- 1State Key Laboratory of Mariculture Biobreeding and Sustainable Goods, Yellow Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences, Qingdao, China
- 2Adisseo Life Science (Shanghai) Co., Ltd, Shanghai, China
Vibrio genus is a common pathogen in aquaculture and causes acute hepatopancreatic necrosis disease (AHPND) and massive mortality of shrimp. Many studies have suggested that a single functional ingredient such as plant extract or organic acid can reduce the dependence on antibiotics and promote the growth and immunity of aquatic animals. In this study, we evaluated the effects of a phytobiotic-based compound additive (Sanacore® GM, SNGM), which had a successful trajectory of commercial application in fish farming. However, its effects on the hepatopancreas health and intestinal microbiota of shrimp after Vibrio challenge have not been well evaluated. In the present study, Pacific white shrimp were fed diets with or without supplementation of SNGM, and the SNGM grades were 0-g/kg (CON), 3-g/kg (SNGM3), and 5-g/kg (SNGM5) diets. The feed trial lasted 60 days, after which a Vibrio parahaemolyticus challenge was performed. The results showed that compared to the CON group, both the SNGM3 and SNGM5 groups had a significantly higher weight gain and a lower feed conversion ratio as well as higher survival after Vibrio parahaemolyticus challenge. In the growth trial, the SNGM3 group had a significantly increased total protein, albumin concentration, and acid phosphatase activity in hemolymph compared to the CON group. In the challenge experiment, the SNGM3 and SNGM5 groups had increased albumin and glucose contents as well as the activities of phenoloxidase, lysozyme, alkaline phosphatase, and superoxide dismutase in hemolymph. Both the SNGM3 and SNGM5 groups had improved morphology of the hepatopancreas and intestine. The SNGM5 group had alleviated gut microbiota dysbiosis induced by Vibrio infection by increasing the potential probiotic bacterium abundance (Shewanella) and decreasing the potential pathogenic bacteria abundance (Vibrio, Photobacteriuma, Pseudoalteromonas, and Candidatus_Bacilloplasma). In conclusion, the dietary phytobiotic-based additive at 3-g/kg level increased the growth and Vibrio parahaemolyticus resistance of Pacific white shrimp by promoting immune-related enzyme activities and improving the morphological structure of the hepatopancreas and intestine and the intestinal microbiota composition.
1 Introduction
Pacific white shrimp (Litopenaeus vannamei) is the most popular aquaculture crustacean due to its high nutritional and economic value. With the development of high-density intensive farming, bacterial and viral diseases in shrimp have become a limiting factor for production. Vibrio genus is a common pathogen in aquaculture and can result in serious economic loss (1). Currently, acute hepatopancreatic necrosis disease (AHPND), caused by Vibrio parahaemolyticus, is a severe epidemic disease in white shrimp aquaculture and results in high mortality (2). Antibiotic has been a main solution for this disease threat. However, frequent and massive use of antibiotics can lead to the emergence of antibiotic-resistant bacteria. In particular, the antibiotic resistance of Vibrio, such as ampicillin, ceftriaxone, and imipenem, isolated from farmed shrimp has been widely reported (3). Meanwhile, antibiotics also have some negative effects on animals and threaten human health via their residue in aquatic food products (4). Therefore, searching for strategies which can reduce the dependence on antibiotics has become an urgent task in shrimp farming.
Unlike antibiotics, health additives based on natural components such as plant extracts, organic acids, and yeast extracts have less impact on the antibiotic resistance of microorganisms and are therefore being more and more frequently used in aquaculture (5, 6). The main effective components of plant extracts are polysaccharides, phenolic compounds, flavonoids, alkaloids, terpenoids, caffeic acid, saponins, and essential oils. They are effective for disease control and have also been widely used in human medicine (7–9). In Pacific white shrimp, feeding with diets supplemented with 20 g/kg Asteraceae plant (Bidens alba) or Indian borage (Plectranthus amboinicus) extract for 7 days significantly increased the total hemocyte count, phenoloxidase activity, superoxide anion production, phagocytic activity, immune gene expression, and resistance to Vibrio alginolyticus (10). In black tiger shrimp (Penaeus monodon) and freshwater crab (Paratelphusa hydrodomous), the injection treatment with five Indian traditional medicinal plant extracts (Aegle marmelos, Cynodon dactylon, Lantana camara, Momordica charantia, and Phyllanthus amarus, 100 or 150 mg/kg body weight) individually resulted in a higher antiviral activity against white spot syndrome virus (WSSV) (11). Studies have demonstrated that individually adding Allium sativum, Olea europaea, Argemone mexicana, Sargassum cristaefolium, Nigella sativa, Phyllanthus amarus, or Eleutherine bulbosa extract in the diet all promoted the immunity and resistance to WSSV or Vibrio genus in Pacific white shrimp (12). Organic acids, including formic acid, butyric acid, citric acid, malic acid, fumaric acid, etc., have been commonly used in the food industry and animal feeds. Adding formic acid (0.023%), acetic acid (0.041%), propionic acid (0.03%), and butyric acid (0.066%) could effectively inhibit the growth of Vibrio harveyi isolated from sick shrimp on Muller–Hinton agar medium (13). In black tiger shrimp, a novel microencapsulated organic acid blend supplementation in the diet increased the digestibility, survival after Vibrio harveyi challenge, and phenol oxidase activity and reduced hepatopancreatic damage, total viable bacteria, and presumptive Vibrio spp. counts in the hepatopancreas and intestine (14). Yeast extracts contain nucleotides, amino acids, polysaccharides, and trace elements and have been widely used in animal feeds (15). In kuruma shrimp (Marsupenaeus japonicus), the oral administration or injection of a nucleotide-rich baker’s yeast extract increased the gene expressions of anti-microbial peptides/proteins, such as penaeidin, crustin, and lysozyme, and resistance to Vibrio nigripulchritudo infection (16). In Pacific white shrimp, low-fish meal diets supplemented with yeast extract (500–1,500 mg/kg) improved the growth, antioxidant enzyme activities, and intestinal morphology (17). In summary, plant extract, organic acid, and yeast extract have been widely used in aquaculture as antibiotic alternative disease control strategies.
Nonetheless, many studies have evidenced that the individual use of plant extract, organic acid, yeast extract, and other immuno-stimulants could increase the disease resistance of shrimp. Effective products, in particular, those based on the combination of functional ingredients, are less studied and applied in the aqua-feed industry. Sanacore® GM (SNGM) is a phytobiotic-based compound additive with a successful trajectory of commercial application in aqua-feeds. Studies on gilthead seabream (Sparus aurata) have evidenced that 0.4–0.5% SNGM supplementation increased the resistance to Enteromyxum leei and Vibrio alginolyticus by improving antioxidation and immunity (18, 19) and also reduced the amount of fishmeal in the feed (20). Meanwhile, dietary 0.2–0.3% SNGM in the Pacific white shrimp also promoted growth performance and resistance to Fusarium solani by improving the feed utilization, antioxidant capacity, non-specific immune response, and intestinal health (21). However, few studies have investigated the effects of SNGM on the hepatopancreas health and intestinal microbiota of Pacific white shrimp, in particular, after Vibrio challenge. Therefore, the present study aimed to evaluate the efficacy of SNGM in the diets of Pacific white shrimp in terms of growth, immunity, hepatopancreas health, intestinal microbiota, and resistance to Vibrio parahaemolyticus challenge. The results of this study could be helpful to the green and healthy development of shrimp farming.
2 Materials and methods
2.1 Experimental diets
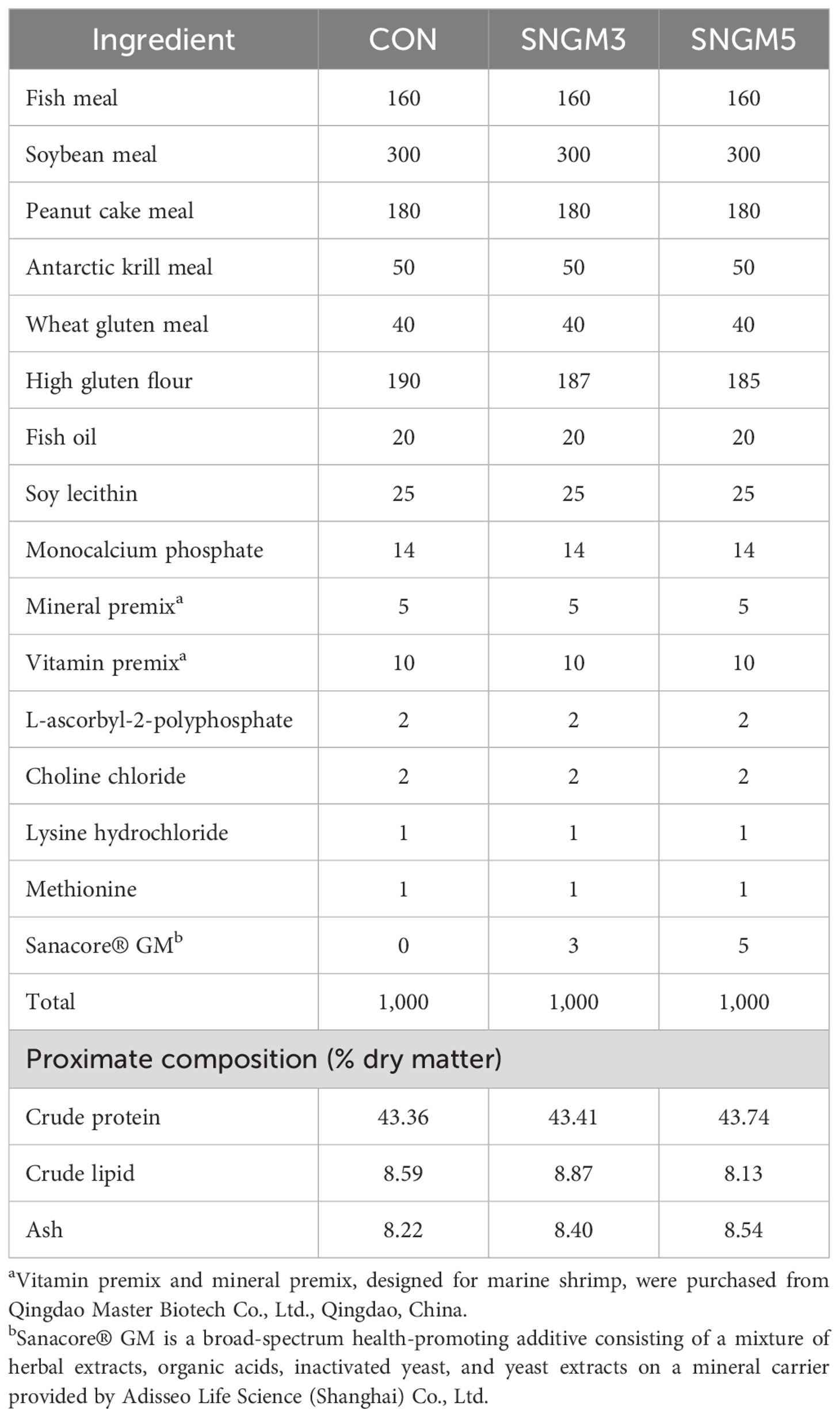
The basal diet (CON) used fishmeal, soybean meal, peanut cake meal, and Antarctic krill meal as main protein sources, and fish oil and soy lecithin as the main lipid sources (Table 1). The control diet contained approximately 43% protein and 8.5% lipid. A phytobiotic-based, health-promoting additive compound, Sanacore® GM [SNGM, Adisseo Life Science (Shanghai) Co., Ltd.], which is a mixture of herbal extracts, organic acids, inactivated yeast, and yeast extracts on a mineral carrier, was added into the control diet at the levels of 0.3% and 0.5%, respectively, to obtain the other two experimental diets (designated as SNGM3 and SNGM5, respectively).
