- 1Division of Pediatric Transplantation & Cellular Therapies, Memorial Sloan Kettering Cancer Center, New York, NY, United States
- 2Division of Oncology, Department of Pediatrics, Children’s Hospital of Philadelphia, Philadelphia, PA, United States
- 3Division of Hematology, Department of Pediatrics, Children’s Hospital of Philadelphia, Philadelphia, PA, United States
Severe congenital neutropenia (SCN) is caused by germline mutations, most commonly in ELANE, impacting neutrophil maturation and leading to high risk of life-threatening infections. Most patients with ELANE-mutant SCN can achieve safe neutrophil counts with chronic Granulocyte-Colony Stimulating Factor (G-CSF). However, up to 10% of patients have neutropenia refractory to G-CSF and require allogeneic stem cell transplant. Traditional conditioning for these patients includes busulfan and cyclophosphamide which is associated with significant toxicities. We present five patients with SCN without myeloid malignancy transplanted using a reduced toxicity regimen of busulfan, fludarabine and thymoglobulin. 5 pediatric patients with SCN underwent matched sibling donor bone marrow transplant (MSD-BMT) between 2014-2022 on or per CHP14BT057 (NCT02928991), a prospective, single center trial testing elimination of cyclophosphamide from conditioning in pediatric patients with single lineage inherited BMF syndromes. All patients had MSDs and no evidence of MDS. Conditioning consisted of PK-adjusted busulfan, fludarabine, and thymoglobulin, with calcineurin inhibitor and mycophenolate mofetil GVHD prophylaxis. With median follow-up of 48.4 months, overall and event-free survival were 100%. There was no acute GVHD and one instance of chronic limited GVHD. Patients exhibited >95% donor myeloid chimerism at 5 years post-BMT. Two patients experienced CMV reactivation without end-organ disease, and no other viral reactivation or significant infections occurred. MSD-BMT with reduced toxicity myeloablation for SCN provides excellent outcomes while minimizing toxicity. These data suggest that busulfan, fludarabine, and ATG can be considered an efficacious, low-toxicity standard of care regimen for patients with SCN undergoing MSD-BMT.
1 Introduction
Severe congenital neutropenia (SCN) defines a rare, heterogeneous group of disorders of neutrophil maturation. Prevalence estimates range from 1-9 cases per million individuals (1). More than thirty genes have been identified in which variants impair neutrophil differentiation, though this is not a universal finding (2–4). The most common of these are heterozygous ELANE mutations, accounting for approximately 50-60% of cases of SCN (5, 6). ELANE encodes neutrophil elastase, a serine protease enzyme contained in neutrophil azurophilic granules that hydrolyzes cell surface proteins and is released during the neutrophil activation process (6–8). Approximately two hundred distinct ELANE mutations have been identified, and the correlation of specific mutations to SCN phenotype are beginning to be understood (9, 10).
SCN typically presents with multiple severe infections in infancy and early childhood. These include omphalitis, pneumonia, bacteremia, abscess, and otitis media. Patients also frequently experience aphthous stomatitis and periodontitis. Historically, SCN had an approximate mortality rate of 50% in the first year of life due to sepsis, with a subsequent mortality of 6-7% per year. Since the advent of G-CSF therapy, patients with SCN have experienced a significant improvement in average lifespan and the mortality rate has been reduced to 0.9% per year (11). Patients with ELANE-mediated SCN on long-term G-CSF, however, have a cumulative risk of 22% for progression to MDS/leukemia after 15 years of treatment (11). Despite this leukemogenic risk, G-CSF is the initial treatment of choice, with a goal of achieving ANCs exceeding 1.0x109/L (12). Approximately 10% of patients are unable to maintain this ANC and remain at risk for morbidity and mortality due to sepsis (13). Allogeneic stem cell transplant (AlloSCT) is the only curative treatment option for SCN. Definitive reasons to pursue HSCT include failure to maintain appropriate ANC with G-CSF or progression to MDS/leukemia. The cumulative risk of malignancy with G-CSF raises questions regarding the advisability of pre-emptive transplant for patients with high G-CSF requirements, recurrent intractable infections, or at some centers high-risk ELANE variants (9). In cases where a MSD is available, transplant is often considered. The appropriateness of pre-emptive alloSCT for patients with only alternative donor options is less clear (14, 15).
