
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Immunol., 14 May 2024
Sec. Cancer Immunity and Immunotherapy
Volume 15 - 2024 | https://doi.org/10.3389/fimmu.2024.1369190
This article is part of the Research TopicCommunity Series in Personalized Immunotherapy: Advancing Processes to Extend Patient Collectives, Volume IIView all 6 articles
 Oskar Krueger1*
Oskar Krueger1* Robin Eisenburger1
Robin Eisenburger1 Alpaslan Tasdogan1
Alpaslan Tasdogan1 Lisa Zimmer1
Lisa Zimmer1 Elisabeth Livingstone1
Elisabeth Livingstone1 Eva Hadaschik1
Eva Hadaschik1 Sarah Theurer2
Sarah Theurer2 Berthold Brodin3
Berthold Brodin3 Dirk Schadendorf1
Dirk Schadendorf1 Selma Ugurel1
Selma Ugurel1Melanoma causes the majority of skin cancer-related deaths. Despite novel therapy options, metastatic melanoma still has a poor prognosis. Immune checkpoint inhibition (ICI) therapy has been shown to prolong overall survival in patients with advanced melanoma, but mucosal melanomas respond less favorably compared to melanomas of cutaneous origin. We report on a patient with a mucosal melanoma of the rectum diagnosed in June 2020. Since a surgical intervention in order to achieve a tumor-free situation would have required an amputation of the rectum, a neo-adjuvant systemic immunotherapy with ipilimumab and nivolumab was initiated. As restaging and colonoscopy after four doses of this combination immunotherapy showed a partial response, the patient decided against the pre-planned surgery and a maintenance therapy with nivolumab was started. Repeated colonoscopy showed a complete response after four doses of nivolumab. After ongoing ICI therapy with nivolumab and no evidence of tumor relapse, immunotherapy was stopped in July 2022 after nearly 2 years of continuous treatment. The patient remained tumor-free during further follow-up. Neo-adjuvant immunotherapy is getting more explored in advanced melanoma. By administering ICI therapy before surgical resection of an essentially operable tumor, a stronger and more diverse immunological response is supposed to be achieved. Our reported case demonstrates that this approach could also be effective in mucosal melanoma despite of its generally lower response to immunotherapy.
The neoadjuvant use of immune checkpoint inhibitor (ICI) therapy in resectable skin cancers, particularly melanoma, is a major subject of current research. By administering ICI therapy before surgical resection of an essentially operable tumor, a stronger and more diverse immunological response is supposed to be achieved. Blank and coworkers showed favorable response and survival outcomes in stage III cutaneous melanoma patients treated with the CTLA-4 inhibitor ipilimumab plus the PD-1 inhibitor nivolumab in a neoadjuvant versus an adjuvant setting (1). Patel et al. demonstrated that a higher proportion of patients with resectable stage III/IV cutaneous melanoma achieved a 2-year event-free survival with combined neoadjuvant and subsequent adjuvant therapy with the PD-1 inhibitor pembrolizumab, compared to patients receiving only adjuvant pembrolizumab (2). A similar perioperative treatment regimen has been successfully tested using nivolumab plus the LAG-3 inhibitor relatlimab (3).
However, the efficacy of neoadjuvant ICI therapy in patients with mucosal melanoma are not as well studied. We here report a complete and durable response of an intestinal mucosal melanoma of the rectum upon neoadjuvant treatment with ipilimumab plus nivolumab.
During follow-up care for rectal cancer of a 76-year-old male patient diagnosed in January 2015 and treated with partial rectum amputation and FOLFOX chemotherapy, a new non-pigmented tumor lesion of 15 mm diameter was discovered localized on site of the previous rectocolostomy in June 2020 by colonoscopy (Figure 1A). As a recurrence of rectal cancer was primarily suspected, the coarse, polypoid tumor was biopsied. Histological work-up revealed mucosal melanoma with immunohistochemical positivity of S100, HMB45, melan A and SOX10 (Figure 2). PD-L1 expression was negative (0% of tumor cells stained). Molecular pathology showed no mutations in NRAS, NF-1 and KIT, but in BRAF (V600G) and SF3B1 (R625C). Disease staging by CT, MRI, and PET-MRI scan showed no evidence of metastases. The dermatological examination revealed no primary tumor on the skin or the visible mucosa. Serum LDH and S100B levels were within normal range.