The experimental diets were made using a customized single-screw pelleting machine in the laboratory. Before drying, the pellets (2.0 × 2.0 mm) were steamed for 10 min to enhance their stability in water. The diets were then oven-dried to a moisture content of around 5% and were stored at -20°C.
2.2 Experimental shrimp and feeding procedure
The juvenile shrimp used in the study were purchased from Rizhao Tengyun Aquaculture Co. Ltd. (Rizhao, Shandong Province, China), where the growth trial was conducted. Flow-through deep-well seawater was used in both the acclimating period and the whole growth trial period. A total of 1,200 experimental shrimp with an average initial body weight of approximately 4.5 g was distributed into 12 polyethylene tanks (300 L). Each diet was randomly assigned to for replicate tanks, and each tank was stocked with 100 shrimp. The growth trial lasted 60 days, and the experimental shrimps were hand-fed to apparent satiation four times each day (6:00, 11:30, 17:00, and 21:30). Dead shrimps were taken out when found, and the number and the weight of dead shrimp were recorded. The residual feed and feces were siphoned out 30 min after every feeding (after the shrimp stopped feeding). During the feeding trial, the water temperature ranged from 24°C to 27°C, salinity was 18 to 20, pH was 8.0 to 8.4, dissolved oxygen was 6 to 7 mg L-1, and ammonia was <0.2 mg L-1.
2.3 Challenge experiment
At the end of the growth trial, 30 shrimps/tank were transferred to separate tanks for the challenge experiment with Vibrio parahaemolyticus. A pre-experiment was conducted to determine the most suitable challenging method and corresponding semi-lethal concentration (22). The final challenge method was as follows: shrimps were immersed in 2-L bacteria solution (at a concentration of 1 × 108 cfu/mL) for 30 min. After that, the shrimp-and-bacteria solution was poured into 200 L of clean seawater (the final bacteria concentration was approximately 1×106 cfu/mL). During the challenge experiment, no more clean water was added into the tanks. The mortality was recorded daily continuously for 7 days.
2.4 Sample collection
Samplings were conducted for both the growth trial and the challenge experiment. At the end of the growth trial, the weight and the number of shrimps in each tank were recorded after having been anesthetized with eugenol (1:10,000). From each tank, six shrimps were randomly sampled to collect the hemolymph from pericardial cavity using syringes. The hemolymph was centrifuged at 4°C to obtain the supernatant. The hemolymph of three shrimps from each tank was pooled and gently mixed with anticoagulant to count the hemocyte. After that, these nine shrimps were dissected on ice. The hepatopancreas, intestine, and muscle were collected and frozen immediately with liquid nitrogen and then stored at -80°C for further processing. The sampling process after the 7-day challenge experiment was the same as described above.
2.5 Proximate composition analysis
The proximate composition analysis of experimental diets, whole fish, and muscle was performed according to the standard methods of the Association of Official Analytical Chemists (2005). Briefly, samples were oven-dried at 105°C to a constant weight for moisture assay. Crude protein content (N × 6.25) was measured by the Kjeldahl method using the UDK142 automatic distillation unit (VELP, Usmate, MB, Italy). The crude lipid content was detected by petroleum ether extraction using the Soxhlet method (Foss Tecator, Hoganas, Sweden). The ash content was measured by incinerating the sample in a muffle furnace at 550°C to constant weight.
2.6 Biochemical indexes of the hemolymph, hepatopancreas, and intestine
The contents of total soluble protein (TP, A045–4-2), albumin (ALB, A028–2-1), glucose (GLU, F006–1-1), total cholesterol (T-CHO, A111–1-1), triglycerides (TG, A110–1-1), malonaldehyde (MDA, A003–1-1), and protein carbonyl (PC, A087–1-1) and the activities of alkaline phosphatase (AKP, A059–2-1), acid phosphatase (ACP, A060–2-1), lysozyme (LZM, A050–1-1), phenol oxidase (PO, H247–1-1), and superoxide dismutase (SOD, A001–3-2) in the hemolymph, hepatopancreas or intestine were analyzed by using commercial kits purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). All measurement steps were based on the manufacturer’s protocols (http://www.njjcbio.com/).
2.7 Histological analysis of the hepatopancreas and intestine
The middle intestine and hepatopancreas were collected and fixed in 4% paraformaldehyde immediately. Then, the samples were conducted with a graded ethanol series and xylene solution. After paraffin embedding, the molds were sectioned for 5-μm thickness by a semiautomatic microtome. The sections were stained with the Harris hematoxylin-eosin mixture (H&E) and photographed under a light microscope (Nikon Ds-Ri2, Boston, USA).
2.8 Intestinal microbiota analysis
The whole intestines of three shrimps from the same tank were pooled as one sample (three tanks were randomly selected from each treatment) under sterile conditions. The total bacterial DNA of the intestine was isolated using marine animals DNA kit (Tiangen, China). The quantity and the quality of extracted DNA were tested by using Titertek-Berthold Colibri spectrometer (Colibri, Germany). The ratio of 260/280 nm of DNA ranged from 1.8 to 1.9 and was confirmed by agarose gel electrophoresis. 338F (5′-GTGCCAGCMGCCGCGGTAA-3′) and 806R (5′-CCGTCAATTCCTTTRAGTTT-3′) were selected as the forward and reverse primer, respectively, and the V3–V4 regions of the bacteria 16S ribosomal RNA genes were amplified by PCR using the DNA as template. The PCR (25 μL volume) was performed by mixing 20 ng DNA, 0.4 μL Fast PfuPolymerase (TransGen Biotech, China), 1 μL of each primer (5 μM), 4 μL 5 × Fast Pfu Buffer, and 2 μL dNTPs (2.5 mM). The PCR program was as follows: 95°C for 5 min, 30 cycles of 95°C for 30 s, 55°C for 30 s, and 72°C for 50 s and 72°C for 10 min. Then, Illumina high-throughput sequencing was carried out by Majorbio Bio-Pharm Technology Co., Ltd. (Shanghai, China). All sequencing data were uploaded to the NCBI database under accession number SUB14101752/PRJNA1055185 and bioinformation cloud platform of Majorbio for further analysis (http://www.i-sanger.com). Cluster analysis was carried out for non-repeating sequences (excluding single sequences) according to 97% similarity using Uparse software, and chimeras were removed in the clustering process to obtain the representative sequences of operational taxonomic units (OTUs). The taxonomic analysis of OTU representative sequences was performed by using RDP classifier. The richness and the diversity of bacteria at each taxonomic level were analyzed by using Qiime and Mothur software.
2.9 Statistical analysis
The calculations used in this study include the following:
where W0 = initial body weight and W1 = final body weight.
All data were subjected to one-way analysis of variance (ANOVA) using the SPSS Statistics 20.0 software for Windows. Shapiro–Wilk and Levene’s tests were performed to evaluate the normality and homogeneity of data. Significant differences between the means were detected by using Tukey’s multiple-range test. The results were presented as means ± standard errors of means (SEM), and the level of significance was chosen at P < 0.05. In the normal growth experiment, values with different lowercase superscript letters are significantly different (P < 0.05). In the challenge experiment, values with different capital superscript letters are significantly different (P < 0.05).
3 Results
3.1 Growth performance and Vibrio parahaemolyticus resistance
After the 60-day growth trial, shrimps fed SNGM3 and SNGM5 had significantly higher final body weight (FBW), weight gain (WG), and survival and significantly lower feed conversion ratio (FCR) compared to the CON group (P < 0.05) (Figures 1A, B, D, F). There were no significant differences in initial body weight, feed intake, and condition factor between the CON and SNGM groups (Figures 1A, C, E).