Given the rarity of SCN, there remains a paucity of data on the most appropriate conditioning regimen. To date, most patients who have had alloSCT for SCN have received myeloablative conditioning with busulfan and cyclophosphamide (Bu/Cy), and a small minority have received varied other regimens (16). This Bu/Cy myeloablative regimen carries numerous well-documented risks, including increased risk of veno-occlusive disease, pulmonary toxicity, infertility, and secondary malignancy (17). Busulfan and fludarabine has been proposed in this patient population as an alternative conditioning that can mitigate the severe side effects associated with cyclophosphamide (18–21). Our institutional experience supports this regimen, and we currently have an open phase I clinical trial to assess the efficacy of a busulfan/fludarabine-based conditioning regimen in bone marrow failure (BMF) patients. We present five pediatric patients with SCN transplanted using a conditioning regimen of busulfan, fludarabine, and ATG.
2 Methods
2.1 Patient characteristics
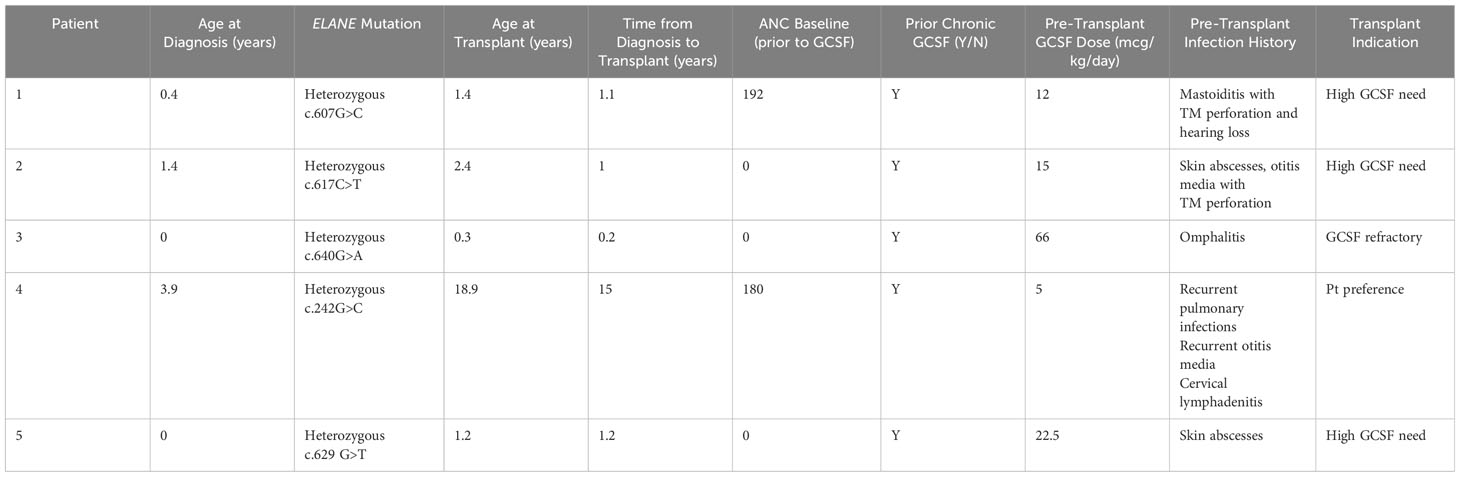
Five patients with ELANE-mediated SCN received MSD-BMT between 2014 and 2022 at our institution. Patient characteristics are listed in Table 1. All patients met phenotypic criteria for ELANE-related SCN prior to transplant, including ANC <0.2x109/L in the absence of G-CSF treatment and a pathogenic ELANE variant. All patients were treated on or per CHP14BT057 (NCT02928991), a prospective, single center trial testing elimination of cyclophosphamide from conditioning in pediatric patients with single lineage BMF syndromes.