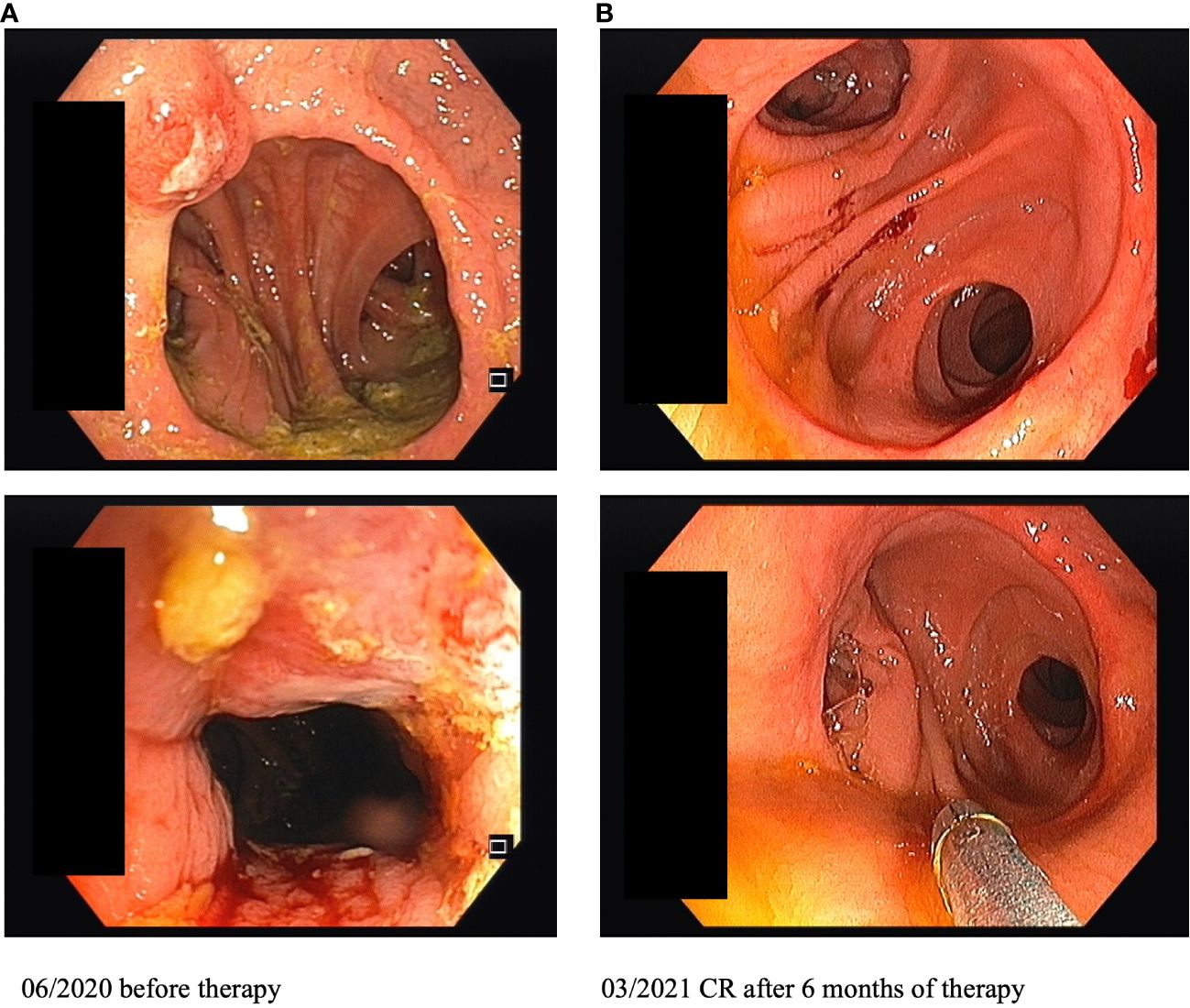
Figure 1 Images of colonoscopy on site of the previous rectocolostomy before and after 6 months of immunotherapy (CR, complete response).
