi: 10.1210/jc.2009-2009
72. Negro R, Schwartz A, Gismondi R, Tinelli A, Mangieri T, Stagnaro-Green A. Thyroid antibody positivity in the first trimester of pregnancy is associated with negative pregnancy outcomes. J Clin Endocrinol Metab. (2011) 96:E920–24. doi: 10.1210/jc.2011-0026
73. Stagnaro-Green A, Glinoer D. Thyroid autoimmunity and the risk of miscarriage. Best Pract Res Clin Endocrinol Metab. (2004) 18:167–81. doi: 10.1016/j.beem.2004.03.007
74. Stagnaro-Green A, Chen X, Bogden JD, Davies TF, Scholl TO. The thyroid and pregnancy: a novel risk factor for very preterm delivery. Thyroid. (2005) 15:351–57. doi: 10.1089/thy.2005.15.351
75. Negro R, Formoso G, Mangieri T, Pezzarossa A, Dazzi D, Hassan H. Levothyroxine treatment in euthyroid pregnant women with autoimmune thyroid disease: effects on obstetrical complications. J Clin Endocrinol Metab. (2006) 91:2587–91. doi: 10.1210/jc.2005-1603
76. Lazarus J, Brown RS, Daumerie C, Hubalewska-Dydejczyk A, Negro R, Vaidya B. 2014 European thyroid association guidelines for the management of subclinical hypothyroidism in pregnancy and in children. Eur Thyroid J. (2014) 3:76–94. doi: 10.1159/000362597
77. Yang M, Sun M, Jiang C, Wu Q, Jiang Y, Xu J, et al. Thyroid hormones and carnitine in the second trimester negatively affect neonate birth weight: A prospective cohort study. Front Endocrinol (Lausanne). (2023) 14:1080969. doi: 10.3389/fendo.2023.1080969
78. Komendova I, Spitalnikova S. Intellectual performance of children of mothers with an untreated thyroid disorder in the first trimester of pregnancy. Endokrynol Pol. (2018) 69:241–45. doi: 10.5603/EP.a2018.0025
79. Opazo MC, Gianini A, Pancetti F, Azkcona G, Alarcon L, Lizana R, et al. Maternal hypothyroxinemia impairs spatial learning and synaptic nature and function in the offspring. Endocrinology. (2008) 149:5097–106. doi: 10.1210/en.2008-0560
80. Levie D, Korevaar T, Bath SC, Dalmau-Bueno A, Murcia M, Espada M, et al. Thyroid function in early pregnancy, child IQ, and autistic traits: A meta-analysis of individual participant data. J Clin Endocrinol Metab. (2018) 103:2967–79. doi: 10.1210/jc.2018-00224
81. Pakkila F, Mannisto T, Pouta A, Hartikainen AL, Ruokonen A, Surcel HM, et al. The impact of gestational thyroid hormone concentrations on ADHD symptoms of the child. J Clin Endocrinol Metab. (2014) 99:E1–08. doi: 10.1210/jc.2013-2943
82. Hou Q, Zou H, Zhang S, Lin J, Nie W, Cui Y, et al. Association of maternal TSH and neonatal metabolism: A large prospective cohort study in China. Front Endocrinol (Lausanne). (2022) 13:1052836. doi: 10.3389/fendo.2022.1052836
83. Janczewska I, Cichon-Kotek M, Glinska M, Deptulska-Hurko K, Basinski K, Wozniak M, et al. Contributors to preterm birth: data from a single polish perinatal center. Children (Basel). (2023) 10. doi: 10.3390/children10030447
84. Wang J, Gong XH, Peng T, Wu JN. Association of thyroid function during pregnancy with the risk of pre-eclampsia and gestational diabetes mellitus. Endocr Pract. (2021) 27:819–25. doi: 10.1016/j.eprac.2021.03.014
85. Su X, Liu Y, Li G, Liu X, Huang S, Duan T, et al. Associations of hypothyroxinemia with risk of preeclampsia-eclampsia and gestational hypertension. Front Endocrinol (Lausanne). (2021) 12:777152. doi: 10.3389/fendo.2021.777152