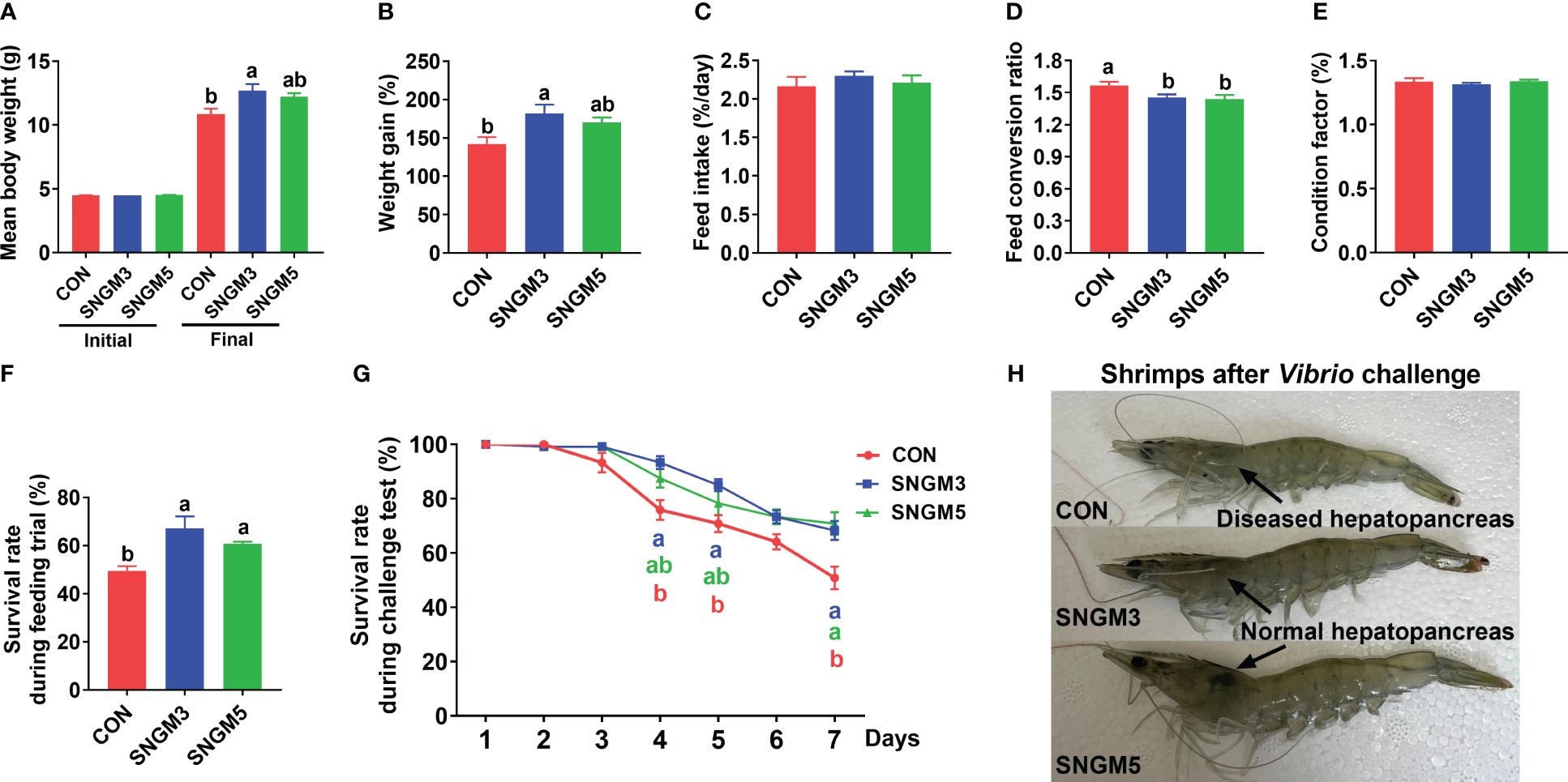
Figure 1. Effects of SNGM on growth performance and Vibrio parahaemolyticus resistance. (A) Initial and final body weight of the shrimp. (B) Weight gain. (C) Feed intake. (D) Feed conversion ratio. (E) Condition factor. (F) Survival during growth trial. (G) Survival during the 7-day challenge test. (H) Color of the hepatopancreas and the appearance of the shrimps after the 7-day challenge test. Values are means ± SEM and different lowercase letters indicate significant difference (P < 0.05) between groups.
After the 7-day challenge experiment, the survival rates in the SNGM3 and SNGM5 groups were significantly higher than in the CON group (P < 0.05) (Figure 1G). As shown in Figure 1H, the color of the hepatopancreas in the CON group turned white after Vibrio parahaemolyticus infection, but the SNGM groups, especially the SNGM5 group, had more normal and healthier (darker color) hepatopancreas after the 7-day challenge experiment.
3.2 The body proximate compositions
As shown in Figure 2, the proximate compositions of whole shrimp and muscle were measured. There were no significant effects on moisture, crude lipid, and crude protein contents of whole shrimp and muscle between the CON and SNGM groups (P > 0.05).
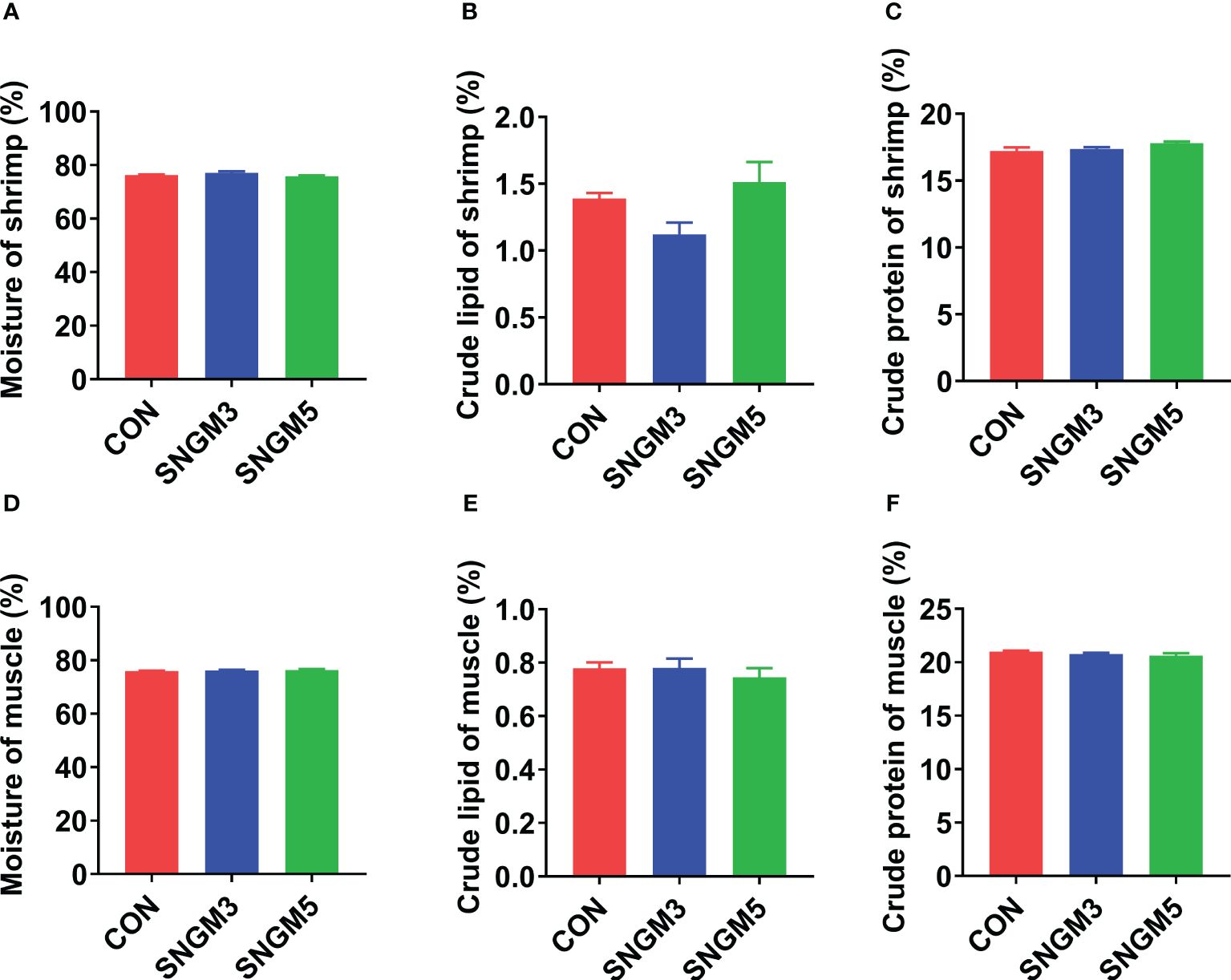
Figure 2. Effects of SNGM on the proximate compositions of the whole shrimp and muscle. (A) Moisture content of the whole shrimp. (B) Crude lipid content of whole shrimp. (C) Crude protein content of the whole shrimp. (D) Moisture content of the muscle. (E) Crude lipid content of the muscle. (F) Crude protein content of the muscle. Values are means ± SEM.
3.3 The immune parameters of hemolymph
At the end of the growth trial, compared to the CON group, the acid phosphatase and alkaline phosphatase activities were significantly increased in the SNGM3 group (Figures 3D, E). The phenol oxidase and alkaline phosphatase activities were significantly increased in the SNGM5 group (P < 0.05) (Figures 3B, E). There were no significant differences in hemocyte count, protein carbonyl, and malonaldehyde contents as well as lysozyme and superoxide dismutase activities between the CON and SNGM groups (P > 0.05) (Figures 3A, C, F, G, H).
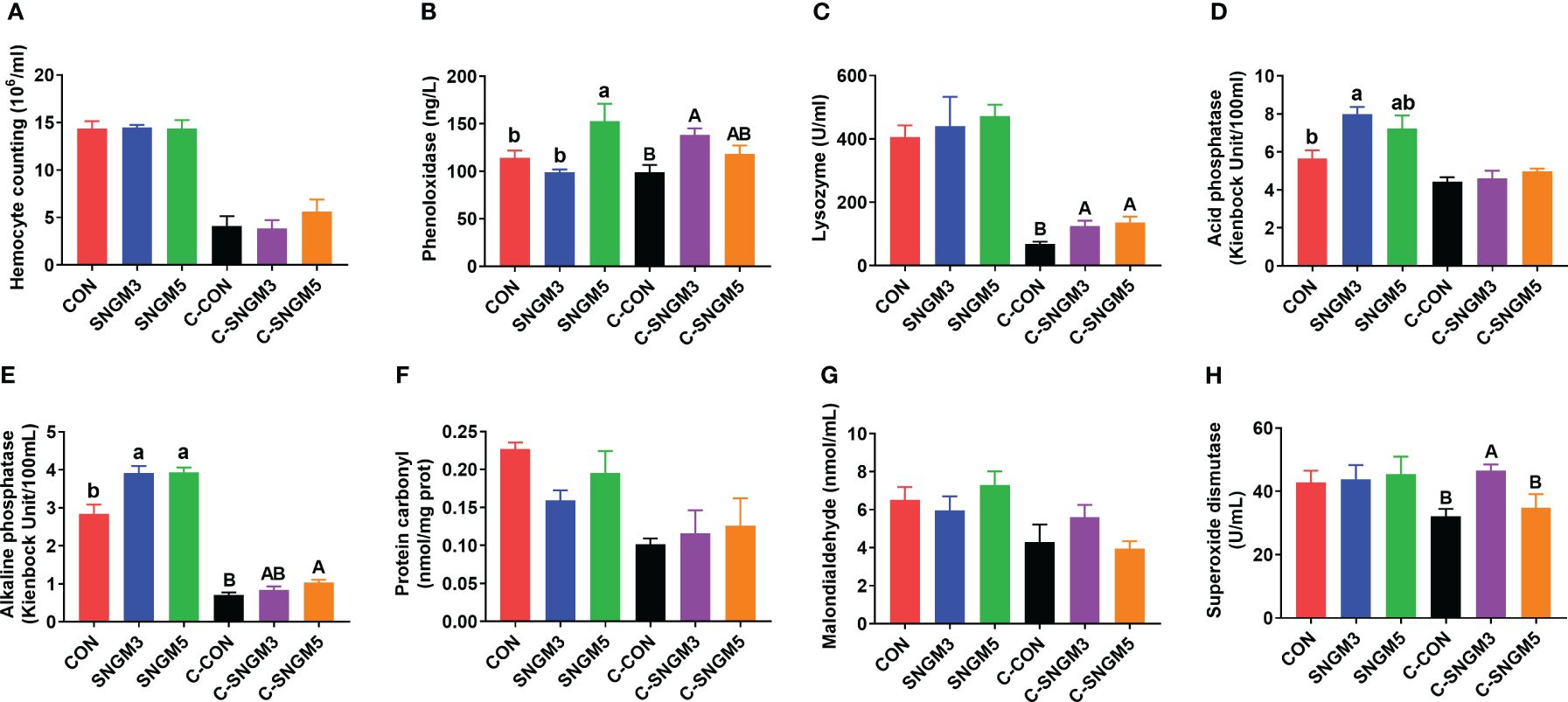
Figure 3. Effects of SNGM on the immune parameters of the hemolymph. (A) Hemocyte count. (B) Phenol oxidase activity. (C) Lysozyme activity. (D) Acid phosphatase activity. (E) Alkaline phosphatase activity. (F) Protein carbonyl content. (G) Malonaldehyde content. (H) Superoxide dismutase activity. Growth experiment groups: CON, SNGM3, and SNGM5. Challenge experiment groups: C-CON, C-SNGM3, and C-SNGM5. Values are means ± SEM. In the growth experiment, values with different lowercase letters are significantly different (P < 0.05). In the challenge experiment, values with different capital letters are significantly different (P < 0.05).