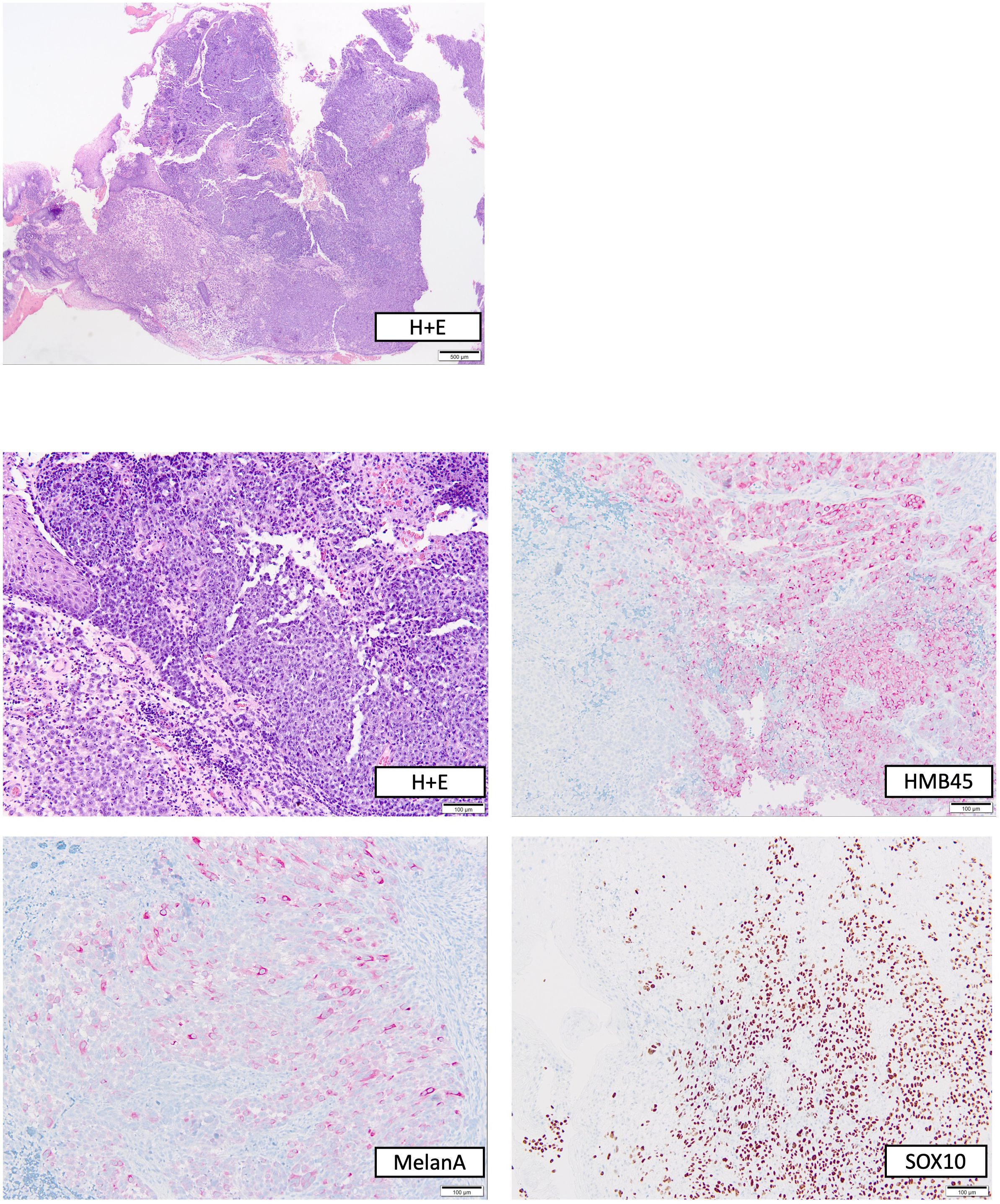
Figure 2 Histopathological work-up of the polypoid rectal tumor biopsied before start of immunotherapy. Top, overview stained with hematoxylin + eosin (H+E); bottom, H+E and immunohistochemical stainings in higher magnification.
The case was discussed in our interdisciplinary tumor board. Since a surgical intervention in order to achieve a tumor-free situation in this preoperated area would have required an amputation of the rectum, a neoadjuvant systemic immunotherapy was recommended. After shared decision making, we initiated a neoadjuvant ICI combination therapy with ipilimumab (3 mg/kg) plus nivolumab (1 mg/kg) Q3W in August 2020 with the aim to improve surgical options and hereby maintain the patient’s bowel continence.
Restaging and colonoscopy after four doses of ipilimumab plus nivolumab showed a partial response with a marked shrinkage of the polypoid tumor. Apart from immune-related thyroiditis and hypophysitis, which were both treated by hormone substitution (L-thyroxine, hydrocortisone), treatment was well tolerated. There was no delay in treatment, but the patient is still dependent on oral substitution therapy. Since at that time a significant partial response was already achieved, the patient decided against the surgery which was originally planned in the neoadjuvant approach. Thus, a maintenance therapy with nivolumab (480 mg Q4W) was started in November 2020. In March 2021, after four doses of nivolumab, repeated colonoscopy showed a complete response with total remission of the previously polypoid tumor lesion (Figure 1B). After ongoing ICI therapy with nivolumab and no evidence of tumor relapse, immunotherapy was stopped in July 2022 after nearly 2 years of continuous treatment. The patient is undergoing regular follow-up care including CT/MRI restaging as well as repeated colonoscopies. Until October 2023 the patient remained tumor-free. Written informed consent was obtained from the patient for the publication of this Case Report.