2.2 Transplant characteristics
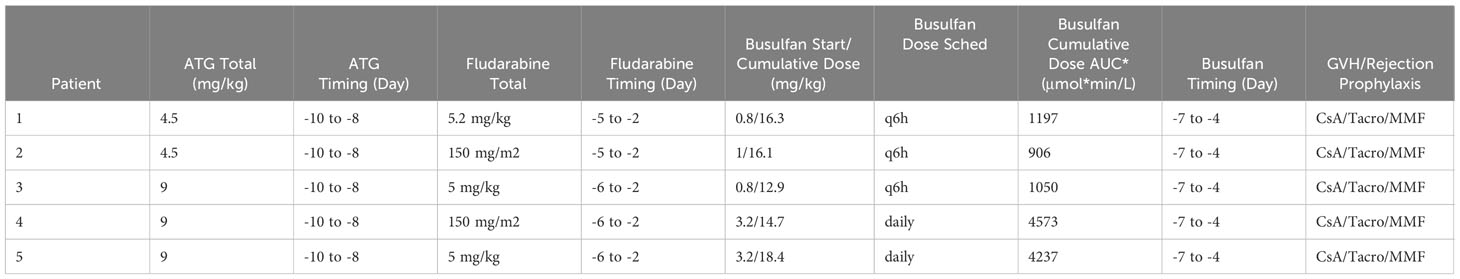
All patients received a bone marrow graft from a 10/10 MSD. G-CSF was discontinued prior to start of conditioning. All patients received busulfan, fludarabine and ATG. Busulfan was PK-adjusted, and starting doses are listed in Table 2. For patients <1 year at time of transplant and for those treated before 2016, busulfan was administered every 6 hours, with target AUC of 900-1500 μmol*min/L (3.69-6.16 mg*h/L). The dose was adjusted based on the first dose PK per institutional SOP. Based on change in institutional SOP, for patients >1 year of age treated in or after 2016, daily dosing of busulfan was used targeting an AUC of 3600-6000 μmol*min/L (14.8-24.6 mg*h/L). Fludarabine was given at a dose of 150 mg/m2 or 5.2 mg/kg for infants <10kg over 4 days for patients treated before 2016, and 150 mg/m2 or 5 mg/kg for infants <10kg over 5 days for patients treated after 2016. This difference was due to change in institutional SOP. ATG dose was either 4.5 or 9 mg/kg, with difference again based on era of transplant due to a change in institutional SOP. The full conditioning schedule is listed in Table 2. For GVHD prophylaxis, all patients received IV cyclosporine (CsA) infusion followed by transition to oral tacrolimus once engraftment was achieved for a minimum of 3 months followed by taper. Patients also received mycophenolate (MMF) for a duration up to 45 days. Time to neutrophil and platelet engraftment was assessed per CIBMTR criteria. Peripheral blood donor chimerism was assessed by variable nucleotide tandem repeat analysis methods per institutional standard practice.
3 Results
3.1 Disease history
ELANE variants for the 5 patients with SCN undergoing MSD-BMT are listed in Table 1, along with age at diagnosis, baseline ANC ranges (prior to G-CSF initiation) and history of pre-transplant complications. Notable pre-transplant infections included: omphalitis, recurrent otitis media, mastoiditis, skin infections, pulmonary infections, and lymphadenitis. No patients had evidence of MDS or leukemia at the time of transplant. Weight based G-CSF dosing at the time of transplant ranged from 5 mcg/kg/day to 66 mcg/kg/day. The indications for transplant included need for high doses of G-CSF (n=3), failure to achieve safe ANC with G-CSF therapy (n=1) and patient preference (n=1). Age at time of BMT ranged from 0.3-18.9 years.