In the challenge experiment, the phenol oxidase, lysozyme, alkaline phosphatase, and superoxide dismutase activities in the SNGM groups were higher than in the CON group (P < 0.05) (Figures 3B, C, E, H), and SNGM did not affect the hemocyte count, acid phosphatase activity, protein carbonyl content, and malonaldehyde content (P > 0.05) (Figures 3A, D, F, G). Compared with normal-growth groups, Vibrio challenge reduced the hemocyte count, and activities of lysozyme, acid phosphatase, and alkaline phosphatase (Figures 3A, C-E).
3.4 The immune parameters and health status of the hepatopancreas
At the end of the growth trial, the hepatopancreas cells in all groups are star-shaped and regularly arranged, but there was an increase in histological status in group SNGM5 compared to the control (Figure 4A). After the challenge experiment, the hepatic corpuscles deformed to some extent, the tubule epithelial cells fell off, and the lumen was irregular and atrophied, but the SNGM3 and SNGM5 groups revealed better conditions (Figure 4A). At the growth trial, there were no significant differences in the activity of acid phosphatase, alkaline phosphatase, and superoxide dismutase, respectively, between the CON and SNGM groups (P > 0.05) (Figures 4B–D). In the challenge experiment, the SNGM5 group had a higher acid phosphatase activity and a lower superoxide dismutase activity than the CON group (P < 0.05) (Figures 4B, D).
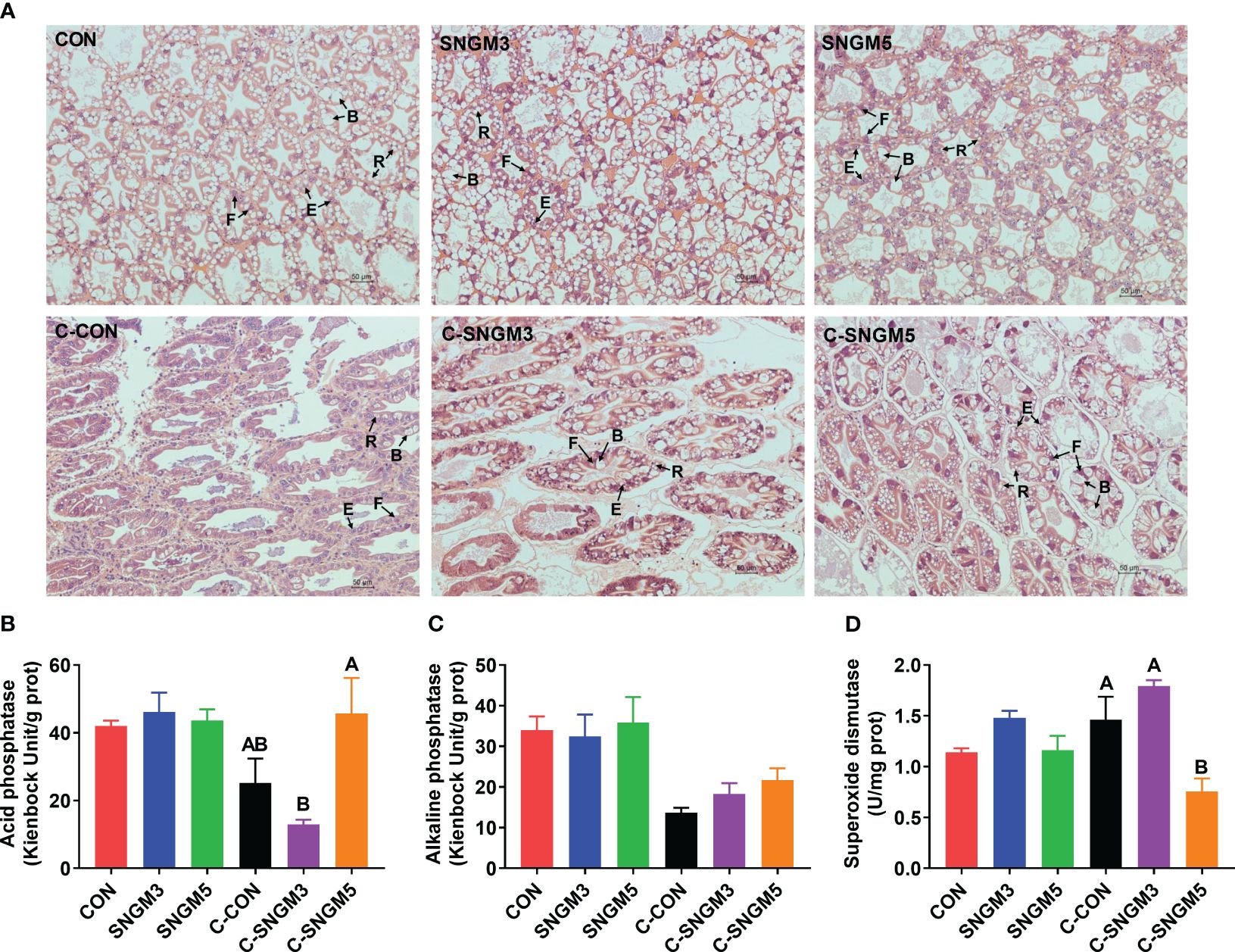
Figure 4. Effects of SNGM on the histology and immune parameters of the hepatopancreas. (A) Histological analysis (H&E staining) of the hepatopancreas. Black arrows mark the four cell types of tubules, including B (“blasenzellen”) cells that typically contain a single, large secretory vesicle, R (“restzellen”) cells that usually contain variable-sized lipid vacuoles, F (“fibrillenzellen” or fibrous) cells that contain a large number of ribosomes and a well-developed endoplasmic reticulum, and E (“embryonalzellen” or embryonic) cells that are the main cell types at the tip of the distal hepatopancreatic tubule. (B) Acid phosphatase activity. (C) Alkaline phosphatase activity. (D) Superoxide dismutase activity. Growth experiment groups: CON, SNGM3, and SNGM5. Challenge experiment groups: C-CON, C-SNGM3, and C-SNGM5. Values are means ± SEM. In the challenge experiment, values with different capital letters are significantly different (P < 0.05).
3.5 Nutrient levels of the hemolymph and hepatopancreas
At the growth trial, compared with the CON group, the total soluble protein and albumin concentrations in the hemolymph were significantly increased in the SNGM3 group (P < 0.05). The SNGM5 group also resulted in an increase in numerical values, but no significant difference was observed (P > 0.05) (Figures 5A, B). Compared with the CON group, dietary SNGM significantly increased the hemolymph glucose content in both the growth trial and the challenge experiments (P < 0.05) (Figure 5D). In the challenge experiment, compared with the CON group, the SNGM supplementation increased the total soluble protein and albumin concentrations in both hemolymph and hepatopancreas (Figures 5A, E, F), and a significant difference was found in the hemolymph albumin concentration (P < 0.05) (Figure 5B). In addition, the SNGM supplementation did not affect the triglyceride content in the hemolymph and hepatopancreas as well as the total cholesterol content in the hepatopancreas (P > 0.05) (Figures 5C, G, H).