To date, neoadjuvant therapy regimens have not been approved by the EMA or FDA for skin tumors in general, but are being investigated with great interest in clinical trials (4, 5). The combination regimen of ipilimumab plus nivolumab showed promising results in the neoadjuvant treatment of melanoma (1), so that its use is currently being compared with adjuvant nivolumab monotherapy in a clinical phase 3 trial in stage III cutaneous or unknown primary melanoma patients (NCT04949113).
The present case of a durable complete remission of a mucosal melanoma of the rectum upon neoadjuvant immunotherapy serves as a prime example for the immediate use of immune checkpoint blockade in cases of difficult operability, and the decision to forego primary tumor resection. Surprisingly, the mucosal melanoma of the patient exhibited a BRAF mutation, which would have made a neoadjuvant targeted therapy also a potential option for treatment (6). However, a pooled analysis of six clinical trials in patients with resectable stage III cutaneous malignant melanoma, tumor response was more durable after neoadjuvant immunotherapy, in particular with the combination of ipilimumab plus nivolumab, than with neoadjuvant BRAF/MEK inhibition (7). With the introduction of triple therapy regimens (combined BRAF/MEK inhibition plus PD-1 blockade) in the metastatic setting of cutaneous melanoma (8), trials using triple therapy in the neoadjuvant setting for BRAF-mutant melanoma may be seen in the future (9).
Mucosal melanomas in general respond less favorably to immunotherapy compared to cutaneous melanomas (10, 11). Therefore, it is surprising that the patient reported here responded so well to the neoadjuvant immunotherapy. However, study findings signal further use of neoadjuvant strategy with immune checkpoint inhibitor therapy in mucosal melanoma (11). In the end, the patient decided against a final surgical intervention to remove any residual tumor after clinical complete response, and thus ultimately received a definitive immunotherapy. Despite the rejection of the surgery a histological confirmation using a sample biopsy would have been useful in order to distinguish between complete and partial remission. However, the patient declined a biopsy due to concerns about anastomotic leakage.
This is a weakness of the neoadjuvant therapy approach, as patients can ultimately object to surgical therapy and therefore do not receive a neoadjuvant therapy concept as previously discussed, but rather definitive immunotherapy. As a result, baseline biomarkers such as tumor mutational status, tumor infiltrating lymphocytes and PD-L1 expression are also not available to correlate to outcome.
In the event of tumor progression, we would have switched the systemic therapy to a targeted therapy with combined BRAF and MEK inhibition as the mucosal melanoma of the patient exhibited a BRAF mutation.
There is compelling study data suggesting the switch from an adjuvant to a neoadjuvant approach in resectable stage III/IV melanoma. Further studies will be necessary to clarify whether a neoadjuvant versus adjuvant versus perioperative approach should be preferred dependent on relapse-free and overall survival outcomes of the patients.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethical approval was not required for the studies involving humans because there was informed consent from the patient and it was just a case report. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
OK: Writing – original draft. RE: Writing – review & editing. AT: Writing – review & editing. LZ: Writing – review & editing. EL: Writing – review & editing. EH: Writing – review & editing. ST: Writing – review & editing. BB: Writing – review & editing. DS: Writing – review & editing. SU: Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