3.2 Engraftment, survival, and donor chimerism
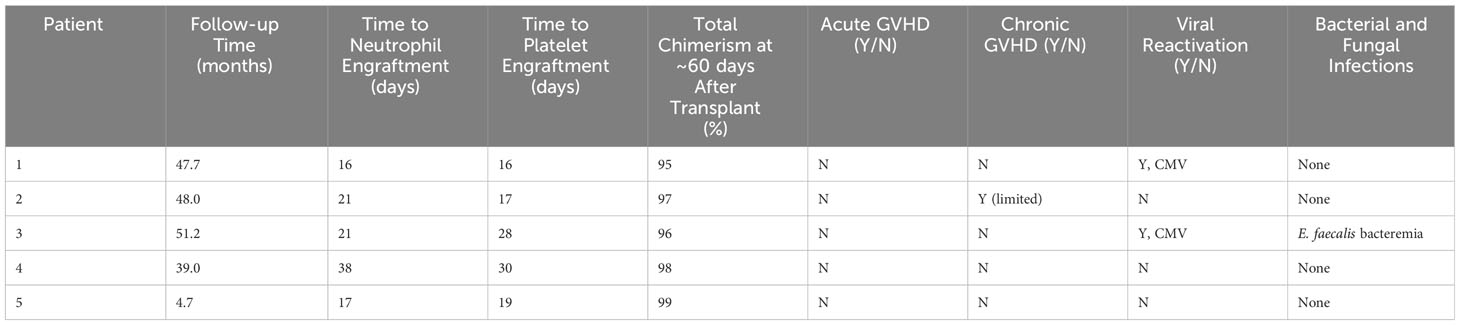
Median post-transplant follow-up ranged was 48.4 months (12-51.9 months). Median time to neutrophil engraftment was 21 days (16-38 days) (see Table 3). Two patients received G-CSF starting at Day +15 and Day +17 based on clinician preference and was continued for 4 days before stopping based on engraftment. Median time to platelet engraftment was 19 days (16-30 days). Overall and event-free survival were 100%. No patient required G-CSF after engraftment. All patients reached at least 95% total donor chimerism by 60 days after transplant and maintained >90% total chimerism throughout their entire period of follow-up (Figure 1). All patients reached 100% donor myeloid chimerism by day 36 after transplant and maintained >95% chimerism for the remainder of follow-up. T cell chimerism increased consistently in the first six months after transplant and remained at or above 89% in all patients.
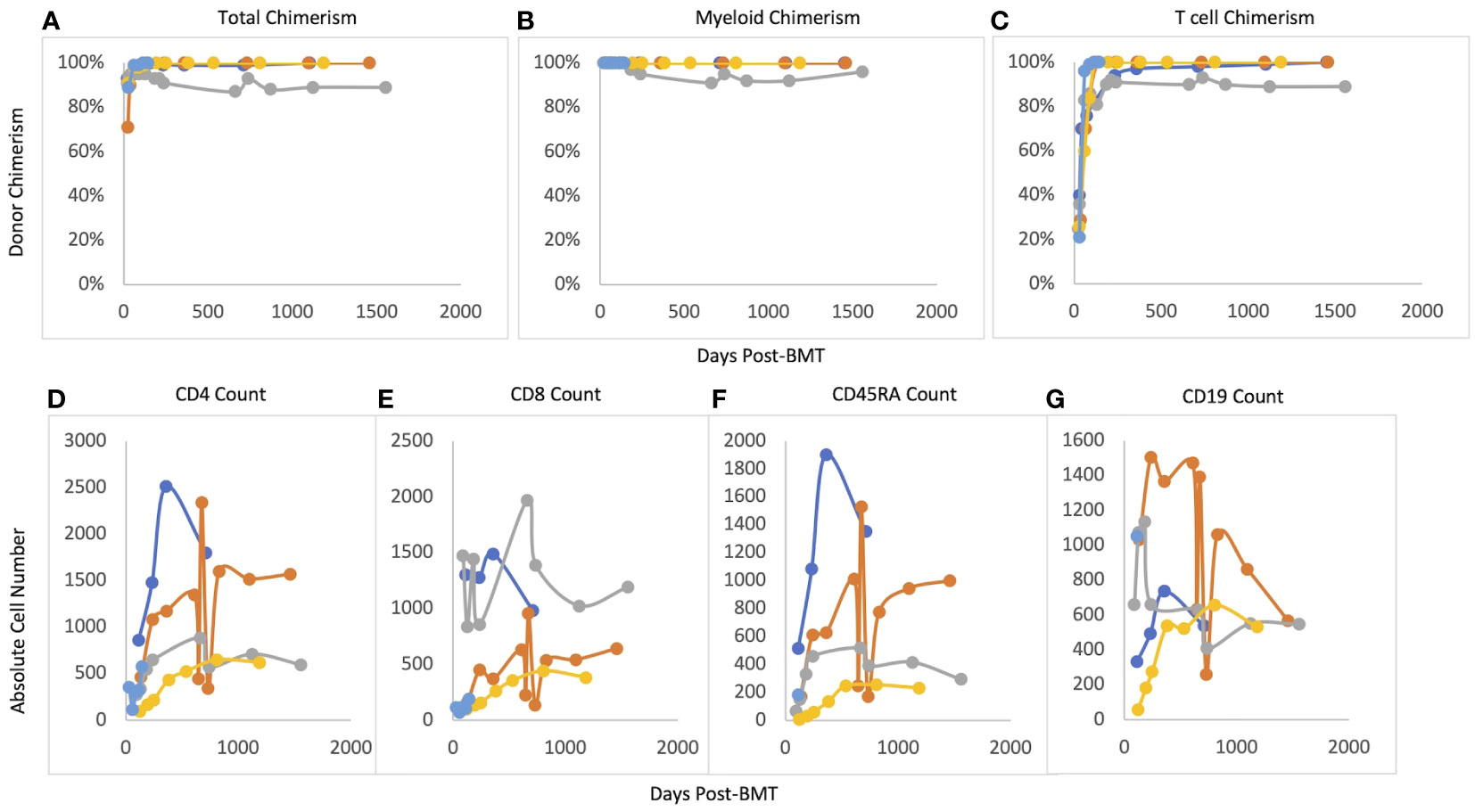
Figure 1 Post-BMT chimerism and immune reconstitution. (A) Total chimerism, (B) Myeloid chimerism, (C) T cell chimerism, (D) CD4 reconsitution; (E) CD8 reconstitution, (F) CD45RA reconstitution, (G) CD19 reconstitution.
3.3 GVHD, infections, and other adverse events
There were no cases of acute GVHD. One patient developed chronic limited GVHD which manifested as fasciitis limited to hands and wrists. These symptoms were treated with a course of prednisone and imatinib with full symptom resolution. No other patients required systemic GVHD therapy. Two patients developed low-level CMV reactivation managed with anti-viral medications. Neither developed CMV disease and time to resolution of CMV was 53 days post-transplant. There were no other viral reactivations. Bacterial and fungal infections during the peri- and post-transplant periods included E. faecalis bacteremia in one patient. No severe organ complications occurred. One patient had transient electrolyte wasting without evidence of renal dysfunction. No patients developed hepatic veno-occlusive disease, transplant-associated thrombotic microangiopathy, or chronic lung function impairment.