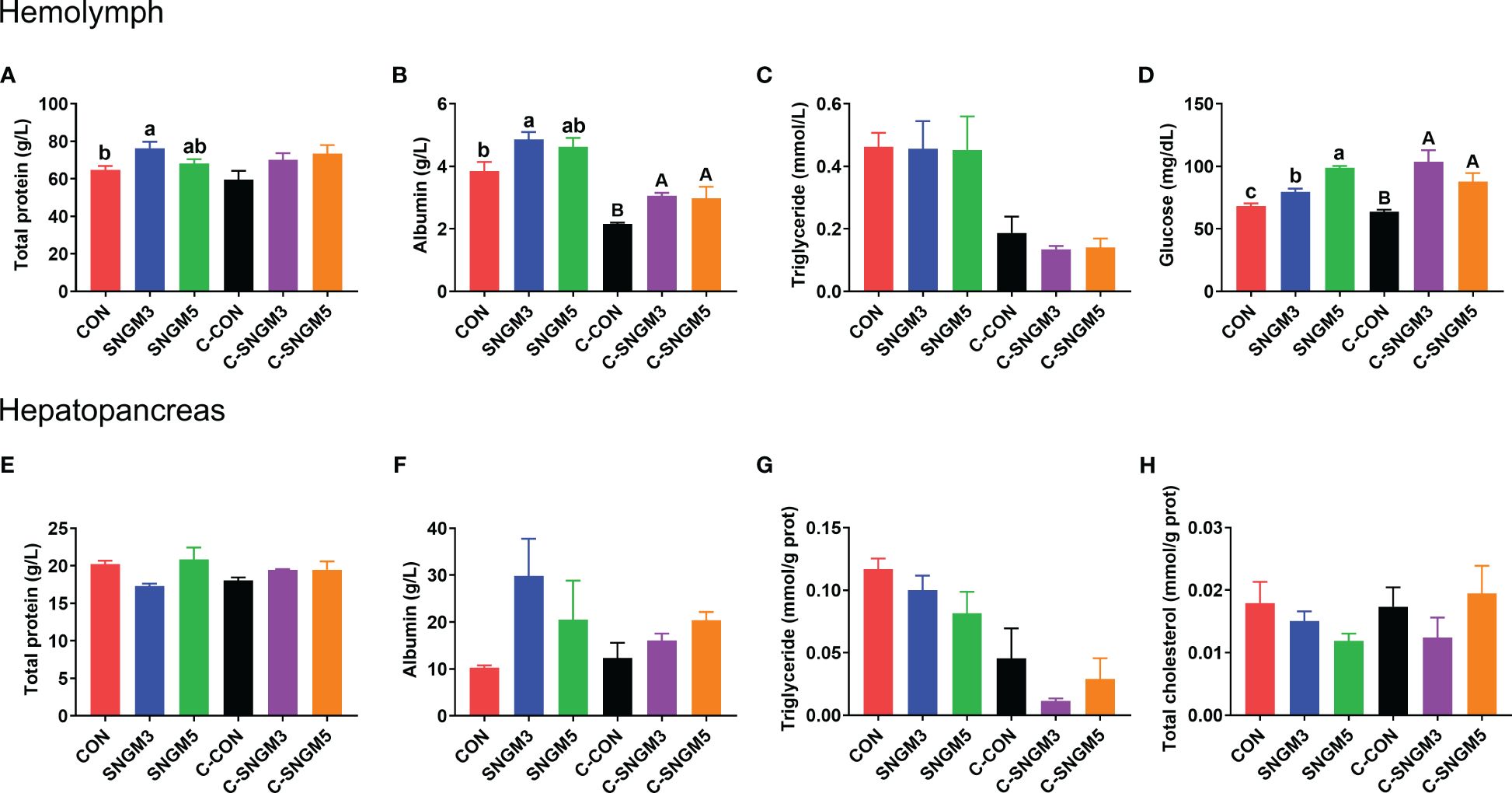
Figure 5. Effects of SNGM on nutrient metabolism of the hemolymph and hepatopancreas. (A) Total soluble protein content in the hemolymph. (B) Albumin content in the hemolymph. (C) Triglyceride content in the hemolymph. (D) Glucose content in the hemolymph. (E) Total soluble protein content in the hepatopancreas. (F) Albumin content in the hepatopancreas. (G) Triglyceride content in the hepatopancreas. (H) Total cholesterol content in the hepatopancreas. Growth experiment groups: CON, SNGM3, and SNGM5. Challenge experiment groups: C-CON, C-SNGM3, and C-SNGM5. Values are means ± SEM. In the growth experiment, values with different lowercase letters are significantly different (P < 0.05). In the challenge experiment, values with different capital letters are significantly different (P < 0.05).
3.6 The immune parameters and morphology of the intestine
The representative slices of middle intestine histology are shown below. In the growth trial, there were no significant differences in the intestinal morphology among the CON, SNGM3, and SNGM5 groups (P > 0.05) (Figure 6A). However, after the challenge experiment, the intestinal folds and epithelial cells of the CON group were damaged, atrophied, and exfoliated, but the SNGM alleviated the adverse effects induced by Vibrio parahaemolyticus. In the growth trial, compared to the CON group, the SNGM supplementation reduced the lysozyme activity in the intestine, and significance was found between CON and SNGM3 (P < 0.05) (Figure 6D). In the challenge experiment, SNGM5 significantly improved the acid phosphatase and lysozyme activities in the intestine (P < 0.05) (Figures 6B, D). Besides, the SNGM supplementation did not affect the alkaline phosphatase activity in the intestine (Figure 6C).
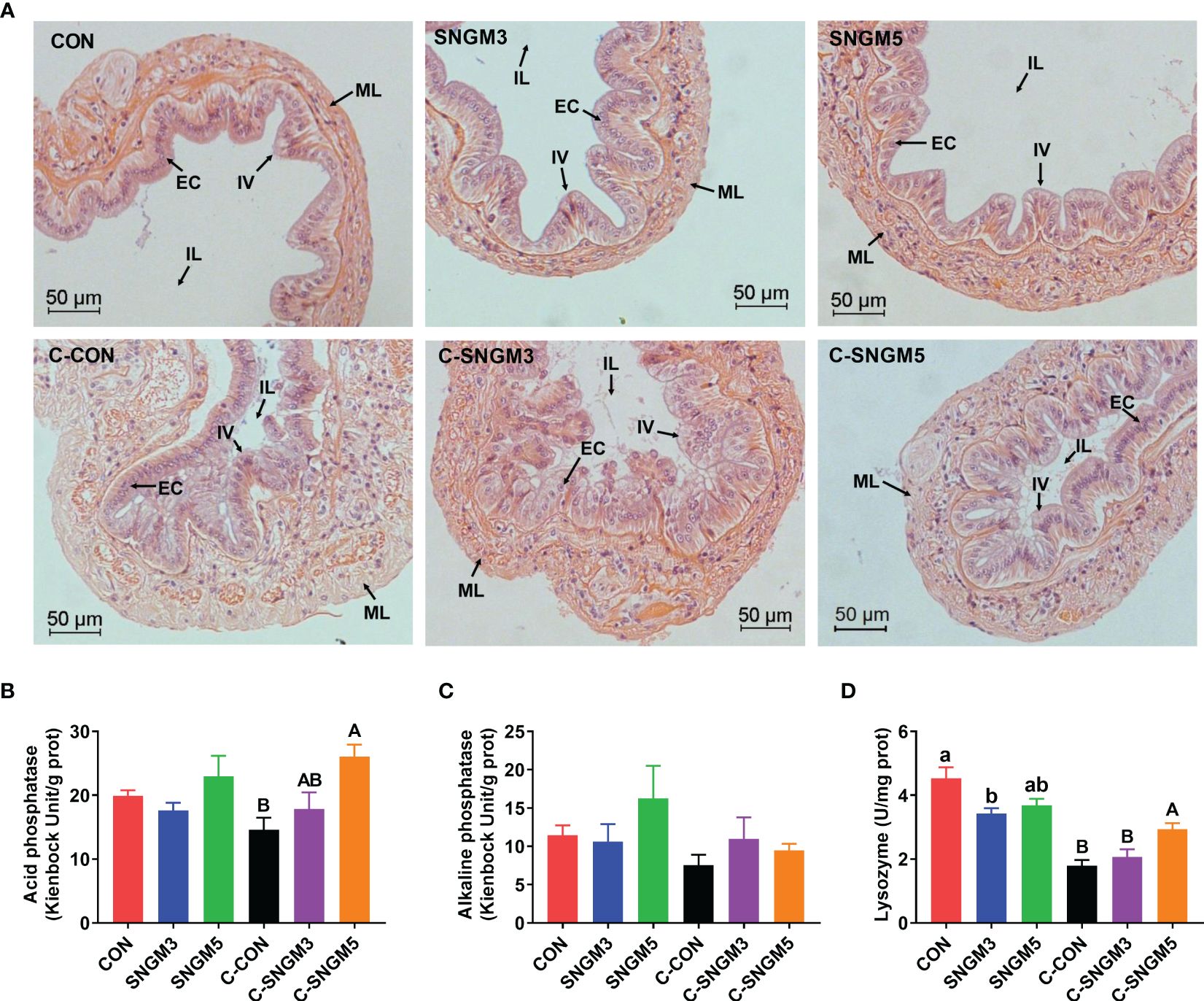
Figure 6. Effects of SNGM on the histology and immune parameters of the intestine. (A) Histological analysis (H&E staining) of the intestine. Black arrows mark the intestinal villi (IV), epithelial cell (EC), intestinal lumen (IL), and muscle layer (ML) of the intestine. (B) Acid phosphatase activity. (C) Alkaline phosphatase activity. (D) Lysozyme activity. Growth experiment groups: CON, SNGM3, and SNGM5. Challenge experiment groups: C-CON, C-SNGM3, and C-SNGM5. Values are means ± SEM. In the growth experiment, values with different lowercase letters are significantly different (P < 0.05). In the challenge experiment, values with different capital letters are significantly different (P < 0.05).
3.7 The composition of intestinal microbiota
The percent of community abundance on the phylum level (Figure 7A) showed that Proteobacteria, Bacteroidota, Firmicutes, and Actinobacteria were dominant in the gut of shrimps. At the growth trial, compared with the CON group, the abundance of Proteobacteria and Actinobacteria increased while those of Bacteroidota and Firmicutes decreased in the SNGM3 and SNGM5 groups. Compared with the growth groups, after the challenge experiment, the abundance of Proteobacteria increased, but those of Bacteroidota, Firmicutes, and Actinobacteria all decreased (Figure 7A). There was no significant difference in these parameters among the control, SNGM3, and SNGM5 groups in both the growth trial and the challenge experiments (P > 0.05). The PCA analysis suggested that the composition of intestinal microbiota was similar among the CON, SNGM3, and SNGM5 groups in the growth trial, but it was obviously different after the challenge experiment, especially between the C-CON and C-SNGM5 groups (Figure 7B). Compared to C-CON, the C-SNGM5 group showed a shorter distance to the normal growth trial groups, which indicates generally normal intestinal microbiota.