86. Lazarus JH. Thyroid function in pregnancy. Br Med Bull. (2011) 97:137–48. doi: 10.1093/bmb/ldq039
87. Tudela CM, Casey BM, McIntire DD, Cunningham FG. Relationship of subclinical thyroid disease to the incidence of gestational diabetes. Obstet Gynecol. (2012) 119:983–88. doi: 10.1097/AOG.0b013e318250aeeb
88. Ying H, Tang YP, Bao YR, Su XJ, Cai X, Li YH, et al. Maternal TSH level and TPOAb status in early pregnancy and their relationship to the risk of gestational diabetes mellitus. Endocrine. (2016) 54:742–50. doi: 10.1007/s12020-016-1022-6
89. Breathnach FM, Donnelly J, Cooley SM, Geary M, Malone FD. Subclinical hypothyroidism as a risk factor for placental abruption: evidence from a low-risk primigravid population. Aust N Z J Obstet Gynaecol. (2013) 53:553–60. doi: 10.1111/ajo.12131
90. Casey BM, Dashe JS, Wells CE, McIntire DD, Byrd W, Leveno KJ, et al. Subclinical hypothyroidism and pregnancy outcomes. Obstet Gynecol. (2005) 105:239–45. doi: 10.1097/01.AOG.0000152345.99421.22
91. Galofre JC, Haber RS, Mitchell AA, Pessah R, Davies TF. Increased postpartum thyroxine replacement in Hashimoto's thyroiditis. Thyroid. (2010) 20:901–08. doi: 10.1089/thy.2009.0391
92. Wang W, Teng W, Shan Z, Wang S, Li J, Zhu L, et al. The prevalence of thyroid disorders during early pregnancy in China: the benefits of universal screening in the first trimester of pregnancy. Eur J Endocrinol. (2011) 164:263–68. doi: 10.1530/EJE-10-0660
93. Laurberg P, Andersen SL, Hindersson P, Nohr EA, Olsen J. Dynamics and predictors of serum TSH and fT4 reference limits in early pregnancy: A study within the danish national birth cohort. J Clin Endocrinol Metab. (2016) 101:2484–92. doi: 10.1210/jc.2016-1387
94. Marwaha RK, Chopra S, Gopalakrishnan S, Sharma B, Kanwar RS, Sastry A, et al. Establishment of reference range for thyroid hormones in normal pregnant Indian women. Bjog. (2008) 115:602–06. doi: 10.1111/j.1471-0528.2008.01673.x
95. Kostecka-Matyja M, Fedorowicz A, Bar-Andziak E, Bednarczuk T, Buziak-Bereza M, Dumnicka P, et al. Reference values for TSH and free thyroid hormones in healthy pregnant women in Poland: A prospective, multicenter study. Eur Thyroid J. (2017) 6:82–8. doi: 10.1159/000453061
96. Rosario PW, Carvalho M, Calsolari MR. TSH reference values in the first trimester of gestation and correlation between maternal TSH and obstetric and neonatal outcomes: a prospective Brazilian study. Arch Endocrinol Metab. (2016) 60:314–18. doi: 10.1590/2359-3997000000132
97. Amouzegar A, Ainy E, Khazan M, Mehran L, Hedayati M, Azizi F. Local versus international recommended TSH references in the assessment of thyroid function during pregnancy. Horm Metab Res. (2014) 46:206–10. doi: 10.1055/s-0033-1363277
98. Benhadi N, Wiersinga WM, Reitsma JB, Vrijkotte TG, Bonsel GJ. Higher maternal TSH levels in pregnancy are associated with increased risk for miscarriage, fetal or neonatal death. Eur J Endocrinol. (2009) 160:985–91. doi: 10.1530/EJE-08-0953
99. Aktas ON, Gursoy T, Soysal E, Esencan E, Ercin S. Thyroid hormone levels in late preterm, early term and term infants: a study with healthy neonates revealing reference values and factors affecting thyroid hormones. J Pediatr Endocrinol Metab. (2017) 30:1191–96. doi: 10.1515/jpem-2017-0215