4 Discussion
ELANE-related SCN is a rare disorder of neutrophil maturation that carries a high risk of life-threatening infections. It requires lifelong G-CSF therapy to mitigate this risk, but G-CSF may cause severe long-term side effects, including significant osteopenia, splenomegaly, and risk of myeloid malignancies. AlloSCT is the only currently available curative therapy for SCN. The discussion to consider alloSCT becomes more acute in patients who develop clonal evolution involving somatic RUNX1 mutations or cytogenetic abnormalities and many centers will consider using best available alternative donor in these cases. Thus, the question of which patients should consider pre-emptive transplant in the absence of myeloid malignancies is much debated. Most centers consider patients who require high standing doses of G-CSF (≥8 mcg/kg/day) and who have a suitable available MSD to be excellent candidates for pre-emptive transplant, but whether this approach should be offered to patients with lower daily G-CSF requirements or for those whose options are limited to alternative donor transplant is less certain.
There are no defined standards of care for conditioning in alloSCT for ELANE-related SCN. Busulfan and cyclophosphamide is the most common regimen used in published cases (16), but poses significant risks from dual alkylating agents. We present 5 patients that received MSD-BMT using a reduced toxicity busulfan and fludarabine conditioning regimen for ELANE-mutant SCN without myeloid malignancy. None of the patients in this study developed significant lung, liver, or kidney toxicity, and overall survival was 100%. This strategy also did not compromise efficacy. All patients exhibited event-free survival, time to neutrophil and platelet engraftment and total donor chimerism comparable to busulfan and cyclophosphamide-based regimens (21–23). Our patients exhibited low rates of GVHD with no cases requiring ongoing systemic therapy. Viral reactivation occurred in a minority of patients and resolved without end-organ damage in all cases.
MSD-BMT with myeloablative but reduced-toxicity conditioning for ELANE-mutant SCN provides excellent outcomes with minimal toxicity. Additional reduced-toxicity regimens could also be considered, including treosulfan/fludarabine. Treosulfan has demonstrated comparable efficacy and a favorable toxicity profile compared to busulfan (24–26), and may represent an opportunity to further optimize outcomes. However, the currently enrolling clinical trial BMT-CTN 1904 examining a treosulfan-based conditioning regimen for patients with bone marrow failure excludes patients with SCN due to a paucity of data using similar regimens including busulfan/fludarabine. As our study demonstrates excellent engraftment for a busulfan/fludarabine/ATG regimen, we theorize that treosulfan might perform similarly and should be tested in patients with SCN.
Our study is limited in scope by the small size and lack of control group receiving traditional busulfan/cyclophosphamide regimens for comparison; however, comparisons to cases in the literature are favorable. These pilot data suggest that this busulfan and fludarabine-based approach should be studied in multicenter clinical trials, where any patient with ELANE-related SCN who has a MSD and daily G-CSF requirement would be eligible.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Children’s Hospital of Philadelphia Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