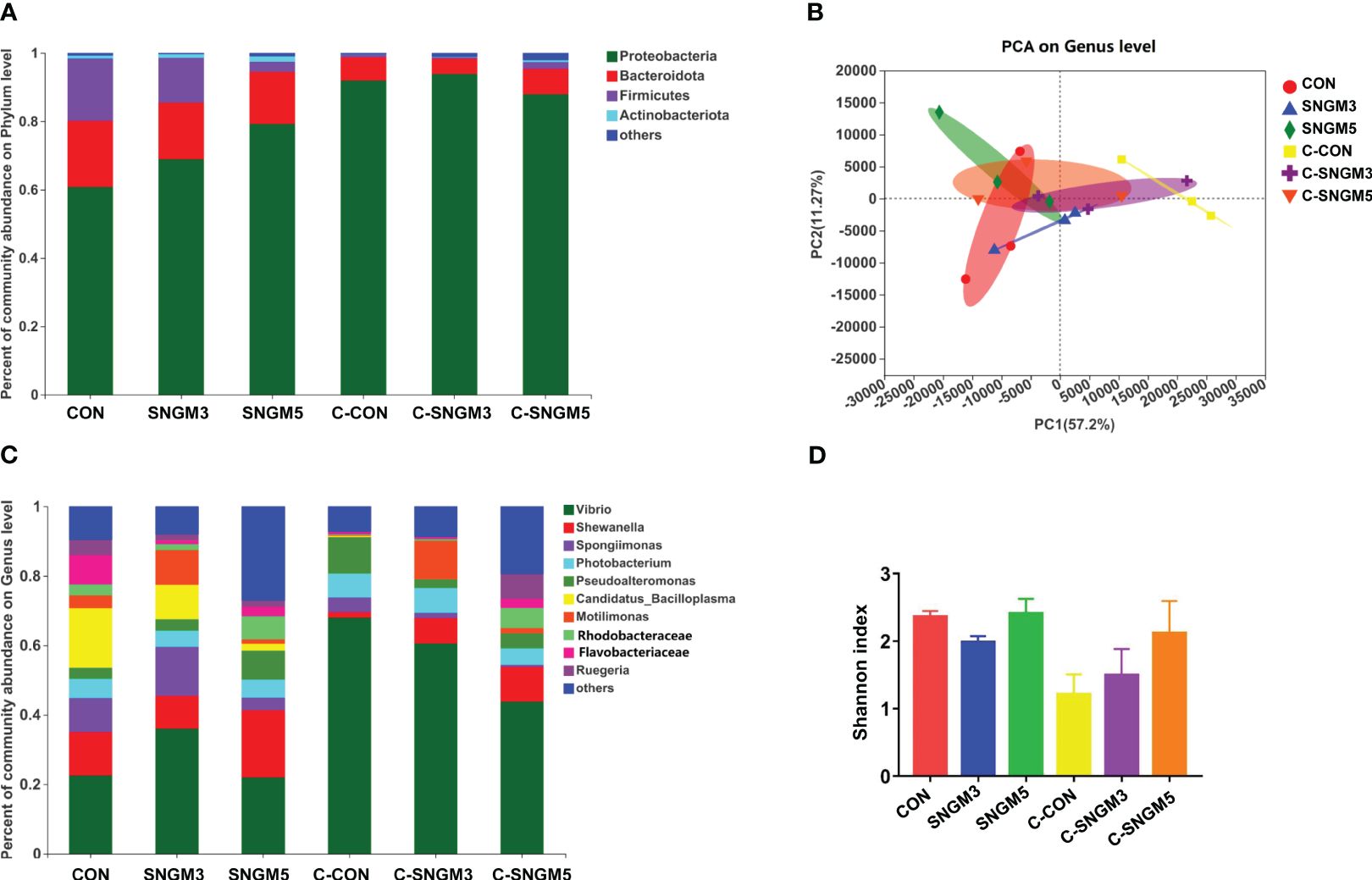
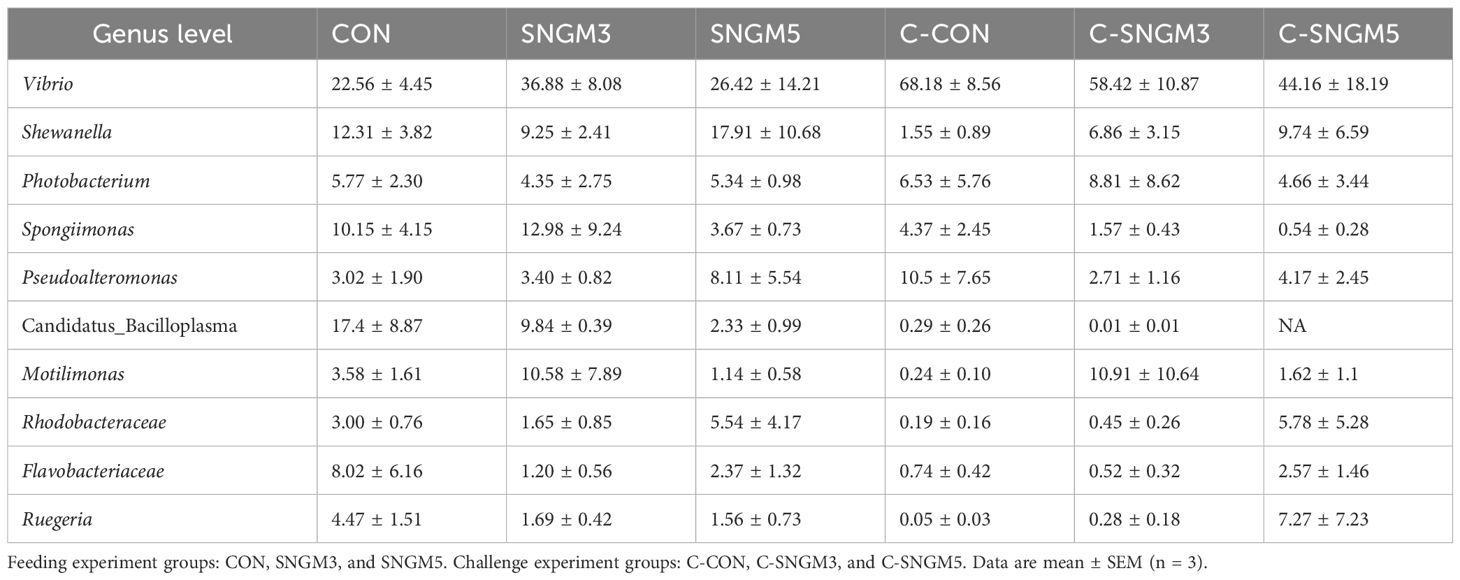
Figure 7. Effects of SNGM on intestinal microbiota. (A) Percent of community abundance on the phylum level. (B) Principal component analysis (PCA) on the genus level. (C) Percent of community abundance on genus the level. (D) Shannon index on the genus level. Growth experiment groups: CON, SNGM3, and SNGM5. Challenge experiment groups: C-CON, C-SNGM3, and C-SNGM5.
The dominant bacteria genus abundance (top 10) is shown in Figure 7C; Table 2. At the growth trial, there was no difference in the abundance of the 10 bacteria between the CON group and SNGM groups (P > 0.05). Compared with the growth trial, the Vibrio abundance was increased in all challenge experiment groups. After the challenge experiment, the abundance of Vibrio, Photobacteriuma, Pseudoalteromonas, and Candidatus_Bacilloplasma, which belong to pathogenic bacteria, in the C-SNGM3 and C-SNGM5 groups, respectively, was lower compared to the C-CON group, while the abundance of Shewanella, which belongs to probiotic bacteria, was increased by SNGM. Compared with the growth trial, the community diversity (Shannon index) in the challenge experiment decreased slightly. In the challenge experiment, the community diversity of SNGM groups was slightly higher compared to the CON group (Figure 7D).
4 Discussion
In recent years, with the development of intensive aquaculture, aquatic animals are more vulnerable to environmental stresses and pathogen infection, which impaired the animal growth, metabolism, antioxidation capacity, and immunity (2, 23, 24). The AHPND outbreak took place firstly in China in 2009 and then spread to Vietnam, Malaysia, Thailand, and Mexico, which caused enormous economic losses (25). Health-promoting additives have been used in animal feeds for decades as antimicrobial and growth promoter. However, few information was available about their combination use in the shrimp farming industry. In the present study, we evaluated the use of SNGM, a commercially available functional feed additive, in Pacific white shrimp diets. Our results showed that dietary SNGM, especially at 3 g/kg feed, promoted growth and resistance to Vibrio. After challenge by Vibrio, the SNGM also resulted in higher activities of phenol oxidase, lysozyme, alkaline phosphatase, and superoxide dismutase than the CON group. These data all clearly demonstrated that the phytobiotic-based compound additive (Sanacore® GM) could improve the growth, immunity, antioxidant capacity, and resistance to pathogenic bacteria in Pacific white shrimp. Similar results were observed in other previous studies. Plant extracts have been widely used in aquaculture to reduce the dependence on antibiotics and promote the health status of shrimp (12, 26, 27). Pacific white shrimp immersed in the seawater containing 400 or 600 mg/L plant (Gracilaria tenuistipitata) extract for 3 h had significantly improved levels of hemocyte count, respiratory burst, lysozyme, phenoloxidase, and superoxide dismutase as well as higher survival after WSSV challenge (28). In black tiger shrimp (Penaeus monodon), compared with control diet, feeding diets with methanolic extracts of nine plants at 2.5 mL/kg for 60 days resulted in higher survival during the growth process, specific growth rate, feed efficiency, and survival after Vibrio harveyi infection (29). In Pacific white shrimp, oral (10 g/kg feed for 14 days) or intramuscular (50 ug/g body weight for 48 h) administration of galangal extract all significantly increased the relative expression of immune-related genes in the hemocytes and survival after Vibrio harveyi infection (30). Organic acids also play beneficial roles by inhibiting intestinal pathogens’ growth. Sodium acetate, sodium butyrate, and sodium propionate could inhibit the growth of three Vibrio species (Vibrio harveyi, V. alginolyticus, and V. anguillarum) in vitro (TCBS agar). Pacific white shrimp diets containing 2% sodium propionate reduced the Vibrio species concentration in the intestinal microbiota and increased the feed palatability and apparent digestibility of dry matter and phosphorus (31). Yeast extract is also recognized as immune-stimulant. In juvenile Pacific white shrimp, dietary yeast extract supplementation at 2.0% increased the weight gain, specific growth rate, condition factor, total antioxidant capacity, catalase and glutathione peroxidase activities, and microbiota diversity (Shannon indexes) (32). Yeast extract could replace 45% of the fish meal in Pacific white shrimp diet (containing 25% fish meal) and did not affect weight gain and muscle composition (33). In particular, our results proved that the combination of functional ingredients improved the growth and immunity in Pacific white shrimp.