AT declares speakers honoraria from Merck Sharp & Dohme. LZ declares speakers and advisory board honoraria from Bristol-Myers Squibb, Merck Sharp & Dohme, Novartis, Pierre Fabre, Sanofi, Sunpharma, research support from Novartis and travel support from Merck Sharp & Dohme, Bristol-Myers Squibb, Pierre Fabre, Sanofi, Sunpharma and Novartis; outside the submitted work. EL served as consultant and/or has received honoraria from Bristol-Myers Squibb, Merck Sharp & Dohme, Novartis, Pierre-Fabre, Sanofi, Sunpharma, Takeda and travel support from Bristol-Myers Squibb, Pierre- Fabre, Sunpharma and Novartis, outside the submitted work. DS reports personal fees and non-financial support from Roche/Genentech, grants, personal fees, non-financial support and other from BMS, personal fees from Merck Sharp & Dohme, personal fees and non-financial support from Merck Serono, grant, personal fees and non-financial support from Amgen, personal fees from Immunocore, personal fees from Incyte, personal fees from 4SC, personal fees from Pierre Fabre, personal fees and non-financial support from Sanofi/Regeneron, personal fees from Array BioPharma, personal fees from Pfizer, personal fees from Philogen, personal fees from Regeneron, personal fees from Nektar, personal fees from Sandoz, grants, personal fees and non-financial support from Novartis, personal fees and non-financial support from SunPharma, Neracare, Replimune, Helsinn, OncoSec and InFlaRx outside the submitted work. SU declares research support from Bristol Myers Squibb and Merck Serono; speakers and advisory board honoraria from Bristol Myers Squibb, Merck Sharp & Dohme, Merck Serono, and Novartis; and meeting and travel support from Almirall, Bristol-Myers Squibb, IGEA Clinical Biophysics, Merck Sharp & Dohme, Novartis, Pierre Fabre, and Sun Pharma.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Blank CU, Rozeman EA, Fanchi LF, Sikorska K, van de Wiel B, Kvistborg P, et al. Neoadjuvant versus adjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma. Nat Med. (2018) 24:1655–61. doi: 10.1038/s41591-018-0198-0
2. Patel SP, Othus M, Chen Y, Wright GP Jr., Yost KJ, Hyngstrom JR, et al. Neoadjuvant-adjuvant or adjuvant-only pembrolizumab in advanced melanoma. N Engl J Med. (2023) 388:813–23. doi: 10.1056/NEJMoa2211437
3. Amaria RN, Postow M, Burton EM, Tetzlaff MT, Ross MI, Torres-Cabala C, et al. Neoadjuvant relatlimab and nivolumab in resectable melanoma. Nature. (2022) 611:155–60. doi: 10.1038/s41586-022-05368-8
4. Ugurel S, Röhmel J, Ascierto PA, Becker JC, Flaherty KT, Grob JJ, et al. Survival of patients with advanced metastatic melanoma: The impact of MAP kinase pathway inhibition and immune checkpoint inhibition - Update 2019. Eur J Cancer. (2020) 130:126–38. doi: 10.1016/j.ejca.2020.02.021
5. SChadendorf D, van Akkooi ACJ, Berking C, Griewank KG, Gutzmer R, Hauschild A, et al. Melanoma. Lancet. (2018) 392:971–84. doi: 10.1016/S0140-6736(18)31559-9
6. Nassar KW, Tan AC. The mutational landscape of mucosal melanoma. Semin Cancer Biol. (2020) 61:139–48. doi: 10.1016/j.semcancer.2019.09.013
7. Menzies AM, Amaria RN, Rozeman EA, Huang AC, Tetzlaff MT, van de Wiel BA, et al. Pathological response and survival with neoadjuvant therapy in melanoma: a pooled analysis from the International Neoadjuvant Melanoma Consortium (INMC). Nat Med. (2021) 27:301–9. doi: 10.1038/s41591-020-01188-3
8. Welti M, Dimitriou F, Gutzmer R, Dummer R. Triple combination of immune checkpoint inhibitors and BRAF/MEK inhibitors in BRAFV600 melanoma: current status and future perspectives. Cancers (Basel). (2022) 14. doi: 10.3390/cancers14225489
9. Long GV, Carlino MS, Au-Yeung G, Spillane AJ, Shannon KF, Gyorki DE, et al. NeoTrio: Randomized trial of neoadjuvant (NAT) pembrolizumab (Pembro) alone, in sequence (SEQ) with, or concurrent (CON) with dabrafenib plus trametinib (D+T) in resectable BRAF-mutant stage III melanoma to determine optimal combination of therapy. J Clin Oncol. (2022) 40:9503. doi: 10.1200/JCO.2022.40.16_suppl.9503
10. Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Lao CD, et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. (2019) 381:1535–46. doi: 10.1056/NEJMoa1910836
Keywords: mucosal melanoma, immunotherapy, complete response, checkpoint inhibitors, neoadjuvant
Citation: Krueger O, Eisenburger R, Tasdogan A, Zimmer L, Livingstone E, Hadaschik E, Theurer S, Brodin B, Schadendorf D and Ugurel S (2024) Case report: Durable complete response of a mucosal melanoma of the rectum after neoadjuvant immunotherapy with ipilimumab plus nivolumab. Front. Immunol. 15:1369190. doi: 10.3389/fimmu.2024.1369190
Received: 11 January 2024; Accepted: 29 April 2024;
Published: 14 May 2024.
Edited by:
Stefan B. Eichmüller, German Cancer Research Center (DKFZ), GermanyReviewed by:
Upendra Hegde, University of Connecticut, United StatesCopyright © 2024 Krueger, Eisenburger, Tasdogan, Zimmer, Livingstone, Hadaschik, Theurer, Brodin, Schadendorf and Ugurel. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Oskar Krueger, b3NrYXIua3J1ZWdlckB1ay1lc3Nlbi5kZQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.