JO: Writing – original draft, Writing – review & editing, Conceptualization, Methodology. NG: Writing – original draft, Writing – review & editing, Data curation, Formal analysis. KV: Investigation, Writing – review & editing, Resources, Project administration. CE: Conceptualization, Resources, Supervision, Writing – review & editing. LW: Conceptualization, Resources, Writing – review & editing. JW: Supervision, Writing – review & editing. TO: Conceptualization, Supervision, Writing – original draft, Writing – review & editing, Methodology.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
JO consults for Emendo Biotherapeutics.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Donadieu J, Beaupain B, Mahlaoui N, Bellanné-Chantelot C. Epidemiology of congenital neutropenia. Hematol Oncol Clin North Am. (2013) 27:1–17. doi: 10.1016/j.hoc.2012.11.003
2. Donadieu J, Bellanné-Chantelot C. Genetics of severe congenital neutropenia as a gateway to personalized therapy. Hematol Am Soc Hematol Educ Program. (2022) 2022:658–65. doi: 10.1182/hematology.2022000392
3. Willemsen M, Barber JS, Nieuwenhove EV, Staels F, Gerbaux M, Neumann J, et al. Homozygous DBF4 mutation as a cause of severe congenital neutropenia. J Allergy Clin Immunol. (2023) 152:266–77. doi: 10.1016/j.jaci.2023.02.016
4. Linder MI, Mizoguchi Y, Hesse S, Csaba G, Tatematsu M, Lyskiewicz M, et al. Human genetic defects in SRP19 and SRPRA cause severe congenital neutropenia with distinctive proteome changes. Blood. (2023) 141:645–58. doi: 10.1182/blood.2022016783
5. Rosenberg PS, Alter BP, Link DC, Stein S, Rodger E, Bolyard AA, et al. Neutrophil elastase mutations and risk of leukaemia in severe congenital neutropenia. Br J Haematol. (2008) 140:210–3. doi: 10.1111/j.1365-2141.2007.06897.x
6. Horwitz MS, Duan Z, Korkmaz B, Lee HH, Mealiffe ME, Salipante SJ. Neutrophil elastase in cyclic and severe congenital neutropenia. Blood. (2007) 109:1817–24. doi: 10.1182/blood-2006-08-019166
7. Horwitz M, Benson KF, Person RE, Aprikyan AG, Dale DC. Mutations in ELA2, encoding neutrophil elastase, define a 21-day biological clock in cyclic haematopoiesis. Nat Genet. (1999) 23:433–6. doi: 10.1038/70544
8. Skokowa J, Dale DC, Touw IP, Zeidler C, Welte K. Severe congenital neutropenias. Nat Rev Dis Primers. (2017) 3:17032. doi: 10.1038/nrdp.2017.32
9. Makaryan V, Zeidler C, Bolyard AA, Bonilla MA, Boxer LA, Cham B, et al. The diversity of mutations and clinical outcomes for ELANE-associated neutropenia. Curr Opin Hematol. (2015) 22:3–11. doi: 10.1097/moh.0000000000000105
10. Germeshausen M, Deerberg S, Peter Y, Reimer C, Kratz CP, Ballmaier M. The spectrum of ELANE mutations and their implications in severe congenital and cyclic neutropenia. Hum Mutat. (2013) 34:905–14. doi: 10.1002/humu.22308
11. Rosenberg PS, Alter BP, Bolyard AA, Bonilla MA, Boxer LA, Cham B, et al. The incidence of leukemia and mortality from sepsis in patients with severe congenital neutropenia receiving long-term G-CSF therapy. Blood. (2006) 107:4628–35. doi: 10.1182/blood-2005-11-4370
12. Bonilla MA, Gillio AP, Ruggeiro M, ernan NA, Brochstein JA, Abboud M, et al. Effects of recombinant human granulocyte colony-stimulating factor on neutropenia in patients with congenital agranulocytosis. N Engl J Med. (1989) 320:1574–80. doi: 10.1056/nejm198906153202402
13. Dale DC, Bolyard AA, Shannon JA, Connelly JA, Link DC, Bonilla MA, et al. The severe chronic neutropenia international registry: 10-year follow-up report. Support Cancer Ther. (2006) 3:220–31. doi: 10.3816/SCT.2006.n.020
14. Choi SW, Levine J. Indications for hematopoietic cell transplantation for children with severe congenital neutropenia. Pediatr Transplant. (2010) 14:937–9. doi: 10.1111/j.1399-3046.2010.01386.x
15. Connelly JA, Walkovich K. Diagnosis and therapeutic decision-making for the neutropenic patient. Hematol Am Soc Hematol Educ Program. (2021) 2021:492–503. doi: 10.1182/hematology.2021000284