Disease outbreak is a major threat to the sustainable development of shrimp farming. At present, among shrimp pathogens, Vibrio parahaemolyticus is the most severe one, which could result in AHPND. This disease, which causes a pale and atrophied hepatopancreas with an empty gastrointestinal tract in shrimp and further causes mortality, has led to a large economic loss in white shrimp farming (2, 34). In the study, SNGM showed promising performance in resistance to Vibrio parahaemolyticus infection. In Pacific white shrimp, the histological analysis of AHPND showed that the tubule epithelial cells of hepatopancreas fell off into the tubule lumens (22). Similar results were also found in the challenge experiment of the present study. After challenge by Vibrio parahaemolyticus, the hepatic corpuscles deformed to some extent, the tubule epithelial cells fell off, and the lumen was irregular and atrophied, but the use of SNGM alleviated the adverse conditions and increased the total soluble protein and albumin concentrations in the hepatopancreas. This was similar to the previous studies demonstrating the relevant efficacy of individual use of health additives in shrimp and, more broadly, fish (35, 36). These results suggest that SNGM could improve shrimp health by maintaining the morphological structure of both hepatopancreas and intestine. In yellow drum (Nibea albiflora), dietary Bacillus subtilis B0E9 and Enterococcus faecalis AT5 increased the resistance to red-head disease challenged by Vibrio harveyi B0003 by improving the liver morphology, serum and skin immunity, and intestine and skin mucosal microbiota composition (37). There have been few studies investigating the relationship between health additives and gut microbiota after Vibrio challenge in shrimp. We found that Proteobacteria, Bacteroidota, Firmicutes, and Actinobacteria were the dominant phyla in the intestine of Pacific white shrimp. In the growth trial, the SNGM addition increased the abundance of Proteobacteria and Actinobacteria but decreased the abundance of Bacteroidota and Firmicutes. The Vibrio parahaemolyticus challenge increased the Proteobacteria abundance but reduced the abundance of Bacteroidota, Firmicutes, and Actinobacteria. Meanwhile, the community diversity (Shannon index) was decreased slightly after challenge. A study on Chinese mitten crab (Eriocheir sinensis) showed that the hepatopancreas necrosis disease also reduced the abundance of phyla Firmicutes (38). Regulation of intestinal microbiota by plant extracts or organic acids has been reported in limited studies. The addition of bamboo leaf flavonoids (500 and 1,000 mg/kg) in crab diets decreased the Shannon (diversity) and Chao (richness) indexes as well as the phyla Bacteroidota abundance (39). A study on Pacific white shrimp showed that diets supplemented with a mixture of organic acids increased the microbiota diversity and richness in the gut and the abundance of Firmicutes and Lactobacillus but reduced the abundance of Proteobacteria (40). In shrimp primary cells, a mixture of organic acids increased the probiotic Faecalibacterium prausnitzii content, short-chain fatty acid level in the gut, and resistance to V. parahaemolyticus (41).
In the present study, compared with the growth trial, the Vibrio abundance was increased in all challenge experiment groups. After the challenge experiment, the SNGM groups had lower Vibrio, Photobacteriuma, Pseudoalteromonas, and Candidatus_Bacilloplasma abundance in the gut than the C-CON group, while these had higher Shewanella abundance and community diversity than the C-CON group. The PCA analysis also showed that the intestinal microbiota of the C-SNGM5 group showed a shorter distance to the normal growth trial groups than the C-CON group. Previous studies have shown that Vibrio, Photobacteriuma, Pseudoalteromonas, and Candidatus_Bacilloplasma are pathogenic bacterium and usually accumulate in the gut of diseased shrimp, but the Shewanella, Chitinibacter, and Rhodobacter abundance is higher in healthy shrimp (42). In shrimp with white feces syndrome (WFS), the intestinal Paracoccus and Lactococcus abundance and the bacterial diversity were reduced, whereas the abundance of Candidatus, Bacilloplasma, and Phascolarctobacterium was increased (43). Dietary sulfate-based alginate polysaccharide (2% to 3% for 56 days) improved the intestinal health and Vibrio parahaemolyticus resistance by decreasing the Vibrio, Pseudoalteromonas, and Candidatus Bacilloplasma abundance in Pacific white shrimp fed low-fishmeal diets (44). All of these results clearly indicate that the use of SNGM could promote intestinal health by increasing the probiotics level and reducing the level of harmful bacteria.
5 Conclusion
Dietary phytobiotic-based additive at 3-g/kg level significantly increased the growth and survival of Pacific white shrimp after Vibrio parahaemolyticus challenge. The immune-related enzyme activities, morphological structure of hepatopancreas and intestine, hepatopancreas metabolism and intestinal microbiota composition were also improved by adding 3–5 g/kg of additive. The results suggest that the suitable dosage of the phytobiotic-based additive (Sanacore® GM) is 3-g/kg feed to promote shrimp growth and disease resistance in practical applications.
Data availability statement
The data presented in the study are deposited in the NCBI repository, accession number PRJNA1055185. Further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was approved by Experimental Animal Care, Ethics and Safety Inspection Committee of the Yellow Sea Fisheries Research Institute, CAFS (IACUC-20220928001). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
QM: Conceptualization, Data curation, Formal analysis, Writing – original draft, Writing – review & editing. GZ: Investigation, Methodology, Supervision, Writing – review & editing. JL: Methodology, Software, Writing – review & editing. IC: Validation, Visualization, Writing – review & editing. YW: Data curation, Software, Writing – review & editing. ML: Formal analysis, Funding acquisition, Writing – review & editing. PD: Methodology, Software, Writing – review & editing. WN: Project administration, Supervision, Writing – review & editing. HX: Conceptualization, Methodology, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Key Research and Development Program of China (2022YFD2400205), Central Public-interest Scientific Institution Basal Research Fund, CAFS (2024CG01) and Adisseo Life Science (Shanghai) Co., Ltd. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Acknowledgments
We thank Mei Duan for technical assistance in the study.
Conflict of interest
Authors GZ, I-TC, and WN-O were employed by the company Adisseo Life Science Shanghai Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Thillaichidambaram M, Narayanan K, Selvaraj S, Sundararaju S, Muthiah RC, Figge MJ. Isolation and characterization of Vibrio owensii from Palk Bay and its infection study against post larvae of Litopenaeus vannamei. Microb Pathogenesis. (2022) 172:105751. doi: 10.1016/j.micpath.2022.105751
2. Reyes G, Betancourt I, Andrade B, Panchana F, Roman R, Sorroza L, et al. Microbiome of Penaeus vannamei larvae and potential biomarkers associated with high and low survival in shrimp hatchery tanks affected by acute hepatopancreatic necrosis disease. Front Microbiol. (2022) 13:838640. doi: 10.3389/fmicb.2022.838640
3. Costa RA, Araujo RL, Souza OV, Silva dos Fernandes Vieira RH. Antibiotic-resistant Vibrios in farmed shrimp. BioMed Res Int. (2015) 2015:505914. doi: 10.1155/2015/505914
4. Li K, Liu L, Zhan J, Scippo ML, Hvidtfeldt K, Liu Y, et al. Sources and fate of antimicrobials in integrated fish-pig and non-integrated tilapia farms. Sci Total Environ. (2017) 595:393–9. doi: 10.1016/j.scitotenv.2017.01.124
5. Ahmadifar E, Pourmohammadi Fallah H, Yousefi M, Dawood MAO, Hoseinifar SH, Adineh H, et al. The gene regulatory roles of herbal extracts on the growth, immune system, and reproduction of fish. Animals. (2021) 11:2167. doi: 10.3390/ani11082167
6. Zhou S, Dong J, Liu Y, Yang Q, Xu N, Yang Y, et al. Antiparasitic efficacy of herbal extracts and active compound against gyrodactylus kobayashii in. Carassius Auratus Front Vet Sci. (2021) 8:665072. doi: 10.3389/fvets.2021.665072
7. Chen Z, Ye SY. Research progress on antiviral constituents in traditional Chinese medicines and their mechanisms of action. Pharm Biol. (2022) 60:1063–76. doi: 10.1080/13880209.2022.2074053
8. Cor D, Knez Z, Hrncic MK. Antitumour, antimicrobial, antioxidant and antiacetylcholinesterase effect of ganoderma lucidum terpenoids and polysaccharides: A review. Molecules. (2018) 23:649. doi: 10.3390/molecules23030649
9. Ti H. Phytochemical profiles and their anti-inflammatory responses against influenza from traditional Chinese medicine or herbs. Mini-Rev Med Chem. (2020) 20:2153–64. doi: 10.2174/1389557520666200807134921
10. Huang HT, Liao ZH, Wu YS, Lin YJ, Kang YS, Nan FH. Effects of Bidens alba and Plectranthus amboinicus dietary supplements on nonspecific immune responses, growth, and resistance to Vibrio alginolyticus in white leg shrimp (Penaeus vannamei). Aquaculture. (2022) 546:737306. doi: 10.1016/j.aquaculture.2021.737306
11. Balasubramanian G, Sarathi M, Kumar SR, Hameed ASS. Screening the antiviral activity of Indian medicinal plants against white spot syndrome virus in shrimp. Aquaculture. (2007) 263:15–9. doi: 10.1016/j.aquaculture.2006.09.037
12. Tadese DA, Song C, Sun C, Liu B, Liu B, Zhou Q, et al. The role of currently used medicinal plants in aquaculture and their action mechanisms: A review. Rev Aquacult. (2022) 14:816–47. doi: 10.1111/raq.12626