100. Mehari A, Challa F, Gebreyesus G, Alemayehu D, Seifu D. Establishment of reference intervals of thyroid function tests from cord blood of neonates in two selected hospitals, Addis Ababa, Ethiopia. BMC Pediatr. (2016) 16:118. doi: 10.1186/s12887-016-0654-2
101. Ziegler GM, Slaughter JL, Chaudhari M, Singh H, Sanchez PJ, Bunch DR. Preterm to term infant postmenstrual age reference intervals for thyroid-stimulating hormone and free thyroxine. Pediatr Res. (2022) 91:1130–35. doi: 10.1038/s41390-021-01838-3
102. Wassner AJ. Pediatric hypothyroidism: diagnosis and treatment. Paediatr Drugs. (2017) 19:291–301. doi: 10.1007/s40272-017-0238-0
103. Alexander EK, Marqusee E, Lawrence J, Jarolim P, Fischer GA, Larsen PR. Timing and magnitude of increases in levothyroxine requirements during pregnancy in women with hypothyroidism. N Engl J Med. (2004) 351:241–49. doi: 10.1056/NEJMoa040079
104. Sullivan SD, Downs E, Popoveniuc G, Zeymo A, Jonklaas J, Burman KD. Randomized trial comparing two algorithms for levothyroxine dose adjustment in pregnant women with primary hypothyroidism. J Clin Endocrinol Metab. (2017) 102:3499–507. doi: 10.1210/jc.2017-01086
105. Kiran Z, Khalid W, Sheikh A, Islam N. Levothyroxine dosages during pregnancy among hypothyroid women. An experience from a tertiary care center of Karachi, Pakistan, based on data from Maternal Hypothyroidism on Pregnancy Outcomes Study (MHPO-5). BMC Res Notes. (2022) 15:92. doi: 10.1186/s13104-022-05984-7
106. Yu YH, Filion KB, Reynier P, Platt RW, Yu O, Grandi SM. Use of levothyroxine among pregnant women with subclinical hypothyroidism in the United Kingdom: A population-based assessment. Pharmacol Res Perspect. (2021) 9:e848. doi: 10.1002/prp2.848
107. Markantes GK, Dimitropoulos K, Mamali I, Tseti I, Sakellaropoulos G, Markou KB, et al. Therapeutic equivalence of a new preparation of liquid levothyroxine with tablets in patients with overt primary hypothyroidism. Eur Thyroid J. (2021) 10:59–64. doi: 10.1159/000508216
108. Nazarpour S, Ramezani TF, Simbar M, Tohidi M, Alavi MH, Azizi F. Effects of levothyroxine treatment on pregnancy outcomes in pregnant women with autoimmune thyroid disease. Eur J Endocrinol. (2017) 176:253–65. doi: 10.1530/EJE-16-0548
109. Nazarpour S, Ramezani TF, Simbar M, Tohidi M, Minooee S, Rahmati M, et al. Effects of levothyroxine on pregnant women with subclinical hypothyroidism, Negative for thyroid peroxidase antibodie. J Clin Endocrinol Metab. (2018) 103:926–35. doi: 10.1210/jc.2017-01850
110. Kraut E, Farahani P. A Systematic Review of Clinical Practice Guidelines' Recommendations on Levothyroxine Therapy Alone versus Combination Therapy (LT4 plus LT3) for Hypothyroidism. Clin Invest Med. (2015) 38:E305–13. doi: 10.25011/cim.v38i6.26194
111. Han L, Ma Y, Liang Z, Chen D. Laboratory characteristics analysis of the efficacy of levothyroxine on subclinical hypothyroidism during pregnancy: a single-center retrospective study. Bioengineered. (2021) 12:4183–90. doi: 10.1080/21655979.2021.1955589