16. Connelly JA, Choi SW, Levine JE. Hematopoietic stem cell transplantation for severe congenital neutropenia. Curr Opin Hematol. (2012) 19:44–51. doi: 10.1097/MOH.0b013e32834da96e
17. Ben-Barouch S, Cohen O, Vidal L, Avivi I, Ram R. Busulfan fludarabine vs busulfan cyclophosphamide as a preparative regimen before allogeneic hematopoietic cell transplantation: systematic review and meta-analysis. Bone Marrow Transplant. (2016) 51:232–40. doi: 10.1038/bmt.2015.238
18. Hashem H, Abu-Arja R, Auletta JJ, Rangarajan HG, Varga E, Rose MJ, et al. Successful second hematopoietic cell transplantation in severe congenital neutropenia. Pediatr Transplant. (2018) 22. doi: 10.1111/petr.13078
19. Carlsson G, Winiarski J, Ljungman P, Ringden O, Mattsson J, Nordenskjold M, et al. Hematopoietic stem cell transplantation in severe congenital neutropenia. Pediatr Blood Cancer. (2011) 56:444–51. doi: 10.1002/pbc.22836
20. Oshima K, Hanada R, Kobayashi R, Kato K, Nagatoshi Y, Tabuchi K, et al. Hematopoietic stem cell transplantation in patients with severe congenital neutropenia: an analysis of 18 Japanese cases. Pediatr Transplant. (2010) 14:657–63. doi: 10.1111/j.1399-3046.2010.01319.x
21. Rotulo GA, Beaupain B, Rialland F, Paillard C, Nachit O, Galambrun C, et al. HSCT may lower leukemia risk in ELANE neutropenia: a before-after study from the French Severe Congenital Neutropenia Registry. Bone Marrow Transplant. (2020) 55:1614–22. doi: 10.1038/s41409-020-0800-1
22. Ferry C, Ouachée M, Leblanc T, Michel G, Notz-Carrere A, Tabrizi R, et al. Hematopoietic stem cell transplantation in severe congenital neutropenia: experience of the French SCN register. Bone Marrow Transplant. (2005) 35:45–50. doi: 10.1038/sj.bmt.1704718
23. Zeidler C, Welte K, Barak Y, Barriga F, Bolyard AA, Boxer L, et al. Stem cell transplantation in patients with severe congenital neutropenia without evidence of leukemic transformation. Blood. (2000) 95:1195–8. doi: 10.1182/blood.V95.4.1195.004k36_1195_1198
24. van der Stoep M, Bertaina A, Moes D, Algeri M, Bredius RGM, Smiers FJW. Impact of treosulfan exposure on early and long-term clinical outcomes in pediatric allogeneic hematopoietic stem cell transplantation recipients: A prospective multicenter study. Transplant Cell Ther. (2022) 28:99.e1–7. doi: 10.1016/j.jtct.2021.09.018
25. Olivas-Mazón R, Bueno D, Sisinni L, Mozo Y, Casado-Abad G, Martínez AP. A retrospective study of treosulfan versus busulfan-based conditioning in pediatric patients. Eur J Haematol. (2022) 109:474–82. doi: 10.1111/ejh.13828
Keywords: severe congenital neutropenia, stem cell transplant, conditioning regimen, busulfan and fludarabine, bone marrow failure, primary immunodeficiency
Citation: Oved JH, Gibson NM, Venella K, Elgarten CW, Wray L, Warren JT and Olson TS (2024) Reduced toxicity matched sibling bone marrow transplant results in excellent outcomes for severe congenital neutropenia. Front. Immunol. 15:1369243. doi: 10.3389/fimmu.2024.1369243
Received: 11 January 2024; Accepted: 12 February 2024;
Published: 26 February 2024.
Edited by:
Andrew R. Gennery, Newcastle University, United KingdomReviewed by:
Sharat Chandra, Cincinnati Children’s Hospital Medical Center, United StatesMaria Isabel Benitez-Carabante, Vall d’Hebron University Hospital, Spain
Copyright © 2024 Oved, Gibson, Venella, Elgarten, Wray, Warren and Olson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nora M. Gibson, Z2lic29ubjFAY2hvcC5lZHU=
†These authors have contributed equally to this work and share first authorship
 Joseph H. Oved
Joseph H. Oved Nora M. Gibson
Nora M. Gibson Kimberly Venella2
Kimberly Venella2 Julia T. Warren
Julia T. Warren Timothy S. Olson
Timothy S. Olson