13. Mine S, Boopathy R. Effect of organic acids on shrimp pathogen. Vibrio Harveyi Curr Microbiol. (2011) 63:1–7. doi: 10.1007/s00284-011-9932-2
14. Ng WK, Koh CB, Teoh CY, Romano N. Farm-raised tiger shrimp, Penaeus monodon, fed commercial feeds with added organic acids showed enhanced nutrient utilization, immune response and resistance to Vibrio harveyi challenge. Aquaculture. (2015) 449:69–77. doi: 10.1016/j.aquaculture.2015.02.006
15. Tao Z, Yuan H, Liu M, Liu Q, Zhang S, Liu H, et al. Yeast extract: Characteristics, production, applications and future perspectives. J Microbiol Biotechn. (2023) 33:151–66. doi: 10.4014/jmb.2207.07057
16. Biswas G, Korenaga H, Nagamine R, Kono T, Shimokawa H, Itami T, et al. Immune stimulant effects of a nucleotide-rich baker’s yeast extract in the kuruma shrimp. Marsupenaeus Japonicus Aquacult. (2012) 366:40–5. doi: 10.1016/j.aquaculture.2012.09.001
17. Ma S, Wang X, Gao W, Zhang W, Mai K. Supplementation of yeast extract to practical diet improves the growth, anti-oxidative capacity and intestinal morphology of shrimp Litopenaeus vannamei. J Ocean U China. (2019) 18:933–38. doi: 10.1007/s11802-019-3970-y
18. Abdel-Tawwab M, Selema TAMA, Abotaleb MM, Khalil RH, Sabry NM, Soliman AM, et al. Effects of a commercial feed additive (Sanacore (R) GM) on immune-antioxidant profile and resistance of gilthead seabream (Sparus aurata) against Vibrio alginolyticus infection. Ann Anim Sci. (2023) 23:185–93. doi: 10.2478/aoas-2022-0053
19. Palenzuela O, Del Pozo R, Piazzon MC, Isern-Subich MM, Ceulemans S, Coutteau P, et al. Effect of a functional feed additive on mitigation of experimentally induced gilthead sea bream Sparus aurata enteromyxosis. Dis Aquat Organ. (2020) 138:111–20. doi: 10.3354/dao03453
20. Piazzon MC, Naya-Catala F, Pereira GV, Estensoro I, Del Pozo R, Calduch-Giner JA, et al. A novel fish meal-free diet formulation supports proper growth and does not impair intestinal parasite susceptibility in gilthead sea bream (Sparus aurata) with a reshape of gut microbiota and tissue-specific gene expression patterns. Aquaculture. (2022) 558:738362. doi: 10.1016/j.aquaculture.2022.738362
21. Eissa EH, Elbahnaswy S, El-Baz AH, El-Haroun E, Ashour M, Mansour AT, et al. Effects of dietary commercial phytobiotic “Sanacore (R) GM” on Pacific white shrimp (Litopenaeus vannamei) growth, immune response, redux status, intestinal health, and disease resistance against Fusarium solani. Aquacult Int. (2023) 32:3041–60. doi: 10.1007/s10499-023-01310-5
22. Loc T, Nunan L, Redman RM, Mohney LL, Pantoja CR, Fitzsimmons K, et al. Determination of the infectious nature of the agent of acute hepatopancreatic necrosis syndrome affecting penaeid shrimp. Dis Aquat Organ. (2013) 105:45–55. doi: 10.3354/dao02621
23. Bu XY, Li YR, Lai WC, Yao CW, Liu YT, Wang Z, et al. Innovation and development of the aquaculture nutrition research and feed industry in China. Rev Aquac. (2023) 16:759–74. doi: 10.1111/raq.12865
24. Cheng W, Chen JC. Effects of pH, temperature and salinity on immune parameters of the freshwater prawn Macrobrachium rosenbergii. Fish Shellfish Immun. (2000) 10:387–91. doi: 10.1006/fsim.2000.0264
25. Nunan L, Lightner D, Pantoja C, Gomez-Jimenez S. Detection of acute hepatopancreatic necrosis disease (AHPND) in Mexico. Dis Aquat Organ. (2014) 111:81–6. doi: 10.3354/dao02776
26. Yin GJ, Jeney G, Racz T, Xu P, Jun M, Jeney Z. Effect of two Chinese herbs (Astragalus radix and Scutellaria radix) on non-specific immune response of tilapia, Oreochromis niloticus. Aquaculture. (2006) 253:39–47. doi: 10.1016/j.aquaculture.2005.06.038
27. Robertsen B. Modulation of the non-specific defence of fish by structurally conserved microbial polymers. Fish Shellfish Immun. (1999) 9:269–90. doi: 10.1006/fsim.1998.0186
28. Lin YC, Yeh ST, Li CC, Chen LL, Cheng AC, Chen JC. An immersion of Gracilaria tenuistipitata extract improves the immunity and survival of white shrimp Litopenaeus vannamei challenged with white spot syndrome virus. Fish Shellfish Immun. (2011) 31:1239–46. doi: 10.1016/j.fsi.2011.07.021
29. AftabUddin S, Siddique MAM, Romkey SS, Shelton WL. Antibacterial function of herbal extracts on growth, survival and immunoprotection in the black tiger shrimp Penaeus monodon. Fish Shellfish Immun. (2017) 65:52–8. doi: 10.1016/j.fsi.2017.03.050
30. Chaweepack T, Chaweepack S, Muenthaisong B, Ruangpan L, Nagata K, Kamei K. Effect of galangal (Alpinia galanga Linn.) extract on the expression of immune-related genes and Vibrio harveyi resistance in Pacific white shrimp (Litopenaeus vannamei). Aquacult Int. (2015) 23:385–99. doi: 10.1007/s10499-014-9822-2
31. Da Silva BC, Vieira F, Pedreira Mourino JL, Ferreira GS, Seiffert WQ. Salts of organic acids selection by multiple characteristics for marine shrimp nutrition. Aquaculture. (2013) 384:104–10. doi: 10.1016/j.aquaculture.2012.12.017
32. Zheng L, Xie S, Zhuang Z, Liu Y, Tian L, Niu J. Effects of yeast and yeast extract on growth performance, antioxidant ability and intestinal microbiota of juvenile Pacific white shrimp (Litopenaeus vannamei). Aquaculture. (2021) 530:735941. doi: 10.1016/j.aquaculture.2020.735941
33. Zhao L, Wang W, Huang X, Guo T, Wen W, Feng L, et al. The effect of replacement of fish meal by yeast extract on the digestibility, growth and muscle composition of the shrimp. Litopenaeus Vannamei Aquac Res. (2017) 48:311–20. doi: 10.1111/are.2017.48.issue-1
34. Lee CT, Chen IT, Yang YT, Ko TP, Huang YT, Huang JY, et al. The opportunistic marine pathogen Vibrio parahaemolyticus becomes virulent by acquiring a plasmid that expresses a deadly toxin. P Natl Acad Sci USA. (2015) 112:10798–803. doi: 10.1073/pnas.1503129112
35. Romano N, Koh CB, Ng WK. Dietary microencapsulated organic acids blend enhances growth, phosphorus utilization, immune response, hepatopancreatic integrity and resistance against Vibrio harveyi in white shrimp, Litopenaeus vannamei. Aquaculture. (2015) 435:228–36. doi: 10.1016/j.aquaculture.2014.09.037
36. Song C, Liu B, Xu P, Ge X, Zhang H. Emodin ameliorates metabolic and antioxidant capacity inhibited by dietary oxidized fish oil through PPARs and Nrf2-Keap1 signaling in Wuchang bream (Megalobrama amblycephala). Fish Shellfish Immun. (2019) 94:842–51. doi: 10.1016/j.fsi.2019.10.001
37. Ding XY, Wei CY, Liu ZY, Yang HL, Han F, Sun YZ. Autochthonous Bacillus subtilis and Enterococcus faecalis improved liver health, immune response, mucosal microbiota and red-head disease resistance of yellow drum (Nibea albiflora). Fish Shellfish Immun. (2023) 134:108575. doi: 10.1016/j.fsi.2023.108575
38. Zhan M, Xi C, Gong J, Zhu M, Shui Y, Xu Z, et al. 16S rRNA gene sequencing analysis reveals an imbalance in the intestinal flora of Eriocheir sinensis with hepatopancreatic necrosis disease. Comp Biochem Phys D. (2022) 42:100988. doi: 10.1016/j.cbd.2022.100988
39. Zhang R, Shi X, Liu J, Jiang Y, Wu Y, Xu Y, et al. The effects of bamboo leaf flavonoids on growth performance, immunity, antioxidant status, and intestinal microflora of Chinese mitten crabs (Eriocheir sinensis). Anim Feed Sci Tech. (2022) 288:115297. doi: 10.1016/j.anifeedsci.2022.115297
40. He W, Rahimnejad S, Wang L, Song K, Lu K, Zhang C. Effects of organic acids and essential oils blend on growth, gut microbiota, immune response and disease resistance of Pacific white shrimp (Litopenaeus vannamei) against Vibrio parahaemolyticus. Fish Shellfish Immun. (2017) 70:164–73. doi: 10.1016/j.fsi.2017.09.007
41. Butucel E, Balta I, McCleery D, Marcu A, Stef D, Pet I, et al. The prebiotic effect of an organic acid mixture on faecalibacterium prausnitzii metabolism and its anti-pathogenic role against Vibrio parahaemolyticus in Shrimp. Biology-Basel. (2023) 12:57. doi: 10.3390/biology12010057
42. Huang Z, Zeng S, Xiong J, Hou D, Zhou R, Xing C, et al. Microecological Koch’s postulates reveal that intestinal microbiota dysbiosis contributes to shrimp white feces syndrome. Microbiome. (2020) 8:32. doi: 10.1186/s40168-020-00802-3
43. Hou D, Huang Z, Zeng S, Liu J, Wei D, Deng X, et al. Intestinal bacterial signatures of white feces syndrome in shrimp. Appl Microbiol Biot. (2018) 102:3701–9. doi: 10.1007/s00253-018-8855-2
44. Chen G, Liu B, Chen J, Liu H, Tan B, Dong X, et al. Supplementing sulfate-Based alginate polysaccharide improves Pacific white shrimp (Litopenaeus vannamei) fed fishmeal replacement with cottonseed protein concentrate: Effects on growth, intestinal health, and disease resistance. Aquacult Nutr. (2022) 2022:7132362. doi: 10.1155/2022/7132362
Keywords: shrimp, Immunity, hepatopancreas health, intestinal microbiota, Vibrio
Citation: Ma Q, Zhao G, Liu J, Chen I-T, Wei Y, Liang M, Dai P, Nuez-Ortin WG and Xu H (2024) Effects of a phytobiotic-based additive on the growth, hepatopancreas health, intestinal microbiota, and Vibrio parahaemolyticus resistance of Pacific white shrimp, Litopenaeus vannamei. Front. Immunol. 15:1368444. doi: 10.3389/fimmu.2024.1368444
Received: 10 January 2024; Accepted: 16 July 2024;
Published: 09 August 2024.
Edited by:
Samad Rahimnejad, University of Murcia, SpainReviewed by:
Jing Xing, Ocean University of China, ChinaXianyong Bu, Ocean University of China, China
Yun-Zhang Sun, Jimei University, China
Copyright © 2024 Ma, Zhao, Liu, Chen, Wei, Liang, Dai, Nuez-Ortin and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Houguo Xu, eHVoZ0B5c2ZyaS5hYy5jbg==
 Qiang Ma
Qiang Ma Guiping Zhao
Guiping Zhao Jiahao Liu
Jiahao Liu I-Tung Chen
I-Tung Chen Yuliang Wei
Yuliang Wei Mengqing Liang
Mengqing Liang Ping Dai1
Ping Dai1 Waldo G. Nuez-Ortin
Waldo G. Nuez-Ortin Houguo Xu
Houguo Xu