112. Laurberg P, Cerqueira C, Ovesen L, Rasmussen LB, Perrild H, Andersen S, et al. Iodine intake as a determinant of thyroid disorders in populations. Best Pract Res Clin Endocrinol Metab. (2010) 24:13–27. doi: 10.1016/j.beem.2009.08.013
113. Yang J, Liu Y, Liu H, Zheng H, Li X, Zhu L, et al. Associations of maternal iodine status and thyroid function with adverse pregnancy outcomes in Henan Province of China. J Trace Elem Med Biol. (2018) 47:104–10. doi: 10.1016/j.jtemb.2018.01.013
114. Charoenratana C, Leelapat P, Traisrisilp K, Tongsong T. Maternal iodine insufficiency and adverse pregnancy outcomes. Matern Child Nutr. (2016) 12:680–87. doi: 10.1111/mcn.12211
115. Murcia M, Espada M, Julvez J, Llop S, Lopez-Espinosa MJ, Vioque J, et al. Iodine intake from supplements and diet during pregnancy and child cognitive and motor development: the INMA Mother and Child Cohort Study. J Epidemiol Community Health. (2018) 72:216–22. doi: 10.1136/jech-2017-209830
116. Moleti M, Trimarchi F, Tortorella G, Candia LA, Giorgianni G, Sturniolo G, et al. Effects of maternal iodine nutrition and thyroid status on cognitive development in offspring: A pilot study. Thyroid. (2016) 26:296–305. doi: 10.1089/thy.2015.0336
117. Thomas JV, Collett-Solberg PF. Perinatal goiter with increased iodine uptake and hypothyroidism due to excess maternal iodine ingestion. Horm Res. (2009) 72:344–47. doi: 10.1159/000249162
118. Levie D, Derakhshan A, Shu H, Broeren M, de Poortere RA, Peeters RP, et al. The association of maternal iodine status in early pregnancy with thyroid Function in the swedish environmental longitudinal, mother and child, asthma and Allergy study. Thyroid. (2019) 29:1660–68. doi: 10.1089/thy.2019.0164
119. Wu Y, Yang J, Su Q, Gu H, Qin L. Urinary iodine concentration and its associations with thyroid function in pregnant women of Shanghai. Front Endocrinol (Lausanne). (2023) 14:1184747. doi: 10.3389/fendo.2023.1184747
120. Aakre I, Morseth MS, Dahl L, Henjum S, Kjellevold M, Moe V, et al. Iodine status during pregnancy and at 6 weeks, 6, 12 and 18 months post-partum. Matern Child Nutr. (2021) 17:e13050. doi: 10.1111/mcn.13050
121. Threapleton DE, Waiblinger D, Snart C, Taylor E, Keeble C, Ashraf S, et al. Prenatal and postpartum maternal iodide intake from diet and supplements, urinary Iodine and thyroid hormone concentrations in a region of the United Kingdom with Mild-to-moderate iodine deficiency. Nutrients. (2021) 13. doi: 10.3390/nu13010230
Keywords: maternal hypothyroidism, bibliometrics, CiteSpace, VOSviewer, visualization, emerging topics, research focus
Citation: Chen A, Luo Z, Zhang J and Cao X (2024) Emerging research themes in maternal hypothyroidism: a bibliometric exploration. Front. Immunol. 15:1370707. doi: 10.3389/fimmu.2024.1370707
Received: 15 January 2024; Accepted: 12 March 2024;
Published: 26 March 2024.
Edited by:
Jose De Jesus Garduno Garcia, Universidad Autónoma del Estado de México, MexicoReviewed by:
Yantong Wan, Southern Medical University, ChinaCopyright © 2024 Chen, Luo, Zhang and Cao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinqiu Zhang, OTk1Njg3NDU1QHFxLmNvbQ==; Xiaohui Cao, Y2FveGlhb2h1aTE5ODVAc2luYS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.